Extracellular Vesicles in Alzheimer’s Disease: Dual Roles in Pathogenesis, Promising Avenues for Diagnosis and Therapy
Abstract
1. Introduction
| EV Subtype | Size Range | Key Surface Markers | Typical Cargoes | Primary Functions | References |
|---|---|---|---|---|---|
| Exosomes | 30–150 nm | Tetraspanins (CD9, CD63, CD81), ALIX, TSG101, HSP70 | Proteins, lipids, mRNAs, miRNAs, other non-coding RNAs | Intercellular signaling, immune modulation, synaptic plasticity. In AD, heavily implicated in Aβ/tau propagation and neuroinflammation. | [33,34] |
| Microvesicles | 100–1000 nm | Integrins, selectins, CD40, ARF6, tissue factor | Cytosolic proteins, lipids, RNAs, organelles | Intercellular communication, Stimulate synaptic activity. | [24,35] |
| Apoptotic Bodies | 1–5 μm | Phosphatidylserine (exposed), Annexin V, calreticulin, calnexin | Plasma membrane, fragmented organelles, various biomolecules (RNA and DNA) | Clearance of apoptotic debris; intercellular communication. | [21,26] |
| NDEVs | 144–230 nm | L1CAM, GluR2/3, GAP43, NLGN3 | Synaptic proteins, lipids, regulatory miRNAs (e.g., miR-21-5p), and pathogenic molecules (tau and Aβ) | Propagation of pathological proteins, participating in synaptic communication and plasticity. | [36,37,38] |
| ADEVs | 100–160 nm | EAAT1/GLAST, GFAP | Pathogenic molecules (tau and Aβ), PAR-4 and ceramide, heat shock proteins and NTPDases | Promote neurite outgrowth, dendritic branching, neuronal survival, and synaptic plasticity, impair neuronal excitability and neurite growth. | [39,40,41] |
| MDEVs | 98 ± 24.1 nm | CD9, CD81, CD63, MHC class II molecules | Pathogenic molecules (tau and Aβ), TRME2, Inflammatory factors (IL-1β and TNF-α), synaptogenic proteins | Propagation of Aß and Tau, release of inflammatory factors, synaptic dysfunction, alleviation of oxidative stress, neurite outgrowth. | [42,43,44] |
| ODEVs | 30–80 nm | ALIX, TSG101, Myelin-specific proteins (PLP, CNO, MBP, MOG) | PLP, CNP, MBP, MOG, and characteristic myelin lipids | Trigger of neuronal hyperactivity, alleviation of oxidative stress synaptic preservation. | [45,46,47,48] |
| MSC-EVs | 40–100 nm | CD9, ALIX, TSG101, CD90, CD73, and CD44 | Growth factors (e.g., VEGF, TGF-β, HGF), immunomodulatory molecules (IL-10), lipids, and microRNAs (e.g., miR-21, miR-122, miR-146a) | Clearance of Aß and Tau, alleviation of oxidative stress, synaptic preservation, reduction of neuroinflammation, metabolic modulation and folate delivery. | [49,50,51,52] |
2. Classification and Functional Heterogeneity
2.1. Neuron-Derived Extracellular Vesicles (NDEVs)
2.2. Astrocyte-Derived Extracellular Vesicles (ADEVs)
2.3. Microglia-Derived Extracellular Vesicles (MDEVs)
2.4. Oligodendrocyte-Derived Extracellular Vesicles (ODEVs)
2.5. Mesenchymal Stem Cell-Derived Extracellular Vesicles (MSC-EVs)
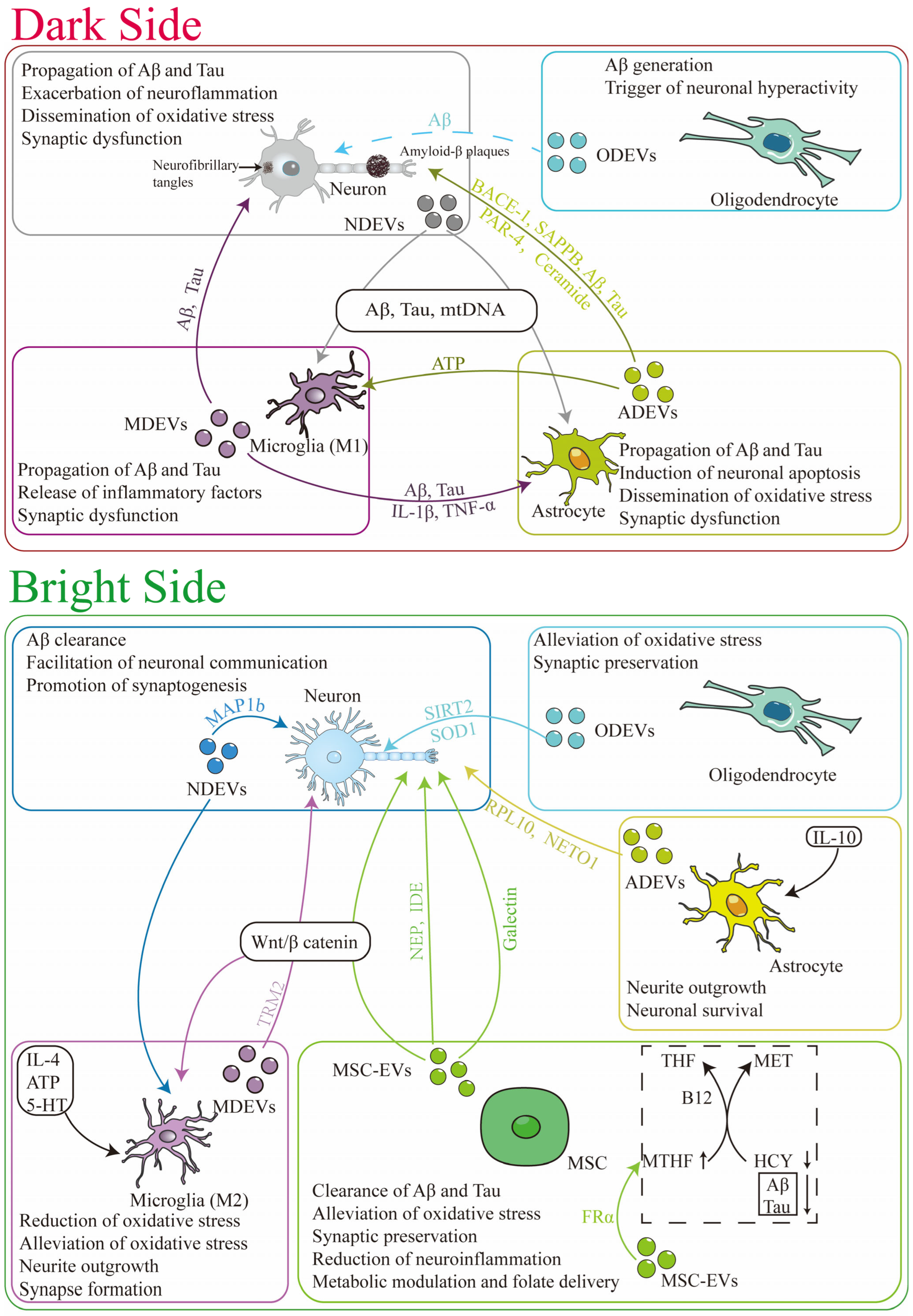
3. The Dual Role of EVs in Mediating AD Pathogenesis
3.1. The Dark Side: EVs as Mediators of AD Progression
3.1.1. Propagation of Aβ and Tau
3.1.2. Amplification of Neuroinflammation
3.1.3. Dissemination of Oxidative Stress
3.2. The Bright Side: Endogenous Protective Functions of EVs in AD
3.2.1. Clearance of Pathogenic Proteins
3.2.2. Neuroprotection and Immunomodulation
3.2.3. Synaptic Preservation
3.2.4. Alleviation of Oxidative Stress
3.2.5. Metabolic Modulation and Folate Delivery
4. Harnessing EVs for Therapy: Targeting Core AD Pathologies
4.1. Engineered EVs for Targeting Aβ and Tau Pathology
4.2. Suppressing Neuroinflammation with Engineered EVs
4.3. Advanced Engineering: EVs as Delivery Systems for Genetic and Epigenetic Therapy
5. EVs as a Diagnostic Tool
5.1. Neuron-Derived EVs: Capturing Core Pathology and Synaptic Integrity
5.2. The Diagnostic Potential of miRNA and Glial Cells-Derived EVs
6. Promising Therapeutic Strategy: Link to Clinical Treatment
6.1. Biocompatibility and Low Immunogenicity
6.2. BBB Penetration
6.3. Regenerative Potential via Wnt/β-Catenin Signaling
6.4. Clinical Translation: Ongoing Trails and Early Outcomes
6.5. Industrial Development and Commercialization of Exosome-Based Therapeutics for AD
7. Challenges and Limitations on the Path to Translation
7.1. Heterogeneity and Standardization
7.2. Scalable Manufacturing and GMP Compliance
7.3. Targeting Specificity and Delivery Efficiency
7.4. Long-Term Safety and Clinical Validation
8. Future Perspectives
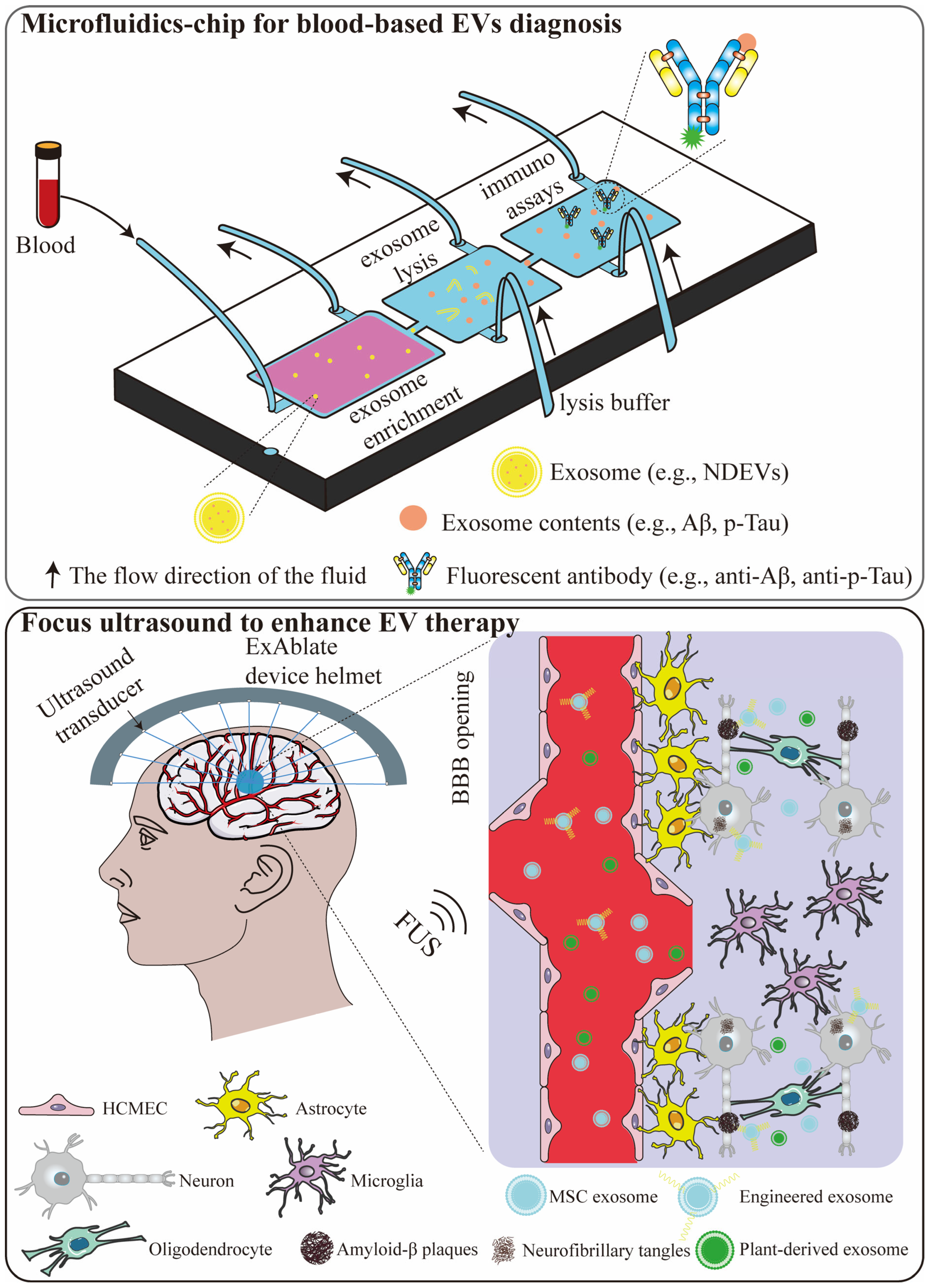
8.1. Microfluidic Chip for Blood-Based EVs Diagnostics
8.2. Focused Ultrasound to Enhance EV Therapy
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| Aβ | amyloid-β |
| APP | amyloid precursor protein |
| ADEVs | Astrocyte-derived extracellular vesicles |
| ALS | amyotrophic lateral sclerosis |
| ATN | amyloid beta (A), tau (T) and neurodegeneration (N) |
| ahaMSCs-Exos | administered allogeneic adipose-derived MSC-EVs |
| BD-tau | brain-derived tau |
| BDENs | broccoli-derived exosomes-like nanoparticles |
| BACE-1 | β-secretase |
| CNS | central nervous system |
| CNP | 2′,3′-cyclic nucleotide 3′-phosphodiesterase |
| CSF | cerebrospinal fluid |
| CNC | cognitively normal controls |
| DAMPs | damage-associated molecular patterns |
| Exo-cur | EVs derived from curcumin-primed macrophages |
| EVs | extracellular vesicles |
| EAAT1 | excitatory amino acid transporter 1 |
| FRα | folate-receptor α |
| FUS | focused ultrasound |
| GFAP | glial fibrillary acidic protein |
| GSK-3β | glycogen synthase kinase-3β |
| GMP | good manufacturing practice |
| GAL-3 | galectin |
| hUCB-MSCs | human umbilical cord blood-derived mesenchymal stem cells |
| hUC-MSCs-EVs | exosomes from human umbilical cord mesenchymal stem cells |
| hiPSC-NSCs | human induced pluripotent stem cells |
| HHcy | hyperhomocysteinemia |
| Hcy | homocysteine |
| HTL | hcy-thiolactone |
| HDS-R | Hasegawa revised dementia scale |
| IDE | insulin-degrading enzyme |
| ILVs | intraluminal vesicles |
| IL-4 | interleukin-4 |
| L1CAM | L1 cell adhesion molecule |
| MSC | mesenchymal stem cell |
| MVBs | multivesicular bodies |
| MDEVs | microglia-derived extracellular vesicles |
| MHC | major histocompatibility complex |
| MBP | myelin basic protein |
| MSC-EVs | mesenchymal stem cell-derived extracellular vesicles |
| mtDNA | mitochondrial DNA |
| MOG | myelin oligodendrocyte glycoprotein |
| MSCs | mesenchymal stem cells |
| MAP1b | microtubule-associated protein 1b |
| MExo-Gem | mannose-modified exosomes loaded with gemfibrozil |
| MSC-RVG-Exo | MSC-derived exosomes engineered with the brain-targeting RVG peptide |
| MAPLEX | a novel photoinducible exosome system |
| MRI | magnetic resonance imaging |
| miRNAs | microRNAs |
| MCI | mild cognitive impairment |
| MCIC | patients with MCI who converted to AD dementia |
| MCIS | stable MCI |
| NDEVs | neuron-derived extracellular vesicles |
| NEP | neprilysin |
| NIA-AA | National Institute on aging-Alzheimer’s association |
| NRGN | neurogranin |
| NFTs | neurofibrillary tangles |
| NTPDases | nucleoside triphosphate diphosphohydrolases |
| ODEVs | oligodendrocyte-derived extracellular vesicles |
| PLP | proteolipid protein |
| p-tau | phosphorylated tau |
| PET | positron emission tomography |
| ROS | reactive oxygen species |
| RVG | rabies viral glycoprotein |
| REST | repressor element 1-silencing transcriptional |
| sAPPβ | soluble amyloid precursor protein β |
| SVZ | subventricular zone |
| SCD | subjective cognitive decline |
| VaD | vascular dementia |
| 5-MTHF | 5-Methyltetrahydrofolic acid |
| 5-HT | serotonin |
References
- Better, M.A. 2024 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2024, 20, 3708–3821. [Google Scholar]
- Malaha, K.A.; Thebaut, C.; Achille, D.; Preux, P.M.; Guerchet, M. Costs of Dementia in Low- and Middle-Income Countries: A Systematic Review. J. Alzheimer’s Dis. 2023, 91, 115–128. [Google Scholar] [CrossRef]
- Rayaprolu, S.; Higginbotham, L.; Bagchi, P.; Watson, C.M.; Zhang, T.; Levey, A.I.; Rangaraju, S.; Seyfried, N.T. Systems-based proteomics to resolve the biology of Alzheimer’s disease beyond amyloid and tau. Neuropsychopharmacology 2020, 46, 98–115. [Google Scholar] [CrossRef]
- Abdelnour, C.; Agosta, F.; Bozzali, M.; Fougere, B.; Iwata, A.; Nilforooshan, R.; Takada, L.T.; Vinuela, F.; Traber, M. Perspectives and challenges in patient stratification in Alzheimer’s disease. Alzheimer’s Res. Ther. 2022, 14, 112. [Google Scholar] [CrossRef]
- Bloom, G.S. Amyloid-beta and tau: The trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. 2014, 71, 505–508. [Google Scholar] [CrossRef]
- Zhang, J.F.; Zhang, Y.L.; Wang, J.X.; Xia, Y.L.; Zhang, J.X.; Chen, L. Recent advances in Alzheimer’s disease: Mechanisms, clinical trials and new drug development strategies. Signal Transduct. Target. Ther. 2024, 9, 211. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Wang, X. Alzheimer’s disease: Insights into pathology, molecular mechanisms, and therapy. Protein Cell 2025, 16, 83–120. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, H.; Li, R.; Sterling, K.; Song, W. Amyloid beta-based therapy for Alzheimer’s disease: Challenges, successes and future. Signal Transduct. Target. Ther. 2023, 8, 248. [Google Scholar] [CrossRef]
- Twarowski, B.; Herbet, M. Inflammatory Processes in Alzheimer’s Disease—Pathomechanism, Diagnosis and Treatment: A Review. Int. J. Mol. Sci. 2023, 24, 6518. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; van der Flier, W.M.; Jessen, F.; Hoozemanns, J.; Thal, D.R.; Boche, D.; Brosseron, F.; Teunissen, C.; Zetterberg, H.; Jacobs, A.H.; et al. Neuroinflammation in Alzheimer disease. Nat. Rev. Immunol. 2024, 25, 321–352. [Google Scholar] [CrossRef]
- Leng, F.; Edison, P. Neuroinflammation and microglial activation in Alzheimer disease: Where do we go from here? Nat. Rev. Neurol. 2020, 17, 157–172. [Google Scholar] [CrossRef]
- Klegeris, A.; Bajwa, E. Neuroinflammation as a mechanism linking hypertension with the increased risk of Alzheimer’s disease. Neural Regen. Res. 2022, 17, 2342–2346. [Google Scholar] [CrossRef] [PubMed]
- Busche, M.A.; Hyman, B.T. Synergy between amyloid-β and tau in Alzheimer’s disease. Nat. Neurosci. 2020, 23, 1183–1193. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wei, W.; Zhao, M.; Ma, L.; Jiang, X.; Pei, H.; Cao, Y.; Li, H. Interaction between Aβ and Tau in the Pathogenesis of Alzheimer’s Disease. Int. J. Biol. Sci. 2021, 17, 2181–2192. [Google Scholar] [CrossRef]
- Kumar, M.A.; Baba, S.K.; Sadida, H.Q.; Marzooqi, S.A.; Jerobin, J.; Altemani, F.H.; Algehainy, N.; Alanazi, M.A.; Abou-Samra, A.-B.; Kumar, R.; et al. Extracellular vesicles as tools and targets in therapy for diseases. Signal Transduct. Target. Ther. 2024, 9, 27. [Google Scholar] [CrossRef]
- Belkozhayev, A.M.; Al-Yozbaki, M.; George, A.; Ye Niyazova, R.; Sharipov, K.O.; Byrne, L.J.; Wilson, C.M. Extracellular Vesicles, Stem Cells and the Role of miRNAs in Neurodegeneration. Curr. Neuropharmacol. 2022, 20, 1450–1478. [Google Scholar] [CrossRef]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Marar, C.; Starich, B.; Wirtz, D. Extracellular vesicles in immunomodulation and tumor progression. Nat. Immunol. 2021, 22, 560–570. [Google Scholar] [CrossRef]
- Buratta, S.; Tancini, B.; Sagini, K.; Delo, F.; Chiaradia, E.; Urbanelli, L.; Emiliani, C. Lysosomal Exocytosis, Exosome Release and Secretory Autophagy: The Autophagic- and Endo-Lysosomal Systems Go Extracellular. Int. J. Mol. Sci. 2020, 21, 2576. [Google Scholar] [CrossRef]
- Lee, Y.; Andaloussi, S.E.; Wood, M.J. Exosomes and microvesicles: Extracellular vesicles for genetic information transfer and gene therapy. Hum. Mol. Genet. 2012, 21, R125–R134. [Google Scholar] [CrossRef]
- Jeppesen, D.K.; Fenix, A.M.; Franklin, J.L.; Higginbotham, J.N.; Zhang, Q.; Zimmerman, L.J.; Liebler, D.C.; Ping, J.; Liu, Q.; Evans, R.; et al. Reassessment of Exosome Composition. Cell 2019, 177, 428–445.e18. [Google Scholar] [CrossRef]
- Wang, X.; Tian, L.; Lu, J.G.; Ng, I.O.L. Exosomes and cancer—Diagnostic and prognostic biomarkers and therapeutic vehicle. Oncogenesis 2022, 11, 54. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, F.; Baur, A. Biogenesis and Functions of Exosomes and Extracellular Vesicles. Methods Mol. Biol. 2016, 1448, 201–216. [Google Scholar]
- Ratajczak, M.Z.; Ratajczak, J. Extracellular microvesicles/exosomes: Discovery, disbelief, acceptance, and the future? Leukemia 2020, 34, 3126–3135. [Google Scholar] [CrossRef]
- Poon, I.K.H.; Parkes, M.A.F.; Jiang, L.; Atkin-Smith, G.K.; Tixeira, R.; Gregory, C.D.; Ozkocak, D.C.; Rutter, S.F.; Caruso, S.; Santavanond, J.P.; et al. Moving beyond size and phosphatidylserine exposure: Evidence for a diversity of apoptotic cell-derived extracellular vesicles in vitro. J. Extracell Vesicles 2019, 8, 1608786. [Google Scholar] [CrossRef]
- Santavanond, J.P.; Rutter, S.F.; Atkin-Smith, G.K.; Poon, I.K.H. Apoptotic Bodies: Mechanism of Formation, Isolation and Functional Relevance. Subcell Biochem. 2021, 97, 61–88. [Google Scholar]
- Estevez-Souto, V.; Da Silva-Alvarez, S.; Collado, M. The role of extracellular vesicles in cellular senescence. FEBS J. 2023, 290, 1203–1211. [Google Scholar] [CrossRef]
- Dixson, A.; Dawson, T.R.; Di Vizio, D.; Weaver, A.M. Context-specific regulation of extracellular vesicle biogenesis and cargo selection. Nat. Rev. Mol. Cell Biol. 2023, 24, 454–476. [Google Scholar] [CrossRef] [PubMed]
- Joshi, P.; Benussi, L.; Furlan, R.; Ghidoni, R.; Verderio, C. Extracellular vesicles in Alzheimer’s disease: Friends or foes? Focus on abeta-vesicle interaction. Int. J. Mol. Sci. 2015, 16, 4800–4813. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, X.; Wang, X.; Yi, X.; Wong, Y.K.; Wu, J.; Xie, F.; Hu, D.; Wang, Q.; Wang, J.; et al. Research progress on the role of extracellular vesicles in neurodegenerative diseases. Transl. Neurodegener. 2023, 12, 43. [Google Scholar] [CrossRef]
- Kalluri, R. The biology and function of extracellular vesicles in immune response and immunity. Immunity 2024, 57, 1752–1768. [Google Scholar] [CrossRef]
- Chen, J.; Tian, C.; Xiong, X.; Yang, Y.; Zhang, J. Extracellular vesicles: New horizons in neurodegeneration. eBioMedicine 2025, 113, 105605. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef] [PubMed]
- Mathivanan, S.; Ji, H.; Simpson, R.J. Exosomes: Extracellular organelles important in intercellular communication. J. Proteomics 2010, 73, 1907–1920. [Google Scholar] [CrossRef] [PubMed]
- Antonucci, F.; Turola, E.; Riganti, L.; Caleo, M.; Gabrielli, M.; Perrotta, C.; Novellino, L.; Clementi, E.; Giussani, P.; Viani, P.; et al. Microvesicles released from microglia stimulate synaptic activity via enhanced sphingolipid metabolism. EMBO J. 2012, 31, 1231–1240. [Google Scholar] [CrossRef] [PubMed]
- Manolopoulos, A.; Delgado-Peraza, F.; Mustapic, M.; Pucha, K.A.; Nogueras-Ortiz, C.; Daskalopoulos, A.; Knight, D.D.; Leoutsakos, J.M.; Oh, E.S.; Lyketsos, C.G.; et al. Comparative assessment of Alzheimer’s disease-related biomarkers in plasma and neuron-derived extracellular vesicles: A nested case-control study. Front. Mol. Biosci. 2023, 10, 1254834. [Google Scholar] [CrossRef]
- Gonul, C.P.; Karacicek, B.; Genc, S. Neuron-Derived Extracellular Vesicles: Emerging Biomarkers and Functional Mediators in Alzheimer’s Disease, with Comparative Insights into Neurodevelopment and Aging. Dev. Neurobiol. 2025, 85, e22984. [Google Scholar] [CrossRef]
- Winston, C.N.; Goetzl, E.J.; Akers, J.C.; Carter, B.S.; Rockenstein, E.M.; Galasko, D.; Masliah, E.; Rissman, R.A. Prediction of conversion from mild cognitive impairment to dementia with neuronally derived blood exosome protein profile. Alzheimer’s Dement. 2016, 3, 63–72. [Google Scholar] [CrossRef]
- Gonzalez-Molina, L.A.; Villar-Vesga, J.; Henao-Restrepo, J.; Villegas, A.; Lopera, F.; Cardona-Gomez, G.P.; Posada-Duque, R. Extracellular Vesicles From 3xTg-AD Mouse and Alzheimer’s Disease Patient Astrocytes Impair Neuroglial and Vascular Components. Front. Aging Neurosci. 2021, 13, 593927. [Google Scholar] [CrossRef]
- Kawata, K.; Mitsuhashi, M.; Aldret, R. A Preliminary Report on Brain-Derived Extracellular Vesicle as Novel Blood Biomarkers for Sport-Related Concussions. Front. Neurol. 2018, 9, 239. [Google Scholar] [CrossRef]
- Li, B.; Ma, Z.; Li, Z. A novel regulator in Alzheimer’s disease progression: The astrocyte-derived extracellular vesicles. Ageing Res. Rev. 2023, 86, 101871. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, C.; Nan, Y.; Nan, S. Microglia-Derived Extracellular Vesicles Carrying miR-711 Alleviate Neurodegeneration in a Murine Alzheimer’s Disease Model by Binding to Itpkb. Front. Cell Dev. Biol. 2020, 8, 566530. [Google Scholar] [CrossRef]
- Potolicchio, I.; Carven, G.J.; Xu, X.; Stipp, C.; Riese, R.J.; Stern, L.J.; Santambrogio, L. Proteomic analysis of microglia-derived exosomes: Metabolic role of the aminopeptidase CD13 in neuropeptide catabolism. J. Immunol. 2005, 175, 2237–2243. [Google Scholar] [CrossRef]
- Hanisch, U.K.; Kettenmann, H. Microglia: Active sensor and versatile effector cells in the normal and pathologic brain. Nat. Neurosci. 2007, 10, 1387–1394. [Google Scholar] [CrossRef]
- Kramer-Albers, E.M.; Bretz, N.; Tenzer, S.; Winterstein, C.; Mobius, W.; Berger, H.; Nave, K.A.; Schild, H.; Trotter, J. Oligodendrocytes secrete exosomes containing major myelin and stress-protective proteins: Trophic support for axons? PROTEOMICS Clin. Appl. 2007, 1, 1446–1461. [Google Scholar] [CrossRef]
- Bakhti, M.; Winter, C.; Simons, M. Inhibition of myelin membrane sheath formation by oligodendrocyte-derived exosome-like vesicles. J. Biol. Chem. 2011, 286, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Kramer-Albers, E.M.; Werner, H.B. Mechanisms of axonal support by oligodendrocyte-derived extracellular vesicles. Nat. Rev. Neurosci. 2023, 24, 474–486. [Google Scholar] [CrossRef] [PubMed]
- Frohlich, D.; Kuo, W.P.; Fruhbeis, C.; Sun, J.J.; Zehendner, C.M.; Luhmann, H.J.; Pinto, S.; Toedling, J.; Trotter, J.; Kramer-Albers, E.M. Multifaceted effects of oligodendroglial exosomes on neurons: Impact on neuronal firing rate, signal transduction and gene regulation. Philos. Trans. R Soc. Lond. B Biol. Sci. 2014, 369, 20130510. [Google Scholar] [CrossRef] [PubMed]
- Bodart-Santos, V.; de Carvalho, L.R.P.; de Godoy, M.A.; Batista, A.F.; Saraiva, L.M.; Lima, L.G.; Abreu, C.A.; De Felice, F.G.; Galina, A.; Mendez-Otero, R.; et al. Extracellular vesicles derived from human Wharton’s jelly mesenchymal stem cells protect hippocampal neurons from oxidative stress and synapse damage induced by amyloid-beta oligomers. Stem. Cell Res. Ther. 2019, 10, 332. [Google Scholar] [CrossRef]
- Gorabi, A.M.; Kiaie, N.; Barreto, G.E.; Read, M.I.; Tafti, H.A.; Sahebkar, A. The Therapeutic Potential of Mesenchymal Stem Cell-Derived Exosomes in Treatment of Neurodegenerative Diseases. Mol. Neurobiol. 2019, 56, 8157–8167. [Google Scholar] [CrossRef]
- Zhang, X.; Che, X.; Zhang, S.; Wang, R.; Li, M.; Jin, Y.; Wang, T.; Song, Y. Mesenchymal stem cell-derived extracellular vesicles for human diseases. Extracell Vesicles Circ. Nucl. Acids 2024, 5, 64–82. [Google Scholar] [CrossRef] [PubMed]
- Elia, C.A.; Tamborini, M.; Rasile, M.; Desiato, G.; Marchetti, S.; Swuec, P.; Mazzitelli, S.; Clemente, F.; Anselmo, A.; Matteoli, M.; et al. Intracerebral Injection of Extracellular Vesicles from Mesenchymal Stem Cells Exerts Reduced Abeta Plaque Burden in Early Stages of a Preclinical Model of Alzheimer’s Disease. Cells 2019, 8, 1059. [Google Scholar] [CrossRef]
- Bravo-Miana, R.D.C.; Arizaga-Echebarria, J.K.; Otaegui, D. Central nervous system-derived extracellular vesicles: The next generation of neural circulating biomarkers? Transl. Neurodegener. 2024, 13, 32. [Google Scholar] [CrossRef]
- Amin, S.; Massoumi, H.; Tewari, D.; Roy, A.; Chaudhuri, M.; Jazayerli, C.; Krishan, A.; Singh, M.; Soleimani, M.; Karaca, E.E.; et al. Cell Type-Specific Extracellular Vesicles and Their Impact on Health and Disease. Int. J. Mol. Sci. 2024, 25, 2730. [Google Scholar] [CrossRef]
- Lemaire, Q.; Duhamel, M.; Raffo-Romero, A.; Salzet, M.; Lefebvre, C. Characterization of Immune Cell-derived Extracellular Vesicles and Studying Functional Impact on Cell Environment. J. Vis. Exp. 2020, 160, e60118. [Google Scholar] [CrossRef]
- Jana, K.; Ghosh, S.; Parua, P.; Debnath, B.; Halder, J.; Sahoo, R.K.; Rai, V.K.; Pradhan, D.; Dash, P.; Das, C.; et al. Insights into the Versatile Role of Extracellular Vesicles in the Treatment of CNS Disorders. Mol. Neurobiol. 2025, 63, 14. [Google Scholar] [CrossRef] [PubMed]
- Chinnathambi, S.; Kumarappan, M.; Malik, S.; Velmurugan, G.; Chandrashekar, M. Neuron-derived extracellular vesicle-based diagnostics for Tau and Alzheimer’s disease. Brain Netw. Disord. 2025, 1, 150–166. [Google Scholar] [CrossRef]
- Huo, L.; Du, X.; Li, X.; Liu, S.; Xu, Y. The Emerging Role of Neural Cell-Derived Exosomes in Intercellular Communication in Health and Neurodegenerative Diseases. Front. Neurosci. 2021, 15, 738442. [Google Scholar] [CrossRef]
- Solana-Balaguer, J.; Campoy-Campos, G.; Martin-Flores, N.; Perez-Sisques, L.; Sitja-Roqueta, L.; Kucukerden, M.; Gamez-Valero, A.; Coll-Manzano, A.; Marti, E.; Perez-Navarro, E.; et al. Neuron-derived extracellular vesicles contain synaptic proteins, promote spine formation, activate TrkB-mediated signalling and preserve neuronal complexity. J. Extracell Vesicles 2023, 12, e12355. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, A.; Fan, H.; Li, Y.; Yang, N.; Tang, Y. Astrocyte-Derived Extracellular Vesicles for Ischemic Stroke: Therapeutic Potential and Prospective. Aging Dis. 2024, 15, 1227–1254. [Google Scholar]
- Chaudhuri, A.D.; Dasgheyb, R.M.; DeVine, L.R.; Bi, H.; Cole, R.N.; Haughey, N.J. Stimulus-dependent modifications in astrocyte-derived extracellular vesicle cargo regulate neuronal excitability. Glia 2020, 68, 128–144. [Google Scholar] [CrossRef]
- Li, J.; Xu, H.; Zhang, K.; Liu, Y.; Zeng, C.; Fu, Y.; Li, Y. Astrocyte-derived exosomes-transported miRNA-26a-5p ameliorates sevoflurane-induced cognitive dysfunction in aged mice. Transl. Res. 2024, 268, 79–96. [Google Scholar] [CrossRef]
- Long, X.; Yao, X.; Jiang, Q.; Yang, Y.; He, X.; Tian, W.; Zhao, K.; Zhang, H. Astrocyte-derived exosomes enriched with miR-873a-5p inhibit neuroinflammation via microglia phenotype modulation after traumatic brain injury. J. Neuroinflamm. 2020, 17, 89. [Google Scholar] [CrossRef]
- Basso, M.; Pozzi, S.; Tortarolo, M.; Fiordaliso, F.; Bisighini, C.; Pasetto, L.; Spaltro, G.; Lidonnici, D.; Gensano, F.; Battaglia, E.; et al. Mutant copper-zinc superoxide dismutase (SOD1) induces protein secretion pathway alterations and exosome release in astrocytes: Implications for disease spreading and motor neuron pathology in amyotrophic lateral sclerosis. J. Biol. Chem. 2013, 288, 15699–15711. [Google Scholar] [CrossRef] [PubMed]
- Goetzl, E.J.; Mustapic, M.; Kapogiannis, D.; Eitan, E.; Lobach, I.V.; Goetzl, L.; Schwartz, J.B.; Miller, B.L. Cargo proteins of plasma astrocyte-derived exosomes in Alzheimer’s disease. FASEB J. 2016, 30, 3853–3859. [Google Scholar] [CrossRef]
- Kowal, J.; Arras, G.; Colombo, M.; Jouve, M.; Morath, J.P.; Primdal-Bengtson, B.; Dingli, F.; Loew, D.; Tkach, M.; Thery, C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl. Acad. Sci. USA 2016, 113, E968–E977. [Google Scholar] [CrossRef]
- Zhu, L.; Zhou, T.; Wu, L.; Zhu, X.; Chen, L.; Zhang, M.; Zhou, J.; Wang, F. Microglial exosome TREM2 ameliorates ferroptosis and neuroinflammation in alzheimer’s disease by activating the Wnt/beta-catenin signaling. Sci. Rep. 2025, 15, 24968. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Wang, J.; Zhao, Y.; Feng, Y.; Han, S.; Dong, Q.; Cui, M.; Tieu, K. Microglial exosomes facilitate alpha-synuclein transmission in Parkinson’s disease. Brain 2020, 143, 1476–1497. [Google Scholar] [CrossRef]
- Wu, J.; Lu, J.; Pan, M.Z.; Gu, X.C.; Dai, L.; Wang, Y.; Shen, B.; Zhang, X.B. Update on the roles and applications of extracellular vesicles in depression. World J. Psychiatry 2025, 15, 102643. [Google Scholar] [CrossRef]
- Fruhbeis, C.; Frohlich, D.; Kuo, W.P.; Amphornrat, J.; Thilemann, S.; Saab, A.S.; Kirchhoff, F.; Mobius, W.; Goebbels, S.; Nave, K.A.; et al. Neurotransmitter-triggered transfer of exosomes mediates oligodendrocyte-neuron communication. PLoS Biol. 2013, 11, e1001604. [Google Scholar] [CrossRef] [PubMed]
- Fruhbeis, C.; Kuo-Elsner, W.P.; Muller, C.; Barth, K.; Peris, L.; Tenzer, S.; Mobius, W.; Werner, H.B.; Nave, K.A.; Frohlich, D.; et al. Oligodendrocytes support axonal transport and maintenance via exosome secretion. PLoS Biol. 2020, 18, e3000621. [Google Scholar] [CrossRef] [PubMed]
- Simons, M.; Nave, K.A. Oligodendrocytes: Myelination and Axonal Support. Cold Spring Harb. Perspect. Biol. 2015, 8, a020479. [Google Scholar] [CrossRef]
- Padinharayil, H.; Varghese, J.; Wilson, C.; George, A. Mesenchymal stem cell-derived exosomes: Characteristics and applications in disease pathology and management. Life Sci. 2024, 342, 122542. [Google Scholar] [CrossRef]
- Lu, C.H.; Chen, Y.A.; Ke, C.C.; Liu, R.S. Mesenchymal Stem Cell-Derived Extracellular Vesicle: A Promising Alternative Therapy for Osteoporosis. Int. J. Mol. Sci. 2021, 22, 12750. [Google Scholar] [CrossRef]
- Ahn, S.Y.; Park, W.S.; Kim, Y.E.; Sung, D.K.; Sung, S.I.; Ahn, J.Y.; Chang, Y.S. Vascular endothelial growth factor mediates the therapeutic efficacy of mesenchymal stem cell-derived extracellular vesicles against neonatal hyperoxic lung injury. Exp. Mol. Med. 2018, 50, 1–12. [Google Scholar] [CrossRef]
- Kou, M.; Huang, L.; Yang, J.; Chiang, Z.; Chen, S.; Liu, J.; Guo, L.; Zhang, X.; Zhou, X.; Xu, X.; et al. Mesenchymal stem cell-derived extracellular vesicles for immunomodulation and regeneration: A next generation therapeutic tool? Cell Death Dis. 2022, 13, 580. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, X.; Zhang, H.; Song, P.; Pan, W.; Xu, P.; Wang, G.; Hu, P.; Wang, Z.; Huang, K.; et al. Mesenchymal Stem Cells Derived Extracellular Vesicles Alleviate Traumatic Hemorrhagic Shock Induced Hepatic Injury via IL-10/PTPN22-Mediated M2 Kupffer Cell Polarization. Front. Immunol. 2021, 12, 811164. [Google Scholar] [CrossRef]
- Racchetti, G.; Meldolesi, J. Extracellular Vesicles of Mesenchymal Stem Cells: Therapeutic Properties Discovered with Extraordinary Success. Biomedicines 2021, 9, 667. [Google Scholar] [CrossRef]
- Qiu, G.; Zheng, G.; Ge, M.; Wang, J.; Huang, R.; Shu, Q.; Xu, J. Mesenchymal stem cell-derived extracellular vesicles affect disease outcomes via transfer of microRNAs. Stem. Cell Res. Ther. 2018, 9, 320. [Google Scholar] [CrossRef]
- Lai, R.C.; Tan, S.S.; Yeo, R.W.; Choo, A.B.; Reiner, A.T.; Su, Y.; Shen, Y.; Fu, Z.; Alexander, L.; Sze, S.K.; et al. MSC secretes at least 3 EV types each with a unique permutation of membrane lipid, protein and RNA. J. Extracell Vesicles 2016, 5, 29828. [Google Scholar] [CrossRef] [PubMed]
- Watson, L.S.; Hamlett, E.D.; Stone, T.D.; Sims-Robinson, C. Neuronally derived extracellular vesicles: An emerging tool for understanding Alzheimer’s disease. Mol. Neurodegener. 2019, 14, 22. [Google Scholar] [CrossRef]
- Upadhya, R.; Zingg, W.; Shetty, S.; Shetty, A.K. Astrocyte-derived extracellular vesicles: Neuroreparative properties and role in the pathogenesis of neurodegenerative disorders. J. Control Release 2020, 323, 225–239. [Google Scholar] [CrossRef]
- Wang, L.; Lin, Y.; Yang, Z.; Zhang, K.; Gong, H.; Zheng, Y.; Wang, B.; Zhang, X.; Sun, M. Microglia-derived extracellular vesicles mediate fine particulate matter-induced Alzheimer’s disease-like behaviors through the miR-34a-5p/DUSP10/p-p38 MAPK pathway. J. Hazard Mater. 2025, 495, 138853. [Google Scholar] [CrossRef]
- Zhao, S.; Sheng, S.; Wang, Y.; Ding, L.; Xu, X.; Xia, X.; Zheng, J.C. Astrocyte-derived extracellular vesicles: A double-edged sword in central nervous system disorders. Neurosci. Biobehav. Rev. 2021, 125, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Hao, Y.; Feng, Y.; Li, H.; Mao, Y.; Dong, Q.; Cui, M. Microglial Exosomes in Neurodegenerative Disease. Front. Mol. Neurosci. 2021, 14, 630808. [Google Scholar] [CrossRef]
- Sasmita, A.O.; Depp, C.; Nazarenko, T.; Sun, T.; Siems, S.B.; Ong, E.C.; Nkeh, Y.B.; Bohler, C.; Yu, X.; Bues, B.; et al. Oligodendrocytes produce amyloid-beta and contribute to plaque formation alongside neurons in Alzheimer’s disease model mice. Nat. Neurosci. 2024, 27, 1668–1674. [Google Scholar] [CrossRef]
- Rajani, R.M.; Ellingford, R.; Hellmuth, M.; Harris, S.S.; Taso, O.S.; Graykowski, D.; Lam, F.K.W.; Arber, C.; Fertan, E.; Danial, J.S.H.; et al. Selective suppression of oligodendrocyte-derived amyloid beta rescues neuronal dysfunction in Alzheimer’s disease. PLoS Biol. 2024, 22, e3002727. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Pulliam, L. Exosomes as mediators of neuroinflammation. J. Neuroinflamm. 2014, 11, 68. [Google Scholar] [CrossRef]
- Lombardi, M.; Gabrielli, M.; Adinolfi, E.; Verderio, C. Role of ATP in Extracellular Vesicle Biogenesis and Dynamics. Front. Pharmacol. 2021, 12, 654023. [Google Scholar] [CrossRef] [PubMed]
- Bianco, F.; Pravettoni, E.; Colombo, A.; Schenk, U.; Möller, T.; Matteoli, M.; Verderio, C. Astrocyte-Derived ATP Induces Vesicle Shedding and IL-1 β Release from Microglia. J. Immunol. 2005, 174, 7268–7277. [Google Scholar] [CrossRef]
- Wang, G.; Dinkins, M.; He, Q.; Zhu, G.; Poirier, C.; Campbell, A.; Mayer-Proschel, M.; Bieberich, E. Astrocytes secrete exosomes enriched with proapoptotic ceramide and prostate apoptosis response 4 (PAR-4): Potential mechanism of apoptosis induction in Alzheimer disease (AD). J. Biol. Chem. 2012, 287, 21384–21395. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, L.; Bali, J.; Barr, M.M.; Court, F.A.; Kramer-Albers, E.M.; Picou, F.; Raposo, G.; van der Vos, K.E.; van Niel, G.; Wang, J.; et al. Emerging roles of extracellular vesicles in the nervous system. J. Neurosci. 2014, 34, 15482–15489. [Google Scholar] [CrossRef]
- Dickens, A.M.; Tovar, Y.R.L.B.; Yoo, S.W.; Trout, A.L.; Bae, M.; Kanmogne, M.; Megra, B.; Williams, D.W.; Witwer, K.W.; Gacias, M.; et al. Astrocyte-shed extracellular vesicles regulate the peripheral leukocyte response to inflammatory brain lesions. Sci. Signal 2017, 10, eaai7696. [Google Scholar] [CrossRef]
- Yu, Z.; Shi, M.; Stewart, T.; Fernagut, P.O.; Huang, Y.; Tian, C.; Dehay, B.; Atik, A.; Yang, D.; De Giorgi, F.; et al. Reduced oligodendrocyte exosome secretion in multiple system atrophy involves SNARE dysfunction. Brain 2020, 143, 1780–1797. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Halliwell, B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat. Rev. Neurosci. 2019, 20, 148–160. [Google Scholar] [CrossRef]
- Swerdlow, R.H. Mitochondria and Mitochondrial Cascades in Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 62, 1403–1416. [Google Scholar] [CrossRef]
- Passos, J.F.; Saretzki, G.; Ahmed, S.; Nelson, G.; Richter, T.; Peters, H.; Wappler, I.; Birket, M.J.; Harold, G.; Schaeuble, K.; et al. Mitochondrial dysfunction accounts for the stochastic heterogeneity in telomere-dependent senescence. PLoS Biol. 2007, 5, e110. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.E.; Starkov, A.; Blass, J.P.; Ratan, R.R.; Beal, M.F. Cause and consequence: Mitochondrial dysfunction initiates and propagates neuronal dysfunction, neuronal death and behavioral abnormalities in age-associated neurodegenerative diseases. Biochim. Biophys. Acta 2010, 1802, 122–134. [Google Scholar] [CrossRef]
- Bir, A.; Ghosh, A.; Chauhan, A.; Saha, S.; Saini, A.K.; Bisaglia, M.; Chakrabarti, S. Exosomal Dynamics and Brain Redox Imbalance: Implications in Alzheimer’s Disease Pathology and Diagnosis. Antioxidants 2024, 13, 316. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, B.; Xu, L.; Yu, S.; Fu, J.; Wang, J.; Yan, X.; Su, J. ROS-Induced mtDNA Release: The Emerging Messenger for Communication between Neurons and Innate Immune Cells during Neurodegenerative Disorder Progression. Antioxidants 2021, 10, 1917. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, B.; Duan, R.; Liu, Y. Mitochondrial DNA Leakage and cGas/STING Pathway in Microglia: Crosstalk Between Neuroinflammation and Neurodegeneration. Neuroscience 2024, 548, 1–8. [Google Scholar] [CrossRef]
- Brites, D.; Fernandes, A. Neuroinflammation and Depression: Microglia Activation, Extracellular Microvesicles and microRNA Dysregulation. Front. Cell Neurosci. 2015, 9, 476. [Google Scholar] [CrossRef] [PubMed]
- Goetzl, E.J.; Kapogiannis, D.; Schwartz, J.B.; Lobach, I.V.; Goetzl, L.; Abner, E.L.; Jicha, G.A.; Karydas, A.M.; Boxer, A.; Miller, B.L. Decreased synaptic proteins in neuronal exosomes of frontotemporal dementia and Alzheimer’s disease. FASEB J. 2016, 30, 4141–4148. [Google Scholar] [CrossRef] [PubMed]
- Goetzl, E.J.; Boxer, A.; Schwartz, J.B.; Abner, E.L.; Petersen, R.C.; Miller, B.L.; Carlson, O.D.; Mustapic, M.; Kapogiannis, D. Low neural exosomal levels of cellular survival factors in Alzheimer’s disease. Ann. Clin. Transl. Neurol. 2015, 2, 769–773. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.L.; Yang, Y.M.; Zhang, X.Y.; Shi, Z.Q.; Gao, H.L.; Zhong, M.L.; Fan, Y.G.; Zhang, H.Y.; Liu, B.; Qing, G.Y. Secreted endogenous macrosomes reduce Aβ burden and ameliorate Alzheimer’s disease. Sci. Adv. 2023, 21, eade0293. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, L.; Yang, J.; Meng, L.; Chen, J.; Zhou, L.; Wang, J.; Xiong, M.; Zhang, Z. Soluble TREM2 ameliorates tau phosphorylation and cognitive deficits through activating transgelin-2 in Alzheimer’s disease. Nat. Commun. 2023, 14, 6670. [Google Scholar] [CrossRef]
- Yuyama, K.; Igarashi, Y. Exosomes as Carriers of Alzheimer’s Amyloid-ss. Front. Neurosci. 2017, 11, 229. [Google Scholar] [CrossRef]
- Tamboli, I.Y.; Barth, E.; Christian, L.; Siepmann, M.; Kumar, S.; Singh, S.; Tolksdorf, K.; Heneka, M.T.; Lutjohann, D.; Wunderlich, P.; et al. Statins promote the degradation of extracellular amyloid beta-peptide by microglia via stimulation of exosome-associated insulin-degrading enzyme (IDE) secretion. J. Biol. Chem. 2010, 285, 37405–37414. [Google Scholar] [CrossRef]
- Xiong, W.P.; Yao, W.Q.; Wang, B.; Liu, K. BMSCs-exosomes containing GDF-15 alleviated SH-SY5Y cell injury model of Alzheimer’s disease via AKT/GSK-3beta/beta-catenin. Brain Res. Bull. 2021, 177, 92–102. [Google Scholar] [CrossRef]
- Feng, X.; Fan, H. Ningbo Institute of Life and Health Industry, UCAS, Ningbo, Zhejiang Province, China. 2026; manuscript in preparation. [Google Scholar]
- Santos, R.T.; Braga, C.L.; de Sa Freire Onofre, M.E.; da Silva, C.M.; de Novaes Rocha, N.; Veras, R.G.; de Souza Serra, S.S.; Teixeira, D.E.; Dos Santos Alves, S.A.; Miranda, B.T.; et al. Cardioprotective effects of extracellular vesicles from hypoxia-preconditioned mesenchymal stromal cells in experimental pulmonary arterial hypertension. Stem Cell Res. Ther. 2025, 16, 466. [Google Scholar] [CrossRef]
- Wang, H.; Sui, H.; Zheng, Y.; Jiang, Y.; Shi, Y.; Liang, J.; Zhao, L. Curcumin-primed exosomes potently ameliorate cognitive function in AD mice by inhibiting hyperphosphorylation of the Tau protein through the AKT/GSK-3beta pathway. Nanoscale 2019, 11, 7481–7496. [Google Scholar] [CrossRef]
- Rao, S.; Madhu, L.N.; Babu, R.S.; Shankar, G.; Kotian, S.; Nagarajan, A.; Upadhya, R.; Narvekar, E.; Cai, J.J.; Shetty, A.K. Extracellular vesicles from hiPSC-derived NSCs protect human neurons against Abeta-42 oligomers induced neurodegeneration, mitochondrial dysfunction and tau phosphorylation. Stem Cell Res. Ther. 2025, 16, 191. [Google Scholar] [CrossRef]
- Lim, H.; Lee, D.; Choi, W.K.; Choi, S.J.; Oh, W.; Kim, D.H. Galectin-3 Secreted by Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells Reduces Aberrant Tau Phosphorylation in an Alzheimer Disease Model. Stem Cells Int. 2020, 2020, 8878412. [Google Scholar] [CrossRef] [PubMed]
- de Godoy, M.A.; Saraiva, L.M.; de Carvalho, L.R.P.; Vasconcelos-Dos-Santos, A.; Beiral, H.J.V.; Ramos, A.B.; Silva, L.R.P.; Leal, R.B.; Monteiro, V.H.S.; Braga, C.V.; et al. Mesenchymal stem cells and cell-derived extracellular vesicles protect hippocampal neurons from oxidative stress and synapse damage induced by amyloid-beta oligomers. J. Biol. Chem. 2018, 293, 1957–1975. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Huang, M.; Zheng, M.; Dai, C.; Song, Q.; Zhang, Q.; Li, Q.; Gu, X.; Chen, H.; Jiang, G.; et al. ADSCs-derived extracellular vesicles alleviate neuronal damage, promote neurogenesis and rescue memory loss in mice with Alzheimer’s disease. J. Control Release 2020, 327, 688–702. [Google Scholar] [CrossRef] [PubMed]
- Sha, S.; Shen, X.L.; Cao, Y.P.; Qu, L. Mesenchymal stem cells-derived extracellular vesicles ameliorate Alzheimer’s disease in rat models via the microRNA-29c-3p/BACE1 axis and the Wnt/β-catenin pathway. Aging 2021, 13, 15285–15306. [Google Scholar] [CrossRef]
- Ding, M.; Shen, Y.; Wang, P.; Xie, Z.; Xu, S.; Zhu, Z.; Wang, Y.; Lyu, Y.; Wang, D.; Xu, L.; et al. Exosomes Isolated From Human Umbilical Cord Mesenchymal Stem Cells Alleviate Neuroinflammation and Reduce Amyloid-Beta Deposition by Modulating Microglial Activation in Alzheimer’s Disease. Neurochem. Res. 2018, 43, 2165–2177. [Google Scholar] [CrossRef]
- Long, Q.; Upadhya, D.; Hattiangady, B.; Kim, D.K.; An, S.Y.; Shuai, B.; Prockop, D.J.; Shetty, A.K. Intranasal MSC-derived A1-exosomes ease inflammation, and prevent abnormal neurogenesis and memory dysfunction after status epilepticus. Proc. Natl. Acad. Sci. USA 2017, 114, E3536–E3545. [Google Scholar] [CrossRef]
- Reza-Zaldivar, E.E.; Hernandez-Sapiens, M.A.; Gutierrez-Mercado, Y.K.; Sandoval-Avila, S.; Gomez-Pinedo, U.; Marquez-Aguirre, A.L.; Vazquez-Mendez, E.; Padilla-Camberos, E.; Canales-Aguirre, A.A. Mesenchymal stem cell-derived exosomes promote neurogenesis and cognitive function recovery in a mouse model of Alzheimer’s disease. Neural Regen. Res. 2019, 14, 1626–1634. [Google Scholar]
- Tzioras, M.; McGeachan, R.I.; Durrant, C.S.; Spires-Jones, T.L. Synaptic degeneration in Alzheimer disease. Nat. Rev. Neurol. 2022, 19, 19–38. [Google Scholar] [CrossRef]
- Antoniou, A.; Auderset, L.; Kaurani, L.; Sebastian, E.; Zeng, Y.; Allahham, M.; Cases-Cunillera, S.; Schoch, S.; Grundemann, J.; Fischer, A.; et al. Neuronal extracellular vesicles and associated microRNAs induce circuit connectivity downstream BDNF. Cell Rep. 2023, 42, 112063. [Google Scholar] [CrossRef] [PubMed]
- An, K.; Klyubin, L.; Kim, Y.; Jung, J.H.; Mably, J.A.; O’Dowd, Y.S.; Lynch, T.; Kanmert, D.; Lemere, A.C.; Finan, M.G.; et al. Exosomes neutralize synaptic-plasticity-disrupting activity of Aβ assemblies in vivo. Mol. Brain 2013, 6, 47. [Google Scholar] [CrossRef]
- Goldie, B.J.; Dun, M.D.; Lin, M.; Smith, N.D.; Verrills, N.M.; Dayas, C.V.; Cairns, M.J. Activity-associated miRNA are packaged in Map1b-enriched exosomes released from depolarized neurons. Nucleic Acids Res. 2014, 42, 9195–9208. [Google Scholar] [CrossRef]
- Zhang, H.; Xie, X.H.; Xu, S.X.; Wang, C.; Sun, S.; Song, X.; Li, R.; Li, N.; Feng, Y.; Duan, H.; et al. Oligodendrocyte-derived exosomes-containing SIRT2 ameliorates depressive-like behaviors and restores hippocampal neurogenesis and synaptic plasticity via the AKT/GSK-3beta pathway in depressed mice. CNS Neurosci. Ther. 2024, 30, e14661. [Google Scholar] [CrossRef]
- Mancini, W.V.S.B.; Mattera, V.S.; Pasquini, J.M.; Pasquini, L.A.; Correale, J.D. Microglia-derived extracellular vesicles in homeostasis and demyelination/remyelination processes. J. Neurochem. 2024, 168, 3–25. [Google Scholar]
- Glebov, K.; Lochner, M.; Jabs, R.; Lau, T.; Merkel, O.; Schloss, P.; Steinhauser, C.; Walter, J. Serotonin stimulates secretion of exosomes from microglia cells. Glia 2015, 63, 626–634. [Google Scholar] [CrossRef]
- Orihuela, R.; McPherson, C.A.; Harry, G.J. Microglial M1/M2 polarization and metabolic states. Br. J. Pharmacol. 2016, 173, 649–665. [Google Scholar] [CrossRef]
- Bai, R.; Guo, J.; Ye, X.Y.; Xie, Y.; Xie, T. Oxidative stress: The core pathogenesis and mechanism of Alzheimer’s disease. Ageing Res. Rev. 2022, 77, 101619. [Google Scholar] [CrossRef]
- Li, S.; Sheng, Z.H. Oligodendrocyte-derived transcellular signaling regulates axonal energy metabolism. Curr. Opin. Neurobiol. 2023, 80, 102722. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Wan, L.; Luo, Z.; Xie, Y.; Liu, Y.; Huang, T.; Lu, H.; Hu, J.; Xiong, Y. Microglia-Derived Exosomes Improve Spinal Cord Functional Recovery after Injury via Inhibiting Oxidative Stress and Promoting the Survival and Function of Endothelia Cells. Oxid. Med. Cell. Longev. 2021, 2021, 1695087. [Google Scholar] [CrossRef] [PubMed]
- Ceruti, S.; Colombo, L.; Magni, G.; Vigano, F.; Boccazzi, M.; Deli, M.A.; Sperlagh, B.; Abbracchio, M.P.; Kittel, A. Oxygen-glucose deprivation increases the enzymatic activity and the microvesicle-mediated release of ectonucleotidases in the cells composing the blood-brain barrier. Neurochem. Int. 2011, 59, 259–271. [Google Scholar] [CrossRef]
- Zeng, P.; Shi, Y.; Wang, X.M.; Lin, L.; Du, Y.J.; Tang, N.; Wang, Q.; Fang, Y.Y.; Wang, J.Z.; Zhou, X.W.; et al. Emodin Rescued Hyperhomocysteinemia-Induced Dementia and Alzheimer’s Disease-Like Features in Rats. Int. J. Neuropsychopharmacol. 2019, 22, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xie, X.; Sun, Y.; Zhou, F. Blood and CSF Homocysteine Levels in Alzheimer’s Disease: A Meta-Analysis and Meta-Regression of Case-Control Studies. Neuropsychiatr. Dis. Treat. 2022, 18, 2391–2403. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, A.; Samra, Y.A.; Elsherbiny, N.M.; Al-Shabrawey, M. Implication of Hyperhomocysteinemia in Blood Retinal Barrier (BRB) Dysfunction. Biomolecules 2020, 10, 1119. [Google Scholar] [CrossRef]
- Persichilli, S.; Gervasoni, J.; Di Napoli, A.; Fuso, A.; Nicolia, V.; Giardina, B.; Scarpa, S.; Desiderio, C.; Cavallaro, R.A. Plasma thiols levels in Alzheimer’s disease mice under diet-induced hyperhomocysteinemia: Effect of S-adenosylmethionine and superoxide-dismutase supplementation. J. Alzheimer’s Dis. 2015, 44, 1323–1331. [Google Scholar] [CrossRef]
- Jakubowski, H. Homocysteine Thiolactone Detoxifying Enzymes and Alzheimer’s Disease. Int. J. Mol. Sci. 2024, 25, 8095. [Google Scholar] [CrossRef]
- Witucki, L.; Borowczyk, K.; Suszynska-Zajczyk, J.; Warzych, E.; Pawlak, P.; Jakubowski, H. Deletion of the Homocysteine Thiolactone Detoxifying Enzyme Bleomycin Hydrolase, in Mice, Causes Memory and Neurological Deficits and Worsens Alzheimer’s Disease-Related Behavioral and Biochemical Traits in the 5xFAD Model of Alzheimer’s Disease. J. Alzheimer’s Dis. 2023, 95, 1735–1755. [Google Scholar] [CrossRef]
- Chen, H.; Liu, S.; Ji, L.; Wu, T.; Ma, F.; Ji, Y.; Zhou, Y.; Zheng, M.; Zhang, M.; Huang, G. Associations between Alzheimer’s Disease and Blood Homocysteine, Vitamin B12, and Folate: A Case-Control Study. Curr. Alzheimer Res. 2015, 12, 88–94. [Google Scholar] [CrossRef]
- Gu, Y.; Nieves, W.J.; Stern, Y.; Luchsinger, A.P.; Scarmeas, N. Food Combination and Alzheimer Disease Risk. Arch. Neurol. 2010, 7, 699–706. [Google Scholar] [CrossRef]
- Li, F.; Li, Y. Ningbo Institute of Life and Health Industry, UCAS, Ningbo, Zhejiang Province, China. 2026; manuscript in preparation. [Google Scholar]
- Hao, Y.; Su, C.; Liu, X.; Sui, H.; Shi, Y.; Zhao, L. Bioengineered microglia-targeted exosomes facilitate Abeta clearance via enhancing activity of microglial lysosome for promoting cognitive recovery in Alzheimer’s disease. Biomater. Adv. 2022, 136, 212770. [Google Scholar] [CrossRef] [PubMed]
- Iyaswamy, A.; Thakur, A.; Guan, X.J.; Krishnamoorthi, S.; Fung, T.Y.; Lu, K.; Gaurav, I.; Yang, Z.; Su, C.F.; Lau, K.F.; et al. Fe65-engineered neuronal exosomes encapsulating corynoxine-B ameliorate cognition and pathology of Alzheimer’s disease. Signal Transduct. Target. Ther. 2023, 8, 404. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xu, Z.; Yu, J.; Zang, X.; Jiang, S.; Xu, S.; Wang, W.; Hong, S. MiR-146a-5p engineered hucMSC-derived extracellular vesicles attenuate Dermatophagoides farinae-induced allergic airway epithelial cell inflammation. Front. Immunol. 2024, 15, 1443166. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.H.; Guo, H.D.; Li, H.; Zhai, Y.; Gong, Z.B.; Wu, J.; Liu, J.S.; Dong, Y.R.; Hou, S.X.; Liu, J.R. RVG-modified exosomes derived from mesenchymal stem cells rescue memory deficits by regulating inflammatory responses in a mouse model of Alzheimer’s disease. Immun. Ageing 2019, 16, 10. [Google Scholar] [CrossRef]
- Cui, G.H.; Wu, J.; Mou, F.F.; Xie, W.H.; Wang, F.B.; Wang, Q.L.; Fang, J.; Xu, Y.W.; Dong, Y.R.; Liu, J.R.; et al. Exosomes derived from hypoxia-preconditioned mesenchymal stromal cells ameliorate cognitive decline by rescuing synaptic dysfunction and regulating inflammatory responses in APP/PS1 mice. FASEB J. 2018, 32, 654–668. [Google Scholar] [CrossRef] [PubMed]
- Ni, H.; Yang, S.; Siaw-Debrah, F.; Hu, J.; Wu, K.; He, Z.; Yang, J.; Pan, S.; Lin, X.; Ye, H.; et al. Exosomes Derived From Bone Mesenchymal Stem Cells Ameliorate Early Inflammatory Responses Following Traumatic Brain Injury. Front. Neurosci. 2019, 13, 14. [Google Scholar] [CrossRef]
- Bhardwaj, S.; Kesari, K.K.; Rachamalla, M.; Mani, S.; Ashraf, G.M.; Jha, S.K.; Kumar, P.; Ambasta, R.K.; Dureja, H.; Devkota, H.P.; et al. CRISPR/Cas9 gene editing: New hope for Alzheimer’s disease therapeutics. J. Adv. Res. 2022, 40, 207–221. [Google Scholar] [CrossRef]
- Akyuz, E.; Aslan, F.S.; Gokce, E.; Ilmaz, O.; Topcu, F.; Kakac, S. Extracellular vesicle and CRISPR gene therapy: Current applications in Alzheimer’s disease, Parkinson’s disease, amyotrophic lateral sclerosis, and Huntington’s disease. Eur. J. Neurosci. 2024, 60, 6057–6090. [Google Scholar] [CrossRef]
- Hanafy, A.S.; Schoch, S.; Lamprecht, A. CRISPR/Cas9 Delivery Potentials in Alzheimer’s Disease Management: A Mini Review. Pharmaceutics 2020, 12, 801. [Google Scholar] [CrossRef]
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011, 29, 341–345. [Google Scholar] [CrossRef]
- Han, J.; Sul, H.J.; Lee, J.; Kim, E.; Kim, K.H.; Chae, M.; Lim, J.; Kim, J.; Kim, C.; Kim, J.K.; et al. Engineered exosomes with a photoinducible protein delivery system enable CRISPR-Cas–based epigenome editing in Alzheimer’s disease. Drug Deliv. 2024, 16, eadi4830. [Google Scholar] [CrossRef]
- Kostyushev, D.; Kostyusheva, A.; Brezgin, S.; Smirnov, V.; Volchkova, E.; Lukashev, A.; Chulanov, V. Gene Editing by Extracellular Vesicles. Int. J. Mol. Sci. 2020, 21, 7362. [Google Scholar] [CrossRef]
- Jack, C.R., Jr.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. Contributors, NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimer’s Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef]
- Keshavan, A.; Pannee, J.; Karikari, T.K.; Rodriguez, J.L.; Ashton, N.J.; Nicholas, J.M.; Cash, D.M.; Coath, W.; Lane, C.A.; Parker, T.D.; et al. Population-based blood screening for preclinical Alzheimer’s disease in a British birth cohort at age 70. Brain 2021, 144, 434–449. [Google Scholar] [PubMed]
- Janelidze, S.; Teunissen, C.E.; Zetterberg, H.; Allue, J.A.; Sarasa, L.; Eichenlaub, U.; Bittner, T.; Ovod, V.; Verberk, I.M.W.; Toba, K.; et al. Head-to-Head Comparison of 8 Plasma Amyloid-beta 42/40 Assays in Alzheimer Disease. JAMA Neurol. 2021, 78, 1375–1382. [Google Scholar] [CrossRef]
- Nakamura, A.; Kaneko, N.; Villemagne, V.L.; Kato, T.; Doecke, J.; Dore, V.; Fowler, C.; Li, Q.X.; Martins, R.; Rowe, C.; et al. High performance plasma amyloid-beta biomarkers for Alzheimer’s disease. Nature 2018, 554, 249–254. [Google Scholar] [CrossRef]
- Schindler, S.E.; Bollinger, J.G.; Ovod, V.; Mawuenyega, K.G.; Li, Y.; Gordon, B.A.; Holtzman, D.M.; Morris, J.C.; Benzinger, T.L.S.; Xiong, C.; et al. High-precision plasma beta-amyloid 42/40 predicts current and future brain amyloidosis. Neurology 2019, 93, e1647–e1659. [Google Scholar] [CrossRef] [PubMed]
- Karikari, T.K.; Pascoal, T.A.; Ashton, N.J.; Janelidze, S.; Benedet, A.L.; Rodriguez, J.L.; Chamoun, M.; Savard, M.; Kang, M.S.; Therriault, J.; et al. Blood phosphorylated tau 181 as a biomarker for Alzheimer’s disease: A diagnostic performance and prediction modelling study using data from four prospective cohorts. Lancet Neurol. 2020, 19, 422–433. [Google Scholar] [CrossRef]
- Palmqvist, S.; Janelidze, S.; Quiroz, Y.T.; Zetterberg, H.; Lopera, F.; Stomrud, E.; Su, Y.; Chen, Y.; Serrano, G.E.; Leuzy, A.; et al. Discriminative Accuracy of Plasma Phospho-tau217 for Alzheimer Disease vs Other Neurodegenerative Disorders. JAMA 2020, 324, 772–781. [Google Scholar] [CrossRef]
- Janelidze, S.; Mattsson, N.; Palmqvist, S.; Smith, R.; Beach, T.G.; Serrano, G.E.; Chai, X.; Proctor, N.K.; Eichenlaub, U.; Zetterberg, H.; et al. Plasma P-tau181 in Alzheimer’s disease: Relationship to other biomarkers, differential diagnosis, neuropathology and longitudinal progression to Alzheimer’s dementia. Nat. Med. 2020, 26, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Thijssen, E.H.; La Joie, R.; Wolf, A.; Strom, A.; Wang, P.; Iaccarino, L.; Bourakova, V.; Cobigo, Y.; Heuer, H.; Spina, S.; et al. Treatment for Frontotemporal Lobar Degeneration, i., Diagnostic value of plasma phosphorylated tau181 in Alzheimer’s disease and frontotemporal lobar degeneration. Nat. Med. 2020, 26, 387–397. [Google Scholar] [CrossRef]
- Jiao, B.; Ouyang, Z.; Xiao, X.; Zhang, C.; Xu, T.; Yang, Q.; Zhu, Y.; Liu, Y.; Liu, X.; Zhou, Y.; et al. Development and validation of machine learning models with blood-based digital biomarkers for Alzheimer’s disease diagnosis: A multicohort diagnostic study. eClinicalMedicine 2025, 81, 103142. [Google Scholar] [CrossRef]
- Ashton, N.J.; Pascoal, T.A.; Karikari, T.K.; Benedet, A.L.; Lantero-Rodriguez, J.; Brinkmalm, G.; Snellman, A.; Scholl, M.; Troakes, C.; Hye, A.; et al. Plasma p-tau231: A new biomarker for incipient Alzheimer’s disease pathology. Acta Neuropathol. 2021, 141, 709–724. [Google Scholar] [CrossRef]
- Karikari, T.K.; Ashton, N.J.; Brinkmalm, G.; Brum, W.S.; Benedet, A.L.; Montoliu-Gaya, L.; Lantero-Rodriguez, J.; Pascoal, T.A.; Suarez-Calvet, M.; Rosa-Neto, P.; et al. Blood phospho-tau in Alzheimer disease: Analysis, interpretation, and clinical utility. Nat. Rev. Neurol. 2022, 18, 400–418. [Google Scholar] [CrossRef]
- Ashton, N.J.; Leuzy, A.; Karikari, T.K.; Mattsson-Carlgren, N.; Dodich, A.; Boccardi, M.; Corre, J.; Drzezga, A.; Nordberg, A.; Ossenkoppele, R.; et al. The validation status of blood biomarkers of amyloid and phospho-tau assessed with the 5-phase development framework for AD biomarkers. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2140–2156. [Google Scholar] [CrossRef]
- Gonzalez-Ortiz, F.; Kirsebom, B.E.; Contador, J.; Tanley, J.E.; Selnes, P.; Gisladottir, B.; Palhaugen, L.; Suhr Hemminghyth, M.; Jarholm, J.; Skogseth, R.; et al. Plasma brain-derived tau is an amyloid-associated neurodegeneration biomarker in Alzheimer’s disease. Nat. Commun. 2024, 15, 2908. [Google Scholar] [CrossRef] [PubMed]
- Grande, G.; Qiu, C.; Valletta, M.; Orsini4, N.; Rizzuto, D.; Dale, M.; Fredolini, C.; Winblad, B.; Vetrano, D.L. Blood-based biomarkers of Alzheimer’s disease and incident dementia in the community. Nat. Med. 2025, 31, 2027–2035. [Google Scholar] [CrossRef]
- Olsson, B.; Lautner, R.; Andreasson, U.; Öhrfelt, A.; Portelius, E.; Bjerke, M.; Hölttä, M.; Rosén, C.; Olsson, C.; Strobel, G.; et al. CSF and blood biomarkers for the diagnosis of Alzheimer’s disease: A systematic review and meta-analysis. Lancet Neurol. 2016, 15, 673–684. [Google Scholar] [CrossRef]
- Cleary, A.J.; Kumar, A.; Craft, S. Neuron-derived extracellular vesicles as a liquid biopsy for brain insulin dysregulation in Alzheimer’s disease and related disorders. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2024, 21, e14497. [Google Scholar] [CrossRef]
- Zetterberg, H.; Blennow, K. Moving fluid biomarkers for Alzheimer’s disease from research tools to routine clinical diagnostics. Mol. Neurodegener. 2021, 16, 10. [Google Scholar] [CrossRef] [PubMed]
- Hagey, D.W.; El Andaloussi, S. The promise and challenges of extracellular vesicles in the diagnosis of neurodegenerative diseases. Handb. Clin. Neurol. 2023, 193, 227–241. [Google Scholar] [PubMed]
- Kandeel, M.; Morsy, M.A.; Alkhodair, K.M.; Alhojaily, S. Mesenchymal Stem Cell-Derived Extracellular Vesicles: An Emerging Diagnostic and Therapeutic Biomolecules for Neurodegenerative Disabilities. Biomolecules 2023, 13, 1250. [Google Scholar] [CrossRef]
- Jia, L.; Qiu, Q.; Zhang, H.; Chu, L.; Du, Y.; Zhang, J.; Zhou, C.; Liang, F.; Shi, S.; Wang, S.; et al. Concordance between the assessment of Ab42, T-tau, and P-T181-tau in peripheral blood neuronal-derived exosomes and cerebrospinal fluid. Alzheimer’s Dement. 2019, 15, 1071–1080. [Google Scholar] [CrossRef]
- Li, T.R.; Yao, Y.; Jiang, X.; Dong, Q.; Yu, W.; Wang, T.; Cai, Y.; Han, Y. β-Amyloid in blood neuronal-derived extracellular vesicles is elevated in cognitively normal adults at risk of Alzheimer’s disease and predicts cerebral amyloidosis. Alzheimer’s Res. Ther. 2022, 14, 66. [Google Scholar] [CrossRef] [PubMed]
- Fiandacaa, M.S.; Kapogiannisb, D.; Mapstonec, M.; Boxerd, A.; Eitanb, E.; Schwartze, J.B.; Abnerf, E.L.; Peterseng, R.C.; Federoffa, H.J.; Millerd, B.L.; et al. Identification of pre-clinical Alzheimer’s disease by a profile of pathogenic proteins in neurally-derived blood exosomes: A case-control study. Alzheimer’s Dement. 2014, 11, 600–607. [Google Scholar] [CrossRef]
- Winston, C.N.; Goetzl, E.J.; Baker, L.D.; Vitiello, M.V.; Rissman, R.A. Growth Hormone-Releasing Hormone Modulation of Neuronal Exosome Biomarkers in Mild Cognitive Impairment. J. Alzheimer’s Dis. 2018, 66, 971–981. [Google Scholar] [CrossRef]
- Eitan, E.; Thornton-Wells, T.; Elgart, K.; Erden, E.; Gershun, E.; Levine, A.; Volpert, O.; Azadeh, M.; Smith, D.G.; Kapogiannis, D. Synaptic proteins in neuron-derived extracellular vesicles as biomarkers for Alzheimer’s disease: Novel methodology and clinical proof of concept. Extracell. Vesicles Circ. Nucleic Acids 2023, 4, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yang, H.; Zhang, C.; Jing, Y.; Wang, C.; Liu, C.; Zhang, R.; Wang, J.; Zhang, J.; Zen, K.; et al. Investigation of microRNA expression in human serum during the aging process. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2015, 70, 102–109. [Google Scholar] [CrossRef]
- Taha, H.B. Alzheimer’s disease and related dementias diagnosis: A biomarkers meta-analysis of general and CNS extracellular vesicles. npj Dement. 2025, 1, 3. [Google Scholar] [CrossRef]
- Bhatnagar, D.; Ladhe, S.; Kumar, D. Discerning the Prospects of miRNAs as a Multi-Target Therapeutic and Diagnostic for Alzheimer’s Disease. Mol. Neurobiol. 2023, 60, 5954–5974. [Google Scholar] [CrossRef] [PubMed]
- Da Conceicao, A.R.R.; Marinatto, J.; Pinheiro, L.S.; Rody, T.; De Felice, F.G. The Multifaceted Role of Extracellular Vesicles in Alzheimer’s Disease. J. Neurochem. 2025, 169, e70209. [Google Scholar] [CrossRef]
- Winston, C.N.; Goetzl, E.J.; Schwartz, J.B.; Elahi, F.M.; Rissman, R.A. Complement protein levels in plasma astrocyte-derived exosomes are abnormal in conversion from mild cognitive impairment to Alzheimer’s disease dementia. Alzheimer’s Dement. 2019, 11, 61–66. [Google Scholar] [CrossRef]
- TaŞDelen, E.; Kizil, E.T.Ö.; AydemİR, S.T.; Yalap, Ö.E.; BİNgÖL, A.P.; Kutlay, N. Determination of miR-373 and miR-204 levels in neuronal exosomes in Alzheimer?s disease. Turk. J. Med. Sci. 2022, 52, 1458–1467. [Google Scholar] [CrossRef]
- Tian, C.; Stewart, T.; Hong, Z.; Guo, Z.; Aro, P.; Soltys, D.; Pan, C.; Peskind, E.R.; Zabetian, C.P.; Shaw, L.M.; et al. Blood extracellular vesicles carrying synaptic function- and brain-related proteins as potential biomarkers for Alzheimer’s disease. Alzheimer’s Dement. 2022, 19, 909–923. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Zhu, M.; Kong, C.; Pang, Y.; Zhang, H.; Qiu, Q.; Wei, C.; Tang, Y.; Wang, Q.; Li, Y.; et al. Blood neuro-exosomal synaptic proteins predict Alzheimer’s disease at the asymptomatic stage. Alzheimer’s Dement. 2021, 17, 49–60. [Google Scholar] [CrossRef]
- Zhao, A.; Li, Y.; Yan, Y.; Qiu, Y.; Li, B.; Xu, W.; Wang, Y.; Liu, J.; Deng, Y. Increased prediction value of biomarker combinations for the conversion of mild cognitive impairment to Alzheimer’s dementia. Transl. Neurodegener. 2020, 9, 30. [Google Scholar] [CrossRef]
- Kumar, A.; Su, Y.; Sharma, M.; Singh, S.; Kim, S.; Peavey, J.J.; Suerken, C.K.; Lockhart, S.N.; Whitlow, C.T.; Craft, S.; et al. MicroRNA expression in extracellular vesicles as a novel blood-based biomarker for Alzheimer’s disease. Alzheimer’s Dement. 2023, 19, 4952–4966. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xia, M.; Meng, S.; Wu, D.; Ling, S.; Chen, X.; Liu, C. MicroRNA-29c-3p in dual-labeled exosome is a potential diagnostic marker of subjective cognitive decline. Neurobiol. Dis. 2022, 171, 105800. [Google Scholar] [CrossRef] [PubMed]
- Durur, D.Y.; Tastan, B.; Ugur Tufekci, K.; Olcum, M.; Uzuner, H.; Karakulah, G.; Yener, G.; Genc, S. Alteration of miRNAs in Small Neuron-Derived Extracellular Vesicles of Alzheimer’s Disease Patients and the Effect of Extracellular Vesicles on Microglial Immune Responses. J. Mol. Neurosci. 2022, 72, 1182–1194. [Google Scholar] [CrossRef]
- Witwer, K.W.; Wolfram, J. Extracellular vesicles versus synthetic nanoparticles for drug delivery. Nat. Rev. Mater. 2021, 6, 103–106. [Google Scholar] [CrossRef]
- Elsharkasy, O.M.; Nordin, J.Z.; Hagey, D.W.; de Jong, O.G.; Schiffelers, R.M.; Andaloussi, S.E.; Vader, P. Extracellular vesicles as drug delivery systems: Why and how? Adv. Drug. Deliv. Rev. 2020, 159, 332–343. [Google Scholar] [CrossRef]
- Patil, S.M.; Sawant, S.S.; Kunda, N.K. Exosomes as drug delivery systems: A brief overview and progress update. Eur. J. Pharm. Biopharm. 2020, 154, 259–269. [Google Scholar] [CrossRef]
- Bruno, S.; Collino, F.; Deregibus, M.C.; Grange, C.; Tetta, C.; Camussi, G. Microvesicles derived from human bone marrow mesenchymal stem cells inhibit tumor growth. Stem Cells Dev. 2013, 22, 758–771. [Google Scholar] [CrossRef]
- Xia, Y.; Zhang, J.; Liu, G.; Wolfram, J. Immunogenicity of Extracellular Vesicles. Adv. Mater. 2024, 36, e2403199. [Google Scholar] [CrossRef]
- Han, G.; Zhang, Y.; Zhong, L.; Wang, B.; Qiu, S.; Song, J.; Lin, C.; Zou, F.; Wu, J.; Yu, H.; et al. Generalizable anchor aptamer strategy for loading nucleic acid therapeutics on exosomes. EMBO Mol. Med. 2024, 16, 1027–1045. [Google Scholar] [CrossRef]
- Pang, C.; Zhang, J.; Gu, Y.; Zhang, Q.; Zhao, Y. The biological roles of exosome-encapsulated traditional Chinese medicine monomers in neuronal disorders. J. Pharm. Anal. 2025, 15, 101131. [Google Scholar] [CrossRef] [PubMed]
- Shaffi, C.S.; Hairuddin, O.N.; Mansor, S.F.; Syafiq, T.M.F.; Yahaya, B.H. Unlocking the Potential of Extracellular Vesicles as the Next Generation Therapy: Challenges and Opportunities. Tissue Eng. Regen. Med. 2024, 21, 513–527. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lu, S.; Wan, M.; Xie, H.; Wang, Z. Peripheral extracellular vesicles in neurodegeneration: Pathogenic influencers and therapeutic vehicles. J. Nanobiotechnol. 2024, 22, 170. [Google Scholar] [CrossRef] [PubMed]
- Skotland, T.; Sagini, K.; Sandvig, K.; Llorente, A. An emerging focus on lipids in extracellular vesicles. Adv. Drug Deliv. Rev. 2020, 159, 308–321. [Google Scholar] [CrossRef]
- Williams, A.; Branscome, H.; Kashanchi, F.; Batrakova, E.V. Targeting of Extracellular Vesicle-Based Therapeutics to the Brain. Cells 2025, 14, 548. [Google Scholar] [CrossRef]
- Grange, C.; Tapparo, M.; Bruno, S.; Chatterjee, D.; Quesenberry, P.J.; Tetta, C.; Camussi, G. Biodistribution of mesenchymal stem cell-derived extracellular vesicles in a model of acute kidney injury monitored by optical imaging. Int. J. Mol. Med. 2014, 33, 1055–1063. [Google Scholar] [CrossRef]
- Qin, Q.; Shan, Z.; Xing, L.; Jiang, Y.; Li, M.; Fan, L.; Zeng, X.; Ma, X.; Zheng, D.; Wang, H.; et al. Synergistic effect of mesenchymal stem cell-derived extracellular vesicle and miR-137 alleviates autism-like behaviors by modulating the NF-kappaB pathway. J. Transl. Med. 2024, 22, 446. [Google Scholar] [CrossRef] [PubMed]
- Macyczko, J.R.; Wang, N.; Zhao, J.; Ren, Y.; Lu, W.; Ikezu, T.C.; Zhao, N.; Liu, C.C.; Bu, G.; Li, Y. Suppression of Wnt/beta-Catenin Signaling Is Associated with Downregulation of Wnt1, PORCN, and Rspo2 in Alzheimer’s Disease. Mol. Neurobiol. 2023, 60, 26–35. [Google Scholar] [CrossRef]
- Tapia-Rojas, C.; Inestrosa, N.C. Loss of canonical Wnt signaling is involved in the pathogenesis of Alzheimer’s disease. Neural Regen. Res. 2018, 13, 1705–1710. [Google Scholar]
- Wang, Q.; Huang, X.; Su, Y.; Yin, G.; Wang, S.; Yu, B.; Li, H.; Qi, J.; Chen, H.; Zeng, W.; et al. Activation of Wnt/beta-catenin pathway mitigates blood-brain barrier dysfunction in Alzheimer’s disease. Brain 2022, 145, 4474–4488. [Google Scholar] [CrossRef]
- Warrier, S.; Marimuthu, R.; Sekhar, S.; Bhuvanalakshmi, G.; Arfuso, F.; Das, A.K.; Bhonde, R.; Martins, R.; Dharmarajan, A. sFRP-mediated Wnt sequestration as a potential therapeutic target for Alzheimer’s disease. Int. J. Biochem. Cell Biol. 2016, 75, 104–111. [Google Scholar] [CrossRef]
- Vargas, J.Y.; Fuenzalida, M.; Inestrosa, N.C. In vivo activation of Wnt signaling pathway enhances cognitive function of adult mice and reverses cognitive deficits in an Alzheimer’s disease model. J. Neurosci. 2014, 34, 2191–2202. [Google Scholar] [CrossRef]
- Varshini, M.S.; Reddy, R.A.; Krishnamurthy, P.T.; Wadhwani, A. Harmony of Wnt pathway in Alzheimer’s: Navigating the multidimensional progression from preclinical to clinical stages. Neurosci. Biobehav. Rev. 2024, 165, 105863. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Jiao, G.; Wu, W.; Wang, H.; Ren, S.; Zhang, L.; Zhou, H.; Liu, H.; Chen, Y. Exosomes from Bone Marrow Mesenchymal Stem Cells Inhibit Neuronal Apoptosis and Promote Motor Function Recovery via the Wnt/beta-catenin Signaling Pathway. Cell Transplant. 2019, 28, 1373–1383. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, Q.; Xiao, J.; Niu, C.; Li, Y.; Zhang, X.; Zhou, Z.; Shu, G.; Yin, G. Wnt/beta-catenin signalling: Function, biological mechanisms, and therapeutic opportunities. Signal Transduct. Target. Ther. 2022, 7, 3. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, X.; Zhang, X.; Sun, Y.; Yan, Y.; Shi, H.; Zhu, Y.; Wu, L.; Pan, Z.; Zhu, W.; et al. Human umbilical cord mesenchymal stem cell exosomes enhance angiogenesis through the Wnt4/beta-catenin pathway. Stem Cells Transl. Med. 2015, 4, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, J.; Ma, B.; Li, N.; Wang, S.; Sun, Z.; Xue, C.; Han, H.; Wei, J.; Zhao, R.C. MSC-derived exosomes promote recovery from traumatic brain injury via microglia/macrophages in rat. Aging 2020, 12, 18274–18296. [Google Scholar] [CrossRef]
- Dolen, D.; Ahmadov, T.; Dolas, I.; Unal, T.C.; Aydoseli, A.; Ozturk, M.; Sabanci, P.A.; Aras, Y.; Bilgic, M.B.; Sencer, A. Analysis of the Prognosis of High-Grade gliomas in the View of New Immunohistochemistry Markers and 2016 WHO Classification. Turk. Neurosurg. 2022, 32, 500–507. [Google Scholar] [CrossRef]
- Zuo, R.; Liu, M.; Wang, Y.; Li, J.; Wang, W.; Wu, J.; Sun, C.; Li, B.; Wang, Z.; Lan, W.; et al. BM-MSC-derived exosomes alleviate radiation-induced bone loss by restoring the function of recipient BM-MSCs and activating Wnt/beta-catenin signaling. Stem Cell Res. Ther. 2019, 10, 30. [Google Scholar] [CrossRef]
- Yu, A.; Zhang, Y.; Zhong, S.; Yang, Z.; Xie, M. Human umbilical cord mesenchymal stem cell-derived exosomes enhance follicular regeneration in androgenetic alopecia via activation of Wnt/beta-catenin pathway. Stem Cell Res. Ther. 2025, 16, 418. [Google Scholar] [CrossRef]
- Xie, X.; Song, Q.; Dai, C.; Cui, S.; Tang, R.; Li, S.; Chang, J.; Li, P.; Wang, J.; Li, J.; et al. Clinical safety and efficacy of allogenic human adipose mesenchymal stromal cells-derived exosomes in patients with mild to moderate Alzheimer’s disease: A phase I/II clinical trial. Gen. Psychiatry 2023, 36, e101143. [Google Scholar] [CrossRef]
- Morita, J.; Hirano, A.; Izawa, H. Safety and clinical efficacy on intranasal administration of mesenchymal stem cell-derived secretome in patients with Alzheimer’s disease and its future prospect. Glycative Stress Res. 2024, 11, 103–110. [Google Scholar]
- AEGLE Therapeutics. Available online: https://aegletherapeutics.com/ev-therapy/ (accessed on 30 December 2025).
- Codiak BioSciences, Inc. Available online: https://www.biospace.com/codiak-presents-preclinical-data-on-exoaso-stat6-and-exoaso-c-ebp%CE%B2-programs-at-the-society-for-immunotherapy-of-cancer-sitc-2022-annual-meeting (accessed on 30 December 2025).
- Therapeutics, E. Available online: https://www.evoxtherapeutics.com/pipeline/ (accessed on 30 December 2025).
- Serrano, D.R.; Juste, F.; Anaya, B.J.; Ramirez, B.I.; Sanchez-Guirales, S.A.; Quispillo, J.M.; Hernandez, E.M.; Simon, J.A.; Trallero, J.M.; Serrano, C.; et al. Exosome-Based Drug Delivery: A Next-Generation Platform for Cancer, Infection, Neurological and Immunological Diseases, Gene Therapy and Regenerative Medicine. Pharmaceutics 2025, 17, 1336. [Google Scholar] [CrossRef] [PubMed]
- EXO Biologics. Available online: https://exoxpert.com/exoxpert-an-exosome-cdmo-launched-by-exo-biologics/?utm_source=chatgpt.com (accessed on 30 December 2025).
- Li, Y.; Chen, Y.H.; Liu, B.Y.; Nie, Q.; Li, L.J.; Duan, X.; Wu, L.Z.; Chen, G. Deciphering the Heterogeneity Landscape of Mesenchymal Stem/Stromal Cell-Derived Extracellular Vesicles for Precise Selection in Translational Medicine. Adv. Healthc. Mater. 2023, 12, e2202453. [Google Scholar] [CrossRef]
- Ioannou, S.; Kay, A.G.; Stone, A.P.; Rand, E.; Elberfeld, S.; Bolton, W.; Larson, T.; Crossland, R.E.; Kehoe, O.; Mentlak, D.A.; et al. Extracellular vesicle bioactivity and potential for clinical development are determined by mesenchymal stromal cell clonal subtype. Stem Cell Res. Ther. 2025, 16, 571. [Google Scholar] [CrossRef]
- Hagey, D.W.; Ojansivu, M.; Bostancioglu, B.R.; Saher, O.; Bost, J.P.; Gustafsson, M.O.; Gramignoli, R.; Svahn, M.; Gupta, D.; Stevens, M.M.; et al. The cellular response to extracellular vesicles is dependent on their cell source and dose. Sci. Adv. 2023, 9, eadh1168. [Google Scholar] [CrossRef]
- Ahn, S.H.; Ryu, S.W.; Choi, H.; You, S.; Park, J.; Choi, C. Manufacturing Therapeutic Exosomes: From Bench to Industry. Mol. Cells 2022, 45, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Konoshenko, M.Y.; Lekchnov, E.A.; Vlassov, A.V.; Laktionov, P.P. Isolation of Extracellular Vesicles: General Methodologies and Latest Trends. BioMed Res. Int. 2018, 2018, 8545347. [Google Scholar] [CrossRef] [PubMed]
- Andriolo, G.; Provasi, E.; Brambilla, A.; Panella, S.; Soncin, S.; Cicero, V.L.; Radrizzani, M.; Turchetto, L.; Barile, L. Methodologies for Scalable Production of High-Quality Purified Small Extracellular Vesicles from Conditioned Medium. Methods Mol. Biol. 2023, 2668, 69–98. [Google Scholar]
- Visan, K.S.; Lobb, R.J.; Ham, S.; Lima, L.G.; Palma, C.; Edna, C.P.Z.; Wu, L.Y.; Gowda, H.; Datta, K.K.; Hartel, G.; et al. Comparative analysis of tangential flow filtration and ultracentrifugation, both combined with subsequent size exclusion chromatography, for the isolation of small extracellular vesicles. J. Extracell. Vesicles 2022, 11, 12266. [Google Scholar] [CrossRef]
- Huang, J.; Chen, H.; Li, N.; Liu, P.; Yang, J.; Zhao, Y. Emerging technologies towards extracellular vesicles large-scale production. Bioact. Mater. 2025, 52, 338–365. [Google Scholar] [CrossRef]
- Murphy, D.E.; de Jong, O.G.; Brouwer, M.; Wood, M.J.; Lavieu, G.; Schiffelers, R.M.; Vader, P. Extracellular vesicle-based therapeutics: Natural versus engineered targeting and trafficking. Exp. Mol. Med. 2019, 51, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Massa, M.; Croce, S.; Campanelli, R.; Abba, C.; Lenta, E.; Valsecchi, C.; Avanzini, M.A. Clinical Applications of Mesenchymal Stem/Stromal Cell Derived Extracellular Vesicles: Therapeutic Potential of an Acellular Product. Diagnostics 2020, 10, 999. [Google Scholar] [CrossRef]
- Chu, M.; Wang, H.; Bian, L.; Huang, J.; Wu, D.; Zhang, R.; Fei, F.; Chen, Y.; Xia, J. Nebulization Therapy with Umbilical Cord Mesenchymal Stem Cell-Derived Exosomes for COVID-19 Pneumonia. Stem Cell Rev. Rep. 2022, 18, 2152–2163. [Google Scholar] [CrossRef]
- Sengupta, V.; Sengupta, S.; Lazo, A.; Woods, P.; Nolan, A.; Bremer, N. Exosomes Derived from Bone Marrow Mesenchymal Stem Cells as Treatment for Severe COVID-19. Stem Cells Dev. 2020, 29, 747–754. [Google Scholar] [CrossRef]
- Fang, S.; Tian, H.; Li, X.; Jin, D.; Li, X.; Kong, J.; Yang, C.; Yang, X.; Lu, Y.; Luo, Y.; et al. Clinical application of a microfluidic chip for immunocapture and quantification of circulating exosomes to assist breast cancer diagnosis and molecular classification. PLoS ONE 2017, 12, e0175050. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, H.J.; Fine, D.; Schmulen, J.; Hu, Y.; Godin, B.; Zhang, J.X.; Liu, X. Ciliated micropillars for the microfluidic-based isolation of nanoscale lipid vesicles. Lab Chip 2013, 13, 2879–2882. [Google Scholar] [CrossRef]
- He, M.; Crow, J.; Roth, M.; Zeng, Y.; Godwin, A.K. Integrated immunoisolation and protein analysis of circulating exosomes using microfluidic technology. Lab Chip 2014, 14, 3773–3780. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Yang, Y.; Zeng, Y.; He, M. A microfluidic ExoSearch chip for multiplexed exosome detection towards blood-based ovarian cancer diagnosis. Lab Chip 2016, 16, 489–496. [Google Scholar] [CrossRef]
- Park, E.J.; Zhang, Y.Z.; Vykhodtseva, N.; McDannold, N. Ultrasound-mediated blood-brain/blood-tumor barrier disruption improves outcomes with trastuzumab in a breast cancer brain metastasis model. J. Control Release 2012, 163, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Lipsman, N.; Meng, Y.; Bethune, A.J.; Huang, Y.; Lam, B.; Masellis, M.; Herrmann, N.; Heyn, C.; Aubert, I.; Boutet, A.; et al. Blood-brain barrier opening in Alzheimer’s disease using MR-guided focused ultrasound. Nat. Commun. 2018, 9, 2336. [Google Scholar] [CrossRef]
- Wu, L.; Lin, B.; Fan, H.; Li, Y. Establishment of an ultrasound-responsive microfluidic chip BBB-glioblastoma model for studying sonodynamic therapy-enhanced nanodrug delivery. Lab Chip 2025, 25, 6673–6687. [Google Scholar] [CrossRef] [PubMed]
- Leinenga, G.; Götz, J. Scanning ultrasound removes amyloid-b and restores memory in an Alzheimer’s disease mouse model. Sci. Transl. Med. 2015, 7, 278ra33. [Google Scholar] [CrossRef]
- Shen, Y.; Hua, L.; Yeh, C.K.; Shen, L.; Ying, M.; Zhang, Z.; Liu, G.; Li, S.; Chen, S.; Chen, X.; et al. Ultrasound with microbubbles improves memory, ameliorates pathology and modulates hippocampal proteomic changes in a triple transgenic mouse model of Alzheimer’s disease. Theranostics 2020, 10, 11794–11819. [Google Scholar] [CrossRef]
- Rezai, A.R.; D’Haese, P.F.; Finomore, V.; Carpenter, J.; Ranjan, M.; Wilhelmsen, K.; Mehta, R.I.; Wang, P.; Najib, U.; Vieira Ligo Teixeira, C.; et al. Ultrasound Blood-Brain Barrier Opening and Aducanumab in Alzheimer’s Disease. N. Engl. J. Med. 2024, 390, 55–62. [Google Scholar] [CrossRef]
- Jordao, J.F.; Thevenot, E.; Markham-Coultes, K.; Scarcelli, T.; Weng, Y.Q.; Xhima, K.; O’Reilly, M.; Huang, Y.; McLaurin, J.; Hynynen, K.; et al. Amyloid-beta plaque reduction, endogenous antibody delivery and glial activation by brain-targeted, transcranial focused ultrasound. Exp. Neurol. 2013, 248, 16–29. [Google Scholar] [CrossRef]
- Deng, Z.; Wang, J.; Xiao, Y.; Li, F.; Niu, L.; Liu, X.; Meng, L.; Zheng, H. Ultrasound-mediated augmented exosome release from astrocytes alleviates amyloid-beta-induced neurotoxicity. Theranostics 2021, 11, 4351–4362. [Google Scholar] [CrossRef] [PubMed]
- Ye, D.; Chen, S.; Liu, Y.; Weixel, C.; Hu, Z.; Yuan, J.; Chen, H. Mechanically manipulating glymphatic transport by ultrasound combined with microbubbles. Proc. Natl. Acad. Sci. USA 2023, 120, e2212933120. [Google Scholar] [CrossRef]
| EV Subtype | Source | Changes in the Components of EVs | Disease | Conclusion | References |
|---|---|---|---|---|---|
| NDEVs | Neuron | Aβ42, T-tau, and p-tau T181 ↑ | AD aMCI | Blood exosomal Aβ42, T-tau, and p-tau T181 show strong concordance with CSF and offer comparable diagnostic power for AD/aMCI. | [174] |
| Aβ42 ↑ | AD aMCI | NDEVs levels of Aβ42 increase progressively across the AD continuum—from cognitively normal amyloid-negative individuals to amyloid-positive individuals, to those with MCI. | [175] | ||
| Aβ42, T-tau, p-tau T181, p-tau S396 ↑ | AD FID | NDEVs levels of p-tau T181, p-tau S396, and Aβ1–42 achieves 96.4% accuracy in classifying AD patients and predict disease onset 10 years prior to clinical diagnosis. | [176] | ||
| p-tau T181,p-tau S396 ↑ NRGN, EST ↓ | AD MCI | The combined profile of p-tau, Aβ1–42, NRGN and REST in plasma NDEVs serves as an accurate predictor for the conversion from MCI to AD dementia. | [38] | ||
| Aβ1–42 ↑ NRGN, synaptophysin ↓ synaptotagmin, synaptopodin ↓ | MCI | Aβ1–42, NRGN, synaptophysin, synapsin, and synaptopodin in NDEVs accurately differentiate patients MCI from CNC. | [177] | ||
| p-tau T181, Aβ42, NRGN ↑ ProBDNF, GluR2, PSD95 ↓ GAP43, Syntaxin-1 ↓ | AD | A model incorporating GluR2, proBDNF, NRGN, and GAP43 achieved an 81.3% accuracy for AD classification. Their levels correlated with cognitive scores (MMSE and COR-SOB), supporting utility for both diagnosis and progression monitoring. | [178] | ||
| miR-373, miR-204 ↓ | AD | The significant reduction in miR-204 and miR-373 in NDEVs positions them as potential biomarkers for AD. | [184] | ||
| NMDAR2A | AD | Analysis of synaptic protein profiles in CNS-derived plasma EVs provides a robust, liquid biopsy biomarker for synaptic dysfunction on AD | [185] | ||
| GAP43, neurogranin, SNAP25, synaptotagmin1 ↓ | AD | NDEV proteins levels (GAP43) correlate with CSF, serving as effective biomarkers and enabling the prediction of AD onset 5 to 7 years prior to the emergence of cognitive impairment. | [186] | ||
| Aβ1–42 ↑ | AD MCI | The combination of Aβ1–42 levels in NDEVs accurately predicts conversion from MCI to AD dementia. | [187] | ||
| miR-9-5p, miR-106b-5p, miR-125b-5p ↑ | AD MCI MCI-AD | MiR-106b-5p is significantly overexpressed in the AD group and shows perfect accuracy in distinguish AD from cognitive normal (CN) group. | [188] | ||
| Aβ42/40, Tau, P-tau-T181, Aβ42, miR-29c-3p ↑ | SCD aMCI AD VaD | The level of miR-29c-3p in NCAM/amphiphysin 1 dual-labeled exosomes (NDEVs) of patients with subjective cognitive decline (SCD) is higher than that in healthy controls and the vascular dementia (VaD) group, and it demonstrates the best performance in diagnosing SCD, holding potential advantages for early diagnosis. The biomarkers such as Aβ42 in single-labeled exosomes have diagnostic value for aMCI and AD, but limited diagnostic value for SCD. | [189] | ||
| let-7e-5p, miR-96-5p, miR-484 ↑ miR-99b-5p, miR-100-5p, miR-30e-5p ↓ miR-378i, miR-145-5p, miR-378c, miR-451a ↓ | AD | Compared with the HC, plasma levels of sNDEV let-7e-5p, miR-96-5p, and miR-484 were significantly increased in AD group, while levels of miR-99b-5p, miR-100-5p, miR-30e-5p et al. were significantly decreased. Let-7e expressed in NDEVs could serve as a potential biomarker for AD diagnosis (AUC value of 0.9214). | [190] | ||
| ADEVs | Astrocyte | BACE-1, g-secretase, soluble Aβ42, sAPPβ↓ sAPPa, GDNF, P-tau T181, p-tau S396 ↑ | AD FID | The levels of BACE-1 and aAPPβ in ADEVs can effectively distinguish AD patients from healthy controls, with AUC values of 0.78 and 0.83, respectively. | [65] |
| C1q, C4b, factor D, fragment ↑ Bb, C5b, C3b, C5b-C9 ↑ CD46, CD59, type 1 complement receptor ↓ | MCI | The changes in complement protein levels in ADEVs not only distinguish AD patients from the control group but also demonstrate high diagnostic sensitivity in identifying MCI patients at high risk of converting to AD. | [183] | ||
| miR-29a-5p, miR-132-5p, miR-107 ↑ | AD MCI MCI-AD | The expression of miR-107 shows an increasing trend among the three patient groups and can perfectly predict the incidence of AD dementia (AUC = 1.000). | [188] | ||
| MDEVs | Microglia | miR-132-5p, miR-106b-5p ↑ miR-29a-5p, miR-125b-5p ↓ | AD MCI MCI-AD | The levels of miR-29a-5p and miR-106-5p is significantly reduced across all impairment groups (AUC = 0.925). MiR-132-5p and miR-125b-5p together can perfectly predict AD (AUC = 1.000). | [188] |
| ODEVs | Oligodendrocytes | miR-29a-5p, miR-107, miR-135b-5p ↑ | AD MCI MCI-AD | miR-29a-5p, miR-107, and miR-135b-5p were significantly overexpressed in the AD group. Mir-29a-5p showed AUC = 1.000 in predicting AD incidence. | [188] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Li, F.; Wu, L.; Feng, X.; Li, Y.; Fan, H. Extracellular Vesicles in Alzheimer’s Disease: Dual Roles in Pathogenesis, Promising Avenues for Diagnosis and Therapy. Pharmaceutics 2026, 18, 70. https://doi.org/10.3390/pharmaceutics18010070
Li F, Wu L, Feng X, Li Y, Fan H. Extracellular Vesicles in Alzheimer’s Disease: Dual Roles in Pathogenesis, Promising Avenues for Diagnosis and Therapy. Pharmaceutics. 2026; 18(1):70. https://doi.org/10.3390/pharmaceutics18010070
Chicago/Turabian StyleLi, Feng, Liyang Wu, Xin Feng, Yihong Li, and Huadong Fan. 2026. "Extracellular Vesicles in Alzheimer’s Disease: Dual Roles in Pathogenesis, Promising Avenues for Diagnosis and Therapy" Pharmaceutics 18, no. 1: 70. https://doi.org/10.3390/pharmaceutics18010070
APA StyleLi, F., Wu, L., Feng, X., Li, Y., & Fan, H. (2026). Extracellular Vesicles in Alzheimer’s Disease: Dual Roles in Pathogenesis, Promising Avenues for Diagnosis and Therapy. Pharmaceutics, 18(1), 70. https://doi.org/10.3390/pharmaceutics18010070










