Abstract
Cardiovascular disorders are the main complication in autosomal dominant polycystic kidney disease (ADPKD). contributing to both morbidity and mortality. This review considers clinical studies unveiling cardiovascular features in patients with ADPKD. Additionally, it focuses on basic science studies addressing the dysfunction of the polycystin proteins located in the cardiovascular system as a contributing factor to cardiovascular abnormalities. In particular, the effects of polycystin proteins’ deficiency on the cardiomyocyte function have been considered.
1. Epidemiology, Genetics and Pathogenetsis
Autosomal dominant polycystic kidney disease (ADPKD) is the most common monogenic renal disorder, implies a progressive loss of renal function and accounts for 4% to 10% of patients with end-stage renal disease (ESRD) [1,2]. The disease, which occurs worldwide with an estimated prevalence of 1:400 to 1:100, is characterized by the age-dependent growth of renal cysts such that ESRD typically ensues during mid adulthood [3,4,5].
Mutations in Polycystic Kidney Disease 1 and 2 (PKD-1 and PKD-2, respectively) genes have a well-defined role in uncontrolled cystogenesis in both kidneys, the hallmark of the disease, as well as in the liver, and less frequently in the pancreas, seminal vesicles and arachnoid membrane [1,2,6,7,8,9,10,11,12,13,14]. Approximately 85% of ADPKD cases are caused by mutations in the PKD1 gene [MIM 601313], which is located on chromosome 16p13.3, while the remaining cases are due to mutations in PKD2 [MIM 173910], located on chromosome 4q21 [9,10].
The protein products of PKD1 and PKD2, polycystin-1 (PC1) and polycystin-2 (PC2), are membrane proteins that probably form a functional complex [15,16,17,18,19]. They have been shown to localize predominantly, although not exclusively, in primary cilia and mediate the sensitivity of kidney epithelial cells to fluid shear stress [6,7,8]. PC1 has been proposed to be a mechanosensitive molecule, given its large extracellular domain and remarkable mechanical strength [1]. The distribution of the polycystin proteins at different subcellular locations is required for them to orchestrate a network of signaling pathways that have been implicated in the pathogenesis of PKD [8,20].
PC1 or PC2 dysfunction may result in decreased intracellular calcium inflow with increased 3′, 5′-cyclic adenosinemonophosphate (cAMP) production and the activation of mechanisms which contribute to cyst cell growth by stimulating both epithelial cell proliferation and transepithelial fluid secretion [21]. Indeed, relevant downstream responses of the changed calcium signaling ultimately lead to increased proliferation and increased apoptosis [22]. Factors impacting upon disease progression include the level of PKD protein expression and the penetrance of pathogenic alleles [23,24]. However, both ADPKD type 1 and type 2 share the full spectrum of renal and extrarenal manifestations, although type 2 has a delayed onset compared to type 1 [1,2,25,26,27]. In type 2 patients, the common complications of ADPKD, such as hypertension, hematuria and urinary-tract infection, seem to be milder than in type 1 patients at the same age. The median age at death or onset of end-stage renal disease has been reported as 53 years in type 1 patients and 69 years in type 2 [25].
1.1. Kidney Polycystic Disease
The renal phenotype in patients with ADPKD ranges from elderly patients without renal failure to rare cases of enlarged kidneys that are detected in utero [28]. The disease course is highly variable, as a significant minority of patients do not reach ESRD even in old age, while a small number exhibit early-onset disease, with a diagnosis made in infancy by the identification of enlarged echogenic kidneys.
Renal cyst expansion results from aberrant proliferation of the cyst wall epithelial cells and the accumulation of fluid within the cavity of the cyst. There is increased extracellular matrix remodeling as the cyst invades the adjacent parenchyma, leading to abnormal matrix deposition and fibrosis. The gradually growing renal cysts start to develop in utero and can originate from all areas of the kidneys, although cysts usually form in the distal regions of the nephron and the collecting duct [29,30,31,32,33].
Impaired urinary concentrating capacity has been well documented as common even in early stages [1,26,27,28].
Another important feature of ADPKD is the increase in plasma vasopressin concentrations. This defect is often attributed to the disruption of the medullary architecture, because its presence and severity correlate with the extent of the cystic disease. There is substantial evidence that the urinary concentrating defect and raised vasopressin concentrations could contribute to cystogenesis [34,35,36]. They might also contribute to the glomerular hyperfiltration seen in children and young adults, the development of hypertension and chronic kidney-disease progression [37].
Another early functional defect is a reduction in renal blood flow, likely due to the development of cysts with changes in intrarenal pressures, and to neuro humoral or local mediators [38,39,40]. As mentioned above, the identity of the mutated gene in patients with ADPKD explains part of the phenotypic variability observed in clinical practice, so that patients with a mutation in PKD1 have earlier-onset ESRD, lower glomerular filtration rate (GFR) and larger kidney volumes than patients with a mutation in PKD2 [1,25,41].
1.2. Arterial Hypertension
Even though enlarged cystic kidneys are the most obvious phenotype in ADPKD, cardiovascular disorders are the main complication contributing to both morbidity and mortality [42]. Systemic arterial hypertension is an early finding, occurring in about 60% of patients prior to a significant decline in GFR [3]. In particular, hypertension is observed in patients with ADPKD about a decade earlier than in the general population and even before the loss of renal function [43,44,45]. Although the mechanism underlying hypertension is not completely understood, the up-regulation of the renin angiotensin aldosterone system (RAAS), due to cystic compression of the renal vasculature, is considered a key mechanism in the pathogenesis of hypertension in ADPKD, as it leads to an increase in vascular resistance, and contributes to cardiac hypertrophy [46,47]. The RAAS pathway is the subject of clinical trials in patients with ADPKD in the HALT study, a US multicenter trial conducted on about 1000 ADPKD patients for 5.5 years. The benefits of blood-pressure control by antihypertensive drugs on patients with ADPKD have been largely demonstrated [48]. In patients with eGFR above 60 mL/min/1.73 m2, a target blood pressure of 110/70 mm Hg was suggested, especially in young patients with a high progression risk [49], while a target blood pressure below 120/80 mm Hg was recommended in chronic kidney disease stage 3 [50]. Thus, the aggressive control of hypertension in ADPKD patients appears to be clinically necessary and practically relevant. Nevertheless, as hypertension is likely dependent on the interaction of hemodynamic, endocrine and neurogenic factors, it has been postulated that mechanisms including increased sympathetic nerve activity [51], increased plasma endothelin-1 concentrations [52] and insulin resistance [53,54] contribute to the pathogenesis of hypertension in ADPKD. These theories can account for the high prevalence of hypertension but fail to explain why hypertension is frequently observed in patients with ADPKD, including children, even before the onset of renal insufficiency [55,56].
The PKD genes are expressed in a wide range of tissues beyond the kidney, and their expression is developmentally regulated in most of these tissues [57,58]. The fact that PC1 and PC2 are expressed in vascular smooth muscle and endothelial cells suggests that a reduced polycystin protein function in the vasculature could play a primary role in the early development of hypertension [59,60].
The primary dysfunction of the PC1 and PC2 complex results in decreased intracellular calcium inflow, with enhanced vascular smooth muscle contractility in response to adrenergic stimulation [61], increased muscular cell proliferation and apoptosis [62], and impaired endothelial-dependent vasorelaxation [63], functional abnormalities that might play a role in vascular remodeling and the early development of hypertension [64].
1.3. Vascular Abnormalities
The reduced expression of the polycystin proteins (haploinsufficiency) in the endothelial cells and vascular smooth muscle cells of most blood vessels, including the aorta and cerebral arteries, are likely to cause the vascular abnormalities in patients with ADPKD [59,65].
The functions of PC1 and PC2 in the vasculature indicate that they have a crucial role in mechano-sensation [66,67,68,69]. In endothelial cells, the proteins are involved in fluid-shear stress sensing, thereby regulating calcium signaling, and there is NO release or vasodilatation in response to the increased blood flow [70,71,72,73,74,75,76,77]. In vascular smooth muscle cells, the polycystins regulate pressure sensing by modulating the activity of the stretch-activated cation channels and myogenic contraction [78,79,80]. A loss of myogenic tone may contribute to aneurysm formation owing to an increase in arterial wall stress [81,82].
Indeed, the risk of cerebral aneurysms is higher in families with a positive family history of ADPKD than in those without, probably because of modifying genes [83,84]. Moreover, patients with ADPKD are more prone to coronary arteries aneurisms and acute dissection, which are a source of coronary syndromes and death [85,86,87]. Likewise, primary defects in vascular structure may lead to acute aortic dissection, that occurs in ADPKD patients more frequently than in the general population [88,89,90].
1.4. The Heart in ADPKD
ADPKD is a systemic disease associated with several extrarenal manifestations, including aortic root dilatation and cardiac valvular abnormalities, mostly mitral valve prolapse [91,92]. The early development of hypertension leads to LV hypertrophy, a major cardiovascular risk factor, and to LV diastolic dysfunction [67]. The occurrence of advanced kidney failure contributes to the increased frequency of cardiovascular events seen in ADPKD, as it is a well-known key factor in the development of cardiac function impairment.
The relevance of LV hypertrophy in ADPKD has been established by clinical studies [93,94,95,96,97,98]. Among 116 consecutive ADPKD patients, LVH was found in 46% of males and 37% of females, and the LV mass, as measured by two-dimensional echocardiography and indexed by body surface area, correlated significantly with the blood pressure level. An early onset of hypertension and a frequently inadequate treatment may account for the role of blood pressure as a contributing factor to LV hypertrophy in ADPKD patients. In the HALTPKD study (NCT0028368), the rigorous blood pressure control obtained with the use of RAAS inhibitors was able to achieve a significant reduction of LV mass as measured by MR imaging [48]. It is worth noting that LV hypertrophy can also be observed in normotensive ADPKD patients [56,91,92,93,94]. In a single-site study [99] including 126 ADPKD patients (78% with hypertension), the prevalence of LV hypertrophy was not influenced by the co-diagnosis of hypertension (21% vs. 17%). Factors other than hypertension, including anemia, obesity and sodium intake, as well as the increased activity of the RAAS, vitamin D deficiency [100] and secondary hyperparathyroidism, might be involved in the increase of the LV mass in ADPKD. Note that the vitamin D receptor is expressed in renal juxtaglomerular cells, vascular smooth muscle cells and, most interestingly, cardiac myocytes. Insulin resistance could be a further factor responsible for the onset of cardiac hypertrophy in ADPKD patients [53,54]. Endothelial dysfunction, oxidative stress and nitric oxide deficiency are also related to cardiac hypertrophy owing to arterial hypertension. Alterations in fluid shear stress mechanosensitivity are evident early on in ADPKD [71,72,73,74,77]. Normotensive ADPKD patients display a loss of nitric oxide release and an associated reduction in the endothelium-dependent dilation of conduit arteries during sustained flow increase (hand skin heating), notwithstanding a preserved flow mediated dilation during transient flow stimulation (postischemic hyperemia) [77]. However, the occurrence of LV hypertrophy in young adult patients before the onset of hypertension [56,94,95] and even in children [101] suggests a role of ADPKD’s specific mechanisms in the pathogenesis of cardiac involvement. It has been reported that cardiac diastolic dysfunction is an early feature of ADPKD, as it is often present prior to the loss of kidney function and even in normotensive individuals. Bi-ventricular diastolic dysfunction has been documented even in young ADPKD patients with still normal blood pressure [102].
A single-site study utilized speckle-tracking echocardiography to assess the LV function in a cohort of ADPKD patients with normal ejection fraction as compared to matched healthy subjects as well as to matched chronic kidney disease (other than ADPKD) patients [103,104]. ADPKD patients were found to have subclinical systolic dysfunction with reduced LV global longitudinal strain and twist in association with LV diastolic dysfunction. Interestingly, the impairment of the torsional function was associated to the increase in diastolic filling pressures as evaluated by Doppler measures of E/e’, that is, the velocity of the mitral valve inflow compared to the mitral annulus velocity. The subclinical changes in the LV function were considered as due to myocardial cell dysfunction, which was directly related to the disease rather than to confounding factors such as hypertension or renal insufficiency.
Understanding the mechanisms involved in ADPKD-associated cardiac manifestations may open up specific therapeutic perspectives; nevertheless, the precise pathways and interactions remain largely unknown. Several in vivo studies have attempted to clarify the role of polycystin proteins in heart cells. To gain insight into the disease mechanisms and pathogenesis of ADPKD, various mouse models have been genetically engineered, including inducible, conditional knockout mice and mouse models with hypomorphic or hypermutable alleles of PKD1 or PKD2. Otherwise, the ablation of both alleles of PKD1 or PKD2 results in embryonic lethality, and heterozygous mice develop only a very mild form of the disease [105,106,107].
PC1 and PC2, the PKD1 andPKD2 products, are essential for the development of the heart. One of the major sites of PKD1 expression is in the developing and mature cardiovascular system [105]. Moreover, PC1 has been found to regulate L-type calcium channel protein levels through the AKT pathway and cardiomyocyte contractility in a mouse model, suggesting a crucial role for this protein in cardiac function [108,109,110]. PC1 has been shown to act as a mechanosensor in cardiomyocytes, required for stretch-induced cardiomyocyte hypertrophy as well as for pressure overload-induced hypertrophy [108]. PC1 is a critical modulator of apoptosis and fibrosis. Cardiomyocyte PC1 plays a pivotal role in regulating fibroblast-to-myofibroblast differentiation, with implications for cardiac fibrosis and remodeling [111]. Also noteworthy is the fact that mice harboring a cardiomyocyte-selective conditional silencing of PC1 do not develop fibrosis after being subjected to transverse aortic constriction [108], supporting the role of this protein in cardiac remodeling. Moreover, PC1 mitigates cardiac damage during ischemia/reperfusion, likely through AKT activation, and regulates the connective tissue growth factor expression in cardiomyocytes [111]. The cardiomyocyte-specific deletion of PKD1 impairs the systolic and diastolic functions in mice [112].
PC2 is involved in the cardiomyocyte regulation of ion transport and calcium signaling [113,114,115,116]. Heart function has been shown to depend on PKD2 activity, as evidenced by the fact that a lack of PC2 in zebrafish led to signs of heart failure. Moreover, patients with ADPKD due to reduced PC2 activity are particularly prone to develop idiopathic dilated cardiomyopathy [113]. While PC2 regulates intracellular calcium cycling, a follow-up report showed that PKD2-haploinsufficient mice present a desensitized calcium contraction coupling in cardiomyocytes and an altered response to adrenergic stimulus [114]. PC2 is also important for the regulation of ryanodine receptors’ RyR2 function, and, thus, the loss of RyR2 regulation that occurs when PC2 is mutated results in altered calcium signaling in the heart [107]. Moreover, cardiomyocyte specific PC2 knockout mice manifest an impaired autophagic flux in the setting of nutrient deprivation, due to the altered control of intracellular calcium homeostasis [115].The role of polycystin proteins for autophagy, through which the cell can remove the buildup of deleterious protein components, might affect cardiomyocytes’ sarcomeric function and impair the ability of the cardiomyocytes to contract, and ultimately results in a stiffer and less compliant heart [117]. This mechanism may in part explain the decreased torsional compliance of ADPKD patients’ hearts [104].
A study that used ADPKD patient-specific induced pluripotent stem cells differentiated toward ventricular-like cardiomyocytes, confirmed the PC1 and PC2 cardiomyocyte expression and showed that the PKD mutation per se is a cause of cardiomyocyte calcium cycling abnormality and is proarrhythmogenic [118]. These findings are consistent with previous observations in mouse models. The most relevant result was the close correspondence between the iPSC-derived cardiomyocytes behavior and the donor patient’s clinical phenotype.
Nevertheless, unraveling the organ-specific pathophysiology of a human disease with multiple organ involvement such as ADPKD is very challenging. Figure 1 provides an overview of the mechanisms of cardiac involvement.
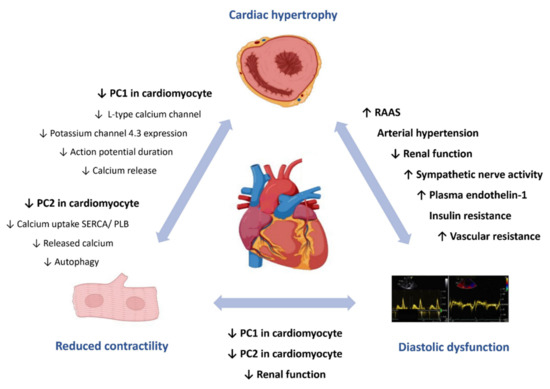
Figure 1.
Autosomal dominant polycystic kidney disease and the heart. Current understanding of pathophysiological mechanisms underlying cardiac involvement in autosomal dominant polycystic kidney disease. Activation of RAAS and other mechanisms, including the increased activity of the sympathetic nervous system, the decline of renal function, insulin resistance, disturbances in the fine- tuning of vascular tone and arterial hypertension, are responsible for cardiac hypertrophy and left ventricular diastolic dysfunction. A reduced activity of cardiomyocyte PC1 or PC2 is thought to reduce myocardial contractility and contribute to diastolic dysfunction. PC, polycystin; RAAS, renin–angiotensin–aldosterone system; SERCA, sarco/endoplasmic reticulum calcium-ATPase; PLB, phospholamban.
A relatively frequent coexistence of ADPKD and inherited cardiomyopathies such as idiopathic dilated cardiomyopathy and hypertrophic obstructive cardiomyopathy has been found out by reviewing the ADPKD database of a large tertiary center [119]. A genetic interaction between the PKD genes and the genes mutated in inherited cardiomyopathies can be hypothesized, rather than ADPKD being the direct cause of the cardiomyopathies.
2. Conclusions
Taken together, data from clinical and experimental studies provide compelling evidence that the cardiovascular complications of ADPKD are due at least in part to primary manifestations of the mutant proteins. Considering the importance of the polycystin proteins in cardiac development and myocardial function, the heart should be regarded as a target organ in ADPKD patients. Understanding the pathophysiologic mechanisms of heart involvement may be the foundation of novel therapy development.
Author Contributions
L.S.: proposed the idea, proposed the structure of the paper, wrote the paper; G.G.: provided a substantial contribution to the interpretation of data from the research literature; G.E.: critically appraised the paper, made final suggestions. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Torres, V.E.; Harris, P.C.; Pirson, Y. Autosomal dominant polycystic kidney disease. Lancet 2007, 369, 1287–1301. [Google Scholar] [CrossRef]
- Bergmann, C.; Guay-Woodford, L.M.; Harris, P.C.; Horie, S.; Peters, D.J.M.; Torres, V.E. Polycystic kidney disease. Nat. Rev. Dis. Primers 2018, 4, 50. [Google Scholar] [CrossRef]
- Schrier, R.W.; Mcfann, K.K.; Johnson, A.M. Epidemiological study of kidney survival in autosomal dominant polycystic kidney disease. Kidney Int. 2003, 63, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Willey, C.J.; Blais, J.D.; Hall, A.K.; Krasa, H.B.; Makin, A.J.; Czerwiec, F.S. Prevalence of autosomal dominant polycystic kidney disease in the European Union. Nephrol. Dial. Transplant. 2017, 32, 1356–1363. [Google Scholar] [CrossRef] [PubMed]
- Willey, C.; Kamat, S.; Stellhorn, R.; Blais, J. Analysis of nationwide data to determine the incidence and diagnosed prevalence of autosomal dominant polycystic kidney disease in the USA: 2013–2015. Kidney Dis. 2019, 5, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Kimberling, W.J.; Fain, P.R.; Kenyon, J.B.; Goldgar, D.; Sujansky, E.; Gabow, P.A. Linkage heterogeneity of autosomal dominant polycystic kidney disease. N. Engl. J. Med. 1988, 319, 913–918. [Google Scholar] [CrossRef]
- Ward, C.J.; Turley, H.; Ong, A.C.; Comley, M.; Biddolph, S.; Chetty, R.; Ratcliffe, P.J.; Gattner, K.; Harris, P.C. Polycystin, the polycystic kidney disease 1 protein, is expressed by epithelial cells in fetal, adult, and polycystic kidney. Proc. Natl. Acad. Sci. USA 1996, 93, 1524–1528. [Google Scholar] [CrossRef] [PubMed]
- Ibraghimov-Beskrovnaya, O.; Dackowski, W.R.; Foggensteiner, L.; Coleman, N.; Thiru, S.; Petry, L.R.; Burn, T.C.; Connors, T.D.; van Raay, T.; Bradley, J.; et al. Polycystin: In vitro synthesis, in vivo tissue expression, and subcellular localization identifies a large membrane-associated protein. Proc. Natl. Acad. Sci. USA 1997, 94, 6397–6402. [Google Scholar] [CrossRef]
- Peters, D.J.; Spruit, L.; Saris, J.J.; Ravine, D.; Sandkuijl, L.A.; Fossdal, R.; van Eijk, R.; Norby, S.; Constantinou-Deltas, C.D.; Pierides, A.; et al. Chromosome 4 localization of a second gene for autosomal dominant polycystic kidney disease. Nat. Genet. 1993, 5, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, T.; Wu, G.; Hayashi, T.; Xenophontos, S.L.; Veldhuisen, B.; Saris, J.J.; Reynolds, D.M.; Cai, Y.; Gabow, P.A.; Pierides, A.; et al. PKD2, a gene for polycystic kidney disease that encodes an integral membrane protein. Science 1996, 272, 1339–1342. [Google Scholar] [CrossRef]
- Calvet, J.P.; Grantham, J.J. The genetics and physiology of polycystic kidney disease. Semin. Nephrol. 2001, 21, 107–123. [Google Scholar] [CrossRef]
- Grantham, J.J. Polycystic kidney disease: From the bedside to the gene and back. Curr. Opin. Nephrol. Hypertens. 2001, 10, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Cornec-Le Gall, E.; Torres, V.E.; Harris, P.C. Genetic complexity of autosomal dominant polycystic kidney and liver diseases. J. Am. Soc. Nephrol. 2018, 29, 13–23. [Google Scholar] [CrossRef]
- Fedeles, S.V.; Tian, X.; Gallagher, A.R.; Mitobe, M.; Nishio, S.; Lee, S.H.; Cai, Y.; Geng, L.; Crews, C.M.; Somlo, S.A. Genetic interaction network of five genes for human polycystic kidney and liver diseases defines polycystin-1 as the central determinant of cyst formation. Nat. Genet. 2011, 43, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Qian, F.; Germino, F.J.; Cai, Y.; Zhang, X.; Somlo, S.; Germino, G.G. PKD1 interacts with PKD2 through a probable coiled-coil domain. Nat. Genet. 1997, 16, 179–183. [Google Scholar] [CrossRef]
- Tsiokas, L.; Kim, E.; Arnould, T.; Sukhatme, V.P.; Walz, G. Homo- and heterodimeric interactions between the gene products of PKD1 and PKD2. Proc. Natl. Acad. Sci. USA 1997, 94, 6965–6970. [Google Scholar] [CrossRef] [PubMed]
- Hanaoka, K.; Qian, F.; Boletta, A.; Bhunia, A.K.; Piontek, K.; Tsiokas, L.; Sukhatme, V.P.; Guggino, W.B.; Germino, G.G. Co-assembly of polycystin-1 and -2 produces unique cation-permeable currents. Nature 2000, 408, 990–994. [Google Scholar] [CrossRef]
- Chauvet, V.; Tian, X.; Husson, H.; Grimm, D.H.; Wang, T.; Hiesberger, T.; Igarashi, P.; Bennett, A.M.; Ibraghimov-Beskrovnaya, O.; Somlo, S.; et al. Mechanical stimuli induce cleavage and nuclear translocation of the polycystin–1 C terminus. J. Clin. Investig. 2004, 114, 1433–1443. [Google Scholar] [CrossRef]
- Low, S.H.; Vasanth, S.; Larson, C.H.; Mukherjee, S.; Sharma, N.; Kinter, M.T.; Kane, M.E.; Obara, T.; Weimbs, T. Polycystin-1, STAT6, and P100 function in a pathway that transduces ciliary mechanosensation and is activated in polycystic kidney disease. Dev. Cell 2006, 10, 57–69. [Google Scholar] [CrossRef]
- Harris, P.C.; Torres, V.E. Genetic mechanisms and signaling pathways in autosomal dominant polycystic kidney disease (ADPKD). J. Clin. Investig. 2014, 124, 2315–2324. [Google Scholar] [CrossRef]
- Wallace, D.P. Cyclic AMP-mediated cyst expansion. Biochim. Biophys. Acta 2011, 1812, 1291–1300. [Google Scholar] [CrossRef] [PubMed]
- Mekahli, D.; Parys, J.B.; Bultynck, G.; Missiaen, L.; de Smedt, H. Polycystins and cellular Ca2+ signaling. Cell. Mol. Life Sci. 2013, 70, 2697–2712. [Google Scholar] [CrossRef] [PubMed]
- Rossetti, S.; Kubly, V.J.; Consugar, M.B.; Hopp, K.; Roy, S.; Horsley, S.W.; Chauveau, D.; Rees, L.; Barratt, T.M.; van’t Hoff, W.G.; et al. Incompletely penetrant PKD1 alleles suggest a role for gene dosage in cyst initiation in polycystic kidney disease. Kidney Int. 2009, 75, 848–855. [Google Scholar] [CrossRef]
- Chapin, H.C.; Caplan, M.J. The cell biology of polycystic kidney disease. J. Cell Biol. 2010, 191, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Hateboer, N.; V Dijk, M.A.; Bogdanova, N.; Coto, E.; Saggar-Malik, A.K.; San Millan, J.L.; Torra, R.; Breuning, M.; Ravine, D. Comparison of phenotypes of polycystic kidney disease types 1 and 2. European PKD1–PKD2 study group. Lancet 1999, 353, 103–107. [Google Scholar] [CrossRef]
- Audrézet, M.P.; Cornec-Le Gall, E.; Chen, J.M.; Redon, S.; Quéré, I.; Creff, J.; Bénech, C.; Maestri, S.; Le Meur, Y.; Férec, C. Autosomal dominant polycystic kidney disease: Comprehensive mutation analysis of PKD1 and PKD2 in 700 unrelated patients. Hum. Mutat. 2012, 33, 1239–1250. [Google Scholar] [CrossRef]
- Heyer, C.M.; Sundsbak, J.L.; Abebe, K.Z.; Chapman, A.B.; Torres, V.E.; Grantham, J.J.; Bae, K.T.; Schrier, R.W.; Perrone, R.D.; Braun, W.E.; et al. Predicted mutation strength of nontruncating PKD1 mutations aids genotype-phenotype correlations in autosomal dominant polycystic kidney disease. J. Am. Soc. Nephrol. 2016, 27, 2872–2884. [Google Scholar] [CrossRef]
- MacDermot, K.D.; Saggar-Malik, A.K.; Economides, D.L. Prenatal diagnosis of autosomal dominant polycystic kidney disease (PKD1) presenting in utero and prognosis for very early onset disease. J. Med. Genet. 1998, 35, 13–16. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Igarashi, P.; Somlo, S. Genetics and pathogenesis of polycystic kidney disease. J. Am. Soc. Nephrol. 2002, 13, 2384–2398. [Google Scholar] [CrossRef]
- Wilson, P.D. Polycystic kidney disease. N. Engl. J. Med. 2004, 350, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Grantham, J.J. Clinical practice. Autosomal dominant polycystic kidney disease. N. Engl. J. Med. 2008, 359, 1477–1485. [Google Scholar] [CrossRef] [PubMed]
- Zerres, K.; Rudnik-Schöneborn, S.; Deget, F. Childhood onset autosomal dominant polycystic kidney disease in sibs: Clinical picture and recurrence risk. German working group on paediatric nephrology (arbeitsgemeinschaft für pädiatrische nephrology). J. Med. Genet. 1993, 30, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Shamshirsaz, A.A.; Reza Bekheirnia, M.; Kamgar, M.; Johnson, A.M.; McFann, K.; Cadnapaphornchai, M.; Nobakhthaghighi, N.; Schrier, R.W. Autosomal-dominant polycystic kidney disease in infancy and childhood: Progression and outcome. Kidney Int. 2005, 68, 2218–2224. [Google Scholar] [CrossRef] [PubMed]
- Gattone, V.H.; Wang, X.; Harris, P.C.; Torres, V.E. Inhibition of renal cystic disease development and progression by a vasopressin V2 receptor antagonist. Nat. Med. 2003, 9, 1323–1326. [Google Scholar] [CrossRef] [PubMed]
- Torres, V.E.; Wang, X.; Qian, Q.; Somlo, S.; Harris, P.C.; Gattone, V.H. Effective treatment of an orthologous model of autosomal dominant polycystic kidney disease. Nat. Med. 2004, 10, 363–364. [Google Scholar] [CrossRef]
- Nagao, S.; Nishii, K.; Katsuyama, M.; Kurahashi, H.; Marunouchi, T.; Takahashi, H.; Wallace, D.P. Increased water intake decreases progression of polycystic kidney disease in the PCK rat. J. Am. Soc. Nephrol. 2006, 17, 228–235. [Google Scholar] [CrossRef]
- Wong, H.; Vivian, L.; Weiler, G.; Filler, G. Patients with autosomal dominant polycystic kidney disease hyperfiltrate early in their disease. Am. J. Kidney Dis. 2004, 43, 624–628. [Google Scholar] [CrossRef]
- Chapman, A.B.; Johnson, A.; Gabow, P.A.; Schrier, R.W. The renin-angiotensin-aldosterone system and autosomal dominant polycystic kidney disease. N. Engl. J. Med. 1990, 323, 1091–1096. [Google Scholar] [CrossRef]
- Torres, V.E.; Wilson, D.M.; Burnett, J.C.J.; Johnson, C.M.; Offord, K.P. Effect of inhibition of converting enzyme on renal hemodynamics and sodium management in polycystic kidney disease. Mayo Clin. Proc. 1991, 66, 1010–1017. [Google Scholar] [CrossRef]
- Watson, M.; Macnicol, A.; Allan, P.; Wright, A. Effects of angiotensin-converting enzyme inhibition in adult polycystic kidney disease. Kidney Int. 1992, 41, 206–210. [Google Scholar] [CrossRef][Green Version]
- Harris, P.C.; Bae, K.T.; Rossetti, S.; Torres, V.E.; Grantham, J.J.; Chapman, A.B.; Guay-Woodford, L.M.; King, B.F.; Wetzel, L.H.; Baumgarten, D.A.; et al. Cyst number but not the rate of cystic growth is associated with the mutated gene in autosomal dominant polycystic kidney disease. J. Am. Soc. Nephrol. 2006, 17, 3013–3019. [Google Scholar] [CrossRef] [PubMed]
- Helal, I.; Reed, B.; Mettler, P.; Mc Fann, K.; Tkachenko, O.; Yan, X.D.; Schrier, R.W. Prevalence of cardiovascular events in patients with autosomal dominant polycystic kidney disease. Am. J. Nephrol. 2012, 36, 362–370. [Google Scholar] [CrossRef]
- Kelleher, C.L.; McFann, K.K.; Johnson, A.M.; Schrier, R.W. Characteristics of hypertension in young adults with autosomal dominant polycystic kidney disease compared with the general US population. Am. J. Hypertens. 2004, 17, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Seeman, T.; Dusek, J.; Vondrák, K.; Bláhová, K.; Simková, E.; Kreisinger, J.; Dvorák, P.; Kyncl, M.; Hríbal, Z.; Janda, J. Renal concentrating capacity is linked to blood pressure in children with autosomal dominant polycystic kidney disease. Physiol. Res. 2004, 53, 629–634. [Google Scholar]
- Schrier, R.W. Hypertension and autosomal dominant polycystic kidney disease. Am. J. Kidney Dis. 2011, 57, 811–813. [Google Scholar] [CrossRef] [PubMed]
- Harrap, S.B.; Davies, D.L.; Macnicol, A.M.; Dominiczak, A.F.; Fraser, R.; Wright, A.F.; Watson, M.L.; Briggs, J.D. Renal, cardiovascular and hormonal characteristics of young adults with autosomal dominant polycystic kidney disease. Kidney Int. 1991, 40, 501–508. [Google Scholar] [CrossRef]
- Rahbari-Oskoui, F.; Williams, O.; Chapman, A. Mechanisms and management of hypertension in autosomal dominant polycystic kidney disease. Nephrol. Dial. Transplant. 2014, 29, 2194–2201. [Google Scholar] [CrossRef]
- Perrone, R.D.; Abebe, K.Z.; Schrier, R.W.; Chapman, A.B.; Torres, V.E.; Bost, J.; Kaya, D.; Miskulin, D.C.; Steinman, T.I.; Braun, W.; et al. Cardiac magnetic resonance assessment of left ventricular mass in autosomal dominant polycystic kidney disease. Clin. J. Am. Soc. Nephrol. 2011, 6, 2508–2515. [Google Scholar] [CrossRef] [PubMed]
- Schrier, R.W.; Abebe, K.Z.; Perrone, R.D.; Torres, V.E.; Braun, W.E.; Steinman, T.I.; Winklhofer, F.T.; Brosnahan, G.; Czarnecki, P.G.; Hogan, M.C.; et al. Blood pressure in early autosomal dominant polycystic kidney disease. N. Engl. J. Med. 2014, 371, 2255–2266. [Google Scholar] [CrossRef]
- Torres, V.E.; Abebe, K.Z.; Chapman, A.B.; Schrier, R.W.; Braun, W.E.; Steinman, T.I.; Winklhofer, F.T.; Brosnahan, G.; Czarnecki, P.G.; Hogan, M.C.; et al. Angiotensin blockade in late autosomal dominant polycystic kidney disease. N. Engl. J. Med. 2014, 371, 2267–2276. [Google Scholar] [CrossRef]
- Klein, I.H.; Ligtenberg, G.; Oey, P.L.; Koomans, H.A.; Blankestijn, P.J. Sympathetic activity is increased in polycystic kidney disease and associated with hypertension. J. Am. Soc. Nephrol. 2001, 12, 2427–2433. [Google Scholar] [PubMed]
- Merta, M.; Reiterová, J.; Rysavá, R.; Tesar, V.; Závada, J.; Jáchymová, M.; Zima, T. Role of endothelin and nitric oxide in the pathogenesis of arterial hypertension in autosomal dominant polycystic kidney disease. Physiol. Res. 2003, 52, 433–437. [Google Scholar]
- Vareesangthip, K.; Tong, P.; Wilkinson, R.; Thomas, T.H. Insulin resistance in adult polycystic kidney disease. Kidney Int. 1997, 52, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Lumiaho, A.; Pihlajamäki, J.; Hartikainen, J.; Ikäheimo, R.; Miettinen, R.; Niemitukia, L.; Lampainen, E.; Laakso, M. Insulin resistance is related to left ventricular hypertrophy in patients with polycystic kidney disease type 1. Am. J. Kidney Dis. 2003, 41, 1219–1224. [Google Scholar] [CrossRef]
- Zeier, M.; Geberth, S.; Schmidt, K.G.; Mandelbaum, A.; Ritz, E. Elevated blood pressure profile and left ventricular mass in children and young adults with autosomal dominant polycystic kidney disease. J. Am. Soc. Nephrol. 1993, 3, 1451–1457. [Google Scholar]
- Martinez-Vea, A.; Bardaj, A.; Gutierrez, C.; Garca, C.; Peralta, C.; Marcas, L.; Oliver, J.A. Exercise blood pressure, cardiac structure, and diastolic dysfunction in young normotensive patients with polycystic kidney disease: A prehypertensive state. Am. J. Kidney Dis. 2004, 44, 216–223. [Google Scholar] [CrossRef]
- Geng, L.; Segal, Y.; Pavlova, A.; Barros, E.J.; Löhning, C.; Lu, W.; Nigam, S.K.; Frischauf, A.M.; Reeders, S.T.; Zhou, J. Distribution and developmentally regulated expression of murine polycystin. Am. J. Physiol. 1997, 272, F451–F459. [Google Scholar] [CrossRef]
- Markowitz, G.S.; Cai, Y.; Li, L.; Wu, G.; Ward, L.C.; Somlo, S.; D’Agati, V.D. Polycystin-2 expression is developmentally regulated. Am. J. Physiol. 1999, 277, F17–F25. [Google Scholar] [CrossRef]
- Griffin, M.D.; Torres, V.E.; Grande, J.P.; Kumar, R. Vascular expression of polycystin. J. Am. Soc. Nephrol. 1997, 8, 616–626. [Google Scholar] [PubMed]
- Torres, V.E.; Cai, Y.; Chen, X.; Wu, G.Q.; Geng, L.; Cleghorn, K.A.; Johnson, C.M.; Somlo, S. Vascular expression of polycystin 2. J. Am. Soc. Nephrol. 2001, 12, 1–9. [Google Scholar] [PubMed]
- Qian, Q.; Hunter, L.W.; Du, H.; Ren, Q.; Han, Y.; Sieck, G.C. PKD2+/− vascular smooth muscles develop exaggerated vasocontraction in response to phenylephrine stimulation. J. Am. Soc. Nephrol. 2007, 18, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Kip, S.N.; Hunter, L.W.; Ren, Q.; Harris, P.C.; Somlo, S.; Torres, V.E.; Sieck, G.C.; Qian, Q. [Ca2+] i reduction increases cellular proliferation and apoptosis in vascular smooth muscle cells: Relevance to the ADPKD phenotype. Circ. Res. 2005, 96, 873–880. [Google Scholar] [CrossRef]
- Wang, D.; Iversen, J.; Wilcox, C.S.; Strandgaard, S. Endothelial dysfunction and reduced nitric oxide in resistance arteries in autosomal-dominant polycystic kidney disease. Kidney Int. 2003, 64, 1381–1388. [Google Scholar] [CrossRef] [PubMed]
- Bichet, D.; Peters, D.; Patel, A.J.; Delmas, P.; Honoré, E. Cardiovascular polycystins: Insights from autosomal dominant polycystic kidney disease and transgenic animal models. Trends Cardiovasc. Med. 2006, 16, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Wang, C.J.; Judge, D.P.; Halushka, M.K.; Ni, J.; Habashi, J.P.; Moslehi, J.; Bedja, D.; Gabrielson, K.L.; Xu, H.; et al. A Pkd1-Fbn1 genetic interaction implicates TGF-beta signaling in the pathogenesis of vascular complications in autosomal dominant polycystic kidney disease. J. Am. Soc. Nephrol. 2014, 25, 81–91. [Google Scholar] [CrossRef]
- Boulter, C.; Mulroy, S.; Webb, S.; Fleming, S.; Brindle, K.; Sandford, R. Cardiovascular, skeletal, and renal defects in mice with a targeted disruption of the Pkd1 gene. Proc. Natl. Acad. Sci. USA 2001, 98, 12174–12179. [Google Scholar] [CrossRef]
- Ecder, T.; Schrier, R.W. Cardiovascular abnormalities in autosomal-dominant polycystic kidney disease. Nat. Rev. Nephrol. 2009, 5, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Honoré, E. Polycystins and renovascular mechanosensory transduction. Nat. Rev. Nephrol. 2010, 6, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Nowak, K.L.; Farmer, H.; Cadnapaphornchai, M.A.; Gitomer, B.; Chonchol, M. Vascular dysfunction in children and young adults with autosomal dominant polycystic kidney disease. Nephrol. Dial. Transplant. 2017, 32, 342–347. [Google Scholar] [CrossRef]
- Sharif-Naeini, R.; Folgering, J.H.; Bichet, D.; Duprat, F.; Lauritzen, I.; Arhatte, M.; Jodar, M.; Dedman, A.; Chatelain, F.C.; Schulte, U.; et al. Polycystin-1 and -2 dosage regulates pressure sensing. Cell 2009, 139, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Clausen, P.; Feldt-Rasmussen, B.; Iversen, J.; Lange, M.; Eidemak, I.; Strandgaard, S. Flow-associated dilatory capacity of the brachial artery is intact in early autosomal dominant polycystic kidney disease. Am. J. Nephrol. 2006, 26, 335–339. [Google Scholar] [CrossRef]
- Kocaman, O.; Oflaz, H.; Yekeler, E.; Dursun, M.; Erdogan, D.; Demirel, S.; Alisir, S.; Turgut, F.; Mercanoglu, F.; Ecder, T. Endothelial dysfunction and increased carotid intima-media thickness in patients with autosomal dominant polycystic kidney disease. Am. J. Kidney Dis. 2004, 43, 854–860. [Google Scholar] [CrossRef] [PubMed]
- Peterson, K.M.; Franchi, F.; Loeffler, D.L.; Psaltis, P.J.; Harris, P.C.; Lerman, L.O.; Lerman, A.; Rodriguez-Porcel, M. Endothelial dysfunction occurs prior to clinical evidence of polycystic kidney disease. Am. J. Nephrol. 2013, 38, 233–240. [Google Scholar] [CrossRef]
- Klawitter, J.; Reed-Gitomer, B.Y.; McFann, K.; Pennington, A.; Klawitter, J.; Abebe, K.Z.; Klepacki, J.; Cadnapaphornchai, M.A.; Brosnahan, G.; Chonchol, M.; et al. Endothelial dysfunction and oxidative stress in polycystic kidney disease. Am. J. Physiol. Renal Physiol. 2014, 307, F1198–F1206. [Google Scholar] [CrossRef]
- Huang, J.L.; Woolf, A.S.; Kolatsi-Joannou, M.; Baluk, P.; Sandford, R.N.; Peters, D.J.; McDonald, D.M.; Price, K.L.; Winyard, P.J.; Long, D.A. Vascular endothelial growth factor C for polycystic kidney diseases. J. Am. Soc. Nephrol. 2016, 27, 69–77. [Google Scholar] [CrossRef]
- Outeda, P.; Huso, D.L.; Fisher, S.A.; Halushka, M.K.; Kim, H.; Qian, F.; Germino, G.G.; Watnick, T. Polycystin signaling is required for directed endothelial cell migration and lymphatic development. Cell Rep. 2014, 7, 634–644. [Google Scholar] [CrossRef] [PubMed]
- Lorthioir, A.; Joannidès, R.; Rémy-Jouet, I.; Fréguin-Bouilland, C.; Iacob, M.; Roche, C.; Monteil, C.; Lucas, D.; Renet, S.; Audrézet, M.P.; et al. Polycystin deficiency induces dopamine-reversible alterations in flow-mediated dilatation and vascular nitric oxide release in humans. Kidney Int. 2015, 87, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Morel, N.; Vandenberg, G.; Ahrabi, A.K.; Caron, N.; Desjardins, F.; Balligand, J.L.; Horie, S.; Devuyst, O. PKD1 haploinsufficiency is associated with altered vascular reactivity and abnormal calcium signaling in the mouse aorta. Pflugers Archiv 2009, 457, 845–856. [Google Scholar] [CrossRef] [PubMed]
- Qian, Q.; Hunter, L.W.; Li, M.; Marin-Padilla, M.; Prakash, Y.S.; Somlo, S.; Harris, P.C.; Torres, V.E.; Sieck, G.C. Pkd2 haploinsufficiency alters intracellular calcium regulation in vascular smooth muscle cells. Hum. Mol. Genet. 2003, 12, 1875–1880. [Google Scholar] [CrossRef] [PubMed]
- Qian, Q.; Li, M.; Cai, Y.; Ward, C.J.; Somlo, S.; Harris, P.C.; Torres, V.E. Analysis of the polycystins in aortic vascular smooth muscle cells. J. Am. Soc. Nephrol. 2003, 14, 2280–2287. [Google Scholar] [CrossRef] [PubMed]
- Hassane, S.; Claij, N.; Lantinga-van Leeuwen, I.S.; van Munsteren, J.C.; van Lent, N.; Hanemaaijer, R.; Breuning, M.H.; Peters, D.J.; DeRuiter, M.C. Pathogenic sequence for dissecting aneurysm formation in a hypomorphic polycystic kidney disease 1 mouse model. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2177–2183. [Google Scholar] [CrossRef]
- Hadimeri, H.; Lamm, C.; Nyberg, G. Coronary aneurysms in patients with autosomal dominant polycystic kidney disease. J. Am. Soc. Nephrol. 1998, 9, 837–841. [Google Scholar]
- Belz, M.M.; Hughes, R.L.; Kaehny, W.D.; Johnson, A.M.; Fick-Brosnahan, G.M.; Earnest, M.P.; Gabow, P.A. Familial clustering of ruptured intracranial aneurysms in autosomal dominant polycystic kidney disease. Am. J. Kidney Dis. 2001, 38, 770–776. [Google Scholar] [CrossRef]
- Biagini, A.; Maffei, S.; Baroni, M.; Piacenti, M.; Terrazzi, M.; Paoli, F.; Trianni, G.; Picano, E.; Salvatore, L. Familiar clustering of aortic dissection in polycystic kidney disease. Am. J. Cardiol. 1993, 72, 741–742. [Google Scholar] [CrossRef]
- Neves, J.B.; Rodrigues, F.B.; Lopes, J.A. Autosomal dominant polycystic kidney disease and coronary artery dissection or aneurysm: A systematic review. Ren. Fail. 2016, 38, 493–502. [Google Scholar] [CrossRef]
- Jiang, L.C.; Cao, J.Y.; Chen, M. Coronary artery aneurysm combined with other multiple aneurysms at multiple locations: A case report and systematic review. Medicine 2017, 96, e9230. [Google Scholar] [CrossRef] [PubMed]
- Oomman, A.; Ramachandran, P.; Kumar, N.S. Combined presence of coronary artery ectasia and descending aortic dissection in polycystic kidney disease presenting as acute coronary syndrome. Indian Heart J. 2003, 55, 646–648. [Google Scholar] [PubMed]
- Lee, C.C.; Chang, W.T.; Fang, C.C.; Tsai, I.L.; Chen, W.J. Sudden death caused by dissecting thoracic aortic aneurysm in a patient with autosomal dominant polycystic kidney disease. Resuscitation 2004, 63, 93–96. [Google Scholar] [CrossRef]
- Nacasch, N.; Werner, M.; Golan, E.; Korzets, Z. Arterial dissections in autosomal dominant polycystic kidney disease—Chance association or part of the disease spectrum? Clin. Nephrol. 2010, 73, 478–481. [Google Scholar] [CrossRef]
- Silverio, A.; Prota, C.; Di Maio, M.; Polito, M.V.; Cogliani, F.M.; Citro, R.; Gigantino, A.; Iesu, S.; Piscione, F. Aortic dissection in patients with autosomal dominant polycystic kidney disease: A series of two cases and a review of the literature. Nephrology 2015, 20, 229–235. [Google Scholar] [CrossRef]
- Ivy, D.D.; Shaffer, E.M.; Johnson, A.M.; Kimberling, W.J.; Dobin, A.; Gabow, P.A. Cardiovascular abnormalities in children with autosomal dominant polycystic kidney disease. J. Am. Soc. Nephrol. 1995, 5, 2032–2036. [Google Scholar] [PubMed]
- Lumiaho, A.; Ikäheimo, R.; Miettinen, R.; Niemitukia, L.; Laitinen, T.; Rantala, A.; Lampainen, E.; Laakso, M.; Hartikainen, J. Mitral valve prolapse and mitral regurgitation are common in patients with polycystic kidney disease type 1. Am. J. Kidney Dis. 2001, 38, 1208–1216. [Google Scholar] [CrossRef]
- Chapman, A.B.; Johnson, A.M.; Rainguet, S.; Hossack, K.; Gabow, P.; Schrier, R.W. Left ventricular hypertrophy in autosomal dominant polycystic kidney disease. J. Am. Soc. Nephrol. 1997, 8, 1292–1297. [Google Scholar]
- Saggar-Malik, A.K.; Missouris, C.G.; Gill, J.S.; Singer, D.R.; Markandu, N.D.; MacGregor, G.A. Left ventricular mass in normotensive subjects with autosomal dominant polycystic kidney disease. BMJ 1994, 309, 1617–1618. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Vea, A.; Valero, F.A.; Bardaji, A.; Gutierrez, C.; Broch, M.; Garcia, C.; Richart, C.; Oliver, J.A. Left ventricular hypertrophy in hypertensive patients with autosomal dominant polycystic kidney disease: Influence of blood pressure and humoral and neurohormonal factors. Am. J. Nephrol. 2000, 20, 193–200. [Google Scholar] [CrossRef]
- Bardají, A.; Vea, A.M.; Gutierrez, C.; Ridao, C.; Richart, C.; Oliver, J.A. Left ventricular mass and diastolic function in normotensive young adults with autosomal dominant polycystic kidney disease. Am. J. Kidney Dis. 1998, 32, 970–975. [Google Scholar] [CrossRef]
- Valero, F.A.; Martinez-Vea, A.; Bardají, A.; Gutierrez, C.; Garcia, C.; Richart, C.; Oliver, J.A. Ambulatory blood pressure and left ventricular mass in normotensive patients with autosomal dominant polycystic kidney disease. J. Am. Soc. Nephrol. 1999, 10, 1020–1026. [Google Scholar]
- Schrier, R.W. Renal volume; renin-angiotensin-aldosterone system; hypertension; and left ventricular hypertrophy in patients with autosomal dominant polycystic kidney disease. J. Am. Soc. Nephrol. 2009, 20, 1888–1893. [Google Scholar] [CrossRef]
- Chen, H.; Watnick, T.; Hong, S.N.; Daly, B.; Li, Y.; Seliger, S.L. Left ventricular hypertrophy in a contemporary cohort of autosomal dominant polycystic kidney disease patients. BMC Nephrol. 2019, 20, 386. [Google Scholar] [CrossRef]
- Mancuso, P.; Rahman, A.; Hershey, S.D.; Dandu, L.; Nibbelink, K.A.; Simpson, R.U. 1,25-Dihydroxyvitamin-D3 treatment reduces cardiac hypertrophy and left ventricular diameter in spontaneously hypertensive heart failure-prone (cp/+) rats independent of changes in serum leptin. J. Cardiovasc. Pharmacol. 2008, 51, 559–564. [Google Scholar] [CrossRef]
- Cadnapaphornchai, M.A.; McFann, K.; Strain, J.D.; Masoumi, A.; Schrier, R.W. Increased left ventricular mass in children with autosomal dominant polycystic kidney disease and borderline hypertension. Kidney Int. 2008, 74, 1192–1196. [Google Scholar] [CrossRef]
- Oflaz, H.; Alisir, S.; Buyukaydin, B.; Kocaman, O.; Turgut, F.; Namli, S.; Pamukcu, B.; Oncul, A.; Ecder, T. Biventricular diastolic dysfunction in patients with autosomal dominant polycystic kidney disease. Kidney Int. 2005, 68, 2244–2249. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, L.; Pisani, A.; Giugliano, G.; Trimarco, B.; Riccio, E.; Visciano, B.; Remuzzi, G.; Ruggenenti, P. Data on the assessment of LV mechanics by speckle tracking echocardiography in ADPKD patients. Data Brief 2018, 21, 2075–2081. [Google Scholar] [CrossRef]
- Spinelli, L.; Pisani, A.; Giugliano, G.; Trimarco, B.; Riccio, E.; Visciano, B.; Remuzzi, G.; Ruggenenti, P.; ALADIN-Cardiovascular Study Group. Left ventricular dysfunction in ADPKD and effects of octreotide-LAR: A cross-sectional and longitudinal substudy of the ALADIN trial. Int. J. Cardiol. 2019, 275, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Somlo, S. Molecular genetics and mechanism of autosomal dominant polycystic kidney disease. Mol. Genet. Metab. 2000, 69, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Markowitz, G.S.; Li, L.; D’Agati, V.D.; Factor, S.M.; Geng, L.; Tibara, S.; Tuchman, J.; Cai, Y.; Park, J.H.; et al. Cardiac defects and renal failure in mice with targeted mutations in Pkd2. Nat. Genet. 2000, 24, 75–78. [Google Scholar] [CrossRef]
- Anyatonwu, G.I.; Estrada, M.; Tian, X.; Somlo, S.; Ehrlich, B.E. Regulation of ryanodine receptor dependent calcium signaling by polycystin-2. Proc. Natl. Acad. Sci. USA 2007, 104, 6454–6459. [Google Scholar] [CrossRef] [PubMed]
- Pedrozo, Z.; Criollo, A.; Battiprolu, P.K.; Morales, C.R.; Contreras-Ferrat, A.; Fernández, C.; Jiang, N.; Luo, X.; Caplan, M.J.; Somlo, S.; et al. Polycystin-1 is a cardiomyocyte mechanosensor that governs L-type Ca2+ channel protein stability. Circulation 2015, 131, 2131–2142. [Google Scholar] [CrossRef]
- Córdova-Casanova, A.; Olmedo, I.; Riquelme, J.A.; Barrientos, G.; Sánchez, G.; Gillette, T.G.; Lavandero, S.; Chiong, M.; Donoso, P.; Pedrozo, Z. Mechanical stretch increases L-type calcium channel stability in cardiomyocytes through a polycystin-1/AKT-dependent mechanism. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 289–296. [Google Scholar] [CrossRef]
- Balbo, B.E.; Amaral, A.G.; Fonseca, J.M.; de Castro, I.; Salemi, V.M.; Souza, L.E.; Dos Santos, F.; Irigoyen, M.C.; Qian, F.; Chammas, R.; et al. Cardiac dysfunction in Pkd1-deficient mice with phenotype rescue by galectin-3 knockout. Kidney Int. 2016, 90, 580–597. [Google Scholar] [CrossRef]
- Aránguiz, P.; Romero, P.; Vásquez, F.; Flores-Vergara, R.; Aravena, D.; Sánchez, G.; González, M.; Olmedo, I.; Pedrozo, Z. Polycystin-1 mitigates damage and regulates CTGF expression through AKT activation during cardiac ischemia/reperfusion. Biochim. Biophys. Acta Mol. Basis Dis. 2021, 1867, 165986. [Google Scholar] [CrossRef] [PubMed]
- Altamirano, F.; Schiattarella, G.G.; French, K.M.; Kim, S.Y.; Engelberger, F.; Kyrychenko, S.; Villalobos, E.; Tong, D.; Schneider, J.W.; Ramirez-Sarmiento, C.A.; et al. Polycystin-1 assembles with Kv channels to govern cardiomyocyte repolarization and contractility. Circulation 2019, 140, 921–936. [Google Scholar] [CrossRef] [PubMed]
- Paavola, J.; Schliffke, S.; Rossetti, S.; Kuo, I.Y.; Yuan, S.; Sun, Z.; Harris, P.C.; Torres, V.E.; Ehrlich, B.E. Polycystin-2 mutations lead to impaired calcium cycling in the heart and predispose to dilated cardiomyopathy. J. Mol. Cell Cardiol. 2013, 58, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Volk, T.; Schwoerer, A.P.; Thiessen, S.; Schultz, J.H.; Ehmke, H. A polycystin-2-like large conductance cation channel in rat left ventricular myocytes. Cardiovasc. Res. 2003, 58, 76–88. [Google Scholar] [CrossRef]
- Kuo, I.Y.; Kwaczala, A.T.; Nguyen, L.; Russell, K.S.; Campbell, S.G.; Ehrlich, B.E. Decreased polycystin 2 expression alters calcium-contraction coupling and changes β-adrenergic signaling pathways. Proc. Natl. Acad. Sci. USA 2014, 111, 16604–16609. [Google Scholar] [CrossRef] [PubMed]
- Criollo, A.; Altamirano, F.; Pedrozo, Z.; Schiattarella, G.G.; Li, D.L.; Rivera-Mejías, P.; Sotomayor-Flores, C.; Parra, V.; Villalobos, E.; Battiprolu, P.K.; et al. Polycystin-2-dependent control of cardiomyocyte autophagy. J. Mol. Cell Cardiol. 2018, 118, 110–121. [Google Scholar] [CrossRef]
- Kuo, I.Y.; Chapman, A.B. Polycystins, ADPKD, and cardiovascular disease. Kidney Int. Rep. 2019, 5, 396–406. [Google Scholar] [CrossRef]
- Lee, J.J.; Cheng, S.J.; Huang, C.Y.; Chen, C.Y.; Feng, L.; Hwang, D.Y.; Kamp, T.J.; Chen, H.C.; Hsieh, P.C.H. Primary cardiac manifestation of autosomal dominant polycystic kidney disease revealed by patient induced pluripotent stem cell-derived cardiomyocytes. EBioMedicine 2019, 40, 675–684. [Google Scholar] [CrossRef]
- Chebib, F.T.; Hogan, M.C.; El-Zoghby, Z.M.; Irazabal, M.V.; Senum, S.R.; Heyer, C.M.; Madsen, C.D.; Cornec-Le Gall, E.; Behfar, A.; Harris, P.C.; et al. Autosomal dominant polycystic kidney patients may be predisposed to various cardiomyopathies. Kidney Int. Rep. 2017, 2, 913–923. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).