Surface Modifications of High-Performance Polymer Polyetheretherketone (PEEK) to Improve Its Biological Performance in Dentistry
Abstract
1. Introduction
2. High-Performance Polymer PEEK in Dentistry
2.1. The Superior Properties of PEEK
2.2. Drawbacks of PEEK
3. Desired Properties of High-Performance Polymers in Dentistry
3.1. Adhesiveness-Bonding Strength
3.2. Bioactivity (Osseointegration and Antibacterial Activity)
3.2.1. Osseointegration
3.2.2. Antibacterial Activity
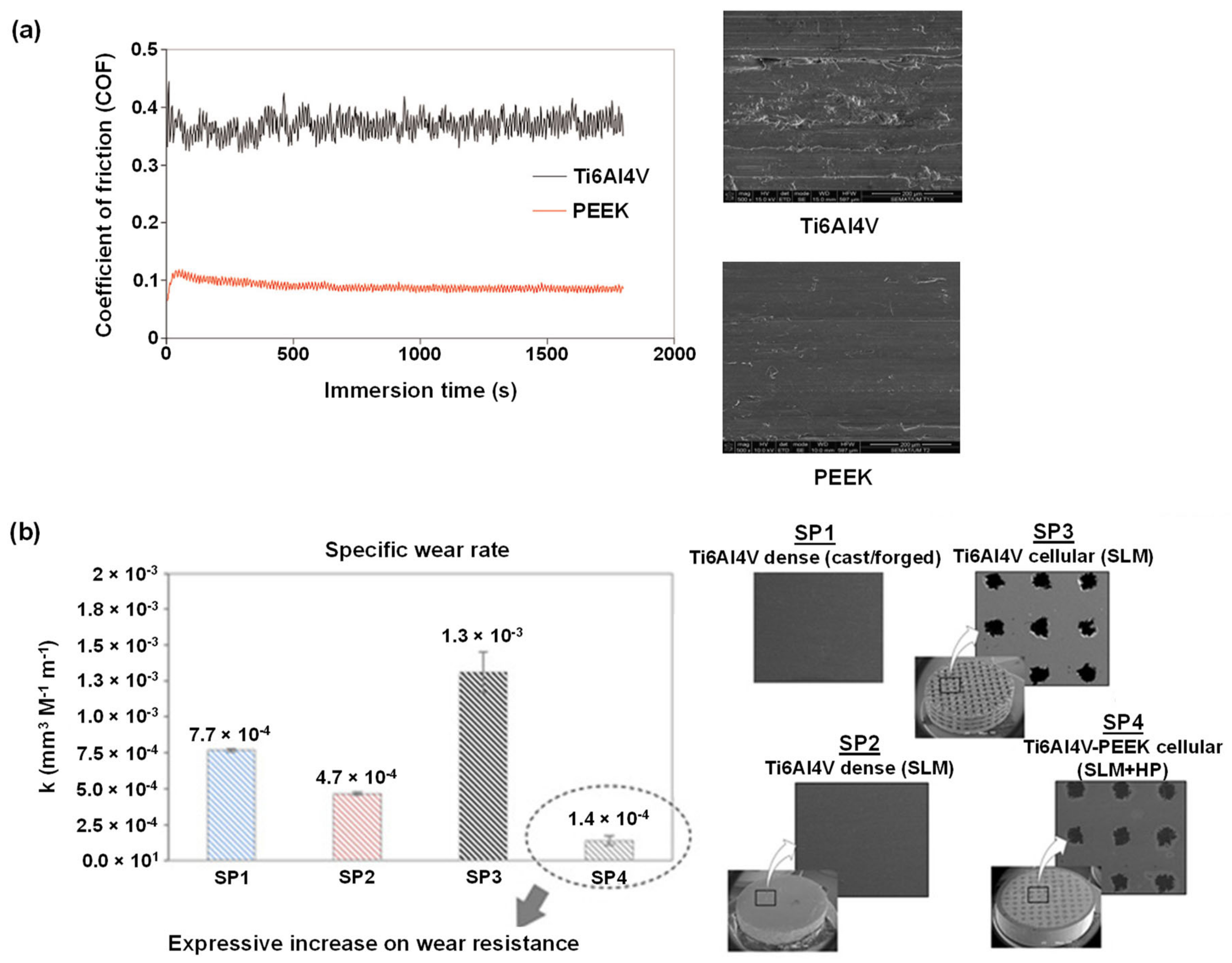
3.3. Tribology-Resistance to Tribocorrosion
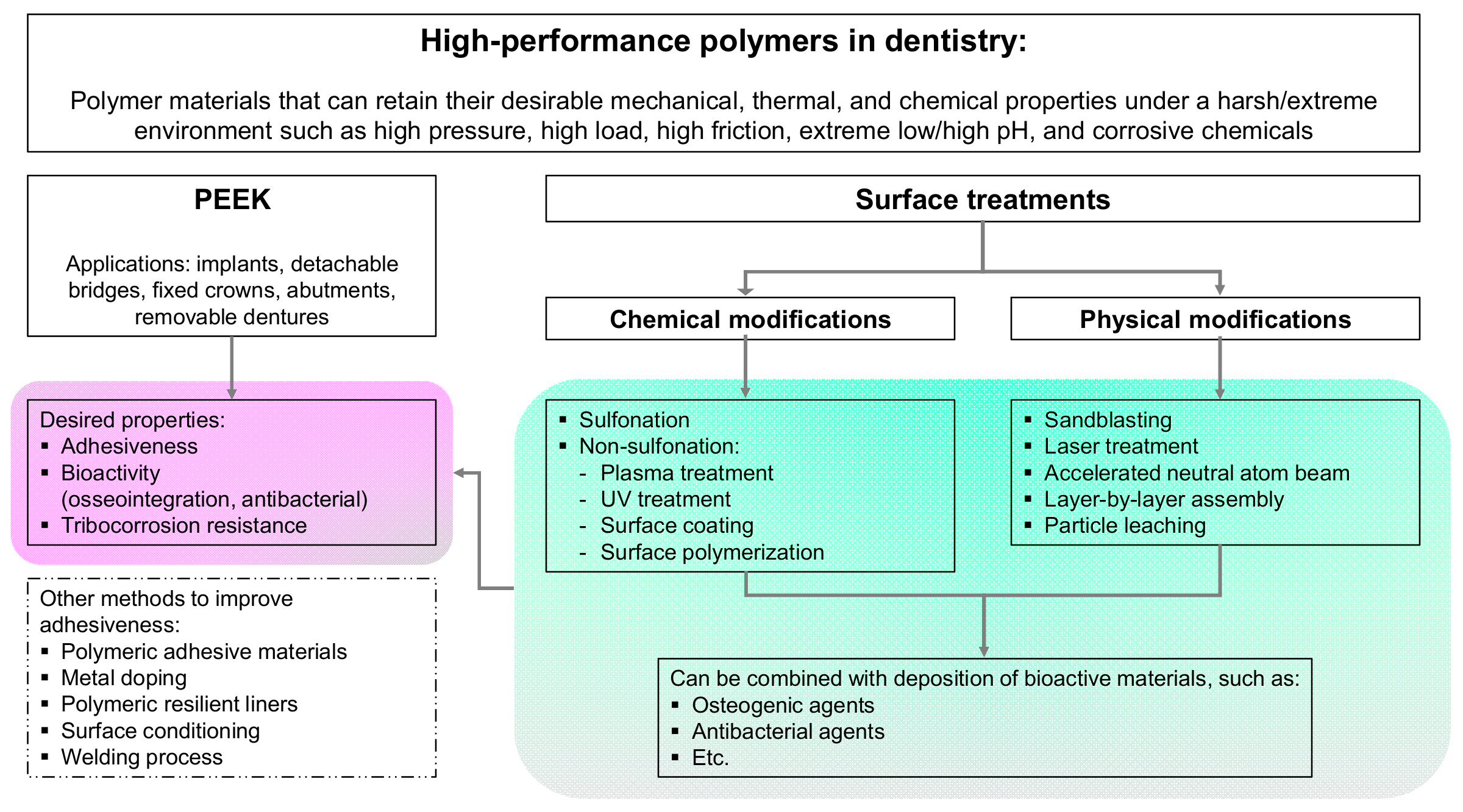
4. PEEK Surface Modifications
4.1. The Importance of Surface Wettability
4.2. The importance of Surface Topography
4.3. Chemical Surface Modifications of PEEK
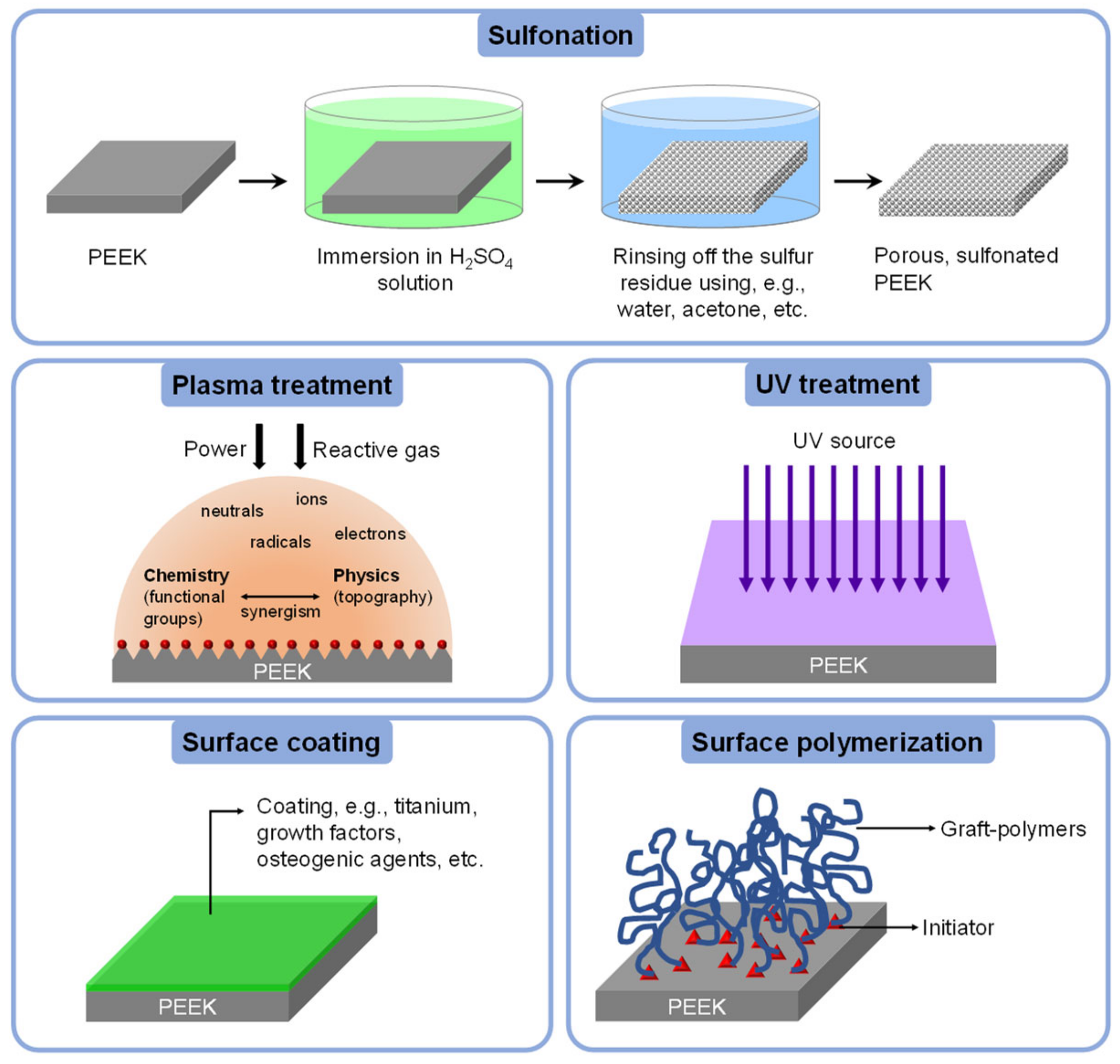
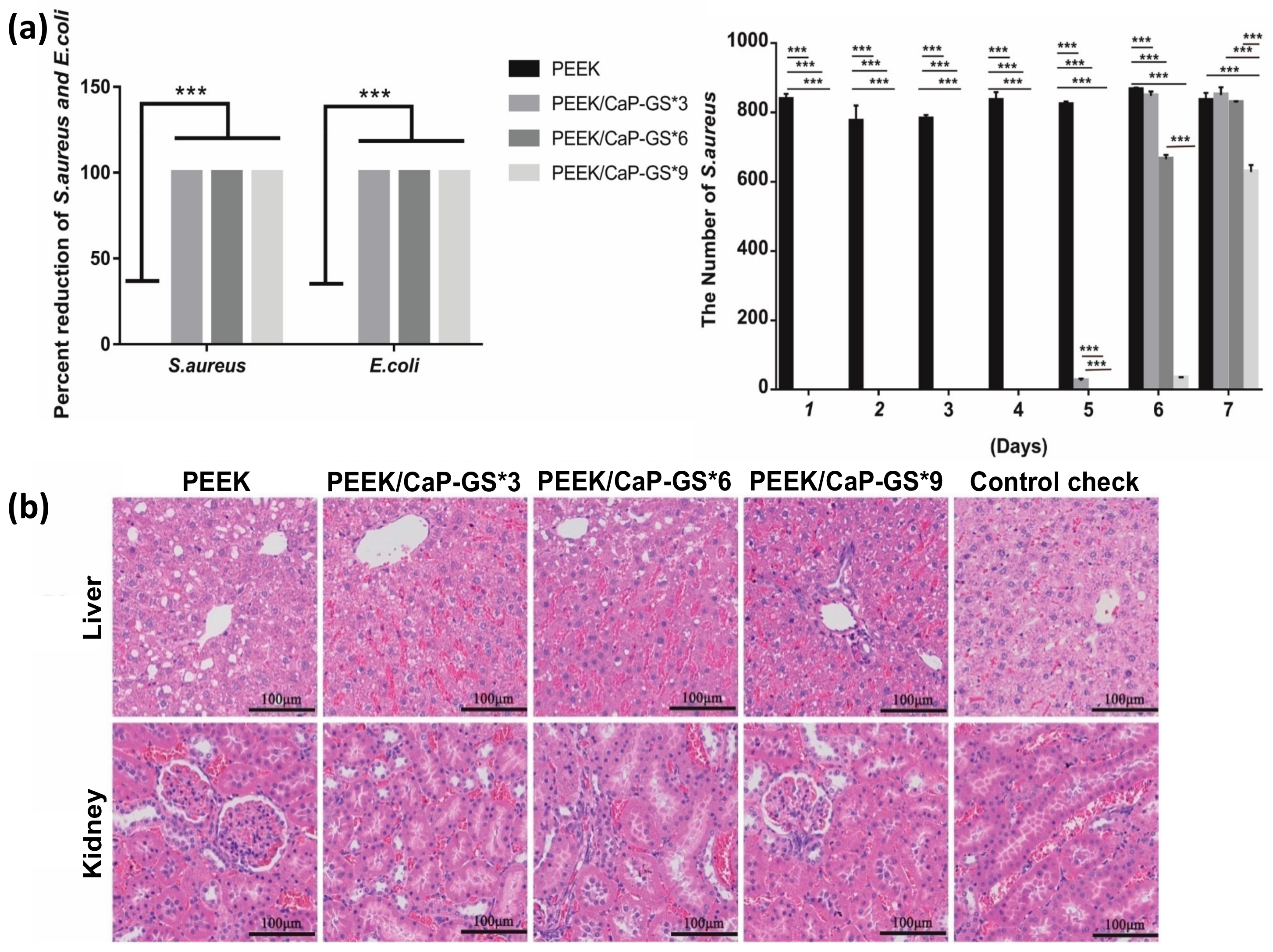
4.3.1. Sulfonation
4.3.2. Non-Sulfonation
Plasma Treatment
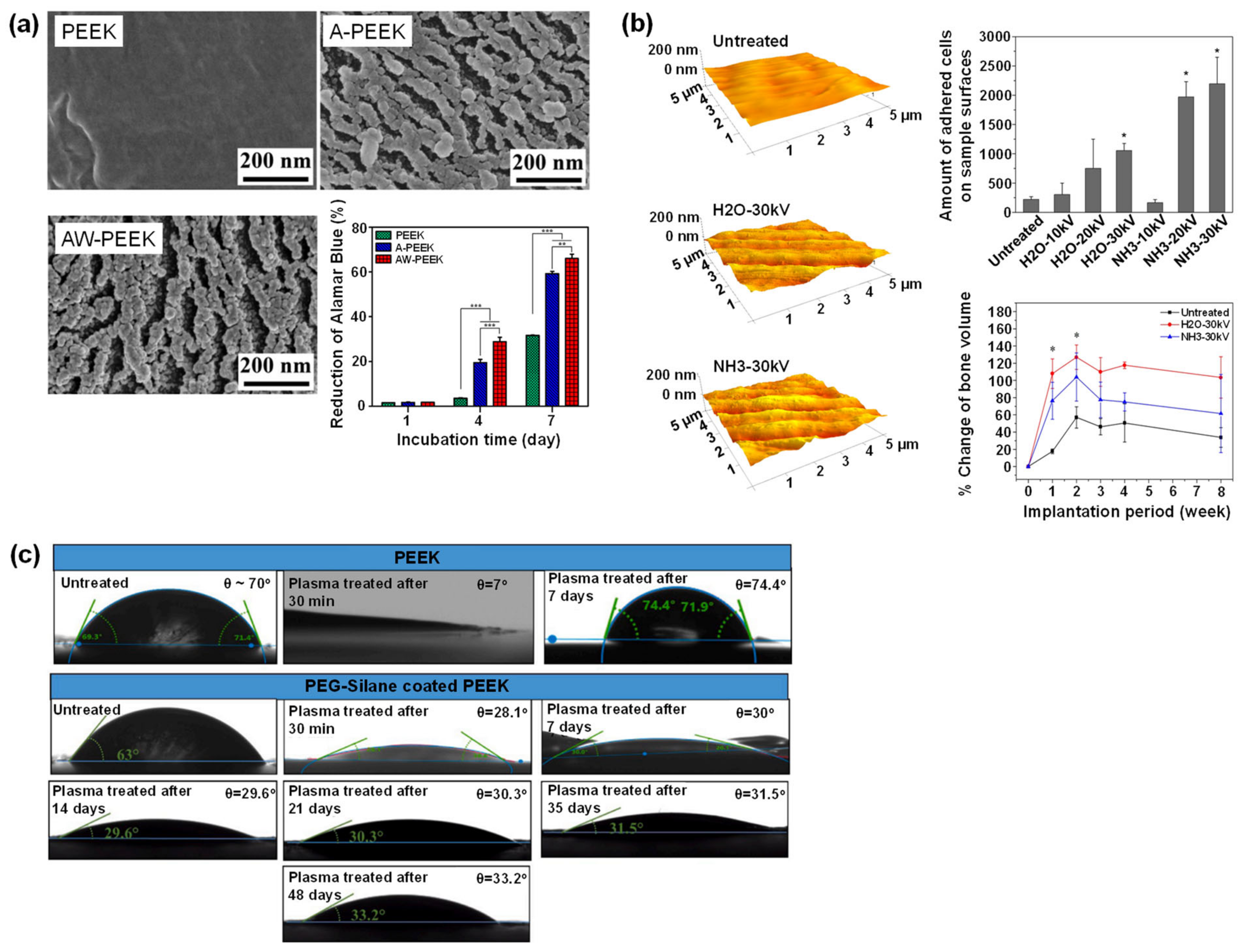
UV Treatment
Surface Coating
Surface Polymerization
4.4. Physical Surface Modifications of PEEK
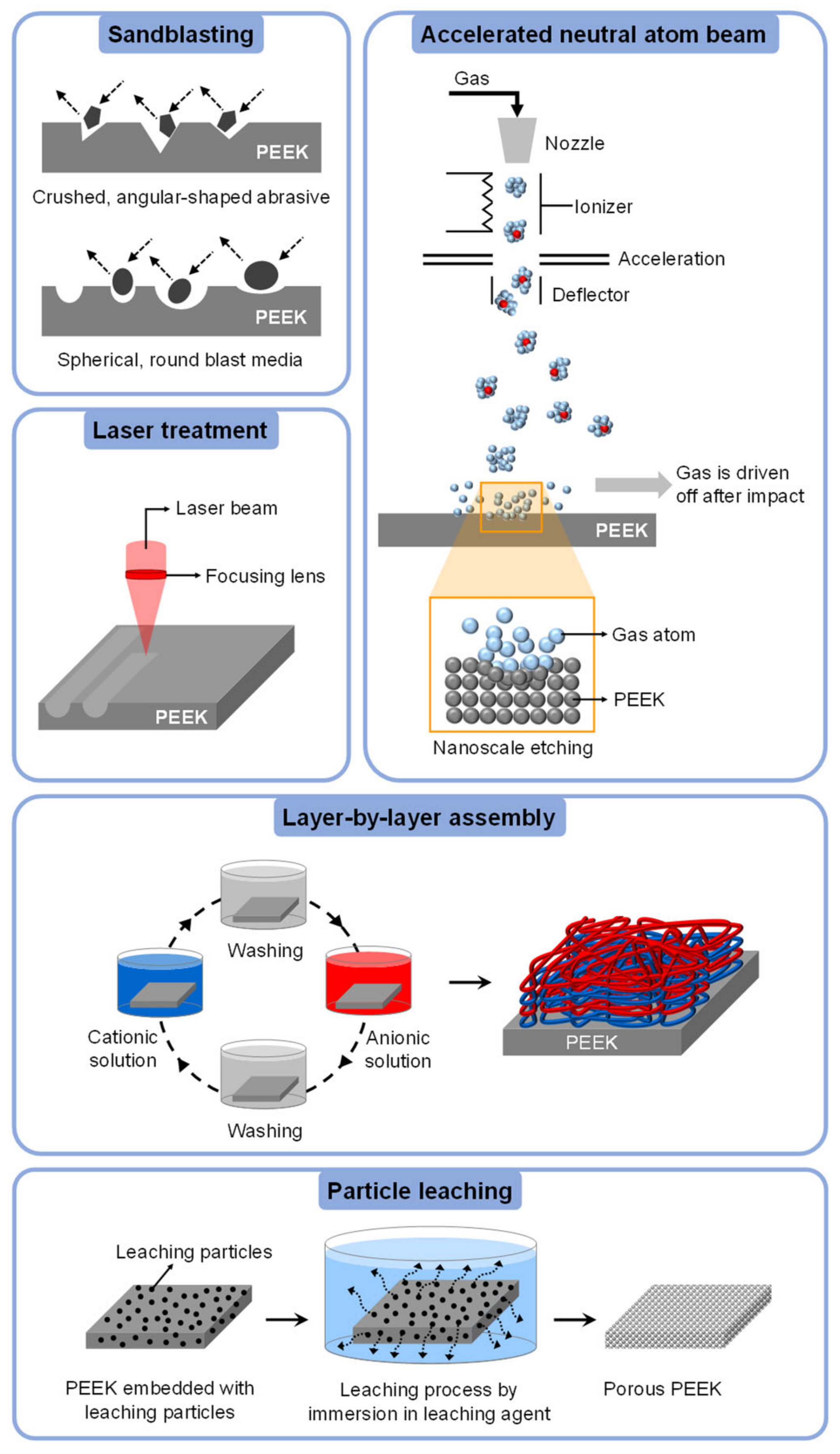
4.4.1. Sandblasting
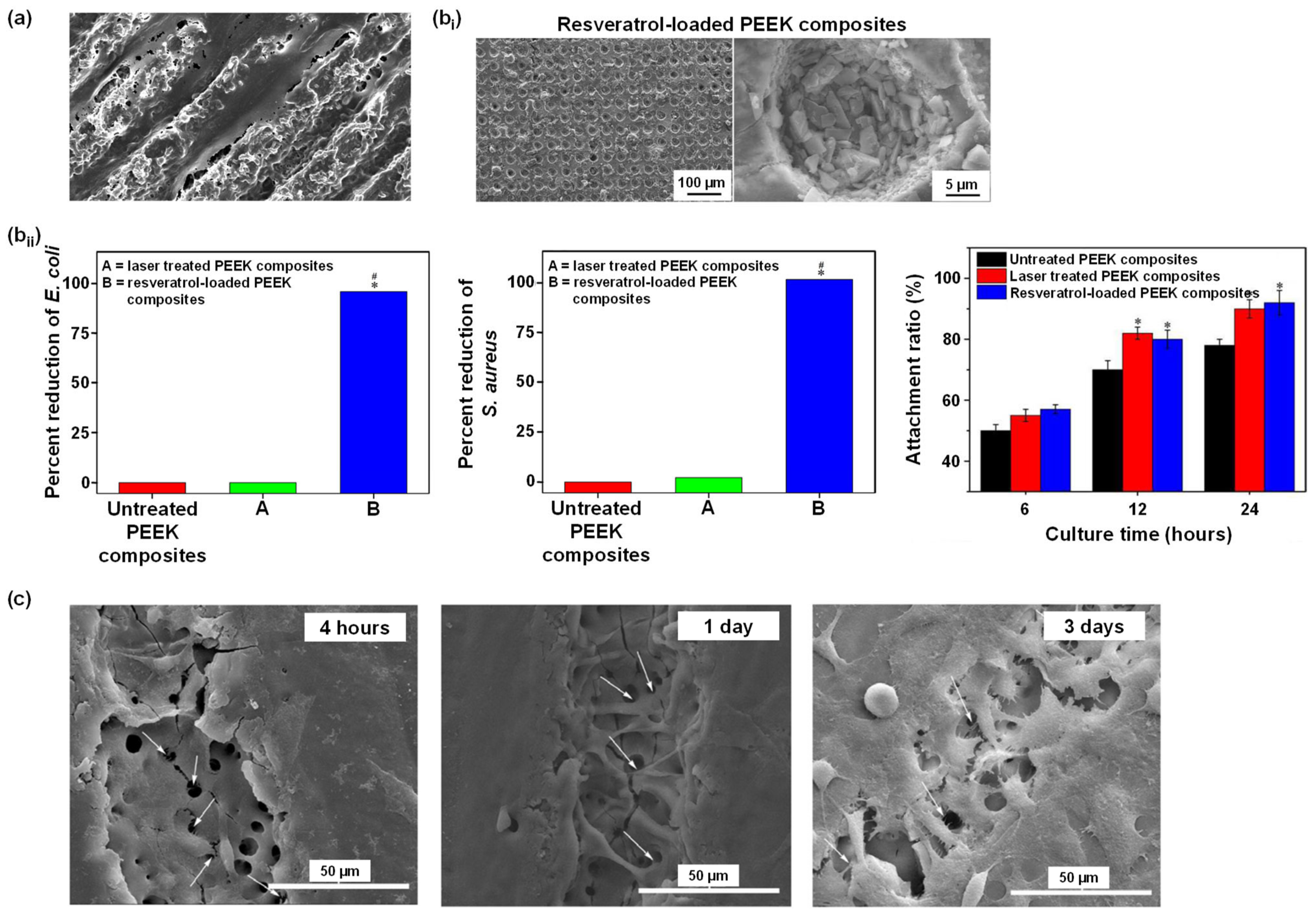
4.4.2. Laser Treatment
4.4.3. Accelerated Neutral Atom Beam (ANAB)
4.4.4. Layer-by-Layer (LbL) Assembly
4.4.5. Particle Leaching
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- De Leon, A.C.; Chen, Q.; Palaganas, N.B.; Palaganas, J.O.; Manapat, J.; Advincula, R.C. High performance polymer nanocomposites for additive manufacturing applications. React. Funct. Polym. 2016, 103, 141–155. [Google Scholar] [CrossRef]
- Xu, X.; He, L.; Zhu, B.; Li, J.; Li, J. Advances in polymeric materials for dental applications. Polym. Chem. 2017, 8, 807–823. [Google Scholar] [CrossRef]
- Wang, X.; Gao, G.; Song, H.B.; Zhang, X.; Stansbury, J.W.; Bowman, C.N. Evaluation of a photo-initiated copper(I)-catalyzed azide-alkyne cycloaddition polymer network with improved water stability and high mechanical performance as an ester-free dental restorative. Dent. Mater. 2021, 37, 1592–1600. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, P.K.; Negi, Y.S.; Goel, N.K.; Diwan, R.K.; Rattan, S. Chemical initiator-free synthesis of poly (acrylic acid-co-itaconic acid) using radiation-induced polymerization for application in dental cements. Radiat. Phys. Chem. 2022, 198, 110243. [Google Scholar] [CrossRef]
- Xing, A.; Sun, Q.; Meng, Y.; Zhang, Y.; Li, X.; Han, B. A hydroxyl-containing hyperbranched polymer as a multi-purpose modifier for a dental epoxy. React. Funct. Polym. 2020, 149, 104505. [Google Scholar] [CrossRef]
- Gama, L.T.; Duque, T.M.; Özcan, M.; Philippi, A.G.; Mezzomo, L.A.M.; Gonçalves, T.M.S.V. Adhesion to high-performance polymers applied in dentistry: A systematic review. Dent. Mater. 2020, 36, e93–e108. [Google Scholar] [CrossRef]
- Kadambi, P.; Luniya, P.; Dhatrak, P. Current advancements in polymer/polymer matrix composites for dental implants: A systematic review. Mater. Today Proc. 2021, 46, 740–745. [Google Scholar] [CrossRef]
- Oladapo, B.I.; Kayode, J.F.; Karagiannidis, P.; Naveed, N.; Mehrabi, H.; Ogundipe, K.O. Polymeric composites of cubic-octahedron and gyroid lattice for biomimetic dental implants. Mater. Chem. Phys. 2022, 289, 126454. [Google Scholar] [CrossRef]
- Haleem, A.; Javaid, M. Polyether ether ketone (peek) and its manufacturing of customised 3D printed dentistry parts using additive manufacturing. Clin. Epidemiol. Glob. Health 2019, 7, 654–660. [Google Scholar] [CrossRef]
- Liu, C.; Lin, J.; Tang, L.; Liu, Z.; Jiang, Z.; Lian, K. Design of metal-polymer structure for dental implants with stiffness adaptable to alveolar bone. Compos. Commun. 2021, 24, 100660. [Google Scholar] [CrossRef]
- Schönhoff, L.M.; Mayinger, F.; Eichberger, M.; Reznikova, E.; Stawarczyk, B. 3D printing of dental restorations: Mechanical properties of thermoplastic polymer materials. J. Mech. Behav. Biomed. Mater. 2021, 119, 104544. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Thakur, A. Applications of poly(methyl methacrylate) polymer in dentistry: A review. Mater. Today Proc. 2022, 50, 1619–1625. [Google Scholar] [CrossRef]
- Vaidyanathan, T.K.; Vaidyanathan, J. Validity of predictive models of stress relaxation in selected dental polymers. Dent. Mater. 2015, 31, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, M. Mechanical properties enhancement of self-cured PMMA reinforced with zirconia and boron nitride nanopowders for high-performance dental materials. J. Mech. Behav. Biomed. Mater. 2020, 110, 103937. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Guo, J.; Li, K.; Sun, J.; Zeng, Y.; Ning, C. High strength polymer/silicon nitride composites for dental restorations. Dent. Mater. 2019, 35, 1254–1263. [Google Scholar] [CrossRef]
- Kirschner, J.; Szillat, F.; Bouzrati-Zerelli, M.; Becht, J.-M.; Klee, J.E.; Lalevée, J. Sulfinates and sulfonates as high performance co-initiators in CQ based systems: Towards aromatic amine-free systems for dental restorative materials. Dent. Mater. 2020, 36, 187–196. [Google Scholar] [CrossRef]
- Bortolatto, J.F.; Buzalaf, M.R.A.; Ebrahimi, J.; Floros, M.C.; Ho, M.; Prakki, A. Methacrylation of epigallocatechin-gallate for covalent attachment with a dental polymer. Dent. Mater. 2021, 37, 1751–1760. [Google Scholar] [CrossRef]
- González-López, J.A.; Pérez-Mondragón, A.A.; Cuevas-Suárez, C.E.; Esparza González, S.C.; Herrera-González, A.M. Dental composite resins with low polymerization stress based on a new allyl carbonate monomer. J. Mech. Behav. Biomed. Mater. 2020, 110, 103955. [Google Scholar] [CrossRef]
- Shah, P.K.; Stansbury, J.W. Photopolymerization shrinkage-stress reduction in polymer-based dental restoratives by surface modification of fillers. Dent. Mater. 2021, 37, 578–587. [Google Scholar] [CrossRef]
- Pratap, B.; Ravi Kant, G.; Denis, L.; Goswami, D. Evaluation of polymerization shrinkage and Vickers hardness for restorative dental composites. Mater. Today Proc. 2020, 21, 1563–1565. [Google Scholar] [CrossRef]
- Pratap, B.; Gupta, R.K.; Bhardwaj, B.; Nag, M. Modeling based experimental investigation on polymerization shrinkage and micro-hardness of nano alumina filled resin based dental material. J. Mech. Behav. Biomed. Mater. 2019, 99, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Huyang, G.; Palagummi, S.V.; Liu, X.; Skrtic, D.; Beauchamp, C.; Bowen, R.; Sun, J. High performance dental resin composites with hydrolytically stable monomers. Dent. Mater. 2018, 34, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Ilie, N.; Erich Serfözö, N.; Prodan, D.; Diegelmann, J.; Moldovan, M. Synthesis and performance of experimental resin-based dental adhesives reinforced with functionalized graphene and hydroxyapatite fillers. Mater. Des. 2022, 221, 110985. [Google Scholar] [CrossRef]
- Bathala, L.; Majeti, V.; Rachuri, N.; Singh, N.; Gedela, S. The role of polyether ether ketone (PEEK) in DEntistry—A review. J Med. Life 2019, 12, 5–9. [Google Scholar] [CrossRef]
- Yu, D.; Lei, X.; Zhu, H. Modification of polyetheretherketone (PEEK) physical features to improve osteointegration. J. Zhejiang Univ.—Sci. B 2022, 23, 189–203. [Google Scholar] [CrossRef]
- Zheng, Z.; Liu, P.; Zhang, X.; Zou, X.; Mei, X.; Zhang, S.; Zhang, S. Strategies to improve bioactive and antibacterial properties of polyetheretherketone (PEEK) for use as orthopedic implants. Mater. Today Bio 2022, 16, 100402. [Google Scholar] [CrossRef]
- Alexakou, E.; Damanaki, M.; Zoidis, P.; Bakiri, E.; Mouzis, N.; Smidt, G.; Kourtis, S. PEEK high performance polymers: A review of properties and clinical applications in prosthodontics and restorative dentistry. Eur. J. Prosthodont. Restor. Dent. 2019, 27, 113–121. [Google Scholar] [CrossRef]
- May, R. Polyetheretherketones. In Encyclopedia of Polymer Science and Engineering; Mark, H., Bikales, N., Overberger, C., Menges, G., Kroschiwitz, J., Eds.; Wiley: New York, NY, USA, 1988; pp. 313–320. [Google Scholar]
- Batak, B.; Çakmak, G.; Johnston, W.M.; Yilmaz, B. Surface roughness of high-performance polymers used for fixed implant-supported prostheses. J. Prosthet. Dent. 2021, 126, 254.e1–254.e6. [Google Scholar] [CrossRef]
- Ma, R.; Tang, T. Current strategies to improve the bioactivity of peek. Int. J. Mol. Sci. 2014, 15, 5426–5445. [Google Scholar] [CrossRef]
- Najeeb, S.; Zafar, M.S.; Khurshid, Z.; Siddiqui, F. Applications of polyetheretherketone (PEEK) in oral implantology and prosthodontics. J. Prosthodont. Res. 2016, 60, 12–19. [Google Scholar] [CrossRef]
- Qin, L.; Yao, S.; Zhao, J.; Zhou, C.; Oates, T.W.; Weir, M.D.; Wu, J.; Xu, H.H. Review on development and dental applications of polyetheretherketone-based biomaterials and restorations. Materials 2021, 14, 408. [Google Scholar] [CrossRef] [PubMed]
- Najeeb, S.; Bds, Z.K.; Bds, S.Z.; Bds, M.S.Z. Bioactivity and osseointegration of PEEK are inferior to those of titanium: A systematic review. J. Oral Implantol. 2016, 42, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Çayır Bozoğlu, Ü.; Kiremitçi, A.; Yurtsever, M.Ç.; Gümüşderelioğlu, M. Peek dental implants coated with boron-doped nano-hydroxyapatites: Investigation of in-vitro osteogenic activity. J. Trace Elem. Med. Biol. 2022, 73, 127026. [Google Scholar] [CrossRef] [PubMed]
- Di Maggio, B.; Sessa, P.; Mantelli, P.; Maniscalco, P.; Rivera, F.; Calori, G.M.; Bisogno, L.; Scaravilli, G.; Caforio, M. PEEK radiolucent plate for distal radius fractures: Multicentre clinical results at 12 months follow up. Injury 2017, 48, S34–S38. [Google Scholar] [CrossRef]
- Mishra, S.; Chowdhary, R. PEEK materials as an alternative to titanium in dental implants: A systematic review. Clin. Implant Dent. Relat. Res. 2019, 21, 208–222. [Google Scholar] [CrossRef]
- Krätzig, T.; Mende, K.C.; Mohme, M.; Kniep, H.; Dreimann, M.; Stangenberg, M.; Westphal, M.; Gauer, T.; Eicker, S.O. Carbon fiber–reinforced PEEK versus titanium implants: An in vitro comparison of susceptibility artifacts in CT and MR imaging. Neurosurg. Rev. 2021, 44, 2163–2170. [Google Scholar] [CrossRef]
- da Cruz, M.B.; Marques, J.F.; Peñarrieta-Juanito, G.M.; Costa, M.; Souza, J.C.M.; Magini, R.S.; Miranda, G.; Silva, F.S.; Caramês, J.M.M.; da Mata, A.D.S.P. Bioactive-enhanced polyetheretherketone dental implant materials: Mechanical characterization and cellular responses. J. Oral Implantol. 2020, 47, 9–17. [Google Scholar] [CrossRef]
- Peng, T.-Y.; Shih, Y.-H.; Hsia, S.-M.; Wang, T.-H.; Li, P.-J.; Lin, D.-J.; Sun, K.-T.; Chiu, K.-C.; Shieh, T.-M. In vitro assessment of the cell metabolic activity, cytotoxicity, cell attachment, and inflammatory reaction of human oral fibroblasts on polyetheretherketone (PEEK) implant–abutment. Polymers 2021, 13, 2995. [Google Scholar] [CrossRef]
- Gheisarifar, M.; Thompson, G.A.; Drago, C.; Tabatabaei, F.; Rasoulianboroujeni, M. In vitro study of surface alterations to polyetheretherketone and titanium and their effect upon human gingival fibroblasts. J. Prosthet. Dent. 2021, 125, 155–164. [Google Scholar] [CrossRef]
- Torstrick, F.B.; Lin, A.S.P.; Potter, D.; Safranski, D.L.; Sulchek, T.A.; Gall, K.; Guldberg, R.E. Porous PEEK improves the bone-implant interface compared to plasma-sprayed titanium coating on PEEK. Biomaterials 2018, 185, 106–116. [Google Scholar] [CrossRef]
- Briem, D.; Strametz, S.; Schröoder, K.; Meenen, N.M.; Lehmann, W.; Linhart, W.; Ohl, A.; Rueger, J.M. Response of primary fibroblasts and osteoblasts to plasma treated polyetheretherketone (PEEK) surfaces. J. Mater. Sci. Mater. Med. 2005, 16, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xu, M.; Zhang, W.; Kwok, D.T.K.; Jiang, J.; Wu, Z.; Chu, P.K. Mechanical and biological characteristics of diamond-like carbon coated poly aryl-ether-ether-ketone. Biomaterials 2010, 31, 8181–8187. [Google Scholar] [CrossRef] [PubMed]
- Nieminen, T.; Kallela, I.; Wuolijoki, E.; Kainulainen, H.; Hiidenheimo, I.; Rantala, I. Amorphous and crystalline polyetheretherketone: Mechanical properties and tissue reactions during a 3-year follow-up. J. Biomed. Mater. Res. Part A 2008, 84, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Toth, J.M.; Wang, M.; Estes, B.T.; Scifert, J.L.; Seim, H.B.; Turner, A.S. Polyetheretherketone as a biomaterial for spinal applications. Biomaterials 2006, 27, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Sagomonyants, K.B.; Jarman-Smith, M.L.; Devine, J.N.; Aronow, M.S.; Gronowicz, G.A. The in vitro response of human osteoblasts to polyetheretherketone (PEEK) substrates compared to commercially pure titanium. Biomaterials 2008, 29, 1563–1572. [Google Scholar] [CrossRef]
- Olivares-Navarrete, R.; Gittens, R.A.; Schneider, J.M.; Hyzy, S.L.; Haithcock, D.A.; Ullrich, P.F.; Schwartz, Z.; Boyan, B.D. Osteoblasts exhibit a more differentiated phenotype and increased bone morphogenetic protein production on titanium alloy substrates than on poly-ether-ether-ketone. Spine J 2012, 12, 265–272. [Google Scholar] [CrossRef]
- Olivares-Navarrete, R.; Hyzy, S.L.; Gittens, R.A.s.; Schneider, J.M.; Haithcock, D.A.; Ullrich, P.F.; Slosar, P.J.; Schwartz, Z.; Boyan, B.D. Rough titanium alloys regulate osteoblast production of angiogenic factors. Spine J 2013, 13, 1563–1570. [Google Scholar] [CrossRef]
- Zhao, M.; An, M.; Wang, Q.; Liu, X.; Lai, W.; Zhao, X.; Wei, S.; Ji, J. Quantitative proteomic analysis of human osteoblast-like MG-63 cells in response to bioinert implant material titanium and polyetheretherketone. J Proteom. 2012, 75, 3560–3573. [Google Scholar] [CrossRef]
- Olivares-Navarrete, R.; Hyzy, S.L.; Slosar, P.J.; Schneider, J.M.; Schwartz, Z.; Boyan, B.D. Implant materials generate different peri-implant inflammatory factors: Poly-ether-ether-ketone promotes fibrosis and microtextured titanium promotes osteogenic factors. Spine (Phila Pa 1976) 2015, 40, 399–404. [Google Scholar] [CrossRef]
- Koch, F.P.; Weng, D.; Krämer, S.; Biesterfeld, S.; Jahn-Eimermacher, A.; Wagner, W. Osseointegration of one-piece zirconia implants compared with a titanium implant of identical design: A histomorphometric study in the dog. Clin. Oral Implant Res. 2010, 21, 350–356. [Google Scholar] [CrossRef]
- Nakahara, I.; Takao, M.; Goto, T.; Ohtsuki, C.; Hibino, S.; Sugano, N. Interfacial shear strength of bioactive-coated carbon fiber reinforced polyetheretherketone after in vivo implantation. J. Orthop. Res. 2012, 30, 1618–1625. [Google Scholar] [CrossRef] [PubMed]
- Webster, T.J.; Patel, A.A.; Rahaman, M.N.; Sonny Bal, B. Anti-infective and osteointegration properties of silicon nitride, poly(ether ether ketone), and titanium implants. Acta Biomater. 2012, 8, 4447–4454. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.H.; Li, Y.; Zhang, Y.Q.; Li, X.K.; Yuan, C.F.; Hao, Y.L.; Zhang, Z.Y.; Guo, Z. Porous titanium-6 aluminum-4 vanadium cage has better osseointegration and less micromotion than a poly-ether-ether-ketone cage in sheep vertebral fusion. Artif. Organs 2013, 37, E191–E201. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S.M.; Devine, J.N. PEEK biomaterials in trauma, orthopedic, and spinal implants. Biomaterials 2007, 28, 4845–4869. [Google Scholar] [CrossRef] [PubMed]
- Caglar, I.; Ates, S.M.; Yesil Duymus, Z. An in vitro evaluation of the effect of various adhesives and surface treatments on bond strength of resin cement to polyetheretherketone. J. Prosthodont. 2019, 28, e342–e349. [Google Scholar] [CrossRef] [PubMed]
- Stawarczyk, B.; Jordan, P.; Schmidlin, P.R.; Roos, M.; Eichberger, M.; Gernet, W.; Keul, C. PEEK surface treatment effects on tensile bond strength to veneering resins. J. Prosthet. Dent. 2014, 112, 1278–1288. [Google Scholar] [CrossRef]
- Zhou, L.; Qian, Y.; Zhu, Y.; Liu, H.; Gan, K.; Guo, J. The effect of different surface treatments on the bond strength of PEEK composite materials. Dent. Mater. 2014, 30, e209–e215. [Google Scholar] [CrossRef]
- Toledano, M.; Vallecillo-Rivas, M.; Aguilera, F.S.; Osorio, M.T.; Osorio, E.; Osorio, R. Polymeric zinc-doped nanoparticles for high performance in restorative dentistry. J. Dent. 2021, 107, 103616. [Google Scholar] [CrossRef]
- Van Landuyt, K.L.; Snauwaert, J.; De Munck, J.; Peumans, M.; Yoshida, Y.; Poitevin, A.; Coutinho, E.; Suzuki, K.; Lambrechts, P.; Van Meerbeek, B. Systematic review of the chemical composition of contemporary dental adhesives. Biomaterials 2007, 28, 3757–3785. [Google Scholar] [CrossRef]
- Yilmaz, B.; Gouveia, D.; Schimmel, M.; Lu, W.-E.; Özcan, M.; Abou-Ayash, S. Effect of adhesive system, resin cement, heat-pressing technique, and thermomechanical aging on the adhesion between titanium base and a high-performance polymer. J. Prosthet. Dent. 2022. [Google Scholar] [CrossRef]
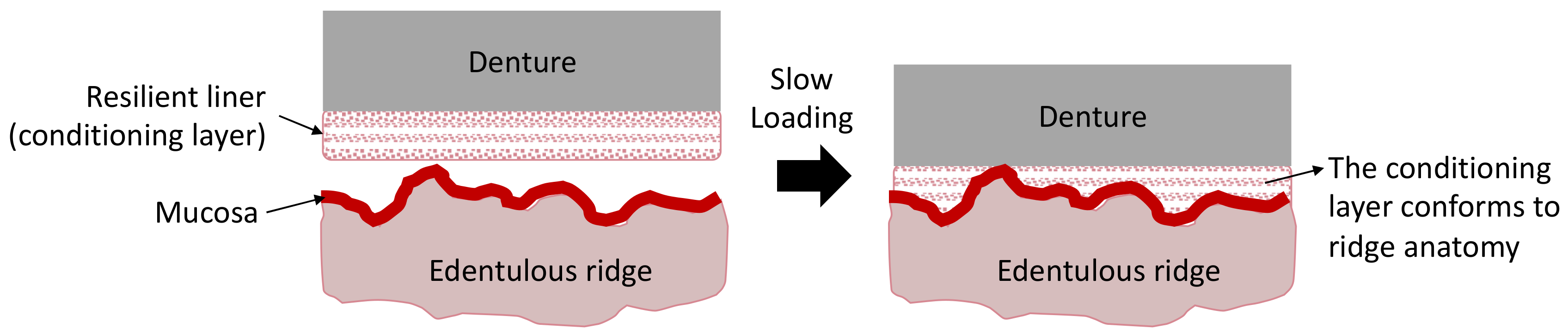
- Rathi, S.; Verma, A. Resilient liners in prosthetic dentistry: An update. Int. J. Appl. Dent. Sci. 2018, 4, 34–38. [Google Scholar]
- Azpiazu-Flores, F.X.; Schricker, S.R.; Seghi, R.R.; Johnston, W.M.; Leyva del Rio, D. Adhesive strength of 3 long-term resilient liners to CAD-CAM denture base polymers and heat-polymerized polymethyl methacrylate with thermocycling. J. Prosthet. Dent. 2022. [Google Scholar] [CrossRef] [PubMed]
- Stawarczyk, B.; Taufall, S.; Roos, M.; Schmidlin, P.R.; Lümkemann, N. Bonding of composite resins to PEEK: The influence of adhesive systems and air-abrasion parameters. Clin. Oral Investig. 2018, 22, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Kern, M.; Lehmann, F. Influence of surface conditioning on bonding to polyetheretherketon (PEEK). Dent. Mater. 2012, 28, 1280–1283. [Google Scholar] [CrossRef]
- Rosentritt, M.; Preis, V.; Behr, M.; Sereno, N.; Kolbeck, C. Shear bond strength between veneering composite and PEEK after different surface modifications. Clin. Oral Investig. 2015, 19, 739–744. [Google Scholar] [CrossRef]
- Escobar, M.; Souza, J.C.M.; Barra, G.M.O.; Fredel, M.C.; Özcan, M.; Henriques, B. On the synergistic effect of sulfonic functionalization and acidic adhesive conditioning to enhance the adhesion of PEEK to resin-matrix composites. Dent. Mater. 2021, 37, 741–754. [Google Scholar] [CrossRef]
- Abdulfattah, N.; Schmidt, F.; Wang, Y.; Bötticher, N.; Konzack, N.; Giuliano, M.; Müller, W.-D.; Schwitalla, A.D. Ultrasonic welding of polyetheretherketone for dental applications. J. Mech. Behav. Biomed. Mater. 2022, 130, 105225. [Google Scholar] [CrossRef]
- Hudecki, A.; Kiryczyński, G.; Łos, M.J. Chapter 7—Biomaterials, definition, overview. In Stem Cells and Biomaterials for Regenerative Medicine; Łos, M.J., Hudecki, A., Wiecheć, E., Eds.; Academic Press: Boston, MA, USA, 2019; pp. 85–98. [Google Scholar] [CrossRef]
- Albrektsson, T.; Johansson, C. Osteoinduction, osteoconduction and osseointegration. Eur. Spine J. 2001, 10, S96–S101. [Google Scholar]
- Terheyden, H.; Lang, N.P.; Bierbaum, S.; Stadlinger, B. Osseointegration—Communication of cells. Clin. Oral Implant Res. 2012, 23, 1127–1135. [Google Scholar] [CrossRef]
- Gruber, R.; Bosshardt, D.D. Chapter 54—Dental implantology and implants—Tissue interface. In Stem Cell Biology and Tissue Engineering in Dental Sciences; Vishwakarma, A., Sharpe, P., Shi, S., Ramalingam, M., Eds.; Academic Press: Boston, MA, USA, 2015; pp. 735–747. [Google Scholar] [CrossRef]
- Boschetto, F.; Marin, E.; Ohgitani, E.; Adachi, T.; Zanocco, M.; Horiguchi, S.; Zhu, W.; McEntire, B.J.; Mazda, O.; Bal, B.S.; et al. Surface functionalization of PEEK with silicon nitride. Biomed. Mater. 2020, 16, 015015. [Google Scholar] [CrossRef]
- Zhang, J.; Wei, W.; Yang, L.; Pan, Y.; Wang, X.; Wang, T.; Tang, S.; Yao, Y.; Hong, H.; Wei, J. Stimulation of cell responses and bone ingrowth into macro-microporous implants of nano-bioglass/polyetheretherketone composite and enhanced antibacterial activity by release of hinokitiol. Colloids Surf. B Biointerfaces 2018, 164, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, K.; Hao, Y.; Liu, M.; Wu, W. Osteoblast/bone-tissue responses to porous surface of polyetheretherketone–nanoporous lithium-doped magnesium silicate blends’ integration with polyetheretherketone. Int. J. Nanomed. 2019, 14, 4975–4989. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Pan, Y.; Tang, S.; Li, Q.; Tang, T.; Zheng, K.; Boccaccini, A.R.; Wei, S.; Wei, J.; Su, J. Macro-mesoporous composites containing PEEK and mesoporous diopside as bone implants: Characterization, in vitro mineralization, cytocompatibility, and vascularization potential and osteogenesis in vivo. J. Mater. Chem. B 2017, 5, 8337–8352. [Google Scholar] [CrossRef]
- Yu, H.; Chen, Y.; Mao, M.; Liu, D.; Ai, J.; Leng, W. PEEK-biphasic bioceramic composites promote mandibular defect repair and upregulate BMP-2 expression in rabbits. Mol. Med. Rep. 2018, 17, 8221–8227. [Google Scholar] [CrossRef] [PubMed]
- Busscher, H.J.; Rinastiti, M.; Siswomihardjo, W.; van der Mei, H.C. Biofilm formation on dental restorative and implant materials. J. Dent. Res. 2010, 89, 657–665. [Google Scholar] [CrossRef]
- Larsen, T.; Fiehn, N.-E. Dental biofilm infections—An update. APMIS 2017, 125, 376–384. [Google Scholar] [CrossRef]
- Øilo, M.; Bakken, V. Biofilm and Dental Biomaterials. Materials 2015, 8, 2887–2900. [Google Scholar] [CrossRef]
- Kligman, S.; Ren, Z.; Chung, C.-H.; Perillo, M.A.; Chang, Y.-C.; Koo, H.; Zheng, Z.; Li, C. The impact of dental implant surface modifications on osseointegration and biofilm formation. J. Clin. Med. 2021, 10, 1641. [Google Scholar] [CrossRef]
- Hickok, N.J.; Shapiro, I.M.; Chen, A.F. The impact of incorporating antimicrobials into implant surfaces. J. Dent. Res. 2017, 97, 14–22. [Google Scholar] [CrossRef]
- Lewis, K. Persister cells: Molecular mechanisms related to antibiotic tolerance. In Antibiotic Resistance, Part of the Handbook of Experimental Pharmacology; Coates, A.R.M., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; Volume 211, pp. 121–133. [Google Scholar]
- Bormann, N.; Schwabe, P.; Smith, M.D.; Wildemann, B. Analysis of parameters influencing the release of antibiotics mixed with bone grafting material using a reliable mixing procedure. Bone 2014, 59, 162–172. [Google Scholar] [CrossRef]
- Widyaya, V.T.; Müller, C.; Al-Ahmad, A.; Lienkamp, K. Three-dimensional, bifunctional microstructured polymer hydrogels made from polyzwitterions and antimicrobial polymers. Langmuir 2019, 35, 1211–1226. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Liu, C.; Chen, T.; Liu, H. The study of PEEK composites as the dental implant materials. J. Simul. 2017, 5, 5–7. [Google Scholar]
- Sampaio, M.; Buciumeanu, M.; Henriques, B.; Silva, F.S.; Souza, J.C.M.; Gomes, J.R. Tribocorrosion behavior of veneering biomedical PEEK to Ti6Al4V structures. J. Mech. Behav. Biomed. Mater. 2016, 54, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Pei, X.-Q.; Lin, L.; Schlarb, A.K.; Bennewitz, R. Correlation of friction and wear across length scales for PEEK sliding against steel. Tribol. Int. 2019, 136, 462–468. [Google Scholar] [CrossRef]
- Kucher, M.; Dannemann, M.; Füßel, R.; Weber, M.-T.; Modler, N. Sliding friction and wear of human teeth against biocompatible polyether ether ketone (PEEK) under various wear conditions. Wear 2021, 486–487, 204110. [Google Scholar] [CrossRef]
- Souza, J.C.M.; Correia, M.S.T.; Oliveira, M.N.; Silva, F.S.; Henriques, B.; Novaes de Oliveira, A.P.; Gomes, J.R. PEEK-matrix composites containing different content of natural silica fibers or particulate lithium-zirconium silicate glass fillers: Coefficient of friction and wear volume measurements. Biotribology 2020, 24, 100147. [Google Scholar] [CrossRef]
- Sampaio, M.; Buciumeanu, M.; Henriques, B.; Silva, F.S.; Souza, J.C.M.; Gomes, J.R. Comparison between PEEK and Ti6Al4V concerning micro-scale abrasion wear on dental applications. J. Mech. Behav. Biomed. Mater. 2016, 60, 212–219. [Google Scholar] [CrossRef]
- Babaier, R.; Watts, D.C.; Silikas, N. Effects of three food-simulating liquids on the roughness and hardness of CAD/CAM polymer composites. Dent. Mater. 2022, 38, 874–885. [Google Scholar] [CrossRef]
- Souza, J.C.M.; Barbosa, S.L.; Ariza, E.; Celis, J.P.; Rocha, L.A. Simultaneous degradation by corrosion and wear of titanium in artificial saliva containing fluorides. Wear 2012, 292–293, 82–88. [Google Scholar] [CrossRef]
- Sansone, V.; Pagani, D.; Melato, M. The effects on bone cells of metal ions released from orthopaedic implants. A review. Clin. Cases Miner. Bone Metab. 2013, 10, 34–40. [Google Scholar] [CrossRef]
- Goodman, S.B. Wear particles, periprosthetic osteolysis and the immune system. Biomaterials 2007, 28, 5044–5048. [Google Scholar] [CrossRef] [PubMed]
- Bartolomeu, F.; Buciumeanu, M.; Costa, M.M.; Alves, N.; Gasik, M.; Silva, F.S.; Miranda, G. Multi-material Ti6Al4V & PEEK cellular structures produced by Selective Laser Melting and Hot Pressing: A tribocorrosion study targeting orthopedic applications. J. Mech. Behav. Biomed. Mater. 2019, 89, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Lang, N.P.; Salvi, G.E.; Huynh-Ba, G.; Ivanovski, S.; Donos, N.; Bosshardt, D.D. Early osseointegration to hydrophilic and hydrophobic implant surfaces in humans. Clin. Oral Implant. Res. 2011, 22, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Sartoretto, S.C.; Alves, A.T.N.N.; Resende, R.F.B.; Calasans-Maia, J.; Granjeiro, J.M.; Calasans-Maia, M.D. Early osseointegration driven by the surface chemistry and wettability of dental implants. J. Appl. Oral Sci. 2015, 23, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Polo-Corrales, L.; Latorre-Esteves, M.; Ramirez-Vick, J.E. Scaffold design for bone regeneration. J. Nanosci. Nanotechnol. 2014, 14, 15–56. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Herten, M.; Sager, M.; Wieland, M.; Dard, M.; Becker, J. Histological and immunohistochemical analysis of initial and early osseous integration at chemically modified and conventional SLA® titanium implants: Preliminary results of a pilot study in dogs. Clin. Oral Implant Res. 2007, 18, 481–488. [Google Scholar] [CrossRef]
- Achinas, S.; Charalampogiannis, N.; Euverink, G.J.W. A Brief Recap of Microbial Adhesion and Biofilms. Appl. Sci. 2019, 9, 2801. [Google Scholar] [CrossRef]
- How, K.Y.; Song, K.P.; Chan, K.G. Porphyromonas gingivalis: An overview of periodontopathic pathogen below the gum line. Front. Microbiol. 2016, 7, 53. [Google Scholar] [CrossRef]
- Naito, Y.; Tohda, H.; Okuda, K.; Takazoe, I. Adherence and hydrophobicity of invasive and noninvasive strains of Porphyromonas gingivalis. Oral Microbiol. Immunol. 1993, 8, 195–202. [Google Scholar] [CrossRef]
- Song, F.; Koo, H.; Ren, D. Effects of material properties on bacterial adhesion and biofilm formation. J. Dent. Res. 2015, 94, 1027–1034. [Google Scholar] [CrossRef]
- Çulhaoğlu, A.K.; Özkır, S.E.; Şahin, V.; Yılmaz, B.; Kılıçarslan, M.A. Effect of various treatment modalities on surface characteristics and shear bond strengths of polyetheretherketone-based core materials. J. Prosthodont. 2020, 29, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Ates, S.M.; Caglar, I.; Yesil Duymus, Z. The effect of different surface pretreatments on the bond strength of veneering resin to polyetheretherketone. J. Adhes. Sci. Technol. 2018, 32, 2220–2231. [Google Scholar] [CrossRef]
- Bötel, F.; Zimmermann, T.; Sütel, M.; Müller, W.-D.; Schwitalla, A.D. Influence of different low-pressure plasma process parameters on shear bond strength between veneering composites and PEEK materials. Dent. Mater. 2018, 34, e246–e254. [Google Scholar] [CrossRef] [PubMed]
- Fokas, G.; Guo, C.Y.; Tsoi, J.K.H. The effects of surface treatments on tensile bond strength of polyether-ketone-ketone (PEKK) to veneering resin. J. Mech. Behav. Biomed. Mater. 2019, 93, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Keul, C.; Liebermann, A.; Schmidlin, P.; Roos, M.; Sener, B.; Stawarczyk, B. Influence of PEEK Surface Modification on Surface Properties and Bond Strength to Veneering Resin Composites. J. Adhes. Dent. 2014, 16, 383–392. [Google Scholar] [CrossRef]
- Lee, K.-S.; Shin, M.-S.; Lee, J.-Y.; Ryu, J.-J.; Shin, S.-W. Shear bond strength of composite resin to high performance polymer PEKK according to surface treatments and bonding materials. J. Adv. Prosthodont. 2017, 9, 350–357. [Google Scholar] [CrossRef]
- Schwitalla, A.D.; Bötel, F.; Zimmermann, T.; Sütel, M.; Müller, W.-D. The impact of argon/oxygen low-pressure plasma on shear bond strength between a veneering composite and different PEEK materials. Dent. Mater. 2017, 33, 990–994. [Google Scholar] [CrossRef]
- Stawarczyk, B.; Silla, M.; Roos, M.; Eichberger, M.; Lümkemann, N. bonding behaviour of polyetherketoneketone to methylmethacrylate- and dimethacrylate-based polymers. J. Adhes. Dent. 2017, 19, 331–338. [Google Scholar] [CrossRef]
- Shabib, S. Use of Nd:YVO4 laser, photodynamic therapy, sulfuric acid and sand blasting on improving bond integrity of PEEK to resin cement with adhesive. Photodiagn. Photodyn. Ther. 2022, 39, 102865. [Google Scholar] [CrossRef]
- Luo, S.; Wang, P.; Ma, M.; Pan, Z.; Lu, L.; Yin, F.; Cai, J. Genistein loaded into microporous surface of nano tantalum/PEEK composite with antibacterial effect regulating cellular response in vitro, and promoting osseointegration in vivo. J. Mech. Behav. Biomed. Mater. 2022, 125, 104972. [Google Scholar] [CrossRef]
- Hieda, A.; Uemura, N.; Hashimoto, Y.; Toda, I.; Baba, S. In vivo bioactivity of porous polyetheretherketone with a foamed surface. Dent. Mater. J. 2017, 36, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Cheng, Q.; Zhao, R.; Zhu, X.; Yang, X.; Yang, X.; Zhang, K.; Song, Y.; Zhang, X. Comparison of osteointegration property between PEKK and PEEK: Effects of surface structure and chemistry. Biomaterials 2018, 170, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Evans, N.T.; Torstrick, F.B.; Lee, C.S.; Dupont, K.M.; Safranski, D.L.; Chang, W.A.; Macedo, A.E.; Lin, A.S.; Boothby, J.M.; Whittingslow, D.C.; et al. High-strength, surface-porous polyether-ether-ketone for load-bearing orthopedic implants. Acta Biomater 2015, 13, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Vaezi, M.; Yang, S. Extrusion-based additive manufacturing of PEEK for biomedical applications. Virtual Phys. Prototyp. 2015, 10, 123–135. [Google Scholar] [CrossRef]
- Jarman-Smith, M.; Brady, M.; Kurtz, S.M.; Cordaro, N.M.; Walsh, W.R. Chapter 12—Porosity in Polyaryletheretherketone. In PEEK Biomaterials Handbook; Kurtz, S.M., Ed.; William Andrew Publishing: Oxford, UK, 2012; pp. 181–199. [Google Scholar] [CrossRef]
- Yu, D.; Wang, J.; Qian, K.-J.; Yu, J.; Zhu, H.-Y. Effects of nanofibers on mesenchymal stem cells: Environmental factors affecting cell adhesion and osteogenic differentiation and their mechanisms. J. Zhejiang Univ.—Sci. B 2020, 21, 871–884. [Google Scholar] [CrossRef]
- Converse, G.L.; Conrad, T.L.; Roeder, R.K. Mechanical properties of hydroxyapatite whisker reinforced polyetherketoneketone composite scaffolds. J. Mech. Behav. Biomed. Mater. 2009, 2, 627–635. [Google Scholar] [CrossRef]
- Uddin, M.N.; Dhanasekaran, P.S.; Asmatulu, R. Mechanical properties of highly porous PEEK bionanocomposites incorporated with carbon and hydroxyapatite nanoparticles for scaffold applications. Prog. Biomater. 2019, 8, 211–221. [Google Scholar] [CrossRef]
- Cheng, K.-J.; Liu, Y.-F.; Wang, R.; Zhang, J.-X.; Jiang, X.-F.; Dong, X.-T.; Xu, X. Topological optimization of 3D printed bone analog with PEKK for surgical mandibular reconstruction. J. Mech. Behav. Biomed. Mater. 2020, 107, 103758. [Google Scholar] [CrossRef]
- Davies, J.E. Understanding peri-implant endosseous healing. J. Dent. Educ. 2003, 67, 932–949. [Google Scholar] [CrossRef]
- Pachauri, P.; Bathala, L.R.; Sangur, R. Techniques for dental implant nanosurface modifications. J. Adv. Prosthodont. 2014, 6, 498–504. [Google Scholar] [CrossRef]
- Hoang, D.; Perrault, D.; Stevanovic, M.; Ghiassi, A. Surgical applications of three-dimensional printing: A review of the current literature & how to get started. Ann. Transl. Med. 2016, 4, 456. [Google Scholar] [PubMed]
- Han, X.; Yang, D.; Yang, C.; Spintzyk, S.; Scheideler, L.; Li, P.; Li, D.; Geis-Gerstorfer, J.; Rupp, F. Carbon fiber reinforced PEEK composites based on 3D-printing technology for orthopedic and dental applications. J. Clin. Med. 2019, 8, 240. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Sharma, N.; Xu, Z.; Scheideler, L.; Geis-Gerstorfer, J.; Rupp, F.; Thieringer, F.M.; Spintzyk, S. An in vitro study of osteoblast response on fused-filament fabrication 3D printed PEEK for dental and cranio-maxillofacial implants. J. Clin. Med. 2019, 8, 771. [Google Scholar] [CrossRef] [PubMed]
- Berent, Z.T.; Wagoner Johnson, A.J. Cell seeding simulation on micropatterned islands shows cell density depends on area to perimeter ratio, not on island size or shape. Acta Biomater. 2020, 107, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wehrle, E.; Adamek, P.; Paul, G.R.; Qin, X.-H.; Rubert, M.; Müller, R. Optimization of mechanical stiffness and cell density of 3D bioprinted cell-laden scaffolds improves extracellular matrix mineralization and cellular organization for bone tissue engineering. Acta Biomater. 2020, 114, 307–322. [Google Scholar] [CrossRef]
- Ardhani, R.; Diana, R.; Pidhatika, B. How porphyromonas gingivalis navigate the map: The effect of surface topography on the adhesion of porphyromonas gingivalis on biomaterials. Materials 2022, 15, 4988. [Google Scholar] [CrossRef]
- Palmer, J.; Flint, S.; Brooks, J. Bacterial cell attachment, the beginning of a biofilm. J. Ind. Microbiol. Biotechnol. 2007, 34, 577–588. [Google Scholar] [CrossRef]
- Heimer, S.; Schmidlin, P.R.; Roos, M.; Stawarczyk, B. Surface properties of polyetheretherketone after different laboratory and chairside polishing protocols. J. Prosthet. Dent. 2017, 117, 419–425. [Google Scholar] [CrossRef]
- Alam, F.; Varadarajan, K.M.; Koo, J.H.; Wardle, B.L.; Kumar, S. Additively manufactured polyetheretherketone (PEEK) with carbon nanostructure reinforcement for biomedical structural applications. Adv. Eng. Mater. 2020, 22, 2000483. [Google Scholar] [CrossRef]
- Quan, D.; Alderliesten, R.; Dransfeld, C.; Tsakoniatis, I.; Teixeira De Freitas, S.; Scarselli, G.; Murphy, N.; Ivanković, A.; Benedictus, R. Significantly enhanced structural integrity of adhesively bonded PPS and PEEK composite joints by rapidly UV-irradiating the substrates. Compos. Sci. Technol. 2020, 199, 108358. [Google Scholar] [CrossRef]
- Li, D.; Zheng, Q.; Wang, Y.; Chen, H. Combining surface topography with polymer chemistry: Exploring new interfacial biological phenomena. Polym. Chem. 2014, 5, 14–24. [Google Scholar] [CrossRef]
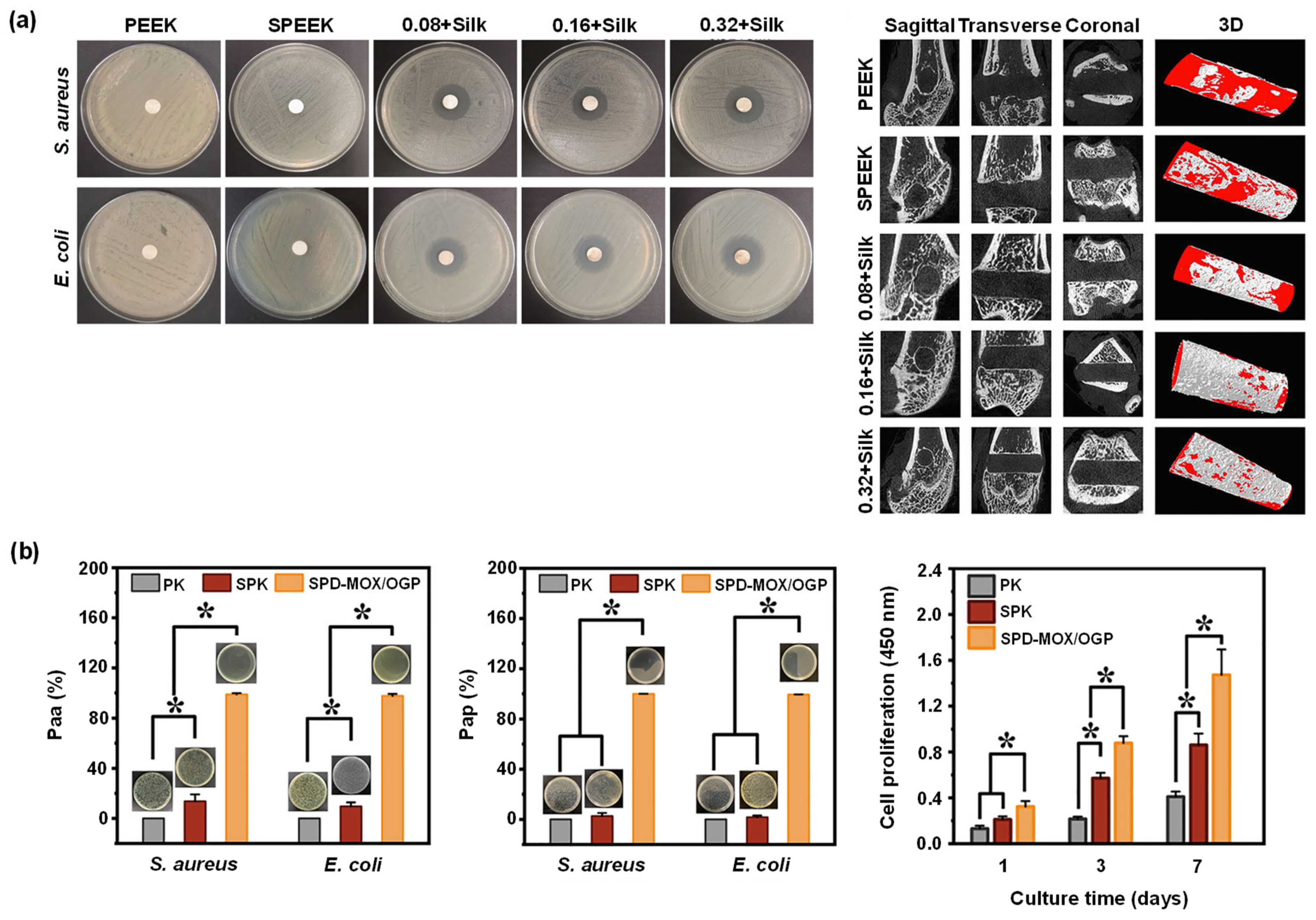
- Brum, R.S.; Monich, P.R.; Berti, F.; Fredel, M.C.; Porto, L.M.; Benfatti, C.A.M.; Souza, J.C.M. On the sulphonated PEEK for implant dentistry: Biological and physicochemical assessment. Mater. Chem. Phys. 2019, 223, 542–547. [Google Scholar] [CrossRef]
- Sang, S.; Yang, C.; Chai, H.; Yuan, X.; Liu, W.; Zhang, X. The sulfonated polyetheretherketone with 3D structure modified by two bio-inspired methods shows osteogenic and antibacterial functions. Chem. Eng. J. 2021, 420, 130059. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, M.; Wang, Z.; Wang, Y.; Dong, W.; Ma, W.; Zhao, S.; Sun, D. 3D-printed porous PEEK scaffold combined with CSMA/POSS bioactive surface: A strategy for enhancing osseointegration of PEEK implants. Compos. Part B Eng. 2022, 230, 109512. [Google Scholar] [CrossRef]
- Gao, C.; Wang, Z.; Jiao, Z.; Wu, Z.; Guo, M.; Wang, Y.; Liu, J.; Zhang, P. Enhancing antibacterial capability and osseointegration of polyetheretherketone (PEEK) implants by dual-functional surface modification. Mater. Des. 2021, 205, 109733. [Google Scholar] [CrossRef]
- Cheng, Q.; Yuan, B.; Chen, X.; Yang, X.; Lin, H.; Zhu, X.; Zhang, K.; Zhang, X. Regulation of surface micro/nano structure and composition of polyetheretherketone and their influence on the behavior of MC3T3-E1 pre-osteoblasts. J. Mater. Chem. B 2019, 7, 5713–5724. [Google Scholar] [CrossRef]
- He, X.; Deng, Y.; Yu, Y.; Lyu, H.; Liao, L. Drug-loaded/grafted peptide-modified porous PEEK to promote bone tissue repair and eliminate bacteria. Colloids Surf. B Biointerfaces 2019, 181, 767–777. [Google Scholar] [CrossRef]
- Wang, W.; Luo, C.J.; Huang, J.; Edirisinghe, M. PEEK surface modification by fast ambient-temperature sulfonation for bone implant applications. J. R. Soc. Interface 2019, 16, 20180955. [Google Scholar] [CrossRef]
- Ma, R.; Wang, J.; Li, C.; Ma, K.; Wei, J.; Yang, P.; Guo, D.; Wang, K.; Wang, W. Effects of different sulfonation times and post-treatment methods on the characterization and cytocompatibility of sulfonated PEEK. J. Biomater. Appl. 2020, 35, 342–352. [Google Scholar] [CrossRef]
- Zhao, Y.; Wong, H.M.; Wang, W.; Li, P.; Xu, Z.; Chong, E.Y.W.; Yan, C.H.; Yeung, K.W.K.; Chu, P.K. Cytocompatibility, osseointegration, and bioactivity of three-dimensional porous and nanostructured network on polyetheretherketone. Biomaterials 2013, 34, 9264–9277. [Google Scholar] [CrossRef]
- Miyazaki, T.; Matsunami, C.; Shirosaki, Y. Bioactive carbon–PEEK composites prepared by chemical surface treatment. Mater. Sci. Eng. C 2017, 70, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Yabutsuka, T.; Fukushima, K.; Hiruta, T.; Takai, S.; Yao, T. Effect of pores formation process and oxygen plasma treatment to hydroxyapatite formation on bioactive PEEK prepared by incorporation of precursor of apatite. Mater. Sci. Eng. C 2017, 81, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Deng, Y.; Yang, L.; Shi, X.; Yang, W.; Chen, Z.-G. Enhanced antibacterial property and osteo-differentiation activity on plasma treated porous polyetheretherketone with hierarchical micro/nano-topography. J. Biomater. Sci. Polym. Ed. 2018, 29, 520–542. [Google Scholar] [CrossRef]
- Saidak, Z.; Marie, P.J. Strontium signaling: Molecular mechanisms and therapeutic implications in osteoporosis. Pharmacol. Ther. 2012, 136, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Jiang, X.; Chen, X.; Shao, Z.; Yang, W. Doxorubicin-Loaded Magnetic Silk Fibroin Nanoparticles for Targeted Therapy of Multidrug-Resistant Cancer. Adv. Mater. 2014, 26, 7393–7398. [Google Scholar] [CrossRef] [PubMed]
- Ekambaram, R.; Paraman, V.; Raja, L.; Suresh, M.K.; Dharmalingam, S. Design and development of electrospun SPEEK incorporated with aminated zirconia and curcumin nanofibers for periodontal regeneration. J. Mech. Behav. Biomed. Mater. 2021, 123, 104796. [Google Scholar] [CrossRef] [PubMed]
- Chaijareenont, P.; Prakhamsai, S.; Silthampitag, P.; Takahashi, H.; Arksornnukit, M. Effects of different sulfuric acid etching concentrations on PEEK surface bonding to resin composite. Dent. Mater. J. 2018, 37, 385–392. [Google Scholar] [CrossRef]
- Zhang, J.; Yi, Y.; Wang, C.; Ding, L.; Wang, R.; Wu, G. Effect of acid-etching duration on the adhesive performance of printed polyetheretherketone to veneering resin. Polymers 2021, 13, 3509. [Google Scholar] [CrossRef]
- Xue, Z.; Wang, Z.; Sun, A.a.; Huang, J.; Wu, W.; Chen, M.; Hao, X.; Huang, Z.; Lin, X.; Weng, S. Rapid construction of polyetheretherketone (PEEK) biological implants incorporated with brushite (CaHPO4·2H2O) and antibiotics for anti-infection and enhanced osseointegration. Mater. Sci. Eng. C 2020, 111, 110782. [Google Scholar] [CrossRef]
- Wei, X.; Zhou, W.; Tang, Z.; Wu, H.; Liu, Y.; Dong, H.; Wang, N.; Huang, H.; Bao, S.; Shi, L.; et al. Magnesium surface-activated 3D printed porous PEEK scaffolds for in vivo osseointegration by promoting angiogenesis and osteogenesis. Bioact. Mater. 2023, 20, 16–28. [Google Scholar] [CrossRef]
- Zhao, Y.; Wong, H.M.; Lui, S.C.; Chong, E.Y.W.; Wu, G.; Zhao, X.; Wang, C.; Pan, H.; Cheung, K.M.C.; Wu, S.; et al. Plasma surface functionalized polyetheretherketone for enhanced osseo-integration at bone-implant interface. ACS Appl. Mater. Interfaces 2016, 8, 3901–3911. [Google Scholar] [CrossRef]
- Senatov, F.; Maksimkin, A.; Chubrik, A.; Kolesnikov, E.; Orlova, P.; Krivozubov, M.; Nikitin, K.; Gromov, A.; Karyagina, A. Osseointegration evaluation of UHMWPE and PEEK-based scaffolds with BMP-2 using model of critical-size cranial defect in mice and push-out test. J. Mech. Behav. Biomed. Mater. 2021, 119, 104477. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lu, T.; Meng, F.; Zhu, H.; Liu, X. Enhanced osteoblast responses to poly ether ether ketone surface modified by water plasma immersion ion implantation. Colloids Surf. B Biointerfaces 2014, 117, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Gabriel, M.; Schmidt, F.; Müller, W.-D.; Schwitalla, A.D. The impact of different low-pressure plasma types on the physical, chemical and biological surface properties of PEEK. Dent. Mater. 2021, 37, e15–e22. [Google Scholar] [CrossRef] [PubMed]
- Waser-Althaus, J.; Salamon, A.; Waser, M.; Padeste, C.; Kreutzer, M.; Pieles, U.; Müller, B.; Peters, K. Differentiation of human mesenchymal stem cells on plasma-treated polyetheretherketone. J. Mater. Sci. Mater. Med. 2014, 25, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Gan, K.; Liu, H.; Jiang, L.; Liu, X.; Song, X.; Niu, D.; Chen, T.; Liu, C. Bioactivity and antibacterial effect of nitrogen plasma immersion ion implantation on polyetheretherketone. Dent. Mater. 2016, 32, e263–e274. [Google Scholar] [CrossRef]
- Wang, B.; Huang, M.; Dang, P.; Xie, J.; Zhang, X.; Yan, X. PEEK in fixed dental prostheses: Application and adhesion improvement. Polymers 2022, 14, 2323. [Google Scholar] [CrossRef]
- Younis, M.; Unkovskiy, A.; ElAyouti, A.; Geis-Gerstorfer, J.; Spintzyk, S. The effect of various plasma gases on the shear bond strength between unfilled polyetheretherketone (PEEK) and veneering composite following artificial aging. Materials 2019, 12, 1447. [Google Scholar] [CrossRef]
- Przykaza, K.; Jurak, M.; Wiącek, A.E.; Mroczka, R. Characteristics of hybrid chitosan/phospholipid-sterol, peptide coatings on plasma activated PEEK polymer. Mater. Sci. Eng. C 2021, 120, 111658. [Google Scholar] [CrossRef]
- Chen, M.; Ouyang, L.; Lu, T.; Wang, H.; Meng, F.; Yang, Y.; Ning, C.; Ma, J.; Liu, X. enhanced bioactivity and bacteriostasis of surface fluorinated polyetheretherketone. ACS Appl. Mater. Interfaces 2017, 9, 16824–16833. [Google Scholar] [CrossRef]
- Sundriyal, P.; Sahu, M.; Prakash, O.; Bhattacharya, S. Long-term surface modification of PEEK polymer using plasma and PEG silane treatment. Surf. Interfaces 2021, 25, 101253. [Google Scholar] [CrossRef]
- Al Qahtani, M.S.A.; Wu, Y.; Spintzyk, S.; Krieg, P.; Killinger, A.; Schweizer, E.; Stephan, I.; Scheideler, L.; Geis-Gerstorfer, J.; Rupp, F. UV-A and UV-C light induced hydrophilization of dental implants. Dent. Mater. 2015, 31, e157–e167. [Google Scholar] [CrossRef] [PubMed]
- Mathieson, I.; Bradley, R.H. Improved adhesion to polymers by UV/ozone surface oxidation. Int. J. Adhes. Adhes. 1996, 16, 29–31. [Google Scholar] [CrossRef]
- Guo, L.; Smeets, R.; Kluwe, L.; Hartjen, P.; Barbeck, M.; Cacaci, C.; Gosau, M.; Henningsen, A. Cytocompatibility of titanium, zirconia and modified PEEK after surface treatment using UV light or non-thermal plasma. Int. J. Mol. Sci. 2019, 20, 5596. [Google Scholar] [CrossRef]
- Guo, L.; Zou, Z.; Smeets, R.; Kluwe, L.; Hartjen, P.; Gosau, M.; Henningsen, A. Attachment and osteogenic potential of dental pulp stem cells on non-thermal plasma and UV light treated titanium, zirconia and modified PEEK surfaces. Materials 2022, 15, 2215. [Google Scholar] [CrossRef]
- Najeeb, S.; Khurshid, Z.; Matinlinna, J.P.; Siddiqui, F.; Nassani, M.Z.; Baroudi, K. nanomodified peek dental implants: Bioactive composites and surface modification—A review. Int. J. Dent. 2015, 2015, 381759. [Google Scholar] [CrossRef]
- Oladapo, B.I.; Ismail, S.O.; Bowoto, O.K.; Omigbodun, F.T.; Olawumi, M.A.; Muhammad, M.A. Lattice design and 3D-printing of PEEK with Ca10(OH)(PO4)3 and in-vitro bio-composite for bone implant. Int. J. Biol. Macromol. 2020, 165, 50–62. [Google Scholar] [CrossRef]
- Johansson, P.; Jimbo, R.; Kjellin, P.; Currie, F.; Chrcanovic, B.R.; Wennerberg, A. Biomechanical evaluation and surface characterization of a nano-modified surface on PEEK implants: A study in the rabbit tibia. Int. J. Nanomed. 2014, 9, 3903–3911. [Google Scholar] [CrossRef]
- Han, C.-M.; Lee, E.-J.; Kim, H.-E.; Koh, Y.-H.; Kim, K.N.; Ha, Y.; Kuh, S.-U. The electron beam deposition of titanium on polyetheretherketone (PEEK) and the resulting enhanced biological properties. Biomaterials 2010, 31, 3465–3470. [Google Scholar] [CrossRef]
- Macdonald, M.L.; Samuel, R.E.; Shah, N.J.; Padera, R.F.; Beben, Y.M.; Hammond, P.T. Tissue integration of growth factor-eluting layer-by-layer polyelectrolyte multilayer coated implants. Biomaterials 2011, 32, 1446–1453. [Google Scholar] [CrossRef]
- Han, C.-M.; Jang, T.-S.; Kim, H.-E.; Koh, Y.-H. Creation of nanoporous TiO2 surface onto polyetheretherketone for effective immobilization and delivery of bone morphogenetic protein. J. Biomed. Mater. Res. Part A 2014, 102, 793–800. [Google Scholar] [CrossRef]
- Khabibullin, A.; Mastan, E.; Matyjaszewski, K.; Zhu, S. Surface-initiated atom transfer radical polymerization. In Controlled Radical Polymerization at and from Solid Surfaces; Vana, P., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 29–76. [Google Scholar] [CrossRef]
- Flejszar, M.; Chmielarz, P. Surface modifications of poly(ether ether ketone) via polymerization methods—Current status and future prospects. Materials 2020, 13, 999. [Google Scholar] [CrossRef] [PubMed]
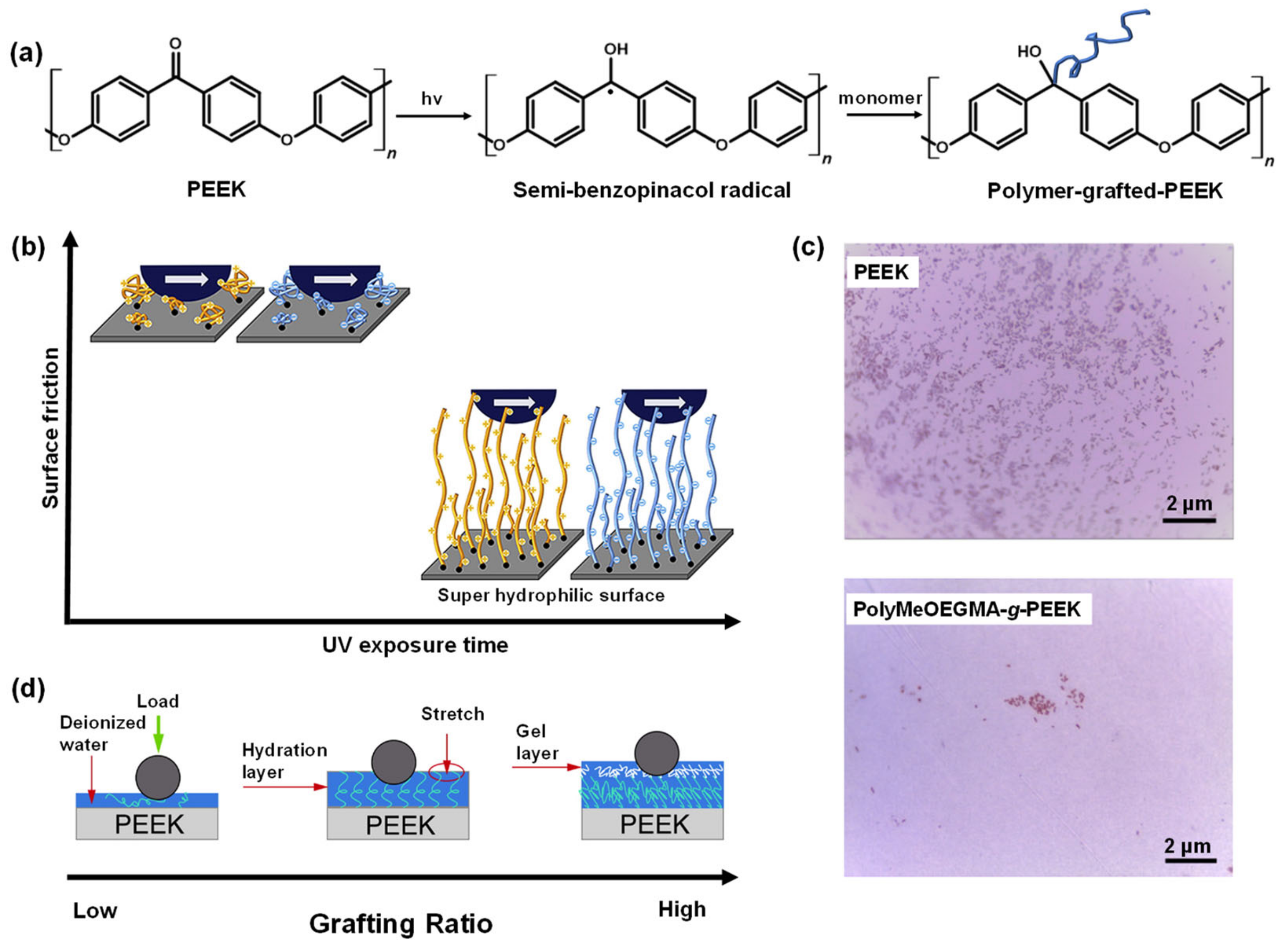
- Yameen, B.; Álvarez, M.; Azzaroni, O.; Jonas, U.; Knoll, W. Tailoring of poly(ether ether ketone) surface properties via surface-initiated atom transfer radical polymerization. Langmuir 2009, 25, 6214–6220. [Google Scholar] [CrossRef]
- Fristrup, C.J.; Jankova, K.; Hvilsted, S. Hydrophilization of poly(ether ether ketone) films by surface-initiated atom transfer radical polymerization. Polym. Chem. 2010, 1, 1696–1701. [Google Scholar] [CrossRef]
- Kyomoto, M.; Ishihara, K. Self-initiated surface graft polymerization of 2-methacryloyloxyethyl phosphorylcholine on poly(ether ether ketone) by photoirradiation. ACS Appl. Mater. Interfaces 2009, 1, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Kyomoto, M.; Moro, T.; Takatori, Y.; Kawaguchi, H.; Nakamura, K.; Ishihara, K. Self-initiated surface grafting with poly(2-methacryloyloxyethyl phosphorylcholine) on poly(ether-ether-ketone). Biomaterials 2010, 31, 1017–1024. [Google Scholar] [CrossRef] [PubMed]
- Chouwatat, P.; Hirai, T.; Higaki, K.; Higaki, Y.; Sue, H.-J.; Takahara, A. Aqueous lubrication of poly(etheretherketone) via surface-initiated polymerization of electrolyte monomers. Polymer 2017, 116, 549–555. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, L.; Xiao, L.; Zhang, Q.; Liu, Y. Enhanced osteogenic activity of phosphorylated polyetheretherketone via surface-initiated grafting polymerization of vinylphosphonic acid. Colloids Surf. B Biointerfaces 2019, 173, 591–598. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, L.; Ma, Y.; Xiao, L.; Liu, Y. Enhanced osteoblasts responses to surface-sulfonated polyetheretherketone via a single-step ultraviolet-initiated graft polymerization. Ind. Eng. Chem. Res. 2018, 57, 10403–10410. [Google Scholar] [CrossRef]
- Yousaf, A.; Farrukh, A.; Oluz, Z.; Tuncel, E.; Duran, H.; Doğan, S.Y.; Tekinay, T.; Rehman, H.u.; Yameen, B. UV-light assisted single step route to functional PEEK surfaces. React. Funct. Polym. 2014, 83, 70–75. [Google Scholar] [CrossRef]
- Zhao, X.; Xiong, D.; Wang, K.; Wang, N. Improved biotribological properties of PEEK by photo-induced graft polymerization of acrylic acid. Mater. Sci. Eng. C 2017, 75, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhu, Y.; Gao, H.; Ge, P.; Ren, K.; Gao, J.; Cao, Y.; Han, D.; Zhang, J. One-step fabrication of functionalized poly(etheretherketone) surfaces with enhanced biocompatibility and osteogenic activity. Mater. Sci. Eng. C 2018, 88, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Khoury, J.; Edelman, E.R.; Talmo, C.; Webster, T.J. Accelerated neutral atom beam (ANAB) modified polyethylene for decreased wear and reduced bacteria colonization: An in vitro study. Nanomed. Nanotechnol. Biol. Med. 2022, 42, 102540. [Google Scholar] [CrossRef] [PubMed]
- Koch, F.P.; Weng, D.; Kramer, S.; Wagner, W. Soft tissue healing at one-piece zirconia implants compared to titanium and PEEK implants of identical design: A histomorphometric study in the dog. Int. J. Periodont. Restor. Dent. 2013, 33, 669–677. [Google Scholar] [CrossRef]
- Tang, X.; Huang, K.; Dai, J.; Wu, Z.; Cai, L.; Yang, L.; Wei, J.; Sun, H. Influences of surface treatments with abrasive paper and sand-blasting on surface morphology, hydrophilicity, mineralization and osteoblasts behaviors of n-CS/PK composite. Sci. Rep. 2017, 7, 568. [Google Scholar] [CrossRef]
- Deng, Y.; Liu, X.; Xu, A.; Wang, L.; Luo, Z.; Zheng, Y.; Deng, F.; Wei, J.; Tang, Z.; Wei, S. Effect of surface roughness on osteogenesis in vitro and osseointegration in vivo of carbon fiber-reinforced polyetheretherketone–nanohydroxyapatite composite. Int. J. Nanomed. 2015, 10, 1425–1447. [Google Scholar]
- Ourahmoune, R.; Salvia, M.; Mathia, T.G.; Mesrati, N. Surface morphology and wettability of sandblasted PEEK and its composites. Scanning 2014, 36, 64–75. [Google Scholar] [CrossRef]
- Sunarso; Tsuchiya, A.; Fukuda, N.; Toita, R.; Tsuru, K.; Ishikawa, K. Effect of micro-roughening of poly(ether ether ketone) on bone marrow derived stem cell and macrophage responses, and osseointegration. J. Biomater. Sci. Polym. Ed. 2018, 29, 1375–1388. [Google Scholar] [CrossRef]
- Shehab-Eldin, A.M.G.; El-Sayed, M.; Osman, M.F. Effect of surface pretreatments on the surface roughness and shear bond strength of a modified polyetheretherketone (PEEK) material. Mansoura J. Dent. 2020, 7, 8–16. [Google Scholar] [CrossRef]
- Gouveia, D.d.N.M.; Razzoog, M.E.; Sierraalta, M.; Alfaro, M.F. Effect of surface treatment and manufacturing process on the shear bond strength of veneering composite resin to polyetherketoneketone (PEKK) and polyetheretherketone (PEEK). J. Prosthet. Dent. 2022, 128, 1061–1066. [Google Scholar] [CrossRef]
- Spyropoulos, D.; Kamposiora, P.; Zoidis, P. The effect of surface pretreatment and water storage on the bonding strength of a resin composite cement to modified PEEK. Eur. J. Prosthodont. Restor. Dent. 2020, 28, 121–127. [Google Scholar] [PubMed]
- Adem, N.; Bal, B.; Kazazoğlu, E. Comparative study of chemical and mechanical surface treatment effects on the shear bond strength of polyether-ether-ketone to veneering resin. Int. J. Prosthodont. 2021, 35, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Tosun, B.; Yanıkoğlu, N. Evaluation of the effects of different surface modification methods on the bond strength of high-performance polymers and resin matrix ceramics. Clin. Oral Investig. 2022, 26, 3781–3790. [Google Scholar] [CrossRef] [PubMed]
- Laurens, P.; Sadras, B.; Decobert, F.; Arefi-Khonsari, F.; Amouroux, J. Enhancement of the adhesive bonding properties of PEEK by excimer laser treatment. Int. J. Adhes. Adhes. 1998, 18, 19–27. [Google Scholar] [CrossRef]
- Cordero, D.; López-Álvarez, M.; Rodríguez-Valencia, C.; Serra, J.; Chiussi, S.; González, P. In vitro response of pre-osteoblastic cells to laser microgrooved PEEK. Biomed. Mater. 2013, 8, 055006. [Google Scholar] [CrossRef]
- Tsuka, H.; Morita, K.; Kato, K.; Kimura, H.; Abekura, H.; Hirata, I.; Kato, K.; Tsuga, K. Effect of laser groove treatment on shear bond strength of resin-based luting agent to polyetheretherketone (PEEK). J. Prosthodont. Res. 2019, 63, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Wan, Y.; Zhu, X.; Zhang, P.; Yang, Z.; Yao, F.; Luo, H. Simultaneous engineering of nanofillers and patterned surface macropores of graphene/hydroxyapatite/polyetheretherketone ternary composites for potential bone implants. Mater. Sci. Eng. C 2021, 123, 111967. [Google Scholar] [CrossRef]
- Riveiro, A.; Soto, R.; Comesaña, R.; Boutinguiza, M.; del Val, J.; Quintero, F.; Lusquiños, F.; Pou, J. Laser surface modification of PEEK. Appl. Surf. Sci. 2012, 258, 9437–9442. [Google Scholar] [CrossRef]
- Cai, G.; Wang, H.; Jung, Y.K.; Xu, Z.; Zhang, J.; He, J.; Wang, D.; Shin, J.-W.; Kaewmanee, R.; Nabanita, S.; et al. Hierarchically porous surface of PEEK/nMCS composite created by femtosecond laser and incorporation of resveratrol exhibiting antibacterial performances and osteogenic activity in vitro. Compos. Part B Eng. 2020, 186, 107802. [Google Scholar] [CrossRef]
- Zheng, Y.; Xiong, C.; Wang, Z.; Li, X.; Zhang, L. A combination of CO2 laser and plasma surface modification of poly(etheretherketone) to enhance osteoblast response. Appl. Surf. Sci. 2015, 344, 79–88. [Google Scholar] [CrossRef]
- Ulgey, M.; Gorler, O.; Karahan Gunduz, C. Effects of laser modalities on shear bond strengths of composite superstructure to zirconia and PEEK infrastructures: An in vitro study. Odontology 2021, 109, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Jahandideh, Y.; Falahchai, M.; Pourkhalili, H. Effect of Surface Treatment With Er:YAG and CO2 Lasers on Shear Bond Strength of Polyether Ether Ketone to Composite Resin Veneers. J. Lasers Med. Sci. 2020, 11, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Taha, D.; Safwat, F.; Wahsh, M. Effect of combining different surface treatments on the surface characteristics of polyetheretherketone-based core materials and shear bond strength to a veneering composite resin. J. Prosthet. Dent. 2022, 127, 599.e1–599.e7. [Google Scholar] [CrossRef] [PubMed]
- Henriques, B.; Fabris, D.; Mesquita-Guimarães, J.; Sousa, A.C.; Hammes, N.; Souza, J.C.M.; Silva, F.S.; Fredel, M.C. Influence of laser structuring of PEEK, PEEK-GF30 and PEEK-CF30 surfaces on the shear bond strength to a resin cement. J. Mech. Behav. Biomed. Mater. 2018, 84, 225–234. [Google Scholar] [CrossRef]
- Hammouti, S.; Pascale-Hamri, A.; Faure, N.; Beaugiraud, B.; Guibert, M.; Mauclair, C.; Benayoun, S.; Valette, S. Wear rate control of peek surfaces modified by femtosecond laser. Appl. Surf. Sci. 2015, 357, 1541–1551. [Google Scholar] [CrossRef]
- Wang, M. The tribological performance of engineered micro-surface topography by picosecond laser on PEEK. Ind. Lubr. Tribol. 2020, 72, 172–179. [Google Scholar] [CrossRef]
- Wyatt, H.; Elliott, M.; Revill, P.; Clarke, A. The effect of engineered surface topography on the tribology of CFR-PEEK for novel hip implant materials. Biotribology 2016, 7, 22–30. [Google Scholar] [CrossRef]
- Dufils, J.; Faverjon, F.; Héau, C.; Donnet, C.; Benayoun, S.; Valette, S. Combination of laser surface texturing and DLC coating on PEEK for enhanced tribological properties. Surf. Coat. Technol. 2017, 329, 29–41. [Google Scholar] [CrossRef]
- Kirkpatrick, A.; Kirkpatrick, S.; Walsh, M.; Chau, S.; Mack, M.; Harrison, S.; Svrluga, R.; Khoury, J. Investigation of accelerated neutral atom beams created from gas cluster ion beams. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2013, 307, 281–289. [Google Scholar] [CrossRef]
- Khoury, J.; Selezneva, I.; Pestov, S.; Tarassov, V.; Ermakov, A.; Mikheev, A.; Lazov, M.; Kirkpatrick, S.R.; Shashkov, D.; Smolkov, A. Surface bioactivation of PEEK by neutral atom beam technology. Bioact. Mater. 2019, 4, 132–141. [Google Scholar] [CrossRef]
- Khoury, J.; Maxwell, M.; Cherian, R.E.; Bachand, J.; Kurz, A.C.; Walsh, M.; Assad, M.; Svrluga, R.C. Enhanced bioactivity and osseointegration of PEEK with accelerated neutral atom beam technology. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 531–543. [Google Scholar] [CrossRef] [PubMed]
- Ajami, S.; Coathup, M.J.; Khoury, J.; Blunn, G.W. Augmenting the bioactivity of polyetheretherketone using a novel accelerated neutral atom beam technique. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 1438–1446. [Google Scholar] [CrossRef] [PubMed]
- Webster, T.J.; Shallenberger, J.R.; Edelman, E.R.; Khoury, J. Accelerated neutral atom beam (ANAB) modified poly-ether-ether-ketone for increasing in vitro bone cell functions and reducing bacteria colonization without drugs or antibiotics. J. Biomed. Nanotechnol. 2022, 18, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Michel, M.; Toniazzo, V.; Ruch, D.; Ball, V. Deposition mechanisms in layer-by-layer or step-by-step deposition methods: From elastic and impermeable films to soft membranes with ion exchange properties. ISRN Mater. Sci. 2012, 2012, 701695. [Google Scholar] [CrossRef]
- Schuh, K.; Prucker, O.; Rühe, J. Tailor-made polymer multilayers. Adv. Funct. Mater. 2013, 23, 6019–6023. [Google Scholar] [CrossRef]
- Richardson, J.J.; Björnmalm, M.; Caruso, F. Technology-driven layer-by-layer assembly of nanofilms. Science 2015, 348, aaa2491. [Google Scholar] [CrossRef]
- Tang, Z.; Wang, Y.; Podsiadlo, P.; Kotov, N.A. Biomedical applications of layer-by-layer assembly: From biomimetics to tissue engineering. Adv. Mater. 2006, 18, 3203–3224. [Google Scholar] [CrossRef]
- Amani, H.; Arzaghi, H.; Bayandori, M.; Dezfuli, A.S.; Pazoki-Toroudi, H.; Shafiee, A.; Moradi, L. Controlling cell behavior through the design of biomaterial surfaces: A focus on surface modification techniques. Adv. Mater. Interfaces 2019, 6, 1900572. [Google Scholar] [CrossRef]
- Liu, X.; Han, F.; Zhao, P.; Lin, C.; Wen, X.; Ye, X. Layer-by-layer self-assembled multilayers on PEEK implants improve osseointegration in an osteoporosis rabbit model. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1423–1433. [Google Scholar] [CrossRef]
- Deng, Y.; Yang, L.; Huang, X.; Chen, J.; Shi, X.; Yang, W.; Hong, M.; Wang, Y.; Dargusch, M.S.; Chen, Z.-G. Dual Ag/ZnO-decorated micro-/nanoporous sulfonated polyetheretherketone with superior antibacterial capability and biocompatibility via layer-by-layer self-assembly strategy. Macromol. Biosci. 2018, 18, 1800028. [Google Scholar] [CrossRef]
- dos Santos, F.S.F.; de Lima, G.G.; de Cássia Alves Leal Cruz, R.; Pimentel, C.A.; Ferreira, V.P.; Cardoso, M.J.B.; Rezende, R.A.; Fook, M.V.L. PEEK physical surface modification: Evaluation of particles leaching process. Mater. Res. [Online] 2019, 22, e20180520. [Google Scholar] [CrossRef]
- Torstrick, B.F.; Evans, N.T.; Stevens, H.Y.; Gall, K.; Guldberg, R.E. Do surface porosity and pore size influence mechanical properties and cellular response to PEEK? Clin. Orthop. Relat. Res.® 2016, 474, 2373–2383. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pidhatika, B.; Widyaya, V.T.; Nalam, P.C.; Swasono, Y.A.; Ardhani, R. Surface Modifications of High-Performance Polymer Polyetheretherketone (PEEK) to Improve Its Biological Performance in Dentistry. Polymers 2022, 14, 5526. https://doi.org/10.3390/polym14245526
Pidhatika B, Widyaya VT, Nalam PC, Swasono YA, Ardhani R. Surface Modifications of High-Performance Polymer Polyetheretherketone (PEEK) to Improve Its Biological Performance in Dentistry. Polymers. 2022; 14(24):5526. https://doi.org/10.3390/polym14245526
Chicago/Turabian StylePidhatika, Bidhari, Vania Tanda Widyaya, Prathima C. Nalam, Yogi Angga Swasono, and Retno Ardhani. 2022. "Surface Modifications of High-Performance Polymer Polyetheretherketone (PEEK) to Improve Its Biological Performance in Dentistry" Polymers 14, no. 24: 5526. https://doi.org/10.3390/polym14245526
APA StylePidhatika, B., Widyaya, V. T., Nalam, P. C., Swasono, Y. A., & Ardhani, R. (2022). Surface Modifications of High-Performance Polymer Polyetheretherketone (PEEK) to Improve Its Biological Performance in Dentistry. Polymers, 14(24), 5526. https://doi.org/10.3390/polym14245526






