Validating the Predictions of a Dynamic Transmission Model Using Real-World Data from a Universal Varicella Vaccination Program in Germany
Abstract
1. Introduction
2. The Model
3. Data
3.1. Natural Varicella Infection, Demography, and Social Contact Patterns
3.2. Varicella Prevalence and Incidence
3.3. Vaccination
- 4 years for the first dose (administered at the age of 1);
- 3 years for the second dose (administered at the age of 2).
3.4. Other Parameters
| Parameter | Value | Description | Source |
|---|---|---|---|
| 100 | number of annual age strata (age groups); age groups are indexed by numbers | assumption | |
| see Figure 2 | number of births in year | UN data [36] | |
| see Figure 2 | mortality rate in age group | UN data [36] | |
| rate at which an exposed individual becomes infectious (assumed to be equal to , where is the average duration of the latent period of the disease) | Heininger and Seward, 2006 [34], CDC [35] | ||
| rate at which an infectious individual recovers (assumed to be equal to , where is the average duration of the infection) | Heininger and Seward, 2006 [34], CDC [35] | ||
| 1 | age of vaccination with the first dose | assumption, Siedler and Arndt [44] | |
| 2 | age of vaccination with the second dose | assumption, Siedler and Arndt [44] | |
| see Figure 2 | coverage of the first dose of vaccination in year | RKI reports [47], information about school entry age [48,49] | |
| see Figure 2 | coverage of the second dose of vaccination in year | RKI reports [47], information about school entry age [48,49] | |
| 81.9% | effectiveness of the first vaccine dose | Liese et al. (2013) [45] and Siedler et al. (2016) [46] | |
| 94.4% | effectiveness of the second vaccine dose | Siedler et al. (2016) [46] | |
| 0.5 | rate of infectiousness of breakthrough infections (in vaccinated individuals) compared to natural infections (in unvaccinated individuals) | Brisson et al. (2000) [30] | |
| 0.73 | rate of susceptibility of vaccinated individuals who are susceptible for the infection compared to unvaccinated individuals | Brisson et al. (2000) [30] | |
| waning rate of the immunity induced by single-dose vaccination | assumption, Horn, 2016 [24] and Horn, 2018 [25] | ||
| waning rate of the immunity induced by two-dose vaccination | assumption, Horn, 2016 [24] and Horn, 2018 [25] | ||
| age-specific values in the range between 1.86 and 73.52 | parameter describing social contact rate: is the average number of contacts made by a specific individual from age group with a specific individual from age group per unit time () is the total population size) | computed based on data derived from Mossong et al. (2008) [37]; see Appendix A.4 | |
| case importation constant representing the contribution of imported varicella cases to the force of infection () | assumption, Ouwens et al. (2015) [32] and Akpo et al. (2020) [33] | ||
| age-specific values in the range between and | disease prevalence observed within age group , used for calibrating the values of | computed based on seroprevalence derived from Wiese-Posselt et al. (2017) [38], Bollaerts et al. (2017) [39], and Wutzler et al. (2001) [40]; see Appendix A.1 | |
| age-specific values in the range between 0.033 and 0.429 | transmissibility of the virus representing the probability that contact between an infectious individual from age group and a susceptible individual from age group results in infection transmission | calibrated; see Appendix A.3 |
4. Results
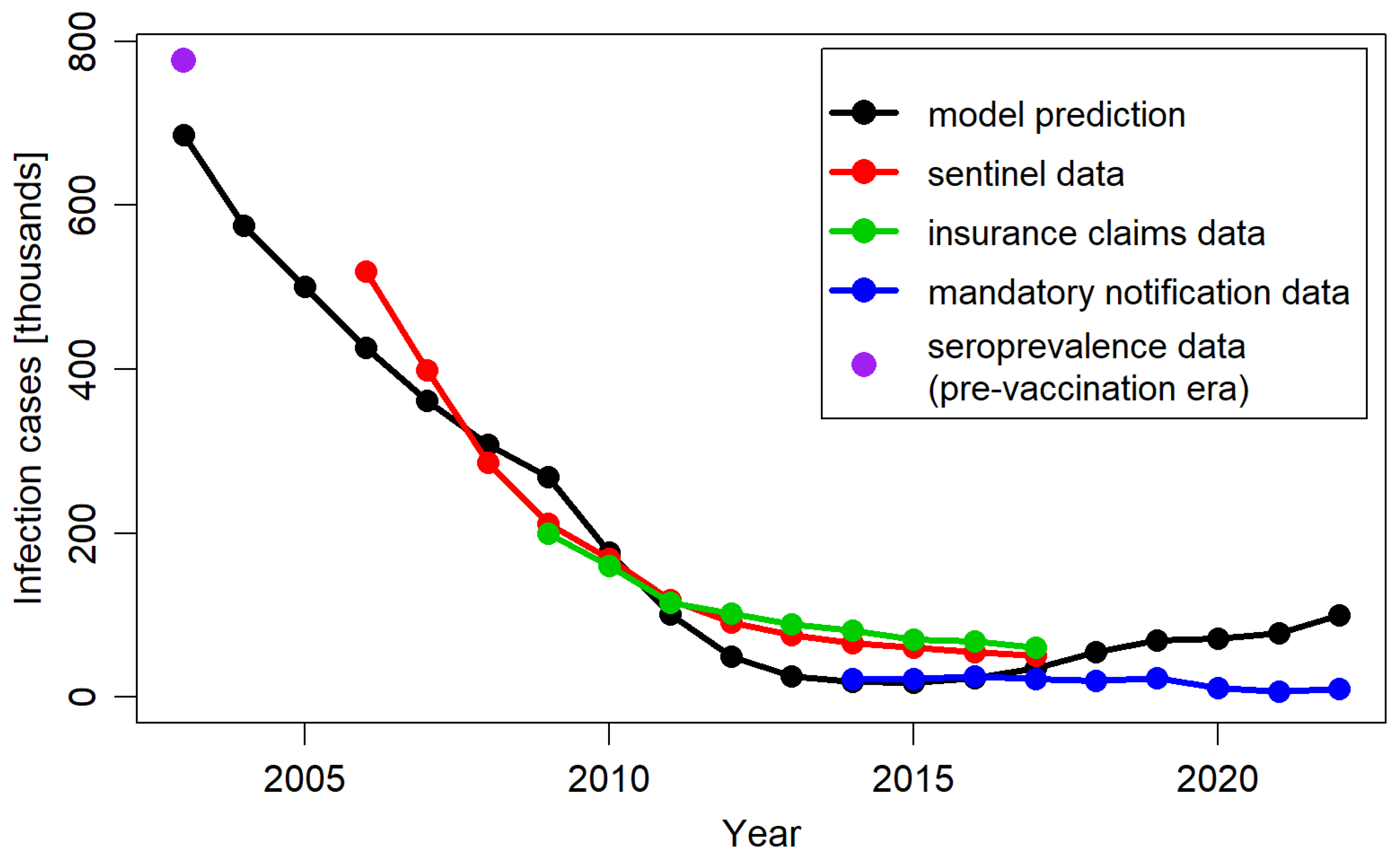
4.1. Validation of Model Predictions Against Reported Data
4.2. Identification of the Model Key Drivers
5. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix A.1. Deriving Prevalence from Seroprevalence, Including Maternal Protection
Appendix A.2. Model Equations
- is assigned the value equal to the number of births in the studied year and the remaining compartments corresponding to the first age group are set to 0.
- For each compartment pertaining to the age group is assigned the value of the same compartment pertaining to the age group .
- From the compartment (susceptible individuals at the age of first vaccine dose uptake), a fraction given by (the coverage of the first vaccine dose in year ) is removed; the fraction of the removed individuals given by (the effectiveness of the first vaccine dose) is added to the compartment, and the fraction of the removed individuals given by is added to the compartment.
- From the compartment (susceptible individuals vaccinated with one dose, at the age of second vaccine dose uptake), a fraction given by (the coverage of the second vaccine dose in year ) is removed; a fraction of the removed individuals given by (the effectiveness of the second vaccine dose) is added to the compartment, and a fraction of the removed individuals given by is added to the compartment.
- From the compartment (individuals vaccinated and protected with one vaccine dose at the age of second dose uptake), a fraction given by is moved into the compartment. The form of this transition reflects the assumption that the vaccine’s second dose has the efficacy of 100% for the individuals who are successfully vaccinated with the first dose.
Appendix A.3. Calibration
Appendix A.4. Social Contact Matrix
References
- Pitman, R.; Fisman, D.; Zaric, G.S.; Postma, M.; Kretzschmar, M.; Edmunds, J.; Brisson, M. Dynamic Transmission Modeling: A Report of the ISPOR-SMDM Modeling Good Research Practices Task Force-5. Value Health 2012, 15, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Drake, T.L.; Devine, A.; Yeung, S.; Day, N.P.J.; White, L.J.; Lubell, Y. Dynamic Transmission Economic Evaluation of Infectious Disease Interventions in Low- and Middle-Income Countries: A Systematic Literature Review. Health Econ. 2016, 25, 124–139. [Google Scholar] [CrossRef] [PubMed]
- Løchen, A.; Anderson, R. Dynamic transmission models and economic evaluations of pneumococcal conjugate vaccines: A quality appraisal and limitations. Clin. Microbiol. Infect. 2019, 26, 60–70. [Google Scholar] [CrossRef]
- Advisory Committee on Immunization Practices (ACIP). Evidence to Recommendations for Use of Hepatitis A Vaccine Catch-Up. Available online: https://www.cdc.gov/acip/evidence-to-recommendations/hep-a-catchup-etr.html (accessed on 20 May 2024).
- Taychakhoonavudh, S.; Chumchujan, W.; Hutubessy, R.; Chaiyakunapruk, N. Landscape of vaccine access and health technology assessment role in decision-making process in ASEAN countries. Hum. Vaccines Immunother. 2020, 16, 1728–1737. [Google Scholar] [CrossRef]
- National Advisory Committee on Immunization (NACI). Guidelines for the Economic Evaluation of Vaccination Programs in Canada; Public Health Agency of Canada: Ottawa, ON, Canada, 2023. [Google Scholar]
- World Health Organization. Who Guide for Standardization of Economic Evaluations of Immunization Programmes; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Sander, B.; Krahn, M.; Prosser, L.; Bryan, S.; Brouwer, W.; Jit, M.; Lee, K.; Naus, M.; Ozawa, S.; Lathia, N.; et al. EE211 Guidelines for the Economic Evaluation of Vaccination Programs in Canada. Value Health 2023, 26, S97. [Google Scholar] [CrossRef]
- Australian Technical Advisory Group on Immunisation (ATAGI). Guidelines for Preparing a Request for Advice from the Australian Technical Advisory Group on Immunisation (ATAGI) to Support Pharmaceutical Benefits Advisory Committee (PBAC) Consideration of Vaccines. 2019. Available online: https://www.health.gov.au/resources/publications/atagi-pre-submission-advice-for-industry-sponsors-wishing-to-make-a-pbac-submission (accessed on 20 May 2024).
- Dooling, K.; Marin, M.; Gershon, A.A. Clinical Manifestations of Varicella: Disease Is Largely Forgotten, but It’s Not Gone. J. Infect. Dis. 2022, 226, S380–S384. [Google Scholar] [CrossRef]
- Marin, M.; Güris, D.; Chaves, S.S.; Schmid, S.; Seward, J.F.; Advisory Committee on Immunization Practices; Centers for Disease Control and Prevention. Prevention of varicella: Recommendations of the Advisory Committee on Immunization Practices (ACIP). Morb. Mortal. Wkly. Report. Recomm. Rep. 2007, 56, 1–40. [Google Scholar]
- Gershon, A.A.; Breuer, J.; Cohen, J.I.; Cohrs, R.J.; Gershon, M.D.; Gilden, D.; Grose, C.; Hambleton, S.; Kennedy, P.G.E.; Oxman, M.N.; et al. Varicella zoster virus infection. Nat. Rev. Dis. Primers 2015, 2, 15016. [Google Scholar] [CrossRef]
- Kennedy, P.G.E.; Gershon, A.A. Clinical Features of Varicella-Zoster Virus Infection. Viruses 2018, 10, 609. [Google Scholar] [CrossRef]
- Pawaskar, M.; Méroc, E.; Samant, S.; Flem, E.; Bencina, G.; Riera-Montes, M.; Heininger, U. Economic burden of varicella in Europe in the absence of universal varicella vaccination. BMC Public Health 2021, 21, 2312. [Google Scholar] [CrossRef]
- Zhou, F.; Leung, J.; Marin, M.; Dooling, K.L.; Anderson, T.C.; Ortega-Sanchez, I.R. Health and Economic Impact of the United States Varicella Vaccination Program, 1996–2020. J. Infect. Dis. 2022, 226, S463–S469. [Google Scholar] [CrossRef] [PubMed]
- Wutzler, P.; Bonanni, P.; Burgess, M.; Gershon, A.; Sáfadi, M.A.; Casabona, G. Varicella vaccination—The global experience. Expert Rev. Vaccines 2017, 16, 833–843. [Google Scholar] [CrossRef] [PubMed]
- Moek, F.; Siedler, A. Trends in age-specific varicella incidences following the introduction of the general recommendation for varicella immunization in Germany, 2006–2022. BMC Public Health 2023, 23, 2191. [Google Scholar] [CrossRef] [PubMed]
- DEGLI Atti, M.L.C.; Rota, M.C.; Mandolini, D.; Bella, A.; Gabutti, G.; Crovari, P.; Salmaso, S. Assessment of varicella underreporting in Italy. Epidemiol. Infect. 2002, 128, 479–484. [Google Scholar] [CrossRef]
- Bonhoeffer, J.; Baer, G.; Muehleisen, B.; Aebi, C.; Nadal, D.; Schaad, U.B.; Heininger, U. Prospective surveillance of hospitalisations associated with varicella-zoster virus infections in children and adolescents. Eur. J. Pediatr. 2005, 164, 366–370. [Google Scholar] [CrossRef]
- Sočan, M. Evaluation of Mandatory Case-based Reporting System for Varicella in the Prevaccine Era. Central Eur. J. Public Health 2010, 18, 99–103. [Google Scholar] [CrossRef]
- Banz, K.; Wagenpfeil, S.; Neiss, A.; Goertz, A.; Staginnus, U.; Vollmar, J.; Wutzler, P. The cost-effectiveness of routine childhood varicella vaccination in Germany. Vaccine 2002, 21, 1256–1267. [Google Scholar] [CrossRef]
- Banz, K.; Wagenpfeil, S.; Neiss, A.; Hammerschmidt, T.; Wutzler, P. The burden of varicella in Germany. Eur. J. Health Econ. 2004, 5, 46–53. [Google Scholar] [CrossRef]
- Hammerschmidt, T.; Bisanz, H.; Wutzler, P. Universal mass vaccination against varicella in Germany using an MMRV combination vaccine with a two-dose schedule: An economic analysis. Vaccine 2007, 25, 7307–7312. [Google Scholar] [CrossRef]
- Horn, J.; Karch, A.; Damm, O.; Kretzschmar, M.E.; Siedler, A.; Ultsch, B.; Weidemann, F.; Wichmann, O.; Hengel, H.; Greiner, W.; et al. Current and future effects of varicella and herpes zoster vaccination in Germany—Insights from a mathematical model in a country with universal varicella vaccination. Hum. Vaccines Immunother. 2016, 12, 1766–1776. [Google Scholar] [CrossRef]
- Horn, J.; Damm, O.; Greiner, W.; Hengel, H.; Kretzschmar, M.E.; Siedler, A.; Ultsch, B.; Weidemann, F.; Wichmann, O.; Karch, A.; et al. Influence of demographic changes on the impact of vaccination against varicella and herpes zoster in Germany—A mathematical modelling study. BMC Med. 2018, 16, 3. [Google Scholar] [CrossRef] [PubMed]
- Ramos, I.C.; Feenstra, T.; Ghabri, S.; Al, M. Evaluating the Validation Process: Embracing Complexity and Transparency in Health Economic Modelling. PharmacoEconomics 2024, 42, 715–719. [Google Scholar] [CrossRef] [PubMed]
- Schenzle, D. An Age-Structured Model of Pre- and Post-Vaccination Measles Transmission. Math. Med. Biol. A J. IMA 1984, 1, 169–191. [Google Scholar] [CrossRef] [PubMed]
- Bolker, B.M.; Grenfell, B.T. Chaos and biological complexity in measles dynamics. Proc. R. Soc. B Biol. Sci. 1993, 251, 75–81. [Google Scholar] [CrossRef]
- Schuette, M.C.; Hethcote, H.W. Modeling the Effects of Varicella Vaccination Programs on the Incidence of Chickenpox and Shingles. Bull. Math. Biol. 1999, 61, 1031–1064. [Google Scholar] [CrossRef]
- Brisson, M.; Edmunds, W.J.; Gay, N.J.; Law, B.; De Serres, G. Modelling the impact of immunization on the epidemiology of varicella zoster virus. Epidemiol. Infect. 2000, 125, 651–669. [Google Scholar] [CrossRef]
- Brisson, M.; Melkonyan, G.; Drolet, M.; De Serres, G.; Thibeault, R.; De Wals, P. Modeling the impact of one- and two-dose varicella vaccination on the epidemiology of varicella and zoster. Vaccine 2010, 28, 3385–3397. [Google Scholar] [CrossRef]
- Ouwens, M.J.; Littlewood, K.J.; Sauboin, C.; Téhard, B.; Denis, F.; Boëlle, P.-Y.; Alain, S. The Impact of 2-Dose Routine Measles, Mumps, Rubella, and Varicella Vaccination in France on the Epidemiology of Varicella and Zoster Using a Dynamic Model With an Empirical Contact Matrix. Clin. Ther. 2015, 37, 816–829.e10. [Google Scholar] [CrossRef]
- Akpo, E.I.H.; Cristeau, O.; Hunjan, M.; Casabona, G. Epidemiological Impact and Cost-Effectiveness of Varicella Vaccination Strategies in the United Kingdom. Clin. Infect. Dis. 2020, 73, e3617–e3626. [Google Scholar] [CrossRef]
- Heininger, U.; Seward, J.F. Varicella. Lancet 2006, 368, 1365–1376. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Chickenpox (Varicella). Available online: https://www.cdc.gov/chickenpox/index.html (accessed on 6 February 2024).
- United Nations, Department of Economic and Social Affairs, Population Division. World Population Prospects 2022. 2022. Available online: https://www.un.org/development/desa/pd/ (accessed on 14 October 2021).
- Mossong, J.; Hens, N.; Jit, M.; Beutels, P.; Auranen, K.; Mikolajczyk, R.; Massari, M.; Salmaso, S.; Tomba, G.S.; Wallinga, J.; et al. Social Contacts and Mixing Patterns Relevant to the Spread of Infectious Diseases. PLoS Med. 2008, 5, e74. [Google Scholar] [CrossRef]
- Wiese-Posselt, M.; Siedler, A.; Mankertz, A.; Sauerbrei, A.; Hengel, H.; Wichmann, O.; Poethko-Müller, C. Varicella-zoster virus seroprevalence in children and adolescents in the pre-varicella vaccine era, Germany. BMC Infect. Dis. 2017, 17, 356. [Google Scholar] [CrossRef] [PubMed]
- Bollaerts, K.; Riera-Montes, M.; Heininger, U.; Hens, N.; Souverain, A.; Verstraeten, T.; Hartwig, S. A systematic review of varicella seroprevalence in European countries before universal childhood immunization: Deriving incidence from seroprevalence data. Epidemiol. Infect. 2017, 145, 2666–2677. [Google Scholar] [CrossRef]
- Wutzler, P.; Färber, I.; Wagenpfeil, S.; Bisanz, H.; Tischer, A. Seroprevalence of varicella-zoster virus in the German population. Vaccine 2001, 20, 121–124. [Google Scholar] [CrossRef]
- RKI: Epidemiologisches Bulletin 3/2020. Available online: https://www.rki.de/DE/Content/Infekt/EpidBull/Archiv/2020/Ausgaben/03_20 (accessed on 8 February 2024).
- Rieck, T.; Feig, M.; Eckmanns, T.; Benzler, J.; Siedler, A.; Wichmann, O. Vaccination coverage among children in Germany estimated by analysis of health insurance claims data. Hum. Vaccines Immunother. 2013, 10, 476–484. [Google Scholar] [CrossRef]
- Das Informationssystem der Gesundheitsberichterstattung des Bundes. 2022. Available online: https://www.gbe-bund.de/gbe/ (accessed on 8 February 2024).
- Siedler, A.; Arndt, U. Impact of the routine varicella vaccination programme on varicella epidemiology in Germany. Eurosurveillance 2010, 15, 19530. [Google Scholar] [CrossRef]
- Liese, J.G.; Cohen, C.; Rack, A.; Pirzer, K.; Eber, S.; Blum, M.; Greenberg, M.; Streng, A. The Effectiveness of Varicella Vaccination in Children in Germany: A Case-control Study. Pediatr. Infect. Dis. J. 2013, 32, 998–1004. [Google Scholar] [CrossRef]
- Siedler, A.; Rieck, T.; Tolksdorf, K. Strong Additional Effect of a Second Varicella Vaccine Dose in Children in Germany, 2009-2014. J. Pediatr. 2016, 173, 202–206.e2. [Google Scholar] [CrossRef]
- RKI: Impfquoten bei den Schuleingangs-Untersuchungen in Deutschland. Available online: https://www.rki.de/DE/Content/Infekt/Impfen/Impfstatus/schulanfaenger/schuleingangsuntersuchungen_node (accessed on 24 August 2023).
- Handbook Germany: School Entry Health Checks. Available online: https://handbookgermany.de/en/school-entry-health-checks (accessed on 19 February 2024).
- Führer, A.; Wienke, A.; Wiermann, S.; Gröger, C.; Tiller, D. Risk-based approach to school entry examinations in Germany—A validation study. BMC Pediatr. 2019, 19, 448. [Google Scholar] [CrossRef]
- Vynnycky, E.; White, R.G. An Introduction to Infectious Disease Modelling, Reprint ed; Oxford University Press: Oxford, UK, 2011. [Google Scholar]
- Eddy, D.M.; Hollingworth, W.; Caro, J.J.; Tsevat, J.; McDonald, K.M.; Wong, J.B. Model Transparency and Validation: A Report of the ISPOR-SMDM Modeling Good Research Practices Task Force-7. Value Health 2012, 15, 843–850. [Google Scholar] [CrossRef]
- Wolfson, L.J.; Daniels, V.J.; Pillsbury, M.; Kurugöl, Z.; Yardimci, C.; Kyle, J.; Dinleyici, E.C. Cost-effectiveness analysis of universal varicella vaccination in Turkey using a dynamic transmission model. PLoS ONE 2019, 14, e0220921. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Zhang, H.; Ma, C.; Zhang, H.; Yin, D.; Fang, H. National and provincial burden of varicella disease and cost-effectiveness of childhood varicella vaccination in China from 2019 to 2049: A modelling analysis. Lancet Reg. Health West. Pac. 2022, 32, 100639. [Google Scholar] [CrossRef] [PubMed]
- Papaloukas, O.; Giannouli, G.; Papaevangelou, V. Successes and challenges in varicella vaccine. Ther. Adv. Vaccines 2014, 2, 39–55. [Google Scholar] [CrossRef]
- World Health Organization. Weekly Epidemiological Record, 1998, vol. 73, 32. Wkly. Epidemiol. Rec. Relev. Épidémiologique Hebd. 1998, 73, 241–248. [Google Scholar]
- van Hoek, A.J.; Melegaro, A.; Zagheni, E.; Edmunds, W.J.; Gay, N. Modelling the impact of a combined varicella and zoster vaccination programme on the epidemiology of varicella zoster virus in England. Vaccine 2011, 29, 2411–2420. [Google Scholar] [CrossRef]
- Burgess, C.; Samant, S.; Lefevre, T.; Larsen, C.S.; Pawaskar, M. Universal varicella vaccination in Denmark: Modeling public health impact, age-shift, and cost-effectiveness. PLoS Glob. Public Health 2023, 3, e0001743. [Google Scholar]
- Bialek, S.R.; Perella, D.; Zhang, J.; Mascola, L.; Viner, K.; Jackson, C.; Lopez, A.S.; Watson, B.; Civen, R. Impact of a Routine Two-Dose Varicella Vaccination Program on Varicella Epidemiology. Pediatrics 2013, 132, e1134–e1140. [Google Scholar] [CrossRef]
- Baxter, R.; Tran, T.N.; Ray, P.; Lewis, E.; Fireman, B.; Black, S.; Shinefield, H.R.; Coplan, P.M.; Saddier, P. Impact of Vaccination on the Epidemiology of Varicella: 1995–2009. Pediatrics 2014, 134, 24–30. [Google Scholar] [CrossRef]
- Varela, F.H.; Pinto, L.A.; Scotta, M.C. Global impact of varicella vaccination programs. Hum. Vaccines Immunother. 2019, 15, 645–657. [Google Scholar] [CrossRef]
- Poletti, P.; Melegaro, A.; Ajelli, M.; del Fava, E.; Guzzetta, G.; Faustini, L.; Tomba, G.S.; Lopalco, P.; Rizzo, C.; Merler, S.; et al. Perspectives on the Impact of Varicella Immunization on Herpes Zoster. A Model-Based Evaluation from Three European Countries. PLoS ONE 2013, 8, e60732. [Google Scholar] [CrossRef]
- Santermans, E.; Goeyvaerts, N.; Melegaro, A.; Edmunds, W.; Faes, C.; Aerts, M.; Beutels, P.; Hens, N. The social contact hypothesis under the assumption of endemic equilibrium: Elucidating the transmission potential of VZV in Europe. Epidemics 2015, 11, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Arregui, S.; Aleta, A.; Sanz, J.; Moreno, Y. Projecting social contact matrices to different demographic structures. PLoS Comput. Biol. 2018, 14, e1006638. [Google Scholar] [CrossRef] [PubMed]
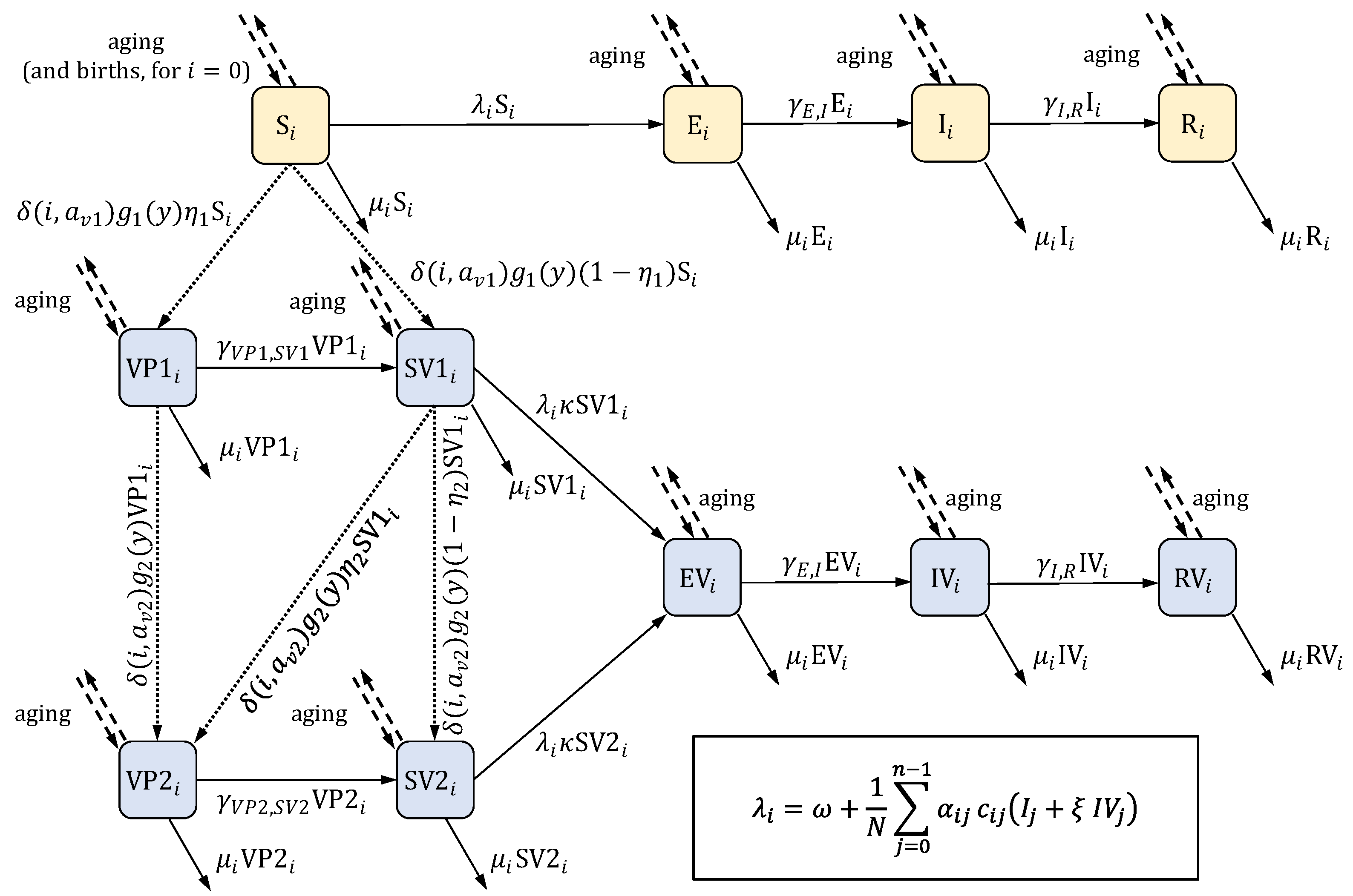



| State | Description |
|---|---|
| S | Susceptible, unvaccinated |
| E | Exposed (infected but not yet infectious for others), unvaccinated |
| I | Infectious, unvaccinated |
| R | Recovered, unvaccinated |
| SV1 | Vaccinated with one dose and susceptible |
| SV2 | Vaccinated with two doses and susceptible |
| VP1 | Vaccinated with one dose and protected (immune) |
| VP2 | Vaccinated with two doses and protected |
| EV | Vaccinated and exposed |
| IV | Vaccinated and infectious |
| RV | Vaccinated and recovered |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Market Access Society. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Żerda, I.; Stanisz, T.; Fundament, T.; Chełmikowski, F.; Kłębczyk, W.; Pochopień, M.; Clay, E.; Aballéa, S.; Toumi, M. Validating the Predictions of a Dynamic Transmission Model Using Real-World Data from a Universal Varicella Vaccination Program in Germany. J. Mark. Access Health Policy 2025, 13, 20. https://doi.org/10.3390/jmahp13020020
Żerda I, Stanisz T, Fundament T, Chełmikowski F, Kłębczyk W, Pochopień M, Clay E, Aballéa S, Toumi M. Validating the Predictions of a Dynamic Transmission Model Using Real-World Data from a Universal Varicella Vaccination Program in Germany. Journal of Market Access & Health Policy. 2025; 13(2):20. https://doi.org/10.3390/jmahp13020020
Chicago/Turabian StyleŻerda, Iwona, Tomasz Stanisz, Tomasz Fundament, Filip Chełmikowski, Wioletta Kłębczyk, Michał Pochopień, Emilie Clay, Samuel Aballéa, and Mondher Toumi. 2025. "Validating the Predictions of a Dynamic Transmission Model Using Real-World Data from a Universal Varicella Vaccination Program in Germany" Journal of Market Access & Health Policy 13, no. 2: 20. https://doi.org/10.3390/jmahp13020020
APA StyleŻerda, I., Stanisz, T., Fundament, T., Chełmikowski, F., Kłębczyk, W., Pochopień, M., Clay, E., Aballéa, S., & Toumi, M. (2025). Validating the Predictions of a Dynamic Transmission Model Using Real-World Data from a Universal Varicella Vaccination Program in Germany. Journal of Market Access & Health Policy, 13(2), 20. https://doi.org/10.3390/jmahp13020020






