Application of Pharmacokinetic and Pharmacodynamic Analysis to the Development of Liposomal Formulations for Oncology
Abstract
:1. Introduction
| Approved liposomal anticancer chemotherapeutics | |||
|---|---|---|---|
| Liposomal anticancer drug | Brand name | Indications | References |
| Pegylated liposomal DXR | Doxil® | AIDS-related Kaposi’s sarcoma | [28,29,30] |
| Metastatic ovarian cancer | |||
| Metastatic breast cancer | [31,32,33,34] | ||
| Multiple myeloma | [35,36,37,38] | ||
| Non-pegylated liposomal DXR | Myocet® | Same indications as Doxil® | [39,40,41] |
| Liposomal daunorubicin | DaunoXome® | AIDS-related Kaposi’s sarcoma | [42] |
| Liposomal cytarabine | Acute myeloid leukemia | [43] | |
| DepoCyte® | Lymphomas and leukemia with meningeal spread | [44] | |
| Liposomal anticancer drugs in development | |||
| Drug name | Encapsulated drug | Stage of development | References |
| Liposomal annamycin | Annamycin | Phase II | [45,46] |
| SPI-77 | Cisplatin | Phase II | [16,47,48,49] |
| Lipoplatin | Cisplatin | Phase III | [50] |
| LiPlaCis | Cisplatin | Phase I | [51] |
| l-NDDP/aroplatin | Cisplatin analogue | Phase II | [17,52] |
| ThermoDox® | Doxorubicin | Phase II | [53] |
| JNS002 | Doxorubicin | Phase II | [54] |
| TLI | Topotecan | Trial | www.clinicaltrials.gov |
| OSI211 | Lurtotecan | Phase III | [52,55] |
| LEM | Mitoxantrone | Preclinical | [56] |
| NL CPT-11 | Camptothecin | Trial | www.clinicaltrials.gov |
| L9NC | 9-Nitro-20-( S)-camptothecin | Trial | www.clinicaltrials.gov |
| PNU-93914 | Paclitaxel | Trial | www.clinicaltrials.gov |
| LEP-ETU | Paclitaxel | Trial | www.clinicaltrials.gov |
| IHL-305 | Irinotecan | Phase I | [57] |
| PEP02 | Irinotecan | Phase I | [58] |
| MBP426 | Oxaliplatin | Phase I | [59] |
| LE-SN38 | Active metabolite of Irinotecan | Trial | www.clinicaltrials.gov |
| Marqibo® | Vinscristine | Phase II | [60] |
| VLI | Vinorelbine | Trial | www.clinicaltrials.gov |
| CPX-1 | Combination: Irinotecan + Floxuridine | Phase I | [61] |
| CPX-351 | Combination: Cytarabine + Daunorubicin | Phase I | [62] |
2. Requisite Drug and Carrier Properties
3. Integration of in Vivo Factors Influencing Pharmacokinetic (PK) and Performance of Liposomal Formulations
4. Physicochemical Properties of Liposomal Formulations and Their Effects upon Pharmacokinetics
4.1. Particle Size
4.2. Membrane Charge
4.3. Membrane Lipid Composition and Surface Properties
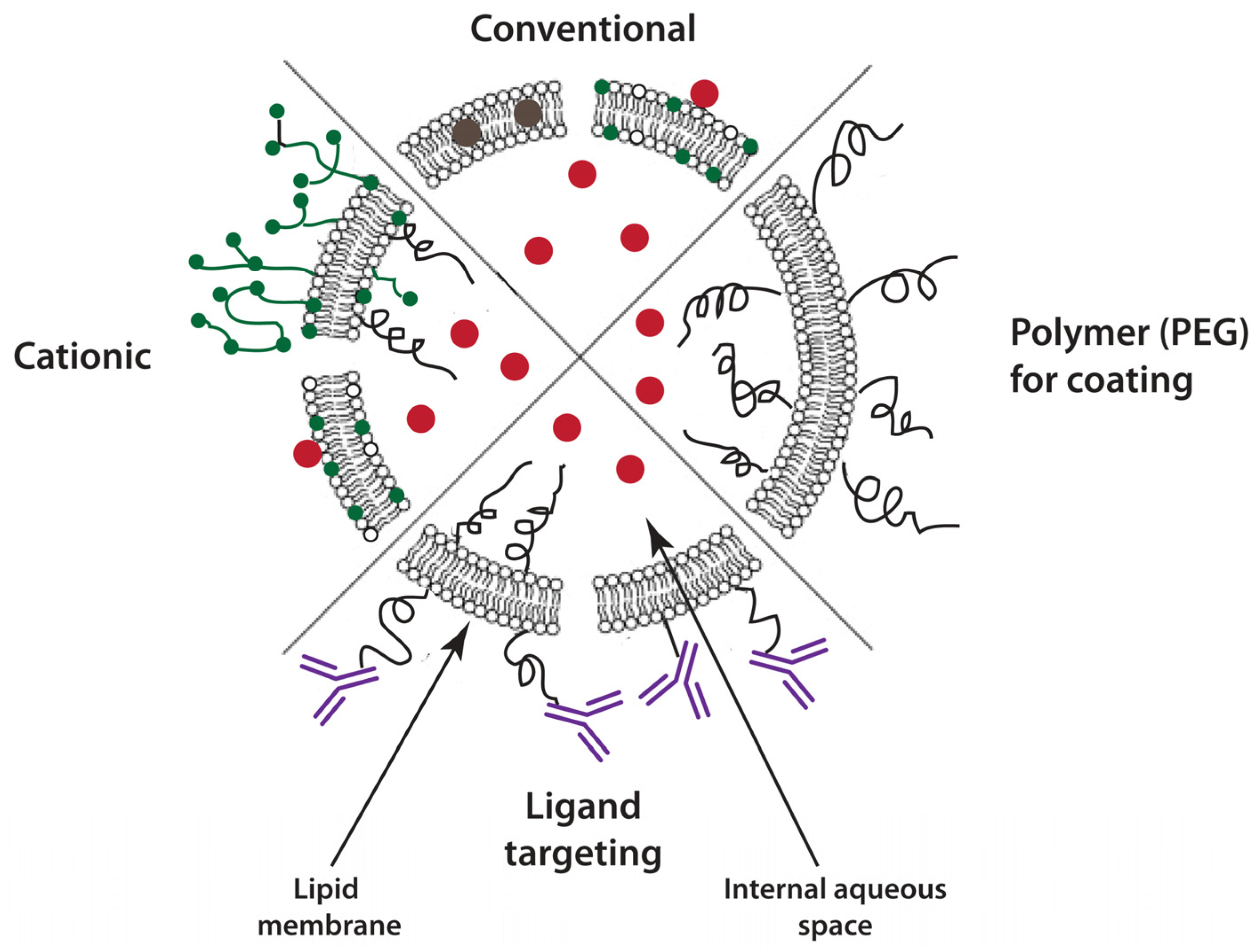
4.4. Operational Categorization of Liposomes
4.4.1. Conventional Liposomes
4.4.2. Sterically-Stabilized Liposomes (SSL)
4.4.3. Immunoliposomes
4.4.4. Cationic Liposomes
5. PK/ Pharmacodynamic (PD) Analysis of Liposomal Formulations
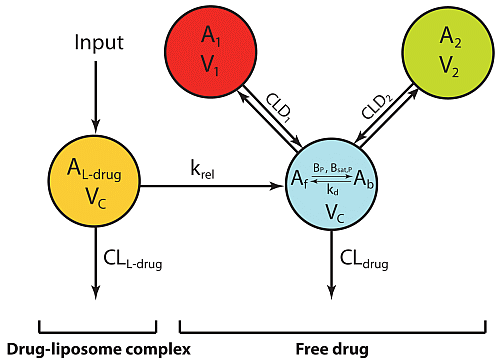
5.1. Mathematical Modeling of Liposomal Anticancer Drugs

5.2. Effect of Drug Release Rate on Plasma PK
5.3. Effect of Drug Release Rate on Tumor PK
5.4. Effect of Drug Release Rate on Antitumor Efficacy
5.5. Influence of Liposomal Drug Deposition on Antitumor Efficacy
5.6. Interrelationships of Release Rates, Deposition, and Efficacy
5.7. Analysis of Tumor Priming that Promotes Liposome Deposition
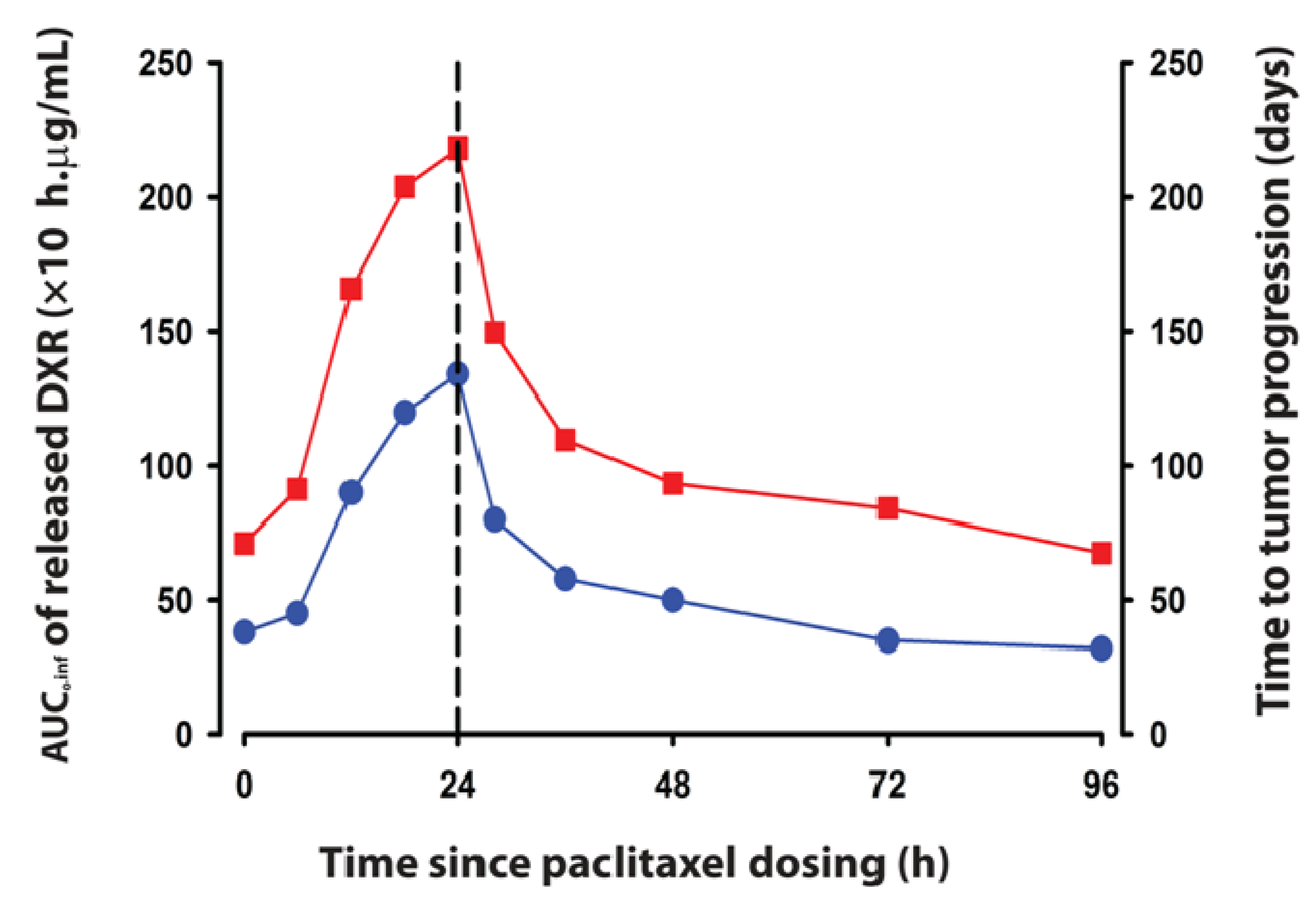
6. Translation of PK System Parameters from Animal Models to Humans
| Parameter (unit) | Definition | 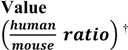 | References |
|---|---|---|---|
| Mouse DXR | |||
| k12_DXR (1/min) | Rate constant of DXR transport from central to peripheral compartment | 0.74 (0.1) | [158] |
| k21_DXR (1/min) | Rate constant of DXR transport from peripheral to central compartment | 5.5 × 10−3 (0.2) | [158] |
| kel_DXR (1/min) | Rate constant of DXR elimination from central compartment | 0.36 (0.2) | [158] |
| tvf_in_DXR (cm/min) | Transvascular flux per surface area for DXR from capillary to interstitial space | 2.96 × 10−4 (12.3) | [158] |
| tvf_out_DXR (cm/min) | Transvascular flux per surface area for DXR from interstitial to capillary space | 1.18 × 10−3 (7.2) | [158] |
| Mouse Liposome | |||
| kel_lipo (1/min) | Rate constant of liposome elimination from central compartment | 1.14 × 10−3 (0.1) | [158] |
| tvf_in_lipo (cm/min) | Transvascular flux per surface area for liposome from capillary to interstitial space | 2.64 × 10−6 (1.0) | [158] |
| tvf_out_lipo (cm/min) | Transvascular flux per surface area for liposome from interstitial to capillary space | 7.14 × 10−6 (1.0) | [158] |
| Human DXR | |||
| k12_DXR (1/min) | Rate constant of DXR transport from central to peripheral compartment | 4.75 × 10−2 (0.1) | [158] |
| k21_DXR (1/min) | Rate constant of DXR transport from peripheral to central compartment | 1.25 × 10−3 (0.2) | [158] |
| kel_DXR (1/min) | Rate constant of DXR elimination from central compartment | 8.2 × 10−2 (0.2) | [158] |
| tvf_in_DXR (cm/min) | Rate constant of DXR elimination from central compartment | 3.63 × 10−3 (12.3) | [186,187,188] |
| tvf_out_DXR (cm/min) | Rate constant of liposome elimination from central compartment | 8.45 × 10−3 (7.2) | [158] |
| Human Liposome | |||
| kel_lipo (1/min) | Rate constant of liposome elimination from central compartment | 1.67 × 10−4 (0.1) | [47] |
| tvf_in_lipo (cm/min) | Transvascular flux per surface area for liposome from capillary to interstitial space | 2.64 × 10−6 (1.0) | [158] |
| tvf_out_lipo (cm/min) | Transvascular flux per surface area for liposome from interstitial to capillary space | 7.14 × 10−6 (1.0) | [158] |
| Tumor | |||
| Qtumor (L/min/kg) | Blood flow into tumor | 2.82 × 10−2 (0.1) | [176,190] |
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Torchilin, V.P. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 2005, 4, 145–160. [Google Scholar] [CrossRef]
- Slingerland, M.; Guchelaar, H.J.; Gelderblom, H. Liposomal drug formulations in cancer therapy: 15 years along the road. Drug Discov. Today 2012, 17, 160–166. [Google Scholar] [CrossRef]
- Drummond, D.C.; Meyer, O.; Hong, K.; Kirpotin, D.B.; Papahadjopoulos, D. Optimizing liposomes for delivery of chemotherapeutic agents to solid tumors. Pharmacol. Rev. 1999, 51, 691–743. [Google Scholar]
- Storm, G.; ten Kate, M.T.; Working, P.K.; Bakker-Woudenberg, I.A. Doxorubicin entrapped in sterically stabilized liposomes: Effects on bacterial blood clearance capacity of the mononuclear phagocyte system. Clin. Cancer Res. 1998, 4, 111–115. [Google Scholar]
- Gregoriadis, G. Overview of liposomes. J. Antimicrob. Chemother. 1991, 28, 39–48. [Google Scholar] [CrossRef]
- Drummond, D.C.; Noble, C.O.; Hayes, M.E.; Park, J.W.; Kirpotin, D.B. Pharmacokinetics and in vivo drug release rates in liposomal nanocarrier development. J. Pharm. Sci. 2008, 97, 4696–4740. [Google Scholar] [CrossRef]
- Oude Blenke, E.; Mastrobattista, E.; Schiffelers, R.M. Strategies for triggered drug release from tumor targeted liposomes. Expert Opin. Drug Deliv. 2013, 10, 1399–1410. [Google Scholar] [CrossRef]
- Li, L.; ten Hagen, T.L.; Bolkestein, M.; Gasselhuber, A.; Yatvin, J.; van Rhoon, G.C.; Eggermont, A.M.; Haemmerich, D.; Koning, G.A. Improved intratumoral nanoparticle extravasation and penetration by mild hyperthermia. J. Control. Release 2013, 167, 130–137. [Google Scholar] [CrossRef]
- Li, L.; ten Hagen, T.L.; Hossann, M.; Suss, R.; van Rhoon, G.C.; Eggermont, A.M.; Haemmerich, D.; Koning, G.A. Mild hyperthermia triggered doxorubicin release from optimized stealth thermosensitive liposomes improves intratumoral drug delivery and efficacy. J. Control. Release 2013, 168, 142–150. [Google Scholar] [CrossRef]
- Chang, H.I.; Yeh, M.K. Clinical development of liposome-based drugs: Formulation, characterization, and therapeutic efficacy. Int. J. Nanomedicine 2012, 7, 49–60. [Google Scholar]
- Guo, W.; Johnson, J.L.; Khan, S.; Ahmad, A.; Ahmad, I. Paclitaxel quantification in mouse plasma and tissues containing liposome-entrapped paclitaxel by liquid chromatography-tandem mass spectrometry: Application to a pharmacokinetics study. Anal. Biochem. 2005, 336, 213–220. [Google Scholar] [CrossRef]
- Sharma, A.; Mayhew, E.; Bolcsak, L.; Cavanaugh, C.; Harmon, P.; Janoff, A.; Bernacki, R.J. Activity of paclitaxel liposome formulations against human ovarian tumor xenografts. Int. J. Cancer 1997, 71, 103–107. [Google Scholar] [CrossRef]
- Sharma, A.; Mayhew, E.; Straubinger, R.M. Antitumor effect of taxol-containing liposomes in a taxol-resistant murine tumor model. Cancer Res. 1993, 53, 5877–5881. [Google Scholar]
- Lim, H.J.; Masin, D.; Madden, T.D.; Bally, M.B. Influence of drug release characteristics on the therapeutic activity of liposomal mitoxantrone. J. Pharmacol. Exp Ther. 1997, 281, 566–573. [Google Scholar]
- Forssen, E.A.; Male-Brune, R.; Adler-Moore, J.P.; Lee, M.J.; Schmidt, P.G.; Krasieva, T.B.; Shimizu, S.; Tromberg, B.J. Fluorescence imaging studies for the disposition of daunorubicin liposomes (DaunoXome) within tumor tissue. Cancer Res. 1996, 56, 2066–2075. [Google Scholar]
- Kim, E.S.; Lu, C.; Khuri, F.R.; Tonda, M.; Glisson, B.S.; Liu, D.; Jung, M.; Hong, W.K.; Herbst, R.S. A phase II study of STEALTH cisplatin (SPI-77) in patients with advanced non-small cell lung cancer. Lung Cancer 2001, 34, 427–432. [Google Scholar] [CrossRef]
- Lu, C.; Perez-Soler, R.; Piperdi, B.; Walsh, G.L.; Swisher, S.G.; Smythe, W.R.; Shin, H.J.; Ro, J.Y.; Feng, L.; Truong, M.; et al. Phase II study of a liposome-entrapped cisplatin analog (L-NDDP) administered intrapleurally and pathologic response rates in patients with malignant pleural mesothelioma. J. Clin. Oncol. 2005, 23, 3495–3501. [Google Scholar] [CrossRef]
- Tardi, P.; Choice, E.; Masin, D.; Redelmeier, T.; Bally, M.; Madden, T.D. Liposomal encapsulation of topotecan enhances anticancer efficacy in murine and human xenograft models. Cancer Res. 2000, 60, 3389–3393. [Google Scholar]
- Pal, A.; Khan, S.; Wang, Y.F.; Kamath, N.; Sarkar, A.K.; Ahmad, A.; Sheikh, S.; Ali, S.; Carbonaro, D.; Zhang, A.; et al. Preclinical safety, pharmacokinetics and antitumor efficacy profile of liposome-entrapped SN-38 formulation. Anticancer Res. 2005, 25, 331–341. [Google Scholar]
- Emerson, D.L.; Bendele, R.; Brown, E.; Chiang, S.; Desjardins, J.P.; Dihel, L.C.; Gill, S.C.; Hamilton, M.; LeRay, J.D.; Moon-McDermott, L.; et al. Antitumor efficacy, pharmacokinetics, and biodistribution of NX 211: A low-clearance liposomal formulation of lurtotecan. Clin. Cancer Res. 2000, 6, 2903–2912. [Google Scholar]
- Drummond, D.C.; Noble, C.O.; Guo, Z.; Hong, K.; Park, J.W.; Kirpotin, D.B. Development of a highly active nanoliposomal irinotecan using a novel intraliposomal stabilization strategy. Cancer Res. 2006, 66, 3271–3277. [Google Scholar] [CrossRef]
- Krishna, R.; Webb, M.S.; St Onge, G.; Mayer, L.D. Liposomal and nonliposomal drug pharmacokinetics after administration of liposome-encapsulated vincristine and their contribution to drug tissue distribution properties. J. Pharmacol. Exp. Ther. 2001, 298, 1206–1212. [Google Scholar]
- Zhigaltsev, I.V.; Maurer, N.; Akhong, Q.F.; Leone, R.; Leng, E.; Wang, J.; Semple, S.C.; Cullis, P.R. Liposome-encapsulated vincristine, vinblastine and vinorelbine: A comparative study of drug loading and retention. J. Control. Release 2005, 104, 103–111. [Google Scholar] [CrossRef]
- Cosco, D.; Paolino, D.; Muzzalupo, R.; Celia, C.; Citraro, R.; Caponio, D.; Picci, N.; Fresta, M. Novel PEG-coated niosomes based on bola-surfactant as drug carriers for 5-fluorouracil. Biomed. Microdevices 2009, 11, 1115–1125. [Google Scholar] [CrossRef]
- Storm, G.; Crommelin, D.J.A. Liposomes: Quo vadis? Pharm. Sci. Technol. Today 1998, 1, 19–31. [Google Scholar] [CrossRef]
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar]
- Celia, C.; Cosco, D.; Paolino, D.; Fresta, M. Gemcitabine-loaded innovative nanocarriers vs. GEMZAR: Biodistribution, pharmacokinetic features and in vivo antitumor activity. Expert Opin. Drug Deliv. 2011, 8, 1609–1629. [Google Scholar] [CrossRef]
- Stewart, S.; Jablonowski, H.; Goebel, F.D.; Arasteh, K.; Spittle, M.; Rios, A.; Aboulafia, D.; Galleshaw, J.; Dezube, B.J. Randomized comparative trial of pegylated liposomal doxorubicin versus bleomycin and vincristine in the treatment of AIDS-related Kaposi’s sarcoma. J. Clin. Oncol. 1998, 16, 683–691. [Google Scholar]
- Northfelt, D.W.; Dezube, B.J.; Thommes, J.A.; Miller, B.J.; Fischl, M.A.; Friedman-Kien, A.; Kaplan, L.D.; Du Mond, C.; Mamelok, R.D.; Henry, D.H. Pegylated-liposomal doxorubicin versus doxorubicin, bleomycin, and vincristine in the treatment of AIDS-related Kaposi’s sarcoma: results of a randomized phase III clinical trial. J. Clin. Oncol. 1998, 16, 2445–2451. [Google Scholar]
- Cianfrocca, M.; Lee, S.; von Roenn, J.; Tulpule, A.; Dezube, B.J.; Aboulafia, D.M.; Ambinder, R.F.; Lee, J.Y.; Krown, S.E.; Sparano, J.A. Randomized trial of paclitaxel versus pegylated liposomal doxorubicin for advanced human immunodeficiency virus-associated Kaposi sarcoma: Evidence of symptom palliation from chemotherapy. Cancer 2010, 116, 3969–3977. [Google Scholar] [CrossRef]
- Gordon, A.N.; Fleagle, J.T.; Guthrie, D.; Parkin, D.E.; Gore, M.E.; Lacave, A.J. Recurrent epithelial ovarian carcinoma: A randomized phase III study of pegylated liposomal doxorubicin versus topotecan. J. Clin. Oncol. 2001, 19, 3312–3322. [Google Scholar]
- Pignata, S.; Scambia, G.; Savarese, A.; Breda, E.; Sorio, R.; Pisano, C.; Lorusso, D.; Cognetti, F.; Vernaglia Lombardi, A.; Gebbia, V.; et al. Carboplatin and pegylated liposomal doxorubicin for advanced ovarian cancer: Preliminary activity results of the MITO-2 phase III trial. Oncology 2009, 76, 49–54. [Google Scholar] [CrossRef]
- Markman, M.; Moon, J.; Wilczynski, S.; Lopez, A.M.; Rowland, K.M., Jr.; Michelin, D.P.; Lanzotti, V.J.; Anderson, G.L.; Alberts, D.S. Single agent carboplatin versus carboplatin plus pegylated liposomal doxorubicin in recurrent ovarian cancer: Final survival results of a SWOG (S0200) phase 3 randomized trial. Gynecol. Oncol. 2010, 116, 323–325. [Google Scholar] [CrossRef]
- Pujade-Lauraine, E.; Wagner, U.; Aavall-Lundqvist, E.; Gebski, V.; Heywood, M.; Vasey, P.A.; Volgger, B.; Vergote, I.; Pignata, S.; Ferrero, A.; et al. Pegylated liposomal Doxorubicin and Carboplatin compared with Paclitaxel and Carboplatin for patients with platinum-sensitive ovarian cancer in late relapse. J. Clin. Oncol. 2010, 28, 3323–3329. [Google Scholar] [CrossRef]
- Keller, A.M.; Mennel, R.G.; Georgoulias, V.A.; Nabholtz, J.M.; Erazo, A.; Lluch, A.; Vogel, C.L.; Kaufmann, M.; von Minckwitz, G.; Henderson, I.C.; et al. Randomized phase III trial of pegylated liposomal doxorubicin versus vinorelbine or mitomycin C plus vinblastine in women with taxane-refractory advanced breast cancer. J. Clin. Oncol. 2004, 22, 3893–3901. [Google Scholar] [CrossRef]
- Chan, S.; Davidson, N.; Juozaityte, E.; Erdkamp, F.; Pluzanska, A.; Azarnia, N.; Lee, L.W. Phase III trial of liposomal doxorubicin and cyclophosphamide compared with epirubicin and cyclophosphamide as first-line therapy for metastatic breast cancer. Ann. Oncol. 2004, 15, 1527–1534. [Google Scholar]
- Sparano, J.A.; Makhson, A.N.; Semiglazov, V.F.; Tjulandin, S.A.; Balashova, O.I.; Bondarenko, I.N.; Bogdanova, N.V.; Manikhas, G.M.; Oliynychenko, G.P.; Chatikhine, V.A.; et al. Pegylated liposomal doxorubicin plus docetaxel significantly improves time to progression without additive cardiotoxicity compared with docetaxel monotherapy in patients with advanced breast cancer previously treated with neoadjuvant-adjuvant anthracycline therapy: Results from a randomized phase III study. J. Clin. Oncol. 2009, 27, 4522–4529. [Google Scholar] [CrossRef]
- Alba, E.; Ruiz-Borrego, M.; Margeli, M.; Rodriguez-Lescure, A.; Sanchez-Rovira, P.; Ruiz, A.; Mel-Lorenzo, J.R.; Ramos-Vazquez, M.; Ribelles, N.; Calvo, E.; et al. Maintenance treatment with pegylated liposomal doxorubicin versus observation following induction chemotherapy for metastatic breast cancer: GEICAM 2001-01 study. Breast Cancer Res. Treat. 2010, 122, 169–176. [Google Scholar] [CrossRef]
- Rifkin, R.M.; Gregory, S.A.; Mohrbacher, A.; Hussein, M.A. Pegylated liposomal doxorubicin, vincristine, and dexamethasone provide significant reduction in toxicity compared with doxorubicin, vincristine, and dexamethasone in patients with newly diagnosed multiple myeloma: A Phase III multicenter randomized trail. Cancer 2006, 106, 848–858. [Google Scholar] [CrossRef]
- Orlowski, R.Z.; Nagler, A.; Sonneveld, P.; Blade, J.; Hajek, R.; Spencer, A.; San Miguel, J.; Robak, T.; Dmoszynska, A.; Horvath, N.; et al. Randomized phase III study of pegylated liposomal doxorubicin plus bortezomib compared with bortezomib alone in relapsed or refractory multiple myeloma: Combination therapy improves time to progression. J. Clin. Oncol. 2007, 25, 3892–3901. [Google Scholar] [CrossRef]
- Sonneveld, P.; Hajek, R.; Nagler, A.; Spencer, A.; Blade, J.; Robak, T.; Zhuang, S.H.; Harousseau, J.L.; Orlowski, R.Z. Combined pegylated liposomal doxorubicin and bortezomib is highly effective in patients with recurrent or refractory multiple myeloma who received prior thalidomide/lenalidomide therapy. Cancer 2008, 112, 1529–1537. [Google Scholar] [CrossRef]
- Gill, P.S.; Wernz, J.; Scadden, D.T.; Cohen, P.; Mukwaya, G.M.; von Roenn, J.H.; Jacobs, M.; Kempin, S.; Silverberg, I.; Gonzales, G.; et al. Randomized phase III trial of liposomal daunorubicin versus doxorubicin, bleomycin, and vincristine in AIDS-related Kaposi’s sarcoma. J. Clin. Oncol. 1996, 14, 2353–2364. [Google Scholar]
- Latagliata, R.; Breccia, M.; Fazi, P.; Iacobelli, S.; Martinelli, G.; di Raimondo, F.; Sborgia, M.; Fabbiano, F.; Pirrotta, M.T.; Zaccaria, A.; et al. Liposomal daunorubicin versus standard daunorubicin: Long term follow-up of the GIMEMA GSI 103 AMLE randomized trial in patients older than 60 years with acute myelogenous leukaemia. Br. J. Haematol. 2008, 143, 681–689. [Google Scholar] [CrossRef]
- Glantz, M.J.; LaFollette, S.; Jaeckle, K.A.; Shapiro, W.; Swinnen, L.; Rozental, J.R.; Phuphanich, S.; Rogers, L.R.; Gutheil, J.C.; Batchelor, T.; et al. Randomized trial of a slow-release versus a standard formulation of cytarabine for the intrathecal treatment of lymphomatous meningitis. J. Clin. Oncol. 1999, 17, 3110–3116. [Google Scholar]
- Booser, D.J.; Esteva, F.J.; Rivera, E.; Valero, V.; Esparza-Guerra, L.; Priebe, W.; Hortobagyi, G.N. Phase II study of liposomal annamycin in the treatment of doxorubicin-resistant breast cancer. Cancer Chemother. Pharmacol. 2002, 50, 6–8. [Google Scholar] [CrossRef]
- Wetzler, M.; Thomas, D.A.; Wang, E.S.; Shepard, R.; Ford, L.A.; Heffner, T.L.; Parekh, S.; Andreeff, M.; O’Brien, S.; Kantarjian, H.M. Phase I/II trial of nanomolecular liposomal annamycin in adult patients with relapsed/refractory acute lymphoblastic leukemia. Clin. Lymphoma Myeloma Leuk. 2013, 13, 430–434. [Google Scholar] [CrossRef]
- Harrington, K.J.; Lewanski, C.R.; Northcote, A.D.; Whittaker, J.; Wellbank, H.; Vile, R.G.; Peters, A.M.; Stewart, J.S. Phase I–II study of pegylated liposomal cisplatin (SPI-077) in patients with inoperable head and neck cancer. Ann. Oncol. 2001, 12, 493–496. [Google Scholar] [CrossRef]
- Seetharamu, N.; Kim, E.; Hochster, H.; Martin, F.; Muggia, F. Phase II study of liposomal cisplatin (SPI-77) in platinum-sensitive recurrences of ovarian cancer. Anticancer Res. 2010, 30, 541–545. [Google Scholar]
- White, S.C.; Lorigan, P.; Margison, G.P.; Margison, J.M.; Martin, F.; Thatcher, N.; Anderson, H.; Ranson, M. Phase II study of SPI-77 (sterically stabilised liposomal cisplatin) in advanced non-small-cell lung cancer. Br. J. Cancer 2006, 95, 822–828. [Google Scholar] [CrossRef]
- Stathopoulos, G.P.; Antoniou, D.; Dimitroulis, J.; Stathopoulos, J.; Marosis, K.; Michalopoulou, P. Comparison of liposomal cisplatin versus cisplatin in non-squamous cell non-small-cell lung cancer. Cancer Chemother. Pharmacol. 2011, 68, 945–950. [Google Scholar] [CrossRef]
- De Jonge, M.J.; Slingerland, M.; Loos, W.J.; Wiemer, E.A.; Burger, H.; Mathijssen, R.H.; Kroep, J.R.; den Hollander, M.A.; van der Biessen, D.; Lam, M.H.; et al. Early cessation of the clinical development of LiPlaCis, a liposomal cisplatin formulation. Eur. J. Cancer 2010, 46, 3016–3021. [Google Scholar] [CrossRef]
- Dragovich, T.; Mendelson, D.; Kurtin, S.; Richardson, K.; von Hoff, D.; Hoos, A. A Phase II trial of the liposomal DACH platinum L-NDDP in patients with therapy-refractory advanced colorectal cancer. Cancer Chemother. Pharmacol. 2006, 58, 759–764. [Google Scholar] [CrossRef]
- Celsion coporation. Available online: http://celsion.com/docs/technology_thermodox (accessed on 10 March 2014).
- Katsumata, N.; Fujiwara, Y.; Kamura, T.; Nakanishi, T.; Hatae, M.; Aoki, D.; Tanaka, K.; Tsuda, H.; Kamiura, S.; Takehara, K.; et al. Phase II clinical trial of pegylated liposomal doxorubicin (JNS002) in Japanese patients with mullerian carcinoma (epithelial ovarian carcinoma, primary carcinoma of fallopian tube, peritoneal carcinoma) having a therapeutic history of platinum-based chemotherapy: A Phase II study of the Japanese Gynecologic Oncology Group. Jpn. J. Clin. Oncol. 2008, 38, 777–785. [Google Scholar] [CrossRef]
- Duffaud, F.; Borner, M.; Chollet, P.; Vermorken, J.B.; Bloch, J.; Degardin, M.; Rolland, F.; Dittrich, C.; Baron, B.; Lacombe, D.; et al. Phase II study of OSI-211 (liposomal lurtotecan) in patients with metastatic or loco-regional recurrent squamous cell carcinoma of the head and neck. An EORTC new drug development group study. Eur. J. Cancer 2004, 40, 2748–2752. [Google Scholar]
- Gokhale, P.C.; Pei, J.; Zhang, C.; Ahmad, I.; Rahman, A.; Kasid, U. Improved safety, pharmacokinetics and therapeutic efficacy profiles of a novel liposomal formulation of mitoxantrone. Anticancer Res. 2001, 21, 3313–3321. [Google Scholar]
- Infante, J.R.; Keedy, V.L.; Jones, S.F.; Zamboni, W.C.; Chan, E.; Bendell, J.C.; Lee, W.; Wu, H.; Ikeda, S.; Kodaira, H.; et al. Phase I and pharmacokinetic study of IHL-305 (PEGylated liposomal irinotecan) in patients with advanced solid tumors. Cancer Chemother. Pharmacol. 2012, 70, 699–705. [Google Scholar] [CrossRef]
- Roy, A.C.; Park, S.R.; Cunningham, D.; Kang, Y.K.; Chao, Y.; Chen, L.T.; Rees, C.; Lim, H.Y.; Tabernero, J.; Ramos, F.J.; et al. A randomized phase II study of PEP02 (MM-398), irinotecan or docetaxel as a second-line therapy in patients with locally advanced or metastatic gastric or gastro-oesophageal junction adenocarcinoma. Ann. Oncol. 2013, 24, 1567–1573. [Google Scholar] [CrossRef]
- Senzer, N.M.; Matsuno, K.; Yamagata, N.; Fujisawa, T.; Wasserman, E.; Sutherland, W.; Sharma, S.; Phan, A. MBP-426, a novel liposome-encapsulated oxaliplatin, in combination with 5-FU/leucovorin (LV): Phase I results of a Phase I/II study in gastro-esophageal adenocarcinoma, with pharmacokinetics. Mol. Cancer Ther. 2009, 8, 12–19. [Google Scholar] [CrossRef]
- Rodriguez, M.A.; Pytlik, R.; Kozak, T.; Chhanabhai, M.; Gascoyne, R.; Lu, B.; Deitcher, S.R.; Winter, J.N. Vincristine sulfate liposomes injection (Marqibo) in heavily pretreated patients with refractory aggressive non-Hodgkin lymphoma: Report of the pivotal phase II study. Cancer 2009, 115, 3475–3482. [Google Scholar] [CrossRef]
- Batist, G.; Gelmon, K.A.; Chi, K.N.; Miller, W.H., Jr.; Chia, S.K.; Mayer, L.D.; Swenson, C.E.; Janoff, A.S.; Louie, A.C. Safety, pharmacokinetics, and efficacy of CPX-1 liposome injection in patients with advanced solid tumors. Clin. Cancer Res. 2009, 15, 692–700. [Google Scholar] [CrossRef]
- Feldman, E.J.; Lancet, J.E.; Kolitz, J.E.; Ritchie, E.K.; Roboz, G.J.; List, A.F.; Allen, S.L.; Asatiani, E.; Mayer, L.D.; Swenson, C.; et al. First-in-man study of CPX-351: A liposomal carrier containing cytarabine and daunorubicin in a fixed 5:1 molar ratio for the treatment of relapsed and refractory acute myeloid leukemia. J. Clin. Oncol. 2011, 29, 979–985. [Google Scholar] [CrossRef]
- Young, R.C.; Ozols, R.F.; Myers, C.E. The anthracycline antineoplastic drugs. N. Engl. J. Med. 1981, 305, 139–153. [Google Scholar] [CrossRef]
- Nichols, J.W.; Deamer, D.W. Catecholamine uptake and concentration by liposomes maintaining pH gradients. Biochim. Biophys. Acta 1976, 455, 269–271. [Google Scholar] [CrossRef]
- Mayer, L.D.; Bally, M.B.; Hope, M.J.; Cullis, P.R. Uptake of antineoplastic agents into large unilamellar vesicles in response to a membrane potential. Biochim. Biophys. Acta 1985, 816, 294–302. [Google Scholar] [CrossRef]
- Madden, T.D.; Harrigan, P.R.; Tai, L.C.; Bally, M.B.; Mayer, L.D.; Redelmeier, T.E.; Loughrey, H.C.; Tilcock, C.P.; Reinish, L.W.; Cullis, P.R. The accumulation of drugs within large unilamellar vesicles exhibiting a proton gradient: A survey. Chem. Phys. Lipids 1990, 53, 37–46. [Google Scholar] [CrossRef]
- Lasic, D.D.; Frederik, P.M.; Stuart, M.C.; Barenholz, Y.; McIntosh, T.J. Gelation of liposome interior. A novel method for drug encapsulation. FEBS Lett. 1992, 312, 255–258. [Google Scholar] [CrossRef]
- Haran, G.; Cohen, R.; Bar, L.K.; Barenholz, Y. Transmembrane ammonium sulfate gradients in liposomes produce efficient and stable entrapment of amphipathic weak bases. Biochim. Biophys. Acta 1993, 1151, 201–215. [Google Scholar] [CrossRef]
- Cullis, P.R.; Hope, M.J.; Bally, M.B.; Madden, T.D.; Mayer, L.D.; Fenske, D.B. Influence of pH gradients on the transbilayer transport of drugs, lipids, peptides and metal ions into large unilamellar vesicles. Biochim. Biophys. Acta 1997, 1331, 187–211. [Google Scholar] [CrossRef]
- Zucker, D.; Marcus, D.; Barenholz, Y.; Goldblum, A. Liposome drugs’ loading efficiency: A working model based on loading conditions and drug’s physicochemical properties. J. Control. Release 2009, 139, 73–80. [Google Scholar]
- Charrois, G.J.; Allen, T.M. Drug release rate influences the pharmacokinetics, biodistribution, therapeutic activity, and toxicity of pegylated liposomal doxorubicin formulations in murine breast cancer. Biochim. Biophys. Acta 2004, 1663, 167–177. [Google Scholar] [CrossRef]
- Johnston, M.J.; Edwards, K.; Karlsson, G.; Cullis, P.R. Influence of drug-to-lipid ratio on drug release properties and liposome integrity in liposomal doxorubicin formulations. J. Liposome Res. 2008, 18, 145–157. [Google Scholar] [CrossRef]
- Laginha, K.M.; Verwoert, S.; Charrois, G.J.; Allen, T.M. Determination of doxorubicin levels in whole tumor and tumor nuclei in murine breast cancer tumors. Clin. Cancer Res. 2005, 11, 6944–6949. [Google Scholar] [CrossRef]
- Lim, H.J.; Masin, D.; McIntosh, N.L.; Madden, T.D.; Bally, M.B. Role of drug release and liposome-mediated drug delivery in governing the therapeutic activity of liposomal mitoxantrone used to treat human A431 and LS180 solid tumors. J. Pharmacol. Exp. Ther. 2000, 292, 337–345. [Google Scholar]
- Ishida, T.; Harashima, H.; Kiwada, H. Liposome clearance. Biosci. Rep. 2002, 22, 197–224. [Google Scholar] [CrossRef]
- Moghimi, S.M.; Hunter, A.C.; Andresen, T.L. Factors controlling nanoparticle pharmacokinetics: An integrated analysis and perspective. Annu. Rev. Pharmacol. Toxicol. 2012, 52, 481–503. [Google Scholar] [CrossRef]
- Senior, J.H. Fate and behavior of liposomes in vivo: A review of controlling factors. Crit. Rev. Ther. Drug Carrier Syst. 1987, 3, 123–193. [Google Scholar]
- Weinstein, J.N. Liposomes as drug carriers in cancer therapy. Cancer Treat. Rep. 1984, 68, 127–135. [Google Scholar]
- Gregoriadis, G. The carrier potential of liposomes in biology and medicine (second of two parts). N. Engl. J. Med. 1976, 295, 765–770. [Google Scholar] [CrossRef]
- Gregoriadis, G. The carrier potential of liposomes in biology and medicine (first of two parts). N. Engl. J. Med. 1976, 295, 704–710. [Google Scholar] [CrossRef]
- Patel, H.M. Influence of lipid composition on opsonophagocytosis of liposomes. Res. Immunol. 1992, 143, 242–244. [Google Scholar] [CrossRef]
- Devine, D.V.; Marjan, J.M. The role of immunoproteins in the survival of liposomes in the circulation. Crit. Rev. Ther. Drug Carrier Syst. 1997, 14, 105–131. [Google Scholar]
- Ohlson, M.; Sorensson, J.; Haraldsson, B. Glomerular size and charge selectivity in the rat as revealed by FITC-ficoll and albumin. Am. J. Physiol. Renal Physiol. 2000, 279, F84–F91. [Google Scholar]
- Lund, U.; Rippe, A.; Venturoli, D.; Tenstad, O.; Grubb, A.; Rippe, B. Glomerular filtration rate dependence of sieving of albumin and some neutral proteins in rat kidneys. Am. J. Physiol. Renal Physiol. 2003, 284, F1226–F1234. [Google Scholar]
- Allen, T.M.; Cheng, W.W.; Hare, J.I.; Laginha, K.M. Pharmacokinetics and pharmacodynamics of lipidic nano-particles in cancer. Anticancer Agents Med. Chem. 2006, 6, 513–523. [Google Scholar] [CrossRef]
- Adlakha-Hutcheon, G.; Bally, M.B.; Shew, C.R.; Madden, T.D. Controlled destabilization of a liposomal drug delivery system enhances mitoxantrone antitumor activity. Nat. Biotechnol. 1999, 17, 775–779. [Google Scholar] [CrossRef]
- Hitzman, C.J.; Wiedmann, T.S.; Dai, H.; Elmquist, W.F. Measurement of drug release from microcarriers by microdialysis. J. Pharm. Sci. 2005, 94, 1456–1466. [Google Scholar] [CrossRef]
- Musteata, F.M.; Pawliszyn, J.; Qian, M.G.; Wu, J.T.; Miwa, G.T. Determination of drug plasma protein binding by solid phase microextraction. J. Pharm. Sci. 2006, 95, 1712–1722. [Google Scholar] [CrossRef]
- Webb, M.S.; Harasym, T.O.; Masin, D.; Bally, M.B.; Mayer, L.D. Sphingomyelin-cholesterol liposomes significantly enhance the pharmacokinetic and therapeutic properties of vincristine in murine and human tumour models. Br. J. Cancer 1995, 72, 896–904. [Google Scholar] [CrossRef]
- Bulitta, J.B.; Zhao, P.; Arnold, R.D.; Kessler, D.R.; Daifuku, R.; Pratt, J.; Luciano, G.; Hanauske, A.R.; Gelderblom, H.; Awada, A.; et al. Mechanistic population pharmacokinetics of total and unbound paclitaxel for a new nanodroplet formulation versus Taxol in cancer patients. Cancer Chemother. Pharmacol. 2009, 63, 1049–1063. [Google Scholar] [CrossRef]
- Kagan, L.; Gershkovich, P.; Wasan, K.M.; Mager, D.E. Physiologically based pharmacokinetic model of amphotericin B disposition in rats following administration of deoxycholate formulation (Fungizone(R)): Pooled analysis of published data. AAPS J. 2011, 13, 255–264. [Google Scholar] [CrossRef]
- Jain, R.K. Transport of molecules in the tumor interstitium: A review. Cancer Res. 1987, 47, 3039–3051. [Google Scholar]
- Jain, R.K. Delivery of novel therapeutic agents in tumors: Physiological barriers and strategies. J. Natl. Cancer Inst. 1989, 81, 570–576. [Google Scholar] [CrossRef]
- Jain, R.K. Vascular and interstitial barriers to delivery of therapeutic agents in tumors. Cancer Metastasis Rev. 1990, 9, 253–266. [Google Scholar] [CrossRef]
- Jain, R.K. Physiological barriers to delivery of monoclonal antibodies and other macromolecules in tumors. Cancer Res. 1990, 50, 814–819. [Google Scholar]
- Papahadjopoulos, D.; Allen, T.M.; Gabizon, A.; Mayhew, E.; Matthay, K.; Huang, S.K.; Lee, K.D.; Woodle, M.C.; Lasic, D.D.; Redemann, C.; et al. Sterically stabilized liposomes: Improvements in pharmacokinetics and antitumor therapeutic efficacy. Proc. Natl. Acad. Sci. USA 1991, 88, 11460–11464. [Google Scholar] [CrossRef]
- Yuan, F.; Leunig, M.; Huang, S.K.; Berk, D.A.; Papahadjopoulos, D.; Jain, R.K. Microvascular permeability and interstitial penetration of sterically stabilized (STEALTH) liposomes in a human tumor xenograft. Cancer Res. 1994, 54, 3352–3356. [Google Scholar]
- Jain, R.K. Delivery of molecular medicine to solid tumors. Science 1996, 271, 1079–1080. [Google Scholar] [CrossRef]
- Yuan, F.; Salehi, H.A.; Boucher, Y.; Vasthare, U.S.; Tuma, R.F.; Jain, R.K. Vascular permeability and microcirculation of gliomas and mammary carcinomas transplanted in rat and mouse cranial windows. Cancer Res. 1994, 54, 4564–4568. [Google Scholar]
- Seymour, L.W. Passive tumor targeting of soluble macromolecules and drug conjugates. Crit. Rev. Ther. Drug Carrier Syst. 1992, 9, 135–187. [Google Scholar]
- Huang, S.K.; Martin, F.J.; Jay, G.; Vogel, J.; Papahadjopoulos, D.; Friend, D.S. Extravasation and transcytosis of liposomes in Kaposi’s sarcoma-like dermal lesions of transgenic mice bearing the HIV tat gene. Am. J. Pathol. 1993, 143, 10–14. [Google Scholar]
- Sarti, P.; Ginobbi, P.; D’Agostino, I.; Arancia, G.; Lendaro, E.; Molinari, A.; Ippoliti, R.; Citro, G. Liposomal targeting of leukaemia HL60 cells induced by transferrin-receptor endocytosis. Biotechnol. Appl. Biochem. 1996, 24, 269–276. [Google Scholar]
- Park, J.W.; Hong, K.; Carter, P.; Asgari, H.; Guo, L.Y.; Keller, G.A.; Wirth, C.; Shalaby, R.; Kotts, C.; Wood, W.I.; et al. Development of anti-p185HER2 immunoliposomes for cancer therapy. Proc. Natl. Acad. Sci. USA 1995, 92, 1327–1331. [Google Scholar] [CrossRef]
- Straubinger, R.M.; Hong, K.; Friend, D.S.; Papahadjopoulos, D. Endocytosis of liposomes and intracellular fate of encapsulated molecules: Encounter with a low pH compartment after internalization in coated vesicles. Cell 1983, 32, 1069–1079. [Google Scholar] [CrossRef]
- Heath, T.; Lopez, N.; Stern, W.; Papahadjopoulos, D. 5-Fluoroorotate: A new liposome-dependent cytotoxic agent. FEBS Lett. 1985, 187, 73–75. [Google Scholar] [CrossRef]
- Heath, T.D.; Brown, C.S. Liposome dependent delivery of N-phosphonacetyl-l-aspartic acid to cells in vitro. J. Liposome Res. 1989, 1, 303–317. [Google Scholar] [CrossRef]
- Matthay, K.K.; Abai, A.M.; Cobb, S.; Hong, K.; Papahadjopoulos, D.; Straubinger, R.M. Role of ligand in antibody-directed endocytosis of liposomes by human T-leukemia cells. Cancer Res. 1989, 49, 4879–4886. [Google Scholar]
- Kirpotin, D.B.; Drummond, D.C.; Shao, Y.; Shalaby, M.R.; Hong, K.; Nielsen, U.B.; Marks, J.D.; Benz, C.C.; Park, J.W. Antibody targeting of long-circulating lipidic nanoparticles does not increase tumor localization but does increase internalization in animal models. Cancer Res. 2006, 66, 6732–6740. [Google Scholar] [CrossRef]
- Drummond, D.C.; Noble, C.O.; Guo, Z.; Hayes, M.E.; Connolly-Ingram, C.; Gabriel, B.S.; Hann, B.; Liu, B.; Park, J.W.; Hong, K.; et al. Development of a highly stable and targetable nanoliposomal formulation of topotecan. J. Control. Release 2010, 141, 13–21. [Google Scholar]
- Hendriks, B.S.; Klinz, S.G.; Reynolds, J.G.; Espelin, C.W.; Gaddy, D.F.; Wickham, T.J. Impact of tumor HER2/ERBB2 expression level on HER2-targeted liposomal doxorubicin-mediated drug delivery: Multiple low-affinity interactions lead to a threshold effect. Mol. Cancer Ther. 2013, 12, 1816–1828. [Google Scholar] [CrossRef]
- Connor, J.; Huang, L. Efficient cytoplasmic delivery of a fluorescent dye by pH-sensitive immunoliposomes. J. Cell Biol. 1985, 101, 582–589. [Google Scholar] [CrossRef]
- Straubinger, R.M.; Düzgünes, N.; Papahadjopoulos, D. pH-Sensitive liposomes mediate cytoplasmic delivery of encapsulated macromolecules. FEBS Lett. 1985, 179, 148–154. [Google Scholar] [CrossRef]
- Chu, C.-J.; Dijkstra, J.; Lai, M.-Z.; Hong, K.; Szoka, F.C., Jr. Efficiency of cytoplasmic delivery by pH-sensitive liposomes to cells in culture. Pharm. Res. 1990, 7, 824–834. [Google Scholar] [CrossRef]
- Heath, T.; Montgomery, J.; Piper, J.; Papahadjopoulos, D. Antibody-targeted liposomes: Increase in specific toxicity of methotrexate-g-aspartate. Proc. Natl. Acad. Sci. USA. 1983, 80, 1377–1381. [Google Scholar] [CrossRef]
- Charrois, G.J.; Allen, T.M. Rate of biodistribution of STEALTH liposomes to tumor and skin: Influence of liposome diameter and implications for toxicity and therapeutic activity. Biochim. Biophys. Acta 1609, 102–108. [Google Scholar]
- Nagayasu, A.; Uchiyama, K.; Kiwada, H. The size of liposomes: A factor which affects their targeting efficiency to tumors and therapeutic activity of liposomal antitumor drugs. Adv. Drug Deliv. Rev. 1999, 40, 75–87. [Google Scholar] [CrossRef]
- Hobbs, S.K.; Monsky, W.L.; Yuan, F.; Roberts, W.G.; Griffith, L.; Torchilin, V.P.; Jain, R.K. Regulation of transport pathways in tumor vessels: Role of tumor type and microenvironment. Proc. Natl. Acad. Sci. USA 1998, 95, 4607–4612. [Google Scholar]
- Allen, T.M.; Hansen, C.; Rutledge, J. Liposomes with prolonged circulation times: Factors affecting uptake by reticuloendothelial and other tissues. Biochim. Biophys. Acta 1989, 981, 27–35. [Google Scholar] [CrossRef]
- Woodle, M.C.; Matthay, K.K.; Newman, M.S.; Hidayat, J.E.; Collins, L.R.; Redemann, C.; Martin, F.J.; Papahadjopoulos, D. Versatility in lipid compositions showing prolonged circulation with sterically stabilized liposomes. Biochim. Biophys. Acta 1992, 1105, 193–200. [Google Scholar] [CrossRef]
- Gabizon, A.; Papahadjopoulos, D. Liposome formulations with prolonged circulation time in blood and enhanced uptake by tumors. Proc. Natl. Acad. Sci. USA 1988, 85, 6949–6953. [Google Scholar] [CrossRef]
- Allen, T.M.; Chonn, A. Large unilamellar liposomes with low uptake into the reticuloendothelial system. FEBS Lett. 1987, 223, 42–46. [Google Scholar] [CrossRef]
- Eastman, S.J.; Wilschut, J.; Cullis, P.R.; Hope, M.J. Intervesicular exchange of lipids with weak acid and weak base characteristics: Influence of transmembrane pH gradients. Biochim. Biophys. Acta 1989, 981, 178–184. [Google Scholar] [CrossRef]
- Mayhew, E.; Ito, M.; Lazo, R. Toxicity of non-drug-containing liposomes for cultured human cells. Exp. Cell Res. 1987, 171, 195–202. [Google Scholar] [CrossRef]
- Senior, J.H.; Trimble, K.R.; Maskiewicz, R. Interaction of positively-charged liposomes with blood: Implications for their application in vivo. Biochim. Biophys. Acta 1991, 1070, 173–179. [Google Scholar] [CrossRef]
- Gabizon, A.; Papahadjopoulos, D. The role of surface charge and hydrophilic groups on liposome clearance in vivo. Biochim. Biophys. Acta 1992, 1103, 94–100. [Google Scholar] [CrossRef]
- Litzinger, D.C.; Brown, J.M.; Wala, I.; Kaufman, S.A.; Van, G.Y.; Farrell, C.L.; Collins, D. Fate of cationic liposomes and their complex with oligonucleotide in vivo. Biochim. Biophys. Acta 1996, 1281, 139–149. [Google Scholar] [CrossRef]
- Thurston, G.; McLean, J.W.; Rizen, M.; Baluk, P.; Haskell, A.; Murphy, T.J.; Hanahan, D.; McDonald, D.M. Cationic liposomes target angiogenic endothelial cells in tumors and chronic inflammation in mice. J. Clin. Investig. 1998, 101, 1401–1413. [Google Scholar] [CrossRef]
- Campbell, R.B.; Fukumura, D.; Brown, E.B.; Mazzola, L.M.; Izumi, Y.; Jain, R.K.; Torchilin, V.P.; Munn, L.L. Cationic charge determines the distribution of liposomes between the vascular and extravascular compartments of tumors. Cancer Res. 2002, 62, 6831–6836. [Google Scholar]
- Senior, J.; Gregoriadis, G. Stability of small unilamellar liposomes in serum and clearance from the circulation: The effect of the phospholipid and cholesterol components. Life Sci. 1982, 30, 2123–2136. [Google Scholar] [CrossRef]
- Gregoriadis, G.; Davis, C. Stability of liposomes in vivo and in vitro is promoted by their cholesterol content and the presence of blood cells. Biochem. Biophys. Res. Commun. 1979, 89, 1287–1293. [Google Scholar] [CrossRef]
- Allen, T.M.; Hansen, C.; Martin, F.; Redemann, C.; Yau-Young, A. Liposomes containing synthetic lipid derivatives of poly (ethylene glycol) show prolonged circulation half-lives in vivo. Biochim. Biophys. Acta 1991, 1066, 29–36. [Google Scholar] [CrossRef]
- Daemen, T.; Hofstede, G.; Ten Kate, M.T.; Bakker-Woudenberg, I.A.; Scherphof, G.L. Liposomal doxorubicin-induced toxicity: Depletion and impairment of phagocytic activity of liver macrophages. Int. J. Cancer 1995, 61, 716–721. [Google Scholar] [CrossRef]
- Woodle, M.C.; Lasic, D.D. Sterically stabilized liposomes. Biochim. Biophys. Acta 1992, 1113, 171–199. [Google Scholar] [CrossRef]
- Hong, K.; Kirpotin, D.B.; Park, J.W.; Shao, Y.; Shalaby, R.; Colbern, G.; Benz, C.C.; Papahadjopoulos, D. Anti-HER2 immunoliposomes for targeted drug delivery. Ann. N.Y. Acad. Sci. 1999, 886, 293–296. [Google Scholar] [CrossRef]
- Sapra, P.; Allen, T.M. Internalizing antibodies are necessary for improved therapeutic efficacy of antibody-targeted liposomal drugs. Cancer Res. 2002, 62, 7190–7194. [Google Scholar]
- Sugano, M.; Egilmez, N.K.; Yokota, S.J.; Chen, F.A.; Harding, J.; Huang, S.K.; Bankert, R.B. Antibody targeting of doxorubicin-loaded liposomes suppresses the growth and metastatic spread of established human lung tumor xenografts in severe combined immunodeficient mice. Cancer Res. 2000, 60, 6942–6949. [Google Scholar]
- Nielsen, U.B.; Kirpotin, D.B.; Pickering, E.M.; Hong, K.; Park, J.W.; Refaat Shalaby, M.; Shao, Y.; Benz, C.C.; Marks, J.D. Therapeutic efficacy of anti-ErbB2 immunoliposomes targeted by a phage antibody selected for cellular endocytosis. Biochim. Biophys. Acta 2002, 1591, 109–118. [Google Scholar] [CrossRef]
- Lopes de Menezes, D.E.; Pilarski, L.M.; Allen, T.M. In vitro and in vivo targeting of immunoliposomal doxorubicin to human B-cell lymphoma. Cancer Res. 1998, 58, 3320–3330. [Google Scholar]
- Park, J.W.; Hong, K.; Kirpotin, D.B.; Colbern, G.; Shalaby, R.; Baselga, J.; Shao, Y.; Nielsen, U.B.; Marks, J.D.; Moore, D.; et al. Anti-HER2 immunoliposomes: Enhanced efficacy attributable to targeted delivery. Clin. Cancer Res. 2002, 8, 1172–1181. [Google Scholar]
- Mamot, C.; Drummond, D.C.; Noble, C.O.; Kallab, V.; Guo, Z.; Hong, K.; Kirpotin, D.B.; Park, J.W. Epidermal growth factor receptor-targeted immunoliposomes significantly enhance the efficacy of multiple anticancer drugs in vivo. Cancer Res. 2005, 65, 11631–11638. [Google Scholar]
- Heath, T.D.; Fraley, R.T.; Bentz, J.; Voss, E.W., Jr.; Herron, J.N.; Papahadjopoulos, D. Antibody-directed liposomes. Determination of affinity constants for soluble and liposome-bound antifluorescein. Biochim. Biophys. Acta 1984, 770, 148–158. [Google Scholar] [CrossRef]
- Allen, T.M.; Newman, M.S.; Woodle, M.C.; Mayhew, E.; Uster, P.S. Pharmacokinetics and anti-tumor activity of vincristine encapsulated in sterically stabilized liposomes. Int. J. Cancer 1995, 62, 199–204. [Google Scholar] [CrossRef]
- Moghimi, S.M.; Szebeni, J. Stealth liposomes and long circulating nanoparticles: Critical issues in pharmacokinetics, opsonization and protein-binding properties. Prog. Lipid Res. 2003, 42, 463–478. [Google Scholar] [CrossRef]
- Zhou, R.; Mazurchuk, R.; Straubinger, R.M. Antivasculature effects of doxorubicin-containing liposomes in an intracranial rat brain tumor model. Cancer Res. 2002, 62, 2561–2566. [Google Scholar]
- Laverman, P.; Brouwers, A.H.; Dams, E.T.; Oyen, W.J.; Storm, G.; van Rooijen, N.; Corstens, F.H.; Boerman, O.C. Preclinical and clinical evidence for disappearance of long-circulating characteristics of polyethylene glycol liposomes at low lipid dose. J. Pharmacol. Exp. Ther. 2000, 293, 996–1001. [Google Scholar]
- Ishida, T.; Ichihara, M.; Wang, X.; Yamamoto, K.; Kimura, J.; Majima, E.; Kiwada, H. Injection of PEGylated liposomes in rats elicits PEG-specific IgM, which is responsible for rapid elimination of a second dose of PEGylated liposomes. J. Control. Release 2006, 112, 15–25. [Google Scholar]
- Ishida, T.; Atobe, K.; Wang, X.; Kiwada, H. Accelerated blood clearance of PEGylated liposomes upon repeated injections: Effect of doxorubicin-encapsulation and high-dose first injection. J. Control. Release 2006, 115, 251–258. [Google Scholar] [CrossRef]
- Ishida, T.; Maeda, R.; Ichihara, M.; Irimura, K.; Kiwada, H. Accelerated clearance of PEGylated liposomes in rats after repeated injections. J. Control. Release 2003, 88, 35–42. [Google Scholar] [CrossRef]
- Gabizon, A.; Shiota, R.; Papahadjopoulos, D. Pharmacokinetics and tissue distribution of doxorubicin encapsulated in stable liposomes with long circulation times. J. Natl. Cancer Inst. 1989, 81, 1484–1488. [Google Scholar] [CrossRef]
- Lyass, O.; Uziely, B.; Ben-Yosef, R.; Tzemach, D.; Heshing, N.I.; Lotem, M.; Brufman, G.; Gabizon, A. Correlation of toxicity with pharmacokinetics of pegylated liposomal doxorubicin (Doxil) in metastatic breast carcinoma. Cancer 2000, 89, 1037–1047. [Google Scholar] [CrossRef]
- Lorusso, D.; di Stefano, A.; Carone, V.; Fagotti, A.; Pisconti, S.; Scambia, G. Pegylated liposomal doxorubicin-related palmar-plantar erythrodysesthesia (“hand-foot” syndrome). Ann. Oncol. 2007, 18, 1159–1164. [Google Scholar] [CrossRef]
- Arnold, R.D.; Mager, D.E.; Slack, J.E.; Straubinger, R.M. Effect of repetitive administration of Doxorubicin-containing liposomes on plasma pharmacokinetics and drug biodistribution in a rat brain tumor model. Clin. Cancer Res. 2005, 11, 8856–8865. [Google Scholar] [CrossRef]
- Ait-Oudhia, S.; Straubinger, R.M.; Mager, D.E. Systems pharmacological analysis of paclitaxel-mediated tumor priming that enhances nanocarrier deposition and efficacy. J. Pharmacol. Exp. Ther. 2013, 344, 103–112. [Google Scholar] [CrossRef]
- Jang, S.H.; Wientjes, M.G.; Au, J.L. Enhancement of paclitaxel delivery to solid tumors by apoptosis-inducing pretreatment: Effect of treatment schedule. J. Pharmacol. Exp. Ther. 2001, 296, 1035–1042. [Google Scholar]
- Xiong, X.B.; Huang, Y.; Lu, W.L.; Zhang, H.; Zhang, X.; Zhang, Q. Enhanced intracellular uptake of sterically stabilized liposomal Doxorubicin in vitro resulting in improved antitumor activity in vivo. Pharm. Res. 2005, 22, 933–939. [Google Scholar] [CrossRef]
- Harashima, H.; Tsuchihashi, M.; Iida, S.; Doi, H.; Kiwada, H. Pharmacokinetic/pharmacodynamic modeling of antitumor agents encapsulated into liposomes. Adv. Drug Deliv. Rev. 1999, 40, 39–61. [Google Scholar] [CrossRef]
- Harashima, H.; Iida, S.; Urakami, Y.; Tsuchihashi, M.; Kiwada, H. Optimization of antitumor effect of liposomally encapsulated doxorubicin based on simulations by pharmacokinetic/pharmacodynamic modeling. J. Control. Release 1999, 61, 93–106. [Google Scholar]
- Hendriks, B.S.; Reynolds, J.G.; Klinz, S.G.; Geretti, E.; Lee, H.; Leonard, S.C.; Gaddy, D.F.; Espelin, C.W.; Nielsen, U.B.; Wickham, T.J. Multiscale kinetic modeling of liposomal doxorubicin delivery quantifies the role of tumor and drug-specific parameters in local delivery to tumors. Clin. Pharmacol. Ther. 2012, 1, 1–11. [Google Scholar]
- Ait-Oudhia, S.; Straubinger, R.M.; Mager, D.E. Meta-analysis of nanoparticulate paclitaxel delivery system pharmacokinetics and model prediction of associated neutropenia. Pharm. Res. 2012, 29, 2833–2844. [Google Scholar] [CrossRef]
- Mayer, L.D.; Bally, M.B.; Cullis, P.R. Uptake of adriamycin into large unilamellar vesicles in response to a pH gradient. Biochim. Biophys. Acta 1986, 857, 123–126. [Google Scholar] [CrossRef]
- Harrigan, P.R.; Wong, K.F.; Redelmeier, T.E.; Wheeler, J.J.; Cullis, P.R. Accumulation of doxorubicin and other lipophilic amines into large unilamellar vesicles in response to transmembrane pH-gradients. Biochim. Biophys. Acta 1993, 1149, 329–348. [Google Scholar]
- Oh, Y.-K.; Straubinger, R.M. Cellular retention of liposome-delivered compounds modulated by a probenecid-sensitive anion transporter. Pharm. Res. 1997, 14, 1203–1209. [Google Scholar] [CrossRef]
- Straubinger, R.M.; Lopez, N.G.; Debs, R.; Hong, K.; Papahadjopoulos, D. Liposome-based therapy of human ovarian cancer: Parameters determining potency of negatively-charged and antibody-targeted liposomes. Cancer Res. 1988, 48, 5237–5245. [Google Scholar]
- Paolino, D.; Cosco, D.; Racanicchi, L.; Trapasso, E.; Celia, C.; Iannone, M.; Puxeddu, E.; Costante, G.; Filetti, S.; Russo, D.; et al. Gemcitabine-loaded PEGylated unilamellar liposomes vs. GEMZAR: Biodistribution, pharmacokinetic features and in vivo antitumor activity. J. Control. Release 2010, 144, 144–150. [Google Scholar]
- Pattillo, C.B.; Sari-Sarraf, F.; Nallamothu, R.; Moore, B.M.; Wood, G.C.; Kiani, M.F. Targeting of the antivascular drug combretastatin to irradiated tumors results in tumor growth delay. Pharm. Res. 2005, 22, 1117–1120. [Google Scholar] [CrossRef]
- Mori, A.; Kennel, S.J.; van Borssum Waalkes, M.; Scherphof, G.L.; Huang, L. Characterization of organ-specific immunoliposomes for delivery of 3',5'-O-dipalmitoyl-5-fluoro-2'-deoxyuridine in a mouse lung-metastasis model. Cancer Chemother. Pharmacol. 1995, 35, 447–456. [Google Scholar] [CrossRef]
- Talbott, C.M.; Vorobyov, I.; Borchman, D.; Taylor, K.G.; DuPre, D.B.; Yappert, M.C. Conformational studies of sphingolipids by NMR spectroscopy. II. Sphingomyelin. Biochim. Biophys. Acta 2000, 1467, 326–337. [Google Scholar]
- Allen, T.M.; Mumbengegwi, D.R.; Charrois, G.J. Anti-CD19-targeted liposomal doxorubicin improves the therapeutic efficacy in murine B-cell lymphoma and ameliorates the toxicity of liposomes with varying drug release rates. Clin. Cancer Res. 2005, 11, 3567–3573. [Google Scholar] [CrossRef]
- Working, P.K.; Dayan, A.D. Pharmacological-toxicological expert report. CAELYX. (Stealth liposomal doxorubicin HCl). Hum. Exp. Toxicol. 1996, 15, 751–785. [Google Scholar]
- Hong, R.L.; Huang, C.J.; Tseng, Y.L.; Pang, V.F.; Chen, S.T.; Liu, J.J.; Chang, F.H. Direct comparison of liposomal doxorubicin with or without polyethylene glycol coating in C-26 tumor-bearing mice: Is surface coating with polyethylene glycol beneficial? Clin. Cancer Res. 1999, 5, 3645–3652. [Google Scholar]
- Gabizon, A.; Martin, F. Polyethylene glycol-coated (pegylated) liposomal doxorubicin. Rationale for use in solid tumours. Drugs 1997, 54, 15–21. [Google Scholar] [CrossRef]
- Gabizon, A.; Shmeeda, H.; Barenholz, Y. Pharmacokinetics of pegylated liposomal Doxorubicin: Review of animal and human studies. Clin. Pharmacokinet. 2003, 42, 419–436. [Google Scholar] [CrossRef]
- Working, P.K.; Newman, M.S.; Sullivan, T.; Yarrington, J. Reduction of the cardiotoxicity of doxorubicin in rabbits and dogs by encapsulation in long-circulating, pegylated liposomes. J. Pharmacol. Exp. Ther. 1999, 289, 1128–1133. [Google Scholar]
- Jain, R.K.; Stylianopoulos, T. Delivering nanomedicine to solid tumors. Nat. Rev. Clin. Oncol. 2010, 7, 653–664. [Google Scholar] [CrossRef]
- Minchinton, A.I.; Tannock, I.F. Drug penetration in solid tumours. Nat. Rev. Cancer 2006, 6, 583–592. [Google Scholar] [CrossRef]
- Baxter, L.T.; Zhu, H.; Mackensen, D.G.; Butler, W.F.; Jain, R.K. Biodistribution of monoclonal antibodies: Scale-up from mouse to human using a physiologically based pharmacokinetic model. Cancer Res. 1995, 55, 4611–4622. [Google Scholar]
- Mager, D.E.; Jusko, W.J. Development of translational pharmacokinetic-pharmacodynamic models. Clin. Pharmacol. Ther. 2008, 83, 909–912. [Google Scholar] [CrossRef]
- El-Kareh, A.W.; Secomb, T.W. A mathematical model for comparison of bolus injection, continuous infusion, and liposomal delivery of doxorubicin to tumor cells. Neoplasia 2000, 2, 325–338. [Google Scholar]
- Gianni, L.; Kearns, C.M.; Giani, A.; Capri, G.; Vigano, L.; Lacatelli, A.; Bonadonna, G.; Egorin, M.J. Nonlinear pharmacokinetics and metabolism of paclitaxel and its pharmacokinetic/pharmacodynamic relationships in humans. J. Clin. Oncol. 1995, 13, 180–190. [Google Scholar]
- Griffon-Etienne, G.; Boucher, Y.; Brekken, C.; Suit, H.D.; Jain, R.K. Taxane-induced apoptosis decompresses blood vessels and lowers interstitial fluid pressure in solid tumors: Clinical implications. Cancer Res. 1999, 59, 3776–3782. [Google Scholar]
- Lu, D.; Wientjes, M.G.; Lu, Z.; Au, J.L. Tumor priming enhances delivery and efficacy of nanomedicines. J. Pharmacol. Exp. Ther. 2007, 322, 80–88. [Google Scholar] [CrossRef]
- Aroui, S.; Brahim, S.; de Waard, M.; Breard, J.; Kenani, A. Efficient induction of apoptosis by doxorubicin coupled to cell-penetrating peptides compared to unconjugated doxorubicin in the human breast cancer cell line MDA-MB 231. Cancer Lett. 2009, 285, 28–38. [Google Scholar] [CrossRef]
- Desai, N.; Trieu, V.; Yao, Z.; Louie, L.; Ci, S.; Yang, A.; Tao, C.; De, T.; Beals, B.; Dykes, D.; et al. Increased antitumor activity, intratumor paclitaxel concentrations, and endothelial cell transport of cremophor-free, albumin-bound paclitaxel, ABI-007, compared with cremophor-based paclitaxel. Clin. Cancer Res. 2006, 12, 1317–1324. [Google Scholar] [CrossRef]
- Milas, L.; Hunter, N.R.; Kurdoglu, B.; Mason, K.A.; Meyn, R.E.; Stephens, L.C.; Peters, L.J. Kinetics of mitotic arrest and apoptosis in murine mammary and ovarian tumors treated with taxol. Cancer Chemother. Pharmacol. 1995, 35, 297–303. [Google Scholar] [CrossRef]
- Sparreboom, A.; van Tellingen, O.; Nooijen, W.J.; Beijnen, J.H. Nonlinear pharmacokinetics of paclitaxel in mice results from the pharmaceutical vehicle Cremophor EL. Cancer Res. 1996, 56, 2112–2115. [Google Scholar]
- Ah-See, M.L.; Makris, A.; Taylor, N.J.; Harrison, M.; Richman, P.I.; Burcombe, R.J.; Stirling, J.J.; d’Arcy, J.A.; Collins, D.J.; Pittam, M.R.; et al. Early changes in functional dynamic magnetic resonance imaging predict for pathologic response to neoadjuvant chemotherapy in primary breast cancer. Clin. Cancer Res. 2008, 14, 6580–6589. [Google Scholar] [CrossRef]
- Baek, H.M.; Yu, H.J.; Chen, J.H.; Nalcioglu, O.; Su, M.Y. Quantitative correlation between 1H MRS and dynamic contrast-enhanced MRI of human breast cancer. Magn. Reson. Imaging 2008, 26, 523–531. [Google Scholar] [CrossRef]
- Yankeelov, T.E.; Lepage, M.; Chakravarthy, A.; Broome, E.E.; Niermann, K.J.; Kelley, M.C.; Meszoely, I.; Mayer, I.A.; Herman, C.R.; McManus, K.; et al. Integration of quantitative DCE-MRI and ADC mapping to monitor treatment response in human breast cancer: Initial results. Magn. Reson. Imaging 2007, 25, 1–13. [Google Scholar]
- Eytan, G.D.; Kuchel, P.W. Mechanism of action of P-glycoprotein in relation to passive membrane permeation. Int. Rev. Cytol. 1999, 190, 175–250. [Google Scholar] [CrossRef]
- Baxter, L.T.; Zhu, H.; Mackensen, D.G.; Jain, R.K. Physiologically based pharmacokinetic model for specific and nonspecific monoclonal antibodies and fragments in normal tissues and human tumor xenografts in nude mice. Cancer Res. 1994, 54, 1517–1528. [Google Scholar]
- Stewart, D.J. Making and using DNA microarrays: A short course at Cold Spring Harbor Laboratory. Genome Res. 2000, 10, 1–3. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ait-Oudhia, S.; Mager, D.E.; Straubinger, R.M. Application of Pharmacokinetic and Pharmacodynamic Analysis to the Development of Liposomal Formulations for Oncology. Pharmaceutics 2014, 6, 137-174. https://doi.org/10.3390/pharmaceutics6010137
Ait-Oudhia S, Mager DE, Straubinger RM. Application of Pharmacokinetic and Pharmacodynamic Analysis to the Development of Liposomal Formulations for Oncology. Pharmaceutics. 2014; 6(1):137-174. https://doi.org/10.3390/pharmaceutics6010137
Chicago/Turabian StyleAit-Oudhia, Sihem, Donald E. Mager, and Robert M. Straubinger. 2014. "Application of Pharmacokinetic and Pharmacodynamic Analysis to the Development of Liposomal Formulations for Oncology" Pharmaceutics 6, no. 1: 137-174. https://doi.org/10.3390/pharmaceutics6010137
APA StyleAit-Oudhia, S., Mager, D. E., & Straubinger, R. M. (2014). Application of Pharmacokinetic and Pharmacodynamic Analysis to the Development of Liposomal Formulations for Oncology. Pharmaceutics, 6(1), 137-174. https://doi.org/10.3390/pharmaceutics6010137