Abstract
The links between chronic kidney disease (CKD) and cardiac conditions such as coronary heart disease or valvular disease are well established in the literature. However, the relationship between hypertrophic cardiomyopathy (HCM) and CKD is not as frequently described or researched. HCM is the most common form of inherited cardiac disease. It is mainly transmitted in an autosomal dominant fashion and caused by mutations in genes encoding sarcomere proteins. HCM is estimated to affect 0.2% of the general population and has an annual mortality rate of between approximately 0.5 and 1%. Our review article aims to summarize the genetics of HCM; discuss the potential clinical mimics that occur concurrently with HCM and CKD, potential interlinks that associate between these two conditions, the role of renal dysfunction as a poor prognostic indicator in HCM; and based on currently available evidence, recommend a management approach that may be suitable when clinicians are faced with this clinical scenario.
1. Introduction
Chronic kidney disease (CKD) is estimated to affect more than 800 million people worldwide and is increasingly being recognized as a public health issue, which burdens healthcare systems worldwide [1,2,3]. All stages of CKD have been shown to be independent risk factors for cardiovascular disease, which may manifest as coronary artery disease, arrhythmia, or sudden cardiac death [4]. Hypertrophic cardiomyopathy (HCM) is the most commonly inherited form of cardiac disease with an estimated prevalence of 1 in 500 in the general population [5]. HCM is primarily inherited in an autosomal dominant fashion and several genes, mostly encoding the sarcomere protein, have been shown to be associated with the disease [6].
The European Society of Cardiology (ESC) describes HCM as “the presence of an increase in left ventricular wall thickness (with or without right ventricular hypertrophy) or mass that is not solely explained by abnormal loading conditions” such as hypertension or valve diseases [7]. Left ventricular hypertrophy (LVH) is, however, very common and in clinical practice, it is sometimes difficult to differentiate HCM from other mimics such as hypertensive heart disease, cardiac amyloidosis, or other genetic diseases such as Fabry disease or Danon disease [8]. Despite being an important cardiac condition to consider, the relationship between HCM and CKD has not been well described in the literature.
2. What Is Hypertrophic Cardiomyopathy?
2.1. Classification of Hypertrophic Cardiomyopathy
HCM is commonly subdivided into an obstructive or a non-obstructive form. In HCM, hypertrophy can affect any part of the left ventricle; however, in two-thirds of cases, it is the interventricular septum that is involved. Hypertrophic obstructive cardiomyopathy (HOCM) is the most common form, estimated to represent 70% of cases [9,10]. Obstruction can typically occur at two levels: at the left ventricular outflow tract or at the midventricular level [11]. The former is thought to result from dynamic systolic anterior motion of the anterior mitral valve leaflet whereas the latter is believed to be caused by obstruction at the papillary muscle level and has been shown to have a strong association with apical aneurysm formation [12,13]. Left ventricular outflow tract obstruction (LVOTO) can be diagnosed on an echocardiogram and is considered to be significant when the peak gradient is >30 mmHg. About one-third of patients have LVOTO at rest and another third of patients have LVOTO after provoked maneuvers [14]. Several studies have linked LVOTO with a higher risk of sudden cardiac death [15].
Non-obstructive HCM (NO-HCM) is characterized by the absence of LVOTO. This accounts for approximately 30% of HCM cases. It is defined by an LVOT gradient of <30 mmHg on an echocardiogram under exertion or at rest. The non-obstructive type can be further subdivided according to the systolic function: normal systolic function vs. impaired systolic function [11]. Previously, it was believed that patients with NO-HCM were at a low risk of mortality compared to patients with HOCM. Recent studies have shown that the long-term mortality of patients with NO-HCM is not significantly different from patients with HOCM [16,17].
2.2. Genetics of Hypertrophic Cardiomyopathy
Sarcomere proteins are the fundamental contractile unit of the cardiac muscle cells and are composed of two main myofilaments: a thick filament (myosin) and a thin filament (actin) [18]. Movement of the two myofilaments against each other results in lengthening or shortening of the sarcomere, resulting in muscle contraction (refer to Figure 1).
HCM is primarily inherited in an autosomal dominant fashion. Several genes have been identified to be the cause of HCM, the most common ones encoding for sarcomere proteins constitute 40% of cases. MYH7, encoding for the β myosin heavy chain (β-MyHC), was the first gene to be discovered in the late 1990s [19]. Together with MYBPC3, which encodes for myosin binding protein C, the two genes, encoding for thick filaments of the sarcomere proteins, are believed to be responsible for the majority of HCM cases associated with sarcomere protein mutations. Other genes encoding for thick filament proteins include MYL2 (regulatory myosin light chain), MYL3 (essential myosin light chain), and MYH6 (myosin heavy chain 6). In total, 5% of cases are due to genes encoding the thin filament components and the following genes have been discovered in relation to these proteins: ACTC1 (cardiac α-actin 1), TNNC1 (troponin/tropomyosin complex formed by cardiac troponin C), TNNI3 (cardiac troponin I), TNNT2 (cardiac troponin T), TPM1 (tropomyosin 1). More rarely, mutations in genes encoding for the Z disc protein (ACTN2, Alpha-actinin-2; MYOZ2, Myozenin-2) as well as the M line proteins (MuRF1— Muscle RING Finger, OBSCN—Obscurin, and MYOM2—Myomesin 2) have been associated with HCM [20,21]. Studies have shown that the presence of a sarcomere protein mutation is a strong predictor of adverse outcomes in patients with HCM [22] (refer to Figure 1).
In the remaining 60% of cases, the causative mutations remain undetermined. However, following the development of DNA sequencing technologies, some mutations in non-sarcomere protein genes have been discovered to be associated with the disease in a small number of patients with HCM and sometimes a combination of mutations has been linked to the disease. Some of the non-sarcomere protein genes include CSRP3 (cysteine- and glycine-rich protein 3), FHL1 (four and a half LIM domains 1), FLNC (filamin C), FHOD3 (formin homology 2 domain-containing 3), JPH2 (junctophilin 2), PLN (phospholamban), TRIM63 (Tripartite Motif-Containing 63), and KLHL24 (Kelch-like protein 24) [23].
Phenotypes of HCM are varied—ranging from patients who have a gene mutation but are asymptomatic and have no clinical manifestation of the disease to patients who have significant hypertrophy with left ventricular outflow tract obstruction, resulting in end-stage heart failure. This has especially been noted in families where members who share common mutations display different levels of cardiac hypertrophy [24,25].
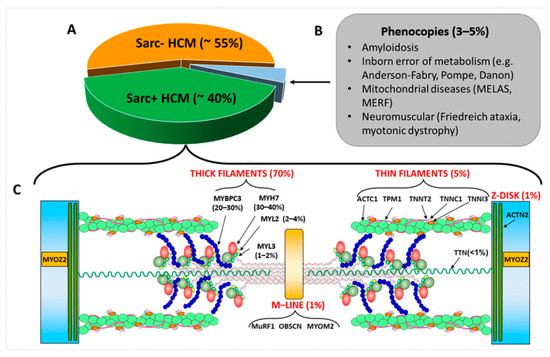

Figure 1.
(A): Percentage of sarcomere-mutation-positive and sarcomere-mutation-negative HCM and of phenocopies; the latter are listed in Panel (B). (C): sarcomeric genes associated with sarcomere-positive HCM with the relative percentages of affected individuals. The image also shows the coded proteins and their localization in the sarcomere. Abbreviation as in the text from Borrelli et al. [23].
2.3. Mimics of Hypertrophic Cardiomyopathy
Left ventricular hypertrophy (LVH) is estimated to be present in about 15–20% of the general population [26,27]. A variety of conditions can cause LVH and are sometimes referred to as mimics of HCM. Some examples include hypertension where LVH happens due to a chronic workload of the heart, due to a pressure overload induced by systemic hypertension; chronic kidney disease; physiological LVH, which is reported to occur in about 2% of athletes; and several genetic conditions, which can resemble the HCM phenotype in the absence of a sarcomere protein mutation (Danon disease, PRKAG2 cardiomyopathy, Pompe disease, Forbes disease), lysosomal storage disorders (Anderson–Fabry disease), cardiac amyloidosis, mitochondrial cytopathies, and other inborn metabolic disorders’ infiltrative cardiac processes [28,29,30]. In clinical practice, it can sometimes be challenging to differentiate HCM from these other causes of LVH, but it is crucial to do so, as the appropriate management strategy for the various scenarios may be different [31].
2.4. Diagnosing Hypertrophic Cardiomyopathy
As described, HCM can be difficult to differentiate from other conditions of a similar clinical presentation. The ESC recommends a systematic and multiparametric approach toward the clinical diagnosis of cardiomyopathy. This includes history and examination, pedigree analysis, resting 12-lead electrocardiogram, ambulatory electrocardiogram monitoring, blood tests, and multimodality imaging (echocardiogram, cardiac magnetic resonance imaging, computer tomography, and nuclear medicine-based techniques). Finally, as HCM is known to be a mostly inherited condition, a genetic analysis plays an important role in the diagnostic process [32].
Common symptoms reported on initial presentation may include shortness of breath, chest pain, palpitations, presyncope, and syncope, although some individuals can be asymptomatic. A number of non-cardiac symptoms and signs can also point toward HCM mimics, for example, learning difficulties or visual impairment, which can be associated with conditions such as Fabry’s or mitochondrial cytopathies. Family history is essential to establish if there is a Mendelian pattern of inheritance but also to determine if other family members are at risk of the disease. HCM is typically acquired in an autosomal dominant fashion and family history pointing toward other modes of inheritance would be suggestive of other HCM mimics. For instance, X-linked transmission should be suspected if there is a family history of males being strongly affected and can also point toward other mimics such as Fabry or Danon disease [33].
An electrocardiogram would be an ideal initial investigation to screen for HCM. Common findings are patterns of left ventricular hypertrophy or arrhythmias detected on resting or ambulatory electrocardiogram monitoring [32].
Non-invasive imaging is considered the backbone of the diagnosis and follow up in patients with HCM. An echocardiogram should assess for left ventricular hypertrophy, LVOTO, systolic anterior motion, systolic and diastolic dysfunction, as well as left atrial size. Cardiac magnetic resonance or nuclear imaging may also assist in the diagnosis of HCM via their ability to better characterize cardiac tissue. They can provide important etiological clues pointing toward HCM mimics including physiological LVH. With a higher image resolution and ability to identify hypertrophied areas not visualized on an echocardiogram, cardiac magnetic resonance imaging is emerging with consideration as the gold standard modality in the diagnostic assessment of HCM. It has been deemed capable of differentiating between types of cardiomyopathies with a sensitivity and specificity of up to 87% and 82%, respectively [34]. Findings on cardiac magnetic resonance imaging can sometimes provide important cues regarding the etiology of cardiomyopathy. For instance, findings of patchy mid-wall enhancement in hypertrophied areas are characteristic of sarcomeric HCM whereas posterolateral late gadolinium enhancement and concentric left ventricular hypertrophy can point toward a diagnosis of Fabry disease instead. Diffuse subendocardial late gadolinium enhancement on the other hand can raise suspicion of cardiac amyloidosis. On the other hand, nuclear medicine techniques can also be helpful in identifying conditions such as amyloidosis and sarcoidosis [33,35,36,37].
Finally, genetic testing plays a pivotal role and in the case of HCM, genes robustly associated with the presenting phenotype should be investigated first. Genetic testing that aids in confirming the diagnosis may inform the prognosis and appropriate management, and future family planning.
3. Association between Hypertrophic Cardiomyopathy and Chronic Kidney Disease
S.D., H.H.L.W., and R.C. each independently conducted an electronic search on the PubMed database using the following key words: ‘Hypertrophic Cardiomyopathy’ AND ‘Chronic Kidney Disease’. It was concurred that this search yielded a total of 122 articles. After scanning through titles and abstracts, several articles, which were not directly applicable to the research topic of this review paper, were excluded. Exclusion criteria included studies that were conducted in populations with HCM mimics such as Fabry disease, mitochondrial cardiomyopathy, or Danon disease, studies conducted in animal models, and studies that did not look at the association between HCM and CKD. This narrowed down the search to nine articles, which were analyzed in more extensive detail. Current conclusions from available studies suggest that a bidirectional relationship exists between HCM and CKD. A summary of the studies that have evaluated the association between HCM and CKD from our literature search is presented in Table 1.

Table 1.
Summary of studies looking at the association between HCM and CKD.
A nationwide population-based cohort study conducted in South Korea, which investigated the incidence of end-stage kidney disease (ESKD) during the follow up in 10,300 patients with HCM compared to 51,500 age- and sex-matched controls, found that the incidence rate of ESKD was higher in the HCM group (4.14 per 1000) compared to the control group (0.60 per 1000). Additionally, the study found that the HCM group had a lower glomerular filtration rate than the control group (80.7 vs. 87.0 mL/min/1.73 m2). The study also highlighted that HCM was an independent predictor for ESKD development when adjusted for other generally accepted prognosticators such as age, hypertension, diabetes, pre-existing renal disease, or prior use of a renin–angiotensin system blocker. Finally, the study also highlighted those patients with HCM under the age of 60 had a 20-fold increased risk for incident ESKD. This study, published in 2019, claimed to be the first epidemiologic study to demonstrate the link between renal impairment and HCM. Of note, in this paper, HCM was defined by a diagnostic code given after reviewing clinical records and imaging modalities, which was validated by a previous study and was considered reliable by the authors of the paper [38].
Another single-center cohort study of 581 patients diagnosed with HCM reported that 15% of patients with HCM had moderate to severe renal dysfunction. In this study, the estimated glomerular filtration rate (eGFR) was strongly associated with the prognosis in patients with HCM. The all-cause mortality rate was found to increase with declining eGFR and was reported to be 9.4% in patients with an eGFR < 60 mL/min/1.73 m2 compared to 1.9% in patients with an eGFR > 60 mL/min/1.73 m2. Of note, this association between renal dysfunction and all-cause mortality was found to be significant among patients under the age of 55. In this cohort study, HCM was defined by echocardiography findings of left ventricular wall thickness ≥15 mm in the absence of any cardiac or systemic etiology that could explain hypertrophy, a similar definition to the one from the European Society of Cardiology [39]. Similarly, the association between renal dysfunction and prognostic values was reported in another paper. This study, which included 450 patients with HCM who were followed up with over a median period of 8.8 years, found that patients with eGFR < 60 mL/min/1.73m2 had a higher risk of sudden death compared to patients without renal dysfunction. Similarly, in this study, HCM was diagnosed using echocardiographic criteria [40].
When looking at patients with CKD who progressed to ESKD, and more specifically the dialysis population, few studies have investigated the presence of left ventricular hypertrophy and HCM. In a comparative study published in 1994, patients on hemodialysis were found to have a left ventricular diastolic dysfunction similar to what is observed in patients with hypertensive heart disease but was less severe than that found in patients with HCM. However, this study was limited by its small sample size [41]. Another prospective study, which followed 150 dialysis patients with congestive heart failure over a period of 3 to 5 years, found that the survival rate was worse in patients with an echocardiographic diagnosis of dilated cardiomyopathy (67% 2-year survival rate) compared to patients with a normal echocardiogram finding (90% 2-year survival rate). Moreover, in patients with hypertrophic hyperkinetic cardiomyopathy, the 2-year survival rate was 30% after the start of the study, and 43% after the first congestive heart failure episode [42]. Finally, a comparative study published in 1983 hypothesized hypertrophic cardiomyopathy to be an acquired disease of ESKD. It looked at the results of echocardiograms of 52 patients on hemodialysis and reported 9.6% of patients to have asymmetric septal hypertrophy (ASH) of the heart, considered a specific indicator of hypertrophic cardiomyopathy. It also reported one case of the development of ASH over time by analyzing serial echocardiogram images. In this study, patients with ASH were found to have more frequent hypotensive episodes on dialysis. Limitations of this paper include its small sample size as well as an old-fashioned definition of HCM with echocardiographic criteria different to the one used nowadays [43]. A similar finding was also present in a case series of 10 pediatric patients on hemodialysis, which reported an impressive 40% incidence of echocardiographic features of HCM in the studied patients. This paper was published in 1981 and like the previous paper, it is limited by its small sample size and defined HCM by echocardiographic findings of ASH [44]. Another small study looked at a subset of four patients with refractory heart failure and recurrent episodes of hypotension on dialysis and reported that these patients have echocardiographic features of HCM with preserved systolic function and that the use of verapamil was associated with a reduction in the number of hypotensive episodes on dialysis. The authors of this study reported that the potential benefit from calcium channel blockade might be related to the prolongation of left ventricular diastolic relaxation time, which in turn caused an increase in filling of the left ventricle and systolic ejection volume [45].
Interestingly, we also found a study of 24 patients with ESKD and LVH whose echocardiograms were compared before and after starting peritoneal dialysis. In this study, seven patients showed features of asymmetric septal hypertrophy and after initiation of peritoneal dialysis, the degree of septal hypertrophy was reported to have been reduced [46].
Most of the studies that looked at the association between HCM and CKD that we reviewed were limited by their small sample sizes and their definition of HCM, which did not always match the most recent one, for instance, the one used by the European Society of Cardiology. Additionally, out of nine papers, five of them were published before 1995. Larger prospective studies are therefore required in order to confirm and quantify the relationship between the two diseases.
Although the mechanisms that may relate chronic kidney disease to HCM have not fully been established, they are likely to be multifactorial. Left ventricular diastolic dysfunction in various cardiovascular diseases has often been linked to renal impairment [47]. By increasing left ventricular filling pressure, central venous pressure is subsequently increased, leading to renal resistance and renal venous hypertension, which results in impaired renal function. Additionally, in the case of HCM with LVOTO, chronic renal hypoperfusion and hemodynamic instability have been hypothesized to cause chronic renal injury, which can also result in progressive renal impairment. Finally, due to its association with thromboembolism-associated renal infarction, atrial fibrillation, which is one of the most common arrhythmias present in HCM, is also believed to be part of the mechanisms leading to renal impairment in patients with HCM [38,39].
During our literature search, we came across a few studies looking at the association between Fabry’s disease (FD), a common HCM mimic, and CKD. A multicentric study involving 10 referral centers in India looked at 54 patients from 37 families of proven FD. In total, 70.8% of patients were found to have renal impairment, which was described as the predominant manifestation of the disease [48]. In a cross-sectional study, all adult patients undergoing dialysis in Western Australia without previously known FD were screened for the disease as they were deemed to be high-risk. The analysis of this population showed a prevalence of 0% undiagnosed FD [49]. Similarly, a single-center study in Taiwan conducted FD screening in male patients above 20 years of age from a pre-ESKD dialysis program and found a prevalence rate of FD at 0.16% [50].
4. Renal Dysfunction as a Poor Prognostic Indicator in Hypertrophic Cardiomyopathy
As mentioned previously, a few studies have reported lower eGFR as being a poor prognostic factor in HCM and associated with an increase in all-cause mortality. This is not surprising as CKD by itself has been linked to an increased risk for all-cause and cardiovascular death in several studies [51]. Additionally, all stages of CKD have been shown to be independent risk factors for cardiovascular disease, which can manifest as coronary artery disease, arrhythmia, or sudden cardiac death [4].
Progression of kidney disease leads to cardiac remodeling including the development of left ventricular hypertrophy, and studies have demonstrated that lower eGFR is associated with increased left ventricular wall thickness [52,53]. In hypertrophic cardiomyopathy, hypertrophy of the ventricle is already present, and one can note how a declining eGFR could exacerbate the degree of ventricular hypertrophy and increase the risk of adverse outcomes. Additionally, hypertension is commonly present in patients with CKD and is known to promote a hypercontractile cardiac state, which can induce hypertrophy of the ventricle in patients with HCM [54]. Further studies looking at renal function in the HCM population specifically and its association with adverse outcomes are needed to establish the relationship between the two conditions.
5. Management of Hypertrophic Cardiomyopathy in Chronic Kidney Disease
The standard approach toward the management of HCM is usually categorized between the management of symptomatic patients with LVOTO and those without LVOTO.
Patients with LVOTO can be managed medically or surgically. First-line medical management may include beta-blockers or non-dihydropyridine calcium channel blockers uptitrated to the maximum dose. Disopyramide and Macavatam (cardiac myosin ATPase inhibitors) also have a role in the treatment of patients who remain symptomatic following first-line treatment. Invasive treatment in the form of surgery (ventricular septal myectomy) or septal alcohol ablation to reduce LVOTO is to be considered in patients in the following cases: LVOTO gradient ≥ 50 mmHg, severe symptoms (New York Heart Association functional class III–IV heart failure), and/or exertional or unexplained recurrent syncope despite maximum tolerated medical therapy [55].
Management of symptomatic patients without LVOTO focuses on the treatment of heart failure, arrhythmias, and chest pain. This is achieved via the usual drug therapy recommendations for the treatment of angina and heart failure. Additionally, there is a role for cardiac resynchronization therapy as well as implantable cardiac cardioverter defibrillators in certain cases for the prevention of sudden cardiac death [56].
When it comes to therapies that can be useful to the management of patients with HCM without LVOTO as well as CKD, several drugs used in the treatment of heart failure have been shown to have renoprotective effects, e.g., renin angiotensin aldosterone system antagonists (RAASi) and sodium–glucose co-transporter 2 inhibitors (SGLT2i).
RAASi such as angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, and angiotensin receptor-neprilysin inhibitors play an important role in heart failure by decreasing the left ventricular afterload, increasing cardiac output, and decreasing both right and left heart filling pressures [57]. Randomized control trials have shown their benefit in the management of chronic heart failure, especially heart failure with a reduced ejection fraction, and demonstrated the benefits of these drugs in symptom control and survival [58]. Similarly, RAASi have been shown to reduce urinary albumin excretion and optimize blood pressure control, resulting in delayed progression of CKD to ESKD [59]. Common side effects of RAASi include a reduction in eGFR or hyperkalemia, causing them to be underused in patients with ESKD. Whilst the benefit of RAASi has been demonstrated in CKD 2-3, the benefit in the later CKD stages is conflicting due to this population not being fully represented in studies [60,61,62].
SGLT2i were originally developed for the management of diabetes, but several studies have shown their benefit across many patient sub-groups, including those with and without type 2 diabetes and at different stages of CKD, and in patients with heart failure with a preserved or reduced ejection fraction [63].
When it comes to patients with ESKD, the impact of dialysis on LVH has been studied and multiple randomized control studies have shown an intensive-hemodialysis-reduced left ventricular mass, a measure of LVH [64]. However, the exact impact of dialysis on patients with HCM is not fully known.
6. Conclusions
From our review, renal dysfunction in patients with HCM was a common finding and associated with adverse outcomes including increasing the risk of mortality. Patients on dialysis with HCM were found to have worse clinical outcomes. These findings highlight the importance of monitoring renal function in the HCM population. However, most studies were conducted three decades ago, and their definition of HCM did not always match the one that is defined nowadays. Larger prospective studies looking at renal dysfunction in patients with HCM are needed to confirm and quantify this association. Additionally, it would be interesting to study the role of dialysis in the HCM population with ESKD in order to determine if it has any impact on left ventricular hypertrophy.
Author Contributions
Conceptualization, S.D., H.H.L.W. and R.C.; methodology, S.D., H.H.L.W. and R.C.; validation, H.H.L.W. and R.C.; investigation, S.D.; resources, S.D.; writing—original draft preparation, S.D.; writing—review and editing, S.D., H.H.L.W., M.D. and R.C.; visualization, H.H.L.W., M.D. and R.C.; supervision, R.C.; project administration, R.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hill, N.R.; Fatoba, S.T.; Oke, J.L.; Hirst, J.A.; O’Callaghan, C.A.; Lasserson, D.S.; Hobbs, F.D.R. Global Prevalence of Chronic Kidney Disease—A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0158765. [Google Scholar] [CrossRef] [PubMed]
- Jankowski, J.; Floege, J.; Fliser, D.; Bohm, M.; Marx, N. Cardiovascular Disease in Chronic Kidney Disease. Circulation 2021, 143, 1157–1172. [Google Scholar] [CrossRef]
- Kovesdy, C.P. Epidemiology of chronic kidney disease: An update 2022. Kidney Int. Suppl. 2022, 12, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Van Biesen, W.; De Bacquer, D.; Verbeke, F.; Delanghe, J.; Lameire, N.; Vanholder, R. The glomerular filtration rate in an apparently healthy population and its relation with cardiovascular mortality during 10 years. Eur. Heart J. 2007, 28, 478–483. [Google Scholar] [CrossRef] [PubMed]
- McKenna, W.J.; Judge, D.P. Epidemiology of the inherited cardiomyopathies. Nat. Rev. Cardiol. 2021, 18, 22–36. [Google Scholar] [CrossRef] [PubMed]
- Richard, P.; Charron, P.; Carrier, L.; Ledeuil, C.; Cheav, T.; Pichereau, C.; Benaiche, A.; Isnard, R.; Dubourg, O.; Burban, M.; et al. Hypertrophic cardiomyopathy: Distribution of disease genes, spectrum of mutations, and implications for a molecular diagnosis strategy. Circulation 2003, 107, 2227–2232. [Google Scholar] [CrossRef] [PubMed]
- Elliott, P.; Andersson, B.; Arbustini, E.; Bilinska, Z.; Cecchi, F.; Charron, P.; Dubourg, O.; Kühl, U.; Maisch, B.; McKenna, W.J.; et al. Classification of the cardiomyopathies: A position statement from the European Society Of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2008, 29, 270–276. [Google Scholar] [CrossRef]
- Sankaranarayanan, R.; Fleming, E.J.; Garratt, C.J. Mimics of Hypertrophic Cardiomyopathy—Diagnostic Clues to Aid Early Identification of Phenocopies. Arrhythmia Electrophysiol. Rev. 2013, 2, 36–40. [Google Scholar] [CrossRef]
- Raj, M.A.; Ranka, S.; Goyal, A. Hypertrophic Obstructive Cardiomyopathy; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Prinz, C.; Farr, M.; Hering, D.; Horstkotte, D.; Faber, L. The diagnosis and treatment of hypertrophic cardiomyopathy. Dtsch. Arztebl. Int. 2011, 108, 209–215. [Google Scholar] [CrossRef]
- Albakri, A. Hypertrophic cardiomyopathy: A review of literature on clinical status and meta-analysis of diagnosis and clinical management methods. Clin. Med. Investig. 2018, 3, 3–16. [Google Scholar] [CrossRef]
- Wigle, E.D.; Rakowski, H.; Kimball, B.P.; Williams, W.G. Hypertrophic cardiomyopathy. Clinical spectrum and treatment. Circulation 1995, 92, 1680–1692. [Google Scholar] [CrossRef]
- Todde, G.; Canciello, G.; Borrelli, F.; Perillo, E.F.; Esposito, G.; Lombardi, R.; Losi, M.A. Diagnosis and Treatment of Obstructive Hypertrophic Cardiomyopathy. Cardiogenetics 2023, 13, 75–91. [Google Scholar] [CrossRef]
- European Society of Cardiology. Available online: https://www.escardio.org/Councils/Council-on-Cardiovascular-Genomics/Cardiovascular-Genomics-Insight/Volume-1/how-to-measure-intraventricular-obstruction-in-hypertrophic-cardiomyopathy (accessed on 10 December 2023).
- Jordà, P.; García-Álvarez, A. Hypertrophic cardiomyopathy: Sudden cardiac death risk stratification in adults. Glob. Cardiol. Sci. Pract. 2018, 2018, 25. [Google Scholar] [CrossRef] [PubMed]
- Pelliccia, F.; Pasceri, V.; Limongelli, G.; Autore, C.; Basso, C.; Corrado, D.; Imazio, M.; Rapezzi, C.; Sinagra, G.; Mercuro, G.; et al. Long-term outcome of nonobstructive versus obstructive hypertrophic cardiomyopathy: A systematic review and meta-analysis. Int. J. Cardiol. 2017, 243, 379–384. [Google Scholar] [CrossRef]
- Pozios, I.; Corona-Villalobos, C.; Sorensen, L.L.; Bravo, P.E.; Canepa, M.; Pisanello, C.; Pinheiro, A.; Dimaano, V.L.; Luo, H.; Dardari, Z.; et al. Comparison of Outcomes in Patients With Nonobstructive, Labile-Obstructive, and Chronically Obstructive Hypertrophic Cardiomyopathy. Am. J. Cardiol. 2015, 116, 938–944. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, O. Direct Sarcomere Modulators Are Promising New Treatments for Cardiomyopathies. Int. J. Mol. Sci. 2020, 21, 226. [Google Scholar] [CrossRef]
- Geisterfer-Lowrance, A.A.; Kass, S.; Tanigawa, G.; Vosberg, H.P.; McKenna, W.; Seidman, C.E.; Seidman, J.G. A molecular basis for familial hypertrophic cardiomyopathy: A beta cardiac myosin heavy chain gene missense mutation. Cell 1990, 62, 999–1006. [Google Scholar] [CrossRef]
- Marian, A.J. Molecular Genetic Basis of Hypertrophic Cardiomyopathy. Circulation 2021, 128, 1533–1553. [Google Scholar] [CrossRef]
- Alfares, A.; Kelly, M.; McDermott, G.; Funke, B.H.; Lebo, M.S.; Baxter, S.B.; Shen, J.; McLaughlin, H.M.; Clark, E.H.; Babb, L.J.; et al. Results of clinical genetic testing of 2912 probands with hypertrophic cardiomyopathy: Expanded panels offer limited additional sensitivity. Genet. Med. 2015, 17, 880–888. [Google Scholar] [CrossRef]
- Ho, C.Y.; Day, S.M.; Ashley, E.A.; Michels, M.; Pereira, A.C.; Jacoby, D.; Cirino, A.L.; Fox, J.C.; Lakdawala, N.K.; Ware, J.S.; et al. Genotype and Lifetime Burden of Disease in Hypertrophic Cardiomyopathy: Insights from the Sarcomeric Human Cardiomyopathy Registry (SHaRe). Circulation 2018, 138, 1387–1398. [Google Scholar] [CrossRef]
- Borrelli, F.; Losi, M.A.; Canciello, G.; Todde, G.; Perillo, E.F.; Ordine, L.; Frisso, G.; Esposito, G.; Lombardi, R. Sarcomeric versus Non-Sarcomeric HCM. Cardiogenetics 2023, 13, 92–105. [Google Scholar] [CrossRef]
- Rowin, E.J.; Maron, M.S.; Chan, R.H.; Hausvater, A.; Wang, W.; Rastegar, H.; Maron, B.J. Interaction of Adverse Disease Related Pathways in Hypertrophic Cardiomyopathy. Am. J. Cardiol. 2017, 120, 2256–2264. [Google Scholar] [CrossRef]
- Rowin, E.; Maron, B.; Carrick, R.; Patel, P.P.; Koethe, B.; Wells, S.; Maron, M.S. Outcomes in Patients With Hypertrophic Cardiomyopathy and Left Ventricular Systolic Dysfunction. J. Am. Coll. Cardiol. 2020, 75, 3033–3043. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.R. Left ventricular hypertrophy: Its Prevalence, Etiology, and Significance. Clin. Cardiol. 1991, 14, 13–17. [Google Scholar] [CrossRef]
- Schirmer, H.; Lunde, P.; Rasmussen, K. Prevalence of left ventricular hypertrophy in a general population; The Tromsø Study. Eur. Heart J. 1999, 20, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Bornstein, A.B.; Rao, S.S.; Marwaha, K. Left Ventricular Hypertrophy; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Ruilope, L.M.; Schmieder, R.E. Left Ventricular Hypertrophy and Clinical Outcomes in Hypertensive Patients. Am. J. Hypertens. 2008, 21, 500–508. [Google Scholar] [CrossRef]
- Maron, B.J.; Pelliccia, A.; Spirito, P. Cardiac disease in young trained athletes. Insights into methods for distinguishing athlete’s heart from structural heart disease, with particular emphasis on hypertrophic cardiomyopathy. Circulation 1995, 91, 1596–1601. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.C.; Rohan, S.; Dastidar, A.G.; Burchell, A.E.; Ratcliffe, L.E.; Hart, E.C.; Paton, J.F.; Hamilton, M.; Nightingale, A.K.; Manghat, N.E. Hypertensive heart disease versus hypertrophic cardiomyopathy: Multi-parametric CMR predictors beyond end-diastolic wall thickness ≥15 mm. J. Cardiovasc. Magn. Reason. 2016, 18, 264. [Google Scholar] [CrossRef]
- Rapezzi, C.; Arbustini, E.; Caforio, A.L.; Charron, P.; Gimeno-Blanes, J.; Heliö, T.; Linhart, A.; Mogensen, J.; Pinto, Y.; Ristic, A.; et al. Diagnostic work-up in cardiomyopathies: Bridging the gap between clinical phenotypes and final diagnosis. A position statement from the ESC Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2013, 34, 1448–1458. [Google Scholar] [CrossRef]
- Arbelo, E.; Protonotarios, A.; Gimeno, J.R.; Arbustini, E.; Barriales-Villa, R.; Basso, C.; Bezzina, C.R.; Biagini, E.; Blom, N.A.; de Boer, R.A.; et al. 2023 ESC Guidelines for the management of cardiomyopathies. Eur. Heart J. 2023, 44, 3503–3626. [Google Scholar] [CrossRef]
- Mayala, H.A.; Bakari, K.H.; Wang, Z. The role of cardiac magnetic resonance (CMR) in the diagnosis of cardiomyopathy: A systematic review. Malawi Med. J. 2018, 30, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Mitevksa, I.P. Focus on echocardiography in hypertrophic cardiomyopathy—Fourth in series. E-J. Counc. Cardiol Pract. 2015, 13, 20. [Google Scholar]
- Brenes, J.C.; Doltra, A.; Prat, S. Cardiac magnetic resonance imaging in the evaluation of patients with hypertrophic cardiomyopathy. Glob. Cardiol. Sci. Pract. 2018, 2018, 22. [Google Scholar] [CrossRef]
- Sivalokanathan, S. The Role of Cardiovascular Magnetic Resonance Imaging in the Evaluation of Hypertrophic Cardiomyopathy. Diagnostics 2022, 12, 314. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Han, K.; Park, J.B.; Hwang, I.C.; Yoon, Y.E.; Park, H.E.; Choi, S.Y.; Kim, Y.J.; Cho, G.Y.; Kim, H.K.; et al. Risk of end-stage renal disease in patients with hypertrophic cardiomyopathy: A nationwide population-based cohort study. Sci. Rep. 2019, 9, 14565. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.Y.; Zhang, J.L.; Huang, B.T.; Peng, Y.; Chen, S.J.; Chen, M. Renal function as a predictor of outcomes in patients with hypertrophic cardiomyopathy: A cohort study of a hospitalized population. Clin. Chim. Acta Int. J. Clin. Chem. 2021, 512, 92–99. [Google Scholar] [CrossRef]
- Higuchi, S.; Minami, Y.; Shoda, M.; Shirotani, S.; Kanai, M.; Kataoka, S.; Yazaki, K.; Saito, C.; Haruki, S.; Yagishita, D.; et al. Effect of Renal Dysfunction on Risk of Sudden Cardiac Death in Patients With Hypertrophic Cardiomyopathy. Am. J. Cardiol. 2021, 144, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, S.; Kagoshima, T.; Hashimoto, T.; Nakajima, T.; Dohi, K. Left ventricular diastolic function in patients on maintenance hemodialysis: Comparison with hypertensive heart disease and hypertrophic cardiomyopathy. Clin. Nephrol. 1994, 42, 109–116. [Google Scholar]
- Parfrey, P.S.; Griffiths, S.M.; Harnett, J.D.; Taylor, R.; King, A.; Hand, J.; Barre, P.E. Outcome of congestive heart failure, dilated cardiomyopathy, hypertrophic hyperkinetic disease, and ischemic heart disease in dialysis patients. Am. J. Nephrol. 1990, 10, 213–221. [Google Scholar] [CrossRef]
- Klein, J.; McLeish, K.; Hodsden, J.; Lordon, R. Hypertrophic cardiomyopathy: An acquired disorder of end–stage renal disease. Trans. Am. Soc. Artif. Intern. Organs 1983, 29, 120–123. [Google Scholar]
- Drukker, A.; Urbach, J.; Glaser, J. Hypertrophic cardiomyopathy in children with end-stage renal disease and hypertension. Proc. Eur. Dial. Transpl. Assoc. 1981, 18, 542–547. [Google Scholar]
- Whelton, P.K.; Watson, A.J.; Kone, B.; Fortuin, N.J. Calcium channel blockade in dialysis patients with left ventricular hypertrophy and well-preserved systolic function. J. Cardiovasc. Pharmacol. 1987, 10, 185–186. [Google Scholar] [CrossRef]
- Timio, M.; Martini, F.; Venanzi, S.; Ronconi, M.; Lippi, G.; Pippi, C. La funzione ventricolare sinistra nei pazienti in trattamento dialitico peritoneale [Left ventricular function in patients under peritoneal dialysis treatment]. G. Ital. Di Cardiol. 1984, 14, 570–576. [Google Scholar]
- Verma, A.; Anavekar, N.S.; Meris, A.; Thune, J.J.; Arnold, J.M.; Ghali, J.K.; Velazquez, E.J.; McMurray, J.J.; Pfeffer, M.A.; Solomon, S.D. The relationship between renal function and cardiac structure, function, and prognosis after myocardial infarction: The VALIANT Echo Study. J. Am. Coll. Cardiol. 2007, 50, 1238–1245. [Google Scholar] [CrossRef] [PubMed]
- Nampoothiri, S.; Yesodharan, D.; Bhattacherjee, A.; Ahamed, H.; Puri, R.D.; Gupta, N.; Kabra, M.; Ranganath, P.; Bhat, M.; Phadke, S.; et al. Fabry disease in India: A multicenter study of the clinical and mutation spectrum in 54 patients. JIMD Rep. 2020, 56, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Jahan, S.; Sarathchandran, S.; Akhter, S.; Goldblatt, J.; Stark, S.; Crawford, D.; Mallett, A.; Thomas, M. Prevalence of Fabry disease in dialysis patients: Western Australia Fabry disease screening study—The FoRWARD study. Orphanet J. Rare Dis. 2020, 15, 10. [Google Scholar] [CrossRef] [PubMed]
- Shih, C.Y.; You, Z.H.; Tsai, S.F.; Wu, M.J.; Yu, T.M.; Chuang, Y.W.; Chen, C.H. Diagnosis and Management of Fabry Disease in High-Risk Renal Disease Patients in Taiwan: A Single Center Study. Transplant. Proc. 2023, 55, 788–791. [Google Scholar] [CrossRef]
- Tonelli, M.; Wiebe, N.; Culleton, B.; House, A.; Rabbat, C.; Fok, M.; McAlister, F.; Garg, A. Chronic Kidney Disease and Mortality Risk: A Systematic Review. J. Am. Soc. Nephrol. 2006, 17, 2034–2047. [Google Scholar] [CrossRef]
- Izumaru, K.; Hata, J.; Nakano, T.; Nakashima, Y.; Nagata, M.; Fukuhara, M.; Oda, Y.; Kitazono, T.; Ninomiya, T. Reduced Estimated GFR and Cardiac Remodeling: A Population-Based Autopsy Study. Am. J. Kidney Dis. 2019, 74, 373–381. [Google Scholar] [CrossRef]
- Moran, A.; Katz, R.; Jenny, N.S.; Astor, B.; Bluemke, D.A.; Lima, J.A.; Siscovick, D.; Bertoni, A.G.; Shlipak, M.G. Left ventricular hypertrophy in mild and moderate reduction in kidney function determined using cardiac magnetic resonance imaging and cystatin C: The multi-ethnic study of atherosclerosis (MESA). Am. J. Kidney Dis. 2008, 52, 839–848. [Google Scholar] [CrossRef]
- Mace, H.; Rizwan, A.; Lutz, W.; Hamid, A.; Campbell, W.F.; McMullan, M.R.; Hall, M.E. Hypertension in patients with hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2023, 81, 1605. [Google Scholar] [CrossRef]
- UpToDate. Available online: https://www.uptodate.com/contents/hypertrophic-cardiomyopathy-management-of-patients-with-outflow-tract-obstruction?search=hcm%20management (accessed on 11 December 2023).
- UpToDate. Available online: https://www.uptodate.com/contents/hypertrophic-cardiomyopathy-management-of-patients-without-outflow-tract-obstruction?search=hcm%20management&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1 (accessed on 11 December 2023).
- UpToDate. Available online: https://www.uptodate.com/contents/renal-effects-of-ace-inhibitors-in-heart-failure (accessed on 11 December 2023).
- Singhania, N.; Bansal, S.; Mohandas, S.; Nimmatoori, D.P.; Ejaz, A.A.; Singhania, G. Role of renin-angiotensin-aldosterone system inhibitors in heart failure and chronic kidney disease. Drugs Context 2020, 9. [Google Scholar] [CrossRef]
- Cho, I.J.; Kang, S.M. Angiotensin receptor-neprilysin inhibitor in patients with heart failure and chronic kidney disease. Kidney Res. Clin. Pract. 2021, 40, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Weir, M.R.; Lakkis, J.I.; Jaar, B.; Rocco, M.V.; Choi, M.J.; Kramer, H.J.; Ku, E. Use of Renin-Angiotensin System Blockade in Advanced CKD: An NKF-KDOQI Controversies Report. Am. J. Kidney Dis. 2018, 72, 873–884. [Google Scholar] [CrossRef]
- Walther, C.P.; Winkelmayer, W.C.; Richardson, P.A.; Virani, S.S.; Navaneethan, S.D. Renin-angiotensin system blocker discontinuation and adverse outcomes in chronic kidney disease. Nephrol. Dial. Transplant. 2021, 36, 1893–1899. [Google Scholar] [CrossRef] [PubMed]
- Mukoyama, M.; Kuwabara, T. Role of renin-angiotensin system blockade in advanced CKD: To use or not to use? Hypertens. Res. 2022, 45, 1072–1075. [Google Scholar] [CrossRef]
- Van der Aart-van der Beek, A.B.; de Boer, R.A.; Heerspink, H.J.L. Kidney and heart failure outcomes associated with SGLT2 inhibitor use. Nat. Rev. Nephrol. 2022, 18, 294–306. [Google Scholar] [CrossRef]
- McCullough, P.A.; Chan, C.T.; Weinhandl, E.D.; Burkart, J.M.; Bakris, G.L. Intensive Hemodialysis, Left Ventricular Hypertrophy, and Cardiovascular Disease. Am. J. Kidney Dis. 2016, 68, 5–14. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).