Precision Adjuvant Strategies in Vaccine Development for Substance Use Disorders: Variability and Mechanistic Insights
Abstract
1. Introduction
2. Adjuvant Platforms in SUD Vaccinology
2.1. Aluminum Salt-Based Adjuvants
2.2. Emulsion-Based Adjuvants
2.3. Toll-like Receptor Agonists
2.4. Protein-Based Adjuvants
2.5. Cytokine Modulators
3. Variability of Adjuvant Performance Across Drug Classes
4. Mechanistic Insights Linking Drug Biology to Adjuvant Efficacy
5. Translational Barriers for SUD Vaccines
6. Conclusions and Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AH | aluminum hydroxide (aluminum oxyhydroxide, AlOOH) adjuvant |
| AlOOH | aluminum oxyhydroxide |
| Alum | aluminum salt-based adjuvants in general or when the specific salt is unknown |
| AP | aluminum phosphate adjuvant |
| APC | antigen-presenting cell |
| ASC | apoptosis-associated speck-like protein containing a CARD |
| AS03 | squalene oil-in-water emulsion adjuvant |
| ATP | adenosine triphosphate |
| Bcl-6 | B-cell lymphoma 6 (Tfh lineage-defining transcription factor) |
| BGG | bovine gamma globulin |
| BSA | bovine serum albumin |
| CARD | caspase-recruitment domain |
| CCL2 | C-C motif chemokine ligand 2 |
| CCL3/4 | C-C motif chemokine ligand 3/4 |
| CCR2 | C-C motif chemokine receptor 2 |
| CFA | Complete Freund’s Adjuvant |
| COC | cocaine |
| CpG ODN | CpG oligodeoxynucleotide (TLR9 agonist) |
| CRM197 | cross-reactive material 197 (nontoxic diphtheria toxin mutant) |
| DAMP | damage-associated molecular pattern |
| DC | dendritic cell |
| dmLT | double-mutant E. coli heat-labile enterotoxin (LT) |
| dsRNA | double-stranded RNA (TLR3 agonist) |
| FEN | fentanyl |
| GLA-SE | glucopyranosyl lipid A in squalene emulsion (TLR4 agonist) |
| IFN | interferon (e.g., IFN-β, IFN-γ) |
| IgG | immunoglobulin G (e.g., IgG2a, IgG3) |
| IL | interleukin (e.g., IL-1β, IL-6, IL-12p70) |
| I.V. | intravenous |
| KLH | keyhole limpet hemocyanin |
| LP | liposome |
| LPS | lipopolysaccharide |
| LT | E. coli heat-labile enterotoxin |
| LTA1 | A1 domain of E. coli heat-labile enterotoxin (LT) |
| MF59 | squalene oil-in-water emulsion adjuvant |
| MHC | major histocompatibility complex |
| MPLA | monophosphoryl lipid A (TLR4 agonist) |
| MyD88 | myeloid differentiation primary response protein 88 (TLR adaptor) |
| NanoNicVac | nicotine nanovaccine with a PLGA core and liposomal shell |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NLRP3 | NOD-like receptor protein 3 inflammasome |
| NP | nanoparticle |
| O/W | oil-in-water (emulsion) |
| OUD | opioid use disorder |
| OXY | oxycodone |
| PADRE | Pan DR-binding epitope (universal CD4+ T-helper epitope) |
| PAMP | pathogen-associated molecular pattern |
| PLGA | poly(lactic-co-glycolic acid) |
| PRR | pattern-recognition receptor |
| R848 | resiquimod (TLR7/8 agonist) |
| rEPA | recombinant Pseudomonas exoprotein A |
| SAS | Sigma Adjuvant System (O/W emulsion) |
| SUD | substance use disorder |
| Th1/Th2 | T helper type 1/type 2 |
| Tfh | T follicular helper (cell) |
| TLR | toll-like receptor |
| TNF-α | tumor necrosis factor-alpha |
| TRAF6 | TNF receptor-associated factor 6 |
| TRIF | TIR-domain-containing adaptor inducing IFN-β (TLR adaptor) |
| TT | tetanus toxoid |
| UM-3006 | small-molecule TLR7/8 agonist |
| W/O | water-in-oil (emulsion) |
References
- APA. What Is a Substance Use Disorder? 2024. Available online: https://www.psychiatry.org/patients-families/addiction-substance-use-disorders/what-is-a-substance-use-disorder (accessed on 25 June 2025).
- JHM. Substance Use Disorder. Available online: https://www.hopkinsmedicine.org/health/conditions-and-diseases/substance-abuse-chemical-dependency (accessed on 25 June 2025).
- Sanchez-Roige, S.; Kember, R.L.; Agrawal, A. Substance use and common contributors to morbidity: A genetics perspective. EBioMedicine 2022, 83, 104212. [Google Scholar] [CrossRef]
- Connery, H.S.; McHugh, R.K.; Reilly, M.B.; Shin, S.; Greenfield, S.F. Substance Use Disorders in Global Mental Health Delivery: Epidemiology, Treatment Gap, and Implementation of Evidence-Based Treatments. Harv. Rev. Psychiatry 2020, 28, 316–327. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Qi, X.; Wang, Y.; Fang, K. Global burden of drug use disorders by region and country, 1990–2021. Front. Public Health 2024, 12, 1470809. [Google Scholar] [CrossRef]
- UNODC. World Drug Report 2023; United Nations Office on Drugs and Crime: Vienna, Austria, 2023. [Google Scholar]
- Congdon, P. Geographical Aspects of Recent Trends in Drug-Related Deaths, with a Focus on Intra-National Contextual Variation. Int. J. Environ. Res. Public Health 2020, 17, 8081. [Google Scholar] [CrossRef]
- NCHS. Provisional Drug Overdose Death Counts; National Center for Health Statistics: Hyattsville, MD, USA, 2025.
- Florence, C.; Luo, F.; Rice, K. The economic burden of opioid use disorder and fatal opioid overdose in the United States, 2017. Drug Alcohol. Depend. 2021, 218, 108350. [Google Scholar] [CrossRef] [PubMed]
- JEC. The Economic Toll of the Opioid Crisis Reached Nearly $1.5 Trillion in 2020. 2022. Available online: https://www.jec.senate.gov/public/index.cfm/democrats/issue-briefs?ID=CE55E977-B473-414F-8B88-53EB55EB7C7C (accessed on 25 June 2025).
- Carley, J.A.; Oesterle, T. Therapeutic Approaches to Opioid Use Disorder: What is the Current Standard of Care? Int. J. Gen. Med. 2021, 14, 2305–2311. [Google Scholar] [CrossRef]
- Santo, T., Jr.; Clark, B.; Hickman, M.; Grebely, J.; Campbell, G.; Sordo, L.; Chen, A.; Tran, L.T.; Bharat, C.; Padmanathan, P.; et al. Association of Opioid Agonist Treatment With All-Cause Mortality and Specific Causes of Death Among People With Opioid Dependence: A Systematic Review and Meta-analysis. JAMA Psychiatry 2021, 78, 979–993. [Google Scholar] [CrossRef] [PubMed]
- Hall, N.Y.; Le, L.; Majmudar, I.; Mihalopoulos, C. Barriers to accessing opioid substitution treatment for opioid use disorder: A systematic review from the client perspective. Drug Alcohol. Depend. 2021, 221, 108651. [Google Scholar] [CrossRef]
- Math, S.B.; Mohan, A.; Kumar, N.C. Opioid substitution therapy: Legal challenges. Indian J. Psychiatry 2018, 60, 271–277. [Google Scholar] [CrossRef]
- Zweben, J.E.; Sorensen, J.L.; Shingle, M.; Blazes, C.K. Discontinuing Methadone and Buprenorphine: A Review and Clinical Challenges. J. Addict. Med. 2021, 15, 454–460. [Google Scholar] [CrossRef]
- Singh, D.; Saadabadi, A. Naltrexone. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Shulman, M.; Greiner, M.G.; Tafessu, H.M.; Opara, O.; Ohrtman, K.; Potter, K.; Hefner, K.; Jelstrom, E.; Rosenthal, R.N.; Wenzel, K.; et al. Rapid Initiation of Injection Naltrexone for Opioid Use Disorder: A Stepped-Wedge Cluster Randomized Clinical Trial. JAMA Netw. Open 2024, 7, e249744. [Google Scholar] [CrossRef]
- SAMHSA. Chapter 2—How Stimulants Affect the Brain and Behavior. In Treatment for Stimulant Use Disorders; Substance Abuse and Mental Health Services Administration: Rockville, MD, USA, 2021. [Google Scholar]
- Han, B.; Jones, C.M.; Volkow, N.D.; Rikard, S.M.; Dowell, D.; Einstein, E.B.; Guy, G.P.; Tomoyasu, N.; Ko, J.; Baldwin, G.; et al. Prescription Stimulant Use, Misuse, and Use Disorder Among US Adults Aged 18 to 64 Years. JAMA Psychiatry 2025, 82, 572–581. [Google Scholar] [CrossRef]
- Urits, I.; Gress, K.; Charipova, K.; Li, N.; Berger, A.A.; Cornett, E.M.; Hasoon, J.; Kassem, H.; Kaye, A.D.; Viswanath, O. Cannabis Use and its Association with Psychological Disorders. Psychopharmacol. Bull. 2020, 50, 56–67. [Google Scholar]
- Young, E.J.; Radnai, L.; Prikhodko, V.; Miller, C.A. Novel therapeutics in development for the treatment of stimulant-use disorder. Curr. Opin. Neurobiol. 2024, 87, 102898. [Google Scholar] [CrossRef]
- Elkrief, L.; Sharafi, H.; Bakouni, H.; McAnulty, C.; Bastien, G.; Dubreucq, S.; Garel, N.; Trépanier, A.; Ziegler, D.; Jutras-Aswad, D. Efficacy and Safety of Modafinil for Treatment of Amphetamine-Type Stimulant Use Disorder: A Systematic Review and Meta-Analysis of Randomized Placebo-Controlled Trials: Efficacité et innocuité du modafinil pour le traitement des troubles liés à l’usage de stimulants de type amphétamine: Revue systématique et méta-analyse d’essais randomisés contrôlés par placebo. Can. J. Psychiatry 2024, 69, 793–805. [Google Scholar]
- Bahji, A. Limitations and Future Directions in Pharmacological Treatment for Amphetamine-Type Stimulant Use Disorder. Can. J. Psychiatry 2025, 70, 136–137. [Google Scholar] [CrossRef] [PubMed]
- Connor, J.P.; Stjepanović, D.; Le Foll, B.; Hoch, E.; Budney, A.J.; Hall, W.D. Cannabis use and cannabis use disorder. Nat. Rev. Dis. Primers 2021, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Rømer Thomsen, K.; Thylstrup, B.; Kenyon, E.A.; Lees, R.; Baandrup, L.; Feldstein Ewing, S.W.; Freeman, T.P. Cannabinoids for the treatment of cannabis use disorder: New avenues for reaching and helping youth? Neurosci. Biobehav. Rev. 2022, 132, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Tikka, S.K.; Bhute, A.R.; Bastia, B.K. N-acetyl cysteine in the treatment of cannabis use disorder: A systematic review of clinical trials. Addict. Behav. 2022, 129, 107283. [Google Scholar] [CrossRef]
- Ronsley, C.; Nolan, S.; Knight, R.; Hayashi, K.; Klimas, J.; Walley, A.; Wood, E.; Fairbairn, N. Treatment of stimulant use disorder: A systematic review of reviews. PLoS ONE 2020, 15, e0234809. [Google Scholar] [CrossRef]
- Rawson, R.A.; Erath, T.G.; Chalk, M.; Clark, H.W.; McDaid, C.; Wattenberg, S.A.; Roll, J.M.; McDonell, M.G.; Parent, S.; Freese, T.E. Contingency Management for Stimulant Use Disorder: Progress, Challenges, and Recommendations. J. Ambul. Care Manag. 2023, 46, 152–159. [Google Scholar] [CrossRef]
- Ciccarone, D.; Shoptaw, S. Understanding Stimulant Use and Use Disorders in a New Era. Med. Clin. N. Am. 2022, 106, 81–97. [Google Scholar] [CrossRef]
- Le Foll, B.; Tang, V.M.; Rueda, S.; Trick, L.V.; Boileau, I. Cannabis use disorder: From neurobiology to treatment. J. Clin. Investig. 2024, 134, e172887. [Google Scholar] [CrossRef]
- Budney, A.J.; Fearer, S.; Walker, D.D.; Stanger, C.; Thostenson, J.; Grabinski, M.; Bickel, W.K. An initial trial of a computerized behavioral intervention for cannabis use disorder. Drug Alcohol. Depend. 2011, 115, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Coughlin, L.N.; Bonar, E.E.; Wieringa, J.; Zhang, L.; Rostker, M.J.; Augustiniak, A.N.; Goodman, G.J.; Lin, L.A. Pilot trial of a telehealth-delivered behavioral economic intervention promoting cannabis-free activities among adults with cannabis use disorder. J. Psychiatr. Res. 2023, 163, 202–210. [Google Scholar] [CrossRef]
- Sinha, R. New Findings on Biological Factors Predicting Addiction Relapse Vulnerability. Curr. Psychiatry Rep. 2011, 13, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Chalana, H.; Kundal, T.; Gupta, V.; Malhari, A.S. Predictors of Relapse after Inpatient Opioid Detoxification during 1-Year Follow-Up. J. Addict. 2016, 2016, 7620860. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, M.; Tremblay, J.; Baudry, C.; Pearson, J.; Bertrand, K. A systematic review and meta-analysis of the efficacy of the long-term treatment and support of substance use disorders. Social. Sci. Med. 2021, 285, 114289. [Google Scholar] [CrossRef]
- Nagy, N.E.S.; Ella, E.I.A.; Shorab, E.M.; Moneam, M.H.E.-D.A.; Tohamy, A.A. Assessment of addiction management program and predictors of relapse among inpatients of the Psychiatric Institute at Ain Shams University Hospital. Middle East. Curr. Psychiatry 2022, 29, 80. [Google Scholar] [CrossRef]
- Theriot, J.; Sabir, S.; Azadfard, M. Opioid Antagonists. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Lu, T.; Li, X.; Zheng, W.; Kuang, C.; Wu, B.; Liu, X.; Xue, Y.; Shi, J.; Lu, L.; Han, Y. Vaccines to Treat Substance Use Disorders: Current Status and Future Directions. Pharmaceutics 2024, 16, 84. [Google Scholar] [CrossRef]
- Pravetoni, M.; Comer, S.D. Development of vaccines to treat opioid use disorders and reduce incidence of overdose. Neuropharmacology 2019, 158, 107662. [Google Scholar] [CrossRef]
- Hossain, M.K.; Davidson, M.; Kypreos, E.; Feehan, J.; Muir, J.A.; Nurgali, K.; Apostolopoulos, V. Immunotherapies for the Treatment of Drug Addiction. Vaccines 2022, 10, 1778. [Google Scholar] [CrossRef]
- Pravetoni, M. Biologics to treat substance use disorders: Current status and new directions. Hum. Vaccin. Immunother. 2016, 12, 3005–3019. [Google Scholar] [CrossRef] [PubMed]
- Bonese, K.F.; Wainer, B.H.; Fitch, F.W.; Rothberg, R.M.; Schuster, C.R. Changes in heroin self-administration by a rhesus monkey after morphine immunisation. Nature 1974, 252, 708–710. [Google Scholar] [CrossRef] [PubMed]
- Collins, K.C.; Schlosburg, J.E.; Bremer, P.T.; Janda, K.D. Methamphetamine Vaccines: Improvement through Hapten Design. J. Med. Chem. 2016, 59, 3878–3885. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.K.; Davidson, M.; Feehan, J.; Deraos, G.; Nurgali, K.; Matsoukas, J.; Apostolopoulos, V. Development of Methamphetamine Conjugated Vaccine through Hapten Design: In Vitro and In Vivo Characterization. Vaccines 2023, 11, 340. [Google Scholar] [CrossRef]
- Hosztafi, S.; Galambos, A.R.; Köteles, I.; Karádi, D.; Fürst, S.; Al-Khrasani, M. Opioid-Based Haptens: Development of Immunotherapy. Int. J. Mol. Sci. 2024, 25, 7781. [Google Scholar] [CrossRef]
- Baruffaldi, F.; Kelcher, A.H.; Laudenbach, M.; Gradinati, V.; Limkar, A.; Roslawski, M.; Birnbaum, A.; Lees, A.; Hassler, C.; Runyon, S.; et al. Preclinical Efficacy and Characterization of Candidate Vaccines for Treatment of Opioid Use Disorders Using Clinically Viable Carrier Proteins. Mol. Pharm. 2018, 15, 4947–4962. [Google Scholar] [CrossRef]
- Zhao, Z.; Hu, Y.; Harmon, T.; Pentel, P.R.; Ehrich, M.; Zhang, C. Hybrid nanoparticle-based nicotine nanovaccines: Boosting the immunological efficacy by conjugation of potent carrier proteins. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1655–1665. [Google Scholar] [CrossRef]
- Alving, C.R.; Matyas, G.R.; Torres, O.; Jalah, R.; Beck, Z. Adjuvants for vaccines to drugs of abuse and addiction. Vaccine 2014, 32, 5382–5389. [Google Scholar] [CrossRef]
- Zhao, Z.; Harris, B.; Hu, Y.; Harmon, T.; Pentel, P.R.; Ehrich, M.; Zhang, C. Rational incorporation of molecular adjuvants into a hybrid nanoparticle-based nicotine vaccine for immunotherapy against nicotine addiction. Biomaterials 2018, 155, 165–175. [Google Scholar] [CrossRef]
- Cornuz, J.; Zwahlen, S.; Jungi, W.F.; Osterwalder, J.; Klingler, K.; van Melle, G.; Bangala, Y.; Guessous, I.; Müller, P.; Willers, J.; et al. A vaccine against nicotine for smoking cessation: A randomized controlled trial. PLoS ONE 2008, 3, e2547. [Google Scholar] [CrossRef]
- Wagena, E.J.; de Vos, A.; Horwith, G.; van Schayck, C.P. The immunogenicity and safety of a nicotine vaccine in smokers and nonsmokers: Results of a randomized, placebo-controlled phase 1/2 trial. Nicotine Tob. Res. 2008, 10, 213–218. [Google Scholar] [CrossRef]
- Martell, B.A.; Orson, F.M.; Poling, J.; Mitchell, E.; Rossen, R.D.; Gardner, T.; Kosten, T.R. Cocaine vaccine for the treatment of cocaine dependence in methadone-maintained patients: A randomized, double-blind, placebo-controlled efficacy trial. Arch. Gen. Psychiatry 2009, 66, 1116–1123. [Google Scholar] [CrossRef]
- Lavelle, E.C.; McEntee, C.P. Vaccine adjuvants: Tailoring innate recognition to send the right message. Immunity 2024, 57, 772–789. [Google Scholar] [CrossRef]
- Levast, B.; Awate, S.; Babiuk, L.; Mutwiri, G.; Gerdts, V.; van Drunen Littel-van den Hurk, S. Vaccine Potentiation by Combination Adjuvants. Vaccines 2014, 2, 297–322. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.K.; Mahajan, P.; Singh, N.K.; Gupta, A.; Aggarwal, R.; Rappuoli, R.; Johri, A.K. New-age vaccine adjuvants, their development, and future perspective. Front. Immunol. 2023, 14, 1043109. [Google Scholar] [CrossRef]
- Zhao, T.; Cai, Y.; Jiang, Y.; He, X.; Wei, Y.; Yu, Y.; Tian, X. Vaccine adjuvants: Mechanisms and platforms. Signal Transduct. Target. Ther. 2023, 8, 283. [Google Scholar] [CrossRef]
- Ben-Akiva, E.; Chapman, A.; Mao, T.; Irvine, D.J. Linking vaccine adjuvant mechanisms of action to function. Sci. Immunol. 2025, 10, eado5937. [Google Scholar] [CrossRef] [PubMed]
- Hoogsteder, P.H.J.; Kotz, D.; van Spiegel, P.I.; Viechtbauer, W.; Brauer, R.; Kessler, P.D.; Kalnik, M.W.; Fahim, R.E.F.; van Schayck, O.C.P. The efficacy and safety of a nicotine conjugate vaccine (NicVAX®) or placebo co-administered with varenicline (Champix®) for smoking cessation: Study protocol of a phase IIb, double blind, randomized, placebo controlled trial. BMC Public Health 2012, 12, 1052. [Google Scholar] [CrossRef] [PubMed]
- Tonstad, S.; Heggen, E.; Giljam, H.; Lagerbäck, P.-Å.; Tønnesen, P.; Wikingsson, L.D.; Lindblom, N.; de Villiers, S.; Svensson, T.H.; Fagerström, K.-O. Niccine®, a Nicotine Vaccine, for Relapse Prevention: A Phase II, Randomized, Placebo-Controlled, Multicenter Clinical Trial. Nicotine Tob. Res. 2013, 15, 1492–1501. [Google Scholar] [CrossRef]
- Hoogsteder, P.H.J.; Kotz, D.; van Spiegel, P.I.; Viechtbauer, W.; van Schayck, O.C.P. Efficacy of the nicotine vaccine 3′-AmNic-rEPA (NicVAX) co-administered with varenicline and counselling for smoking cessation: A randomized placebo-controlled trial. Addiction 2014, 109, 1252–1259. [Google Scholar] [CrossRef]
- Bremer, P.T.; Schlosburg, J.E.; Lively, J.M.; Janda, K.D. Injection Route and TLR9 Agonist Addition Significantly Impact Heroin Vaccine Efficacy. Mol. Pharm. 2014, 11, 1075–1080. [Google Scholar] [CrossRef]
- Crouse, B.; Miller, S.M.; Muelken, P.; Hicks, L.; Vigliaturo, J.R.; Marker, C.L.; Guedes, A.G.P.; Pentel, P.R.; Evans, J.T.; LeSage, M.G.; et al. A TLR7/8 agonist increases efficacy of anti-fentanyl vaccines in rodent and porcine models. npj Vaccines 2023, 8, 107. [Google Scholar] [CrossRef] [PubMed]
- Stone, A.E.; Scheuermann, S.E.; Haile, C.N.; Cuny, G.D.; Velasquez, M.L.; Linhuber, J.P.; Duddupudi, A.L.; Vigliaturo, J.R.; Pravetoni, M.; Kosten, T.A.; et al. Fentanyl conjugate vaccine by injected or mucosal delivery with dmLT or LTA1 adjuvants implicates IgA in protection from drug challenge. npj Vaccines 2021, 6, 69. [Google Scholar] [CrossRef] [PubMed]
- Bremer, P.T.; Janda, K.D. Conjugate Vaccine Immunotherapy for Substance Use Disorder. Pharmacol. Rev. 2017, 69, 298–315. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Lee, J.C.; Blake, S.; Ellis, B.; Eubanks, L.M.; Janda, K.D. Broadly Neutralizing Synthetic Cannabinoid Vaccines. JACS Au 2021, 1, 31–40. [Google Scholar] [CrossRef]
- Worob, A.; Wenthur, C.J. Development of Cross-Reactive Antibodies for the Identification and Treatment of Synthetic Cannabinoid Receptor Agonist Toxicity. Vaccines 2022, 10, 1253. [Google Scholar] [CrossRef]
- Ghimire, T.R. The mechanisms of action of vaccines containing aluminum adjuvants: An in vitro vs in vivo paradigm. Springerplus 2015, 4, 181. [Google Scholar] [CrossRef]
- Laera, D.; HogenEsch, H.; O’Hagan, D.T. Aluminum Adjuvants-‘Back to the Future’. Pharmaceutics 2023, 15, 1884. [Google Scholar] [CrossRef]
- Pulendran, B.; Arunachalam, P.S.; O’Hagan, D.T. Emerging concepts in the science of vaccine adjuvants. Nat. Rev. Drug Discov. 2021, 20, 454–475. [Google Scholar] [CrossRef] [PubMed]
- HogenEsch, H.; O’Hagan, D.T.; Fox, C.B. Optimizing the utilization of aluminum adjuvants in vaccines: You might just get what you want. npj Vaccines 2018, 3, 51. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Zou, Y.; Hu, Z. Advances in aluminum hydroxide-based adjuvant research and its mechanism. Hum. Vaccin. Immunother. 2015, 11, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Mei, C.; Deshmukh, S.; Cronin, J.; Cong, S.; Chapman, D.; Lazaris, N.; Sampaleanu, L.; Schacht, U.; Drolet-Vives, K.; Ore, M.; et al. Aluminum Phosphate Vaccine Adjuvant: Analysis of Composition and Size Using Off-Line and In-Line Tools. Comput. Struct. Biotechnol. J. 2019, 17, 1184–1194. [Google Scholar] [CrossRef]
- Zhang, T.; He, P.; Guo, D.; Chen, K.; Hu, Z.; Zou, Y. Research Progress of Aluminum Phosphate Adjuvants and Their Action Mechanisms. Pharmaceutics 2023, 15, 1756. [Google Scholar] [CrossRef]
- Eisenbarth, S.C.; Colegio, O.R.; O’Connor, W.; Sutterwala, F.S.; Flavell, R.A. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature 2008, 453, 1122–1126. [Google Scholar] [CrossRef]
- Kool, M.; Pétrilli, V.; De Smedt, T.; Rolaz, A.; Hammad, H.; van Nimwegen, M.; Bergen, I.M.; Castillo, R.; Lambrecht, B.N.; Tschopp, J. Cutting edge: Alum adjuvant stimulates inflammatory dendritic cells through activation of the NALP3 inflammasome. J. Immunol. 2008, 181, 3755–3759. [Google Scholar] [CrossRef]
- Li, H.; Willingham, S.B.; Ting, J.P.; Re, F. Cutting edge: Inflammasome activation by alum and alum’s adjuvant effect are mediated by NLRP3. J. Immunol. 2008, 181, 17–21. [Google Scholar] [CrossRef]
- Rüedi-Bettschen, D.; Wood, S.L.; Gunnell, M.G.; West, C.M.; Pidaparthi, R.R.; Carroll, F.I.; Blough, B.E.; Owens, S.M. Vaccination protects rats from methamphetamine-induced impairment of behavioral responding for food. Vaccine 2013, 31, 4596–4602. [Google Scholar] [CrossRef]
- Kosten, T.A.; Shen, X.Y.; O’Malley, P.W.; Kinsey, B.M.; Lykissa, E.D.; Orson, F.M.; Kosten, T.R. A morphine conjugate vaccine attenuates the behavioral effects of morphine in rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 45, 223–229. [Google Scholar] [CrossRef]
- Pravetoni, M.; Le Naour, M.; Tucker, A.M.; Harmon, T.M.; Hawley, T.M.; Portoghese, P.S.; Pentel, P.R. Reduced antinociception of opioids in rats and mice by vaccination with immunogens containing oxycodone and hydrocodone haptens. J. Med. Chem. 2013, 56, 915–923. [Google Scholar] [CrossRef]
- Walter, D.L.; Bian, Y.; Hu, H.; Hamid, F.A.; Rostamizadeh, K.; Vigliaturo, J.R.; DeHority, R.; Ehrich, M.; Runyon, S.; Pravetoni, M.; et al. The immunological and pharmacokinetic evaluation of Lipid-PLGA hybrid nanoparticle-based oxycodone vaccines. Biomaterials 2025, 313, 122758. [Google Scholar] [CrossRef]
- de Villiers, S.H.; Cornish, K.E.; Troska, A.J.; Pravetoni, M.; Pentel, P.R. Increased efficacy of a trivalent nicotine vaccine compared to a dose-matched monovalent vaccine when formulated with alum. Vaccine 2013, 31, 6185–6193. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Crouse, B.; Vigliaturo, J.; Wu, M.M.; Heimisdottir, D.; Kassick, A.J.; Averick, S.E.; Raleigh, M.D.; Pravetoni, M. Multivalent Vaccination Strategies Protect against Exposure to Polydrug Opioid and Stimulant Mixtures in Mice and Rats. ACS Pharmacol. Transl. Sci. 2024, 7, 363–374. [Google Scholar] [CrossRef]
- Hatsukami, D.K.; Rennard, S.; Jorenby, D.; Fiore, M.; Koopmeiners, J.; de Vos, A.; Horwith, G.; Pentel, P.R. Safety and immunogenicity of a nicotine conjugate vaccine in current smokers. Clin. Pharmacol. Ther. 2005, 78, 456–467. [Google Scholar] [CrossRef]
- Kosten, T.R.; Domingo, C.B.; Shorter, D.; Orson, F.; Green, C.; Somoza, E.; Sekerka, R.; Levin, F.R.; Mariani, J.J.; Stitzer, M.; et al. Vaccine for cocaine dependence: A randomized double-blind placebo-controlled efficacy trial. Drug Alcohol. Depend. 2014, 140, 42–47. [Google Scholar] [CrossRef]
- Kool, M.; Fierens, K.; Lambrecht, B.N. Alum adjuvant: Some of the tricks of the oldest adjuvant. J. Med. Microbiol. 2012, 61 Pt 7, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Lan, J.; Feng, D.; He, X.; Zhang, Q.; Zhang, R. Basic Properties and Development Status of Aluminum Adjuvants Used for Vaccines. Vaccines 2024, 12, 1187. [Google Scholar] [CrossRef]
- O’Hagan, D.T.; van der Most, R.; Lodaya, R.N.; Coccia, M.; Lofano, G. “World in motion”—Emulsion adjuvants rising to meet the pandemic challenges. NPJ Vaccines 2021, 6, 158. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Gong, H.; Sun, Q.; Yang, J.; Yan, X.; Xu, F. Research progress on emulsion vaccine adjuvants. Heliyon 2024, 10, e24662. [Google Scholar] [CrossRef]
- Freund, J.; McDermott, K. Sensitization to Horse Serum by Means of Adjuvants. Proc. Soc. Exp. Biol. Med. 1942, 49, 548–553. [Google Scholar] [CrossRef]
- Cai, X.; Tsuchikama, K.; Janda, K.D. Modulating cocaine vaccine potency through hapten fluorination. J. Am. Chem. Soc. 2013, 135, 2971–2974. [Google Scholar] [CrossRef]
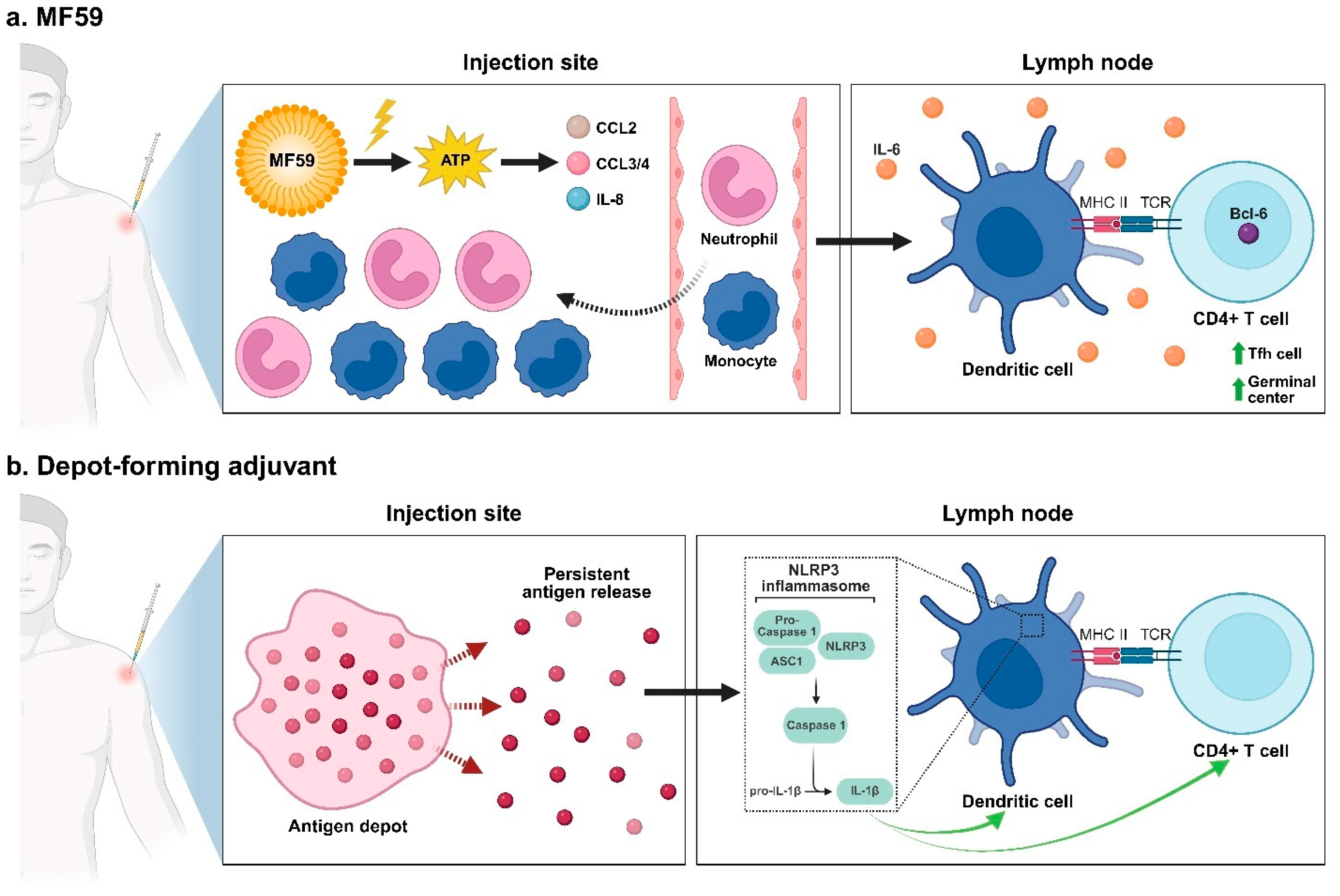
- O’Hagan, D.T.; Ott, G.S.; De Gregorio, E.; Seubert, A. The mechanism of action of MF59—An innately attractive adjuvant formulation. Vaccine 2012, 30, 4341–4348. [Google Scholar] [CrossRef] [PubMed]
- Morel, S.; Didierlaurent, A.; Bourguignon, P.; Delhaye, S.; Baras, B.; Jacob, V.; Planty, C.; Elouahabi, A.; Harvengt, P.; Carlsen, H.; et al. Adjuvant System AS03 containing α-tocopherol modulates innate immune response and leads to improved adaptive immunity. Vaccine 2011, 29, 2461–2473. [Google Scholar] [CrossRef]
- Vono, M.; Taccone, M.; Caccin, P.; Gallotta, M.; Donvito, G.; Falzoni, S.; Palmieri, E.; Pallaoro, M.; Rappuoli, R.; Di Virgilio, F.; et al. The adjuvant MF59 induces ATP release from muscle that potentiates response to vaccination. Proc. Natl. Acad. Sci. USA 2013, 110, 21095–21100. [Google Scholar] [CrossRef]
- Moni, S.S.; Abdelwahab, S.I.; Jabeen, A.; Elmobark, M.E.; Aqaili, D.; Ghoal, G.; Oraibi, B.; Farasani, A.M.; Jerah, A.A.; Alnajai, M.M.A.; et al. Advancements in Vaccine Adjuvants: The Journey from Alum to Nano Formulations. Vaccines 2023, 11, 1704. [Google Scholar] [CrossRef]
- Mastelic Gavillet, B.; Eberhardt, C.S.; Auderset, F.; Castellino, F.; Seubert, A.; Tregoning, J.S.; Lambert, P.H.; de Gregorio, E.; Del Giudice, G.; Siegrist, C.A. MF59 Mediates Its B Cell Adjuvanticity by Promoting T Follicular Helper Cells and Thus Germinal Center Responses in Adult and Early Life. J. Immunol. 2015, 194, 4836–4845. [Google Scholar] [CrossRef]
- Torten, M.; Miller, C.H.; Eisele, J.H.; Henderson, G.L.; Benjamini, E. Prevention of the effects of fentanyl by immunological means. Nature 1975, 253, 565–566. [Google Scholar] [CrossRef] [PubMed]
- Fox, B.S.; Kantak, K.M.; Edwards, M.A.; Black, K.M.; Bollinger, B.K.; Botka, A.J.; French, T.L.; Thompson, T.L.; Schad, V.C.; Greenstein, J.L.; et al. Efficacy of a therapeutic cocaine vaccine in rodent models. Nat. Med. 1996, 2, 1129–1132. [Google Scholar] [CrossRef]
- Pravetoni, M.; Keyler, D.E.; Raleigh, M.D.; Harris, A.C.; Lesage, M.G.; Mattson, C.K.; Pettersson, S.; Pentel, P.R. Vaccination against nicotine alters the distribution of nicotine delivered via cigarette smoke inhalation to rats. Biochem. Pharmacol. 2011, 81, 1164–1170. [Google Scholar] [CrossRef] [PubMed]
- Moreno, A.Y.; Mayorov, A.V.; Janda, K.D. Impact of distinct chemical structures for the development of a methamphetamine vaccine. J. Am. Chem. Soc. 2011, 133, 6587–6595. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.L.; Moreno, A.Y.; Aarde, S.M.; Creehan, K.M.; Vandewater, S.A.; Vaillancourt, B.D.; Wright, M.J., Jr.; Janda, K.D.; Taffe, M.A. A methamphetamine vaccine attenuates methamphetamine-induced disruptions in thermoregulation and activity in rats. Biol. Psychiatry 2013, 73, 721–728. [Google Scholar] [CrossRef]
- Collins, K.C.; Janda, K.D. Investigating hapten clustering as a strategy to enhance vaccines against drugs of abuse. Bioconjug Chem. 2014, 25, 593–600. [Google Scholar] [CrossRef]
- Madge, H.Y.R.; Alexander, S.; Azuar, A.; Zhang, J.; Koirala, P.; Burne, T.H.; Toth, I.; Stephenson, R.J. Synthetic Anti-Cocaine Nanoaccine Successfully Prevents Cocaine-Induced Hyperlocomotion. J. Med. Chem. 2023, 66, 12407–12419. [Google Scholar] [CrossRef]
- Robinson, C.; Baehr, C.; Schmiel, S.E.; Accetturo, C.; Mueller, D.L.; Pravetoni, M. Alum adjuvant is more effective than MF59 at prompting early germinal center formation in response to peptide-protein conjugates and enhancing efficacy of a vaccine against opioid use disorders. Hum. Vaccin. Immunother. 2019, 15, 909–917. [Google Scholar] [CrossRef] [PubMed]
- Moreno, A.Y.; Azar, M.R.; Warren, N.A.; Dickerson, T.J.; Koob, G.F.; Janda, K.D. A Critical Evaluation of a Nicotine Vaccine within a Self-Administration Behavioral Model. Mol. Pharm. 2010, 7, 431–441. [Google Scholar] [CrossRef]
- O’Hagan, D.T.; Friedland, L.R.; Hanon, E.; Didierlaurent, A.M. Towards an evidence based approach for the development of adjuvanted vaccines. Curr. Opin. Immunol. 2017, 47, 93–102. [Google Scholar] [CrossRef]
- Gnjatic, S.; Sawhney, N.B.; Bhardwaj, N. Toll-like receptor agonists: Are they good adjuvants? Cancer J. 2010, 16, 382–391. [Google Scholar] [CrossRef]
- Banstola, A.; Jeong, J.H.; Yook, S. Immunoadjuvants for cancer immunotherapy: A review of recent developments. Acta Biomater. 2020, 114, 16–30. [Google Scholar] [CrossRef]
- Chang, Z.L. Important aspects of Toll-like receptors, ligands and their signaling pathways. Inflamm. Res. 2010, 59, 791–808. [Google Scholar] [CrossRef] [PubMed]
- Gay, N.J.; Symmons, M.F.; Gangloff, M.; Bryant, C.E. Assembly and localization of Toll-like receptor signalling complexes. Nat. Rev. Immunol. 2014, 14, 546–558. [Google Scholar] [CrossRef]
- Kawasaki, T.; Kawai, T. Toll-like receptor signaling pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. TLR signaling. Cell Death Differ. 2006, 13, 816–825. [Google Scholar] [CrossRef]
- Honda, K.; Taniguchi, T. IRFs: Master regulators of signalling by Toll-like receptors and cytosolic pattern-recognition receptors. Nat. Rev. Immunol. 2006, 6, 644–658. [Google Scholar] [CrossRef]
- Matyas, G.R.; Mayorov, A.V.; Rice, K.C.; Jacobson, A.E.; Cheng, K.; Iyer, M.R.; Li, F.; Beck, Z.; Janda, K.D.; Alving, C.R. Liposomes containing monophosphoryl lipid A: A potent adjuvant system for inducing antibodies to heroin hapten analogs. Vaccine 2013, 31, 2804–2810. [Google Scholar] [CrossRef]
- Bremer, P.T.; Kimishima, A.; Schlosburg, J.E.; Zhou, B.; Collins, K.C.; Janda, K.D. Combatting Synthetic Designer Opioids: A Conjugate Vaccine Ablates Lethal Doses of Fentanyl Class Drugs. Angew. Chem. Int. Ed. Engl. 2016, 55, 3772–3775. [Google Scholar] [CrossRef]
- Bremer, P.T.; Schlosburg, J.E.; Banks, M.L.; Steele, F.F.; Zhou, B.; Poklis, J.L.; Janda, K.D. Development of a Clinically Viable Heroin Vaccine. J. Am. Chem. Soc. 2017, 139, 8601–8611. [Google Scholar] [CrossRef] [PubMed]
- Kimishima, A.; Wenthur, C.J.; Eubanks, L.M.; Sato, S.; Janda, K.D. Cocaine Vaccine Development: Evaluation of Carrier and Adjuvant Combinations That Activate Multiple Toll-Like Receptors. Mol. Pharm. 2016, 13, 3884–3890. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.K.; Davidson, M.; Feehan, J.; Matsoukas, J.M.; Nurgali, K.; Apostolopoulos, V. A methamphetamine vaccine using short monoamine and diamine peptide linkers and poly-mannose. Bioorganic Med. Chem. 2024, 113, 117930. [Google Scholar] [CrossRef] [PubMed]
- Haile, C.N.; Varner, K.J.; Huijing, X.; Arora, R.; Orson, F.M.; Kosten, T.R.; Kosten, T.A. Active and Passive Immunization with an Anti-Methamphetamine Vaccine Attenuates the Behavioral and Cardiovascular Effects of Methamphetamine. Vaccines 2022, 10, 1508. [Google Scholar] [CrossRef]
- Barrientos, R.C.; Whalen, C.; Torres, O.B.; Sulima, A.; Bow, E.W.; Komla, E.; Beck, Z.; Jacobson, A.E.; Rice, K.C.; Matyas, G.R. Bivalent Conjugate Vaccine Induces Dual Immunogenic Response That Attenuates Heroin and Fentanyl Effects in Mice. Bioconjugate Chem. 2021, 32, 2295–2306. [Google Scholar] [CrossRef] [PubMed]
- Lockner, J.W.; Ho, S.O.; McCague, K.C.; Chiang, S.M.; Do, T.Q.; Fujii, G.; Janda, K.D. Enhancing nicotine vaccine immunogenicity with liposomes. Bioorg Med. Chem. Lett. 2013, 23, 975–978. [Google Scholar] [CrossRef]
- Powers, N.; Massena, C.; Crouse, B.; Smith, M.; Hicks, L.; Evans, J.T.; Miller, S.; Pravetoni, M.; Burkhart, D. Self-Adjuvanting TLR7/8 Agonist and Fentanyl Hapten Co-Conjugate Achieves Enhanced Protection against Fentanyl Challenge. Bioconjug Chem. 2023, 34, 1811–1821. [Google Scholar] [CrossRef]
- Hwang, C.S.; Bremer, P.T.; Wenthur, C.J.; Ho, S.O.; Chiang, S.; Ellis, B.; Zhou, B.; Fujii, G.; Janda, K.D. Enhancing Efficacy and Stability of an Antiheroin Vaccine: Examination of Antinociception, Opioid Binding Profile, and Lethality. Mol. Pharm. 2018, 15, 1062–1072. [Google Scholar] [CrossRef]
- Stevens, M.W.; Gunnell, M.G.; Tawney, R.; Owens, S.M. Optimization of a methamphetamine conjugate vaccine for antibody production in mice. Int. Immunopharmacol. 2016, 35, 137–141. [Google Scholar] [CrossRef]
- Kumar, S.; Sunagar, R.; Gosselin, E. Bacterial Protein Toll-Like-Receptor Agonists: A Novel Perspective on Vaccine Adjuvants. Front. Immunol. 2019, 10, 1144. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Dinamarca, D.A.; Salazar, M.L.; Castillo, B.N.; Manubens, A.; Vasquez, A.E.; Salazar, F.; Becker, M.I. Protein-Based Adjuvants for Vaccines as Immunomodulators of the Innate and Adaptive Immune Response: Current Knowledge, Challenges, and Future Opportunities. Pharmaceutics 2022, 14, 1671. [Google Scholar] [CrossRef]
- Taylor, D.N.; Treanor, J.J.; Sheldon, E.A.; Johnson, C.; Umlauf, S.; Song, L.; Kavita, U.; Liu, G.; Tussey, L.; Ozer, K.; et al. Development of VAX128, a recombinant hemagglutinin (HA) influenza-flagellin fusion vaccine with improved safety and immune response. Vaccine 2012, 30, 5761–5769. [Google Scholar] [CrossRef]
- Deng, J.; Yu, X.-Q.; Wang, P.-H. Inflammasome activation and Th17 responses. Mol. Immunol. 2019, 107, 142–164. [Google Scholar] [CrossRef]
- Lisk, C.; Yuen, R.; Kuniholm, J.; Antos, D.; Reiser, M.L.; Wetzler, L.M. Toll-Like Receptor Ligand Based Adjuvant, PorB, Increases Antigen Deposition on Germinal Center Follicular Dendritic Cells While Enhancing the Follicular Dendritic Cells Network. Front. Immunol. 2020, 11, 1254. [Google Scholar] [CrossRef] [PubMed]
- Reiser, M.L.; Mosaheb, M.M.; Lisk, C.; Platt, A.; Wetzler, L.M. The TLR2 Binding Neisserial Porin PorB Enhances Antigen Presenting Cell Trafficking and Cross-presentation. Sci. Rep. 2017, 7, 736. [Google Scholar] [CrossRef] [PubMed]
- Lamontagne, F.; Khatri, V.; St-Louis, P.; Bourgault, S.; Archambault, D. Vaccination Strategies Based on Bacterial Self-Assembling Proteins as Antigen Delivery Nanoscaffolds. Vaccines 2022, 10, 1920. [Google Scholar] [CrossRef]
- Brouwer, P.J.M.; Antanasijevic, A.; Berndsen, Z.; Yasmeen, A.; Fiala, B.; Bijl, T.P.L.; Bontjer, I.; Bale, J.B.; Sheffler, W.; Allen, J.D.; et al. Enhancing and shaping the immunogenicity of native-like HIV-1 envelope trimers with a two-component protein nanoparticle. Nat. Commun. 2019, 10, 4272. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, X.; Bian, Y.; Wang, S.; Chai, Q.; Guo, Z.; Wang, Z.; Zhu, P.; Peng, H.; Yan, X.; et al. Dual-targeting nanoparticle vaccine elicits a therapeutic antibody response against chronic hepatitis B. Nat. Nanotechnol. 2020, 15, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Marcandalli, J.; Fiala, B.; Ols, S.; Perotti, M.; de van der Schueren, W.; Snijder, J.; Hodge, E.; Benhaim, M.; Ravichandran, R.; Carter, L.; et al. Induction of Potent Neutralizing Antibody Responses by a Designed Protein Nanoparticle Vaccine for Respiratory Syncytial Virus. Cell 2019, 176, 1420–1431.e17. [Google Scholar] [CrossRef]
- Cox, J.R.; Blazeck, J. Protein engineering: A driving force toward synthetic immunology. Trends Biotechnol. 2022, 40, 509–521. [Google Scholar] [CrossRef] [PubMed]
- J, L.A.A.; Pa, P.; Seng, C.Y.; Rhee, J.H.; Lee, S.E. Protein nanocages: A new frontier in mucosal vaccine delivery and immune activation. Hum. Vaccin. Immunother. 2025, 21, 2492906. [Google Scholar] [CrossRef]
- Lockner, J.W.; Eubanks, L.M.; Choi, J.L.; Lively, J.M.; Schlosburg, J.E.; Collins, K.C.; Globisch, D.; Rosenfeld-Gunn, R.J.; Wilson, I.A.; Janda, K.D. Flagellin as carrier and adjuvant in cocaine vaccine development. Mol. Pharm. 2015, 12, 653–662. [Google Scholar] [CrossRef]
- Sanderson, S.D.; Cheruku, S.R.; Padmanilayam, M.P.; Vennerstrom, J.L.; Thiele, G.M.; Palmatier, M.I.; Bevins, R.A. Immunization to nicotine with a peptide-based vaccine composed of a conformationally biased agonist of C5a as a molecular adjuvant. Int. Immunopharmacol. 2003, 3, 137–146. [Google Scholar] [CrossRef]
- Rudra, J.S.; Ding, Y.; Neelakantan, H.; Ding, C.; Appavu, R.; Stutz, S.; Snook, J.D.; Chen, H.; Cunningham, K.A.; Zhou, J. Suppression of Cocaine-Evoked Hyperactivity by Self-Adjuvanting and Multivalent Peptide Nanofiber Vaccines. ACS Chem. Neurosci. 2016, 7, 546–552. [Google Scholar] [CrossRef]
- Liang, X.; Liu, R.; Chen, C.; Ji, F.; Li, T. Opioid System Modulates the Immune Function: A Review. Transl. Perioper. Pain. Med. 2016, 1, 5–13. [Google Scholar]
- Cui, A.; Huang, T.; Li, S.; Ma, A.; Pérez, J.L.; Sander, C.; Keskin, D.B.; Wu, C.J.; Fraenkel, E.; Hacohen, N. Dictionary of immune responses to cytokines at single-cell resolution. Nature 2024, 625, 377–384. [Google Scholar] [CrossRef]
- Zhang, J.M.; An, J. Cytokines, inflammation, and pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef]
- Dinarello, C.A. Historical insights into cytokines. Eur. J. Immunol. 2007, 37 (Suppl. S1), S34–S45. [Google Scholar] [CrossRef] [PubMed]
- Cameron, M.J.; Kelvin, D.J. Cytokines and chemokines--their receptors and their genes: An overview. Adv. Exp. Med. Biol. 2003, 520, 8–32. [Google Scholar]
- Justiz Vaillant, A.A.; Qurie, A. Interleukin. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Bachmann, M.F.; Oxenius, A. Interleukin 2: From immunostimulation to immunoregulation and back again. EMBO Rep. 2007, 8, 1142–1148. [Google Scholar] [CrossRef] [PubMed]
- Ghiasi, H.; Cai, S.; Slanina, S.M.; Perng, G.C.; Nesburn, A.B.; Wechsler, S.L. The role of interleukin (IL)-2 and IL-4 in herpes simplex virus type 1 ocular replication and eye disease. J. Infect. Dis. 1999, 179, 1086–1093. [Google Scholar] [CrossRef]
- Akdis, M.; Burgler, S.; Crameri, R.; Eiwegger, T.; Fujita, H.; Gomez, E.; Klunker, S.; Meyer, N.; O’Mahony, L.; Palomares, O.; et al. Interleukins, from 1 to 37, and interferon-γ: Receptors, functions, and roles in diseases. J. Allergy Clin. Immunol. 2011, 127, 701–721.e70. [Google Scholar] [CrossRef] [PubMed]
- Vignali, D.A.; Kuchroo, V.K. IL-12 family cytokines: Immunological playmakers. Nat. Immunol. 2012, 13, 722–728. [Google Scholar] [CrossRef]
- Yasuda, K.; Nakanishi, K.; Tsutsui, H. Interleukin-18 in Health and Disease. Int. J. Mol. Sci. 2019, 20, 649. [Google Scholar] [CrossRef]
- Mihaescu, G.; Chifiriuc, M.C.; Filip, R.; Bleotu, C.; Ditu, L.M.; Constantin, M.; Cristian, R.-E.; Grigore, R.; Bertesteanu, S.V.; Bertesteanu, G.; et al. Role of interferons in the antiviral battle: From virus-host crosstalk to prophylactic and therapeutic potential in SARS-CoV-2 infection. Front. Immunol. 2024, 14, 1273604. [Google Scholar] [CrossRef]
- McNab, F.; Mayer-Barber, K.; Sher, A.; Wack, A.; O’Garra, A. Type I interferons in infectious disease. Nat. Rev. Immunol. 2015, 15, 87–103. [Google Scholar] [CrossRef]
- Tovey, M.G.; Lallemand, C. Adjuvant activity of cytokines. Methods Mol. Biol. 2010, 626, 287–309. [Google Scholar] [PubMed]
- Rock, K.L.; Reits, E.; Neefjes, J. Present Yourself! By MHC Class I and MHC Class II Molecules. Trends Immunol. 2016, 37, 724–737. [Google Scholar] [CrossRef] [PubMed]
- Schoenborn, J.R.; Wilson, C.B. Regulation of interferon-gamma during innate and adaptive immune responses. Adv. Immunol. 2007, 96, 41–101. [Google Scholar]
- Bian, Y.; Walter, D.L.; Zhang, C. Efficiency of Interferon-γ in Activating Dendritic Cells and Its Potential Synergy with Toll-like Receptor Agonists. Viruses 2023, 15, 1198. [Google Scholar] [CrossRef]
- Parameswaran, N.; Patial, S. Tumor necrosis factor-α signaling in macrophages. Crit. Rev. Eukaryot. Gene Expr. 2010, 20, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Sedger, L.M.; McDermott, M.F. TNF and TNF-receptors: From mediators of cell death and inflammation to therapeutic giants—past, present and future. Cytokine Growth Factor. Rev. 2014, 25, 453–472. [Google Scholar] [CrossRef]
- Wajant, H.; Siegmund, D. TNFR1 and TNFR2 in the Control of the Life and Death Balance of Macrophages. Front. Cell Dev. Biol. 2019, 7, 91. [Google Scholar] [CrossRef]
- Rahman, T.; Das, A.; Abir, M.H.; Nafiz, I.H.; Mahmud, A.R.; Sarker, M.R.; Emran, T.B.; Hassan, M.M. Cytokines and their role as immunotherapeutics and vaccine Adjuvants: The emerging concepts. Cytokine 2023, 169, 156268. [Google Scholar] [CrossRef]
- Capitini, C.M.; Fry, T.J.; Mackall, C.L. Cytokines as Adjuvants for Vaccine and Cellular Therapies for Cancer. Am. J. Immunol. 2009, 5, 65–83. [Google Scholar] [CrossRef] [PubMed]
- Kayamuro, H.; Yoshioka, Y.; Abe, Y.; Arita, S.; Katayama, K.; Nomura, T.; Yoshikawa, T.; Kubota-Koketsu, R.; Ikuta, K.; Okamoto, S.; et al. Interleukin-1 Family Cytokines as Mucosal Vaccine Adjuvants for Induction of Protective Immunity against Influenza Virus. J. Virol. 2010, 84, 12703–12712. [Google Scholar] [CrossRef]
- Laudenbach, M.; Baruffaldi, F.; Robinson, C.; Carter, P.; Seelig, D.; Baehr, C.; Pravetoni, M. Blocking interleukin-4 enhances efficacy of vaccines for treatment of opioid abuse and prevention of opioid overdose. Sci. Rep. 2018, 8, 5508. [Google Scholar] [CrossRef]
- Crouse, B.; Robinson, C.; Huseby Kelcher, A.; Laudenbach, M.; Abrahante, J.E.; Pravetoni, M. Mechanisms of interleukin 4 mediated increase in efficacy of vaccines against opioid use disorders. npj Vaccines 2020, 5, 99. [Google Scholar] [CrossRef]
- Crouse, B.; Baehr, C.; Hicks, D.; Pravetoni, M. IL-4 Predicts the Efficacy of a Candidate Antioxycodone Vaccine and Alters Vaccine-Specific Antibody-Secreting Cell Proliferation in Mice. J. Immunol. 2023, 210, 1272–1280. [Google Scholar] [CrossRef] [PubMed]
- Kvistad, D.; Pallikkuth, S.; Sirupangi, T.; Pahwa, R.; Kizhner, A.; Petrovas, C.; Villinger, F.; Pahwa, S. IL-21 enhances influenza vaccine responses in aged macaques with suppressed SIV infection. JCI Insight 2021, 6, e150888. [Google Scholar] [CrossRef]
- Quast, I.; Dvorscek, A.R.; Pattaroni, C.; Steiner, T.M.; McKenzie, C.I.; Pitt, C.; O’Donnell, K.; Ding, Z.; Hill, D.L.; Brink, R.; et al. Interleukin-21, acting beyond the immunological synapse, independently controls T follicular helper and germinal center B cells. Immunity 2022, 55, 1414–1430.e5. [Google Scholar] [CrossRef]
- Cheng, E.M.; Tsarovsky, N.W.; Sondel, P.M.; Rakhmilevich, A.L. Interleukin-12 as an in situ cancer vaccine component: A review. Cancer Immunol. Immunother. 2022, 71, 2057–2065. [Google Scholar] [CrossRef]
- Jia, Z.; Ragoonanan, D.; Mahadeo, K.M.; Gill, J.; Gorlick, R.; Shpal, E.; Li, S. IL12 immune therapy clinical trial review: Novel strategies for avoiding CRS-associated cytokines. Front. Immunol. 2022, 13, 952231. [Google Scholar] [CrossRef]
- Bracci, L.; Canini, I.; Venditti, M.; Spada, M.; Puzelli, S.; Donatelli, I.; Belardelli, F.; Proietti, E. Type I IFN as a vaccine adjuvant for both systemic and mucosal vaccination against influenza virus. Vaccine 2006, 24, S56–S57. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Zhang, L.; Cao, L.; Jiang, J.; Shi, Y.; Guo, H.; Wang, Y.; Li, H.; Zhang, Y. Preliminary Study on Type I Interferon as a Mucosal Adjuvant for Human Respiratory Syncytial Virus F Protein. Vaccines 2024, 12, 1297. [Google Scholar] [CrossRef]
- Petrina, M.; Martin, J.; Basta, S. Granulocyte macrophage colony-stimulating factor has come of age: From a vaccine adjuvant to antiviral immunotherapy. Cytokine Growth Factor. Rev. 2021, 59, 101–110. [Google Scholar] [CrossRef]
- Zhao, W.; Zhao, G.; Wang, B. Revisiting GM-CSF as an adjuvant for therapeutic vaccines. Cell Mol. Immunol. 2018, 15, 187–189. [Google Scholar] [CrossRef]
- Brooks, D.G.; Lee, A.M.; Elsaesser, H.; McGavern, D.B.; Oldstone, M.B.A. IL-10 blockade facilitates DNA vaccine-induced T cell responses and enhances clearance of persistent virus infection. J. Exp. Med. 2008, 205, 533–541. [Google Scholar] [CrossRef]
- Ni, G.; Wang, T.; Walton, S.; Zhu, B.; Chen, S.; Wu, X.; Wang, Y.; Wei, M.Q.; Liu, X. Manipulating IL-10 signalling blockade for better immunotherapy. Cell. Immunol. 2015, 293, 126–129. [Google Scholar] [CrossRef]
- Kelly, A.M.; McCarthy, K.N.; Claxton, T.J.; Carlile, S.R.; O’Brien, E.C.; Vozza, E.G.; Mills, K.H.; McLoughlin, R.M. IL-10 inhibition during immunization improves vaccine-induced protection against Staphylococcus aureus infection. JCI Insight 2024, 9, e178216. [Google Scholar] [CrossRef]
- Cerutti, A. The regulation of IgA class switching. Nat. Rev. Immunol. 2008, 8, 421–434. [Google Scholar] [CrossRef]
- Deng, Z.; Fan, T.; Xiao, C.; Tian, H.; Zheng, Y.; Li, C.; He, J. TGF-β signaling in health, disease and therapeutics. Signal Transduct. Target. Ther. 2024, 9, 61. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Smith, D.; Zhao, Z.; Harmon, T.; Pentel, P.R.; Ehrich, M.; Zhang, C. Alum as an adjuvant for nanoparticle based vaccines: A case study with a hybrid nanoparticle-based nicotine vaccine. Nanomed. Nanotechnol. Biol. Med. 2019, 20, 102023. [Google Scholar] [CrossRef] [PubMed]
- Pravetoni, M.; Vervacke, J.S.; Distefano, M.D.; Tucker, A.M.; Laudenbach, M.; Pentel, P.R. Effect of currently approved carriers and adjuvants on the pre-clinical efficacy of a conjugate vaccine against oxycodone in mice and rats. PLoS ONE 2014, 9, e96547. [Google Scholar] [CrossRef] [PubMed]
- Mata-Haro, V.; Cekic, C.; Martin, M.; Chilton, P.M.; Casella, C.R.; Mitchell, T.C. The Vaccine Adjuvant Monophosphoryl Lipid A as a TRIF-Biased Agonist of TLR4. Science 2007, 316, 1628–1632. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.S.; Ho, B.; Leung, B.P.; Ding, J.L. TLR cross-talk confers specificity to innate immunity. Int. Rev. Immunol. 2014, 33, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Napolitani, G.; Rinaldi, A.; Bertoni, F.; Sallusto, F.; Lanzavecchia, A. Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1–polarizing program in dendritic cells. Nat. Immunol. 2005, 6, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Bohnenkamp, H.R.; Papazisis, K.T.; Burchell, J.M.; Taylor-Papadimitriou, J. Synergism of Toll-like receptor-induced interleukin-12p70 secretion by monocyte-derived dendritic cells is mediated through p38 MAPK and lowers the threshold of T-helper cell type I responses. Cell. Immunol. 2007, 247, 72–84. [Google Scholar] [CrossRef]
- Mäkelä, S.M.; Strengell, M.; Pietilä, T.E.; Österlund, P.; Julkunen, I. Multiple signaling pathways contribute to synergistic TLR ligand-dependent cytokine gene expression in human monocyte-derived macrophages and dendritic cells. J. Leukoc. Biol. 2009, 85, 664–672. [Google Scholar] [CrossRef]
- Antúnez, L.R.; Livingston, A.; Berkland, C.; Dhar, P. Physiochemical Properties of Aluminum Adjuvants Elicit Differing Reorganization of Phospholipid Domains in Model Membranes. Mol. Pharm. 2016, 13, 1731–1737. [Google Scholar] [CrossRef]
- Badran, G.; Angrand, L.; Masson, J.-D.; Crépeaux, G.; David, M.-O. Physico-chemical properties of aluminum adjuvants in vaccines: Implications for toxicological evaluation. Vaccine 2022, 40, 4881–4888. [Google Scholar] [CrossRef]
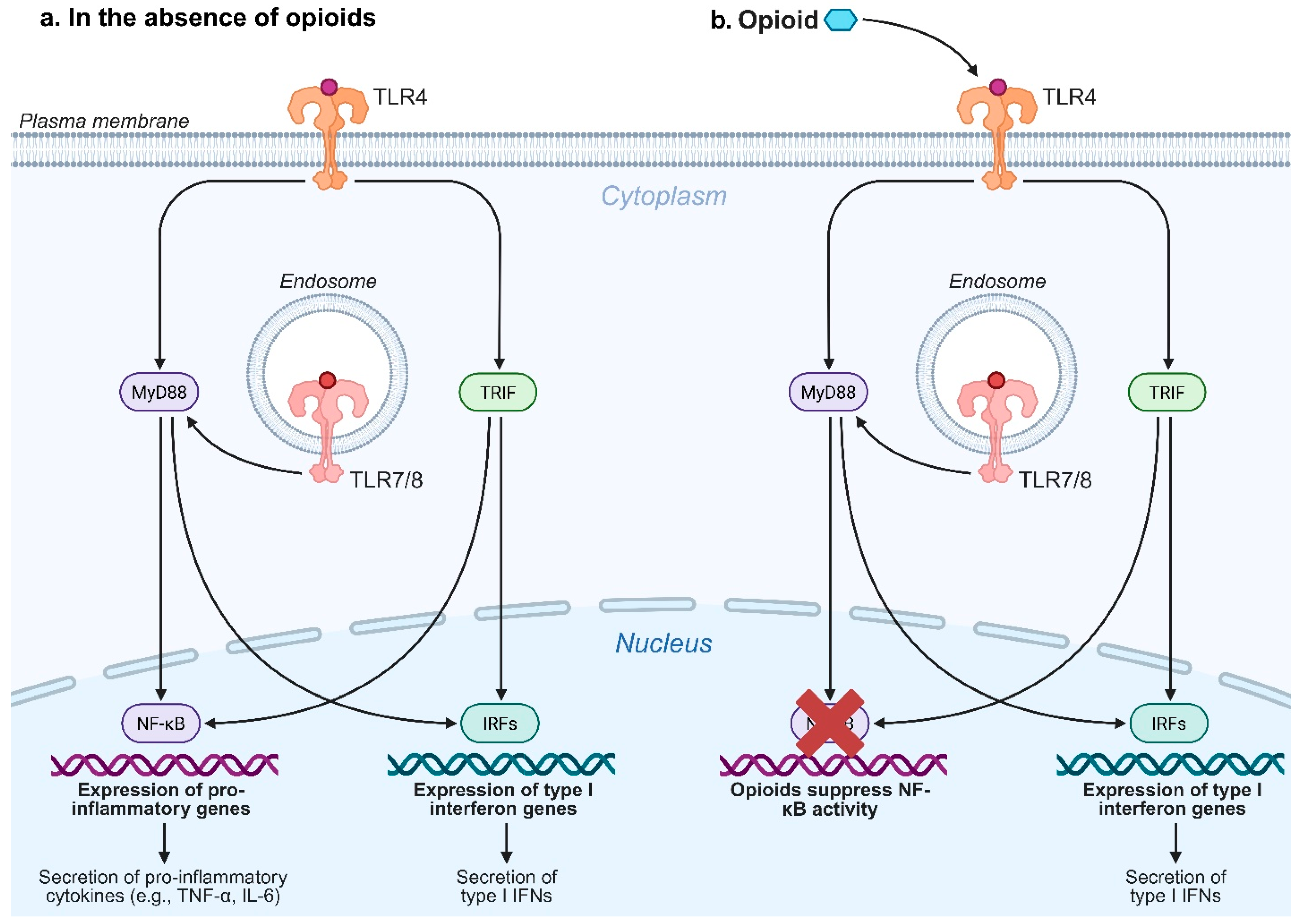
- Zhang, P.; Yang, M.; Chen, C.; Liu, L.; Wei, X.; Zeng, S. Toll-Like Receptor 4 (TLR4)/Opioid Receptor Pathway Crosstalk and Impact on Opioid Analgesia, Immune Function, and Gastrointestinal Motility. Front. Immunol. 2020, 11, 1455. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, C.; Li, R.; Wang, Z.; Yuan, Y.; Li, H.; Fu, Z.; Zhou, M.; Zhao, L. Monophosphoryl-Lipid A (MPLA) is an Efficacious Adjuvant for Inactivated Rabies Vaccines. Viruses 2019, 11, 1118. [Google Scholar] [CrossRef]
- Stevens, C.; Aravind, S.; Das, S.; Davis, R. Pharmacological characterization of LPS and opioid interactions at the toll-like receptor 4. Br. J. Pharmacol. 2013, 168, 1421–1429. [Google Scholar] [CrossRef]
- Madera-Salcedo, I.K.; Cruz, S.L.; Gonzalez-Espinosa, C. Morphine Prevents Lipopolysaccharide-Induced TNF Secretion in Mast Cells Blocking IκB Kinase Activation and SNAP-23 Phosphorylation: Correlation with the Formation of a β-Arrestin/TRAF6 Complex. J. Immunol. 2013, 191, 3400–3409. [Google Scholar] [CrossRef]
- Witherow, D.S.; Garrison, T.R.; Miller, W.E.; Lefkowitz, R.J. β-Arrestin inhibits NF-κB activity by means of its interaction with the NF-κB inhibitor IκBα. Proc. Natl. Acad. Sci. USA 2004, 101, 8603–8607. [Google Scholar] [CrossRef]
- Bencsics, A.; Elenkov, I.J.; Vizi, E.S. Effect of morphine on lipopolysaccharide-induced tumor necrosis factor-α production in vivo: Involvement of the sympathetic nervous system. J. Neuroimmunol. 1997, 73, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Seubert, A.; Calabro, S.; Santini, L.; Galli, B.; Genovese, A.; Valentini, S.; Aprea, S.; Colaprico, A.; D’Oro, U.; Giuliani, M.M.; et al. Adjuvanticity of the oil-in-water emulsion MF59 is independent of Nlrp3 inflammasome but requires the adaptor protein MyD88. Proc. Natl. Acad. Sci. USA 2011, 108, 11169–11174. [Google Scholar] [CrossRef]
- Seubert, A.; Monaci, E.; Pizza, M.; O’Hagan, D.T.; Wack, A. The Adjuvants Aluminum Hydroxide and MF59 Induce Monocyte and Granulocyte Chemoattractants and Enhance Monocyte Differentiation toward Dendritic Cells1. J. Immunol. 2008, 180, 5402–5412. [Google Scholar] [CrossRef] [PubMed]
- Calabro, S.; Tortoli, M.; Baudner, B.C.; Pacitto, A.; Cortese, M.; O’Hagan, D.T.; De Gregorio, E.; Seubert, A.; Wack, A. Vaccine adjuvants alum and MF59 induce rapid recruitment of neutrophils and monocytes that participate in antigen transport to draining lymph nodes. Vaccine 2011, 29, 1812–1823. [Google Scholar] [CrossRef]
- Ellebedy, A.H.; Lupfer, C.; Ghoneim, H.E.; DeBeauchamp, J.; Kanneganti, T.D.; Webby, R.J. Inflammasome-independent role of the apoptosis-associated speck-like protein containing CARD (ASC) in the adjuvant effect of MF59. Proc. Natl. Acad. Sci. USA 2011, 108, 2927–2932. [Google Scholar] [CrossRef]
- Chakarov, S.; Fazilleau, N. Monocyte-derived dendritic cells promote T follicular helper cell differentiation. EMBO Mol. Med. 2014, 6, 590–603. [Google Scholar] [CrossRef]
- Choi, J.; Diao, H.; Faliti, C.E.; Truong, J.; Rossi, M.; Bélanger, S.; Yu, B.; Goldrath, A.W.; Pipkin, M.E.; Crotty, S. Bcl-6 is the nexus transcription factor of T follicular helper cells via repressor-of-repressor circuits. Nat. Immunol. 2020, 21, 777–789. [Google Scholar] [CrossRef]
- AWATE, S.; Babiuk, L.A.; Mutwiri, G. Mechanisms of Action of Adjuvants. Front. Immunol. 2013, 4, 114. [Google Scholar] [CrossRef] [PubMed]
- Shenderov, K.; Barber, D.L.; Mayer-Barber, K.D.; Gurcha, S.S.; Jankovic, D.; Feng, C.G.; Oland, S.; Hieny, S.; Caspar, P.; Yamasaki, S.; et al. Cord factor and peptidoglycan recapitulate the Th17-promoting adjuvant activity of mycobacteria through mincle/CARD9 signaling and the inflammasome. J. Immunol. 2013, 190, 5722–5730. [Google Scholar] [CrossRef]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef]
- Plein, L.M.; Rittner, H.L. Opioids and the immune system—Friend or foe. Br. J. Pharmacol. 2018, 175, 2717–2725. [Google Scholar] [CrossRef] [PubMed]
- Franchi, S.; Moschetti, G.; Amodeo, G.; Sacerdote, P. Do All Opioid Drugs Share the Same Immunomodulatory Properties? A Review From Animal and Human Studies. Front. Immunol. 2019, 10, 2914. [Google Scholar] [CrossRef]
- Wen, S.; Jiang, Y.; Liang, S.; Cheng, Z.; Zhu, X.; Guo, Q. Opioids Regulate the Immune System: Focusing on Macrophages and Their Organelles. Front. Pharmacol. 2021, 12, 814241. [Google Scholar] [CrossRef]
- Manolova, V.; Flace, A.; Bauer, M.; Schwarz, K.; Saudan, P.; Bachmann, M.F. Nanoparticles target distinct dendritic cell populations according to their size. Eur. J. Immunol. 2008, 38, 1404–1413. [Google Scholar] [CrossRef]
- Howard, G.P.; Verma, G.; Ke, X.; Thayer, W.M.; Hamerly, T.; Baxter, V.K.; Lee, J.E.; Dinglasan, R.R.; Mao, H.-Q. Critical size limit of biodegradable nanoparticles for enhanced lymph node trafficking and paracortex penetration. Nano Res. 2019, 12, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Catenacci, L.; Rossi, R.; Sechi, F.; Buonocore, D.; Sorrenti, M.; Perteghella, S.; Peviani, M.; Bonferoni, M.C. Effect of Lipid Nanoparticle Physico-Chemical Properties and Composition on Their Interaction with the Immune System. Pharmaceutics 2024, 16, 1521. [Google Scholar] [CrossRef] [PubMed]
- Lonez, C.; Vandenbranden, M.; Ruysschaert, J.-M. Cationic lipids activate intracellular signaling pathways. Adv. Drug Deliv. Rev. 2012, 64, 1749–1758. [Google Scholar] [CrossRef]
- Kasturi, S.P.; Skountzou, I.; Albrecht, R.A.; Koutsonanos, D.; Hua, T.; Nakaya, H.I.; Ravindran, R.; Stewart, S.; Alam, M.; Kwissa, M.; et al. Programming the magnitude and persistence of antibody responses with innate immunity. Nature 2011, 470, 543–547. [Google Scholar] [CrossRef]
- Liu, H.; Moynihan, K.D.; Zheng, Y.; Szeto, G.L.; Li, A.V.; Huang, B.; Van Egeren, D.S.; Park, C.; Irvine, D.J. Structure-based programming of lymph-node targeting in molecular vaccines. Nature 2014, 507, 519–522. [Google Scholar] [CrossRef]
- Phung, I.; Rodrigues, K.A.; Marina-Zárate, E.; Maiorino, L.; Pahar, B.; Lee, W.H.; Melo, M.; Kaur, A.; Allers, C.; Fahlberg, M.; et al. A combined adjuvant approach primes robust germinal center responses and humoral immunity in non-human primates. Nat. Commun. 2023, 14, 7107. [Google Scholar] [CrossRef] [PubMed]
- Hartmann-Boyce, J.; Cahill, K.; Hatsukami, D.; Cornuz, J. Nicotine vaccines for smoking cessation. Cochrane Database Syst. Rev. 2012, 2012, Cd007072. [Google Scholar] [CrossRef] [PubMed]
- Kosten, T.R. Vaccines as Immunotherapies for Substance Use Disorders. Am. J. Psychiatry 2024, 181, 362–371. [Google Scholar] [CrossRef]
- Hall, W.; Carter, L. Ethical issues in using a cocaine vaccine to treat and prevent cocaine abuse and dependence. J. Med. Ethics 2004, 30, 337–340. [Google Scholar] [CrossRef]
- Hall, W.; Gartner, C. Ethical and policy issues in using vaccines to treat and prevent cocaine and nicotine dependence. Curr. Opin. Psychiatry 2011, 24, 191–196. [Google Scholar] [CrossRef]
- Young, M.J.; Sisti, D.A.; Rimon-Greenspan, H.; Schwartz, J.L.; Caplan, A.L. Immune to addiction: The ethical dimensions of vaccines against substance abuse. Nat. Immunol. 2012, 13, 521–524. [Google Scholar] [CrossRef]
- IOM/NRC. 4 Behavioral Responses and Consent. In New Treatments for Addiction: Behavioral, Ethical, Legal, and Social Questions; The National Academies Press: Washington, DC, USA, 2004. [Google Scholar]
- Wartenweiler, V.; Chung, G.; Stewart, A.; Wenthur, C. Pharmacy stakeholder reports on ethical and logistical considerations in anti-opioid vaccine development. BMC Med. Ethics 2021, 22, 30. [Google Scholar] [CrossRef]
- van Delden, J.J.M.; van der Graaf, R. Revised CIOMS International Ethical Guidelines for Health-Related Research Involving Humans. JAMA 2017, 317, 135–136. [Google Scholar] [CrossRef]
- International Ethical Guidelines for Health-related Research Involving Humans: Prepared by the Council for International Organizations of Medical Sciences (CIOMS) in Collaboration with the World Health Organization (WHO); WHO: Geneva, Switzerland, 2016.
- Bloom, B.T.; Bushell, M.J. Vaccines against Drug Abuse-Are We There Yet? Vaccines 2022, 10, 860. [Google Scholar] [CrossRef] [PubMed]
- Vasiliu, O. Current Trends and Perspectives in the Immune Therapy for Substance Use Disorders. Front. Psychiatry 2022, 13, 882491. [Google Scholar] [CrossRef] [PubMed]


| Adjuvant | Vaccine Target | Species Tested | Performance | References |
|---|---|---|---|---|
| Aluminum hydroxide (AH) | Nicotine (NicVAX®, Niccine®), Cocaine (TA-CD) | Human | Safe with moderate immunogenicity; limited efficacy in sustaining abstinence; high responders showed partial benefits | Hatsukami et al. [83], Hoogsteder et al. [58,60], Tonstad et al. [59], Martell et al. [52], Kosten et al. [84] |
| Oxycodone | Mouse | Alum elicited a more robust antibody response than the TLR agonists R848 and MPLA | Walter et al. [80] | |
| Methamphetamine (ICKLH-SMO9), morphine (KLH-6-SM), nicotine (trivalent) | Rat | Effective in terms of immunogenicity and protective efficacy; used in multivalent constructs | Rüedi-Bettschen et al. [77], Kosten et al. [78], de Villiers et al. [81] | |
| Multivalent against fentanyl, carfentanil, oxycodone, heroin, methamphetamine, and their analogs or metabolites | Mouse, rat | Effective in inducing independent antibody responses against the respective targets | Song et al. [82] | |
| Alum adjuvant not specified | Oxycodone (6OXY(Gly)4-KLH) | Mouse, rat | Effective against both oxycodone and hydrocodone | Pravetoni et al. [79] |
| Adjuvant | Vaccine Target | Species Tested | Performance | References |
|---|---|---|---|---|
| Freund’s Adjuvant | Fentanyl (FEN-BGG), Cocaine (COC-BSA), Nicotine (3′-AmNic-rEPA) | Mouse, rat | Strong antibody responses; not approved for human use due to safety | Torten et al. [96], Fox et al. [97], Pravetoni et al. [98] |
| Sigma Adjuvant System® (SAS) | Methamphetamine (MH6), Nicotine (triAM1(Gly)2) | Mouse | Elicited high titers and strong affinity; trivalent formulation outperformed monovalent | Moreno et al. [99], Miller et al. [100], Collins and Janda [101] |
| MF59 | Cocaine (COC-5+MF59), Oxycodone (OXY(Gly)4-KLH) | Mouse | Enhanced efficacy in peptide-based vaccines but not in conjugates | Madge et al. [102], Robinson et al. [103] |
| AS03 | Nicotine (AM1-TT) | Mouse, rat | Enhanced immunogenicity in mice and rats; reduced nicotine self-administration in rats | Moreno et al. [104] |
| Adjuvant | Vaccine Target | Species Tested | Performance | References |
|---|---|---|---|---|
| TLR2 agonist (Pam3CAG) | Nicotine | Mouse | Effective when co-administered with MPLA | Lockner et al. [120] |
| TLR3 agonist (dsRNA) | Heroin | Mouse | Effective when co-formulated with AH | Hwang et al. [122] |
| TLR4 agonist (MPLA) | Nicotine, Heroin, Oxycodone, heroin and fentanyl bivalent | Mouse, rat | Synergistic with R848 for nicotine; ineffective for oxycodone due to TLR4 suppression; effective in bivalent vaccine when co-formulated with AH | Zhao et al. [49], Matyas et al. [113], Walter et al. [80], Barrientos et al. [119] |
| TLR 4 agonist (GLA-SE) | Methamphetamine | Mouse | Higher efficacy compared to AH | Stevens et al. [123] |
| TLR5 agonist (entolimod) | Methamphetamine | Mouse, rat | Effective when co-administered with AH | Haile et al. [118] |
| TLR7/8 agonist (R848) | Nicotine, Oxycodone | Mouse | Strong synergy with MPLA in nicotine vaccines | Zhao et al. [49], Walter et al. [80] |
| TLR7/8 agonist (UM-3006) | Fentanyl | Mouse | Effective when co-conjugating hapten and UM-3006 to carrier; synergizes with AH to increase efficacy | Powers et al. [121] |
| TLR9 agonist (CpG ODN) | Fentanyl, Heroin, Cocaine, Methamphetamine | Mouse, rhesus monkey | Synergizes with AH to increase efficacy | Bremer et al. [114,115], Hwang et al. [122], Kimishima et al. [116], Hossain et al. [117] |
| Adjuvant | Vaccine Target | Species Tested | Performance | References |
|---|---|---|---|---|
| Flagellin | Cocaine (GNE-FliC) | Mouse | Dual role as carrier and adjuvant; synergizes with AH to increase efficacy | Lockner et al. [136] |
| E. coli enterotoxin-derived dmLT and LTA1 | Fentanyl | Mouse | Efficacious; mucosal routes induced IgA | Stone et al. [63] |
| B subunit of cholera toxin (CTB) | Cocaine (TA-CD) | Human | Safe with moderate immunogenicity; limited efficacy in sustaining abstinence; high responders showed partial benefits | Kosten et al. [84] |
| Peptide containing a B cell epitope (YKQGGFLGL) and a conformationally biased C5a receptor agonist (YSFKPMPLaR) | Nicotine | Rat | Dual role as carrier and adjuvant; effective without external adjuvants | Sanderson et al. [137] |
| Self-assembling peptide nanofiber (KFE8) | Cocaine | Mouse | Dual role as carrier and adjuvant; effective without external adjuvants | Rudra et al. [138] |
| Adjuvant | Vaccine Target | Species Tested | Performance | References |
|---|---|---|---|---|
| IFN-γ | Oxycodone | Mouse | Enhanced titers and brain protection when paired with TLR agonists | Bian et al. (manuscript in preparation) |
| IL-4 neutralization (anti-IL-4 mAb) | Oxycodone, Fentanyl | Mouse | Enhanced IgG2a and IgG3, improved germinal center response and protection | Laudenbach et al. [162], Crouse et al. [163,164] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bian, Y.; Ci, Q.; Luo, X.M.; Zhang, C. Precision Adjuvant Strategies in Vaccine Development for Substance Use Disorders: Variability and Mechanistic Insights. Pharmaceutics 2025, 17, 1223. https://doi.org/10.3390/pharmaceutics17091223
Bian Y, Ci Q, Luo XM, Zhang C. Precision Adjuvant Strategies in Vaccine Development for Substance Use Disorders: Variability and Mechanistic Insights. Pharmaceutics. 2025; 17(9):1223. https://doi.org/10.3390/pharmaceutics17091223
Chicago/Turabian StyleBian, Yuanzhi, Qiaoqiao Ci, Xin M. Luo, and Chenming Zhang. 2025. "Precision Adjuvant Strategies in Vaccine Development for Substance Use Disorders: Variability and Mechanistic Insights" Pharmaceutics 17, no. 9: 1223. https://doi.org/10.3390/pharmaceutics17091223
APA StyleBian, Y., Ci, Q., Luo, X. M., & Zhang, C. (2025). Precision Adjuvant Strategies in Vaccine Development for Substance Use Disorders: Variability and Mechanistic Insights. Pharmaceutics, 17(9), 1223. https://doi.org/10.3390/pharmaceutics17091223







