Abstract
Research on lithium-ion batteries has been driven by the growing demand for electric vehicles to mitigate greenhouse gas emissions. Despite advances, batteries still face significant challenges in efficiency, lifetime, safety, and material optimization. In this context, the objective of this research is to develop a predictive model based on Deep deep-Learning learning techniques. Based on Deep Learning techniques that combine Transformer and Physicsphysics-Informed informed approaches for the optimization and design of electrochemical parameters that improve the performance of lithium batteries. Also, we present a training database consisting of three key components: numerical simulation using the Doyle–Fuller–Newman (DFN) mathematical model, experimentation with a lithium half-cell configured with a zinc oxide anode, and a set of commercial battery discharge curves using electronic monitoring. The results show that the developed Transformer–Physics physics-Informed informed model can effectively integrate deep deep-learning DNF to make predictions of the electrochemical behavior of lithium-ion batteries. The model can estimate the battery battery-charge capacity with an average error of 2.5% concerning the experimental data. In addition, it was observed that the Transformer could explore new electrochemical parameters that allow the evaluation of the behavior of batteries without requiring invasive analysis of their internal structure. This suggests that the Transformer model can assess and optimize lithium-ion battery performance in various applications, which could significantly impact the battery industry and its use in Electric Vehicles vehicles (EVs).
1. Introduction
The climate crisis and its environmental impact have sounded alarm bells as a problem that requires an immediate response to preserve balance and avoid irreversible damage to the planet [1]. In response to the issue of global warming, research has built a conceptual framework to understand the environmental crisis and its effects on biodiversity loss, highlighting the importance of addressing them as a unified and interconnected problem, thus coining the term planetary health. For example, increasing global temperatures can cause droughts, forest fires, and rising sea levels, lead to biodiversity loss, which is a negative indicator of planet health [2,3].
The issue of global warming has led to research into the integration of renewable energy sources and technologies that mitigate greenhouse gas emissions. As a solution to this problem, researchers have proposed electric vehicles and clean-source storage technologies [4]. However, a paradigm transition requires efficient management of energy demand and adequate infrastructure to support the growing demand for electric vehicles powered by renewable energy sources [5]. Thus, various research directions are being pursued, including evaluating the efficacy of energy response strategies to address the fluctuating nature of renewable energy production and regulate the charging behavior of electric vehicles; optimizing energy consumption and maintaining grid stability; and addressing fluctuations in renewable energy generation and managing the charging patterns of electric vehicles [6,7].
An important point in the transition to electric vehicles is electrochemical batteries, which play a crucial role in the mastery of energy storage systems that drive the driving power of vehicles. Generally speaking, batteries store electrical energy from chemical rations in a potential difference, divided by three elements: —anode, cathode, and electrolyte, —which can then be used to power engines or other systems [8]. However, batteries have limited the transition to electric vehicles due to the degradation of the battery components. In general, several factors contribute to degradation, including ambient temperature, discharge current and cutoff voltage, and electrochemical interactions of their operation [9,10]. A detailed analysis reveals that the intensive use of batteries in energy storage applications can affect their useful life [11].
Modern technologies have generated an increasing demand for energy efficiency and electromobility, driving the development of new generations of batteries for portable electronic devices, household appliances, and electric vehicles. In this context, batteries such as lead-acid, nickel-cadmium and, more recently, lithium-ion batteries have been designed and produced [12,13]. Among the latter, LFP (Lithium Ferro Phosphate), NMC (Lithium Nickel Manganese Cobalt Oxide), LMO (Lithium Manganese Oxide), and NCA (Lithium Nickel Cobalt Aluminum) technologies stand out. Various technologies come with their own sets of pros and cons: LFP batteries are characterized by excellent thermal stability and safety, making them suitable for electric vehicles; NMC batteries provide a good equilibrium between energy density and safety, making them a popular choice in portable devices and electric cars; LMO batteries are recognized for delivering high power, but they do face issues related to thermal stability; while NCA batteries, known for their high energy density, are used in specialized fields [14,15]. The proper Li-ion battery type depends on safety, cost, required energy density, and particular specifications. Therefore, it is essential to understand the characteristics and limitations of each technology to optimize its use in different contexts and ensure optimal performance.
The development of lithium-ion batteries has an energy density that is higher than that of other storage systems. Research has presented significant advances in the development of lithium-ion battery anodes and cathodes with high charge and discharge and discharge rates [16]. Incorporating a uniformly implanted nanometric amorphous silicon layer, which increases battery performance, stands out. However, the state-of-the-art has focused on thermal regulation during fast charging as an effective strategy to achieve optimal performance [17]. In conclusion, exploring key opportunities to improve lithium battery efficiency and increase energy density by optimizing design parameters and advanced material selection is challenging in the transition to electromobility [16,18].
To accelerate the transition to electric vehicles worldwide, optimizing and designing improvements in battery energy storage is essential. In this context, research has shown that artificial intelligence can be an ally to optimization. In particular, key areas of artificial intelligence, such as supervised and unsupervised machine learning, metaheuristic optimization, and deep learning, have made important contributions [19,20]. Based on artificial intelligence, battery design can be studied and optimized through exploitation and exploration. Exploitation involves training models with data generated on performance, lifetime, failures, and other key aspects of battery operation [21]. On the other hand, exploration focuses on the search for new materials or components to improve energy storage or battery life, opening up new possibilities for battery design and optimization [10].
Applying machine learning and optimization techniques to the operation of energy-storage batteries has improved their performance and lifetime. In this regard, the use of the Krill Herd algorithm to manage energy in microgrids with dynamic reconfiguration has proven to be particularly effective, as it improves efficiency and significantly reduces costs [22]. Similarly, the application of machine learning to fault detection in energy storage and lithium ion batteries has reduced costs and improved efficiency, resulting in higher reliability and lower risk [23]. On the other hand, Q-learning techniques have been the key to optimizing battery management in electric vehicles, improving efficiency, and reducing costs [24]. Consequently, recent research has shown that algorithms such as Black Widow Optimization (BWO) can optimize power generation in microgrids, considering factors such as battery lifetime and CO2 emissions [25]. This allows for maintaining an economical, reliable, and environmentally sustainable balance. Finally, the Compact Cyclone-Based Manta-Ray Foraging Optimization (C-CMRFO) algorithm is designed specifically for sustainable microgrids [26]. This algorithm not only improves power distribution efficiency and optimizes resource allocation, but also facilitates the integration of renewable energy sources, ensuring accurate voltage and frequency regulation that supports grid stability.
In exploring research on lithium-ion batteries, both optimization and design, as well as the application of Deep Learning techniques to improve their management, have been addressed. On the one hand, the Physics-Informed Informed Neural Neural Network network has provided a method for accurately estimating the useful state of batteries, taking advantage of the combination of physical models and neural networks to capture the dynamics of battery degradation [27]. This allows estimating its useful life with an average error [28]. However, Deep Learning techniques have been applied to estimate batteries’ state-of-charge (SoC), taking advantage of available field and test data. The methodology developed allows us to study different battery chemistries and electrochemical parameter conditions and their correlation with the state of charge [29]. In summary, the combination of models based on the laws of physics and artificial neural networks has proven effective in accurately estimating the utility state and the state of charge of batteries, which can significantly improve their management [30].
Exploiting lithium batteries performance for improving or monitoring their system has led researchers to apply classical artificial intelligence techniques such as artificial neural networks (ANN), fuzzy logic, and machine learning [31,32,33,34]. For their part, ANNs have shown effectiveness in processing the state of charge of lithium batteries, allowing the monitoring of the battery and its correlation with its state, which allows estimating the behavior of the voltage curve [35,36,37]. From a fuzzy logic perspective, they have focused on monitoring and analyzing lithium batteries to improve the efficiency and performance of electric vehicles and optimize the charging capacity at low temperatures using external heating systems [38,39,40]. Recently, machine learning has emerged as a tool for improving electric vehicle performance in several areas, including range, charging and charging conditions, battery health status, and design of new lithium batteries [41,42,43].
The application of machine learning techniques to operate energy storage batteries has significantly optimized their performance and lifetime. Implementing the Krill Herd algorithm for energy management in dynamic reconfiguration microgrids has resulted in improved efficiency and substantial cost savings [22]. Furthermore, machine learning applied to fault detection in energy storage batteries has shown promising results, minimizing the risk of unforeseen failures and enhancing efficiency [23]. In addition, Q-learning methods have successfully optimized battery management in electric vehicles, markedly improving efficiency and lowering costs [24]. In short, machine learning has revolutionized battery optimization, significantly improving battery performance and lifetime [44].
In exploring research on lithium-ion batteries, both optimization and design and the application of Deep Learning techniques to improve their management have been addressed. By integrating physical models with neural networks, the Physicsphysics-Informed informed Neural neural Network network offers a precise approach to estimating the operational state of batteKPOJries, effectively capturing the nuances of battery degradation [27]. This allows estimating its useful life with an average error [28]. However, Deep Learning techniques have been applied to estimate batteries’ state-of-charge (SoC) state, taking advantage of available field and test data. The methodology developed allows us to study different battery chemistries and electrochemical parameter conditions and their correlation with the state of charge [29].
One of the challenges in electric vehicles and electromobility systems is estimating of the State of Charge (SoC) and State of Health (SoH) of lithium-ion batteries. This precision is critical to ensuring electric vehicles’ optimal performance and safety, driving a growing interest in developing deep learning models. Recently, research has explored transformers as predictors in the estimation of SoC and SoH, focusing primarily on introducing relevant battery values into the encoder-decoder and feedforward-forward network mechanisms to estimate SoC and SoH [45]. In addition to the above, the state-of-the-art has focused on SoH estimation using Deep Learning approaches that incorporate internal DC resistance [46], significantly improving prediction accuracy and training convergence. Finally, designing specialized transformer networks for SoC estimation under complex operating conditions has achieved prediction accuracy by proposing a multi-attenuation head that allows correlating different estimated battery values [47].
In the experimental exploration of lithium batteries, research has highlighted the importance of discovering new materials for the different components of their structure, such as the anode, cathode, and electrolyte. These components form a set of physicochemical properties that increase the charge density in lithium batteries. Regarding the characterization of materials, electrochemical impedance spectroscopy has been shown to allow modeling and describing the behavior of materials under different conditions; the results show that it is possible to predict the deterioration and behavior of the battery with this technique [48]. In addition, excess dopants have been found in specific areas of materials such as that can significantly improve their ionic conductivity and chemical stability, making them suitable for use as electrolytes in lithium-metal batteries [49]. However, the electrochemical properties of the batteries have been characterized by voltage-current-cyclic measurement techniques, which have allowed prediction of the behavior of the charge density. In summary, the state -of -the -art has shown that different configurations in battery structures improve or optimize their performance, which opens new frontiers in exploring the physical-chemical component space.
This work presents a framework for the analyzing and optimizing-ion batteries based on a deep deep-learning application using a Transformer Deep-Learning learning scheme [50,51].
This framework allows us to accurately analyze the state of charge and discharge of batteries, which is fundamental to their optimization and improvement. The main contributions of this work are as follows.
- An electronic system designed specifically for data acquisition and graphical representation of commercial lithium-ion batteries charge and discharge and discharge voltage curves was developed. This system allows for accurate information on the electrical behavior of batteries under various operating conditions, providing essential data to understand their performance and dynamic characteristics, including the measurement of critical parameters such as voltage, current, and temperature during the charge and discharge and discharge processes. This information is valuable for the analysis and optimization of commercial batteries and for developing predictive models that improve their management and allow progress in designing more efficient and long-lasting energy management systems.
- We propose a database of 425 batteries created using three key components: numerical simulations based on the DFN model, experimentation with a lithium half-cell and a zinc oxide anode, and a set of discharge curves of commercially used batteries from an electronic data acquisition system. Each battery includes its time series of discharge voltage and electrochemical parameters, covering a variety of current storage behaviors and configurations. The database is available in an open-access repository.
- We present a neural network trained and refined to simulate lithium-ion batteries’ behavior accurately. This network has been developed by integrating the Informed Physics technique with Pseudo-2D models, which allows for capturing the complex and varied patterns in the responses of these batteries under changes in their electrochemical parameters.
2. Materials and Methods
2.1. Physico-Chemical Model of the Lithium-Ion Battery
The study of the charge/dischargecharge–discharge cycles of lithium batteries in a sandwich unit cell is the starting point of our analysis, since the battery voltage will depend on the configuration of its unit cell. This analysis allows us to understand better the underlying phenomena affecting the cell system’s charge density and cycle life, which are critical in applications such as lithium-ion batteries. In particular, we focus on the physicochemical models that allow us to describe the electrochemical parameters that, in design or laboratory experimentation, determine battery-charge and -discharge potentials [52].
The base model for the simulation of a lithium cell was proposed by Doyle, Fuller, and Newman (DFN), which focuses on the idealization of the one-dimensional transport of the lithium-ion from the anode through the separator to the cathode. In particular, DFN deals with lithium-ion diffusion without introducing unnecessary complexity, The model eliminates the generation of additional film elements in the separator zone and volumetric variations during cell operation. The delimitations proposed above allow us to describe the differential equations in a standard model. However, it is important to note that the diffusion spaces of the lithium ions are modeled as spherical elements for simplicity and identification.
In the following, we describe the fundamental equations that model under a physicochemical approach of a lithium cell; the first equation is characterized by Fick’s law, which studies the diffusion of the lithium ion between the electrodes in spherical coordinates given by Equation (1):
In agreement with previous works by Doyle et al. [52] and Chen et al. [53], which provide detailed descriptions of the boundary and initial conditions for Equations (1)–(4), the objective is to present only the models used in the present investigation. In particular, for Equation (1), the solid phase ion diffusion coefficient is defined as the proportionality constant between Lithium Ion concentration .
The second governing Equation for a lithium battery is defined by the application of Ohm’s law, considering the solid-state regions of the cell. In this context, Equation (2) describes the relationship between the electric potential () and the reaction ratio in the cell (), which is characteristic of the effective electrical conductivity () of the solid phases of the cell.
The third equation, Equation (3), estimates the concentration of lithium ions in the electrolyte zone, which is influenced by the electrochemical parameters that determine the charge density of the battery. In this context, the lithiumi-ion comes in contact with a dynamic medium involving the diffusion coefficient (), the amount of porosity of the medium (), the Faraday constant (F) and the ionic transfer value ():
The fourth equation, Equation (4), describes the electrolyte potential estimation, which is the main factor in this investigation. Since we seek to model the charge/dischargecharge–discharge curve of the battery, Equation (4) plays a crucial role in this context:
The phases of the cell determine the total potential, which is a fundamental magnitude for understanding the battery’s behavior. Equation (4) is determined by the conductivity coefficient in the medium (), which describes the ability to conduct electricity.
2.2. ZnO Half-Cell
Zinc oxide (ZnO) was obtained by chemical precipitation using the methodology described in a previous work [54]. Synthesis was performed using zinc nitrate hexahydrate and NaOH, both at 0.2 M in a molar ratio of 1:2 . The solution was added dropwise to the NaOH solution for 45 min and at a temperature of 50 °C, with constant stirring. The solution was then vigorously agitated with ultrasonic for 30 min at 50 °C. Finally, the temperature was reduced to room temperature and the precipitate was washed with distilled water and dried at 100 ° C for 24 h.
The structural characterization was performed with X-ray diffraction (XRD), using a Bruker D8Advance equipment, Raman microspectroscopy equipment was carried out in Horiba Olympus BX41 (50 to 3000 cm−1) Greeneen laser whose wavelength is 532 nm.
However, the electrochemical characterization of ZnO was performed by assembling a coin-type half-cell. The assembly was made by depositing the anode material on a copper foil. The composition of the anode is a weight ratio of 80|10|10, which is made up of ZnO|carbon super P|polyvinylidine fluoride, respectively. (1 M, 150 µm) was used as an electrolyte, which was impregnated in a fiberglass separator (0.6 mm thick), and for the cathode, a metallic Li foil was used whose diameter is 13 mm. Charge and discharge measurements were made for a current density of 100 mAg−1 in a voltage range of 0.01–3.0 V versus at 25 °C.
2.3. Battery Management System
The electric vehicle industry (EVI) has received significant attention due to its high performance and efficiency, which presents a viable solution to mitigate emissions and address global pollution problems. Vehicles of this kind use lithium-ion batteries as their power source, enabling engines to operate in an eco-friendly and sustainable manner. However, the automotive sector is confronting a pressing necessity: the creation of effective Battery Management Systembattery management systems (BMSs). These systems play a crucial role in accurately gauging and estimating the electrical parameters of batteries and accurately regulating theirState of Charge (SoC), thereby guaranteeing optimal and safe operation of electric vehicles [55].
The advancement of Battery battery Management management Systems systems (BMSs) for electric vehicle batteries is presently in an investigation phase. BMS created for portable electronic devices like cell phones and computers are not directly transferable or adaptable to these applications. Lithium-ion batteries employed in electric vehicles exhibit electrical properties that exceed those of batteries in electronic devices by a factor of at least 100, introducing novel challenges with respect to this technology’s design, efficiency, and scalability [56].
A Battery Management management System system (BMS) comprises various electronic elements that execute several essential tasks. Sensing stages, electronic conditioning stages, control stages, actuators, and a processing stage are included for the real-time programming of control algorithms. These phases handle measurement signals that reflect the battery’s charge/dischargecharge–discharge electrical conditions, allowing accurate control of its performance, and thus guaranteeing its safe and efficient operation [57].
To design a standardized BMS for monitoring and assessing various lithium lithium-ion batteries, it is essential to consider that such systems utilize the constant voltage/constant current (CV/CC) method for charging. As a result, a simplified model, such as an equivalent circuit model (ECM), is used.
However, ECM presents several limitations, particularly in capturing lithium-ion batteries’ nonlinear and dynamic behavior under real-world conditions. These models often rely on predefined parameters that may not adapt well to varying temperatures, load conditions, or aging effects. To address these challenges, this research incorporates Deep Learning techniques into BMS to enhance battery state estimation. Using large data sets and advanced machine-learning algorithms, the proposed model offers improved accuracy, adaptability, and robustness compared to traditional ECM-based approaches. In Figure 1 illustrates an equivalent circuit, which captures the electronic mathematical representation of a second-order linear dynamic system.
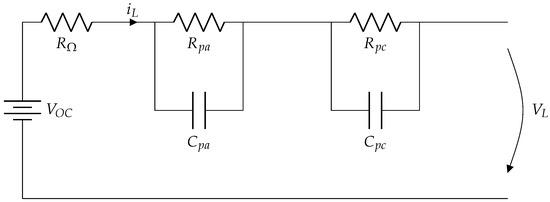
Figure 1.
Equivalent Circuit Model Schematic.
As a result, a simplified model, such as an equivalent circuit model (ECM), is used. Below is a schematic that illustrates an equivalent circuit, which captures the electronic mathematical representation of a second-order linear dynamic system.
2.3.1. Equivalent Circuit Model (ECM)
Electric vehicles (EVs) use battery packs as key components for their performance. A battery pack is composed of a significant number of individual cells, each of which is a complete battery with two terminals [58]. In this context, the equivalent Thévenin circuit model, introduced mainly by Hart et al. [59] and later modified by Pillai et al. [60], is proposed to estimate theState of Charge (SoC) of a lithium lithium-ion battery in electric vehicle (EV) applications using an adaptive Kalman filter as a tool to represent the electrical behavior of cells. The resistance and capacitance parameters presented in the following model were obtained through eElectrochemical iImpedance sSpectroscopy (EIS) tests on the battery cells. However, this model has limitations in terms of accuracy, as each of the elements that make up it can experience significant variations depending on operating conditions, handling, and battery status.
The values of the ECM components—such as ohmic resistance (), charge transfer resistance (), and double-layer capacitances (, )—are typically determined through experimental techniques and parameter estimation methods [61]. One of the most common approaches is eElectrochemical iImpedance sSpectroscopy (EIS), where an alternating current (AC) signal is applied across the cell at different frequencies to measure its impedance response. By fitting the impedance data to the ECM, researchers can extract the values of resistive and capacitive elements. In addition, current pulse testing is employed, in which a step current is introduced, and the resulting voltage response is analyzed to estimate the transient resistance and capacitance values. Since these parameters evolve due to aging and operational conditions, adaptive estimation techniques are often necessary to maintain accuracy in battery modeling.
In the equivalent circuit model shown in Figure 1, the open-circuit voltageOpen Circuit Voltage () represents theState of Charge (SoC) of the battery and can be considered an ideal approximation of a constant voltage source. This parameter reflects the level of energy stored in the battery.
The resistive and capacitive elements of the circuit model the internal phenomena of the battery:
- : represents the ohmic resistance of the cell, associated with the opposition to the passage of current due to the conductive materials of the battery.
- : is resistance to electrochemical polarization, which models the losses related to electrochemical processes during charging and discharging.
- : represents the concentration polarization resistance, which describes the losses due to ion transport in the electrolyte.
The equivalent capacitances of the circuit include:
- : The capacitance associated with electrochemical polarization, which reflects the battery’s ability to respond to rapid changes in charge.
- : The capacitance associated with the concentration polarization, which models the transient response due to the movement of ions lithium in the electrolyte.
This set of resistances and capacitances describes the dynamic behavior of the battery, especially during charging and discharging, and allows the capture of its transient response. Together, the model reflects how the internal characteristics of the battery influence itsState of Charge (SoC) and overall performance.
2.3.2. Variability of ECM Components with SoC and Temperature
The components of the ECM are not constant; they vary with the state of charge (SoC) and temperature (T) due to changes in battery chemistry and material properties.
- Ohmic Resistance ()
- −
- It increases with low SoC due to reduced ion availability.
- −
- It decreases with increasing temperature because ion mobility improves.
- Charge Transfer Resistance ()
- −
- It increases in low SoC due to reduced active reaction sites.
- −
- It decreases at higher temperatures due to faster electrochemical kinetics.
- Electrolyte Resistance ()
- −
- Higher at low temperatures due to increased electrolyte viscosity.
- −
- Lower in higher SoC as ionic conduction improves.
- Capacitive Elements (, )
- −
- Depend on the available double-layer charge storage.
- −
- Decrease at low SoC as fewer charge carriers participate.
2.3.3. Modeling the Dependence on SoC and Temperature
To incorporate these variations, empirical equations or lookup tables are often used to express the values of the components as functions of SoC and temperature. A common formulation for ohmic resistance is
Similarly, the charge transfer resistance can be expressed as:
where , , , and are empirical constants derived from experimental data. These equations allow the model to dynamically adjust to variations in battery conditions, enhancing accuracy in predicting performance and degradation over time.
2.3.4. Mathematical Equivalent Circuit Model
The described proposed equivalent circuit (EMC) appears to model the electrical behavior of an electrochemical cell by considering several elements that represent specific physical and chemical effects. The general description using Kirchhoff’s second law (or voltage law) can be represented as follows in Equation (7):
Utilizing Equation (7) in the ECM diagram:
where:
Cell voltage (): It is modeled as an ideal voltage source that represents the cell’s state of charge (SoC).
Ohmic resistance (): Immediately after the voltage source, a potential drop is due to cell ohmic resistance. This includes internal resistances, such as those associated with electrical contacts.
First resonant circuit (): This parallel circuit, composed of a resistor () and a capacitor (), models the resistance of the solid electrolyte. It represents the capacitive and resistive response of the electrolyte.
Second resonant circuit (): Similar to the first, this parallel circuit is made up of a resistor () and a capacitor (), representing the resistance associated with charge transfer at electrochemical interfaces.
External load (): Finally, the voltage drop caused by the load attached to the cell is represented as resistance.
where:
: Cell open-circuit voltage, representing the state of charge (SoC). : The load current, the current flowing through the external load. : Ohmic resistance, accounting for internal resistances such as electrical contacts. : Voltage drop across each circuit element . : First resonant circuit, modeling the resistance of the solid electrolyte.
: Resistance associated with the solid electrolyte. : Capacitance associated with the solid electrolyte.
: Second resonant circuit, modeling charge transfer resistance.
: Resistance associated with charge transfer. : Capacitance associated with charge transfer.
: External load resistance, representing the power-consuming device. : Voltage across the external load. : Nominal capacity of the battery. t: Time. : Charge state, representing the available capacity of the battery. T: Temperature, which affects battery performance and degradation.
Finding the solution for in Equation (9):
Expressing the voltage-on-load in Equation (10). () as a function of the state of charge (SoC) and temperature (T) is crucial because it effectively reflects the performance dynamics under various operating conditions. This is particularly pertinent because, despite the emergence of numerous approaches for estimating SoC since the 1980s, a comprehensive definition accommodating its full complexity remains unrealized. Since SoC is crucial for vital operations, such as predicting remaining life and estimating capacity, directly associating it with variables such as T and helps to address intrinsic challenges, such as nonlinear battery degradation and accumulation of inaccuracies in terminal measurements. SoC, as defined in classical terms, is represented as:
where denotes the battery current, represents the nominal capacity, and t signifies the time [62]. Equation (11), indicates that changes in external load and internal chemical reactions cause a slow decrease in nominal capacity over time, leading to nonlinear and nonstationary degradation characteristics. Consequently, representing as a function of and temperature T enhances the precision of the model and allows dynamic adaptations needed to address these fluctuations, thus increasing the robustness and dependability of the EMS.
2.4. Informed Physics–Chemestry Chemistry-Informed Deep Learning
Physico-chemistry chemistry is a constantly evolving field, where technological advances and mathematical modeling allow complex problems to be tackled more effectively. Deep learning is one of the frontier approaches that is revolutionizing how physics problem simulations are performed. In this context, we use deep neural networks to solve numerical problems and model physicochemical phenomena, such as the simulation of lithium-ion batteries. In a definitional context, Informed Physicsphysics–Chemestrychemistry-informed Deep Learning is the area of deep learning neural networks that allow solving problems utilizing the information of the mathematical field (Equations (1)–(4)) that characterize each problem (conservative laws), for example, the simulation of electrochemical reactions that exist in the transport of lithium ions, where deep learning can go deep at different scales, both atomic, molecular, and macroscopic.
The Physicsphysics-Informed informed approach lies in incorporating knowledge of fundamental physical laws to effectively reduce the search space and adjust the weights of the deep learning neural network, which in turn favors convergence to the desired solution, which, in our case, corresponds to an approximation between the experimental measurement data mentioned in Section 2.2 and Section 2.3. In this context, we have employed the DNF model described in Equations (1)–(4) as a basis for deriving and defining Equations (12)–(15), thus establishing a solid connection between the physical principles of lithium batteries and the architecture of neural networks.
Equations (12)–(15) represent the four fundamental equations of the DNF model described in a quadratic error that compares the electrochemical parameters as input variables to estimate the behavior of the discharge curve in terms of the potential per battery cell. In the ideal case, Equations (5)–(8) would have a global optimum at zero, which favors convergence for the gradient descent algorithm implemented in deep learning. On the other hand, in our methodology, we have introduced boundary conditions () and initial (, [52]) corresponding to the cell characteristics present in Section 2.2 and Section 2.3, which are derived in Equation (16) and are a function of cell parameters, which will be important factors in the attention mechanisms of Section 2.5.
2.5. Transformer Deep-Learning
In this section, we will address the implications of Transformer Deep Learning in the context of the design and optimization of Lithium Ion batteries from their sequence of electrochemical parameters. As a starting point, historically, the concept of attention has become a fundamental tool in machine learning since its introduction in the 1990s [63]. At the time, attention was successfully used in computer vision tasks to study critical regions of images. However, its potential was limited because, at the beginning of the 21st century, specific applications dominated the concept of attention, and it could not be extended to other areas, such as natural language processing or applications in laws of electrochemistry.
2017 was a turning point in the development of artificial intelligence because Vaswani et al. [50] coined the term “Attention Is All You Need”, which presents the fundamental structure of Transformers. A Transformer, Figure 2, comprises an attention layer, which is the central part of the learning model, since it allows neural networks to pay attention to certain parts of the input data. The attention mechanism was proposed at the end of the 20th century to allow neural networks to focus their attention on particular areas of the input data; consequently, the state-of-the-art has improved the accuracy and efficiency of deep learning.
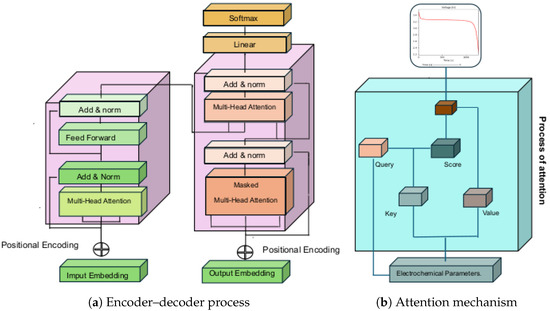
Figure 2.
Transformer deep deep-learning model for estimating the charge/dischargecharge–discharge curve from electrochemical parameters: (a) encoder–decoder process for input patterns (electrochemical parameters); (b) attention mechanism describing the learning curve based on the battery’s physicochemical properties.
Critical parameters, such as lithium-ion diffusion, modify the storage density of batteries in the context of the simulation and design of lithium batteries and their charge-discharge curve. This is where the concept of attention comes into play; Figure 2b, allows deep learning to focus on the most relevant characteristics of the most relevant parameters in energy storage. In the next subsection, we will address the architecture of a Deep Learning Transformer in battery design and simulation; then, we will focus on encoder and decoder processing to provide a complete framework for battery behavioral learning.
2.5.1. Encoder
The encoder, Figure 2a is one of the components of the Transformers Transformer model for Deep Learning, which is constituted by a set of layers divided into two main elements: the first, the multi-headed attention, is used to process the input information and obtain a compact representation of the data, this layer uses three matrices constituted by Query Q, Key K and Value V; the second, the forward-feed layer, is used to process the information obtained in the multi-headed attention layer and obtain a complete representation of the data. The forward-feed layer works as follows: the output of the multihead attention layer of the electrochemical parameters is used, and a dense neural network processes the information to obtain a complete representation representative of the main elements that modulate the lithium batteries.
The crucial procedure in the encoder is to extract the relevant information from the time series stored in the database, which represents the discharge characteristics of the lithium battery in the three environments described in Section 2.1, Section 2.2 and Section 2.3. In this sense, pooling is applied to identify relevant data sequences and project the resulting vectors onto battery-specific electrochemical parameters. Thus, the encoder is responsible for inferring the electrochemical parameters modeled by the battery.
2.5.2. Decoder
The Decoder, Figure 2b, is a key system in the transformer Transformer architecture. In tThe practice of deep learning on lithium battery values, it allows the translation of the sequence of information in terms of responses of interest, for example, charge–discharge curves. In contrast to the encoder, the Decoder includes layers such as masked multi-head attention, multi-head attention (In the context of electrochemical parameter characterization, this mechanism is understood to aim at controlling the relevance of the parameters that modulate the battery, discarding non-relevant parameters; that is, masking is understood as the attention mechanism that ignores atypical electrochemical parameters in the inference process), feedforward, and residual connection. These layers use the scaled product to exclude estimates of electrochemical parameters and consider only outputs of interest in the discharge curve. In electrochemical parameter learning practice, the attention mechanism is applied twice in the Decoder: first, to estimate the attention between output elements in the lithium-ion battery discharge and second, to determine the attention between the parameter and the charge curve.
3. Results
3.1. Experimental Setup
A series of experiments were designed to characterize the properties of lithium batteries and evaluate their behavior during the charging and discharging process. To achieve this, we carried out a cell-level experimentation based on material analysis to characterize the charge and discharge of lithium batteries and determine their characteristic curves, an analysis of electronic instrumentation for data acquisition, and finally, Deep Learning training, focusing on the behavior of the voltage discharge curve about the electrochemical parameters of the battery:
However, in the context of the development of lithium batteries, it is essential to characterize their behavior during the charge -and -discharge process. We developed an electronic design based on data acquisition circuits to measure and calculate the voltage versus time curve to achieve this goal. This tool allows collect accurate data on the behavior of batteries during the charge and discharge process, particularly in the discharge cycle. In this approach, several types were tested: CR2032, CR1616, CR2025, CR2016, CR2050, CR1620, CR1632, and CR2430. In addition, we built a database of rechargeable batteries, such as CRG-255, 18650, CR2, CRP2, CRP2, 14500, and CR123. As a contribution to the community, we have provided access to the data generated in this study; our database is available on GitHub (https://github.com/Optimization-litihum-Baterry/electrochemistry_database-.git, (accessed on 30 December 2024)).
Several batteries of CR2032, CR1616, CR2025, CR2016, CR2050, CR1620, CR1632, and CR2430 were analyzed during their discharge cycle to evaluate their behavior for failure in a deep learning environment. The created database contains detailed information about the discharge of each of these batteries. This database validates the transformer transformer in design environments or optimizes electrochemical parameters. The electronic parameters of the voltage measurement captured during the coin cell batteries’ discharge are described below. The battery is connected to a resistive load of 15 k, showing a typical discharge curve with an initial voltage of V that gradually decreases to 2 V after a time of 1100 h under standard room temperature conditions (23 °C). These results are available in the database for analysis and comparison with other types of batteries with different electrical characteristics.
Finally, we developed a database that contains the experimental results of 425 stable batteries, which were obtained from the numerical estimation presented in Section 2.1; according to the standards presented in the state of aArt, our contribution is to generate a set of electrochemical parameters, cell discharge time series, and simulation time. These database elements were generated to train a machine learning model based on Transformer Deep Learning, which is used to design or optimize lithium-ion batteries with a nominal voltage standard of 3.9 to 4.2 Volts.
3.2. Half-Cell
Figure 3a shows the ZnO diffraction pattern in which only the signals corresponding to the crystalline phase of this material are observed (COD-2300450). The results show only the characteristic planes of the , which are: (101), (100), (002), and (102), s. Since no other signal is observed, it can be assumed that the was only obtained in the expected phase. Furthermore, in Figure 3b, a micro-Raman spectramicro-Raman spectrum of is shown where only the vibrational modes at 98, 202, 330, 380, 437 and 581 cm−1 are perceived. These results corroborate the results obtained by XRD since no signals that do not correspond to ZnO are observed either.
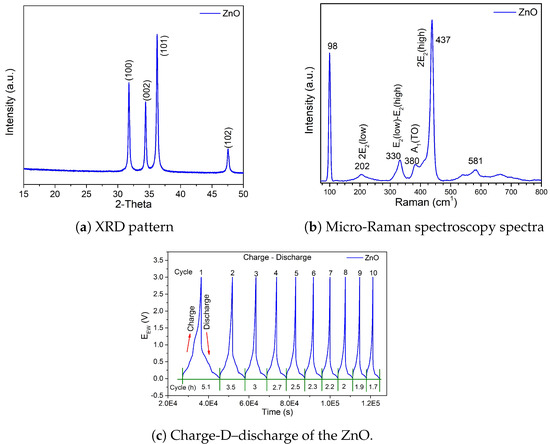
Figure 3.
Structural characterization of , (a) XRD pattern, (b) Raman spectra and electrochemical characterization (c) Charge and discharge profile of the ZnO Half-Cell.
Charge–discharge measurements are shown in Figure 3c, and the battery, which in this case is a half-cell, was measured under the conditions described above, i.e., a current density of 100 mAg−1 in a voltage range of 0.01–3.0 V vs. in 25 °C. Ten charge and discharge cycles were performed as observed at the peaks of each signal. However, in the same figure, each cycle is carried out at different times, since it is known that as the cycles increase, every battery loses capacity and, therefore, the charging and discharging times for each cycle are reduced. This is shown in Figure 3c, where the first cycle is performed in 5.1 h and gradually reduced to 1.7 h in cycle 10. In addition, Table 1 shows the capacity values that decrease considerably in the first 10 cycles for the half-cell.

Table 1.
Capacity values and storage percentage in 10 cycles for the ZnO half-cell.
The measurements were made in a voltage range of 0.01—3.0 V to provide additional data to those that were experimentally measured at 4.0 V with commercial batteries (18500).
Additionally, another reason to analyze a half-cell is to demonstrate that Transformer-based Deep deep-lLearning models can be used to predict the charging and discharging behavior of a lithium-ion electrochemical cell, whether commercial, such as 18500 types or a new materials cell. This means that the models proposed here could be adapted to different battery technologies of the automotive electric vehicle industry.
3.3. Battery Management Acquisition System
The block diagram of the data acquisition system is presented in this investigation in Figure 4. The data obtained through this system are crucial to collecting reliable and accurate experimental data on commercial rechargeable lithium batteries such as BAT-Li-AAA, BAT-Li14500 and LIR18500. This system allows monitoring, controlling, and recording the voltage discharge curves as a function of time, as well as other relevant electrochemical parameters under different operating conditions. The importance of obtaining these curves is that they provide a detailed view of the behavior of the batteries.
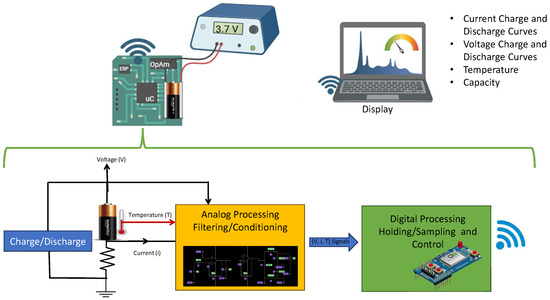
Figure 4.
Data Acquisition System Proposal.
This database covers common configurations in the current market and undesired behaviors, which enriches the range of data available for model training. In this context, the diversity of data collected ensures that simulation and design models, such as those based on Deep Learning schemes with Transformers, can be rigorously trained, covering a wide variety of real and potential scenarios.
Furthermore, the information obtained from these curves is essential for developing and refining neural networks capable of accurately simulating the behavior of lithium batteries. The integration of techniques such as Informed Physics and Pseudo-2D models allows for capturing the complex and varied patterns in the responses of these batteries to changes in their electrochemical parameters. This is essential for designing more efficient and long-lasting batteries and improving simulations that inform critical decisions in energy storage and management systems.
The following is a detailed description of the data acquisition system for obtaining charge and discharge graphs for commercial lithium-ion batteries.
3.3.1. Data Acquisition Process Details of the Sensing Stage
High-precision sensors were used to capture variations in battery parameters. These sensors were selected based on the specific nature of each variable.
- Current: Measured with a Hall effect-based sensor, capable of recording both alternating currents (AC) and direct currents (DC).
- Voltage: The signal was conditioned using attenuation/amplification and filtering circuits to ensure an accurate reading even in the presence of noise.
- Temperature: Recorded using a thermistor, whose resistance change proportional to temperature allowed reliable measurements of thermal conditions.
3.3.2. Sampling and Holding Process
The analog data wasdata were digitized using an ADC converter integrated into an ESP32 microcontroller. This device was configured for an appropriate sampling rate, ensuring accurate and continuous real-time data capture In addition, standard communication protocols were used to transfer the data to the central processing system.
3.3.3. Processing and Storage
Once acquired, the data were processed using algorithms designed to calculate parameters such as the state of charge (SoC), available capacity, charge/dischargecharge–discharge rates, and thermal behavior. These data were stored in a structured database to facilitate later analysis and visualization.
3.3.4. Utility of Experimental Data
Detailed analysis of the charge/dischargecharge–discharge graph allows identifying important patterns and trends in battery performance. This includes:
The efficiency of the charge and discharge process under different operating conditions. The influence of factors such as temperature and usage cycles on the remaining battery capacity. The detection of possible anomalies, such as overheating or abrupt voltage drops, could compromise the battery’s lifespan.
In Figure 5, the charge and discharge behavior of an LIR18500 type battery, obtained from experimental data collected using our Battery Management Systembattery management system (BMS), is shown.
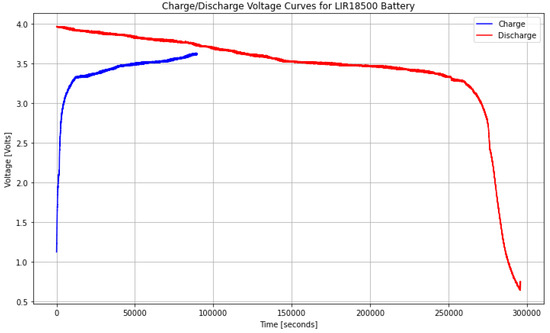
Figure 5.
Experimental Dataset For Commercial Batteries (Charge/Discharge Voltage Curves).
This experimental database was generated by constantly monitoring critical battery parameters such as voltage, current, and temperature during operation. The first charge and discharge cycle of a new LIR18500 battery cell provides critical insights into its initial performance, efficiency, and potential long-term behavior. The charge curve follows the expected lithium-ion profile, with a constant current (CC) phase followed by a constant voltage (CV) phase, while the discharge curve exhibits a steady voltage decline until reaching the cutoff voltage, indicating stable internal resistance. Coulombic efficiency is high, with minimal energy loss, and the recorded capacity aligns closely with the nominal specification, suggesting optimal initial performance [64]. Thermal stability was maintained throughout the cycle, with no abrupt voltage drops or anomalies, confirming proper electrolyte and electrode interface formation. This first cycle establishes a baseline for future performance analysis, where any capacity loss or efficiency loss over subsequent cycles will indicate aging effects such as electrode wear or Solid Electrolyte Interphase (SEI) layer formation.
3.4. Deep-Learning Transformer Training Database
The Deep Learning canon divides the validation of a model into three main stages: first, setting up the training dataset, which contains the necessary information to highlight the main data; second, the training process, where the learning structures are fitted to the database; third, data validation, a crucial step to know the level of confusion or uncertainty in the database. Consequently, Transformer deep learning is a structure adapted following the canon of learning, and therefore, in the following sections, we will follow this methodology.
As a first stage of deep learning, we have designed and built a set of simulations and experimental analysis of lithium-battery-discharge measurements for the training of models based on Transformer and other techniques following the same methodology; the simulations have been configured by the model of Section 2.1, focusing the attention on the electrochemical parameters that coincide with the configurations that determine a lithium battery. This set of simulations is bounded to a voltage boundary between 4.2 and 2 volts, constituting a fundamental basis for training. This process is crucial because it allows the Deep Learning structures to adjust to the information of the electrochemical parameters, resulting in more accurate and effective models.
We have released a set of lithium battery simulation experiments, which is available in our GitHub repository for access and exploitation. This set of experiments is made up of three fundamental elements: the electrochemical parameters that define the experimental configuration of a battery, the time window plots derived from the experimental design simulation, and the lithium lithium-battery battery-discharge voltage data. The selection of the electrochemical parameters involved has been carried out from the latest research for their level of importance, which has identified 45 fundamental parameters that form the basis of the study Appendix A details these essential elements, providing crucial information for their understanding and application in future research (https://github.com/Optimization-litihum-Baterry/electrochemistry_database-.git, (accessed on 30 December 2024)).
To illustrate the database proposed in this work, Figure 6 highlights two significant simulation experiments for lithium batteries, each describing a particular behavior with variations in electrochemical parameters. In Figure 6a, a battery is observed that starts with an initial voltage of 4.1 V and discharges following a linear trend until it reaches 3.3 V, highlighted by a discharge slope. This pattern suggests a controlled response to discharge. In contrast, Figure 6b shows a different experiment where the initial voltage is 4 V and decreases slowly, where the discharge slope is maintained with a horizontal trend; finally, at about 0.8 h, the discharge behavior changes exponentially until it reaches 2.8 V.
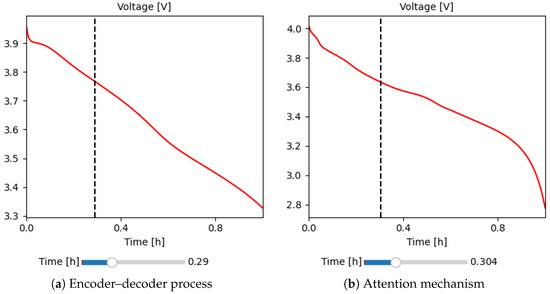
Figure 6.
Representation of the Transformer Deep Learning model for estimating the charge/dischargecharge–discharge curve based on the electrochemical parameters of stable batteries. (a) Shows the encoder–decoder process for input patterns (electrochemical parameters). (b) Illustrates the attention mechanism for learning the curve from the battery’s physicochemical properties.
The experiments presented in the database provide a training corpus for analyzing different scenarios and battery behaviors, enriching the variations of electrochemical parameters and their response to the voltage density. HavingWith access to these variations of results, the training process can dive into different deep learning methodologies to create new models and develop effective strategies to predict and understand the discharge voltages in real experiments. This not only allows a deeper understanding of the operation of lithium batteries, but also provides valuable tools for developing solutions in electric vehicles.
3.5. Transformer Deep Learning Training and Validation
In deep learning methodology, the second stage of the processing lies in training the specific system or model to be implemented. In our particular case, we are working with Deep Learning Transformers, a new paradigm of deep artificial neural networks, and advanced attention techniques. The main objective of this stage is to prepare the model for specialized learning tasks and to pay attention to the relevant electrochemical parameters that characterize a lithium battery. To achieve this, experiments were performed using the databases in Section 3.2, Section 3.3 and Section 3.4, taking into account experimental material measurements, computational simulation, and measurements of commercial lithium batteries.
Figure 7 shows the convergence behavior of the Transformer-based Deep Learning model for time series (charge/dischargecharge–discharge curve), which we have implemented as part of our methodology. Initially, embedded inputs feed the electrochemical parameters, time, and discharge curve through the neural network layers. Next, the combination of embeddings occurs, where these electrochemical parameters are added to the discharge curve and time. Thirdly, the Paddings mask operates by discarding unnecessary patterns and readying the data for the attention stage. Fourthly, Transformer encoding focuses on the self-attention mechanism via a series of intermediate dense networks. Finally, the output layer is responsible for configuring the result into charge/dischargecharge–discharge time series.
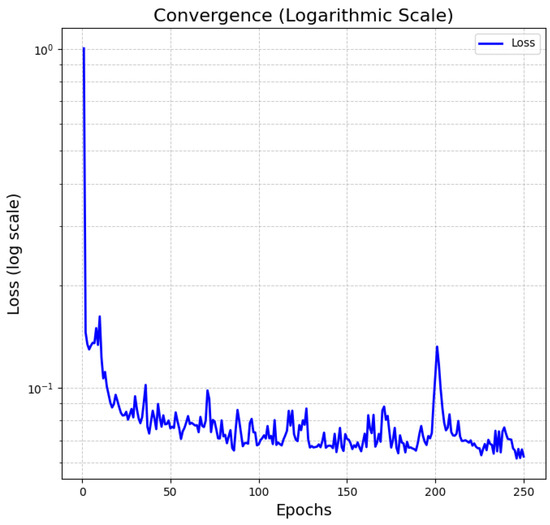
Figure 7.
Convergence of Deep-Learning Transformer training on a logarithmic scale: the convergence process is approached with a database of 425 stable battery cells (Section 3.4).
Once the Transformer-based model training process has been achieved, the next step in the deep-learning methodology is to validate the learning results with the experimental databases described above. Figure 8 shows a comparative approximation between an experimental measurement of the rechargeable lithium battery in a time window of 3600 s; the predictive approach is characterized by fitting the discharge curve to the transform and then estimating the electrochemical parameters of the battery. For example, we show the validation of the results in experimental measurements for two commercial batteries, the 18650 and another one of the same type, but with serial number 14500, both characterized by providing a used voltage of 3.7 V.
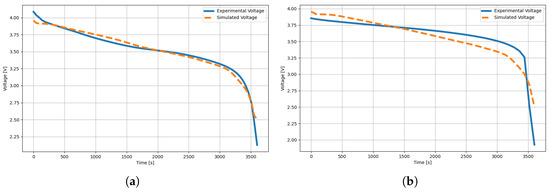
Figure 8.
Prediction of electrochemical parameters using the Deep Learningdeep-learning Transformer trained in Section 2: (a) Shows a fit of a cell discharge potential drop from experimental samples taken for an 18650 battery; (b) depicts a 3600 s time window of the potential drop for a 14500 battery.
In the first battery, Figure 8a, we observed that the model managed to fit adequately along the experimental voltage curve, which implies an approximate confidence level in the electrochemical parameters modeling the behavior of the 18650 battery. In summary, the parameters obtained in Table 2 can be described as a good representation of the battery behavior without the need for invasive experimentation of the structure and its components. In Table 2, the parameters relevant to manufacturing are reported and delimited for practicality. In comparison, the state-of-the-art results of this type of battery allow us to verify that the Transformer has achieved competitive results in describing a battery from its discharge curve measurement.

Table 2.
Electrochemical parameters approximated by Transformer Deep-Learning for one discharge cycle of an 18650 battery.
On the other hand, in the battery of Figure 8b, we observed that the model was adequately fitted during the first units of time up to approximately 1500 s. However, the abrupt change that the battery takes after 3000 s generates uncertainty in the model and cannot achieve a good approximation of the electrochemical parameters described by this battery. In Table 3, we present the electrochemical parameters. Although they are valid, these parameters have certain levels of approximation; nevertheless, before the design of a lithium battery, they are elements to begin its configuration, as well as the optimization of batteries where a starting solution set is needed.

Table 3.
Electrochemical parameters approximated by Transformer Deep-Learning for one discharge cycle of an 14500 battery.
4. Discussion
One of the most important aspects of the present research is the proposal of a database that includes experimental results from 425 stable batteries, which are available to open an area of research in the design and optimization of lithium batteries from dDeep-learning Transformers. This database was used to train a Transformers-based deep learning model, which allowed accurate prediction of discharge curves of both commercial and matter-design lithium batteries. In addition, the research discusses the importance of characterizing the behavior of lithium batteries during their charging and discharging process. This concluded that this characterization is fundamental to improving the efficiency and durability of these batteries without an exhaustive laboratory analysis.
Our research proves how predictive models based on machine-learning techniques can improve lithium battery management and advance the design of more efficient and durable energy management systems based on electrochemical parameters. The results demonstrate that the fusion of Informed Physics and the Transformer Attention process enables the prediction of battery behavior by focusing the study on the noninvasive determination of battery material components, which can improve energy efficiency and reduce battery degradation. This is particularly important in applications like electric vehicle fleet management, where energy efficiency and battery durability are crucial. Further, our findings suggest that predictive models can be used to identify opportunities for improvement in battery management and develop optimization strategies that can be applied in various contexts, both in electric vehicles and systems relying on electric energy storage. In summary, our research provides a solid foundation for developing more accurate and robust predictive deep-learning models that can be used to improve lithium battery management.
5. Conclusions
In this research, we have presented an experimental characterization of material and time-series acquisition of lithium batteries to be applied to deep learning models, particularly Transformer deep learning, which are applied to the simulation and prediction of lithium charging and discharging behavior. The main objective of the research was achieved through the characterization of the measurement and monitoring of lithium lithium ion batteries, resulting in a set of electrochemical parameters that characterize the behavior of the battery; from the experimental database, we have trained a model based on deep learning Transformers that can accurately predict the behavior of these parameters without the need for invasive analysis of the battery.
We contribute to lithium-ion battery design and optimization by characterizing and training a deep learning-based model using the Transformers Transformer approach. This approach integrates experimental material measurements, computational simulations, and commercial battery data to describe the electrochemical parameters that govern the electrical energy storage density performance. The results obtained demonstrate that it is possible to train a deep learning model that predicts the behavior of lithium-ion batteries, which implies a methodology that allows the discovery of new descriptive parameters from the experimental and market knowledge surrounding lithium batteries. Significantly, our research reveals that integrating the attention mechanism into the experimental data set and computational simulations can generate a comprehensive and accurate understanding of the electrochemical mechanisms underlying the charging and discharging of lithium ion batteries, which could revolutionize the design and development of more efficient batteries.
From another perspective, the research results show that the proposed Transformer model can achieve adequate training convergence to approximate experimental voltage discharges. This indicates that the electrochemical parameters that model the battery behavior are reliable in predicting battery discharge. In the first case study, the Transformer model reliably fits the experimental voltage time series for the 18650 battery, Section 3.5.
We list the key results of the present research on the estimation of electrochemical parameters of lithium batteries from the Transformers Transformer deep learning model and the Physics-Iinformed approach. The key discoveries include an attention model meticulously designed to forecast parameters that represent battery attributes and elements, allowing for the detection of complex patterns and nonlinear dynamics in battery charge and discharge data. Comprehensive experimental analysis of crucial components in lithium battery modeling, aligned with battery time series, has enabled the identification and evaluation the most significant electrochemical factors affecting battery performance. The blend of attention mechanisms with physics-informed components in the model allowed a concentration on critical parameters, significantly boosting prediction accuracy and revealing patterns crucial for developing and enhancing new or existing batteries. Furthermore, a notable addition to this study is the proposed database dedicated to designing and optimizing new batteries or improving current ones. This database provides a compilation of modeling parameters related to electrical storage behavior, It includes experimental, commercial, and design factors, significantly aiding laboratory experiments by reducing time and cost.
The research we present opens new perspectives for the development of battery technologies. Specifically, it focuses on design and optimization, which are critical in the fundamentals of electric vehicles in transition toward the eradication of the use of fossil fuels. To consolidate the results obtained and promote their implementation in practice, we envision future work to deepen the exploration of new elements for solid-state electrolyte batteries and the exploration of more specialized battery banks around electromobility, which marks a new era in the development of lithium batteries. However, in the academic and research approach, the results of our proposal modify the frontier of knowledge, demonstrating that deep learning transformations that were initially developed for natural language processing can be implemented in charge and discharge time-series prediction environments, in addition to generating attention mechanisms that coincide with the laws of physics.
Author Contributions
Conceptualization, J.d.A.-S., G.P.-Z. and J.L.L.-R.; methodology, J.d.A.-S., G.P.-Z. and J.L.L.-R.; software, J.d.A.-S., G.P.-Z. and J.L.L.-R.; formal analysis, J.d.A.-S., G.P.-Z. and J.L.L.-R.; investigation, J.d.A.-S., G.P.-Z. and J.L.L.-R.; data curation, G.H.P., I.Z.G. and J.Y.V.G.; writing—original draft preparation, J.d.A.-S., G.P.-Z. and J.L.L.-R., G.H.P., I.Z.G. and J.Y.V.G.; writing—review and editing, G.H.P., I.Z.G. and J.Y.V.G.; visualization, G.H.P., I.Z.G. and J.Y.V.G. All authors have read and agreed to the published version of the manuscript.
Funding
We thank the Consejo Nacional de Humanidades, Ciencias y Tecnologías for supporting and motivating the development, the Tecnológico Nacional de México for approving the project financed with key: 19918.24-PD, which made possible the experimentation and the results with its contributions, and the Instituto Tecnológico Superior de Purísima del Ricón for the time provided to work on the project.
Data Availability Statement
The original data presented in the study are openly available in repository at https://github.com/Optimization-litihum-Baterry/electrochemistry_database-.git (accessed on 30 December 2024).
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A
Appendix A.1
Table A1 summarizes the electrochemical parameters selected from the literature related to the characteristics that modulate and characterize the behavior of a lithium-ion battery. The objective of Table A1 is to construct a database containing experiments on stable lithium lithium-ion batteries to train deep learning methodologies, which are deposited on the Github GitHub repository (https://github.com/Optimization-litihum-Baterry/electrochemistry_database-.git) (accessed on 30 December 2024). Some of the parameters listed are constant variables that are part of the design and manipulation of lithium-ion batteries. However, an additional set of independent variables modulates battery behavior. Consequently, these parameters are listed in the database.

Table A1.
Lithium-ion batteries parameters.
Table A1.
Lithium-ion batteries parameters.
| Name | Variable |
|---|---|
| Ideal gas constant [J·K−1·mol−1] | 7.300 |
| Faraday constant [C·mol−1] | 70,194.084 |
| Boltzmann constant [J·K−1] | 0.000 |
| Electron charge [C] | 0.000 |
| Negative electrode thickness [m] | 0.000 |
| Separator thickness [m] | 0.000 |
| Positive electrode thickness [m] | 0.000 |
| Electrode height [m] | 0.500 |
| Electrode width [m] | 0.200 |
| Nominal cell capacity [A·h] | 2.748 |
| Current function [A] | 1.545 |
| Contact resistance [Ohm] | 0.000 |
| Negative electrode conductivity [S·m−1] | 173.022 |
| Maximum concentration in negative electrode [mol·m−3] | 43,023.260 |
| Negative electrode diffusivity [m2·s−1] | 0.000 |
| Negative electrode porosity | 0.350 |
| Negative electrode active material volume fraction | 0.737 |
| Negative particle radius [m] | 0.000 |
| Negative electrode Bruggeman coefficient (electrolyte) | 1.410 |
| Negative electrode Bruggeman coefficient (electrode) | 1.415 |
| Negative electrode charge transfer coefficient | 0.468 |
| Negative electrode double-layer capacity [F·m−2] | 0.174 |
| Negative electrode OCP entropic change [V·K−1] | 0.000 |
| Positive electrode conductivity [S·m−1] | 0.306 |
| Maximum concentration in positive electrode [mol·m−3] | 26,597.640 |
| Positive electrode diffusivity [m2·s−1] | 0.000 |
| Positive electrode porosity | 0.379 |
| Positive electrode active material volume fraction | 0.233 |
| Positive particle radius [m] | 0.000 |
| Positive electrode Bruggeman coefficient (electrode) | 1.134 |
| Positive electrode Bruggeman coefficient (electrolyte) | 2.444 |
| Positive electrode charge transfer coefficient | 0.539 |
| Positive electrode double-layer capacity [F·m−2] | 0.260 |
| Positive electrode OCP entropic change [V·K−1] | 0.000 |
| Separator porosity | 0.457 |
| Separator Bruggeman coefficient (electrolyte) | 1.221 |
| Initial concentration in electrolyte [mol·m−3] | 1216.002 |
| Cation transference number | 0.495 |
| Thermodynamic factor | 2.030 |
| Electrolyte diffusivity [m2·s−1] | 0.000 |
| Reference temperature [K] | 439.879 |
| Ambient temperature [K] | 311.921 |
| Electrodes connected in parallel to make a cell | 0.629 |
| Number of cells connected in series to make a battery | 0.980 |
| Lower voltage cutoff-off [V] | 1.610 |
| Upper voltage cutoff-off [V] | 3.658 |
| Open-circuit voltage at 0% SoC [V] | 1.911 |
| Open-circuit voltage at 100% SoC [V] | 4.108 |
| Initial concentration in negative electrode [mol·m−3] | 24,340.483 |
| Initial concentration in positive electrode [mol·m−3] | 90.190 |
| Initial temperature [K] | 275.012 |
References
- Abbasi, K.; Ali, P.; Barbour, V.; Benfield, T.; Bibbins-Domingo, K.; Hancocks, S.; Horton, R.; Laybourn-Langton, L.; Mash, R.; Sahni, P.; et al. Time to treat the climate and nature crisis as one indivisible global health emergency. npj Prim. Care Respir. Med. 2023, 33, 37. [Google Scholar] [CrossRef]
- Gabric, A.J. The Climate Change Crisis: A Review of Its Causes and Possible Responses. Atmosphere 2023, 14, 1081. [Google Scholar] [CrossRef]
- Kumar, S.; Chatterjee, U.; David Raj, A.; Sooryamol, K.R. Global Warming and Climate Crisis/Extreme Events. In Climate Crisis: Adaptive Approaches and Sustainability; Chatterjee, U., Shaw, R., Kumar, S., Raj, A.D., Das, S., Eds.; Springer Nature: Cham, Switzerland, 2023; pp. 3–18. [Google Scholar] [CrossRef]
- Fu, Y.; Bai, H.; Cai, Y.; Yang, W.; Li, Y. Optimal configuration method of demand-side flexible resources for enhancing renewable energy integration. Sci. Rep. 2024, 14, 7658. [Google Scholar] [CrossRef] [PubMed]
- Chatuanramtharnghaka, B.; Deb, S.; Singh, K.R.; Ustun, T.S.; Kalam, A. Reviewing Demand Response for Energy Management with Consideration of Renewable Energy Sources and Electric Vehicles. World Electr. Veh. J. 2024, 15, 412. [Google Scholar] [CrossRef]
- Tomczyk, M.; Wojtaszek, H.; Chackiewicz, M.; Orłowska, M. Electromobility and Renewable Energy Sources: Comparison of Attitudes and Infrastructure in Poland and Germany. Energies 2023, 16, 7935. [Google Scholar] [CrossRef]
- Habib, K.; Hansdóttir, S.T.; Habib, H. Critical metals for electromobility: Global demand scenarios for passenger vehicles, 2015–2050. Resour. Conserv. Recycl. 2020, 154, 104603. [Google Scholar] [CrossRef]
- Kostenko, G.; Zaporozhets, A. Transition from Electric Vehicles to Energy Storage: Review on Targeted Lithium-Ion Battery Diagnostics. Energies 2024, 17, 5132. [Google Scholar] [CrossRef]
- Alanazi, F. Electric Vehicles: Benefits, Challenges, and Potential Solutions for Widespread Adaptation. Appl. Sci. 2023, 13, 6016. [Google Scholar] [CrossRef]
- Suanpang, P.; Jamjuntr, P. Optimal Electric Vehicle Battery Management Using Q-learning for Sustainability. Sustainability 2024, 16, 7180. [Google Scholar] [CrossRef]
- Lin, Z.; Li, D.; Zou, Y. Energy efficiency of lithium-ion batteries: Influential factors and long-term degradation. J. Energy Storage 2023, 74, 109386. [Google Scholar] [CrossRef]
- Nyamathulla, S.; Dhanamjayulu, C. A review of battery energy storage systems and advanced battery management system for different applications: Challenges and recommendations. J. Energy Storage 2024, 86, 111179. [Google Scholar] [CrossRef]
- Ajibade, H.; Ujah, C.O.; Nnakwo, K.C.; Kallon, D.V.V. Improvement in battery technologies as panacea for renewable energy crisis. Discov. Appl. Sci. 2024, 6, 374. [Google Scholar] [CrossRef]
- Tran, M.K.; DaCosta, A.; Mevawalla, A.; Panchal, S.; Fowler, M. Comparative Study of Equivalent Circuit Models Performance in Four Common Lithium-Ion Batteries: LFP, NMC, LMO, NCA. Batteries 2021, 7, 51. [Google Scholar] [CrossRef]
- Assi, M.; Amer, M. A Comparative Analysis of Lithium-Ion Batteries Using a Proposed Electrothermal Model Based on Numerical Simulation. World Electr. Veh. J. 2025, 16, 60. [Google Scholar] [CrossRef]
- Ghani, F.; An, K.; Lee, D. A Review on Design Parameters for the Full-Cell Lithium-Ion Batteries. Batteries 2024, 10, 340. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhang, B.; Fu, Y.; Shen, F.; Zheng, Q.; Chalise, D.; Miao, R.; Kaur, S.; Lubner, S.D.; Tucker, M.C.; et al. Extreme fast charging of commercial Li-ion batteries via combined thermal switching and self-heating approaches. Nat. Commun. 2023, 14, 3229. [Google Scholar] [CrossRef]
- Chen, G.J.; Chung, W.H. Evaluation of Charging Methods for Lithium-Ion Batteries. Electronics 2023, 12, 4095. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Jiang, B.; He, H.; Huang, S.; Wang, C.; Zhang, Y.; Han, X.; Guo, D.; He, G.; et al. Realistic fault detection of li-ion battery via dynamical deep learning. Nat. Commun. 2023, 14, 5940. [Google Scholar] [CrossRef]
- de Anda-Suárez, J.; Rico-García, E.D.; Pérez-Zúñiga, G.; López-Ramírez, J.L. Optimization of Lithium-Ion Batteries Using Boltzmann Metaheuristics Systems: Towards a Green Artificial Intelligence. In New Horizons for Fuzzy Logic, Neural Networks and Metaheuristics; Castillo, O., Melin, P., Eds.; Springer Nature: Cham, Switzerland, 2024; pp. 133–148. [Google Scholar] [CrossRef]
- Karthick, K.; Ravivarman, S.; Priyanka, R. Optimizing Electric Vehicle Battery Life: A Machine Learning Approach for Sustainable Transportation. World Electr. Veh. J. 2024, 15, 60. [Google Scholar] [CrossRef]
- Hassan, M. Machine learning optimization for hybrid electric vehicle charging in renewable microgrids. Sci. Rep. 2024, 14, 13973. [Google Scholar] [CrossRef]
- Samanta, A.; Chowdhuri, S.; Williamson, S.S. Machine Learning-Based Data-Driven Fault Detection/Diagnosis of Lithium-Ion Battery: A Critical Review. Electronics 2021, 10, 1309. [Google Scholar] [CrossRef]
- Jiang, Y.; Song, W. Predicting the Cycle Life of Lithium-Ion Batteries Using Data-Driven Machine Learning Based on Discharge Voltage Curves. Batteries 2023, 9, 413. [Google Scholar] [CrossRef]
- Ayub, M.A.; Hussan, U.; Rasheed, H.; Liu, Y.; Peng, J. Optimal energy management of MG for cost-effective operations and battery scheduling using BWO. Energy Rep. 2024, 12, 294–304. [Google Scholar] [CrossRef]
- Majeed, M.A.; Phichaisawat, S.; Asghar, F.; Hussan, U. Dynamic Resource Management in Microgrids: Optimizing Efficiency Through Renewable Penetration and Resource Allocation With C-CMRFO. IEEE Access 2024, 12, 155391–155407. [Google Scholar] [CrossRef]
- Wang, F.; Zhai, Z.; Zhao, Z.; Di, Y.; Chen, X. Physics-informed neural network for lithium-ion battery degradation stable modeling and prognosis. Nat. Commun. 2024, 15, 4332. [Google Scholar] [CrossRef]
- Lu, J.; Xiong, R.; Tian, J.; Wang, C.; Sun, F. Deep learning to estimate lithium-ion battery state of health without additional degradation experiments. Nat. Commun. 2023, 14, 2760. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Chen, C.; Shen, W.; Sun, F.; Xiong, R. Deep Learning Framework for Lithium-ion Battery State of Charge Estimation: Recent Advances and Future Perspectives. Energy Storage Mater. 2023, 61, 102883. [Google Scholar] [CrossRef]
- Madani, S.S.; Ziebert, C.; Vahdatkhah, P.; Sadrnezhaad, S.K. Recent Progress of Deep Learning Methods for Health Monitoring of Lithium-Ion Batteries. Batteries 2024, 10, 204. [Google Scholar] [CrossRef]
- Sumanasena, V.; Gunasekara, L.; Kahawala, S.; Mills, N.; De Silva, D.; Jalili, M.; Sierla, S.; Jennings, A. Artificial Intelligence for Electric Vehicle Infrastructure: Demand Profiling, Data Augmentation, Demand Forecasting, Demand Explainability and Charge Optimisation. Energies 2023, 16, 2245. [Google Scholar] [CrossRef]
- Ai, Z.; Nee, A.Y.C.; Ong, S.K. Artificial Intelligence in Electric Vehicle Battery Disassembly: A Systematic Review. Automation 2024, 5, 484–507. [Google Scholar] [CrossRef]
- Miraftabzadeh, S.M.; Longo, M.; Di Martino, A.; Saldarini, A.; Faranda, R.S. Exploring the Synergy of Artificial Intelligence in Energy Storage Systems for Electric Vehicles. Electronics 2024, 13, 1973. [Google Scholar] [CrossRef]
- Mądziel, M.; Campisi, T. Predictive Artificial Intelligence Models for Energy Efficiency in Hybrid and Electric Vehicles: Analysis for Enna, Sicily. Energies 2024, 17, 4913. [Google Scholar] [CrossRef]
- Castro, J.F.C.; Venerando, A.C.; Rosas, P.A.C.; Neto, R.C.; Limongi, L.R.; Xavier, F.L.; Rhoden, W.M.; Spader, N.; Simões, A.P.; Dantas, N.K.L.; et al. Operation Model Based on Artificial Neural Network and Economic Feasibility Assessment of an EV Fast Charging Hub. Energies 2024, 17, 3354. [Google Scholar] [CrossRef]
- Garud, K.S.; Han, J.W.; Hwang, S.G.; Lee, M.Y. Artificial Neural Network Modeling to Predict Thermal and Electrical Performances of Batteries with Direct Oil Cooling. Batteries 2023, 9, 559. [Google Scholar] [CrossRef]
- Olcay, K.; Çetinkaya, N. Analysis of the Electric Vehicle Charging Stations Effects on the Electricity Network with Artificial Neural Network. Energies 2023, 16, 1282. [Google Scholar] [CrossRef]
- Hussan, U.; Majeed, M.A.; Asghar, F.; Waleed, A.; Khan, A.; Javed, M.R. Fuzzy logic-based voltage regulation of hybrid energy storage system in hybrid electric vehicles. Electr. Eng. 2022, 104, 485–495. [Google Scholar] [CrossRef]
- Şen, M.; Özcan, M.; Eker, Y.R. Fuzzy Logic-Based Energy Management System for Regenerative Braking of Electric Vehicles with Hybrid Energy Storage System. Appl. Sci. 2024, 14, 3077. [Google Scholar] [CrossRef]
- Omakor, J.; Alzayed, M.; Chaoui, H. Particle Swarm-Optimized Fuzzy Logic Energy Management of Hybrid Energy Storage in Electric Vehicles. Energies 2024, 17, 2163. [Google Scholar] [CrossRef]
- Shan, C.; Chin, C.S.; Mohan, V.; Zhang, C. Review of Various Machine Learning Approaches for Predicting Parameters of Lithium-Ion Batteries in Electric Vehicles. Batteries 2024, 10, 181. [Google Scholar] [CrossRef]
- Adnane, M.; Khoumsi, A.; Trovão, J.P.F. Efficient Management of Energy Consumption of Electric Vehicles Using Machine Learning—A Systematic and Comprehensive Survey. Energies 2023, 16, 4897. [Google Scholar] [CrossRef]
- Ariche, S.; Boulghasoul, Z.; El Ouardi, A.; Elbacha, A.; Tajer, A.; Espié, S. A Comparative Study of Electric Vehicles Battery State of Charge Estimation Based on Machine Learning and Real Driving Data. J. Low Power Electron. Appl. 2024, 14, 59. [Google Scholar] [CrossRef]
- Nozarijouybari, Z.; Fathy, H.K. Machine learning for battery systems applications: Progress, challenges, and opportunities. J. Power Sources 2024, 601, 234272. [Google Scholar] [CrossRef]
- Guirguis, J.; Ahmed, R. Transformer-Based Deep Learning Models for State of Charge and State of Health Estimation of Li-Ion Batteries: A Survey Study. Energies 2024, 17, 3502. [Google Scholar] [CrossRef]
- Sun, Z.; He, W.; Wang, J.; He, X. State of Health Estimation for Lithium-Ion Batteries with Deep Learning Approach and Direct Current Internal Resistance. Energies 2024, 17, 2487. [Google Scholar] [CrossRef]
- Shi, D.; Zhao, J.; Wang, Z.; Zhao, H.; Wang, J.; Lian, Y.; Burke, A.F. Spatial-Temporal Self-Attention Transformer Networks for Battery State of Charge Estimation. Electronics 2023, 12, 2598. [Google Scholar] [CrossRef]
- Xiao, Y.; Huang, X.; Meng, J.; Zhang, Y.; Knap, V.; Stroe, D.I. Electrochemical Impedance Spectroscopy-Based Characterization and Modeling of Lithium-Ion Batteries Based on Frequency Selection. Batteries 2025, 11, 11. [Google Scholar] [CrossRef]
- Yao, Z.; Qi, F.; Sun, Q.; Ye, L.; Yang, X.; Chao, G.; Tang, P.; Zhu, K. Preparation and Electrochemical Characterization of Y-Doped Li1.3Al0.3Ti1.7(PO4)3 Solid Electrolytes for Lithium-Metal Batteries. Crystals 2025, 15, 31. [Google Scholar] [CrossRef]
- Vaswani, A.; Shazeer, N.; Parmar, N.; Uszkoreit, J.; Jones, L.; Gomez, A.N.; Kaiser, L.; Polosukhin, I. Attention Is All You Need. arXiv 2017, arXiv:1706.03762. [Google Scholar]
- Nayak, G.H.; Alam, M.W.; Avinash, G.; Kumar, R.R.; Ray, M.; Barman, S.; Singh, K.; Naik, B.S.; Alam, N.M.; Pal, P.; et al. Transformer-based deep learning architecture for time series forecasting. Softw. Impacts 2024, 22, 100716. [Google Scholar] [CrossRef]
- Doyle, M.; Fuller, T.F.; Newman, J. Modeling of Galvanostatic Charge and Discharge of the Lithium/Polymer/Insertion Cell. J. Electrochem. Soc. 1993, 140, 1526. [Google Scholar] [CrossRef]
- Chen, C.H.; Planella, F.B.; O’Regan, K.; Gastol, D.; Widanage, W.D.; Kendrick, E. Development of Experimental Techniques for Parameterization of Multi-scale Lithium-ion Battery Models. J. Electrochem. Soc. 2020, 167, 080534. [Google Scholar] [CrossRef]
- Pérez-Zúñiga, G.; Herrera-Pérez, G.; Verde-Gómez, Y.; Valenzuela-Muñiz, A.M. Self-assembled ZnO-rGO nanocomposite, a solid-state transformation to control its crystallite size. J. Alloy Compd. 2021, 875, 159992. [Google Scholar] [CrossRef]
- Young, K.; Wang, C.; Wang, L.Y.; Strunz, K. Electric Vehicle Battery Technologies; Springer: New York, NY, USA, 2013; pp. 15–56. [Google Scholar] [CrossRef]
- Olabi, A.G.; Abbas, Q.; Shinde, P.A.; Abdelkareem, M.A. Rechargeable batteries: Technological advancement, challenges, current and emerging applications. Energy 2023, 266, 126408. [Google Scholar] [CrossRef]
- Waseem, M.; Ahmad, M.; Parveen, A.; Suhaib, M. Battery technologies and functionality of battery management system for EVs: Current status, key challenges, and future prospectives. J. Power Sources 2023, 580, 233349. [Google Scholar] [CrossRef]
- Waseem, M.; Lakshmi, G.S.; Sreeshobha, E.; Khan, S. An Electric Vehicle Battery and Management Techniques: Comprehensive Review of Important Obstacles, New Advancements, and Recommendations. Energy Storage Sav. 2024, in press. [Google Scholar] [CrossRef]
- Hart, P.; Kollmeyer, P.; Juang, L.; Lasseter, R.; Jahns, T. Modeling of second-life batteries for use in a CERTS microgrid. In Proceedings of the 2014 Power and Energy Conference at Illinois (PECI), Champaign, IL, USA, 28 February–1 March 2014; pp. 1–8. [Google Scholar] [CrossRef]
- Pillai, P.; Nguyen, J.; Balasingam, B. Performance Analysis of Empirical Open-Circuit Voltage Modeling in Lithium Ion Batteries, Part-2: Data Collection Procedure. IEEE Trans. Transp. Electrif. 2023, 11, 153–162. [Google Scholar] [CrossRef]
- Salek, F.; Resalati, S.; Babaie, M.; Henshall, P.; Morrey, D.; Yao, L. A Review of the Technical Challenges and Solutions in Maximising the Potential Use of Second Life Batteries from Electric Vehicles. Batteries 2024, 10, 79. [Google Scholar] [CrossRef]
- Hannan, M.A.; Lipu, M.S.; Hussain, A.; Mohamed, A. A review of lithium-ion battery state of charge estimation and management system in electric vehicle applications: Challenges and recommendations. Batteries 2017, 10, 79. [Google Scholar] [CrossRef]
- Niu, Z.; Zhong, G.; Yu, H. A review on the attention mechanism of deep learning. Neurocomputing 2021, 452, 48–62. [Google Scholar] [CrossRef]
- Wan, Y.; Liu, Y.; Chao, D.; Li, W.; Zhao, D. Recent advances in hard carbon anodes with high initial Coulombic efficiency for sodium-ion batteries. Nano Mater. Sci. 2022, 5, 189–201. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the World Electric Vehicle Association. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).