Artificial Intelligence Applications in Smart Healthcare: A Survey
Abstract
1. Introduction
2. Definition and Characteristics
2.1. Definition
2.2. Characteristics
- (1)
- Data collection and integration part
- (2)
- Unstructured data processing part
- (3)
- Data preprocessing part
- (4)
- Algorithm and model part
- (5)
- Prediction analysis part
- (6)
- Decision support part
- (7)
- Feedback and optimization part
3. Opportunities
4. Applications
4.1. Disease Prediction and Prevention
4.2. Diagnostic Imaging
4.3. Personalized Treatment Plans
4.4. Virtual Health Assistant
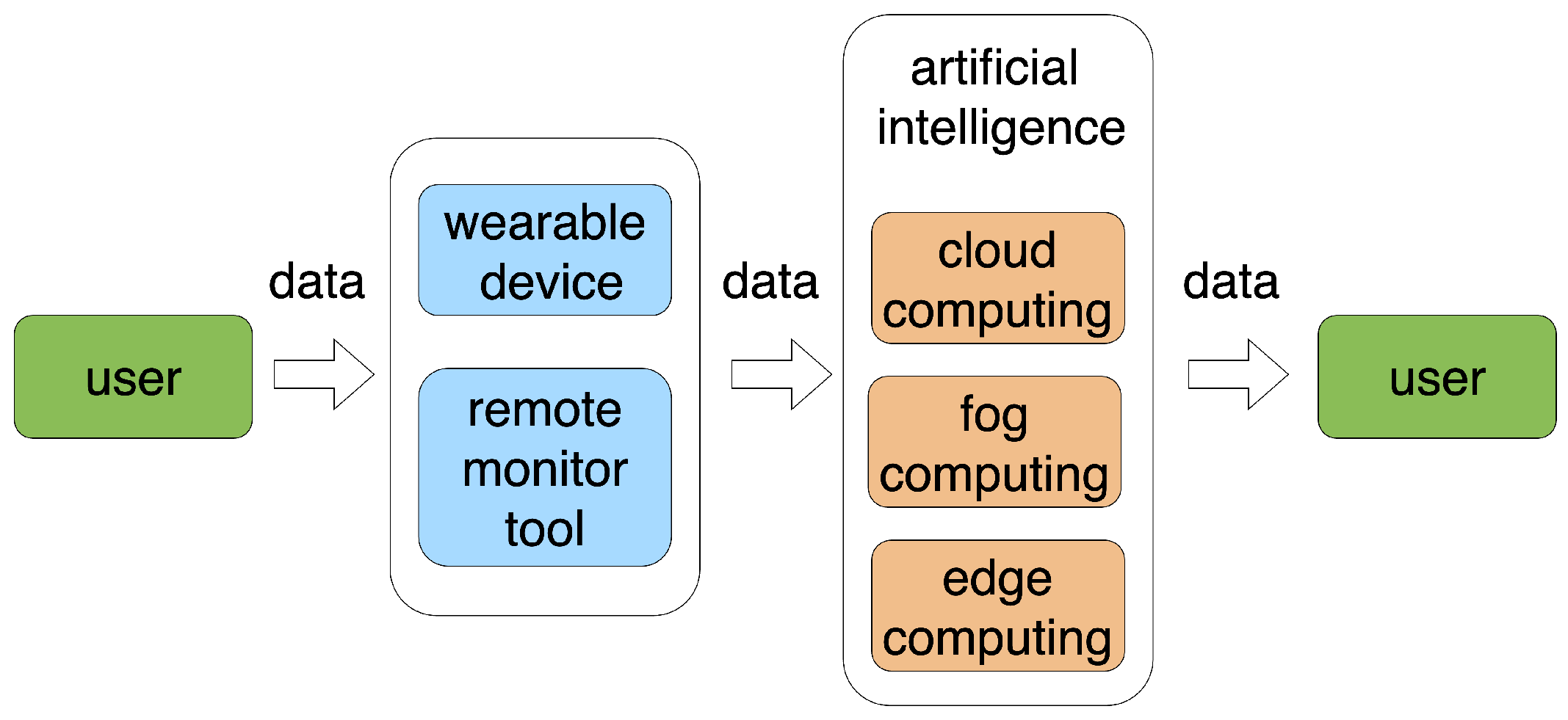
4.5. Remote Patient Monitoring
4.6. Drug Discovery and Development
4.7. Robotic Surgery
4.8. NLP for Electronic Health Records
4.9. Behavioral Health Support
4.10. Clinical Trial Matching
5. Challenges and Existing Solutions
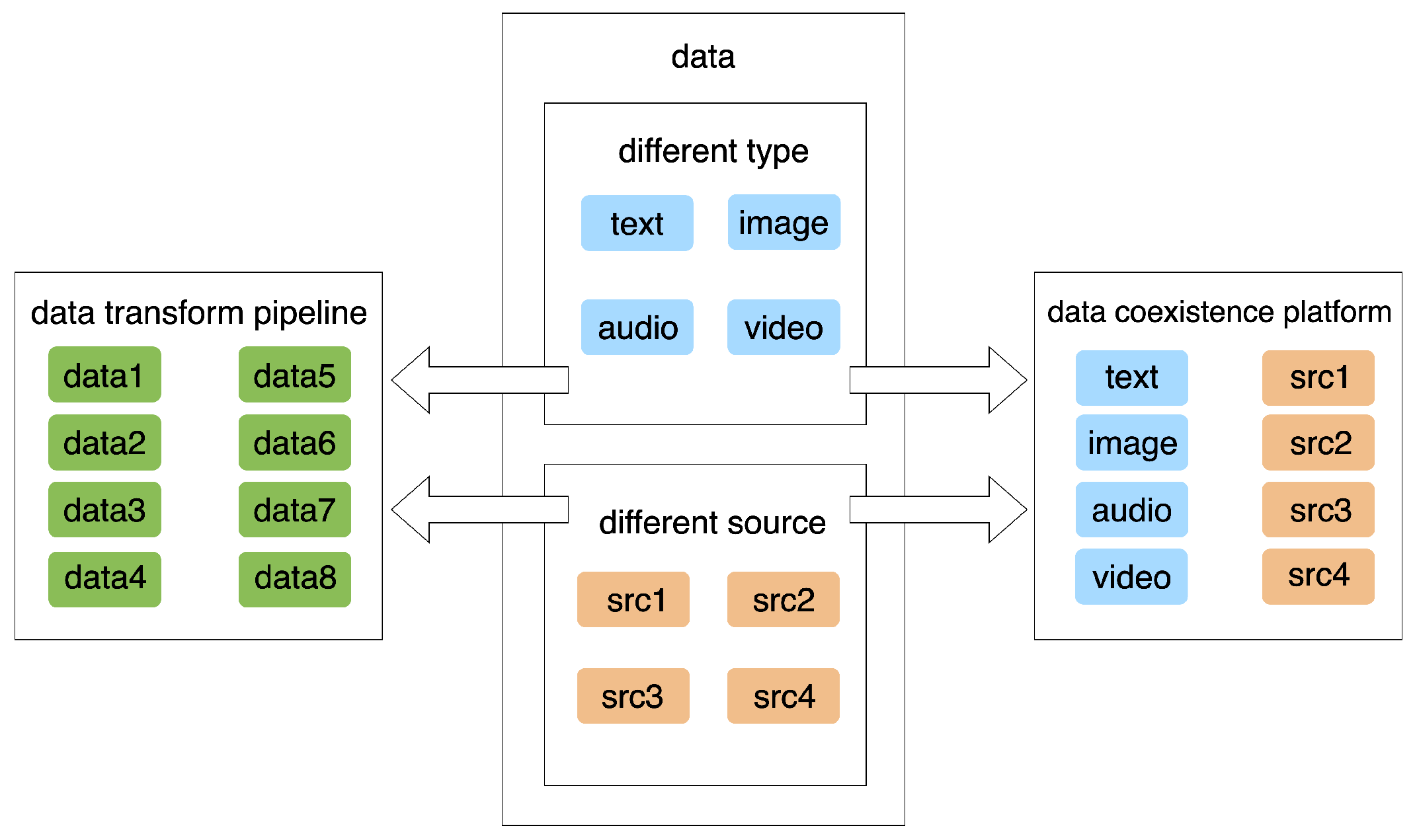
5.1. Data Integration and Interoperability
5.1.1. Challenges
5.1.2. Existing Solutions
5.1.3. Evaluation of the Existing Solutions
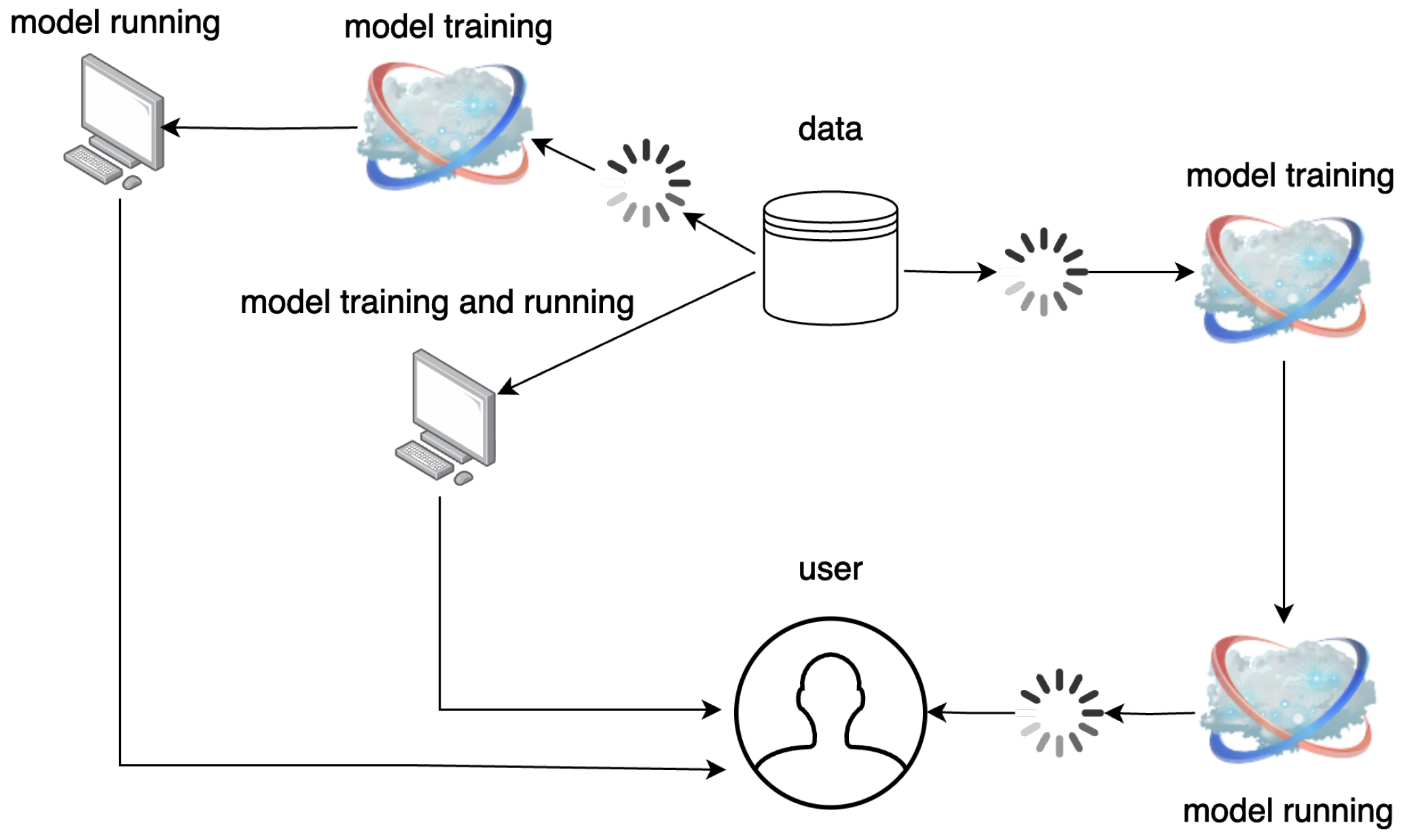
5.2. Large-Scale Data Handling
5.2.1. Challenges
5.2.2. Existing Solutions
5.2.3. Evaluation of the Existing Solutions
5.3. Real-Time Processing
5.3.1. Challenges
5.3.2. Existing Solutions
5.3.3. Evaluation of the Existing Solutions
5.4. Model Interpretability
5.4.1. Challenges
5.4.2. Existing Solutions
5.4.3. Evaluation of the Existing Solutions
5.5. Continuous Learning and Adaptability
5.5.1. Challenges
5.5.2. Existing Solutions
5.5.3. Evaluation of the Existing Solutions
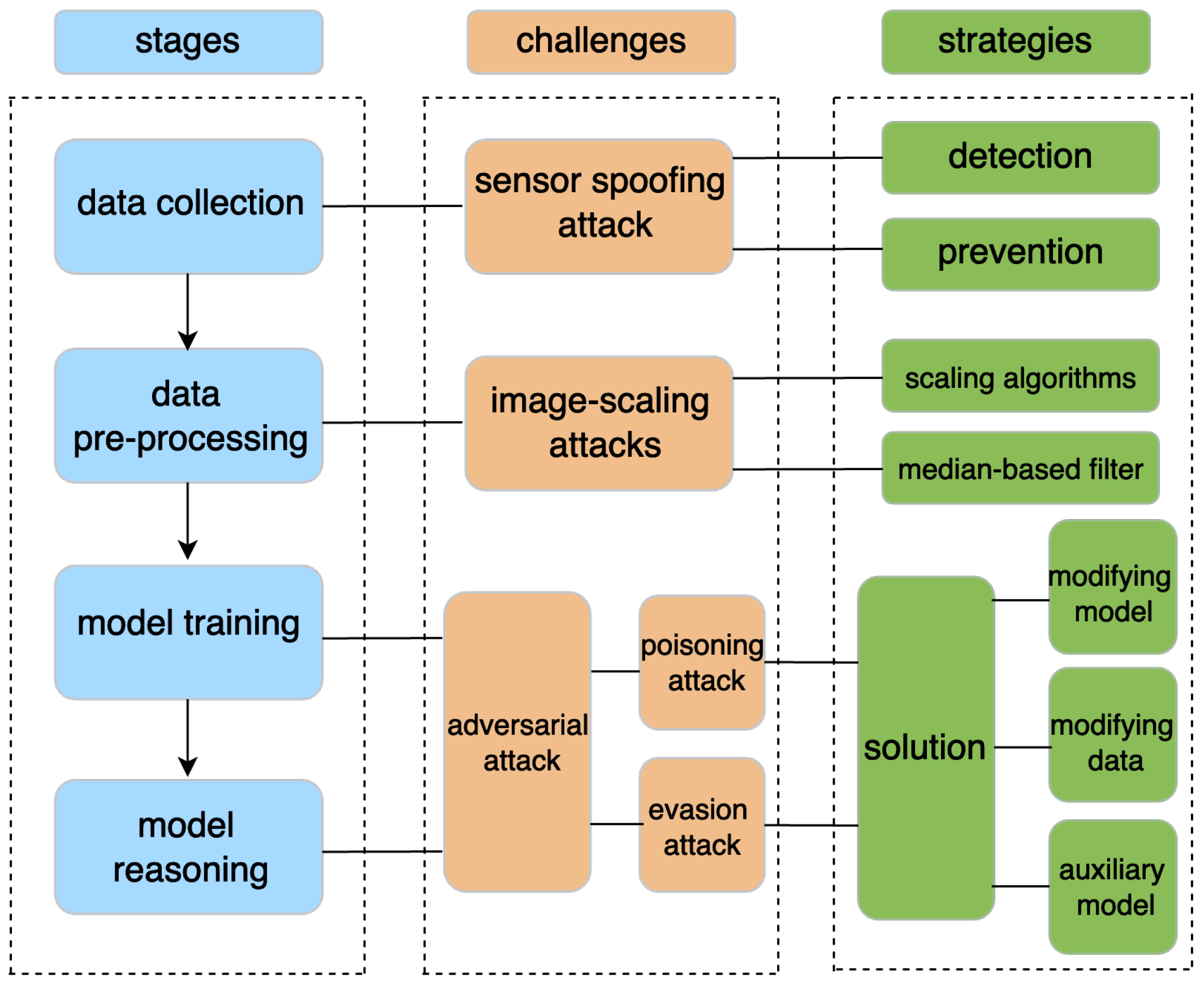
5.6. Security of AI Models
5.6.1. Challenges
5.6.2. Existing Solutions
5.6.3. Evaluation of the Existing Solutions
5.7. Ethical AI Design
5.7.1. Challenges
5.7.2. Existing Solutions
5.7.3. The Impact of AI on HIPAA
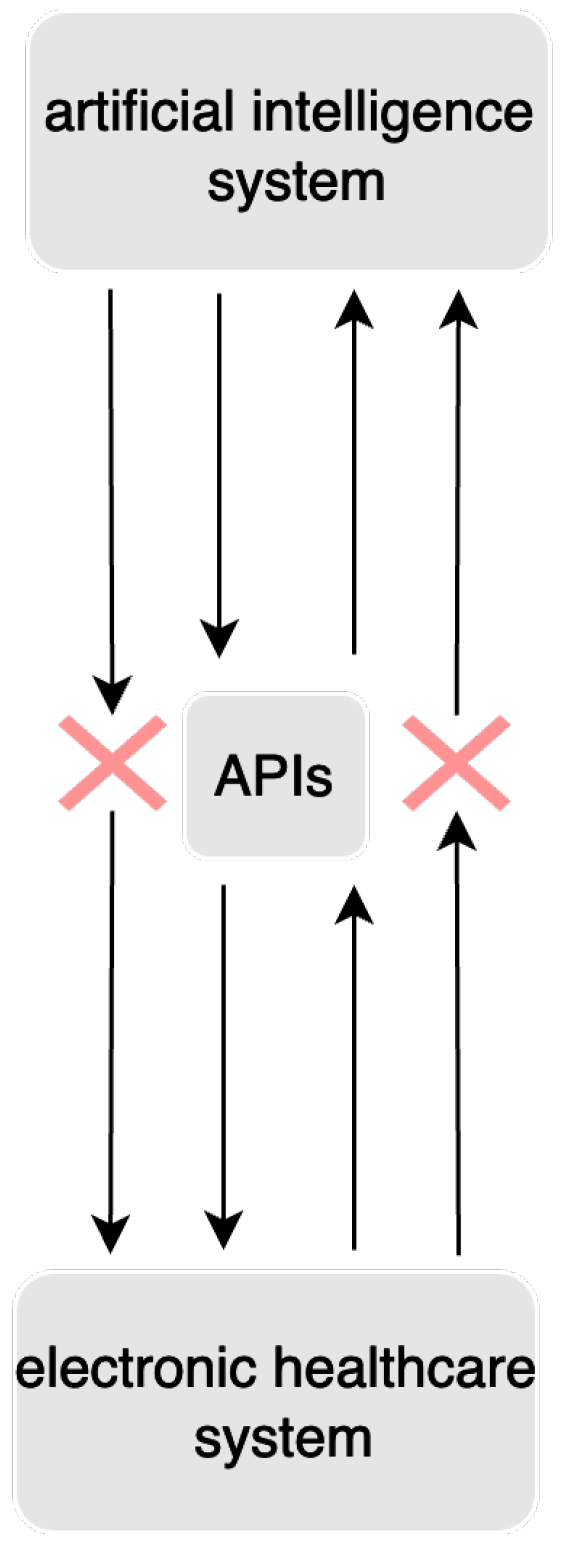
5.8. Integration with Electronic Health Records
5.8.1. Challenges
5.8.2. Existing Solutions
5.8.3. Evaluation of the Existing Solutions
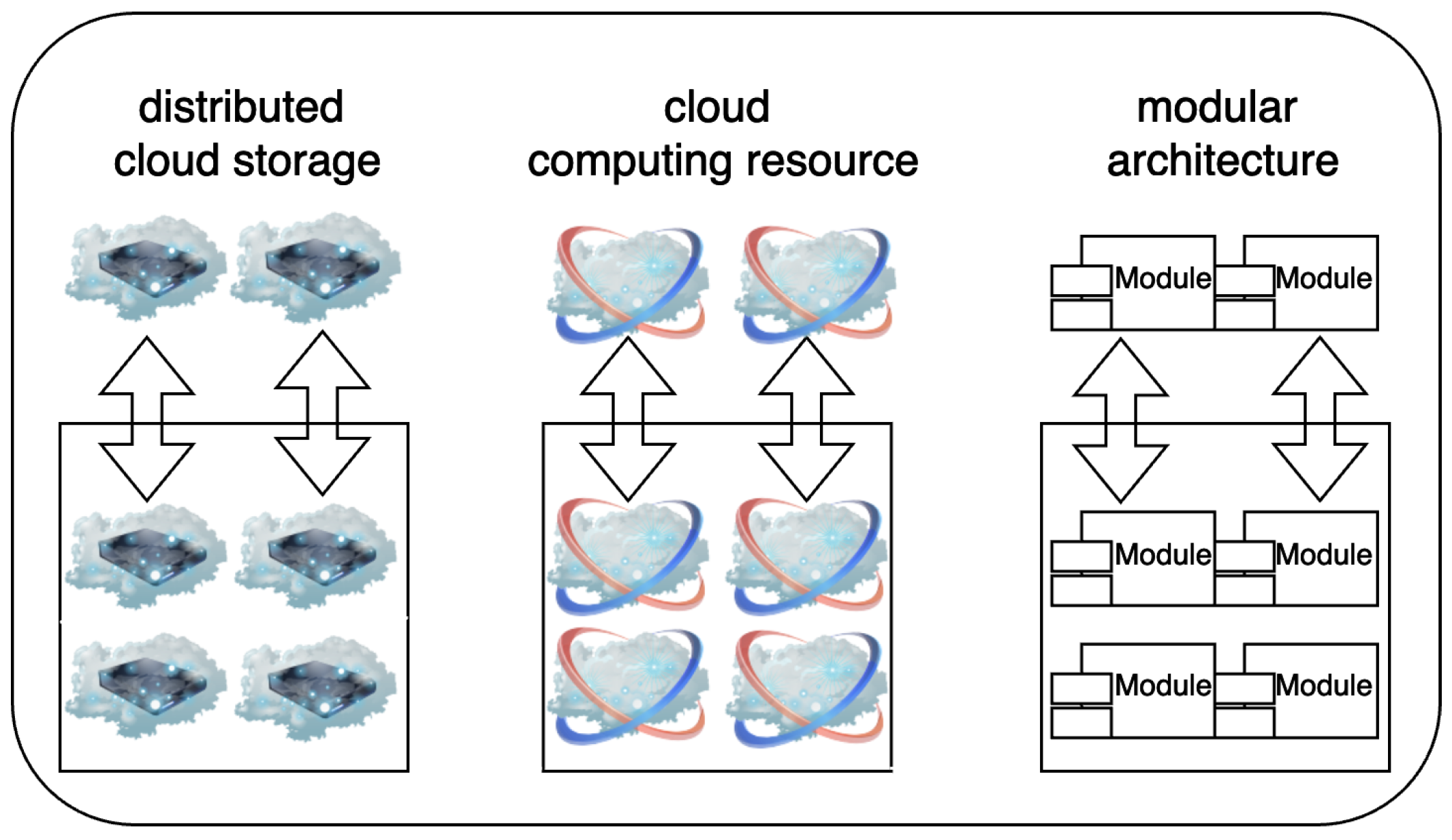
5.9. Scalability
5.9.1. Challenges
5.9.2. Existing Solutions
5.9.3. Evaluation of the Existing Solutions
5.10. Underserved and Remote Areas with Limited Connectivity
5.10.1. Challenges
5.10.2. Existing Solutions
5.10.3. Evaluation of the Existing Solutions
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AI | Artificial intelligence |
| EHR | Electric healthcare record |
| ML | Machine learning |
| DL | Deep learning |
| NLP | Natural language processing |
| CNN | Convolutional neural network |
| RNN | Recurrent neural network |
| LSTM | Long short-term memory |
| SVM | Support vector machine |
| ANN | Artificial neural network |
| XAI | Explainable AI |
| API | Application programming interface |
| FHIR | Fast Healthcare Interoperability Resources |
References
- Tian, S.; Yang, W.; Le Grange, J.M.; Wang, P.; Huang, W.; Ye, Z. Smart healthcare: Making medical care more intelligent. Glob. Health J. 2019, 3, 62–65. [Google Scholar] [CrossRef]
- Nasr, M.; Islam, M.M.; Shehata, S.; Karray, F.; Quintana, Y. Smart healthcare in the age of AI: Recent advances, challenges, and future prospects. IEEE Access 2021, 9, 145248–145270. [Google Scholar] [CrossRef]
- Chaudhary, S.; Kakkar, R.; Jadav, N.K.; Nair, A.; Gupta, R.; Tanwar, S.; Agrawal, S.; Alshehri, M.D.; Sharma, R.; Sharma, G.; et al. A taxonomy on smart healthcare technologies: Security framework, case study, and future directions. J. Sensors 2022, 2022, 1863838. [Google Scholar] [CrossRef]
- Merative, L.P. Official Website. Available online: https://www.merative.com/company (accessed on 1 August 2024).
- Tempus Official Website. Available online: https://www.tempus.com/ (accessed on 1 August 2024).
- Aidoc Official Website. Available online: https://www.aidoc.com/ (accessed on 1 August 2024).
- PathAI Official Website. Available online: https://www.pathai.com/ (accessed on 1 August 2024).
- Solanas, A.; Casino, F.; Batista, E.; Rallo, R. Trends and challenges in smart healthcare research: A journey from data to wisdom. In Proceedings of the 2017 IEEE 3rd International Forum on Research and Technologies for Society and Industry (RTSI), Modena, Italy, 11–13 September 2017; pp. 1–6. [Google Scholar]
- Chui, K.T.; Alhalabi, W.; Pang, S.S.H.; Pablos, P.O.d.; Liu, R.W.; Zhao, M. Disease diagnosis in smart healthcare: Innovation, technologies and applications. Sustainability 2017, 9, 2309. [Google Scholar] [CrossRef]
- Olawade, D.B.; Wada, O.J.; Ling, J. Using artificial intelligence to improve public health: A narrative review. Front. Public Health 2023, 11, 1196397. [Google Scholar] [CrossRef]
- Xie, S.; Yu, Z.; Lv, Z. Multi-Disease Prediction Based on Deep Learning: A Survey. CMES-Comput. Model. Eng. Sci. 2021, 128, 489–522. [Google Scholar] [CrossRef]
- Paul, D.; Sanap, G.; Shenoy, S.; Kalyane, D.; Kalia, K.; Tekade, R.K. Artificial intelligence in drug discovery and development. Drug Discov. Today 2021, 26, 80. [Google Scholar] [CrossRef]
- Panesar, S.; Cagle, Y.; Chander, D.; Morey, J.; Fernandez-Miranda, J.; Kliot, M. Artificial intelligence and the future of surgical robotics. Ann. Surg. 2019, 270, 223–226. [Google Scholar] [CrossRef]
- Renukappa, S.; Mudiyi, P.; Suresh, S.; Abdalla, W.; Subbarao, C. Evaluation of challenges for adoption of smart healthcare strategies. Smart Health 2022, 26, 100330. [Google Scholar] [CrossRef]
- Defination Source 1. Available online: https://www.hpe.com/us/en/what-is/ai-healthcare.html/ (accessed on 1 August 2024).
- Defination Source 2. Available online: https://www.arm.com/glossary/ai-in-healthcare/ (accessed on 1 August 2024).
- Wikipedia Official Website. Available online: https://en.wikipedia.org/wiki/Artificial_intelligence_in_healthcare/ (accessed on 1 August 2024).
- The American Medical Association Official Website. Available online: https://www.ama-assn.org/practice-management/digital/augmented-intelligence-medicine/ (accessed on 1 August 2024).
- Chen, M.; Decary, M. Artificial intelligence in healthcare: An essential guide for health leaders. Healthc. Manag. Forum 2020, 33, 10–18. [Google Scholar] [CrossRef]
- The Amazon Website Service Official Website. Available online: https://aws.amazon.com/what-is/structured-data/?nc1=h_ls/ (accessed on 1 August 2024).
- Kamruzzaman, M. Architecture of smart health care system using artificial intelligence. In Proceedings of the 2020 IEEE International Conference on Multimedia & Expo Workshops (ICMEW), London, UK, 6–10 July 2020; pp. 1–6. [Google Scholar]
- Jiang, F.; Jiang, Y.; Zhi, H.; Dong, Y.; Li, H.; Ma, S.; Wang, Y.; Dong, Q.; Shen, H.; Wang, Y. Artificial intelligence in healthcare: Past, present and future. Stroke Vasc. Neurol. 2017, 2. [Google Scholar] [CrossRef]
- Lytras, M.D.; Chui, K.T.; Visvizi, A. Data analytics in smart healthcare: The recent developments and beyond. Appl. Sci. 2019, 9, 2812. [Google Scholar] [CrossRef]
- Tayefi, M.; Ngo, P.; Chomutare, T.; Dalianis, H.; Salvi, E.; Budrionis, A.; Godtliebsen, F. Challenges and opportunities beyond structured data in analysis of electronic health records. Wiley Interdiscip. Rev. Comput. Stat. 2021, 13, e1549. [Google Scholar] [CrossRef]
- Ali, F.; El-Sappagh, S.; Islam, S.R.; Kwak, D.; Ali, A.; Imran, M.; Kwak, K.S. A smart healthcare monitoring system for heart disease prediction based on ensemble deep learning and feature fusion. Inf. Fusion 2020, 63, 208–222. [Google Scholar] [CrossRef]
- Ahmad, N.F.; Hoang, D.B.; Phung, M.H. Robust preprocessing for health care monitoring framework. In Proceedings of the 2009 11th International Conference on e-Health Networking, Applications and Services (Healthcom), Sydney, Australia, 16–18 December 2009; pp. 169–174. [Google Scholar]
- Bohr, A.; Memarzadeh, K. The rise of artificial intelligence in healthcare applications. In Artificial Intelligence in Healthcare; Elsevier: Amsterdam, The Netherlands, 2020; pp. 25–60. [Google Scholar]
- Kim, J.C.; Chung, K. Recurrent neural network-based multimodal deep learning for estimating missing values in healthcare. Appl. Sci. 2022, 12, 7477. [Google Scholar] [CrossRef]
- Abdelfattah, S.; Baza, M.; Mahmoud, M.; Fouda, M.M.; Abualsaud, K.; Yaacoub, E.; Alsabaan, M.; Guizani, M. Lightweight Multi-Class Support Vector Machine-Based Medical Diagnosis System with Privacy Preservation. Sensors 2023, 23, 9033. [Google Scholar] [CrossRef]
- Sheng, J.Q.; Hu, P.J.H.; Liu, X.; Huang, T.S.; Chen, Y.H. Predictive analytics for care and management of patients with acute diseases: Deep learning-based method to predict crucial complication phenotypes. J. Med. Internet Res. 2021, 23, e18372. [Google Scholar] [CrossRef]
- Sloane, E.B.; Silva, R.J. Artificial intelligence in medical devices and clinical decision support systems. In Clinical Engineering Handbook; Elsevier: Amsterdam, The Netherlands, 2020; pp. 556–568. [Google Scholar]
- Smith, A.; Severn, M. An Overview of Continuous Learning Artificial Intelligence-Enabled Medical Devices. Can. J. Health Technol. 2022, 2. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C. Trends in using deep learning algorithms in biomedical prediction systems. Front. Neurosci. 2023, 17, 1256351. [Google Scholar] [CrossRef]
- Yelne, S.; Chaudhary, M.; Dod, K.; Sayyad, A.; Sharma, R. Harnessing the power of AI: A comprehensive review of its impact and challenges in nursing science and healthcare. Cureus 2023, 15, e49252. [Google Scholar] [CrossRef]
- Johnson, K.B.; Wei, W.Q.; Weeraratne, D.; Frisse, M.E.; Misulis, K.; Rhee, K.; Zhao, J.; Snowdon, J.L. Precision medicine, AI, and the future of personalized health care. Clin. Transl. Sci. 2021, 14, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Joshi, M. Adaptive Learning through Artificial Intelligence. Int. J. Innov. Res. Sci. Eng. Technol. 2023, 4, 1–2. [Google Scholar] [CrossRef]
- Bajwa, J.; Munir, U.; Nori, A.; Williams, B. Artificial intelligence in healthcare: Transforming the practice of medicine. Future Healthc. J. 2021, 8, e188. [Google Scholar] [CrossRef] [PubMed]
- Ghaffar Nia, N.; Kaplanoglu, E.; Nasab, A. Evaluation of artificial intelligence techniques in disease diagnosis and prediction. Discov. Artif. Intell. 2023, 3, 5. [Google Scholar] [CrossRef]
- Umapathy, V.R.; Raj, R.D.S.; Yadav, S.; Munavarah, S.A.; Anandapandian, P.A.; Mary, A.V.; Padmavathy, K.; Akshay, R.; Rajkumar, D.S.R.; Anandapandian, P.A., IV; et al. Perspective of Artificial Intelligence in Disease Diagnosis: A Review of Current and Future Endeavours in the Medical Field. Cureus 2023, 15, e45684. [Google Scholar] [CrossRef]
- Vaishya, R.; Javaid, M.; Khan, I.H.; Haleem, A. Artificial Intelligence (AI) applications for COVID-19 pandemic. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 337–339. [Google Scholar] [CrossRef]
- Nam, D.; Chapiro, J.; Paradis, V.; Seraphin, T.P.; Kather, J.N. Artificial intelligence in liver diseases: Improving diagnostics, prognostics and response prediction. JHEP Rep. 2022, 4, 100443. [Google Scholar] [CrossRef]
- Dlamini, Z.; Francies, F.Z.; Hull, R.; Marima, R. Artificial intelligence (AI) and big data in cancer and precision oncology. Comput. Struct. Biotechnol. J. 2020, 18, 2300–2311. [Google Scholar] [CrossRef]
- Kumar, Y.; Koul, A.; Singla, R.; Ijaz, M.F. Artificial intelligence in disease diagnosis: A systematic literature review, synthesizing framework and future research agenda. J. Ambient Intell. Humaniz. Comput. 2023, 14, 8459–8486. [Google Scholar] [CrossRef]
- Potočnik, J.; Foley, S.; Thomas, E. Current and potential applications of artificial intelligence in medical imaging practice: A narrative review. J. Med. Imaging Radiat. Sci. 2023, 54, 376–385. [Google Scholar] [CrossRef]
- Binczyk, F.; Prazuch, W.; Bozek, P.; Polanska, J. Radiomics and artificial intelligence in lung cancer screening. Transl. Lung Cancer Res. 2021, 10, 1186. [Google Scholar] [CrossRef] [PubMed]
- Frizzell, T.O.; Glashutter, M.; Liu, C.C.; Zeng, A.; Pan, D.; Hajra, S.G.; D’Arcy, R.C.; Song, X. Artificial intelligence in brain MRI analysis of Alzheimer’s disease over the past 12 years: A systematic review. Ageing Res. Rev. 2022, 77, 101614. [Google Scholar] [CrossRef] [PubMed]
- Guermazi, A.; Tannoury, C.; Kompel, A.J.; Murakami, A.M.; Ducarouge, A.; Gillibert, A.; Li, X.; Tournier, A.; Lahoud, Y.; Jarraya, M.; et al. Improving radiographic fracture recognition performance and efficiency using artificial intelligence. Radiology 2022, 302, 627–636. [Google Scholar] [CrossRef]
- Najjar, R. Redefining radiology: A review of artificial intelligence integration in medical imaging. Diagnostics 2023, 13, 2760. [Google Scholar] [CrossRef]
- Pinto-Coelho, L. How Artificial Intelligence Is Shaping Medical Imaging Technology: A Survey of Innovations and Applications. Bioengineering 2023, 10, 1435. [Google Scholar] [CrossRef]
- Gupta, N.S.; Kumar, P. Perspective of artificial intelligence in healthcare data management: A journey towards precision medicine. Comput. Biol. Med. 2023, 162, 107051. [Google Scholar] [CrossRef]
- Das, S.; Mazumder, S.; Alam, N.; Vernekar, M.; Dam, A.; Bhowmick, A.K.; Hajra, S.; Das, J.K.; Basu, B. Precision Oncology in the Era of Genomics and Artificial Intelligence. J. Curr. Oncol. Trends 2024, 1, 22–30. [Google Scholar]
- Nosrati, H.; Nosrati, M. Artificial intelligence in regenerative medicine: Applications and implications. Biomimetics 2023, 8, 442. [Google Scholar] [CrossRef] [PubMed]
- Vettoretti, M.; Cappon, G.; Facchinetti, A.; Sparacino, G. Advanced diabetes management using artificial intelligence and continuous glucose monitoring sensors. Sensors 2020, 20, 3870. [Google Scholar] [CrossRef]
- Alowais, S.A.; Alghamdi, S.S.; Alsuhebany, N.; Alqahtani, T.; Alshaya, A.I.; Almohareb, S.N.; Aldairem, A.; Alrashed, M.; Bin Saleh, K.; Badreldin, H.A.; et al. Revolutionizing healthcare: The role of artificial intelligence in clinical practice. BMC Med. Educ. 2023, 23, 689. [Google Scholar] [CrossRef]
- Adnan, M.; Kalra, S.; Cresswell, J.C.; Taylor, G.W.; Tizhoosh, H.R. Federated learning and differential privacy for medical image analysis. Sci. Rep. 2022, 12, 1953. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Jain, S.; Mallik, S.; Pandey, A.; Chouhan, R. VIRTUAL AI HEALTH ASSISTANCE. Available online: https://www.researchgate.net/publication/369084637_VIRTUAL_AI_HEALTH_ASSISTANCE (accessed on 1 August 2024).
- Van Bulck, L.; Couturier, R.; Moons, P. Applications of artificial intelligence for nursing: Has a new era arrived? Eur. J. Cardiovasc. Nurs. 2023, 22, e19–e20. [Google Scholar] [CrossRef]
- Kanimozhi, J.; Preethi, G.; Mohanasuganthi, N.; Abi Ayshwariya, S.; Jaffrin, L.C. Virtual Medical Assistant System for Diseases Detection using Machine Learning. In Proceedings of the 2023 2nd International Conference on Smart Technologies and Systems for Next Generation Computing (ICSTSN), Villupuram, India, 21–22 April 2023; pp. 1–6. [Google Scholar]
- Harry, A. The Future of Medicine: Harnessing the Power of AI for Revolutionizing Healthcare. Int. J. Multidiscip. Sci. Arts 2023, 2, 36–47. [Google Scholar] [CrossRef]
- Milne-Ives, M.; de Cock, C.; Lim, E.; Shehadeh, M.H.; de Pennington, N.; Mole, G.; Normando, E.; Meinert, E. The effectiveness of artificial intelligence conversational agents in health care: Systematic review. J. Med. Internet Res. 2020, 22, e20346. [Google Scholar] [CrossRef] [PubMed]
- Balsa, J.; Félix, I.; Cláudio, A.P.; Carmo, M.B.; Silva, I.C.e.; Guerreiro, A.; Guedes, M.; Henriques, A.; Guerreiro, M.P. Usability of an intelligent virtual assistant for promoting behavior change and self-care in older people with type 2 diabetes. J. Med. Syst. 2020, 44, 1–12. [Google Scholar] [CrossRef]
- Boucher, E.M.; Harake, N.R.; Ward, H.E.; Stoeckl, S.E.; Vargas, J.; Minkel, J.; Parks, A.C.; Zilca, R. Artificially intelligent chatbots in digital mental health interventions: A review. Expert Rev. Med. Devices 2021, 18, 37–49. [Google Scholar] [CrossRef]
- Pendy, B. Artificial Intelligence in Health Sector of USA. J. Indones. Sos. Sains 2023, 4, 200–208. [Google Scholar] [CrossRef]
- Ali, O.; Abdelbaki, W.; Shrestha, A.; Elbasi, E.; Alryalat, M.A.A.; Dwivedi, Y.K. A systematic literature review of artificial intelligence in the healthcare sector: Benefits, challenges, methodologies, and functionalities. J. Innov. Knowl. 2023, 8, 100333. [Google Scholar] [CrossRef]
- Shaik, T.; Tao, X.; Higgins, N.; Li, L.; Gururajan, R.; Zhou, X.; Acharya, U.R. Remote patient monitoring using artificial intelligence: Current state, applications, and challenges. Wiley Interdiscip. Rev. Data Min. Knowl. Discov. 2023, 13, e1485. [Google Scholar] [CrossRef]
- Anu Shilvya, J.; George, S.T.; Subathra, M.; Manimegalai, P.; Mohammed, M.A.; Jaber, M.M.; Kazemzadeh, A.; Al-Andoli, M.N. Home based monitoring for smart health-care systems: A survey. Wirel. Commun. Mob. Comput. 2022, 2022, 1829876. [Google Scholar] [CrossRef]
- Shastry, K.A.; Shastry, A. An integrated deep learning and natural language processing approach for continuous remote monitoring in digital health. Decis. Anal. J. 2023, 8, 100301. [Google Scholar] [CrossRef]
- Vora, L.K.; Gholap, A.D.; Jetha, K.; Thakur, R.R.S.; Solanki, H.K.; Chavda, V.P. Artificial intelligence in pharmaceutical technology and drug delivery design. Pharmaceutics 2023, 15, 1916. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Liu, X.; Zhang, S.; Chen, S. Artificial intelligence for drug discovery: Resources, methods, and applications. Mol. Ther.-Nucleic Acids 2023, 31, 691–702. [Google Scholar] [CrossRef]
- Sarkar, C.; Das, B.; Rawat, V.S.; Wahlang, J.B.; Nongpiur, A.; Tiewsoh, I.; Lyngdoh, N.M.; Das, D.; Bidarolli, M.; Sony, H.T. Artificial intelligence and machine learning technology driven modern drug discovery and development. Int. J. Mol. Sci. 2023, 24, 2026. [Google Scholar] [CrossRef]
- Pérez Santín, E.; Rodríguez Solana, R.; González García, M.; García Suárez, M.D.M.; Blanco Díaz, G.D.; Cima Cabal, M.D.; Moreno Rojas, J.M.; López Sánchez, J.I. Toxicity prediction based on artificial intelligence: A multidisciplinary overview. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2021, 11, e1516. [Google Scholar] [CrossRef]
- Mak, K.K.; Wong, Y.H.; Pichika, M.R. Artificial intelligence in drug discovery and development. In Drug Discovery and Evaluation: Safety and Pharmacokinetic Assays; Springer Science & Business Media: Berlin, Germany, 2023; pp. 1–38. [Google Scholar]
- Bhattamisra, S.K.; Banerjee, P.; Gupta, P.; Mayuren, J.; Patra, S.; Candasamy, M. Artificial Intelligence in Pharmaceutical and Healthcare Research. Big Data Cogn. Comput. 2023, 7, 10. [Google Scholar] [CrossRef]
- Charles, Y.P.; Lamas, V.; Ntilikina, Y. Artificial intelligence and treatment algorithms in spine surgery. Orthop. Traumatol. Surg. Res. 2023, 109, 103456. [Google Scholar] [CrossRef]
- D’Ettorre, C.; Mariani, A.; Stilli, A.; y Baena, F.R.; Valdastri, P.; Deguet, A.; Kazanzides, P.; Taylor, R.H.; Fischer, G.S.; DiMaio, S.P.; et al. Accelerating surgical robotics research: A review of 10 years with the da vinci research kit. IEEE Robot. Autom. Mag. 2021, 28, 56–78. [Google Scholar] [CrossRef]
- Denecke, K.; Baudoin, C.R. A review of artificial intelligence and robotics in transformed health ecosystems. Front. Med. 2022, 9, 795957. [Google Scholar] [CrossRef]
- Lee, K.S.; Jung, S.H.; Kim, D.H.; Chung, S.W.; Yoon, J.P. Artificial intelligence-and computer-assisted navigation for shoulder surgery. J. Orthop. Surg. 2024, 32, 10225536241243166. [Google Scholar] [CrossRef]
- Han, J.; Davids, J.; Ashrafian, H.; Darzi, A.; Elson, D.S.; Sodergren, M. A systematic review of robotic surgery: From supervised paradigms to fully autonomous robotic approaches. Int. J. Med Robot. Comput. Assist. Surg. 2022, 18, e2358. [Google Scholar] [CrossRef] [PubMed]
- Loftus, T.J.; Filiberto, A.C.; Balch, J.; Ayzengart, A.L.; Tighe, P.J.; Rashidi, P.; Bihorac, A.; Upchurch, G.R., Jr. Intelligent, autonomous machines in surgery. J. Surg. Res. 2020, 253, 92–99. [Google Scholar] [CrossRef]
- El Kah, A.; Zeroual, I. A review on applied natural language processing to electronic health records. In Proceedings of the 2021 1st International Conference on Emerging Smart Technologies and Applications (eSmarTA), Sana’a, Yemen, 10–12 August 2021; pp. 1–6. [Google Scholar]
- Ahmad, P.N.; Shah, A.M.; Lee, K. A Review on Electronic Health Record Text-Mining for Biomedical Name Entity Recognition in Healthcare Domain. Healthcare 2023, 11, 1268. [Google Scholar] [CrossRef]
- Kormilitzin, A.; Vaci, N.; Liu, Q.; Nevado-Holgado, A. Med7: A transferable clinical natural language processing model for electronic health records. Artif. Intell. Med. 2021, 118, 102086. [Google Scholar] [CrossRef]
- Minerva, F.; Giubilini, A. Is AI the Future of Mental Healthcare? Topoi 2023, 42, 809–817. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.E.; Torous, J.; De Choudhury, M.; Depp, C.A.; Graham, S.A.; Kim, H.C.; Paulus, M.P.; Krystal, J.H.; Jeste, D.V. Artificial intelligence for mental health care: Clinical applications, barriers, facilitators, and artificial wisdom. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2021, 6, 856–864. [Google Scholar] [CrossRef]
- Babu, N.V.; Kanaga, E.G.M. Sentiment analysis in social media data for depression detection using artificial intelligence: A review. SN Comput. Sci. 2022, 3, 74. [Google Scholar] [CrossRef] [PubMed]
- Guillodo, E.; Lemey, C.; Simonnet, M.; Walter, M.; Baca-García, E.; Masetti, V.; Moga, S.; Larsen, M.; Network, H.; Ropars, J.; et al. Clinical applications of mobile health wearable–based sleep monitoring: Systematic review. JMIR mHealth uHealth 2020, 8, e10733. [Google Scholar] [CrossRef]
- Denecke, K.; Schmid, N.; Nüssli, S. Implementation of cognitive behavioral therapy in e–mental health apps: Literature review. J. Med. Internet Res. 2022, 24, e27791. [Google Scholar] [CrossRef]
- Ranson, J.M.; Bucholc, M.; Lyall, D.; Newby, D.; Winchester, L.; Oxtoby, N.P.; Veldsman, M.; Rittman, T.; Marzi, S.; Skene, N.; et al. Harnessing the potential of machine learning and artificial intelligence for dementia research. Brain Inform. 2023, 10, 6. [Google Scholar] [CrossRef]
- Kolluri, S.; Lin, J.; Liu, R.; Zhang, Y.; Zhang, W. Machine learning and artificial intelligence in pharmaceutical research and development: A review. AAPS J. 2022, 24, 19. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, G.; Singh, S.; Pathania, M.; Gosavi, S.; Abhishek, S.; Parchani, A.; Dhar, M. Artificial intelligence in clinical medicine: Catalyzing a sustainable global healthcare paradigm. Front. Artif. Intell. 2023, 6, 1227091. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Wang, Z.; Floudas, C.S.; Chen, F.; Gong, C.; Bracken-Clarke, D.; Xue, E.; Yang, Y.; Sun, J.; Lu, Z. Matching patients to clinical trials with large language models. arXiv 2023, arXiv:2307.15051v4. [Google Scholar]
- Meystre, S.M.; Heider, P.M.; Cates, A.; Bastian, G.; Pittman, T.; Gentilin, S.; Kelechi, T.J. Piloting an automated clinical trial eligibility surveillance and provider alert system based on artificial intelligence and standard data models. BMC Med. Res. Methodol. 2023, 23, 88. [Google Scholar] [CrossRef]
- Chow, R.; Midroni, J.; Kaur, J.; Boldt, G.; Liu, G.; Eng, L.; Liu, F.F.; Haibe-Kains, B.; Lock, M.; Raman, S. Use of artificial intelligence for cancer clinical trial enrollment: A systematic review and meta-analysis. J. Natl. Cancer Inst. 2023, 115, 365–374. [Google Scholar] [CrossRef]
- Ortega-Calvo, A.S.; Morcillo-Jimenez, R.; Fernandez-Basso, C.; Gutiérrez-Batista, K.; Vila, M.A.; Martin-Bautista, M.J. AIMDP: An Artificial Intelligence Modern Data Platform. Use case for Spanish national health service data silo. Future Gener. Comput. Syst. 2023, 143, 248–264. [Google Scholar] [CrossRef]
- Williams, E.; Kienast, M.; Medawar, E.; Reinelt, J.; Merola, A.; Klopfenstein, S.A.I.; Flint, A.R.; Heeren, P.; Poncette, A.S.; Balzer, F.; et al. A Standardized Clinical Data Harmonization Pipeline for Scalable AI Application Deployment (FHIR-DHP): Validation and Usability Study. JMIR Med. Inform. 2023, 11, e43847. [Google Scholar] [CrossRef]
- Sinaci, A.A.; Gencturk, M.; Teoman, H.A.; Laleci Erturkmen, G.B.; Alvarez-Romero, C.; Martinez-Garcia, A.; Poblador-Plou, B.; Carmona-Pírez, J.; Löbe, M.; Parra-Calderon, C.L. A Data Transformation Methodology to Create Findable, Accessible, Interoperable, and Reusable Health Data: Software Design, Development, and Evaluation Study. J. Med. Internet Res. 2023, 25, e42822. [Google Scholar] [CrossRef] [PubMed]
- Setyawan, R.; Hidayanto, A.N.; Sensuse, D.I.; Kautsarina; Suryono, R.R.; Abilowo, K. Data integration and interoperability problems of HL7 FHIR implementation and potential solutions: A systematic literature review. In Proceedings of the 2021 5th International Conference on Informatics and Computational Sciences (ICICoS), Semarang, Indonesia, 24–25 November 2021; pp. 293–298. [Google Scholar]
- Cai, Q.; Wang, H.; Li, Z.; Liu, X. A survey on multimodal data-driven smart healthcare systems: Approaches and applications. IEEE Access 2019, 7, 133583–133599. [Google Scholar] [CrossRef]
- Pashazadeh, A.; Navimipour, N.J. Big data handling mechanisms in the healthcare applications: A comprehensive and systematic literature review. J. Biomed. Inform. 2018, 82, 47–62. [Google Scholar] [CrossRef]
- Kalia, K.; Gupta, N. Analysis of hadoop MapReduce scheduling in heterogeneous environment. Ain Shams Eng. J. 2021, 12, 1101–1110. [Google Scholar] [CrossRef]
- Khalil, W.A.; Torkey, H.; Attiya, G. Survey of Apache Spark optimized job scheduling in Big Data. Int. J. Ind. Sustain. Dev. 2020, 1, 39–48. [Google Scholar] [CrossRef][Green Version]
- Dash, S.; Shakyawar, S.K.; Sharma, M.; Kaushik, S. Big data in healthcare: Management, analysis and future prospects. J. Big Data 2019, 6, 1–25. [Google Scholar] [CrossRef]
- Nazir, S.; Khan, S.; Khan, H.U.; Ali, S.; Garcia-Magarino, I.; Atan, R.B.; Nawaz, M. A comprehensive analysis of healthcare big data management, analytics and scientific programming. IEEE Access 2020, 8, 95714–95733. [Google Scholar] [CrossRef]
- Gupta, M.K.; Chandra, P. A comprehensive survey of data mining. Int. J. Inf. Technol. 2020, 12, 1243–1257. [Google Scholar] [CrossRef]
- Ahmed, N.; Barczak, A.L.; Susnjak, T.; Rashid, M.A. A comprehensive performance analysis of Apache Hadoop and Apache Spark for large scale data sets using HiBench. J. Big Data 2020, 7, 110. [Google Scholar] [CrossRef]
- Ibtisum, S.; Bazgir, E.; Rahman, S.A.; Hossain, S.S. A comparative analysis of big data processing paradigms: Mapreduce vs. apache spark. World J. Adv. Res. Rev. 2023, 20, 1089–1098. [Google Scholar] [CrossRef]
- Mavridis, I.; Karatza, H. Performance evaluation of cloud-based log file analysis with Apache Hadoop and Apache Spark. J. Syst. Softw. 2017, 125, 133–151. [Google Scholar] [CrossRef]
- Surianarayanan, C.; Lawrence, J.J.; Chelliah, P.R.; Prakash, E.; Hewage, C. A survey on optimization techniques for edge artificial intelligence (ai). Sensors 2023, 23, 1279. [Google Scholar] [CrossRef]
- Hua, H.; Li, Y.; Wang, T.; Dong, N.; Li, W.; Cao, J. Edge computing with artificial intelligence: A machine learning perspective. ACM Comput. Surv. 2023, 55, 184. [Google Scholar] [CrossRef]
- Bourechak, A.; Zedadra, O.; Kouahla, M.N.; Guerrieri, A.; Seridi, H.; Fortino, G. At the Confluence of Artificial Intelligence and Edge Computing in IoT-Based Applications: A Review and New Perspectives. Sensors 2023, 23, 1639. [Google Scholar] [CrossRef] [PubMed]
- Khanh, Q.V.; Hoai, N.V.; Van, A.D.; Minh, Q.N. An integrating computing framework based on edge-fog-cloud for internet of healthcare things applications. Internet Things 2023, 23, 100907. [Google Scholar] [CrossRef]
- Tripathy, S.S.; Rath, M.; Tripathy, N.; Roy, D.S.; Francis, J.S.A.; Bebortta, S. An Intelligent Health Care System in Fog Platform with Optimized Performance. Sustainability 2023, 15, 1862. [Google Scholar] [CrossRef]
- Hazra, A.; Rana, P.; Adhikari, M.; Amgoth, T. Fog computing for next-generation internet of things: Fundamental, state-of-the-art and research challenges. Comput. Sci. Rev. 2023, 48, 100549. [Google Scholar] [CrossRef]
- Wani, N.A.; Kumar, R.; Bedi, J. DeepXplainer: An interpretable deep learning based approach for lung cancer detection using explainable artificial intelligence. Comput. Methods Programs Biomed. 2024, 243, 107879. [Google Scholar] [CrossRef]
- Kök, İ.; Okay, F.Y.; Muyanlı, Ö.; Özdemir, S. Explainable artificial intelligence (xai) for internet of things: A survey. IEEE Internet Things J. 2023, 10. [Google Scholar] [CrossRef]
- Band, S.S.; Yarahmadi, A.; Hsu, C.C.; Biyari, M.; Sookhak, M.; Ameri, R.; Dehzangi, I.; Chronopoulos, A.T.; Liang, H.W. Application of explainable artificial intelligence in medical health: A systematic review of interpretability methods. Inform. Med. Unlocked 2023, 40, 101286. [Google Scholar] [CrossRef]
- Frasca, M.; La Torre, D.; Pravettoni, G.; Cutica, I. Explainable and interpretable artificial intelligence in medicine: A systematic bibliometric review. Discov. Artif. Intell. 2024, 4, 15. [Google Scholar] [CrossRef]
- Dieber, J.; Kirrane, S. Why model why? Assessing the strengths and limitations of LIME. arXiv 2020, arXiv:2012.00093. [Google Scholar]
- Prendin, F.; Pavan, J.; Cappon, G.; Del Favero, S.; Sparacino, G.; Facchinetti, A. The importance of interpreting machine learning models for blood glucose prediction in diabetes: An analysis using SHAP. Sci. Rep. 2023, 13, 16865. [Google Scholar] [CrossRef]
- Suara, S.; Jha, A.; Sinha, P.; Sekh, A.A. Is grad-CAM explainable in medical images? In Proceedings of the International Conference on Computer Vision and Image Processing, Jammu, India, 3–5 November 2023; pp. 124–135. [Google Scholar]
- Couplet, E.; Lambert, P.; Verleysen, M.; Mulders, D.; Lee, J.A.; De Bodt, C. Natively Interpretable t-SNE. AIMLAI Workshop 2023, 1, 1–16. [Google Scholar]
- Liu, B. Lifelong machine learning: A paradigm for continuous learning. Front. Comput. Sci. 2017, 11, 359–361. [Google Scholar] [CrossRef]
- Feng, J.; Phillips, R.V.; Malenica, I.; Bishara, A.; Hubbard, A.E.; Celi, L.A.; Pirracchio, R. Clinical artificial intelligence quality improvement: Towards continual monitoring and updating of AI algorithms in healthcare. NPJ Digit. Med. 2022, 5, 66. [Google Scholar] [CrossRef] [PubMed]
- Jie, Z.; Zhiying, Z.; Li, L. A meta-analysis of Watson for Oncology in clinical application. Sci. Rep. 2021, 11, 5792. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.K.; Kipnes, M.; Castorino, K.; Bailey, T.S.; Akturk, H.K.; Welsh, J.B.; Christiansen, M.P.; Balo, A.K.; Brown, S.A.; Reid, J.L.; et al. Accuracy and safety of Dexcom G7 continuous glucose monitoring in adults with diabetes. Diabetes Technol. Ther. 2022, 24, 373–380. [Google Scholar] [CrossRef]
- Allam, Z. The rise of machine intelligence in the COVID-19 pandemic and its impact on health policy. In Surveying the COVID-19 Pandemic and Its Implications; Elsevier: Amsterdam, The Netherlands, 2020; p. 89. [Google Scholar]
- Parisi, G.I.; Kemker, R.; Part, J.L.; Kanan, C.; Wermter, S. Continual lifelong learning with neural networks: A review. Neural Netw. 2019, 113, 54–71. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, X.; Su, H.; Zhu, J. A comprehensive survey of continual learning: Theory, method and application. IEEE Trans. Pattern Anal. Mach. Intell. 2024, 46, 5362–5383. [Google Scholar] [CrossRef]
- Prabhu, A.; Cai, Z.; Dokania, P.; Torr, P.; Koltun, V.; Sener, O. Online continual learning without the storage constraint. arXiv 2023, arXiv:2305.09253. [Google Scholar]
- Gupta, S.; Singh, P.; Chang, K.; Qu, L.; Aggarwal, M.; Arun, N.; Vaswani, A.; Raghavan, S.; Agarwal, V.; Gidwani, M.; et al. Addressing catastrophic forgetting for medical domain expansion. arXiv 2021, arXiv:2103.13511. [Google Scholar]
- Hu, Y.; Kuang, W.; Qin, Z.; Li, K.; Zhang, J.; Gao, Y.; Li, W.; Li, K. Artificial intelligence security: Threats and countermeasures. ACM Comput. Surv. (CSUR) 2021, 55, 1–36. [Google Scholar] [CrossRef]
- Xu, Y.; Han, X.; Deng, G.; Li, J.; Liu, Y.; Zhang, T. SoK: Rethinking sensor spoofing attacks against robotic vehicles from a systematic view. In Proceedings of the 2023 IEEE 8th European Symposium on Security and Privacy (EuroS&P), Delft, The Netherlands, 3–7 July 2023; pp. 1082–1100. [Google Scholar]
- Quiring, E.; Klein, D.; Arp, D.; Johns, M.; Rieck, K. Adversarial preprocessing: Understanding and preventing Image-Scaling attacks in machine learning. In Proceedings of the 29th USENIX Security Symposium (USENIX Security 20), Online, 12–14 August 2020; pp. 1363–1380. [Google Scholar]
- Qayyum, A.; Qadir, J.; Bilal, M.; Al-Fuqaha, A. Secure and robust machine learning for healthcare: A survey. IEEE Rev. Biomed. Eng. 2020, 14, 156–180. [Google Scholar] [CrossRef] [PubMed]
- Alabdulatif, A.; Khalil, I.; Saidur Rahman, M. Security of blockchain and AI-empowered smart healthcare: Application-based analysis. Appl. Sci. 2022, 12, 11039. [Google Scholar] [CrossRef]
- Kaissis, G.A.; Makowski, M.R.; Rückert, D.; Braren, R.F. Secure, privacy-preserving and federated machine learning in medical imaging. Nat. Mach. Intell. 2020, 2, 305–311. [Google Scholar] [CrossRef]
- Kaviani, S.; Han, K.J.; Sohn, I. Adversarial attacks and defenses on AI in medical imaging informatics: A survey. Expert Syst. Appl. 2022, 198, 116815. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Z.M. Ethics and governance of trustworthy medical artificial intelligence. BMC Med. Inform. Decis. Mak. 2023, 23, 7. [Google Scholar] [CrossRef] [PubMed]
- Ueda, D.; Kakinuma, T.; Fujita, S.; Kamagata, K.; Fushimi, Y.; Ito, R.; Matsui, Y.; Nozaki, T.; Nakaura, T.; Fujima, N.; et al. Fairness of artificial intelligence in healthcare: Review and recommendations. Jpn. J. Radiol. 2024, 42, 3–15. [Google Scholar] [CrossRef]
- Khan, B.; Fatima, H.; Qureshi, A.; Kumar, S.; Hanan, A.; Hussain, J.; Abdullah, S. Drawbacks of artificial intelligence and their potential solutions in the healthcare sector. Biomed. Mater. Devices 2023, 1, 731–738. [Google Scholar] [CrossRef]
- Aggarwal, P.; Papay, F.A. Artificial intelligence image recognition of melanoma and basal cell carcinoma in racially diverse populations. J. Dermatol. Treat. 2022, 33, 2257–2262. [Google Scholar] [CrossRef]
- Bhatia, S.; Lassmann, B.; Cohn, E.; Desai, A.N.; Carrion, M.; Kraemer, M.U.; Herringer, M.; Brownstein, J.; Madoff, L.; Cori, A.; et al. Using digital surveillance tools for near real-time mapping of the risk of infectious disease spread. NPJ Digit. Med. 2021, 4, 73. [Google Scholar] [CrossRef]
- Abràmoff, M.D.; Tarver, M.E.; Loyo-Berrios, N.; Trujillo, S.; Char, D.; Obermeyer, Z.; Eydelman, M.B.; Foundational Principles of Ophthalmic Imaging and Algorithmic Interpretation Working Group of the Collaborative Community for Ophthalmic Imaging Foundation, Washington, D.C.; Maisel, W.H. Considerations for addressing bias in artificial intelligence for health equity. NPJ Digit. Med. 2023, 6, 170. [Google Scholar] [CrossRef]
- Pourzolfaghar, Z.; Alfano, M.; Helfert, M. Application of ethical AI requirements to an AI solution use-case in healthcare domain. Am. J. Bus. 2023, 38, 112–128. [Google Scholar] [CrossRef]
- Chikhaoui, E.; Alajmi, A.; Larabi-Marie-Sainte, S. Artificial intelligence applications in healthcare sector: Ethical and legal challenges. Emerg. Sci. J. 2022, 6, 717–738. [Google Scholar] [CrossRef]
- N’gbesso, Y. Integration of Artificial Intelligence in electronic health records: Impacts and challenges. Comput. Sustain. Soc. 2020. Available online: https://www.researchgate.net/profile/Yolande-Ngbesso/publication/347447047_Integration_of_Artificial_Intelligence_in_electronic_health_records_Impacts_and_challenges/links/60269d7c45851589399ec526/Integration-of-Artificial-Intelligence-in-electronic-health-records-Impacts-and-challenges.pdf (accessed on 1 August 2024).
- Lin, A.L.; Chen, W.C.; Hong, J.C. Electronic health record data mining for artificial intelligence healthcare. In Artificial Intelligence in Medicine; Elsevier: Amsterdam, The Netherlands, 2021; pp. 133–150. [Google Scholar]
- Chishtie, J.; Sapiro, N.; Wiebe, N.; Rabatach, L.; Lorenzetti, D.; Leung, A.A.; Rabi, D.; Quan, H.; Eastwood, C.A. Use of Epic Electronic health record system for health care research: Scoping review. J. Med. Internet Res. 2023, 25, e51003. [Google Scholar] [CrossRef] [PubMed]
- Patil, A.P.; Chakrabarti, N. A review into the evolution of HIPAA in response to evolving technological environments. Full Length Artic. 2021, 4, 5–15. [Google Scholar] [CrossRef]
- Kawamoto, K.; Finkelstein, J.; Del Fiol, G. Implementing Machine Learning in the Electronic Health Record: Checklist of Essential Considerations. Mayo Clin. Proc. 2023, 98, 366–369. [Google Scholar] [CrossRef] [PubMed]
- Gordon, W.J.; Rudin, R.S. Why APIs? Anticipated value, barriers, and opportunities for standards-based application programming interfaces in healthcare: Perspectives of US thought leaders. JAMIA Open 2022, 5, ooac023. [Google Scholar] [CrossRef] [PubMed]
- Barmer, H.; Dzombak, R.; Gaston, M.; Palat, V.; Redner, F.; Smith, T.; Wohlbier, J. Scalable AI. 2021. Available online: https://insights.sei.cmu.edu/documents/608/2021_019_001_735330.pdf (accessed on 1 August 2024).
- Cohen, R.Y.; Kovacheva, V.P. A Methodology for a Scalable, Collaborative, and Resource-Efficient Platform to Facilitate Healthcare AI Research. arXiv 2021, arXiv:2112.06883. [Google Scholar] [CrossRef]
- Saiyeda, A.; Mir, M.A. Cloud computing for deep learning analytics: A survey of current trends and challenges. Int. J. Adv. Res. Comput. Sci. 2017, 8, 68. [Google Scholar]
- Borra, P. A Survey of Google Cloud Platform (GCP): Features, Services, and Applications. Int. J. Adv. Res. Sci. Commun. Technol. (IJARSCT) 2024, 4, 191–199. [Google Scholar] [CrossRef]
- Wittig, A.; Wittig, M. Amazon Web Services in Action: An In-Depth Guide to AWS; Simon and Schuster: New York, NY, USA, 2023. [Google Scholar]
- Liang, M.; Fu, W.; Feng, L.; Lin, Z.; Panakanti, P.; Zheng, S.; Sridharan, S.; Delimitrou, C. Mystique: Enabling Accurate and Scalable Generation of Production AI Benchmarks. In Proceedings of the 50th Annual International Symposium on Computer Architecture, Orlando, FL, USA, 17–21 June 2023; pp. 1–13. [Google Scholar]
- Gao, W.; Zhan, J.; Wang, L.; Luo, C.; Zheng, D.; Wen, X.; Ren, R.; Zheng, C.; He, X.; Ye, H.; et al. Bigdatabench: A scalable and unified big data and ai benchmark suite. arXiv 2018, arXiv:1802.08254. [Google Scholar]
- Mittal, S.; Bengio, Y.; Lajoie, G. Is a modular architecture enough? Adv. Neural Inf. Process. Syst. 2022, 35, 28747–28760. [Google Scholar]
- Amajuoyi, C.P.; Nwobodo, L.K.; Adegbola, M.D. Transforming business scalability and operational flexibility with advanced cloud computing technologies. Comput. Sci. IT Res. J. 2024, 5, 1469–1487. [Google Scholar] [CrossRef]
- Kindratenko, V.; Mu, D.; Zhan, Y.; Maloney, J.; Hashemi, S.H.; Rabe, B.; Xu, K.; Campbell, R.; Peng, J.; Gropp, W. Hal: Computer system for scalable deep learning. In Proceedings of the Practice and Experience in Advanced Research Computing, Portland, OR, USA, 26–30 July 2020; pp. 41–48. [Google Scholar]
- Amjad, A.; Kordel, P.; Fernandes, G. A review on innovation in healthcare sector (telehealth) through artificial intelligence. Sustainability 2023, 15, 6655. [Google Scholar] [CrossRef]
- Uschnig, C.; Recker, F.; Blaivas, M.; Dong, Y.; Dietrich, C.F. Tele-ultrasound in the era of COVID-19: A practical guide. Ultrasound Med. Biol. 2022, 48, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Rao, D.P.; Shroff, S.; Savoy, F.M.; S, S.; Hsu, C.K.; Negiloni, K.; Pradhan, Z.S.; PV, J.; Sivaraman, A.; Rao, H.L. Evaluation of an offline, artificial intelligence system for referable glaucoma screening using a smartphone-based fundus camera: A prospective study. Eye 2024, 38, 1104–1111. [Google Scholar] [CrossRef]
- Yang, Y.; Mandt, S.; Theis, L. An introduction to neural data compression. Found. Trends Comput. Graph. Vis. 2023, 15, 113–200. [Google Scholar] [CrossRef]
- Wang, C.H.; Huang, K.Y.; Yao, Y.; Chen, J.C.; Shuai, H.H.; Cheng, W.H. Lightweight deep learning: An overview. IEEE Consum. Electron. Mag. 2022, 13, 51–64. [Google Scholar] [CrossRef]
- Eng, R.I.M.I.; Mustafa, D.A.B.N. Optimization Technologies for Low-Bandwidth Networks. IOSR J. Electron. Commun. Eng. 2015, 10, 9–17. [Google Scholar]
- Jain, A.; Krishnan, R.; Rogye, A.; Natarajan, S. Use of offline artificial intelligence in a smartphone-based fundus camera for community screening of diabetic retinopathy. Indian J. Ophthalmol. 2021, 69, 3150–3154. [Google Scholar]
- Kahdim, A.N.; Manaa, M.E. Design an efficient internet of things data compression for healthcare applications. Bull. Electr. Eng. Inform. 2022, 11, 1678–1686. [Google Scholar] [CrossRef]
- Malibari, A.A. An efficient IoT-Artificial intelligence-based disease prediction using lightweight CNN in healthcare system. Meas. Sensors 2023, 26, 100695. [Google Scholar] [CrossRef]
- Routray, S.K.; Javali, A.; Sahoo, A.; Semunigus, W.; Pappa, M. Lossless compression techniques for low bandwidth io ts. In Proceedings of the 2020 Fourth International Conference on I-SMAC (IoT in Social, Mobile, Analytics and Cloud) (I-SMAC), Palladam, India, 7–9 October 2020; pp. 177–181. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, X.; He, P.; Zhou, Y.; Qin, X. Artificial Intelligence Applications in Smart Healthcare: A Survey. Future Internet 2024, 16, 308. https://doi.org/10.3390/fi16090308
Gao X, He P, Zhou Y, Qin X. Artificial Intelligence Applications in Smart Healthcare: A Survey. Future Internet. 2024; 16(9):308. https://doi.org/10.3390/fi16090308
Chicago/Turabian StyleGao, Xian, Peixiong He, Yi Zhou, and Xiao Qin. 2024. "Artificial Intelligence Applications in Smart Healthcare: A Survey" Future Internet 16, no. 9: 308. https://doi.org/10.3390/fi16090308
APA StyleGao, X., He, P., Zhou, Y., & Qin, X. (2024). Artificial Intelligence Applications in Smart Healthcare: A Survey. Future Internet, 16(9), 308. https://doi.org/10.3390/fi16090308






