Dual Core-Shell Loaded Lipid-Polymer Hybrid Nanoparticles as Combination Anti-Infective Delivery Platforms
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Empty LPHNP Manufacture and Characterization
2.3. CTX-Loaded LPHNP Manufacture and Characterization
2.4. CTX and RN7IN6 Co-Loaded LPHNP Manufacturing and Characterization
2.5. Physical Characterization of Nanoparticles
2.6. Nanoparticle Purification
2.7. Quantification of CTX and RN7IN6
2.8. Evaluation of CTX and RN7IN6 EE% and Incorporated Drug Concentration
2.9. CTX and RN7IN6 Release Kinetics
2.10. Antimicrobial Efficacy Assessment
2.10.1. Bacteria
2.10.2. Free CTX and RN7IN6 Antibacterial Activity
2.10.3. CTX and RN7IN6 Synergism Assessment (Microdilution Checkerboard Assay)
- FICI < 0.5: synergistic activity;
- 0.5 < FICI < 1: partial synergism;
- FICI = 1: addition;
- 1 < FICI < 4: indifference;
- FICI ≥ 4: antagonism.
2.10.4. Antimicrobial Activity of Empty and Loaded LPHNPs
- CTX-CNPs and CTX-LPHNPs were diluted to reach a CTX concentration of 512 μg/mL for S. aureus and 16 μg/mL for E. coli;
- Co-loaded LPHNPs were diluted based on RN7IN6 content to 32× MIC of free RN7IN6, corresponding to 512 μg/mL for S. aureus and 1024 μg/mL for E. coli. The corresponding CTX concentration was 765 μg/mL and 1530 μg/mL for S. aureus and E. coli, respectively;
- Empty LPHNPs were diluted in the same manner as for CTX-CNPs and CTX-LPHNPs.
2.11. Statistical Analysis
3. Results and Discussion
3.1. Empty LPHNP Manufacture and Characterization
3.2. Fabrication and Characterization of CTX-Loaded LPHNPs
3.3. Co-Loaded LPHNP Production: Optimization of RN7IN6 Loading Concentration
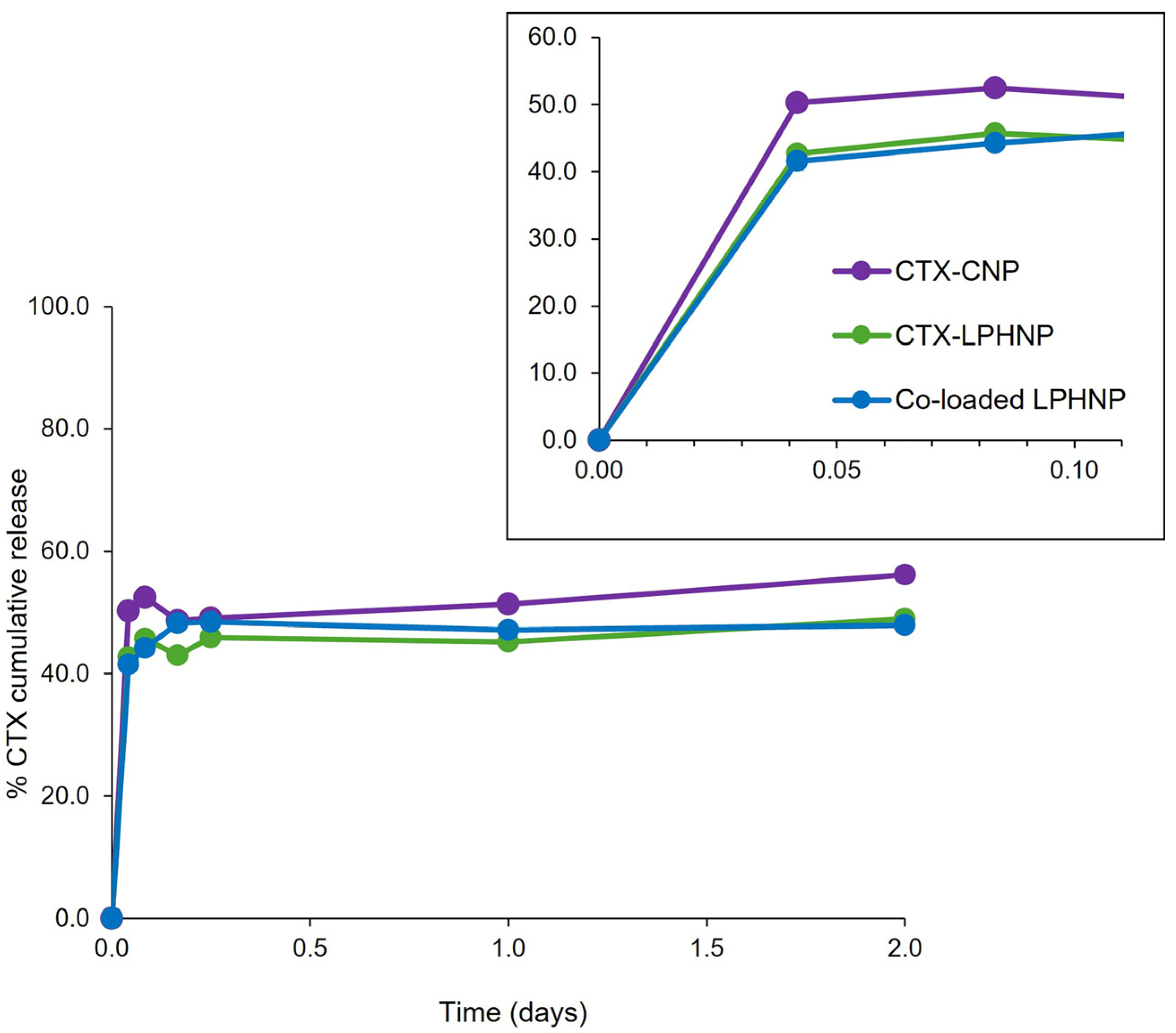
3.4. CTX and RN7IN6 Release Kinetics
3.5. Antibacterial Efficacy of CTX and RN7IN6 in Combination
3.6. Co-Loaded LPHNPs: Preliminary Assessment of Antibacterial Efficacy
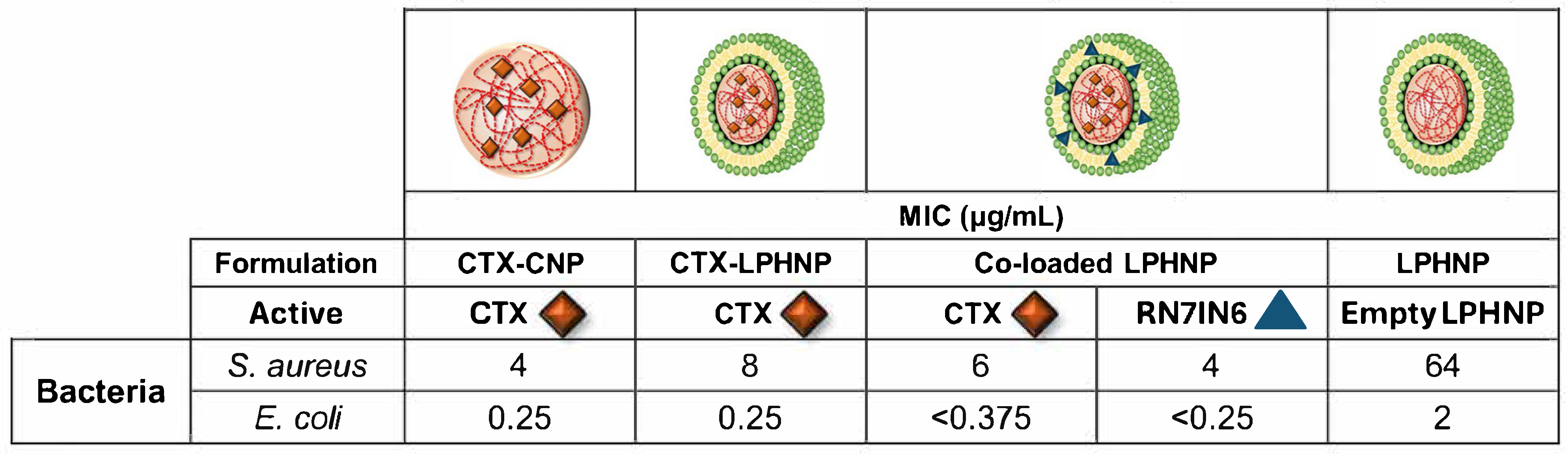
3.6.1. Impact of CNPs and LPHNPs on CTX Activity
3.6.2. Impact of LPHNP Lipid Shell on CTX Activity
3.6.3. Impact of RN7IN6 on CTX Activity in Co-Loaded LPHNPs
3.6.4. Impact of Co-Loaded LPHNP Delivery on RN7IN6
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMP | Antimicrobial peptide |
| AMR | Antimicrobial resistance |
| CL | 1′,3′-bis [1,2-dioleoyl-sn-glycero-3-phospho]- glycerol, tetraoleoyl cardiolipin |
| CNP | Chitosan nanoparticle |
| CTX | Cefotaxime |
| DMF | N,N-dimethylformamide |
| EE% | Encapsulation efficiency |
| FIC | Fractional inhibitory concentration |
| FICI | Fractional inhibitory concentration index |
| FRR | Flow rate ratio |
| LPHNP | Lipid-polymer hybrid nanoparticle |
| MHB2 | Mueller Hinton broth 2 |
| MIC | Minimum inhibitory concentration |
| PBS | Phosphate-buffered saline |
| PDI | Polydispersity index |
| POPE | 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine |
| POPG | 1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-(1′-rac-glycerol) (sodium salt) |
| TFA | Trifluoroacetic acid |
| TFR | Total flow rate |
References
- O’Neill, J. Review on Antimicrobial Resistance. Tackling Drug Resistant Infections Globally: Final Report and Recommendations; UK Prime Minister: London, UK, 2016. [Google Scholar]
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Maher, C.; Hassan, K.A. The Gram-negative permeability barrier: Tipping the balance of the in and the out. mBio 2023, 14, e01205-23. [Google Scholar] [CrossRef]
- Gargate, N.; Laws, M.; Rahman, K.M. Current economic and regulatory challenges in developing antibiotics for Gram-negative bacteria. npj Antimicrob. Resist. 2025, 3, 50. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. 2023 Antibacterial Agents in Clinical and Preclinical Development: An Overview and Analysis; World Health Organisation: Geneva, Switzerland, 2024. [Google Scholar]
- Gajbhiye, K.R.; Salve, R.; Narwade, M.; Sheikh, A.; Kesharwani, P.; Gajbhiye, V. Lipid polymer hybrid nanoparticles: A custom-tailored next-generation approach for cancer therapeutics. Mol. Cancer 2023, 22, 160. [Google Scholar] [CrossRef]
- Mohanty, A.; Uthaman, S.; Park, I.-K. Utilization of polymer-lipid hybrid nanoparticles for targeted anti-cancer therapy. Molecules 2020, 25, 4377. [Google Scholar] [CrossRef]
- Shen, S.; Li, T.; Fan, J.; Shao, Q.; Dong, H.; Xu, X.; Mo, R. Lipid-polymer hybrid nanoparticle with cell-distinct drug release for treatment of stemness-derived resistant tumor. Acta Pharm. Sin. B 2023, 13, 1262–1273. [Google Scholar] [CrossRef]
- Baek, J.-S.; Tan, C.H.; Ng, N.K.J.; Yeo, Y.P.; Rice, S.A.; Loo, S.C.J. A programmable lipid-polymer hybrid nanoparticle system for localized, sustained antibiotic delivery to Gram-positive and Gram-negative bacterial biofilms. Nanoscale Horiz. 2018, 3, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Borbolla, A.; García-Hevia, L.; Fanarraga, M.L. Cell membrane-coated nanoparticles for precision medicine: A comprehensive review of coating techniques for tissue-specific therapeutics. Int. J. Mol. Sci. 2024, 25, 2071. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Xu, L.; Yang, B.; Fan, F.; Yang, L. Kill the real with the fake: Eliminate intracellular Staphylococcus aureus using nanoparticle coated with its extracellular vesicle membrane as active-targeting drug carrier. ACS Infect. Dis. 2019, 5, 218–227. [Google Scholar] [CrossRef]
- Tomlinson, S.; Taylor, P.W.; Luzio, J.P. Transfer of phospholipid and protein into the envelope of Gram-negative bacteria by liposome fusion. Biochemistry 1989, 28, 8303–8311. [Google Scholar] [CrossRef]
- Wu, S.; Huang, Y.; Yan, J.; Li, Y.; Wang, J.; Yang, Y.Y.; Yuan, P.; Ding, X. Bacterial outer membrane-coated mesoporous silica nanoparticles for targeted delivery of antibiotic rifampicin against Gram-negative bacterial infection in vivo. Adv. Funct. Mater. 2021, 31, 2103442. [Google Scholar] [CrossRef]
- Khan, M.; Madni, A.; Tahir, N.; Parveen, F.; Khan, S.; Jan, N.; Ali, A.; Abdurrahim, M.; Farooq, U.; Khan, M. Co-delivery of curcumin and cisplatin to enhance cytotoxicity of cisplatin using lipid-chitosan hybrid nanoparticles. Int. J. Nanomed. 2020, 15, 2207–2217. [Google Scholar] [CrossRef] [PubMed]
- Murugaiyan, J.; Kumar, P.A.; Rao, G.S.; Iskandar, K.; Hawser, S.; Hays, J.P.; Mohsen, Y.; Adukkadukkam, S.; Awuah, W.A.; Jose, R.A.M.; et al. Progress in alternative strategies to combat antimicrobial resistance: Focus on antibiotics. Antibiotics 2022, 11, 200. [Google Scholar] [CrossRef]
- Rajamanickam, K.; Yang, J.; Sakharkar, M.K. A novel antimicrobial–phytochemical conjugate with antimicrobial activity against Streptococcus uberis, Enterococcus faecium, and Enterococcus faecalis. Front. Pharmacol. 2019, 10, 1405. [Google Scholar] [CrossRef] [PubMed]
- Jindal, H.M.; Le, C.F.; Mohd Yusof, M.Y.; Velayuthan, R.D.; Lee, V.S.; Zain, S.M.; Isa, D.M.; Sekaran, S.D. Antimicrobial activity of novel synthetic peptides serived from indolicidin and ranalexin against Streptococcus pneumoniae. PLoS ONE 2015, 10, e0128532. [Google Scholar] [CrossRef]
- D’Aloisio, V.; Schofield, A.; Kendall, D.A.; Hutcheon, G.A.; Coxon, C.R. The development and optimisation of an HPLC-based in vitro serum stability assay for a calcitonin gene-related peptide receptor antagonist peptide. J. Pept. Sci. 2024, 30, e3539. [Google Scholar] [CrossRef]
- Graef, F.; Gordon, S.; Lehr, C.-M. Anti-infectives in drug delivery—Overcoming the Gram-negative bacterial cell envelope. Curr. Top. Microbiol. Immunol. 2016, 398, 475–496. [Google Scholar] [CrossRef]
- Roces, C.B.; Lou, G.; Jain, N.; Abraham, S.; Thomas, A.; Halbert, G.W.; Perrie, Y. Manufacturing considerations for the development of lipid nanoparticles using microfluidics. Pharmaceutics 2020, 12, 1095. [Google Scholar] [CrossRef] [PubMed]
- Needs, S.H.; Saiprom, N.; Rafaque, Z.; Imtiaz, W.; Chantratita, N.; Runcharoen, C.; Thammachote, J.; Anun, S.; Peacock, S.J.; Ray, P.; et al. Miniaturised broth microdilution for simplified antibiotic susceptibility testing of Gram negative clinical isolates using microcapillary devices. Analyst 2022, 147, 3558–3569. [Google Scholar] [CrossRef]
- Bellio, P.; Fagnani, L.; Nazzicone, L.; Celenza, G. New and simplified method for drug combination studies by checkerboard assay. MethodsX 2021, 8, 101543. [Google Scholar] [CrossRef]
- Fatsis-Kavalopoulos, N.; Sánchez-Hevia, D.L.; Andersson, D.I. Beyond the FIC index: The extended information from fractional inhibitory concentrations (FICs). J. Antimicrob. Chemother. 2024, 79, 2394–2396. [Google Scholar] [CrossRef]
- Gui, K.; Zhang, X.; Chen, F.; Ge, Z.; Zhang, S.; Qi, X.; Sun, J.; Yu, Z. Lipid-polymer nanoparticles with CD133 aptamers for targeted delivery of all-trans retinoic acid to osteosarcoma initiating cells. Biomed. Pharmacother. 2019, 111, 751–764. [Google Scholar] [CrossRef]
- Li, P.; Chen, X.; Shen, Y.; Li, H.; Zou, Y.; Yuan, G.; Hu, P.; Hu, H. Mucus penetration enhanced lipid polymer nanoparticles improve the eradication rate of Helicobacter pylori biofilm. J. Control. Release 2019, 300, 52–63. [Google Scholar] [CrossRef]
- Lin, J.; Cai, Q.; Tang, Y.; Xu, Y.; Wang, Q.; Li, T.; Xu, H.; Wang, S.; Fan, K.; Liu, Z.; et al. PEGylated lipid bilayer coated mesoporous silica nanoparticles for co-delivery of paclitaxel and curcumin: Design, characterization and its cytotoxic effect. Int. J. Pharm. 2018, 536, 272–282. [Google Scholar] [CrossRef]
- Liu, D.; Lian, Y.; Fang, Q.; Liu, L.; Zhang, J.; Li, J. Hyaluronic-acid-modified lipid-polymer hybrid nanoparticles as an efficient ocular delivery platform for moxifloxacin hydrochloride. Int. J. Biol. Macromol. 2018, 116, 1026–1036. [Google Scholar] [CrossRef]
- Hu, Y.; Hoerle, R.; Ehrich, M.; Zhang, C. Engineering the lipid layer of lipid–PLGA hybrid nanoparticles for enhanced in vitro cellular uptake and improved stability. Acta Biomater. 2015, 28, 149–159. [Google Scholar] [CrossRef]
- Wan, F.; Nylander, T.; Klodzinska, S.N.; Foged, C.; Yang, M.; Baldursdottir, S.G.; Nielsen, H.M. Lipid shell-enveloped polymeric nanoparticles with high integrity of lipid shells improve mucus penetration and interaction with cystic fibrosis-related bacterial biofilms. ACS Appl. Mater. Interfaces 2018, 10, 10678–10687. [Google Scholar] [CrossRef]
- Jiang, L.; Lee, H.W.; Loo, S.C.J. Therapeutic lipid-coated hybrid nanoparticles against bacterial infections. RSC Adv. 2020, 10, 8497–8517. [Google Scholar] [CrossRef]
- Tormena, N.; Pilizota, T.; Voïtchovsky, K. A minimalist model lipid system mimicking the biophysical properties of Escherichia coli’s inner membrane. Langmuir 2025, 41, 12301–12310. [Google Scholar] [CrossRef]
- Drost, M.; Diamanti, E.; Fuhrmann, K.; Goes, A.; Shams, A.; Haupenthal, J.; Koch, M.; Hirsch, A.K.H.; Fuhrmann, G. Bacteriomimetic liposomes improve antibiotic activity of a novel energy-coupling factor transporter inhibitor. Pharmaceutics 2022, 14, 4. [Google Scholar] [CrossRef]
- Paez-Perez, M.; Russell, I.A.; Cicuta, P.; Di Michele, L. Modulating membrane fusion through the design of fusogenic DNA circuits and bilayer composition. Soft Matter 2022, 18, 7035–7044. [Google Scholar] [CrossRef]
- Zhang, L.; Chan, J.M.; Gu, F.X.; Rhee, J.-W.; Wang, A.Z.; Radovic-Moreno, A.F.; Alexis, F.; Langer, R.; Farokhzad, O.C. Self-assembled lipid–polymer hybrid nanoparticles: A robust drug delivery platform. ACS Nano 2008, 2, 1696–1702. [Google Scholar] [CrossRef]
- Anwer, M.K.; Ali, E.A.; Iqbal, M.; Ahmed, M.M.; Aldawsari, M.F.; Saqr, A.A.; Ansari, M.N.; Aboudzadeh, M.A. Development of sustained release baricitinib loaded lipid-polymer hybrid nanoparticles with improved oral bioavailability. Molecules 2022, 27, 168. [Google Scholar] [CrossRef]
- Javaid, S.; Ahmad, N.M.; Mahmood, A.; Nasir, H.; Iqbal, M.; Ahmad, N.; Irshad, S. Cefotaxime loaded polycaprolactone based polymeric nanoparticles with antifouling properties for in-vitro drug release applications. Polymers 2021, 13, 2180. [Google Scholar] [CrossRef]
- Shafique, M.; Ur Rehman, M.; Kamal, Z.; Alzhrani, R.M.; Alshehri, S.; Alamri, A.H.; Bakkari, M.A.; Sabei, F.Y.; Safhi, A.Y.; Mohammed, A.M.; et al. Formulation development of lipid polymer hybrid nanoparticles of doxorubicin and its in-vitro, in-vivo and computational evaluation. Front. Pharmacol. 2023, 14, 1025013. [Google Scholar] [CrossRef]
- Jindal, H.M.; Zandi, K.; Ong, K.C.; Velayuthan, R.D.; Rasid, S.M.; Samudi Raju, C.; Sekaran, S.D. Mechanisms of action and in vivo antibacterial efficacy assessment of five novel hybrid peptides derived from indolicidin and ranalexin against Streptococcus pneumoniae. PeerJ 2017, 5, e3887. [Google Scholar] [CrossRef]
- Lee, A.L.Z.; Wang, Y.; Ye, W.-H.; Yoon, H.S.; Chan, S.Y.; Yang, Y.-Y. Efficient intracellular delivery of functional proteins using cationic polymer core/shell nanoparticles. Biomaterials 2008, 29, 1224–1232. [Google Scholar] [CrossRef]
- Parashar, S.; Chauhan, C.; Rajasekharan, A.; Rautela, J.; Jain, T.; Raza, K. An augmented method for collecting PLGA nanoparticles and the fabrication of 1,3,4,6-tetra-O-acetyl-2-azido-2-deoxy-D-glucopyranose (Ac42AzGlc)-loaded PLGA nanoparticles for efficient and prospective in vivo metabolic processing. Front. Bioeng. Biotechnol. 2022, 10, 833456. [Google Scholar] [CrossRef]
- Filippov, S.K.; Khusnutdinov, R.; Murmiliuk, A.; Inam, W.; Zakharova, L.Y.; Zhang, H.; Khutoryanskiy, V.V. Dynamic light scattering and transmission electron microscopy in drug delivery: A roadmap for correct characterization of nanoparticles and interpretation of results. Mater. Horiz. 2023, 10, 5354–5370. [Google Scholar] [CrossRef]
- Maguire, C.M.; Rösslein, M.; Wick, P.; Prina-Mello, A. Characterisation of particles in solution—A perspective on light scattering and comparative technologies. Sci. Technol. Adv. Mater. 2018, 19, 732–745. [Google Scholar] [CrossRef]
- Torcato, I.M.; Huang, Y.-H.; Franquelim, H.G.; Gaspar, D.; Craik, D.J.; Castanho, M.A.R.B.; Troeira Henriques, S. Design and characterization of novel antimicrobial peptides, R-BP100 and RW-BP100, with activity against Gram-negative and Gram-positive bacteria. Biochim. Biophys. Acta Biomembr. 2013, 1828, 944–955. [Google Scholar] [CrossRef]
- Hsu, J.C.Y.; Yip, C.M. Molecular dynamics simulations of indolicidin association with model lipid bilayers. Biophys. J. 2007, 92, L100–L102. [Google Scholar] [CrossRef]
- Nielsen, J.E.; Bjørnestad, V.A.; Lund, R. Resolving the structural interactions between antimicrobial peptides and lipid membranes using small-angle scattering methods: The case of indolicidin. Biophys. J. 2018, 14, 8750–8763. [Google Scholar] [CrossRef]
- Lee, H.W.; Kharel, S.; Loo, S.C.J. Lipid-coated hybrid nanoparticles for enhanced bacterial biofilm penetration and antibiofilm efficacy. ACS Omega 2022, 7, 35814–35824. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, P.; Su, W.; Wang, S.; Liao, Z.; Niu, R.; Chang, J. PLGA/polymeric liposome for targeted drug and gene co-delivery. Biomaterials 2010, 31, 8741–8748. [Google Scholar] [CrossRef]
- Murzyn, K.; Róg, T.; Pasenkiewicz-Gierula, M. Phosphatidylethanolamine-phosphatidylglycerol bilayer as a model of the inner bacterial membrane. Biophys. J. 2005, 88, 1091–1103. [Google Scholar] [CrossRef]
- Unsay, J.D.; Cosentino, K.; Subburaj, Y.; García-Sáez, A.J. Cardiolipin effects on membrane structure and dynamics. Langmuir 2013, 29, 15878–15887. [Google Scholar] [CrossRef]
- World Health Organisation. WHO Bacterial Priority Pathogens List, 2024: Bacterial Pathogens of Public Health Importance to Guide Research, Development and Strategies to Prevent and Control Antimicrobial Resistance; World Health Organisation: Geneva, Switzerland, 2024. [Google Scholar]
- Roque-Borda, C.A.; Zhang, Q.; Nguyen, T.P.T.; Nguyen, T.T.H.; Medhi, H.; de Souza Rodrigues, H.L.; Canales Carnero, C.S.; Sutherland, D.; Helmy, N.M.; Araveti, P.B.; et al. Synergistic combinations of antimicrobial peptides and conventional antibiotics: A strategy to delay resistance emergence in WHO priority bacteria. Pharmacol. Rev. 2025, 78, 100104. [Google Scholar] [CrossRef] [PubMed]
- Tong, Z.; Zhang, Y.; Ling, J.; Ma, J.; Huang, L.; Zhang, L. An in vitro study on the effects of nisin on the antibacterial activities of 18 antibiotics against Enterococcus faecalis. PLoS ONE 2014, 9, e89209. [Google Scholar] [CrossRef]
- Tabcheh, J.; Vergalli, J.; Davin-Régli, A.; Ghanem, N.; Pages, J.-M.; Al-Bayssari, C.; Brunel, J.M. Rejuvenating the activity of usual antibiotics on resistant Gram-negative bacteria: Recent issues and perspectives. Int. J. Mol. Sci. 2023, 24, 1515. [Google Scholar] [CrossRef]
- Mugabe, C.; Halwani, M.; Azghani, A.O.; Lafrenie, R.M.; Omri, A. Mechanism of enhanced activity of liposome-entrapped aminoglycosides against resistant strains of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2006, 50, 2016–2022. [Google Scholar] [CrossRef] [PubMed]
- Sachetelli, S.; Khalil, H.; Chen, T.; Beaulac, C.; Sénéchal, S.; Lagacé, J. Demonstration of a fusion mechanism between a fluid bactericidal liposomal formulation and bacterial cells. Biochim. Biophys. Acta 2000, 1463, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Cheow, W.S.; Chang, M.W.; Hadinoto, K. Antibacterial efficacy of inhalable levofloxacin-loaded polymeric nanoparticles against E. coli biofilm cells: The effect of antibiotic release profile. Pharm. Res. 2010, 27, 1597–1609. [Google Scholar] [CrossRef]
- Jiang, L.; Greene, M.K.; Insua, J.L.; Pessoa, J.S.; Small, D.M.; Smyth, P.; McCann, A.P.; Cogo, F.; Bengoechea, J.A.; Taggart, C.C.; et al. Clearance of intracellular Klebsiella pneumoniae infection using gentamicin-loaded nanoparticles. J. Control. Release 2018, 279, 316–325. [Google Scholar] [CrossRef]
- Graef, F.; Vukosavljevic, B.; Michel, J.-P.; Wirth, M.; Ries, O.; De Rossi, C.; Windbergs, M.; Rosilio, V.; Ducho, C.; Gordon, S.; et al. The bacterial cell envelope as delimiter of anti-infective bioavailability—An in vitro permeation model of the Gram-negative bacterial inner membrane. J. Control. Release 2016, 243, 214–224. [Google Scholar] [CrossRef]
- Macesic, N.; Uhlemann, A.-C.; Peleg, A.Y. Multidrug-resistant Gram-negative bacterial infections. Lancet 2025, 405, 257–272. [Google Scholar] [CrossRef] [PubMed]
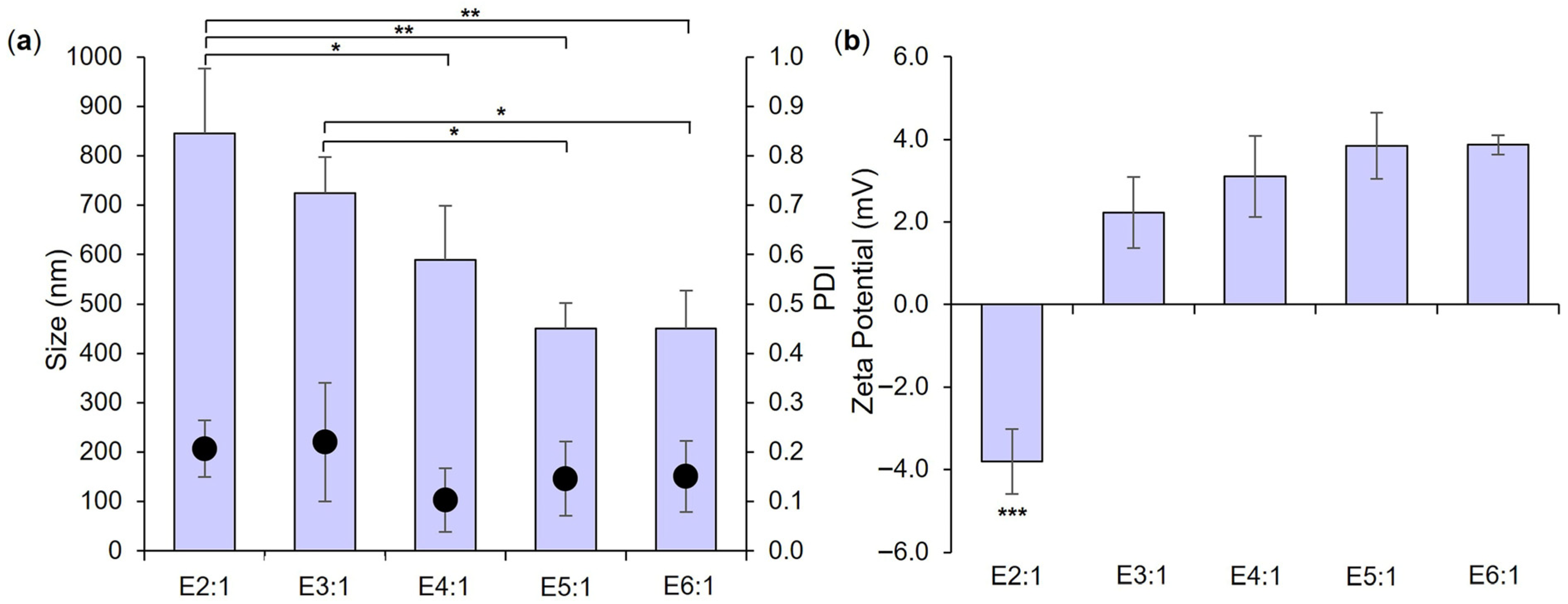
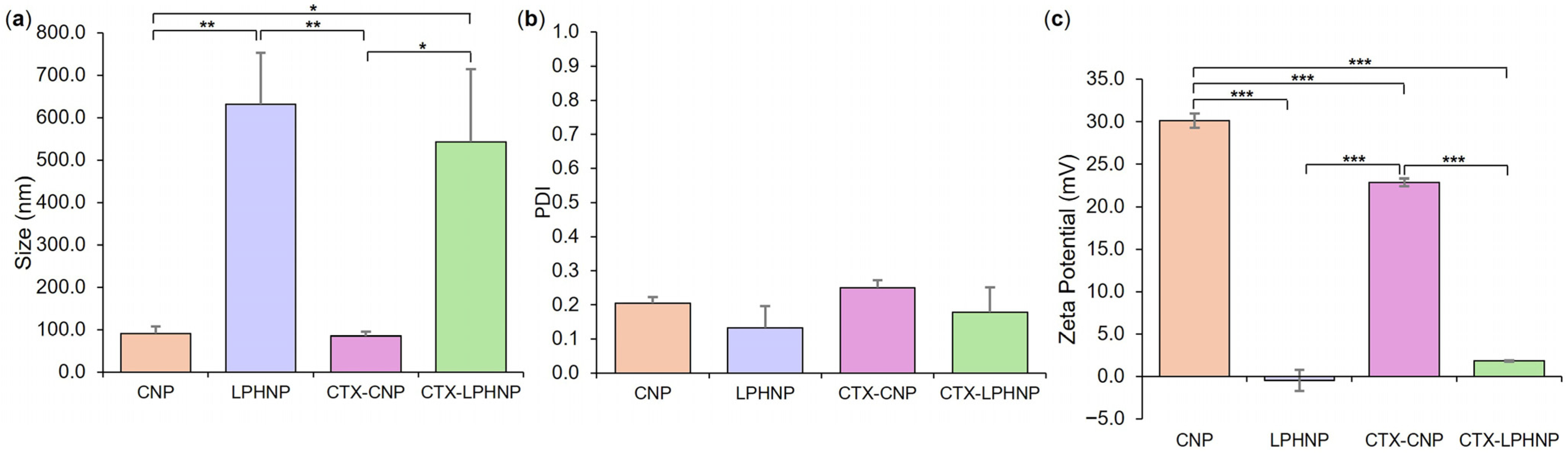
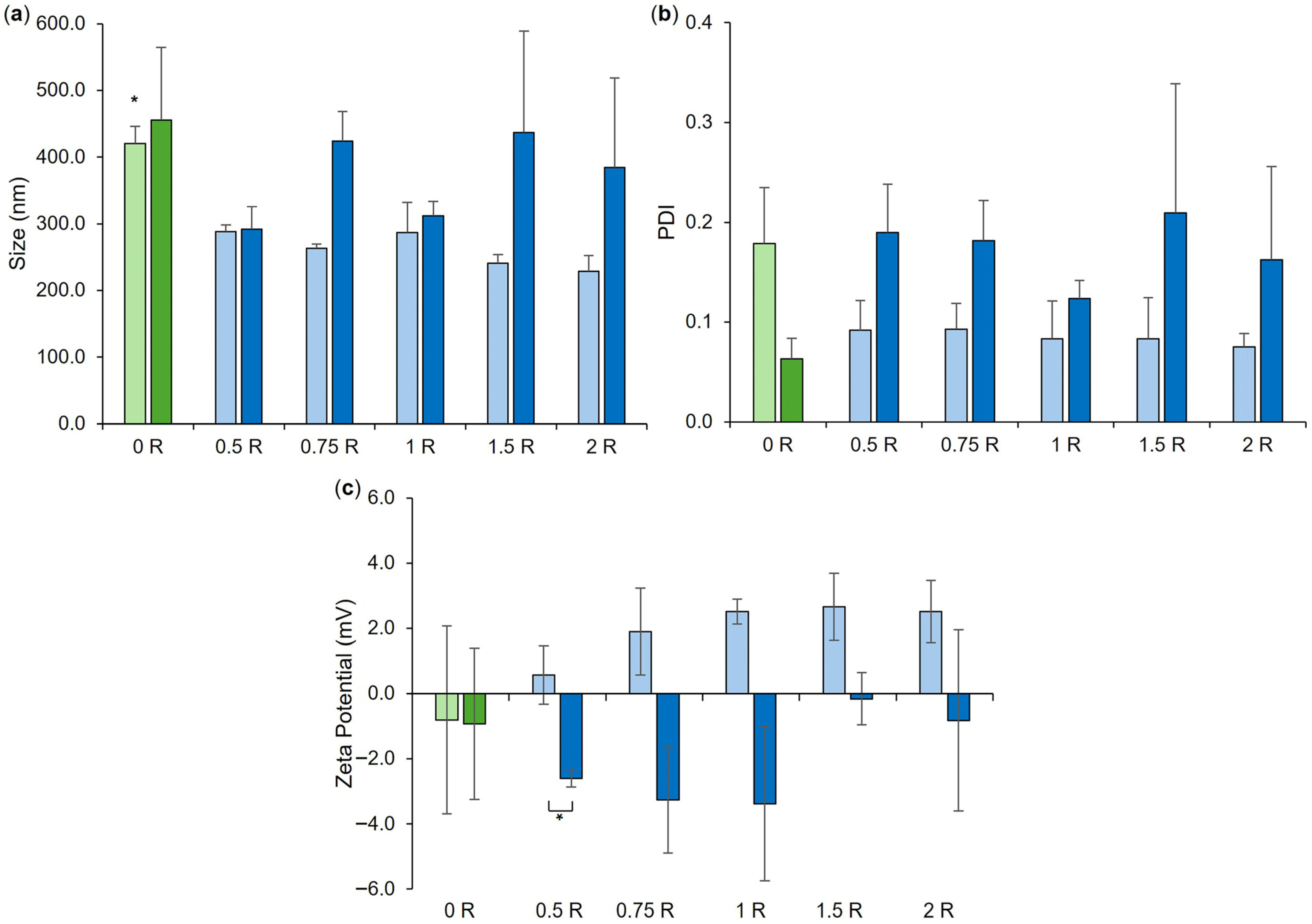
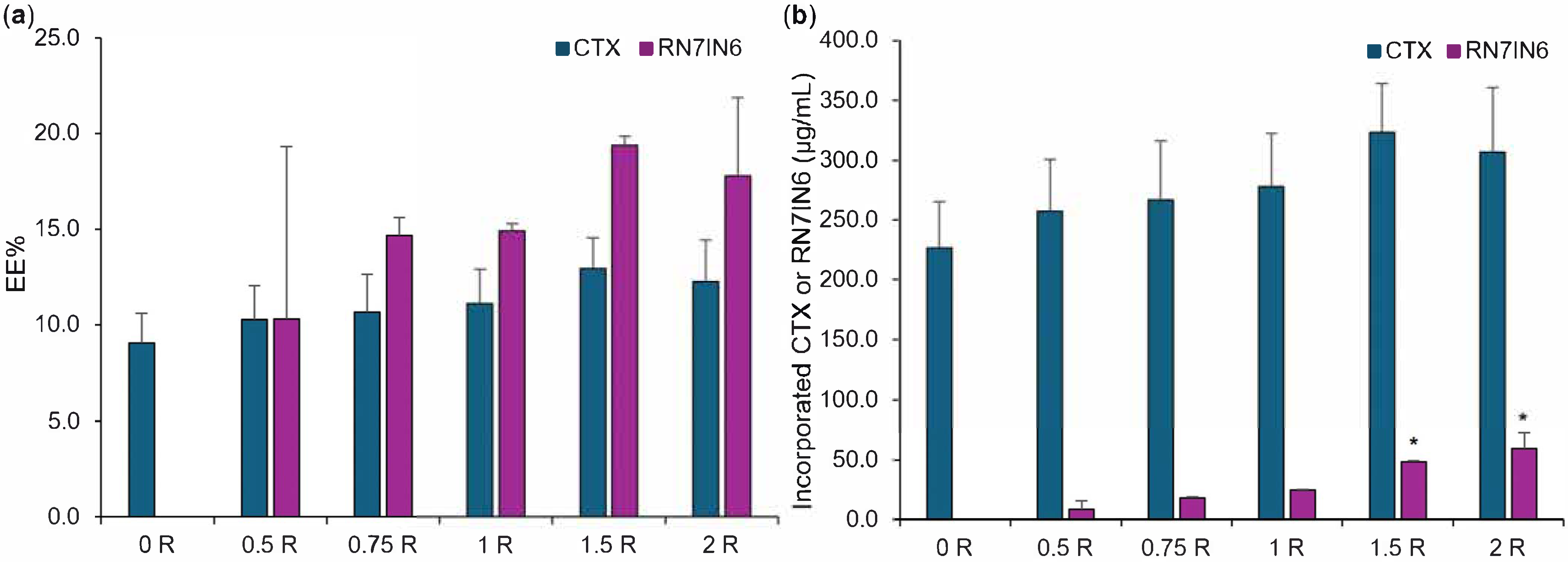
| Formulation | Lipid Concentration (mg/mL) | Weight Ratio (Lipid:Chitosan) | TFR (mL/min) | FRR (CNP:Lipid) |
|---|---|---|---|---|
| E2:1 | 0.5 | 0.43:1 | 20 | 2:1 |
| E3:1 | 0.5 | 0.32:1 | 20 | 3:1 |
| E4:1 | 0.5 | 0.26:1 | 20 | 4:1 |
| E5:1 | 0.5 | 0.21:1 | 20 | 5:1 |
| E6:1 | 0.5 | 0.18:1 | 20 | 6:1 |
| Organic Phase | |||||
|---|---|---|---|---|---|
| Formulation | Aqueous Phase | RN7IN6 (mg/mL) | Lipid (mg/mL) | TFR (mL/min) | FRR (CTX-Loaded CNPs:RN7IN6 in Lipid |
| 0.5 R | CTX-CNP | 0.5 | 0.5 | 20 | 5:1 |
| 0.75 R | 0.75 | ||||
| 1 R | 1 | ||||
| 1.5 R | 1.5 | ||||
| 2 R | 2 | ||||
| MIC-Separate (μg/mL) | MIC-in Combination (μg/mL) | |||||
|---|---|---|---|---|---|---|
| Bacteria | RN7IN6 | CTX | RN7IN6 | CTX | FICI | Effect |
| S. aureus | 16.00 ± 0.00 | 2.00 ± 0.00 | 6.00 ± 2.83 | 0.750 ± 0.353 | 0.75 ± 0.00 | Partial synergism |
| E. coli | 32.00 ± 0.00 | 0.10 ± 0.04 | 5.33 ± 2.31 | 0.052 ± 0.018 | 0.67 ± 0.07 | Partial synergism |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Carini, V.; Scagnetti, G.; Foulkes, J.; Evans, K.; Saleem, I.; Gordon, S. Dual Core-Shell Loaded Lipid-Polymer Hybrid Nanoparticles as Combination Anti-Infective Delivery Platforms. Pharmaceutics 2026, 18, 13. https://doi.org/10.3390/pharmaceutics18010013
Carini V, Scagnetti G, Foulkes J, Evans K, Saleem I, Gordon S. Dual Core-Shell Loaded Lipid-Polymer Hybrid Nanoparticles as Combination Anti-Infective Delivery Platforms. Pharmaceutics. 2026; 18(1):13. https://doi.org/10.3390/pharmaceutics18010013
Chicago/Turabian StyleCarini, Valeria, Giulia Scagnetti, Joanne Foulkes, Katie Evans, Imran Saleem, and Sarah Gordon. 2026. "Dual Core-Shell Loaded Lipid-Polymer Hybrid Nanoparticles as Combination Anti-Infective Delivery Platforms" Pharmaceutics 18, no. 1: 13. https://doi.org/10.3390/pharmaceutics18010013
APA StyleCarini, V., Scagnetti, G., Foulkes, J., Evans, K., Saleem, I., & Gordon, S. (2026). Dual Core-Shell Loaded Lipid-Polymer Hybrid Nanoparticles as Combination Anti-Infective Delivery Platforms. Pharmaceutics, 18(1), 13. https://doi.org/10.3390/pharmaceutics18010013








