An Overview of Lithium-Ion Battery Recycling: A Comparison of Brazilian and International Scenarios
Abstract
1. Introduction
2. Lithium-Ion Batteries
3. Battery Recycling Processes
3.1. Pyrometallurgy
3.2. Hydrometallurgy
3.3. Recycling Processes Under Development
3.3.1. Direct Process
3.3.2. Biometallurgy
3.3.3. Comparison of Battery Recycling Processes
4. International Recycling Legislation and Scenario
5. Brazilian Recycling Legislation and Scenario
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EVs | Electric Vehicles |
| BEVs | Battery electric vehicles |
| PHEVs | Plug-in Hybrids |
| ABVE | Brazilian Electric Vehicle Association |
| GHG | Greenhouse gases |
| NMC | Nickel Manganese Cobalt Oxide |
| LIB | Lithium-ion battery |
| LFP | Lithium Iron Phosphate |
| SEI | Solid Electrolyte Interface |
| MIIT | Ministry of Industry and Information Technology |
| EPR | Extended Producer Warranty |
| EPA | Environmental Protection Agency |
References
- International Energy Agency (IEA). Global EV Outlook; International Energy Agency: Paris, France, 2024. [Google Scholar]
- Zhan, W.; Wang, Z.; Deng, J.; Liu, P.; Cui, D. Integrating System Dynamics and Agent-Based Modeling: A Data-Driven Framework for Predicting Electric Vehicle Market Penetration and GHG Emissions Reduction under Various Incentives Scenarios. Appl. Energy 2024, 372, 123749. [Google Scholar] [CrossRef]
- Liu, W.; Placke, T.; Chau, K.T. Overview of Batteries and Battery Management for Electric Vehicles. Energy Rep. 2022, 8, 4058–4084. [Google Scholar] [CrossRef]
- Wagh, K.; Dhatrak, P. A Review on Powertrain Subsystems and Charging Technology in Battery Electric Vehicles: Current and Future Trends. Proc. Inst. Mech. Eng. Part D J. Automob. Eng. 2022, 236, 479–496. [Google Scholar] [CrossRef]
- PNME 3o Anuário Brasileiro da Mobilidade Elétrica. Available online: https://pnme.org.br/ (accessed on 13 April 2025).
- Xiang, J.; Wei, Y.; Zhong, Y.; Yang, Y.; Cheng, H.; Yuan, L.; Xu, H.; Huang, Y. Building Practical High-Voltage Cathode Materials for Lithium-Ion Batteries. Adv. Mater. 2022, 34, 2200912. [Google Scholar] [CrossRef]
- Miao, Y.; Hynan, P.; von Jouanne, A.; Yokochi, A. Current Li-Ion Battery Technologies in Electric Vehicles and Opportunities for Advancements. Energies 2019, 12, 1074. [Google Scholar] [CrossRef]
- Li, X. A Review of the Possible Ways to Increase the Energy Density of Lithium-Ion Battery. J. Phys. Conf. Ser. 2023, 2608, 012013. [Google Scholar] [CrossRef]
- Nájera, J.; Arribas, J.R.; de Castro, R.M.; Núñez, C.S. Semi-Empirical Ageing Model for LFP and NMC Li-Ion Battery Chemistries. J. Energy Storage 2023, 72, 108016. [Google Scholar] [CrossRef]
- Moreira, Y.H.B.; Mantegazini, D.Z.; Andrade, G.R.S.; Bacelos, M.S. Reciclagem de Baterias de Íon-Lítio: Uma Breve Revisão Sobre Os Processos, Avanços e Perspectivas. Braz. J. Prod. Eng. 2024, 10, 36–52. [Google Scholar] [CrossRef]
- Han, X.; Lu, L.; Zheng, Y.; Feng, X.; Li, Z.; Li, J.; Ouyang, M. A Review on the Key Issues of the Lithium Ion Battery Degradation among the Whole Life Cycle. eTransportation 2019, 1, 100005. [Google Scholar] [CrossRef]
- Dambros Telli, G.; Gungor, S.; Lorente, S. Counterflow Canopy-to-Canopy and U-Turn Liquid Cooling Solutions for Battery Modules in Stationary Battery Energy Storage Systems. Appl. Therm. Eng. 2024, 238, 121997. [Google Scholar] [CrossRef]
- Gungor, S.; Telli, G.D.; Lorente, S. Characterizing Li-Ion Battery Thermal Behavior; a Methodology When Little Information Is Available. Int. Commun. Heat. Mass. Transf. 2023, 148, 107076. [Google Scholar] [CrossRef]
- Rahman, T.; Alharbi, T. Exploring Lithium-Ion Battery Degradation: A Concise Review of Critical Factors, Impacts, Data-Driven Degradation Estimation Techniques, and Sustainable Directions for Energy Storage Systems. Batteries 2024, 10, 220. [Google Scholar] [CrossRef]
- Noura, N.; Boulon, L.; Jemeï, S. A Review of Battery State of Health Estimation Methods: Hybrid Electric Vehicle Challenges. World Electr. Veh. J. 2020, 11, 66. [Google Scholar] [CrossRef]
- Zhang, M.; Fan, X. Review on the State of Charge Estimation Methods for Electric Vehicle Battery. World Electr. Veh. J. 2020, 11, 23. [Google Scholar] [CrossRef]
- European Parliament and of the Council. Regulation (EU) 2023/1542—12 of July 2023; European Union: Brussels, Belgium, 2023. [Google Scholar]
- Kader, Z.A.; Marshall, A.; Kennedy, J. A Review on Sustainable Recycling Technologies for Lithium-Ion Batteries. Emergent Mater. 2021, 4, 725–735. [Google Scholar] [CrossRef]
- Kala, S.; Mishra, A. Battery Recycling Opportunity and Challenges in India. Mater. Today Proc. 2021, 46, 1543–1556. [Google Scholar] [CrossRef]
- Sita, L.E.; Sommerville, R.; Alsofi, G.; Lima da Silva, W.; Gastol, D.; Scarminio, J.; Kendrick, E. Direct Recycling of LixNi0.5Mn0.3Co0.2O2 from Production Scrap and End-Of-Life Batteries, Using Solid-State Relithiation. Batter. Supercaps 2025, 8, e202400536. [Google Scholar] [CrossRef]
- Srinivasan, S.; Shanthakumar, S.; Ashok, B. Sustainable Lithium-Ion Battery Recycling: A Review on Technologies, Regulatory Approaches and Future Trends. Energy Rep. 2025, 13, 789–812. [Google Scholar] [CrossRef]
- Hannan, M.A.; Lipu, M.S.H.; Hussain, A.; Mohamed, A. A Review of Lithium-Ion Battery State of Charge Estimation and Management System in Electric Vehicle Applications: Challenges and Recommendations. Renew. Sustain. Energy Rev. 2017, 78, 834–854. [Google Scholar] [CrossRef]
- Zhang, Y.; Huo, Q.; Du, P.; Wang, L.; Zhang, A.; Song, Y.; Lv, Y.; Li, G. Advances in New Cathode Material LiFePO4 for Lithium-Ion Batteries. Synth. Met. 2012, 162, 1315–1326. [Google Scholar] [CrossRef]
- Takami, N.; Inagaki, H.; Tatebayashi, Y.; Saruwatari, H.; Honda, K.; Egusa, S. High-Power and Long-Life Lithium-Ion Batteries Using Lithium Titanium Oxide Anode for Automotive and Stationary Power Applications. J. Power Sources 2013, 244, 469–475. [Google Scholar] [CrossRef]
- Silveira, A.V.M.; Santana, M.P.; Tanabe, E.H.; Bertuol, D.A. Recovery of Valuable Materials from Spent Lithium Ion Batteries Using Electrostatic Separation. Int. J. Miner. Process 2017, 169, 91–98. [Google Scholar] [CrossRef]
- Julien, C.; Mauger, A.; Vijh, A.; Zaghib, K. Lithium Batteries; Springer International Publishing: Cham, Switzerland, 2016; ISBN 978-3-319-19107-2. [Google Scholar]
- Meyers, R.A. (Ed.) Encyclopedia of Sustainability Science and Technology; Springer: New York, NY, USA, 2012; ISBN 978-0-387-89469-0. [Google Scholar]
- Jiang, Q.; Wang, X.; Zhang, H. One-Pot Hydrothermal Synthesis of LiMn2O4 Cathode Material with Excellent High-Rate and Cycling Properties. J. Electron. Mater. 2016, 45, 4350–4356. [Google Scholar] [CrossRef]
- Zhou, P.; Meng, H.; Zhang, Z.; Chen, C.; Lu, Y.; Cao, J.; Cheng, F.; Chen, J. Stable Layered Ni-Rich LiNi0.9Co0.07Al0.03O2 Microspheres Assembled with Nanoparticles as High-Performance Cathode Materials for Lithium-Ion Batteries. J. Mater. Chem. A Mater. 2017, 5, 2724–2731. [Google Scholar] [CrossRef]
- Larouche, F.; Tedjar, F.; Amouzegar, K.; Houlachi, G.; Bouchard, P.; Demopoulos, G.P.; Zaghib, K. Progress and Status of Hydrometallurgical and Direct Recycling of Li-Ion Batteries and Beyond. Materials 2020, 13, 801. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-H. Global Patent Analysis of Battery Recycling Technologies: A Comparative Study of Korea, China, and the United States. World Electr. Veh. J. 2024, 15, 260. [Google Scholar] [CrossRef]
- Hassan, S.S.M.; Fathy, M.A. Novel Paper-Based Potentiometric Combined Sensors Using Coumarin Derivatives Modified with Vanadium Pentoxide Nanoparticles for the Selective Determination of Trace Levels of Lead Ions. Microchim. Acta 2024, 191, 427. [Google Scholar] [CrossRef]
- Yoo, E.; Lee, U.; Kelly, J.C.; Wang, M. Life-Cycle Analysis of Battery Metal Recycling with Lithium Recovery from a Spent Lithium-Ion Battery. Resour. Conserv. Recycl. 2023, 196, 107040. [Google Scholar] [CrossRef]
- Zheng, X.; Zhu, Z.; Lin, X.; Zhang, Y.; He, Y.; Cao, H.; Sun, Z. A Mini-Review on Metal Recycling from Spent Lithium Ion Batteries. Engineering 2018, 4, 361–370. [Google Scholar] [CrossRef]
- Dorella, G.; Mansur, M.B. A Study of the Separation of Cobalt from Spent Li-Ion Battery Residues. J. Power Sources 2007, 170, 210–215. [Google Scholar] [CrossRef]
- Zeng, X.; Li, J.; Singh, N. Recycling of Spent Lithium-Ion Battery: A Critical Review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 1129–1165. [Google Scholar] [CrossRef]
- Feng, J.; Liu, W.; Chen, F. Moving towards a Circular Economy: A Systematic Review of Barriers to Electric Vehicle Battery Recycling. Sustain. Prod. Consum. 2025, 54, 241–260. [Google Scholar] [CrossRef]
- Harper, G.; Sommerville, R.; Kendrick, E.; Driscoll, L.; Slater, P.; Stolkin, R.; Walton, A.; Christensen, P.; Heidrich, O.; Lambert, S.; et al. Recycling Lithium-Ion Batteries from Electric Vehicles. Nature 2019, 575, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, Y.; Zhang, G. Summary of Pretreatment of Waste Lithium-Ion Batteries and Recycling of Valuable Metal Materials: A Review. Separations 2024, 11, 196. [Google Scholar] [CrossRef]
- Makuza, B.; Tian, Q.; Guo, X.; Chattopadhyay, K.; Yu, D. Pyrometallurgical Options for Recycling Spent Lithium-Ion Batteries: A Comprehensive Review. J. Power Sources 2021, 491, 229622. [Google Scholar] [CrossRef]
- Lombardo, G.; Ebin, B.; Foreman, M.R.S.J.; Steenari, B.M.; Petranikova, M. Incineration of EV Lithium-Ion Batteries as a Pretreatment for Recycling—Determination of the Potential Formation of Hazardous by-Products and Effects on Metal Compounds. J. Hazard. Mater. 2020, 393, 122372. [Google Scholar] [CrossRef]
- Bridgwater, A.V. Waste Incineration and Pyrolysis. Resour. Recovery Conserv. 1980, 5, 99–115. [Google Scholar] [CrossRef]
- Sun, L.; Qiu, K. Vacuum Pyrolysis and Hydrometallurgical Process for the Recovery of Valuable Metals from Spent Lithium-Ion Batteries. J. Hazard. Mater. 2011, 194, 378–384. [Google Scholar] [CrossRef]
- Xiao, J.; Li, J.; Xu, Z. Challenges to Future Development of Spent Lithium Ion Batteries Recovery from Environmental and Technological Perspectives. Environ. Sci. Technol. 2020, 54, 9–25. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, C.; He, W.; Li, G.; Huang, J. A Review on Management of Spent Lithium Ion Batteries and Strategy for Resource Recycling of All Components from Them. Waste Manag. Res. J. A Sustain. Circ. Econ. 2018, 36, 99–112. [Google Scholar] [CrossRef]
- Zhang, G.; Du, Z.; He, Y.; Wang, H.; Xie, W.; Zhang, T. A Sustainable Process for the Recovery of Anode and Cathode Materials Derived from Spent Lithium-Ion Batteries. Sustainability 2019, 11, 2363. [Google Scholar] [CrossRef]
- Diaz, F.; Wang, Y.; Moorthy, T.; Friedrich, B. Degradation Mechanism of Nickel-Cobalt-Aluminum (NCA) Cathode Material from Spent Lithium-Ion Batteries in Microwave-Assisted Pyrolysis. Metals 2018, 8, 565. [Google Scholar] [CrossRef]
- Mao, J.; Li, J.; Xu, Z. Coupling Reactions and Collapsing Model in the Roasting Process of Recycling Metals from LiCoO2 Batteries. J. Clean. Prod. 2018, 205, 923–929. [Google Scholar] [CrossRef]
- Liu, P.; Xiao, L.; Tang, Y.; Chen, Y.; Ye, L.; Zhu, Y. Study on the Reduction Roasting of Spent LiNixCoyMnzO2 Lithium-Ion Battery Cathode Materials. J. Therm. Anal. Calorim. 2019, 136, 1323–1332. [Google Scholar] [CrossRef]
- Li, J.; Wang, G.; Xu, Z. Environmentally-Friendly Oxygen-Free Roasting/Wet Magnetic Separation Technology for in Situ Recycling Cobalt, Lithium Carbonate and Graphite from Spent LiCoO2/Graphite Lithium Batteries. J. Hazard. Mater. 2016, 302, 97–104. [Google Scholar] [CrossRef]
- Atia, T.A.; Elia, G.; Hahn, R.; Altimari, P.; Pagnanelli, F. Closed-Loop Hydrometallurgical Treatment of End-of-Life Lithium-Ion Batteries: Towards Zero-Waste Process and Metal Recycling in Advanced Batteries. J. Energy Chem. 2019, 35, 220–227. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, B.; Zhang, L.; Guo, S. Microwave-Absorbing Properties of Cathode Material during Reduction Roasting for Spent Lithium-Ion Battery Recycling. J. Hazard Mater. 2020, 384, 121487. [Google Scholar] [CrossRef]
- Sohn, H.Y.; Wadsworth, M.E. (Eds.) Rate Processes of Extractive Metallurgy; Springer: Boston, MA, USA, 1979; ISBN 978-1-4684-9119-7. [Google Scholar]
- Qu, G.; Yang, J.; Wang, H.; Ran, Y.; Li, B.; Wei, Y. Applicability of the Reduction Smelting Recycling Process to Different Types of Spent Lithium-Ion Batteries Cathode Materials. Waste Manag. 2023, 166, 222–232. [Google Scholar] [CrossRef]
- Guoxing, R.; Songwen, X.; Meiqiu, X.; Bing, P.; Youqi, F.; Fenggang, W.; Xing, X. Recovery of Valuable Metals from Spent Lithium-Ion Batteries by Smelting Reduction Process Based on MnO-SiO2-Al2O3 Slag System. In Advances in Molten Slags, Fluxes, and Salts; Wiley: Hoboken, NJ, USA, 2016; pp. 211–218. [Google Scholar]
- Lo Sardo, C.; Cacciatore, G.; Cappuccino, G.; Aiello, D.; Napoli, A. Spent Lithium Battery Recycling: Traditional and Innovative Approaches. Processes 2025, 13, 950. [Google Scholar] [CrossRef]
- Yan, Z.; Sattar, A.; Li, Z. Priority Lithium Recovery from Spent Li-Ion Batteries via Carbothermal Reduction with Water Leaching. Resour. Conserv. Recycl. 2023, 192, 106937. [Google Scholar] [CrossRef]
- Fu, Y.; He, Y.; Yang, Y.; Qu, L.; Li, J.; Zhou, R. Microwave Reduction Enhanced Leaching of Valuable Metals from Spent Lithium-Ion Batteries. J. Alloys Compd. 2020, 832, 154920. [Google Scholar] [CrossRef]
- Ren, G.; Xiao, S.; Xie, M.; Pan, B.; Chen, J.; Wang, F.; Xia, X. Recovery of Valuable Metals from Spent Lithium Ion Batteries by Smelting Reduction Process Based on FeO–SiO2–Al2O3 Slag System. Trans. Nonferrous Met. Soc. China 2017, 27, 450–456. [Google Scholar] [CrossRef]
- Ahmed, S.; Haleem, N.; Jamal, Y.; Khan, S.J.; Yang, X. Recovery of Lithium and Cobalt from Used Lithium-Ion Cell Phone Batteries through a Pyro-Hydrometallurgical Hybrid Extraction Process and Chemical Precipitation. J. Mater. Cycles Waste Manag. 2025, 27, 925–936. [Google Scholar] [CrossRef]
- Feng, S.; Li, D.; Deng, J.; Yang, Z.; Zhang, J.; Zhou, Y. Closed-Loop Recovery of Spent Lithium-Ion Batteries Based on Preferentially Selective Extraction of Lithium Strategy. Sep. Purif. Technol. 2025, 354, 128953. [Google Scholar] [CrossRef]
- Shi, J.; Hou, C.; Dong, J.; Chen, D.; Li, J. Low-Temperature Chlorination Roasting Technology for the Simultaneous Recovery of Valuable Metals from Spent LiCoO2 Cathode Material. Int. J. Miner. Metall. Mater. 2025, 32, 80–91. [Google Scholar] [CrossRef]
- Fan, E.; Li, L.; Lin, J.; Wu, J.; Yang, J.; Wu, F.; Chen, R. Low-Temperature Molten-Salt-Assisted Recovery of Valuable Metals from Spent Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2019, 7, 16144–16150. [Google Scholar] [CrossRef]
- Lv, W.; Wang, Z.; Cao, H.; Sun, Y.; Zhang, Y.; Sun, Z. A Critical Review and Analysis on the Recycling of Spent Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2018, 6, 1504–1521. [Google Scholar] [CrossRef]
- Velázquez-Martínez, O.; Valio, J.; Santasalo-Aarnio, A.; Reuter, M.; Serna-Guerrero, R. A Critical Review of Lithium-Ion Battery Recycling Processes from a Circular Economy Perspective. Batteries 2019, 5, 68. [Google Scholar] [CrossRef]
- Georgi-Maschler, T.; Friedrich, B.; Weyhe, R.; Heegn, H.; Rutz, M. Development of a Recycling Process for Li-Ion Batteries. J. Power Sources 2012, 207, 173–182. [Google Scholar] [CrossRef]
- Assefi, M.; Maroufi, S.; Yamauchi, Y.; Sahajwalla, V. Pyrometallurgical Recycling of Li-Ion, Ni–Cd and Ni–MH Batteries: A Minireview. Curr. Opin. Green. Sustain. Chem. 2020, 24, 26–31. [Google Scholar] [CrossRef]
- Jie, Y.; Yang, S.; Li, Y.; Zhao, D.; Lai, Y.; Chen, Y. Oxidizing Roasting Behavior and Leaching Performance for the Recovery of Spent LiFePO4 Batteries. Minerals 2020, 10, 949. [Google Scholar] [CrossRef]
- Träger, T.; Friedrich, B.; Weyhe, R. Recovery Concept of Value Metals from Automotive Lithium-Ion Batteries. Chem. Ing. Tech. 2015, 87, 1550–1557. [Google Scholar] [CrossRef]
- Li, L.; Lu, J.; Ren, Y.; Zhang, X.X.; Chen, R.J.; Wu, F.; Amine, K. Ascorbic-Acid-Assisted Recovery of Cobalt and Lithium from Spent Li-Ion Batteries. J. Power Sources 2012, 218, 21–27. [Google Scholar] [CrossRef]
- Joulié, M.; Laucournet, R.; Billy, E. Hydrometallurgical Process for the Recovery of High Value Metals from Spent Lithium Nickel Cobalt Aluminum Oxide Based Lithium-Ion Batteries. J. Power Sources 2014, 247, 551–555. [Google Scholar] [CrossRef]
- Davis, K.; Demopoulos, G.P. Hydrometallurgical Recycling Technologies for NMC Li-Ion Battery Cathodes: Current Industrial Practice and New R&D Trends. RSC Sustain. 2023, 1, 1932–1951. [Google Scholar] [CrossRef]
- Chen, L.; Tang, X.; Zhang, Y.; Li, L.; Zeng, Z.; Zhang, Y. Process for the Recovery of Cobalt Oxalate from Spent Lithium-Ion Batteries. Hydrometallurgy 2011, 108, 80–86. [Google Scholar] [CrossRef]
- Chen, X.; Fan, B.; Xu, L.; Zhou, T.; Kong, J. An Atom-Economic Process for the Recovery of High Value-Added Metals from Spent Lithium-Ion Batteries. J. Clean. Prod. 2016, 112, 3562–3570. [Google Scholar] [CrossRef]
- Guzolu, J.S.; Gharabaghi, M.; Mobin, M.; Alilo, H. Extraction of Li and Co from Li-Ion Batteries by Chemical Methods. J. Inst. Eng. Ser. D 2017, 98, 43–48. [Google Scholar] [CrossRef]
- Su, F.; Zhou, X.; Liu, X.; Yang, J.; Tang, J.; Yang, W.; Li, Z.; Wang, H.; Ma, Y. Efficient Recovery of Valuable Metals from Spent Lithium-Ion Batteries by Pyrite Method with Hydrometallurgy Process. Chem. Eng. J. 2023, 455, 140914. [Google Scholar] [CrossRef]
- Lee, C.K.; Rhee, K.-I. Reductive Leaching of Cathodic Active Materials from Lithium Ion Battery Wastes. Hydrometallurgy 2003, 68, 5–10. [Google Scholar] [CrossRef]
- Almeida, J.R.; Moura, M.N.; Barrada, R.V.; Barbieri, E.M.S.; Carneiro, M.T.W.D.; Ferreira, S.A.D.; Lelis, M.d.F.F.; de Freitas, M.B.J.G.; Brandão, G.P. Composition Analysis of the Cathode Active Material of Spent Li-Ion Batteries Leached in Citric Acid Solution: A Study to Monitor and Assist Recycling Processes. Sci. Total Environ. 2019, 685, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, C.; Lai, F.; Yan, F.; Zhang, Z. Reduction-Ammoniacal Leaching to Recycle Lithium, Cobalt, and Nickel from Spent Lithium-Ion Batteries with a Hydrothermal Method: Effect of Reductants and Ammonium Salts. Waste Manag. 2020, 102, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Qiao, W.; Zhang, R.; Wen, Y.; Wang, X.; Wang, Z.; Tang, G.; Liu, M.; Kang, H.; Said, Z.; Hwang, J.-Y.; et al. Green Solvents in Battery Recycling: Status and Challenges. J. Mater. Chem. A Mater. 2024, 12, 11235–11265. [Google Scholar] [CrossRef]
- Shen, J.; Zhou, M.; Liu, W.; Shi, Y.; Tang, W.; Deng, Y.; Liu, R.; Zuo, Y.; Zhang, J. Advanced Direct Recycling Technology Enables a Second Life of Spent Lithium-Ion Battery. Energy Storage Mater. 2025, 74, 103964. [Google Scholar] [CrossRef]
- Mao, J.; Ye, C.; Zhang, S.; Xie, F.; Zeng, R.; Davey, K.; Guo, Z.; Qiao, S. Toward Practical Lithium-Ion Battery Recycling: Adding Value, Tackling Circularity and Recycling-Oriented Design. Energy Environ. Sci. 2022, 15, 2732–2752. [Google Scholar] [CrossRef]
- Yu, X.; Li, W.; Gupta, V.; Gao, H.; Tran, D.; Sarwar, S.; Chen, Z. Current Challenges in Efficient Lithium-Ion Batteries’ Recycling: A Perspective. Glob. Chall. 2022, 6, 2200099. [Google Scholar] [CrossRef]
- Dobó, Z.; Dinh, T.; Kulcsár, T. A Review on Recycling of Spent Lithium-Ion Batteries. Energy Rep. 2023, 9, 6362–6395. [Google Scholar] [CrossRef]
- Wang, Y.; Goikolea, E.; de Larramendi, I.R.; Lanceros-Méndez, S.; Zhang, Q. Recycling Methods for Different Cathode Chemistries—A Critical Review. J. Energy Storage 2022, 56, 106053. [Google Scholar] [CrossRef]
- Milian, Y.E.; Jamett, N.; Cruz, C.; Herrera-León, S.; Chacana-Olivares, J. A Comprehensive Review of Emerging Technologies for Recycling Spent Lithium-Ion Batteries. Sci. Total Environ. 2024, 910, 168543. [Google Scholar] [CrossRef]
- Gupta, V.; Yu, X.; Gao, H.; Brooks, C.; Li, W.; Chen, Z. Scalable Direct Recycling of Cathode Black Mass from Spent Lithium-Ion Batteries. Adv. Energy Mater. 2023, 13, 2203093. [Google Scholar] [CrossRef]
- Sommerville, R.; Shaw-Stewart, J.; Goodship, V.; Rowson, N.; Kendrick, E. A Review of Physical Processes Used in the Safe Recycling of Lithium Ion Batteries. Sustain. Mater. Technol. 2020, 25, e00197. [Google Scholar] [CrossRef]
- Nasser, O.A.; Petranikova, M. Review of Achieved Purities after Li-Ion Batteries Hydrometallurgical Treatment and Impurities Effects on the Cathode Performance. Batteries 2021, 7, 60. [Google Scholar] [CrossRef]
- Sloop, S.; Crandon, L.; Allen, M.; Koetje, K.; Reed, L.; Gaines, L.; Sirisaksoontorn, W.; Lerner, M. A Direct Recycling Case Study from a Lithium-Ion Battery Recall. Sustain. Mater. Technol. 2020, 25, e00152. [Google Scholar] [CrossRef]
- Ji, Y.; Kpodzro, E.E.; Jafvert, C.T.; Zhao, F. Direct Recycling Technologies of Cathode in Spent Lithium-Ion Batteries. Clean. Technol. Recycl. 2021, 1, 124–151. [Google Scholar] [CrossRef]
- Park, K.; Yu, J.; Coyle, J.; Dai, Q.; Frisco, S.; Zhou, M.; Burrell, A. Direct Cathode Recycling of End-Of-Life Li-Ion Batteries Enabled by Redox Mediation. ACS Sustain. Chem. Eng. 2021, 9, 8214–8221. [Google Scholar] [CrossRef]
- Bahaloo-Horeh, N.; Mousavi, S.M. Enhanced Recovery of Valuable Metals from Spent Lithium-Ion Batteries through Optimization of Organic Acids Produced by Aspergillus Niger. Waste Manag. 2017, 60, 666–679. [Google Scholar] [CrossRef]
- Mishra, D.; Kim, D.-J.; Ralph, D.E.; Ahn, J.-G.; Rhee, Y.-H. Bioleaching of Metals from Spent Lithium Ion Secondary Batteries Using Acidithiobacillus Ferrooxidans. Waste Manag. 2008, 28, 333–338. [Google Scholar] [CrossRef]
- Xin, B.; Zhang, D.; Zhang, X.; Xia, Y.; Wu, F.; Chen, S.; Li, L. Bioleaching Mechanism of Co and Li from Spent Lithium-Ion Battery by the Mixed Culture of Acidophilic Sulfur-Oxidizing and Iron-Oxidizing Bacteria. Bioresour. Technol. 2009, 100, 6163–6169. [Google Scholar] [CrossRef]
- Xin, Y.; Guo, X.; Chen, S.; Wang, J.; Wu, F.; Xin, B. Bioleaching of Valuable Metals Li, Co, Ni and Mn from Spent Electric Vehicle Li-Ion Batteries for the Purpose of Recovery. J. Clean. Prod. 2016, 116, 249–258. [Google Scholar] [CrossRef]
- Niu, Z.; Zou, Y.; Xin, B.; Chen, S.; Liu, C.; Li, Y. Process Controls for Improving Bioleaching Performance of Both Li and Co from Spent Lithium Ion Batteries at High Pulp Density and Its Thermodynamics and Kinetics Exploration. Chemosphere 2014, 109, 92–98. [Google Scholar] [CrossRef]
- Zeng, G.; Luo, S.; Deng, X.; Li, L.; Au, C. Influence of Silver Ions on Bioleaching of Cobalt from Spent Lithium Batteries. Miner. Eng. 2013, 49, 40–44. [Google Scholar] [CrossRef]
- Roy, J.J.; Cao, B.; Madhavi, S. A Review on the Recycling of Spent Lithium-Ion Batteries (LIBs) by the Bioleaching Approach. Chemosphere 2021, 282, 130944. [Google Scholar] [CrossRef] [PubMed]
- Ghassa, S.; Farzanegan, A.; Gharabaghi, M.; Abdollahi, H. Novel Bioleaching of Waste Lithium Ion Batteries by Mixed Moderate Thermophilic Microorganisms, Using Iron Scrap as Energy Source and Reducing Agent. Hydrometallurgy 2020, 197, 105465. [Google Scholar] [CrossRef]
- Naseri, T.; Mousavi, S.M. Improvement of Li and Mn Bioleaching from Spent Lithium-Ion Batteries, Using Step-Wise Addition of Biogenic Sulfuric Acid by Acidithiobacillus Thiooxidans. Heliyon 2024, 10, e37447. [Google Scholar] [CrossRef]
- Jegan Roy, J.; Srinivasan, M.; Cao, B. Bioleaching as an Eco-Friendly Approach for Metal Recovery from Spent NMC-Based Lithium-Ion Batteries at a High Pulp Density. ACS Sustain. Chem. Eng. 2021, 9, 3060–3069. [Google Scholar] [CrossRef]
- Liao, X.; Ye, M.; Liang, J.; Li, S.; Liu, Z.; Deng, Y.; Guan, Z.; Gan, Q.; Fang, X.; Sun, S. Synergistic Enhancement of Metal Extraction from Spent Li-Ion Batteries by Mixed Culture Bioleaching Process Mediated by Ascorbic Acid: Performance and Mechanism. J. Clean. Prod. 2022, 380, 134991. [Google Scholar] [CrossRef]
- Chandakhiaw, T.; Teaumroong, N.; Piromyou, P.; Songwattana, P.; Tanthanuch, W.; Tancharakorn, S.; Khumkoa, S. Efficiency of Penicillium sp. and Aspergillus sp. for Bioleaching Lithium Cobalt Oxide from Battery Wastes in Potato Dextrose Broth and Sucrose Medium. Results Eng. 2024, 24, 103170. [Google Scholar] [CrossRef]
- Bahaloo-Horeh, N.; Mousavi, S.M.; Baniasadi, M. Use of Adapted Metal Tolerant Aspergillus Niger to Enhance Bioleaching Efficiency of Valuable Metals from Spent Lithium-Ion Mobile Phone Batteries. J. Clean. Prod. 2018, 197, 1546–1557. [Google Scholar] [CrossRef]
- Paul, S.; Shrotriya, P. Efficient Recycling Processes for Lithium-Ion Batteries. Materials 2025, 18, 613. [Google Scholar] [CrossRef]
- Raj, T.; Chandrasekhar, K.; Kumar, A.N.; Sharma, P.; Pandey, A.; Jang, M.; Jeon, B.H.; Varjani, S.; Kim, S.H. Recycling of Cathode Material from Spent Lithium-Ion Batteries: Challenges and Future Perspectives. J. Hazard. Mater. 2022, 429, 128312. [Google Scholar] [CrossRef]
- Vierunketo, M.; Klemettinen, A.; Reuter, M.A.; Santasalo-Aarnio, A.; Serna-Guerrero, R. A Grave-to-Cradle Analysis of Lithium-Ion Battery Cathode Materials Using Material and Energy Circularity Indicators. J. Clean. Prod. 2024, 471, 143435. [Google Scholar] [CrossRef]
- Kallitsis, E.; Korre, A.; Kelsall, G.H. Life Cycle Assessment of Recycling Options for Automotive Li-Ion Battery Packs. J. Clean. Prod. 2022, 371, 133636. [Google Scholar] [CrossRef]
- Mayyas, A.; Moawad, K.; Chadly, A.; Alhseinat, E. Can Circular Economy and Cathode Chemistry Evolution Stabilize the Supply Chain of Li-Ion Batteries? Extr. Ind. Soc. 2023, 14, 101253. [Google Scholar] [CrossRef]
- Tan, J.; Keiding, J.K. Mapping the Cobalt and Lithium Supply Chains for E-Mobility Transition: Significance of Overseas Investments and Vertical Integration in Evaluating Mineral Supply Risks. Resour. Conserv. Recycl. 2024, 209, 107788. [Google Scholar] [CrossRef]
- Li, J.; Wang, Z.; Li, H.; Jiao, J. Which Policy Can Effectively Promote the Formal Recycling of Power Batteries in China? Energy 2024, 299, 131445. [Google Scholar] [CrossRef]
- International Energy Agency (IEA). Specifications for the Comprehensive Utilisation of Waste EV Batteries; International Energy Agency: Paris, France, 2024. [Google Scholar]
- Yang, H.; Hu, X.; Zhang, G.; Dou, B.; Cui, G.; Yang, Q.; Yan, X. Life Cycle Assessment of Secondary Use and Physical Recycling of Lithium-Ion Batteries Retired from Electric Vehicles in China. Waste Manag. 2024, 178, 168–175. [Google Scholar] [CrossRef]
- Patel, P.; Ellis, T.; Howes, J. How Green Is Your Electric Vehicle? MRS Bull. 2017, 42, 416–417. [Google Scholar] [CrossRef]
- Seika, J.; Kubli, M. Repurpose or Recycle? Simulating End-of-Life Scenarios for Electric Vehicle Batteries under the EU Battery Regulation. Sustain. Prod. Consum. 2024, 51, 644–656. [Google Scholar] [CrossRef]
- Kendall, A.; Dayemo, K.; Helal, N.; Iskakov, G.; Pares, F.; Slattery, M.; Fulton, L. Electric Vehicle Lithium-Ion Batteries in Lower- and Middle-Income Countries: Life Cycle Impacts and Issues, 1st ed.; ITS—Institute of Transportation Studies: Berkeley, CA, USA, 2023. [Google Scholar]
- Trost, J.N.; Dunn, J.B. Assessing the Feasibility of the Inflation Reduction Act’s EV Critical Mineral Targets. Nat. Sustain. 2023, 6, 639–643. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Roy, S.; Vajtai, R. Emerging Processes for Sustainable Li-Ion Battery Cathode Recycling. Small 2024, 2400557. [Google Scholar] [CrossRef]
- Montavalli, J. Closing the Loop on EV Battery Recycling. Available online: www.sae.org (accessed on 19 April 2025).
- Buchholz, K. Honda Taps Recycling Firms for Future EV Batteries. Available online: https://www.sae.org/news/2023/07/honda-battery-recycling (accessed on 31 May 2025).
- Chen, W.; Li, J.; Ji, H.; Shi, R.; Wang, J.; Zhu, Y.; Liu, J.; Zhang, R.; Wu, Z.; Xiao, X.; et al. Efficient and Scalable Direct Regeneration of Spent Layered Cathode Materials via Advanced Oxidation. Adv. Mater. 2025, 37, 2416818. [Google Scholar] [CrossRef] [PubMed]
- Chigbu, B.I.; Nekhwevha, F.H.; Umejesi, I. Electric Vehicle Battery Remanufacturing: Circular Economy Leadership and Workforce Development. World Electr. Veh. J. 2024, 15, 441. [Google Scholar] [CrossRef]
- PNME 4o Anuário Brasileiro Da Mobilidade Elétrica. Available online: https://pnme.org.br/ (accessed on 19 April 2025).
- Branco, J.E.H.; da Rocha, F.V.; Péra, T.G.; de Bastiani, F.P.; Bartholomeu, D.B.; Costa, E.L.; Grilo Junior, I. Assessing Greenhouse Gas Emissions and Costs of Brazilian Light-Duty Vehicles. Renew. Sustain. Energy Rev. 2024, 206, 114845. [Google Scholar] [CrossRef]
- Telli, G.D.; Altafini, C.R.; Costa, C.A.; Rosa, J.S.; Rocha, L.A.O.; Lorenzini, G. Experimental Study of a Dual-Fuel Generator Set Operating on Diesel Fuel Direct Injected and Hydrous Ethanol Fumigation at Different Loads. Int. J. Des. Nat. Ecodynam. 2020, 15, 777–784. [Google Scholar] [CrossRef]
- Telli, G.D.; Altafini, C.R.; Rosa, J.S.; Costa, C.A. Experimental Analysis of a Small Engine Operating on Diesel–Natural Gas and Soybean Vegetable Oil–Natural Gas. J. Braz. Soc. Mech. Sci. Eng. 2018, 40, 547. [Google Scholar] [CrossRef]
- Presidência da República - Secretaria Especial para Assuntos Jurídicos Casa Civil. Lei No 14.902. Available online: www.planalto.gov.br (accessed on 18 April 2025).
- ABREMA Falta Legislação Específica Sobre Reciclagem e Descarte. Available online: www.abrema.org.br (accessed on 19 April 2025).
- Wilke, C.; Kaas, A.; Peuker, U.A. Influence of the Cell Type on the Physical Processes of the Mechanical Recycling of Automotive Lithium-Ion Batteries. Metals 2023, 13, 1901. [Google Scholar] [CrossRef]
- Sistema de Estimativas de Emissões e Remoções de Gases de Efeito Estufa (SEEG). Análise Das Emissões de Gases de Efeito Estufa e Suas Implicações Para as Metas Climáticas Do Brasil. Available online: https://seeg.eco.br/ (accessed on 10 June 2025).

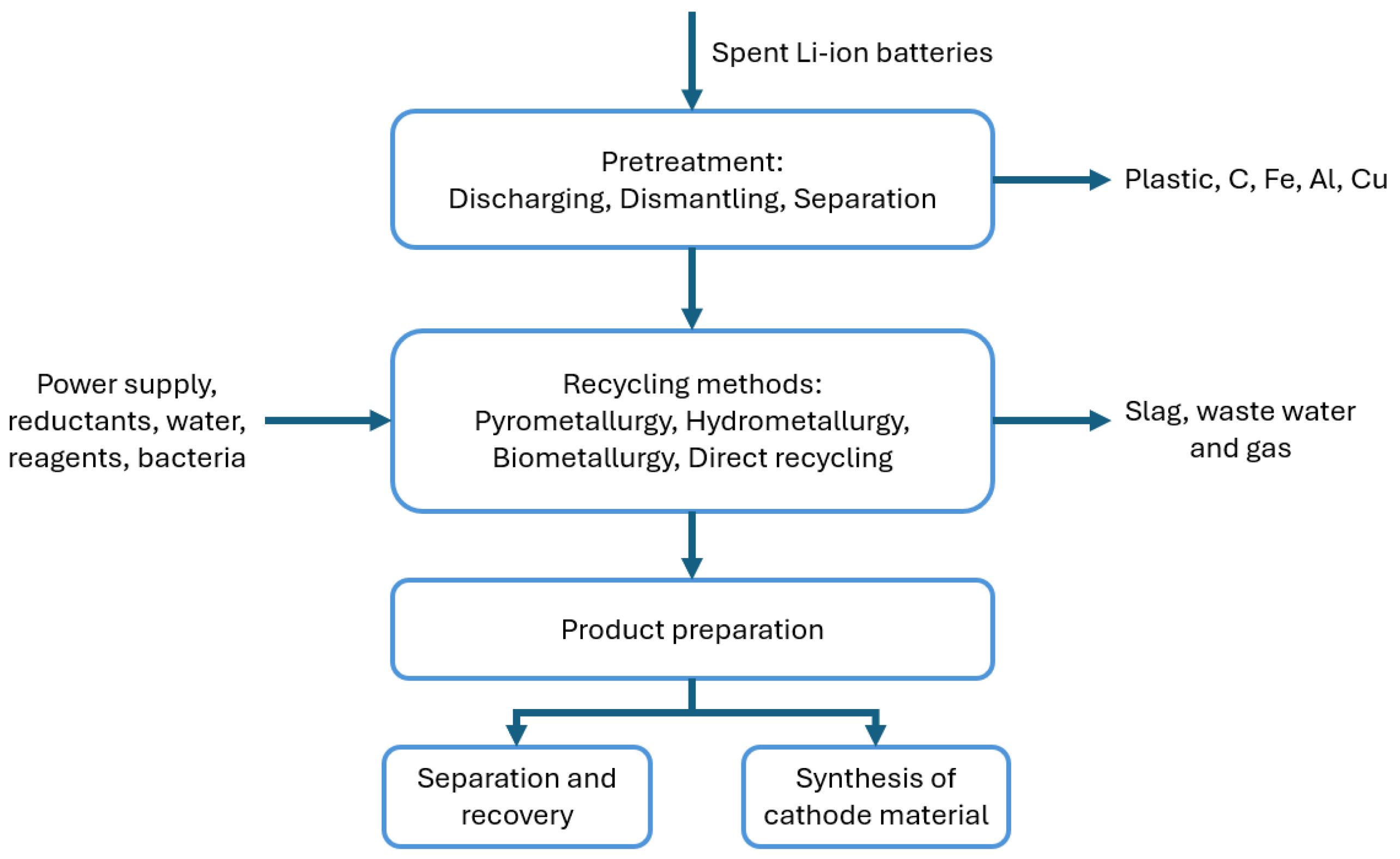
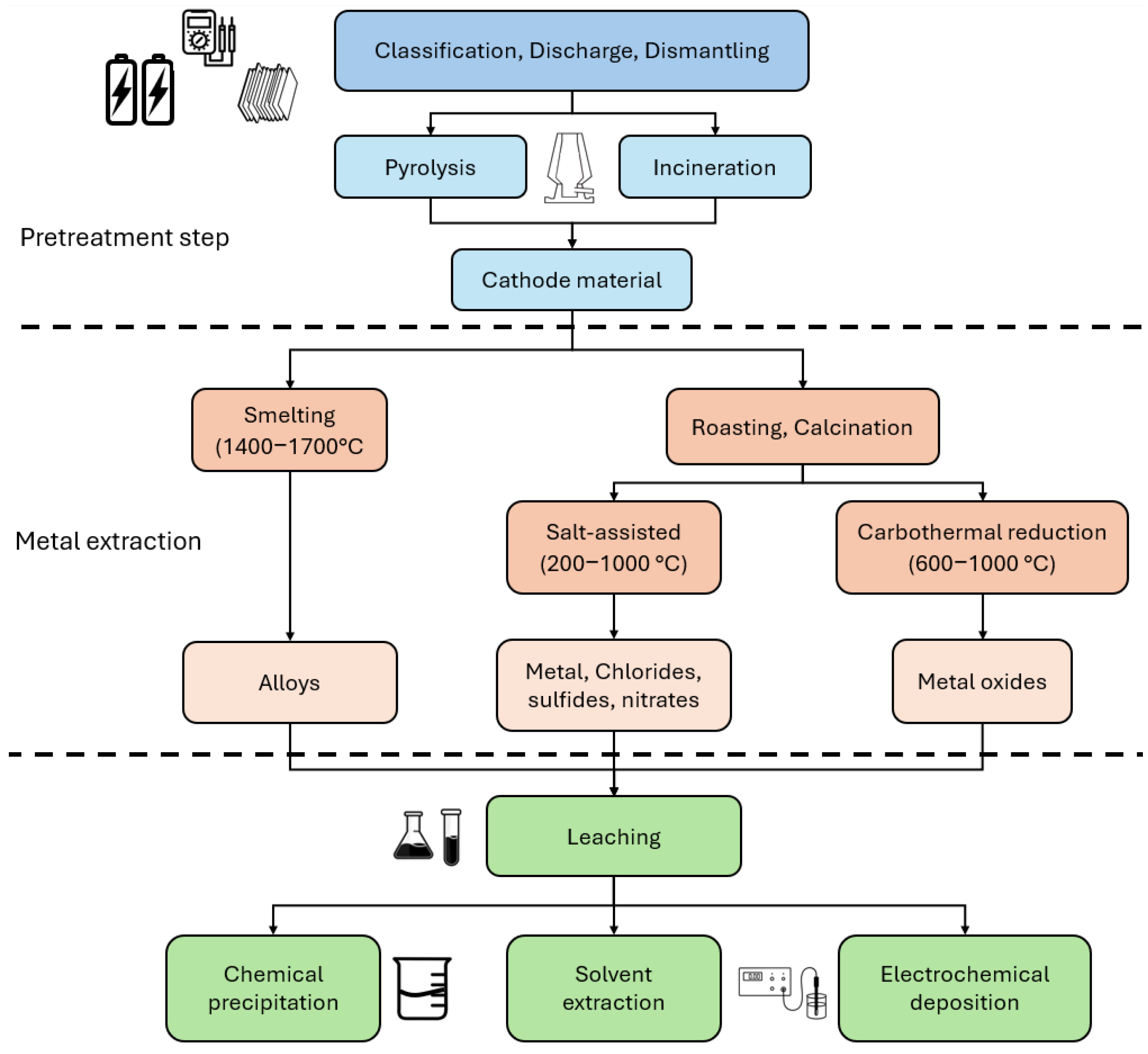
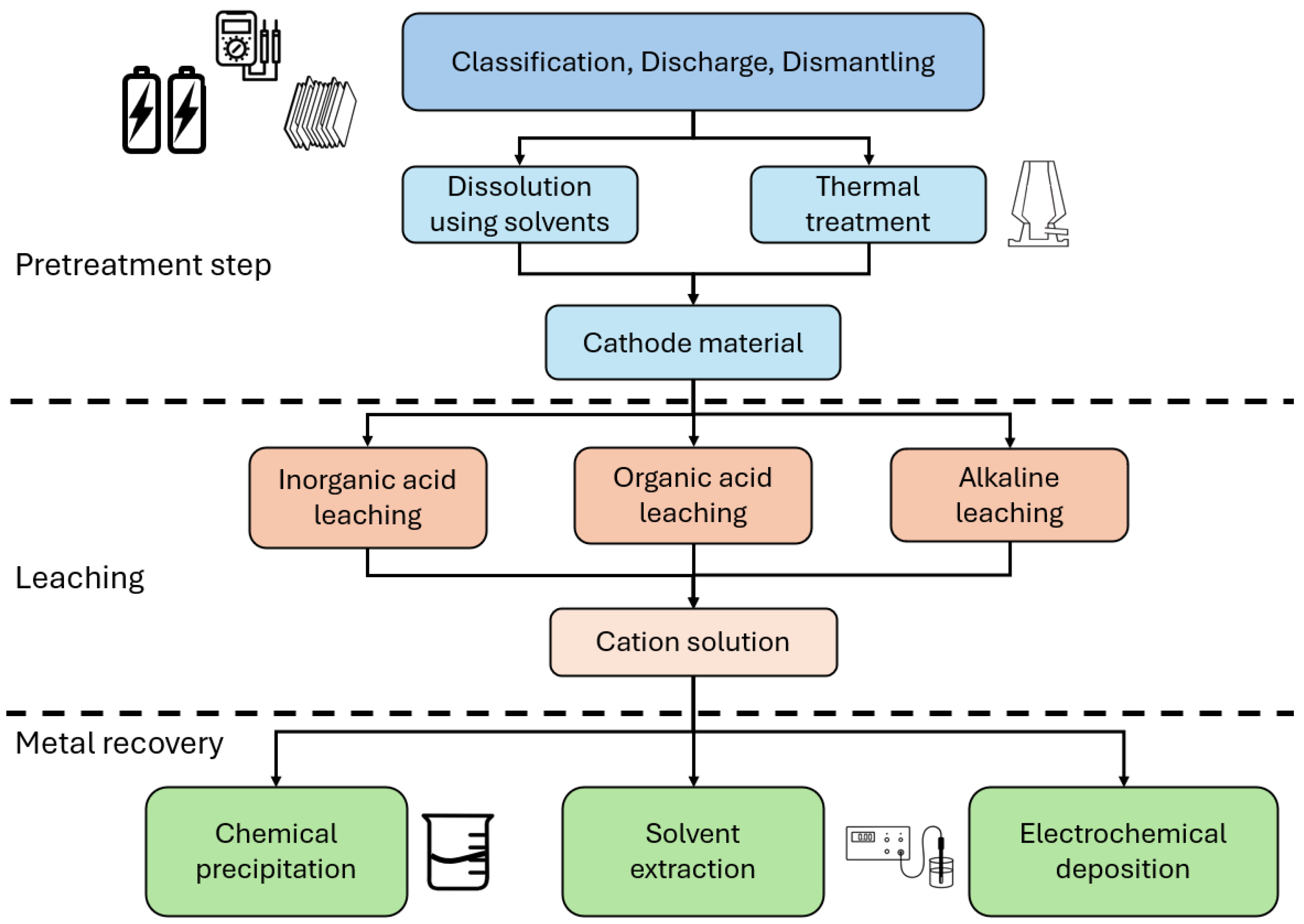
| Cathode Material | Potential (V vs. Li0) | Discharge Capacity (mAh/g at 0.1C) | Specific Energy (Wh/kg) | Capacity Retention, 100 Cycles (%) | Advantages | Disadvantages |
|---|---|---|---|---|---|---|
| LCO (LiCoO2) | 3.7–3.9 | 140 | 520 | 97–98 | High specific energy | Short service life, Limited load capacity and safety |
| NCA (LiNiCoAlO2) | 3.8 | 180–200 | 680–760 | 93 | High energy, High power density, Good service life | High cost, Low security |
| NMC (LiNiMnCoO2) | 3.3 | 170 | 560 | 95 | Good performance in all properties | High cost |
| LMO (LiMn2O4) | 3.8 | 120 | 455 | 89–93 | High specific power, Safety, Long service life | Average performance across all properties |
| LFP (LiFePO4) | 3.3 | 155–160 | 560 | >99 | Good thermal stability, Excellent safety, Long service life | Moderate specific energy, Low voltage, Reduced performance at low temperature |
| Cathode Material | Methodology | Process Conditions | Efficiency | Refs. |
|---|---|---|---|---|
| LiNixCoyMnzO2 | Roasting | 650 °C, 30 min, coke dosage of 10% | Li: 93,67%, Ni: 93,33%, Co: 98.08%, Mn: 98.68% | [49] |
| Li(NixMnyCo1−x−y)O2 | Carbothermal reduction; water leaching | 700–1200 °C, 1 h | Li: 93% | [57] |
| LiNiMnCoO2 | Microwave carbothermal reduction; acid leaching | 900 °C, 30 min | Li: 99.68%, Co: 97.85%, Ni: 97.65%, Mn: 96.73% | [58] |
| LiCoNiO2 | Smelting | 1450 °C, 30 min | Co: 98.83%, Ni: 98.39%, Cu: 93.57% | [59] |
| Mixed materials | Calcination; organic acid leaching | 700 °C, 2 h | Li: 91.5%, Co: 95.02% | [60] |
| LiFePO4 | Salt-assisted roasting (Na2CO3); inorganic acid leaching | 600 °C, 2 h | Li: 99.2% | [61] |
| LiCoO2 | Chlorination roasting (NH4Cl); water leaching | 400 °C, 20 min | Li: 99.43%, Co: 99.05% | [62] |
| LiCoO2 | Chlorination roasting (NH4Cl); water leaching | 350 °C, 20 min | Li: 99.18%, Co: 99.3% | [63] |
| Cathode Material | Leaching Reagents | Process Conditions | Efficiency | Refs. |
|---|---|---|---|---|
| LiCoO2 | HCl (5 M) | 95 °C, 70 min, S/L: 10 g/L | Li: 98%, Co: 99% | [75] |
| LiNixCoᵧMn_zO2 | H2SO4 (3 M), FeS2 | 80 °C, 2 h, S/L: 40 g/L | Li: 99.9%, Co: 99.5%, Mn: 98%, Ni: 98.9% | [76] |
| LiCoO2 | HNO3 (1 M), H2O2 (1.7 vol%) | 75 °C, 30 min, S/L: 10–20 g/L | Li: 99%, Co: 99% | [77] |
| Cathode material | Citric acid (2 M), H2O2 (0.25 M) | 80 °C, 2 h, S/L: 20 g/L | Li: 99%, Co: 99%, Mn: 92%, Ni: 90% | [78] |
| LiNixCoᵧMn_zO2 | NH3·H2O (6 M), (NH4)2CO3 (0.5 M), Na2SO3 (0.5 M) | 150 °C, 30 min, S/L: 10 g/L | Li: 87.0%, Co: 99.5%, Ni: 91.1% | [79] |
| Microorganism | Type of Material | Conditions | Efficiency | Refs. |
|---|---|---|---|---|
| A. ferrooxidans | LiCoO2-based spent LIBs | pH 2; 10% (v/v) Modified 9K medium; 100 g/L; 30 °C; 160 rpm; 72 h | Co: 94%, Li: 60% | [99] |
| Consortium of thermophilic bacteria | Waste LIB cells | pH 1.8; 10% inoculation; 45 °C; 130 rpm | Co: 99.9%, Ni: 99.7%, Li: 84% | [100] |
| Aspergillus niger | Spent LIBs | 26.478 g/L sucrose, 3.45% (v/v) inoculum; pH 5.44 | Cu: 100%, Li: 100%, Mn: 77%, Al: 75%, Co: 64%, Ni: 54% | [93] |
| Acidithiobacillus thiooxidans | LiMnO2 cathode | 30 °C, 8 days, S/L ratio: 60 g/L | Li: 93%, Mn: 53% | [101] |
| Acidithiobacillus ferrooxidans | LiNixCoᵧMnxO2 cathode | 30 °C, 72 h, S/L ratio: 100 g/L | Li: 89%, Co: 82%, Mn: 92%, Ni: 90% | [102] |
| A. caldus & S. thermosulfidooxidans | LiCoO2 cathode | 30 °C, 2 days, S/L ratio: 20 g/L | Li: 94%, Co: 95% | [103] |
| Penicillium | LiCoO2 cathode | 25 °C, 30 days | Li: 99.88%, Co: 77.87% | [104] |
| Aspergillus niger | Mixed cathode materials | 30 °C, 30 days, 1% (w/v) pulp density, adapted strain | Li: 100%, Cu: 94%, Mn: 72%, Al: 62%, Ni: 45%, Co: 38% | [105] |
| Recycling Processes | Current Scale of Application | Recycling Cost/Time | Advantages | Disadvantages |
|---|---|---|---|---|
| Pyrometallurgy | Industrial [21]. | High/Fast | Application flexibility for different types of batteries [21]. | High emission of greenhouse gases in the process [19]. |
| Simple pre-treatment, without the need for disassembly and separation of materials [10]. | High energy demand for process execution [19]. | |||
| Hydrometallurgy | Industrial [18]. | Moderate/Moderate | Application flexibility for different types of batteries [18]. | High demand for equipment maintenance, due to degradation caused by chemical reagents used in the process [10,21] |
| Low energy demand for process execution [18]. | Need for post-treatment of effluents generated in the process, aiming to reduce environmental impact [10,21] | |||
| Direct Process | Laboratory [21]. | Low/Fast | Low emission of greenhouse gases in the process [20]. | Low robustness of the process, with many variables for its execution, depending on the battery to be recycled [87]. |
| Low energy demand for process execution [20]. | Difficulty in obtaining the purity of materials required by the industry [87]. | |||
| Biometallurgy | Laboratory [18] | Low/Slow | Low emission of greenhouse gases in the process [21]. | Long period required to execute the process, due to the time required for the bacteria to react [18]. |
| Low energy demand for process execution [18]. | Low robustness of the process, due to the sensitivity of the bacterial cultivation stage [18]. |
| Country/ Region | Number of EVs (BEVs + PHEVs) on the Road in 2024 | Lithium-Ion Batteries Recycling Legislation | Minimum Material Recovery Targets Stipulated | Examples of Recycling Industries | Average Material Recovery Rate on Industries |
|---|---|---|---|---|---|
| China | 34.00 million | Specifications for the Comprehensive Utilization of Waste EV Batteries 2024 (MIIT) | 90% for lithium; 98% for nickel, cobalt and manganese. | Huayou Cobalt; Brunp Recycling; GEM; GHTECH. | 92% for lithium; 98% for nickel, cobalt and manganese; 89% for copper. |
| European Union | 10.20 million | Battery Regulation 2023/1542 | 50% for lithium; 90% for cobalt, copper, lead and nickel. | Hydrovolt (NO); Altilium (UK); Librec (CH). | 94% for lithium, nickel, cobalt and manganese and copper. |
| United States | 6.30 million | - | - | Redwood Materials; Ascend Elements; Cirba Solutions; Cox Automotive. | 95% for lithium, nickel, cobalt and manganese and copper. |
| Brazil | 214,000 | - | - | Energy Source; Lorene; Tupy. | 90% for lithium, nickel, cobalt and manganese and copper. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the World Electric Vehicle Association. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furlanetto, J.; de Lara, M.V.C.; Simionato, M.; Nascimento, V.d.; Telli, G.D. An Overview of Lithium-Ion Battery Recycling: A Comparison of Brazilian and International Scenarios. World Electr. Veh. J. 2025, 16, 371. https://doi.org/10.3390/wevj16070371
Furlanetto J, de Lara MVC, Simionato M, Nascimento Vd, Telli GD. An Overview of Lithium-Ion Battery Recycling: A Comparison of Brazilian and International Scenarios. World Electric Vehicle Journal. 2025; 16(7):371. https://doi.org/10.3390/wevj16070371
Chicago/Turabian StyleFurlanetto, Jean, Marcus V. C. de Lara, Murilo Simionato, Vagner do Nascimento, and Giovani Dambros Telli. 2025. "An Overview of Lithium-Ion Battery Recycling: A Comparison of Brazilian and International Scenarios" World Electric Vehicle Journal 16, no. 7: 371. https://doi.org/10.3390/wevj16070371
APA StyleFurlanetto, J., de Lara, M. V. C., Simionato, M., Nascimento, V. d., & Telli, G. D. (2025). An Overview of Lithium-Ion Battery Recycling: A Comparison of Brazilian and International Scenarios. World Electric Vehicle Journal, 16(7), 371. https://doi.org/10.3390/wevj16070371







