Insertion Process of Ceramic Nanoporous Microneedles by Means of a Novel Mechanical Applicator Design
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Methods
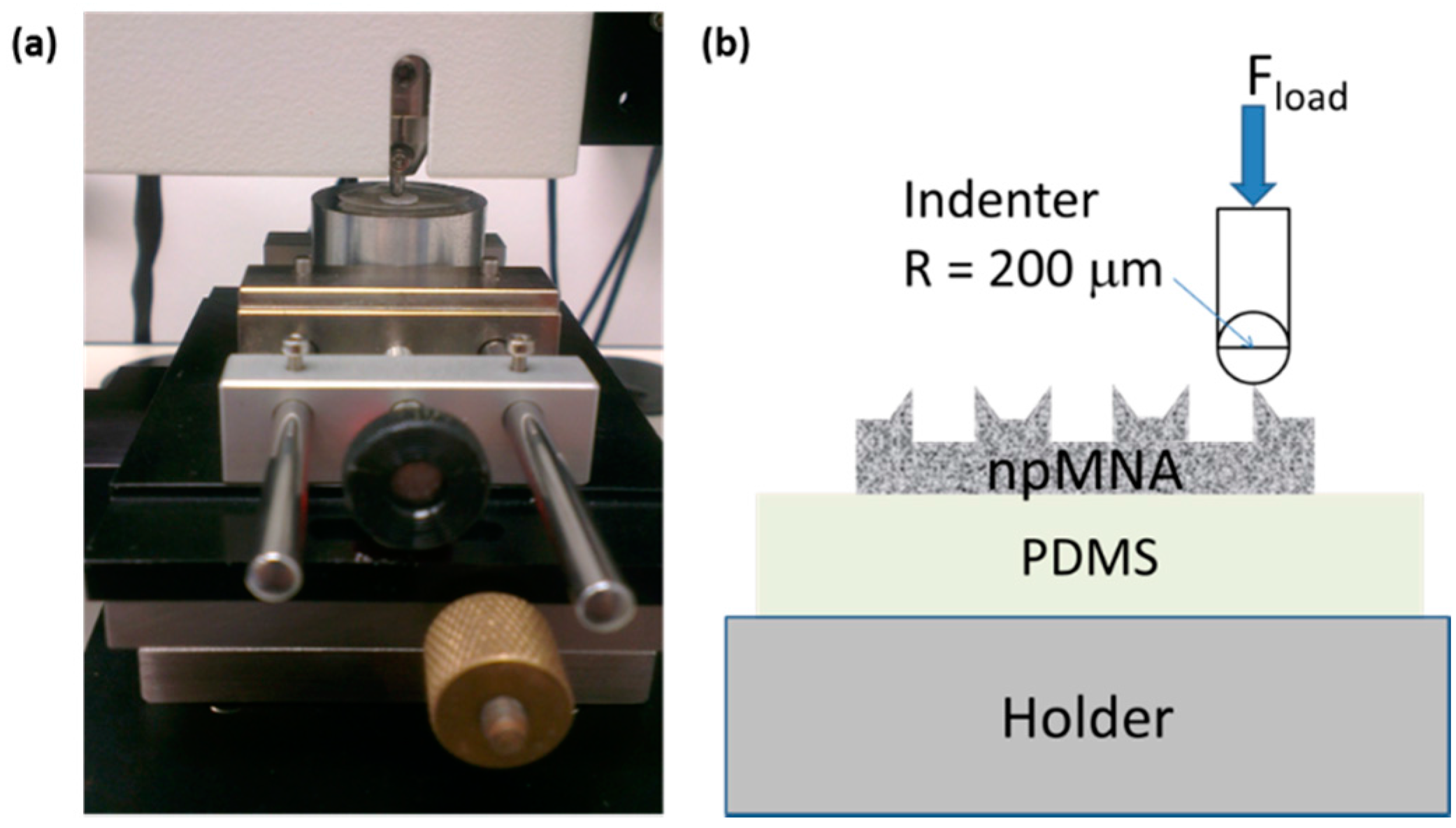
2.2.1. Fracture Force Measurements
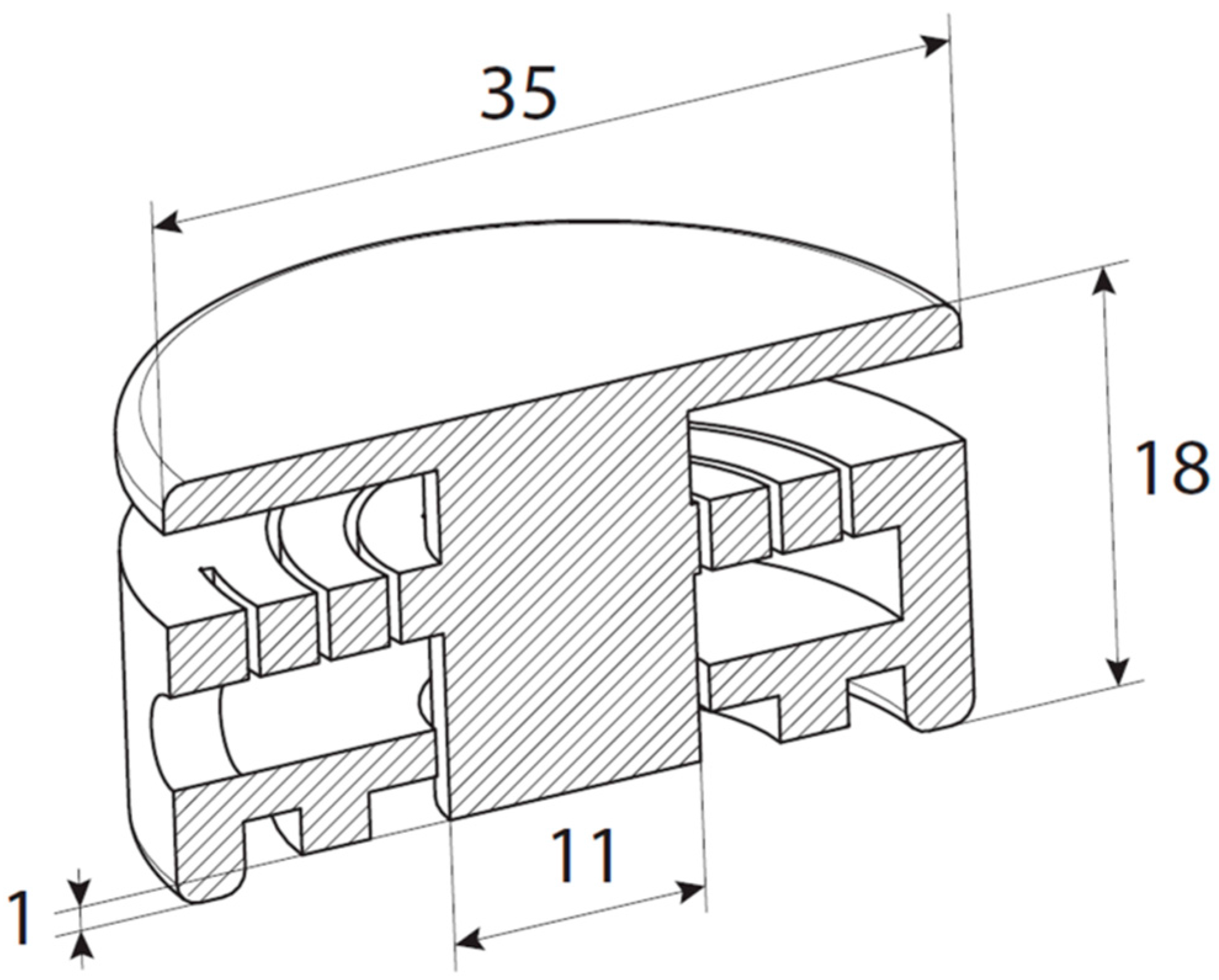
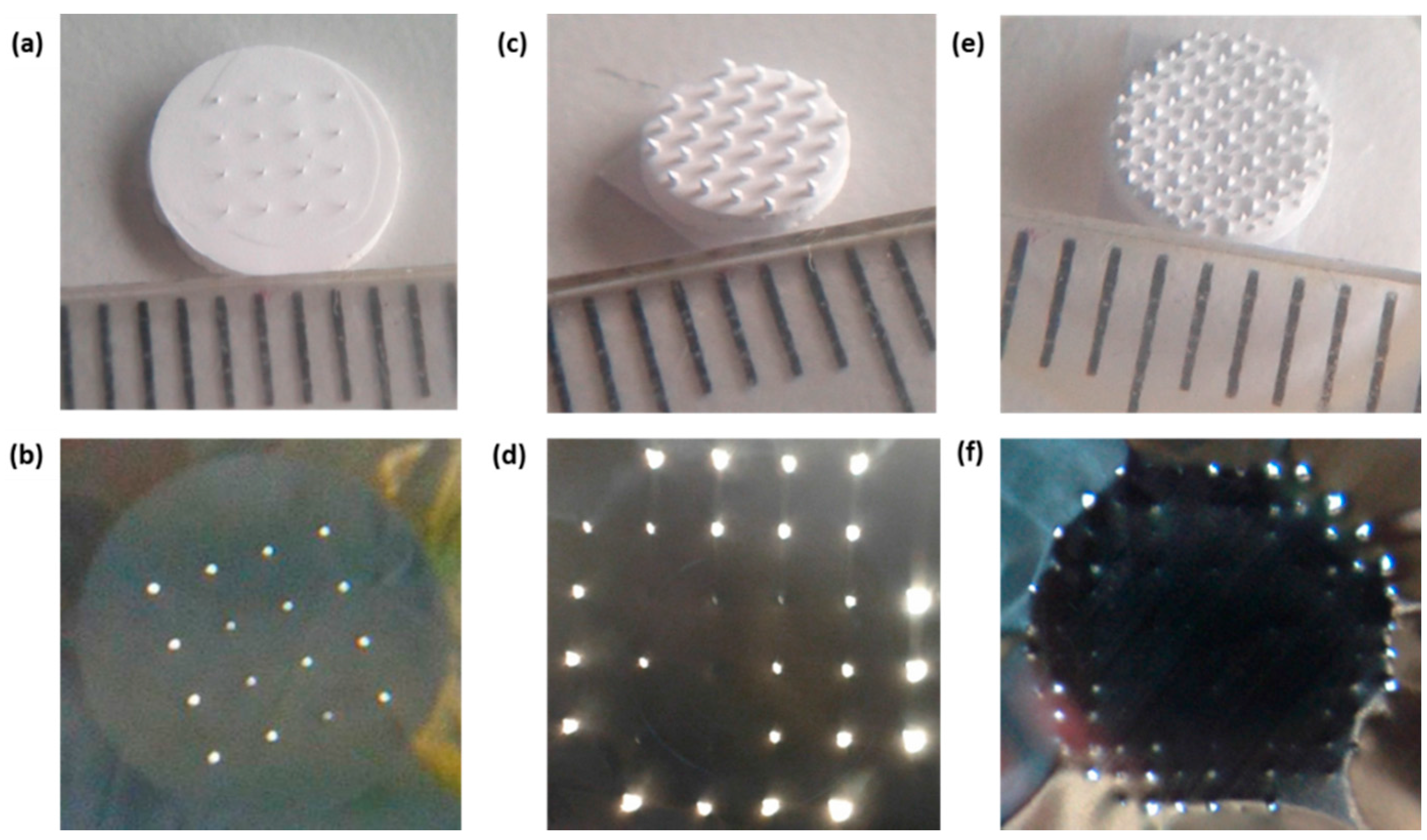
2.2.2. Applicator Fabrication and Design
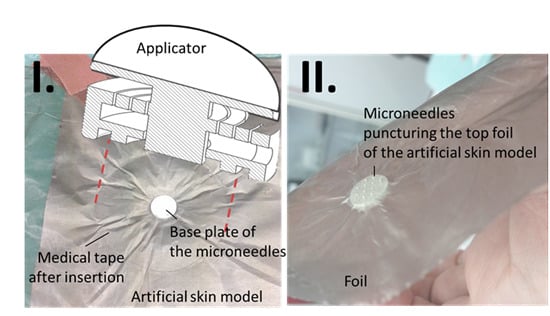
2.2.3. Experimental Evaluation of the Applicator-Assisted Insertion Method
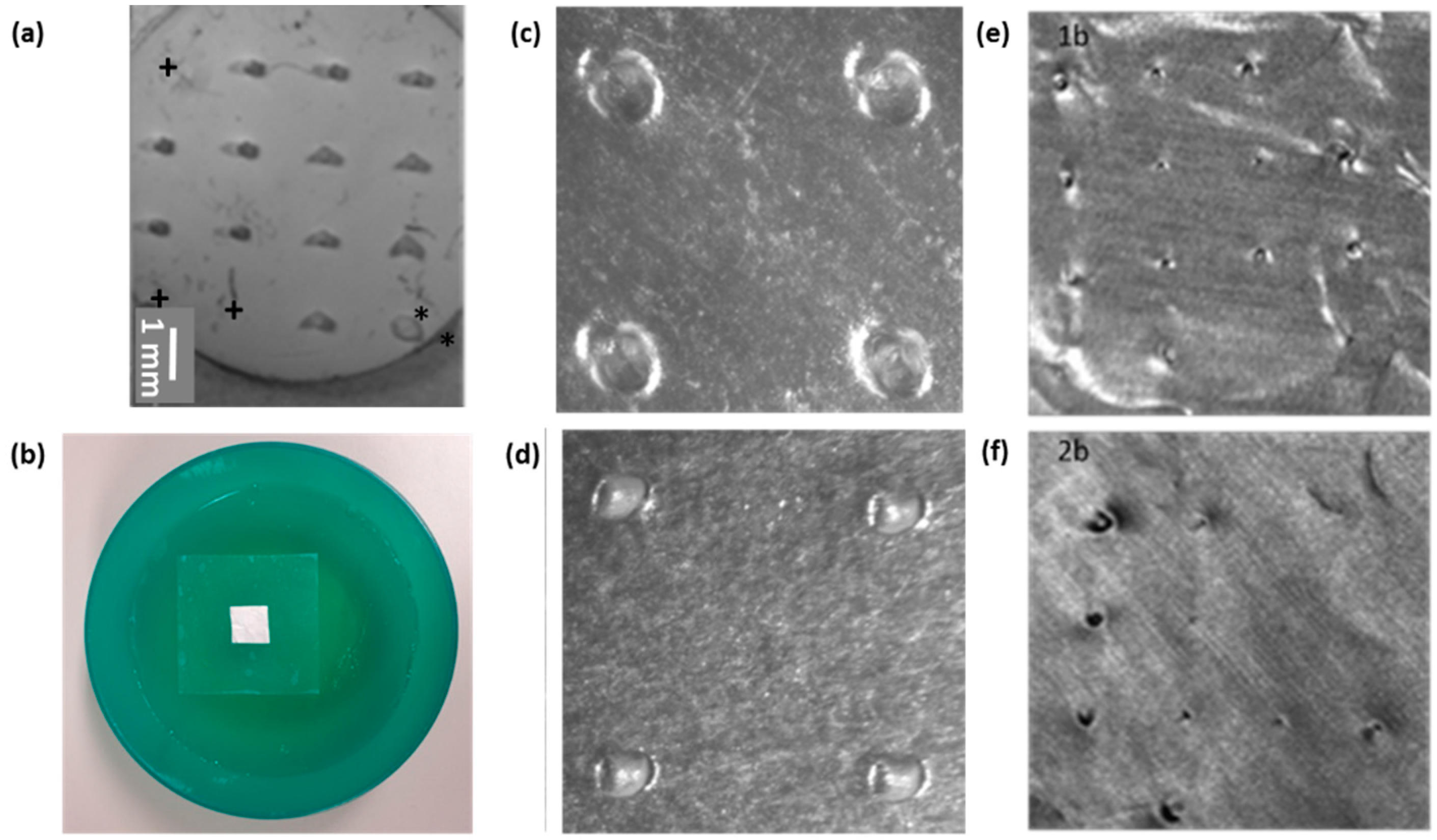
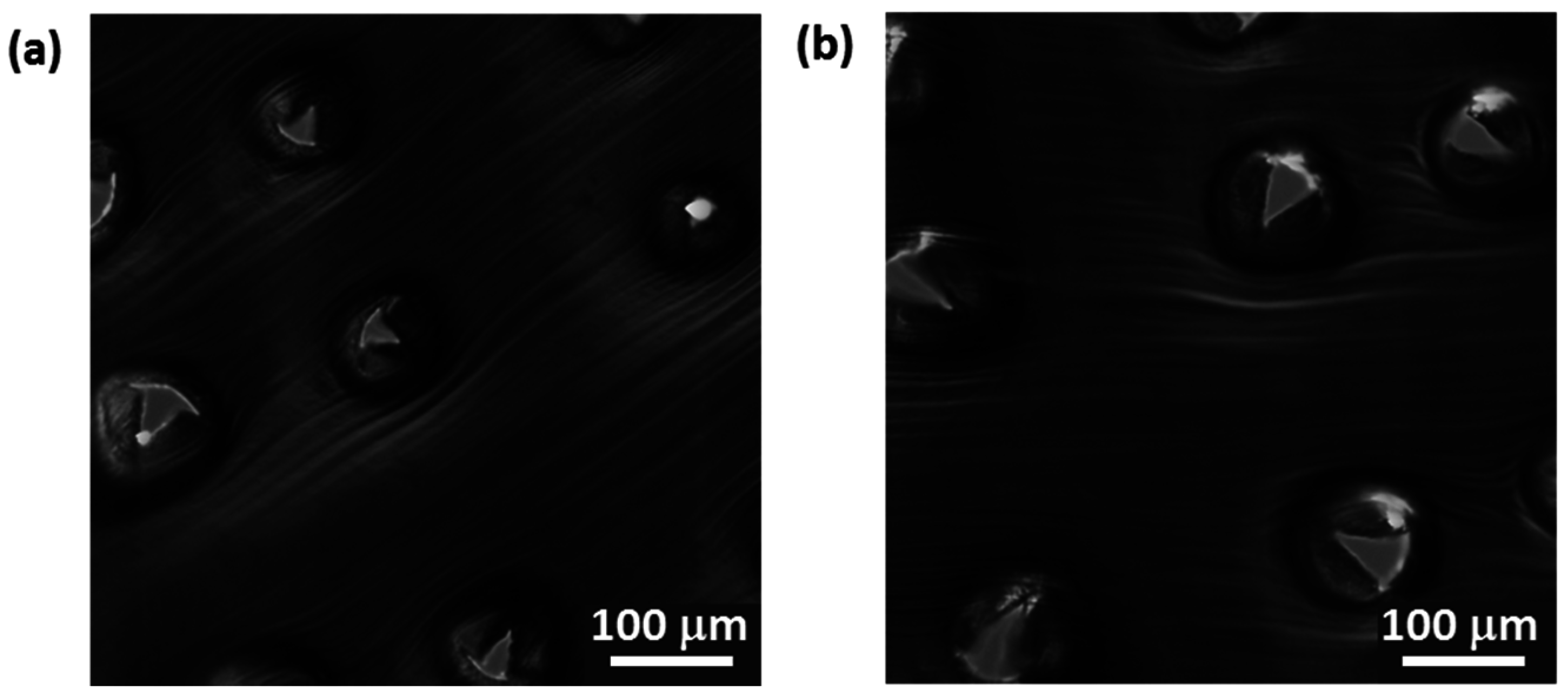
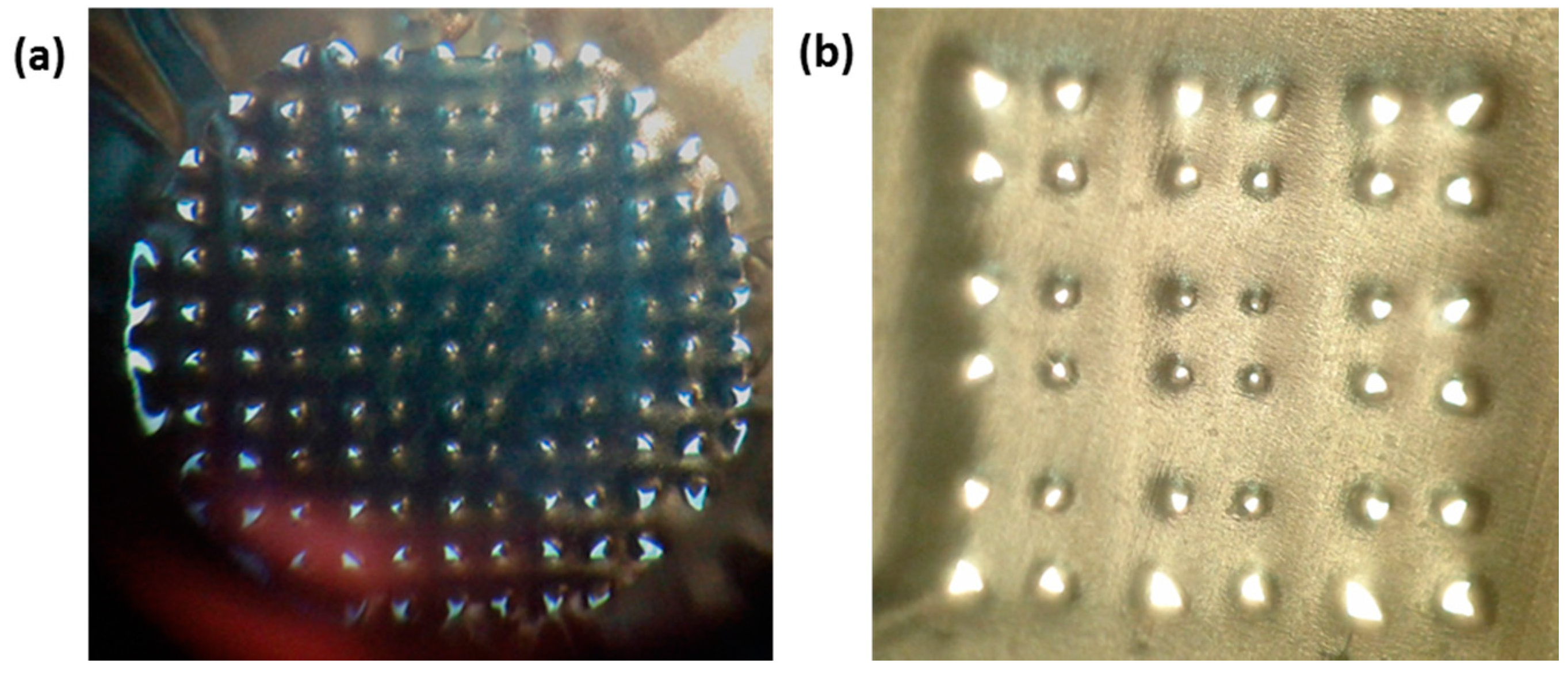
2.2.4. Evaluation of Puncture Efficiency
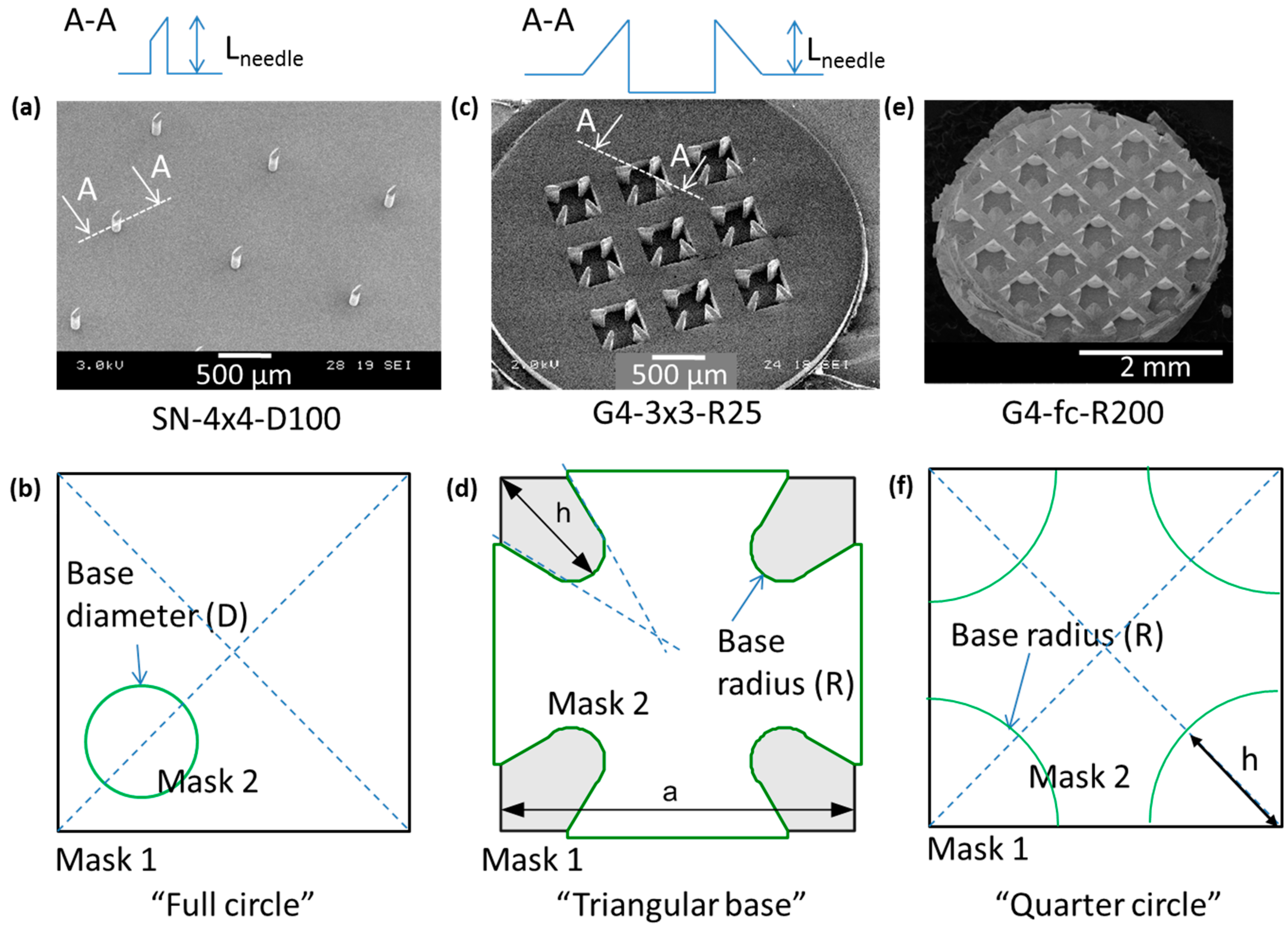
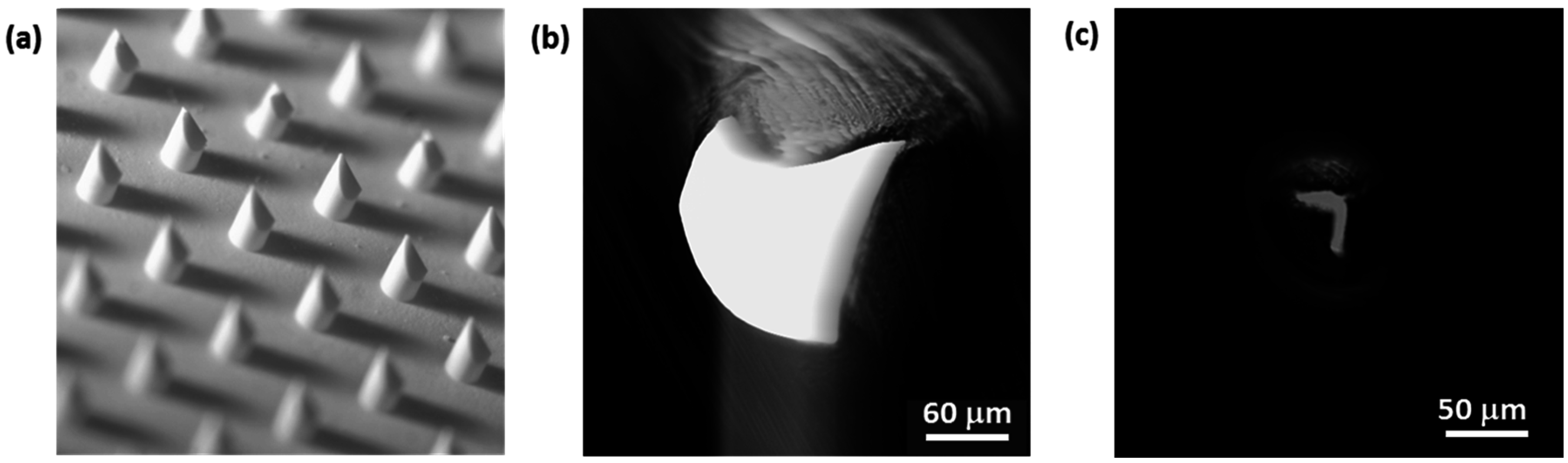
2.2.5. npMNA Images
3. Results and Discussion
3.1. Indicative Measurement of Crack-Fracture Force
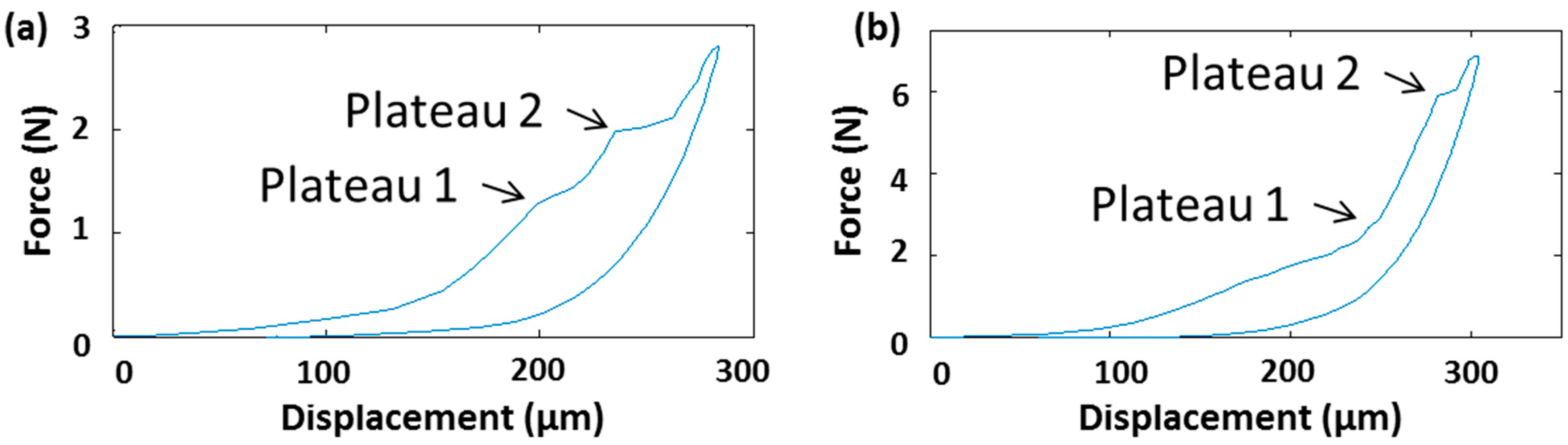
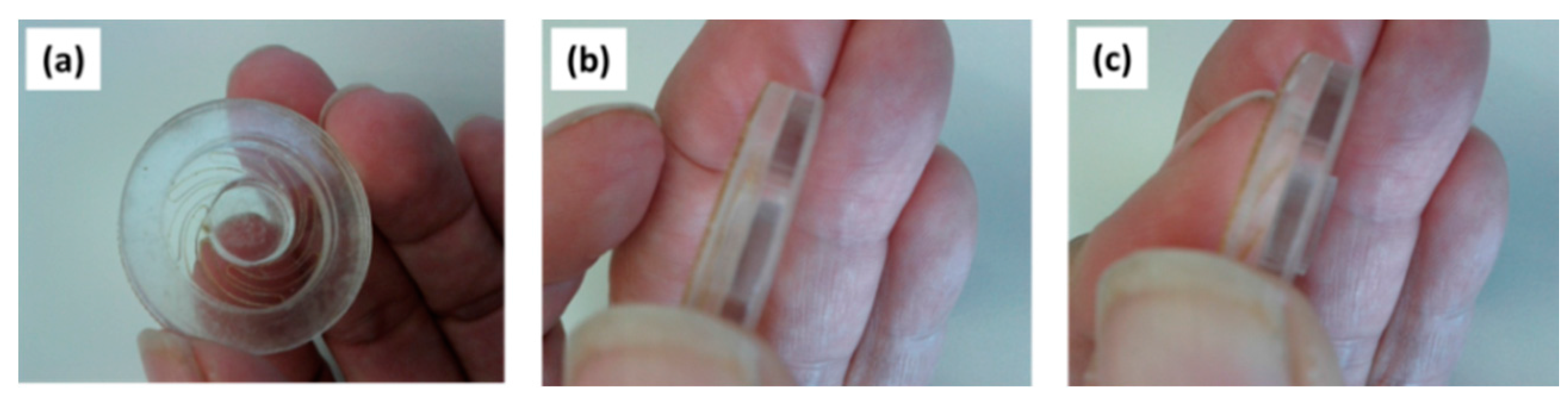
3.2. npMNA Patch Applicator Design Study

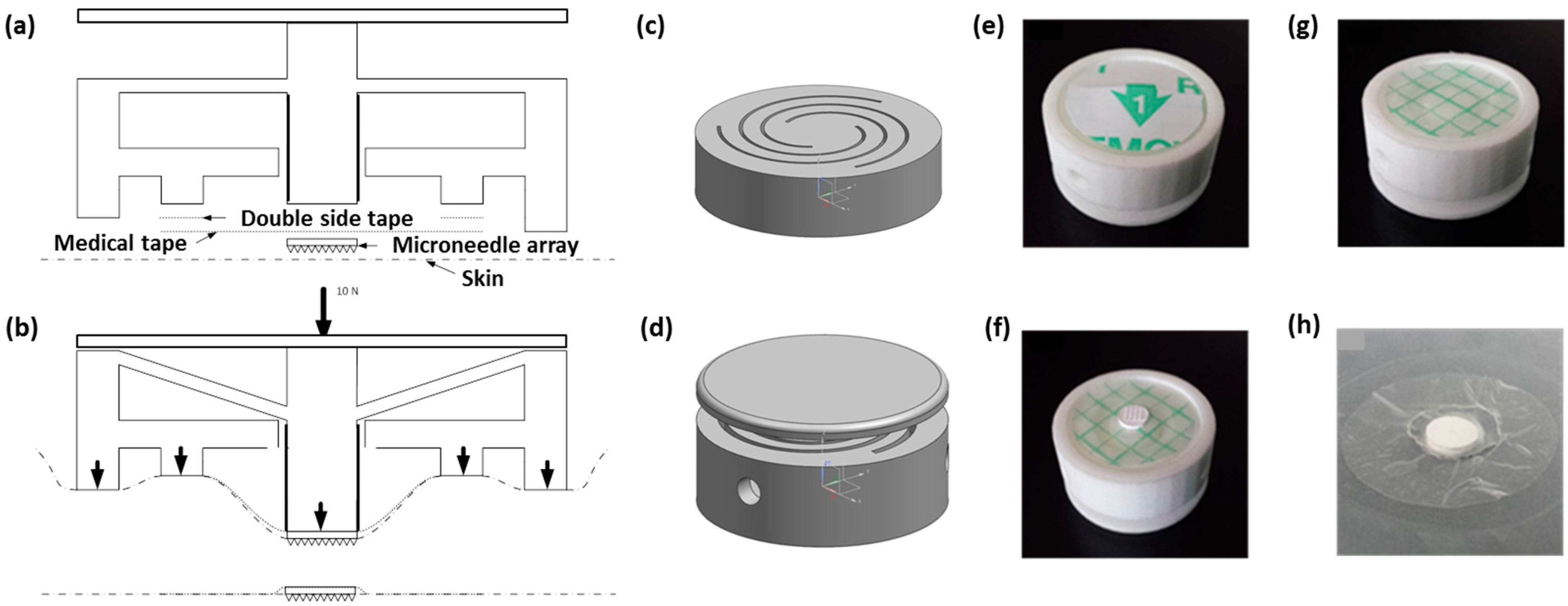
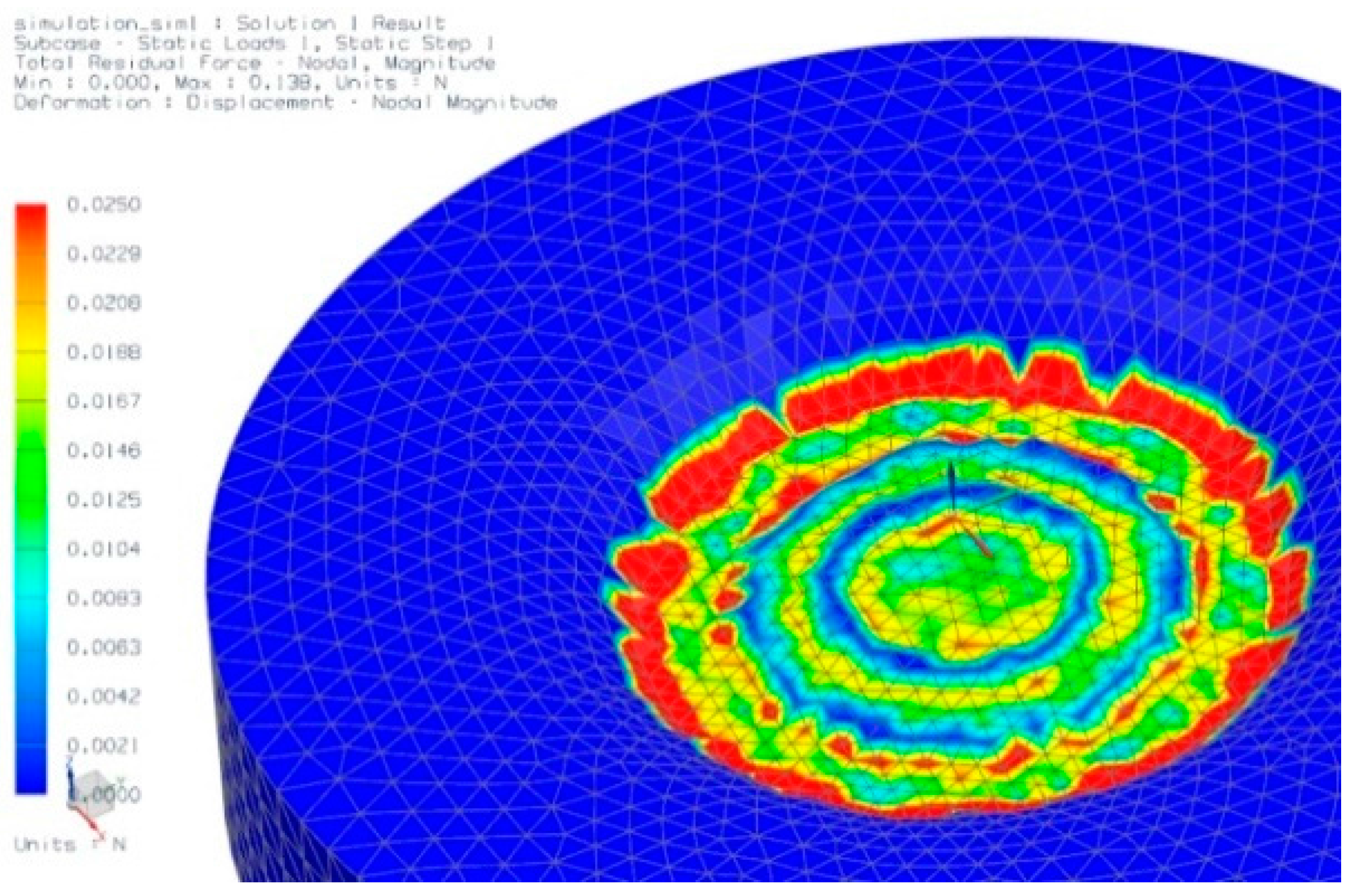
3.3. Testing the Penetration Efficiency of the npMNA Insertion Method
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- ZosanoPharma. Available online: http://www.zosanopharma.com/ (accessed on 20 August 2015).
- Corium. Available online: http://www.coriumgroup.com/ (accessed on 20 August 2015).
- Debiotech. Available online: http://www.debiotech.com (accessed on 20 August 2015).
- Mylife Technologies. Available online: http://www.mylifetechnologies.nl/ (accessed on 20 August 2015).
- Boks, M.A.; Unger, W.W.J.; Engels, S.; Ambrosini, M.; van Kooyk, Y.; Luttge, R. Controlled release of a model vaccine by nanoporous ceramic microneedle arrays. Int. J. Pharm. 2015, 491, 375–383. [Google Scholar] [CrossRef] [PubMed]
- van der Maaden, K.; Luttge, R.; Vos, P.J.; Bouwstra, J.; Kersten, G.; Ploemen, I. Microneedle-based drug and vaccine delivery via nanoporous microneedle arrays. Drug Deliv. Transl. Res. 2015, 5, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Cormier, M.; Johnson, B.; Ameri, M.; Nyam, K.; Libiran, L.; Zhang, D.D.; Daddona, P. Transdermal delivery of desmopressin using a coated microneedle array patch system. J. Control. Release 2004, 9, 503–511. [Google Scholar] [CrossRef]
- Gittard, S.D.; Ovsianikov, A.; Chichkov, B.N.; Doraiswamy, A.; Narayan, R.J. Two Photon Polymerization of Microneedles for Transdermal Drug Delivery. Expert Opin. Drug Deliv. 2010, 7, 513–533. [Google Scholar] [CrossRef] [PubMed]
- Gardeniers, H.J.G.E.; Luttge, R.; Berenschot, E.J.W.; de Boer, M.J.; Yeshurun, S.Y.; Hefetz, M.; van’t Oever, R.; van den Berg, A. Silicon micromachined hollow microneedles for transdermal liquid transport. J. Microelectromech. Syst. 2003, 12, 855–862. [Google Scholar] [CrossRef]
- Griss, P.; Stemme, G. Side-opened out-of-plane microneedles for microfluidic transdermal liquid transfer. J. Microelectromech. Syst. 2003, 12, 296–301. [Google Scholar] [CrossRef]
- Henry, S.; McAllister, D.V.; Allen, M.G.; Prausnitz, M.R. Microfabricated microneedles: A novel approach to transdermal drug delivery. J. Pharm. Sci. 1998, 87, 922–925. [Google Scholar] [CrossRef] [PubMed]
- Bystrova, S.N.; Luttge, R. Micromolding for ceramic microneedle arrays. Microelectro. Eng. 2011, 88, 1681–1684. [Google Scholar] [CrossRef]
- Ita, K. Transdermal delivery of drugs with microneedles—Potential and challenges. Phamaceutics 2015, 7, 90–105. [Google Scholar] [CrossRef] [PubMed]
- van der Maaden, K.; Jiskoot, W.; Bouwstra, J. Microneedle technologies for (trans)dermal drug and vaccine delivery. J. Control. Release 2012, 161, 645–655. [Google Scholar] [CrossRef] [PubMed]
- McAllister, D.V.; Wang, P.M.; Davis, S.P.; Park, J.-H.; Canatella, P.J.; Allen, M.G.; Prausnitz, M.R. Microfabricated needles for transdermal delivery of macromolecules and nanoparticles: Fabrication methods and transport studies. Proc. Natl. Acad. Sci. USA 2003, 100, 13755–13760. [Google Scholar] [CrossRef] [PubMed]
- Tuan-Mahmood, T.-M.; McCrudden, M.T.C.; Torrisi, B.M.; McAlister, E.; Garland, M.J.; Singh, T.R.R.; Donnelly, R.F. Microneedles for intradermal and transdermal delivery. Eur. J. Pharm. Sci. 2013, 50, 623–637. [Google Scholar] [CrossRef] [PubMed]
- Sivamania, R.K.; Liepmann, D.; Maibach, H.I. Microneedles and transdermal applications. Expert Opin. Drug Deliv. 2007, 4, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Levin, Y.; Kochba, E.; Hung, I.; Kenney, R. Intradermal vaccination using the novel microneedle device MicronJet600: Past, present, and future. Hum. Vaccin. Immunother. 2015, 11, 991–997. [Google Scholar] [CrossRef] [PubMed]
- Levin, Y.; Kochba, E.; Kenney, R. Clinical evaluation of a novel microneedle device for intradermal delivery of an influenza vaccine: are all delivery methods the same? Vaccine 2014, 32, 4249–4252. [Google Scholar] [CrossRef] [PubMed]
- Prausnitz, M.R.; Mikszta, J.A.; Cormier, M.; Andrianov, A.K. Microneedle-based vaccines. Curr. Top. Microbiol. Immunol. 2009, 333, 369–393. [Google Scholar] [PubMed]
- Kommareddy, S.; Baudner, B.C.; Bonificio, A.; Gallorini, S.; Palladino, G.; Determan, A.S.; Dohmeier, D.M.; Kroells, K.D.; Sternjohn, J.R.; Singh, M.; et al. Influenza subunit vaccine coated microneedle patches elicit comparable immune responses to intramuscular injection in guinea pigs. Vaccine 2013, 31, 3435–3441. [Google Scholar] [CrossRef] [PubMed]
- Scopus. Available online: http://www.scopus.com/ (accessed on 8 August 2015).
- Park, J.-H.; Allen, M.G.; Prausnitz, M.R. Polymer microneedles for controlled-release drug delivery. Pharm. Res. 2006, 23, 1008–1019. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Choi, S.-O.; Felner, E.I.; Prausnitz, M.R. Dissolving microneedle patch for transdermal delivery of human growth hormone. Small 2011, 7, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.F.; Moffatt, K.; Alkilani, Z.A.; Vicente-Pérez, E.M.; Barry, J.; McCrudden, M.T.C.; Woolfson, A.D. Hydrogel-forming microneedle array can be effectively inserted in skin by self-application: A pilot study centred on pharmacists Intervention and a patient information leaflet. Pharm. Res. 2014, 31, 1989–1999. [Google Scholar] [CrossRef] [PubMed]
- Norman, J.J.; Arya, J.M.; McClain, M.A.; Frew, P.M.; Meltzer, M.I.; Prausnitz, M.R. Microneedle patches: Usability and acceptability for self-vaccination against influenza. Vaccine 2014, 32, 1856–1862. [Google Scholar] [CrossRef] [PubMed]
- Lüttge, R.; Bystrova, S.N.; van Bennekom, J.G.; Domanski, M.; Loeters, P.W.H.; Lammertink, R.G.H.; Winnubst, A.J.A. Integrated microneedle array and a method for manufacturing thereof. Eur. Pat. Appl. 08152571.9, 2008. [Google Scholar]
- McDonald, J.C.; Whitesides, G.M. Poly(dimethylsiloxane) as a Material for Fabricating Microfluidic Devices. Acc. Chem. Res. 2002, 35, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Verbaan, F.J.; Bal, S.M.; van den Berg, D.J.; Groenink, W.H.H.; Verpoorten, H.; Lüttge, R.; Bouwstra, J.A. Assembled microneedle arrays enhance the transport of compounds varying over a large range of molecular weight across human dermatomed skin. J. Control. Release 2007, 117, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Teo, M.A.L.; Shearwood, C.; Ng, K.C.; Lu, J.; Moochhala, S. In vitro and in vivo characterization of MEMS microneedles. Biomed. Microdevices 2005, 7, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Verbaan, F.J.; Bal, S.M.; van den Berg, D.J.; Dijksman, J.A.; van Hecke, M.; Verpoorten, H.; van den Berg, A.; Luttge, R.; Bouwstra, J.A. Improved piercing of microneedle arrays in dermatomed human skin by an impact insertion method. J. Control. Release 2008, 128, 80–88. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hartmann, X.H.M.; Van der Linde, P.; Homburg, E.F.G.A.; Van Breemen, L.C.A.; De Jong, A.M.; Luttge, R. Insertion Process of Ceramic Nanoporous Microneedles by Means of a Novel Mechanical Applicator Design. Pharmaceutics 2015, 7, 503-522. https://doi.org/10.3390/pharmaceutics7040503
Hartmann XHM, Van der Linde P, Homburg EFGA, Van Breemen LCA, De Jong AM, Luttge R. Insertion Process of Ceramic Nanoporous Microneedles by Means of a Novel Mechanical Applicator Design. Pharmaceutics. 2015; 7(4):503-522. https://doi.org/10.3390/pharmaceutics7040503
Chicago/Turabian StyleHartmann, Xavier H. M., Peter Van der Linde, Erik F. G. A. Homburg, Lambert C. A. Van Breemen, Arthur M. De Jong, and Regina Luttge. 2015. "Insertion Process of Ceramic Nanoporous Microneedles by Means of a Novel Mechanical Applicator Design" Pharmaceutics 7, no. 4: 503-522. https://doi.org/10.3390/pharmaceutics7040503
APA StyleHartmann, X. H. M., Van der Linde, P., Homburg, E. F. G. A., Van Breemen, L. C. A., De Jong, A. M., & Luttge, R. (2015). Insertion Process of Ceramic Nanoporous Microneedles by Means of a Novel Mechanical Applicator Design. Pharmaceutics, 7(4), 503-522. https://doi.org/10.3390/pharmaceutics7040503