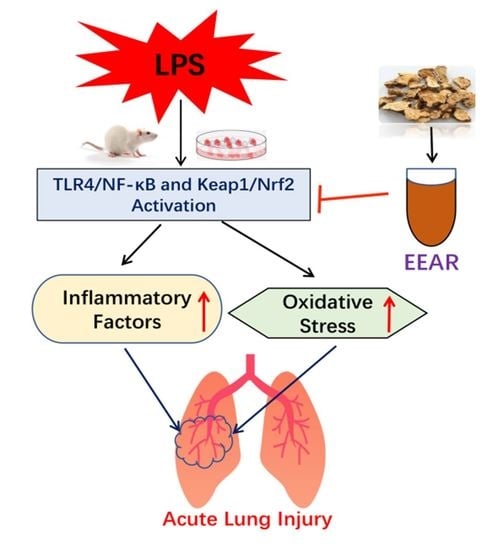
Protective Effects of Atractylodis lancea Rhizoma on Lipopolysaccharide-Induced Acute Lung Injury via TLR4/NF-κB and Keap1/Nrf2 Signaling Pathways In Vitro and In Vivo
Abstract
1. Introduction
2. Results
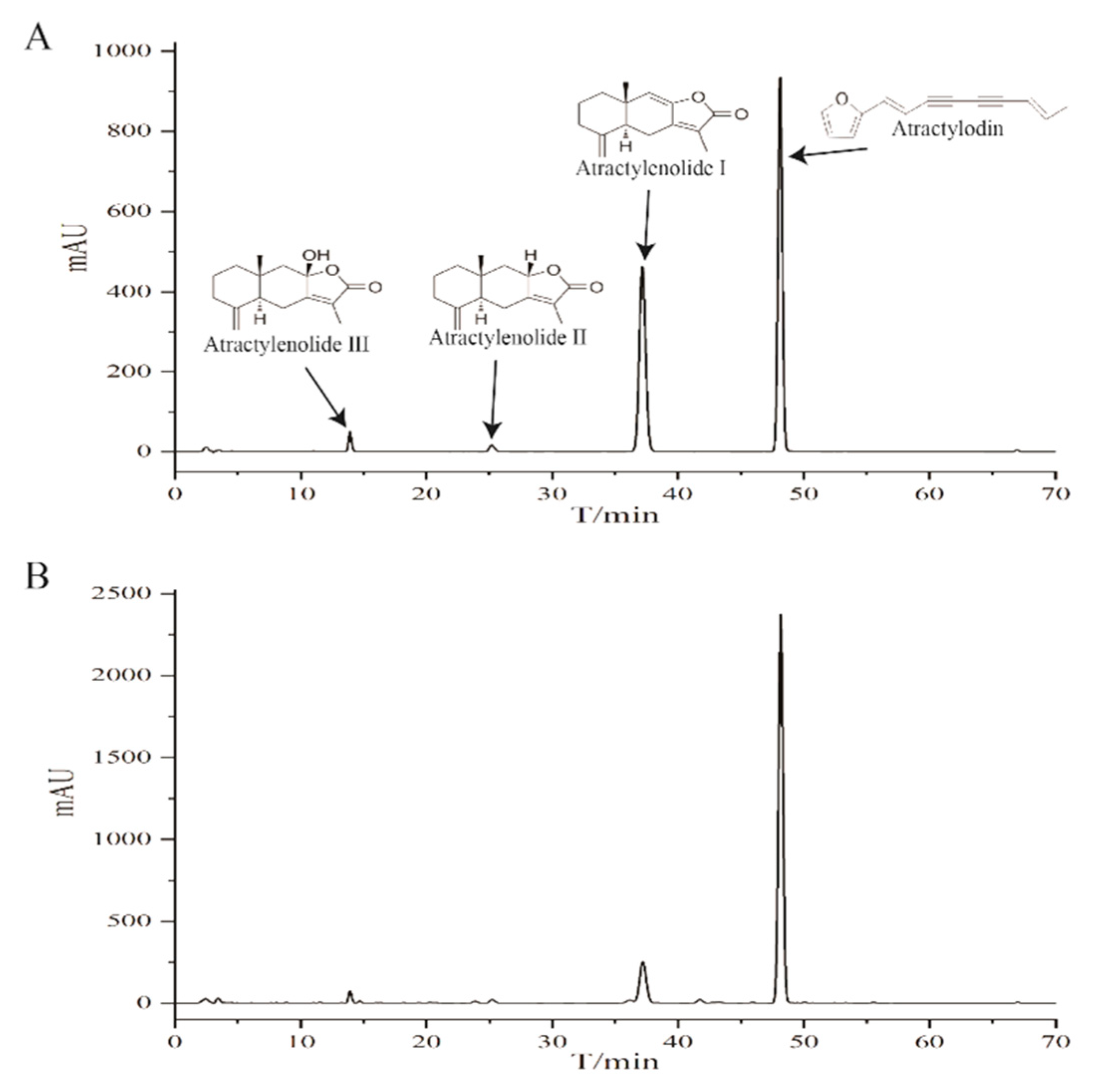
2.1. HPLC Profile of the Chemical Composition of EEAR
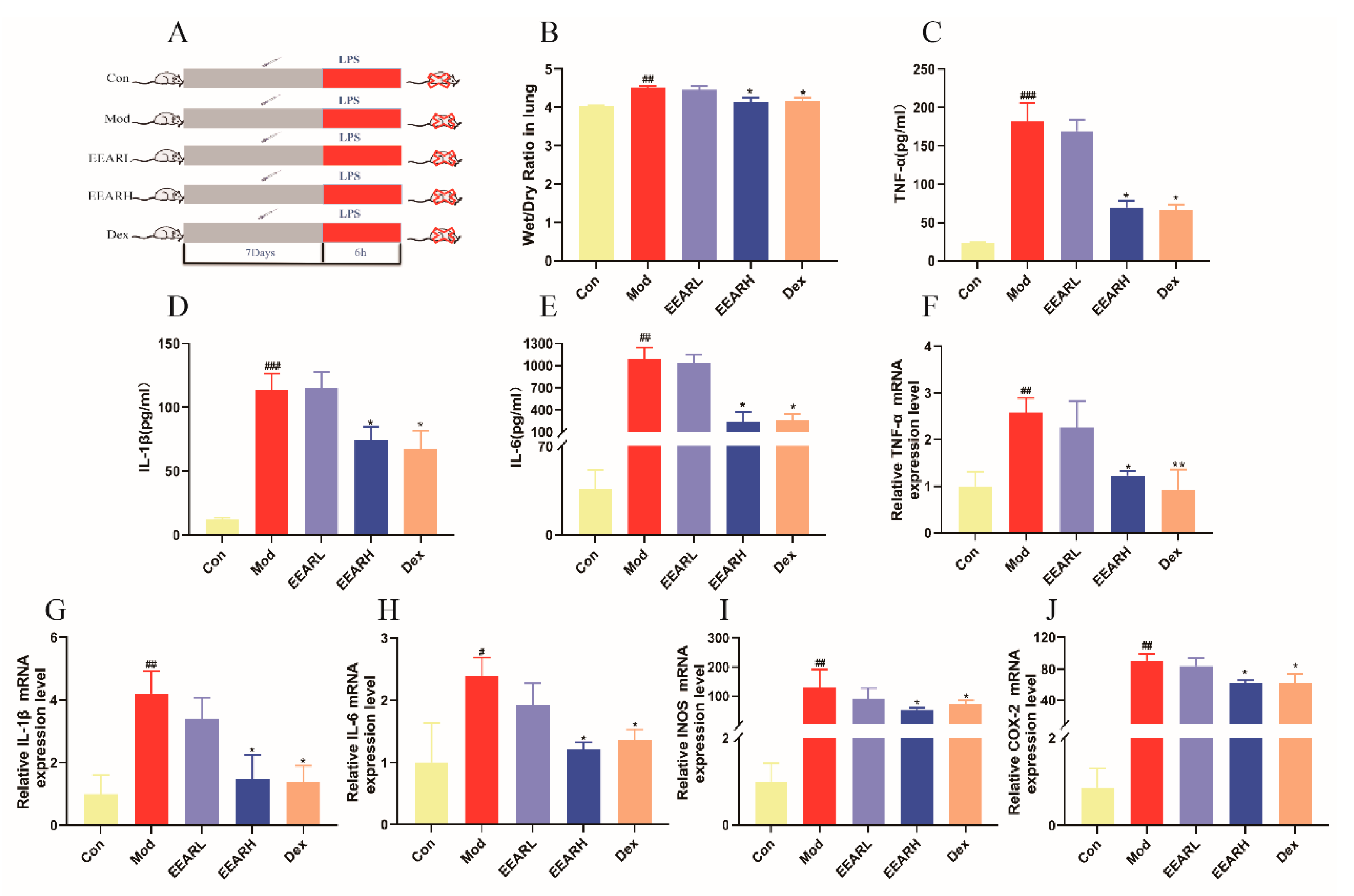
2.2. Effects of EEAR on Wet-Dry (W/D) Specific Gravity and the Inflammatory Response in the Lungs of Rats with ALI
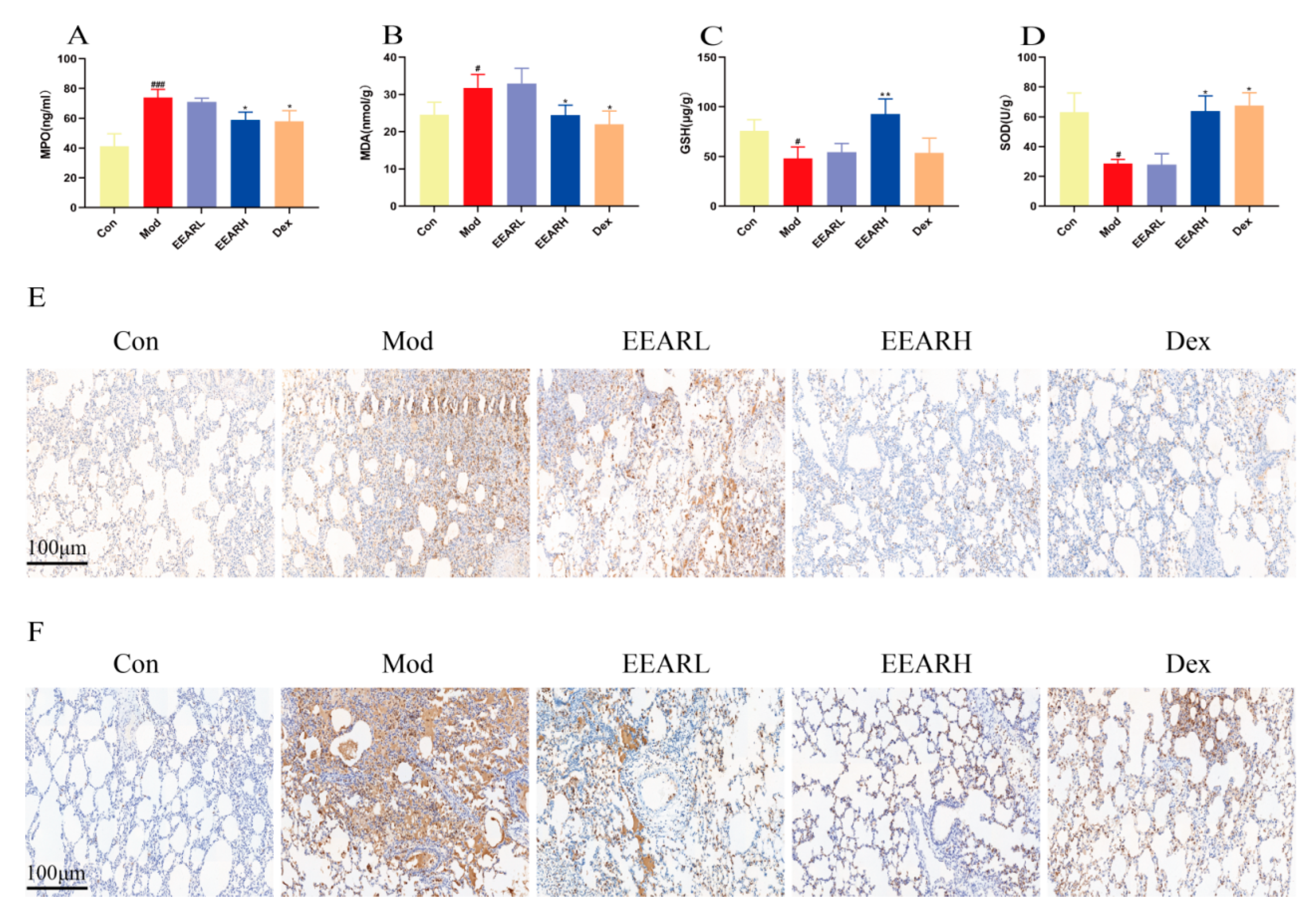
2.3. Effects of EEAR on Neutrophils and Oxidative Stress Levels in the Lungs of Rats with ALI
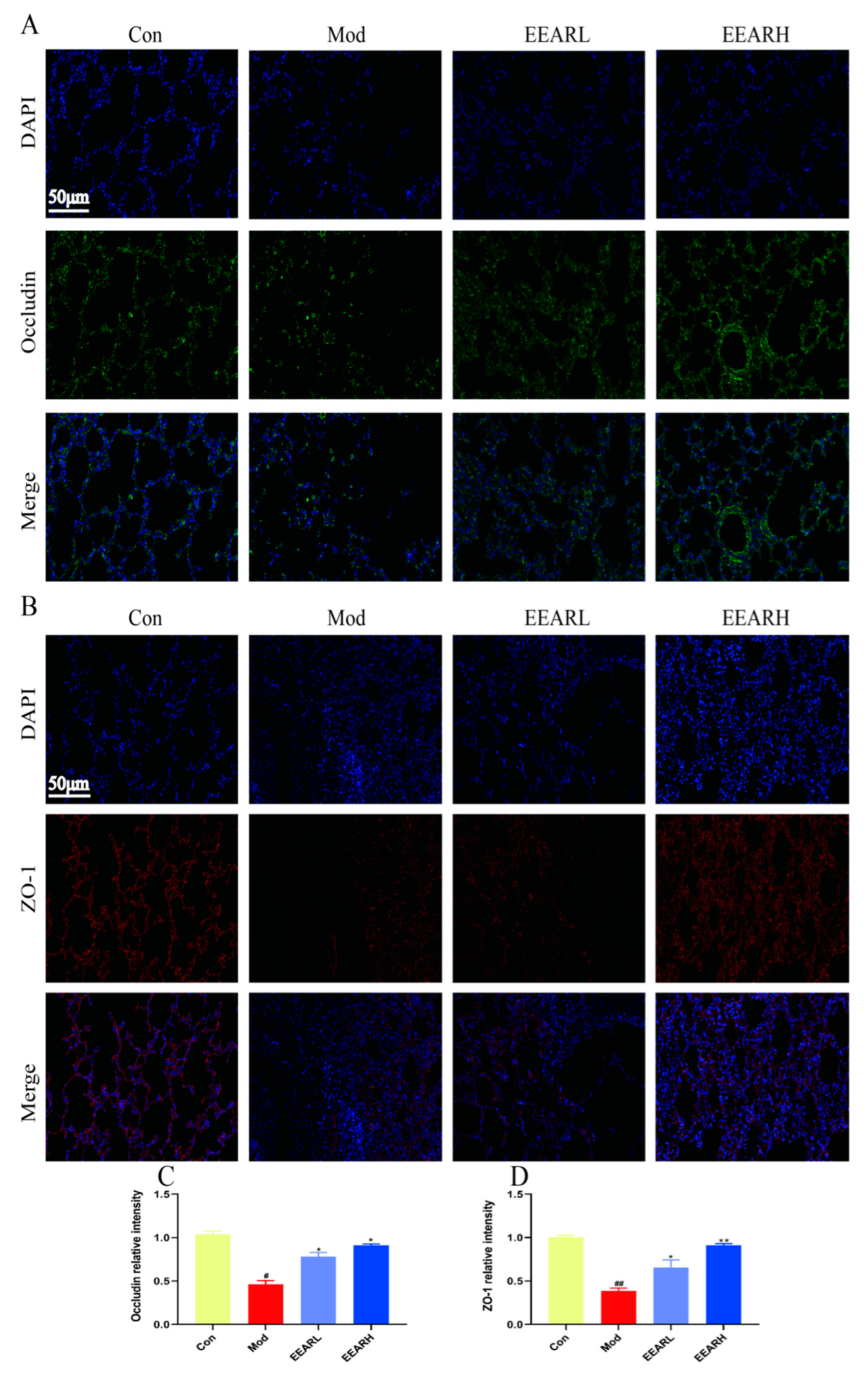
2.4. Effect of EEAR on Lung Barrier Function in Rats with ALI
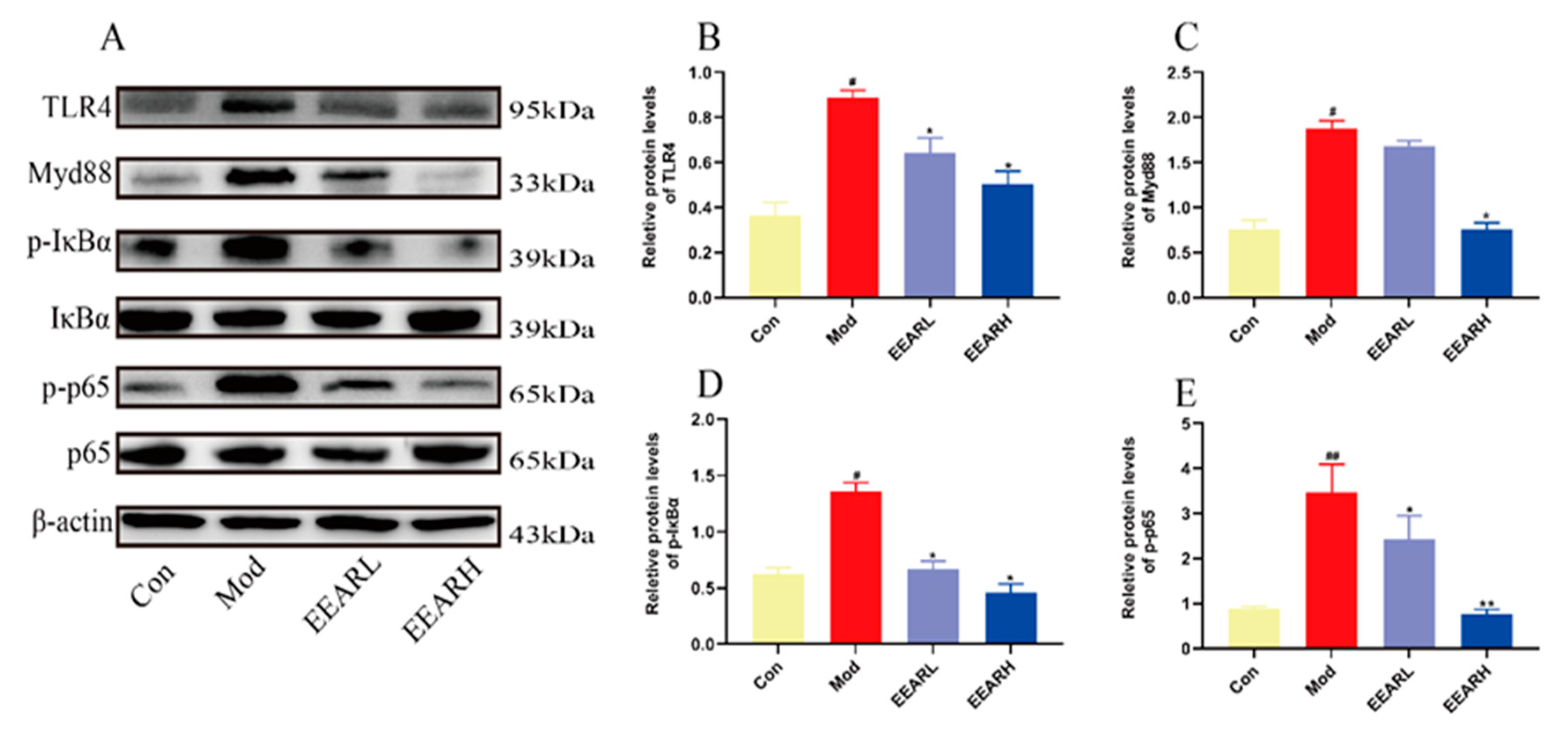
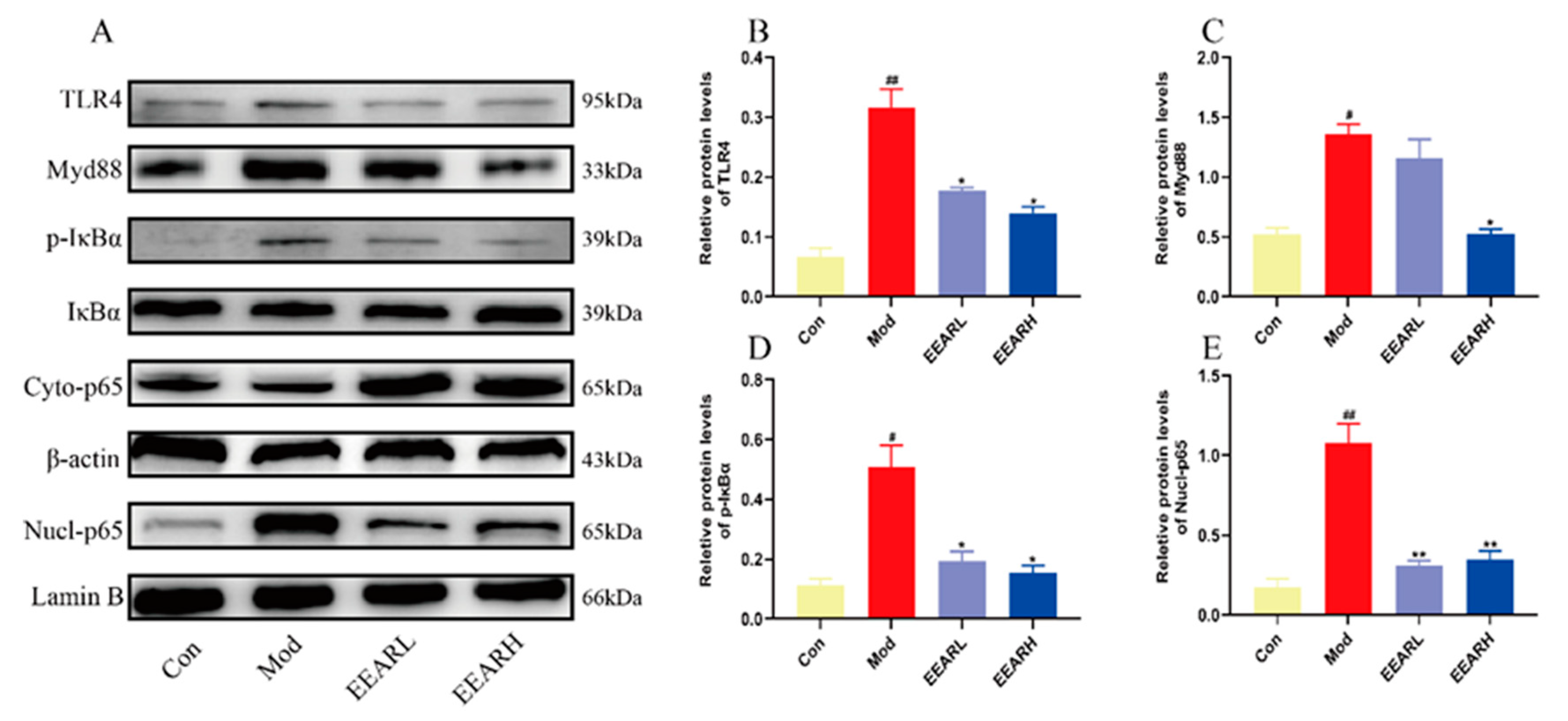
2.5. Effects of EEAR on the TLR4/NF-κB Signaling Pathway
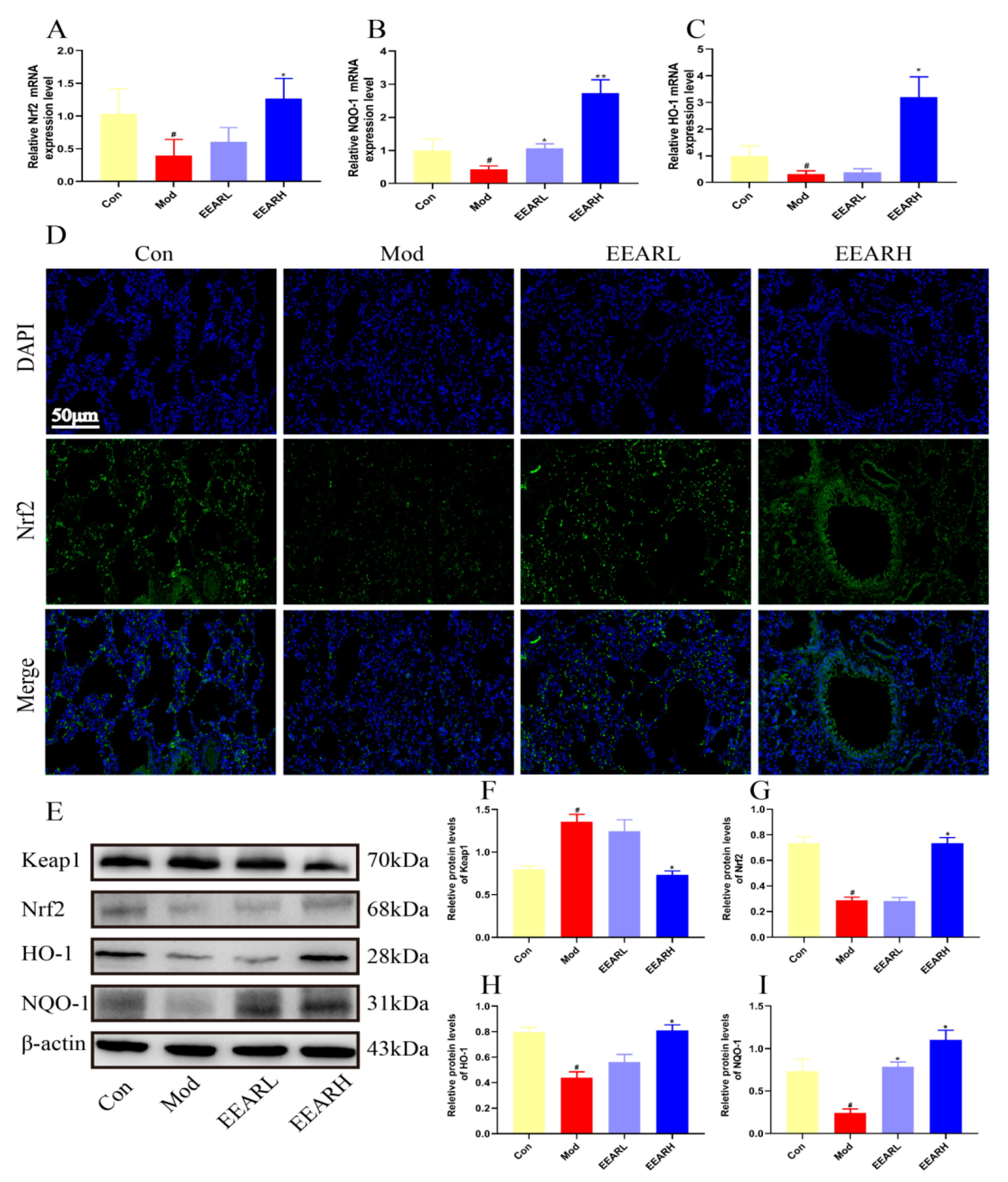
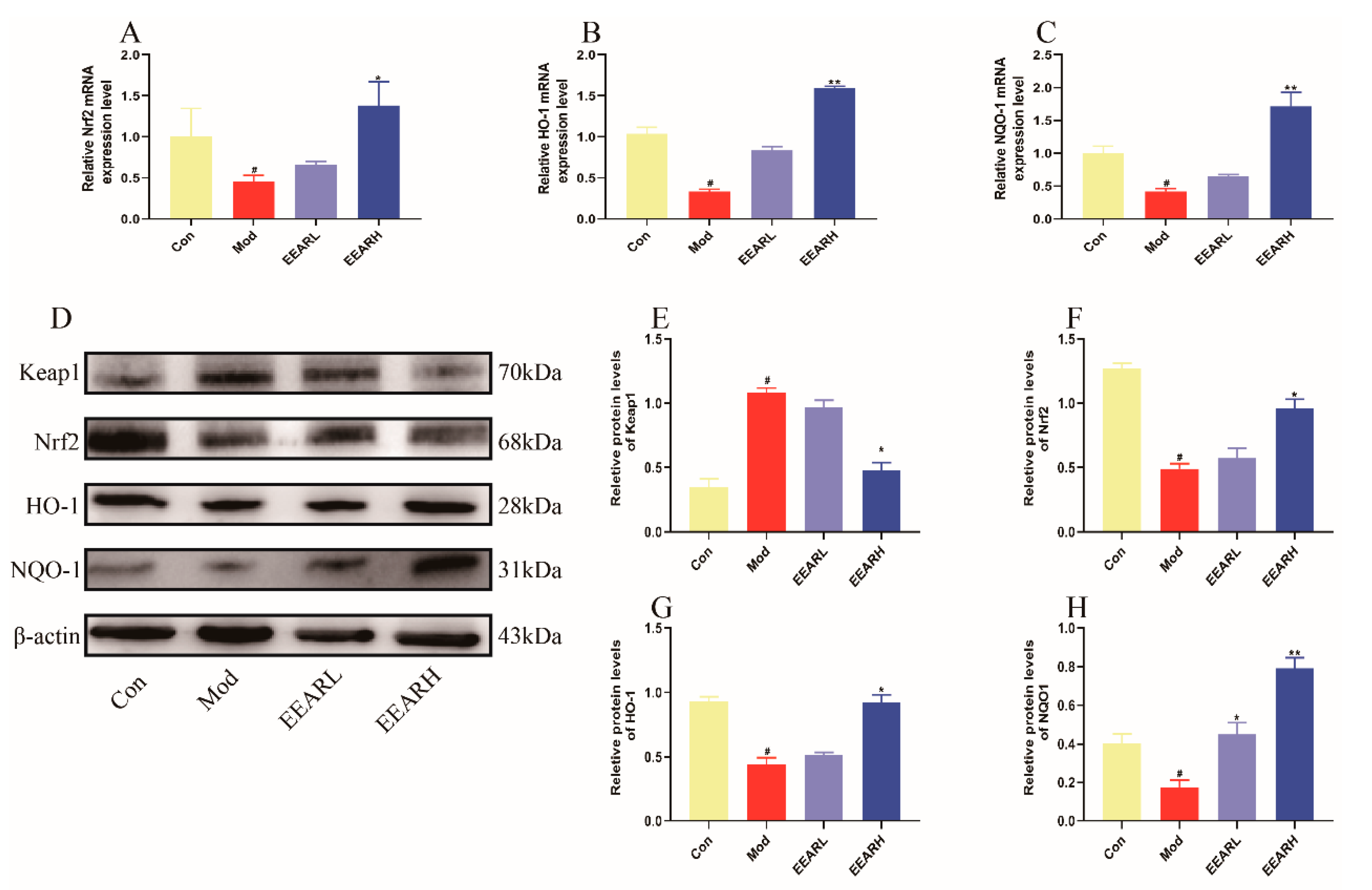
2.6. Effects of EEAR on the Keap1/Nrf2 Signaling Pathway
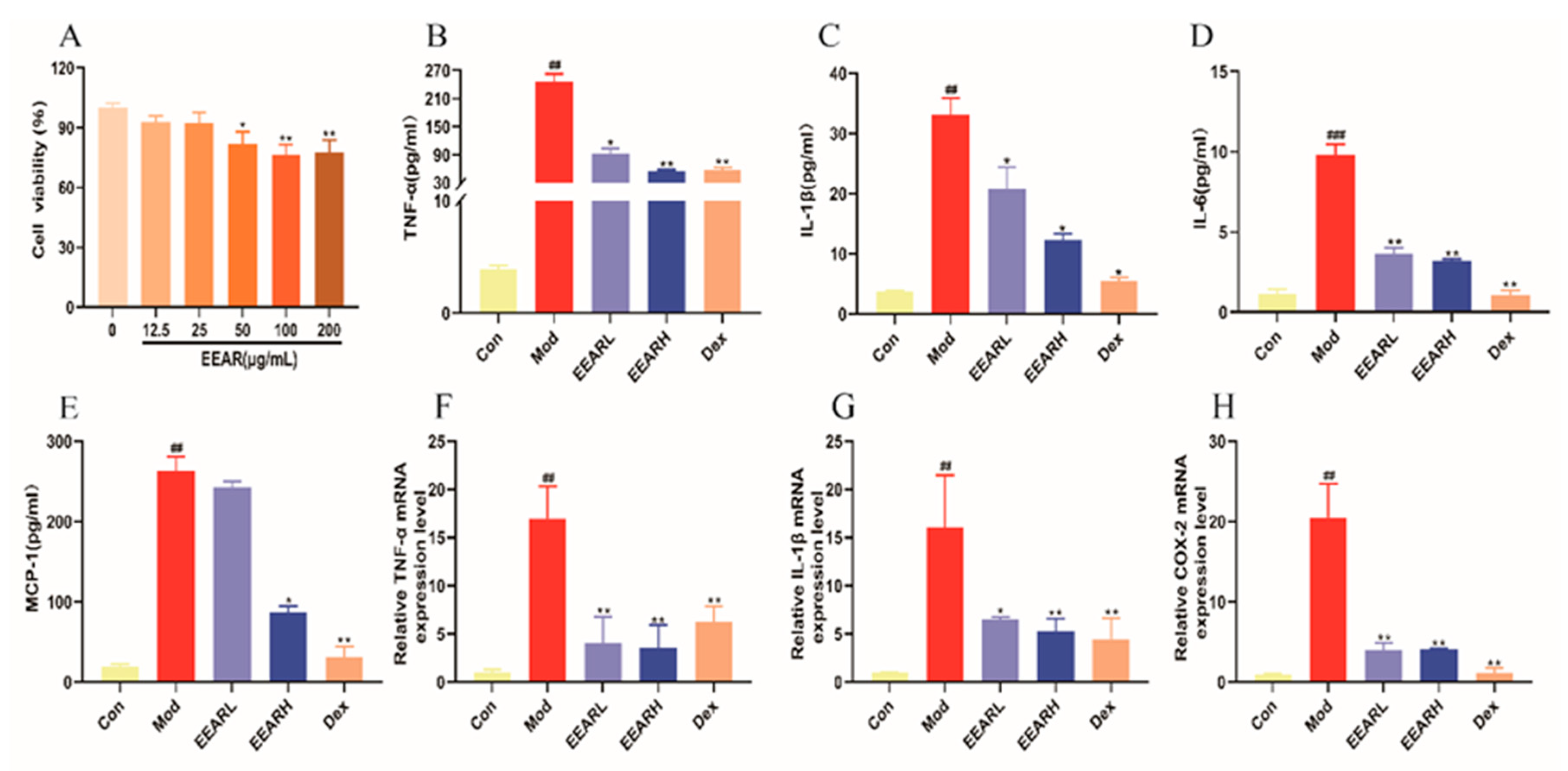
2.7. Effect of EEAR on LPS-Induced Inflammatory Factor Expression in THP-1 Cells In Vitro
2.8. Effects of EEAR on the TLR4/NF-κB Signaling Pathway In Vitro
2.9. Effects of EEAR on the Keap1/Nrf2 Signaling Pathway In Vitro
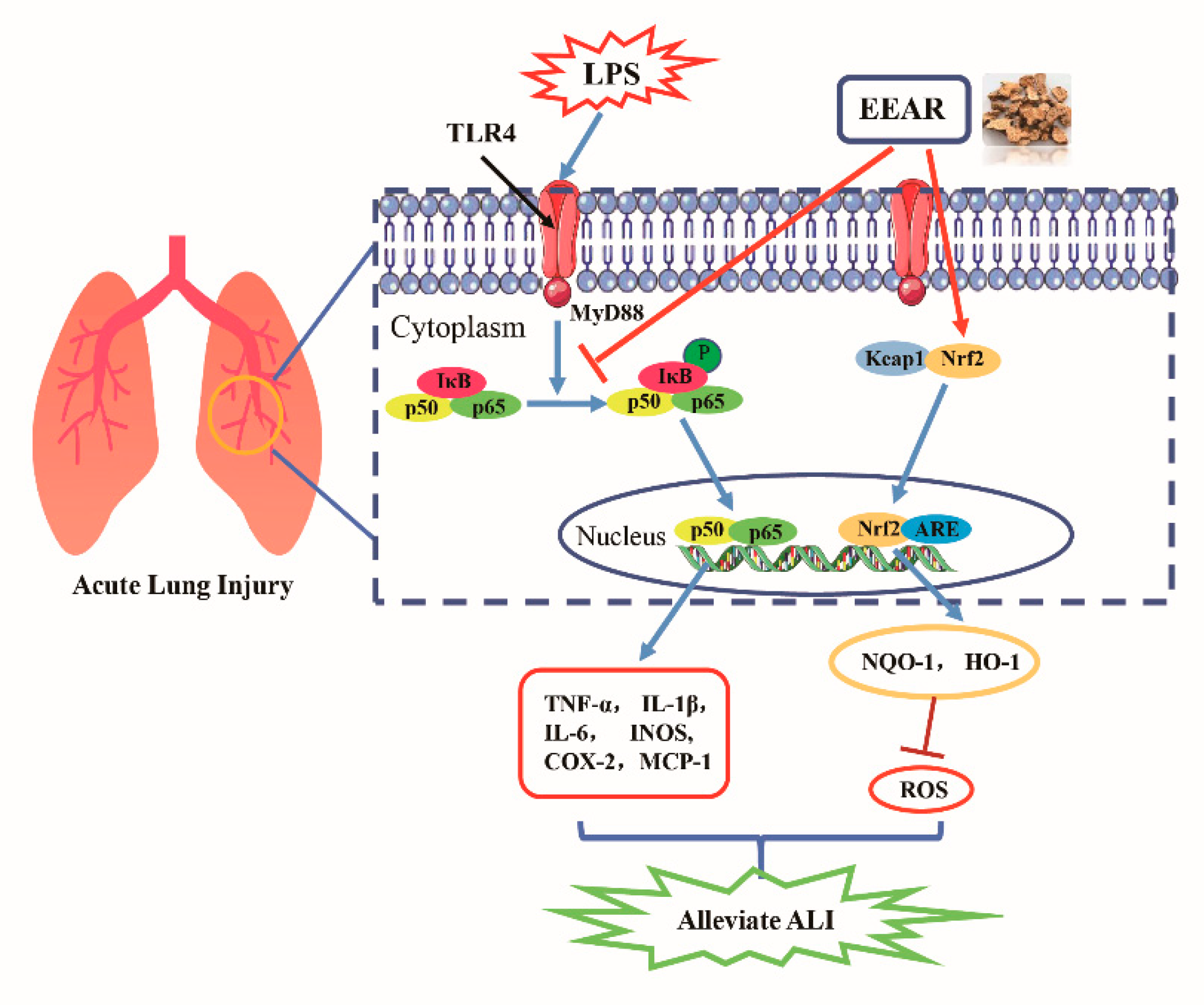
3. Discussion
4. Materials and Methods
4.1. Extract of Atractylodis rhizoma
4.2. Conversion of Dosage
4.3. Sample Preparation and HPLC Chromatographic Conditions
4.4. In Vivo Experimental Design
4.5. Lung Wet/Dry Ratios
4.6. ELISA Assays
4.7. Measurement of MDA, GSH, and SOD Levels in Lung Tissue
4.8. Immunohistochemical Staining
4.9. Immunofluorescence
4.10. RNA Extraction and RT-qPCR
4.11. Cell Culture and CCK-8 Assay
4.12. Western Blot Analysis
4.13. Isolation of Nuclear and Cytosolic Fractions
4.14. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nieman, G.F.; Andrews, P.; Satalin, J.; Wilcox, K.; Kollisch-Singule, M.; Madden, M.; Aiash, H.; Blair, S.J.; Gatto, L.A.; Habashi, N.M. Acute lung injury: How to stabilize a broken lung. Crit. Care 2018, 22, 136. [Google Scholar] [CrossRef] [PubMed]
- Killien, E.Y.; Mills, B.; Watson, R.S.; Vavilala, M.S.; Rivara, F.P. Morbidity and Mortality Among Critically Injured Children With Acute Respiratory Distress Syndrome. Crit. Care Med. 2019, 47, e112–e119. [Google Scholar] [CrossRef] [PubMed]
- Fan, E.; Brodie, D.; Slutsky, A.S. Acute Respiratory Distress Syndrome: Advances in Diagnosis and Treatment. Jama 2018, 319, 698–710. [Google Scholar] [CrossRef] [PubMed]
- Prasertsan, P.; Anuntaseree, W.; Ruangnapa, K.; Saelim, K.; Geater, A. Severity and Mortality Predictors of Pediatric Acute Respiratory Distress Syndrome According to the Pediatric Acute Lung Injury Consensus Conference Definition. Pediatr. Crit. Care Med. 2019, 20, e464–e472. [Google Scholar] [CrossRef]
- Ma, X.; Li, X.; Di, Q.; Zhao, X.; Zhang, R.; Xiao, Y.; Sun, P.; Tang, H.; Quan, J.; Xiao, W.; et al. Natural molecule Munronoid I attenuates LPS-induced acute lung injury by promoting the K48-linked ubiquitination and degradation of TAK1. Biomed. Pharmacother. 2021, 138, 111543. [Google Scholar] [CrossRef]
- Fisher, A.B.; Dodia, C.; Chatterjee, S.; Feinstein, S.I. A Peptide Inhibitor of NADPH Oxidase (NOX2) Activation Markedly Decreases Mouse Lung Injury and Mortality Following Administration of Lipopolysaccharide (LPS). Int. J. Mol. Sci. 2019, 20, 2395. [Google Scholar] [CrossRef]
- Kumar, V. Pulmonary Innate Immune Response Determines the Outcome of Inflammation During Pneumonia and Sepsis-Associated Acute Lung Injury. Front. Immunol. 2020, 11, 1722. [Google Scholar] [CrossRef]
- Englert, J.A.; Bobba, C.; Baron, R.M. Integrating molecular pathogenesis and clinical translation in sepsis-induced acute respiratory distress syndrome. JCI Insight 2019, 4, e124061. [Google Scholar] [CrossRef]
- Dupuis, C.; Sonneville, R.; Adrie, C.; Gros, A.; Darmon, M.; Bouadma, L.; Timsit, J.F. Impact of transfusion on patients with sepsis admitted in intensive care unit: A systematic review and meta-analysis. Ann. Intensive Care 2017, 7, 5. [Google Scholar] [CrossRef]
- Tossetta, G.; Marzioni, D. Natural and synthetic compounds in Ovarian Cancer: A focus on NRF2/KEAP1 pathway. Pharmacol. Res. 2022, 183, 106365. [Google Scholar] [CrossRef]
- Marzioni, D.; Mazzucchelli, R.; Fantone, S.; Tossetta, G. NRF2 modulation in TRAMP mice: An in vivo model of prostate cancer. Mol. Biol. Rep. 2022. [Google Scholar] [CrossRef] [PubMed]
- Ghareghomi, S.; Habibi-Rezaei, M.; Arese, M.; Saso, L.; Moosavi-Movahedi, A.A. Nrf2 Modulation in Breast Cancer. Biomedicines 2022, 10, 2668. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Donquiles, C.; Alonso-Molero, J.; Fernandez-Villa, T.; Vilorio-Marqués, L.; Molina, A.J.; Martín, V. The NRF2 transcription factor plays a dual role in colorectal cancer: A systematic review. PLoS ONE 2017, 12, e0177549. [Google Scholar] [CrossRef]
- Fortenberry, J.D. Walk this way. Crit. Care Med. 2011, 39, 2752–2753. [Google Scholar] [CrossRef]
- Yeh, C.H.; Yang, J.J.; Yang, M.L.; Li, Y.C.; Kuan, Y.H. Rutin decreases lipopolysaccharide-induced acute lung injury via inhibition of oxidative stress and the MAPK-NF-kappaB pathway. Free Radic. Biol. Med. 2014, 69, 249–257. [Google Scholar] [CrossRef]
- Xia, W.; Pan, Z.; Zhang, H.; Zhou, Q.; Liu, Y. Inhibition of ERRalpha Aggravates Sepsis-Induced Acute Lung Injury in Rats via Provoking Inflammation and Oxidative Stress. Oxidative Med. Cell. Longev. 2020, 2020, 2048632. [Google Scholar] [CrossRef] [PubMed]
- Lorne, E.; Dupont, H.; Abraham, E. Toll-like receptors 2 and 4: Initiators of non-septic inflammation in critical care medicine? Intensive Care Med. 2010, 36, 1826–1835. [Google Scholar] [CrossRef]
- Letourneau, P.A.; Menge, T.D.; Wataha, K.A.; Wade, C.E.; Cox, C.S., Jr.; Holcomb, J.B.; Pati, S. Human Bone Marrow Derived Mesenchymal Stem Cells Regulate Leukocyte-Endothelial Interactions and Activation of Transcription Factor NF-Kappa B. J. Tissue Sci. Eng. 2011, S3, 001. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zabell, T.; Creasy, A.; Yang, X.; Chatterjee, V.; Villalba, N.; Kistler, E.B.; Wu, M.H.; Yuan, S.Y. Gut Ischemia Reperfusion Injury Induces Lung Inflammation via Mesenteric Lymph-Mediated Neutrophil Activation. Front. Immunol. 2020, 11, 586685. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Zhou, L.; Gai, S.; Zhai, Y.; Gou, N.; Wang, X.; Zhang, X.; Cui, M.; Wang, L.; Wang, S. Acacia catechu (L.f.) Willd and Scutellaria baicalensis Georgi extracts suppress LPS-induced pro-inflammatory responses through NF-small ka, CyrillicB, MAPK, and PI3K-Akt signaling pathways in alveolar epithelial type II cells. Phytother. Res. 2019, 33, 3251–3260. [Google Scholar] [CrossRef]
- Chen, G.; Ge, D.; Zhu, B.; Shi, H.; Ma, Q. Salvia miltiorrhiza Injection Alleviates LPS-Induced Acute Lung Injury by Adjusting the Balance of MMPs/TIMPs Ratio. Evid. Based Complement. Altern. Med. 2020, 2020, 9617081. [Google Scholar] [CrossRef]
- Shen, B.; Zhao, C.; Chen, C.; Li, Z.; Li, Y.; Tian, Y.; Feng, H. Picroside II Protects Rat Lung and A549 Cell Against LPS-Induced Inflammation by the NF-kappaB Pathway. Inflammation 2017, 40, 752–761. [Google Scholar] [CrossRef]
- Ding, Z.; Zhong, R.; Xia, T.; Yang, Y.; Xing, N.; Wang, W.; Wang, Y.; Yang, B.; Sun, X.; Shu, Z. Advances in research into the mechanisms of Chinese Materia Medica against acute lung injury. Biomed. Pharmacother. 2020, 122, 109706. [Google Scholar] [CrossRef]
- Hossen, M.J.; Amin, A.; Fu, X.Q.; Chou, J.Y.; Wu, J.Y.; Wang, X.Q.; Chen, Y.J.; Wu, Y.; Li, J.; Yin, C.L.; et al. The anti-inflammatory effects of an ethanolic extract of the rhizome of Atractylodes lancea, involves Akt/NF-kappaB signaling pathway inhibition. J. Ethnopharmacol. 2021, 277, 114183. [Google Scholar] [CrossRef]
- Shi, K.; Qu, L.; Lin, X.; Xie, Y.; Tu, J.; Liu, X.; Zhou, Z.; Cao, G.; Li, S.; Liu, Y. Deep-Fried Atractylodis rhizoma Protects against Spleen Deficiency-Induced Diarrhea through Regulating Intestinal Inflammatory Response and Gut Microbiota. Int. J. Mol. Sci. 2019, 21, 124. [Google Scholar] [CrossRef]
- Grommes, J.; Soehnlein, O. Contribution of neutrophils to acute lung injury. Mol. Med. 2011, 17, 293–307. [Google Scholar] [CrossRef]
- Neudecker, V.; Brodsky, K.S.; Clambey, E.T.; Schmidt, E.P.; Packard, T.A.; Davenport, B.; Standiford, T.J.; Weng, T.; Fletcher, A.A.; Barthel, L.; et al. Neutrophil transfer of miR-223 to lung epithelial cells dampens acute lung injury in mice. Sci. Transl. Med. 2017, 9, eaah5360. [Google Scholar] [CrossRef]
- Kurdowska, A.K.; Florence, J.M. Promoting Neutrophil Apoptosis to Treat Acute Lung Injury. Am. J. Respir. Crit. Care Med. 2019, 200, 399–400. [Google Scholar] [CrossRef]
- Kellner, M.; Noonepalle, S.; Lu, Q.; Srivastava, A.; Zemskov, E.; Black, S.M. ROS Signaling in the Pathogenesis of Acute Lung Injury (ALI) and Acute Respiratory Distress Syndrome (ARDS). Adv. Exp. Med. Biol. 2017, 967, 105–137. [Google Scholar] [CrossRef]
- van Berlo, D.; Wessels, A.; Boots, A.W.; Wilhelmi, V.; Scherbart, A.M.; Gerloff, K.; van Schooten, F.J.; Albrecht, C.; Schins, R.P. Neutrophil-derived ROS contribute to oxidative DNA damage induction by quartz particles. Free Radic. Biol. Med. 2010, 49, 1685–1693. [Google Scholar] [CrossRef]
- Herold, S.; Gabrielli, N.M.; Vadasz, I. Novel concepts of acute lung injury and alveolar-capillary barrier dysfunction. Am. J. Physiol. Lung Cell. Mol. Physiol. 2013, 305, L665–L681. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, J.; Matthay, M.A. Regulation and repair of the alveolar-capillary barrier in acute lung injury. Annu. Rev. Physiol. 2013, 75, 593–615. [Google Scholar] [CrossRef] [PubMed]
- Mu, S.; Liu, Y.; Jiang, J.; Ding, R.; Li, X.; Li, X.; Ma, X. Unfractionated heparin ameliorates pulmonary microvascular endothelial barrier dysfunction via microtubule stabilization in acute lung injury. Respir. Res. 2018, 19, 220. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Lou, J.; Mao, Y.Y.; Lai, T.W.; Liu, L.Y.; Zhu, C.; Zhang, C.; Liu, J.; Li, Y.Y.; Zhang, F.; et al. Activation of MTOR in pulmonary epithelium promotes LPS-induced acute lung injury. Autophagy 2016, 12, 2286–2299. [Google Scholar] [CrossRef]
- Gross, C.M.; Kellner, M.; Wang, T.; Lu, Q.; Sun, X.; Zemskov, E.A.; Noonepalle, S.; Kangath, A.; Kumar, S.; Gonzalez-Garay, M.; et al. LPS-induced Acute Lung Injury Involves NF-kappaB-mediated Downregulation of SOX18. Am. J. Respir. Cell. Mol. Biol. 2018, 58, 614–624. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, Z.Y.; Li, Y.Y.; Luo, Y.; Liu, M.L.; Dong, H.Y.; Wang, Y.X.; Liu, Y.; Zhao, P.T.; Jin, F.G.; et al. Antiinflammatory effects of matrine in LPS-induced acute lung injury in mice. Eur. J. Pharm. Sci. 2011, 44, 573–579. [Google Scholar] [CrossRef]
- Du, L.; Hu, X.; Chen, C.; Kuang, T.; Yin, H.; Wan, L. Seabuckthorn Paste Protects Lipopolysaccharide-Induced Acute Lung Injury in Mice through Attenuation of Oxidative Stress. Oxidative Med. Cell. Longev. 2017, 2017, 4130967. [Google Scholar] [CrossRef]
- Davidson, B.A.; Vethanayagam, R.R.; Grimm, M.J.; Mullan, B.A.; Raghavendran, K.; Blackwell, T.S.; Freeman, M.L.; Ayyasamy, V.; Singh, K.K.; Sporn, M.B.; et al. NADPH oxidase and Nrf2 regulate gastric aspiration-induced inflammation and acute lung injury. J. Immunol. 2013, 190, 1714–1724. [Google Scholar] [CrossRef]
- de la Vega, M.R.; Dodson, M.; Gross, C.; Mansour, H.M.; Lantz, R.C.; Chapman, E.; Wang, T.; Black, S.M.; Garcia, J.G.; Zhang, D.D. Role of Nrf2 and Autophagy in Acute Lung Injury. Curr. Pharmacol. Rep. 2016, 2, 91–101. [Google Scholar] [CrossRef]
- Yao, W.; Luo, G.; Zhu, G.; Chi, X.; Zhang, A.; Xia, Z.; Hei, Z. Propofol activation of the Nrf2 pathway is associated with amelioration of acute lung injury in a rat liver transplantation model. Oxidative Med. Cell. Longev. 2014, 2014, 258567. [Google Scholar] [CrossRef]
- Silva, P.L.; Moraes, L.; Santos, R.S.; Samary, C.; Ramos, M.B.; Santos, C.L.; Morales, M.M.; Capelozzi, V.L.; Garcia, C.S.; de Abreu, M.G.; et al. Recruitment maneuvers modulate epithelial and endothelial cell response according to acute lung injury etiology. Crit. Care Med. 2013, 41, e256–e265. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Lv, L.; Zhai, P.; Long, T.; Zhou, Q.; Pan, H.; Botwe, G.; Wang, L.; Wang, Q.; Tan, L.; et al. Connexin 40 regulates lung endothelial permeability in acute lung injury via the ROCK1-MYPT1- MLC20 pathway. Am. J. Physiol. Lung Cell. Mol. Physiol. 2019, 316, L35–L44. [Google Scholar] [CrossRef] [PubMed]
- Fanelli, V.; Ranieri, V.M. Mechanisms and clinical consequences of acute lung injury. Ann. Am. Thorac. Soc. 2015, 12 (Suppl. 1), S3–S8. [Google Scholar] [CrossRef] [PubMed]
- Reiss, L.K.; Uhlig, U.; Uhlig, S. Models and mechanisms of acute lung injury caused by direct insults. Eur. J. Cell Biol. 2012, 91, 590–601. [Google Scholar] [CrossRef]
- Ryter, S.W.; Choi, A.M. Gaseous therapeutics in acute lung injury. Compr. Physiol. 2011, 1, 105–121. [Google Scholar] [CrossRef]
- Diaz, J.V.; Brower, R.; Calfee, C.S.; Matthay, M.A. Therapeutic strategies for severe acute lung injury. Crit. Care Med. 2010, 38, 1644–1650. [Google Scholar] [CrossRef]
- von Dossow-Hanfstingl, V. Advances in therapy for acute lung injury. Anesthesiol. Clin. 2012, 30, 629–639. [Google Scholar] [CrossRef]
- Qu, L.; Shi, K.; Xu, J.; Liu, C.; Ke, C.; Zhan, X.; Xu, K.; Liu, Y. Atractylenolide-1 targets SPHK1 and B4GALT2 to regulate intestinal metabolism and flora composition to improve inflammation in mice with colitis. Phytomedicine 2022, 98, 153945. [Google Scholar] [CrossRef]
- Hakansson, H.F.; Smailagic, A.; Brunmark, C.; Miller-Larsson, A.; Lal, H. Altered lung function relates to inflammation in an acute LPS mouse model. Pulm. Pharmacol. Ther. 2012, 25, 399–406. [Google Scholar] [CrossRef]
- Gao, P.; Zhao, Z.; Zhang, C.; Wang, C.; Long, K.; Guo, L.; Li, B. The therapeutic effects of traditional Chinese medicine Fusu agent in LPS-induced acute lung injury model rats. Drug Des. Dev. Ther. 2018, 12, 3867–3878. [Google Scholar] [CrossRef]
- Zhi, H.J.; Zhu, H.Y.; Zhang, Y.Y.; Lu, Y.; Li, H.; Chen, D.F. In vivo effect of quantified flavonoids-enriched extract of Scutellaria baicalensis root on acute lung injury induced by influenza A virus. Phytomedicine 2019, 57, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Xu, L.; Zeng, Y.; Gong, F. Effect of gut microbiota on LPS-induced acute lung injury by regulating the TLR4/NF-kB signaling pathway. Int. Immunopharmacol. 2021, 91, 107272. [Google Scholar] [CrossRef] [PubMed]
- Shaukat, A.; Guo, Y.F.; Jiang, K.; Zhao, G.; Wu, H.; Zhang, T.; Yang, Y.; Guo, S.; Yang, C.; Zahoor, A.; et al. Ginsenoside Rb1 ameliorates Staphylococcus aureus-induced Acute Lung Injury through attenuating NF-κB and MAPK activation. Microb. Pathog. 2019, 132, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Shan, S.; Shen, Z.; Zhang, C.; Kou, R.; Xie, K.; Song, F. Mitophagy protects against acetaminophen-induced acute liver injury in mice through inhibiting NLRP3 inflammasome activation. Biochem. Pharmacol. 2019, 169, 113643. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Huang, S.; Meng, X.; Zhang, J.; Yu, S.; Li, J.; Shi, M.; Fan, H.; Zhao, Y. Mechanism of Phosgene-Induced Acute Lung Injury and Treatment Strategy. Int. J. Mol. Sci. 2021, 22, 10933. [Google Scholar] [CrossRef]
- Li, J.; Lu, K.; Sun, F.; Tan, S.; Zhang, X.; Sheng, W.; Hao, W.; Liu, M.; Lv, W.; Han, W. Panaxydol attenuates ferroptosis against LPS-induced acute lung injury in mice by Keap1-Nrf2/HO-1 pathway. J. Transl. Med. 2021, 19, 96. [Google Scholar] [CrossRef]
- Huang, C.Y.; Deng, J.S.; Huang, W.C.; Jiang, W.P.; Huang, G.J. Attenuation of Lipopolysaccharide-Induced Acute Lung Injury by Hispolon in Mice, Through Regulating the TLR4/PI3K/Akt/mTOR and Keap1/Nrf2/HO-1 Pathways, and Suppressing Oxidative Stress-Mediated ER Stress-Induced Apoptosis and Autophagy. Nutrients 2020, 12, 1742. [Google Scholar] [CrossRef]
- Lin, X.; Guo, X.; Qu, L.; Tu, J.; Li, S.; Cao, G.; Liu, Y. Preventive effect of Atractylodis rhizoma extract on DSS-induced acute ulcerative colitis through the regulation of the MAPK/NF-κB signals in vivo and in vitro. J. Ethnopharmacol. 2022, 292, 115211. [Google Scholar] [CrossRef]
- Lin, Z.; Gan, T.; Huang, Y.; Bao, L.; Liu, S.; Cui, X.; Wang, H.; Jiao, F.; Zhang, M.; Su, C.; et al. Anti-Inflammatory Activity of Mulberry Leaf Flavonoids In Vitro and In Vivo. Int. J. Mol. Sci. 2022, 23, 7694. [Google Scholar] [CrossRef]
- Zhao, X.L.; Yu, L.; Zhang, S.D.; Ping, K.; Ni, H.Y.; Qin, X.Y.; Zhao, C.J.; Wang, W.; Efferth, T.; Fu, Y.J. Cryptochlorogenic acid attenuates LPS-induced inflammatory response and oxidative stress via upregulation of the Nrf2/HO-1 signaling pathway in RAW 264.7 macrophages. Int. Immunopharmacol. 2020, 83, 106436. [Google Scholar] [CrossRef]
- Pei, X.; Zhang, X.J.; Chen, H.M. Bardoxolone treatment alleviates lipopolysaccharide (LPS)-induced acute lung injury through suppressing inflammation and oxidative stress regulated by Nrf2 signaling. Biochem. Biophys. Res. Commun. 2019, 516, 270–277. [Google Scholar] [CrossRef] [PubMed]
| Name | Primer Sequences (from 5′→3′) | |
|---|---|---|
| Rat-TNF-α | Forward | GGAGGGAGAACAGCAACTCC |
| Reverse | GCCAGTGTATGAGAGGGACG | |
| Rat-IL-1β | Forward | AGGCTGACAGACCCCAAAAG |
| Reverse | GGTCGTCATCATCCCACGAG | |
| Rat-IL-6 | Forward | AGAGACTTCCAGCCAGTTGC |
| Reverse | AGTCTCCTCTCCGGACTTGT | |
| Rat-INOS | Forward | GGGGACTGGACTTTTAGAGACG |
| Reverse | CCGTGGGGCTTGTAGTTGAC | |
| Rat-COX-2 | Forward | GTTCATCCCGGATCCCCAAG |
| Reverse | ACGTGGGGAGGGTAGATCAT | |
| Rat-Nrf2 | Forward | GGTTGCCCACATTCCCAAAC |
| Reverse | CAGGGCAAGCGACTGAAATG | |
| Rat-NQO-1 | Forward | CGGCTCCATGTACTCTCTGC |
| Reverse | GAGTGGTGACTCCTCCCAGA | |
| Rat-HO-1 | Forward | GCCTGGTTCAAGATACTACCTCT |
| Reverse | CTGAGTGTGAGGACCCATCG | |
| Rat-β-actin | Forward | GCAGGAGTACGATGAGTCCG |
| Reverse | ACGCAGCTCAGTAACAGTCC | |
| Human-TNF-α | Forward | TCTTCTCGAACCCCGAGTGA |
| Reverse | TATCTCTCAGCTCCACGCCA | |
| Human-IL-1β | Forward | GGCTGCTCTGGGATTCTCTT |
| Reverse | ATTTCACTGGCGAGCTCAGG | |
| Human-COX-2 | Forward | TGCTGGTGGAAAAACCTCGT |
| Reverse | AAAACCCACTTCGCCTCCAA | |
| Human-Nrf2 | Forward | GATCTTGGAGTTGCCCACATTC |
| Reverse | CAAGTGACTGAAACGTAGCCG | |
| Human-NOQ-1 | Forward | TCCCCCTGCAGTGGTTTG |
| Reverse | CATGTCCCCGTGGATCCCTT | |
| Human-HO-1 | Forward | CTCCGGCAGTCAACGCCT |
| Reverse | CTCTGACAAATCCTGGGGCA | |
| Human-β-actin | Forward | GGATTCCTATGTGGGCGACGA |
| Reverse | GCGTACAGGGATAGCACAGC | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, K.; Xiao, Y.; Dong, Y.; Wang, D.; Xie, Y.; Tu, J.; Xu, K.; Zhou, Z.; Cao, G.; Liu, Y. Protective Effects of Atractylodis lancea Rhizoma on Lipopolysaccharide-Induced Acute Lung Injury via TLR4/NF-κB and Keap1/Nrf2 Signaling Pathways In Vitro and In Vivo. Int. J. Mol. Sci. 2022, 23, 16134. https://doi.org/10.3390/ijms232416134
Shi K, Xiao Y, Dong Y, Wang D, Xie Y, Tu J, Xu K, Zhou Z, Cao G, Liu Y. Protective Effects of Atractylodis lancea Rhizoma on Lipopolysaccharide-Induced Acute Lung Injury via TLR4/NF-κB and Keap1/Nrf2 Signaling Pathways In Vitro and In Vivo. International Journal of Molecular Sciences. 2022; 23(24):16134. https://doi.org/10.3390/ijms232416134
Chicago/Turabian StyleShi, Kun, Yangxin Xiao, Yan Dong, Dongpeng Wang, Ying Xie, Jiyuan Tu, Kang Xu, Zhongshi Zhou, Guosheng Cao, and Yanju Liu. 2022. "Protective Effects of Atractylodis lancea Rhizoma on Lipopolysaccharide-Induced Acute Lung Injury via TLR4/NF-κB and Keap1/Nrf2 Signaling Pathways In Vitro and In Vivo" International Journal of Molecular Sciences 23, no. 24: 16134. https://doi.org/10.3390/ijms232416134
APA StyleShi, K., Xiao, Y., Dong, Y., Wang, D., Xie, Y., Tu, J., Xu, K., Zhou, Z., Cao, G., & Liu, Y. (2022). Protective Effects of Atractylodis lancea Rhizoma on Lipopolysaccharide-Induced Acute Lung Injury via TLR4/NF-κB and Keap1/Nrf2 Signaling Pathways In Vitro and In Vivo. International Journal of Molecular Sciences, 23(24), 16134. https://doi.org/10.3390/ijms232416134