Omics Approaches in Drug Development against Leishmaniasis: Current Scenario and Future Prospects
Abstract
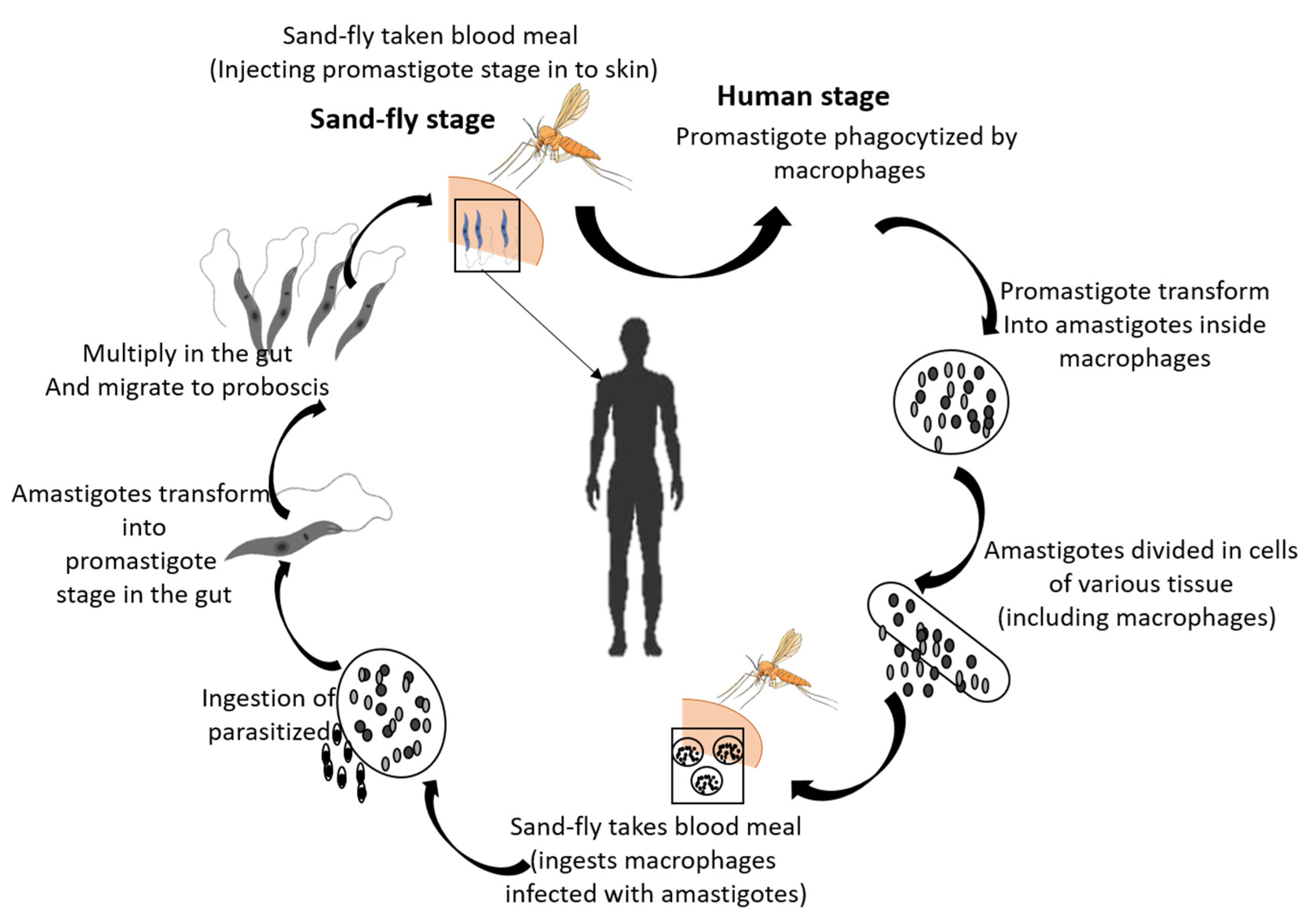
:1. Introduction
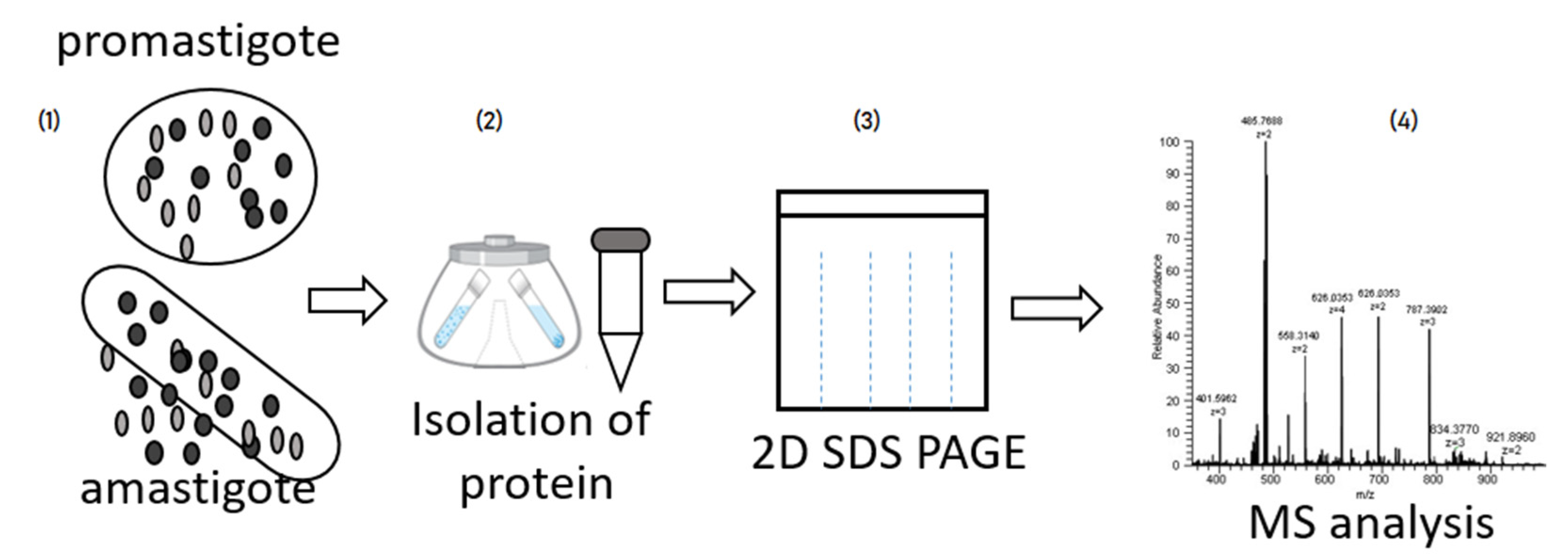
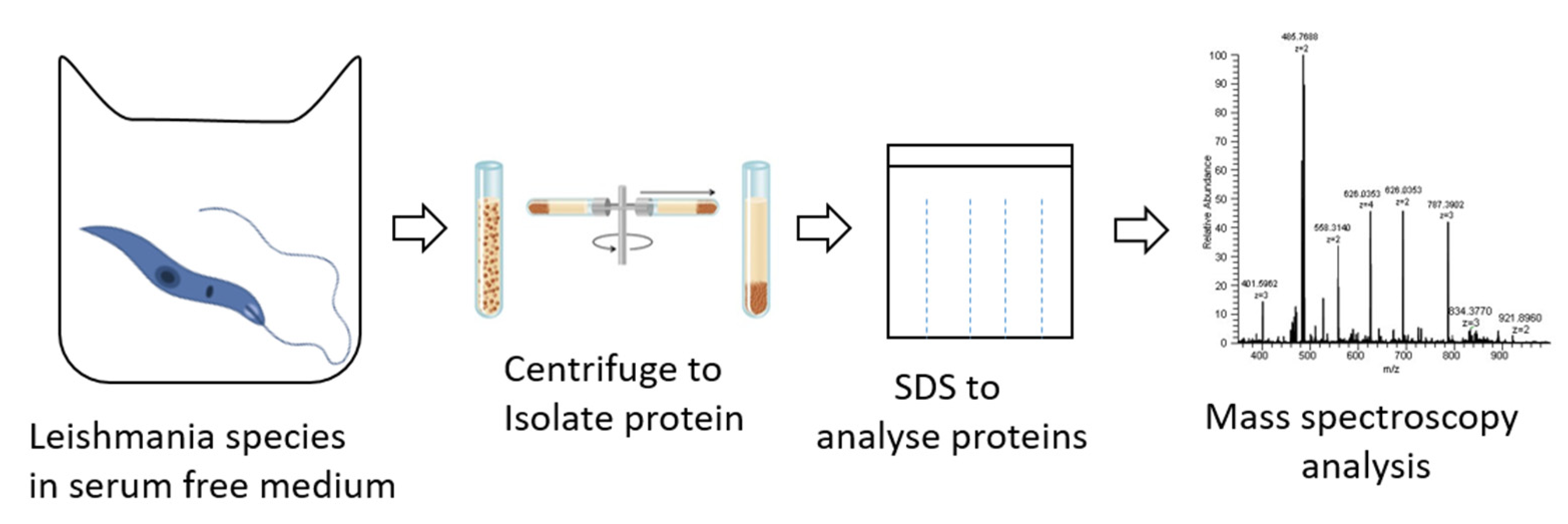
2. General Proteomic Approach in Different Type of Leishmaniasis
3. Proteomic Analysis of Different Leishmania Species
4. Proteomic Approach to Host Cell Constituents and Their Relationship with the Immune Response
4.1. Role of Macrophages
4.2. Role of Neutrophils
4.3. Role of Lymphocytes
5. Proteomic Approach to Secreted Proteins in Leishmaniasis
| Leishmania Species | Target | UniProt ID/PDB ID | Drug Design Approach | Designed Drug Molecule | References |
|---|---|---|---|---|---|
| L. major | Pteridine reductase 1(PTR1) | Q01782/2bfo | Molecular docking | Dihydropyrimidine | [82] |
| L. infantum | NADH dehydrogenase 2 (LiNDH2) | 4g6g, 4g73 | Homology modelling | 6-methoxy-quinalidine | [83] |
| L. donavani | Pteridine reductase 1(PTR1) and dihydrofolate reductase-thymidylate synthase enzyme (DHFR-TS) | Molecular docking | Withaferin-A | [84] | |
| L. major | Glycylpeptide n-tetradecanoyltransferase | 4cgn | HTS | 2-(4-fluorophenyl)-N-(3-piperidin-4-yl-1H-indol-5-yl)ethanamide (di diastereoisomer | [85] |
| Leishmaniasis spp. | Inositol phosphorylceramide synthase (IPCS) | Strcture based drug desiging | 3-(1,3-Benzodioxol-5-yl)-6-{[(1E)-2 | [86] | |
| Leishmaniasis spp. | Trypanothione reductase | 2JK6 | Strcture based drug desiging | 5-Nitrothiophene-2-carboxamides | |
| L. donavani | Leishmania Sperimidine synthase | 3bwb | Molecular dynamic simulation | 4-{[(2R)-2-[(2-oxo-4-phenyl-2 H-chromen-7-yl) oxy] propanamide] methyl}pyrdine-1-ium)., (1r, 4r)-4-{[4-(azaniumylmethyl)-1H-1, 2, 3-triazol-1-yl] methyl}-1-({ \2-oxo-1-azatricyclo [7.3.1.05.13] trideca-3,5,7,9 (13)-tetraen-4-yl} methyl) piperidin-1-ium). | [87] |
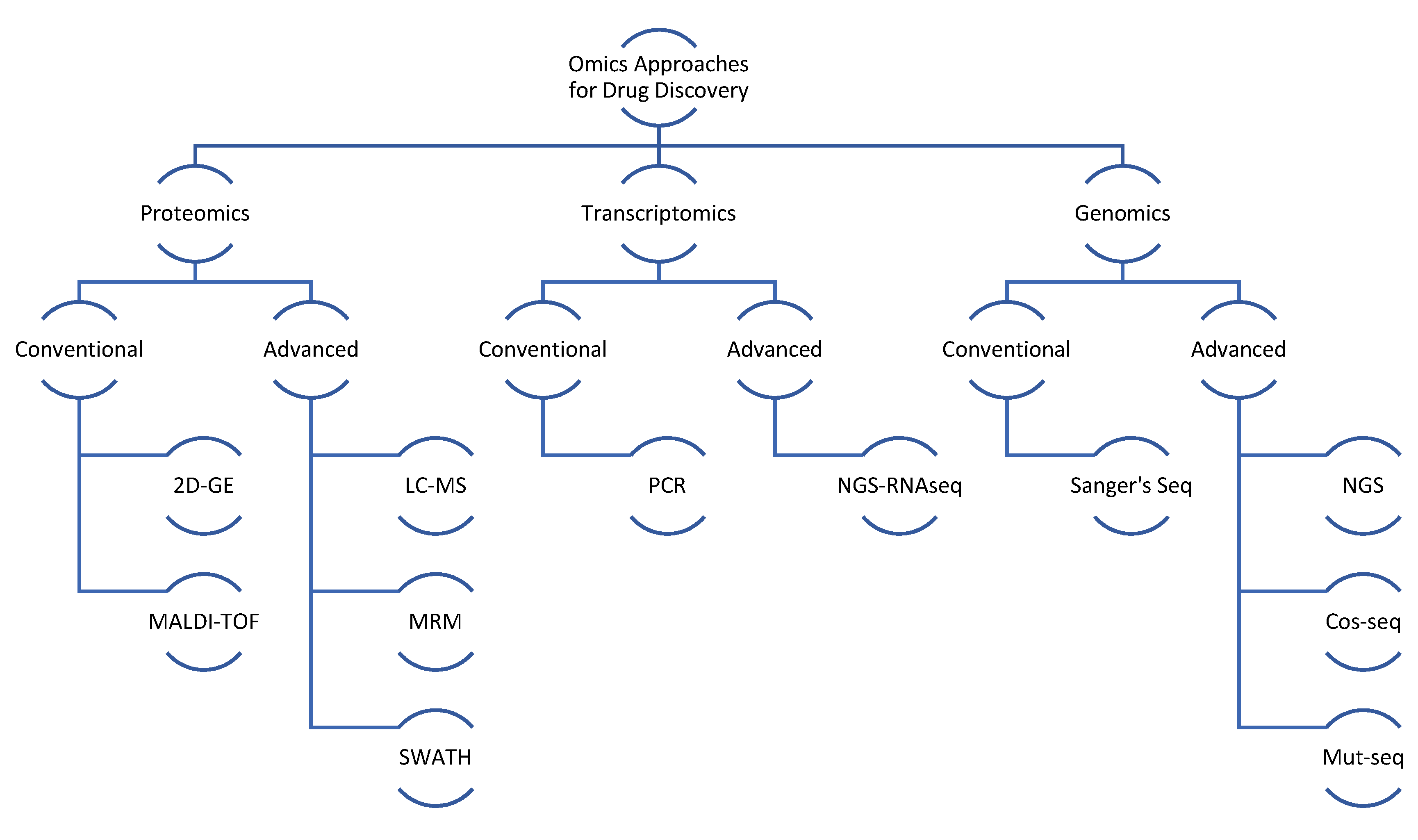
6. Advanced Proteomics Approaches in Leishmaniasis Drug Discovery
7. Genomic and Transcriptomic Studies on Leishmaniasis Drug Discovery
8. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Torres-Guerrero, E.; Quintanilla-Cedillo, M.R.; Ruiz-Esmenjaud, J.; Arenas, R. Leishmaniasis: A Review. F1000Research 2017, 6, 750. [Google Scholar] [CrossRef]
- Piscopo, T.V.; Mallia Azzopardi, C. Leishmaniasis. Postgrad. Med. J. 2007, 83, 649–657. [Google Scholar] [CrossRef] [Green Version]
- Brannigan, J.A.; Wilkinson, A.J. Drug discovery in leishmaniasis using protein lipidation as a target. Biophys. Rev. 2021, 13, 1139–1146. [Google Scholar] [CrossRef] [PubMed]
- Alvar, J.; Vélez, I.D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Jannin, J.; den Boer, M. The WHO Leishmaniasis Control Team Leishmaniasis Worldwide and Global Estimates of Its Incidence. PLoS ONE 2012, 7, e35671. [Google Scholar] [CrossRef] [PubMed]
- Prevention, C.-C. For D.C. and CDC-Leishmaniasis-Epidemiology & Risk Factors. Available online: https://www.cdc.gov/parasites/leishmaniasis/epi.html (accessed on 25 July 2022).
- Murray, H.W. Kala-Azar—Progress against a Neglected Disease. N. Engl. J. Med. 2002, 347, 1793–1794. [Google Scholar] [CrossRef]
- Guerin, P.J.; Olliaro, P.; Sundar, S.; Boelaert, M.; Croft, S.L.; Desjeux, P.; Wasunna, M.K.; Bryceson, A.D. Visceral leishmaniasis: Current status of control, diagnosis, and treatment, and a proposed research and development agenda. Lancet Infect. Dis. 2002, 2, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Gossage, S.M.; Rogers, M.E.; Bates, P.A. Two separate growth phases during the development of Leishmania in sand flies: Implications for understanding the life cycle. Int. J. Parasitol. 2003, 33, 1027–1034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teixeira, D.E.; Benchimol, M.; Rodrigues, J.C.F.; Crepaldi, P.H.; Pimenta, P.F.P.; de Souza, W. The Cell Biology of Leishmania: How to Teach Using Animations. PLoS Pathog. 2013, 9, e1003594. [Google Scholar] [CrossRef] [Green Version]
- Negrão, F.; Eberlin, M.N.; Giorgio, S. Proteomic approaches for drug discovery against tegumentary leishmaniasis. Biomed. Pharmacother. 2017, 95, 577–582. [Google Scholar] [CrossRef]
- Srivastava, P.; Dayama, A.; Mehrotra, S.; Sundar, S. Diagnosis of visceral leishmaniasis. Trans. R. Soc. Trop. Med. Hyg. 2011, 105, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Aronson, N.; Herwaldt, B.L.; Libman, M.; Pearson, R.; Lopez-Velez, R.; Weina, P.; Carvalho, E.; Ephros, M.; Jeronimo, S.; Magill, A. Diagnosis and Treatment of Leishmaniasis: Clinical Practice Guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Am. J. Trop. Med. Hyg. 2017, 96, 24–45. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.; Joshi, J.; Kaur, S. Leishmaniasis diagnosis: An update on the use of parasitological, immunological and molecular methods. J. Parasit. Dis. 2020, 44, 253–272. [Google Scholar] [CrossRef] [PubMed]
- Lockwood, D.; Moore, E. Treatment of visceral leishmaniasis. J. Glob. Infect. Dis. 2010, 2, 151. [Google Scholar] [CrossRef] [PubMed]
- Eiras, D.P.; Kirkman, L.A.; Murray, H.W. Cutaneous Leishmaniasis: Current Treatment Practices in the USA for Returning Travelers. Curr. Treat. Options Infect. Dis. 2015, 7, 52–62. [Google Scholar] [CrossRef] [Green Version]
- Frézard, F.; Demicheli, C.; Ribeiro, R. Pentavalent Antimonials: New Perspectives for Old Drugs. Molecules 2009, 14, 2317–2336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brito, N.C.; Rabello, A.; Cota, G.F. Efficacy of pentavalent antimoniate intralesional infiltration therapy for cutaneous leishmaniasis: A systematic review. PLoS ONE 2017, 12, e0184777. [Google Scholar] [CrossRef]
- Prevention, C.-C. For D.C. and CDC-Leishmaniasis-Resources for Health Professionals. Available online: https://www.cdc.gov/parasites/leishmaniasis/health_professionals/index.html (accessed on 25 July 2022).
- Handman, E. Leishmaniasis: Current Status of Vaccine Development. Clin. Microbiol. Rev. 2001, 14, 229–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghorbani, M.; Farhoudi, R. Leishmaniasis in humans: Drug or vaccine therapy? Drug Des. Devel. Ther. 2017, 12, 25–40. [Google Scholar] [CrossRef] [Green Version]
- Coutinho De Oliveira, B.; Duthie, M.S.; Alves Pereira, V.R. Vaccines for leishmaniasis and the implications of their development for American tegumentary leishmaniasis. Hum. Vaccines Immunother. 2020, 16, 919–930. [Google Scholar] [CrossRef]
- Srivastava, S.; Shankar, P.; Mishra, J.; Singh, S. Possibilities and challenges for developing a successful vaccine for leishmaniasis. Parasit. Vectors 2016, 9, 277. [Google Scholar] [CrossRef]
- Pijpers, J.; den Boer, M.L.; Essink, D.R.; Ritmeijer, K. The safety and efficacy of miltefosine in the long-term treatment of post-kala-azar dermal leishmaniasis in South Asia—A review and meta-analysis. PLoS Negl. Trop. Dis. 2019, 13, e0007173. [Google Scholar] [CrossRef]
- Omollo, R.; Alexander, N.; Edwards, T.; Khalil, E.A.; Younis, B.M.; Abuzaid, A.A.; Wasunna, M.; Njoroge, N.; Kinoti, D.; Kirigi, G.; et al. Safety and Efficacy of miltefosine alone and in combination with sodium stibogluconate and liposomal amphotericin B for the treatment of primary visceral leishmaniasis in East Africa: Study protocol for a randomized controlled trial. Trials 2011, 12, 166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorlo, T.P.C.; Balasegaram, M.; Beijnen, J.H.; de Vries, P.J. Miltefosine: A review of its pharmacology and therapeutic efficacy in the treatment of leishmaniasis. J. Antimicrob. Chemother. 2012, 67, 2576–2597. [Google Scholar] [CrossRef] [PubMed]
- Veras, P.; Bezerra de Menezes, J. Using Proteomics to Understand How Leishmania Parasites Survive inside the Host and Establish Infection. Int. J. Mol. Sci. 2016, 17, 1270. [Google Scholar] [CrossRef]
- Sundar, S.; Singh, B. Understanding Leishmania parasites through proteomics and implications for the clinic. Expert Rev. Proteom. 2018, 15, 371–390. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Misra, P.; Sisodia, B.; Shasany, A.K.; Sundar, S.; Dube, A. Mass spectrometry-based proteomic analysis of Leishmania donovani soluble proteins in Indian clinical isolate. Pathog. Dis. 2014, 70, 84–87. [Google Scholar] [CrossRef] [Green Version]
- Walgren, J. Application of proteomic technologies in the drug development process. Toxicol. Lett. 2004, 149, 377–385. [Google Scholar] [CrossRef]
- Amiri-Dashatan, N.; Koushki, M.; Abbaszadeh, H.-A.; Rostami-Nejad, M.; Rezaei-Tavirani, M. Proteomics Applications in Health: Biomarker and Drug Discovery and Food Industry. Iran. J. Pharm. Res. IJPR 2018, 17, 1523–1536. [Google Scholar] [PubMed]
- Cho, W.C.S. Proteomics Technologies and Challenges. Genom. Proteom. Bioinform. 2007, 5, 77–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuzyk, M.A.; Parker, C.E.; Domanski, D.; Borchers, C.H. Development of MRM-based assays for the absolute quantitation of plasma proteins. In The Low Molecular Weight Proteome; Springer: New York, NY, USA, 2013; pp. 53–82. [Google Scholar]
- Miao, Z.; Chen, H.; Liu, P.; Liu, Y. Development of submillisecond time-resolved mass spectrometry using desorption electrospray ionization. Anal. Chem. 2011, 83, 3994–3997. [Google Scholar] [CrossRef]
- Huang, Q.; Yang, L.; Luo, J.; Guo, L.; Wang, Z.; Yang, X.; Jin, W.; Fang, Y.; Ye, J.; Shan, B. SWATH enables precise label-free quantification on proteome scale. Proteomics 2015, 15, 1215–1223. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Matsudaira, P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J. Biol. Chem. 1987, 262, 10035–10038. [Google Scholar] [CrossRef]
- Rashidi, S.; Kalantar, K.; Department of Immunology, Shiraz University of Medical Sciences, Shiraz, Iran; Rostamzadeh, D.; Immunology Research Center, Tabriz University of Medical Sciences, Tabriz, Iran; Hatam, G.; Basic Sciences in Infectious Diseases Research Center, Shiraz University of Medical Sciences, Shiraz, Iran. The Importance of Checking Leishmania Promastigotes Viability in the Proteomics Analysis of Secretions. Turk. J. Parasitol. 2018, 42, 245–248. [Google Scholar] [CrossRef]
- Gupta, S.K.; Sisodia, B.S.; Sinha, S.; Hajela, K.; Naik, S.; Shasany, A.K.; Dube, A. Proteomic approach for identification and characterization of novel immunostimulatory proteins from soluble antigens ofLeishmania donovani promastigotes. Proteomics 2007, 7, 816–823. [Google Scholar] [CrossRef] [PubMed]
- Scott, P.; Novais, F.O. Cutaneous leishmaniasis: Immune responses in protection and pathogenesis. Nat. Rev. Immunol. 2016, 16, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Negrão, F.; Diedrich, J.K.; Giorgio, S.; Eberlin, M.N.; Yates, J.R. Tandem Mass Tag Proteomic Analysis of in Vitro and in Vivo Models of Cutaneous Leishmaniasis Reveals Parasite-Specific and Nonspecific Modulation of Proteins in the Host. ACS Infect. Dis. 2019, 5, 2136–2147. [Google Scholar] [CrossRef] [PubMed]
- Montoya, A.; López, M.C.; Vélez, I.D.; Robledo, S.M. Label-free quantitative proteomic analysis reveals potential biomarkers for early healing in cutaneous leishmaniasis. PeerJ 2019, 6, e6228. [Google Scholar] [CrossRef]
- Wright, M.H.; Paape, D.; Storck, E.M.; Serwa, R.A.; Smith, D.F.; Tate, E.W. Global Analysis of Protein N-Myristoylation and Exploration of N-Myristoyltransferase as a Drug Target in the Neglected Human Pathogen Leishmania donovani. Chem. Biol. 2015, 22, 342–354. [Google Scholar] [CrossRef] [Green Version]
- Corpas-Lopez, V.; Moniz, S.; Thomas, M.; Wall, R.J.; Torrie, L.S.; Zander-Dinse, D.; Tinti, M.; Brand, S.; Stojanovski, L.; Manthri, S.; et al. Pharmacological Validation of N-Myristoyltransferase as a Drug Target in Leishmania donovani. ACS Infect. Dis. 2019, 5, 111–122. [Google Scholar] [CrossRef]
- Amiri Dashatan, N.; Rezaie Tavirani, M.; Zali, H.; Koushki, M.; Ahmadi, N. Prediction of Leishmania Major Key Proteins via Topological Analysis of Protein-Protein Interaction Network. Galen Med. J. 2018, 7, e1129. [Google Scholar] [CrossRef] [PubMed]
- Lari, N.; Jalal, R.; Minuchehr, Z.; Rajabian Noghondar, M. Identifying miltefosine-resistant key genes in protein–protein interactions network and experimental verification in Iranian Leishmania major. Mol. Biol. Rep. 2019, 46, 5371–5388. [Google Scholar] [CrossRef] [PubMed]
- Flórez, A.F.; Park, D.; Bhak, J.; Kim, B.-C.; Kuchinsky, A.; Morris, J.H.; Espinosa, J.; Muskus, C. Protein network prediction and topological analysis in Leishmania major as a tool for drug target selection. BMC Bioinform. 2010, 11, 484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flórez, A.F.; Watowich, S.; Muskus, C. Current Advances in Computational Strategies for Drug Discovery in Leishmaniasis. In Current Topics in Tropical Medicine; InTech, InTech Open: Rijeka, Croatia, 2012. [Google Scholar]
- Krobthong, S.; Yingchutrakul, Y.; Samutrtai, P.; Hitakarun, A.; Siripattanapipong, S.; Leelayoova, S.; Mungthin, M.; Choowongkomon, K. Utilizing Quantitative Proteomics to Identify Species-Specific Protein Therapeutic Targets for the Treatment of Leishmaniasis. ACS Omega 2022, 7, 12580–12588. [Google Scholar] [CrossRef] [PubMed]
- Serafim, T.D.; Coutinho-Abreu, I.V.; Dey, R.; Kissinger, R.; Valenzuela, J.G.; Oliveira, F.; Kamhawi, S. Leishmaniasis: The act of transmission. Trends Parasitol. 2021, 37, 976–987. [Google Scholar] [CrossRef]
- Forrest, D.M.; Batista, M.; Marchini, F.K.; Tempone, A.J.; Traub-Csekö, Y.M. Proteomic analysis of exosomes derived from procyclic and metacyclic-like cultured Leishmania infantum chagasi. J. Proteom. 2020, 227, 103902. [Google Scholar] [CrossRef] [PubMed]
- Yasmin, H.; Adhikary, A.; Al-Ahdal, M.N.; Roy, S.; Kishore, U. Host–Pathogen Interaction in Leishmaniasis: Immune Response and Vaccination Strategies. Immuno 2022, 2, 218–254. [Google Scholar] [CrossRef]
- Giraud, E.; Lestinova, T.; Derrick, T.; Martin, O.; Dillon, R.J.; Volf, P.; Műller, I.; Bates, P.A.; Rogers, M.E. Leishmania proteophosphoglycans regurgitated from infected sand flies accelerate dermal wound repair and exacerbate leishmaniasis via insulin-like growth factor 1-dependent signalling. PLoS Pathog. 2018, 14, e1006794. [Google Scholar]
- de Jesus, J.B.; Mesquita-Rodrigues, C.; Cuervo, P. Proteomics Advances in the Study of Leishmania Parasites and Leishmaniasis. In Proteins and Proteomics of Leishmania and Trypanosoma; Santos, A.L.S., Branquinha, M.H., d’Avila-Levy, C.M., Kneipp, L.F., Sodré, C.L., Eds.; Springer: Dordrecht, The Netherlands, 2014; Volume 74, pp. 323–349. [Google Scholar]
- Lynn, M.A.; Marr, A.K.; McMaster, W.R. Differential quantitative proteomic profiling of Leishmania infantum and Leishmania mexicana density gradient separated membranous fractions. J. Proteom. 2013, 82, 179–192. [Google Scholar] [CrossRef]
- McNicoll, F.; Drummelsmith, J.; Müller, M.; Madore, É.; Boilard, N.; Ouellette, M.; Papadopoulou, B. A combined proteomic and transcriptomic approach to the study of stage differentiation in Leishmania infantum. Proteomics 2006, 6, 3567–3581. [Google Scholar] [CrossRef]
- Garcia, G.C.; Carvalho, A.M.R.S.; Duarte, M.C.; Silva, M.F.C.; Medeiros, F.A.C.; Coelho, E.A.F.; de Moura Franco, D.M.; Gonçalves, D.U.; de Oliveira Mendes, T.A.; Menezes-Souza, D. Development of a chimeric protein based on a proteomic approach for the serological diagnosis of human tegumentary leishmaniasis. Appl. Microbiol. Biotechnol. 2021, 105, 6805–6817. [Google Scholar] [CrossRef]
- Cuervo, P.; de Jesus, J.B.; Junqueira, M.; Mendonça-Lima, L.; González, L.J.; Betancourt, L.; Grimaldi, G.; Domont, G.B.; Fernandes, O.; Cupolillo, E. Proteome analysis of Leishmania (Viannia) braziliensis by two-dimensional gel electrophoresis and mass spectrometry. Mol. Biochem. Parasitol. 2007, 154, 6–21. [Google Scholar] [CrossRef] [PubMed]
- Leifso, K.; Cohen-Freue, G.; Dogra, N.; Murray, A.; McMaster, W.R. Genomic and proteomic expression analysis of Leishmania promastigote and amastigote life stages: The Leishmania genome is constitutively expressed. Mol. Biochem. Parasitol. 2007, 152, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.M.; Andrade, H.M.; Bartholomeu, D.C.; Freitas, L.M.; Pires, S.F.; Chapeaurouge, A.D.; Perales, J.; Ferreira, A.T.; Giusta, M.S.; Melo, M.N.; et al. Analysis of Leishmania chagasi by 2-D Difference Gel Eletrophoresis (2-D DIGE) and Immunoproteomic: Identification of Novel Candidate Antigens for Diagnostic Tests and Vaccine. J. Proteome Res. 2011, 10, 2172–2184. [Google Scholar] [CrossRef]
- Ashrafmansouri, M.; Amiri-Dashatan, N.; Ahmadi, N.; Rezaei-Tavirani, M.; SeyyedTabaei, S.; Haghighi, A. Quantitative proteomic analysis to determine differentially expressed proteins in axenic amastigotes of Leishmania tropica and Leishmania major. IUBMB Life 2020, 72, 1715–1724. [Google Scholar] [CrossRef] [PubMed]
- Bayer-Santos, E.; Aguilar-Bonavides, C.; Rodrigues, S.P.; Cordero, E.M.; Marques, A.F.; Varela-Ramirez, A.; Choi, H.; Yoshida, N.; da Silveira, J.F.; Almeida, I.C. Proteomic Analysis of Trypanosoma cruzi Secretome: Characterization of Two Populations of Extracellular Vesicles and Soluble Proteins. J. Proteome Res. 2013, 12, 883–897. [Google Scholar] [CrossRef] [PubMed]
- Brotherton, M.-C.; Racine, G.; Ouameur, A.A.; Leprohon, P.; Papadopoulou, B.; Ouellette, M. Analysis of Membrane-Enriched and High Molecular Weight Proteins in Leishmania infantum Promastigotes and Axenic Amastigotes. J. Proteome Res. 2012, 11, 3974–3985. [Google Scholar] [CrossRef]
- Fialho Junior, L.; da Fonseca Pires, S.; Burchmore, R.; McGill, S.; Weidt, S.; Ruiz, J.C.; Guimarães, F.G.; Chapeourouge, A.; Perales, J.; de Andrade, H.M. Proteomic analysis reveals differentially abundant proteins probably involved in the virulence of amastigote and promastigote forms of Leishmania infantum. Parasitol. Res. 2021, 120, 679–692. [Google Scholar] [CrossRef] [PubMed]
- Brotherton, M.-C.; Racine, G.; Foucher, A.L.; Drummelsmith, J.; Papadopoulou, B.; Ouellette, M. Analysis of Stage-Specific Expression of Basic Proteins in Leishmania infantum. J. Proteome Res. 2010, 9, 3842–3853. [Google Scholar] [CrossRef]
- Ashrafmansouri, M.; Sadjjadi, F.S.; Seyyedtabaei, S.; Haghighi, A.; Rezaei-Tavirani, M.; Ahmadi, N. Comparative Two-dimensional Gel Electrophoresis Maps for Amastigote-like Proteomes of Iranian Leishmania Tropica and Leishmania major Isolates. Galen Med. J. 2019, 8, 1520. [Google Scholar] [CrossRef]
- Elhelu, M.A. The role of macrophages in immunology. J. Natl. Med. Assoc. 1983, 75, 314–317. [Google Scholar] [PubMed]
- Arango Duque, G.; Descoteaux, A. Leishmania survival in the macrophage: Where the ends justify the means. Curr. Opin. Microbiol. 2015, 26, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Menezes, J.P.B.; Almeida, T.F.; Petersen, A.L.O.A.; Guedes, C.E.S.; Mota, M.S.V.; Lima, J.G.B.; Palma, L.C.; Buck, G.A.; Krieger, M.A.; Probst, C.M.; et al. Proteomic analysis reveals differentially expressed proteins in macrophages infected with Leishmania amazonensis or Leishmania major. Microbes Infect. 2013, 15, 579–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verma, S.; Deep, D.K.; Gautam, P.; Singh, R.; Salotra, P. Proteomic Analysis of Leishmania donovani Membrane Components Reveals the Role of Activated Protein C Kinase in Host-Parasite Interaction. Pathogens 2021, 10, 1194. [Google Scholar] [CrossRef] [PubMed]
- Smirlis, D.; Dingli, F.; Pescher, P.; Prina, E.; Loew, D.; Rachidi, N.; Späth, G.F. SILAC-based quantitative proteomics reveals pleiotropic, phenotypic modulation in primary murine macrophages infected with the protozoan pathogen Leishmania donovani. J. Proteom. 2020, 213, 103617. [Google Scholar] [CrossRef] [PubMed]
- Salotra, P.; Verma, S.; Deep, D.K.; Singh, R. Proteomic analysis of membrane proteins involved in Leishmania-macrophage interaction. FASEB J. 2020, 34, 1. [Google Scholar] [CrossRef]
- Peters, N.C.; Egen, J.G.; Secundino, N.; Debrabant, A.; Kimblin, N.; Kamhawi, S.; Lawyer, P.; Fay, M.P.; Germain, R.N.; Sacks, D. In Vivo Imaging Reveals an Essential Role for Neutrophils in Leishmaniasis Transmitted by Sand Flies. Science 2008, 321, 970–974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubie, T.; Mohammed, Y. Review on the Role of Host Immune Response in Protection and Immunopathogenesis during Cutaneous Leishmaniasis Infection. J. Immunol. Res. 2020, 2020, 2496713. [Google Scholar] [CrossRef]
- Carreira, J.C.A.; da Silva, A.V.M. The Role of Neutrophils in the Interaction with Leishmania: Far beyond a Simple Trojan Horse? Open J. Anim. Sci. 2021, 11, 399–421. [Google Scholar] [CrossRef]
- Musa, M.A.; Nakamura, R.; Hena, A.; Varikuti, S.; Nakhasi, H.L.; Goto, Y.; Satoskar, A.R.; Hamano, S. Lymphocytes influence Leishmania major pathogenesis in a strain-dependent manner. PLoS Negl. Trop. Dis. 2019, 13, e0007865. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, P.; Singh, V.; Naik, S. Immune response to leishmania: Paradox rather than paradigm. FEMS Immunol. Med. Microbiol. 2007, 51, 229–242. [Google Scholar] [CrossRef]
- da Silva Santos, C.; Attarha, S.; Saini, R.K.; Boaventura, V.; Costa, J.; Khouri, R.; Barral-Netto, M.; Brodskyn, C.I.; Souchelnytskyi, S. Proteome Profiling of Human Cutaneous Leishmaniasis Lesion. J. Investig. Dermatol. 2015, 135, 400–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, M.; Tegel, H.; Sivertsson, Å.; Hober, S.; Snijder, A.; Ormö, M.; Strömstedt, P.-E.; Davies, R.; Holmberg Schiavone, L. Secretome-Based Screening in Target Discovery. SLAS Discov. 2020, 25, 535–551. [Google Scholar] [CrossRef]
- Pissarra, J.; Pagniez, J.; Petitdidier, E.; Séveno, M.; Vigy, O.; Bras-Gonçalves, R.; Lemesre, J.-L.; Holzmuller, P. Proteomic Analysis of the Promastigote Secretome of Seven Leishmania Species. J. Proteome Res. 2022, 21, 30–48. [Google Scholar] [CrossRef]
- Rashidi, S.; Nguewa, P.; Mojtahedi, Z.; Shahriari, B.; Kalantar, K.; Hatam, G. Identification of immunoreactive proteins in secretions of Leishmania infantum promastigotes: An immunoproteomic approach. East. Mediterr. Health J. 2020, 26, 1548–1555. [Google Scholar] [CrossRef]
- Silverman, J.M.; Chan, S.K.; Robinson, D.P.; Dwyer, D.M.; Nandan, D.; Foster, L.J.; Reiner, N.E. Proteomic analysis of the secretome of Leishmania donovani. Genome Biol. 2008, 9, R35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rashid, U.; Sultana, R.; Shaheen, N.; Hassan, S.F.; Yaqoob, F.; Ahmad, M.J.; Iftikhar, F.; Sultana, N.; Asghar, S.; Yasinzai, M.; et al. Structure based medicinal chemistry-driven strategy to design substituted dihydropyrimidines as potential antileishmanial agents. Eur. J. Med. Chem. 2016, 115, 230–244. [Google Scholar] [CrossRef]
- Stevanović, S.; Perdih, A.; Senćanski, M.; Glišić, S.; Duarte, M.; Tomás, A.M.; Sena, F.V.; Sousa, F.M.; Pereira, M.M.; Solmajer, T. In Silico Discovery of a Substituted 6-Methoxy-quinalidine with Leishmanicidal Activity in Leishmania infantum. Molecules 2018, 23, 772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vadloori, B.; Sharath, A.K.; Prabhu, N.P.; Maurya, R. Homology modelling, molecular docking, and molecular dynamics simulations reveal the inhibition of Leishmania donovani dihydrofolate reductase-thymidylate synthase enzyme by Withaferin-A. BMC Res. Notes 2018, 11, 246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jennie, A. Hutton Structure-Based Design of Potent and Selective Leishmania N-Myristoyltransferase Inhibitors|Journal of Medicinal Chemistry. Available online: https://pubs.acs.org/doi/10.1021/jm5011397 (accessed on 14 September 2022).
- Mandlik, V.; Singh, S. Molecular docking and molecular dynamics simulation study of inositol phosphorylceramide synthase-inhibitor complex in leishmaniasis: Insight into the structure based drug design. F1000Research 2016, 5, 1610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grover, A.; Katiyar, S.P.; Singh, S.K.; Dubey, V.K.; Sundar, D. A leishmaniasis study: Structure-based screening and molecular dynamics mechanistic analysis for discovering potent inhibitors of spermidine synthase. Biochim. Biophys. Acta BBA-Proteins Proteom. 2012, 1824, 1476–1483. [Google Scholar] [CrossRef]
- Routaray, C.B.; Bhor, R.; Bai, S.; Kadam, N.S.; Jagtap, S.; Doshi, P.J.; Sundar, S.; Sawant, S.; Kulkarni, M.J.; Pai, K. SWATH-MS based quantitative proteomics analysis to evaluate the antileishmanial effect of Commiphora wightii-Guggul and Amphotericin B on a clinical isolate of Leishmania donovani. J. Proteom. 2020, 223, 103800. [Google Scholar] [CrossRef] [PubMed]
- Roseboom, I.C.; Thijssen, B.; Rosing, H.; Alves, F.; Mondal, D.; Teunissen, M.B.; Beijnen, J.H.; Dorlo, T.P. Development and validation of an HPLC-MS/MS method for the quantification of the anti-leishmanial drug miltefosine in human skin tissue. J. Pharm. Biomed. Anal. 2022, 207, 114402. [Google Scholar] [CrossRef] [PubMed]
- SIu, A.; Dorosevich, A. The treatment of biological material by Falck’s method and its modifications using a device of original design. Biulleten Eksperimentalnoi Biol. Meditsiny 1993, 116, 633–636. [Google Scholar]
- West, G.M.; Tucker, C.L.; Xu, T.; Park, S.K.; Han, X.; Yates III, J.R.; Fitzgerald, M.C. Quantitative proteomics approach for identifying protein–drug interactions in complex mixtures using protein stability measurements. Proc. Natl. Acad. Sci. USA 2010, 107, 9078–9082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Menezes, J.P.B.; Guedes, C.E.S.; Petersen, A.L.D.O.A.; Fraga, D.B.M.; Veras, P.S.T. Advances in development of new treatment for leishmaniasis. BioMed Res. Int. 2015, 2015, 815023. [Google Scholar] [CrossRef] [PubMed]
- Cantacessi, C.; Dantas-Torres, F.; Nolan, M.J.; Otranto, D. The past, present, and future of Leishmania genomics and transcriptomics. Trends Parasitol. 2015, 31, 100–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blackwell, J.M.; Fakiola, M.; Singh, O.P. Genetics, Transcriptomics and Meta-Taxonomics in Visceral Leishmaniasis. Front. Cell. Infect. Microbiol. 2020, 10, 590888. [Google Scholar] [CrossRef] [PubMed]
- Cruz, A.K.; Freitas-Castro, F. Genome and transcriptome analyses of Leishmania spp.: Opening Pandora’s box. Curr. Opin. Microbiol. 2019, 52, 64–69. [Google Scholar] [CrossRef]
- Dillon, L.A.L.; Okrah, K.; Hughitt, V.K.; Suresh, R.; Li, Y.; Fernandes, M.C.; Belew, A.T.; Corrada Bravo, H.; Mosser, D.M.; El-Sayed, N.M. Transcriptomic profiling of gene expression and RNA processing during Leishmania major differentiation. Nucleic Acids Res. 2015, 43, 6799–6813. [Google Scholar] [CrossRef] [Green Version]
- Gazanion, É.; Fernández-Prada, C.; Papadopoulou, B.; Leprohon, P.; Ouellette, M. Cos-Seq for high-throughput identification of drug target and resistance mechanisms in the protozoan parasite Leishmania. Proc. Natl. Acad. Sci. USA 2016, 113, E3012–E3021. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharya, A.; Bigot, S.; Padmanabhan, P.K.; Mukherjee, A.; Coelho, A.; Leprohon, P.; Papadopoulou, B.; Ouellette, M. New insights in the mode of action of anti-leishmanial drugs by using chemical mutagenesis screens coupled to next-generation sequencing. Microb. Cell 2020, 7, 59. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rabaan, A.A.; Bakhrebah, M.A.; Mohapatra, R.K.; Farahat, R.A.; Dhawan, M.; Alwarthan, S.; Aljeldah, M.; Al Shammari, B.R.; Al-Najjar, A.H.; Alhusayyen, M.A.; et al. Omics Approaches in Drug Development against Leishmaniasis: Current Scenario and Future Prospects. Pathogens 2023, 12, 39. https://doi.org/10.3390/pathogens12010039
Rabaan AA, Bakhrebah MA, Mohapatra RK, Farahat RA, Dhawan M, Alwarthan S, Aljeldah M, Al Shammari BR, Al-Najjar AH, Alhusayyen MA, et al. Omics Approaches in Drug Development against Leishmaniasis: Current Scenario and Future Prospects. Pathogens. 2023; 12(1):39. https://doi.org/10.3390/pathogens12010039
Chicago/Turabian StyleRabaan, Ali A., Muhammed A. Bakhrebah, Ranjan K. Mohapatra, Ramadan Abdelmoez Farahat, Manish Dhawan, Sara Alwarthan, Mohammed Aljeldah, Basim R. Al Shammari, Amal H. Al-Najjar, Mona A. Alhusayyen, and et al. 2023. "Omics Approaches in Drug Development against Leishmaniasis: Current Scenario and Future Prospects" Pathogens 12, no. 1: 39. https://doi.org/10.3390/pathogens12010039
APA StyleRabaan, A. A., Bakhrebah, M. A., Mohapatra, R. K., Farahat, R. A., Dhawan, M., Alwarthan, S., Aljeldah, M., Al Shammari, B. R., Al-Najjar, A. H., Alhusayyen, M. A., Al-Absi, G. H., Aldawood, Y., Alsaleh, A. A., Alshamrani, S. A., Almuthree, S. A., Alawfi, A., Alshengeti, A., Alwashmi, A. S. S., Hajissa, K., & Nassar, M. S. (2023). Omics Approaches in Drug Development against Leishmaniasis: Current Scenario and Future Prospects. Pathogens, 12(1), 39. https://doi.org/10.3390/pathogens12010039









