Tissue Specific Modulation of cyp2c and cyp3a mRNA Levels and Activities by Diet-Induced Obesity in Mice: The Impact of Type 2 Diabetes on Drug Metabolizing Enzymes in Liver and Extra-Hepatic Tissues
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animals
2.3. CYP450 mRNA Levels
2.3.1. Isolation of RNA and Preparation of cDNA
2.3.2. RT-qPCR Analysis
2.4. In Vitro CYP450 Metabolism in Liver and Extra-Hepatic Organs
2.4.1. Preparation of Microsomes
2.4.2. Effects of the HFD on Hepatic and Extra-Hepatic cyp450 Activities
2.5. High Performance Chromatography–Mass Spectrometry Analytical Methods
2.5.1. Chromatographic Conditions for the Metabolites of Bupropion, Midazolam and Ebastine
2.5.2. Chromatographic Conditions for the Metabolites of Chlorzoxazone, Tolbutamide, Dodecanoic Acid, Bufuralol and Repaglinide
2.6. Statistical Analysis
3. Results
3.1. Animal Model
3.2. Effects of HFD on cyp450 mRNA Expression Levels
3.2.1. General Pattern of cyp450 Expression
3.2.2. Modulation of cyp450 mRNA Expression by HFD
3.3. Modulation of cyp450 Hepatic Activities by DIO Mouse as a Model of T2D
3.3.1. Hepatic Activities
3.3.2. Extra-Hepatic Activities
4. Discussion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| DIO | diet-induced obesity |
| DMSO | dimethyl sulfoxide |
| G6P | glucose-6-phosphate |
| G6PD | glucose-6-phosphate dehydrogenase |
| HDR | high-diet responders |
| HFD | high-fat diet |
| IFN-γ | interferon-γ |
| IL-1β | interleukin-1β |
| IL-6 | interleukin-6 |
| LC-MS/MS | liquid chromatography–tandem mass spectrometry |
| LDR | low-diet responders |
| NADP | Nicotinamide-Adenine Dinucleotide Phosphate |
| P450 | cytochrome P450 |
| PMSF | phenylmethanesulfonyl |
| RT-qPCR | real time quantitative polymerase chain reaction |
| T2D | type 2 diabetes |
| VHDR | very high-diet responders |
References
- Whiting, D.R.; Guariguata, L.; Weil, C.; Shaw, J. IDF diabetes atlas: Global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res. Clin. Pract. 2011, 94, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2014; U.S. Department of Health and Human Services: Atlanta, GA, USA, 2014.
- American Diabetes Association. Standards of medical care in diabetes—2015: summary of revisions. Diabetes Care 2015, 38 (Suppl. 1), S4. [Google Scholar]
- Pacanowski, M.A.; Hopley, C.W.; Aquilante, C.L. Interindividual variability in oral antidiabetic drug disposition and response: The role of drug transporter polymorphisms. Expert Opin. Drug Metab. Toxicol. 2008, 4, 529–544. [Google Scholar] [CrossRef] [PubMed]
- Nathan, D.M.; Buse, J.B.; Davidson, M.B.; Ferrannini, E.; Holman, R.R.; Sherwin, R.; Zinman, B. Medical management of hyperglycaemia in type 2 diabetes mellitus: A consensus algorithm for the initiation and adjustment of therapy: A consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia 2009, 52, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Morrish, G.A.; Pai, M.P.; Green, B. The effects of obesity on drug pharmacokinetics in humans. Expert Opin. Drug Metab. Toxicol. 2011, 7, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Cheymol, G. Effects of obesity on pharmacokinetics implications for drug therapy. Clin. Pharmacokinet. 2000, 39, 215–231. [Google Scholar] [CrossRef] [PubMed]
- Akhlaghi, F.; Dostalek, M.; Falck, P.; Mendonza, A.E.; Amundsen, R.; Gohh, R.Y.; Asberg, A. The concentration of cyclosporine metabolites is significantly lower in kidney transplant recipients with diabetes mellitus. Ther. Drug Monit. 2012, 34, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Marques, M.P.; Coelho, E.B.; Dos Santos, N.A.; Geleilete, T.J.; Lanchote, V.L. Dynamic and kinetic disposition of nisoldipine enantiomers in hypertensive patients presenting with type-2 diabetes mellitus. Eur. J. Clin. Pharmacol. 2002, 58, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Kazui, M.; Nishiya, Y.; Ishizuka, T.; Hagihara, K.; Farid, N.A.; Okazaki, O.; Ikeda, T.; Kurihara, A. Identification of the human cytochrome P450 enzymes involved in the two oxidative steps in the bioactivation of clopidogrel to its pharmacologically active metabolite. Drug Metab. Dispos. 2010, 38, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Lenzini, P.; Wadelius, M.; Kimmel, S.; Anderson, J.L.; Jorgensen, A.L.; Pirmohamed, M.; Caldwell, M.D.; Limdi, N.; Burmester, J.K.; Dowd, M.B.; et al. Integration of genetic, clinical, and INR data to refine warfarin dosing. Clin. Pharmacol. Ther. 2010, 87, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Hall, H.M.; Banerjee, S.; McGuire, D.K. Variability of clopidogrel response in patients with type 2 diabetes mellitus. Diabetes Vasc. Dis. Res. 2011, 8, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, P.A.; Oetting, W.S.; Brearley, A.M.; Leduc, R.; Guan, W.; Schladt, D.; Matas, A.J.; Lamba, V.; Julian, B.A.; Mannon, R.B.; et al. Novel polymorphisms associated with tacrolimus trough concentrations: Results from a multicenter kidney transplant consortium. Transplantation 2011, 91, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Dostalek, M.; Sam, W.J.; Paryani, K.R.; Macwan, J.S.; Gohh, R.Y.; Akhlaghi, F. Diabetes mellitus reduces the clearance of atorvastatin lactone: Results of a population pharmacokinetic analysis in renal transplant recipients and in vitro studies using human liver microsomes. Clin. Pharmacokinet. 2012, 51, 591–606. [Google Scholar] [CrossRef] [PubMed]
- Manolopoulos, V.G.; Ragia, G.; Tavridou, A. Pharmacogenomics of oral antidiabetic medications: Current data and pharmacoepigenomic perspective. Pharmacogenomics 2011, 12, 1161–1191. [Google Scholar] [CrossRef] [PubMed]
- Manolopoulos, V.G. Pharmacogenomics and adverse drug reactions in diagnostic and clinical practice. Clin. Chem. Lab. Med. 2007, 45, 801–814. [Google Scholar] [CrossRef] [PubMed]
- Holstein, A.; Beil, W. Oral antidiabetic drug metabolism: Pharmacogenomics and drug interactions. Expert Opin. Drug Metab. Toxicol. 2009, 5, 225–241. [Google Scholar] [CrossRef] [PubMed]
- Bano, G. Glucose homeostasis, obesity and diabetes. Best Pract. Res. Clin. Obstet. Gynaecol. 2013, 27, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Paragh, G.; Seres, I.; Harangi, M.; Fulop, P. Dynamic interplay between metabolic syndrome and immunity. Adv. Exp. Med. Biol. 2014, 824, 171–190. [Google Scholar] [PubMed]
- Dandona, P.; Aljada, A.; Bandyopadhyay, A. Inflammation: The link between insulin resistance, obesity and diabetes. Trends Immunol. 2004, 25, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E.T. Impact of infectious and inflammatory disease on cytochrome P450-mediated drug metabolism and pharmacokinetics. Clin. Pharmacol. Ther. 2009, 85, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Rendic, S.; Guengerich, F.P. Update information on drug metabolism systems—2009, part II: Summary of information on the effects of diseases and environmental factors on human cytochrome P450 (CYP) enzymes and transporters. Curr. Drug Metab. 2010, 11, 4–84. [Google Scholar] [CrossRef] [PubMed]
- Du Souich, P.; Fradette, C. The effect and clinical consequences of hypoxia on cytochrome P450, membrane carrier proteins activity and expression. Expert Opin. Drug Metab. Toxicol. 2011, 7, 1083–1100. [Google Scholar] [CrossRef] [PubMed]
- Hameed, I.; Masoodi, S.R.; Mir, S.A.; Nabi, M.; Ghazanfar, K.; Ganai, B.A. Type 2 diabetes mellitus: From a metabolic disorder to an inflammatory condition. World J. Diabetes 2015, 6, 598–612. [Google Scholar] [PubMed]
- Sunman, J.A.; Hawke, R.L.; LeCluyse, E.L.; Kashuba, A.D. Kupffer cell-mediated IL-2 suppression of CYP3A activity in human hepatocytes. Drug Metab. Dispos. 2004, 32, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Donato, M.T.; Guillen, M.I.; Jover, R.; Castell, J.V.; Gomez-Lechon, M.J. Nitric oxide-mediated inhibition of cytochrome P450 by interferon-gamma in human hepatocytes. J. Pharmacol. Exp. Ther. 1997, 281, 484–490. [Google Scholar] [PubMed]
- Jover, R.; Bort, R.; Gomez-Lechon, M.J.; Castell, J.V. Down-regulation of human CYP3A4 by the inflammatory signal interleukin-6: Molecular mechanism and transcription factors involved. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2002, 16, 1799–1801. [Google Scholar] [CrossRef] [PubMed]
- Kotlyar, M.; Carson, S.W. Effects of obesity on the cytochrome P450 enzyme system. Int. J. Clin. Pharmacol. Ther. 1999, 37, 8–19. [Google Scholar] [PubMed]
- Cheng, P.Y.; Morgan, E.T. Hepatic cytochrome P450 regulation in disease states. Curr. Drug Metab. 2001, 2, 165–183. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Tian, X.; Leung, L.; Wang, J.; Houvig, N.; Xiang, J.; Wan, Z.K.; Saiah, E.; Hahm, S.; Suri, V.; et al. Comparative pharmacokinetics and metabolism studies in lean and diet- induced obese mice: An animal efficacy model for 11beta-hydroxysteroid dehydrogenase type 1 (11beta-HSD1) inhibitors. Drug Metab. Lett. 2011, 5, 55–63. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, D.; Davis, S.N.; Kim, R.B.; Wilkinson, G.R. Effect of fasting and obesity in humans on the 6-hydroxylation of chlorzoxazone: A putative probe of CYP2E1 activity. Clin. Pharmacol. Ther. 1994, 56, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Emery, M.G.; Fisher, J.M.; Chien, J.Y.; Kharasch, E.D.; Dellinger, E.P.; Kowdley, K.V.; Thummel, K.E. CYP2E1 activity before and after weight loss in morbidly obese subjects with nonalcoholic fatty liver disease. Hepatology 2003, 38, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Hall, S.D.; Maya, J.F.; Li, L.; Asghar, A.; Gorski, J.C. Diabetes mellitus increases the in vivo activity of cytochrome P450 2E1 in humans. Br. J. Clin. Pharmacol. 2003, 55, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Fisher, C.D.; Lickteig, A.J.; Augustine, L.M.; Ranger-Moore, J.; Jackson, J.P.; Ferguson, S.S.; Cherrington, N.J. Hepatic cytochrome P450 enzyme alterations in humans with progressive stages of nonalcoholic fatty liver disease. Drug Metab. Dispos. 2009, 37, 2087–2094. [Google Scholar] [CrossRef] [PubMed]
- Woolsey, S.J.; Mansell, S.E.; Kim, R.B.; Tirona, R.G.; Beaton, M.D. CYP3A activity and expression in nonalcoholic fatty liver disease. Drug Metab. Dispos. 2015, 43, 1484–1490. [Google Scholar] [CrossRef] [PubMed]
- Kudo, T.; Shimada, T.; Toda, T.; Igeta, S.; Suzuki, W.; Ikarashi, N.; Ochiai, W.; Ito, K.; Aburada, M.; Sugiyama, K. Altered expression of CYP in TSOD mice: A model of type 2 diabetes and obesity. Xenobiotica 2009, 39, 889–902. [Google Scholar] [CrossRef] [PubMed]
- Lam, J.L.; Jiang, Y.; Zhang, T.; Zhang, E.Y.; Smith, B.J. Expression and functional analysis of hepatic cytochromes P450, nuclear receptors, and membrane transporters in 10- and 25-week-old db/db mice. Drug Metab. Dispos. 2010, 38, 2252–2258. [Google Scholar] [CrossRef] [PubMed]
- Ghose, R.; Omoluabi, O.; Gandhi, A.; Shah, P.; Strohacker, K.; Carpenter, K.C.; McFarlin, B.; Guo, T. Role of high-fat diet in regulation of gene expression of drug metabolizing enzymes and transporters. Life Sci. 2011, 89, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Patoine, D.; Petit, M.; Pilote, S.; Picard, F.; Drolet, B.; Simard, C. Modulation of CYP3a expression and activity in mice models of type 1 and type 2 diabetes. Pharmacol. Res. Perspect. 2014, 2, e00082. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Rougee, L.R.; Bedwell, D.W.; Cramer, J.W.; Mohutsky, M.A.; Calvert, N.A.; Moulton, R.D.; Cassidy, K.C.; Yumibe, N.P.; Adams, L.A.; et al. Difference in the pharmacokinetics and hepatic metabolism of antidiabetic drugs in zucker diabetic fatty and sprague-dawley rats. Drug Metab. Dispos. 2016, 44, 1184–1192. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, T.; Honma, R.; Maguchi, S.; Tamaki, H.; Nemoto, N. Different expression of hepatic and renal cytochrome P450s between the streptozotocin-induced diabetic mouse and rat. Xenobiotica 2001, 31, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Khemawoot, P.; Yokogawa, K.; Shimada, T.; Miyamoto, K. Obesity-induced increase of CYP2E1 activity and its effect on disposition kinetics of chlorzoxazone in Zucker rats. Biochem. Pharmacol. 2007, 73, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.J.; Choi, J.M.; Yun, K.U.; Oh, J.M.; Kwak, H.C.; Oh, J.G.; Lee, K.S.; Kim, B.H.; Heo, T.H.; Kim, S.K. Hepatic expression of cytochrome P450 in type 2 diabetic Goto-Kakizaki rats. Chem. Biol. Interact. 2012, 195, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Song, B.J.; Matsunaga, T.; Hardwick, J.P.; Park, S.S.; Veech, R.L.; Yang, C.S.; Gelboin, H.V.; Gonzalez, F.J. Stabilization of cytochrome P450j messenger ribonucleic acid in the diabetic rat. Mol. Endocrinol. 1987, 1, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.G.; Hong, J.Y.; Ma, Q.A.; Li, D.C.; Bullock, J.; Gonzalez, F.J.; Park, S.S.; Gelboin, H.V.; Yang, C.S. Mechanism of induction of cytochrome P-450ac (P-450j) in chemically induced and spontaneously diabetic rats. Arch. Biochem. Biophys. 1988, 263, 29–35. [Google Scholar] [CrossRef]
- Yamazoe, Y.; Murayama, N.; Shimada, M.; Yamauchi, K.; Kato, R. Cytochrome P450 in livers of diabetic rats: Regulation by growth hormone and insulin. Arch. Biochem. Biophys. 1989, 268, 567–575. [Google Scholar] [CrossRef]
- Thummel, K.E.; Schenkman, J.B. Effects of testosterone and growth hormone treatment on hepatic microsomal P450 expression in the diabetic rat. Mol. Pharmacol. 1990, 37, 119–129. [Google Scholar] [PubMed]
- Raza, H.; Ahmed, I.; Lakhani, M.S.; Sharma, A.K.; Pallot, D.; Montague, W. Effect of bitter melon (Momordica charantia) fruit juice on the hepatic cytochrome P450-dependent monooxygenases and glutathione S-transferases in streptozotocin-induced diabetic rats. Biochem. Pharmacol. 1996, 52, 1639–1642. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y. Changes of CYP2E1 activity in diabetic rat model. Acta Pharm. Sin. 1998, 33, 891–895. [Google Scholar]
- Karlgren, M.; Miura, S.; Ingelman-Sundberg, M. Novel extrahepatic cytochrome P450s. Toxicol. Appl. Pharmacol. 2005, 207, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Peyot, M.L.; Pepin, E.; Lamontagne, J.; Latour, M.G.; Zarrouki, B.; Lussier, R.; Pineda, M.; Jetton, T.L.; Madiraju, S.R.; Joly, E.; et al. Beta-cell failure in diet-induced obese mice stratified according to body weight gain: Secretory dysfunction and altered islet lipid metabolism without steatosis or reduced beta-cell mass. Diabetes 2010, 59, 2178–2187. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Td, S. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Michaud, V.; Frappier, M.; Dumas, M.C.; Turgeon, J. Metabolic activity and mRNA levels of human cardiac CYP450s involved in drug metabolism. PLoS ONE 2010, 5, e15666. [Google Scholar] [CrossRef] [PubMed]
- Shayeganpour, A.; Korashy, H.; Patel, J.P.; El-Kadi, A.O.; Brocks, D.R. The impact of experimental hyperlipidemia on the distribution and metabolism of amiodarone in rat. Int. J. Pharm. 2008, 361, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Dostalek, M.; Court, M.H.; Yan, B.; Akhlaghi, F. Significantly reduced cytochrome P450 3A4 expression and activity in liver from humans with diabetes mellitus. Br. J. Pharmacol. 2011, 163, 937–947. [Google Scholar] [CrossRef] [PubMed]
- Patoine, D.; Levac, X.; Pilote, S.; Drolet, B.; Simard, C. Decreased CYP3A expression and activity in guinea pig models of diet-induced metabolic syndrome: Is fatty liver infiltration involved? Drug Metab. Dispos. 2013, 41, 952–957. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.S. Opioid Metabolism. Mayo Clin. Proc. 2009, 84, 613–624. [Google Scholar] [CrossRef]
- Yoshinari, K.; Takagi, S.; Yoshimasa, T.; Sugatani, J.; Miwa, M. Hepatic CYP3A expression is attenuated in obese mice fed a high-fat diet. Pharm. Res. 2006, 23, 1188–1200. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Cui, J.Y.; Lu, H.; Klaassen, C.D. Effect of various diets on the expression of phase-I drug-metabolizing enzymes in livers of mice. Xenobiotica 2015, 45, 586–597. [Google Scholar] [CrossRef] [PubMed]
- Gravel, S.; Grangeon, A.; Gaudette, F.; Chiasson, J.-L.; Dallaire, S.; Langelier, H.; Turgeon, J.; Michaud, V. Type 2 Diabetes modulates CYP450 metabolic activities; an important variability factor in drug response. In Proceedings of the ASCPT 2016, San Diego, CA, USA, 8–12 March 2016. [Google Scholar]
- Kim, Y.C.; Oh, E.Y.; Kim, S.H.; Lee, M.G. Pharmacokinetics of diclofenac in rat model of diabetes mellitus induced by alloxan or steptozotocin. Biopharm. Drug Dispos. 2006, 27, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.; Muroya, Y.; Roman, R.J. Cytochrome P450 eicosanoids in hypertension and renal disease. Curr. Opin. Nephrol. Hypertens. 2015, 24, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.H.; Smith, A.; Zhou, Y.; Chang, H.H.; Lin, S.; Zhao, X.; Imig, J.D.; Dorrance, A.M. Downregulation of renal CYP-derived eicosanoid synthesis in rats with diet-induced hypertension. Hypertension 2003, 42, 594–599. [Google Scholar] [CrossRef] [PubMed]
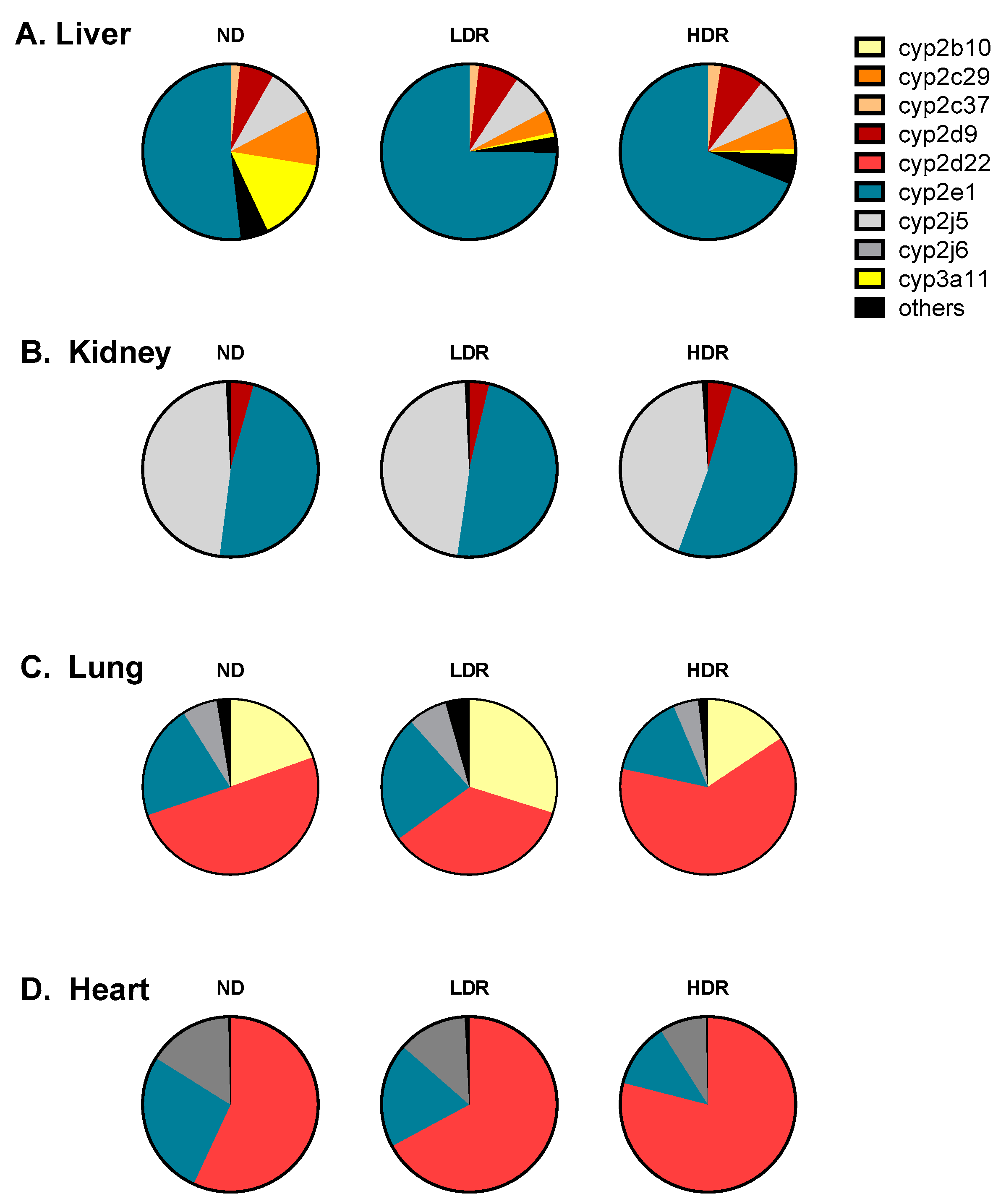


| Tissue | Diet | cyp2b9 | cyp2b10 | cyp2c29 | cyp2c37 | cyp2c39 | cyp2c40 | cyp2d9 | cyp2d22 | cyp2e1 | cyp2j5 | cyp2j6 | cyp3a11 | cyp3a13 | cyp3a25 | cyp4a10 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Liver | ND | 0.01 | 0.02 | 0.49 | 1.31 | 0.01 | 0.44 | 0.55 | 0.53 | 0.56 | 0.98 | 1.08 | 0.58 | 2.40 | 0.44 | 1.25 |
| LDR | 4.89 * | NQ | 0.25 * | 1.77 | 0.01 | 0.20 | 0.81 | 0.59 | 1.02 | 1.05 | 0.88 | 0.04 ᵵ | 3.07 | 0.17 | 1.22 | |
| HDR | 8.51 ᵵ | 0.03 | 0.30 | 2.15 | 0.02 ** | 0.37 | 0.71 | 1.03 | 0.79 | 0.90 | 1.09 | 0.04 ᵵ | 2.96 | 0.21 | 3.72 | |
| Kidney | ND | ND | 0.54 | 2.35 | ND | ND | 8.62 | 1.65 | 1.32 | 1.28 | 4.89 | 4.56 | 1.41 | 1.37 | 2.00 | 1.42 |
| LDR | ND | 0.46 | 3.08 | ND | ND | 3.08 | 1.35 | 1.38 | 1.29 | 4.68 | 4.62 | 0.57 | 2.12 | 1.79 | 1.13 | |
| HDR | ND | 0.42 | 1.59 | ND | ND | 5.92 | 1.65 | 1.50 | 1.29 | 4.18 | 4.03 | 0.72 | 2.41 | 0.95 | 2.43 | |
| Heart | ND | ND | 1.21 | 0.26 | ND | ND | ND | 0.05 | 3.24 | 0.96 | 0.09 | 2.19 | ND | NQ | ND | 1.09 |
| LDR | ND | 2.16 | 0.49 | ND | ND | ND | 0.07 | 3.40 | 0.62 | 0.19 | 1.85 | ND | NQ | ND | 2.15 | |
| HDR | ND | 1.68 | 0.30 | ND | ND | ND | 0.07 | 5.03 | 0.51 * | 0.19 | 1.67 | ND | NQ | NQ | 1.47 | |
| Lung | ND | ND | 0.73 | 1.11 | ND | ND | ND | 0.07 | 2.06 | 0.21 | 0.13 | 1.20 | ND | 3.26 | 0.06 | 3.28 |
| LDR | ND | 1.66 ᵵ | 2.96 | ND | ND | ND | 0.08 | 2.05 | 0.33 ** | 0.47 | 1.88 | ND | 8.15 ** | 0.07 | 9.90 * | |
| HDR | ND | 1.17 ** | 1.00 | ND | ND | ND | ND | 5.76 | 0.32 * | 0.83 | 1.69 | ND | 5.39 | 0.11 | 3.43 |
| Liver | ND | LDR | HDR |
|---|---|---|---|
| (nmol/mg protein/min) | |||
| Bupropion → Hydroxybupropion | 0.030 ± 0.004 | 0.030 ± 0.004 | 0.023 ± 0.002 ** |
| Tolbutamide → Hydroxytolbutamide | 0.22 ± 0.01 | 0.15 ± 0.01 ᵵ | 0.17 ± 0.02 ᵵ |
| Repaglinide → M1-repaglinide | 0.050 ± 0.006 | 0.007 ± 0.002 ᵵ | 0.0081 ± 0.0003 ᵵ |
| Repaglinide → Hydroxyrepaglinide | 0.0020 ± 0.0001 | 0.0010 ± 0.0002 ᵵ | 0.0013 ± 0.0004 ** |
| Bufuralol → Hydroxybufuralol | 0.26 ± 0.05 | 0.19 ± 0.06 | 0.21 ± 0.06 |
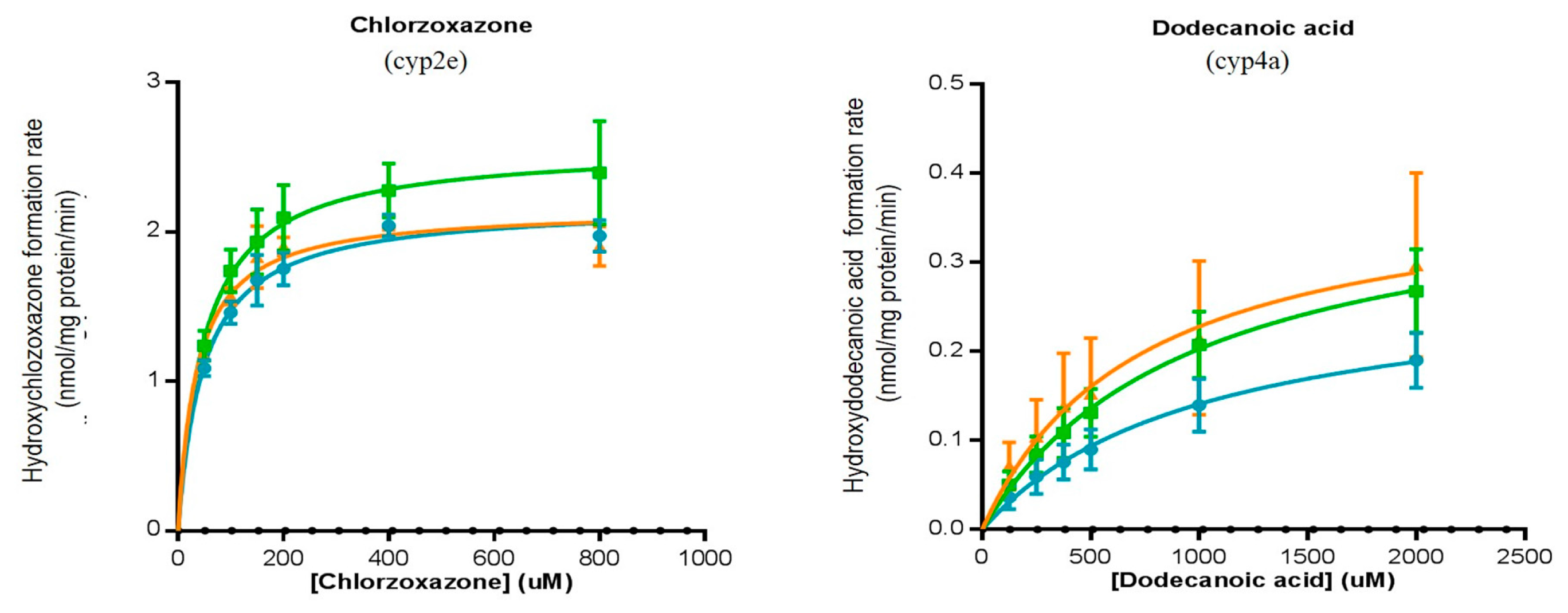
| Chlorzoxazone → Hydroxychlorzoxazone | 2.0 ± 0.1 | 2.4 ± 0.3 ** | 1.9 ± 0.1 |
| Ebastine → Hydroxyebastine and carebastine | 0.044 ± 0.007 | 0.07 ± 0.01 ᵵ | 0.057 ± 0.008 * |
| Midazolam → 1′-hydroxymidazolam | 0.32 ± 0.06 | 0.06 ± 0.01 ᵵ | 0.06 ± 0.01 ᵵ |
| Dodecanoic acid → 12-hydroxydecanoic acid | 0.19 ± 0.03 | 0.27 ± 0.05 | 0.3 ± 0.1 * |
| (A) | |||
| Kidney | ND | LDR | HDR |
| (pmol/mg protein/min) | |||
| Bupropion → Hydroxybupropion | 12.5 ± 0.6 | 12.6 ± 0.4 | 8 ± 1 ** |
| Tolbutamide → Hydroxytolbutamide | 1.76 ± 0.06 | 2.63 ± 0.01 ᵵ | 2.10 ± 0.04 ** |
| Repaglinide → M1-repaglinide | 0.09 ± 0.01 | 0.105 ± 0.002 | 0.073 ± 0.003 |
| Bufuralol → Hydroxybufuralol | 3.50 ± 0.03 | 7.7 ± 0.4 ᵵ | 7.4 ± 0.2 ᵵ |
| Chlorzoxazone → Hydroxychlorzoxazone | 130 ± 6 | 144.6 ± 0.2 ** | 108 ± 3 ** |
| Ebastine → Hydroxyebastine and carebastine | 13 ± 1 | 13.9 ± 0.3 | 9 ± 1 * |
| Midazolam → 1′-hydroxymidazolam | 0.24 ± 0.02 | 0.065 ± 0.002 ᵵ | 0.047 ± 0.008 ᵵ |
| Dodecanoic acid → 12-hydroxydecanoic acid | 173 ± 27 | 193 ± 3 | 118 ± 24 * |
| (B) | |||
| Lung | ND | LDR | HDR |
| (pmol/mg protein/min) | |||
| Bupropion → Hydroxybupropion | 538 ± 26 | 476 ± 4 * | 467 ± 14 ** |
| Tolbutamide → Hydroxytolbutamide | 0.64 ± 0.02 | 0.59 ± 0.03 | 0.54 ± 0.01 ** |
| Repaglinide → M1-repaglinide | N/F | N/F | N/F |
| Bufuralol → Hydroxybufuralol | 0.59 ± 0.02 | 0.55 ± 0.02 * | 0.425 ± 0.001 ᵵ |
| Chlorzoxazone → Hydroxychlorzoxazone | 477 ± 16 | 629 ± 79 ** | 535 ± 19 |
| Ebastine → Hydroxyebastine and carebastine | 17 ± 1 | 12.6 ± 0.9 * | 7 ± 3 ᵵ |
| Midazolam → 1′-hydroxymidazolam | 0.51 ± 0.01 | 0.47 ± 0.04 | 0.51 ± 0.01 |
| Dodecanoic acid → 12-hydroxydecanoic acid | 93 ± 6 | 78 ± 1 * | 68 ± 4 ᵵ |
| (C) | |||
| Heart | ND | LDR | HDR |
| (pmol/mg protein/min) | |||
| Bupropion → Hydroxybupropion | 0.15 ± 0.01 | 0.037 ± 0.004 ᵵ | 0.025 ± 0.003 ᵵ |
| Tolbutamide → Hydroxytolbutamide | N/F | N/F | N/F |
| Repaglinide → M1-repaglinide | N/A | N/A | N/A |
| Bufuralol → Hydroxybufuralol | N/A | N/A | N/A |
| Chlorzoxazone → Hydroxychlorzoxazone | 1.7 ± 0.1 | 1.2 ± 0.1 * | 1.7 ± 0.2 |
| Ebastine → Hydroxyebastine and carebastine | 0.8 ± 0.1 | 0.76 ± 0.06 | 0.78 ± 0.06 |
| Midazolam → 1′-hydroxymidazolam | N/F | N/F | N/F |
| Dodecanoic acid → 12-hydroxydecanoic acid | N/F | N/F | N/F |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maximos, S.; Chamoun, M.; Gravel, S.; Turgeon, J.; Michaud, V. Tissue Specific Modulation of cyp2c and cyp3a mRNA Levels and Activities by Diet-Induced Obesity in Mice: The Impact of Type 2 Diabetes on Drug Metabolizing Enzymes in Liver and Extra-Hepatic Tissues. Pharmaceutics 2017, 9, 40. https://doi.org/10.3390/pharmaceutics9040040
Maximos S, Chamoun M, Gravel S, Turgeon J, Michaud V. Tissue Specific Modulation of cyp2c and cyp3a mRNA Levels and Activities by Diet-Induced Obesity in Mice: The Impact of Type 2 Diabetes on Drug Metabolizing Enzymes in Liver and Extra-Hepatic Tissues. Pharmaceutics. 2017; 9(4):40. https://doi.org/10.3390/pharmaceutics9040040
Chicago/Turabian StyleMaximos, Sarah, Michel Chamoun, Sophie Gravel, Jacques Turgeon, and Veronique Michaud. 2017. "Tissue Specific Modulation of cyp2c and cyp3a mRNA Levels and Activities by Diet-Induced Obesity in Mice: The Impact of Type 2 Diabetes on Drug Metabolizing Enzymes in Liver and Extra-Hepatic Tissues" Pharmaceutics 9, no. 4: 40. https://doi.org/10.3390/pharmaceutics9040040
APA StyleMaximos, S., Chamoun, M., Gravel, S., Turgeon, J., & Michaud, V. (2017). Tissue Specific Modulation of cyp2c and cyp3a mRNA Levels and Activities by Diet-Induced Obesity in Mice: The Impact of Type 2 Diabetes on Drug Metabolizing Enzymes in Liver and Extra-Hepatic Tissues. Pharmaceutics, 9(4), 40. https://doi.org/10.3390/pharmaceutics9040040





