Epigallocatechin Gallate and Glutathione Attenuate Aflatoxin B1-Induced Acute Liver Injury in Ducklings via Mitochondria-Mediated Apoptosis and the Nrf2 Signalling Pathway
Abstract
:1. Introduction
2. Results
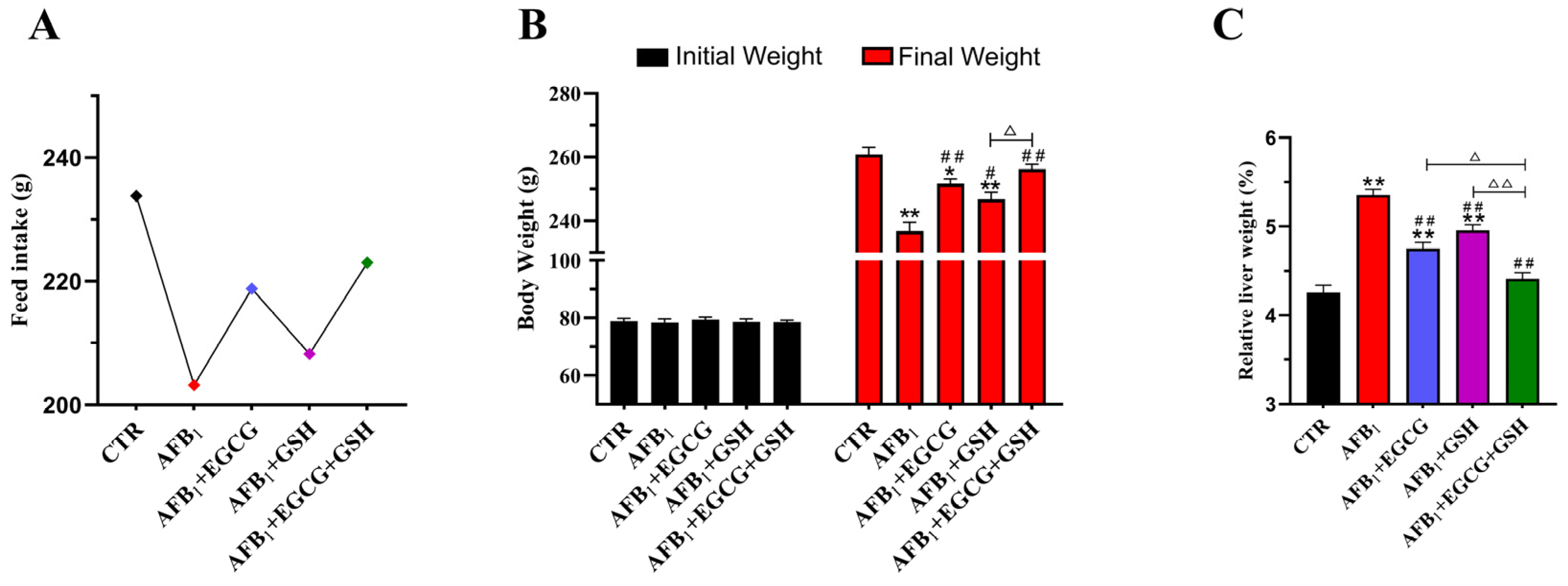
2.1. EGCG and GSH Inhibit AFB1-Induced Changes in Growth Performance and Liver Index of Ducklings
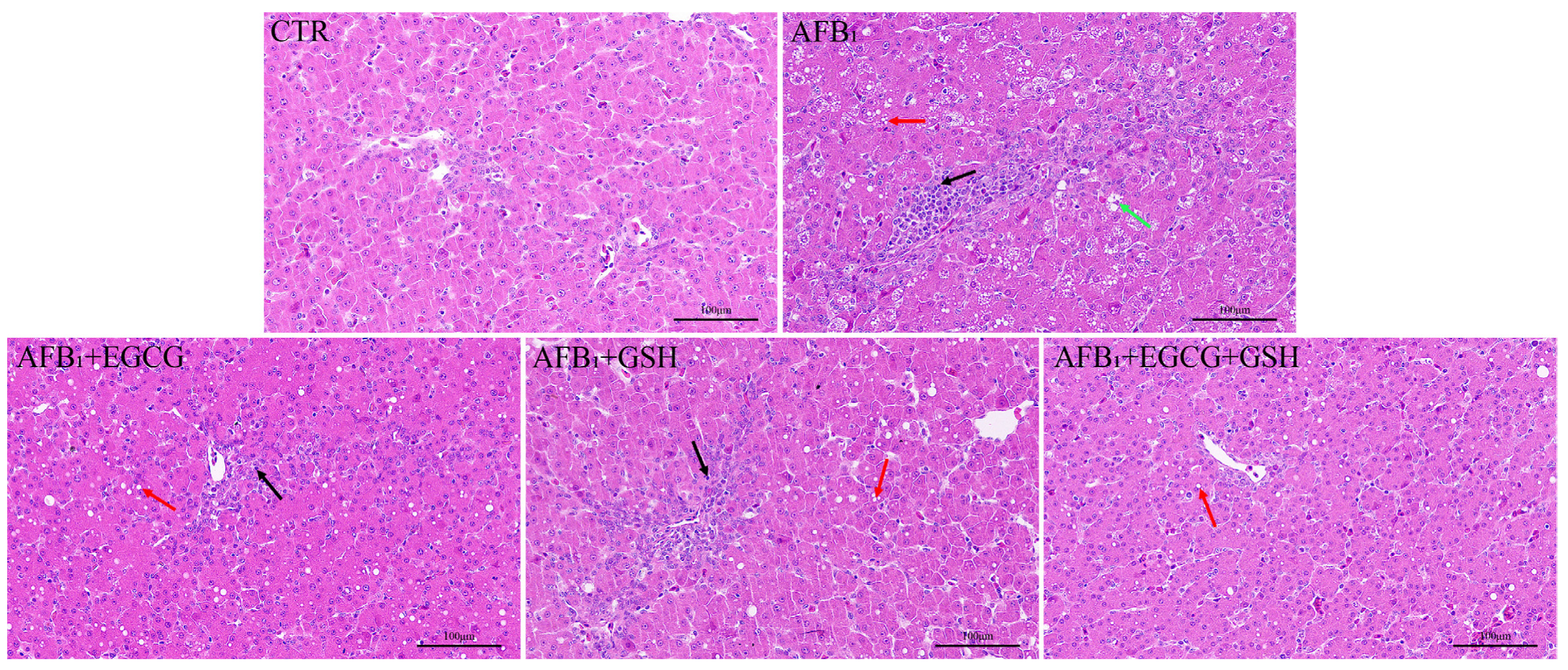
2.2. EGCG and GSH Protect against AFB1-Induced Liver Damage in Ducklings
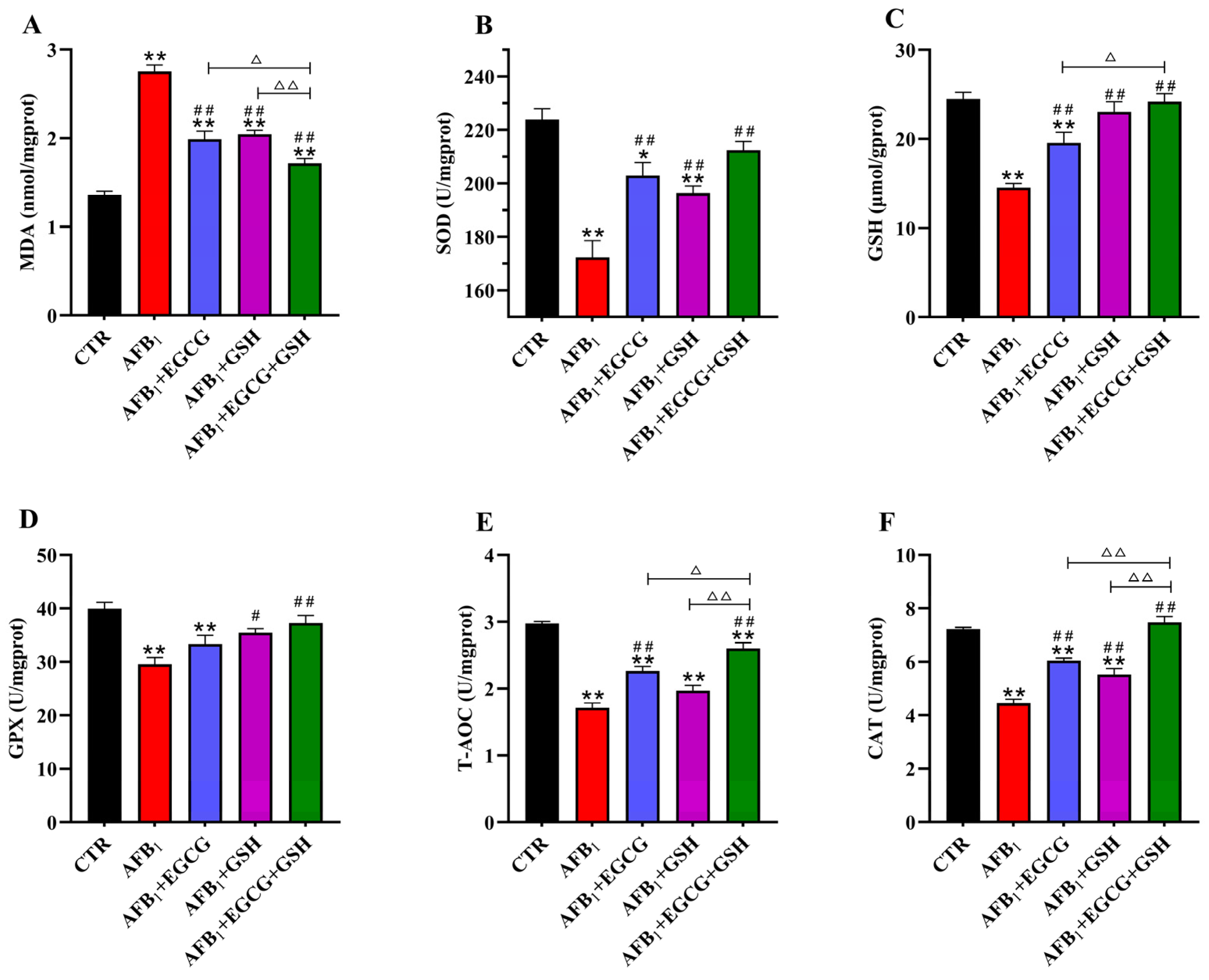
2.3. EGCG and GSH Mitigate AFB1-Induced Oxidative Stress in the Livers of Ducklings
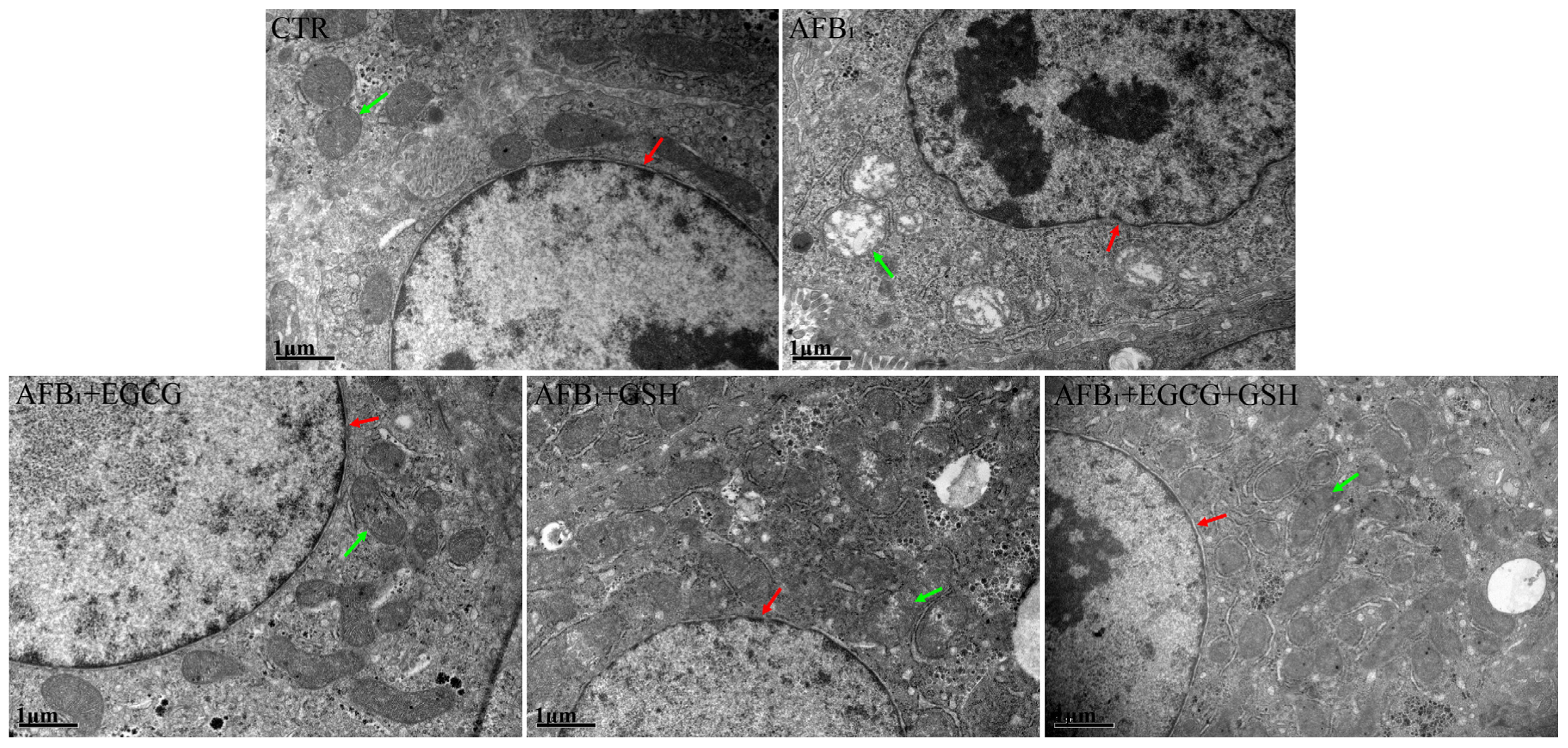
2.4. EGCG and GSH Prevent AFB1-Induced Alterations in the Microstructure and Ultrastructure of Duckling Livers
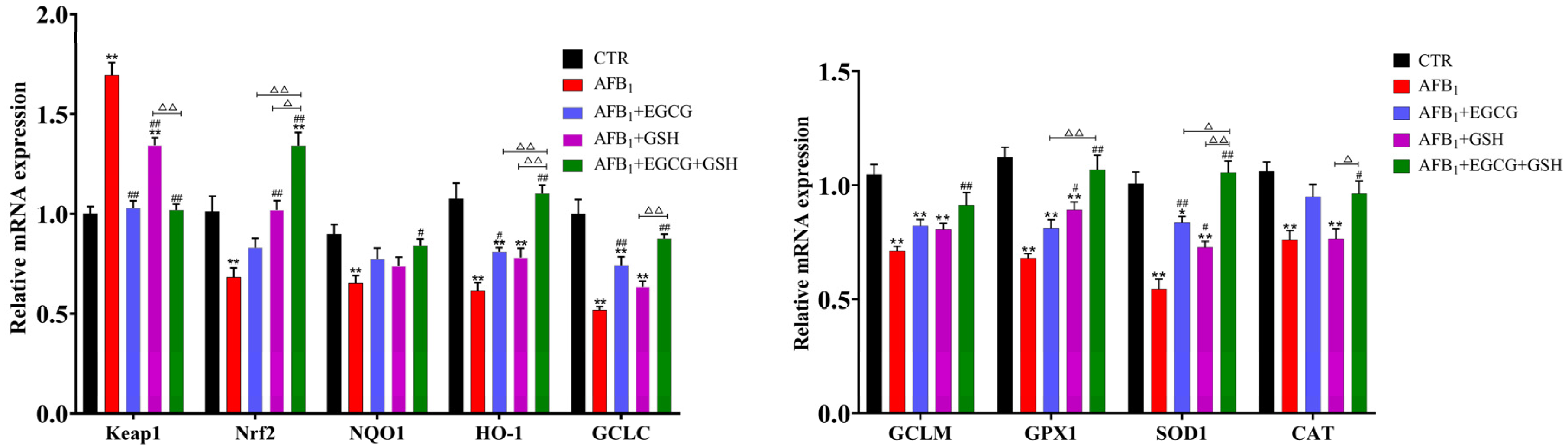
2.5. EGCG and GSH Alleviate the Interference of AFB1 on the Keap1-Nrf2 Antioxidant Signalling Pathway
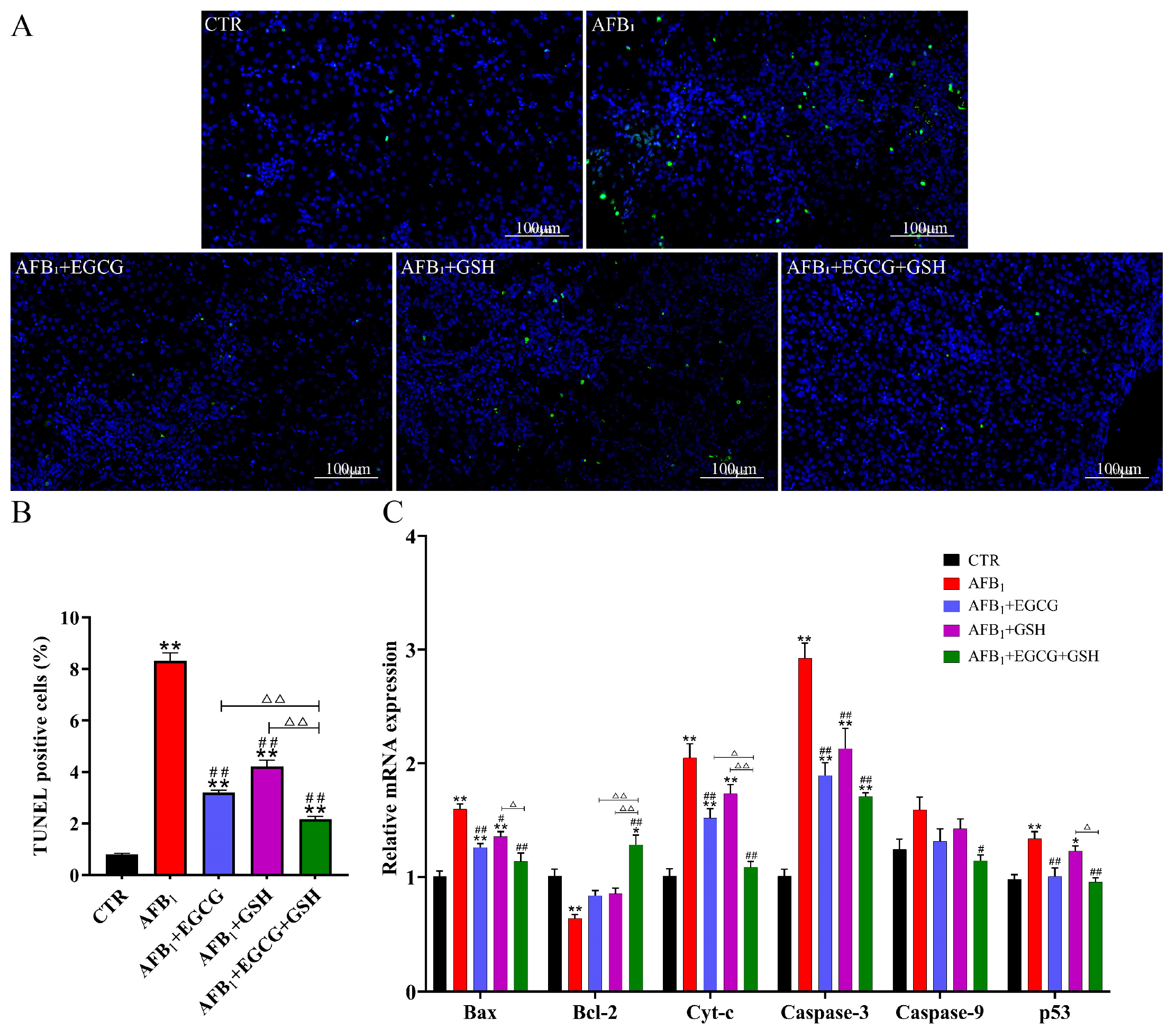
2.6. Protective Effects of EGCG and GSH on AFB1-Induced Apoptosis of Duckling Hepatocytes
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Animals and Experimental Design
5.2. Sample Collection
5.3. Determination of Serum Biochemical Indicators
5.4. Detection of Antioxidant Capacity of the Liver
5.5. Histopathological Analysis
5.6. Ultrastructural Pathology Observation
5.7. Detection of Apoptosis by TUNEL Staining
5.8. Quantitative Real-Time PCR
5.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Ingredients | Content (%) | Nutrition Component 2 | Content (%) |
|---|---|---|---|
| Corn | 52.98 | Crude protein | 20.16 |
| Soybean meal (44% CP) | 30.36 | ME (MJ/kg) | 12.34 |
| Fish meal | 2.50 | Calcium | 0.91 |
| Wheat bran | 3.00 | Available phosphorus | 0.42 |
| Rice bran | 5.25 | Methionine | 0.45 |
| Soybean oil | 1.91 | Lysine | 1.12 |
| Premix 1 | 4.00 | AFB1 (μg/kg) | 2.1 |
| Total | 100.00 |
| Genes | Primer Sequences (5′ to 3′) | Product Lengths (bp) | Accession No. |
|---|---|---|---|
| Keap1 | F: TCAAGACCTCACCCTCCATAAACCC R: AGTAGCCCAAGGACTGCCGATAG | 110 | KU048807.1 |
| Nrf2 | F: TGGCTGAGGTAAGCACAATCACAAG R: GGCTCTCAACAGTCTCCAGGAAATC | 138 | NM_001310777.1 |
| NQO1 | F: TGTCACCATCTCCGACCTCTATGC R: TCCTTCCACGCTTCTCCCATCTC | 126 | XM_027466610.2 |
| HO-1 | F: ATGAATGCCCTTGAGATGGACCTTG R: GTGACCGTTCTCCTGGCTCTTTG | 132 | XM_005015345.5 |
| GCLC | F: TTCAGGTGACATTCCAGGCTTGC R: AGAACGGAGATGCAGCACTCAATG | 108 | XM_027455103.2 |
| GCLM | F: TGTTGTGTGATGCCACCTGATCTC R: GTGCTTTGACGTTCTGGATGCTTTC | 142 | XM_027462629.2 |
| SOD1 | F: TCATCTCTCTGACTGGACCACACTG R: GTTAGCGTGCTCTCGTTGTCTCC | 103 | KU048808.1 |
| GPX1 | F: CCACCAGGAGAATGCCACCAAC R: CTTCCCGTTCACCTCGCACTTC | 115 | XM_027467953.2 |
| CAT | F: ATGGACCAATGTGCGTGACTGAC R: CATGCGGCTCTCCTTCACAACAG | 104 | XM_027458335.2 |
| Cyt-c | F: CCAGTGCCATACGGTTGAGAAGG R: TCTGTGTAGGAGAAGCCCTCAGC | 105 | XM_027447873.2 |
| Bax | F: TCGTCGCCCTCTTCTACTTCGC R: CAGGAGACGATGGTGCGGAAAAG | 88 | KY788660.1 |
| Bcl-2 | F: CATGTGCGTGGAGAGCGTCAAC R: ACTGATCCAGCCTCCGTTGTCC | 124 | XM_027451679.2 |
| Caspase-3 | F: TGAGGCAGACAGTGGACCAGATG R: TCCTTCAGCACCCTACACAGAGAC | 156 | XM_021279218.3 |
| Caspase-9 | F: TGGATTGCGATTCACCCGAAGATG R: ATTACCCGAGGGAGCCTGGAAAG | 83 | XM_038166520.1 |
| p53 | F: AGGAGGAGAACTTCCGCAAGAGG R: GCAGGCAGAAGATCTCGTTGTCG | 129 | XM_038171818.1 |
| GAPDH | F: GTCTCCTGCGACTTCAACGG R: CCTTGGATGCCATGTGGACC | 160 | XM_038180584.1 |
References
- Reverberi, M.; Ricelli, A.; Zjalic, S.; Fabbri, A.A.; Fanelli, C. Natural functions of mycotoxins and control of their biosynthesis in fungi. Appl. Microbiol. Biotechnol. 2010, 87, 899–911. [Google Scholar] [CrossRef] [PubMed]
- da Silva, J.B.; Dilkin, P.; Fonseca, H.; Correa, B. Production of aflatoxins by Aspergillus flavus and of fumonisins by Fusarium species isolated from Brazilian sorghum. Braz. J. Microbiol. 2004, 35, 182–186. [Google Scholar] [CrossRef] [Green Version]
- Girolami, F.; Barbarossa, A.; Badino, P.; Ghadiri, S.; Cavallini, D.; Zaghini, A.; Nebbia, C. Effects of turmeric powder on aflatoxin M1 and aflatoxicol excretion in milk from dairy cows exposed to aflatoxin B1 at the EU maximum tolerable levels. Toxins 2022, 14, 430. [Google Scholar] [CrossRef] [PubMed]
- Sana, S.; Anjum, A.A.; Yaqub, T.; Nasir, M.; Ali, M.A.; Abbas, M. Molecular approaches for characterisation of aflatoxin producing Aspergillus flavus isolates from poultry feed. Pak. Vet. J. 2019, 39, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Fang, J.; Peng, X.; Cui, H.; Chen, J.; Wang, F.; Chen, Z.; Zuo, Z.; Deng, J.; Lai, W.; et al. Effect of selenium supplementation on aflatoxin B-1-induced histopathological lesions and apoptosis in bursa of Fabricius in broilers. Food Chem. Toxicol. 2014, 74, 91–97. [Google Scholar] [CrossRef]
- Morrison, D.M.; Ledoux, D.R.; Chester, L.F.; Samuels, C.A.J.V.S. A limited survey of aflatoxins in poultry feed and feed ingredients in Guyana. Vet. Sci. 2017, 4, 60. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Zhao, L.; Ma, Q.; Ji, C. Novel strategies for degradation of aflatoxins in food and feed: A review. Food Res. Int. 2021, 140, 109878. [Google Scholar] [CrossRef]
- Deng, M.; Zhu, F.; Yang, Y.; Yang, F.; Hao, J.; Chen, S.; Hou, Z. Genome-wide association study reveals novel loci associated with body size and carcass yields in Pekin ducks. BMC Genom. 2019, 20, 1. [Google Scholar] [CrossRef]
- Diaz, G.J.; Murcia, H.W. An unusually high production of hepatic aflatoxin B-1-dihydrodiol, the possible explanation for the high susceptibility of ducks to aflatoxin B-1. Sci. Rep. 2019, 9, 8010. [Google Scholar] [CrossRef] [Green Version]
- Rushing, B.R.; Selim, M.I. Aflatoxin B1: A review on metabolism, toxicity, occurrence in food, occupational exposure, and detoxification methods. Food Chem. Toxicol. 2019, 124, 81–100. [Google Scholar] [CrossRef]
- Stepkowski, T.M.; Kruszewski, M.K. Molecular cross-talk between the NRF2/KEAP1 signaling pathway, autophagy, and apoptosis. Free Radic. Biol. Med. 2011, 50, 1186–1195. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Feng, X.; Hu, X.; Sha, J.; Li, B.; Zhang, H.; Fan, H. Dexmedetomidine ameliorates acute stress-induced kidney injury by attenuating oxidative stress and apoptosis through inhibition of the ROS/JNK signaling pathway. Oxid. Med. Cell. Longev. 2018, 2018, 4035310. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, W. Aflatoxin B1 impairs mitochondrial functions, activates ROS generation, induces apoptosis and involves Nrf2 signal pathway in primary broiler hepatocytes. Anim. Sci. J. 2016, 87, 1490–1500. [Google Scholar] [CrossRef]
- Rains, T.M.; Agarwal, S.; Maki, K.C. Antiobesity effects of green tea catechins: A mechanistic review. J. Nutr. Biochem. 2011, 22, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Hack, M.E.; Elnesr, S.S.; Alagawany, M.; Gado, A.; Noreldin, A.E.; Gabr, A.A. Impact of green tea (Camellia sinensis) and epigallocatechin gallate on poultry. World’s Poult. Sci. J. 2020, 76, 49–63. [Google Scholar] [CrossRef]
- Marin, L.; Miguelez, E.M.; Villar, C.J.; Lombo, F. Bioavailability of dietary polyphenols and gut microbiota metabolism: Antimicrobial properties. BioMed Res. Int. 2015, 2015, 905215. [Google Scholar] [CrossRef] [Green Version]
- Chiu, H.; Venkatakrishnan, K.; Wang, C. The role of nutraceuticals as a complementary therapy against various neurodegenerative diseases: A mini-review. J. Tradit. Complement. Med. 2020, 10, 434–439. [Google Scholar] [CrossRef]
- Aydogan, I.; Karsli, M.A.; Basalan, M.; Yildirim, E.; Cinar, M.; Sen, G.; Sumer, T. Effects of supplemental epigallocatechin gallate in the diet of broilers exposed to fluoride intoxication. Biol. Trace Elem. Res. 2018, 186, 258–266. [Google Scholar] [CrossRef]
- Kim, H.-S.; Quon, M.J.; Kim, J.-a. New insights into the mechanisms of polyphenols beyond antioxidant properties; lessons from the green tea polyphenol, epigallocatechin 3-gallate. Redox Biol. 2014, 2, 187–195. [Google Scholar] [CrossRef] [Green Version]
- Sriram, N.; Kalayarasan, S.; Sudhandiran, G. Epigallocatechin-3-gallate augments antioxidant activities and inhibits inflammation during bleomycin-induced experimental pulmonary fibrosis through Nrf2-Keap1 signaling. Pulm. Pharmacol. Ther. 2009, 22, 221–236. [Google Scholar] [CrossRef]
- Orhan, C.; Tuzcu, M.; Gencoglu, H.; Sahin, N.; Hayirli, A.; Sahin, K. Epigallocatechin-3-gallate exerts protective effects against heat stress through modulating stress-responsive transcription factors in poultry. Br. Poult. Sci. 2013, 54, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Lui, S.M.; Eady, S.J. Glutathione: Its implications for animal health, meat quality, and health benefits of consumers. Aust. J. Agric. Res. 2005, 56, 775–780. [Google Scholar] [CrossRef]
- Lomaestro, B.M.; Malone, M. Glutathione in health and disease: Pharmacotherapeutic issues. Ann. Pharmacother. 1995, 29, 1263–1273. [Google Scholar] [CrossRef]
- Saeij, J.P.J.; van Muiswinkel, W.B.; van de Meent, M.; Amaral, C.; Wiegertjes, G.F. Different capacities of carp leukocytes to encounter nitric oxide-mediated stress: A role for the intracellular reduced glutathione pool. Dev. Comp. Immunol. 2003, 27, 555–568. [Google Scholar] [CrossRef]
- Atef, H.A.; El-Shafei, H.M.; Mansour, M.K.; Al Kalamawy, N.M.; Hassan, N.; Lashin, A.J.I.J.C.M.A.S. Prevalence of ochratoxigenic fungi and ochratoxin A residues in animal feeds and modulation of their toxic effects by glutathione. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 2559–2582. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, L.; Zhao, L.; Wang, X.; Wang, D.; Huang, C.; Zhang, J.; Ji, C.; Ma, Q. Influence of Bacillus subtilis ANSB060 on growth, digestive enzyme and aflatoxin residue in Yellow River carp fed diets contaminated with aflatoxin B-1. Food Chem. Toxicol. 2018, 113, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Limaye, A.; Yu, R.; Chou, C.; Liu, J.; Cheng, K. Protective and detoxifying effects conferred by dietary selenium and curcumin against AFB1-mediated toxicity in livestock: A Review. Toxins 2018, 10, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehta, R.; Campbell, J.S.; Laver, G.W.; Stapley, R.; Mueller, R. Acute hepatic response to aflatoxin B1 in rats fed a methyl-deficient, amino acid-defined diet. Cancer Lett. 1993, 69, 93–106. [Google Scholar] [CrossRef]
- Ruggeberg, K.-G.; O’Sullivan, P.; Kovacs, T.J.; Dawson, K.; Capponi, V.J.; Chan, P.P.; Golobish, T.D.; Gruda, M.C. Hemoadsorption improves survival of rats exposed to an acutely lethal dose of aflatoxin B-1. Sci. Rep. 2020, 10, 799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, J.; Chen, L.; Zhang, L.; Zhang, Z.; Zhao, Y.; Wang, Y.; Ou, J. New insights into the persistent effects of acute exposure to AFB(1) on rat liver. Front Microbiol. 2022, 13, 911757. [Google Scholar] [CrossRef]
- Saei, M.M.; Sadeghi, A.A.; Ahmadvand, H. The effect of Myrtus communis oil extract on growth performance, serum biochemistry and humoral immune responses in broiler chicks fed diet containing aflatoxin B1. Arch. Tierz. 2013, 56, 842–850. [Google Scholar] [CrossRef] [Green Version]
- Mendez-Albores, A.; Del Rio-Garcia, J.C.; Moreno-Martinez, E. Decontamination of aflatoxin duckling feed with aqueous citric acid treatment. Anim. Feed Sci. Technol. 2007, 135, 249–262. [Google Scholar] [CrossRef]
- Mojgani, N.; Razmgah, N.; Torshizi, M.A.K.; Sanjabi, M.R. Effects of three Bacillus specious on hatchability, growth performance and serum biochemistry in Japanese quails fed diet contaminated with Aflatoxin B1. Acta Sci. Anim. Sci. 2020, 42, e50184. [Google Scholar] [CrossRef]
- Yener, Z.; Celik, I.; Ilhan, F.; Bal, R. Effects of Urtica dioica L. seed on lipid peroxidation, antioxidants and liver pathology in aflatoxin-induced tissue injury in rats. Food Chem. Toxicol. 2009, 47, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Bai, K.; Huang, Q.; Zhang, J.; He, J.; Zhang, L.; Wang, T. Supplemental effects of probiotic Bacillus subtilis fmbJ on growth performance, antioxidant capacity, and meat quality of broiler chickens. Poult. Sci. 2017, 96, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lv, Z.; Chen, J.; Nepovimova, E.; Long, M.; Wu, W.; Kuca, K. Bacillus amyloliquefaciens B10 can alleviate liver apoptosis and oxidative stress induced by aflatoxin B1. Food Chem. Toxicol. 2021, 151, 112124. [Google Scholar] [CrossRef] [PubMed]
- Cia, D.; Vergnaud-Gauduchon, J.; Jacquemot, N.; Doly, M. Epigallocatechin gallate (EGCG) prevents H2O2-induced oxidative stress in primary rat retinal pigment epithelial cells. Curr. Eye Res. 2014, 39, 944–952. [Google Scholar] [CrossRef]
- Tipoe, G.L.; Leung, T.M.; Liong, E.C.; Lau, T.Y.H.; Fung, M.L.; Nanji, A.A. Epigallocatechin-3-gallate (EGCG) reduces liver inflammation, oxidative stress and fibrosis in carbon tetrachloride (CCl4)-induced liver injury in mice. Toxicology 2010, 273, 45–52. [Google Scholar] [CrossRef]
- Babaeenezhad, E.; Dezfoulian, O.; Hadipour Moradi, F.; Rahimi Monfared, S.; Fattahi, M.D.; Nasri, M.; Amini, A.; Ahmadvand, H.J.D.; Toxicology, C. Exogenous glutathione protects against gentamicin-induced acute kidney injury by inhibiting NF-κB pathway, oxidative stress, and apoptosis and regulating PCNA. Drug Chem. Toxicol. 2022. [Google Scholar] [CrossRef]
- Kovesi, B.; Pelyhe, C.; Zandoki, E.; Mezes, M.; Balogh, K. Changes of lipid peroxidation and glutathione redox system, and expression of glutathione peroxidase regulatory genes as effect of short-term aflatoxin B-1 exposure in common carp. Toxicon 2018, 144, 103–108. [Google Scholar] [CrossRef]
- Suzuki, T.; Yamamoto, M. Molecular basis of the Keap1-Nrf2 system. Free Radic. Biol. Med. 2015, 88, 93–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baird, L.; Yamamoto, M. The molecular mechanisms regulating the KEAP1-NRF2 pathway. Mol. Cell. Biol. 2020, 40, e00099-20. [Google Scholar] [CrossRef] [PubMed]
- Na, H.K.; Surh, Y.J. Modulation of Nrf2-mediated antioxidant and detoxifying enzyme induction by the green tea polyphenol EGCG. Food Chem. Toxicol. 2008, 46, 1271–1278. [Google Scholar] [CrossRef]
- Thangapandiyan, S.; Miltonprabu, S. Epigallocatechin gallate supplementation protects against renal injury induced by fluoride intoxication in rats: Role of Nrf2/HO-1 signaling. Toxicol. Rep. 2014, 1, 12–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, B.K. Targeting apoptosis pathways for cancer therapy. In Cancer Drug Discovery and Development: The Oncogenomics Handbook; LaRochelle, W.J., Shimkets, R.A., Eds.; Humana Press: Totowa, NJ, USA, 2005; pp. 229–244. ISBN 978-1-59259-893-9. [Google Scholar]
- Meki, A.R.M.; Abdel-Ghaffar, S.K.; El-Gibaly, I.J.N.L. Aflatoxin B1 induces apoptosis in rat liver: Protective effect of melatonin. Neuroendocrinol. Lett. 2001, 22, 417–426. [Google Scholar] [PubMed]
- Raj, H.G.; Kohli, E.; Rohil, V.; Dwarakanath, B.S.; Parmar, V.S.; Malik, S.; Adhikari, J.S.; Tyagi, Y.K.; Goel, S.; Gupta, K.; et al. Acetoxy-4-methylcoumarins confer differential protection from aflatoxin B-1-induced micronuclei and apoptosis in lung and bone marrow cells. Mutat. Res., Genet. Toxicol. Environ. Mutagen. 2001, 494, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Bensassi, F.; Gallerne, C.; El Dein, O.S.; Lemaire, C.; Hajlaoui, M.R.; Bacha, H. Involvement of mitochondria-mediated apoptosis in deoxynivalenol cytotoxicity. Food Chem. Toxicol. 2012, 50, 1680–1689. [Google Scholar] [CrossRef]
- Twiddy, D.; Brown, D.G.; Adrain, C.; Jukes, R.; Martin, S.J.; Cohen, G.M.; MacFarlane, M.; Cain, K. Pro-apoptotic proteins released from the mitochondria regulate the protein composition and caspase-processing activity of the native Apaf-1/caspase-9 apoptosome complex. J. Biol. Chem. 2004, 279, 19665–19682. [Google Scholar] [CrossRef] [Green Version]
- Brunelle, J.K.; Letai, A. Control of mitochondrial apoptosis by the Bcl-2 family. J. Cell Sci. 2009, 122, 437–441. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Sun, Z.; Chu, P.; Li, H.; Ahsan, A.; Zhou, Z.; Zhang, Z.; Sun, B.; Wu, J.; Xi, Y.; et al. EGCG protects against homocysteine-induced human umbilical vein endothelial cells apoptosis by modulating mitochondrial-dependent apoptotic signaling and PI3K/Akt/eNOS signaling pathways. Apoptosis 2017, 22, 672–680. [Google Scholar] [CrossRef]
- Chen, Q.; Yu, M.; Tian, Z.; Cui, Y.; Deng, D.; Rong, T.; Liu, Z.; Song, M.; Li, Z.; Ma, X.; et al. Exogenous glutathione protects IPEC-J2 cells against oxidative stress through a mitochondrial mechanism. Molecules 2022, 27, 2416. [Google Scholar] [CrossRef]
- Dohnal, V.; Wu, Q.; Kuca, K. Metabolism of aflatoxins: Key enzymes and interindividual as well as interspecies differences. Arch. Toxicol. 2014, 88, 1635–1644. [Google Scholar] [CrossRef] [PubMed]
- Monson, M.S.; Coulombe, R.A.; Reed, K.M.J.A. Aflatoxicosis: Lessons from toxicity and responses to aflatoxin B1 in poultry. Agriculture 2015, 5, 742–777. [Google Scholar] [CrossRef] [Green Version]
- Yunus, A.W.; Razzazi-Fazeli, E.; Bohm, J. Aflatoxin B-1 in affecting broiler’s performance, immunity, and gastrointestinal tract: A review of history and contemporary issues. Toxins 2011, 3, 566–590. [Google Scholar] [CrossRef] [Green Version]
- Cao, J.; Song, Y.; Wu, H.; Qin, L.; Hu, L.; Hao, R. Ultrastructural studies on the natural leaf senescence of cinnamomum camphora. Scanning 2013, 35, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Zhong, G.; Wan, F.; Lan, J.; Jiang, X.; Wu, S.; Pan, J.; Tang, Z.; Hu, L. Arsenic exposure induces intestinal barrier damage and consequent activation of gut-liver axis leading to inflammation and pyroptosis of liver in ducks. Sci. Total Environ. 2021, 788, 147780. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wu, J.; Wang, L.; Yang, P.; Liu, Z.; Rajput, S.A.; Hassan, M.; Qi, D. Epigallocatechin Gallate and Glutathione Attenuate Aflatoxin B1-Induced Acute Liver Injury in Ducklings via Mitochondria-Mediated Apoptosis and the Nrf2 Signalling Pathway. Toxins 2022, 14, 876. https://doi.org/10.3390/toxins14120876
Wang Y, Wu J, Wang L, Yang P, Liu Z, Rajput SA, Hassan M, Qi D. Epigallocatechin Gallate and Glutathione Attenuate Aflatoxin B1-Induced Acute Liver Injury in Ducklings via Mitochondria-Mediated Apoptosis and the Nrf2 Signalling Pathway. Toxins. 2022; 14(12):876. https://doi.org/10.3390/toxins14120876
Chicago/Turabian StyleWang, Yanan, Jiayu Wu, Lingfeng Wang, Ping Yang, Zuhong Liu, Shahid Ali Rajput, Mubashar Hassan, and Desheng Qi. 2022. "Epigallocatechin Gallate and Glutathione Attenuate Aflatoxin B1-Induced Acute Liver Injury in Ducklings via Mitochondria-Mediated Apoptosis and the Nrf2 Signalling Pathway" Toxins 14, no. 12: 876. https://doi.org/10.3390/toxins14120876
APA StyleWang, Y., Wu, J., Wang, L., Yang, P., Liu, Z., Rajput, S. A., Hassan, M., & Qi, D. (2022). Epigallocatechin Gallate and Glutathione Attenuate Aflatoxin B1-Induced Acute Liver Injury in Ducklings via Mitochondria-Mediated Apoptosis and the Nrf2 Signalling Pathway. Toxins, 14(12), 876. https://doi.org/10.3390/toxins14120876





