Abstract
Skin exfoliators, specifically alpha and beta hydroxy acids, have been shown to improve overall skin health and the clinical signs of aging. A micropeeling cream was developed with hydroxy acids and a brown algae extract and the efficacy and tolerability were evaluated in two parts. In the first part of the pre-clinical investigation, the micropeeling cream and the placebo control were evaluated by ELISA, immunostaining, qPCR and an activity assay using ex vivo and in vitro models. In the second part of the clinical efficacy study, 36 female subjects were enrolled for bioinstrumental measurements, visual imaging and clinical evaluation for 28 days. Fifty percent of subjects had sensitive skin. The ex-vivo study showed an increase in loricrin, superoxide dismutase 2, and extracellular matrix expression, without stimulating inflammatory biomarkers. The dermatologist observed a significant enhancement in all the parameters evaluated at day 28, and radiance, homogeneity, and roughness were significantly better after the first cream application. The homogeneity, desquamation and pore diameter showed significant improvement at day 7. The cream improved markers associated with skin aging and protection ex vivo. It was well tolerated, even on sensitive skin, and provided a significant improvement of fine lines, skin texture, and overall skin characteristics.
Keywords:
chemical peels; sensitive skin; anti-aging; skin barrier; genetic analysis; safety testing 1. Introduction
The use of chemical peels is a common method of skin resurfacing. The first documented use of peels was the use of sour milk rich in lactic acid to improve skin texture [1]. Various acids have since been identified as skin peeling agents and classified based on their chemical structures, effectiveness, and target benefits, which differ based on potency. Their ability to induce uniform and controlled exfoliation, translating into partial destruction of targeted skin layers, is used to initiate healing and promote cell renewal processes [2]. These include stimulating the synthesis of skin structure proteins, such as collagen and elastin, and stimulating keratinocyte growth, resulting in increased epidermal thickness [2]. Clinically, peeling results in a smoother skin surface texture, which appears more even, rejuvenated, and healthier [3].
Currently, chemical peels are indicated to treat skin conditions of varying severity, such as acne, scars, solar keratosis, and melasma, under the supervision of a dermatologist [4]. Based on the depth of penetration, chemical peels are divided into superficial, medium, and deep peels. Those containing trichloroacetic acid (TCA) are highly potent chemical peels, as they can penetrate beyond the epidermis and affect the papillary to reticular dermis. Classified as medium or deep peels, depending on the TCA concentration and/or other blending acids, they are primarily intended to treat moderate to severe photoaging, melasma, and even premalignant skin tumors [4]. Previously, we demonstrated that a professional-grade TCA-blended lactic acid peel is able to restructure extracellular matrix (ECM) components and inhibit melanin pigmentation in skin explants [5]. Medium and deep peels have potential side effects, which range from edema and erythema to burning and blistering, as well as dyspigmentation [4].
Superficial peels, which target the epidermis using weaker acids, generally contain alpha hydroxy acids (AHAs), beta hydroxy acids (BHAs), and/or alpha keto acids (AKAs). One such AHA, glycolic acid (30–50%), is commonly used to treat milder symptoms [2,4]. Malic acid and citric acid are also commonly used chemical peel acids in cosmetics. The combination of these acids is used to treat recalcitrant warts [6]. The occurrence of side effects increases with the use of deeper peels. Therefore, concentrations of acids below 20% are often recommended to further reduce the side effects. Clinical studies have shown that AHA concentrations of 5–20% are efficacious in improving both signs of aging and mild acne [7,8,9]. In vitro and ex vivo studies have also explored the effect of low concentrations of AHAs on molecular targets, and found that they appear to increase cell proliferation [10], as well as the expression of molecular targets such as collagen, elastin, and vascular endothelial growth factor (VEGF) [11,12].
Currently, there are no standards or guidelines for the use of peels at home. Based on where they live, consumers are exposed to a wide range of chemical peels with various concentrations and combinations, without a proper understanding of the usage instructions, potentially resulting in unnecessary side effects. Additionally, a significant proportion of the population has sensitive skin [13]. Therefore, it was important to formulate a gentle micropeeling cream, which was also appropriate for subjects with sensitive skin to use, with a simple application to not limit the usage. A micropeeling cream is generally considered a superficial peel with low acid concentrations, which limits visual skin peeling, while stimulating skin desquamation processes. Given the level of concern attributed to formulation, with side effects occurring for those with sensitive skin [13], combining AHAs and BHA into a single product is a promising strategy to drive chemical peel efficacy while reducing the potential side effects [14,15].
We formulated a micropeeling cream consisting of the following key components: AHAs and a BHA at 4.5% in total, with a soothing brown algae (Laminaria ochroleuca) extract, for everyday use by sensitive skin subjects. They were selected for the efficacy in resurfacing the skin and protection from skin irritation. The smallest AHA, glycolic acid, is one of the most common AHAs used. It is keratolytic, inducing degradation of corneosomes, thereby resulting in cell desquamation [16]. With known benefits in acne, melisma, post-inflammatory hyperpigmentation and photoaging [16], glycolic acid is a critical component of the micropeeling cream. Salicylic acid is desmolytic, and can be safely used by various skin types [17]. Pyruvic acid is antimicrobial, sebostatic and is known to stimulate the production of new collagen and elastic fibers [18]. Pyruvic acid is metabolized into lactic acid, which makes an effective and safe peeling agent [18]. To reduce the potential risks related to peeling acids, a brown algae extract with soothing and anti-inflammatory properties was also added [19]. These key components were formulated into a cream with easy application on the skin.
The current literature lacks comprehensive studies combining clinical, ex vivo, and in vitro assessments demonstrating the efficacy of mild and low acid peels. In this report, the efficacy of this gentle micropeeling cream was assessed in two parts. In the first part, the improvements on the key biomarkers of inflammation, barrier and antioxidant protection, pigmentation, and ECM structure were demonstrated using in vitro and ex vivo methods. The second part of the report showed the efficacy in an open-label, single-arm clinical study with sensitive skin and non-sensitive skin subjects, demonstrating significant improvements in skin, without irritation.
2. Materials and Methods
2.1. Formulations
A cream product (cream, test product, Appendix A.1) containing a mixture of 4.5% total peel acids (AHAs: glycolic acid, malic acid, citric acid, lactic acid, pyruvic acid, and tartaric acid, BHA: salicylic acid), and a brown algae (Laminaria ochroleuca) extract was prepared for topical use. A placebo formulation (negative control formulation, Appendix A.2) was prepared without the peel acids mentioned above and the brown algae extract for the ex vivo experiments. The pH of the cream and placebo formulations was 3.6 and 3.9, respectively.
2.2. Human Skin Explants
Skin explants from two donors were utilized for this study: NativeSkin Access 11 mm (GenoSkin, Salem, MA, USA) and TenSkin 18 mm (Ten Bio, Kannapolis, NC, USA). Both tissues were from female donors aged 32 and 28 years, respectively. Studies were conducted in quadruplicates or triplicates. The test material (placebo or cream) was uniformly applied to the tissue surface every other day, while the tissue culture media were changed every day. Untreated samples received no test material. After eight days of treatment, the tissues were collected and processed. The media were collected for protein expression; the tissues were placed in RNAlater solution (Life Technologies, Carlsbad, CA, USA) for RNA extraction, or fixed in 4% paraformaldehyde for immunostaining. The collected media were used to measure human IL-1α (R&D Systems, Minneapolis, MN, USA) and human pro-collagen I alpha 1 (R&D Systems) by enzyme-linked immunoassays (ELISAs).
2.3. Immunostaining of Human Skin Explants
Fixed samples were embedded in paraffin, sectioned at 5 μm. Three biomarkers were evaluated by immunofluorescence staining: anti-elastin and SOD2 antibodies (Abcam, Waltham, MA, USA) and anti-loricrin (Sigma-Aldrich, St. Louis, MO, USA). Nuclei were visualized with DAPI (Vector Laboratories, Burlingame, CA, USA). For each treatment, three images (for loricrin) and five images (for SOD2 and elastin, respectively) per section/replicate were taken. The mean gray intensity of the images per amount of protein expressed was evaluated using ImageJ (Version 1.53s, NIH, Bethesda, MD, USA).
2.4. Tyrosinase Activity Assay
Human melanocyte lysates were prepared from darkly-pigmented normal human melanocytes (Life Technologies) using an ice-cold tyrosinase assay buffer (0.1% Triton X-100 in PBS). L-DOPA was purchased from Sigma-Aldrich (St. Louis, MO, USA). Human melanocyte lysates were mixed with the placebo or cream to a final of 0.5%, 0.25%, or 0.125% and incubated at room temperature for 15 min. After the addition of 10 mM L-DOPA, the mixture was further incubated at 37 °C for 20–30 min and read at 490 nm (SpectraMax, Molecular Devices, San Jose, CA, USA). A known tyrosinase inhibitor, glycolic acid 0.04%, served as the positive control [20]. The negative control did not receive inhibitors.
2.5. RT-qPCR Analysis
For RT-qPCR, RNA was extracted from human skin explants using Polytron PT 10–35 GT (Kinematica, Bohemia, NY, USA) and a RNeasy Fibrous Tissue Mini Kit (Qiagen, Germantown, MD, USA) following the manufacturer’s protocol. Extracted RNA was quantitated using NanoDrop One (ThermoFisher Scientific, Waltham, MA, USA), followed by cDNA synthesis using a Maxima First Strand cDNA Synthesis kit (ThermoFisher). In order to evaluate skin-specific changes, 48 molecular targets were placed on TaqmanTM array cards, and QuantStudio 7 Flex (ThermoFisher) was used for gene expression analysis. The results are expressed relative to the housekeeping gene and to the untreated or placebo control.
2.6. Human Volunteers and Clinical Study Design
The objective of the study was to assess the micropeeling effect of the test product after 1, 7, and 28 days of application on the face, for the evolution of the skin parameters and skin tolerability. Thirty-six female subjects, aged 30–60 years, were enrolled in this open-label (unblinded) monocentric clinical study (GREDECO, Paris, France), having met the inclusion and exclusion criteria. Phototype II-VI subjects were recruited and 50% of subjects had sensitive skin. The included subjects had wrinkles on the crow’s feet of grade 2–4 [21], dull to very dull complexion, and lack of radiance and evenness of complexion. Subjects were excluded from the study, if they were pregnant or nursing, recently participated or were still participating in another clinical study, recently received facial injection/implantation of any non-resorbable filling product, as well as for not following study instructions. Subjects under medical care related to immunosuppression or inflammation were also excluded. The study had two subgroups: one group included 30 subjects (Caucasian) who went through all study evaluations, and the second group included six subjects (three Asian and three African) for subjective assessments (data not shown) and a skin tolerance evaluation only. The subjects had to perform a wash-out period of at least 14 days, without application of any anti-aging or skincare products. Only moisturizing cream was authorized.
The subjects were instructed to apply the test cream once in the evening on the entire face, neck, and cleavage for 28 days, and on the lips every other day. One subject with sensitive skin from the second group withdrew from the study due to an adverse reaction. The clinical study was performed in accordance with the Good Clinical Practice, French Decree n2016–1537, and the Declaration of Helsinki. The protocols and consent forms were approved by a U.S. Investigational Review Board (IRB). Skin tolerance and adverse event data were collected, and a dermatologist investigator assessed the skin characteristics throughout the study. A final visit assessment was performed on D28.
2.7. Dermatological Assessments and Clinical Scoring
Five clinical parameters (radiance, homogeneity, imperfections, wrinkle smoothing, and roughness) were visually evaluated by a dermatologist investigator on D0, D1, D7, and D28 (Table 1) on 30 Caucasian subjects. Briefly, the radiance score (0–4) evaluates the quantity of light reflected from the skin, color, and relief [22]. The skin homogeneity score (0–3) assesses the presence of pigmented spots. The overall skin imperfection score (0–9) helps to evaluate dilated pores and pimples on the face. The wrinkle smoothing score (0 = young face without visible wrinkles to 9 = very marked face with many deep wrinkles) is based on the subject’s age and severity of wrinkles (GREDECO, Paris, France) [22]. The roughness score (0–3) is determined according to the severity of skin irregularities [22].

Table 1.
Dermatological assessment of the clinical parameters.
2.8. Skin Lightness Measurement
Skin homogeneity or L* (lightness) in the L*a*b* color space was measured using a chromameter (CR/DP-400®, Konica Minolta, Tokyo, Japan) on D0 (baseline), D1, D7, and D28 at baseline and after test product use. Two measurements of L* were performed on the inner and outer cheek. A decrease in the delta between the two measurements demonstrated an increase in the uniformity of the skin.
2.9. Pore Diameter Measurements
Macrophotographs at 30× magnification using a microscope (ProScopeTM HR, Bodelin Technologies, Oregon City, OR, USA) were taken on D0, D1, D7, and D28 on the left cheek area close to the nasal wing on the 30 Caucasian subjects. Six to ten measurements were performed per subject. The morphometric measurements were performed using software to measure the changes in pore diameter.
2.10. Desquamation Assessment
To measure the quantity of removed corneocytes from the skin surface, a 22 mm adhesive disk (D-squame®, Clinical & Derm, Dallas, TX, USA) was applied to the skin with standardized pressure on D0, D1, D7, and D28 on the left cheek area. The material was then placed on a black reading card and compared to a visual scale from 0 to 5.
2.11. Skin Roughness on the Periocular Area
Photographs combined with stereovision technology to produce 3D reproducible images were performed in 24 cm2 of the periocular area (right or left) facial skin (LifeViz® Micro system, Quantificare, Valbonne, France). All 30 subjects were assessed on D0, D7, and D28. The skin roughness measurement corresponds to the addition of the absolute positive volume and the absolute negative volume, divided by the overall surface area, to show changes in the depth of fine lines [23].
2.12. 2D and 3D Photographs
Photographs of the full face of each subject were taken on D0, D1, D7, and D28 using a reproducible 3D photographic system (LifeViz© Mini, Quantificare). Software was used to quantify the skin roughness using morphometric measurements (DermaPix®, Quantificare).
2.13. Statistical Analysis
The data presented are the mean ± SD for the in vitro, ex vivo, and clinical data except for RT-qPCR, where the data are the mean and RQmin and RQmax for the 95% confidence levels. T-tests were used for statistical analysis resulting from in vitro, ex vivo, and clinical data. For RT-qPCR, the p-value was corrected using the Benjamini–Hochberg false discovery rate. For the clinical analysis, D1, D7, and D28 clinical data were compared to D0 data (baseline), and the percentage variation was calculated. A Wilcoxon test was performed in the case of a non-normal distribution.
3. Results
3.1. Ex Vivo Inflammation Assessment
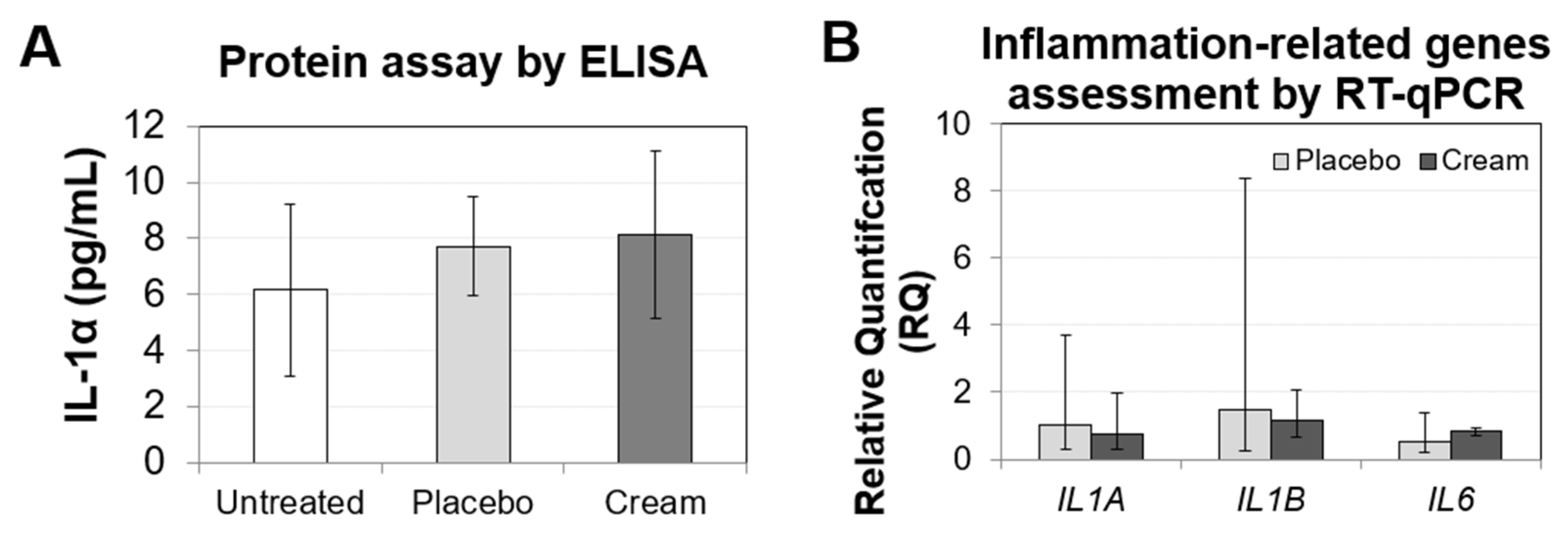
To assess ex vivo skin irritation, biomarkers related to inflammation were evaluated by ELISA (Figure 1A) and RT-qPCR (Figure 1B). The IL-1α levels released by the tissue were low, and there was no significant difference between the untreated and treated samples. The tissue inflammatory response was also assessed by quantifying the gene expression of IL1A, IL1B, and IL6. All three biomarkers in both the placebo and cream treatment showed non-significant differences from the untreated sample. Taken together, these results indicate that the skin irritation potential of this cream is likely low.
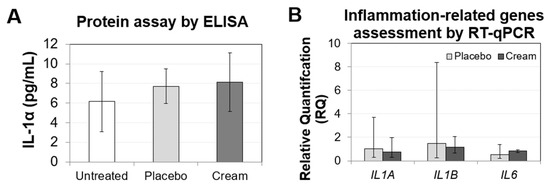
Figure 1.
Assessment of the inflammation biomarkers on human skin explants after eight days of topical treatment. (A) IL-1α ELISA from the untreated, placebo (negative control formulation), and cream (test product) samples. The results are shown as the mean SD. (B) RT-qPCR results from the IL1A, IL1B, and IL6 genes, showing relative quantification compared to the untreated sample. RQmin and RQmax represent the 95% confidence levels. A t-test was performed to evaluate the statistical significance.
3.2. Increased Loricrin and SOD2 Expression for Improved Epidermal Function
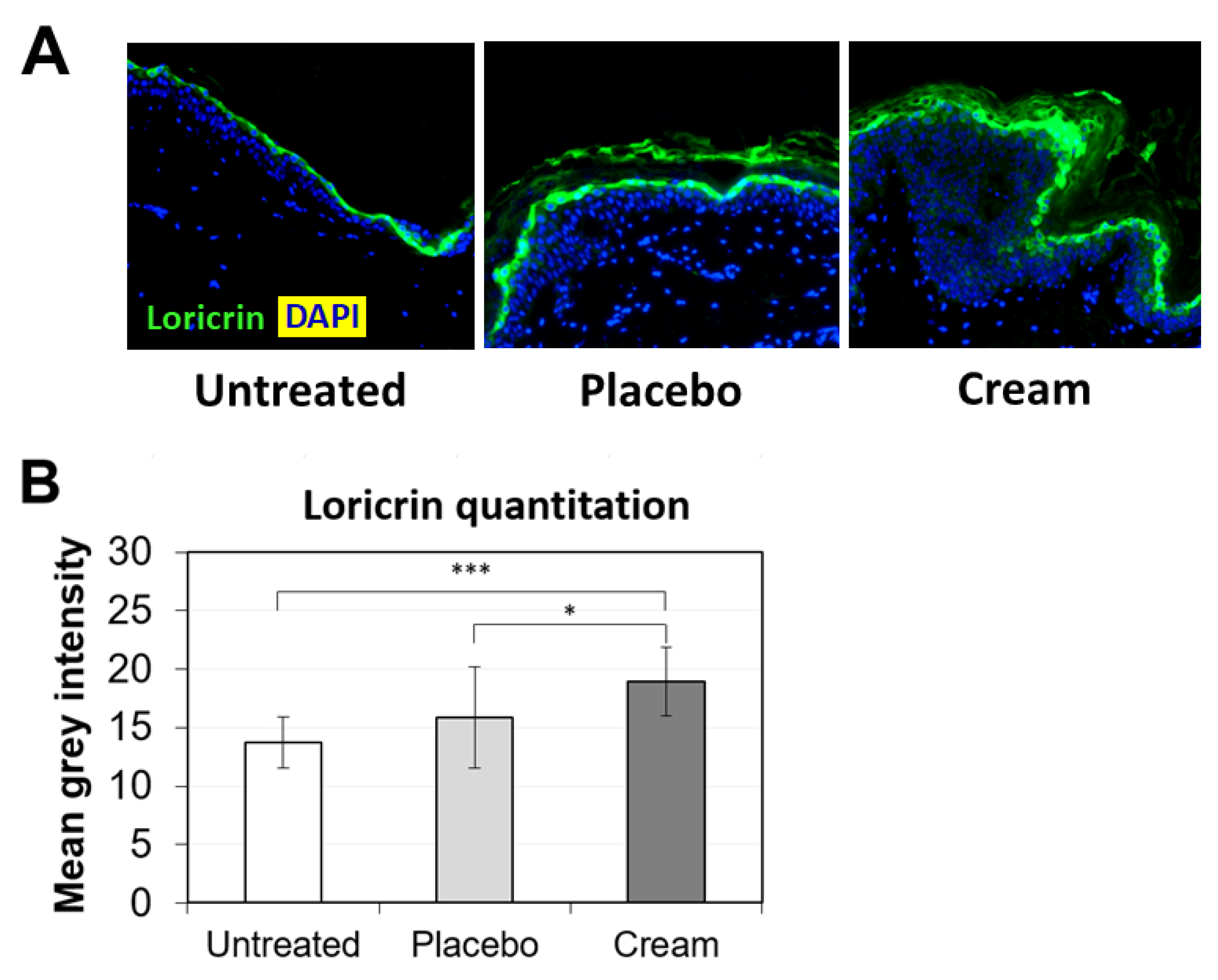
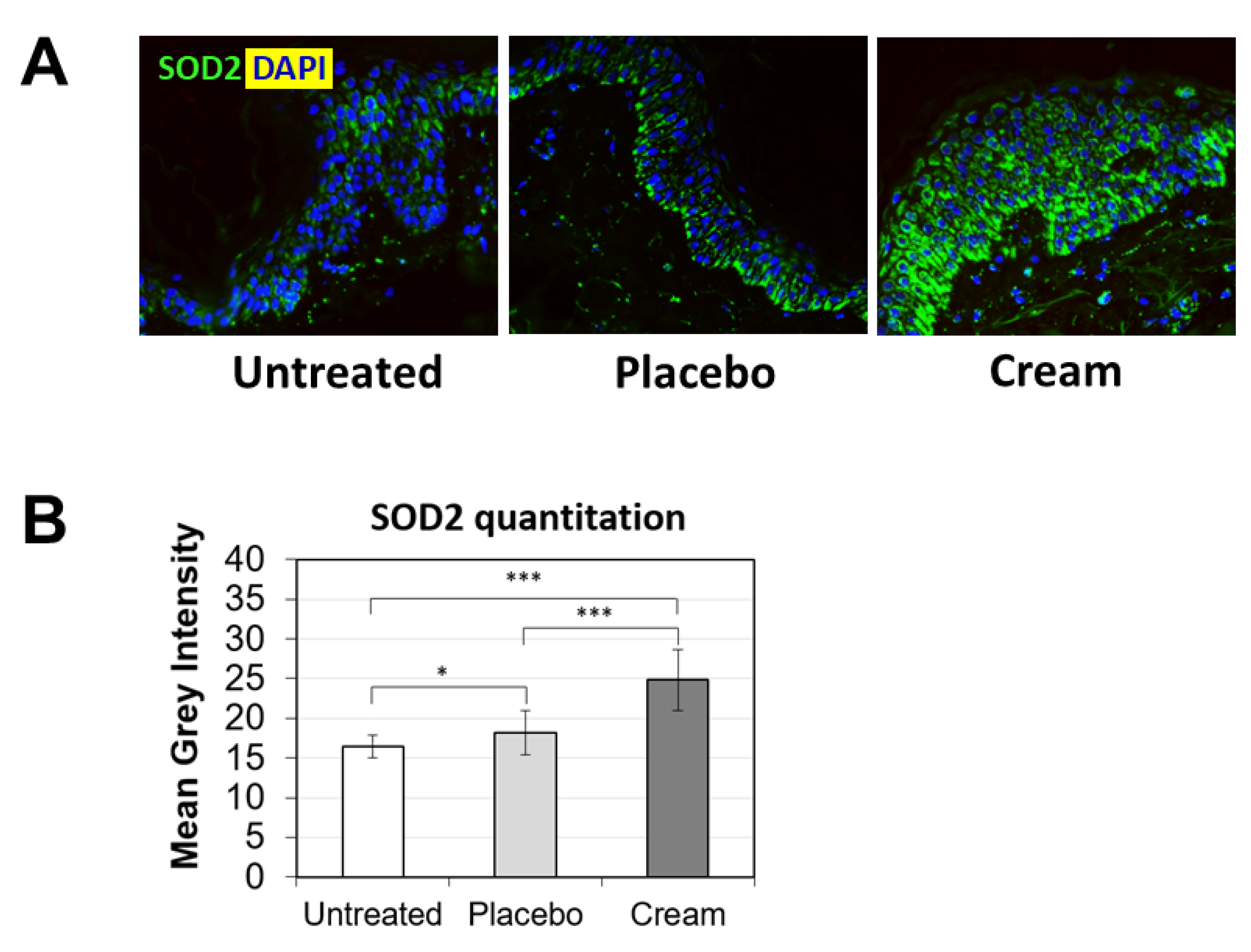
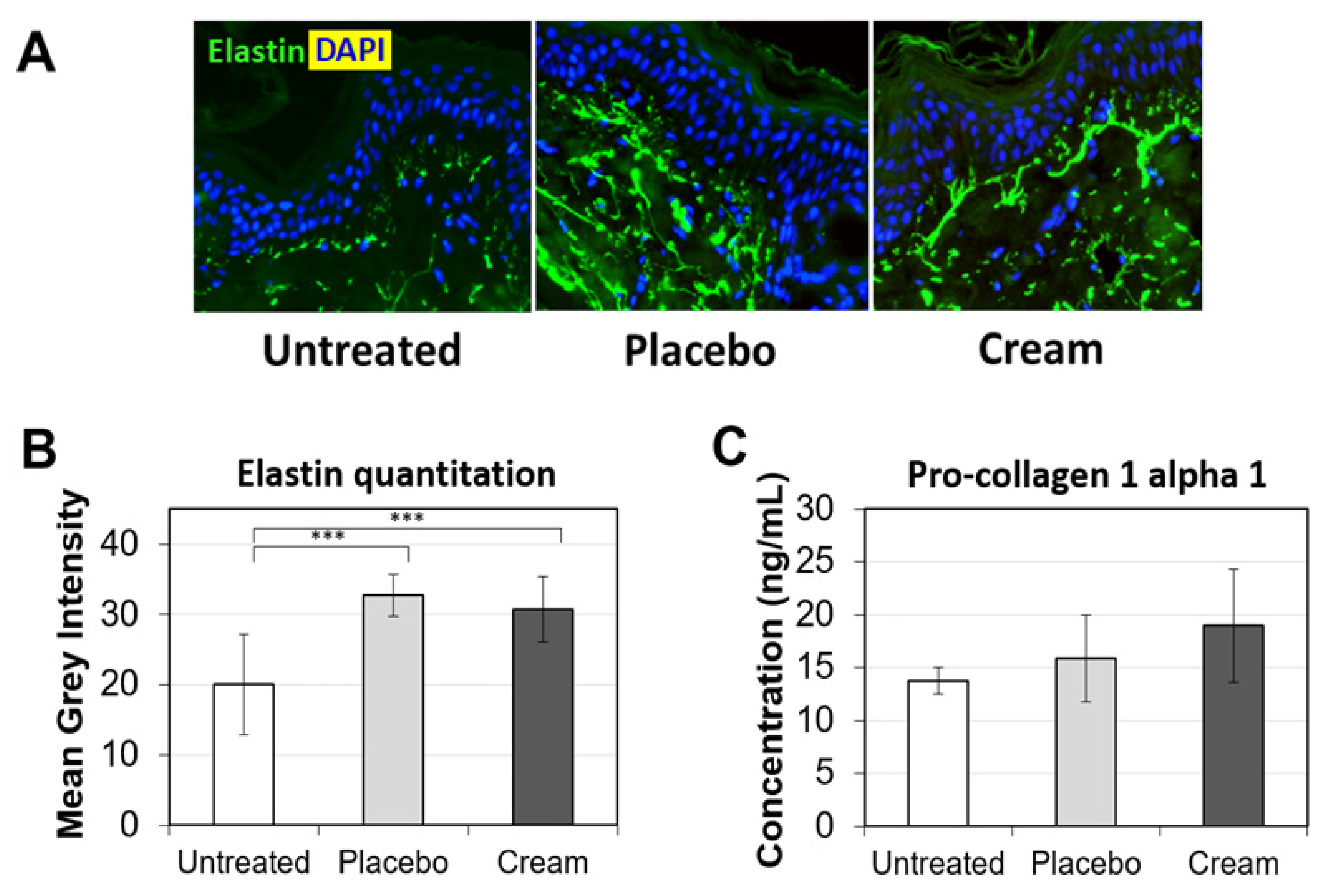
To further examine the potential benefits of the cream on ex vivo tissue, loricrin, a key epidermal barrier biomarker, was evaluated using immunofluorescence staining (Figure 2A). Compared to both the untreated and placebo samples, the cream significantly stimulated loricrin expression, while similar levels were observed for the placebo (Figure 2B). Additionally, the impact on the skin’s antioxidant barrier was assessed by measuring SOD2 protein expression. SOD2 mitigates oxidative stress, specifically associated with superoxide byproducts. As shown visually by immunofluorescence (Figure 3A) and quantified (Figure 3B), the cream strongly stimulated SOD2 expression in the epidermis, while the placebo only slightly increased it. The increase observed following cream application was statistically significant over the placebo.
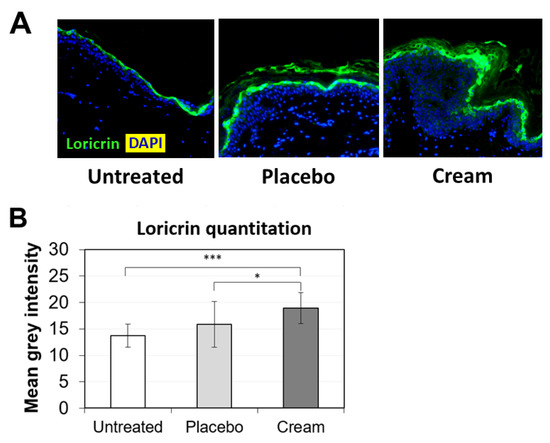
Figure 2.
Assessment of loricrin in the epidermis by immunofluorescence on human skin explants after eight days of topical treatment. (A) Representative images of skin sections of the untreated, placebo (negative control formulation), and cream (test product) groups, where loricrin is visualized in green and the nucleus (DAPI) in blue (biological replicates n = 3). (B) Loricrin expression was quantitated by image processing and analysis of n = 5 images. Statistical comparison of the mean values was achieved using a t-test (*** p < 0.001 and * p < 0.05).
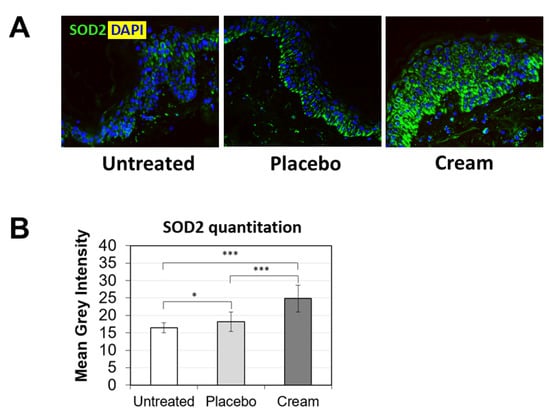
Figure 3.
Assessment of SOD2 in the epidermis by immunofluorescence on human skin explants after eight days of topical treatment. (A) Representative images of skin sections of the untreated, placebo (negative control formulation), and cream (test product) groups, where SOD2 is visualized in green and the nucleus (DAPI) in blue (biological replicates n = 3). (B) SOD2 expression was quantitated by image processing and analysis of n = 5 images. Statistical comparison of the mean values was achieve using a t-test (* p < 0.05 and *** p < 0.001).
3.3. Tyrosinase Activity Inhibition
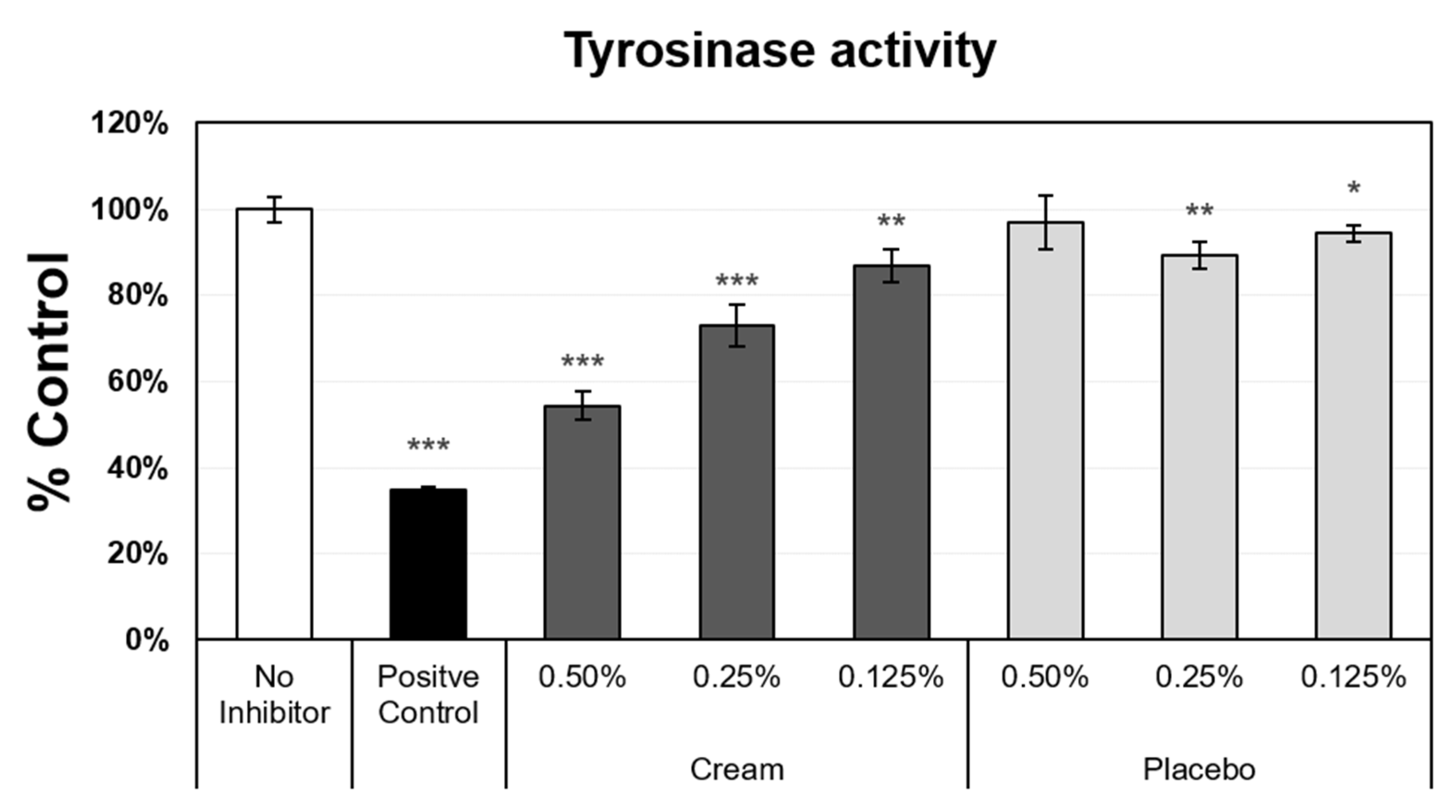
Another measurement of epidermal function and skin aging is the evenness of skin tone, which is driven by melanin synthesis and distribution. Melanin production is a heavily regulated process, with more than 100 genes involved [24]. As skin ages, more discoloration develops, resulting from dysregulation of melanogenesis [25]. To correct these visual changes on the skin, various methods are utilized to reduce melanin production in localized areas. Several molecules are known to inhibit melanin synthesis, through the inhibition or suppression of tyrosinase activity. Tyrosinase is the rate-limiting enzyme in the melanin synthesis pathway, and the reduction in tyrosinase expression and/or activity impacts the melanin content in the skin. Tyrosinase activity was measured in darkly pigmented normal human epidermal melanocytes (Figure 4). The assay included negative (no inhibitor) and positive (glycolic acid) controls, from which the maximum expression level (no inhibitor level set to 100%) and minimum value (38%), respectively, were obtained. The placebo, at 0.25% and 0.125%, only slightly suppressed tyrosinase activity. In contrast, the cream induced a strong inhibition of tyrosinase activity at the 0.5% concentration, and significant dose-dependent inhibition was observed when decreasing the cream concentration from 0.5% to 0.125%.
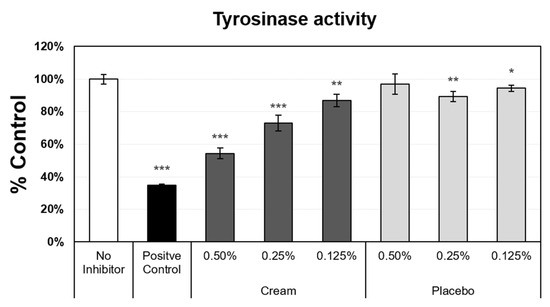
Figure 4.
Tyrosinase activity assay using darkly pigmented normal human melanocytes. Mean data ± SD are presented as % control (n = 4). The negative control did not receive inhibitors (set value, 100%). Glycolic acid was used as the positive control. The placebo (negative control formulation) and cream (test product) formulations were diluted for evaluation. Statistical comparison of the mean values using a t-test (* p < 0.05, ** p < 0.01, and *** p < 0.001).
3.4. Impact on Collagen and Elastin Expression
Young, healthy-looking skin not only displays an even skin tone, but also maintains a robust level of extracellular matrix (ECM) proteins in the dermis. Two of the most well-represented ECM proteins are collagen and elastin. Collagen is a key supporter of skin structure and elastin functions in concert to keep the skin elastic. As skin ages, collagen and elastin expression are reduced, while proteases degrading collagen and elastin are stimulated. Additionally, sun exposure accelerates the manifestation of skin aging, such as glycation of ECM and solar elastosis [26]. These changes may lead to the formation of fine lines and wrinkles. Therefore, stimulating pro-collagen I and elastin expression may improve skin firmness and reduce fine lines and wrinkles. First, elastin expression was assessed by immunofluorescence (Figure 5A). Compared to untreated tissue’s elastin fiber expression (green), the placebo- and cream-treated skin explants expressed longer elastin fibers in the papillary dermis, perpendicular to the basal layer of the epidermis. Tissue elastin quantitation indicated a statistically significant increase versus the untreated sample (Figure 5B). No difference was observed between the placebo and cream, suggesting that the formula backbone influenced the elastin fiber expression in the skin tissues. Pro-collagen I alpha 1 ELISA did not show any statistically significant difference between the treatment groups, although there was a trending increase for tissues treated with the cream (Figure 5C). The lack of statistical significance is likely due to variability in the biological samples.
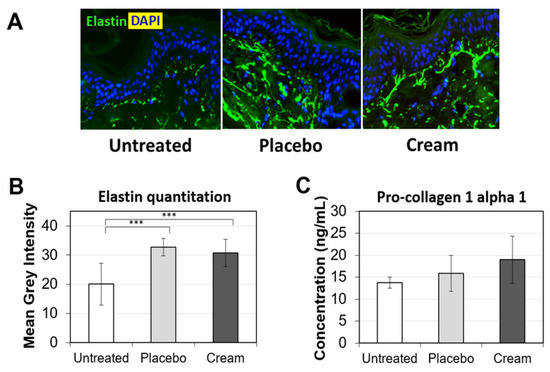
Figure 5.
Evaluation of the ECM on human skin explants after eight days of topical treatment. (A) Representative images of skin sections of the untreated, placebo (negative control formulation), and cream (test product) groups stained for elastin (green) by immunofluorescence. The nucleus (DAPI) is counterstained in blue. (B) Elastin expression is quantitated by image processing and analysis of n = 5 samples. (C) Pro-collagen 1 alpha 1 protein expression was measured by ELISA. The data presented are the mean ± SEM. Statistical comparison of the mean values was achieved using a t-test (*** p < 0.001).
3.5. Clinical Study and Demographic Analysis
Given the observed effects of the micropeeling cream on ex vivo tissue and to evaluate its efficacy in vivo, we performed a clinical study. The study was performed between November and December 2021. Thirty Caucasian subjects, phototypes II–IV, with a mean age of 50.0 ± 6.4 years, completed the efficacy assessment study. Six subjects (three Asian and three African) of phototypes V and VI, participated in the subjective assessments (data not shown) and tolerability of the test formula. All subjects tolerated the test formula, except one Asian subject from the second group, who reported a moderate allergic-type rash on the forehead and neck. The subject self-identified as having sensitive skin. The adverse event spontaneously resolved after discontinuation of the product, and this subject’s participation was discontinued from the study after day 1.
3.6. Improved Dermatological Assessments
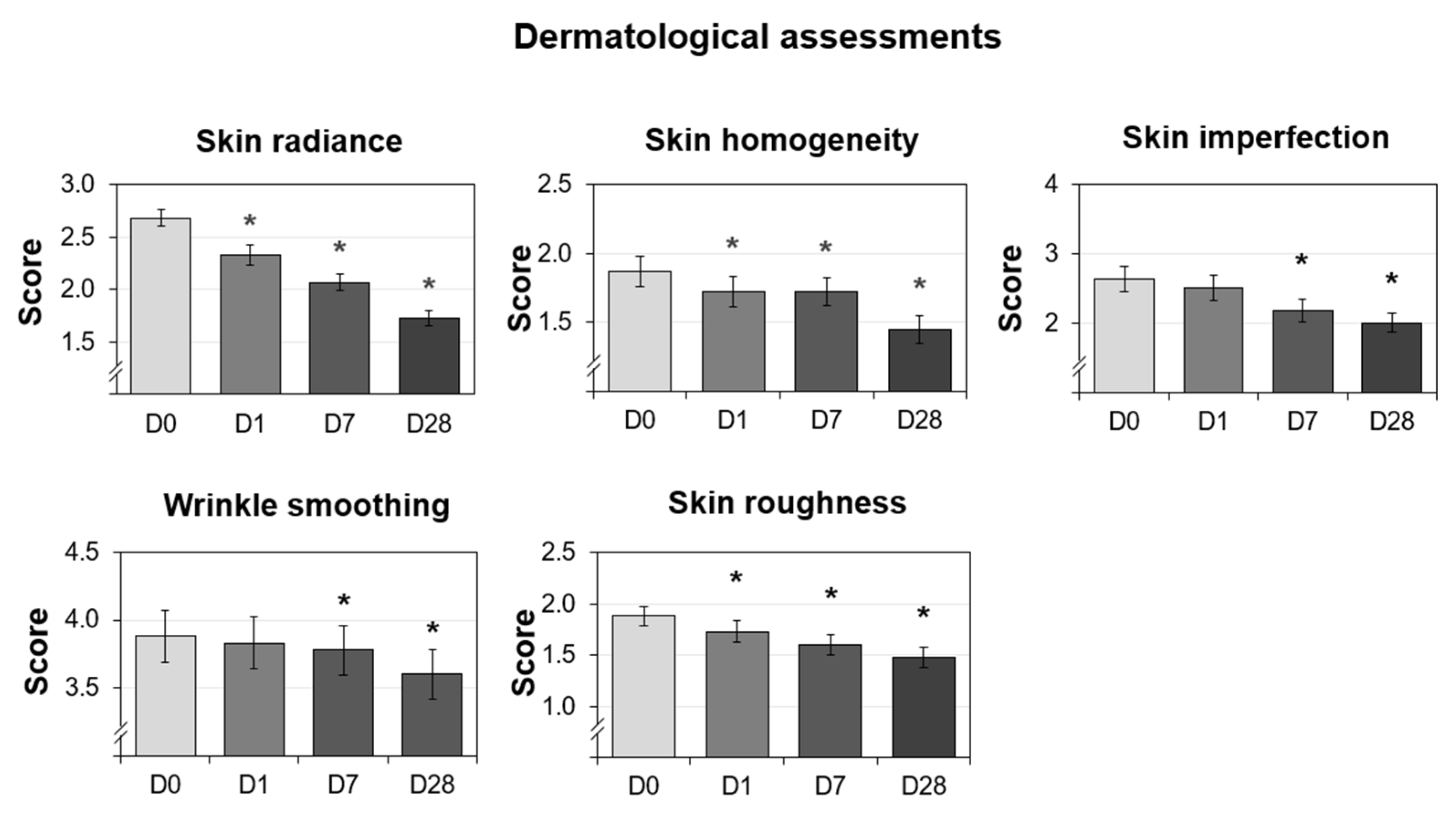
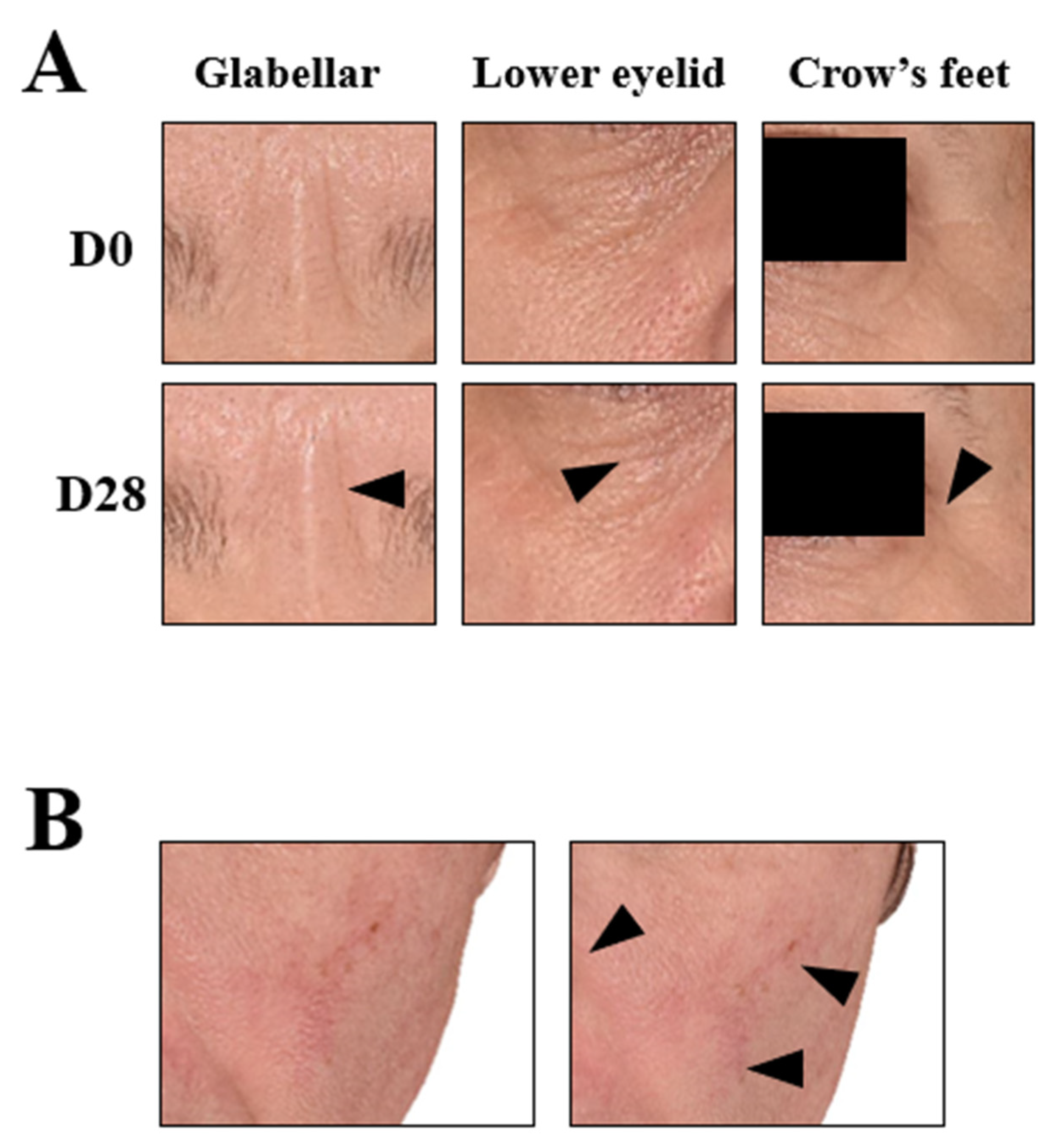
The dermatologist investigator assessed skin radiance, skin homogeneity, wrinkle smoothing, skin imperfections, and skin roughness on D0, D1, D7, and D28. The percent reduction was calculated compared to D0. The resulting graphs are shown in Figure 6. Overall, all of the parameters showed improvements over time. Additionally, the skin radiance, homogeneity, and roughness scores were significantly reduced at all measured time points (D1, D7, and D28), whereas the imperfection and wrinkle smoothing scores were significantly decreased at the last two time points (D7 and D28). The radiance score showed the fastest improvement, where one treatment resulted in a 13% reduction compared to the baseline. The skin roughness and skin homogeneity scores also showed statistically significant improvement after just one use. One of the parameters not easily modulated was wrinkle smoothing, but the use of the cream helped to increase wrinkle smoothing and resulted in statistically significant improvements as measured on D7 and D28, confirming the effectiveness of the long-term use of this micropeeling cream. The photos from a representative subject are shown in Figure 7A to visually demonstrate wrinkle reduction at D28. Overall, all the parameters assessed in the study by the dermatologist investigator showed improvements throughout the four weeks of use.
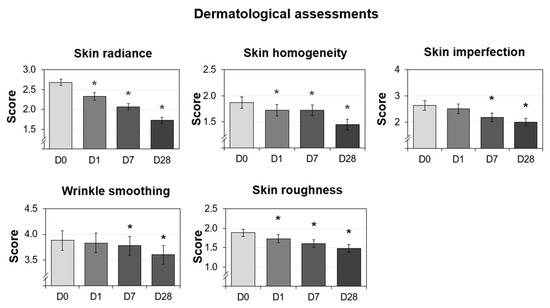
Figure 6.
Dermatological assessments performed on 30 Caucasian subjects after nightly use of the cream (test product) during 28 days. The data presented are the mean ± SD at D0 (baseline), D1, D7, and D28. Statistical comparisons of the mean values to D0 were achieved by a Wilcoxon test (* p < 0.05).
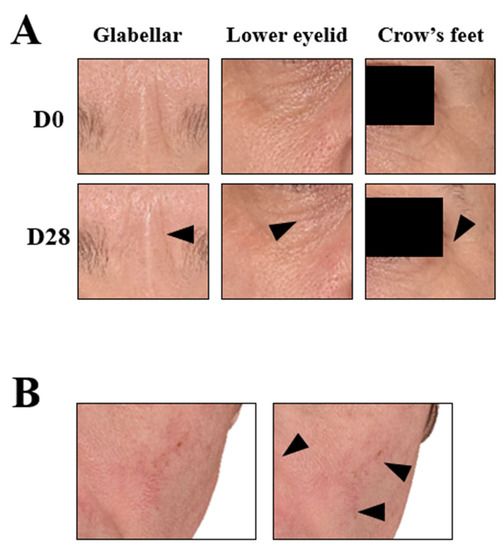
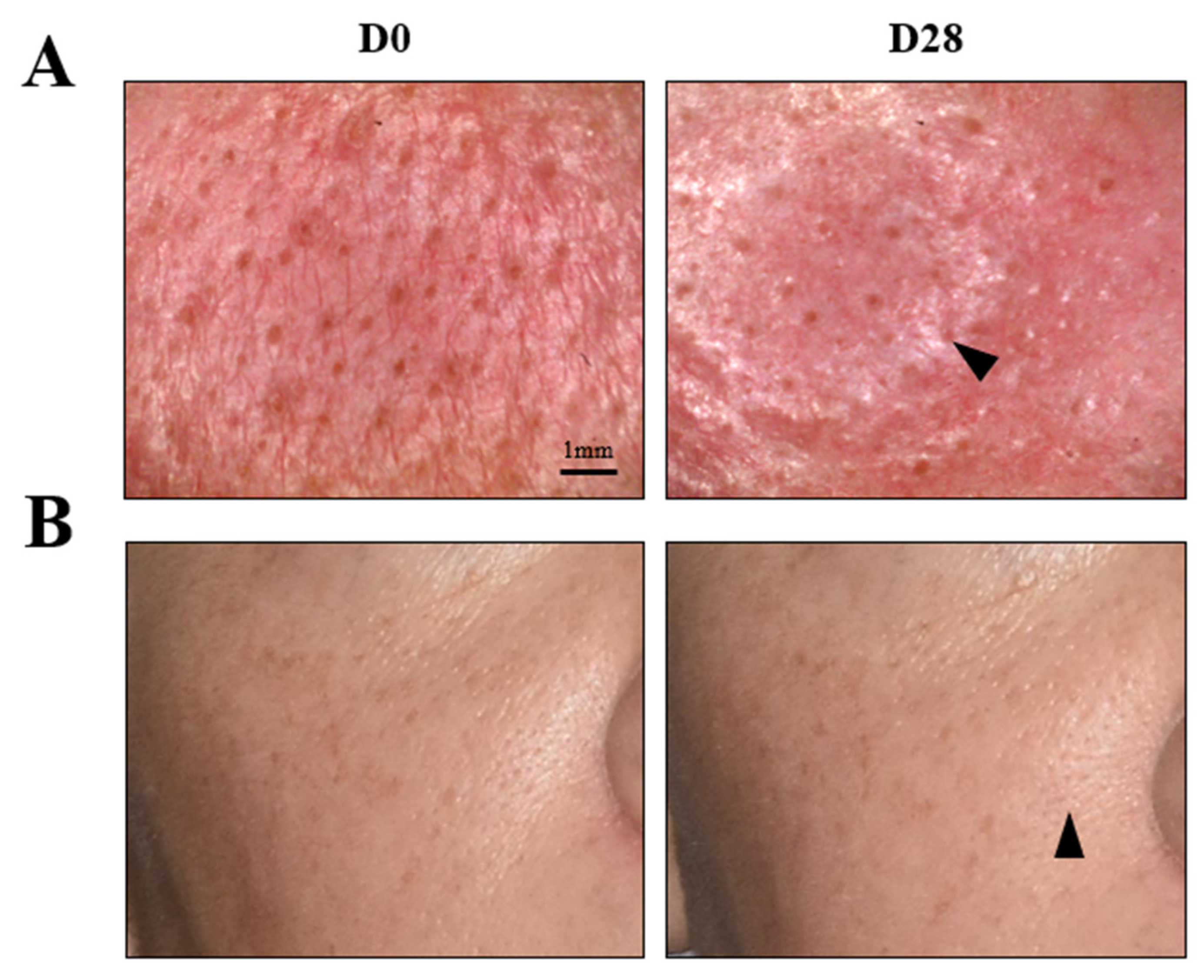
Figure 7.
3D reconstructive images of the face of selected subjects in frontal view. (A) Wrinkle improvement of the periocular area (arrows); representative image of the mean of the wrinkle smoothing score. (B) Discoloration improvement in the nasal wing area and chick (arrows); representative image of the mean of the skin lightness.
3.7. Significant Improvements in the Bioinstrumental Measurements
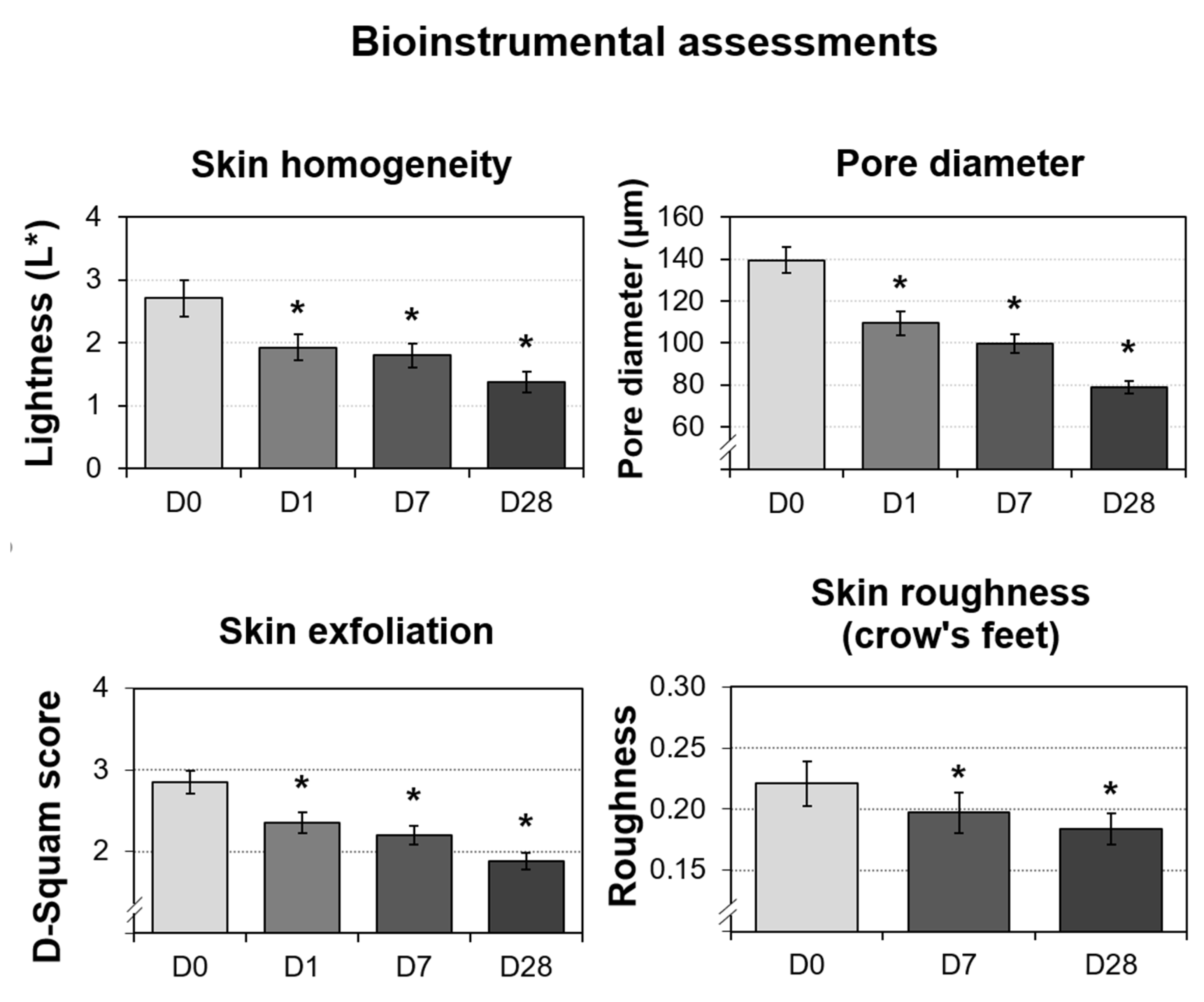
The four parameters evaluated by instruments were skin homogeneity, pore diameter, skin exfoliation, and skin roughness (periocular). As shown in Figure 8, all of the measured skin parameters showed significant improvements over the baseline, as well as throughout the study. Skin homogeneity, measured by colorimetry, was the most significantly impacted. After 28 days of continued topical application, the micropeeling cream improved L* variations by 48.8%. Photographs from one representative subject also confirmed improvements in discoloration, as shown in Figure 7B. Morphometric measurement, pore diameter, was the second parameter that exhibited the most significant changes. From D1, the pore diameter reduction was statistically significant at 21.5%, which continued to 28.5% and 43.3% reductions by D7 and D28, respectively. A representative visualization of pores and photos of the subjects are shown in Figure 9, clearly demonstrating pore diameter reductions. The quantification of the removed corneocytes also revealed significant progressive improvements in skin exfoliation in comparison to the baseline. Lastly, skin roughness on the periocular area was significantly reduced by D7 and D28 with 11% and 17%, respectively, versus the baseline.
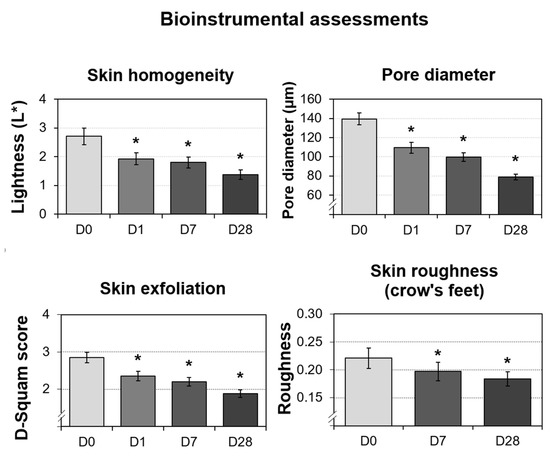
Figure 8.
Bioinstrumental measurements performed on 30 Caucasian subjects after nightly use of the cream (test product) during 28 days. The data presented are the mean ± SD at D0 (baseline), D1, D7, and D28. Statistical comparisons of the mean values to D0 were achieved by Student’s t- or Wilcoxon tests depending on the normality of the distribution (* p < 0.05).
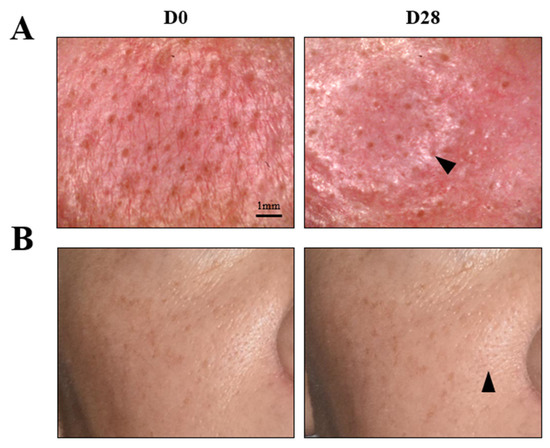
Figure 9.
Pore assessment in the nasal wing area at baseline D0 and D28; representative image of the mean pore diameter. (A) Visualization of pores using a microscope (×30 magnifications). Scale bar: 1 mm. (B) Image of a subject representative of the mean pore diameter at D28. Arrows indicate an improvement in the appearance of pores.
4. Discussion
The micropeeling cream described here is developed for everyday use for skin improvements. The skin benefits associated with the micropeeling cream are skin surface exfoliation, reduced skin roughness and skin texture improvement. These benefits were demonstrated in the clinical study with good tolerance by study subjects, which included sensitive skin subjects.
The skin reactions of consumers with sensitive skin to topical formulations may be heightened, resulting in mild irritation and stinging or burning sensations, while subjects with non-sensitive skin might not feel or develop any adverse events [13]. Sensitivity is often exaggerated by stress, dry or cold weather, and certain inadequate skincare products [13], prompting consumers and physicians to limit topical product use and avoid certain product types. Chemical peels, especially professional peels, are generally considered potential irritants due to their high acid content, low pH, and mechanism of action. Chemical peels exfoliate the skin surface to various degrees and have lasting benefits by improving various signs of aging, such as minimizing discoloration and improving ECM production, both in vivo and by up-regulation of related genes in vitro and ex vivo [5]. Although higher-level benefits are expected following the application of the highest strength peels, the risk of side effects also increases drastically [4], especially in sensitive skin. For these reasons, the peels recommended by healthcare professionals for sensitive skin are superficial peels with low acid concentrations.
Therefore, the micropeeling cream described here constitutes a relevant alternative for sensitive skin subjects. Unlike previously described formulations, which are usually formulated as liquids, a cream format allows for controlled product application with the possibility to apply it only to the desired target sites. The cream is a stable emulsion, containing a total 4.5% AHA/BHA complex complemented with a brown algae extract. This formulation has a final pH of ~3.6, which is much more skin-compatible than higher level acid peels, with a lower pH [11]. More importantly, safety and skin tolerability were key objectives for this study. We assessed the cutaneous tolerance for this product in ex vivo skin models, where both the test and placebo formulas did not induce IL-1α release, compared to the untreated sample. This biomarker assessment suggested a potentially non-irritating formula, which was confirmed in the clinical study. Approximately half of the clinical study subjects (50%) had self-perceived sensitive skin. The safety outcome analysis was that the cream had an overall good safety profile during the 28-day study for 35 subjects. Only one subject, with sensitive skin, developed a moderate allergic-type rash that resolved after product discontinuation. This demonstrates the importance of sequential, low-dose increases in topical products for sensitive skin subjects. The other subjects with sensitive skin tolerated the cream without side effects.
Despite the low level of AHA/BHA present, anti-aging skin benefits associated with chemical peels were still observed. After the first application, skin roughness, determined by clinical scoring and bioinstrumentation, was reduced by 8% and 17.5%, respectively. This decrease continued for the duration of the study to 21% and 34%, respectively. Clinical assessment revealed that improvements in skin roughness were accompanied by improved skin radiance, as well as decreased appearance of pores and wrinkles, even in the periocular area. The micropeeling cream improved skin homogeneity by 22% (clinical grading) and 49% (bioinstrumentation) in four weeks. Previous studies had largely investigated the effect of low single AHA levels on skin over multiple months [7,8,9], and this study showed that a combination of a low-dose AHA/BHA complex may provide measurable skin benefits within less than 28 days of use.
In the accompanying biomarker study, the cream stimulated epidermal function biomarkers, loricrin, and SOD2, improving skin barrier and antioxidant capacity. There was also a dose-dependent inhibition of tyrosinase activity, which may have been reflected in the improved skin homogeneity observed. Additionally, the expression of dermal structure proteins, elastin, and collagen was stimulated significantly or trending, potentially improving skin firmness and elasticity, thus providing better support for the epidermis and possibly improving the appearance of wrinkles. The low concentration of acids in this micropeeling formulation does not appear to have compromised the efficacy of the cream. The presence of various milder acids in an optimized formulation probably helped to maintain the synergistic activities of the peel, with limited occurrence of side effects. The indication for nightly use obtained a high degree of compliance from subjects.
We obtained promising in vitro, ex vivo and in vivo data on efficacy and tolerance, but our study also had some limitations. The skin explants were prepared from abdominoplasty for availability. However, people use chemical peels or a micropeeling cream on their face, which is more sensitive than the body [27]. Therefore, potential skin irritation and concerns were not fully addressed in the first part of the study. Additionally, no methods are available to evaluate skin peeling or smoothing using in vitro or ex vivo methods, which would be a useful tool to differentiate different peeling acids. The clinical study was focused on relatively short-term benefits, up to 28 days. A longer study would have been useful to understand the dermal remodeling after peels or to compare with traditional chemical peels, utilizing the ultrasound probes or evaluating biopsies from the study. Additionally, a different study design would have also been beneficial. For example, a split-face study or a study with a second arm using the placebo formula only would have been a better comparative, controlled study. However, those methods were beyond the scope of this project.
5. Conclusions
This micropeeling cream, developed for daily at-home use for multiple skin types, contains a 4.5% blend of AHAs, BHA, and a soothing botanical extract. During nightly use, we found that the cream was suitable for subjects with sensitive skin. Even with such low acid concentrations, it demonstrated multiple cutaneous improvements in ex vivo and in vitro studies. Skin barrier and antioxidant protection, pigmentation inhibition, and improvements in the ECM structure were the key changes that we observed. In addition, we found that an irritation biomarker was suppressed. The following clinical study confirmed significant improvements in various skin parameters such as wrinkle smoothing, skin texture, overall skin quality, and good skin tolerability in subjects with sensitive and non-sensitive skin, as per assessments conducted by the study dermatologist.
Author Contributions
J.N. and S.G. designed, conducted, analyzed, and interpreted the in vitro and ex vivo data. O.T. designed, conducted, interpreted, and analyzed the data from clinical studies. I.D. supervised the clinical study. J.W. supervised the in vitro and ex vivo research. All the authors contributed to draft preparation and substantively revised it. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by Colgate-Palmolive Company and Filorga. This research received no external funding.
Institutional Review Board Statement
The clinical study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of U.S. IRB, Inc. IRB Number U.S. IRB2021CPS/06.
Informed Consent Statement
Informed consent was obtained from all the subjects involved in the study before participating in any study-related activities. Written informed consent was obtained from the patients to publish this paper.
Data Availability Statement
Data are available on request from the corresponding author.
Acknowledgments
The authors would like to thank Nina Nguon (Laboratoires Filorga, Paris, France) for the technical writing of the manuscript and Alexandra D’Arcangelis (Colgate-Palmolive Company, New York, NY, USA) for editing the manuscript.
Conflicts of Interest
All the authors are employees of Colgate-Palmolive Company, the parent company of Filorga.
Appendix A
Appendix A.1. Cream (Test Product) Ingredient List
Aqua (water), butylene glycol, glycolic acid, propylene glycol dicaprylate/dicaprate, cetearyl alcohol, dimethicone, glycol palmitate, isononyl isononanoate, caprylic/capric triglyceride, glycerin, hydroxyethyl acrylate/sodium acryloyldimethyl taurate copolymer, sodium hydroxide, pseudoalteromonas ferment extract, phenoxyethanol, ceteareth-20, fragrance, hydrolyzed wheat flour, lauroyl lysine, sucrose palmitate, sodium lactate, Aloe barbadensis leaf juice powder, tocopheryl acetate, azelaic acid, Laminaria ochroleuca extract, salicylic acid, polysorbate 60, sorbitan isostearate, glyceryl linoleate, citric acid, sodium PCA, Prunus amygdalus dulcis (sweet almond) oil, sodium chloride, carbomer, lactic acid, malic acid, phytic acid, polysorbate 20, sodium salicylate, glucose, sodium hyaluronate, pyruvic acid, tartaric acid, potassium chloride, potassium sorbate, calcium chloride, magnesium sulfate, glutamine, sodium phosphate, ascorbic acid, sodium acetate, tocopherol, lysine HCl, arginine HCl, palmitoyl tripeptide-1, alanine, histidine HCl, valine, leucine, threonine, isoleucine, palmitoyl tetrapeptide-7, tryptophan, phenylalanine, tyrosine, glycine, polysorbate 80, serine, cystine, cyanocobalamin, glutathione, asparagine, aspartic acid, ornithine HCl, glutamic acid, nicotinamide adenine dinucleotide, proline, methionine, taurine, hydroxyproline, glucosamine HCl, coenzyme a, sodium glucuronate, thiamine diphosphate, retinyl acetate, inositol, niacin, niacinamide, pyridoxine HCl, biotin, calcium pantothenate, riboflavin, sodium tocopheryl phosphate, thiamine HCl and folic acid.
Appendix A.2. Placebo (Negative Control) Ingredient List
Aqua (water), butylene glycol, propylene glycol dicaprylate/dicaprate, cetearyl alcohol, dimethicone, glycol palmitate, isononyl isononanoate, caprylic/capric triglyceride, glycerin, hydroxyethyl acrylate/sodium acryloyldimethyl taurate copolymer, sodium hydroxide, pseudoalteromonas ferment extract, phenoxyethanol, ceteareth-20, fragrance, hydrolyzed wheat flour, lauroyl lysine, sucrose palmitate, sodium lactate, Aloe barbadensis leaf juice powder, tocopheryl acetate, azelaic acid, polysorbate 60, sorbitan isostearate, glyceryl linoleate, sodium PCA, Prunus amygdalus dulcis (sweet almond) oil, sodium chloride, carbomer, phytic acid, polysorbate 20, sodium salicylate, glucose, sodium hyaluronate, potassium chloride, potassium sorbate, calcium chloride, magnesium sulfate, glutamine, sodium phosphate, ascorbic acid, sodium acetate, tocopherol, lysine HCl, arginine HCl, palmitoyl tripeptide-1, alanine, histidine HCl, valine, leucine, threonine, isoleucine, palmitoyl tetrapeptide-7, tryptophan, phenylalanine, tyrosine, glycine, polysorbate 80, serine, cystine, cyanocobalamin, glutathione, asparagine, aspartic acid, ornithine HCl, glutamic acid, nicotinamide adenine dinucleotide, proline, methionine, taurine, hydroxyproline, glucosamine HCl, coenzyme a, sodium glucuronate, thiamine diphosphate, retinyl acetate, inositol, niacin, niacinamide, pyridoxine HCl, biotin, calcium pantothenate, riboflavin, sodium tocopheryl phosphate, thiamine HCl and folic acid.
References
- Rajanala, S.; Vashi, N.A. Cleopatra and Sour Milk-The Ancient Practice of Chemical Peeling. JAMA Dermatol. 2017, 153, 1006. [Google Scholar] [CrossRef] [PubMed]
- Soleymani, T.; Lanoue, J.; Rahman, Z. A Practical Approach to Chemical Peels: A Review of Fundamentals and Step-by-step Algorithmic Protocol for Treatment. J. Clin. Aesthet. Dermatol. 2018, 11, 21–28. [Google Scholar] [PubMed]
- Rendon, M.I.; Berson, D.S.; Cohen, J.L.; Roberts, W.E.; Starker, I.; Wang, B. Evidence and considerations in the application of chemical peels in skin disorders and aesthetic resurfacing. J. Clin. Aesthet. Dermatol. 2010, 3, 32–43. [Google Scholar] [PubMed]
- O’Connor, A.A.; Lowe, P.M.; Shumack, S.; Lim, A.C. Chemical peels: A review of current practice. Australas. J. Dermatol. 2018, 59, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, V.; Sharma, K.; Maksimovic, S.; Fan, A.; Adams-Woodford, A.; Mao, J. Professional-Grade TCA-Lactic Acid Chemical Peel: Elucidating Mode of Action to Treat Photoaging and Hyperpigmentation. Front. Med. 2021, 8, 617068. [Google Scholar] [CrossRef]
- Chiriac, A.; Brzezinski, P. Topical malic acid in combination with citric acid: An option to treat recalcitrant warts. Dermatol. Ther. 2015, 28, 336–338. [Google Scholar] [CrossRef]
- Abels, C.; Kaszuba, A.; Michalak, I.; Werdier, D.; Knie, U.; Kaszuba, A. A 10% glycolic acid containing oil-in-water emulsion improves mild acne: A randomized double-blind placebo-controlled trial. J. Cosmet. Dermatol. 2011, 10, 202–209. [Google Scholar] [CrossRef]
- Bernstein, E.F.; Underhill, C.B.; Lakkakorpi, J.; Ditre, C.M.; Uitto, J.; Yu, R.J.; Scott, E.V. Citric acid increases viable epidermal thickness and glycosaminoglycan content of sun-damaged skin. Dermatol. Surg. 1997, 23, 689–694. [Google Scholar] [CrossRef]
- Smith, W.P. Epidermal and dermal effects of topical lactic acid. J. Am. Acad. Dermatol. 1996, 35, 388–391. [Google Scholar] [CrossRef]
- Denda, S.; Denda, M.; Inoue, K.; Hibino, T. Glycolic acid induces keratinocyte proliferation in a skin equivalent model via TRPV1 activation. J. Dermatol. Sci. 2010, 57, 108–113. [Google Scholar] [CrossRef]
- Narda, M.; Trullas, C.; Brown, A.; Piquero-Casals, J.; Granger, C.; Fabbrocini, G. Glycolic acid adjusted to pH 4 stimulates collagen production and epidermal renewal without affecting levels of proinflammatory TNF-alpha in human skin explants. J. Cosmet. Dermatol. 2021, 20, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Rendl, M.; Mayer, C.; Weninger, W.; Tschachler, E. Topically applied lactic acid increases spontaneous secretion of vascular endothelial growth factor by human reconstructed epidermis. Br. J. Dermatol. 2001, 145, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Berardesca, E.; Farage, M.; Maibach, H. Sensitive skin: An overview. Int. J. Cosmet. Sci. 2013, 35, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Calvisi, L. Efficacy of a combined chemical peel and topical salicylic acid-based gel combination in the treatment of active acne. J. Cosmet. Dermatol. 2021, 20 (Suppl. S2), 2–6. [Google Scholar] [CrossRef] [PubMed]
- Nofal, E.; Nofal, A.; Gharib, K.; Nasr, M.; Abdelshafy, A.; Elsaid, E. Combination chemical peels are more effective than single chemical peel in treatment of mild-to-moderate acne vulgaris: A split face comparative clinical trial. J. Cosmet. Dermatol. 2018, 17, 802–810. [Google Scholar] [CrossRef]
- Sharad, J. Glycolic acid peel therapy—A current review. Clin. Cosmet. Investig. Dermatol. 2013, 6, 281–288. [Google Scholar] [CrossRef]
- Arif, T. Salicylic acid as a peeling agent: A comprehensive review. Clin. Cosmet. Investig. Dermatol. 2015, 8, 455–461. [Google Scholar] [CrossRef]
- Chilicka, K.; Rogowska, A.M.; Szygula, R.; Dziendziora-Urbinska, I.; Taradaj, J. A comparison of the effectiveness of azelaic and pyruvic acid peels in the treatment of female adult acne: A randomized controlled trial. Sci. Rep. 2020, 10, 12612. [Google Scholar] [CrossRef]
- Bonneville, M.; Saint-Mezard, P.; Benetiere, J.; Hennino, A.; Pernet, I.; Denis, A.; Nicolas, J.F. Laminaria ochroleuca extract reduces skin inflammation. J. Eur. Acad. Dermatol. Venereol. 2007, 21, 1124–1125. [Google Scholar] [CrossRef]
- Usuki, A.; Ohashi, A.; Sato, H.; Ochiai, Y.; Ichihashi, M.; Funasaka, Y. The inhibitory effect of glycolic acid and lactic acid on melanin synthesis in melanoma cells. Exp. Dermatol. 2003, 12 (Suppl. S2), 43–50. [Google Scholar] [CrossRef]
- Bazin, R.; Doublet, E. Skin Aging Atlas: Caucasian Type; MED’COM: Paris, France, 2007. [Google Scholar]
- Robin, S.; Fanian, F.; Courderot-Masuyer, C.; Tordjman, M.; Braccini, F.; Boisnic, S.; Philippon, V.; Vincent, A.G.; Salomon, C.; Manfait, M.; et al. Efficacy of a Biorevitalizing-Filler Solution on All Skin Aspects: 10 Years Approach through <i>in Vitro</i> Studies and Clinical Trials. J. Cosmet. Dermatol. Sci. Appl. 2021, 11, 18–37. [Google Scholar] [CrossRef]
- Petit, L.; Zugaj, D.; Bettoli, V.; Dreno, B.; Kang, S.; Tan, J.; Torres, V.; Layton, A.M.; Martel, P. Validation of 3D skin imaging for objective repeatable quantification of severity of atrophic acne scarring. Skin Res. Technol. 2018, 24, 542–550. [Google Scholar] [CrossRef] [PubMed]
- D’Mello, S.A.; Finlay, G.J.; Baguley, B.C.; Askarian-Amiri, M.E. Signaling Pathways in Melanogenesis. Int. J. Mol. Sci. 2016, 17, 1144. [Google Scholar] [CrossRef] [PubMed]
- Skoczynska, A.; Budzisz, E.; Trznadel-Grodzka, E.; Rotsztejn, H. Melanin and lipofuscin as hallmarks of skin aging. Postepy Dermatol. I Alergol. 2017, 34, 97–103. [Google Scholar] [CrossRef]
- Crisan, M.; Taulescu, M.; Crisan, D.; Cosgarea, R.; Parvu, A.; Catoi, C.; Drugan, T. Expression of advanced glycation end-products on sun-exposed and non-exposed cutaneous sites during the ageing process in humans. PLoS ONE 2013, 8, e75003. [Google Scholar] [CrossRef]
- Farage, M.A. The Prevalence of Sensitive Skin. Front. Med. 2019, 6, 98. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).