Nocellara Del Belice (Olea europaea L. Cultivar): Leaf Extract Concentrated in Phenolic Compounds and Its Anti-Inflammatory and Radical Scavenging Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Extraction Procedure
2.3. HPLC Analysis
2.4. Preparation of O. europaea Leaf Extract Concentrated in Polyphenols
2.5. Determination of Total Phenolic Content
2.6. Determination of Total Flavonoid Content
2.7. DPPH Assay: Radical-Scavenging Activity
2.8. Evaluation of Radicals Scavenging by Electron Paramagnetic Spectroscopy (EPR)
2.9. Cell Culture
2.10. Free Fatty Acid (FFA) Exposure and O. europaea Leaf Extract Treatment
2.11. MTT Assay
2.12. Fluorometric Determination of Intracellular Fat Content
2.13. Determination of Intracellular Total Fatty Acid Content
2.14. Fluorescence Image Acquisition
2.15. Cytokines Bioplex Assay
2.16. Statistical Analysis
3. Results
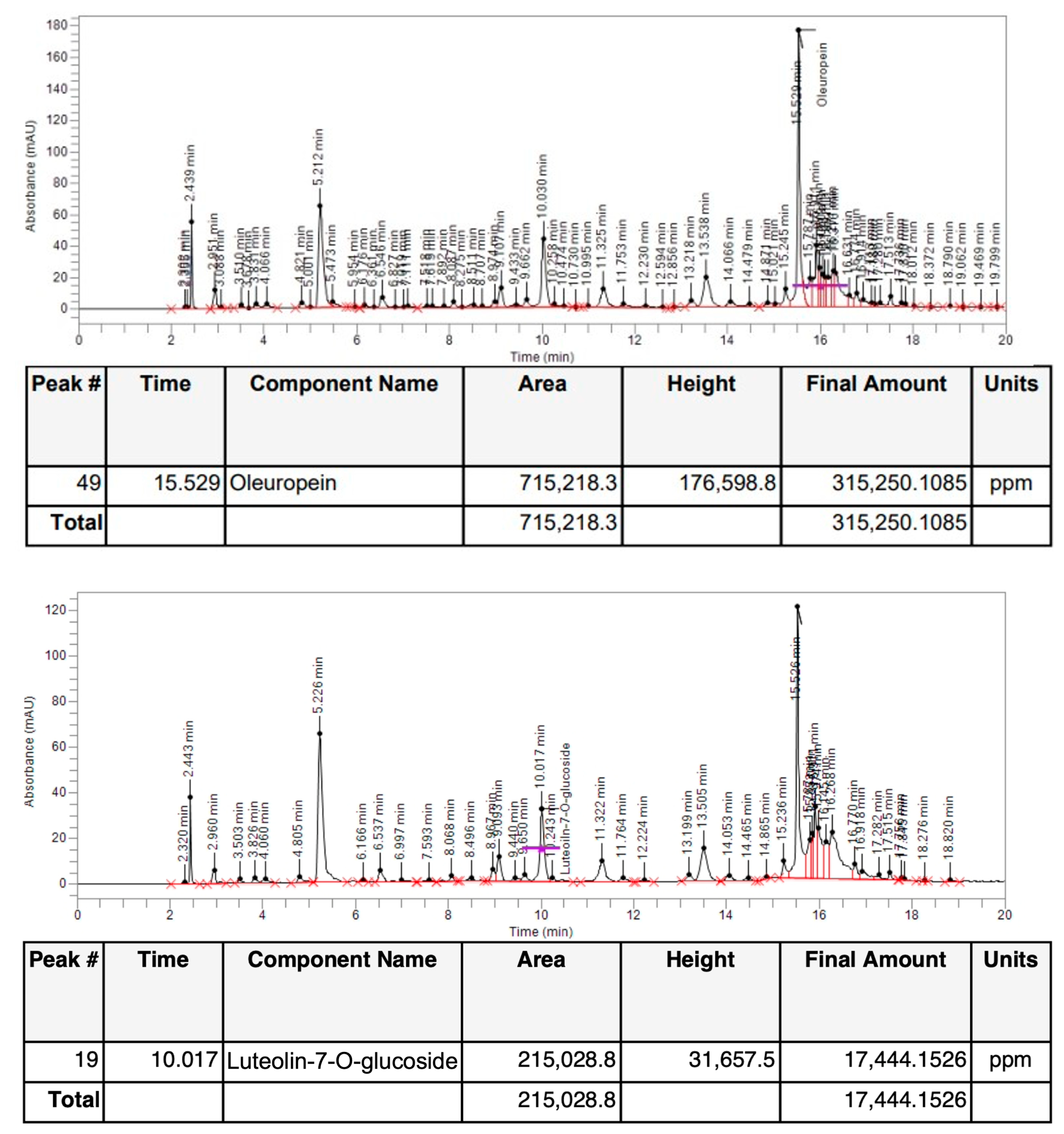
3.1. Identification and Quantification of Oleuropein and Luteolin-7-O-Glucoside Content by HPLC
3.2. Analysis of Olea europaea L. Acetone-Water Leaf Extract Concentrated in Polyphenols
3.3. Characterization of Total Phenolic and Flavonoid Content of Olea europaea L. Leaf Extract Concentrated in Polyphenols
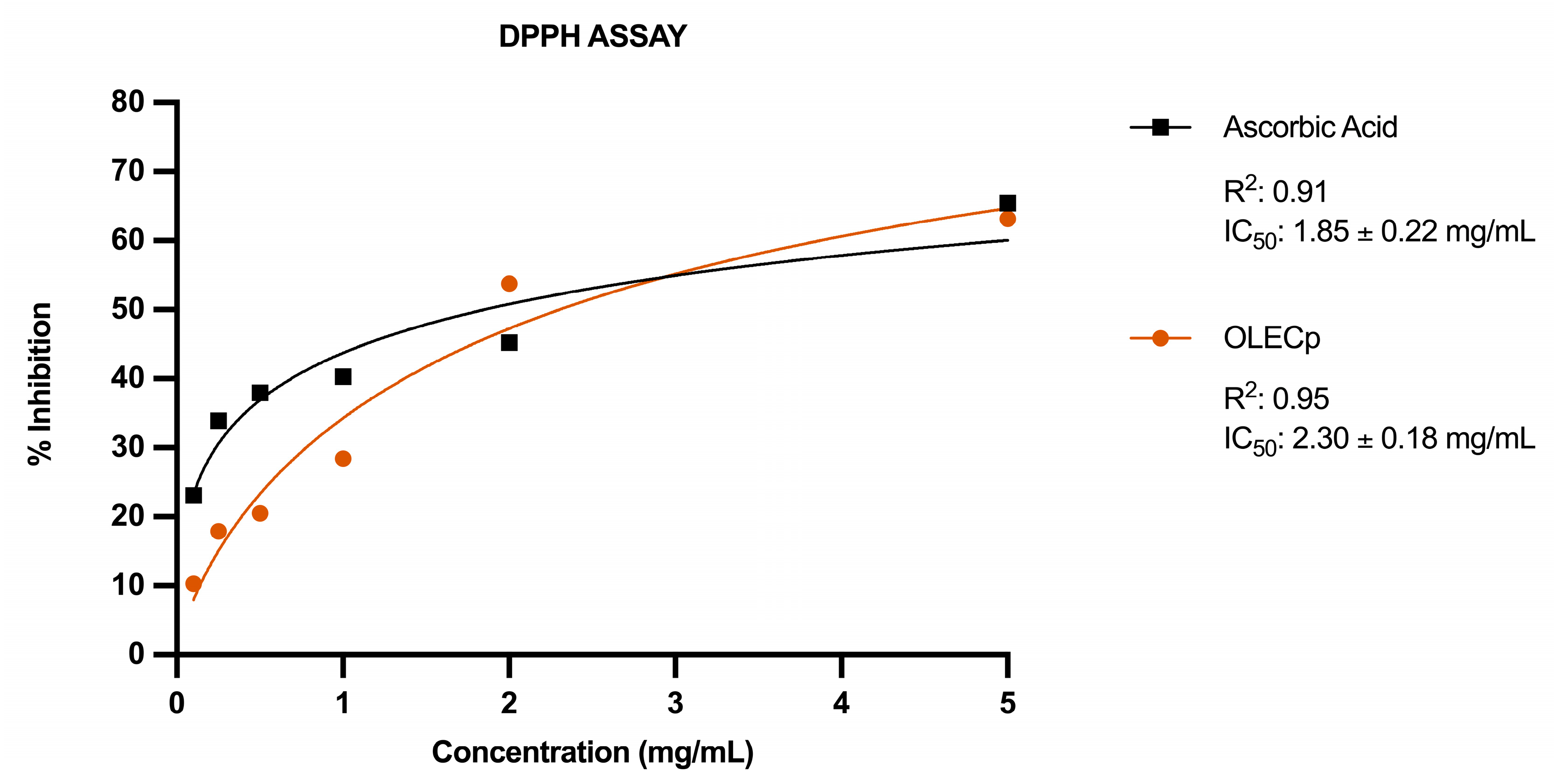
3.4. In Vitro Antioxidant Activity of Olea europaea L. Leaf Extract Concentrated in Polyphenols
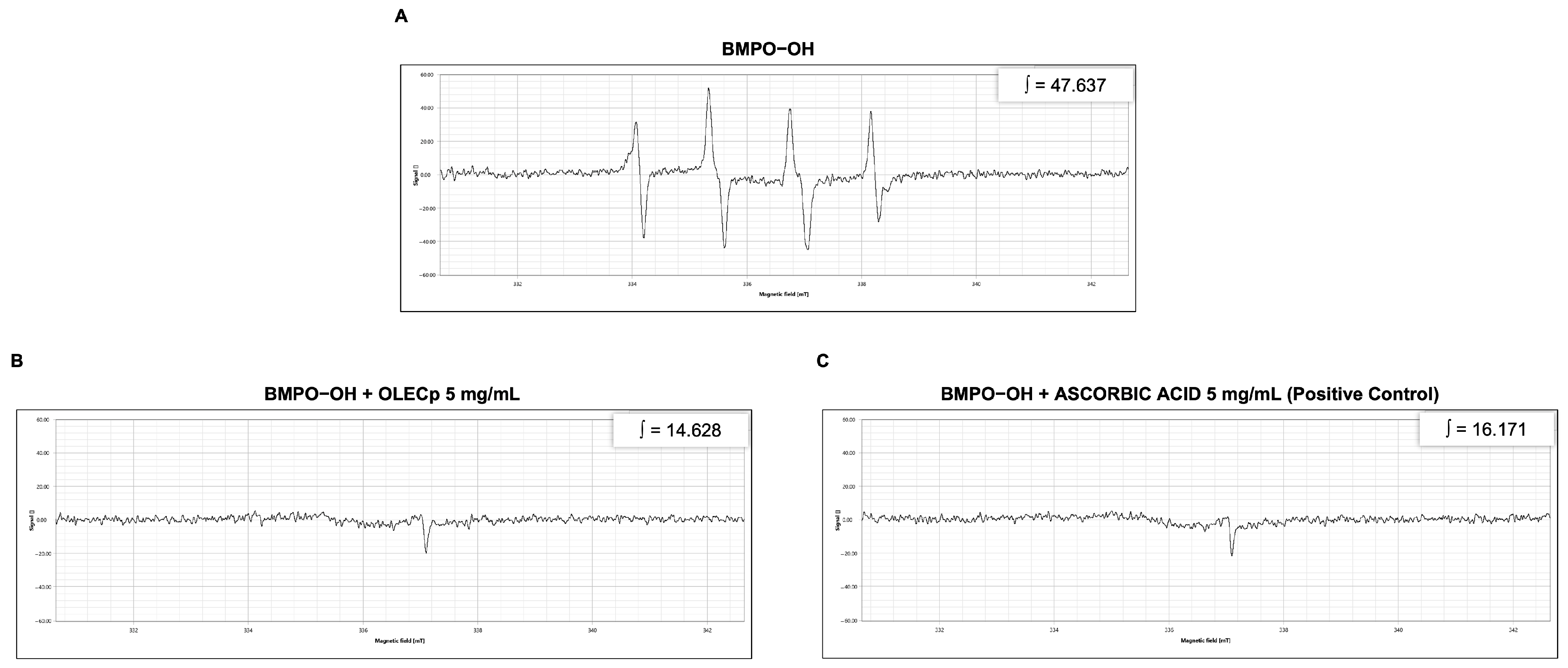
3.5. Evaluation of Radicals Scavenging Activity by EPR Spectroscopy
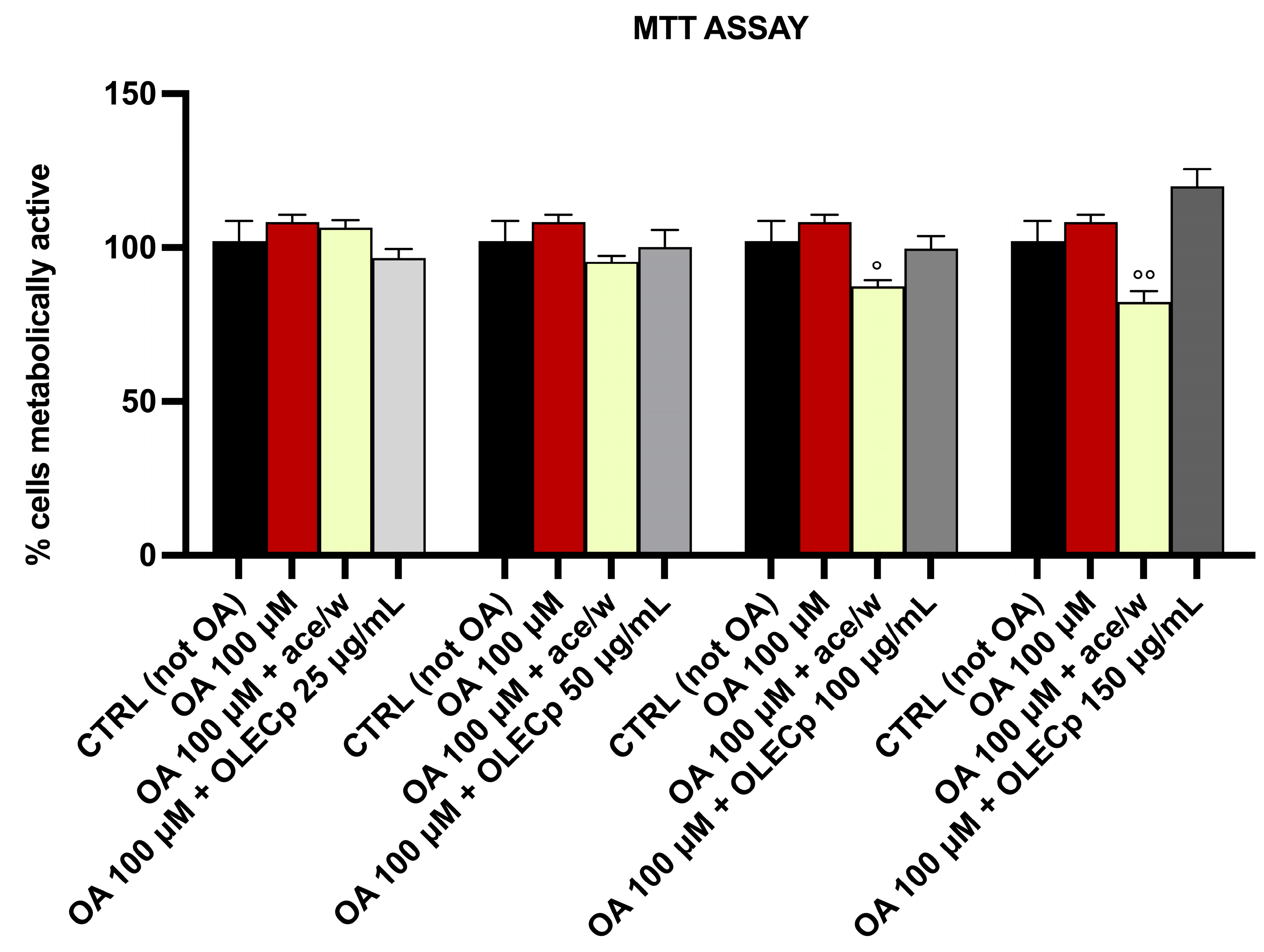
3.6. Olea europaea L. Leaf Extract Treatment Does Not Affect the Cell Viability
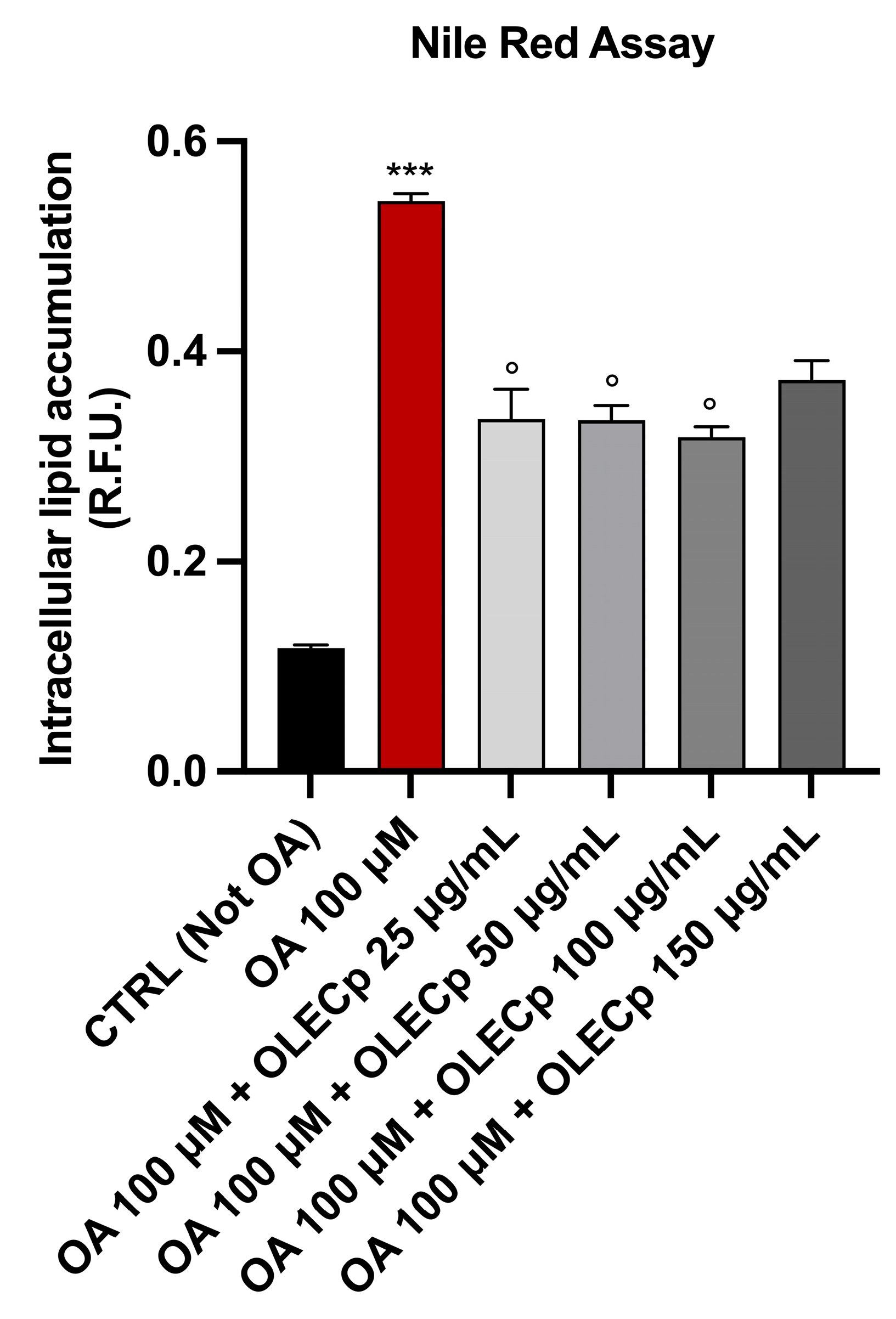
3.7. Olea europaea Leaf Extract Concentrated in Polyphenols Reduces Intracellular Lipid Accumulation
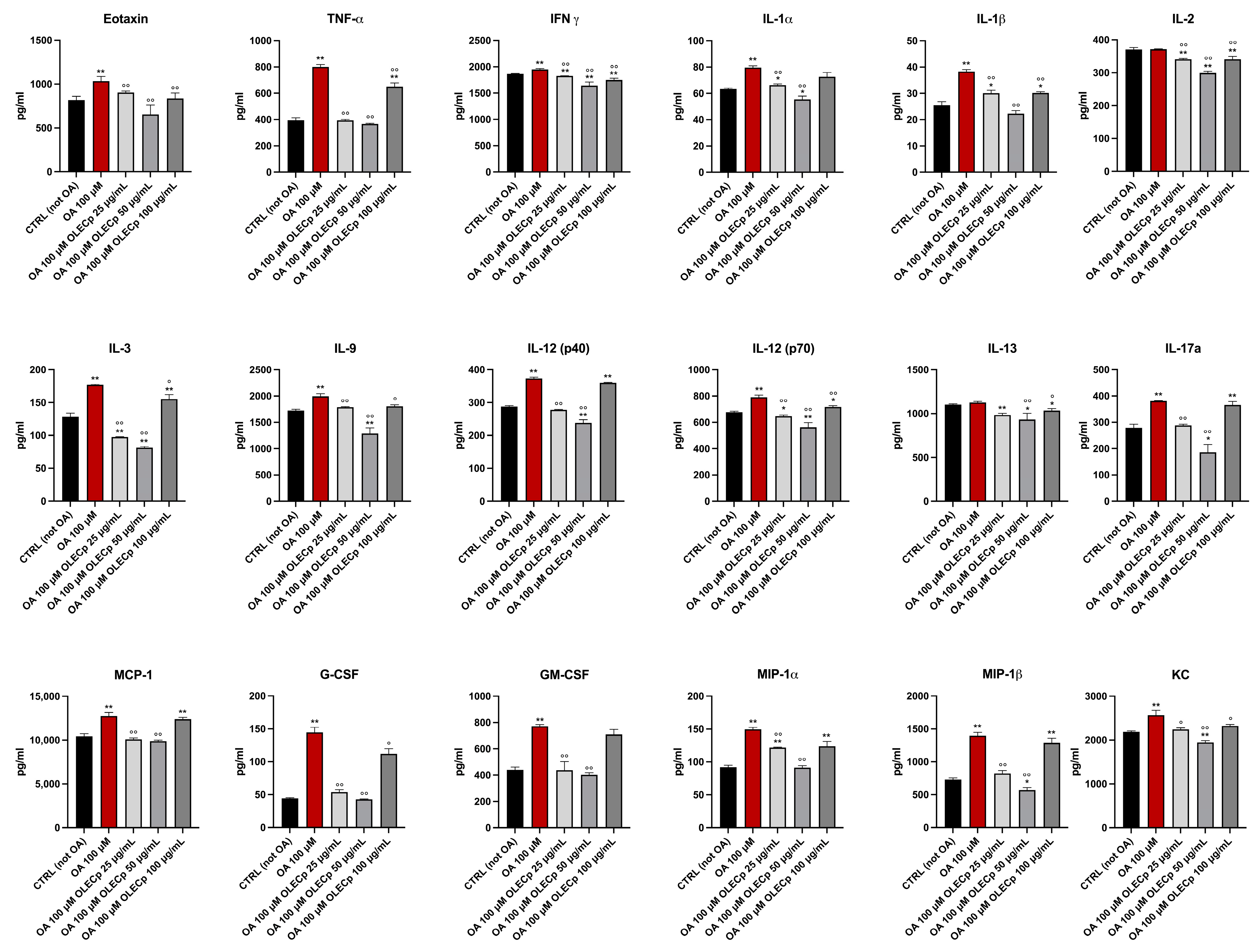
3.8. Olea europaea Leaf Extract Concentrated in Polyphenols Improves Inflammation Status Induced by Exposure to Oleic Acid
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Waterman, E.; Lockwood, B. Active components and clinical applications of olive oil. Altern. Med. Rev. 2007, 12, 331–342. [Google Scholar] [PubMed]
- Uylaşer, V.; Yildiz, G. The historical development and nutritional importance of olive and olive oil constituted an important part of the Mediterranean diet. Crit. Rev. Food Sci. Nutr. 2014, 54, 1092–1101. [Google Scholar] [CrossRef] [PubMed]
- Besnard, G.; Rubio de Casas, R.; Christin, P.A.; Vargas, P. Phylogenetics of Olea (Oleaceae) based on plastid and nuclear ribosomal DNA sequences: Tertiary climatic shifts and lineage differentiation times. Ann. Bot. 2009, 104, 143–160. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.-P.; Sun, J.-H.; Liu, Y.-L.; Xu, C.; Wang, Y.-H.; Suo, Z.-L.; Zhou, S.-L.; Zhang, Z.-X.; Wen, J. Phylogenomic relationships and species identification of the olive genus Olea (Oleaceae). J. Syst. Evol. 2022, 60, 1263–1280. [Google Scholar] [CrossRef]
- Rugini, E.; Baldoni, L.; Muleo, R.; Sebastiani, L. Preface. In The Olive Tree Genome; Rugini, E., Baldoni, L., Muleo, R., Sebastiani, L., Eds.; Springer: Cham, Switzerland, 2016; pp. v–vi. [Google Scholar]
- Giuffrè, A.M. Biometric Evaluation of Twelve Olive Cultivars under Rainfed Conditions in the Region of Calabria, South Italy. Emir. J. Food Agric. 2017, 29, 696–709. [Google Scholar] [CrossRef]
- Sicari, V.; Leporini, M.; Giuffré, A.M.; Aiello, F.; Falco, T.; Pagliuso, M.T.; Ruffolo, A.; Reitano, A.; Romeo, R.; Tundis, R.; et al. Quality parameters, chemical compositions and antioxidant activities of Calabrian (Italy) monovarietal extra virgin olive oils from autochthonous (Ottobratica) and allochthonous (Coratina, Leccino, and Nocellara Del Belice) varieties. Food Meas. 2021, 15, 363–375. [Google Scholar] [CrossRef]
- Ghanbari, R.; Anwar, F.; Alkharfy, K.M.; Gilani, A.H.; Saari, N. Valuable nutrients and functional bioactives in different parts of olive (Olea europaea L.)—A review. Int. J. Mol. Sci. 2012, 13, 3291–3340. [Google Scholar] [CrossRef]
- Silenzi, A.; Giovannini, C.; Scazzocchio, B.; Varì, R.; D’Archivio, M.; Santangelo, C.; Masella, R. Chapter 22—Extra virgin olive oil polyphenols: Biological properties and antioxidant activity. In Pathology; Victor, R.P., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 225–233. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Hoffmann, G. Monounsaturated fatty acids, olive oil and health status: A systematic review and meta-analysis of cohort studies. Lipids Health Dis. 2014, 13, 154. [Google Scholar] [CrossRef]
- Romero-Márquez, J.M.; Navarro-Hortal, M.D.; Jiménez-Trigo, V.; Vera-Ramírez, L.; Forbes-Hernández, T.J.; Esteban-Muñoz, A.; Giampieri, F.; Bullón, P.; Battino, M.; Sánchez-González, C.; et al. An oleuropein rich-olive (Olea europaea L.) leaf extract reduces β-amyloid and tau proteotoxicity through regulation of oxidative- and heat shock-stress responses in Caenorhabditis elegans. Food Chem. Toxicol. 2022, 162, 112914. [Google Scholar] [CrossRef]
- Negro, M.J.; Manzanares, P.; Ruiz, E.; Castro, E.; Ballesteros, M. Chapter 3—The biorefinery concept for the industrial valorization of residues from olive oil industry. In Olive Mill Waste; Charis, M.G., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 57–78. ISBN 9780128053140. [Google Scholar] [CrossRef]
- Nediani, C.; Ruzzolini, J.; Romani, A.; Calorini, L. Oleuropein, a Bioactive Compound from Olea europaea L., as a Potential Preventive and Therapeutic Agent in Non-Communicable Diseases. Antioxidants 2019, 8, 578. [Google Scholar] [CrossRef]
- Fakhraei, N.; Abdolghaffari, A.H.; Delfan, B.; Abbasi, A.; Rahimi, N.; Khansari, A.; Rahimian, R.; Dehpour, A.R. Protective effect of hydroalcoholic olive leaf extract on experimental model of colitis in rat: Involvement of nitrergic and opioidergic systems. Phytother. Res. 2014, 28, 1367–1373. [Google Scholar] [CrossRef]
- Guinda, Á.; Castellano, J.M.; Santos-Lozano, J.M.; Delgado-Hervás, T.; Gutiérrez-Adánez, P.; Rada, M. Determination of major bioactive compounds from olive leaf. Lwt. Food Sci. Technol. 2015, 64, 431–438. [Google Scholar] [CrossRef]
- Antonisamy, P.; Subash-Babu, P.; Albert-Baskar, A.; Alshatwi, A.A.; Aravinthan, A.; Ignacimuthu, S.; Choi, K.; Lee, S.C.; Kim, J. Experimental study on gastroprotective efficacy and mechanisms of luteolin-7-O-glucoside isolated from Ophiorrhiza mungos Linn. in different experimental models. J. Funct. Foods 2016, 25, 302–313. [Google Scholar] [CrossRef]
- Silvan, J.M.; Guerrero-Hurtado, E.; Gutiérrez-Docio, A.; Alarcón-Cavero, T.; Prodanov, M.; Martinez-Rodriguez, A.J. Olive-Leaf Extracts Modulate Inflammation and Oxidative Stress Associated with Human, H. pylori Infection. Antioxidats 2021, 10, 2030. [Google Scholar] [CrossRef]
- Beyaz, S.; Gök, Ö.; Aslan, A. A research paper on the immunomodulatory and anti-inflammatory activities of olive tree (Olea europaea L.) leaf. Int. J. Second. Metab. 2022, 9, 348–359. [Google Scholar] [CrossRef]
- Guex, C.G.; Reginato, F.Z.; de Jesus, P.R.; Brondani, J.C.; Lopes, G.H.H.; Bauermann, L.F. Antidiabetic effects of Olea europaea L. leaves in diabetic rats induced by high-fat diet and low-dose streptozotocin. J. Ethnopharmacol. 2019, 235, 1–7. [Google Scholar] [CrossRef]
- Majumder, D.; Debnath, R.; Nath, P.; Libin Kumar, K.V.; Debnath, M.; Tribedi, P.; Maiti, D. Bromelain and Olea europaea (L.) leaf extract mediated alleviation of benzo(a)pyrene induced lung cancer through Nrf2 and NFκB pathway. Environ. Sci. Pollut. Res. Int. 2021, 28, 47306–47326. [Google Scholar] [CrossRef]
- Hashmi, M.A.; Khan, A.; Hanif, M.; Farooq, U.; Perveen, S. Traditional Uses, Phytochemistry, and Pharmacology of Olea europaea (Olive). Evid. Based Complement. Altern. Med. 2015, 2015, 541591. [Google Scholar] [CrossRef]
- Acar-Tek, N.; Ağagündüz, D. Olive Leaf (Olea europaea L. folium): Potential Effects on Glycemia and Lipidemia. Ann. Nutr. Metab. 2020, 76, 10–15. [Google Scholar] [CrossRef]
- Ahamad, J.; Toufeeq, I.; Khan, M.A.; Ameen, M.S.M.; Anwer, E.T.; Uthirapathy, S.; Mir, S.R.; Ahmad, J. Oleuropein: A natural antioxidant molecule in the treatment of metabolic syndrome. Phytother. Res. 2019, 33, 3112–3128. [Google Scholar] [CrossRef]
- Musolino, V.; Gliozzi, M.; Bombardelli, E.; Nucera, S.; Carresi, C.; Maiuolo, J.; Mollace, R.; Paone, S.; Bosco, F.; Scarano, F.; et al. The synergistic effect of Citrus bergamia and Cynara cardunculus extracts on vascular inflammation and oxidative stress in non-alcoholic fatty liver disease. J. Tradit. Complement. Med. 2020, 10, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Musolino, V.; Gliozzi, M.; Scarano, F.; Bosco, F.; Scicchitano, M.; Nucera, S.; Carresi, C.; Ruga, S.; Zito, M.C.; Maiuolo, J.; et al. Bergamot Polyphenols Improve Dyslipidemia and Pathophysiological Features in a Mouse Model of Non-Alcoholic Fatty Liver Disease. Sci. Rep. 2020, 10, 2565. [Google Scholar] [CrossRef] [PubMed]
- Nucera, S.; Ruga, S.; Cardamone, A.; Coppoletta, A.R.; Guarnieri, L.; Zito, M.C.; Bosco, F.; Macrì, R.; Scarano, F.; Scicchitano, M.; et al. MAFLD progression contributes to altered thalamus metabolism and brain structure. Sci. Rep. 2022, 12, 1207. [Google Scholar] [CrossRef] [PubMed]
- Wani, F.; Albahrawy, A.; Rahiman, S. Hypolipidemic Activity of Olive Oil (Olea europaea) against High Fat Diet-Induced Nonalcoholic Fatty Liver Disease (NAFLD) in Mice. Open J. Pathol. 2015, 5, 73–83. [Google Scholar] [CrossRef]
- Omagari, K.; Kato, S.; Tsuneyama, K.; Hatta, H.; Sato, M.; Hamasaki, M.; Sadakane, Y.; Tashiro, T.; Fukuhata, M.; Miyata, Y.; et al. Olive leaf extract prevents spontaneous occurrence of non-alcoholic steatohepatitis in SHR/NDmcr-cp rats. Pathology 2010, 42, 66–72. [Google Scholar] [CrossRef]
- Zhang, Q.W.; Lin, L.G.; Ye, W.C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef]
- Cifá, D.; Skrt, M.; Pittia, P.; Di Mattia, C.; Poklar Ulrih, N. Enhanced yield of oleuropein from olive leaves using ultrasound-assisted extraction. Food Sci. Nutr. 2018, 6, 1128–1137. [Google Scholar] [CrossRef]
- Alothman, M.; Bhat, R.; Karim, A.A. Antioxidant capacity and phenolic content of selected tropical fruits from Malaysia, extracted with different solvents. Food Chem. 2009, 115, 785–788. [Google Scholar] [CrossRef]
- Wang, B.; Qu, J.; Luo, S.; Feng, S.; Li, T.; Yuan, M.; Huang, Y.; Liao, J.; Yang, R.; Ding, C. Optimization of Ultrasound-Assisted Extraction of Flavonoids from Olive (Olea europaea) Leaves, and Evaluation of Their Antioxidant and Anticancer Activities. Molecules 2018, 23, 2513. [Google Scholar] [CrossRef]
- Irakli, M.; Chatzopoulou, P.; Ekateriniadou, L. Optimization of ultrasound-assisted extraction of phenolic compounds: Oleuropein, phenolic acids, phenolic alcohols and flavonoids from olive leaves and evaluation of its antioxidant activities. Ind. Crops Prod. 2018, 124, 382–388. [Google Scholar] [CrossRef]
- Da Rosa, G.S.; Vanga, S.K.; Gariepy, Y.; Raghavan, V. Comparison of microwave, ultrasonic and conventional techniques for extraction of bioactive compounds from olive leaves (Olea europaea L.). Innov. Food Sci. Emerg. Technol. 2019, 58, 102234. [Google Scholar] [CrossRef]
- Di Meo, M.C.; De Cristofaro, G.A.; Imperatore, R.; Rocco, M.; Giaquinto, D.; Palladino, A.; Zotti, T.; Vito, P.; Paolucci, M.; Varricchio, E. Microwave-Assisted Extraction of Olive Leaf from Five Italian Cultivars: Effects of Harvest-Time and Extraction Conditions on Phenolic Compounds and In Vitro Antioxidant Properties. ACS Food Sci. Technol. 2022, 2, 31–40. [Google Scholar] [CrossRef]
- Rosa, A.D.; Junges, A.; Fernandes, I.A.; Cansian, R.L.; Corazza, M.L.; Franceschi, E.; Backes, G.T.; Valduga, E. High pressure extraction of olive leaves (Olea europaea): Bioactive compounds, bioactivity and kinetic modelling. J Food Sci Technol 2019, 56, 3864–3876. [Google Scholar] [CrossRef]
- Caballero, A.S.; Romero-García, J.M.; Castro, E.; Cardona, C.A. Supercritical fluid extraction for enhancing polyphenolic compounds production from olive waste extracts. J. Chem. Technol. Biotechnol. 2020, 95, 356–362. [Google Scholar] [CrossRef]
- Şahin, S.; Samlı, R. Optimization of olive leaf extract obtained by ultrasound-assisted extraction with response surface methodology. Ultrason. Sonochem. 2013, 20, 595–602. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.G.; Meullemiestre, A.; Fabiano-Tixier, A.S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Giacometti, J.; Žauhar, G.; Žuvić, M. Optimization of Ultrasonic-Assisted Extraction of Major Phenolic Compounds from Olive Leaves (Olea europaea L.) Using Response Surface Methodology. Foods 2018, 7, 149. [Google Scholar] [CrossRef]
- Mollace, R.; Macrì, R.; Tavernese, A.; Gliozzi, M.; Musolino, V.; Carresi, C.; Maiuolo, J.; Fini, M.; Volterrani, M.; Mollace, V. Comparative Effect of Bergamot Polyphenolic Fraction and Red Yeast Rice Extract in Rats Fed a Hyperlipidemic Diet: Role of Antioxidant Properties and PCSK9 Expression. Nutrients 2022, 14, 477. [Google Scholar] [CrossRef]
- Araniti, F.; Marrelli, M.; Lupini, A.; Mercati, F.; Statti, G.A.; Abenavoli, M.R. Phytotoxic activity of Cachrys pungens Jan, a mediterranean species: Separation, identification and quantification of potential allelochemicals. Acta Physiol. Plant. 2014, 36, 1071–1083. [Google Scholar] [CrossRef]
- Rebelo, S.L.; Moniz, T.; Medforth, C.J.; de Castro, B.; Rangel, M. EPR spin trapping studies of H2O2 activation in metaloporphyrin catalyzed oxygenation reactions: Insights on the biomimetic mechanism. Mol. Catal. 2019, 475, 110500. [Google Scholar] [CrossRef]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of Cell Viability by the MTT Assay. Cold Spring Harb. Protoc. 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- De Chiara, F.; Ferret-Miñana, A.; Fernández-Costa, J.M.; Senni, A.; Jalan, R.; Ramón-Azcón, J. Fatty Hepatocytes Induce Skeletal Muscle Atrophy In Vitro: A New 3D Platform to Study the Protective Effect of Albumin in Non-Alcoholic Fatty Liver. Biomedicines 2022, 10, 958. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Kurachi, M.; Yamaguchi, K.; Oda, T. Stimulation of multiple cytokine production in mice by alginate oligosaccharides following intraperitoneal administration. Carbohydr. Res. 2007, 342, 1133–1137. [Google Scholar] [CrossRef]
- Abaza, L.; Taamalli, A.; Nsir, H.; Zarrouk, M. Olive Tree (Olea europeae L.) Leaves: Importance and Advances in the Analysis of Phenolic Compounds. Antioxidants 2015, 4, 682–698. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, A.; Ponnuchamy, M.; Kumar, P.S.; Kapoor, A.; Vo, D.N.; Prabhakar, S. Techniques and modeling of polyphenol extraction from food: A review. Env. Chem. Lett. 2021, 19, 3409–3443. [Google Scholar] [CrossRef]
- Hossain, M.B.; Brunton, N.P.; Patras, A.; Tiwari, B.; O’Donnell, C.P.; Martin-Diana, A.B.; Barry-Ryan, C. Optimization of ultrasound assisted extraction of antioxidant compounds from marjoram (Origanum majorana L.) using response surface methodology. Ultrason. Sonochem. 2012, 19, 582–590. [Google Scholar] [CrossRef]
- Mousavi, S.A.; Nateghi, L.; Javanmard Dakheli, M.; Ramezan, Y.; Piravi-Vanak, Z. Maceration and ultrasound-assisted methods used for extraction of phenolic compounds and antioxidant activity from Ferulago angulata. J. Food Process. Preserv. 2022, 46, e16356. [Google Scholar] [CrossRef]
- Şahin, S.; Bilgin, M. Study on Oleuropein Extraction from Olive Tree (Olea europaea) Leaves by means of SFE: Comparison of Water and Ethanol as Co-Solvent. Sep. Sci. Technol. 2012, 47, 2391–2398. [Google Scholar] [CrossRef]
- Cho, W.Y.; Kim, D.H.; Lee, H.J.; Yeon, S.J.; Lee, C.H. Journal of food quality evaluation of effect of extraction solvent on selected properties of olive leaf extract. J. Food Qual. 2020, 2020, 3013649. [Google Scholar] [CrossRef]
- Zhang, C.; Xin, X.; Zhang, J.; Zhu, S.; Niu, E.; Zhou, Z.; Liu, D. Comparative Evaluation of the Phytochemical Profiles and Antioxidant Potentials of Olive Leaves from 32 Cultivars Grown in China. Molecules 2022, 27, 1292. [Google Scholar] [CrossRef]
- Pasković, I.; Lukić, I.; Žurga, P.; Majetić Germek, V.; Brkljača, M.; Koprivnjak, O.; Major, N.; Grozić, K.; Franić, M.; Ban, D. Temporal Variation of Phenolic and Mineral Composition in Olive Leaves Is Cultivar Dependent. Plants 2020, 9, 1099. [Google Scholar] [CrossRef]
- Nicolì, F.; Negro, C.; Vergine, M.; Aprile, A.; Nutricati, E.; Sabella, E.; Miceli, A.; Luvisi, A.; De Bellis, L. Evaluation of Phytochemical and Antioxidant Properties of 15 Italian Olea europaea L. Cultivar Leaves. Molecules 2019, 24, 1998. [Google Scholar] [CrossRef]
- Ranalli, A.; Contento, S.; Lucera, L.; Di Febo, M.; Marchegiani, D.; Di Fonzo, V. Factors affecting the contents of iridoid oleuropein in olive leaves (Olea europaea L.). J. Agric. Food Chem. 2006, 54, 434–440. [Google Scholar] [CrossRef]
- Vita, F.; Franchina, F.A.; Taiti, C.; Locato, V.; Pennazza, G.; Santonico, M.; Purcaro, G.; De Gara, L.; Mancuso, S.; Mondello, L.; et al. Environmental conditions influence the biochemical properties of the fruiting bodies of Tuber magnatum Pico. Sci. Rep. 2018, 8, 7243. [Google Scholar] [CrossRef]
- Caporali, S.; De Stefano, A.; Calabrese, C.; Giovannelli, A.; Pieri, M.; Savini, I.; Tesauro, M.; Bernardini, S.; Minieri, M.; Terrinoni, A. Anti-Inflammatory and Active Biological Properties of the Plant-Derived Bioactive Compounds Luteolin and Luteolin 7-Glucoside. Nutrients 2022, 14, 1155. [Google Scholar] [CrossRef]
- Sá, C.; Oliveira, A.R.; Machado, C.; Azevedo, M.; Pereira-Wilson, C. Effects on Liver Lipid Metabolism of the Naturally Occurring Dietary Flavone Luteolin-7-glucoside. Evid. Based Complement. Altern. Med. 2015, 2015, 647832. [Google Scholar] [CrossRef]
- Yonekura-Sakakibara, K.; Higashi, Y.; Nakabayashi, R. The Origin and Evolution of Plant Flavonoid Metabolism. Front. Plant Sci. 2019, 10, 943. [Google Scholar] [CrossRef]
- Talhaoui, N.; Gómez-Caravaca, A.M.; León, L.; De la Rosa, R.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Determination of phenolic compounds of ‘Sikitita’ olive leaves by HPLC-DAD-TOF-MS. Comparison with its parents ‘Arbequina’ and ‘Picual’ olive leaves. LWT-Food Sci. Technol. 2014, 58, 28–34. [Google Scholar] [CrossRef]
- Olmo-García, L.; Bajoub, A.; Benlamaalam, S.; Hurtado-Fernández, E.; Bagur-González, M.G.; Chigr, M.; Mbarki, M.; Fernández-Gutiérrez, A.; Carrasco-Pancorbo, A. Establishing the Phenolic Composition of Olea europaea L. Leaves from Cultivars Grown in Morocco as a Crucial Step Towards Their Subsequent Exploitation. Molecules 2018, 23, 2524. [Google Scholar] [CrossRef]
- Benincasa, C.; Romano, E.; Pellegrino, M.; Perri, E. Characterization of Phenolic Profiles of Italian Single Cultivar Olive Leaves (Olea europaea L.) by Mass Spectrometry. Mass. Spectrom. Purif. Tech. 2018, 4, 124. [Google Scholar] [CrossRef]
- Altiok, E.; Bayçin, D.; Bayraktar, O.; Ülkü, S. Isolation of polyphenols from the extracts of olive leaves (Olea europaea L.) by adsorption on silk fibroin. Sep. Purif. Technol. 2008, 62, 342–348. [Google Scholar] [CrossRef]
- Kelly, N.; Kelly, A.L.; O’Mahony, J.A. Strategies for enrichment and purification of polyphenols from fruit-based materials. Trends Food Sci. Technol. 2019, 83, 248–258. [Google Scholar] [CrossRef]
- Díaz-Reinoso, B.; Moure, A.; González, J.A.; Domínguez, H. A membrane process for the recovery of a concentrated phenolic product from white vinasses. Chem. Eng. J. 2017, 327, 210–217. [Google Scholar] [CrossRef]
- Tapia-Quirós, P.; Montenegro-Landívar, M.F.; Reig, M.; Vecino, X.; Cortina, J.L.; Saurina, J.; Granados, M. Recovery of Polyphenols from Agri-Food By-Products: The Olive Oil and Winery Industries Cases. Foods 2022, 11, 362. [Google Scholar] [CrossRef] [PubMed]
- Ntougias, S.; Bourtzis, K.; Tsiamis, G. The microbiology of olive mill wastes. Biomed. Res. Int. 2013, 2013, 784591. [Google Scholar] [CrossRef]
- Pérez-Larrán, P.; Díaz-Reinoso, B.; Moure, A.; Alonso, J.L.; Domínguez, H. Adsorption technologies to recover and concentrate food polyphenols. Curr. Opin. Food Sci. 2018, 23, 165–172. [Google Scholar] [CrossRef]
- Silva, M.; Castellanos, L.; Ottens, M. Capture and Purification of Polyphenols Using Functionalized Hydrophobic Resins. Ind. Eng. Chem. Res. 2018, 57, 5359–5369. [Google Scholar] [CrossRef]
- Li, C.; Zheng, Y.; Wang, X.; Feng, S.; Di, D. Simultaneous separation and purification of flavonoids and oleuropein from Olea europaea L. (olive) leaves using macroporous resin. J. Sci. Food Agric. 2011, 91, 2826–2834. [Google Scholar] [CrossRef]
- Liu, B.; Liu, J.; Huang, D.; Pei, D.; Di, D. Separation and purification of hydroxytysol and oleuropein from Olea europaea L. (olive) leaves using macroporous resins and a novel solvent system. J. Sep. Sci. 2020, 43, 2619–2625. [Google Scholar] [CrossRef]
- Marrelli, M.; Amodeo, V.; Puntillo, D.; Statti, G.; Conforti, F. In vitro antioxidant and anti-denaturation effects of Buglossoides purpurocaerulea (L.) I. M. Johnst. fruit extract. Nat. Prod. Res. 2022, 1–4, Advance online publication. [Google Scholar] [CrossRef]
- Pu-jun, X.; Huang, L.; Zhang, C.; Yao-lei, Z. Phenolic compositions, and antioxidant performance of olive leaf and fruit (Olea europaea L.) extracts and their structure–activity relationships. J. Funct. Foods 2015, 16, 460–471. [Google Scholar]
- Moudache, M.; Colón, M.; Nerín, C.; Zaidi, F. Phenolic content and antioxidant activity of olive by-products and antioxidant film containing olive leaf extract. Food Chem. 2016, 212, 521–527. [Google Scholar] [CrossRef]
- Akar, Z.; Küçük, M.; Doğan, H. A new colorimetric DPPH• scavenging activity method with no need for a spectrophotometer applied on synthetic and natural antioxidants and medicinal herbs. J. Enzym. Inhib. Med. Chem. 2017, 32, 640–647. [Google Scholar] [CrossRef]
- Gęgotek, A.; Skrzydlewska, E. Antioxidative and Anti-Inflammatory Activity of Ascorbic Acid. Antioxidants 2022, 11, 1993. [Google Scholar] [CrossRef]
- Giordano, A.; Morales-Tapia, P.; Moncada-Basualto, M.; Pozo-Martínez, J.; Olea-Azar, C.; Nesic, A.; Cabrera-Barjas, G. Polyphenolic Composition and Antioxidant Activity (ORAC, EPR and Cellular) of Different Extracts of Argylia radiata Vitroplants and Natural Roots. Molecules 2022, 27, 610. [Google Scholar] [CrossRef]
- Mura, F.; Silva, T.; Castro, C.; Borges, F.; Zuñiga, M.C.; Morales, J.; Olea-Azar, C. New insights into the antioxidant activity of hydroxycinnamic and hydroxybenzoic systems: Spectroscopic, electrochemistry, and cellular studies. Free Radic. Res. 2014, 48, 1473–1484. [Google Scholar] [CrossRef]
- Çalişkan, B.; Çalişkan, A.C. EPR Analysis of Antioxidant Compounds. In Free Radicals, Antioxidants and Diseases; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Suzen, S.; Gurer-Orhan, H.; Saso, L. Detection of Reactive Oxygen and Nitrogen Species by Electron Paramagnetic Resonance (EPR) Technique. Molecules 2017, 22, 181. [Google Scholar] [CrossRef]
- Baj, A.; Cedrowski, J.; Olchowik-Grabarek, E.; Ratkiewicz, A.; Witkowski, S. Synthesis, DFT Calculations, and In Vitro Antioxidant Study on Novel Carba-Analogs of Vitamin E. Antioxidants 2019, 8, 589. [Google Scholar] [CrossRef]
- Collin, F. Chemical Basis of Reactive Oxygen Species Reactivity and Involvement in Neurodegenerative Diseases. Int. J. Mol. Sci. 2019, 20, 2407. [Google Scholar] [CrossRef]
- Gullón, B.; Gullón, P.; Eibes, G.; Cara, C.; De Torres, A.; López-Linares, J.C.; Ruiz, E.; Castro, E. Valorisation of olive agro-industrial by-products as a source of bioactive compounds. Sci. Total Environ. 2018, 645, 533–542. [Google Scholar] [CrossRef]
- Nani, A.; Murtaza, B.; Sayed Khan, A.; Khan, N.A.; Hichami, A. Antioxidant and Anti-Inflammatory Potential of Polyphenols Contained in Mediterranean Diet in Obesity: Molecular Mechanisms. Molecules 2021, 26, 985. [Google Scholar] [CrossRef] [PubMed]
- Özcan, M.M.; Matthäus, B. A review: Benefit and bioactive properties of olive (Olea europaea L.) leaves. Eur. Food Res. Technol. 2016, 243, 89–99. [Google Scholar] [CrossRef]
- Umeno, A.; Takashima, M.; Murotomi, K.; Nakajima, Y.; Koike, T.; Matsuo, T.; Yoshida, Y. Radical-scavenging Activity and Antioxidative Effects of Olive Leaf Components Oleuropein and Hydroxytyrosol in Comparison with Homovanillic Alcohol. J. Oleo. Sci. 2015, 64, 793–800. [Google Scholar] [CrossRef]
- Valavanidis, A.; Nisiotou, C.; Papageorgiou, Y.; Kremli, I.; Satravelas, N.; Zinieris, N.; Zygalaki, H. Comparison of the radical scavenging potential of polar and lipidic fractions of olive oil and other vegetable oils under normal conditions and after thermal treatment. J. Agric. Food Chem. 2004, 52, 2358–2365. [Google Scholar] [CrossRef] [PubMed]
- Schlupper, D.; Giesa, S.; Gebhardt, R. Influence of biotransformation of luteolin, luteolin 7-O-glucoside, 3’,4’-dihydroxyflavone and apigenin by cultured rat hepatocytes on antioxidative capacity and inhibition of EGF receptor tyrosine kinase activity. Planta Med. 2006, 72, 596–603. [Google Scholar] [CrossRef]
- Carresi, C.; Musolino, V.; Gliozzi, M.; Maiuolo, J.; Mollace, R.; Nucera, S.; Maretta, A.; Sergi, D.; Muscoli, S.; Gratteri, S.; et al. Anti-oxidant effect of bergamot polyphenolic fraction counteracts doxorubicin-induced cardiomyopathy: Role of autophagy and c-kitposCD45negCD31neg cardiac stem cell activation. J. Mol. Cell Cardiol. 2018, 119, 10–18. [Google Scholar] [CrossRef]
- Fki, I.; Sayadi, S.; Mahmoudi, A.; Daoued, I.; Marrekchi, R.; Ghorbel, H. Comparative Study on Beneficial Effects of Hydroxytyrosol- and Oleuropein-Rich Olive Leaf Extracts on High-Fat Diet-Induced Lipid Metabolism Disturbance and Liver Injury in Rats. Biomed. Res. Int. 2020, 2020, 1315202. [Google Scholar] [CrossRef]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef]
- Hoang, N.A.; Richter, F.; Schubert, M.; Lorkowski, S.; Klotz, L.O.; Steinbrenner, H. Differential capability of metabolic substrates to promote hepatocellular lipid accumulation. Eur. J. Nutr. 2019, 58, 3023–3034. [Google Scholar] [CrossRef]
- Roh, J.S.; Lee, H.; Lim, J.; Kim, J.; Yang, H.; Yoon, Y.; Shin, S.S.; Yoon, M. Effect of Gangjihwan on hepatic steatosis and inflammation in high fat diet-fed mice. J. Ethnopharmacol. 2017, 206, 315–326. [Google Scholar] [CrossRef]
- Duan, Y.; Pan, X.; Luo, J.; Xiao, X.; Li, J.; Bestman, P.L.; Luo, M. Association of Inflammatory Cytokines With Non-Alcoholic Fatty Liver Disease. Front. Immunol. 2022, 13, 880298. [Google Scholar] [CrossRef]
- Lu, S.; Wang, Y.; Liu, J. Tumor necrosis factor-α signaling in nonalcoholic steatohepatitis and targeted therapies. J. Genet. Genom. 2022, 49, 269–278. [Google Scholar] [CrossRef]
- Mu, W.; Cheng, X.F.; Liu, Y.; Lv, Q.Z.; Liu, G.L.; Zhang, J.G.; Li, X.Y. Potential Nexus of Non-alcoholic Fatty Liver Disease and Type 2 Diabetes Mellitus: Insulin Resistance Between Hepatic and Peripheral Tissues. Front. Pharm. 2018, 9, 1566. [Google Scholar] [CrossRef]
- Schroeder, R.A.; Gu, J.S.; Kuo, P.C. Interleukin 1beta-stimulated production of nitric oxide in rat hepatocytes is mediated through endogenous synthesis of interferon gamma. Hepatology 1998, 27, 711–719. [Google Scholar] [CrossRef]
- Després, J.P.; Lemieux, I. Abdominal obesity and metabolic syndrome. Nature 2006, 444, 881–887. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, X.; Liu, P.; Chen, X.; Zhang, J.; Zhang, H.; Li, S.; Chen, Y.; Song, X.; Wang, J.; et al. GCSF deficiency attenuates nonalcoholic fatty liver disease through regulating GCSFR-SOCS3-JAK-STAT3 pathway and immune cells infiltration. Am. J. Physiol. Gastrointest. Liver Physiol. 2021, 320, G531–G542. [Google Scholar] [CrossRef]
- Rafiei, H.; Omidian, K.; Bandy, B. Comparison of dietary polyphenols for protection against molecular mechanisms underlying nonalcoholic fatty liver disease in a cell model of steatosis. Mol. Nutr. Food Res. 2017, 61, 1600781. [Google Scholar] [CrossRef]
- Mirarchi, A.; Mare, R.; Musolino, V.; Nucera, S.; Mollace, V.; Pujia, A.; Montalcini, T.; Romeo, S.; Maurotti, S. Bergamot Polyphenol Extract Reduces Hepatocyte Neutral Fat by Increasing Beta-Oxidation. Nutrients 2022, 14, 3434. [Google Scholar] [CrossRef]
- Vidyashankar, S.; Sandeep Varma, R.; Patki, P.S. Quercetin ameliorate insulin resistance and up-regulates cellular antioxidants during oleic acid induced hepatic steatosis in HepG2 cells. Toxicol. Vitr. 2013, 27, 945–953. [Google Scholar] [CrossRef]
- Park, J.Y.; Kim, Y.; Im, J.A.; Lee, H. Oligonol suppresses lipid accumulation and improves insulin resistance in a palmitate-induced in HepG2 hepatocytes as a cellular steatosis model. BMC Complement. Altern. Med. 2015, 15, 185. [Google Scholar] [CrossRef]
- Izdebska, M.; Piątkowska-Chmiel, I.; Korolczuk, A.; Herbet, M.; Gawrońska-Grzywacz, M.; Gieroba, R.; Sysa, M.; Czajkowska-Bania, K.; Cygal, M.; Korga, A.; et al. The beneficial effects of resveratrol on steatosis and mitochondrial oxidative stress in HepG2 cells. Can. J. Physiol. Pharm. 2017, 95, 1442–1453. [Google Scholar] [CrossRef] [PubMed]
- Svobodova, M.; Andreadou, I.; Skaltsounis, A.L.; Kopecky, J.; Flachs, P. Oleuropein as an inhibitor of peroxisome proliferator-activated receptor gamma. Genes Nutr. 2014, 9, 376. [Google Scholar] [CrossRef] [PubMed]
- Scarano, F.; Gliozzi, M.; Zito, M.C.; Guarnieri, L.; Carresi, C.; Macrì, R.; Nucera, S.; Scicchitano, M.; Bosco, F.; Ruga, S.; et al. Potential of Nutraceutical Supplementation in the Modulation of White and Brown Fat Tissues in Obesity-Associated Disorders: Role of Inflammatory Signalling. Int. J. Mol. Sci. 2021, 22, 3351. [Google Scholar] [CrossRef] [PubMed]
- De Cicco, P.; Maisto, M.; Tenore, G.C.; Ianaro, A. Olive Leaf Extract, from Olea europaea L., Reduces Palmitate-Induced Inflammation via Regulation of Murine Macrophages Polarization. Nutrients 2020, 12, 3663. [Google Scholar] [CrossRef] [PubMed]
- Gentile, D.; Fornai, M.; Pellegrini, C.; Colucci, R.; Benvenuti, L.; Duranti, E.; Masi, S.; Carpi, S.; Nieri, P.; Nericcio, A.; et al. Luteolin Prevents Cardiometabolic Alterations and Vascular Dysfunction in Mice With HFD-Induced Obesity. Front. Pharm. 2018, 9, 1094. [Google Scholar] [CrossRef]
- Mollace, V.; Scicchitano, M.; Paone, S.; Casale, F.; Calandruccio, C.; Gliozzi, M.; Musolino, V.; Carresi, C.; Maiuolo, J.; Nucera, S.; et al. Hypoglycemic and Hypolipemic Effects of a New Lecithin Formulation of Bergamot Polyphenolic Fraction: A Double Blind, Randomized, Placebo- Controlled Study. Endocr. Metab. Immune Disord. Drug Targets 2019, 19, 136–143. [Google Scholar] [CrossRef]
- Gliozzi, M.; Macrì, R.; Coppoletta, A.R.; Musolino, V.; Carresi, C.; Scicchitano, M.; Bosco, F.; Guarnieri, L.; Cardamone, A.; Ruga, S.; et al. From Diabetes Care to Heart Failure Management: A Potential Therapeutic Approach Combining SGLT2 Inhibitors and Plant Extracts. Nutrients 2022, 14, 3737. [Google Scholar] [CrossRef]
- Gliozzi, M.; Musolino, V.; Bosco, F.; Scicchitano, M.; Scarano, F.; Nucera, S.; Zito, M.C.; Ruga, S.; Carresi, C.; Macrì, R.; et al. Cholesterol homeostasis: Researching a dialogue between the brain and peripheral tissues. Pharm. Res. 2021, 163, 105215. [Google Scholar] [CrossRef]
- Carresi, C.; Gliozzi, M.; Musolino, V.; Scicchitano, M.; Scarano, F.; Bosco, F.; Nucera, S.; Maiuolo, J.; Macrì, R.; Ruga, S.; et al. The Effect of Natural Antioxidants in the Development of Metabolic Syndrome: Focus on Bergamot Polyphenolic Fraction. Nutrients 2020, 12, 1504. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musolino, V.; Macrì, R.; Cardamone, A.; Serra, M.; Coppoletta, A.R.; Tucci, L.; Maiuolo, J.; Lupia, C.; Scarano, F.; Carresi, C.; et al. Nocellara Del Belice (Olea europaea L. Cultivar): Leaf Extract Concentrated in Phenolic Compounds and Its Anti-Inflammatory and Radical Scavenging Activity. Plants 2023, 12, 27. https://doi.org/10.3390/plants12010027
Musolino V, Macrì R, Cardamone A, Serra M, Coppoletta AR, Tucci L, Maiuolo J, Lupia C, Scarano F, Carresi C, et al. Nocellara Del Belice (Olea europaea L. Cultivar): Leaf Extract Concentrated in Phenolic Compounds and Its Anti-Inflammatory and Radical Scavenging Activity. Plants. 2023; 12(1):27. https://doi.org/10.3390/plants12010027
Chicago/Turabian StyleMusolino, Vincenzo, Roberta Macrì, Antonio Cardamone, Maria Serra, Anna Rita Coppoletta, Luigi Tucci, Jessica Maiuolo, Carmine Lupia, Federica Scarano, Cristina Carresi, and et al. 2023. "Nocellara Del Belice (Olea europaea L. Cultivar): Leaf Extract Concentrated in Phenolic Compounds and Its Anti-Inflammatory and Radical Scavenging Activity" Plants 12, no. 1: 27. https://doi.org/10.3390/plants12010027
APA StyleMusolino, V., Macrì, R., Cardamone, A., Serra, M., Coppoletta, A. R., Tucci, L., Maiuolo, J., Lupia, C., Scarano, F., Carresi, C., Nucera, S., Bava, I., Marrelli, M., Palma, E., Gliozzi, M., & Mollace, V. (2023). Nocellara Del Belice (Olea europaea L. Cultivar): Leaf Extract Concentrated in Phenolic Compounds and Its Anti-Inflammatory and Radical Scavenging Activity. Plants, 12(1), 27. https://doi.org/10.3390/plants12010027








