Association of GSTT1, GSTM1 and GSTP1 (Ile105Val) mRNA Expression with Cardiometabolic Risk Parameters in Women with Breast Cancer and Comorbidities
Abstract
1. Introduction
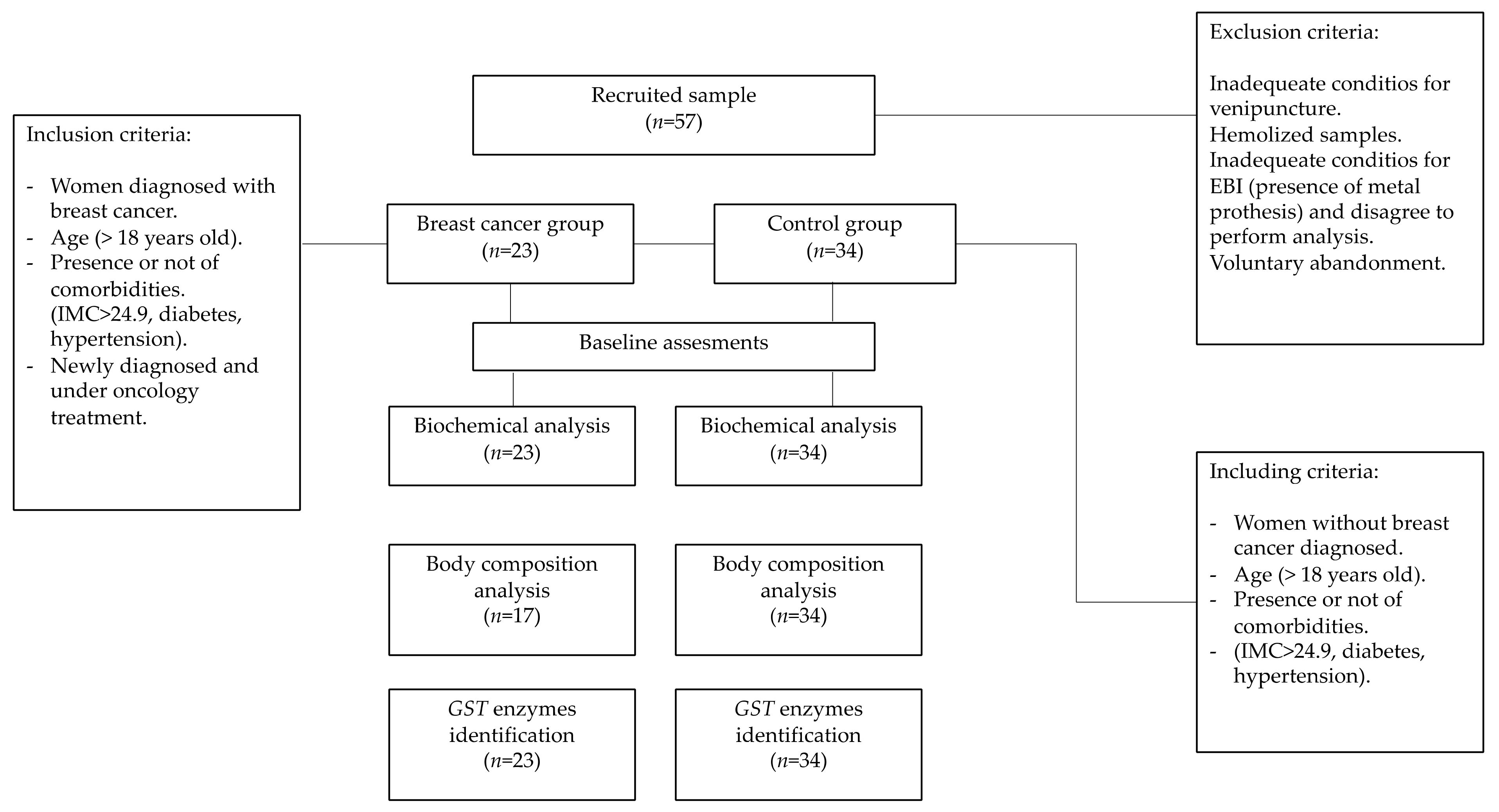
2. Materials and Methods
3. Results
3.1. Detection of GST Polymorphic Variants Expression
3.2. Cardiometabolic Risk Factors and GST Variants Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Koene, R.J.; Prizment, A.E.; Blaes, A.; Konety, S.H. Shared Risk Factors in Cardiovascular Disease and Cancer. Circulation 2016, 133, 1104–1114. [Google Scholar] [CrossRef] [PubMed]
- Figueiras-Graillet, L.M.; García-Jiménez, Y.; Rosas-Munive, E.; Nuriulú-Escobar, P.L.; Sierra-Galán, L.M.; Vásquez-Ortiz, Z.Y.; Parra-Machuca, M.G.; Puente-Barragán, A.C.; Ancona-Vadillo, A.E.; Ruiz-Gastelum, E.D. Abordaje de la enfermedad cardiovascular en mujeres con cáncer de mama. Posición de la Asociación Nacional de Cardiólogos de México (ANCAM). Cardiovasc. Metab. Sci. 2020, 31, 76–103. [Google Scholar] [CrossRef]
- Patnaik, J.L.; Byers, T.; Di Guiseppi, C.; Dabelea, D.; Denberg, T.D. Cardiovascular disease competes with breast cancer as the leading cause of death for older females diagnosed with breast cancer: A retrospective cohort study. Breast Cancer Res. 2011, 13, R64. [Google Scholar] [CrossRef] [PubMed]
- Shamah-Levy, T.; Vielma-Orozco, E.; Heredia-Hernández, O.; Romero-Martínez, M.; Mojica-Cuevas, J.; Cuevas-Nasu, L.; Santaella-Castell, J.A.; Rivera-Dommarco, J. Encuesta Nacional de Salud y Nutrición 2018-19: Resultados Nacionales; Instituto Nacional de Salud Pública: Cuernavaca, Mexico, 2020. [Google Scholar]
- Dirección General de Epidemiología. Panorama Epidemiológico de las Enfermedades No Transmisibles en México; Secretaría de Salud: Mexico City, Mexico, 2021.
- Han, J.H.; Lee, H.J.; Kim, T.S.; Kang, M.H. The effect of glutathione S-transferase M1 and T1 polymorphisms on blood pressure, blood glucose, and lipid profiles following the supplementation of kale (Brassica oleracea acephala) juice in South Korean subclinical hypertensive patients. Nutr. Res. Pract. 2015, 9, 49. [Google Scholar] [CrossRef]
- AL-Eitan, L.N.; Rababa’h, D.M.; Alghamdi, M.A.; Khasawneh, R.H. Association of GSTM1, GSTT1 And GSTP1 Polymorphisms with Breast Cancer Among Jordanian Women. OncoTargets Ther. 2019, 12, 7757–7765. [Google Scholar] [CrossRef]
- Zhu, Q.-L.; Xu, W.-H.; Tao, M.-H. Biomarkers of the Metabolic Syndrome and Breast Cancer Prognosis. Cancers 2010, 2, 721–739. [Google Scholar] [CrossRef]
- Cuevas, S.; Villar, V.A.M.; Jose, P.A. Genetic polymorphisms associated with reactive oxygen species and blood pressure regulation. Pharm. J. 2019, 19, 315–336. [Google Scholar] [CrossRef]
- Singh, R.R.; Reindl, K.M. Glutathione S-Transferases in Cancer. Antioxidants 2021, 10, 701. [Google Scholar] [CrossRef]
- Board, P.G.; Webb, G.C.; Coggan, M. Isolation of a cDNA clone and localization of the human glutathione S-transferase 3 genes to chromosome bands 11q13 and 12q13-14. Ann. Hum. Genet. 1989, 53, 205–213. [Google Scholar] [CrossRef]
- Ma, J.; Zhu, S.-L.; Liu, Y.; Huang, X.-Y.; Su, D.-K. GSTP1 polymorphism predicts treatment outcome and toxicities for breast cancer. Oncotarget 2017, 8, 72939–72949. [Google Scholar] [CrossRef]
- Afrand, M.; Bashardoost, N.; Sheikhha, M.H.; Afkhami-Ardekani, M. Association between Glutathione S-Transferase GSTM1-T1 and P1 Polymorphisms with Metabolic Syndrome in Zoroastrians in Yazd, Iran. Iran. J. Public Health 2015, 44, 673–682. [Google Scholar] [PubMed]
- Wang, G.; Zhang, L.; Li, Q. Genetic polymorphisms of GSTT1, GSTM1, and NQO1 genes and diabetes mellitus risk in Chinese population. Biochem. Biophys. Res. Commun. 2006, 341, 310–313. [Google Scholar] [CrossRef] [PubMed]
- Alriyahee, F.A.A.; Al-Jboori, M.J.; Al-Terehi, M.N.N. Association Serum Lipid Profile with Glutathione S-Transferas M and T genes Polymorphisms in Hypertension of Post-Menopausal Women. Int. J. Pharm. Qual. Assur. 2019, 10, 242–247. [Google Scholar] [CrossRef]
- Oniki, K.; Hori, M.; Takata, K.; Yokoyama, T.; Mihara, S.; Marubayashi, T.; Nakagawa, K. Association between glutathione S-transferase A1, M1 and T1 polymorphisms and hypertension. Pharmacogenet. Genomics 2008, 18, 275–277. [Google Scholar] [CrossRef] [PubMed]
- Rong, S.L.; Zhou, X.D.; Wang, Z.K.; Wang, X.L.; Wang, Y.C.; Xue, C.S.; Li, B. Glutathione S-Transferase M1 and T1 poly-morphisms and hypertension risk: An updated meta-analysis. J. Hum. Hypertens. 2019, 33, 454–465. [Google Scholar] [CrossRef]
- Ge, B.; Song, Y.; Zhang, Y.; Liu, X.; Wen, Y.; Guo, X. Glutathione S-Transferase M1 (GSTM1) and T1 (GSTT1) Null Polymorphisms and the Risk of Hypertension: A Meta-Analysis. PLoS ONE 2015, 10, e0118897. [Google Scholar] [CrossRef]
- Pemble, S.; Schroeder, K.R.; Spencer, S.R.; Meyer, D.J.; Hallier, E.; Bolt, H.M.; Ketterer, B.; Taylor, B. Human glutathione S-transferase theta (GSTT1): cDNA cloning and the characterization of a genetic polymorphism. Biochem. J. 1994, 300, 271–276. [Google Scholar] [CrossRef]
- Turck, N.; Robin, X.; Walter, N.; Fouda, C.; Hainard, A.; Sztajzel, R.; Wagner, G.; Hochstrasser, D.F.; Montaner, J.; Burkhard, P.R.; et al. Blood Glutathione S-Transferase-π as a Time Indicator of Stroke Onset. PLoS ONE 2012, 7, e43830. [Google Scholar] [CrossRef]
- Pahwa, S.; Sharma, R.; Singh, B. Role of Glutathione S-Transferase in Coronary Artery Disease Patients with and Without Type 2 Diabetes Mellitus. J. Clin. Diagn. Res. 2017, 11, BC05–BC08. [Google Scholar] [CrossRef]
- Knight, T.R.; Choudhuri, S.; Klaassen, C.D. Constitutive mRNA Expression of Various Glutathione S-Transferase Isoforms in Different Tissues of Mice. Toxicol. Sci. 2007, 100, 513–524. [Google Scholar] [CrossRef]
- Arand, M.; Mühlbauer, R.; Hengstler, J.; Jäger, E.; Fuchs, J.; Winkler, L.; Oesch, F. A Multiplex Polymerase Chain Reaction Protocol for the Simultaneous Analysis of the GlutathioneS-Transferase GSTM1 and GSTT1 Polymorphisms. Anal. Biochem. 1996, 236, 184–186. [Google Scholar] [CrossRef] [PubMed]
- Salimi, S.; Nakhaee, A.; Jafari, M.; Jahantigh, D.; Sandooghi, M.; Zakeri, Z.; Shahrakipour, M.; Naghavi, A.; Farajian-Mashhadi, F. Combination Effect of GSTM1, GSTT1 and GSTP1 Polymorphisms and Risk of Systemic Lupus Erythematosus. Iran. J. Public Health 2015, 44, 814–821. [Google Scholar] [PubMed]
- Campos, C.Z.; Vitiello, G.A.F.; Dias, F.L.; Hiroki, C.H.; Watanabe, M.A.E.; Guembarovski, R.L.; De Oliveira, C.E.C.; Hirata, B.K.B.; Mazzuco, T.L. Glutathione S-transferases deletions may act as prognosis and therapeutic markers in breast cancer. Clin. Exp. Med. 2017, 18, 27–35. [Google Scholar] [CrossRef]
- Song, Z.; Shao, C.; Feng, C.; Lu, Y.; Gao, Y.; Dong, C. Association of glutathione S-transferase T1, M1, and P1 polymorphisms in the breast cancer risk: A meta-analysis. Ther. Clin. Risk. Manag. 2016, 12, 763. [Google Scholar] [CrossRef]
- Possuelo, L.G.; Peraça, C.F.; Eisenhardt, M.F.; Dotto, M.L.; Cappelletti, L.; Foletto, E.; Valim, A.R.D.M. Polymorphisms of GSTM1 and GSTT1 genes in breast cancer susceptibility: A case-control study. Rev. Bras. Ginecol. Obstetrícia 2013, 35, 569–574. [Google Scholar] [CrossRef]
- Kimi, L.; Ghatak, S.; Yadav, R.P.; Chhuani, L.; Lallawmzuali, D.; Pautu, J.L.; Kumar, N.S. Relevance of GSTM1, GSTT1 and GSTP1 Gene Polymorphism to Breast Cancer Susceptibility in Mizoram Population, Northeast India. Biochem. Genet. 2015, 54, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.; Gupta, S. The multifaceted role of glutathione S-transferases in cancer. Cancer Lett. 2018, 433, 33–42. [Google Scholar] [CrossRef]
- Jaramillo-Rangel, G.; Ortega-Martínez, M.; Cerda-Flores, R.; Barrera-Saldaña, H. Short Communication Polymorphisms in GSTM1, GSTT1, GSTP1, and GSTM3 genes and breast cancer risk in northeastern Mexico. Genet. Mol. Res. 2015, 14, 6465–6471. [Google Scholar] [CrossRef]
- Soto-Quintana, O.; Cabrera-Galeana, P.; Téllez-Trevilla, G.; Barrera-Franco, J.L.; Juárez-Ramiro, A.; Castillo-Cadena, J. Relationship of Polymorphisms of Glutathione S-Transferase GSTT1 and GSTM1 with the Response to Chemotherapy in Mexican Women with Advanced Breast Cancer. J. Cancer Ther. 2011, 2, 354–361. [Google Scholar] [CrossRef][Green Version]
- Canto, P.; Canto-Cetina, T.; Juárez-Velázquez, R.; Rosas-Vargas, H.; Rangel-Villalobos, C.; Canizales-Quinteros, S.; Velázquez-Wong, A.C.; Villareal-Molina, M.T.; Fernandez, G.; Coral-Vázquez, R. Mar Methylenetetrahydrofolate Reductase C677T and Glutathione S-Transferase P1 A313G Are Associated with a Reduced Risk of Preeclampsia in Maya-Mestizo Women. Hypertens. Res. 2008, 31, 1015–1019. [Google Scholar] [CrossRef]
- Pérez-Morales, R.; Castro-Hernández, C.; Gonsebatt, M.E.; Rubio, J. Polymorphism of CYP1A1*2C, GSTM1*0, and GSTT1*0 in a Mexican Mestizo Population: A Similitude Analysis. Hum. Biol. 2008, 80, 457–465. [Google Scholar] [CrossRef]
- Chielle, E.; Trott, A.; da Silva Rosa, B.; Casarin, J.; Fortuna, P.; da Cruz, I.; Moretto, M.B.; Moresco, R.N. Impact of the Ile105Val Polymorphism of the Glutathione S-transferase P1 (GSTP1) Gene on Obesity and Markers of Cardiometabolic Risk in Young Adult Population. Exp. Clin. Endocrinol. Diabetes 2017, 125, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, C.J.; Daviglus, M.L.; Swett, K.; González, H.M.; Gallo, L.C.; Wassertheil-Smoller, S.; Giachello, A.L.; Teng, Y.; Schneiderman, N.; Talavera, G.A.; et al. Dyslipidemia Patterns among Hispanics/Latinos of Diverse Background in the United States. Am. J. Med. 2014, 127, 1186–1194.e1. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Estevez, L.; Moreno-Bueno, G. Updating the role of obesity and cholesterol in breast cancer. Breast Cancer Res. 2019, 21, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Buono, G.; Crispo, A.; Giuliano, M.; De Angelis, C.; Schettini, F.; Forestieri, V.; Lauria, R.; de Laurentis, M.; de Placido, P.; Rea, C.G.; et al. Metabolic syndrome and early stage breast cancer outcome: Results from a prospective observational study. Breast Cancer Res. Treat. 2020, 182, 401–409. [Google Scholar] [CrossRef]
- Cardoso-Peña, E.; Pina, A.E.S.; Villanueva, G.; Chavez, G.E.L.; Martínez, P.R.; Montoya, H.R.; Lechuga, M.G.B.; Arciniega, A.D.B.; Fortepiani, M.D.L.A.; Ramos, R.V.; et al. Visceral Adiposity Index in Breast Cancer Survivors: A Case-Control Study. Int. J. Endocrinol. 2020, 2020, 8874916 . [Google Scholar] [CrossRef]
- Hussain, T.; Alrokayan, S.; Upasna, U.; Pavithrakumari, M.; Jayapriya, J.; Kutala, V.K.; Naushad, S.M. Meta-analysis of genetic polymorphisms in xenobiotic metabolizing enzymes and their association with breast cancer risk. J. Genet. 2018, 97, 523–537. [Google Scholar] [CrossRef]
- Rajagopal, T.; Seshachalam, A.; Rathnam, K.K.; Jothi, A.; Talluri, S.; Venkatabalasubramanian, S.; Dunna, N.R. Impact of xe-nobiotic-metabolizing gene polymorphisms on breast cancer risk in South Indian women. Breast Cancer Res. Treat. 2021, 186, 823–837. [Google Scholar] [CrossRef]
- Hwang, J.; Bae, H.; Choi, S.; Yi, H.; Ko, B.; Kim, N. Impact of air pollution on breast cancer incidence and mortality: A nationwide analysis in South Korea. Sci. Rep. 2020, 10, 1–7. [Google Scholar] [CrossRef]
- Ghosn, B.; Benisi-Kohansal, S.; Ebrahimpour-Kujan, S.; Azadbakht, L.; Esmaillzadeh, A. Association between healthy lifestyle score and breast cancer. Nutr. J. 2020, 19, 1–11. [Google Scholar] [CrossRef]
- Segovia-Mendoza, M.; Morales-Montor, J. Immune Tumor Microenvironment in Breast Cancer and the Participation of Estrogen and Its Receptors in Cancer Physiopathology. Front. Immunol. 2019, 10, 348. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ye, Z.-W.; Chen, W.; Manevich, Y.; Mehrotra, S.; Ball, L.; Janssen-Heininger, Y.M.; Tew, K.D.; Townsend, D.M. S-Glutathionylation of estrogen receptor α affects dendritic cell function. J. Biol. Chem. 2018, 293, 4366–4380. [Google Scholar] [CrossRef] [PubMed]
- Bengtsson, A.K.; Ryan, E.J.; Giordano, D.; Magaletti, D.M.; Clark, E.A. 17β-Estradiol (E2) modulates cytokine and chemokine expression in human monocyte-derived dendritic cells. Blood 2004, 104, 1404–1410. [Google Scholar] [CrossRef] [PubMed]
- Flores-Reséndiz, C.; Soto-Piña, A.E.; Valdés-Ramos, R.; Benítez-Arciniega, A.D.; Tlatempa-Sotelo, P.; Guadarrama-López, A.L.; Martinez-Carillo, B.E.; Pulido-Alvarado, C.C. Association Between Cardiovascular Risk Factors and Stress Hormones with Cognitive Performance in Mexican Adolescents. J. Pediatr. Psychol. 2019, 44, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Costa-Urrutia, P.; Colistro, V.; Franco-Trecu, V.; Granados, J.; Fariña, R.; Rodríguez-Arellano, M.E. Dyslipidemia, Obesity, and Ethnicity in Mexican Children. Int. J. Environ. Res. Public Health 2021, 18, 12659. [Google Scholar] [CrossRef] [PubMed]
- Huerta-Chagoya, A.; Moreno-Macías, H.; Sevilla-González, M.; Rodríguez-Guillén, R.; Ordóñez-Sánchez, M.L.; Gómez-Velasco, D.; Muñóz-Hernández, L.; Segura-Kato, Y.; Arellano-Campos, O.; Cruz-Bautista, I.; et al. Contribution of Known Genetic Risk Variants to Dyslipidemias and Type 2 Diabetes in Mexico: A Population-Based Nationwide Study. Genes 2020, 11, 114. [Google Scholar] [CrossRef]
- Upadhyay, R.K. Emerging Risk Biomarkers in Cardiovascular Diseases and Disorders. J. Lipids 2015, 2015, 971453. [Google Scholar] [CrossRef]
- Madu, C.O.; Wang, S.; Madu, C.O.; Lu, Y. Angiogenesis in Breast Cancer Progression, Diagnosis, and Treatment. J. Cancer 2020, 11, 4474–4494. [Google Scholar] [CrossRef]
- Unger, L.W.; Forstner, B.; Schneglberger, S.; Muckenhuber, M.; Eigenbauer, E.; Scheiner, B.; Mandorfer, M.; Trauner, M.; Reiberger, T. Patterns and preva-lence of dyslipidemia in patients with different etiologies of chronic liver disease. Wien. Klin. Wochenschr. 2019, 131, 395–403. [Google Scholar] [CrossRef]
- Yuan, Y.V.; Kitts, D.D. Dietary (n-3) Fat and Cholesterol Alter Tissue Antioxidant Enzymes and Susceptibility to Oxidation in SHR and WKY Rats. J. Nutr. 2003, 133, 679–688. [Google Scholar] [CrossRef]
- Antonini-Canterin, F.; Di Nora, C.; Pellegrinet, M.; Vriz, O.; La Carrubba, S.; Carerj, S.; Zito, C.; Matescu, A.; Ravasel, A.; Cosei, I.; et al. Effect of uric acid serum levels on carotid arterial stiffness and intima-media thickness: A high resolution Echo-Tracking Study. Monaldi Arch. Chest Dis. 2019, 89. [Google Scholar] [CrossRef] [PubMed]
| Primer Designation | Sequence | Gene ID | SNP Number | Chromosome Position | Nucleotide | Amino Change |
|---|---|---|---|---|---|---|
| GSTM1 forward | 5′-GAACTCCCTGAAAAGCTAAAGC-3′ | 2944 | - | 1:109690472 (GRCh38) 1:110233094 (GRCh37) | - | - |
| GSTM1 reverse | 5′-GTTGGGCTCAAATATACGGTGG-3′ | |||||
| GSTT1 forward | 5′-TTCCTTACTGGTCCTCACATCTC-3′ | 2952 | - | NT_187633.1:270497 (GRCh38) NT_187633.1:24376322 (GRCh37) | - | - |
| GSTT1 reverse | 5′-TCACCGGATCATGGCCAGCA-3′ | |||||
| GSTP1 forward outer primer | 5′-AGGTTACGTAGTTTGCCCAAGGTC-3′ | 2950 | rs1695 | 11:67585218 (GRCh38) 11:67352689 (GRCh37) | A313G | Ile105Val |
| GSTP1 reverse outer primer | 5′-CGTTACTTGGCTGGTTGAT- GTCC-3′ | |||||
| GSTP1 forward inner primer | 5′-GAGGACCTCCGCTGCAAATTCG-3′ | |||||
| GSTP1 reverse inner primer | 5′-CATAGTTGGTGTAGATGAGGGAGCT-3′ |
| Step | Temperature | Duration | Cycles |
|---|---|---|---|
| RT | 50 °C | 10 min | ×1 |
| Primary denaturation | 95 °C | 3 min | ×1 |
| Denaturation | 95 °C | 15 s | ×40 |
| Annealing | 60 °C | 30 s | |
| Melt Curve | 65–95 °C △ 0.05 °C | 0.05 s |
| Indicators | BC Group (n = 23) | Control Group (n = 34) | p |
|---|---|---|---|
| Age (years) | 50.48 (±8.25) | 47.53 (±9.55) | 0.233 |
| Menopause status | |||
| Premenopause | 12 (21.1) | 23 (40.4) | 0.239 |
| Postmenopause | 11 (19.3) | 11 (19.3) | |
| Presence of comorbidities + | |||
| Yes | 9 (15.8) | 15 (26.3) | 0.708 |
| No | 14 (24.6) | 19 (33.3) | |
| SBP (mmHg) | 100.00 (±11.67) | 107.27 (±13.75) | 0.043 * |
| DBP (mmHg) | 66.96 (±7.64) | 70.30 (±8.47) | 0.136 |
| BMI (kg/m2) | 27.96 (±4.72) | 28.40 (±5.16) | 0.746 |
| BFP (%) (n = 17) ~ | 33.86 (±5.54) | 34.54 (±5.32) | 0.672 |
| Glucose (mg/dL) | 117.17 (±66.37) | 102.79 (±30.20) | 0.273 |
| Total cholesterol (mg/dL) | 183.91 (±33.66) | 191.35 (±31.82) | 0.401 |
| HDL-cholesterol (mg/dL) | 40.61 (±11.17) | 43.41 (±8.05) | 0.283 |
| LDL-cholesterol (mg/dL) | 108.61 (±21.94) | 114.28 (±25.88) | 0.392 |
| VLDL-cholesterol (mg/dL) | 34.69 (±27.38) | 33.65 (±10.36) | 0.841 |
| Triacylglycerols (mg/dL) | 214.96 (±192.19) | 215.74 (±93.59) | 0.984 |
| Atherogenic Index | 4.82 (±1.51) | 4.53 (±1.02) | 0.417 |
| GST Variants | BC Group (n = 23) | Control Group (n = 34) | p |
|---|---|---|---|
| GSTT1+ | 14 (60.9) | 20 (58.8) | 0.145 |
| GSTT1− | 9 (39.1) | 14 (41.2) | |
| GSTM1+ | 14 (60.9) | 18 (52.9) | 0.354 |
| GSTM1− | 9 (39.1) | 16 (47.1) | |
| GSTP1 (Ile105Val) | |||
| Negative | 1 (4.3) | - | 0.001 * |
| Ile/Ile | 4 (17.4) | 13 (38.8) | |
| Val/Val | 5 (21.7) | 5 (14.7) | |
| Ile/Val | 13 (56.5) | 16 (47.1) | |
| Breast Cancer Group (n = 23) | |||||||||||
| GSTT1 Expression | p | GSTM1 Expression | p | GSTP1 (Ile105Val) | p | ||||||
| Parameters | Negative | Positive | Negative | Positive | Negative | Ile | Val | Ile/Val | |||
| BP (mmHg) | |||||||||||
| Normal (No) | 8 (34.8) | 10 (43.5) | 0.322 | 6 (26.1) | 12 (52.2) | 0.280 | 1 (4.3) | 3 (13.0) | 2 (8.7) | 12 (52.2) | 0.106 |
| High (Yes) | 1 (4.3) | 4 (17.4) | 3 (13.0) | 2 (8.7) | - | 1 (4.3) | 3 (13.0) | 1 (4.3) | |||
| BMI (kg/m2) | |||||||||||
| Normal | 1 (4.3) | 4 (17.4) | 0.322 | 2 (8.7) | 3 (13.0) | 0.964 | - | 1 (4.3) | - | 4 (17.4) | 0.510 |
| >25 | 8 (34.8) | 10 (43.5) | 7 (30.4) | 11 (47.8) | 1 (4.3) | 3 (13.0) | 5 (21.7) | 9 (39.1) | |||
| BFP (%)a | n = 17 | ||||||||||
| Normal | - | 3 (17.6) | 0.110 | 1 (5.9) | 2 (11.8) | 0.761 | - | - | - | 3 (17.6) | 0.466 |
| High | 7 (41.2) | 7 (41.2) | 6 (35.3) | 8 (47.1) | 1 (5.9) | 1 (5.9) | 5 (29.4) | 7 (41.2) | |||
| Glucose (mg/dL) | |||||||||||
| Normal | 7 (30.4) | 8 (34.8) | 0.311 | 6 (26.1) | 9 (39.1) | 0.907 | 1 (4.3) | 2 (8.7) | 2 (8.7) | 10 (43.5) | 0.372 |
| High | 2 (8.7) | 6 (26.1) | 3 (13.0) | 5 (21.7) | - | 2 (8.7) | 3 (13.0) | 3 (13.0) | |||
| TC (mg/dL) | |||||||||||
| Normal | 8 (34.8) | 10 (43.5) | 0.322 | 6 (26.1) | 12 (52.2) | 0.280 | 1 (4.3) | 3 (13.0) | 3 (13.0) | 11 (47.8) | 0.661 |
| High | 1 (4.3) | 4 (17.4) | 3 (13.0) | 2 (8.7) | - | 1 (4.3) | 2 (8.7) | 2 (8.7) | |||
| HDL-c (mg/dL) | |||||||||||
| Low CVR | - | 1 (4.3) | 0.412 | - | 1 (4.3) | 0.412 | - | 1 (4.3) | - | - | 0.174 |
| High CVR | 9 (39.1) | 13 (56.5) | 9 (39.1) | 13 (56.5) | 1 (4.3) | 3 (13.0) | 5 (21.7) | 13 (56.5) | |||
| LDL-c (mg/dL) | |||||||||||
| Normal | 8 (34.8) | 11 (47.8) | 0.524 | 7 (30.4) | 12 (52.2) | 0.624 | 1 (4.3) | 4 (17.4) | 2 (8.7) | 12 (52.2) | 0.042 * |
| High | 1 (4.3) | 3 (13.0) | 2 (8.7) | 2 (8.7) | - | - | 3 (13.0) | 1 (4.3) | |||
| VLDL-c (mg/dL) | |||||||||||
| Normal | 9 (39.1) | 10 (43.5) | 0.078 | 6 (26.1) | 13 (56.5) | 0.106 | 1 (4.3) | 3 (13.0) | 5 (21.7) | 10 (43.5) | 0.633 |
| High | - | 4 (17.4) | 3 (13.0) | 1 (4.3) | - | 1 (4.3) | - | 3 (13.0) | |||
| TG (mg/dL) | |||||||||||
| Normal | 5 (21.7) | 4 (17.4) | 0.196 | 4 (17.4) | 5 (21.7) | 0.675 | - | 1 (4.3) | 3 (13.0) | 5 (21.7) | 0.595 |
| High | 4 (17.4) | 10 (43.5) | 5 (21.7) | 9 (39.1) | 1 (4.3) | 3 (13.0) | 2 (8.7) | 8 (34.8) | |||
| IA | |||||||||||
| Normal | 5 (21.7) | 8 (34.8) | 0.940 | 3 (13.0) | 10 (43.5) | 0.072 | 1 (4.3) | 3 (13.0) | 2 (8.7) | 7 (30.4) | 0.590 |
| High | 4 (17.4) | 6 (26.1) | 6 (26.1) | 4 (17.4) | - | 1 (4.3) | 3 (13.0) | 6 (26.1) | |||
| Control Group (n = 34) | |||||||||||
| GSTT1 Expression | p | GSTM1 Expression | p | GSTP1 (Ile105Val) | p | ||||||
| Parameters | Negative | Positive | Negative | Positive | Negative | Ile | Val | Ile/Val | |||
| BP (mmHg) | |||||||||||
| Normal (No) | 4 (11.8) | 15 (44.1) | 0.007 * | 8 (23.5) | 11 (32.4) | 0.515 | - | 6 (17.6) | 2 (5.9) | 11 (32.4) | 0.353 |
| High (Yes) | 10 (29.4) | 5 (14.7) | 8 (23.5) | 7 (20.6) | - | 7 (20.6) | 3 (8.8) | 5 (14.7) | |||
| BMI (kg/m2) | |||||||||||
| Normal | 3 (8.8) | 6 (17.6) | 0.577 | 3 (8.8) | 6 (17.6) | 0.336 | - | 4 (11.8) | 2 (5.9) | 3 (8.8) | 0.582 |
| >25 | 11 (32.4) | 14 (41.2) | 13 (38.2) | 12 (35.3) | - | 9 (26.5) | 3 (8.8) | 13 (38.2) | |||
| BFP (%) | |||||||||||
| Normal | 3 (8.8) | 3 (8.8) | 0.628 | 1 (2.9) | 5 (14.7) | 0.100 | - | 3 (8.8) | 2 (5.9) | 1 (2.9) | 0.182 |
| High | 11 (32.4) | 17 (50.0) | 15 (44.1) | 13 (38.2) | - | 10 (29.4) | 3 (8.8) | 15 (44.1) | |||
| Glucose (mg/dL) | |||||||||||
| Normal | 11 (32.4) | 11 (32.4) | 0.157 | 9 (26.5) | 13 (38.2) | 0.331 | - | 9 (26.5) | 3 (8.8) | 10 (29.4) | 0.905 |
| High | 3 (8.8) | 9 (26.5) | 7 (20.6) | 5 (14.7) | - | 4 (11.8) | 2 (5.9) | 6 (17.6) | |||
| TC (mg/dL) | |||||||||||
| Normal | 11 (32.4) | 11 (32.4) | 0.157 | 8 (23.5) | 14 (41.2) | 0.091 | - | 12 (35.3) | 3 (8.8) | 7 (20.6) | 0.024 * |
| High | 9 (25.7) | 9 (26.5) | 8 (23.5) | 4 (11.8) | - | 1 (8.3) | 2 (5.9) | 9 (26.5) | |||
| HDL-c (mg/dL) | |||||||||||
| Low CVR | - | 1 (2.9) | 0.396 | - | 1 (2.9) | 0.339 | - | - | - | 1 (2.9) | 0.560 |
| High CVR | 14 (41.2) | 19 (55.9) | 16 (47.1) | 17 (50.0) | - | 13 (38.2) | 5 (14.7) | 15 (44.1) | |||
| LDL-c (mg/dL) | |||||||||||
| Normal | 13 (38.2) | 13 (38.2) | 0.059 | 12 (35.3) | 14 (41.2) | 0.849 | - | 13 (38.2) | 3 (8.8) | 10 (29.4) | 0.039 * |
| High | 1 (2.9) | 7 (20.6) | 4 (11.8) | 4 (11.8) | - | - | 2 (5.9) | 6 (17.6) | |||
| VLDL-c (mg/dL) | |||||||||||
| Normal | 12 (35.3) | 14 (41.2) | 0.288 | 10 (29.4) | 16 (41.7) | 0.070 | - | 13 (38.2) | 3 (8.8) | 10 (29.4) | 0.039 * |
| High | 2 (5.9) | 6 (17.6) | 6 (17.6) | 2 (5.9) | - | - | 2 (5.9) | 6 (17.6) | |||
| TG (mg/dL) | |||||||||||
| Normal | 6 (17.6) | 4 (11.8) | 0.150 | 3 (8.8) | 7 (20.6) | 0.198 | - | 7 (20.6) | - | 3 (8.8) | 0.035 * |
| High | 8 (23.5) | 16 (47.1) | 13 (38.2) | 11 (32.4) | - | 6 (17.6) | 5 (14.7) | 13 (38.2) | |||
| IA | |||||||||||
| Normal | 9 (26.5) | 9 (26.5) | 0.268 | 6 (17.6) | 12 (35.3) | 0.089 | - | 10 (24.9) | 2 (5.9) | 6 (17.6) | 0.088 |
| High | 5 (14.7) | 11 (32.4) | 10 (29.4) | 6 (17.6) | - | 3 (8.8) | 3 (8.8) | 10 (29.4) | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Becerril Alarcón, Y.; Bastida González, F.; Camacho Beiza, I.R.; Dávila González, E.; Cruz Ramos, J.A.; Benítez Arciniega, A.D.; Valdés Ramos, R.; Soto Piña, A.E. Association of GSTT1, GSTM1 and GSTP1 (Ile105Val) mRNA Expression with Cardiometabolic Risk Parameters in Women with Breast Cancer and Comorbidities. Cardiogenetics 2022, 12, 235-245. https://doi.org/10.3390/cardiogenetics12030022
Becerril Alarcón Y, Bastida González F, Camacho Beiza IR, Dávila González E, Cruz Ramos JA, Benítez Arciniega AD, Valdés Ramos R, Soto Piña AE. Association of GSTT1, GSTM1 and GSTP1 (Ile105Val) mRNA Expression with Cardiometabolic Risk Parameters in Women with Breast Cancer and Comorbidities. Cardiogenetics. 2022; 12(3):235-245. https://doi.org/10.3390/cardiogenetics12030022
Chicago/Turabian StyleBecerril Alarcón, Yizel, Fernando Bastida González, Isidro Roberto Camacho Beiza, Eduardo Dávila González, José Alfonso Cruz Ramos, Alejandra Donají Benítez Arciniega, Roxana Valdés Ramos, and Alexandra Estela Soto Piña. 2022. "Association of GSTT1, GSTM1 and GSTP1 (Ile105Val) mRNA Expression with Cardiometabolic Risk Parameters in Women with Breast Cancer and Comorbidities" Cardiogenetics 12, no. 3: 235-245. https://doi.org/10.3390/cardiogenetics12030022
APA StyleBecerril Alarcón, Y., Bastida González, F., Camacho Beiza, I. R., Dávila González, E., Cruz Ramos, J. A., Benítez Arciniega, A. D., Valdés Ramos, R., & Soto Piña, A. E. (2022). Association of GSTT1, GSTM1 and GSTP1 (Ile105Val) mRNA Expression with Cardiometabolic Risk Parameters in Women with Breast Cancer and Comorbidities. Cardiogenetics, 12(3), 235-245. https://doi.org/10.3390/cardiogenetics12030022






