Predictive Modeling of Drug Product Stability in Pharmaceutical Blister Packs
Abstract
1. Introduction
2. Results and Discussion
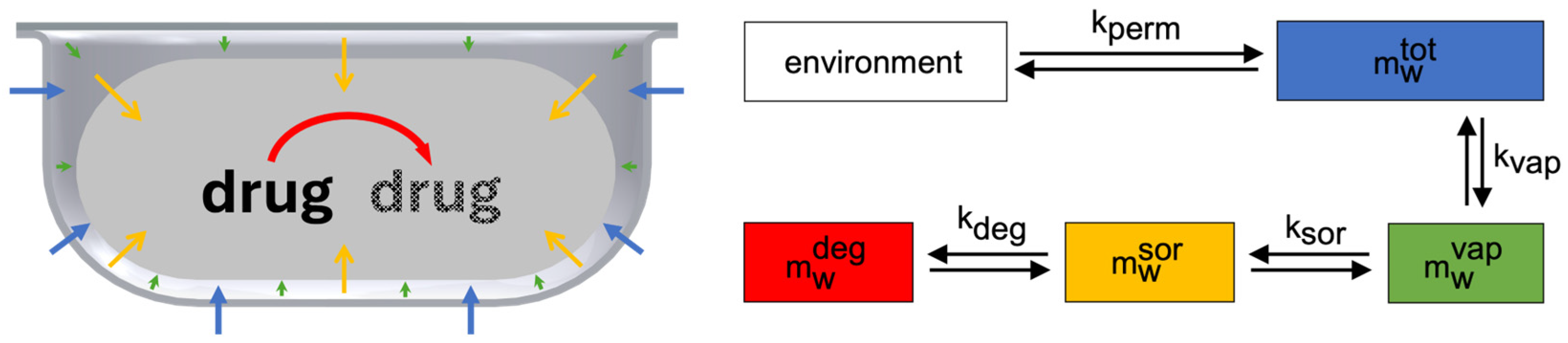
2.1. Model Design
2.2. Model Parametrization
2.2.1. General
2.2.2. Permeation
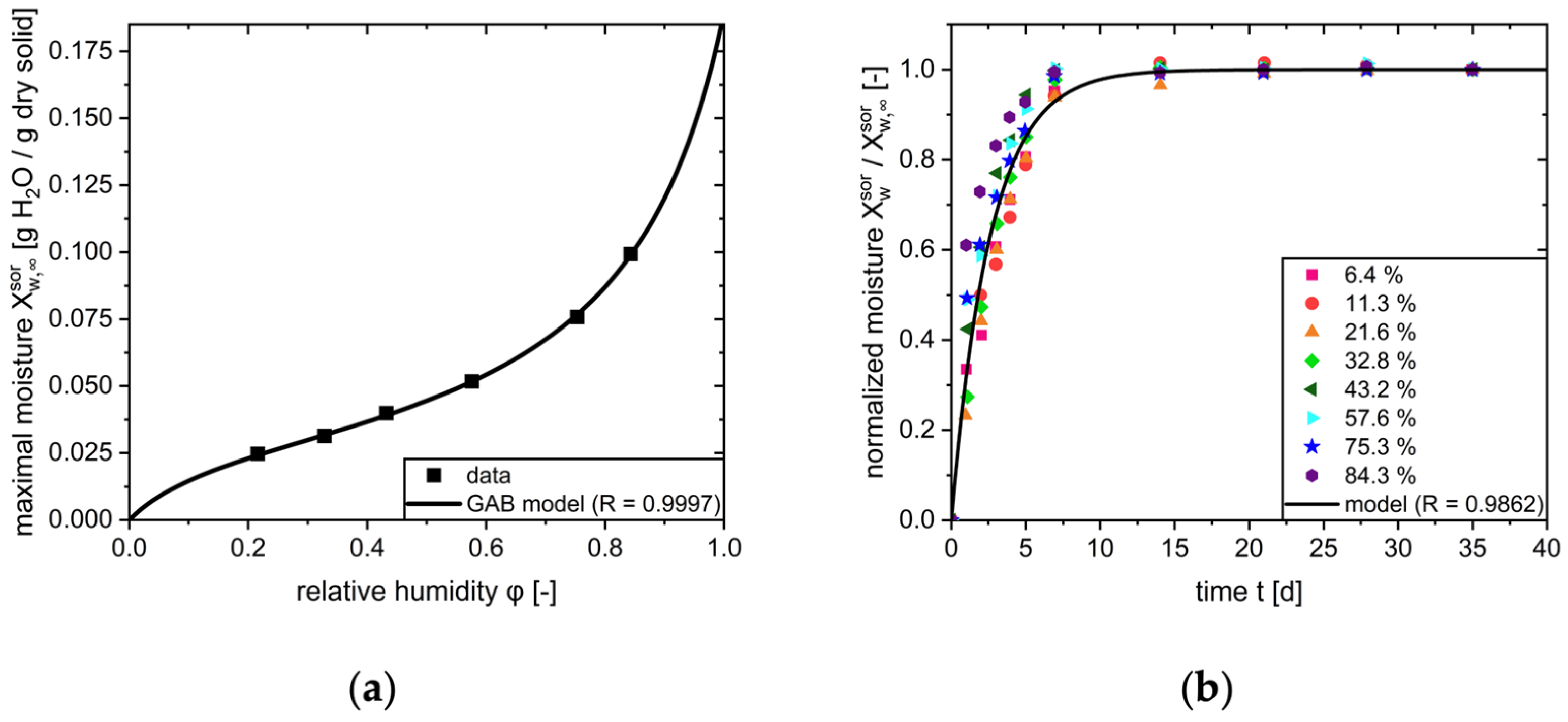
2.2.3. Sorption
2.2.4. Degradation
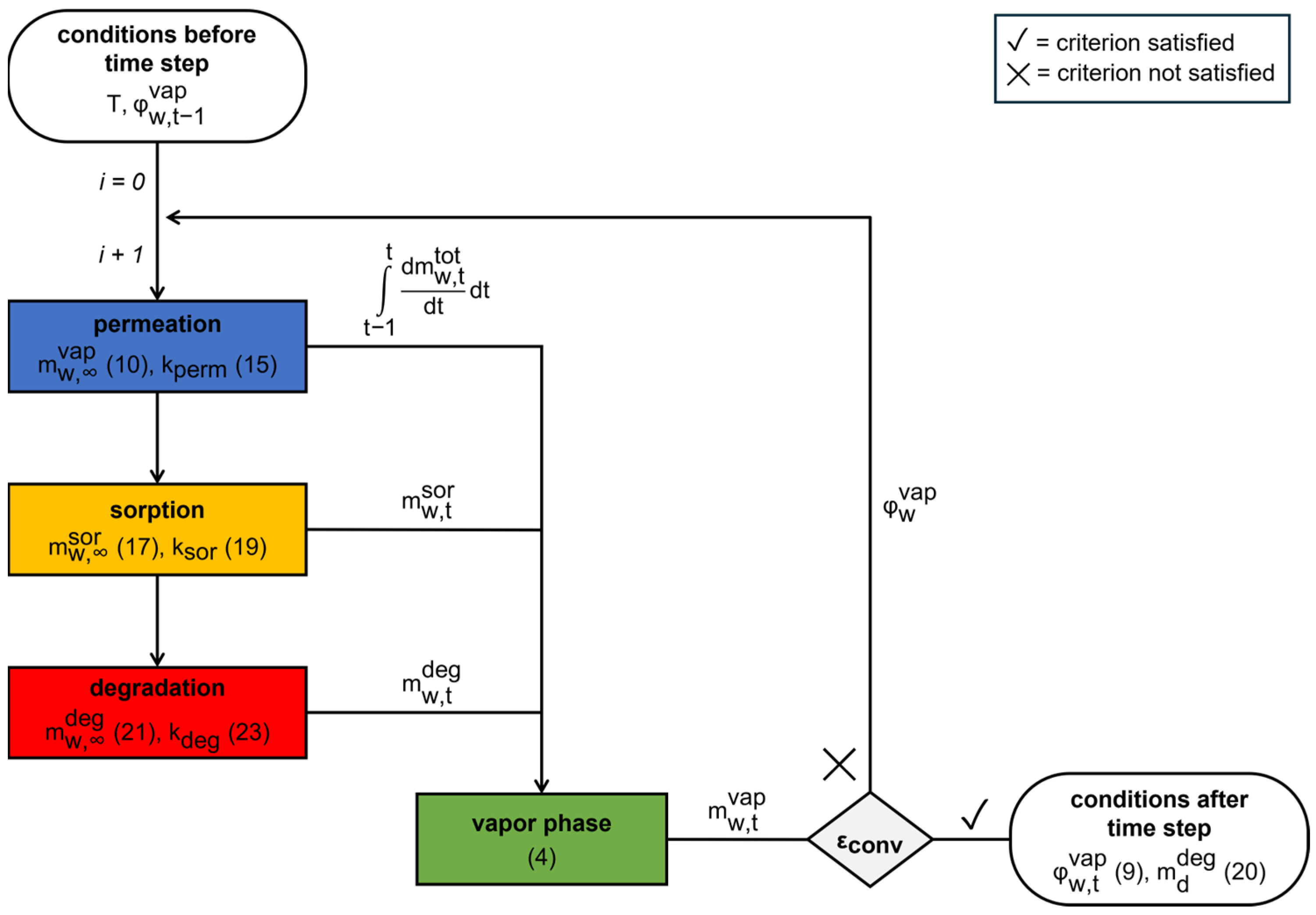
2.3. Model Implementation
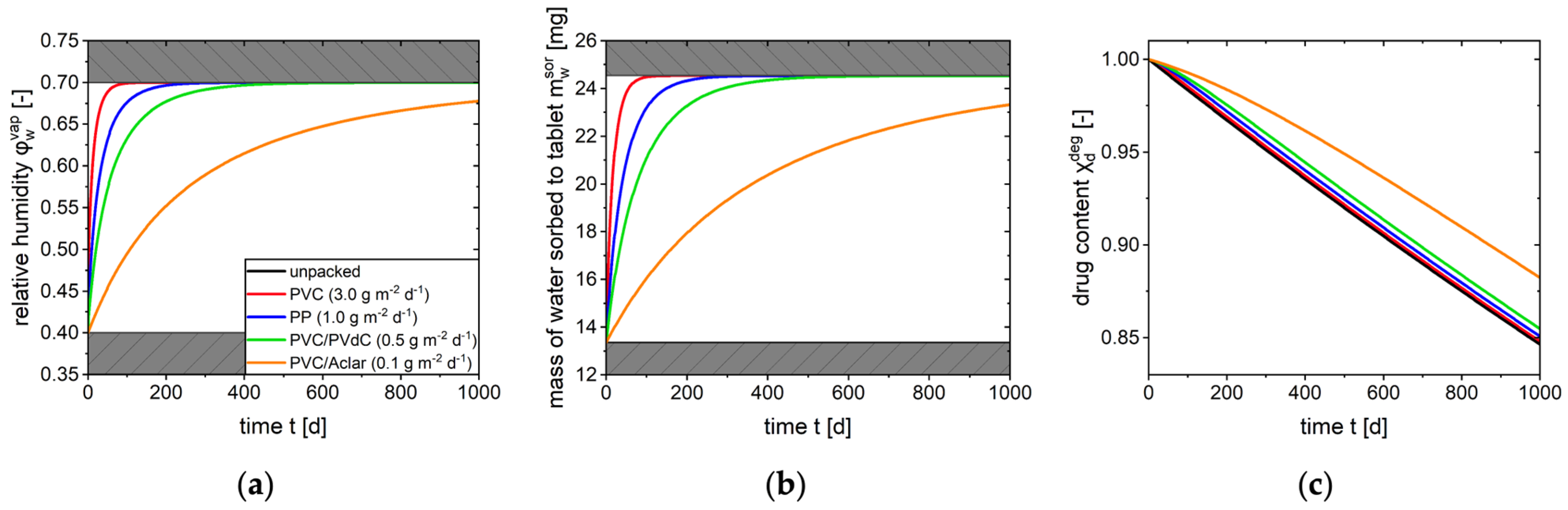
2.4. Model Application
- The barrier properties of the blister, expressed as the rate constant of permeation, are determined in the steady state of water permeation. The saturation of the blister film is neglected with respect to the multi-year time scale of the simulation.
- All rate constants of the different sub-processes are assumed as constants within a time step.
- The sorption properties of the formulation remain constant over the simulation period. As degradation progresses, the composition of the tablet is assumed not be altered due to the accumulation of the degradation product. This may lead to deviations from the originally determined sorption rate constant and sorption isotherm.
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DVS | Dynamic vapor sorption |
| GAB | Guggenheim–Anderson–De Boer |
| PP | Polypropylene |
| PVC | Polyvinyl chloride |
| PVdC | Polyvinylidene chloride |
| WVTR | Water vapor transmission rate |
References
- European Commission. The European Green Deal; European Commission: Brussels, Belgium, 2019; Volume 640. [Google Scholar]
- European Parliament and Council of the European Union. Regulation (EU) 2025/40 of the European Parliament and of the Council of 19 December 2024 on Packaging and Packaging Waste, Amending Regulation (EU) 2019/1020 and Directive (EU) 2019/904, and repealing Directive 94/62/EC; European Union: Brussels, Belgium, 2025; Volume L 40. [Google Scholar]
- Veronica, N.; Heng, P.W.S.; Liew, C.V. Ensuring Product Stability—Choosing the Right Excipients. J. Pharm. Sci. 2022, 111, 2158–2171. [Google Scholar] [CrossRef] [PubMed]
- Ahlneck, C.; Zografi, G. The molecular basis of moisture effects on the physical and chemical stability of drug in the solid state. Int. J. Pharm. 1990, 62, 87–95. [Google Scholar] [CrossRef]
- González-González, O.; Ramirez, I.O.; Ramirez, B.I.; O’Connell, P.; Ballesteros, M.P.; Torrado, J.J.; Serrano, D.R. Drug Stability: ICH versus Accelerated Predictive Stability Studies. Pharmaceutics 2022, 14, 2324. [Google Scholar] [CrossRef] [PubMed]
- Allinson, J.G.; Dansereau, R.J.; Sakr, A. The effects of packaging on the stability of a moisture sensitive compound. Int. J. Pharm. 2001, 221, 49–56. [Google Scholar] [CrossRef]
- Salmenperä, H.; Kauppi, S.; Dahlbo, H.; Fjäder, P. Increasing the Circularity of Packaging along Pharmaceuticals Value Chain. Sustainability 2022, 14, 4715. [Google Scholar] [CrossRef]
- Ding, Q.; Zhu, H. The Key to Solving Plastic Packaging Wastes: Design for Recycling and Recycling Technology. Polymers 2023, 15, 1485. [Google Scholar] [CrossRef]
- Pedrosa de Oliveira, D.; Souza Ribeiro Costa, J.; Oliveira-Nascimento, L. Sustainability of blisters for medicines in tablet form. Sustain. Chem. Pharm. 2021, 21, 100423. [Google Scholar] [CrossRef]
- Pereira, D.A.C.; Ferreira, L.A. Pharmaceutical Blister: Influence of Color and Types of Films in Drug Packaging. Visão Acadêmica 2016, 17, 91–100. [Google Scholar] [CrossRef]
- Nakabayashi, K.; Shimamoto, T.; Mima, H. Stability of Packaged Solid Dosage Forms. II. Shelf-life Prediction for Packaged Sugar-coated Tablets Liable to Moisture and Heat Damage. Chem. Pharm. Bull. 1980, 28, 1099–1106. [Google Scholar] [CrossRef][Green Version]
- Kontny, M.J.; Koppenol, S.; Graham, E.T. Use of the sorption-desorption moisture transfer model to assess the utility of a desiccant in a solid product. Int. J. Pharm. 1992, 84, 261–271. [Google Scholar] [CrossRef]
- Farag Badawy, S.I.; Gawronski, A.J.; Alvarez; Francisco, J. Application of sorption–desorption moisture transfer modeling to the study of chemical stability of a moisture sensitive drug product in different packaging configurations. Int. J. Pharm. 2001, 223, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.N.; Hernandez, R.J.; Burgess, G. Modeling the Moisture Content of a Pharmaceutical Tablet in a Blister Package by Finite Difference Method: Program Development. J. Plast. Film. Sheeting 1998, 14, 152–171. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Y. A new model for predicting moisture uptake by packaged solid pharmaceuticals. Int. J. Pharm. 2003, 255, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Remmelgas, J.; Simonutti, A.-L.; Ronkvist, Å.; Gradinarsky, L.; Löfgren, A. A mechanistic model for the prediction of in-use moisture uptake by packaged dosage forms. Int. J. Pharm. 2013, 441, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Remmelgas, J. Predicting Moisture Uptake in Solid-Dosage Packaging. Pharm. Technol. 2017, 41, 44–49. [Google Scholar]
- Waterman, K.C.; Carella, A.J.; Gumkowski, M.J.; Lukulay, P.; MacDonald, B.C.; Roy, M.C.; Shamblin, S.L. Improved protocol and data analysis for accelerated shelf-life estimation of solid dosage forms. Pharm. Res. 2007, 24, 780–790. [Google Scholar] [CrossRef]
- Genton, D.; Kesselring, U.W. Effect of temperature and relative humidity on nitrazepam stability in solid state. J. Pharm. Sci. 1977, 66, 676–680. [Google Scholar] [CrossRef]
- Waterman, K.C.; MacDonald, B.C. Package Selection for Moisture Protection for Solid, Oral Drug Products. J. Pharm. Sci. 2010, 99, 4437–4452. [Google Scholar] [CrossRef] [PubMed]
- Naversnik, K.; Bohanec, S. Predicting drug hydrolysis based on moisture uptake in various packaging designs. Eur. J. Pharm. Sci. 2008, 35, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Pech, J.; Kaminski, C.; Markus, M.; Hoheisel, W.; Heumann, R.; Winck, J.; Thommes, M. Modeling of Moisture Uptake of Drug Products in Sustainable Blister Packs. In Proceedings of the 24th IAPRI World Packaging Conference 2024, Valencia, Spain, 17–21 June 2024; Volume 2, pp. 329–338, ISBN 978-8-40962-597-0. [Google Scholar]
- Baehr, H.D.; Kabelac, S. Thermodynamik, 15th ed.; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Li, Y.; Sanzgiri, Y.D.; Chen, Y. A study on moisture isotherms of formulations: The Use of polynomial equations to predict the moisture isotherms of tablet products. AAPS PharmSciTech 2003, 4, 461–468. [Google Scholar] [CrossRef]
- PubChem. Cilazapril. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Cilazapril (accessed on 2 May 2025).
- Paszun, S.K.; Stanisz, B.J. Cilazapril decomposition kinetics and mechanism in the solid state versus stability of the other ester pro-drug angiotensin converting enzyme inhibitors. React. Kinet. Mech. Catal. 2013, 109, 285–300. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion, 2nd ed.; Oxford University Press: London, UK, 1975. [Google Scholar]
- ASTM E1249-20; Standard Test Method for Water Vapor Transmission Rate Through Plastic Film and Sheeting Using a Modulated Infrared Sensor. ASTM International: West Conshohocken, PA, USA, 2020. [CrossRef]
- ISO 15106-2:2019; Plastics—Film and Sheeting—Determination of Water Vapour Transmission Rate—Part 2: Infrared Detection Sensor Method. International Organization for Standardization: Geneva, Switzerland, 2019.
- Liveo Research GmbH. Liveo Aclar®. Available online: https://www.liveoresearch.com/en/product/aclar/ (accessed on 2 May 2025).
- Ametek Mocon. PERMATRAN-W Model 3/34 Operator’s Manual; Ametek Mocon: Brooklyn Park, MN, USA, 2022. [Google Scholar]
- Blahovec, J.; Yanniotis, S. GAB Generalized Equation for Sorption Phenomena. Food Bioprocess. Technol. 2008, 1, 82–90. [Google Scholar] [CrossRef]
- Roskar, R.; Kmetec, V. Evaluation of the Moisture Sorption Behaviour of Several Excipients by BET, GAB and Microcalorimetric Approaches. Chem. Pharm. Bull. 2005, 53, 662–665. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kontny, M.J.C.; James, J. Water Sorption of Drugs and Dosage Forms. In Encyclopedia of Pharmaceutical Technology; Swarbrick, J., Ed.; Informa Healthcare, Inc.: New York, NY, USA, 2007; Volume 6, pp. 4049–4065. [Google Scholar][Green Version]
- Legrand, P.; Gahoual, R.; Houzé, P.; Dufaÿ, S. Accelerated Stability Assessment Program to Predict Long-term Stability of Drugs: Application to Ascorbic Acid and to a Cyclic Hexapeptide. AAPS PharmSciTech 2021, 22, 234. [Google Scholar] [CrossRef] [PubMed]
- González-González, O.; Ballesteros, M.P.; Torrado, J.J.; Serrano, D.R. Application of Accelerated Predictive Stability Studies in Extemporaneously Compounded Formulations of Chlorhexidine to Assess the Shelf Life. Molecules 2023, 28, 7925. [Google Scholar] [CrossRef] [PubMed]
- Veronica, N.; Liew, C.V.; Heng, P.W.S. Insights on the role of excipients and tablet matrix porosity on aspirin stability. Int. J. Pharm. 2020, 580, 119218. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.Y.X.; Chow, K.T.; Lui, Y.S.; Salome, A.; Boit, B.; Lefevre, P.; Hiew, T.N.; Gokhale, R.; Heng, P.W.S. Mannitol-Coated Hydroxypropyl Methylcellulose as a Directly Compressible Controlled Release Excipient for Moisture-Sensitive Drugs: A Stability Perspective. Pharm. 2024, 17, 1167. [Google Scholar] [CrossRef] [PubMed]



| Parameter | Symbol | Value | Unit | Reference |
|---|---|---|---|---|
| Permeation | ||||
| Saturation pressure at 40 °C and 100 kPa | 7.39 | kPa | [23] | |
| Molecular mass of water | 18.0 | g mol−1 | - | |
| Gas volume of blister cavity * | 0.41 | ml | - | |
| Ideal gas constant | 8.314 | J mol−1 K−1 | - | |
| Temperature * | 313 | K | - | |
| Surface area of blister cavity * | 3.9 | cm2 | - | |
| Sorption | ||||
| Dry mass of tablet | 364 | mg | [24] | |
| GAB parameter | 0.0296 | - | [24] | |
| GAB parameter | 8.93 | - | [24] | |
| GAB parameter | 0.847 | - | [24] | |
| Rate constant of sorption | 4.41 | s−1 | - | |
| Degradation | ||||
| Molecular mass of cilazapril | 417.5 | g mol−1 | [25] | |
| Mass of cilazapril per tablet * | 10 | mg | - | |
| Pre-exponential factor | 5.4310−18 | s−1 | [26] | |
| Activation energy | 1.71105 | J mol−1 | [26] | |
| Humidity sensitivity factor | 3.4 | - | [26] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pech, J.; Kaminski, C.; Markus, M.; Hoheisel, W.; Heumann, R.; Winck, J.; Thommes, M. Predictive Modeling of Drug Product Stability in Pharmaceutical Blister Packs. Pharmaceutics 2025, 17, 1233. https://doi.org/10.3390/pharmaceutics17091233
Pech J, Kaminski C, Markus M, Hoheisel W, Heumann R, Winck J, Thommes M. Predictive Modeling of Drug Product Stability in Pharmaceutical Blister Packs. Pharmaceutics. 2025; 17(9):1233. https://doi.org/10.3390/pharmaceutics17091233
Chicago/Turabian StylePech, Jan, Christoph Kaminski, Matthias Markus, Werner Hoheisel, Roman Heumann, Judith Winck, and Markus Thommes. 2025. "Predictive Modeling of Drug Product Stability in Pharmaceutical Blister Packs" Pharmaceutics 17, no. 9: 1233. https://doi.org/10.3390/pharmaceutics17091233
APA StylePech, J., Kaminski, C., Markus, M., Hoheisel, W., Heumann, R., Winck, J., & Thommes, M. (2025). Predictive Modeling of Drug Product Stability in Pharmaceutical Blister Packs. Pharmaceutics, 17(9), 1233. https://doi.org/10.3390/pharmaceutics17091233





