Phytochemical Composition, Antioxidant and Antiproliferative Activities of Citrus hystrix, Citrus limon, Citrus pyriformis, and Citrus microcarpa Leaf Essential Oils against Human Cervical Cancer Cell Line
Abstract
:1. Introduction
2. Results and Discussion
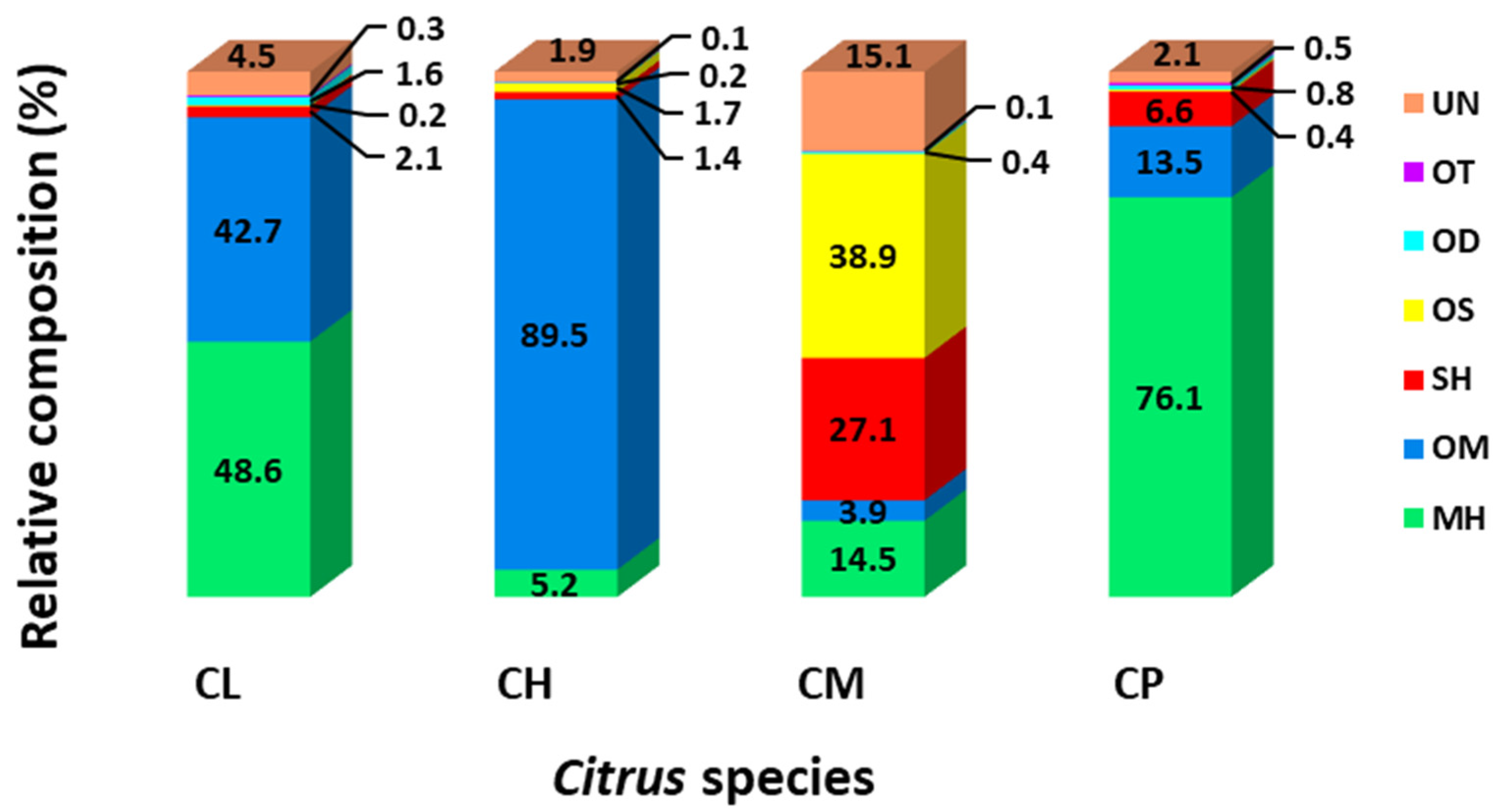
2.1. Phytochemical Compositions
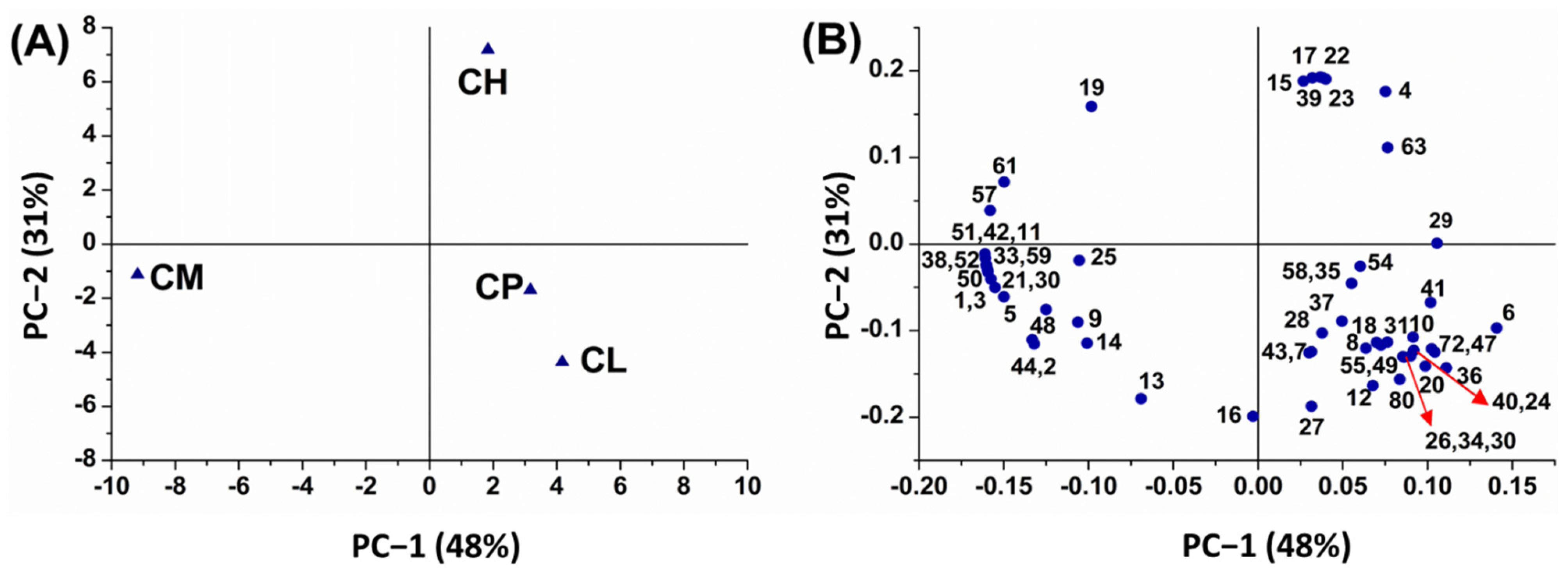
2.2. Discrimination via Principal Component Analysis
2.3. Antioxidant Activity
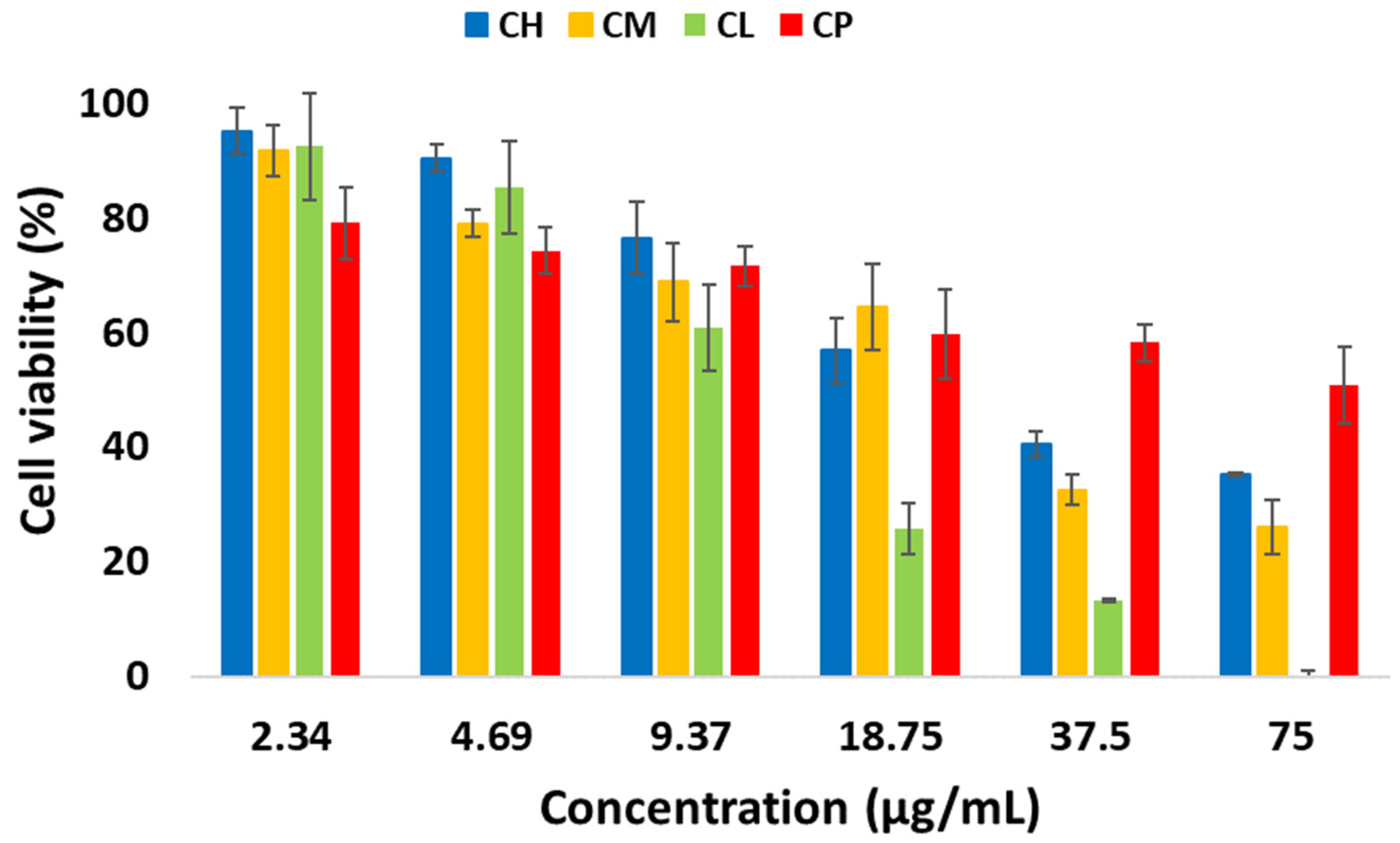
2.4. Antiproliferative Evaluation
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Plant Material and Isolation of Essential Oil
3.3. Gas Chromatography–Quadrupole Mass Spectrometry System
3.4. Data Handling
3.5. Antioxidant Activity by DPPH Assay
3.6. HeLa Cell Culture
3.7. Antiproliferative Activity with 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium Bromide (MTT) Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Talon, M.; Wu, G.A.; Gmitter, F.G., Jr.; Rokhsar, D.S. The Origin of Citrus. In The Genus Citrus; Talon, M., Caruso, M., Gmitter, F.G., Jr., Eds.; Woodhead Publishing: Duxford, UK, 2020; pp. 9–32. ISBN 012812217X. [Google Scholar]
- Tanaka, T. Fundamental Discussion of Citrus Classification. Stud. Citrol. 1977, 14, 1–6. [Google Scholar]
- Palazzolo, E.; Laudicina, V.A.; Germanà, M.A. Current and Potential Use of Citrus Essential Oils. Curr. Org. Chem. 2013, 17, 3042–3049. [Google Scholar] [CrossRef]
- Reddy, D.N. Essential Oils Extracted from Medicinal Plants and Their Applications. In Natural Bio-Active Compounds; Springer: Singapore, 2019; pp. 237–283. [Google Scholar]
- Klimek-szczykutowicz, M.; Szopa, A.; Ekiert, H. Citrus limon (Lemon) Phenomenon—A Review of the Chemistry, Pharmacological Properties, Applications in the Modern Pharmaceutical, Food, and Cosmetics Industries, and Biotechnological Studies. Plants 2020, 9, 119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, X.; Cao, S.; Sun, J.; Lu, D.; Zhong, B.; Chun, J. The Chemical Compositions, and Antibacterial and Antioxidant Activities of Four Types of Citrus Essential Oils. Molecules 2021, 26, 3412. [Google Scholar] [CrossRef]
- Raut, J.S.; Karuppayil, S.M. A Status Review on the Medicinal Properties of Essential Oils. Ind. Crops Prod. 2014, 62, 250–264. [Google Scholar] [CrossRef]
- Spadaro, F.; Circosta, C.; Costa, R.; Pizzimenti, F.; Palumbo, D.R.; Occhiuto, F. Volatile Fraction Composition and Biological Activity of Lemon Oil (Citrus limon L. Burm.): Comparative Study of Oils Extracted from Conventionally Grown and Biological Fruits. J. Essent. Oil Res. 2012, 24, 187–193. [Google Scholar] [CrossRef]
- Alinejhad, D.; Asayesh, M.; Asayesh, M. Determination of the Anti-Inflammatory Property of Tannins from the Rind of Calamansi (Citrus Microcarpa, Rutaceae). J. Int. Oral Health 2016, 8, 546–553. [Google Scholar] [CrossRef]
- Hamdan, D.; El-Readi, M.Z.; Tahrani, A.; Herrmann, F.; Kaufmann, D.; Farrag, N.; El-Shazly, A.; Wink, M. Secondary Metabolites of Ponderosa Lemon (Citrus Pyriformis) and Their Antioxidant, Anti-Inflammatory, and Cytotoxic Activities. Z. Für Naturforsch. C 2011, 66, 385–393. [Google Scholar] [CrossRef] [Green Version]
- Frassinetti, S.; Caltavuturo, L.; Cini, M.; Croce Della, C.M.; Maserti, B.E. Antibacterial and Antioxidant Activity of Essential Oils from Citrus spp. J. Essent. Oil Res. 2011, 23, 27–31. [Google Scholar] [CrossRef]
- Bertuzzi, G. Antioxidative Action of Citrus limonum Essential Oil on Skin. Eur. J. Med. Plants 2013, 3, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.N.T.; Huynh, T.N.N.; Tran, V.T.; Dang, C.H.; Hoang, T.K.D.; Nguyen, T.D. Physicochemical Characterization and Bioactivity Evaluation of Essential Oils from Citrus microcarpa Bunge Leaf and Flower. J. Essent. Oil Res. 2018, 30, 285–292. [Google Scholar] [CrossRef]
- Hosni, K.; Zahed, N.; Chrif, R.; Abid, I.; Medfei, W.; Kallel, M.; Ben Brahim, N.; Sebei, H. Composition of Peel Essential Oils from Four Selected Tunisian Citrus Species: Evidence for the Genotypic Influence. Food Chem. 2010, 123, 1098–1104. [Google Scholar] [CrossRef]
- Butnariu, M. Plants as Source of Essential Oils and Perfumery Applications. In Bioprospecting of Plant Biodiversity for Industrial Molecules; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2021; pp. 261–292. [Google Scholar]
- Ferrer, V.; Costantino, G.; Paoli, M.; Paymal, N.; Quinton, C.; Ollitrault, P.; Tomi, F.; Luro, F. Intercultivar Diversity of Sour Orange (Citrus aurantium L.) Based on Genetic Markers, Phenotypic Characteristics, Aromatic Compounds and Sensorial Analysis. Agronomy 2021, 11, 1084. [Google Scholar] [CrossRef]
- Koziol, A.; Stryjewska, A.; Librowski, T.; Salat, K.; Gawel, M.; Moniczewski, A.; Lochynski, S. An Overview of the Pharmacological Properties and Potential Applications of Natural Monoterpenes. Mini-Rev. Med. Chem. 2014, 14, 1156–1168. [Google Scholar] [CrossRef] [PubMed]
- Koene, R.J.; Prizment, A.E.; Blaes, A.; Konety, S.H. Shared Risk Factors in Cardiovascular Disease and Cancer. Circulation 2016, 133, 1104–1114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paz-Elizur, T.; Sevilya, Z.; Leitner-Dagan, Y.; Elinger, D.; Roisman, L.C.; Livneh, Z. DNA Repair of Oxidative DNA Damage in Human Carcinogenesis: Potential Application for Cancer Risk Assessment and Prevention. Cancer Lett. 2008, 266, 60–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mittal, R.P.; Rana, A.; Jaitak, V. Essential Oils: An Impending Substitute of Synthetic Antimicrobial Agents to Overcome Antimicrobial Resistance. Curr. Drug Targets 2018, 20, 605–624. [Google Scholar] [CrossRef] [PubMed]
- Kokoska, L.; Kloucek, P.; Leuner, O.; Novy, P. Plant-Derived Products as Antibacterial and Antifungal Agents in Human Health Care. Curr. Med. Chem. 2019, 26, 5501–5541. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.; Iqbal, L.; Lateef, M.; Nawab, B.; Saleem, M.; Afza, N. Bio-Reactive Properties of Citrus Waste: An Investigation of Antioxidant and Tyrosinase Inhibitory Activities. Pak. J. Bot. 2011, 43, 2881–2883. [Google Scholar]
- Elansary, H.O.; Abdelgaleil, S.A.M.; Mahmoud, E.A.; Yessoufou, K.; Elhindi, K.; El-Hendawy, S. Effective Antioxidant, Antimicrobial and Anticancer Activities of Essential Oils of Horticultural Aromatic Crops in Northern Egypt. BMC Complement. Altern. Med. 2018, 18, 214. [Google Scholar] [CrossRef]
- Saengha, W.; Karirat, T.; Katisart, T.; Ma, N.L.; Luang-in, V. Cytotoxicity and Antiproliferative Activity of Essential Oils from Lemon, Wild Orange and Petitgrain against MCF-7, HepG2 and HeLa Cancer Cells. Not. Bot. Horti Agrobot. Cluj-Napoca 2022, 50, 12713. [Google Scholar] [CrossRef]
- Jayaprakasha, G.K.; Murthy, K.N.C.; Uckoo, R.M.; Patil, B.S. Chemical Composition of Volatile Oil from Citrus Limettioides and Their Inhibition of Colon Cancer Cell Proliferation. Ind. Crops Prod. 2013, 45, 200–207. [Google Scholar] [CrossRef]
- Yan, H.C.; Hong, P.; Yu, Z.Z.; Jing, S. Evaluation of Antioxidant and Antitumour Activities of Lemon Essential Oil. J. Med. Plants Res. 2010, 4, 1910–1915. [Google Scholar]
- Almas, I.; Innocent, E.; Machumi, F.; Kisinza, W. Effect of Geographical Location on Yield and Chemical Composition of Essential Oils from Three Eucalyptus Species Growing in Tanzania. Asian J. Tradit. Med. 2019, 14, 1–12. [Google Scholar]
- Hammid, S.A.; Ahmad, F. Chemotype of Litsea Cubeba Essential Oil and Its Bioactivity. Nat. Prod. Commun. 2015, 10, 1301–1304. [Google Scholar] [CrossRef] [Green Version]
- Maia, M.F.; Moore, S.J. Plant-Based Insect Repellents: A Review of Their Efficacy, Development and Testing PMD from Lemon Eucalyptus (Corymbia Citriodora) Extract. Malar. J. 2011, 10, S11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Javed, S.; Javaid, A.; Nawaz, S.; Saeed, M.K.; Mahmood, Z.; Siddiqui, S.Z.; Ahmad, R. Phytochemistry, GC-MS Analysis, Antioxidant and Antimicrobial Potential of Essential Oil from Five Citrus Species. J. Agric. Sci. 2014, 6, 201–208. [Google Scholar] [CrossRef] [Green Version]
- Nerio, L.S.; Olivero-Verbel, J.; Stashenko, E. Repellent Activity of Essential Oils: A Review. Bioresour. Technol. 2010, 101, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Smeriglio, A.; Alloisio, S.; Raimondo, F.M.; Denaro, M.; Xiao, J.; Cornara, L.; Trombetta, D. Essential Oil of Citrus Lumia Risso: Phytochemical Profile, Antioxidant Properties and Activity on the Central Nervous System. Food Chem. Toxicol. 2018, 119, 407–416. [Google Scholar] [CrossRef]
- Denkova-Kostova, R.; Teneva, D.; Tomova, T.; Goranov, B.; Denkova, Z.; Shopska, V.; Slavchev, A.; Hristova-Ivanova, Y. Chemical Composition, Antioxidant and Antimicrobial Activity of Essential Oils from Tangerine (Citrus reticulata L.), Grapefruit (Citrus paradisi L.), Lemon (Citrus lemon L.) and Cinnamon (Cinnamomum zeylanicum Blume). Z. Fur Naturforsch.-Sect. C J. Biosci. 2021, 76, 175–185. [Google Scholar] [CrossRef]
- Ambrosio, C.M.S.; Diaz-Arenas, G.L.; Agudelo, L.P.A.; Stashenko, E.; Contreras-Castillo, C.J.; da Gloria, E.M. Chemical Composition and Antibacterial and Antioxidant Activity of a Citrus Essential Oil and Its Fractions. Molecules 2021, 26, 2888. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S.; Sun Song, H.; Ukeda, H.; Sawamura, M. Radical-Scavenging Activities of Citrus Essential Oils and Their Components: Detection Using 1,1-Diphenyl-2-Picrylhydrazyl. J. Agric. Food Chem. 2000, 48, 4156–4161. [Google Scholar] [CrossRef] [PubMed]
- Wungsintaweekul, J.; Sitthithaworn, W.; Putalun, W.; Pfeifhoffer, H.W.; Brantner, A. Antimicrobial, Antioxidant Activities and Chemical Composition of Selected Thai Spices. Songklanakarin J. Sci. Technol. 2010, 32, 589–598. [Google Scholar]
- Loizzo, M.R.; Tundis, R.; Bonesi, M.; Sanzo, G.D.; Verardi, A.; Lopresto, C.G.; Pugliese, A.; Menichini, F.; Balducchi, R.; Calabrò, V. Chemical Profile and Antioxidant Properties of Extracts and Essential Oils from Citrus × Limon (L.) Burm. Cv. Femminello Comune. Chem. Biodivers. 2016, 13, 571–581. [Google Scholar] [CrossRef]
- Hamdan, D.; El-Readi, M.Z.; Nibret, E.; Sporer, F.; Farrag, N.; El-Shazly, A.; Wink, M. Chemical Composition of the Essential Oils of Two Citrus Species and Their Biological Activities. Pharmazie 2010, 65, 141–147. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Navajas, Y.R.; Zapata, E.S.; Fernánde, L. Antioxidant activity of essential oils of five spice plants widely used in a mediterranean diet. Flavour Fragr. J. 2010, 25, 13–19. [Google Scholar] [CrossRef]
- Monajemi, R.; Oryan, S.; Haeri-Roohani, A.; Ghannadi, A.; Jafarian, A. Cytotoxic Effects of Essential Oils of Some Iranian Citrus Peels. Iran. J. Pharm. Res. 2005, 3, 183–187. [Google Scholar]
- Raji, P.; Samrot, A.V.; Dharani, D.; Alexander, B. In Vitro and In Silico Approaches to Study the Bioactivity of Citrus Limon Leaf Extracts. J. Young Pharm. 2017, 9, 290–295. [Google Scholar] [CrossRef] [Green Version]
- Elgendy, E.M.; Ibrahim, H.S.; Elmeherry, H.F.; Sedki, A.G.; Mekhemer, F.U. Chemical and Biological Comparative in Vitro Studies of Cinnamon Bark and Lemon Peel Essential Oils. Food Nutr. Sci. 2017, 08, 110–125. [Google Scholar] [CrossRef] [Green Version]
- Kulig, M.; Galanty, A.; Grabowska, K.; Podolak, I. Assessment of Safety and Health-Benefits of Citrus Hystrix DC. Peel Essential Oil, with Regard to Its Bioactive Constituents in an in Vitro Model of Physiological and Pathological Skin Conditions. Biomed. Pharmacother. 2022, 151, 113151. [Google Scholar] [CrossRef]
- Gyawali, R.; Kim, K.S. Anticancer Phytochemicals of Citrus Fruits—A Review. J. Anim. Res. 2014, 4, 85–95. [Google Scholar] [CrossRef]
- Dosoky, N.S.; Setzer, W.N. Biological Activities and Safety of Citrus spp. Essential Oils. Int. J. Mol. Sci. 2018, 19, 1966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bora, H.; Kamle, M.; Mahato, D.K.; Tiwari, P.; Kumar, P. Citrus Essential Oils (CEOs) and Their Applications in Food: An Overview. Plants 2020, 9, 357. [Google Scholar] [CrossRef] [Green Version]
- Saini, R.K.; Ranjit, A.; Sharma, K.; Prasad, P.; Shang, X.; Gowda, K.G.M.; Keum, Y.S. Bioactive Compounds of Citrus Fruits: A Review of Composition and Health Benefits of Carotenoids, Flavonoids, Limonoids, and Terpenes. Antioxidants 2022, 11, 239. [Google Scholar] [CrossRef]
- Zapata, B.; Durán, C.; Stashenko, E.; Correa-Royero, J.; Betancur-Galvis, L. Actividad Citotóxica de Aceites Esenciales de Lippia Origanoides H.B.K. y Componentes Mayoritarios. Salud UIS 2009, 41, 215–222. [Google Scholar]
- Gómez, L.A.; Stashenko, E.; Ocazionez, R.E. Comparative Study on in Vitro Activities of Citral, Limonene and Essential Oils from Lippia Citriodora and L. Alba on Yellow Fever Virus. Nat. Prod. Commun. 2013, 8, 249–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Si, C.; Ou, Y.; Ma, D.; Hei, L.; Wang, X.; Du, R.; Yang, H.; Liao, Y.; Zhao, J. Cytotoxic Effect of the Essential Oils from Erigeron Canadensis L. on Human Cervical Cancer HeLa Cells in Vitro. Chem. Biodivers. 2022, 19, e202200436. [Google Scholar] [CrossRef] [PubMed]
- Basholli-Salihu, M.; Schuster, R.; Hajdari, A.; Mulla, D.; Viernstein, H.; Mustafa, B.; Mueller, M. Phytochemical Composition, Anti-Inflammatory Activity and Cytotoxic Effects of Essential Oils from Three Pinus spp. Pharm. Biol. 2017, 55, 1553–1560. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, K. Anticancer Effect of Lemongrass Oil and Citral on Cervical Cancer Cell Lines. Pharmacogn. Commun. 2013, 3, 41–48. [Google Scholar] [CrossRef]
- Chang, M.Y.; Shieh, D.E.; Chen, C.C.; Yeh, C.S.; Dong, H.P. Linalool Induces Cell Cycle Arrest and Apoptosis in Leukemia Cells and Cervical Cancer Cells through CDKIs. Int. J. Mol. Sci. 2015, 16, 28169–28179. [Google Scholar] [CrossRef] [Green Version]
- Anuchapreeda, S.; Chueahongthong, F.; Viriyaadhammaa, N.; Panyajai, P.; Anzawa, R.; Tima, S.; Ampasavate, C.; Saiai, A.; Rungrojsakul, M.; Usuki, T.; et al. Antileukemic Cell Proliferation of Active Compounds from Kaffir Lime (Citrus hystrix) Leaves. Molecules 2020, 25, 1300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pejin, B.; Kojic, V.; Bogdanovic, G. An Insight into the Cytotoxic Activity of Phytol at in Vitro Conditions. Nat. Prod. Res. 2014, 28, 2053–2056. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.K.; Tan, L.T.H.; Chan, K.G.; Lee, L.H.; Goh, B.H. Nerolidol: A Sesquiterpene Alcohol with Multi-Faceted Pharmacological and Biological Activities. Molecules 2016, 21, 529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Špičáková, A.; Bazgier, V.; Skálová, L.; Otyepka, M.; Anzenbacher, P. β-Caryophyllene Oxide and Trans-Nerolidol Affect Enzyme Activity of CYP3A4—In Vitro and in Silico Studies. Physiol. Res. 2019, 68, s51–s58. [Google Scholar] [CrossRef]
- Legault, J.; Dahl, W.; Debiton, E.; Pichette, A.; Madelmont, J.C. Antitumor Activity of Balsam Fir Oil: Production of Reactive Oxygen Species Induced by α-Humulene as Possible Mechanism of Action. Planta Med. 2003, 69, 402–407. [Google Scholar] [CrossRef]
- Legault, J.; Pichette, A. Potentiating Effect of β-Caryophyllene on Anticancer Activity of α-Humulene, Isocaryophyllene and Paclitaxel. J. Pharm. Pharmacol. 2010, 59, 1643–1647. [Google Scholar] [CrossRef]
- Adam, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; pp. 10–788. [Google Scholar]
- NIST Chemistry WebBook. Available online: https://webbook.nist.gov/chemistry/ (accessed on 9 September 2022).
- Gursoy, N.; Tepe, B.; Sokmen, M. Evaluation of the Chemical Composition and Antioxidant Activity of the Peel Oil of Citrus Nobilis. Int. J. Food Prop. 2010, 13, 983–991. [Google Scholar] [CrossRef]

| No | Compounds | CASRN | Molecular Formula | Molecular Weight | a Class | b Match Factor (Reverse Match Factor) | RIref | cm/z of Significant Ions (Relative Ion Abundance) | d RIcal | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| e (Relative Percentage Abundance, %) | ||||||||||||
| CL | CH | CM | CP | |||||||||
| 1 | Pinene, α- | 80-56-8 | C10H16 | 136.13 | MH | 948 (949); 940 (943); 952 (954); 945 (948) | 935 | 93.1 (100), 91.1 (48.75), 92.1 (39.17); 93.1 (100), 91.1 (48.10), 92.1 (36.00); 93.1 (100), 91.1 (49.53), 92.1 (38.79); 93.1 (100), 91.1 (47.73), 92.1 (38.09) | 930 (0.67 ± 0.01) | 930 (0.14 ± 0.01) | 931 (1.41 ± 0.03) | 930 (0.60 ± 0.01) |
| 2 | Sabinene | 3387-41-5 | C10H16 | 136.13 | MH | 941 (948); 949 (955); 949 (957) | 971 | 93.1 (100), 91.1 (50.15), 77.1 (38.85); 93.1 (100), 91.1 (51.67), 77.1 (39.52); 93.1 (100), 91.1 (49.63), 77.1 (38.47) | 971 (0.69 ± 0.03) | 972 3.02 ± 0.06) | NA | 971 0.89 ± 0.01) |
| 3 | Pinene, β- | 18172-67-3 | C10H16 | 136.13 | MH | 948 (948); 936 (936); 941 (941); 940 (943) | 973 | 93.1 (100), 91.1 (33.03), 79.1 (26.68); 93.1 (100), 91.1 (33.43), 79.1 (26.28); 93.1 (100), 91.1 (33.39), 79.1 (26.96); 93.1 (100), 91.1 (34.28), 79.1 (25.15) | 973 (1.83 ± 0.02) | 973 (0.16 ± 0.05) | 978 (7.12 ± 0.23) | 973 (0.12 ± 0.02) |
| 4 | Myrcene, β- | 123-35-3 | C10H16 | 136.13 | MH | 939 (953); 949 (964); 940 (956); 949 (962) | 991 | 93.1 (100), 69.1 (61.56), 91.1 (27.58); 93.1 (100), 69.1 (60.67), 91.1 (27.67); 93.1 (100), 69.1 (60.51), 91.1 (27.26); 93.1 (100), 69.1 (60.26), 91.1 (27.74) | 992 (1.78 ± 0.02) | 991 (0.80 ± 0.01) | 991 (0.28 ± 0.01) | 992 (1.63 ± 0.01) |
| 5 | 3-Carene | 13466-78-9 | C10H16 | 136.13 | MH | 934 (934); 926 (933); 904 (921); 897 (906) | 1008 | 93.1 (100), 91.1 (56.18), 77.1 (35.85); 93.1 (100), 91.1 (55.54), 77.1 (44.27); 93.1 (100), 91.1 (51.84), 77.1 (33.96); 93.1 (100), 91.1 (47.73), 77.1 (35.68) | 1010 (5.42 ± 0.08) | 1008 (0.05 ± 0.01) | 1008 (0.38 ± 0.01) | 1008 (0.03 ± 0.01) |
| 6 | Limonene | 138-86-3 | C10H16 | 136.13 | MH | 952 (953); 905 (905); 935 (936); 955 (956) | 1033 | 93.1 (100), 68.2 (98.59), 67.2 (82.02); 93.1 (100), 68.2 (62.14), 67.1 (53.19); 93.1 (100), 68.1 (73.71), 67.2 (63.04); 93.2 (99.09), 68.2 (100), 67.2 (80.60) | 1033 (33.57 ± 0.54) | 1026 (0.21 ± 0.02) | 1027 (1.70 ± 0.03) | 1038 (70.40 ± 0.46) |
| 7 | Ocimene, β- | 13877-91-3 | C10H16 | 136.13 | MH | 946 (946); 939 (939); 952 (952); 945 (945) | 1050 | 93.1 (100), 91.1 (56.62), 79.1 (46.19); 93.1 (100), 91.1 (53.24), 79.1 (44.57); 93.1 (100), 91.1 (53.72), 79.1 (44.23); 93.1 (100), 91.1 (55.17), 79.1 (44.64) | 1050 (1.96 ± 0.02) | 1048 (0.48 ± 0.01) | 1050 (2.36 ± 0.03) | 1051 (1.91 ± 0.01) |
| 8 | Terpinolene | 586-62-9 | C10H16 | 136.13 | MH | 926 (942); 919 (922); 939 (944); 922 (925) | 1087 | 121.1 (100), 93.1 (99.35), 136.2 (87.49); 121.1 (100), 136.1 (93.82), 93.1 (89.14); 121.1 (100), 93.1 (99.73), 136.1 (91.90); 121.1 (100), 93.1 (95.15), 136.2 (82.49) | 1087 (1.54 ± 0.02) | 1086 (0.13 ± 0.01) | 1086 (0.13 ± 0.01) | 1087 (0.08 ± 0.01) |
| 9 | Linalool | 78-70-6 | C10H18O | 154.14 | OM | 932 (932); 912 (913); 946 (946); 946 (946) | 1101 | 71.1 (100), 93.1 (97.92), 55.2 (55.40); 71.1 (100), 93.1 (98.28), 55.1 (54.53); 71.1 (100), 93.1 (98.79), 55.1 (53.45); 71.1 (100), 93.1 (97.83), 55.1 (55.05) | 1100 (0.73 ± 0.01) | 1101 (3.20 ± 0.01) | 1103 (2.90 ± 0.04) | 1101 (1.24 ± 0.01) |
| 10 | Citronellal | 106-23-0 | C10H18O | 154.14 | MA | 925 (925); 920 (931); 909 (909); 923 (923) | 1157 | 69.2 (100), 95.1 (83.95), 55.1 (47.81); 69.2 (100), 95.1 (85.86), 121.2 (48.93); 69.1 (100), 95.1 (84.22), 121.1 (47.39); 69.2 (100), 95.1 (87.03), 121.1 (48.31) | 1155 (1.54 ± 0.01) | 1169 (77.69 ± 0.37) | 1154 (0.28 ± 0.01) | 1157 (5.64 ± 0.02) |
| 11 | Isogeranial | 55722-59-3 | C10H16O | 152.12 | MA | 937 (959) 931 (932) | 1184 | 81.1 (100), 67.1 (84.07), 109.1 (76.29); 81.1 (100), 67.1 (84.18), 109.1 (74.65) | 1184 (1.20 ± 0.01) | NA | NA | 1184 (0.22 ± 0.01) |
| 12 | Citronellol, β- | 106-22-9 | C10H20O | 156.15 | OM | 937 (937); 936 (937); 905 (905); 948 (950) | 1236 | 69.1 (100), 67.1 (47.78), 81.1 (36.60); 69.1 (100), 67.1 (71.79), 81.1 (66.00); 69.1 (100), 67.1 (56.05), 81.2 (59.75); 69.1 (100), 67.1 (70.83), 81.1 (65.03) | 1236 (5.89 ± 0.04) | 1233 (3.75 ± 0.06) | 1229 (0.02 ± 0.01) | 1231 (0.64 ± 0.01) |
| 13 | Citral, β- | 106-26-3 | C10H16O | 152.12 | MA | 949 (950); 942 (943) | 1242 | 69.2 (100), 109.1 (48.27), 94.1 (38.81); 69.2 (100), 109.1 (45.21), 94.1 (37.45) | 1247 (9.11 ± 0.04) | NA | NA | 1243 (1.60 ± 0.01) |
| 14 | Citral, α- | 141-27-5 | C10H16O | 152.12 | MA | 949 (949); 942 (942) | 1287 | 69.2 (100), 84.1 (27.65), 94.1 (19.55); 69.2 (100), 84.1 (28.80), 94.1 (20.16) | 1280 (12.02 ± 0.07) | NA | NA | 1275 (2.03 ± 0.02) |
| 15 | EIemene, δ- | 20307-84-0 | C15H24 | 204.19 | SH | 942 (950); 943 (951); 913 (917) | 1338 | 121.1 (100), 93.1 (53.09), 107.1 (41.12); 121.1 (100), 93.1 (65.01), 136.2 (58.35); 121.2 (100), 93.1 (67.04), 136.2 (58.88) | NA | 1336 (0.03 ± 0.01) | 1338 (3.22 ± 0.01) | 1337 (0.11 ± 0.01) |
| 16 | Citronellol acetate | 150-84-5 | C12H22O2 | 198.17 | MAc | 954 (954); 950 (950); 872 (881); 908 (908) | 1355 | 81.1 (100), 95.1 (97.44), 69.1 (90.52); 95.1 (100), 81.1 (98.43), 69.1 (83.91); 81.1 (100), 95.1 (92.44), 69.1 (87.26); 81.1 (100), 95.1 (96.81), 69.1 (94.12) | 1355 (0.14 ± 0.03) | 1357 (2.81 ± 0.06) | 1355 (0.02 ± 0.01) | 1355 (0.19 ± 0.01) |
| 17 | Nerol acetate | 141-12-8 | C12H20O2 | 196.15 | MAc | 934 (935); 906 (908); 907 (907); 932 (932) | 1367 | 69.2 (100), 93.1 (55.87), 68.2 (38.06); 69.2 (100), 93.2 (53.09), 68.1 (34.91); 69.2 (100), 93.2 (59.57), 68.1 (38.53); 69.1 (100), 93.1 (61.17), 68.1 (41.17) | 1369 (3.57 ± 0.10) | 1366 (0.25 ± 0.03) | 1366 (0.03 ± 0.01) | 1367 (0.73 ± 0.01) |
| 18 | Geranyl acetate | 16409-44-2 | C12H20O2 | 196.15 | MAc | 951 (958); 916 (926); 914 (914) | 1386 | 69.2 (100), 68.2 (36.97), 93.1 (34.17); 69.1 (100), 68.2 (38.07), 93.1 (35.94); 69.2 (100), 68.2 (36.12), 93.1 (35.08) | 1376 (2.92 ± 0.04) | 1386 (0.96 ± 0.02) | NA | 1384 (0.39 ± 0.03) |
| 19 | Elemene, β- | 515-13-9 | C15H24 | 204.19 | SH | 918 (918); 919 (921); 929 (931) | 1393 | 93.1 (100), 81.1 (86.08), 67.1 (83.02); 93.1 (100), 81.1 (82.01), 107.1 (72.88); 93.1 (100), 81.1 (82.01), 107.1 (74.12) | NA | 1391 (0.05 ± 0.01) | 1392 (1.32 ± 0.01) | 1392 (2.78 ± 0.08) |
| 20 | Caryophyllene | 87-44-5 | C15H24 | 204.19 | SH | 923 (923); 952 (952); 937 (937); 950 (950) | 1418 | 91.1 (100), 133.1 (94.72), 93.1 (83.53); 133.1 (100), 91.1 (92.12), 93.1 (87.47); 133.1 (100), 91.1 (95.02), 93.1 (82.61); 133.1 (100), 91.1 (91.34), 93.1 (85.56) | 1418 (1.48 ± 0.02) | 1418 (0.45 ± 0.01) | 1419 (3.29 ± 0.01) | 1418 (1.64 ± 0.03) |
| 21 | Germacrene D | 23986-74-5 | C15H24 | 204.19 | SH | 888 (902); 946 (959); 922 (936) | 1480 | 161.2 (100), 105.1 (50.92), 91.1 (47.97); 161.2 (100), 105.1 (49.83), 91.1 (47.17); 161.2 (100), 105.1 (49.62), 91.1 (47.63) | NA | 1479 (0.04 ± 0.01) | 1486 (13.04 ± 0.25) | 1479 (0.36 ± 0.01) |
| 22 | Selinene, β- | 17066-67-0 | C15H24 | 204.19 | SH | 948 (958); 931 (935) | 1489 | 105.1 (100), 93.2 (93.51), 107.1 (89.08); 93.2 (100), 105.1 (97.15), 107.1 (84.76) | NA | NA | 1489 (2.42 ± 0.17) | 1484 (0.10 ± 0.01) |
| 23 | Bicyclogermacrene | 24703-35-3 | C15H24 | 204.19 | SH | 920 (921); 920 (920); 923 (924); 918 (918) | 1495 | 121.2 (100), 93.1 (65.23), 107.1 (48.60); 121.2 (100), 93.1 (67.06), 107.1 (51.53); 121.1 (100), 93.1 (67.78), 107.1 (51.70); 121.1 (100), 93.1 (86.30), 107.1 (52.65) | 1495 (0.13 ± 0.01) | 1495 (0.33 ± 0.17) | 1497 (2.03 ± 0.38) | 1495 (0.14 ± 0.01) |
| 24 | Elemol | 639-99-6 | C15H26O | 222.19 | OS | 943 (950); 950 (958); 933 (940) | 1549 | 93.1 (100), 161.2 (97.07), 59.1 (93.10); 93.1 (100), 161.2 (94.41), 59.1 (82.73); 93.1 (100), 161.2 (89.99), 59.1 (88.31) | NA | 1548 (0.42 ± 0.05) | 1557 (16.67 ± 0.10) | 1548 (0.19 ± 0.01) |
| 25 | Nerolidol | 40716-66-3 | C15H26O | 222.19 | OS | 863 (863); 931 (938); 941 (950); 906 (911) | 1564 | 69.2 (100), 93.1 (84.83), 107.1 (51.52); 69.2 (100), 93.1 (97.66), 107.1 (66.85); 69.2 (100), 93.1 (93.98), 107.1 (64.33); 69.2 (100), 93.1 (96.15), 107.2 (66.52) | 1564 (0.02 ± 0.01) | 1564 (0.80 ± 0.10) | 1567 (1.53 ± 0.01) | 1564 (0.09 ± 0.01) |
| 26 | Eudesmol, epi-γ- | 117066-77-0 | C15H26O | 222.19 | OS | 933 (951) | 1620 | 189.2 (100), 162.1 (72.76), 204.2 (61.03) | NA | NA | 1520 (1.70 ± 0.04) | NA |
| 27 | Eudesmol, γ- | 1209-71-8 | C15H26O | 222.19 | OS | 932 (933) | 1635 | 189.2 (100), 161.2 (95.46), 204.2 (78.66) | NA | NA | 1635 (5.69 ± 0.20) | NA |
| 28 | Eudesmol, β- | 473-15-4 | C15H26O | 222.19 | OS | 952 (960) | 1656 | 149.2 (100), 59.2 (78.76), 164.2 (44.78) | NA | NA | 1656 (8.58 ± 0.05) | NA |
| 29 | Eudesmol, α- | 473-16-5 | C15H26O | 222.19 | OS | 940 (951) | 1659 | 149.2 (100), 161.2 (98.27), 204.2 (87.13) | NA | NA | 1659 (3.62 ± 0.04) | NA |
| 30 | Phytol | 150-86-7 | C20H40O | 296.31 | OD | 929 (931); 911 (913); 922 (923); 929 (931) | 2113 | 71.1 (100), 123.2 (43.38), 81.1 (33.37); 71.1 (100), 123.1 (44.94), 57.1 (38.08); 71.1 (100), 123.1 (44.15), 81.2 (38.00); 71.1 (100), 123.2 (47.34), 81.2 (39.51) | 2113 (1.62 ± 0.12) | 2113 (0.21 ± 0.02) | 2112 (0.39 ± 0.01) | 2113 (0.78 ± 0.04) |
| Citrus spp. | IC50 (mg/mL) | IC50 (mg/mL) * | References |
|---|---|---|---|
| C. hystrix | 279.03 ± 10.34 | >0.25 | [36] |
| C. limon | 29.14 ± 1.97 | 6.47 | [37] |
| C. pyriformis | 39.99 ± 0.73 | 28.91 | [38] |
| C. microcarpa | 59.42 ± 1.77 | ~0.05 | [13] |
| Ascorbic acid (control) | 0.43 ± 1.70 | 0.42 | [39] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Othman, H.I.A.; Alkatib, H.H.; Zaid, A.; Sasidharan, S.; Rahiman, S.S.F.; Lee, T.P.; Dimitrovski, G.; Althakafy, J.T.; Wong, Y.F. Phytochemical Composition, Antioxidant and Antiproliferative Activities of Citrus hystrix, Citrus limon, Citrus pyriformis, and Citrus microcarpa Leaf Essential Oils against Human Cervical Cancer Cell Line. Plants 2023, 12, 134. https://doi.org/10.3390/plants12010134
Othman HIA, Alkatib HH, Zaid A, Sasidharan S, Rahiman SSF, Lee TP, Dimitrovski G, Althakafy JT, Wong YF. Phytochemical Composition, Antioxidant and Antiproliferative Activities of Citrus hystrix, Citrus limon, Citrus pyriformis, and Citrus microcarpa Leaf Essential Oils against Human Cervical Cancer Cell Line. Plants. 2023; 12(1):134. https://doi.org/10.3390/plants12010134
Chicago/Turabian StyleOthman, Haneen Ibrahim Al, Huda Hisham Alkatib, Atiqah Zaid, Sreenivasan Sasidharan, Siti Sarah Fazalul Rahiman, Tien Ping Lee, George Dimitrovski, Jalal T. Althakafy, and Yong Foo Wong. 2023. "Phytochemical Composition, Antioxidant and Antiproliferative Activities of Citrus hystrix, Citrus limon, Citrus pyriformis, and Citrus microcarpa Leaf Essential Oils against Human Cervical Cancer Cell Line" Plants 12, no. 1: 134. https://doi.org/10.3390/plants12010134







