From Molecular to Nanotechnology Strategies for Delivery of Neurotrophins: Emphasis on Brain-Derived Neurotrophic Factor (BDNF)
Abstract
:1. Introduction
1.1. Burden of Neurodegenerative and Psychiatric Diseases Resulting from Neurotrophin Impairment
1.2. Mechanism of Neurotrophin Action
1.2.1. Structure-Activity Relationships for Neuroprotection

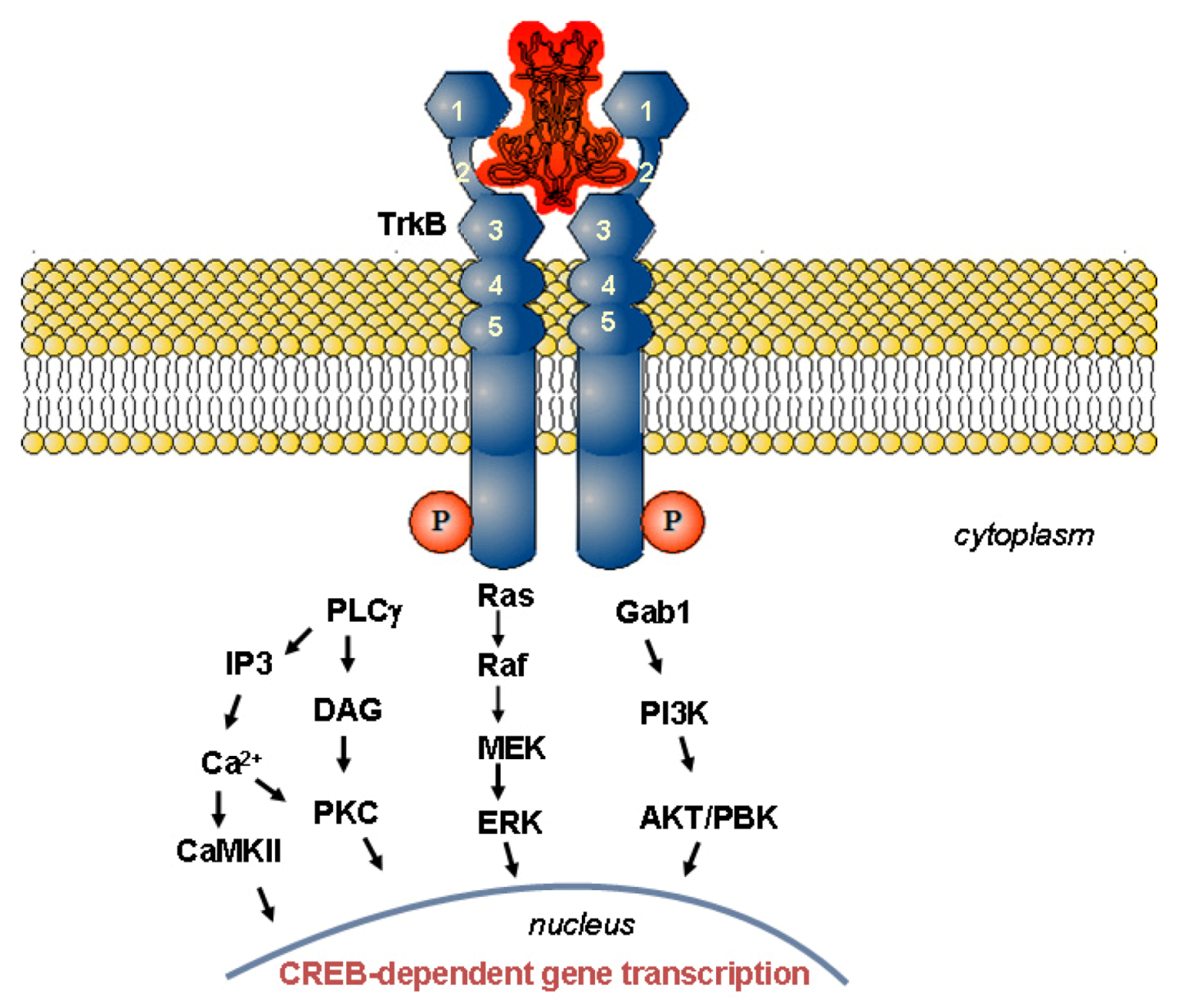
1.2.2. Physiological Role of BDNF in Relation to Neurodegenerative Disorders
2. Therapeutic Strategies Designed for Delivery of Neurotrophins
2.1. Administration of Neurotrophins by Injection
2.2. Neurotrophin Gene and Cell Therapy
| Therapeutic strategy | Neurotrophin | Application | Model | Outcome | Refs. | ||
|---|---|---|---|---|---|---|---|
| I. Administration of recombinant proteins by direct injection | |||||||
| Subcutaneous and intrathecal injection | BDNF | Amyotrophic lateral sclerosis (ALS) | clinical trial phase III | limited BDNF diffusion through the BBB; high dose required to observe survival effects | [47] | ||
| Intracerebroventricular infusion via implanted catheters | GDNF | Parkinson’s disease | clinical trials phase I | GDNF did not reach substantia nigra; side effects | [119,139] | ||
| Direct intraputamenal perfusion via implanted mini-pumps | GDNF | Parkinson’s disease | clinical trials phase I and phase II | clinical improvement of symptoms after 1 year of therapy; GDNF effect on dopamine function | [107,124,159] | ||
| Intraventricular pretreatment | BDNF | Cerebral ischemia | rat | reduced infarct size | [154] | ||
| Intraventricular infusion pumps | BDNF | Cerebral venous ischemia | rat | reduced infarct size; protection of cerebral cortex against apoptosis | [164] | ||
| Intravenous | BDNF | Cerebral ischemia | rat | reduced infarct volume | [155] | ||
| Mini-pump in the cerebral artery | BDNF | Cerebral ischemia | rat | reduced infarct size | [178] | ||
| Intra-hippocampal injection | BDNF | Long-term memory (LTM) storage | rat | memory persistence | [11] | ||
| Cochlear implant of osmotic mini-pump | BDNF | Deafness | guinea pig | enhanced survival of auditory nerves | [88] | ||
| Intracerebroventricular infusion (ICV) | NGF | Alzheimer’s disease | rodents, clinical trials | increased number of axons; prevented degeneration of cholinergic neurons | [220,221,222,223] | ||
| Intranasal | BDNF; NT-4 | CNS disorders; Cerebral ischemia | rat | noninvasive delivery; minimal systemic exposure; enhanced neurogenesis; unknown pharmacokinetics | [53,91,129] | ||
| ICV administration; protein infusion | BDNF | Dependence on psychostimulants | rat | long-lasting antidepressant effects by the use of molecules activating the PI3K/Akt and MAPK/ERK pathways; neuroplasticity | [59,111] | ||
| II. Gene and cell therapy | |||||||
| Gene transfer via adeno-associated viral (AAV) vector | BDNF; GDNF | Huntington’s disease | rat; mice | striatal neuron survival | [12,194,200] | ||
| Ex vivo gene delivery by genetically modified fibroblasts secreting the protein | NGF | Alzheimer’s disease | clinical trial Phase I | cholinergic neuron stimulation; modified disease progression | [203,204] | ||
| Gene transfer via lentivirus or adenovirus followed by protein expression | BDNF | Alzheimer’s disease | mice; rats; monkeys; clinical trials | broad neuroprotective effects | [5,32] | ||
| Lentiviral vectors for local delivery in gene therapy | BDNF; NT-3 | Spinal cord injury | In vitro; rats | bridging axonal regeneration across lesion sides | [165,217] | ||
| Gene transfer via adenovirus | NGF; BDNF | Spinal cord injury | rats | axonal regeneration and collateral sprouting; axonal growth | [41,118,199] | ||
| Herpes simplex virus induced long lasting protein expression | BDNF | Epilepsy | rats | increased neurogenesis; reduced epileptogenesis | [140] | ||
| Gene transfer via cationic liposomes | BDNF; NGF; GDNF | CNS lesion; Spinal cord injury | In vitro | transgene expression at low cellular toxicity | [179,195] | ||
| Gene transfer via genetically-engineered bone marrow stem cells expressing the protein | BDNF; GDNF; NGF; CNTF | Multiple sclerosis; Huntington’s disease; spinal cord injury; glaucoma | mice; rats; In vitro | suppressed demyelination; reduced motor dysfunction; decreased inflammation | [114,196,197,201] | ||
| Transplants of genetically-engineered fibroblasts expressing the protein | BDNF; NGF; NT-3 | Parkinson’s disease; Spinal cord injury | rats | increased nigral dopaminergic neuronal survival responsiveness to axonal regeneration | [103,127,137] | ||
| Neural stem cell transplantation | BDNF | Alzheimer’s disease | mice | improved cognitive function | [224] | ||
| Encapsulated cell biodelivery (ECB) -implanted device with encapsulated protein-secreting cells | NGF | Alzheimer’s disease | Göttingen mini-pigs; clinical trials | persistent NGF secretion; increased neurotrophin levels in the basal forebrain; safety and tolerability; new therapeutic platform in restorative neurosurgery | [102,225] | ||
| Ex vivo gene therapy via protein-expressing BHK cells encapsulated in a device with a semi-permeable polymer membrane | CNTF | Huntington’s disease | clinical trial Phase I | proof of principle for implanted capsules | [184,188] | ||
| Intranigral transplants of mesenchymal stem cells secreting the protein | BDNF | Parkinson’s disease | in vitro; rats | regulated BDNF expression and dopaminergic effect | [161] | ||
| III. Sustained release using polymer systems III.A. Synthetic polymers | |||||||
| Polyethylene glycol (PEG) chain conjugated at the C-terminus of the recombinant protein (intravenous administration) | BDNF | unspecified | in vitro; rats | retained bioactivity of PEGylated neurotrophin | [149] | ||
| Conjugation of a PEG chain and an OX26 antibody (biotin/SA) for targeted delivery (intravenous administration) | BDNF | Cerebral ischemia | in vitro; rats | increased brain uptake of the BDNF construct; minimized rapid clearance upon PEGylation | [141,175,183] | ||
| Covalent coupling with PEG chains (intrathecal injection) | BDNF | Spinal cord injury and diseases | in vitro; rats | improved half-life in the cerebrospinal fluid; increased effect on locomotor activity | [89,160] | ||
| PLGA-PLL-PEG biodegradable microspheres releasing recombinant protein | BDNF | CNS injury | in vitro | sustained release of bioactive human BDNF over 60 days | [94] | ||
| PLGA particles dispersed in a hydrogel of hyaluronic acid (HA) and methylcellulose | Chymotrypsin as a model of NT-3 and five other neuroprotectors | Spinal cord injury | in vitro | sustained release over 28 days from injectable composite hydrogels | [95] | ||
| Poly(lactic-co-glycolic acid) (PLGA) microspheres releasing recombinant protein | NGF | Huntington’s disease; unspecified lesions | rats; in vitro | sustained release over 2.5 months; improved protein stability; reduced striatal lesions | [123,134,143] | ||
| PLGA biodegradable microspheres releasing recombinant protein | GDNF | Parkinson’s disease | in vitro; rats; monkeys | improved dopaminergic graft survival and function | [90,98,104,105,106,107,115,116] | ||
| Ethylene-co-vinyl acetate (EVAc) discs for sustained release | NGF | Alzheimer’s disease | in vitro; rats | controlled release for up to one week; limited NGF transport in the brain tissue; high concentrations near the implant | [121] | ||
| EVAc discs and PLGA microspheres | NGF | unspecified CNS disease | in vitro; rats | high localized doses of recombinant protein near the implants; half-life increased to 1.7 hours | [153] | ||
| PLA-PEG hydrogel | BDNF; NT-3; NGF | Spinal cord or optic nerve injury | in vitro; mice; rats | sustained release over 2 weeks; simultaneous delivery of multiple neurotrophins; stimulated proliferation; enhanced neurite outgrowth | [97,145,173] | ||
| Polyphosphoester (PPE) microspheres incorporated in nerve guide conduits | NGF | Nerve injury | rats | morphological regeneration of sciatic nerve 3 months after the implantation of the conduits | [177] | ||
| Ethylene-vinyl acetate (EVA) nerve guidance channels releasing the protein | GDNF; NGF; NT-3; BDNF | Sciatic nerve injury | in vitro; rats | promoted regeneration of myelinated axons | [92,96,101] | ||
| Poly(lactide-co-glycolide) (PLG) microspheres in nerve guide conduits | NGF | Spinal cord and peripheral nerve injury | in vitro; mice | sustained release over 42 days from the porous constructs allowing for cellular infiltration into the channels; stimulated neurite outgrowth | [180] | ||
| PLA tubular macroporous foam | BDNF | Spinal cord injury | in vitro; rats | low axonal regeneration response; increased angiogenesis | [142] | ||
| Macroporous scaffold of pHEMA and PLL | NGF; NT-3 | Nerve injury | in vitro | minimum concentration gradient of 200 ng/mL required for guidance of the neurite outgrowth | [136] | ||
| Conducting polypyrrole scaffold with surface-conjugated proteins | NGF; NT-3 | Nerve injury | in vitro; rats | nerve fiber growth towards the implant electrode | [108,146,169] | ||
| Implanted EVAc matrices | BDNF | Major depression | rats | dysregulation of BDNF-associated plasticity-related pathways upon sustained release; antidepressant-like effects upon short-term delivery | [158] | ||
| III.B. Naturally occurring polymers | |||||||
| Alginate microspheres | NGF; BDNF | Brain injury; major depression | rats | prevented neuronal degeneration; release over 1-2 days; antidepressant-like behavioral effects of BDNF | [132,158] | ||
| Agarose hydrogels | BDNF | Spinal cord injury | rats | encouraged neurite growth into the channels; axonal regeneration; minimal inflammatory response | [113,163] | ||
| Protein bound to collagen in linearly ordered collagen scaffolds (LOCS) | BDNF | Spinal cord injury | in vitro; rats | improved neuron survival and recovery of spinal cord injury | [110] | ||
| Hyaluronic-acid hydrogel scaffold | BDNF | Spinal cord injury | in vitro; rats | regeneration; improvement in locomotive tests | [218] | ||
| Agarose hydrogel coupled with laminin | NGF | Nerve injury | in vitro | enhanced neurite extension | [182] | ||
| Collagen matrix implants | NT-3 | Spinal cord injury | rats | attraction of corticospinal tract fibers into the graft; recovery function | [112] | ||
| Fibrin matrix containing heparin (or peptide) bound via electrostatic interactions to recombinant protein | BDNF; NGF;NT-3 | Unspecified; Spinal cord injury; Sciatic nerve injury | in vitro; rats | enhanced nerve regeneration across short nerve gaps; localized controlled release up to 7 days; dose-dependent axonal regeneration; affinity-based delivery systems for neural tissue engineering | [126,131,150,166,167,171] | ||
| IV. Lipids and diets variations | |||||||
| Caloric restriction; physical exercise | BDNF; GDNF | Parkinson’s disease | Rhesus monkeys | higher locomotor activity; increased neuronal survival in substantia nigra and striatum | [125,130] | ||
| Potentiation by omega-3 fatty acid | BDNF | cellular model of neurodegeneration | in vitro | increased cell survival | [219] | ||
| Triglyceride matrix implants | BDNF (lysozyme model) | Huntington’s disease | in vitro; rats | controlled release over 1-2 months; preserved protein activity and integrity | [120] | ||
| V. Peptidomimetics, small molecule mimetics and prodrugs | |||||||
| Peptidomimetics | BDNF | Neurodegenerative disorders | in vitro | BDNF-like agonist action; sensory neurons survival | [207,208,209,210,211] | ||
| Small molecule mimetics and modulators | BDNF | Motor trauma; Alzheimer’s disease | rodents; in vitro | TrkB agonists; modulation of the activity of the TrkB receptor; improved motor learning; promoted neurogenesis | [212,213,214,215,216] | ||
| Prodrugs of non-peptide neurotrophin mimetics | non-peptide mimetics of BDNF | Psychiatric disorders | Mice | reduced depression- and anxiety-related behaviors; blood-brain barrier penetration | [213,215] | ||
| Peptidomimetics | NT-3 | Peripheral neuropathies; neurodegenerative diseases | in vitro; animal models | selective inhibition of TrkC-mediated cell survival; neuroprotection | [226,227,228] | ||
2.3. Sustained Release of Neurotrophic Factors by Means of Polymer Carriers
2.3.1. Synthetic Polymers
2.3.2. Natural Polymers
2.4. Dietary Restrictions
2.5. Peptide Mimetics of BDNF

3. Soft Nanotechnologies and Nanocarrier-Mediated Delivery
| Nanosystem | Neurotrophin | Disease/Model | Reference |
|---|---|---|---|
| Polysorbate-coated poly(butyl cyanoacrylate) (PBCA) NPs | NGF | Parkinson’s disease/mouse | [122] |
| Nanoporous poly-L-glutamic acid (PGA) particles | BDNF | Deafness/guinea pig, in vitro | [254] |
| Layer-by-layer (LbL) films on agarose hydrogel scaffolds | BDNF (a lysozyme model) | Spinal cord injury/in vitro | [247] |
| Poly(ethylene glycol)-poly(ε-caprolactone) (PEG-PCL) polymersomes conjugated with OX26 MAb | NC-1900 peptide (an arginine-vasopressin fragment analogue) | Learning and memory impairments/rat | [248] |
| PEG-b-PCL polymersomes with surface-attached polyethylene glycol (PEG) chains | NGF mimetic peptide (hNgfEE) as an alternative of BDNF | In vitro | [255,256] |
| Targeted liposomes | NGF | Alzheimer’s disease/in vitro | [176] |
| Immunoliposomes | Model plasmids (luciferase, β-galactosidase, SV40-lacZ) | Brain disorders/rhesus monkey | [231,249,250,260] |
| Cationic liposomes | Plasmid encoding for GDNF or NGF | Spinal cord injury/in vitro | [179,195] |
| NTS (neurotensin)-polyplex nanocarrier | Neurotrophic genes (GDNF, NRTN, BDNF) | Parkinson’s disease/transfected dopaminergic neurons in vitro, rat | [205] |
| PEGylated cationic lipid NPs | Plasmid encoding for BDNF | In vitro | [257,258] |
| Cubosome NPs containing essential omega-3 fatty acid | BDNF | In vitro | [219] |
| Cubosome NPs | Neuroprotective peptide Gly14-humanin | Alzheimer’s disease/rat | [259] |
| Trojan horse nanocarriers | GDNF; plasmid driven by brain-specific promoter | Parkinson’s disease/rodents, rhesus monkeys | [231,232,245,260] |
| Fusion protein vectors | BDNF-IgG (OX26); NGF-IgG; GDNF-Tat | Ischemial stroke, Parkinson’s disease, Alzheimer’s disease/rats | [231,249,250,251,252,253,261] |

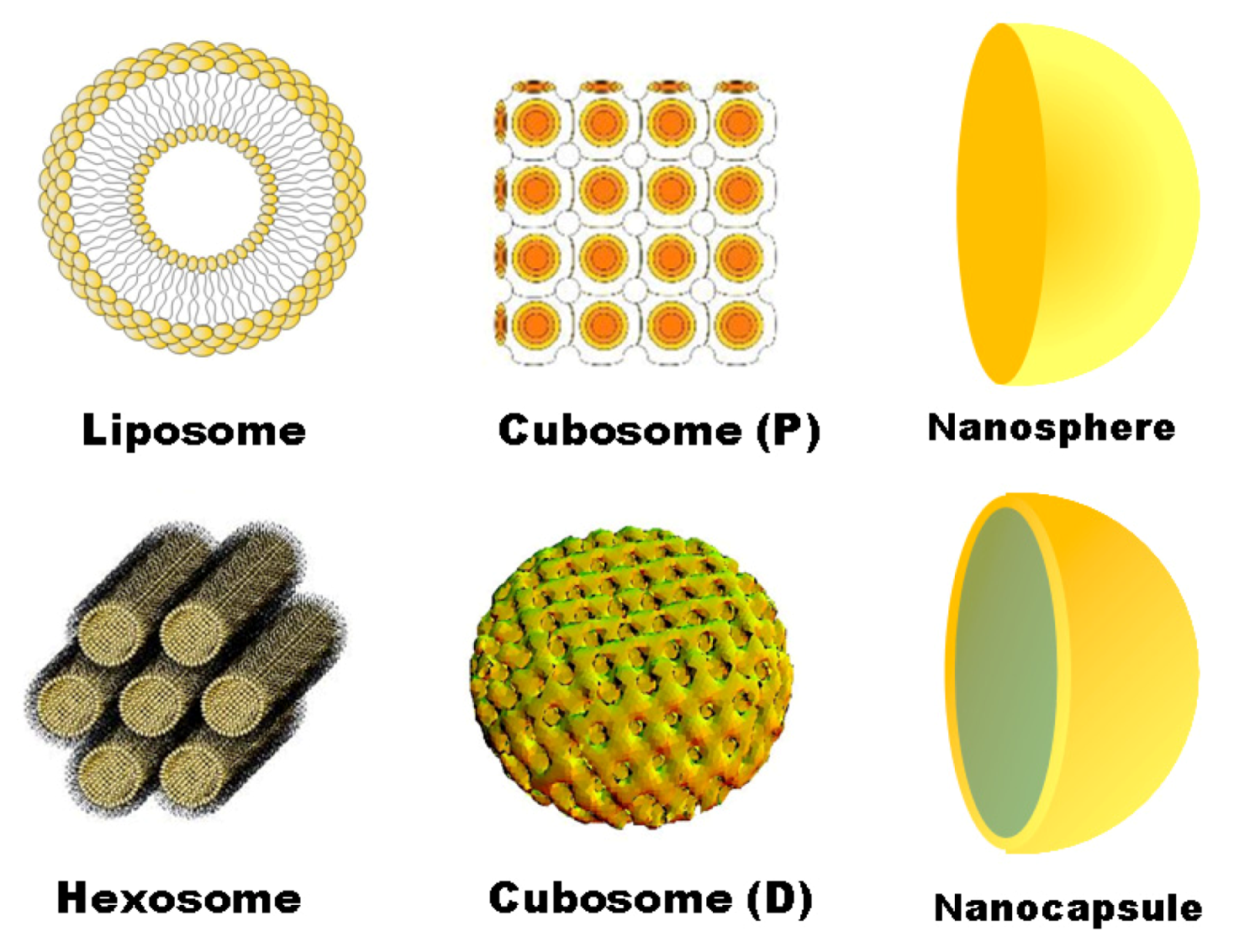
4. Conclusion
Acknowledgments
Conflict of Interest
References
- Zuccato, C.; Cattaneo, E. Brain-derived neurotrophic factor in neurodegenerative diseases. Nat. Rev. Neurol. 2009, 5, 311–322. [Google Scholar] [CrossRef]
- Zuccato, C.; Cattaneo, E. Role of brain-derived neurotrophic factor in Huntington’s disease. Prog. Neurobiol. 2007, 81, 294–330. [Google Scholar] [CrossRef]
- Fumagalli, F.; Molteni, R.; Calabrese, F.; Maj, P.F.; Racagni, G.; Riva, M.A. Neurotrophic factors in neurodegenerative disorders: Potential for therapy. CNS Drugs 2008, 22, 1005–1019. [Google Scholar] [CrossRef]
- Balaratnasingam, S.; Janca, A. Brain derived neurotrophic factor: A novel neurotrophin involved in psychiatric and neurological disorders. Pharmacol. Ther. 2012, 134, 116–124. [Google Scholar] [CrossRef]
- Nagahara, A.H.; Tuszynski, M.H. Potential therapeutic uses of BDNF in neurological and psychiatric disorders. Nat. Rev. Drug Discov. 2011, 10, 209–219. [Google Scholar] [CrossRef]
- Autry, A.E.; Monteggia, L.M. Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacol. Rev. 2012, 64, 238–258. [Google Scholar] [CrossRef]
- Aron, L.; Klein, R. Repairing the parkinsonian brain with neurotrophic factors. TrendsNeurosci. 2011, 34, 88–100. [Google Scholar]
- Chiocco, M.J.; Harvey, B.K.; Wang, Y.; Hoffer, B.J. Neurotrophic factors for the treatment of Parkinson’s disease. Parkinsonism Relat. Disord. 2007, 13, S321–S328. [Google Scholar] [CrossRef]
- Deierborg, T.; Soulet, D.; Roybon, L.; Hall, V.; Brundin, P. Emerging restorative treatments for Parkinson’s disease. Prog. Neurobiol. 2008, 85, 407–432. [Google Scholar] [CrossRef]
- Blesch, A. Neurotrophic factors in neurodegeneration. Brain Pathol. 2006, 16, 295–303. [Google Scholar] [CrossRef]
- Bekinschtein, P.; Cammarota, M.; Katche, C.; Slipczuk, L.; Rossato, J.I.; Goldin, A.; Lzquierdo, I.; Medina, J.H. BDNF is essential to promote persistence of long-term memory storage. Proc. Natl. Acad. Sci. USA 2008, 105, 2711–2716. [Google Scholar]
- Bemelmans, A.P.; Horellou, P.; Pradier, L.; Brunet, I.; Colin, P.; Mallet, J. Brain-derived neurotrophic factor-mediated protection of striatal neurons in an excitotoxic rat model of Huntington’s disease, as demonstrated by adenoviral gene transfer. Hum. Gene Ther. 1999, 10, 2987–2997. [Google Scholar] [CrossRef]
- Byerly, M.S.; Simon, J.; Lebihan-Duval, E.; Duclos, M.J.; Cogburn, L.A.; Porter, T.E. Effects of BDNF, NT-3, and corticosterone on expression of the hypothalamic obesity gene network in vivo and in vitro. Am. J. Physiol-Regul. Integr. Comp. Physiol. 2009, 296, R1180–R1189. [Google Scholar] [CrossRef]
- Canals, J.M.; Pineda, J.R.; Torres-Peraza, J.F.; Bosch, M.; Martin-Ibanez, R.; Munoz, M.T.; Mengod, G.; Ernfors, P.; Alberch, J. Brain-derived neurotrophic factor regulates the onset and severity of motor dysfunction associated with enkephalinergic neuronal degeneration in Huntington’s disease. J. Neurosci. 2004, 24, 7727–7739. [Google Scholar]
- Cannon, T.D.; Yolken, R.; Buka, S.; Torrey, E.F. Collaborative study group on the perinatal origins of severe psychiatric disorders. Decreased neurotrophic response to birth hypoxia in the etiology of schizophrenia. Biol. Psychiatry 2008, 64, 797–802. [Google Scholar] [CrossRef]
- Castrén, E.; Rantamäki, T. The role of BDNF and its receptors in depression and antidepressant drug action: Reactivation of developmental plasticity. Dev. Neurobiol. 2010, 70, 289–297. [Google Scholar] [CrossRef]
- Cattaneo, E.; Zuccato, C.; Tartari, M. Normal huntingtin function: An alternative approach to Huntington’s disease. Nat. Rev. Neurosci. 2005, 6, 919–930. [Google Scholar] [CrossRef]
- Zuccato, C.; Liber, D.; Ramos, C.; Tarditi, A.; Rigamonti, D.; Tartari, M.; Valenza, M.; Cattaneo, E. Progressive loss of BDNF in a mouse model of Huntington’s disease and rescue by BDNF delivery. Pharmacol. Res. 2005, 52, 133–139. [Google Scholar] [CrossRef]
- Fumagalli, F.; Racagni, G.; Riva, M.A. The expanding role of BDNF: A therapeutic target for Alzheimer’s disease? Pharmacogenom. J. 2005, 6, 8–15. [Google Scholar] [CrossRef]
- Galvao, R.; Garcia-Verdugo, J.M.; Alvarez-Buylla, A. Brain-derived neurotrophic factor signaling does not stimulate subventricular zone neurogenesis in adult mice and rats. J. Neurosci. 2008, 28, 13368–13383. [Google Scholar] [CrossRef]
- Gielen, A.; Khademi, M.; Muhallab, S.; Olsson, T.; Piehl, F. Increased brain-derived neurotrophic factor expression in white blood cells of relapsing-remitting multiple sclerosis patients. Scand. J. Immunol. 2003, 57, 493–497. [Google Scholar] [CrossRef]
- Gomez-Pinilla, F. Brain foods: The effects of nutrients on brain function. Nat. Rev. Neurosci. 2008, 9, 568–578. [Google Scholar] [CrossRef]
- Guillin, O.; Griffon, N.; Bezard, E.; Leriche, L.; Diaz, J.; Gross, C.; Sokoloff, P. Brain-derived neurotrophic factor controls dopamine D3 receptor expression: Therapeutic implications in Parkinson’s disease. Eur. J. Pharmacol. 2003, 480, 89–95. [Google Scholar] [CrossRef]
- Liu, Q.R.; Walther, D.; Drgon, T.; Polesskaya, O.; Lesnick, T.G.; Strain, K.J.; de Andrade, M.; Bower, J.H.; Maraganore, D.M.; Uhl, G.R. Human brain derived neurotrophic factor (BDNF) genes, splicing patterns, and assessments of associations with substance abuse and Parkinson’s disease. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2005, 134B, 93–103. [Google Scholar] [CrossRef]
- Lipsky, R.H.; Marini, A.M. Brain-derived neurotrophic factor in neuronal survival and behavior-related plasticity. Ann. N. Y. Acad. Sci. 2007, 1122, 130–143. [Google Scholar] [CrossRef]
- Lu, Y.; Christian, K.; Lu, B. BDNF: A key regulator for protein synthesisdependent LTP and long-term memory? Neurobiol. Learn. Mem. 2008, 89, 312–323. [Google Scholar] [CrossRef]
- Martinowich, K.; Manji, H.; Lu, B. New insights into BDNF function in depression and anxiety. Nat. Neurosci. 2007, 10, 1089–1093. [Google Scholar] [CrossRef]
- Matsumoto, T.; Rauskolb, S.; Polack, M.; Klose, J.; Kolbeck, R.; Korte, M.; Barde, Y.A. Biosynthesis and processing of endogenous BDNF: CNS neurons store and secrete BDNF, not pro-BDNF. Nat. Neurosci. 2008, 11, 131–133. [Google Scholar]
- Matsumoto, K.; Wada, R.K.; Yamashiro, J.M.; Kaplan, D.R.; Thiele, C.J. Expression of brain-derived neurotrophic factor and p145(TrkB) affects survival, differentiation and invasiveness of human neuroblastoma cells. Cancer Res. 1995, 55, 1798–1806. [Google Scholar]
- Monteggia, L.M.; Barrot, M.; Powell, C.M.; Berton, O.; Galanis, V.; Gemelli, T.; Meuth, S.; Nagy, A.; Greene, R.W.; Nestler, E.J. Essential role of brain-derived neurotrophic factor in adult hippocampal function. Proc. Natl. Acad. Sci. USA 2004, 101, 10827–10832. [Google Scholar]
- Murer, M.G.; Yan, Q.; Raisman-Vozari, R. Brain-derived neurotrophic factor in the control humanbrain, and in Alzheimer’s disease and Parkinson’s disease. Prog. Neurobiol. 2001, 63, 71–124. [Google Scholar] [CrossRef]
- Nagahara, A.H.; Merrill, D.A.; Coppola, G.; Tsukada, S.; Schroeder, B.E.; Shaked, G.M.; Wang, L.; Blesch, A.; Kim, A.; Conner, J.M.; et al. Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer’s disease. Nat. Med. 2009, 15, 331–337. [Google Scholar]
- Nakagawa, T.; Ogawa, Y.; Ebihara, K.; Yamanaka, M.; Tsuchida, A.; Taiji, M.; Noguchi, H.; Nakao, K. Antiobesity and antidiabetic effects of brain-derived neurotrophic factor in rodent models of leptin resistance. Int. J. Obes. 2003, 27, 557–565. [Google Scholar] [CrossRef]
- Nosheny, R.L.; Mocchetti, I.; Bachis, A. Brain-derived neurotrophic factor as a prototype neuroprotective factor against HIV-1-associated neuronal degeneration. Neurotox. Res. 2005, 8, 187–198. [Google Scholar] [CrossRef]
- Perez-Navarro, E.; Alberch, J.; Neveu, I.; Arenas, E. Brain-derived neurotrophic factor, neurotrophin-3 and neurotrophin-4/5 differentially regulate the phenotype and prevent degenerative changes in striatal projection neurons after excitotoxicity in vivo. Neuroscience 1999, 91, 1257–1264. [Google Scholar] [CrossRef]
- Pillai, A.; Mahadik, S.P. Increased truncated TrkB receptor expression and decreased BDNF/TrkB signaling in the frontal cortex of reeler mouse model of schizophrenia. Schizophr. Res. 2008, 100, 325–333. [Google Scholar] [CrossRef]
- Pillai, A. Brain-derived neurotropic factor/TrkB signaling in the pathogenesis and novel pharmacotherapy of schizophrenia. Neurosignals 2008, 16, 183–193. [Google Scholar] [CrossRef]
- Rask-Andersen, M.; Olszewski, P.K.; Levine, A.S.; Schiöth, H.B. Molecular mechanisms underlying anorexia nervosa: Focus on human gene association studies and systems controlling food intake. Brain Res. Rev. 2010, 62, 147–164. [Google Scholar] [CrossRef]
- Robinson, R.C.; Radziejewski, C.; Spraggon, G.; Greenwald, J.; Kostura, M.R.; Burtnick, L.D.; Stuart, D.I.; Choe, S.; Jones, E.Y. The structures of the neurotrophin-4 homodimer and the brain-derived neurotrophic factor/neurotrophin-4 heterodimer reveal a common Trk-binding site. Protein Sci. 1999, 8, 2589–2597. [Google Scholar]
- Robinson, R.C.; Radziejewski, C.; Stuart, D.I.; Jones, E.Y. Structure of brain-derived neurotrophic factor/neurotrophin-3 heterodimer. Biochemistry 1995, 34, 4139–4146. [Google Scholar] [CrossRef]
- Romero, M.I.; Rangappa, N.; Garry, M.G.; Smith, G.M. Functional regeneration of chronically injured sensory afferents into adult spinal cord after neurotrophin gene therapy. J. Neurosci. 2001, 21, 8408–8416. [Google Scholar]
- Schabitz, W.R.; Berger, C.; Kollmar, R.; Seitz, M.; Tanay, E.; Kiessling, M.; Schwab, S.; Sommer, C. Effect of brain-derived neurotrophic factor treatment and forced arm use on functional motor recovery after small cortical ischemia. Stroke 2004, 35, 992–997. [Google Scholar] [CrossRef]
- Semkova, I.; Krieglstein, J. Neuroprotection mediated via neurotrophic factors and induction of neurotrophic factors. Brain Res.Brain Res. Rev. 1999, 30, 176–188. [Google Scholar] [CrossRef]
- Spina, M.B.; Squinto, S.P.; Miller, J.; Lindsay, R.M.; Hyman, C. Brain-derived neurotrophic factor protects dopamine neurons against 6-hydroxydopamine and N-methyl-4-phenylpyridinium ion toxicity involvement of the glutathione system. J. Neurochem. 1992, 59, 99–106. [Google Scholar] [CrossRef]
- Stahl, K.; Mylonakou, M.N.; Skare, O.; Amiry-Moghaddam, M.; Torp, R. Cytoprotective effects of growth factors: BDNF more potent than GDNF in an organotypic culture model of Parkinson’s disease. Brain Res. 2011, 1378, 105–118. [Google Scholar] [CrossRef]
- Sun, Y.E.; Wu, H. The ups and downs of BDNF in Rett syndrome. Neuron 2006, 49, 321–323. [Google Scholar] [CrossRef]
- The BDNF Study Group (Phase III). A controlled trial of recombinant methionyl human BDNF in ALS. Neurology 1999, 52, 1427–1433.
- Thompson, R.M.; Weickert, C.S.; Wyatt, E.; Webster, M.J. Decreased BDNF, trkB-TK and GAD67 mRNA expression in the hippocampus of individuals with chizophrenia and mood disorders. J. Psychiatry Neurosci. 2011, 36, 195–203. [Google Scholar] [CrossRef]
- Tsai, S.J. TrkB partial agonists: Potential treatment strategy for epilepsy, mania, and autism. Med. Hypotheses 2006, 6, 173–175. [Google Scholar] [CrossRef]
- Tsai, S.J. TrkB partial agonists: Potential treatment strategy for major depression. Med. Hypotheses 2007, 68, 674–676. [Google Scholar] [CrossRef]
- Tsao, D.; Thomsen, H.K.; Chou, J.; Stratton, J.; Hagen, M.; Loo, C.; Garcia, C.; Sloane, D.L.; Rosenthal, A.; Lin, J.C. TrkB agonists ameliorate obesity and associated metabolic conditions in mice. Endocrinology 2008, 149, 1038–1048. [Google Scholar]
- Vu, T.Q.; Maddipati, R.; Blute, T.A.; Nehilla, B.J.; Nusblat, L.; Desai, T.A. Peptide-conjugated quantum dots activate neuronal receptors and initiate downstream signaling of neurite growth. Nano Lett. 2005, 5, 603–607. [Google Scholar]
- Wang, Z.L.; Cheng, S.M.; Ma, M.M.; Ma, Y.P.; Yang, J.P.; Xu, G.L.; Liu, X.F. Intranasally delivered bFGF enhances neurogenesis in adult rats following cerebral ischemia. Neurosci. Lett. 2008, 446, 30–35. [Google Scholar] [CrossRef]
- Alfa, R.W.; Tuszynski, M.H.; Blesch, A. A novel inducible tyrosine kinase receptor to regulate signal transduction and neurite outgrowth. J. Neurosci. Res. 2009, 87, 2624–2631. [Google Scholar] [CrossRef]
- Bariohay, B.; Roux, J.; Tardivel, C.; Trouslard, J.; Jean, A.; Lebrun, B. Brain-derived neurotrophic factor/tropomyosin-related kinase receptor type B signaling is a downstream effector of the brainstem melanocortin system in food intake control. Endocrinology 2009, 150, 2646–2653. [Google Scholar] [CrossRef]
- Boulle, F.; Kenis, G.; Cazorla, M.; Hamon, H.; Steinbusch, H.W.M.; Lanfumey, L.; van den Hove, D.L.A. TrkB inhibition as a therapeutic target for CNS-related disorders. Prog. Neurobiol. 2012, 98, 197–206. [Google Scholar] [CrossRef]
- Chao, M.V.; Rajagopal, R.; Lee, F.S. Neurotrophin signalling in health anddisease. Clin. Sci. 2006, 110, 167–173. [Google Scholar] [CrossRef]
- Chen, G.; Manji, H.K. The extracellular signal-regulated kinase pathway: An emerging promising target for mood stabilizers. Curr. Opin. Psychiatry 2006, 19, 313–323. [Google Scholar] [CrossRef]
- Corominas, M.; Roncero, C.; Ribases, M.; Castells, X.; Casas, M. Brain-derived neurotrophic factor and its intracellular signaling pathways in cocaine addiction. Neuropsychobiology 2007, 55, 2–13. [Google Scholar] [CrossRef]
- Cowansage, K.K.; LeDoux, J.E.; Monfils, M.H. Brain-derived neurotrophic factor: A dynamic gatekeeper of neural plasticity. Curr. Mol. Pharmacol. 2010, 3, 12–29. [Google Scholar]
- Desmet, C.J.; Peeper, D.S. The neurotrophic receptor TrkB: A drug target in anti-cancer therapy? Cell. Mol. Life Sci. 2006, 63, 755–759. [Google Scholar] [CrossRef]
- Dwivedi, Y.; Rizavi, H.S.; Conley, R.R.; Roberts, R.C.; Tamminga, C.A.; Pandey, G.N. Altered gene expression of brain-derived neurotrophic factor and receptor tyrosine kinase B in postmortem brain of suicide subjects. Arch. Gen. Psychiatry 2003, 60, 804–815. [Google Scholar] [CrossRef]
- Ehrnhoefer, D.E.; Wong, B.K.Y.; Hayden, M.R. Convergent pathogenic pathways in Alzheimer’s and Huntington’s diseases: Shared targets for drug development. Nat. Rev. Drug Discov. 2011, 10, 853–867. [Google Scholar] [CrossRef]
- Elliott, E.; Atlas, R.; Lange, A.; Ginzburg, I. Brain-derived neurotrophic factor induces a rapid dephosphorylation of Tau protein through a PI-3 kinase signalling mechanism. Eur. J. Neurosci. 2005, 22, 1081–1089. [Google Scholar] [CrossRef]
- Hashimoto, K.; Koizumi, H.; Nakazato, M.; Shimizu, E.; Iyo, M. Role of brain-derived neurotrophic factor in eating disorders: Recent findings and its pathophysiological implications. Prog. Neuropsychopharmacol. Biol. Psychiatry 2005, 29, 499–504. [Google Scholar] [CrossRef]
- He, X.P.; Kotloski, R.; Nef, S.; Luikart, B.W.; Parada, L.F.; McNamara, J.O. Conditional deletion of TrkB but not BDNF prevents epileptogenesis in the kindling model. Neuron 2005, 43, 31–42. [Google Scholar]
- Kang, H.; Schuman, E.M. A requirement for local protein synthesis in neurotrophin-induced hippocampal synaptic plasticity. Science 1996, 273, 1402–1406. [Google Scholar]
- Nishida, Y.; Adati, N.; Ozawa, R.; Maeda, A.; Sakaki, Y.; Takeda, T. Identification and classification of genes regulated by phosphatidylinositol 3-kinase- and TrkB-mediated signalling pathways during neuronal differentiation in two subtypes of the human neuroblastoma cell line SH-SY5Y. BMC Res. Notes 2008, 1, 95. [Google Scholar] [CrossRef]
- Ibanez, C.F.; Ilag, L.L.; Murrayrust, J.; Persson, H. An extended surface of binding to Trk tyrosine kinase receptors in NGF and BDNF allows the engineering of a multifunctional pan-neurotrophin. EMBO J. 1993, 12, 2281–2293. [Google Scholar]
- Jaboin, J.; Kim, C.J.; Kaplan, D.R.; Thiele, C.J. Brain-derived neurotrophic factor activation of TrkB protects neuroblastoma cells from chemotherapy-induced apoptosis via phosphatidylinositol 3'-kinase pathway. Cancer Res. 2002, 62, 6756–6763. [Google Scholar]
- Kermani, P.; Rafii, D.; Jin, D.K.; Whitlock, P.; Schaffer, W.; Chiang, A.; Vincent, L.; Friedrich, M.; Shido, K.; Hackett, N.R.; et al. Neurotrophins promote revascularization by local recruitment of TrkB(+) endothelial cells and systemic mobilization of hematopoietic progenitors. J. Clin. Invest. 2005, 115, 653–663. [Google Scholar]
- Lessmann, V.; Gottmann, K.; Malcangio, M. Neurotrophin secretion: Current facts and future prospects. Prog. Neurobiol. 2003, 69, 341–374. [Google Scholar] [CrossRef]
- Malcangio, M.; Lessmann, V. A common thread for pain and memory synapses? Brain-derived neurotrophic factor and TrkB receptors. Trends Pharmacol. Sci. 2003, 24, 116–121. [Google Scholar] [CrossRef]
- Pattarawarapan, M.; Burgess, K. Molecular basis of neurotrophin-receptor interactions. J. Med. Chem. 2003, 46, 5277–5291. [Google Scholar] [CrossRef]
- Rantamaäki, T.; Hendolin, P.; Kankaanpää, A.; Mijatovic, J.; Piepponen, P.; Domenici, E.; Chao, M.V.; Männistö, P.T.; Castrén, E. Pharmacologically diverse antidepressants rapidly activate brain-derived neurotrophic factor receptor TrkB and induce phospholipase-Cgamma signaling pathways in mouse brain. Neuropsychopharmacology 2007, 32, 2152–2162. [Google Scholar] [CrossRef]
- Yang, J.; Siao, C.J.; Nagappan, G.; Marinic, T.; Jing, D.; McGrath, K.; Chen, Z.Y.; Mark, W.; Tessarollo, L.; Lee, F.S.; et al. Neuronal release of proBDNF. Nat. Neurosci. 2009, 12, 113–115. [Google Scholar]
- Reichardt, L.F. Neurotrophin-regulated signalling pathways. Phil. Trans. R. Soc. B 2006, 361, 1545–1564. [Google Scholar]
- Schramm, A.; Schulte, J.H.; Astrahantseff, K.; Apostolov, O.; van Limpt, V.; Sieverts, H.; Kuhfittig-Kulle, S.; Pfeiffer, P.; Versteeg, R.; Eggert, A. Biological effects of TrkA and TrkB receptor signaling in neuroblastoma. Cancer Lett. 2005, 228, 143–153. [Google Scholar] [CrossRef]
- Saarelainen, T.; Hendolin, P.; Lucas, G.; Koponen, E.; Sairanen, M.; MacDonald, E.; Agerman, K.; Haapasalo, A.; Nawa, H.; Aloyz, R.; et al. Activation of the TrkB neurotrophin receptor is induced by antidepressant drugs and is required for antidepressant-induced behavioral effects. J. Neurosci. 2003, 23, 349–357. [Google Scholar]
- Soliman, F.; Glatt, C.E.; Bath, K.G.; Levita, L.; Jones, R.M.; Pattwell, S.S.; Jing, D.; Tottenham, N.; Amso, D.; Somerville, L.H.; et al. A genetic variant BDNF polymorphism alters extinction learning in both mouse and human. Science 2010, 327, 863–866. [Google Scholar]
- Waterhouse, E.G.; Xu, B. New insights into the role of brain-derived neurotrophic factor in synaptic plasticity. Mol. Cell Neurosci. 2009, 42, 81–89. [Google Scholar] [CrossRef]
- Yang, T.; Yin, W.; Derevyanny, V.D.; Moore, L.A.; Longo, F.M. Identification of an ectodomain within the LAR protein tyrosine phosphatase receptor that binds homophilically and activates signalling pathways promoting neurite outgrowth. Eur. J. Neurosci. 2005, 22, 2159–2170. [Google Scholar] [CrossRef]
- Yoshii, A.; Constantine-Paton, M. Postsynaptic BDNF-TrkB signaling in synapse maturation, plasticity, and disease. Dev. Neurobiol. 2010, 70, 304–322. [Google Scholar]
- Zhang, Q.; Liu, G.; Wu, Y.; Sha, H.; Zhang, P.; Jia, J. BDNF promotes EGF-induced proliferation and migration of human fetal neural stem/progenitor cells via the PI3K/Akt pathway. Molecules 2011, 16, 10146–10156. [Google Scholar] [CrossRef]
- Frank, L.; Ventimiglia, R.; Anderson, K.; Lindsay, R.M.; Rudge, J.S. BDNF down-regulates neurotrophin responsiveness, TrkB protein and TrkB mRNA levels in cultured rat hippocampal neurons. Eur. J. Neurosci. 1996, 8, 1220–1230. [Google Scholar] [CrossRef]
- Knusel, B.; Gao, H.; Okazaki, T.; Yoshida, T.; Mori, N.; Hefti, F.; Kaplan, D.R. Ligand-induced down-regulation of Trk messenger RNA, protein and tyrosine phosphorylation in rat cortical neurons. Neuroscience 1997, 78, 851–862. [Google Scholar] [CrossRef]
- Sommerfeld, M.T.; Schweigreiter, R.; Barde, Y.A.; Hoppe, E. Downregulation of the neurotrophin receptor TrkB following ligand binding. Evidence for an involvement of the proteasome and differential regulation of TrkA and TrkB. J. Biol. Chem. 2000, 275, 8982–8990. [Google Scholar]
- Agterberg, M.J.H.; Versnel, H.; van Dijk, L.M.; de Groot, J.; Klis, S.F.L. Enhanced survival of spiral ganglion cells after cessation of treatment with brain-derived neurotrophic factor in deafened guinea pigs. J. Assoc. Res. Otolaryngol. 2009, 10, 355–367. [Google Scholar] [CrossRef]
- Ankeny, D.P.; McTigue, D.M.; Guan, Z.; Yan, Q.; Kinstler, O.; Stokes, B.T.; Jakeman, L.B. Pegylated brain-derived neurotrophic factor shows improved distribution into the spinal cord and stimulates locomotor activity and morphological changes after injury. Exp. Neurol. 2001, 170, 85–100. [Google Scholar]
- Aubert-Pouessel, A.; Venier-Julienne, M.C.; Clavreul, A.; Sergent, M.; Jollivet, C.; Montero-Menei, C.N.; Garcion, E.; Bibby, D.C.; Menei, P.; Benoit, J.P. In vitro study of GDNF release from biodegradable PLGA microspheres. J. Control. Release 2004, 95, 463–475. [Google Scholar] [CrossRef]
- Alcala-Barraza, S.R.; Lee, M.S.; Hanson, L.R.; McDonald, A.A.; Frey, W.H., II; McLoon, L.K. Intranasal delivery of neurotrophic factors BDNF, CNTF, EPO, and NT-4 to the CNS. J. Drug Target. 2010, 18, 179–190. [Google Scholar] [CrossRef]
- Barras, F.M.; Pasche, P.; Bouche, N.; Aebischer, P.; Zurn, A.D. Glial cell line-derived neurotrophic factor released by synthetic guidance channels promotes facial nerve regeneration in the rat. J. Neurosci. Res. 2002, 70, 746–755. [Google Scholar] [CrossRef]
- Benoit, J.P.; Faisant, N.; Venier-Julienne, M.C.; Menei, P. Development of microspheres for neurological disorders: From basics to clinical applications. J. Control. Release 2000, 65, 285–296. [Google Scholar] [CrossRef]
- Bertram, J.P.; Rauch, M.F.; Chang, K.; Lavik, E.B. Using polymer chemistry to modulate the delivery of neurotrophic factors from degradable microspheres: Delivery of BDNF. Pharm. Res. 2010, 27, 82–91. [Google Scholar] [CrossRef]
- Bauman, M.D.; Kang, C.E.; Stanwick, J.C.; Wang, Y.F.; Kim, H.; Lapitsky, Y.; Shoichet, M.S. An injectable dtug delivery platform for sustained combinatory therapy. J. Control. Release 2009, 138, 205–213. [Google Scholar] [CrossRef]
- Bloch, J.; Fine, E.G.; Bouche, N.; Zurn, A.D.; Aebischer, P. Nerve growth factor- and neurotrophin-3-releasing guidance channels promote regeneration of the transected rat dorsal root. Exp. Neurol. 2001, 172, 425–432. [Google Scholar]
- Burdick, J.A.; Ward, M.; Liang, E.; Young, M.J.; Langer, R. Stimulation of neurite outgrowth by neurotrophins delivered from degradable hydrogels. Biomaterials 2006, 27, 452–459. [Google Scholar] [CrossRef]
- Clavreul, A.; Sindji, L.; Aubert-Pouessel, A.; Benoi, J.P.; Menei, P.; Montero-Menei, C.N. Effect of GDNF-releasing biodegradable microspheres on the function and the survival of intrastriatal fetal ventral mesencephalic cell grafts. Eur. J. Pharm. Biopharm. 2006, 63, 221–228. [Google Scholar] [CrossRef]
- Crigler, L.; Robey, R.C.; Asawachaicharn, A.; Gaupp, D.; Phinney, D.G. Human mesenchymal stem cell subpopulations express a variety of neuro-regulatory molecules and promote neuronal cell survival and neuritogenesis. Exp. Neurol. 2006, 198, 54–64. [Google Scholar] [CrossRef]
- Eriksdotter-Jonhagen, M. Local delivery of NGF to basal forebrain in AD patients. Alzheimers Dement. 2010, 6, S147–S148. [Google Scholar]
- Fine, E.G.; Decosterd, I.; Papaloizos, M.; Zurn, A.D.; Aebischer, P. GDNF and NGF released by synthetic guidance channels support sciatic nerve regeneration across a long gap. Eur. J. Neurosci. 2002, 15, 589–601. [Google Scholar] [CrossRef]
- Fjord-Larsen, L.; Kusk, P.; Tornoe, J.; Juliusson, B.; Torp, M.; Bjarkam, C.R.; Nielsen, M.S.; Handberg, A.; Sorensen, J.C.H.; Wahlberg, L.U. Long-term delivery of nerve growth factor by encapsulated cell biodelivery in the Göttingen minipig basal forebrain. Molec. Ther. 2010, 18, 2164–2172. [Google Scholar] [CrossRef]
- Frim, D.M.; Uhler, T.A.; Galpern, W.R.; Beal, M.F.; Breakefield, X.O.; Icasson, O. Implanted fibroblasts genetically engineered to produce brain-derived neurotrophic factor prevent 1-methyl-4-phenylpyridinium toxicity to dopaminergic neurons in the rat. Proc. Natl. Acad. Sci. USA 1994, 91, 5104–5108. [Google Scholar]
- Garbayo, E.; Ansorena, E.; Lanciego, J.L.; Aymerich, M.S.; Blanco-Prieto, M.J. Sustained release of bioactive glycosylated glial cell-line derived neurotrophic factor from biodegradable polymeric microspheres. Eur. J. Pharm. Biopharm. 2008, 69, 844–851. [Google Scholar] [CrossRef]
- Garbayo, E.; Montero-Menei, C.N.; Ansorena, E.; Lanciego, J.L.; Aymerich, M.S.; Blanco-Prieto, M.J. Effective GDNF brain delivery using microspheres—A promising strategy for Parkinson’s disease. J. Control. Release 2009, 135, 119–126. [Google Scholar] [CrossRef] [Green Version]
- Garbayo, E.; Ansorena, E.; Lanciego, J.L.; Blanco-Prieto, M.J.; Aymerich, M.S. Long-term neuroprotection and neurorestoration by glial cell-derived neurotrophic factor microspheres for the treatment of Parkinson's disease. Mov. Disord. 2011, 26, 1943–1947. [Google Scholar] [CrossRef]
- Gill, S.S.; Patel, N.K.; Hotton, G.R.; O’Sullivan, K.; McCarter, R.; Bunnage, M.; Brooks, D.J.; Svendsen, C.N.; Heywood, P. Direct brain infusion of glial cell line-derived neurotrophic factor in Parkinson’s disease. Nat. Med. 2003, 12, 479–479. [Google Scholar]
- Gomez, N.; Schmidt, C.E. Nerve growth factor-immobilized polypyrrole: Bioactive electrically conducting polymer for enhanced neurite extension. J. Biomed. Mater. Res. Part A 2006, 81A, 135–149. [Google Scholar] [CrossRef]
- Guan, J.; Tong, W.; Ding, W.; Du, S.; Xiao, Z.; Han, Q.; Zhu, Z.; Bao, X.; Shi, X.; Wu, C.; et al. Neuronal regeneration and protection by collagen-binding BDNF in the rat middle cerebral artery occlusion model. Biomaterials 2012, 33, 1386–1395. [Google Scholar] [CrossRef]
- Han, Q.Q.; Sun, W.J.; Lin, H.; Zhao, W.X.; Gao, Y.; Zhao, Y.N.; Chen, B.; Xiao, Z.F.; Hu, W.; Li, Y.; et al. Linear ordered collagen scaffolds loaded with collagen-binding brain-derived neurotrophic factor improve the recovery of spinal cord injury in rats. Tissue Eng. Part A 2009, 15, 2927–2935. [Google Scholar]
- Hoshaw, B.A.; Malberg, J.E.; Lucki, I. Central administration of IGF-I and BDNF leads to long-lasting antidepressant-like effects. Brain Res. 2005, 1037, 204–208. [Google Scholar] [CrossRef]
- Houweling, D.A.; Lankhorst, A.J.; Gispen, W.H.; Bar, P.R.; Joosten, E.A.J. Collagen containing neurotrophin-3 (NT-3) attracts regrowing injured corticospinal axons in the adult rat spinal cord and promotes partial functional recovery. Exp. Neurol. 1998, 153, 49–59. [Google Scholar]
- Jain, A.; Kim, Y.T.; McKeon, R.J.; Bellamkonda, R.V. In situ gelling hydrogels for conformal repair of spinal cord defects, and local delivery of BDNF after spinal cord injury. Biomaterials 2006, 27, 497–504. [Google Scholar] [CrossRef]
- Jiang, Y.; Lv, H.; Huang, S.; Tan, H.; Zhang, Y.; Li, H. Bone marrow mesenchymal stem cells can improve the motor function of a Huntington’s disease rat model. Neurol. Res. 2011, 33, 331–337. [Google Scholar] [CrossRef]
- Jollivet, C.; Aubert-Pouessel, A.; Clavreul, A.; Venier-Julienne, M.C.; Montero-Menei, C.N.; Benoit, J.P.; Menei, P. Long-term effect of intra-striatal glial cell line-derived neurotrophic factor-releasing microspheres in a partial rat model of Parkinson's disease. Neurosci. Lett. 2004, 356, 207–210. [Google Scholar] [CrossRef]
- Jollivet, C.; Aubert-Pouessel, A.; Clavreul, A.; Venier-Julienne, M.C.; Remy, S.; Montero-Menei, C.N.; Benoit, J.P.; Menei, P. Striatal implantation of GDNF releasing biodegradable microspheres promotes recovery of motor function in a partial model of Parkinson’s disease. Biomaterials 2004, 25, 933–942. [Google Scholar] [CrossRef]
- Koda, M.; Kamada, T.; Hashimoto, M.; Murakami, M.; Shirasawa, H.; Sakao, S.; Ino, H.; Yoshinaga, K.; Koshizuka, S.; Moriya, H.; et al. Adenovirus vector-mediated ex vivo gene transfer of brain-derived neurotrophic factor to bone marrow stromal cells promotes axonal regeneration after transplantation in completely transected adult rat spinal cord. Eur. Spine J. 2007, 16, 2206–2214. [Google Scholar] [CrossRef]
- Koda, M.; Hashimoto, M.; Murakami, M.; Yoshinaga, K.; Ikeda, O.; Yamazaki, M.; Koshizuka, S.; Kamada, T.; Moriya, H.; Shirasawa, H.; et al. Adenovirus vector-mediated in vivo gene transfer of brain-derived neurotrophic factor (BDNF) promotes rubrospinal axonal regeneration and functional recovery after complete transection of the adult rat spinal cord. J. Neurotrauma 2004, 21, 329–337. [Google Scholar] [CrossRef]
- Kordower, J.H.; Palfi, S.; Chen, E.; Ma, S.; Sendera, T.; Cochran, E.J.; Mufson, E.J.; Penn, R.; Goetz, C.G.; Comella, C.D. Clinico-pathological findings following intraventricular GDNF treatment in patient with Parkinson’s disease. Ann. Neurol. 1999, 46, 419–424. [Google Scholar] [CrossRef]
- Koennings, S.; Sapin, A.; Blunk, T.; Menei, P.; Goepferich, A. Towards controlled release of BDNF-manufacturing strategies for protein-loaded lipid implants and biocompatibility evaluation in the brain. J. Control. Release 2007, 119, 163–172. [Google Scholar] [CrossRef]
- Krewson, C.E.; Klarman, M.L.; Saltzman, W.M. Distribution of nerve growth factor following direct delivery to brain interstitium. Brain Res. 1995, 680, 196–206. [Google Scholar] [CrossRef]
- Kurakhmaeva, K.B.; Djindjikhashvili, I.A.; Petrov, V.E.; Balabanyan, V.U.; Voronina, T.A.; Trofimov, S.S.; Kreuter, J.; Gelperina, S.; Begley, D.; Alyautdin, R.N. Brain targeting of nerve growth factor using poly(butyl cyanoacrylate) nanoparticles. J. Drug Target. 2009, 17, 564–574. [Google Scholar] [CrossRef]
- Lam, X.M.; Duenas, E.T.; Cleland, J.L. Encapsulation and stabilization of nerve growth factor into poly(lactic-co-glycolic) acid microspheres. J. Pharm. Sci. 2001, 90, 1356–1365. [Google Scholar] [CrossRef]
- Lang, A.E.; Gill, S.; Patel, N.K.; Lozano, A.; Nutt, J.G.; Penn, R.; Brooks, D.J.; Hotton, G.; Moro, E.; Heywood, P.; et al. Randomized controlled trial of intraputamenal glial cell line-derived neurotrophic factor infusion in Parkinson’s disease. Ann. Neurol. 2006, 59, 459–466. [Google Scholar] [CrossRef]
- Lebrun, B.; Bariohay, B.; Moyse, E.; Jean, A. Brain-derived neurotrophic factor (BDNF) and food intake regulation. Auton. Neurosci. Basic Clin. 2006, 126, 30–38. [Google Scholar] [CrossRef]
- Lee, A.C.; Yu, V.M.; Lowe, J.B.; Brenner, M.J.; Hunter, D.A.; Mackinnon, S.E.; Sakiyama-Elbert, S.E. Controlled release of nerve growth factor enhances sciatic nerve regeneration. Exp. Neurol. 2003, 184, 295–303. [Google Scholar] [CrossRef]
- Levivier, M.; Przedborski, S.; Bencsics, C.; Kang, U.J. Intrastriatal implantation of fibroblasts genetically engineered to produce brain-derived neurotrophic factor prevents degeneration of dopaminergic neurons in a rat model of Parkinson’s disease. J. Neurosci. 1995, 15, 7810–7820. [Google Scholar]
- Li, L.-Y.; Li, J.-T.; Wu, Q.-Y.; Li, J.; Feng, Z.-T.; Liu, S.; Wang, T.-H. Transplantation of NGF-gene-modified bone marrow stromal cells into a rat model of Alzheimer’s disease. J. Mol. Neurosci. 2008, 34, 157–163. [Google Scholar] [CrossRef]
- Malerba, F.; Paoletti, F.; Capsoni, S.; Cattaneo, A. Intranasal delivery of therapeutic proteins for neurological diseases. Expert Opin. Drug Deliv. 2011, 8, 1277–1296. [Google Scholar] [CrossRef]
- Maswood, N.; Young, J.; Tilmont, E.; Zhang, Z.M.; Gash, D.M.; Gerhardt, G.A.; Grondin, R.; Roth, G.S.; Mattison, J.; Lane, M.A.; et al. Caloric restriction increases neurotrophic factor levels and attenuates neurochemical and behavioral deficits in a primate model of Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2004, 101, 18171–18176. [Google Scholar]
- Maxwell, D.J.; Hicks, B.C.; Parsons, S.; Sakiyama-Elbert, S.E. Development of rationally designed affinity-based drug delivery systems. Acta Biomater. 2005, 1, 101–113. [Google Scholar] [CrossRef]
- Maysinger, D.; Jalsenjak, I.; Cuello, A.C. Microencapsulated nerve growth factor: Effects on the forebrain neurons following devascularizing cortical lesions. Neurosci. Lett. 1992, 140, 71–74. [Google Scholar]
- McGuinness, S.L.; Shepherd, R.K. Exogenous BDNF rescues rat spiral ganglion neurons in vivo. Otol. Neurol. 2005, 26, 1064–1072. [Google Scholar] [CrossRef]
- Menei, P.; Pean, J.M.; Nerriere-Daguin, V.; Jollivet, C.; Brachet, P.; Benoit, J.P. Intracerebral implantation of NGF-releasing biodegradable microspheres protects striatum against excitotoxic damage. Exp. Neurol. 2000, 161, 259–272. [Google Scholar] [CrossRef]
- Moloney, T.C.; Rooney, G.E.; Barry, F.P.; Howard, L.; Dowd, E. Potential of rat bone marrow-derived mesenchymal stem cells as vehicles for delivery of neurotrophins to the Parkinsonian rat brain. Brain Res. 2010, 1359, 33–43. [Google Scholar]
- Moore, K.; Macsween, M.; Shoichet, M. Immobilized concentration gradients of neurotrophic factors guide neurite outgrowth of primary neurons in macroporous scaffolds. Tissue Eng. 2006, 12, 267–278. [Google Scholar] [CrossRef]
- Nakahara, Y.; Gage, F.H.; Tuszynski, M.H. Grafts of fibroblasts genetically modified to secrete NGF, BDNF, NT-3, or basic fgf elicit differential responses in the adult spinal cord. Cell. Transplant. 1996, 5, 191–204. [Google Scholar] [CrossRef]
- Nomura, T.; Honmou, O.; Harada, K.; Houkin, K.; Hamada, H.; Kocsis, J.D. I.v. fusion of brain-derived neurotrophic factor gene-modified human mesenchymal stem cells protects against injury in a cerebral ischemia model in adult rat. Neuroscience 2005, 136, 161–169. [Google Scholar] [CrossRef]
- Nutt, J.G.; Burchiel, K.J.; Comella, C.L.; Jankovic, J.; Lang, A.E.; Laws, E.R., Jr.; Lozano, A.M.; Penn, R.D.; Simpson, R.K., Jr.; et al. Randomized, double-blind trial of glial cell line-derived neurotrophic factor (GDNF) in PD. Neurology 2003, 60, 69–73. [Google Scholar]
- Paradiso, B.; Marconi, P.; Zucchini, S.; Berto, E.; Binaschi, A.; Bozac, A.; Buzzi, A.; Mazzuferi, M.; Magri, E.; Mora, G.N.; et al. Localized delivery of fibroblast growth factor-2 and brain-derived neurotrophic factor reduces spontaneous seizures in an epilepsy model. Proc. Natl. Acad. Sci. USA 2009, 106, 7191–7196. [Google Scholar]
- Pardridge, W.M.; Wu, D.F.; Sakane, T. Combined use of carboxyl-directed protein pegylation and vector-mediated blood-brain barrier drug delivery system optimizes brain uptake of brain-derived neurotrophic factor following intravenous administration. Pharm. Res. 1998, 15, 576–582. [Google Scholar] [CrossRef]
- Patist, C.M.; Mulder, M.B.; Gautier, S.E.; Maquet, V.; Jerome, R.; Oudega, M. Freeze-dried poly(D,L-lactic acid) macroporous guidance scaffolds impregnated with brain-derived neurotrophic factor in the transected adult rat thoracic spinal cord. Biomaterials 2004, 25, 1569–1582. [Google Scholar] [CrossRef]
- Pean, J.M.; Venier-Julienne, M.C.; Boury, F.; Menei, P.; Denizot, B.; Benoit, J.P. NGF release from poly(D,L-lactide-co-glycolide) microspheres: Effect of some formulation parameters on encapsulated NGF stability. J. Control. Release 1998, 56, 175–187. [Google Scholar]
- Pean, J.M.; Venier-Julienne, M.C.; Filmon, R.; Sergent, M.; Phan-Tan-Luu, R.; Benoit, J.P. Optimization of HSA and NGF encapsulation yields in PLGA microparticles. Int. J. Pharm. 1998, 166, 105–115. [Google Scholar] [CrossRef]
- Piantino, J.; Burdick, J.A.; Goldberg, D.; Langer, R.; Benowitz, L.I. An injectable, biodegradable hydrogel for trophic factor delivery enhances axonal rewiring and improves performance after spinal cord injury. Exp. Neurol. 2006, 201, 359–367. [Google Scholar] [CrossRef]
- Richardson, R.T.; Thompson, B.; Moulton, S.; Newbold, C.; Lum, M.G.; Cameron, A.; Wallace, G.; Kapsa, R.; Clark, G.; O’Leary, S. The effect of polypyrrole with incorporated neurotrophin-3 on the promotion of neurite outgrowth from auditory neurons. Biomaterials 2007, 28, 513–523. [Google Scholar] [CrossRef]
- Rosenberg, M.B.; Friedmann, T.; Robertson, R.C.; Tuszynski, M.; Wolff, J.A.; Breakefield, X.O.; Gage, F.H. Grafting genetically modified cells to the damaged brain: Restorative effects of NGF expression. Science 1998, 242, 1575–1578. [Google Scholar]
- Sadan, O.; Bahat-Stromza, M.; Barhum, Y.; Levy, Y.S.; Pisnevsky, A.; Peretz, H.; Ilan, A.B.; Bulvik, S.; Shemesh, N.; Krepel, D.; et al. Protective effects of neurotrophic factors-secreting cells in a 6-OHDA rat model of Parkinson disease. Stem Cells Dev. 2009, 18, 1179–1190. [Google Scholar] [CrossRef]
- Sakane, T.; Pardridge, W.M. Carboxyl-directed pegylation of brain-derived neurotrophic factor markedly reduces systemic clearance with minimal loss of biologic activity. Pharm. Res. 1997, 14, 1085–1091. [Google Scholar] [CrossRef]
- Sakiyama-Elbert, S.E.; Hubbell, J.A. Controlled release of nerve growth factor from a heparin-containing fibrin-based cell ingrowth matrix. J. Control. Release 2000, 69, 149–158. [Google Scholar] [CrossRef]
- Sakiyama-Elbert, S.E.; Hubbell, J.A. Development of fibrin derivatives for controlled release of heparin-binding growth factors. J. Control. Release 2000, 65, 389–402. [Google Scholar] [CrossRef]
- Sakiyama-Elbert, S.E.; Panitch, A.; Hubbell, J.A. Development of growth factor fusion proteins for cell-triggered drug delivery. FASEB J. 2001, 15, 1300–1302. [Google Scholar]
- Saltzman, W.M.; Mak, M.W.; Mahoney, M.J.; Duenas, E.T.; Cleland, J.L. Intracranial delivery of recombinant nerve growth factor: Release kinetics and protein distribution for three delivery systems. Pharm. Res. 1999, 16, 232–240. [Google Scholar] [CrossRef]
- Schabitz, W.R.; Schwab, S.; Spranger, M.; Hacke, W. Intraventricular brain-derived neurotrophic factor reduces infarct size after focal cerebral ischemia in rats. J. Cereb. Blood Flow Metab. 1997, 17, 500–506. [Google Scholar]
- Schabitz, W.R.; Sommer, C.; Zoder, W.; Kiessling, M.; Schwaninger, M.; Schwab, S. Intravenous brain-derived neurotrophic factor reduces infarct size and counterregulates Bax and Bcl-2 expression after temporary focal cerebral ischemia. Stroke 2000, 31, 2212–2217. [Google Scholar] [CrossRef]
- Scharfman, H.; Goodman, J.; Macleod, A.; Phani, S.; Antonelli, C.; Croll, S. Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Exp. Neurol. 2005, 192, 348–356. [Google Scholar] [CrossRef]
- Sharma, H.S.; Sharma, A.; Mössler, H.; Muresanu, D.F. Neuroprotective effects of cerebrolysin, a combination of different active fragments of neurotrophic factors and peptides on the whole body hyperthermia-induced neurotoxicity: Modulatory roles of co-morbidity factors and nanoparticle intoxication. Int. Rev. Neurobiol. 2012, 102, 249–276. [Google Scholar] [CrossRef]
- Sirianni, R.W.; Olausson, P.; Chiu, A.S.; Taylor, J.R.; Saltzman, W.M. The behavioural and biochemical effects of BDNF containing polymers implanted in the hippocampus of rats. Brain Res. 2010, 1321, 40–50. [Google Scholar]
- Slevin, J.T.; Gash, D.M.; Smith, C.D.; Gerhardt, G.A.; Kryscio, R.; Chebrolu, H.; Walton, A.; Wagner, R.; Young, B. Unilateral intraputamenal glial cell line-derived neurotrophic factor in patients with Parkinson disease: Response to 1 year of treatment and 1 year of withdrawal. J. Neurosurg. 2007, 106, 614–620. [Google Scholar] [CrossRef]
- Soderquist, R.G.; Milligan, E.D.; Sloane, E.M.; Harrison, J.A.; Douvas, K.K.; Potter, J.M.; Hughes, T.S.; Chavez, R.A.; Johnson, K.; Watkins, L.R.; et al. PEGylation of brain-derived neurotrophic factor for preserved biological activity and enhanced spinal cord distribution. J. Biomed. Mater. Res. Part A 2009, 91A, 719–729. [Google Scholar] [CrossRef]
- Somoza, R.; Juri, C.; Baes, M.; Wyneken, U.; Rubio, F.J. Intranigral transplantation of epigenetically induced BDNF-secreting human mesenchymal stem cells: Implications for cell-based therapies in Parkinson’s disease. Biol. Blood Marrow Transplant. 2010, 16, 1530–1540. [Google Scholar] [CrossRef]
- Son, J.H.; Chun, H.S.; Joh, T.H.; Cho, S.; Conti, B.; Lee, J.W. Neuroprotection and neuronal differentiation studies using substantia nigra dopaminergic cells derived from transgenic mouse embryos. J. Neurosci. 1999, 19, 10–20. [Google Scholar]
- Stokols, S.; Tuszynski, M.H. Freeze-dried agarose scaffolds with uniaxial channels stimulate and guide linear axonal growth following spinal cord injury. Biomaterials 2006, 27, 443–451. [Google Scholar] [CrossRef]
- Takeshima, Y.; Nakamura, M.; Miyake, H.; Tamaki, R.; Inui, T.; Horiuchi, K.; Wajima, D.; Nakase, H. Neuroprotection with intraventricular brain-derived neurotrophic factor in rat venous occlusion model. Neurosurgery 2011, 68, 1334–1341. [Google Scholar]
- Taylor, L.; Jones, L.; Tuszynski, M.H.; Blesch, A. Neurotrophin-3 gradients established by lentiviral gene delivery promote short-distance axonal bridging beyond cellular grafts in the injured spinal cord. J. Neurosci. 2006, 26, 9713–9721. [Google Scholar] [CrossRef]
- Taylor, S.J.; McDonald, J.W.; Sakiyama-Elbert, S.E. Controlled release of neurotrophin-3 from fibrin gels for spinal cord injury. J. Control. Release 2004, 98, 281–294. [Google Scholar] [CrossRef]
- Taylor, S.J.; Rosenzweig, E.S.; McDonald, J.W.; Sakiyama-Elbert, S.E. Delivery of neurotrophin-3 from fibrin enhances neuronal fiber sprouting after spinal cord injury. J. Control. Release 2006, 113, 226–235. [Google Scholar] [CrossRef]
- Taylor, S.J.; Sakiyama-Elbert, S.E. Effect of controlled delivery of neurotrophin-3 from fibrin on spinal cord injury in a long term model. J. Control. Release 2006, 116, 204–210. [Google Scholar] [CrossRef]
- Thompson, B.C.; Moulton, S.E.; Ding, J.; Richardson, R.; Cameron, A.; O’Leary, S.; Wallace, G.G.; Clark, G.M. Optimising the incorporation and release of a neurotrophic factor using conducting polypyrrole. J. Control. Release 2006, 116, 285–294. [Google Scholar] [CrossRef]
- Vogelin, E.; Baker, J.M.; Gates, J.; Dixit, V.; Constantinescu, M.A.; Jones, N.F. Effects of local continuous release of brain derived neurotrophic factor (BDNF) on peripheral nerve regeneration in a rat model. Exp. Neurol. 2006, 199, 348–353. [Google Scholar] [CrossRef]
- Willerth, S.M.; Johnson, P.J.; Maxwell, D.J.; Parsons, S.R.; Doukas, M.E.; Sakiyama-Elbert, S.E. Rationally designed peptides for controlled release of nerve growth factor from fibrin matrices. J. Biomed. Mater. Res. Part A 2006, 80A, 13–23. [Google Scholar]
- Williams, G.; Williams, E.J.; Maison, P.; Pangalos, M.N.; Walsh, F.S.; Doherty, P. Overcoming the inhibitors of myelin with a novel neurotrophin strategy. J. Biol. Chem. 2005, 280, 5862–5869. [Google Scholar]
- Winter, J.O.; Cogan, S.F.; Rizzo, J.F. Neurotrophin-eluting hydrogel coatings for neural stimulating electrodes. J. Biomed. Mater. Res. Part B 2007, 81B, 551–563. [Google Scholar] [CrossRef]
- Wu, D. Neuroprotection in experimental stroke with targeted neurotrophins. NeuroRx 2005, 2, 120–128. [Google Scholar] [CrossRef]
- Wu, D.F.; Pardridge, W.M. Neuroprotection with noninvasive neurotrophin delivery to the brain. Proc. Natl. Acad. Sci. USA 1999, 96, 254–259. [Google Scholar] [CrossRef]
- Xie, Y.; Ye, L.Y.; Zhang, X.B.; Cui, W.; Lou, J.N.; Nagai, T.; Hou, X.P. Transport of nerve growth factor encapsulated into liposomes across the blood-brain barrier: In vitro and in vivo studies. J. Control. Release 2005, 105, 106–119. [Google Scholar] [CrossRef]
- Xu, X.Y.; Yee, W.C.; Hwang, P.Y.K.; Yu, H.; Wan, A.C.A.; Gao, S.J.; Boon, K.L.; Mao, H.Q.; Leong, K.W.; Wang, S. Peripheral nerve regeneration with sustained release of poly(phosphoester) microencapsulated nerve growth factor within nerve guide conduits. Biomaterials 2003, 24, 2405–2412. [Google Scholar] [CrossRef]
- Yamashita, K.; Wiessner, C.; Lindholm, D.; Thoenen, H.; Hossmann, K.A. Post-occlusion treatment with BDNF reduces infarct size in a model of permanent occlusion of the middle cerebral artery in rat. Metab. Brain Dis. 1997, 12, 271–280. [Google Scholar]
- Yang, K.; Clifton, G.L.; Hayes, R.L. Gene therapy for central nervous system injury: The use of cationic liposomes. J. Neurotrauma 1997, 14, 281–297. [Google Scholar] [CrossRef]
- Yang, Y.; De Laporte, L.; Rives, C.B.; Jang, J.H.; Lin, W.C.; Shull, K.R.; Shea, L.D. Neurotrophin releasing single and multiple lumen nerve conduits. J. Control. Release 2005, 104, 433–446. [Google Scholar] [CrossRef]
- Yu, X.J.; Bellamkonda, R.V. Tissue-engineered scaffolds are effective alternatives to autografts for bridging peripheral nerve gaps. Tissue Eng. 2003, 9, 421–430. [Google Scholar] [CrossRef]
- Yu, X.J.; Dillon, G.P.; Bellamkonda, R.V. A laminin and nerve growth factor-laden three-dimensional scaffold for enhanced neurite extension. Tissue Eng. 1999, 5, 291–304. [Google Scholar] [CrossRef]
- Zhang, Y.; Pardridge, W.M. Conjugation of brain-derived neurotrophic factor to a blood-brain barrier drug targeting system enables neuroprotection in regional brain ischemia following intravenous injection of the neurotrophin. Brain Res. 2001, 889, 49–56. [Google Scholar]
- Bachoud-Levi, A.C.; Deglon, N.; Nguyen, J.P.; Bloch, J.; Bourdet, C.; Winkel, L.; Rémy, P.; Goddard, M.; Lefaucheur, J.-P.; Brugières, P.; et al. Neuroprotective gene therapy for Huntington’s disease using a polymer encapsulated BHK cell line engineered to secrete human CNTF. Hum. Gene Ther. 2000, 11, 1723–1729. [Google Scholar] [CrossRef]
- Bergen, J.M.; Park, I.K.; Horner, P.J.; Pun, S.H. Nonviral approaches for neuronal delivery of nucleic acids. Pharm. Res. 2008, 25, 983–998. [Google Scholar] [CrossRef]
- Blesch, A. Neurotrophin gene therapy for Alzheimer’s disease. Future Neurol. 2006, 1, 179–187. [Google Scholar] [CrossRef]
- Blesch, A. MLV based retroviral and lentiviral vectors for in vitro and in vivo gene transfer. Methods 2004, 33, 164–172. [Google Scholar] [CrossRef]
- Bloch, J.; Bachoud-Levi, A.C.; Deglon, N.; Lefaucheur, J.P.; Winkel, L.; Palfi, S.; Nguyen, J.P.; Bourdet, C.; Gaura, V.; Remy, P.; et al. Neuroprotective gene therapy for Huntington’s disease, using polymer-encapsulated cells engineered to secrete human ciliary neurotrophic factor: Results of a phase I study. Hum. Gene Ther. 2004, 15, 968–975. [Google Scholar] [CrossRef]
- Bjorklund, T.; Kirik, D. Scientific rationale for the development of gene therapy strategies for Parkinson’s disease. Biochim. Biophys. Acta 2009, 1792, 703–713. [Google Scholar] [CrossRef]
- Bjorklund, T.; Kordower, J.H. Gene therapy for Parkinson’s disease. Mov. Disord. 2010, 25, S161–S173. [Google Scholar] [CrossRef]
- Bowers, W.J.; Breakefield, X.O.; Sena-Esteves, M. Genetic therapy for the nervous system. Hum. Molec. Gen. 2011, 20, R28–R41. [Google Scholar] [CrossRef]
- Di Polo, A.; Aigner, L.J.; Dunn, R.J.; Bray, G.M.; Aguayo, A.J. Prolonged delivery of brain-derived neurotrophic factor by adenovirus-infected Muller cells temporarily rescues injured retinal ganglion cells. Proc. Nat. Acad. Sci. USA 1998, 95, 3978–3983. [Google Scholar]
- Henry, R.A.; Hughes, S.M.; Connor, B. AAV-mediated delivery of BDNF augments neurogenesis in the normal and quinolinic acid-lesioned adult rat brain. Eur. J. Neurosci. 2007, 25, 3513–3525. [Google Scholar] [CrossRef]
- Kells, A.P.; Fong, D.M.; Dragunow, M.; During, M.J.; Young, D.; Connoet, B. AAV-mediated gene delivery of BDNF or GDNF is neuroprotective in a model of Huntington disease. Mol. Ther. 2004, 9, 682–688. [Google Scholar] [CrossRef]
- Lu, K.W.; Chen, Z.Y.; Jin, D.D.; Hou, T.S.; Cao, L.; Fu, Q. Cationic liposome-mediated GDNF gene transfer after spinal cord injury. J. Neurotrauma 2002, 19, 1081–1090. [Google Scholar] [CrossRef]
- Lu, P.; Jones, L.L.; Tuszynski, M.H. BDNF-expressing marrow stromal cells support extensive axonal growth at sites of spinal cord injury. Exp. Neurol. 2005, 191, 344–360. [Google Scholar] [CrossRef]
- Makar, T.K.; Bever, C.T.; Singh, I.S.; Royal, W.; Sahu, S.N.; Sura, T.P.; Sultana, S.; Sura, K.T.; Patel, N.; Dhib-Jalbut, S.; et al. Brain-derived neurotrophic factor gene delivery in an animal model of multiple sclerosis using bone marrow stem cells as a vehicle. J. Neuroimmunol. 2009, 210, 40–51. [Google Scholar] [CrossRef]
- Munehisa, S.; Naoyuki, S.; Ryuichi, M. Experimental and clinical application of plasmid DNA in the field of central nervous diseases. Curr. Gene Ther. 2011, 11, 491–500. [Google Scholar] [CrossRef]
- Nakajima, H.; Uchida, K.; Yayama, T.; Kobayashi, S.; Guerrero, A.R.; Furukawa, S.; Baba, H. Targeted retrograde gene delivery of brain-derived neurotrophic factor supresses apoptosis and oligodendrolia after spinal cord injury in rats. Spine 2010, 35, 497–504. [Google Scholar] [CrossRef]
- Ramaswamy, S.; Kordower, J.H. Gene therapy for Huntington’s disease. Neurobiol. Dis. 2012, 48, 243–254. [Google Scholar]
- Park, H.Y.L.; Kim, J.H.K.; Kim, H.S.; Park, C.K. Stem cell-based delivery of brain-derived neurotrophic factor gene in the rat retina. Brain Res. 2012, 1469, 10–23. [Google Scholar] [CrossRef]
- Sayers, S.T.; Khan, N.; Ahmed, Y.; Shahid, R.; Khan, T. Preparation of brain-derived neurotrophic factor- and neurotrophin-3-secreting Schwann cells by infection with a retroviral vector. J. Mol. Neurosci. 1998, 10, 143–160. [Google Scholar] [CrossRef]
- Tuszynski, M.H.; Thal, L.; Pay, M.; Salmon, D.P.; Sang, U.H.; Bakay, R.; Patel, P.; Blesch, A.; Vahlsing, H.L.; Ho, G.; et al. A phase I clinical trial of nerve growth factor gene therapy for Alzheimer disease. Nat. Med. 2005, 11, 551–555. [Google Scholar] [CrossRef]
- Tuszynski, M.H. Nerve growth factor gene therapy in Alzheimer disease. Alzheimer Dis. Assoc. Disord. 2007, 21, 179–189. [Google Scholar] [CrossRef]
- Martinez-Fong, D.; Bannon, M.J.; Trudeau, L.E.; Gonzalez-Barrios, J.A.; Arango-Rodriquez, M.L.; Hernendez-Chan, N.G.; Reyes-Corona, D.; Armendariz-Borunda, J.; Navarro-Quiroqa, I. NTS-Polyplex: A potential nanocarrier for neurotrophic therapy of Parkinson’s disease. Nanomedicine 2012, 8, 1052–1069. [Google Scholar] [CrossRef]
- Yang, L.; Rongqin, H.; Chen, J. Non-viral gene delivery and therapeutics targeting to brain. Curr. Nanosci. 2011, 7, 55–70. [Google Scholar] [CrossRef]
- Fletcher, J.M.; Hughes, R.A. Novel monocyclic and bicyclic loop mimetics of brain-derived neurotrophic factor. J. Pept. Sci. 2006, 12, 515–524. [Google Scholar] [CrossRef]
- Fletcher, J.M.; Hughes, R.A. Modified low molecular weight cyclic peptides as mimetics of BDNF with improved potency, proteolytic stability and transmembrane passage in vitro. Bioorg. Med. Chem. 2009, 17, 2695–2702. [Google Scholar] [CrossRef]
- Fletcher, J.M.; Morton, C.J.; Zwar, R.A.; Murray, S.S.; O’Leary, P.D.; Hughes, R.A. Design of a conformationally defined and proteolytically stable circular mimetic of brain-derived neurotrophic factor. J. Biol. Chem. 2008, 283, 33375–33383. [Google Scholar]
- O’Leary, P.D.; Hughes, R.A. Structure-activity relationships of conformationally constrained peptide analogues of loop 2 of brain-derived neurotrophic factor. J. Neurochem. 1998, 70, 1712–1721. [Google Scholar]
- O’Leary, P.D.; Hughes, R.A. Design of potent peptide mimetics of brain-derived neurotrophic factor. J. Biol. Chem. 2003, 278, 25738–25744. [Google Scholar]
- Longo, F.M.; Yang, T.; Knowles, J.K.; Xie, Y.; Moore, L.A.; Massa, S.M. Small molecule neurotrophin receptor ligands: Novel strategies for targeting Alzheimer’s disease mechanisms. Curr. Alzheimer Res. 2007, 4, 503–506. [Google Scholar]
- Massa, S.M.; Yang, T.; Xie, Y.; Shi, J.; Bilgen, M.; Joyce, J.N.; Nehama, D.; Rajadas, J.; Longo, F.M. Small molecule BDNF mimetics activate TrkB signaling and prevent neuronal degeneration in rodents. J. Clin. Invest. 2010, 120, 1774–1785. [Google Scholar] [CrossRef]
- Jang, S.W.; Liu, X.; Yepes, M.; Shepherd, K.R.; Miller, G.W.; Liu, Y.; Wilson, W.D.; Xiao, G.; Blanchi, B.; Sun, Y.E.; et al. A selective TrkB agonist with potent neurotrophic activities by 7,8-dihydroxyflavone. Proc. Natl. Acad. Sci. USA 2010, 107, 2687–2692. [Google Scholar]
- Monteggia, L.M. Toward neurotrophin-based therapeutics. Am. J. Psychiatry 2011, 168, 114–116. [Google Scholar] [CrossRef]
- Price, R.D.; Milne, S.A.; Sharkey, J.; Matsuoka, N. Advances in small molecules promoting neurotrophic function. Pharmacol. Ther. 2007, 115, 292–306. [Google Scholar] [CrossRef]
- Tuinstra, H.M.; Aviles, M.O.; Shin, S.; Holland, S.J.; Zelivyanskaya, M.L.; Fast, A.G.; Ko, S.Y.; Margul, D.J.; Bartels, A.K.; Boehler, R.M.; et al. Multifunctional, multichannel bridges that deliver neurotrophin encoding lentivirus for regeneration following spinal cord injury. Biomaterials 2012, 33, 1618–1626. [Google Scholar]
- Park, J.; Lim, E.; Back, S.; Na, H.; Park, Y.; Sun, K. Nerve regeneration following spinal cord injury using matrix metalloproteinase-sensitive, hyaluronic acid-based biomimetic hydrogel scaffold containing brain-derived neurotrophic factor. J. Biomed. Mater. Res. A 2010, 93, 1091–1099. [Google Scholar]
- Géral, C.; Angelova, A.; Angelov, B.; Nicolas, V.; Lesieur, S. Multicompartment lipid nanocarriers for targeting of cells expressing brain receptors. In Self-Assembled Supramolecular Architectures: Lyotropic Liquid Crystals; Garti, N., Mezzenga, R., Somasundaran, P., Eds.; John Wiley & Sons, Inc.: New Jersey, NJ, USA, 2012; pp. 319–355. [Google Scholar]
- Eriksdotter-Jönhagen, M.; Nordberg, A.; Amberla, K.; Bäckman, L.; Ebendal, T.; Meyerson, B.; Olson, L.; Seiger, A.; Shigeta, M.; Theodorsson, E.; et al. Intracerebroventricular infusion of nerve growth factor in three patients with Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 1998, 9, 246–257. [Google Scholar] [CrossRef]
- Seiger, A.; Nordberg, A.; von Holst, H.; Bäckman, L.; Ebendal, T.; Alafuzoff, I.; Amberla, K.; Hartvig, P.; Herlitz, A.; Lilja, A.; et al. Intracranial infusion of purified nerve growth factor to an Alzheimer patient: The first attempt of a possible future treatment strategy. Behav. Brain Res. 1993, 57, 255–261. [Google Scholar] [CrossRef]
- Isaacson, L.G.; Saffran, B.N.; Crutcher, K.A. Intracerebral NGF infusion induces hyperinnervation of cerebral blood vessels. Neurobiol. Aging 1990, 11, 51–55. [Google Scholar] [CrossRef]
- Allen, S.J.; Robertson, A.G.S.; Tyler, S.J.; Wilcock, G.K.; Dawbarn, D. Recombinant human nerve growth factor for clinical trials: Protein expression, purification, stability and characterisation of binding to infusion pumps. J. Biochem. Biophys. Methods 2001, 47, 239–255. [Google Scholar] [CrossRef]
- Blurton-Jones, M.; Kitazawa, M.; Martinez-Coria, H.; Castello, N.A.; Mueller, F.-J.; Loring, J.F.; Yamasaki, T.R.; Poon, W.W.; Green, K.N.; LaFerla, F.M. Neural stem cells improve cognition via BDNF in a transgenic model of Alzheimer disease. Proc. Natl. Acad. Sci. USA 2009, 32, 13594–13599. [Google Scholar]
- Wahlberg, L.U.; Lind, G.; Almqvist, P.M.; Kusk, P.; Tornøe, J.; Juliusson, B.; Söderman, M.; Selldén, E.; Seiger, A.; Eriksdotter-Jönhagen, M.; et al. Targeted delivery of nerve growth factor via encapsulated cell biodelivery in Alzheimer disease: A technology platform for restorative neurosurgery. J. Neurosurgery 2012, 117, 340–347. [Google Scholar] [CrossRef]
- Brahimi, F.; Malakhov, A.; Lee, H.B.; Pattarawarapan, M.; Ivanisevic, L.; Burgess, K.; Saragovi, H.U. A peptidomimetic of NT-3 acts as a TrkC antagonist. Peptides 2009, 30, 1833–1839. [Google Scholar] [CrossRef]
- Peleshok, J.; Saragovi, H.U. Functional mimetics of neurotrophins and their receptors. Biochem. Soc. Trans. 2006, 34, 612–617. [Google Scholar] [CrossRef]
- Webster, N.J.G.; Pirrung, M.C. Small molecule activators of the Trk receptors for neuroprotection. BMC Neuroscience 2008, 9, S1:1–S1:8. [Google Scholar]
- Adessi, C.; Soto, C. Converting a peptide into a drug: Strategies to improve stability and bioavailability. Curr. Med. Chem. 2002, 9, 963–978. [Google Scholar] [CrossRef]
- Begley, D.J. Delivery of therapeutic agents to the central nervous system: The problems and the possibilities. Pharmacol. Ther. 2004, 104, 29–45. [Google Scholar] [CrossRef]
- Boado, R.J.; Pardridge, W.M. The Trojan horse liposome technology for nonviral gene transfer across the blood-brain barrier. J. Drug Deliv. 2011, 2011, 296151:1–296151:12. [Google Scholar]
- Xia, C.F.; Boado, R.J.; Zhang, Y.; Chu, C.; Pardridge, W.M. Intravenous glial-derived neurotrophic factor gene therapy of experimental Parkinson’s disease with Trojan horse liposomes and a tyrosine hydroxylase promoter. J. Gene Med. 2008, 10, 306–315. [Google Scholar] [CrossRef]
- Craparo, E.F.; Bondi, M.L.; Pitarresi, G.; Cavallaro, G. Nanoparticulate systems for drug delivery and targeting to the central nervous system. CNS Neurosci. Therapeutics 2011, 17, 670–677. [Google Scholar] [CrossRef]
- Hughes, G.A. Nanostructure-mediated drug delivery. Nanomed. Nanotech. Biol. Med. 2005, 1, 22–30. [Google Scholar] [CrossRef]
- Kanwar, J.R.; Sun, X.; Punj, V.; Sriramoju, B.; Mohan, R.R.; Zhou, S.F.; Chauhan, A.; Kanwar, R.K. Nanoparticles in the treatment and diagnosis of neurological disorders: Untamed dragon with fire power to heal. Nanomed. Nanotech. Biol. Med. 2012, 8, 399–414. [Google Scholar] [CrossRef]
- Koo, O.M.; Rubinstein, I.; Onyuksel, H. Role of nanotechnology in targeted drug delivery and imaging. Nanomed. Nanotech. Biol. Med. 2005, 1, 193–212. [Google Scholar]
- Modi, G.; Pillay, V.; Choonara, Y.E.; Ndesendo, V.M.; du Toit, L.C.; Naidoo, D. Nanotechnologicalapplications for the treatment of neurodegenerative disorders. Prog. Neurobiol. 2009, 88, 272–285. [Google Scholar] [CrossRef]
- Nunes, A.; Al-Jamal, K.T.; Kostarelos, K. Therapeutics, imaging and toxicity of nanomaterials in the central nervous system. J. Control. Release 2012, 161, 290–306. [Google Scholar] [CrossRef]
- Paolino, D.; Cosco, D.; Molinaro, R.; Celia, C.; Fresta, M. Supramolecular devices to improve the treatment of brain diseases. Drug Discov. Today 2011, 16, 311–324. [Google Scholar] [CrossRef]
- Wong, H.L.; Wu, X.Y.; Bendayan, R. Nanotechnological advances for the delivery of CNS therapeutics. Adv. Drug Deliv. Rev. 2012, 64, 686–700. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, L. Multifunctional theranostic nanoparticles for brain tumors. Mol. Ther. 2012, 20, 10–11. [Google Scholar] [CrossRef]
- Patel, M.M.; Goyal, B.R.; Bhadada, S.V.; Bhat, J.S.; Amin, A.F. Getting into the brain: approaches to enhance brain drug delivery. CNS Drugs 2009, 23, 35–58. [Google Scholar]
- Petros, R.A.; DeSimone, J.M. Strategies in the design of nanoparticles for therapeutic applications. Nat. Rev. Drug Discov. 2010, 9, 615–627. [Google Scholar] [CrossRef]
- Suh, W.H.; Suslick, K.S.; Stucky, G.D.; Suh, Y-H. Nanotechnology, nanotoxicology, and neuroscience. Prog. Neurobiol. 2009, 87, 137–170. [Google Scholar]
- Schnyder, A.; Huwyler, J. Drug transport to brain with targeted liposomes. NeuroRx 2005, 2, 99–107. [Google Scholar] [CrossRef]
- Zhang, S.; Uludag, H. Nanoparticulate systems for growth factor delivery. Pharm. Res. 2009, 26, 1561–1580. [Google Scholar] [CrossRef]
- Mehrotra, S.; Lynam, D.; Maloney, R.; Pawelec, K.M.; Tuszynski, M.H.; Lee, I.; Chan, C.; Sakamoto, J. Time controlled protein release from layer-by-layer assembled multilayer functionalized agarose hydrogels. Adv. Funct. Mater. 2010, 20, 247–258. [Google Scholar]
- Pang, Z.; Lu, W.; Gao, H.; Hu, K.; Chen, J.; Zhang, C.; Gao, X.; Jiang, X.; Zhu, C. Preparation and brain delivery property of biodegradable polymersomes conjugated with OX26. J. Control. Release 2008, 128, 120–127. [Google Scholar] [CrossRef]
- Bickel, U.; Yoshikawa, T.; Pardridge, W.M. Delivery of peptides and proteins through the blood-brain barrier. Adv. Drug Deliv. Rev. 2001, 46, 247–279. [Google Scholar] [CrossRef]
- Pardridge, W.M. Vector-mediated peptide drug delivery to the brain. Adv. Drug Deliv. Rev. 1995, 15, 109–146. [Google Scholar] [CrossRef]
- Pardgridge, W.M. Biopharmaceutical drug targeting to the brain. J. Drug Target. 2010, 18, 157–167. [Google Scholar] [CrossRef]
- Pardridge, W.M. Re-engineering bioPharmaceutics for delivery to brain with molecular Trojan horses. Bioconjugate Chem. 2008, 19, 1327–1338. [Google Scholar]
- Boado, R.J.; Zhang, Y.; Zhang, Y.; Pardridge, W.M. Genetic engineering, expression, and activity of a fusion protein of a human neurotrophin and a molecular Trojan horse for delivery across the human blood-brain barrier. Biotechnol. Bioeng. 2007, 97, 1376–1386. [Google Scholar] [CrossRef]
- Tan, J.; Wang, Y.; Yip, X.; Glynn, F.; Shepherd, R.K.; Caruso, F. Nanoporous peptide particles for encapsulating and releasing neurotrophic factors in an animal model of neurodegeneration. Adv. Mater. 2012, 24, 3362–3366. [Google Scholar] [CrossRef]
- Roy, S.; Johnston, A.H.; Moin, S.T.; Dudas, J.; Newman, T.A.; Hausott, B.; Schrott-Fischer, A.; Glueckert, R. Activation of TrkB receptors by NGFβ mimetic peptide conjugated polymersome nanoparticles. Nanomed. Nanotech. Biol. Med. 2012, 8, 271–274. [Google Scholar] [CrossRef]
- Roy, S.; Johnston, A.H.; Newman, T.A.; Glueckert, R.; Dudas, J.; Bitsche, M.; Corbacella, E.; Rieger, G.; Martini, A.; Schrott-Fischer, A. Cell-specific targeting in the mouse inner ear using nanoparticles conjugated with a neurotrophin-derived peptide ligand: Potential tool for drug delivery. Int. J. Pharm. 2010, 390, 214–224. [Google Scholar] [CrossRef]
- Angelov, B.; Angelova, A.; Filippov, S.; Karlsson, G.; Terrill, N.; Lesieur, S.; Štěpánek, P. Topology and internal structure of PEGylated lipid nanocarriers for neuronal transfection: Synchrotron radiation SAXS and cryo-TEM studies. Soft Matter. 2011, 7, 9714–9720. [Google Scholar] [CrossRef]
- Angelov, B.; Angelova, A.; Filippov, S.; Karlsson, G.; Terrill, N.; Lesieur, S.; Štěpánek, P. SAXS study of sterically stabilized lipid nanocarriers functionalized by DNA. J. Phys. Conf. Series 2012, 351, 012004:1–012004:9. [Google Scholar]
- Wu, H.; Li, J.; Zhang, Q.; Yan, X.; Guo, L.; Gao, X.; Qiu, M.; Jiang, X.; Lai, R.; Chen, H. A novel small Odorranalectin-bearing cubosomes: Preparation, brain delivery and pharmacodynamic study on amyloid-b25–35-treated rats following intranasal administration. Eur. J. Pharm. Biopharm. 2012, 80, 368–378. [Google Scholar] [CrossRef]
- Cornford, E.M.; Cornford, M.E. New systems for delivery of drugs to the brain in neurological diseases. Lancet Neurol. 2002, 1, 306–315. [Google Scholar] [CrossRef]
- Kabanov, A.V.; Gendelman, H.E. Nanomedicine in the diagnosis and therapy of neurodegeneratve disorders. Prog. Polym. Sci. 2007, 32, 1054–1082. [Google Scholar] [CrossRef]
- Buse, J.; El-Aneed, A. Properties, engineering and applications of lipid-based nanoparticle drug-delivery systems: Current research and advances. Nanomedicine 2010, 5, 1237–1260. [Google Scholar] [CrossRef]
- Torchilin, V.P. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 2005, 4, 145–160. [Google Scholar] [CrossRef]
- Cortesi, R.; Esposito, E.; Corradini, F.; Sivieri, E.; Drechsler, M.; Rossi, A.; Scatturin, A.; Menegatti, E. Non-phospholipid vesicles as carriers for peptides and proteins: Production, characterization and stability studies. Int. J. Pharm. 2007, 339, 52–60. [Google Scholar]
- Angelova, A.; Angelov, B.; Papahadjopoulos-Sternberg, B.; Ollivon, M.; Bourgaux, C. Proteocubosomes: Nanoporous vehicles with tertiary organized fluid interfaces. Langmuir 2005, 21, 4138–4143. [Google Scholar] [CrossRef]
- Angelova, A.; Ollivon, M.; Campitelli, A.; Bourgaux, C. Lipid cubic phases as stable nanochannel network structures for protein biochip development: X-ray diffraction study. Langmuir 2003, 19, 6928–6935. [Google Scholar]
- Angelova, A.; Angelov, B.; Papahadjopoulos-Sternberg, B.; Bourgaux, C.; Couvreur, P. Protein driven patterning of self-assembled cubosomic nanostructures: Long oriented nanoridges. J. Phys. Chem. B 2005, 109, 3089–3093. [Google Scholar]
- Aota-Nakano, U.; Li, S.J.; Yamazaki, M. Effects of electrostatic interaction on the phase stability and structures of cubic phases of monoolein/oleic acid mixture membranes. Biochim. Biophys. Acta-Biomembr. 1999, 1461, 96–102. [Google Scholar] [CrossRef]
- Barauskas, J.; Johnsson, M.; Johnson, F.; Tiberg, F. Cubic phase nanoparticles (cubosome): Principles for controlling size, structure, and stability. Langmuir 2005, 21, 2569–2577. [Google Scholar] [CrossRef]
- Boomer, J.A.; Qualls, M.M.; Inerowicz, H.D.; Haynes, R.H.; Patri, V.S.; Kim, J.M.; Thompson, D.H. Cytoplasmic delivery of liposomal contents mediated by an acid-labile cholesterol-vinyl ether-PEG conjugate. Bioconjugate Chem. 2009, 20, 47–59. [Google Scholar] [CrossRef]
- Boyd, B.J. Characterisation of drug release from cubosomes using the pressure ultrafiltration method. Int. J. Pharm. 2003, 260, 239–247. [Google Scholar] [CrossRef]
- Briggs, J.; Chung, H.; Caffrey, M. The temperature-composition phase diagram and mesophase structure characterization of the monoolein/water system. J. Phys. II 1996, 6, 723–751. [Google Scholar] [CrossRef]
- Gustafsson, J.; Ljusberg-Wahren, H.; Almgren, M.; Larsson, K. Submicron particles of reversed lipid phases in water stabilized by a nonionic amphiphilic polymer. Langmuir 1997, 13, 6964–6971. [Google Scholar] [CrossRef]
- Hyde, S.T. Bicontinuous structures in lyotropic liquid crystals and crystalline hyperbolic surfaces. Curr. Opin. Solid State Mat. Sci. 1996, 1, 653–662. [Google Scholar] [CrossRef]
- Larsson, K. Cubic lipid-water phases—Structures and biomembrane aspects. J. Phys. Chem. 1989, 93, 7304–7314. [Google Scholar]
- Angelova, A.; Angelov, B.; Papahadjopoulos-Sternberg, B.; Ollivon, M.; Bourgaux, C. Structural organisation of proteocubosome carriers involving medium- and large-size proteins. J. Drug Deliv. Sci. Tech. 2005, 15, 108–112. [Google Scholar]
- Angelova, A.; Angelov, B.; Lesieur, S.; Mutafchieva, R.; Ollivon, M.; Bourgaux, C.; Willumeit, R.; Couvreur, P. Dynamic control of nanofluidic channels in protein drug delivery vehicles. J. Drug Deliv. Sci. Tech. 2008, 18, 41–45. [Google Scholar]
- Angelov, B.; Angelova, A.; Papahadjopoulos-Sternberg, B.; Lesieur, S.; Sadoc, J.-F.; Ollivon, M.; Couvreur, P. Detailed structure of diamond-type lipid cubic nanoparticles. J. Am. Chem. Soc. 2006, 128, 5813–5817. [Google Scholar]
- Angelov, B.; Angelova, A.; Garamus, V.M.; Le Bas, G.; Lesieur, S.; Ollivon, M.; Funari, S.S.; Willumeit, R.; Couvreur, P. Small-angle neutron and X-ray scattering from amphiphilic stimuli-responsive diamond type bicontinuous cubic phase. J. Am. Chem. Soc. 2007, 129, 13474–13479. [Google Scholar]
- Leesajakul, W.; Nakano, M.; Taniguchi, A.; Handa, T. Interaction of cubosomes with plasma components resulting in the destabilization of cubosomes in plasma. Colloid Surf. B Biointerfaces 2004, 34, 253–258. [Google Scholar]
- Luzzati, V. Biological significance of lipid polymorphism: The cubic phases—Commentary. Curr. Opin. Struct. Biol. 1997, 7, 661–668. [Google Scholar] [CrossRef]
- Martins, S.; Sarmento, B.; Ferreira, D.C.; Souto, E.B. Lipid-based colloidal carriers for peptide and protein delivery—liposomes versus lipid nanoparticles. Int. J. Nanomed. 2007, 2, 595–607. [Google Scholar]
- Nylander, T.; Mattisson, C.; Razumas, V.; Miezis, Y.; Hakansson, B. A study of entrapped enzyme stability and substrate diffusion in a monoglyceride-based cubic liquid crystalline phase. Colloid Surf. A Physicochem. Eng. Asp. 1996, 114, 311–320. [Google Scholar]
- Qiu, H.; Caffrey, M. The phase diagram of the monoolein/water system: Metastability and equilibrium aspects. Biomaterials 2000, 21, 223–234. [Google Scholar] [CrossRef]
- Angelov, B.; Angelova, A.; Vainio, U.; Garamus, V.M.; Lesieur, S.; Willumeit, R.; Couvreur, P. Long living intermediates during a lamellar to a diamond-cubic lipid phase transition: A SAXS investigation. Langmuir 2009, 25, 3734–3742. [Google Scholar]
- Angelov, B.; Angelova, A.; Mutafchieva, R.; Lesieur, S.; Vainio, U.; Garamus, V.M.; Jensen, G.V.; Pedersen, J.S. SAXS investigation of a cubic to a sponge (L3) phase transition in self-assembled lipid nanocarriers. Phys. Chem. Chem. Phys. 2011, 13, 3073–3081. [Google Scholar]
- Angelova, A.; Angelov, B.; Garamus, V.M.; Couvreur, P.; Lesieur, S. Small-angle X-ray scattering investigations of biomolecular confinement, loading, and release from liquid crystalline nanochannel assemblies. J. Phys. Chem. Lett. 2012, 3, 445–457. [Google Scholar]
- Angelov, B.; Angelova, A.; Papahadjopoulos-Sternberg, B.; Hoffmann, S.V.; Nicolas, V.; Lesieur, S. Protein-containing PEGylated cubosomic particles: Freeze-fracture electron microscopy and synchrotron radiation circular dichroism study. J. Phys. Chem. B 2012, 116, 7676–7686. [Google Scholar]
- Angelova, A.; Angelov, B.; Mutafchieva, R.; Lesieur, S.; Couvreur, P. Self-assembled multicompartment liquid crystalline lipid carriers for protein, peptide, and nucleic acid drug delivery. Acc. Chem. Res. 2011, 44, 147–156. [Google Scholar] [CrossRef]
- Siekmann, B.; Bunjes, H.; Koch, M.M.H.; Westesen, K. Preparation and structural investigations of colloidal dispersions prepared from cubic monoglyceride-water phases. Int. J. Pharm. 2002, 244, 33–43. [Google Scholar] [CrossRef]
- Yaghmur, A.; Glatter, O. Characterization and potential applications of nanostructured aqueous dispersions. Adv. Colloid Interface Sci. 2009, 147*#x2013;148, 333–342. [Google Scholar] [CrossRef]
- Al-jamal, W.T.; Kostarelos, K. Liposomes: From a clinically established drug delivery system to a nanoparticle platform for theranostic nanomedicine. Acc. Chem. Res. 2011, 44, 1094–1104. [Google Scholar] [CrossRef]
- Angelova, A.; Angelov, B.; Drechsler, M.; Garamus, V.M.; Lesieur, S. Protein entrapment in PEGylated lipid nanoparticles. Int. J. Pharm. 2013. submitted for publication.. [Google Scholar]
- Martina, M.S.; Fortin, J.P.; Ménager, C.; Clément, O.; Barratt, G.; Grabielle-Madelmont, C.; Gazeau, F.; Cabuil, V.; Lesieur, S. Generation of superparamagnetic liposomes revealed as highly efficient MRI contrast agents for in vivo imaging. J. Am. Chem. Soc. 2005, 127, 10676–10685. [Google Scholar]
- Martina, M.S.; Wilhelm, C.; Lesieur, S. The effect of magnetic targeting on the uptake of magnetic-fluid-loaded liposomes by human prostatic adenocarcinoma cells. Biomaterials 2008, 29, 4137–4145. [Google Scholar] [CrossRef]
- Lesieur, S.; Gazeau, F.; Luciani, N.; Ménager, C.; Wilhelm, C. Multifunctional nanovectors based on magnetic nanoparticles coupled with biological vesicles or synthetic liposomes. J. Mater. Chem. 2011, 21, 14387–14393. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Géral, C.; Angelova, A.; Lesieur, S. From Molecular to Nanotechnology Strategies for Delivery of Neurotrophins: Emphasis on Brain-Derived Neurotrophic Factor (BDNF). Pharmaceutics 2013, 5, 127-167. https://doi.org/10.3390/pharmaceutics5010127
Géral C, Angelova A, Lesieur S. From Molecular to Nanotechnology Strategies for Delivery of Neurotrophins: Emphasis on Brain-Derived Neurotrophic Factor (BDNF). Pharmaceutics. 2013; 5(1):127-167. https://doi.org/10.3390/pharmaceutics5010127
Chicago/Turabian StyleGéral, Claire, Angelina Angelova, and Sylviane Lesieur. 2013. "From Molecular to Nanotechnology Strategies for Delivery of Neurotrophins: Emphasis on Brain-Derived Neurotrophic Factor (BDNF)" Pharmaceutics 5, no. 1: 127-167. https://doi.org/10.3390/pharmaceutics5010127
APA StyleGéral, C., Angelova, A., & Lesieur, S. (2013). From Molecular to Nanotechnology Strategies for Delivery of Neurotrophins: Emphasis on Brain-Derived Neurotrophic Factor (BDNF). Pharmaceutics, 5(1), 127-167. https://doi.org/10.3390/pharmaceutics5010127