Microneedle Coating Techniques for Transdermal Drug Delivery
Abstract
:1. Introduction
2. Microneedle Mechanism and Design
3. Microneedle Coating Methods
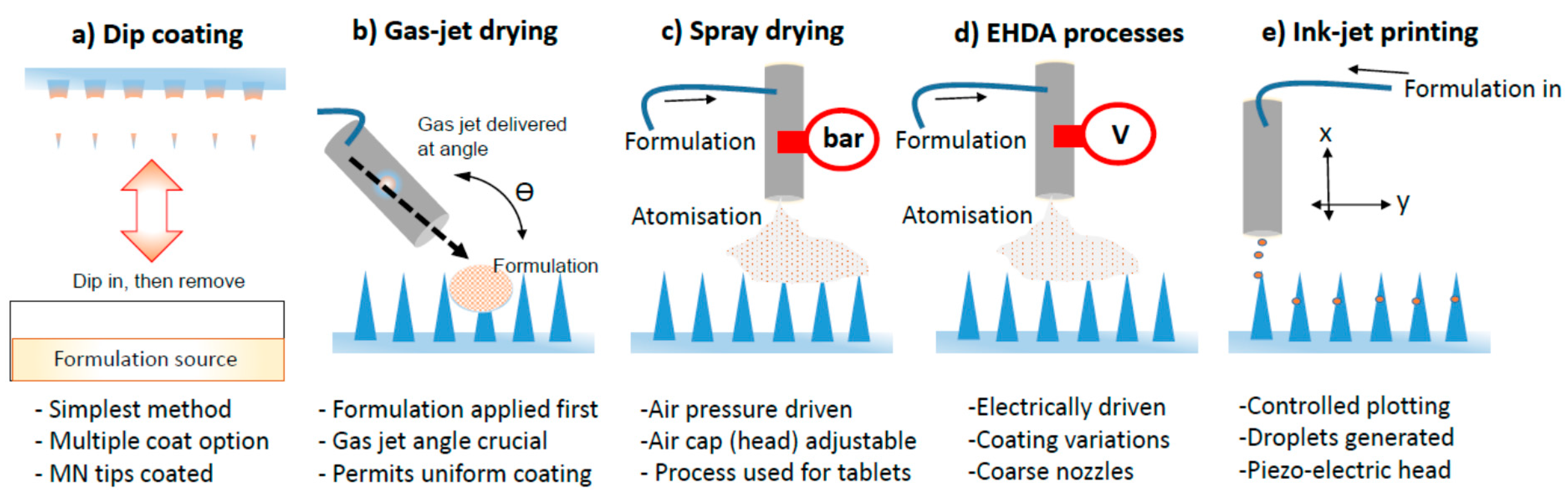
3.1. Dip Coating
3.2. Gas Jet Drying
3.3. Spray Coating
3.4. EHDA Based Processes
3.5. Piezoelectric Inkjet Printing
| Coating Method | Base MN Material | MN Type | Coating Material Type | Excipients | Active or Model | Coating Structure on MN | Points on Process | Comments | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Dip Coating | Stainless Steel | Flat. 700 µm in length | Molten solutions | PEG | Lidocaine | Film | Two main steps (dipping and drying). Additional time required for the preparation of formulation in hot-stage (including mixing) and further mixing using sonication | Lidocaine-PEG coated MNs had significantly higher delivery of drug (in 3 min) as compared with the topical administration of 0.15 g EMLA®. Method can be considered for hydrophobic drugs | [31] |
| Titanium | 340 µm in length | Solutions | Sucrose Polysorbate 20 | rhGH | Film | Two main steps (dipping and drying). Roller drum method used to coat MN tips which were optimised to allow coatings to dry efficiently (5 s) before next dip. Ambient temperature process | Uniform MN coating achieved using high concentration of rhGH. Administered using an applicator. MN tips coated with formulation | [32] | |
| Stainless Steel | Single MNs, in-plane rows of MNs and out-of-plane arrays of MNs Flat. | Solutions and Particles | CMC Sodium salt and Lutrol F-68 NF | Vitamin B, Calcein, gWiz™ luciferase plasmid DNA, Sulforhodamine, BSA, BaSO4 particles and modified Vaccinia Virus | Film and Particles | Two steps (dipping and drying). Modified dipping process using horizontal axis. Process required micro-positioning device to allow MN coating through precision holes which overcomes meniscus rising and subsequent unwanted spreading. Formulation fed into a 2-plate system allowing MNs to be coated. Method monitored in real time through stereo microscope visualisation | The coated materials on the MNs shafts dissolved within 20 s in porcine cadaver skin with complete delivery into the skin. Precision coating and reduced wastage of material due to two plate coating system | [13] | |
| Gas-jet Drying | Silicon | 60 and 90 µm in length, Cone | Solutions | MC, Quil A, Poloxamer | OVA protein vaccine/FLR-dye | Film | Two step process. Includes the application of formulation and then drying based on gas-jet with variable speeds at specific incident angles | Densely packed MN successfully coated using this method. Method can be considered for large molecules. | [17] |
| Silicon | 110 µm in length, Segments | Solutions | MC, Trehalose and 14C-OVA | Human Influenza Vaccine (Fluvax®) | Film | As above. MN patches were rotated to ensure uniformity. A nitrogen gas-jet was used | An improved approach to deliver vaccine to low-resource regions with long time stability. Tracer was incorporated into coating | [35] | |
| Spray Coating | Silicon | 280 µm in length, Contour | Solutions | HPMC, CMC, Tween 80 | Film | Multiple variables can be used for spray optimisation. Coated MNs were dried for 12 h at the ambient temperature. Factorial design used to determine best coating formulation | Various conventional tablet coating polymers deployed for coating MNs. Multiple variables involved which impact spraying time. Surfactant may be required to improve coalescence of droplets | [36] | |
| Silicon | 300 µm in length, Contour | Solutions | CMC, Trehalose, Maltodextrin, Sodium salt, Tween 80 and Lutrol F68 | rADV, modified MVA Vectors and FITC | Relics and Films | Process optimised to control direct deposition on to MNs. This also required careful isolation of viruses during deposition. Multiple variables can be used for spray optimisation. Coated MNs were dried under vacuum (with desiccant) for a further 2–24 h | Uniform coating significantly preserved the virus’s activity which was successfully delivered into the skin and resulted in antibody response equivalent to the response induced by transdermal injection of the same vaccine | [37] | |
| EHDA Process | Stainless Steel | 500 µm in length, Flat | Solutions | PVP | FLR dye | Particles and Fibres | Multiple variables in this process. Reduced drying time due to non-aqueous solvent deployment for formulation. Coating thickness variable—dependent on deposition time. Ambient condition process | Solution properties used to prepare coating formulations are critical to the process and lead to variations in coating structure type | [34] |
| Ink-jet Printing | PMVE-MA | ~800 µm in length | Solutions | DMSO | MNZ | Micro-droplet Film | MNs were exposed to UV light prior to printing with formulation. Ambient temperature process. Six layers of printed patterns applied. 38 µg of MNZ dose per patch prepared | Printing system presents an opportunity for poorly soluble anti-fungal drugs. A multi-mode engineering approach is a valuable for drop on demand system coatings | [50] |
| Stainless Steel | 700 µm in length, Flat | Solutions | De-ionised Water, Ethanol and Soluplus | 5-FU, Curcumin, Cisplatin and Na FLR | Spotted and Micro- droplet Film. | Plotting of droplets on to MNs at 45°. Droplets deposited in continuous jetting cycles to increase coating. Process is computer controlled to determine volumes and real time deposition via imaging | Controlled deposition (of a droplet) using a controlled deposition device. Piezo-electric jet head used. Droplet size correlates with nozzle exit | [51] | |
| PGA | ~800 µm in length, Half conical | Solutions | PMVE-MA, DMSO | VNZ and Methylene blue | Micro-droplet Film | Small quantities of formulations loaded into printer cartridge. 1 µg of the drug onto each MN patch system. Precision controlled deposition. Three layers deposited | VNZ-PGA MNs showed antifungal activity against Candida albicans. Accordingly, this system is ideal for poorly soluble pharmacological agents | [52] |
4. Conclusions
Author Contributions
Conflict of Interests
References
- Prausnitz, M.R.; Langer, R. Transdermal drug delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Ita, K. Transdermal Delivery of Drugs with Microneedles-Potential and Challenges. Pharmaceutics 2015, 7, 90–105. [Google Scholar] [CrossRef] [PubMed]
- More, S.; Ghadge, T.; Dhole, S. Microneedle: An Advanced Technique in Transdermal Drug Delivery System. Asian J. Res. Pharm. Sci. 2013, 3, 141–148. [Google Scholar]
- Kaestli, L.; Wasilewski-Rasca, A.; Bonnabry, P.; Vogt-Ferrier, N. Use of transdermal drug formulations in the elderly. Drugs Aging 2008, 25, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Sivamani, R.K.; Liepmann, D.; Malbach, H.I. Microneedles and transdermal applications. Expert Opin. Drug Deliv. 2007, 4, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Demir, Y.K.; Kerimoglu, O. Novel Use of Pectin as a Microneedle Base. Chem. Pharm. Bull. 2015, 63, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Demir, Y.K.; Akan, Z.; Kerimoglu, O. Characterization of Polymeric Microneedle Arrays for Transdermal Drug Delivery. PLoS ONE 2013, 8, e77289. [Google Scholar] [CrossRef] [PubMed]
- Demir, Y.K.; Akan, Z.; Kerimoglu, O. Sodium Alginate Microneedle Arrays Mediate the Transdermal Delivery of Bovine Serum Albumin. PLoS ONE 2013, 8, e63819. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.F.; Majithiya, R.; Singh, T.R.R.; Morrow, D.I.J.; Garland, M.J.; Demir, Y.K.; Migalska, K.; Ryan, E.; Gillen, D.; Scott, C.J.; et al. Design, Optimization and Characterisation of Polymeric Microneedle Arrays Prepared by a Novel Laser-Based Micromoulding Technique. Pharm. Res. 2011, 28, 41–57. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.R.R.; Garland, M.J.; Cassidy, C.M.; Migalska, K.; Demir, Y.K.; Abdelghany, S.; Ryan, E.; Woolfson, A.D.; Donnelly, R.F. Microporation techniques for enhanced delivery of therapeutic agents. Recent Pat. Drug Deliv. Formul. 2010, 4, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Gill, H.S.; Denson, D.D.; Burris, B.A.; Prausnitz, M.R. Effect of microneedle design on pain in human volunteers. Clin. J. Pain 2008, 24, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Juan Escobar-Chavez, J.; Bonilla-Martinez, D.; Angelica Villegas-Gonzalez, M.; Molina-Trinidad, E.; Casas-Alancaster, N.; Luisa Revilla-Vazquez, A. Microneedles: A Valuable Physical Enhancer to Increase Transdermal Drug Delivery. J. Clin. Pharmacol. 2011, 51, 964–977. [Google Scholar] [CrossRef] [PubMed]
- Gill, H.S.; Prausnitz, M.R. Coated microneedles for transdermal delivery. J. Control. Release 2007, 117, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Cormier, M.; Johnson, B.; Ameri, M.; Nyam, K.; Libiran, L.; Zhang, D.; Daddona, P. Transdermal delivery of desmopressin using a coated microneedle array patch system. J. Control. Release 2004, 97, 503–511. [Google Scholar] [CrossRef]
- Ameri, M.; Fan, S.C.; Maa, Y. Parathyroid Hormone PTH(1–34) Formulation that Enables Uniform Coating on a Novel Transdermal Microprojection Delivery System. Pharm. Res. 2010, 27, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Martanto, W.; Davis, S.P.; Holiday, N.R.; Wang, J.; Gill, H.S.; Prausnitz, M.R. Transdermal delivery of insulin using microneedles in vivo. Pharm Res. 2004, 21, 947–952. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Prow, T.W.; Crichton, M.L.; Jenkins, D.W.K.; Roberts, M.S.; Frazer, I.H.; Fernando, G.J.P.; Kendall, M.A.F. Dry-coated microprojection array patches for targeted delivery of immune-therapeutics to the skin. J. Control. Release 2009, 139, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Andrianov, A.K.; Marin, A.; DeCollibus, D.P. Microneedles with Intrinsic Immunoadjuvant Properties: Microfabrication, Protein Stability, and Modulated Release. Pharm. Res. 2011, 28, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Andrianov, A.K.; DeCollibus, D.P.; Gillis, H.A.; Kha, H.H.; Marin, A.; Prausnitz, M.R.; Babiuk, L.A.; Townsend, H.; Mutwiri, G. Poly[di(carboxylatophenoxy)phosphazene] is a potent adjuvant for intradermal immunization. Proc. Natl. Acad. Sci. USA 2009, 106, 18936–18941. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Quan, F.; Compans, R.W.; Kang, S.; Prausnitz, M.R. Formulation and coating of microneedles with inactivated influenza virus to improve vaccine stability and immunogenicity. J. Control. Release 2010, 142, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Quan, F.; Kim, Y.; Vunnava, A.; Yoo, D.; Song, J.; Prausnitz, M.R.; Compans, R.W.; Kang, S. Intradermal Vaccination with Influenza Virus-Like Particles by Using Microneedles Induces Protection Superior to That with Intramuscular Immunization. J. Virol. 2010, 84, 7760–7769. [Google Scholar] [CrossRef] [PubMed]
- Martanto, W.; Moore, J.; Kashlan, O.; Kamath, R.; Wang, P.; O’Neal, J.; Prausnitz, M. Microinfusion using hollow microneedles. Pharm. Res. 2006, 23, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Bariya, S.H.; Gohel, M.C.; Mehta, T.A.; Sharma, O.P. Microneedles: An emerging transdermal drug delivery system. J. Pharm. Pharmacol. 2012, 64, 11–29. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Choi, S.; Kamath, R.; Yoon, Y.; Allen, M.G.; Prausnitz, M.R. Polymer particle-based micromolding to fabricate novel microstructures. Biomed. Microdevices 2007, 9, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Davis, S.; Yoon, Y.; Prausnitz, M.; Allen, M. Micromachined biodegradable microstructures. In Proceedings of IEEE the Sixteenth Annual International Conference on Micro Electro Mechanical Systems, Kyoto, Japan, 19–23 January 2003; pp. 371–374.
- Fukushima, K.; Ise, A.; Morita, H.; Hasegawa, R.; Ito, Y.; Sugioka, N.; Takada, K. Two-Layered Dissolving Microneedles for Percutaneous Delivery of Peptide/Protein Drugs in Rats. Pharm. Res. 2011, 28, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Murano, H.; Hamasaki, N.; Fukushima, K.; Takada, K. Incidence of low bioavailability of leuprolide acetate after percutaneous administration to rats by dissolving microneedles. Int. J. Pharm. 2011, 407, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Bal, S.M.; Ding, Z.; van Riet, E.; Jiskoot, W.; Bouwstra, J.A. Advances in transcutaneous vaccine delivery: Do all ways lead to Rome? J. Control. Release 2010, 148, 266–282. [Google Scholar] [CrossRef] [PubMed]
- Bubiuk, S.; Baca-Estrada, M.; Babiuk, L.; Ewen, C.; Foldvari, M. Cutaneous vaccination: The skin as an immunologically active tissue and the challenge of antigen delivery. J. Control. Release 2000, 67, 415. [Google Scholar] [CrossRef]
- Gill, H.S.; Prausnitz, M.R. Coating formulations for microneedles. Pharm. Res. 2007, 24, 1369–1380. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Gill, H.S. Coating Solid Dispersions on Microneedles via a Molten Dip-Coating Method: Development and in Vitro Evaluation for Transdermal Delivery of a Water-Insoluble Drug. J. Pharm. Sci. 2014, 103, 3621–3630. [Google Scholar] [CrossRef] [PubMed]
- Ameri, M.; Kadkhodayan, M.; Nguyen, J.; Bravo, J.A.; Su, R.; Chan, K.; Samiee, A.; Daddona, P.E. Human Growth Hormone Delivery with a Microneedle Transdermal System: Preclinical Formulation, Stability, Delivery and PK of Therapeutically Relevant Doses. Pharmaceutics 2014, 6, 220–234. [Google Scholar] [CrossRef] [PubMed]
- Beebe, D.; Mensing, G.; Walker, G. Physics and applications of microfluidics in biology. Annu. Rev. Biomed. Eng. 2002, 4, 261–286. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Mehta, P.; Msallam, H.; Armitage, D.; Ahmad, Z. Smart microneedle coatings for controlled delivery and biomedical analysis. J. Drug Target. 2014, 22, 790–795. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Fernando, G.J.P.; Crichton, M.L.; Flaim, C.; Yukiko, S.R.; Fairmaid, E.J.; Corbett, H.J.; Primiero, C.A.; Ansaldo, A.B.; Frazer, I.H.; et al. Improving the reach of vaccines to low-resource regions, with a needle-free vaccine delivery device and long-term thermostabilization. J. Control. Release 2011, 152, 349–355. [Google Scholar] [CrossRef] [PubMed]
- McGrath, M.G.; Vrdoljak, A.; O’Mahony, C.; Oliveira, J.C.; Moore, A.C.; Crean, A.M. Determination of parameters for successful spray coating of silicon microneedle arrays. Int. J. Pharm. 2011, 415, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Vrdoljak, A.; McGrath, M.G.; Carey, J.B.; Draper, S.J.; Hill, A.V.S.; O’Mahony, C.; Crean, A.M.; Moore, A.C. Coated microneedle arrays for transcutaneous delivery of live virus vaccines. J. Control. Release 2012, 159, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Haj-Ahmad, R.; Rasekh, M.; Nazari, K.; Li, Y.; Fu, Y.; Li, B.; Zhang, Q.; Xia, Z.; Liu, H.; Gu, T.; et al. EHDA Spraying: A Multi-Material Nano-Engineering Route. Curr. Pharm. Des. 2015, 21, 3239–3247. [Google Scholar] [CrossRef] [PubMed]
- Bakhshi, R.; Ahmad, Z.; Soric, M.; Stride, E.; Edirisinghe, M. Nanoparticle Delivery Systems Formed Using Electrically Sprayed Co-Flowing Excipients and Active Agent. J. Biomed. Nanotechnol. 2011, 7, 782–793. [Google Scholar] [CrossRef] [PubMed]
- Bakhshi, P.K.; Nangrejo, M.R.; Stride, E.; Edirisinghe, M. Application of Electrohydrodynamic Technology for Folic Acid Encapsulation. Food Bioprocess Technol. 2013, 6, 1837–1846. [Google Scholar] [CrossRef]
- Halimi, S.U.; Abu Bakar, N.F.; Ismail, S.N.; Hashib, S.A.; Naim, M.N. Electrospray Deposition of Titanium Dioxide (TiO2) Nanoparticles. AIP Conf. Proc. 2014, 1586, 57–62. [Google Scholar]
- Lee, Y.; Wu, B.; Zhuang, W.; Chen, D.; Tang, Y.J. Nanoparticles facilitate gene delivery to microorganisms via an electrospray process. J. Microbiol. Methods 2011, 84, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Ekemen, Z.; Ahmad, Z.; Stride, E.; Kaplan, D.; Edirisinghe, M. Electrohydrodynamic Bubbling: An Alternative Route to Fabricate Porous Structures of Silk Fibroin Based Materials. Biomacromolecules 2013, 14, 1412–1422. [Google Scholar] [CrossRef] [PubMed]
- Saraf, A.; Baggett, L.S.; Raphael, R.M.; Kasper, F.K.; Mikos, A.G. Regulated non-viral gene delivery from coaxial electrospun fiber mesh scaffolds. J. Control. Release 2010, 143, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Ryan, C.N.; Smith, K.L.; Stark, J.P.W. The influence of geometry on the flow rate sensitivity to applied voltage within cone-jet mode electrospray. J. Appl. Phys. 2012, 112, 114510. [Google Scholar] [CrossRef]
- Moghadam, H.; Samimi, M.; Samimi, A.; Khorram, M. Study of Parameters Affecting Size Distribution of Beads Produced from Electro-Spray of High Viscous Liquids. Iran. J. Chem. Eng. 2009, 6, 88–98. [Google Scholar]
- Ahmad, Z.; Zhang, H.B.; Farook, U.; Edirisinghe, M.; Stride, E.; Colombo, P. Generation of multilayered structures for biomedical applications using a novel tri-needle coaxial device and electrohydrodynamic flow. J. R. Soc. Interface 2008, 5, 1255–1261. [Google Scholar] [CrossRef] [PubMed]
- Weber, C. Zum Zerfall eines Flussigkeitsstrahles. ZAMM J. Appl. Math. Mech. 1931, 11, 136–154. (In German) [Google Scholar] [CrossRef]
- Derby, B. Inkjet Printing of Functional and Structural Materials: Fluid Property Requirements, Feature Stability, and Resolution. Ann. Rev. Mater. Res. 2010, 40, 395–414. [Google Scholar] [CrossRef]
- Boehm, R.D.; Miller, P.R.; Daniels, J.; Stafslien, S.; Narayan, R.J. Inkjet printing for pharmaceutical applications. Mater. Today 2014, 17, 247–252. [Google Scholar] [CrossRef]
- Uddin, M.J.; Scoutaris, N.; Klepetsanis, P.; Chowdhry, B.; Prausnitz, M.R.; Douroumis, D. Inkjet printing of transdermal microneedles for the delivery of anticancer agents. Int. J. Pharm. 2015, 494, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Boehm, R.D.; Daniels, J.; Stafslien, S.; Nasir, A.; Lefebvre, J.; Narayan, R.J. Polyglycolic acid microneedles modified with inkjet-deposited antifungal coatings. Biointerphases 2015, 10, 011004. [Google Scholar] [CrossRef] [PubMed]
- Boehm, R.D.; Miller, P.R.; Hayes, S.L.; Monteiro-Riviere, N.A.; Narayan, R.J. Modification of microneedles using inkjet printing. AIP Adv. 2011, 1, 022139. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haj-Ahmad, R.; Khan, H.; Arshad, M.S.; Rasekh, M.; Hussain, A.; Walsh, S.; Li, X.; Chang, M.-W.; Ahmad, Z. Microneedle Coating Techniques for Transdermal Drug Delivery. Pharmaceutics 2015, 7, 486-502. https://doi.org/10.3390/pharmaceutics7040486
Haj-Ahmad R, Khan H, Arshad MS, Rasekh M, Hussain A, Walsh S, Li X, Chang M-W, Ahmad Z. Microneedle Coating Techniques for Transdermal Drug Delivery. Pharmaceutics. 2015; 7(4):486-502. https://doi.org/10.3390/pharmaceutics7040486
Chicago/Turabian StyleHaj-Ahmad, Rita, Hashim Khan, Muhammad Sohail Arshad, Manoochehr Rasekh, Amjad Hussain, Susannah Walsh, Xiang Li, Ming-Wei Chang, and Zeeshan Ahmad. 2015. "Microneedle Coating Techniques for Transdermal Drug Delivery" Pharmaceutics 7, no. 4: 486-502. https://doi.org/10.3390/pharmaceutics7040486
APA StyleHaj-Ahmad, R., Khan, H., Arshad, M. S., Rasekh, M., Hussain, A., Walsh, S., Li, X., Chang, M.-W., & Ahmad, Z. (2015). Microneedle Coating Techniques for Transdermal Drug Delivery. Pharmaceutics, 7(4), 486-502. https://doi.org/10.3390/pharmaceutics7040486