Microglia-Targeted Nanotherapeutics in Major Depressive Disorder: An Integrative Perspective on Neuroinflammation and Drug Delivery
Abstract
1. Introduction
2. Materials and Methods
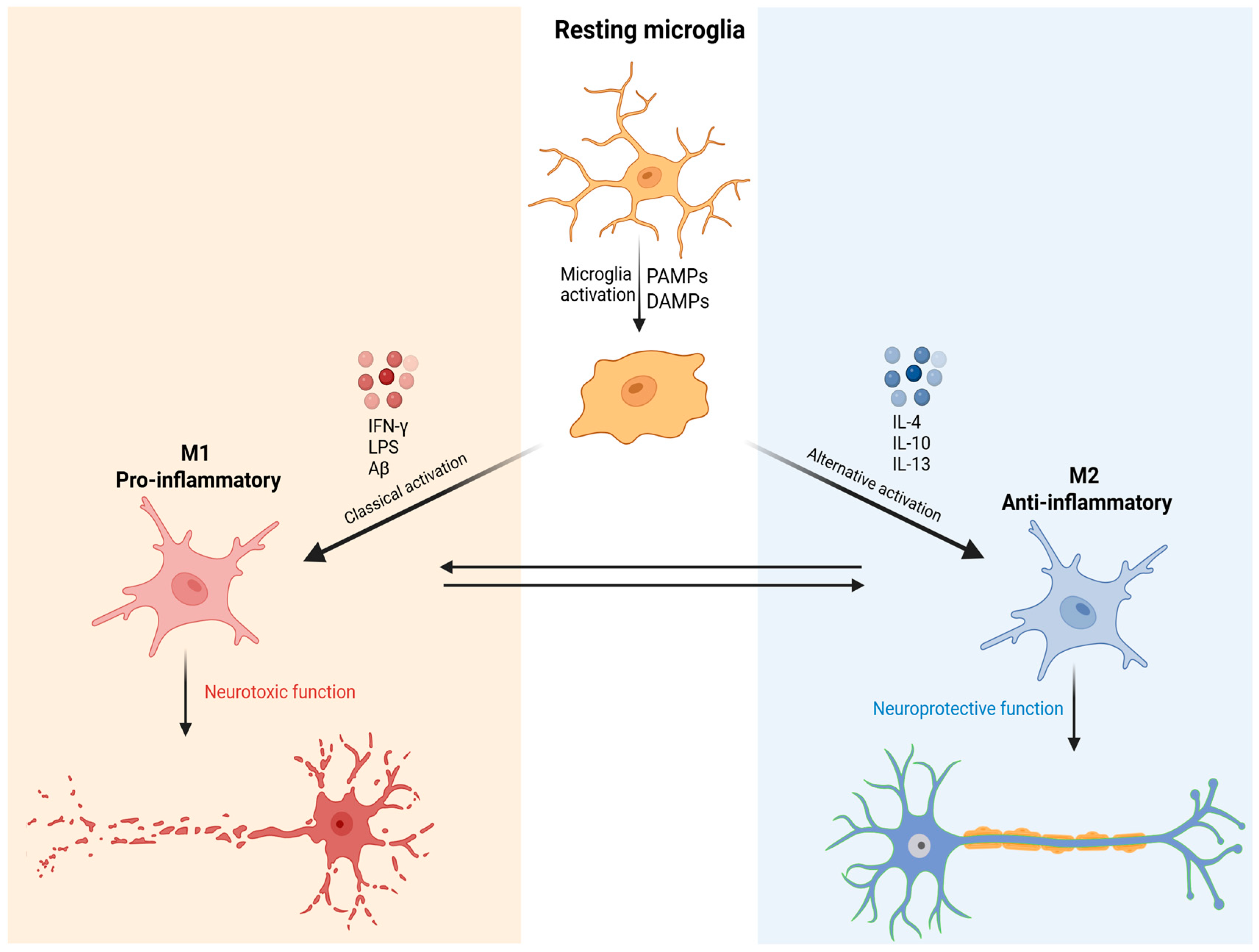
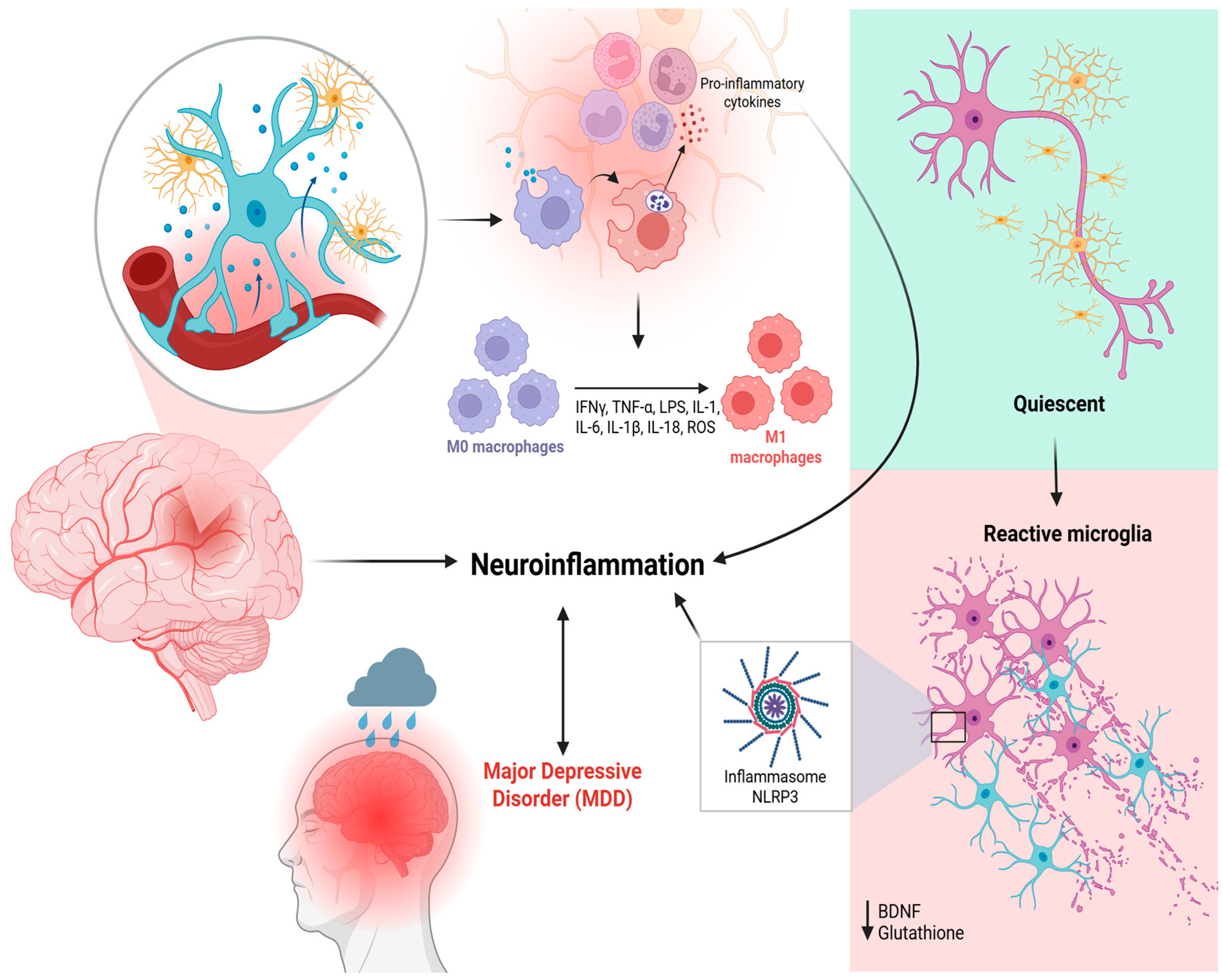
3. The Role of Microglia in MDD Pathophysiology
4. Conventional Therapeutic Approaches and Their Limitations
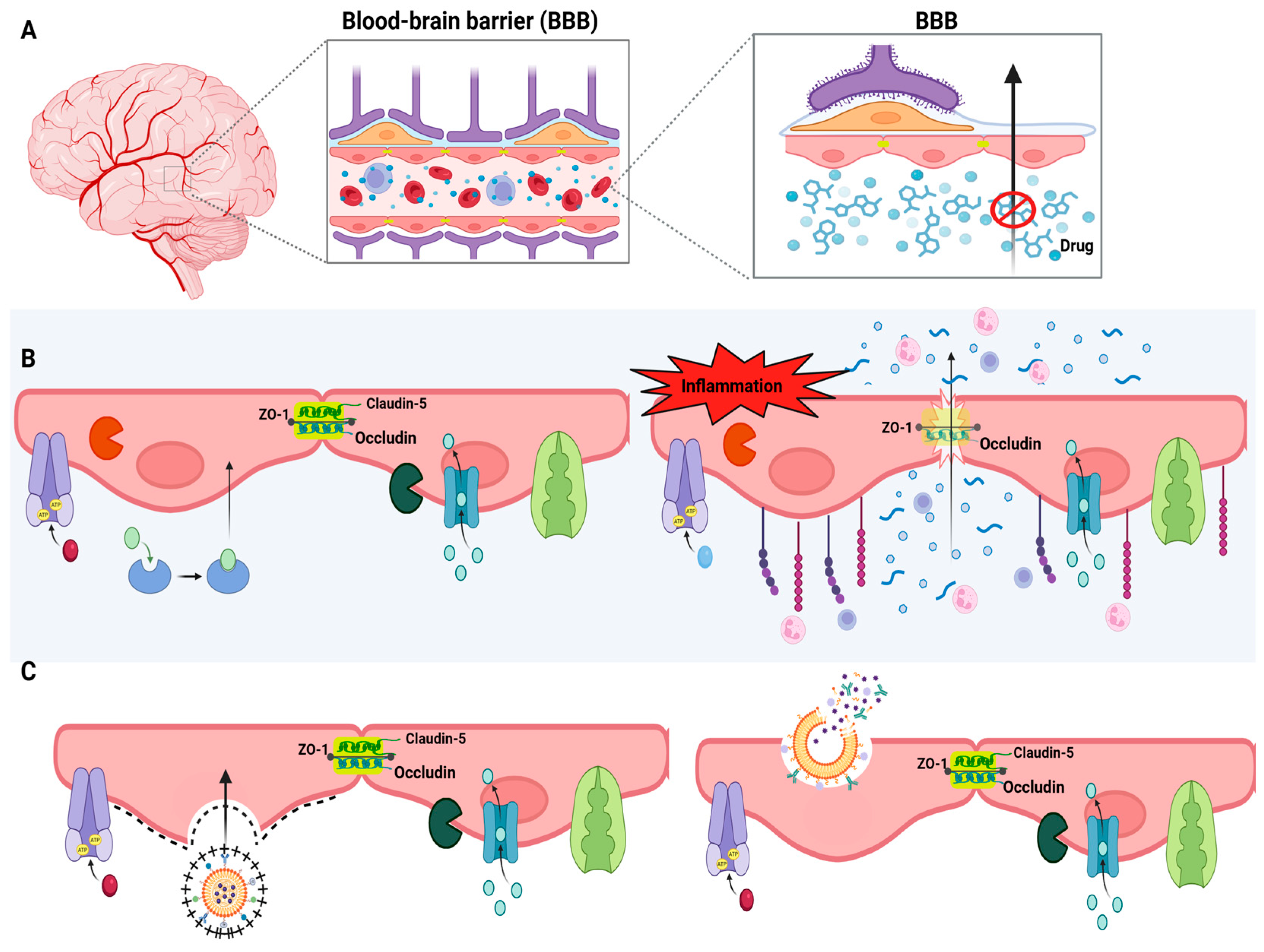
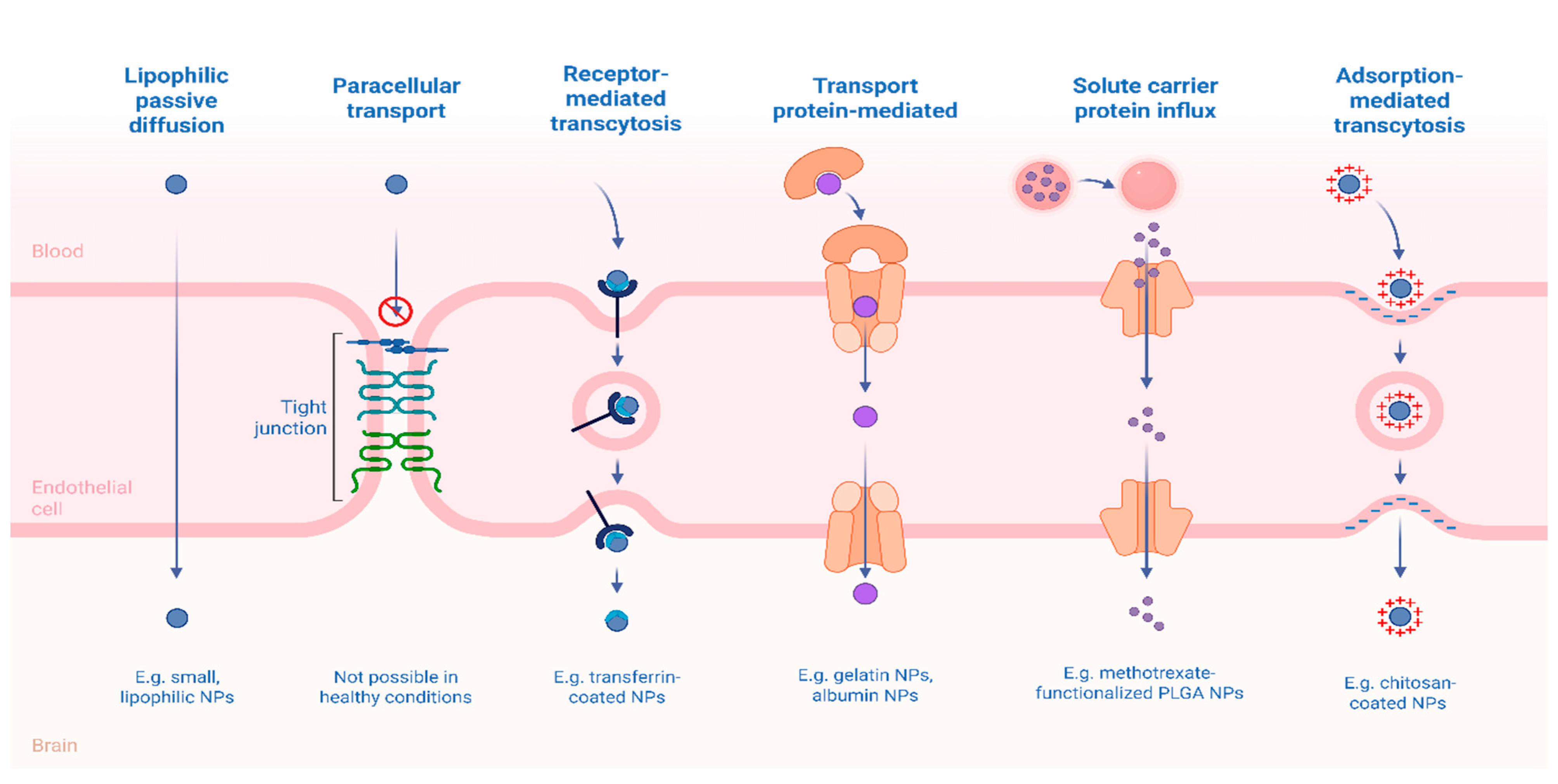
5. Brain-Targeted Drug Delivery Systems
6. Controlled Release Systems
7. Drug Delivery Strategies for Microglial Modulation
7.1. Polymeric Nanoparticles
7.2. Dendrimers
7.3. Lipid-Based Nanoparticles
7.4. Inorganic Nanoparticles
8. Future Perspective
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Maes, M.; Galecki, P.; Chang, Y.S.; Berk, M. A Review on the Oxidative and Nitrosative Stress (O&NS) Pathways in Major Depression and Their Possible Contribution to the (Neuro)Degenerative Processes in That Illness. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 676–692. [Google Scholar] [CrossRef]
- Black, C.N.; Bot, M.; Scheffer, P.G.; Cuijpers, P.; Penninx, B.W.J.H. Is Depression Associated with Increased Oxidative Stress? A Systematic Review and Meta-Analysis. Psychoneuroendocrinology 2015, 51, 164–175. [Google Scholar] [CrossRef]
- Kiani, A.K.; Maltese, P.E.; Dautaj, A.; Paolacci, S.; Kurti, D.; Picotti, P.M.; Bertelli, M. Neurobiological Basis of Chiropractic Manipulative Treatment of the Spine in the Care of Major Depression. Acta Bio Medica Atenei Parm. 2020, 91, e2020006. [Google Scholar] [CrossRef]
- Beurel, E.; Toups, M.; Nemeroff, C.B. The Bidirectional Relationship of Depression and Inflammation: Double Trouble. Neuron 2020, 107, 234–256. [Google Scholar] [CrossRef]
- Worthen, R.J.; Beurel, E. Inflammatory and Neurodegenerative Pathophysiology Implicated in Postpartum Depression. Neurobiol. Dis. 2022, 165, 105646. [Google Scholar] [CrossRef]
- Yirmiya, R.; Rimmerman, N.; Reshef, R. Depression as a Microglial Disease. Trends Neurosci. 2015, 38, 637–658. [Google Scholar] [CrossRef] [PubMed]
- Troubat, R.; Barone, P.; Leman, S.; Desmidt, T.; Cressant, A.; Atanasova, B.; Brizard, B.; El Hage, W.; Surget, A.; Belzung, C.; et al. Neuroinflammation and Depression: A Review. Eur. J. Neurosci. 2021, 53, 151–171. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.H.; Raison, C.L. The Role of Inflammation in Depression: From Evolutionary Imperative to Modern Treatment Target. Nat. Rev. Immunol. 2016, 16, 22–34. [Google Scholar] [CrossRef]
- Miller, A.H.; Haroon, E.; Felger, J.C. Therapeutic Implications of Brain–Immune Interactions: Treatment in Translation. Neuropsychopharmacology 2017, 42, 334–359. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.S.; Jones, G.T.; Hughes, D.M.; Moots, R.J.; Goodson, N.J. Depression and Anxiety Symptoms at TNF Inhibitor Initiation Are Associated with Impaired Treatment Response in Axial Spondyloarthritis. Rheumatology 2021, 60, 5734–5742. [Google Scholar] [CrossRef]
- Woodburn, S.C.; Bollinger, J.L.; Wohleb, E.S. The Semantics of Microglia Activation: Neuroinflammation, Homeostasis, and Stress. J. Neuroinflamm. 2021, 18, 258. [Google Scholar] [CrossRef]
- Zorkina, Y.; Abramova, O.; Ushakova, V.; Morozova, A.; Zubkov, E.; Valikhov, M.; Melnikov, P.; Majouga, A.; Chekhonin, V. Nano Carrier Drug Delivery Systems for the Treatment of Neuropsychiatric Disorders: Advantages and Limitations. Molecules 2020, 25, 5294. [Google Scholar] [CrossRef]
- Fan, Y.; Chen, M.; Zhang, J.; Maincent, P.; Xia, X.; Wu, W. Updated Progress of Nanocarrier-Based Intranasal Drug Delivery Systems for Treatment of Brain Diseases. Crit. Rev. Ther. Drug Carr. Syst. 2018, 35, 433–467. [Google Scholar] [CrossRef] [PubMed]
- Vitorino, C.; Silva, S.; Bicker, J.; Falcão, A.; Fortuna, A. Antidepressants and Nose-to-Brain Delivery: Drivers, Restraints, Opportunities and Challenges. Drug Discov. Today 2019, 24, 1911–1923. [Google Scholar] [CrossRef]
- Liu, J.; Wang, T.; Dong, J.; Lu, Y. The Blood–Brain Barriers: Novel Nanocarriers for Central Nervous System Diseases. J. Nanobiotechnol. 2025, 23, 146. [Google Scholar] [CrossRef]
- Li, H.; Guan, M.; Zhang, N.-N.; Wang, Y.; Liang, T.; Wu, H.; Wang, C.; Sun, T.; Liu, S. Harnessing Nanomedicine for Modulating Microglial States in the Central Nervous System Disorders: Challenges and Opportunities. Biomed. Pharmacother. 2024, 177, 117011. [Google Scholar] [CrossRef]
- Hickman, S.E.; Kingery, N.D.; Ohsumi, T.K.; Borowsky, M.L.; Wang, L.; Means, T.K.; El Khoury, J. The Microglial Sensome Revealed by Direct RNA Sequencing. Nat. Neurosci. 2013, 16, 1896–1905. [Google Scholar] [CrossRef]
- Estes, M.L.; McAllister, A.K. Alterations in Immune Cells and Mediators in the Brain: It’s Not Always Neuroinflammation! Brain Pathol. 2014, 24, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Escoubas, C.C.; Molofsky, A.V. Microglia as Integrators of Brain-Associated Molecular Patterns. Trends Immunol. 2024, 45, 358–370. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; He, Y.; Sun, Z.; Ren, S.; Liu, M.; Wang, G.; Yang, J. Microglia in Depression: An Overview of Microglia in the Pathogenesis and Treatment of Depression. J. Neuroinflamm. 2022, 19, 132. [Google Scholar] [CrossRef]
- Qin, C.; Zhou, L.-Q.; Ma, X.-T.; Hu, Z.-W.; Yang, S.; Chen, M.; Bosco, D.B.; Wu, L.-J.; Tian, D.-S. Dual Functions of Microglia in Ischemic Stroke. Neurosci. Bull. 2019, 35, 921–933. [Google Scholar] [CrossRef] [PubMed]
- Dowlati, Y.; Herrmann, N.; Swardfager, W.; Liu, H.; Sham, L.; Reim, E.K.; Lanctôt, K.L. A Meta-Analysis of Cytokines in Major Depression. Biol. Psychiatry 2010, 67, 446–457. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Li, S.; Wang, S.; Wu, X.; Liu, Y.; Yu, W.; Wang, Y.; Tang, Y.; Xia, M.; Li, B. Major Depressive Disorder: Hypothesis, Mechanism, Prevention and Treatment. Sig Transduct. Target. Ther. 2024, 9, 30. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Yang, W.; Ge, T.; Wang, Y.; Cui, R. Stress Induced Microglial Activation Contributes to Depression. Pharmacol. Res. 2022, 179, 106145. [Google Scholar] [CrossRef]
- Sominsky, L.; De Luca, S.; Spencer, S.J. Microglia: Key Players in Neurodevelopment and Neuronal Plasticity. Int. J. Biochem. Cell Biol. 2018, 94, 56–60. [Google Scholar] [CrossRef]
- Chagas, L.D.S.; Serfaty, C.A. The Influence of Microglia on Neuroplasticity and Long-Term Cognitive Sequelae in Long COVID: Impacts on Brain Development and Beyond. Int. J. Mol. Sci. 2024, 25, 3819. [Google Scholar] [CrossRef]
- Sharma, T.; Guski, L.S.; Freund, N.; Gøtzsche, P.C. Suicidality and Aggression during Antidepressant Treatment: Systematic Review and Meta-Analyses Based on Clinical Study Reports. BMJ 2016, 352, i65. [Google Scholar] [CrossRef]
- Hiemke, C.; Bergemann, N.; Clement, H.; Conca, A.; Deckert, J.; Domschke, K.; Eckermann, G.; Egberts, K.; Gerlach, M.; Greiner, C.; et al. Consensus Guidelines for Therapeutic Drug Monitoring in Neuropsychopharmacology: Update 2017. Pharmacopsychiatry 2018, 51, 9–62. [Google Scholar] [CrossRef]
- Malhi, G.S.; Mann, J.J. Depression. Lancet 2018, 392, 2299–2312. [Google Scholar] [CrossRef]
- Kim, J.-W.; Suzuki, K.; Kavalali, E.T.; Monteggia, L.M. Ketamine: Mechanisms and Relevance to Treatment of Depression. Annu. Rev. Med. 2024, 75, 129–143. [Google Scholar] [CrossRef]
- Cipriani, A.; Furukawa, T.A.; Salanti, G.; Chaimani, A.; Atkinson, L.Z.; Ogawa, Y.; Leucht, S.; Ruhe, H.G.; Turner, E.H.; Higgins, J.P.T.; et al. Comparative Efficacy and Acceptability of 21 Antidepressant Drugs for the Acute Treatment of Adults with Major Depressive Disorder: A Systematic Review and Network Meta-Analysis. Lancet 2018, 391, 1357–1366. [Google Scholar] [CrossRef]
- Sousa, R.D.D.; Rodrigues, A.M.; Gregório, M.J.; Branco, J.D.C.; Gouveia, M.J.; Canhão, H.; Dias, S.S. Anxiety and Depression in the Portuguese Older Adults: Prevalence and Associated Factors. Front. Med. 2017, 4, 196. [Google Scholar] [CrossRef]
- Zakaria, F.H.; Samhani, I.; Mustafa, M.Z.; Shafin, N. Pathophysiology of Depression: Stingless Bee Honey Promising as an Antidepressant. Molecules 2022, 27, 5091. [Google Scholar] [CrossRef] [PubMed]
- Hall, S.; Parr, B.-A.; Hussey, S.; Anoopkumar-Dukie, S.; Arora, D.; Grant, G.D. The Neurodegenerative Hypothesis of Depression and the Influence of Antidepressant Medications. Eur. J. Pharmacol. 2024, 983, 176967. [Google Scholar] [CrossRef] [PubMed]
- Richelson, E. Multi-Modality: A New Approach for the Treatment of Major Depressive Disorder. Int. J. Neuropsychopharmacol. 2013, 16, 1433–1442. [Google Scholar] [CrossRef]
- Severe, J.; Greden, J.F.; Reddy, P. Consequences of Recurrence of Major Depressive Disorder: Is Stopping Effective Antidepressant Medications Ever Safe? Focus 2020, 18, 120–128. [Google Scholar] [CrossRef]
- Cuijpers, P.; Miguel, C.; Ciharova, M.; Ebert, D.; Harrer, M.; Karyotaki, E. Transdiagnostic Treatment of Depression and Anxiety: A Meta-Analysis. Psychol. Med. 2023, 53, 6535–6546. [Google Scholar] [CrossRef]
- Nikolova, V.L.; Cleare, A.J.; Young, A.H.; Stone, J.M. Acceptability, Tolerability, and Estimates of Putative Treatment Effects of Probiotics as Adjunctive Treatment in Patients with Depression: A Randomized Clinical Trial. JAMA Psychiatry 2023, 80, 842. [Google Scholar] [CrossRef]
- Hannestad, J.; DellaGioia, N.; Bloch, M. The Effect of Antidepressant Medication Treatment on Serum Levels of Inflammatory Cytokines: A Meta-Analysis. Neuropsychopharmacology 2011, 36, 2452–2459. [Google Scholar] [CrossRef]
- Warner-Schmidt, J.L.; Vanover, K.E.; Chen, E.Y.; Marshall, J.J.; Greengard, P. Antidepressant Effects of Selective Serotonin Reuptake Inhibitors (SSRIs) Are Attenuated by Antiinflammatory Drugs in Mice and Humans. Proc. Natl. Acad. Sci. USA 2011, 108, 9262–9267. [Google Scholar] [CrossRef] [PubMed]
- Piletz, J.E.; Halaris, A.; Iqbal, O.; Hoppensteadt, D.; Fareed, J.; Zhu, H.; Sinacore, J.; DeVane, C.L. Pro-Inflammatory Biomakers in Depression: Treatment with Venlafaxine. World J. Biol. Psychiatry 2009, 10, 313–323. [Google Scholar] [CrossRef]
- Guo, B.; Zhang, M.; Hao, W.; Wang, Y.; Zhang, T.; Liu, C. Neuroinflammation Mechanisms of Neuromodulation Therapies for Anxiety and Depression. Transl. Psychiatry 2023, 13, 5. [Google Scholar] [CrossRef] [PubMed]
- Zazula, R.; Husain, M.I.; Mohebbi, M.; Walker, A.J.; Chaudhry, I.B.; Khoso, A.B.; Ashton, M.M.; Agustini, B.; Husain, N.; Deakin, J.; et al. Minocycline as Adjunctive Treatment for Major Depressive Disorder: Pooled Data from Two Randomized Controlled Trials. Aust. N. Z. J. Psychiatry 2021, 55, 784–798. [Google Scholar] [CrossRef]
- Shi, J.; Tan, C.; Ge, X.; Qin, Z.; Xiong, H. Recent Advances in Stimuli-Responsive Controlled Release Systems for Neuromodulation. J. Mater. Chem. B 2024, 12, 5769–5786. [Google Scholar] [CrossRef]
- Kadry, H.; Noorani, B.; Cucullo, L. A Blood–Brain Barrier Overview on Structure, Function, Impairment, and Biomarkers of Integrity. Fluids Barriers CNS 2020, 17, 69. [Google Scholar] [CrossRef]
- Lochhead, J.J.; Yang, J.; Ronaldson, P.T.; Davis, T.P. Structure, Function, and Regulation of the Blood-Brain Barrier Tight Junction in Central Nervous System Disorders. Front. Physiol. 2020, 11, 914. [Google Scholar] [CrossRef]
- Liebner, S.; Czupalla, C.J.; Wolburg, H. Current Concepts of Blood-Brain Barrier Development. Int. J. Dev. Biol. 2011, 55, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yang, Y.; Fan, X. Microvascular Pericytes in Brain-Associated Vascular Disease. Biomed. Pharmacother. 2020, 121, 109633. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Ayyadurai, S.; Zlokovic, B.V. Pericytes of the Neurovascular Unit: Key Functions and Signaling Pathways. Nat. Neurosci. 2016, 19, 771–783. [Google Scholar] [CrossRef] [PubMed]
- Alsbrook, D.L.; Di Napoli, M.; Bhatia, K.; Biller, J.; Andalib, S.; Hinduja, A.; Rodrigues, R.; Rodriguez, M.; Sabbagh, S.Y.; Selim, M.; et al. Neuroinflammation in Acute Ischemic and Hemorrhagic Stroke. Curr. Neurol. Neurosci. Rep. 2023, 23, 407–431. [Google Scholar] [CrossRef]
- Varatharaj, A.; Galea, I. The Blood-Brain Barrier in Systemic Inflammation. Brain Behav. Immun. 2017, 60, 1–12. [Google Scholar] [CrossRef]
- Lye, P.; Bloise, E.; Imperio, G.E.; Chitayat, D.; Matthews, S.G. Functional Expression of Multidrug-Resistance (MDR) Transporters in Developing Human Fetal Brain Endothelial Cells. Cells 2022, 11, 2259. [Google Scholar] [CrossRef]
- Loscher, W.; Potschka, H. Blood-Brain Barrier Active Efflux Transporters: ATP-Binding Cassette Gene Family. NeuroRx 2005, 2, 86–98. [Google Scholar] [CrossRef]
- Taskar, K.S.; Yang, X.; Neuhoff, S.; Patel, M.; Yoshida, K.; Paine, M.F.; Brouwer, K.L.R.; Chu, X.; Sugiyama, Y.; Cook, J.; et al. Clinical Relevance of Hepatic and Renal P-gp/BCRP Inhibition of Drugs: An International Transporter Consortium Perspective. Clin. Pharma Ther. 2022, 112, 573–592. [Google Scholar] [CrossRef]
- Radosavljevic, M.; Svob Strac, D.; Jancic, J.; Samardzic, J. The Role of Pharmacogenetics in Personalizing the Antidepressant and Anxiolytic Therapy. Genes 2023, 14, 1095. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, F.E.; Dinan, T.G.; Griffin, B.T.; Cryan, J.F. Interactions between Antidepressants and P-glycoprotein at the Blood–Brain Barrier: Clinical Significance of in Vitro and in Vivo Findings. Br. J. Pharmacol. 2012, 165, 289–312. [Google Scholar] [CrossRef] [PubMed]
- Kuban, W.; Daniel, W.A. Cytochrome P450 Expression and Regulation in the Brain. Drug Metab. Rev. 2021, 53, 1–29. [Google Scholar] [CrossRef]
- Manzoor, S.; Hoda, N. A Comprehensive Review of Monoamine Oxidase Inhibitors as Anti-Alzheimer’s Disease Agents: A Review. Eur. J. Med. Chem. 2020, 206, 112787. [Google Scholar] [CrossRef]
- Boado, R.J.; Tsukamoto, H.; Pardridge, W.M. Drug Delivery of Antisense Molecules to the Brain for Treatment of Alzheimer’s Disease and Cerebral AIDS. J. Pharm. Sci. 1998, 87, 1308–1315. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.A.; Rhea, E.M.; Reed, M.J.; Erickson, M.A. The Penetration of Therapeutics across the Blood-Brain Barrier: Classic Case Studies and Clinical Implications. Cell Rep. Med. 2024, 5, 101760. [Google Scholar] [CrossRef]
- Dichiara, M.; Amata, B.; Turnaturi, R.; Marrazzo, A.; Amata, E. Tuning Properties for Blood–Brain Barrier Permeation: A Statistics-Based Analysis. ACS Chem. Neurosci. 2020, 11, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Huddart, R.; Hicks, J.K.; Ramsey, L.B.; Strawn, J.R.; Smith, D.M.; Bobonis Babilonia, M.; Altman, R.B.; Klein, T.E. PharmGKB Summary: Sertraline Pathway, Pharmacokinetics. Pharmacogenet. Genom. 2020, 30, 26–33. [Google Scholar] [CrossRef]
- Koepsell, H. Glucose Transporters in Brain in Health and Disease. Pflug. Arch. Eur. J. Physiol. 2020, 472, 1299–1343. [Google Scholar] [CrossRef]
- Sun, Z.-W.; Wang, X.; Zhao, Y.; Sun, Z.-X.; Wu, Y.-H.; Hu, H.; Zhang, L.; Wang, S.-D.; Li, F.; Wei, A.-J.; et al. Blood-Brain Barrier Dysfunction Mediated by the EZH2-Claudin-5 Axis Drives Stress-Induced TNF-α Infiltration and Depression-like Behaviors. Brain Behav. Immun. 2024, 115, 143–156. [Google Scholar] [CrossRef]
- Meixensberger, S.; Kuzior, H.; Fiebich, B.L.; Süß, P.; Runge, K.; Berger, B.; Nickel, K.; Denzel, D.; Schiele, M.A.; Michel, M.; et al. Upregulation of sICAM-1 and sVCAM-1 Levels in the Cerebrospinal Fluid of Patients with Schizophrenia Spectrum Disorders. Diagnostics 2021, 11, 1134. [Google Scholar] [CrossRef]
- Haqqani, A.S.; Bélanger, K.; Stanimirovic, D.B. Receptor-Mediated Transcytosis for Brain Delivery of Therapeutics: Receptor Classes and Criteria. Front. Drug Deliv. 2024, 4, 1360302. [Google Scholar] [CrossRef]
- Ding, S.; Khan, A.I.; Cai, X.; Song, Y.; Lyu, Z.; Du, D.; Dutta, P.; Lin, Y. Overcoming Blood–Brain Barrier Transport: Advances in Nanoparticle-Based Drug Delivery Strategies. Mater. Today 2020, 37, 112–125. [Google Scholar] [CrossRef]
- Mubarak, N.; Waqar, M.A.; Khan, A.M.; Asif, Z.; Alvi, A.S.; Virk, A.A.; Amir, S. A Comprehensive Insight of Innovations and Recent Advancements in Nanocarriers for Nose-to-Brain Drug Targeting. Des. Monomers Polym. 2025, 28, 7–29. [Google Scholar] [CrossRef]
- Jiao, Y.; Yang, L.; Wang, R.; Song, G.; Fu, J.; Wang, J.; Gao, N.; Wang, H. Drug Delivery Across the Blood–Brain Barrier: A New Strategy for the Treatment of Neurological Diseases. Pharmaceutics 2024, 16, 1611. [Google Scholar] [CrossRef] [PubMed]
- Adepu, S.; Ramakrishna, S. Controlled Drug Delivery Systems: Current Status and Future Directions. Molecules 2021, 26, 5905. [Google Scholar] [CrossRef] [PubMed]
- Martín-Morales, C.; Caspani, S.; Desco, M.; Tavares De Sousa, C.; Gómez-Gaviro, M.V. Controlled Drug Release Systems for Cerebrovascular Diseases. Adv. Ther. 2025, 8, 2400239. [Google Scholar] [CrossRef]
- Song, Y.H.; De, R.; Lee, K.T. Emerging Strategies to Fabricate Polymeric Nanocarriers for Enhanced Drug Delivery across Blood-Brain Barrier: An Overview. Adv. Colloid. Interface Sci. 2023, 320, 103008. [Google Scholar] [CrossRef]
- Tang, K.; Tang, Z.; Niu, M.; Kuang, Z.; Xue, W.; Wang, X.; Liu, X.; Yu, Y.; Jeong, S.; Ma, Y.; et al. Allosteric Targeted Drug Delivery for Enhanced Blood-Brain Barrier Penetration via Mimicking Transmembrane Domain Interactions. Nat. Commun. 2025, 16, 3410. [Google Scholar] [CrossRef]
- Gao, X.; Xu, J.; Yao, T.; Liu, X.; Zhang, H.; Zhan, C. Peptide-Decorated Nanocarriers Penetrating the Blood-Brain Barrier for Imaging and Therapy of Brain Diseases. Adv. Drug Deliv. Rev. 2022, 187, 114362. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.I.; Lopes, C.M.; Amaral, M.H.; Costa, P.C. Surface-Modified Lipid Nanocarriers for Crossing the Blood-Brain Barrier (BBB): A Current Overview of Active Targeting in Brain Diseases. Colloids Surf. B Biointerfaces 2023, 221, 112999. [Google Scholar] [CrossRef] [PubMed]
- Rafati, N.; Zarepour, A.; Bigham, A.; Khosravi, A.; Naderi-Manesh, H.; Iravani, S.; Zarrabi, A. Nanosystems for Targeted Drug Delivery: Innovations and Challenges in Overcoming the Blood-Brain Barrier for Neurodegenerative Disease and Cancer Therapy. Int. J. Pharm. 2024, 666, 124800. [Google Scholar] [CrossRef]
- Toader, C.; Dumitru, A.V.; Eva, L.; Serban, M.; Covache-Busuioc, R.-A.; Ciurea, A.V. Nanoparticle Strategies for Treating CNS Disorders: A Comprehensive Review of Drug Delivery and Theranostic Applications. Int. J. Mol. Sci. 2024, 25, 13302. [Google Scholar] [CrossRef]
- Yang, J.; Zeng, H.; Luo, Y.; Chen, Y.; Wang, M.; Wu, C.; Hu, P. Recent Applications of PLGA in Drug Delivery Systems. Polymers 2024, 16, 2606. [Google Scholar] [CrossRef]
- Gabold, B.; Adams, F.; Brameyer, S.; Jung, K.; Ried, C.L.; Merdan, T.; Merkel, O.M. Transferrin-Modified Chitosan Nanoparticles for Targeted Nose-to-Brain Delivery of Proteins. Drug Deliv. Transl. Res. 2023, 13, 822–838. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, M.; Tomaro-Duchesneau, C.; Saha, S.; Prakash, S. Intranasal, siRNA Delivery to the Brain by TAT/MGF Tagged PEGylated Chitosan Nanoparticles. J. Pharm. 2013, 2013, 812387. [Google Scholar] [CrossRef]
- Florendo, M.; Figacz, A.; Srinageshwar, B.; Sharma, A.; Swanson, D.; Dunbar, G.L.; Rossignol, J. Use of Polyamidoamine Dendrimers in Brain Diseases. Molecules 2018, 23, 2238. [Google Scholar] [CrossRef]
- Gaitsch, H.; Hersh, A.M.; Alomari, S.; Tyler, B.M. Dendrimer Technology in Glioma: Functional Design and Potential Applications. Cancers 2023, 15, 1075. [Google Scholar] [CrossRef]
- Graván, P.; Aguilera-Garrido, A.; Marchal, J.A.; Navarro-Marchal, S.A.; Galisteo-González, F. Lipid-Core Nanoparticles: Classification, Preparation Methods, Routes of Administration and Recent Advances in Cancer Treatment. Adv. Colloid. Interface Sci. 2023, 314, 102871. [Google Scholar] [CrossRef] [PubMed]
- Eleraky, N.E.; Omar, M.M.; Mahmoud, H.A.; Abou-Taleb, H.A. Nanostructured Lipid Carriers to Mediate Brain Delivery of Temazepam: Design and In Vivo Study. Pharmaceutics 2020, 12, 451. [Google Scholar] [CrossRef] [PubMed]
- Dadkhah, M.; Afshari, S.; Samizadegan, T.; Shirmard, L.R.; Barin, S. Pegylated Chitosan Nanoparticles of Fluoxetine Enhance Cognitive Performance and Hippocampal Brain Derived Neurotrophic Factor Levels in a Rat Model of Local Demyelination. Exp. Gerontol. 2024, 195, 112533. [Google Scholar] [CrossRef]
- Neves, A.R.; Van Der Putten, L.; Queiroz, J.F.; Pinheiro, M.; Reis, S. Transferrin-Functionalized Lipid Nanoparticles for Curcumin Brain Delivery. J. Biotechnol. 2021, 331, 108–117. [Google Scholar] [CrossRef]
- Eldeeb, G.M.; Yousef, M.I.; Helmy, Y.M.; Aboudeya, H.M.; Mahmoud, S.A.; Kamel, M.A. The Protective Effects of Chitosan and Curcumin Nanoparticles against the Hydroxyapatite Nanoparticles-Induced Neurotoxicity in Rats. Sci. Rep. 2024, 14, 21009. [Google Scholar] [CrossRef]
- Yusuf, M.; Khan, M.; Khan, R.A.; Maghrabi, I.A.; Ahmed, B. Polysorbate-80-Coated, Polymeric Curcumin Nanoparticles for in Vivo Anti-Depressant Activity across BBB and Envisaged Biomolecular Mechanism of Action through a Proposed Pharmacophore Model. J. Microencapsul. 2016, 33, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhou, Y.; Jiang, Z.; He, C.; Wang, B.; Wang, Q.; Wang, Z.; Wu, T.; Chen, X.; Deng, Z.; et al. Bioinspired Polydopamine Nanoparticles as Efficient Antioxidative and Anti-Inflammatory Enhancers against UV-Induced Skin Damage. J. Nanobiotechnol. 2023, 21, 354. [Google Scholar] [CrossRef]
- Jiang, C.; Yang, X.; Huang, Q.; Lei, T.; Luo, H.; Wu, D.; Yang, Z.; Xu, Y.; Dou, Y.; Ma, X.; et al. Microglial-Biomimetic Memantine-Loaded Polydopamine Nanomedicines for Alleviating Depression. Adv. Mater. 2025, 37, e2417869. [Google Scholar] [CrossRef]
- Lv, Z.; Zhao, C.; Wu, X.; Chen, Y.; Zheng, C.; Zhang, X.; Xu, Y.; Zhu, L.; Wang, H.; Xie, G.; et al. Facile Engineered Macrophages-Derived Exosomes-Functionalized PLGA Nanocarrier for Targeted Delivery of Dual Drug Formulation against Neuroinflammation by Modulation of Microglial Polarization in a Post-Stroke Depression Rat Model. Biomed. Pharmacother. 2024, 179, 117263. [Google Scholar] [CrossRef]
- Fidelis, E.M.; Savall, A.S.P.; Da Luz Abreu, E.; Carvalho, F.; Teixeira, F.E.G.; Haas, S.E.; Bazanella Sampaio, T.; Pinton, S. Curcumin-Loaded Nanocapsules Reverses the Depressant-Like Behavior and Oxidative Stress Induced by β-Amyloid in Mice. Neuroscience 2019, 423, 122–130. [Google Scholar] [CrossRef]
- He, X.; Yang, L.; Wang, M.; Zhuang, X.; Huang, R.; Zhu, R.; Wang, S. Targeting the Endocannabinoid/CB1 Receptor System For Treating Major Depression Through Antidepressant Activities of Curcumin and Dexanabinol-Loaded Solid Lipid Nanoparticles. Cell Physiol. Biochem. 2017, 42, 2281–2294. [Google Scholar] [CrossRef]
- He, X.; Zhu, Y.; Wang, M.; Jing, G.; Zhu, R.; Wang, S. Antidepressant Effects of Curcumin and HU-211 Coencapsulated Solid Lipid Nanoparticles against Corticosterone-Induced Cellular and Animal Models of Major Depression. Int. J. Nanomed. 2016, 11, 4975–4990. [Google Scholar] [CrossRef] [PubMed]
- Khadrawy, Y.A.; Hosny, E.N.; Magdy, M.; Mohammed, H.S. Antidepressant Effects of Curcumin-Coated Iron Oxide Nanoparticles in a Rat Model of Depression. Eur. J. Pharmacol. 2021, 908, 174384. [Google Scholar] [CrossRef]
- Fahmy, H.M.; Aboalasaad, F.A.; Mohamed, A.S.; Elhusseiny, F.A.; Khadrawy, Y.A.; Elmekawy, A. Evaluation of the Therapeutic Effect of Curcumin-Conjugated Zinc Oxide Nanoparticles on Reserpine-Induced Depression in Wistar Rats. Biol. Trace Elem. Res. 2024, 202, 2630–2644. [Google Scholar] [CrossRef] [PubMed]
- Rubab, S.; Naeem, K.; Rana, I.; Khan, N.; Afridi, M.; Ullah, I.; Shah, F.A.; Sarwar, S.; Din, F.U.; Choi, H.-I.; et al. Enhanced Neuroprotective and Antidepressant Activity of Curcumin-Loaded Nanostructured Lipid Carriers in Lipopolysaccharide-Induced Depression and Anxiety Rat Model. Int. J. Pharm. 2021, 603, 120670. [Google Scholar] [CrossRef]
- Zeb, A.; Rana, I.; Choi, H.-I.; Lee, C.-H.; Baek, S.-W.; Lim, C.-W.; Khan, N.; Arif, S.T.; Sahar, N.U.; Alvi, A.M.; et al. Potential and Applications of Nanocarriers for Efficient Delivery of Biopharmaceuticals. Pharmaceutics 2020, 12, 1184. [Google Scholar] [CrossRef]
- Gul, M.; Shah, F.A.; Sahar, N.; Malik, I.; Din, F.U.; Khan, S.A.; Aman, W.; Choi, H.-I.; Lim, C.-W.; Noh, H.-Y.; et al. Formulation Optimization, in Vitro and in Vivo Evaluation of Agomelatine-Loaded Nanostructured Lipid Carriers for Augmented Antidepressant Effects. Colloids Surf. B Biointerfaces 2022, 216, 112537. [Google Scholar] [CrossRef]
- Zhou, J.-F.; Duan, L.; Wang, Y.-X.; Wang, C.-L.; Tian, M.-L.; Qi, X.-J.; Qiu, F. Design, Characterization of Resveratrol-Thermosensitive Hydrogel System (Res-THS) and Evaluation of Its Anti-Depressant Effect via Intranasal Administration. Mater. Des. 2022, 216, 110597. [Google Scholar] [CrossRef]
- Qi, X.-J.; Liu, X.-Y.; Tang, L.-M.-Y.; Li, P.-F.; Qiu, F.; Yang, A.-H. Anti-Depressant Effect of Curcumin-Loaded Guanidine-Chitosan Thermo-Sensitive Hydrogel by Nasal Delivery. Pharm. Dev. Technol. 2020, 25, 316–325. [Google Scholar] [CrossRef]
- Gao, M.; Hu, P.; Cai, Z.; Wu, Y.; Wang, D.; Hu, W.; Xu, X.; Zhang, Y.; Lu, X.; Chen, D.; et al. Identification of a Microglial Activation-Dependent Antidepressant Effect of Amphotericin B Liposome. Neuropharmacology 2019, 151, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Sheng, L.; Wei, Y.; Pi, C.; Cheng, J.; Su, Z.; Wang, Y.; Chen, T.; Wen, J.; Wei, Y.; Ma, J.; et al. Preparation and Evaluation of Curcumin Derivatives Nanoemulsion Based on Turmeric Extract and Its Antidepressant Effect. Int. J. Nanomed. 2023, 18, 7965–7983. [Google Scholar] [CrossRef] [PubMed]
- Suchiwa, P.-O.; Nudmamud-Thanoi, S.; Phoungpetchara, I.; Tiyaboonchai, W. Comparison of Curcumin-Based Nano-Formulations for Antidepressant Effects in an Animal Model. J. Drug Deliv. Sci. Technol. 2023, 88, 104901. [Google Scholar] [CrossRef]
- Zhang, F.; Nance, E.; Alnasser, Y.; Kannan, R.; Kannan, S. Microglial Migration and Interactions with Dendrimer Nanoparticles Are Altered in the Presence of Neuroinflammation. J. Neuroinflamm. 2016, 13, 65. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-K.; Jeon, S.W. Neuroinflammation and the Immune-Kynurenine Pathway in Anxiety Disorders. Curr. Neuropharmacol. 2018, 16, 574–582. [Google Scholar] [CrossRef]
- Salawi, A. Self-Emulsifying Drug Delivery Systems: A Novel Approach to Deliver Drugs. Drug Deliv. 2022, 29, 1811–1823. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Ettcheto, M.; Egea, M.A.; Espina, M.; Cano, A.; Calpena, A.C.; Camins, A.; Carmona, N.; Silva, A.M.; Souto, E.B.; et al. Memantine Loaded PLGA PEGylated Nanoparticles for Alzheimer’s Disease: In Vitro and in Vivo Characterization. J. Nanobiotechnol. 2018, 16, 32. [Google Scholar] [CrossRef]
- Kannan, S.; Dai, H.; Navath, R.S.; Balakrishnan, B.; Jyoti, A.; Janisse, J.; Romero, R.; Kannan, R.M. Dendrimer-Based Postnatal Therapy for Neuroinflammation and Cerebral Palsy in a Rabbit Model. Sci. Transl. Med. 2012, 4. [Google Scholar] [CrossRef]
- Keshavarz Shahbaz, S.; Koushki, K.; Keshavarz Hedayati, S.; McCloskey, A.P.; Kesharwani, P.; Naderi, Y.; Sahebkar, A. Polymer Nanotherapeutics: A Promising Approach toward Microglial Inhibition in Neurodegenerative Diseases. Med. Res. Rev. 2024, 44, 2793–2824. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Wang, H.; Bian, L.; Gao, F.; Yao, H.; Xie, J. Intranasal Delivery of Hydrophobic AC5216 Loaded Nanoemulsion into Brain To Alleviate Chronic Unpredictable Stress-Induced Depressive-like Behaviors. ACS Appl. Mater. Interfaces 2025, 17, 16533–16547. [Google Scholar] [CrossRef]
- He, J.; Yang, L.; Li, D.; Xie, J.; Zhou, G.; Zhou, R.; Li, Y.; Wei, G.; Gong, Z.; Li, L.; et al. Transferrin-Modified Carboxymethyl Chitosan-Chitosan Nanoparticles as an Efficient Delivery Carrier for Targeted Therapy of Depression. Int. J. Biol. Macromol. 2025, 286, 138352. [Google Scholar] [CrossRef]
- Wang, Y.-F.; Chen, C.-Y.; Lei, L.; Zhang, Y. Regulation of the Microglial Polarization for Alleviating Neuroinflammation in the Pathogenesis and Therapeutics of Major Depressive Disorder. Life Sci. 2025, 362, 123373. [Google Scholar] [CrossRef]
- Patel, R.B.; Rao, H.R.; Thakkar, D.V.; Patel, M.R. Comprehending the Potential of Metallic, Lipid, and Polymer-Based Nanocarriers for Treatment and Management of Depression. Neurochem. Int. 2022, 153, 105259. [Google Scholar] [CrossRef] [PubMed]
- Mutingwende, F.P.; Kondiah, P.P.D.; Ubanako, P.; Marimuthu, T.; Choonara, Y.E. Advances in Nano-Enabled Platforms for the Treatment of Depression. Polymers 2021, 13, 1431. [Google Scholar] [CrossRef]
- Ahmad, S.; Ahmad, S.; Ali, S.; Esa, M.; Khan, A.; Yan, H. Recent Advancements and Unexplored Biomedical Applications of Green Synthesized Ag and Au Nanoparticles: A Review. Int. J. Nanomed. 2024, 19, 3187–3215. [Google Scholar] [CrossRef]
- Leite, J.M.D.S.; Santana, L.M.D.; Nadvorny, D.; Abreu, B.O.D.; Rebouças, J.D.S.; Formiga, F.R.; Soares, M.F.D.L.R.; Soares-Sobrinho, J.L. Nanoparticle Design for Hydrophilic Drugs: Isoniazid Biopolymeric Nanostructure. J. Drug Deliv. Sci. Technol. 2023, 87, 104754. [Google Scholar] [CrossRef]
- Leite, J.M.D.S.; Oliveira, A.C.D.J.; Dourado, D.; Santana, L.M.D.; Medeiros, T.S.; Nadvorny, D.; Silva, M.L.R.; Rolim-Neto, P.J.; Moreira, D.R.M.; Formiga, F.R.; et al. Rifampicin-Loaded Phthalated Cashew Gum Nano-Embedded Microparticles Intended for Pulmonary Administration. Int. J. Biol. Macromol. 2025, 303, 140693. [Google Scholar] [CrossRef]
- Da Silva Leite, J.M.; Patriota, Y.B.G.; De La Roca, M.F.; Soares-Sobrinho, J.L. New Perspectives in Drug Delivery Systems for the Treatment ofTuberculosis. Curr. Med. Chem. 2022, 29, 1936–1958. [Google Scholar] [CrossRef] [PubMed]
- Hnamte, M.; Pulikkal, A.K. Biocompatible Polymeric Nanoparticles as Carriers for Anticancer Phytochemicals. Eur. Polym. J. 2024, 202, 112637. [Google Scholar] [CrossRef]
- Zheng, Y.; He, R.; Wang, P.; Shi, Y.; Zhao, L.; Liang, J. Exosomes from LPS-Stimulated Macrophages Induce Neuroprotection and Functional Improvement after Ischemic Stroke by Modulating Microglial Polarization. Biomater. Sci. 2019, 7, 2037–2049. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, K.; Singh, S.K.; Mishra, D. Evaluation of Safety and Efficacy of Brain Targeted Chitosan Nanoparticles of Minocycline. Int. J. Biol. Macromol. 2013, 59, 20–28. [Google Scholar] [CrossRef]
- Zhu, R.-R.; Cheng, L.-M.; He, X.-L.; Yang, L.; Wang, Z.-J.; Huang, R.-Q. Solid Lipid Nanoparticles Loading with Curcumin and Dexanabinol to Treat Major Depressive Disorder. Neural Regen. Res. 2021, 16, 537. [Google Scholar] [CrossRef]
- Omolo, C.A.; Ismail, E.A.; Amuhaya, E.K.; Fasiku, V.; Nwabuife, J.C.; Mohamed, M.; Gafar, M.A.; Kalhapure, R.; Govender, T. Revolutionizing Drug Delivery: The Promise of Surface Functionalized Dendrimers in Overcoming Conventional Limitations. Eur. Polym. J. 2025, 235, 114079. [Google Scholar] [CrossRef]
- Choudhary, A.J.; Mahajan, S.S.; Majumdar, A.S. Nose to Brain Delivery of Flurbiprofen from a Solid Lipid Nanoparticles-Based Thermosensitive in-Situ Gel. Neurosci. Appl. 2024, 3, 104062. [Google Scholar] [CrossRef]
- Yu, Y.; Yuan, M.; Qin, W.; Bai, H.; Liu, H.; Che, J. A Novel Strategy for Liposomal Drug Separation in Plasma by TiO2 Microspheres and Application in Pharmacokinetics. Int. J. Nanomed. 2023, 18, 1321–1334. [Google Scholar] [CrossRef] [PubMed]
- Zarezadeh, A.; Alizadeh, P.; Yourdkhani, A.; Motesadi Zarandi, F. Synthesis of Copper Ferrite-Decorated Bioglass Nanoparticles: Investigation of Magnetic Properties, Biocompatibility, and Drug Release Behaviour. Appl. Surf. Sci. 2025, 708, 163713. [Google Scholar] [CrossRef]
- Johnsen, K.B. On the Use of the Transferrin Receptor as a Target for Brain Drug Delivery. Ph.D. Thesis, Aalborg University, Aalborg, Denmark, 2017. [Google Scholar]
- Badr, A.; Daily, K.P.; Eltobgy, M.; Estfanous, S.; Tan, M.H.; Chun-Tien Kuo, J.; Whitham, O.; Carafice, C.; Gupta, G.; Amer, H.M.; et al. Microglia-Targeted Inhibition of miR-17 via Mannose-Coated Lipid Nanoparticles Improves Pathology and Behavior in a Mouse Model of Alzheimer’s Disease. Brain Behav. Immun. 2024, 119, 919–944. [Google Scholar] [CrossRef]
- Zhou, F.; He, Y.; Zhang, M.; Gong, X.; Liu, X.; Tu, R.; Yang, B. Polydopamine(PDA)-Coated Diselenide-Bridged Mesoporous Silica-Based Nanoplatform for Neuroprotection by Reducing Oxidative Stress and Targeting Neuroinflammation in Intracerebral Hemorrhage. J. Nanobiotechnol. 2024, 22, 731. [Google Scholar] [CrossRef]
- Tang, T.; Valenzuela, A.; Petit, F.; Chow, S.; Leung, K.; Gorin, F.; Louie, A.Y.; Dhenain, M. In Vivo MRI of Functionalized Iron Oxide Nanoparticles for Brain Inflammation. Contrast Media Mol. Imaging 2018, 2018, 3476476. [Google Scholar] [CrossRef]
- Bhattacharjee, S. Craft of Co-Encapsulation in Nanomedicine: A Struggle To Achieve Synergy through Reciprocity. ACS Pharmacol. Transl. Sci. 2022, 5, 278–298. [Google Scholar] [CrossRef]
- Cerqueira, S.R.; Ayad, N.G.; Lee, J.K. Neuroinflammation Treatment via Targeted Delivery of Nanoparticles. Front. Cell. Neurosci. 2020, 14, 576037. [Google Scholar] [CrossRef]
- Minaiyan, P.; Varshosaz, J.; Minaiyan, M. Colon Delivery of Agomelatine Nanoparticles in the Treatment of TNBS Induced Ulcerative Colitis. Drug Deliv. Transl. Res. 2025, 15, 3137–3148. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, J.T.-W.; Li, N.; Zhu, X.; Li, Y.; Bansal, S.; Wang, Y.; Al-Jamal, K.T. Intranasal Administration of Edaravone Nanoparticles Improves Its Stability and Brain Bioavailability. J. Control. Release 2023, 359, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Hegde, M.; Kumar, A.; Girisa, S.; Alqahtani, M.S.; Abbas, M.; Goel, A.; Hui, K.M.; Sethi, G.; Kunnumakkara, A.B. Exosomal Noncoding RNA-Mediated Spatiotemporal Regulation of Lipid Metabolism: Implications in Immune Evasion and Chronic Inflammation. Cytokine Growth Factor. Rev. 2023, 73, 114–134. [Google Scholar] [CrossRef] [PubMed]
- Altammar, K.A. A Review on Nanoparticles: Characteristics, Synthesis, Applications, and Challenges. Front. Microbiol. 2023, 14, 1155622. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim Khan, K.S.; Khan, I. Nanoparticles: Properties, Applications and Toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]

| Drug Delivery Systems | Compound Used | Physicochemical Parameters | In Vitro Activity | In Vivo Activity | Key Mechanism Related to Microglia/Inflammation | Reference |
|---|---|---|---|---|---|---|
| Polymeric nanoparticle | Curcumin | 194.3 ± 14.8 nm. 19.5 ± 2.6 mV. Drug release: 83.78 ± 7.43 to 95.56 ± 4.67% in 144 h | NA | ↓ immobility (FST/TST), ↑ SOD/catalase activity | NF-κB inhibition; ↓ TNF-α/↓ IL-1β; antioxidant activity (↑SOD/CAT) reducing microglial activation. | Yusuf (2016) [88] |
| Polymeric nanoparticle | Dopamine hydrochloride | 244 nm −48 mV | ≥90% DPPH scavenging at 20 µg/mL; ↓ ROS in LPS-challenged PC12 cells | NA | Reduction of ROS; protection against LPS-induced oxidative stress; attenuation of pro-inflammatory signaling in microglia. | Zhang (2023) [89] |
| Polymeric nanoparticle | Memantine | 163.5 ± 1.5 nm −54.3 ± 2.2 mV | ↓ ROS; polarized microglia from M1 to M2; ↓ CD86, TNF-α and IL-2; ↑ CD206, TGF-β, IL-10 | ↑ Brain/hippocampal uptake (≈1.3× vs. PDA); >memantine monotherapy at lower/less-frequent dosing; minimal toxicity; ↓ ROS/IL-1β/IL-2; ↑ IL-10; ↑neurogenesis; restored synaptic plasticity/neuroprotection. | NMDAR blockade/modulation; ↓ ROS; M1 → M2 reprogramming (↓ CD86, TNF-α; ↑ CD206, IL-10) and neuroprotective effects. | Jiang et al. (2025) [90] |
| Polymeric nanoparticle | Celastrol and minocycline | 132.0 nm −35.5 mV | M1 → M2 shift in LPS-BV-2: ↓ CD80/iNOS; ↑ CD206/Arg1; superior anti-inflammatory efficacy vs. other nanoformulations. | Reversed depressive-like behavior and attenuated weight loss in POSD rats;↓ iNOS/CD86, ↑ Arg-1/CD206 (M1 → M2), consistent with antidepressant efficacy | Induction of M1 → M2 (↓ iNOS/CD80, ↑ Arg-1/CD206); suppression of pro-inflammatory cytokines; inhibition of MAPK/NF-κB pathways. | Lv et al. (2024) [91] |
| Solid lipid nanoparticle | Curcumin | 291 to 312 nm 22–36 mV | NA | Reversed the effects of Aβ25–35 (↑ 663.3% immobility in TST/FST), normalized SOD/CAT levels | Antioxidant effect + SOD/CAT normalization; indirect suppression of inflammatory markers → reduction of microglial activation. | Fidelis et al. (2019) [92] |
| Solid lipid nanoparticle | Curcumin and dexanabinol | −22.6 ± 0.9 mV | ↑ DA/5-HT; reduction in cellular apoptosis | ↑ DA/5-HT levels and mRNA expression of CB1, p-MEK1, and p-ERK1/2 in the hippocampus and striatum | Increased monoamines (DA/5-HT) and reduced apoptosis; modulation of CB1 pathways → indirect impact on microglia and inflammation. | He et al. (2017) [93] |
| Solid lipid nanoparticles | HU-211 and curcumin | −21.7 ± 0.4 mV Drug release: 77% in 7 days | ↑ expression of CB1, p-MEK1, and p-ERK1/2; cellular uptake: 99% | ↑ DA levels | Activation of MAPK/ERK signaling (p-MEK1/p-ERK1/2) and increased DA; reduction of neuronal stress with an indirect anti-inflammatory effect. | He et al. (2016) [94] |
| Magnetic nanoparticles | Curcumin | 15 ± 3 nm −25 mV | NA | ↑ in Na+, K+-ATPase activity and ↑ levels of monoamine neurotransmitters. Prevention of excitotoxicity mediated by NMDA receptor overactivation | Improved enzymatic activity (Na+/K+-ATPase) and increased monoamines; reduced excitotoxicity and indirect anti-inflammatory effect on microglia. | Khadrawy et al. (2021) [95] |
| Magnetic nanoparticles | Curcumin |
342 ± 22.3
−25.6 ± 4.61 mV | NA | ↓ malondialdehyde, ↑ reduced glutathione (GSH) and catalase, and elevated concentrations of 5-HT and NE | Reduction of lipid peroxidation (↓ MDA), ↑ GSH/CAT; normalization of 5-HT and NE, antioxidant effects that attenuate microglial activation. | Fahmy et al. (2024) [96] |
| Nanostructured lipid carriers | Curcumin | 147.8 ± 10.4 nm −32.8 ± 1.4 mV Drug release: ~54% and ~73% at 12 h and 24 h. respectively | NA | Suppression of p-NF-κB, TNF-α, and COX-2 expression | Suppression of p-NF-κB, ↓ TNF-α and ↓ COX-2 (in vivo), direct anti-inflammatory action in brain tissue. | Rubab et al. (2021) [97] |
| Nanostructured lipid carriers | Curcumin | 147.8 ± 10.4 nm −32.8 ± 1.4 mV | NA | ↓ LPS-induced neurodegenerative damage, ↑ tissue architecture and cellular integrity; ↓ p-NF-κB, TNF-α, and COX-2 induced by LPS in the brain | Protection against LPS-induced neurodegenerative damage; ↓ p-NF-κB, TNF-α, COX-2—reduction of neuroinflammation. | Zeb et al. (2020) [98] |
| Nanostructured lipid carriers | Agomelatine | 99.8 ± 2.6 nm 23.2 ± 1.2 mV | NA | ↓ LPS-induced neuroinflammation, TNF-α, and COX-2 | Suppression of LPS-induced inflammation (↓ TNF-α, ↓ COX-2); indirect anti-inflammatory effect mediated by improved neuronal signaling. | Gul et al. (2022) [99] |
| Hydrogel | Resveratrol | Res ~35% and Res-THS ~59% in 10 h | NA | Immobility time in the FST (~180 s → ~95 s); Corticosterone levels (~160 → ~100 ng/mL); 5-HT, DA, and NA levels in the brain. | Antioxidant action + reduction of the HPA axis (↓ corticosterone); normalization of monoamines → anti-inflammatory effect and reduction of microglial activation. | Zhou et al. (2022) [100] |
| Hydrogel | Curcumin | 55.54% release in 10 h. | NA | ↓ immobility time in the forced swim test and tail suspension test; reversal of ptosis and hypothermia; increased levels of 5-HT, NE, and DA in the brain; effect comparable to fluoxetine | ↓ Immobility (FST/TST) with increased monoamines; antioxidant/anti-inflammatory effect that contributes to decreased microglial activation. | Qi et al. (2020) [101] |
| Liposome | Amphotericin B | NA | NA | ↓ depressive behaviors in a dose-dependent manner (not observed with isolated drug) | Activation/modulation of microglia is necessary for the antidepressant effect (minocycline/PLX3397 blocks the effect); microglia-dependent mechanism (↑ IL-1β/IL-6 modulation). | Gao et al. (2019) [102] |
| Nanoemulsion | Curcumin | 116.0 ± 0.30 nm −11.6 ± 1.23 mV Drug release: 36.51 ± 3.24% release in HCl and 44.90 ± 2.47% in PBS over 48 h | TUR exhibited the highest antioxidant activity (ABTS•+), followed by Curcumin and Vitamin C, with IC50 values of 17.9, 29.1, and 57 µg/mL, respectively | TUR-NE (CUMS): ↑ Sucrose preference (77.5% vs. 54.0%); ↓ FST immobility (p < 0.01); ↓ NSFT latency; ↑ 5-HT (plasma 21.7 vs. 16.3 ng/mL; brain 46.9 vs. 44.0 ng/mL) | Increased preference for sucrose and ↓ FST; systemic antioxidant/anti-inflammatory profile that reduces pro-inflammatory brain signals. | Sheng et al. (2023) [103] |
| Self-emulsifying system | Curcumin |
150 nm
Drug 100% released within 5 min | NA | 7.4 ± 0.2 mL (sucrose); 103.4 ± 5.8 s (FST); 247 ± 3.1 g (weight); Partial neuroprotective and hepatoprotective effects | Fast delivery and increased bioavailability of curcumin → antioxidant and anti-inflammatory effects with reduced microglial activation. | Suchiwa Pan et al. (2023) [104] |
| Dendrimers | Polyamidoamine | 4 nm | microglia took up PAMAM dendrimers faster and to a greater extent than healthy controls (~1.6× higher at 4 h; ~80% of microglial cells contained dendrimers) | Selective accumulation in activated microglia; ↑ motor function; ↓ neuroinflammation | Selective accumulation in activated microglia; targeted delivery (PAMAM–NAC) → direct reduction of neuroinflammation and functional improvement via microglial modulation. | Zhang et al. (2016) [105] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Silva, P.R.d.; Barbosa, N.M.M.V.; Leite, J.M.d.S.; Alves, L.P.; Andrade, J.C.d.; Formiga, A.L.D.; Uchôa, A.F.C.; Neri, L.C.D.; Dias, A.L.; Oliveira-Golzio, A.M.F.d.; et al. Microglia-Targeted Nanotherapeutics in Major Depressive Disorder: An Integrative Perspective on Neuroinflammation and Drug Delivery. Pharmaceutics 2026, 18, 27. https://doi.org/10.3390/pharmaceutics18010027
Silva PRd, Barbosa NMMV, Leite JMdS, Alves LP, Andrade JCd, Formiga ALD, Uchôa AFC, Neri LCD, Dias AL, Oliveira-Golzio AMFd, et al. Microglia-Targeted Nanotherapeutics in Major Depressive Disorder: An Integrative Perspective on Neuroinflammation and Drug Delivery. Pharmaceutics. 2026; 18(1):27. https://doi.org/10.3390/pharmaceutics18010027
Chicago/Turabian StyleSilva, Pablo R. da, Nayana M. M. V. Barbosa, Joandra M. da Silva Leite, Larissa P. Alves, Jéssica C. de Andrade, Allessya L. D. Formiga, Ana Flávia C. Uchôa, Luiza C. D. Neri, Arthur Lins Dias, Adriana M. F. de Oliveira-Golzio, and et al. 2026. "Microglia-Targeted Nanotherapeutics in Major Depressive Disorder: An Integrative Perspective on Neuroinflammation and Drug Delivery" Pharmaceutics 18, no. 1: 27. https://doi.org/10.3390/pharmaceutics18010027
APA StyleSilva, P. R. d., Barbosa, N. M. M. V., Leite, J. M. d. S., Alves, L. P., Andrade, J. C. d., Formiga, A. L. D., Uchôa, A. F. C., Neri, L. C. D., Dias, A. L., Oliveira-Golzio, A. M. F. d., Xavier-Júnior, F. H., Castro, R. D. d., Felipe, C. F. B., Scotti, M. T., & Scotti, L. (2026). Microglia-Targeted Nanotherapeutics in Major Depressive Disorder: An Integrative Perspective on Neuroinflammation and Drug Delivery. Pharmaceutics, 18(1), 27. https://doi.org/10.3390/pharmaceutics18010027








