1. Introduction
Prostate cancer is one of the most frequently diagnosed malignancies in men worldwide, representing a major public health concern with a growing global burden. According to the Global Cancer Observatory, it was the second most commonly diagnosed malignancy in men in 2020, with over 1.4 million new cases and approximately 375,000 deaths globally, corresponding to 8% of all deaths caused by cancer in men [
1]. While early-stage prostate cancer can often be managed with surgery or radiation therapy, advanced and metastatic forms require systemic treatment, including hormonal therapy and chemotherapy [
2].
Docetaxel (DTX) is a semi-synthetic taxane widely used in the management of several solid malignancies (including breast, non-small cell lung, gastric, head and neck, ovarian, and prostate cancers) and remains a cornerstone of systemic therapy for advanced prostate cancer, including metastatic castration-resistant disease [
3,
4]. It promotes tubulin polymerization and stabilizes microtubules, thereby inhibiting mitotic cell division. Despite its clinical utility, DTX’s extremely low aqueous solubility, dose-limiting toxicities, and the emergence of drug resistance can compromise tumor exposure and therapeutic benefit [
5,
6].
To address these limitations, various nanocarrier-based drug delivery systems have been explored. Among them, polymeric micelles have attracted considerable attention due to their ability to improve the solubility, stability, and bioavailability of hydrophobic drugs like docetaxel, while enabling passive or active tumor targeting [
7,
8]. By increasing apparent aqueous dispersibility and shielding hydrophobic payloads within a core–shell structure, micelles can improve effective drug availability at the target site and help mitigate off-target exposure. These nanoscale assemblies typically consist of amphiphilic copolymers that self-assemble in water to form core–shell nanostructures, enabling drug encapsulation, protection and (potentially) altered cellular delivery profiles relative to free drug [
7].
Zein, a hydrophobic prolamin derived from maize, has recently emerged as a promising natural polymer for drug delivery applications. Its unique amphiphilic structure, biocompatibility, biodegradability, and Generally Recognized As Safe (GRAS) status make it particularly suitable for formulating micellar systems [
9,
10,
11,
12,
13,
14]. Zein-based micelles have been shown to efficiently encapsulate a range of hydrophobic agents, including chemotherapeutics, and offer enhanced stability and sustained drug release profiles [
10,
15,
16,
17]. Zein-based nanocarriers have also been investigated for enhancing the delivery of other poorly water-soluble anticancer drugs, including docetaxel-loaded zein formulations designed to improve colloidal stability, pharmacokinetics, and antitumor efficacy. For example, chondroitin sulfate-hybridized zein nanoparticles enabled tumor-targeted docetaxel delivery and improved in vivo performance compared with free drug [
18]. In addition, glucose-modified zein nanoparticles have been reported to markedly increase docetaxel oral bioavailability and antitumor activity in vivo, highlighting zein’s versatility as a carrier platform [
19].
However, the hydrophobic nature of zein can limit its colloidal stability and circulation time under physiological conditions. To overcome this, surface modification with polyethylene glycol (PEG) has been widely employed. PEGylation offers multiple benefits: it improves the aqueous dispersibility of zein-based micelles and provides steric stabilization to prevent aggregation [
20]. In some nanoparticle systems, sufficiently dense PEG coatings have been associated with reduced opsonization and clearance by the mononuclear phagocyte system, thereby prolonging systemic circulation [
15,
16,
17,
20,
21,
22,
23,
24]. Additionally, PEG chains on the micelle surface can serve as functional linkers for conjugating targeting ligands such as transferrin, facilitating active targeting without compromising micellar stability. Importantly, the molecular weight of PEG influences these characteristics: longer chains may offer greater steric protection but can also hinder ligand accessibility, while shorter chains provide improved targeting potential but may compromise stability [
21,
25].
While passive targeting through the enhanced permeability and retention (EPR) effect improves tumor accumulation to some extent, it remains insufficient for many solid tumors, including prostate cancer. Therefore, ligand-mediated active targeting strategies have been developed to enhance the selective delivery of nanocarriers to cancer cells. One such approach involves exploiting the transferrin receptor (TfR), which is overexpressed in numerous malignancies, including prostate cancer, due to the increased iron demands of rapidly proliferating cells [
26,
27]. Transferrin-functionalized nanocarriers can bind specifically to TfR, facilitating receptor-mediated endocytosis and intracellular drug delivery, which can enhance the therapeutic index while minimizing off-target toxicity [
26,
28]. Consistent with this rationale, transferrin functionalization has been explored for docetaxel delivery to prostate cancer cells, including transferrin-functionalized liposomes that enhanced interaction with TfR-expressing prostate cancer models [
29,
30,
31,
32]. Moreover, we previously reported transferrin-bearing zein-based hybrid nanoparticles for docetaxel delivery to prostate cancer cells, supporting the feasibility of combining zein and transferrin in ligand-directed taxane delivery systems [
24].
In this study, we investigated the development of PEGylated zein micelles conjugated with transferrin as a targeted nanoplatform for docetaxel delivery in prostate cancer. We evaluated the impact of PEG chain length (5 K vs. 10 K) and transferrin conjugation on micellar characteristics, cellular uptake, and anti-proliferative efficacy across three prostate cancer cell lines, PC-3-Luc, DU145, and LNCaP. Our aim was to elucidate how these physicochemical and biological parameters influence the therapeutic performance of docetaxel-loaded micelles, ultimately contributing to the rational design of more effective nanomedicines for prostate cancer therapy.
2. Materials and Methods
2.1. Cell Lines and Reagents
Yellow zein (from maize), human holo-transferrin (Tf), cupric sulphate (pentahydrate) (CuSO4.5 H2O), 2-Iminothiolane hydrochloride (Traut’s reagent), dimethyl sulfoxide (DMSO), deuterated dimethyl sulfoxide-d6, absolute ethanol (≥99.8%), Folin and Ciocalteu’s phenol reagent, anhydrous sodium carbonate (Na2CO3), sodium potassium tartrate (C4H4KNaO6), bovine serum albumin (BSA), Vivaspin® 6 centrifugal filter tubes with molecular weight cut-offs (MWCO) of 5000 and 100,000 Daltons, citric acid monohydrate, trisodium citrate dihydrate, docetaxel purum (≥97.0%), sodium hydroxide (NaOH), chlorpromazine, colchicine, filipin, poly-L-lysine, phosphate buffer saline, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT), trypsin, Nile red, Triton X-100, and Minimum Essential Eagle’s Medium (MEM) were purchased from Sigma-Aldrich (Poole, UK). Methoxy PEG succinimidyl carboxymethyl esters with molecular weights of 5000 Da (mPEG-SCM-5K) and 10,000 Da (mPEG-SCM-10K) were obtained from JenKem Technology (Plano, TX, USA). Glycine and Snakeskin® dialysis tubing (7–10 kDa MWCO) were supplied by Thermo Fisher Scientific (Loughborough, UK). Sodium pyruvate, L-glutamine, penicillin-streptomycin, 4-(2-hydroxyethyl) piperazine-1-ethanesulfonic acid (HEPES buffer, 10 mM), Roswell Park Memorial Institute (RPMI)-1640 cell culture medium, fetal bovine serum (FBS), and Tubulin Tracker® Green (Oregon Green® 488 Taxol, bis-acetate) were purchased from Life Technologies (Paisley, UK). Vectashield® mounting medium containing 4′,6-diamidino-2-phenylindole (DAPI) was purchased from Vector Laboratories (Peterborough, UK).
The Bioware® PC-3M-luc-C6 human prostate adenocarcinoma cell line that expresses the firefly luciferase and is androgen-independent was obtained from Caliper Life Sciences (Hopkinton, MA, USA). The androgen-independent DU145 and androgen-sensitive LNCaP prostate cancer cell lines were sourced from the European Collection of Cell Cultures (ECACC) (Salisbury, UK).
2.2. Synthesis of Transferrin-Bearing PEGylated Zein-Based Micelles Loading Docetaxel
2.2.1. Synthesis of PEGylated Zein
PEGylated zein was prepared using a method adapted from Podaralla et al. [
23] and Meewan et al. [
16] (
Figure 1). Briefly, yellow zein (0.1 g) was dissolved in 4 mL of 90% (
v/
v) ethanol. Methoxy PEG-succinimidyl carboxymethyl ester (mPEG-SCM) of molecular weight 5000 Da (0.05 g for mPEG5K-zein) or 10,000 Da (0.1 g for mPEG10K-zein) was separately dissolved in 1 mL of 90% (
v/
v) ethanol. The PEG solutions were added to the zein solution and stirred at 100 rpm for 3 h at 25 °C. The reaction was then quenched by adding 1 mL of 1 M glycine buffer, followed by 5 mL of citrate buffer (pH 7.4) to precipitate the PEGylated zein. The resulting mixtures were dialyzed against 2 L of ultrapure Milli-Q water (15.0 MΩ·cm) using dialysis tubing with molecular weight cut-offs of 7000 Da (for mPEG5K-zein) and 10,000 Da (for mPEG10K-zein). Dialysis was carried out for 48 h under continuous stirring (120 rpm) at 25 °C, with the water replaced three times to ensure the removal of free PEG and residual ethanol. The final products were lyophilized using a Christ Epsilon 2-4 LSC
® freeze-dryer (Osterode am Harz, Germany) and stored at −20 °C until use.
2.2.2. Preparation of PEGylated Zein-Based Micelles Loading Docetaxel
PEGylated zein micelles loading docetaxel (mPEG-zein-DTX) were prepared by dissolving 0.3 mg of docetaxel and 50 mg of PEGylated zein (mPEG5K-zein or mPEG10K-zein) in 10 mL of 90% (v/v) ethanol. The solution was stirred overnight at 37 °C to facilitate the incorporation of docetaxel into the micellar structures. Free docetaxel and residual ethanol were removed by dialysis using SnakeSkin® dialysis tubing with molecular weight cut-offs of 7000 Da (for mPEG5K-zein) and 10,000 Da (for mPEG10K-zein). Dialysis was performed against 2 L of ultrapure Milli-Q water under continuous stirring at 25 °C for 48 h, with water replaced three times during the process. The final formulations were lyophilized using a Christ Epsilon 2-4 LSC® freeze-dryer (Osterode am Harz, Germany) and kept at −20 °C until further use.
2.2.3. Conjugation of Transferrin to PEGylated Zein-Based Micelles Loading Docetaxel
Transferrin (Tf) was conjugated to docetaxel-loaded PEGylated zein micelles via a thiol–maleimide “click” reaction, as described by Hermanson [
33], with modifications from Laskar et al. [
34]. Briefly, thiolated transferrin was prepared by dissolving 10 mg of transferrin in 1 mL of 50 mM sodium phosphate buffer containing 0.15 M sodium chloride (pH 8.0). A 10-fold molar excess of Traut’s reagent (2-iminothiolane hydrochloride; 85 µL of a 2 mg/mL solution in deionized water) was added to the transferrin solution and stirred at 350 rpm for 2 h at 25 °C. The thiolated transferrin was then purified to remove unreacted Traut’s reagent by centrifugation at 4200×
g (3140 rpm) for 15 min at 20 °C using Vivaspin
® 6 centrifugal filter units with a 5000 Da molecular weight cut-off (Thermo Scientific Heraeus Megafuge
® 16R, Waltham, MA, USA). Immediately following purification, 1 mL of PEGylated zein micelle solution (prepared by dissolving 35 mg of freeze-dried PEGylated zein in 1 mL of 50 mM sodium phosphate buffer with 0.15 M sodium chloride, pH 8.0) was added to the thiolated transferrin. The mixture was stirred continuously at 350 rpm for 1 h at 25 °C to facilitate conjugation. Unconjugated transferrin was removed using Vivaspin
® 6 centrifugal filter units with a 100,000 Da molecular weight cut-off, by centrifugation at 4200×
g (3140 rpm) for 10 min at 20 °C. The final transferrin-conjugated micelles were collected and adjusted to a final volume of 1 mL using 50 mM sodium phosphate buffer containing 0.15 M sodium chloride (pH 8.0) and stored at 4 °C until further use.
2.3. Characterization of Tf-Bearing, PEGylated Zein-Based Micelles
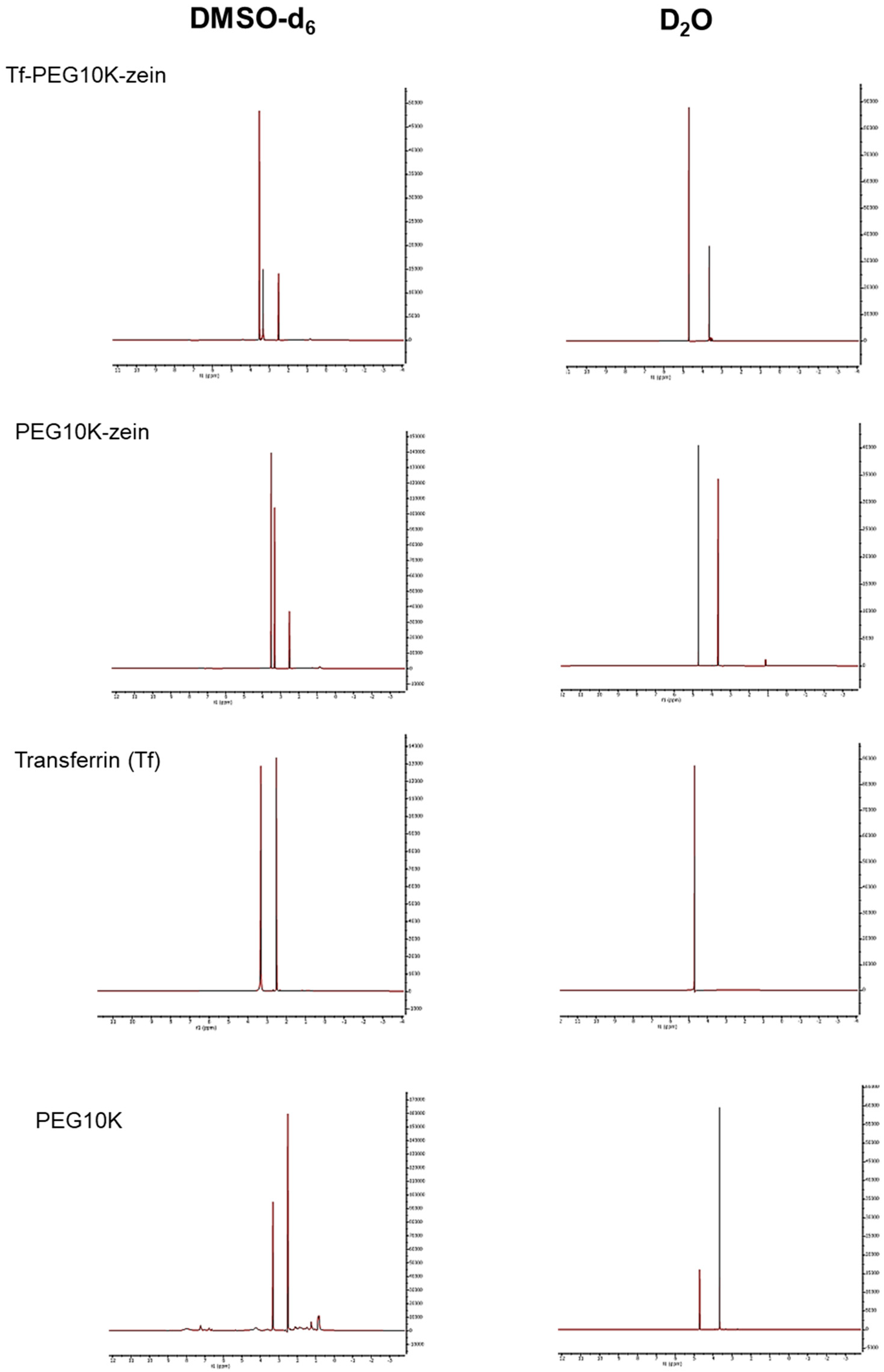
To characterize the core–shell structure of the micelles, Tf-bearing, PEGylated zein-based micelles (5 mg/mL in deuterium oxide (D2O) and deuterated dimethyl sulfoxide (DMSO-d6)) were analyzed by proton nuclear magnetic resonance (1H NMR). 1H NMR spectra were acquired at 500 MHz using a Bruker Avance® III HD500 NMR spectrometer (Billerica, MA, USA). The core–shell structure was confirmed by comparing spectral differences between DMSO-d6 (a good solvent for both core and shell) and D2O (a selective solvent for the hydrophilic shell), indicating the successful formation of micelles with a hydrophobic core and hydrophilic PEG-based shell.
2.3.1. Determination of Critical Micelle Concentration (CMC) of Tf-Bearing, PEGylated Zein-Based Micelles
The self-assembly behavior of transferrin-bearing PEGylated zein (Tf-mPEG-zein) into micelles was assessed using the steady-state fluorescence technique with Nile red as a hydrophobic fluorescent probe. Briefly, 32 µL of Nile red solution (1 mg/mL in methanol) was added to 7 mL plastic vials. Methanol was allowed to completely evaporate over 5 h at room temperature, leaving a thin film of Nile red. Subsequently, 1 mL of Tf-mPEG5K-zein or Tf-mPEG10K-zein solutions (prepared at concentrations ranging from 0.002 to 1 mg/mL in 5% w/v glucose solution) was added to the vials, yielding a final Nile red concentration of 100 µM. Samples were vortexed for 1–2 min and incubated overnight at 25 °C in the dark. On the following day, fluorescence measurements were performed using a Varian Cary Eclipse® spectrofluorometer (Agilent Technologies, Santa Clara, CA, USA) with an excitation wavelength of 550 nm and emission wavelengths from 570 to 800 nm. Excitation and emission slit widths were set at 5 nm and 20 nm, respectively. The ratio of Nile red emission intensity at the wavelength of maximum emission (λmax) in the presence of transferrin-bearing, PEGylated zein micelles (I) over the fluorescence in their absence (I0), in glucose solution (5% w/v) was plotted as a function of transferrin-bearing, PEGylated zein micelle concentration. The critical micelle concentration was determined from the inflection point of the plot of relative fluorescence intensities of Nile red at various micelle concentrations.
2.3.2. Morphological Characterization by Transmission Electron Microscopy
The morphology of conjugated and non-conjugated PEGylated zein micelles (0.4 mg/mL in distilled water) was examined using transmission electron microscopy (TEM). Formvar/carbon-coated copper grids (400 mesh) were glow-discharged to render the surface hydrophilic. A 3–5 μL drop of each micelle sample was placed onto the prepared grids and allowed to air-dry overnight at room temperature. The dried samples were then imaged using a Jeol JEM-1200EX® transmission electron microscope (Jeol, Tokyo, Japan) operating at an accelerating voltage of 80 kV.
2.3.3. Determination of Size and Zeta Potential of the Tf-Bearing PEGylated Zein-Based Micelles
The hydrodynamic size and zeta potential of Tf-bearing and control PEGylated zein micelles (mPEG5K-zein and mPEG10K-zein) loaded with docetaxel were measured using dynamic light scattering (DLS) and laser Doppler electrophoresis, respectively. Measurements were performed with a Zetasizer Nano-ZS® (Malvern Instruments Ltd., Malvern, UK) at 25 °C, with a backscattering detection angle of 173°. All samples were prepared at a concentration of 0.2 mg/mL in 5% (w/v) glucose solution, with a final volume of 1 mL. Prior to analysis, targeted micelles were lyophilized using a Christ Epsilon 2-4 LSC® freeze-dryer (Osterode am Harz, Germany) and reconstituted as described before. Each measurement was conducted in quadruplicate to ensure reproducibility.
2.3.4. Determination of Docetaxel Encapsulation Efficiency and Drug Loading in Tf-Bearing PEGylated Zein-Based Micelles
To determine the encapsulation efficiency of docetaxel, 1 mg of docetaxel-loaded PEGylated zein micelles was dispersed in 1 mL of ultrapure water and centrifuged at 5000×
g for 14 min using an IEC Micromax
® centrifuge (Thermo Life Sciences, Waltham, MA, USA). The supernatant was discarded, and the resulting pellet was dissolved in 1 mL of methanol. An aliquot of the methanolic solution was further diluted with methanol to fall within the calibration range. The fluorescence intensity of fluorescein-labelled docetaxel was measured using a Varian Cary Eclipse
® fluorescence spectrophotometer (Agilent Technologies, Santa Clara, CA, USA) at an excitation wavelength of 495 nm and emission wavelength of 525 nm. Docetaxel concentration was determined by interpolating the fluorescence intensity against a standard calibration curve of fluorescein-labelled docetaxel (0.2–3 μg/mL). Encapsulation efficiency (%EE) was calculated using the following equation, with measurements performed in quadruplicate (n = 4):
For transferrin-conjugated micelles, 10 μL of micellar dispersion was diluted with 990 μL of 50 mM sodium phosphate buffer containing 0.15 M sodium chloride (pH 8.0). The mixture was centrifuged at 5000× g for 14 min, and the supernatant was discarded. The pellet was dissolved in 1 mL of methanol and analyzed following the same procedure as for non-conjugated micelles. Docetaxel loading was expressed both as %EE and as the amount of docetaxel per mg of micelle material.
2.3.5. Quantification of the Amount of Tf Conjugated to the Micelles
The amount of Tf conjugated to PEGylated zein micelles was quantified using the Lowry protein assay, following the original method by Lowry et al. [
35] with modifications based on a previously established protocol [
36]. Briefly, Solution A was prepared by adding 1 mL of 2% (
w/
v) sodium potassium tartrate and 1 mL of 1% (
w/
v) cupric sulphate (both in ultrapure Milli-Q water, 15.0 MΩ·cm) dropwise under continuous stirring into 50 mL of 2% (
w/
v) anhydrous sodium carbonate in 0.1 M NaOH to prevent precipitation. A series of standard protein solutions was prepared using bovine serum albumin (BSA) in 50 mM sodium phosphate buffer containing 0.15 M sodium chloride (pH 8.0), at concentrations ranging from 5 to 500 μg/mL. For each assay, 100 μL of diluted micelle samples (prepared by mixing 5 μL of Tf-bearing or control PEGylated zein micelles with 95 μL of the same phosphate buffer) was mixed with 1 mL of Solution A and incubated at 25 °C in the dark for 10 min. Then, 100 μL of 1N Folin–Ciocalteu’s phenol reagent was added to each sample with immediate vortexing, followed by a 30 min incubation at 25 °C, protected from light. Absorbance was measured at 750 nm using a Varian Cary
® 50 UV-Vis spectrophotometer (Agilent Technologies, Santa Clara, CA, USA). Blank micelles were used as a reference. All measurements were performed in quadruplicate (n = 4).
2.4. In Vitro Studies
2.4.1. Cellular Uptake
The cellular uptake of fluorescein-labelled docetaxel entrapped in transferrin-bearing and control PEGylated zein micelles, as well as in solution, was qualitatively evaluated using confocal microscopy. PC3-Luc, DU145, and LNCaP prostate cancer cells were seeded on sterile coverslips placed in 6-well plates at a density of 2 × 105 cells per well and incubated for 24 h at 37 °C in a humidified atmosphere containing 5% CO2. Following incubation, the medium was removed, and cells were treated with fluorescein-labelled docetaxel (20 μg/well) either in solution or entrapped in the various micellar formulations (Tf-bearing and control PEGylated zein micelles of 5 K and 10 K molecular weights). Incubation with formulations was carried out for 4 h at 37 °C under the same humidified CO2 atmosphere. Untreated cells served as negative controls. After treatment, cells were washed three times with 3 mL of phosphate-buffered saline (PBS) and fixed with 2 mL of methanol for 10 min at 20 °C. Methanol was then removed, and the cells were left to air dry for an additional 10 min at 20 °C. Coverslips were mounted onto glass microscope slides using Vectashield® mounting medium containing DAPI and allowed to stain for 2 h. Fluorescence imaging was performed using a Leica TCS SP5® confocal microscope (Leica Microsystems, Wetzlar, Germany). DAPI-stained nuclei were excited with a 405 nm laser (emission bandwidth: 415–491 nm), while fluorescein-labelled docetaxel was excited with a 494 nm laser (emission bandwidth: 510–530 nm).
The cellular uptake of fluorescein-labelled docetaxel carried by transferrin-bearing and control PEGylated zein micelles, as well as in solution, was also quantitatively assessed by flow cytometry. Uptake was determined by measuring the fluorescence intensity of fluorescein-labelled docetaxel internalized by the cells. Untreated cells served as a negative control. PC3-Luc, DU145, and LNCaP prostate cancer cells were seeded into 6-well plates at a density of 1 × 106 cells/well and incubated at 37 °C in a humidified 5% CO2 atmosphere for 24 h. After incubation, the culture medium was removed and replaced with fresh medium containing fluorescein-labelled docetaxel at a concentration of 20 μg/mL, either free or encapsulated in Tf-bearing or control PEGylated zein micelles. Cells were incubated for an additional 4 h under the same conditions. Following treatment, cells were washed three times with 2 mL of cold PBS, detached using 250 µL of TrypLE® Express, and incubated for 5–10 min at 37 °C. The trypsinization reaction was stopped by adding 500 µL of 1% fetal bovine serum (FBS) in PBS. The cell suspensions were then analyzed using an Attune® NxT Acoustic Focusing Cytometer (Thermo Fisher Scientific, Waltham, MA, USA) equipped with the Attune® NxT Auto Sampler. A total of 30,000 gated events were recorded per sample.
2.4.2. Mechanisms of Cellular Uptake
To elucidate the mechanisms involved in the endocytosis-mediated uptake of fluorescein-labelled docetaxel, PC3-Luc prostate cancer cells were treated with specific endocytic inhibitors. Cells were seeded into 6-well plates at a density of 1 × 106 cells/well and incubated for 24 h at 37 °C in a humidified 5% CO2 atmosphere. Following incubation, the culture medium was removed, and cells were pre-treated with one of the following endocytosis inhibitors for 30 min at 37 °C: transferrin (50 µM; a competitive ligand for transferrin receptors), chlorpromazine (20 µg/mL; clathrin-mediated endocytosis inhibitor), filipin (5 µg/mL; caveolae-mediated endocytosis inhibitor), colchicine (40 µg/mL; microtubule polymerization inhibitor), or poly-L-lysine (40 µg/mL; a general inhibitor of endocytic uptake via charge neutralization). After pre-treatment, the inhibitors were replaced with fresh medium containing both fluorescein-labelled docetaxel (20 µg/well) entrapped in transferrin-bearing PEGylated zein micelles and the corresponding inhibitor at the same concentration. Cells were incubated for an additional 4 h at 37 °C. Following treatment, cells were processed for flow cytometry and confocal microscopy, as described above, to assess changes in cellular uptake.
2.4.3. Anti-Proliferative Efficacy
The anti-proliferative efficacy of docetaxel entrapped in targeted and control PEGylated zein micelles or in solution was assessed using an MTT assay in PC3-Luc, DU145, and LNCaP prostate cancer cells. Cells were seeded into 96-well plates at a density of 2000 cells/well and incubated for 72 h at 37 °C in a humidified atmosphere containing 5% CO2. Following incubation, the medium was replaced with 200 µL of fresh medium containing various concentrations of docetaxel (ranging from 0.000001 to 60 µg/mL), either free or encapsulated in Tf-bearing or control PEGylated zein micelles. The cells were further incubated for 72 h under the same conditions. After treatment, 50 µL of MTT solution (0.5% w/v in PBS) was added to each well, and the plates were incubated for an additional 4 h to allow formation of the formazan product. The MTT solution was then removed, and 200 µL of dimethyl sulfoxide (DMSO) was added to each well to solubilize the purple formazan crystals. The absorbance was measured at 570 nm using a Multiskan Ascent® microplate reader (Thermo Labsystems, Beverly, MA, USA). Percentage cell viability was calculated relative to untreated control cells. Three independent experiments were performed for each formulation, with five replicate wells (n = 5) per concentration. Triton X-100 (0.1% w/v in PBS) served as a positive control, and fresh medium was used as a negative control. Dose–response curves were constructed by plotting percentage cell viability against docetaxel concentration to evaluate the anti-proliferative efficacy of each formulation.
2.5. Statistical Analysis
Results were expressed as the mean ± standard error of the mean (S.E.M.) using Origin 2021 software (OriginLab Corporation, Northampton, MA, USA). Statistical analysis was performed using one-way analysis of variance (ANOVA), followed by Tukey’s multiple comparison post-test to evaluate differences between treatment groups. Analyses were conducted using Minitab® software (version 22.0, Minitab LLC, State College, PA, USA). Differences were considered statistically significant at p < 0.05.
4. Discussion
This study explored the design, synthesis, and performance of transferrin (Tf)-functionalized PEGylated zein micelles as docetaxel (DTX) nanocarriers for prostate cancer, with a specific focus on how PEG chain length (5 kDa vs. 10 kDa) governs ligand presentation and downstream biological performance. PEG5K was selected as a commonly used PEG molecular weight expected to provide steric stabilization while maintaining ligand accessibility, whereas PEG10K was chosen to represent a longer PEG corona expected to increase steric shielding and therefore challenge Tf–receptor engagement [
21,
22]. The results demonstrate successful micelle formation, efficient docetaxel loading, and PEG-length-dependent effects of transferrin conjugation on uptake mechanisms and anti-proliferative activity in PC-3-Luc, DU145, and LNCaP cell lines. Importantly, although PEGylated zein systems and Tf targeting have been explored previously, there remains limited evidence directly comparing how PEG molecular weight modulates Tf display, receptor engagement, and biological performance within the same zein micelle platform. By comparing PEG5K versus PEG10K in otherwise closely matched formulations, we show that PEG length strongly governs Tf conjugation efficiency and functional ligand accessibility, which in turn influences uptake behavior and cytotoxicity outcomes [
16,
24].
NMR analyses supported the successful synthesis of PEGylated zein and subsequent Tf conjugation, consistent with the expected core–shell organization in which zein forms the hydrophobic core and PEG constitutes the hydrophilic corona [
15,
16]. Both PEG5K and PEG10K formulations self-assembled in aqueous medium, as reflected by low critical micelle concentrations and spherical morphologies under TEM. Any discrepancies between DLS- and TEM-derived sizes are expected because DLS reports hydrodynamic diameters in dispersion (including solvated polymer layers), whereas TEM reflects dried particle dimensions on a grid [
7].
PEG chain length strongly influenced micellar size, with PEG10K micelles exhibiting larger hydrodynamic diameters than PEG5K micelles. This trend is consistent with the fact that increasing PEG molecular weight increases the thickness and steric volume of the hydrated corona, thereby increasing the measured hydrodynamic diameter [
7]. Tf conjugation further increased micelle size, consistent with the addition of a surface-associated protein layer and increased interfacial hydration after ligand functionalization. The size increase after Tf attachment appeared more pronounced for the PEG5K formulation, which may reflect a larger relative contribution of the ligand layer to smaller carriers and/or differences in effective ligand presentation. All micelles maintained polydispersity indices below 0.25, indicating that PEGylation and Tf functionalization did not induce broad aggregation and preserved formulation uniformity. In addition, the micelles remained within size regimes commonly discussed as permissive for tumor extravasation in leaky vasculature models [
37], noting that in vivo transport depends strongly on tumor type and physiology.
Zeta potential analysis showed that control (non-targeted) micelles were positively charged, whereas all Tf-bearing micelles carried a negative surface charge (
Table 1). This shift is consistent with successful surface modification because transferrin is an acidic glycoprotein, and its surface presentation can dominate the measured electrophoretic mobility by shifting the effective shear plane and partially shielding the underlying micellar surface [
26,
28]. Beyond confirming conjugation, this change is relevant to nano–bio interactions because a less cationic (or anionic) surface can reduce non-specific electrostatic adsorption to cell membranes and other biological interfaces compared to a strongly cationic surface. This is pertinent here because surface charge can contribute to uptake differences between targeted and non-targeted micelles: cationic nanoparticles/micelles often exhibit enhanced cellular uptake in non-phagocytic cells via electrostatic attraction to the negatively charged plasma membrane, promoting adsorptive interactions and endocytosis [
38,
39,
40]. In contrast, transferrin conjugation shifted the zeta potential to negative values, which may reduce non-specific electrostatic adsorption and thereby contribute to lower uptake for some Tf-bearing formulations, particularly where functional Tf–TfR engagement is limited.
High encapsulation efficiency and consistent drug loading across all formulations indicate that zein micelles are well suited for incorporating hydrophobic drugs such as docetaxel [
9,
11]. All micelles achieved >84% encapsulation efficiency (higher than 252 µg docetaxel), with higher encapsulation efficiency for PEG5K micelles compared with PEG10K micelles. A plausible explanation is that increasing the PEG contribution can alter core–corona packing and reduce the effective hydrophobic microenvironment available for drug partitioning into the zein core [
7]. The slightly lower entrapment efficiency relative to some earlier zein-based reports may also reflect zein source/composition effects; for example, yellow zein contains additional hydrophobic components (e.g., pigments) that can influence solubility and encapsulation behavior compared with more purified forms [
9,
11].
Quantification of Tf conjugation efficiency revealed substantial surface modification, particularly for PEG5K micelles (83.6%) compared with PEG10K micelles (56%). This difference is consistent with steric hindrance imposed by a longer PEG corona, which can obstruct reactive groups during conjugation and can also reduce effective ligand accessibility by partially burying ligands within the PEG brush [
21,
25]. This PEG-length-dependent “shielding versus accessibility” trade-off provides a mechanistic basis for differences in uptake and efficacy between Tf-bearing PEG5K and PEG10K systems.
In vitro uptake studies revealed several key patterns. Across all three cell lines, free docetaxel showed the highest cellular uptake, as expected for a small molecule in 2D monolayer assays where diffusion and rapid membrane access can dominate compared with nanocarriers that require binding/internalization and intracellular release [
7]. Among micellar formulations, uptake varied with PEG length, Tf conjugation, and cell line. In PC-3-Luc cells, control PEG10K micelles displayed the highest uptake among micelle formulations, while Tf-bearing micelles showed reduced internalization; this pattern is consistent with a scenario where cationic surface-driven uptake is prominent for controls, while PEG steric shielding and/or reduced functional ligand exposure limits Tf-mediated enhancement. In DU145 cells, uptake of Tf-bearing micelles was significantly higher than that of their matched controls, consistent with TfR-mediated contributions in a context where receptor availability and endocytic capacity support ligand-driven internalization. In LNCaP cells, uptake levels were more balanced between control and targeted PEG10K micelles, with a modest advantage for Tf-bearing micelles. These observations align with the broader point that receptor density and cell-specific endocytic machinery can both shape nanoparticle uptake, and that receptor expression alone may not fully predict internalization outcomes [
21,
27].
Mechanistic studies using pharmacological inhibitors indicated that internalization of Tf-functionalized micelles involved caveolae- and clathrin-associated pathways as well as transferrin receptor–related uptake, supported by significant inhibition with filipin, chlorpromazine, and free transferrin. Poly-L-lysine also inhibited uptake, consistent with an electrostatic component to interaction/attachment in these systems. By contrast, colchicine produced negligible effects, suggesting macropinocytosis did not play a major role under the conditions tested, consistent with prior observations for PEGylated zein micelles in another cancer model [
16,
17]. Collectively, these data support the view that Tf-bearing micelles exploit multiple endocytic routes, with the relative contribution of receptor-mediated versus charge-mediated interactions depending on formulation surface properties and cellular context [
26,
28,
29,
41].
Anti-proliferative efficacy further clarified the functional consequences of these uptake patterns. Docetaxel in solution consistently showed the lowest IC
50 values across all cell lines, reflecting its rapid availability in 2D assays. When delivered via PEGylated zein micelles, potency was lower than the free drug but remained within the low µg/mL range across formulations. Among micelles, Tf-bearing PEG5K micelles markedly improved cytotoxicity relative to matched control micelles. For example, in PC-3-Luc cells, the IC
50 for targeted PEG5K micelles was 0.09 ± 0.04 µg/mL versus 0.66 ± 0.14 µg/mL for control PEG5K micelles (7.3-fold improvement), and ~2-fold improvements were observed in DU145 and LNCaP cells. These results support the interpretation that shorter PEG chains facilitate better functional accessibility of the Tf ligand and more effective receptor-mediated contributions to uptake and activity [
21,
25]. In contrast, this targeting benefit was not observed for PEG10K micelles: Tf-bearing PEG10K micelles consistently exhibited higher IC
50 values than their matched PEG10K controls across all cell lines, indicating reduced functional efficacy. This counterintuitive outcome is consistent with steric shielding by longer PEG chains, which can diminish ligand–receptor engagement and reduce the functional impact of targeting ligands [
21,
25]. Notably, while longer PEG chains are often used to increase steric shielding in biological media, “stealth” behavior (e.g., reduced opsonization and prolonged circulation) depends strongly on PEG surface architecture and requires dedicated protein adsorption and/or pharmacokinetic evaluation, which was outside the scope of this in vitro study [
21,
42].
Our findings align with the broader literature showing that transferrin functionalization can improve taxane delivery performance relative to non-targeted nanocarriers, while the magnitude and direction of benefit depend on how ligands are presented at the nanoparticle surface. Fernandes et al. reported that transferrin-conjugated liposomes enhanced docetaxel efficacy relative to non-targeted systems [
30], and Zhai et al. described improved delivery efficiency and anti-proliferative activity with transferrin-modified liposomes [
31]. Sahoo et al. similarly showed improved uptake and anti-proliferative efficacy of transferrin-conjugated paclitaxel nanoparticles versus unconjugated nanoparticles and drug in solution in prostate cancer models [
29]. Within zein-based systems, the feasibility of combining zein with transferrin targeting has also been supported by transferrin-bearing zein hybrid lipid nanoparticles for docetaxel delivery to prostate cancer cells [
24]. Against this background, the novelty of the present work lies in directly demonstrating, within the same zein micelle platform, that PEG chain length can invert the apparent benefit of Tf targeting (beneficial at PEG5K, detrimental at PEG10K), providing a clear design rule for balancing PEG-corona steric shielding with functional ligand accessibility.
Overall, these data show that transferrin-functionalized PEGylated zein micelles provide a useful platform for probing how PEG chain length controls transferrin display, receptor engagement, and cellular internalization. While free docetaxel remains the most potent in vitro in the 2D assays used here, Tf-bearing PEG5K micelles improved anti-proliferative efficacy relative to their matched non-targeted micelles, consistent with a functional contribution of transferrin when ligand masking is limited. Considering the higher transferrin conjugation efficiency for PEG5K and the more favorable IC
50 improvements versus matched non-targeted controls, Tf-PEG5K-zein-DTX is identified as the lead targeted formulation in this study, while Tf-PEG10K-zein-DTX serves as a comparator to illustrate PEG-length/steric shielding effects. The experimental design included matched non-targeted micelles (PEG5K-zein-DTX and PEG10K-zein-DTX) as PEG-only controls, enabling separation of PEG chain-length effects from transferrin functionalization effects, and free transferrin competition provided a functional control supporting receptor involvement [
26,
28].
Future work could explore intermediate PEG chain lengths (e.g., PEG2K) and alternative linker/architecture strategies to improve ligand accessibility without compromising micelle integrity in biological media [
21]. Given that transferrin receptor targeting is also widely investigated in other biological barriers, future studies should also consider off-target interactions and biodistribution questions in more complex models. Finally, additional comparator systems such as TfR-low cells would further strengthen selectivity assessment beyond competition assays, and inclusion of blank micelles and orthogonal structural methods could help refine interpretation of drug distribution within the micellar structure [
7,
27]. If development of a dried product is pursued in future, a dedicated lyophilization/redispersion optimization study (including suitable lyoprotectants) would be warranted, as freeze-drying can affect the redispersibility of self-assembled nanocarriers.
5. Conclusions
This study successfully developed and characterized transferrin-conjugated PEGylated zein micelles as a targeted nanocarrier system for docetaxel delivery in prostate cancer. Through physicochemical analysis and in vitro testing across three prostate cancer cell lines (PC-3-Luc, DU145, and LNCaP), the impact of PEG chain length and transferrin surface modification on micellar behavior, cellular uptake, and anti-proliferative efficacy was thoroughly investigated.
The findings demonstrated that PEG chain length plays a pivotal role in modulating micelle size, transferrin conjugation efficiency, and biological performance. Shorter PEG chains (5 K) allowed more efficient transferrin attachment and led to significantly enhanced docetaxel efficacy compared to their non-targeted counterparts. Notably, transferrin-bearing PEG5K micelles reduced IC50 values by up to 7.3-fold compared to control micelles in PC-3-Luc cells, with similar efficacy improvements observed in DU145 and LNCaP cell lines. This improvement was attributed to enhanced cellular uptake through transferrin receptor-mediated endocytosis, as confirmed by uptake inhibition assays using filipin, chlorpromazine, and free transferrin.
In contrast, PEG10K micelles, despite their advantageous colloidal stability and drug loading capacity, showed reduced transferrin conjugation efficiency and diminished targeting capability. The longer PEG chains likely induced steric hindrance, limiting ligand–receptor interactions and resulting in inferior anti-proliferative activity compared to control micelles. These outcomes highlight the critical balance between PEG-mediated steric shielding and targeting efficiency, where overly long PEG chains may compromise the accessibility of targeting ligands.
Overall, transferrin-conjugated PEG5K zein micelles emerged as a more effective nanocarrier formulation of docetaxel for prostate cancer therapy. The study underscores the importance of rational nanocarrier design, where subtle differences in polymer architecture can markedly influence biological behavior and therapeutic performance.