Novel Strategies for Androgenetic Alopecia Therapy: Integrating Multifunctional Plant Extracts with Nanotechnology for Advanced Cutaneous Drug Delivery
Abstract
1. Introduction
2. Pathogenesis and Therapeutic Limitations
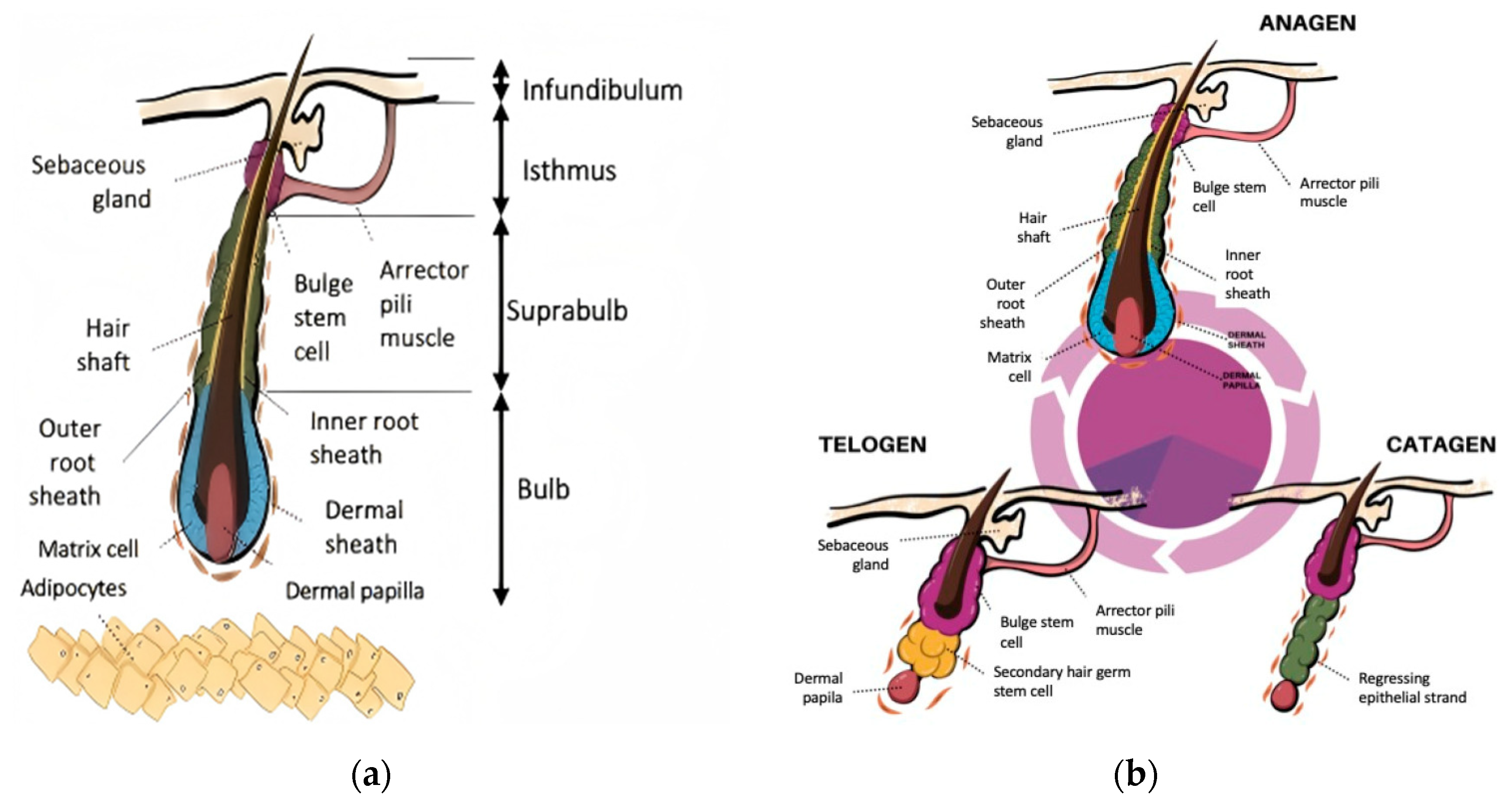
2.1. Follicular Architecture and Hair Follicle Cycling
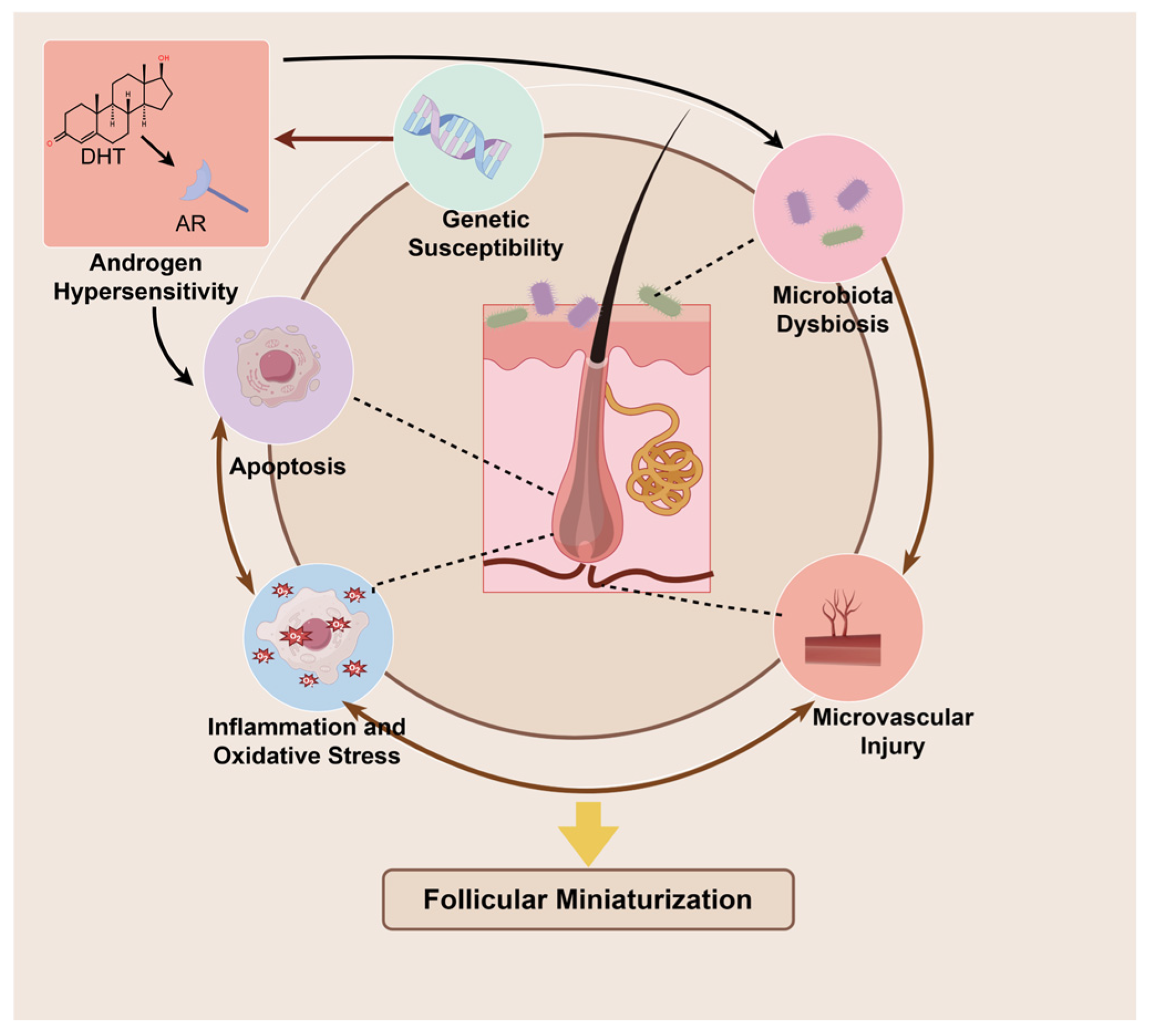
2.2. Pathogenesis of AGA
2.2.1. Androgen Metabolism: Dihydrotestosterone-Driven Pathogenic Cascade in Hair Follicles
2.2.2. Genetic Susceptibility: Polygenic Determinants of Follicular Vulnerability
2.2.3. Microenvironment Deterioration: Multifactorial Perturbations in Inflammation, Microbiota and Microvasculature
2.2.4. Cellular Dysfunction: Oxidative Damage and Senescence as Terminal Pathophysiology
2.3. Topical Treatment of AGA
2.3.1. Minoxidil
2.3.2. Finasteride
3. Therapeutic Potential of Multifunctional Botanicals
3.1. Saw Palmetto Extract
3.2. Cacumen Platycladi Extract
3.3. Panax Ginseng/Red Ginseng Extract
3.4. Rice Bran Extract
4. Nanodelivery Systems: Overcoming Therapeutic Barriers in AGA
4.1. Molecular Challenges: Nanoencapsulation Strategies
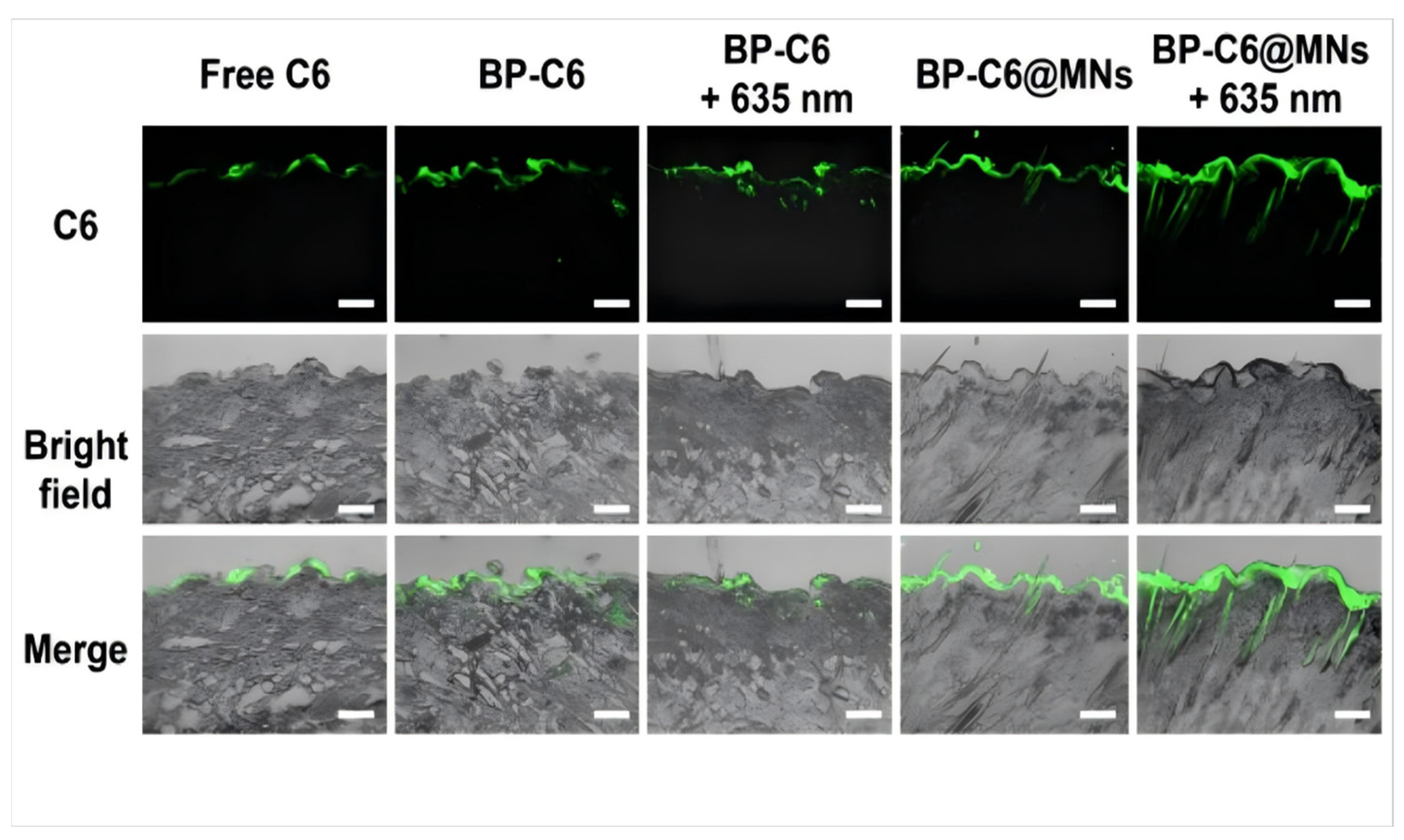
4.2. Cutaneous Barrier Penetration: Precision Follicular Targeting
4.3. Adherence Enhancement: Controlled-Release Platforms
5. Conclusions and Future Trajectories
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AGA | Androgenetic alopecia |
| ALP | Alkaline phosphatase |
| AR | Androgen receptor |
| CP | Cacumen Platycladi |
| DHT | 5α-dihydrotestosterone |
| DKK-1 | Dickkopf-1 |
| DP | Dermal papilla |
| DPCs | Dermal papilla cells |
| FGF5 | Fibroblast growth factor 5 |
| HFSCs | Hair follicle stem cells |
| HIF-1α | Hypoxia inducible factor-1α |
| HMCs | Hair matrix cells |
| HMVECs | Human microvascular endothelial cells |
| HUVECs | Human umbilical vein endothelial cells |
| IGF-1 | Insulin-like growth factor-1 |
| LPS | Lipopolysaccharide |
| ORS | Outer root sheath |
| PG | Panax ginseng |
| PI3K | Phosphatidylinositol 3-kinase |
| RB | Rice bran |
| RG | Red ginseng |
| SHG | Secondary hair germ |
| SP | Saw palmetto |
| TGF-β | Transforming growth factor-β |
| VEGF | Vascular endothelial growth factor |
References
- Gupta, A.K.; Wang, T.; Economopoulos, V. Epidemiological Landscape of Androgenetic Alopecia in the US: An All of Us Cross-Sectional Study. PLoS ONE 2025, 20, e0319040. [Google Scholar] [CrossRef]
- Kanti, V.; Messenger, A.; Dobos, G.; Reygagne, P.; Finner, A.; Blumeyer, A.; Trakatelli, M.; Tosti, A.; Del Marmol, V.; Piraccini, B.M.; et al. Evidence-Based (S3) Guideline for the Treatment of Androgenetic Alopecia in Women and in Men—Short Version. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.L.; Zhou, C.; Shen, Y.W.; Wang, X.Y.; Ding, X.L.; Tian, S.; Liu, Y.; Peng, G.H.; Xue, S.Q.; Zhou, J.E.; et al. Prevalence of Androgenetic Alopecia in China: A Community-Based Study in Six Cities. Br. J. Dermatol. 2010, 162, 843–847. [Google Scholar] [CrossRef] [PubMed]
- Salman, K.E.; Altunay, I.K.; Kucukunal, N.A.; Cerman, A.A. Frequency, Severity and Related Factors of Androgenetic Alopecia in Dermatology Outpatient Clinic: Hospital-Based Cross-Sectional Study in Turkey. An. Bras. Dermatol. 2017, 92, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Aukerman, E.L.; Jafferany, M. The Psychological Consequences of Androgenetic Alopecia: A Systematic Review. J. Cosmet. Dermatol. 2023, 22, 89–95. [Google Scholar] [CrossRef]
- Nilforoushzadeh, M.A.; Golparvaran, M. An Assessment for Measuring Loneliness, Anxiety, and Depression in Male Patients with Androgenetic Alopecia Undergoing Hair Transplantation Surgery: A before-after Study. J. Cosmet. Dermatol. 2022, 21, 7013–7017. [Google Scholar] [CrossRef] [PubMed]
- Nestor, M.S.; Ablon, G.; Gade, A.; Han, H.; Fischer, D.L. Treatment Options for Androgenetic Alopecia: Efficacy, Side Effects, Compliance, Financial Considerations, and Ethics. J. Cosmet. Dermatol. 2021, 20, 3759–3781. [Google Scholar] [CrossRef]
- Feldman, P.R.; Gentile, P.; Piwko, C.; Motswaledi, H.M.; Gorun, S.; Pesachov, J.; Markel, M.; Silver, M.I.; Brenkel, M.; Feldman, O.J.; et al. Hair Regrowth Treatment Efficacy and Resistance in Androgenetic Alopecia: A Systematic Review and Continuous Bayesian Network Meta-Analysis. Front. Med. 2022, 9, 998623. [Google Scholar] [CrossRef]
- Cuevas-Diaz Duran, R.; Martinez-Ledesma, E.; Garcia-Garcia, M.; Bajo Gauzin, D.; Sarro-Ramírez, A.; Gonzalez-Carrillo, C.; Rodríguez-Sardin, D.; Fuentes, A.; Cardenas-Lopez, A. The Biology and Genomics of Human Hair Follicles: A Focus on Androgenetic Alopecia. Int. J. Mol. Sci. 2024, 25, 2542. [Google Scholar] [CrossRef]
- Lin, X.; Zhu, L.; He, J. Morphogenesis, Growth Cycle and Molecular Regulation of Hair Follicles. Front. Cell Dev. Biol. 2022, 10, 899095. [Google Scholar] [CrossRef]
- Pelikh, O.; Eckert, R.W.; Pinnapireddy, S.R.; Keck, C.M. Hair Follicle Targeting with Curcumin Nanocrystals: Influence of the Formulation Properties on the Penetration Efficacy. J. Control. Release 2021, 329, 598–613. [Google Scholar] [CrossRef]
- Hu, S.; Li, Z.; Lutz, H.; Huang, K.; Su, T.; Cores, J.; Dinh, P.-U.C.; Cheng, K. Dermal Exosomes Containing miR-218-5p Promote Hair Regeneration by Regulating β-Catenin Signaling. Sci. Adv. 2020, 6, eaba1685. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Huang, J.; Liu, Z.; Chen, R.; Fu, D.; Yang, L.; Wang, J.; Du, L.; Wen, L.; Miao, Y.; et al. miR-140-5p in Small Extracellular Vesicles from Human Papilla Cells Stimulates Hair Growth by Promoting Proliferation of Outer Root Sheath and Hair Matrix Cells. Front. Cell Dev. Biol. 2020, 8, 593638. [Google Scholar] [CrossRef]
- Ma, S.; Ji, D.; Wang, X.; Yang, Y.; Shi, Y.; Chen, Y. Transcriptomic Analysis Reveals Candidate Ligand-Receptor Pairs and Signaling Networks Mediating Intercellular Communication between Hair Matrix Cells and Dermal Papilla Cells from Cashmere Goats. Cells 2023, 12, 1645. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, M.; Jiang, J.; Li, K.; Liang, H.; Wang, N.; Zou, Y.; Wang, D.; Zhou, S.; Tang, Y.; et al. THSD4 Promotes Hair Growth by Facilitating Dermal Papilla and Hair Matrix Interactions. Theranostics 2025, 15, 3571–3588. [Google Scholar] [CrossRef]
- Martino, P.A.; Heitman, N.; Rendl, M. The Dermal Sheath: An Emerging Component of the Hair Follicle Stem Cell Niche. Exp. Dermatol. 2021, 30, 512–521. [Google Scholar] [CrossRef]
- Martino, P.; Sunkara, R.; Heitman, N.; Rangl, M.; Brown, A.; Saxena, N.; Grisanti, L.; Kohan, D.; Yanagisawa, M.; Rendl, M. Progenitor-Derived Endothelin Controls Dermal Sheath Contraction for Hair Follicle Regression. Nat. Cell Biol. 2023, 25, 222–234. [Google Scholar] [CrossRef]
- Natarelli, N.; Gahoonia, N.; Sivamani, R.K. Integrative and Mechanistic Approach to the Hair Growth Cycle and Hair Loss. J. Clin. Med. 2023, 12, 893. [Google Scholar] [CrossRef]
- Shirokova, V.; Biggs, L.C.; Jussila, M.; Ohyama, T.; Groves, A.K.; Mikkola, M.L. Foxi3 Deficiency Compromises Hair Follicle Stem Cell Specification and Activation. Stem Cells 2016, 34, 1896–1908. [Google Scholar] [CrossRef]
- Morgan, B.A. The Dermal Papilla: An Instructive Niche for Epithelial Stem and Progenitor Cells in Development and Regeneration of the Hair Follicle. Cold Spring Harb. Perspect. Med. 2014, 4, a015180. [Google Scholar] [CrossRef]
- Hawkshaw, N.J.; Hardman, J.A.; Alam, M.; Jimenez, F.; Paus, R. Deciphering the Molecular Morphology of the Human Hair Cycle: Wnt Signalling during the Telogen-Anagen Transformation. Br. J. Dermatol. 2020, 182, 1184–1193. [Google Scholar] [CrossRef]
- Tang, P.; Wang, X.; Zhang, M.; Huang, S.; Lin, C.; Yan, F.; Deng, Y.; Zhang, L.; Zhang, L. Activin B Stimulates Mouse Vibrissae Growth and Regulates Cell Proliferation and Cell Cycle Progression of Hair Matrix Cells through ERK Signaling. Int. J. Mol. Sci. 2019, 20, 853. [Google Scholar] [CrossRef]
- Hébert, J.M.; Rosenquist, T.; Götz, J.; Martin, G.R. FGF5 as a Regulator of the Hair Growth Cycle: Evidence from Targeted and Spontaneous Mutations. Cell 1994, 78, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Lin, H.; Wang, L.; Guo, K.; Jing, R.; Li, X.; Chen, Y.; Hu, Z.; Gao, S.; Xu, N. Suppression of FGF5 and FGF18 Expression by Cholesterol-Modified siRNAs Promotes Hair Growth in Mice. Front. Pharmacol. 2021, 12, 666860. [Google Scholar] [CrossRef] [PubMed]
- Enshell-Seijffers, D.; Lindon, C.; Kashiwagi, M.; Morgan, B.A. Beta-Catenin Activity in the Dermal Papilla Regulates Morphogenesis and Regeneration of Hair. Dev. Cell 2010, 18, 633–642. [Google Scholar] [CrossRef]
- Foitzik, K.; Lindner, G.; Mueller-Roever, S.; Maurer, M.; Botchkareva, N.; Botchkarev, V.; Handjiski, B.; Metz, M.; Hibino, T.; Soma, T.; et al. Control of Murine Hair Follicle Regression (Catagen) by TGF-Beta1 in Vivo. FASEB J. 2000, 14, 752–760. [Google Scholar] [CrossRef]
- Wang, W.; Wang, H.; Long, Y.; Li, Z.; Li, J. Controlling Hair Loss by Regulating Apoptosis in Hair Follicles: A Comprehensive Overview. Biomolecules 2023, 14, 20. [Google Scholar] [CrossRef]
- Heitman, N.; Sennett, R.; Mok, K.-W.; Saxena, N.; Srivastava, D.; Martino, P.; Grisanti, L.; Wang, Z.; Ma’ayan, A.; Rompolas, P.; et al. Dermal Sheath Contraction Powers Stem Cell Niche Relocation during Hair Cycle Regression. Science 2020, 367, 161–166. [Google Scholar] [CrossRef]
- Panteleyev, A.A. Functional Anatomy of the Hair Follicle: The Secondary Hair Germ. Exp. Dermatol. 2018, 27, 701–720. [Google Scholar] [CrossRef]
- Yi, R. Concise Review: Mechanisms of Quiescent Hair Follicle Stem Cell Regulation. Stem Cells 2017, 35, 2323–2330. [Google Scholar] [CrossRef]
- Xing, Y.; Ma, X.; Guo, H.; Deng, F.; Yang, J.; Li, Y. Wnt5a Suppresses β-Catenin Signaling during Hair Follicle Regeneration. Int. J. Med. Sci. 2016, 13, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, F.; Alam, M. The Proportion of Catagen and Telogen Hair Follicles in Occipital Scalp of Male Androgenetic Alopecia Patients: Challenging the Established Dogma. Exp. Dermatol. 2024, 33, e70001. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Chen, D.; Jiang, K.; Song, L.; Qian, N.; Du, Y.; Yang, Y.; Wang, F.; Chen, T. Hair Shaft Miniaturization Causes Stem Cell Depletion through Mechanosensory Signals Mediated by a Piezo1-Calcium-TNF-α Axis. Cell Stem Cell 2022, 29, 70–85.e6. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Yamaga, K.; Tokumasu, R.; Katsuno, T.; Tanaka, H.; Chiba, S.; Yagi, T.; Katayama, I.; Tamura, A.; Murota, H.; et al. Double Mutation of Claudin-1 and Claudin-3 Causes Alopecia in Infant Mice. Ann. N. Y. Acad. Sci. 2023, 1523, 51–61. [Google Scholar] [CrossRef]
- Joko, Y.; Yamamoto, Y.; Kato, S.; Takemoto, T.; Abe, M.; Matsumoto, T.; Fukumoto, S.; Sawatsubashi, S. VDR Is an Essential Regulator of Hair Follicle Regression through the Progression of Cell Death. Life Sci. Alliance 2023, 6, e202302014. [Google Scholar] [CrossRef]
- Ceruti, J.M.; Leirós, G.J.; Balañá, M.E. Androgens and Androgen Receptor Action in Skin and Hair Follicles. Mol. Cell Endocrinol. 2018, 465, 122–133. [Google Scholar] [CrossRef]
- Swerdloff, R.S.; Dudley, R.E.; Page, S.T.; Wang, C.; Salameh, W.A. Dihydrotestosterone: Biochemistry, Physiology, and Clinical Implications of Elevated Blood Levels. Endocr. Rev. 2017, 38, 220–254. [Google Scholar] [CrossRef]
- Leirós, G.J.; Ceruti, J.M.; Castellanos, M.L.; Kusinsky, A.G.; Balañá, M.E. Androgens Modify Wnt Agonists/Antagonists Expression Balance in Dermal Papilla Cells Preventing Hair Follicle Stem Cell Differentiation in Androgenetic Alopecia. Mol. Cell Endocrinol. 2017, 439, 26–34. [Google Scholar] [CrossRef]
- Deng, Z.; Chen, M.; Liu, F.; Wang, Y.; Xu, S.; Sha, K.; Peng, Q.; Wu, Z.; Xiao, W.; Liu, T.; et al. Androgen Receptor-Mediated Paracrine Signaling Induces Regression of Blood Vessels in the Dermal Papilla in Androgenetic Alopecia. J. Investig. Dermatol. 2022, 142, 2088–2099.e9. [Google Scholar] [CrossRef]
- Cobb, J.E.; Wong, N.C.; Yip, L.W.; Martinick, J.; Bosnich, R.; Sinclair, R.D.; Craig, J.M.; Saffery, R.; Harrap, S.B.; Ellis, J.A. Evidence of Increased DNA Methylation of the Androgen Receptor Gene in Occipital Hair Follicles from Men with Androgenetic Alopecia. Br. J. Dermatol. 2011, 165, 210–213. [Google Scholar] [CrossRef]
- Sawaya, M.E.; Price, V.H. Different Levels of 5alpha-Reductase Type I and II, Aromatase, and Androgen Receptor in Hair Follicles of Women and Men with Androgenetic Alopecia. J. Investig. Dermatol. 1997, 109, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Perper, M.; Herskovitz, I.; Tosti, A. Aromatase Inhibitor-Induced Hair Loss in Two Adolescents. Pediatr. Dermatol. 2020, 37, 1125–1127. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.-Y.; Chen, J.Y.-F.; Hsu, W.-L.; Yu, S.; Chen, W.-C.; Chiu, S.-H.; Yang, H.-R.; Lin, S.-Y.; Wu, C.-Y. Female Pattern Hair Loss: An Overview with Focus on the Genetics. Genes 2023, 14, 1326. [Google Scholar] [CrossRef] [PubMed]
- Sadasivam, I.P.; Sambandam, R.; Kaliyaperumal, D.; Dileep, J.E. Androgenetic Alopecia in Men: An Update on Genetics. Indian J. Dermatol. 2024, 69, 282. [Google Scholar] [CrossRef]
- Ambra, R.; Mastroeni, S.; Manca, S.; Mannooranparampil, T.J.; Virgili, F.; Marzani, B.; Pinto, D.; Fortes, C. Genetic Variants and Lifestyle Factors in Androgenetic Alopecia Patients: A Case-Control Study of Single Nucleotide Polymorphisms and Their Contribution to Baldness Risk. Nutrients 2025, 17, 299. [Google Scholar] [CrossRef]
- Liu, F.; Hamer, M.A.; Heilmann, S.; Herold, C.; Moebus, S.; Hofman, A.; Uitterlinden, A.G.; Nöthen, M.M.; van Duijn, C.M.; Nijsten, T.E.; et al. Prediction of Male-Pattern Baldness from Genotypes. Eur. J. Hum. Genet. 2016, 24, 895–902. [Google Scholar] [CrossRef]
- Hagenaars, S.P.; Hill, W.D.; Harris, S.E.; Ritchie, S.J.; Davies, G.; Liewald, D.C.; Gale, C.R.; Porteous, D.J.; Deary, I.J.; Marioni, R.E. Genetic Prediction of Male Pattern Baldness. PLoS Genet. 2017, 13, e1006594. [Google Scholar] [CrossRef]
- Janivara, R.; Hazra, U.; Pfennig, A.; Harlemon, M.; Kim, M.S.; Eaaswarkhanth, M.; Chen, W.C.; Ogunbiyi, A.; Kachambwa, P.; Petersen, L.N.; et al. Uncovering the Genetic Architecture and Evolutionary Roots of Androgenetic Alopecia in African Men. HGG Adv. 2025, 6, 100428. [Google Scholar] [CrossRef]
- Liang, B.; Yang, C.; Zuo, X.; Li, Y.; Ding, Y.; Sheng, Y.; Zhou, F.; Cheng, H.; Zheng, X.; Chen, G.; et al. Genetic Variants at 20p11 Confer Risk to Androgenetic Alopecia in the Chinese Han Population. PLoS ONE 2013, 8, e71771. [Google Scholar] [CrossRef]
- Kim, I.-Y.; Kim, J.-H.; Choi, J.-E.; Yu, S.-J.; Kim, J.H.; Kim, S.R.; Choi, M.S.; Kim, M.H.; Hong, K.-W.; Park, B.-C. The First Broad Replication Study of SNPs and a Pilot Genome-Wide Association Study for Androgenetic Alopecia in Asian Populations. J. Cosmet. Dermatol. 2022, 21, 6174–6183. [Google Scholar] [CrossRef]
- Rui, W.; Sheng, Y.; Hu, R.; Miao, Y.; Han, Y.; Guo, X.; Qi, S.; Xu, F.; Xu, J.; Yang, Q. Association of Single Nucleotide Polymorphisms in the CYP19A1 Gene with Female Pattern Hair Loss in a Chinese Population. Dermatology 2015, 231, 239–244. [Google Scholar] [CrossRef]
- Łukasik, A.; Kozicka, K.; Pisarek, A.; Wojas-Pelc, A. The Role of CYP19A1 and ESR2 Gene Polymorphisms in Female Androgenetic Alopecia in the Polish Population. Adv. Dermatol. Alergol. 2022, 39, 708–713. [Google Scholar] [CrossRef] [PubMed]
- Sueki, H.; Stoudemayer, T.; Kligman, A.M.; Murphy, G.F. Quantitative and Ultrastructural Analysis of Inflammatory Infiltrates in Male Pattern Alopecia. Acta Derm. Venereol. 1999, 79, 347–350. [Google Scholar] [CrossRef] [PubMed]
- El-Domyati, M.; Attia, S.; Saleh, F.; Abdel-Wahab, H. Androgenetic Alopecia in Males: A Histopathological and Ultrastructural Study. J. Cosmet. Dermatol. 2009, 8, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Zhao, W.; Jiang, L.; Shan, S. Single-Nucleus and Bulk RNA Sequencing Reveals the Involvement of Natural Killer and CD8+ T Cells in the Progression of Androgenetic Alopecia. J. Inflamm. Res. 2025, 18, 7033–7046. [Google Scholar] [CrossRef]
- Xiong, H.-D.; Tang, L.-L.; Chen, H.-J.; Wu, Y.; Li, W.-Y.; Wen, S.-J.; Lin, Y.-K. Identification of Immune Microenvironment Changes, Immune-Related Pathways and Genes in Male Androgenetic Alopecia. Medicine 2023, 102, e35242. [Google Scholar] [CrossRef]
- Balık, A.R.; Balık, Z.B.; Aktaş, A.; Neşelioğlu, S.; Karabulut, E.; Karabulut, A.B. Examination of Androgenetic Alopecia with Serum Biomarkers. J. Cosmet. Dermatol. 2021, 20, 1855–1859. [Google Scholar] [CrossRef]
- Kwack, M.H.; Ahn, J.S.; Kim, M.K.; Kim, J.C.; Sung, Y.K. Dihydrotestosterone-Inducible IL-6 Inhibits Elongation of Human Hair Shafts by Suppressing Matrix Cell Proliferation and Promotes Regression of Hair Follicles in Mice. J. Investig. Dermatol. 2012, 132, 43–49. [Google Scholar] [CrossRef]
- Ramos, P.M.; Brianezi, G.; Martins, A.C.P.; da Silva, M.G.; Marques, M.E.A.; Miot, H.A. Apoptosis in Follicles of Individuals with Female Pattern Hair Loss Is Associated with Perifollicular Microinflammation. Int. J. Cosmet. Sci. 2016, 38, 651–654. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, Y.; Deng, S.; Yang, X.; Yao, X. Immune and Non-Immune Interactions in the Pathogenesis of Androgenetic Alopecia. Clin. Rev. Allergy Immunol. 2025, 68, 22. [Google Scholar] [CrossRef]
- Suzuki, K.; Inoue, M.; Cho, O.; Mizutani, R.; Shimizu, Y.; Nagahama, T.; Sugita, T. Scalp Microbiome and Sebum Composition in Japanese Male Individuals with and without Androgenetic Alopecia. Microorganisms 2021, 9, 2132. [Google Scholar] [CrossRef] [PubMed]
- Ho, B.S.-Y.; Ho, E.X.P.; Chu, C.W.; Ramasamy, S.; Bigliardi-Qi, M.; de Sessions, P.F.; Bigliardi, P.L. Microbiome in the Hair Follicle of Androgenetic Alopecia Patients. PLoS ONE 2019, 14, e0216330. [Google Scholar] [CrossRef]
- Tao, K.; Bai, X.; Ji, P.; Zhang, Y.; Cao, T.; Han, F.; Zhang, Z.; Guan, H.; Hu, D. A Composite of Hepatocyte Growth Factor- and 5α-Dihydrotestosterone-Gelatin Microspheres with Adipose-Derived Stem Cells Enhances Wound Healing. Skin Pharmacol. Physiol. 2022, 35, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Kyung, S.; Mun, S.; Yu, B.S.; Yun, K.; Baek, C.; Lee, D.-G.; Kang, S.; Kim, S.R.; Kim, J.-H.; et al. Comparative Analysis of Bacteriome in Hair Follicle Layers of Patients with Female Pattern Androgenic Alopecia. Microorganisms 2025, 13, 1365. [Google Scholar] [CrossRef] [PubMed]
- Li, K.N.; Jain, P.; He, C.H.; Eun, F.C.; Kang, S.; Tumbar, T. Skin Vasculature and Hair Follicle Cross-Talking Associated with Stem Cell Activation and Tissue Homeostasis. eLife 2019, 8, e45977. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, D.; Ma, T.; Liu, Q. Vascular Endothelial Growth Factor Protects CD200-Rich and CD34-Positive Hair Follicle Stem Cells Against Androgen-Induced Apoptosis Through the Phosphoinositide 3-Kinase/Akt Pathway in Patients with Androgenic Alopecia. Dermatol. Surg. 2020, 46, 358–368. [Google Scholar] [CrossRef]
- Seo, J.; Yan, L.; Kageyama, T.; Nanmo, A.; Chun, Y.-S.; Fukuda, J. Hypoxia Inducible Factor-1α Promotes Trichogenic Gene Expression in Human Dermal Papilla Cells. Sci. Rep. 2023, 13, 1478. [Google Scholar] [CrossRef]
- Upton, J.H.; Hannen, R.F.; Bahta, A.W.; Farjo, N.; Farjo, B.; Philpott, M.P. Oxidative Stress-Associated Senescence in Dermal Papilla Cells of Men with Androgenetic Alopecia. J. Investig. Dermatol. 2015, 135, 1244–1252. [Google Scholar] [CrossRef]
- Haslam, I.S.; Jadkauskaite, L.; Szabó, I.L.; Staege, S.; Hesebeck-Brinckmann, J.; Jenkins, G.; Bhogal, R.K.; Lim, F.-L.; Farjo, N.; Farjo, B.; et al. Oxidative Damage Control in a Human (Mini-) Organ: Nrf2 Activation Protects against Oxidative Stress-Induced Hair Growth Inhibition. J. Investig. Dermatol. 2017, 137, 295–304. [Google Scholar] [CrossRef]
- Shirai, K.; Obara, K.; Tohgi, N.; Yamazaki, A.; Aki, R.; Hamada, Y.; Arakawa, N.; Singh, S.R.; Hoffman, R.M.; Amoh, Y. Expression of Anti-Aging Type-XVII Collagen (COL17A1/BP180) in Hair Follicle-Associated Pluripotent (HAP) Stem Cells during Differentiation. Tissue Cell 2019, 59, 33–38. [Google Scholar] [CrossRef]
- Bahta, A.W.; Farjo, N.; Farjo, B.; Philpott, M.P. Premature Senescence of Balding Dermal Papilla Cells in Vitro Is Associated with P16INK4a Expression. J. Investig. Dermatol. 2008, 128, 1088–1094. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, J.; Luo, Y.; Zhao, Z.; Yuan, Y.; Li, J.; Liu, Y.; Yi, Y.; Xu, X.; Lan, Y.; et al. Targeting IGF1-Induced Cellular Senescence to Rejuvenate Hair Follicle Aging. Aging Cell 2025, 24, e70053. [Google Scholar] [CrossRef] [PubMed]
- Pappalardo, A.; Kim, J.Y.; Abaci, H.E.; Christiano, A.M. Restoration of Hair Follicle Inductive Properties by Depletion of Senescent Cells. Aging Cell 2025, 24, e14353. [Google Scholar] [CrossRef]
- Kash, N.; Leavitt, M.; Leavitt, A.; Hawkins, S.D.; Roopani, R.B. Clinical Patterns of Hair Loss in Men: Is Dihydrotestosterone the Only Culprit? Dermatol. Clin. 2021, 39, 361–370. [Google Scholar] [CrossRef]
- Yum, S.; Jeong, S.; Kim, D.; Lee, S.; Kim, W.; Yoo, J.-W.; Kim, J.-A.; Kwon, O.S.; Kim, D.-D.; Min, D.S.; et al. Minoxidil Induction of VEGF Is Mediated by Inhibition of HIF-Prolyl Hydroxylase. Int. J. Mol. Sci. 2017, 19, 53. [Google Scholar] [CrossRef] [PubMed]
- Anjum, M.A.; Zulfiqar, S.; Chaudhary, A.A.; Rehman, I.U.; Bullock, A.J.; Yar, M.; MacNeil, S. Stimulation of Hair Regrowth in an Animal Model of Androgenic Alopecia Using 2-Deoxy-D-Ribose. Front. Pharmacol. 2024, 15, 1370833. [Google Scholar] [CrossRef]
- Kwack, M.H.; Kang, B.M.; Kim, M.K.; Kim, J.C.; Sung, Y.K. Minoxidil Activates β-Catenin Pathway in Human Dermal Papilla Cells: A Possible Explanation for Its Anagen Prolongation Effect. J. Dermatol. Sci. 2011, 62, 154–159. [Google Scholar] [CrossRef]
- Goren, A.; Shapiro, J.; Roberts, J.; McCoy, J.; Desai, N.; Zarrab, Z.; Pietrzak, A.; Lotti, T. Clinical Utility and Validity of Minoxidil Response Testing in Androgenetic Alopecia. Dermatol. Ther. 2015, 28, 13–16. [Google Scholar] [CrossRef]
- Shadi, Z. Compliance to Topical Minoxidil and Reasons for Discontinuation among Patients with Androgenetic Alopecia. Dermatol. Ther. 2023, 13, 1157–1169. [Google Scholar] [CrossRef]
- Suchonwanit, P.; Iamsumang, W.; Leerunyakul, K. Topical Finasteride for the Treatment of Male Androgenetic Alopecia and Female Pattern Hair Loss: A Review of the Current Literature. J. Dermatol. Treat. 2022, 33, 643–648. [Google Scholar] [CrossRef]
- Gupta, A.K.; Talukder, M. Topical Finasteride for Male and Female Pattern Hair Loss: Is It a Safe and Effective Alternative? J. Cosmet. Dermatol. 2022, 21, 1841–1848. [Google Scholar] [CrossRef]
- Choi, J.Y.; Boo, M.Y.; Boo, Y.C. Can Plant Extracts Help Prevent Hair Loss or Promote Hair Growth? A Review Comparing Their Therapeutic Efficacies, Phytochemical Components, and Modulatory Targets. Molecules 2024, 29, 2288. [Google Scholar] [CrossRef] [PubMed]
- Kesika, P.; Sivamaruthi, B.S.; Thangaleela, S.; Bharathi, M.; Chaiyasut, C. Role and Mechanisms of Phytochemicals in Hair Growth and Health. Pharmaceuticals 2023, 16, 206. [Google Scholar] [CrossRef] [PubMed]
- Soe, Z.C.; Ei, Z.Z.; Visuttijai, K.; Chanvorachote, P. Potential Natural Products Regulation of Molecular Signaling Pathway in Dermal Papilla Stem Cells. Molecules 2023, 28, 5517. [Google Scholar] [CrossRef]
- Gupta, A.K.; Talukder, M.; Bamimore, M.A. Natural Products for Male Androgenetic Alopecia. Dermatol. Ther. 2022, 35, e15323. [Google Scholar] [CrossRef] [PubMed]
- Schantz, M.M.; Bedner, M.; Long, S.E.; Molloy, J.L.; Murphy, K.E.; Porter, B.J.; Putzbach, K.; Rimmer, C.A.; Sander, L.C.; Sharpless, K.E.; et al. Development of Saw Palmetto (Serenoa repens) Fruit and Extract Standard Reference Materials. Anal. Bioanal. Chem. 2008, 392, 427–438. [Google Scholar] [CrossRef]
- Zhu, H.-L.; Gao, Y.-H.; Yang, J.-Q.; Li, J.-B.; Gao, J. Serenoa repens Extracts Promote Hair Regeneration and Repair of Hair Loss Mouse Models by Activating TGF-β and Mitochondrial Signaling Pathway. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 4000–4008. [Google Scholar] [CrossRef]
- Bassino, E.; Gasparri, F.; Munaron, L. Serenoa repens and N-Acetyl Glucosamine/Milk Proteins Complex Differentially Affect the Paracrine Communication between Endothelial and Follicle Dermal Papilla Cells. J. Cell Physiol. 2019, 234, 7320–7329. [Google Scholar] [CrossRef]
- Morganti, P.; Fabrizi, G.; James, B.; Bruno, C. Effect of gelatin-cystine and Serenoa repens extract on free radicals level and hair growth. J. Appl. Cosmetol. 1998, 16, 57–64. [Google Scholar]
- Wessagowit, V.; Tangjaturonrusamee, C.; Kootiratrakarn, T.; Bunnag, T.; Pimonrat, T.; Muangdang, N.; Pichai, P. Treatment of Male Androgenetic Alopecia with Topical Products Containing Serenoa repens Extract. Australas. J. Dermatol. 2016, 57, e76–e82. [Google Scholar] [CrossRef]
- Prager, N.; Bickett, K.; French, N.; Marcovici, G. A Randomized, Double-Blind, Placebo-Controlled Trial to Determine the Effectiveness of Botanically Derived Inhibitors of 5-Alpha-Reductase in the Treatment of Androgenetic Alopecia. J. Altern. Complement. Med. 2002, 8, 143–152. [Google Scholar] [CrossRef]
- Rossi, A.; Mari, E.; Scarno, M.; Garelli, V.; Maxia, C.; Scali, E.; Iorio, A.; Carlesimo, M. Comparitive Effectiveness of Finasteride vs Serenoa repens in Male Androgenetic Alopecia: A Two-Year Study. Int. J. Immunopathol. Pharmacol. 2012, 25, 1167–1173. [Google Scholar] [CrossRef]
- Rehman, R.; Hanif, M.A.; Zahid, M.; Qadri, R.W.K. Reporting Effective Extraction Methodology and Chemical Characterization of Bioactive Components of under Explored Platycladus orientalis (L.) Franco from Semi-Arid Climate. Nat. Prod. Res. 2019, 33, 1237–1242. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, B.; Bi, Z.-M.; Wang, Z.-Y.; Duan, L.; Lai, C.-J.-S.; Liu, E.-H. Chemical Profiling and Quantitation of Bioactive Compounds in Platycladi Cacumen by UPLC-Q-TOF-MS/MS and UPLC-DAD. J. Pharm. Biomed. Anal. 2018, 154, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Joo, J.H.; Kim, J.; Rim, H.; Shin, J.Y.; Choi, Y.-H.; Min, K.; Lee, S.Y.; Jun, S.-H.; Kang, N.-G. Platycladus orientalis Leaf Extract Promotes Hair Growth via Non-Receptor Tyrosine Kinase ACK1 Activation. Curr. Issues Mol. Biol. 2024, 46, 11207–11219. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Li, W.; Weng, Z.; Huang, Z.; Liu, J.; Mao, Q.; Ding, B. Water Extract of Cacumen Platycladi Promotes Hair Growth through the Akt/GSK3β/β-Catenin Signaling Pathway. Front. Pharmacol. 2023, 14, 1038039. [Google Scholar] [CrossRef]
- Hong, J.; Xu, B.; Hu, X.; Liu, C.; Liu, H.; Tian, J.; Li, L.; Ding, S.; Zhou, C.; Lu, L. Hyaluronic Acid Microneedles Loaded with Chinese Herbal Extracts as an Intradermal Delivery System for Hair Regeneration. Biomacromolecules 2025, 26, 2945–2959. [Google Scholar] [CrossRef]
- Lin, Z.; Sun, Y.; Li, C.; Zhou, X.; Guo, Y.; Wang, Z.; Li, G. (7E)-7,8-Dehydroheliobuphthalmin from Platycladus orientalis L.: Isolation, Characterization, and Hair Growth Promotion. Int. J. Mol. Sci. 2025, 26, 5189. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, R.; Yin, X.; Lao, Z.; Zhang, Z.; Wu, Q.; Yu, L.; Lai, X.; Wan, Y.; Li, G. Inhibitory Activities of Some Traditional Chinese Herbs against Testosterone 5α-Reductase and Effects of Cacumen Platycladi on Hair Re-Growth in Testosterone-Treated Mice. J. Ethnopharmacol. 2016, 177, 1–9. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, S.; Qu, F.; Su, G.; Zhao, Y. In Vivo and in Vitro Evaluation of Hair Growth Potential of Cacumen Platycladi, and GC-MS Analysis of the Active Constituents of Volatile Oil. J. Ethnopharmacol. 2019, 238, 111835. [Google Scholar] [CrossRef]
- Zhang, N.; Park, D.K.; Park, H.-J. Hair Growth-Promoting Activity of Hot Water Extract of Thuja orientalis. BMC Complement. Altern. Med. 2013, 13, 9. [Google Scholar] [CrossRef] [PubMed]
- Amini, F.; Teh, J.J.; Tan, C.K.; Tan, E.S.S.; Ng, E.S.C. A Pilot Randomized Controlled Trial (RCT) Evaluating the Efficacy of an Exosome-Containing Plant Extract Formulation for Treating Male Alopecia. Life 2025, 15, 500. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Dai, J.; Du, W.; Ji, H. Antioxidant Properties of Platycladus orientalis Flavonoids for Treating UV-Induced Damage in Androgenetic Alopecia Hair. Molecules 2024, 29, 2876. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Han, L.; Chen, S.-S.; Guan, J.; Qu, F.-Z.; Zhao, Y.-Q. Hair Growth Promoting Activity of Cedrol Isolated from the Leaves of Platycladus orientalis. Biomed. Pharmacother. 2016, 83, 641–647. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.-W.; Qu, F.-Z.; Zhang, Y.-M.; Su, G.-Y.; Zhao, Y.-Q. Hair Growth Promotion Effect of Cedrol Cream and Its Dermatopharmacokinetics. RSC Adv. 2018, 8, 42170–42178. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, S.N.; Hong, Y.D.; Park, B.C.; Na, Y. Panax ginseng Extract Antagonizes the Effect of DKK-1-Induced Catagen-like Changes of Hair Follicles. Int. J. Mol. Med. 2017, 40, 1194–1200. [Google Scholar] [CrossRef]
- Kim, S.N.; Kim, S.; Hong, Y.D.; Park, H.; Shin, S.H.; Kim, A.R.; Park, B.C.; Shin, S.S.; Park, J.-S.; Park, M.; et al. The Ginsenosides of Panax ginseng Promote Hair Growth via Similar Mechanism of Minoxidil. J. Dermatol. Sci. 2015, 77, 132–134. [Google Scholar] [CrossRef]
- Jeong, G.; Shin, S.H.; Kim, S.N.; Na, Y.; Park, B.C.; Cho, J.H.; Park, W.-S.; Kim, H.-J. Ginsenoside Re Prevents 3-Methyladenine-Induced Catagen Phase Acceleration by Regulating Wnt/β-Catenin Signaling in Human Dermal Papilla Cells. J. Ginseng Res. 2023, 47, 440–447. [Google Scholar] [CrossRef]
- Li, Z.; Ryu, S.-W.; Lee, J.; Choi, K.; Kim, S.; Choi, C. Protopanaxatirol Type Ginsenoside Re Promotes Cyclic Growth of Hair Follicles via Inhibiting Transforming Growth Factor β Signaling Cascades. Biochem. Biophys. Res. Commun. 2016, 470, 924–929. [Google Scholar] [CrossRef]
- Li, Z.; Li, J.-J.; Gu, L.-J.; Zhang, D.-L.; Wang, Y.-B.; Sung, C.-K. Ginsenosides Rb1 and Rd Regulate Proliferation of Mature Keratinocytes through Induction of P63 Expression in Hair Follicles. Phytother. Res. 2013, 27, 1095–1101. [Google Scholar] [CrossRef]
- Lee, N.-E.; Park, S.-D.; Hwang, H.; Choi, S.-H.; Lee, R.M.; Nam, S.M.; Choi, J.H.; Rhim, H.; Cho, I.-H.; Kim, H.-C.; et al. Effects of a Gintonin-Enriched Fraction on Hair Growth: An in Vitro and in Vivo Study. J. Ginseng Res. 2020, 44, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Lueangarun, S.; Panchaprateep, R. An Herbal Extract Combination (Biochanin A, Acetyl Tetrapeptide-3, and Ginseng Extracts) versus 3% Minoxidil Solution for the Treatment of Androgenetic Alopecia: A 24-Week, Prospective, Randomized, Triple-Blind, Controlled Trial. J. Clin. Aesthet. Dermatol. 2020, 13, 32–37. [Google Scholar] [PubMed]
- Wang, C.-Z.; Zhang, C.-F.; Zhang, Q.-H.; Yuan, C.-S. Phytochemistry of Red Ginseng, a Steam-Processed Panax ginseng. Am. J. Chin. Med. 2024, 52, 35–55. [Google Scholar] [CrossRef]
- Xu, X.-F.; Gao, Y.; Xu, S.-Y.; Liu, H.; Xue, X.; Zhang, Y.; Zhang, H.; Liu, M.-N.; Xiong, H.; Lin, R.-C.; et al. Remarkable Impact of Steam Temperature on Ginsenosides Transformation from Fresh Ginseng to Red Ginseng. J. Ginseng Res. 2018, 42, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Truong, V.-L.; Jeong, W.-S. Hair Growth-Promoting Mechanisms of Red Ginseng Extract through Stimulating Dermal Papilla Cell Proliferation and Enhancing Skin Health. Prev. Nutr. Food Sci. 2021, 26, 275–284. [Google Scholar] [CrossRef]
- Park, G.-H.; Park, K.; Cho, H.; Lee, S.-M.; Han, J.S.; Won, C.H.; Chang, S.E.; Lee, M.W.; Choi, J.H.; Moon, K.C.; et al. Red Ginseng Extract Promotes the Hair Growth in Cultured Human Hair Follicles. J. Med. Food 2015, 18, 354–362. [Google Scholar] [CrossRef]
- Iwabuchi, T.; Ogura, K.; Hagiwara, K.; Ueno, S.; Kitamura, H.; Yamanishi, H.; Tsunekawa, Y.; Kiso, A. Ginsenosides in Panax ginseng Extract Promote Anagen Transition by Suppressing BMP4 Expression and Promote Human Hair Growth by Stimulating Follicle-Cell Proliferation. Biol. Pharm. Bull. 2024, 47, 240–244. [Google Scholar] [CrossRef]
- Shin, D.H.; Cha, Y.J.; Yang, K.E.; Jang, I.-S.; Son, C.-G.; Kim, B.H.; Kim, J.M. Ginsenoside Rg3 Up-Regulates the Expression of Vascular Endothelial Growth Factor in Human Dermal Papilla Cells and Mouse Hair Follicles. Phytother. Res. 2014, 28, 1088–1095. [Google Scholar] [CrossRef]
- Liu, X.; Kong, X.; Xu, L.; Su, Y.; Xu, S.; Pang, X.; Wang, R.; Ma, Y.; Tian, Q.; Han, L. Synergistic Therapeutic Effect of Ginsenoside Rg3 Modified Minoxidil Transfersomes (MXD-Rg3@TFs) on Androgenic Alopecia in C57BL/6 Mice. Int. J. Pharm. 2024, 654, 123963. [Google Scholar] [CrossRef]
- Truong, V.-L.; Keum, Y.-S.; Jeong, W.-S. Red Ginseng Oil Promotes Hair Growth and Protects Skin against UVC Radiation. J. Ginseng Res. 2021, 45, 498–509. [Google Scholar] [CrossRef]
- Truong, V.-L.; Bak, M.J.; Lee, C.; Jun, M.; Jeong, W.-S. Hair Regenerative Mechanisms of Red Ginseng Oil and Its Major Components in the Testosterone-Induced Delay of Anagen Entry in C57BL/6 Mice. Molecules 2017, 22, 1505. [Google Scholar] [CrossRef]
- Ryu, H.J.; Yoo, M.G.; Son, S.W. The Efficacy of 3% Minoxidil vs. Combined 3% Minoxidil and Korean Red Ginseng in Treating Female Pattern Alopecia. Int. J. Dermatol. 2014, 53, e340–e342. [Google Scholar] [CrossRef]
- Hou, J.H.; Shin, H.; Jang, K.H.; Park, C.K.; Koo, B.; Shin, H.; Yuk, S.H.; Lee, K.Y. Anti-Acne Properties of Hydrophobic Fraction of Red Ginseng (Panax ginseng C.A. Meyer) and Its Active Components. Phytother. Res. 2019, 33, 584–590. [Google Scholar] [CrossRef]
- Feng, S.; Wang, L.; Shao, P.; Lu, B.; Chen, Y.; Sun, P. Simultaneous Analysis of Free Phytosterols and Phytosterol Glycosides in Rice Bran by SPE/GC-MS. Food Chem. 2022, 387, 132742. [Google Scholar] [CrossRef]
- Park, H.-Y.; Lee, K.-W.; Choi, H.-D. Rice Bran Constituents: Immunomodulatory and Therapeutic Activities. Food Funct. 2017, 8, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Muangsanguan, A.; Ruksiriwanich, W.; Arjin, C.; Jamjod, S.; Prom-U-Thai, C.; Jantrawut, P.; Rachtanapun, P.; Hnorkaew, P.; Satsook, A.; Sainakham, M.; et al. Comparison of In Vitro Hair Growth Promotion and Anti-Hair Loss Potential of Thai Rice By-Product from Oryza sativa L. Cv. Buebang 3 CMU and Sanpatong. Plants 2024, 13, 3079. [Google Scholar] [CrossRef]
- Kim, Y.-M.; Kwon, S.-J.; Jang, H.-J.; Seo, Y.-K. Rice Bran Mineral Extract Increases the Expression of Anagen-Related Molecules in Human Dermal Papilla through Wnt/Catenin Pathway. Food Nutr. Res. 2017, 61, 1412792. [Google Scholar] [CrossRef][Green Version]
- Khantham, C.; Linsaenkart, P.; Chaitep, T.; Jantrawut, P.; Chittasupho, C.; Rachtanapun, P.; Jantanasakulwong, K.; Phimolsiripol, Y.; Sommano, S.R.; Prom-U-Thai, C.; et al. Antioxidation, Anti-Inflammation, and Regulation of SRD5A Gene Expression of Oryza sativa Cv. Bue Bang 3 CMU Husk and Bran Extracts as Androgenetic Alopecia Molecular Treatment Substances. Plants 2022, 11, 330. [Google Scholar] [CrossRef]
- Ruksiriwanich, W.; Linsaenkart, P.; Khantham, C.; Muangsanguan, A.; Sringarm, K.; Jantrawut, P.; Prom-U-Thai, C.; Jamjod, S.; Yamuangmorn, S.; Arjin, C.; et al. Regulatory Effects of Thai Rice By-Product Extracts from Oryza sativa L. Cv. Bue Bang 3 CMU and Bue Bang 4 CMU on Melanin Production, Nitric Oxide Secretion, and Steroid 5α-Reductase Inhibition. Plants 2023, 12, 653. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-S.; Jeon, M.-H.; Moon, W.-S.; Moon, J.-N.; Cheon, E.J.; Kim, J.-W.; Jung, S.K.; Ji, Y.-H.; Son, S.W.; Kim, M.-R. In Vivo Hair Growth-Promoting Effect of Rice Bran Extract Prepared by Supercritical Carbon Dioxide Fluid. Biol. Pharm. Bull. 2014, 37, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-S.; Park, J.B.; Moon, W.-S.; Moon, J.-N.; Son, S.W.; Kim, M.-R. Safety and Efficacy of Rice Bran Supercritical CO2 Extract for Hair Growth in Androgenic Alopecia: A 16-Week Double-Blind Randomized Controlled Trial. Biol. Pharm. Bull. 2015, 38, 1856–1863. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, Y.; Weber, D.; Yerke, A.; Xue, Y.; Lehman, D.; Williams, T.; Xiao, T.; Haddad, D.; Williams, L. A Substitute Variety for Agronomically and Medicinally Important Serenoa repens (Saw palmetto). Sci. Rep. 2019, 9, 4709. [Google Scholar] [CrossRef] [PubMed]
- Morabito, P.; Miroddi, M.; Giovinazzo, S.; Spina, E.; Calapai, G. Serenoa repens as an Endocrine Disruptor in a 10-Year-Old Young Girl: A New Case Report. Pharmacology 2015, 96, 41–43. [Google Scholar] [CrossRef]
- You, J.; Woo, J.; Roh, K.-B.; Jeon, K.; Jang, Y.; Choi, S.-A.; Ryu, D.; Cho, E.; Park, D.; Lee, J.; et al. Evaluation of Efficacy of Silybum marianum Flower Extract on the Mitigating Hair Loss in Vitro and in Vivo. J. Cosmet. Dermatol. 2024, 23, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Cheon, H.I.; Bae, S.; Ahn, K.J. Flavonoid Silibinin Increases Hair-Inductive Property via Akt and Wnt/β-Catenin Signaling Activation in 3-Dimensional-Spheroid Cultured Human Dermal Papilla Cells. J. Microbiol. Biotechnol. 2019, 29, 321–329. [Google Scholar] [CrossRef]
- Murata, K.; Noguchi, K.; Kondo, M.; Onishi, M.; Watanabe, N.; Okamura, K.; Matsuda, H. Promotion of Hair Growth by Rosmarinus officinalis Leaf Extract. Phytother. Res. 2013, 27, 212–217. [Google Scholar] [CrossRef]
- Eid, A.Y.; Al-Mahdy, D.A.; Sayed, R.H.; Choucry, M.A.; El-Askary, H. Topical Application of Standardized Capsicum and Rosemary Extracts Promotes Hair Growth in Testosterone Induced Alopecia in Wistar Rats: Histological and Morphometric Evaluation. Indian J. Pharmacol. 2025, 57, 134–144. [Google Scholar] [CrossRef]
- Panahi, Y.; Taghizadeh, M.; Marzony, E.T.; Sahebkar, A. Rosemary Oil vs Minoxidil 2% for the Treatment of Androgenetic Alopecia: A Randomized Comparative Trial. Skinmed 2015, 13, 15–21. [Google Scholar]
- Yehia, R.M.; Lamie, C.; Attia, D.A. Microsponges-Mediated Targeted Topical Delivery of Rosemary Oil for Hair Growth Promotion: Optimization and in-Vivo Studies. Pharm. Dev. Technol. 2024, 29, 604–617. [Google Scholar] [CrossRef]
- Shin, H.-S.; Park, S.-Y.; Song, H.-G.; Hwang, E.; Lee, D.-G.; Yi, T.-H. The Androgenic Alopecia Protective Effects of Forsythiaside-A and the Molecular Regulation in a Mouse Model. Phytother. Res. 2015, 29, 870–876. [Google Scholar] [CrossRef]
- Wang, L.; Mo, S.; Zhang, G.; Yue, X.; Qu, Y.; Sun, X.; Wang, K. Natural Phenylethanoid Glycoside Forsythoside A Alleviates Androgenetic Alopecia by Selectively Inhibiting TRPV3 Channels in Mice. Eur. J. Pharmacol. 2025, 990, 177264. [Google Scholar] [CrossRef] [PubMed]
- Telange, D.R.; Patil, A.T.; Pethe, A.M.; Fegade, H.; Anand, S.; Dave, V.S. Formulation and Characterization of an Apigenin-Phospholipid Phytosome (APLC) for Improved Solubility, in Vivo Bioavailability, and Antioxidant Potential. Eur. J. Pharm. Sci. 2017, 108, 36–49. [Google Scholar] [CrossRef] [PubMed]
- van der Vlies, A.J.; Morisaki, M.; Neng, H.I.; Hansen, E.M.; Hasegawa, U. Framboidal Nanoparticles Containing a Curcumin-Phenylboronic Acid Complex with Antiangiogenic and Anticancer Activities. Bioconjug. Chem. 2019, 30, 861–870. [Google Scholar] [CrossRef]
- Donthi, M.R.; Munnangi, S.R.; Krishna, K.V.; Saha, R.N.; Singhvi, G.; Dubey, S.K. Nanoemulgel: A Novel Nano Carrier as a Tool for Topical Drug Delivery. Pharmaceutics 2023, 15, 164. [Google Scholar] [CrossRef]
- Tayeb, H.H.; Felimban, R.; Almaghrabi, S.; Hasaballah, N. Nanoemulsions: Formulation, Characterization, Biological Fate, and Potential Role against COVID-19 and Other Viral Outbreaks. Colloid Interface Sci. Commun. 2021, 45, 100533. [Google Scholar] [CrossRef]
- Abla, K.K.; Alamoudi, M.K.; Soliman, G.A.; Abdel-Kader, M.S.; Aldawsari, M.F.; Mehanna, M.M. Alopecia Management Potential of Rosemary-Based Nanoemulgel Loaded with Metformin: Approach Combining Active Essential Oil and Repurposed Drug. Int. J. Nanomed. 2025, 20, 605–624. [Google Scholar] [CrossRef]
- Shin, K.; Choi, H.; Song, S.K.; Yu, J.W.; Lee, J.Y.; Choi, E.J.; Lee, D.H.; Do, S.H.; Kim, J.W. Nanoemulsion Vehicles as Carriers for Follicular Delivery of Luteolin. ACS Biomater. Sci. Eng. 2018, 4, 1723–1729. [Google Scholar] [CrossRef]
- Chen, B.; Guo, L.; Wang, S.; Xu, J.; Han, H.; Cui, R.; Ding, X.; Cai, G.; He, Y.; Li, D.; et al. Facile Fabrication of Cedrol Nanoemulsions with Deep Transdermal Delivery Ability and High Safety for Effective Androgenic Alopecia Treatment. Colloids Surf. B Biointerfaces 2025, 245, 114259. [Google Scholar] [CrossRef]
- Kumari, L.; Choudhari, Y.; Patel, P.; Gupta, G.D.; Singh, D.; Rosenholm, J.M.; Bansal, K.K.; Kurmi, B.D. Advancement in Solubilization Approaches: A Step towards Bioavailability Enhancement of Poorly Soluble Drugs. Life 2023, 13, 1099. [Google Scholar] [CrossRef]
- Riangjanapatee, P.; Khongkow, M.; Treetong, A.; Unger, O.; Phungbun, C.; Jaemsai, S.; Bootsiri, C.; Okonogi, S. Development of Tea Seed Oil Nanostructured Lipid Carriers and In Vitro Studies on Their Applications in Inducing Human Hair Growth. Pharmaceutics 2022, 14, 984. [Google Scholar] [CrossRef]
- Vanti, G.; Camilla Bergonzi, M.; Rita Bilia, A. Development of Nanoliposomes Loaded with Carbon Dioxide Serenoa repens (Saw palmetto) Extract. J. Nanosci. Nanotechnol. 2021, 21, 2943–2945. [Google Scholar] [CrossRef]
- Liu, Z.; He, Z.; Ai, X.; Guo, T.; Feng, N. Cardamonin-Loaded Liposomal Formulation for Improving Percutaneous Penetration and Follicular Delivery for Androgenetic Alopecia. Drug Deliv. Transl. Res. 2024, 14, 2444–2460. [Google Scholar] [CrossRef]
- Assiri, A.A.; Glover, K.; Mishra, D.; Waite, D.; Vora, L.K.; Thakur, R.R.S. Block Copolymer Micelles as Ocular Drug Delivery Systems. Drug Discov. Today 2024, 29, 104098. [Google Scholar] [CrossRef] [PubMed]
- Luhar, M.; Viradiya, R.; Panjabi, S.; Patel, G. Nanotechnology in Ocular Drug Delivery: The Potential of Polymeric Micelles as a Drug Delivery Vehicle. J. Ocul. Pharmacol. Ther. 2025, 41, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Wang, Z.; Zhu, Z.; Hu, Y.; Wang, Y.; Xue, Y.; Wu, Y.; Guo, Y.; Liang, P.; Chen, H.; et al. Glycyrrhizin Micellar Nanocarriers for Topical Delivery of Baicalin to the Hair Follicles: A Targeted Approach Tailored for Alopecia Treatment. Int. J. Pharm. 2022, 625, 122109. [Google Scholar] [CrossRef]
- Bhattacharjee, S. Craft of Co-Encapsulation in Nanomedicine: A Struggle to Achieve Synergy through Reciprocity. ACS Pharmacol. Transl. Sci. 2022, 5, 278–298. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Zhang, S.; Wang, G.; Yan, Z.; Wu, G.; Tang, L.; Wang, W. Nanocarrier-Based Transdermal Drug Delivery Systems for Dermatological Therapy. Pharmaceutics 2024, 16, 1384. [Google Scholar] [CrossRef]
- Kattou, P.; Lian, G.; Glavin, S.; Sorrell, I.; Chen, T. Development of a Two-Dimensional Model for Predicting Transdermal Permeation with the Follicular Pathway: Demonstration with a Caffeine Study. Pharm. Res. 2017, 34, 2036–2048. [Google Scholar] [CrossRef]
- Zorn-Kruppa, M.; Vidal-Y-Sy, S.; Houdek, P.; Wladykowski, E.; Grzybowski, S.; Gruber, R.; Gorzelanny, C.; Harcup, J.; Schneider, S.W.; Majumdar, A.; et al. Tight Junction Barriers in Human Hair Follicles—Role of Claudin-1. Sci. Rep. 2018, 8, 12800. [Google Scholar] [CrossRef]
- Costa, C.; Cavaco-Paulo, A.; Matamá, T. Mapping Hair Follicle-Targeted Delivery by Particle Systems: What Has Science Accomplished so Far? Int. J. Pharm. 2021, 610, 121273. [Google Scholar] [CrossRef]
- Patzelt, A.; Richter, H.; Knorr, F.; Schäfer, U.; Lehr, C.-M.; Dähne, L.; Sterry, W.; Lademann, J. Selective Follicular Targeting by Modification of the Particle Sizes. J. Control. Release 2011, 150, 45–48. [Google Scholar] [CrossRef]
- Limcharoen, B.; Toprangkobsin, P.; Banlunara, W.; Wanichwecharungruang, S.; Richter, H.; Lademann, J.; Patzelt, A. Increasing the Percutaneous Absorption and Follicular Penetration of Retinal by Topical Application of Proretinal Nanoparticles. Eur. J. Pharm. Biopharm. 2019, 139, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Lima, C.D.S.; Lemes, L.R.; Melo, D.F. Yellow Dots in Trichoscopy: Relevance, Clinical Significance and Peculiarities. An. Bras. Dermatol. 2017, 92, 724–726. [Google Scholar] [CrossRef] [PubMed]
- Prabahar, K.; Udhumansha, U.; Elsherbiny, N.; Qushawy, M. Microneedle Mediated Transdermal Delivery of β-Sitosterol Loaded Nanostructured Lipid Nanoparticles for Androgenic Alopecia. Drug Deliv. 2022, 29, 3022–3034. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Li, Z.; Jiao, S.; Xiao, T.; Wu, Y.; Chen, C.; Guo, S.; Li, X.; Pan, Z.; Li, J.; et al. Black Phosphorus Nanosheets Encapsulated Microneedle for Multifunctional Therapy for Androgenic Alopecia. J. Nanobiotechnol. 2025, 23, 147. [Google Scholar] [CrossRef]
- Wu, X.; Tang, Y.; Shen, N.; Wang, Z. Analysis on the Treatment Compliance of Patients with Androgenetic Alopecia and Its Influencing Factors: Based on the Comparison between Microneedle Therapy and Drug Therapy. Arch. Dermatol. Res. 2025, 317, 670. [Google Scholar] [CrossRef]
- Senthilnathan, A.; Larrondo, J.; De Souza, B.; Harris, T.; Eginli, A.; McMichael, A. Topical Minoxidil Adherence in Patients with Alopecia. J. Drugs Dermatol. 2023, 22, 252–255. [Google Scholar] [CrossRef]
- Suchonwanit, P.; Srisuwanwattana, P.; Chalermroj, N.; Khunkhet, S. A Randomized, Double-Blind Controlled Study of the Efficacy and Safety of Topical Solution of 0.25% Finasteride Admixed with 3% Minoxidil vs. 3% Minoxidil Solution in the Treatment of Male Androgenetic Alopecia. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 2257–2263. [Google Scholar] [CrossRef]
- Heydari, S.; Barzegar-Jalali, M.; Heydari, M.; Radmehr, A.; Paiva-Santos, A.C.; Kouhsoltani, M.; Hamishehkar, H. The Impact of Particle Size of Nanostructured Lipid Carriers on Follicular Drug Delivery: A Comprehensive Analysis of Mouse and Human Hair Follicle Penetration. Bioimpacts 2024, 14, 30243. [Google Scholar] [CrossRef]
- Shan, Y.; Xu, C.; Guo, Y.; Wen, L.; Zhou, S.; Fang, L.; Xu, J.; Zheng, H. Liposomes Enhance the Hair Follicle Delivery of Minoxidil Sulfate with Improved Treatment of Androgenic Alopecia. Int. J. Pharm. 2025, 677, 125642. [Google Scholar] [CrossRef]
- Lee, S.; Kwon, J.A.; Park, K.H.; Jin, C.M.; Joo, J.B.; Choi, I. Controlled Drug Release with Surface-Capped Mesoporous Silica Nanoparticles and Its Label-Free in Situ Raman Monitoring. Eur. J. Pharm. Biopharm. 2018, 131, 232–239. [Google Scholar] [CrossRef]
- Liu, J.; Cabral, H.; Mi, P. Nanocarriers Address Intracellular Barriers for Efficient Drug Delivery, Overcoming Drug Resistance, Subcellular Targeting and Controlled Release. Adv. Drug Deliv. Rev. 2024, 207, 115239. [Google Scholar] [CrossRef]
| Population | Gene/Locus | Function or Pathway | Reported Association Features | Reference(s) |
|---|---|---|---|---|
| European males | AR (X chromosome) | Regulates DHT sensitivity | Sequence variants may enhance follicular sensitivity to DHT, leading to earlier onset | [46,47] |
| Ectodysplasin A2 receptor (X chromo-some) | Involved in ectodermal development and hair follicle morphogenesis | Variants associated with increased susceptibility to AGA | [46] | |
| Asian & African males | X-linked loci | – | Association with AGA not significant in most studies | [48,49,50] |
| Asian males | 20p11 locus | Autosomal | Shows higher predictive value for AGA; mechanisms remain unclear | [49,50] |
| Females | ESR2 | Estrogen receptor | SNPs associated with AGA risk | [52] |
| CYP19A1 | Aromatase (converts androgens to estrogens) | SNPs associated with susceptibility | [51,52] | |
| Multiple populations | Wnt pathway genes | Hair follicle growth regulation | Polymorphisms linked to AGA susceptibility | [44] |
| TGF-β pathway genes | Hair follicle regression signaling | Variants associated with AGA | [44] | |
| HIF-1α | Hypoxia response pathway | Associated with AGA risk in some studies | [44] |
| Saw Palmetto | Cacumen Platycladi | Panax Ginseng | Red Ginseng | Rice Bran | ||
|---|---|---|---|---|---|---|
| Main Bioactive Components | Oleic acid, linoleic acid, palmitic acid, campesterol, β-sitosterol, etc. | α-pinene, α-cedrol, quercitrin, amentoflavone, hinokiflavone (7E)-7,8-Dehydroheliobuphthalmin, etc. | Gintonin, ginsenoside Re, Rb1, Rg1, Rd, etc. | Ginsenoside Re, Rb1, Rg1, Rg3, etc. | β-sitosterol, campesterol, tocotrienols, γ-oryzanol, etc. | |
| In Vitro Studies | HaCaT, HMVECs, DPCs | DPCs, HUVECs | ORS keratinocytes, human HF organ, DPCs | DPCs, human HF organ, OSR cells | DPCs, RAW 264.7, B16F10 | |
| In Vivo Studies | DHT-Induced AGA mouse model | Androgen induced AGA mouse model, C57BL/6 Mice, Wistar rats | Nude mice, C57BL/6 mice | C57BL/6 mice, testosterone-induced AGA mouse model | C57BL/6 mice | |
| Clinical Research Evidence (Topical Application) 1 | Randomized, double-blind, placebo-controlled study; Prospective, open-label, self-controlled study | Randomized, double-blind, placebo-controlled study | Randomized, triple-blind, controlled minoxidil study | Randomized, double-blind, placebo-controlled study | ||
| Mechanism of Action | Anti-Androgenic | 5-α reductase II↓ 2 | AR↓, DHT↓, 5α reductase↓ | AR↓ | SRD5A1↓, SRD5A2↓, SRD5A3↓ | |
| Anti-Inflammatory | Inflammatory cells↓ | NO↓ | ||||
| Antioxidative | Lipid peroxidation↓ | SOD↑, CAT↑, GSH-PX↑, MDA↓ | GPx↑, SOD-2↑, ROS↓ | MDA↓ | ||
| Anti-Apoptotic | Cleaved caspase 3↓, Bcl-2↑, Bax↓ | Survivin↑ | TUNEL-positive cells↓, Bcl-2↑, Bax↓ | Bcl-2↑, Bax↓ | ||
| Pro-Proliferative | Cell viability↑ | Cell viability↑ | Cell viability↑ | Cell viability↑, Ki67↑ | ||
| Pro-Angiogenic | VEGF↑ | Scratch assay (+) | VEGF↑ | VEGF↑ | VEGF↑ | |
| Anti-Aging | p21↓, p16↓ | β-Gal-positive cells↓ | ||||
| Modulation of the Microbiota | Cutibacterium↓ | |||||
| Signaling Pathways | Wnt/β-catenin pathway↑, TGF-β pathway↓ | Wnt/β-catenin pathway↑, tyrosine kinase signaling pathway↑, Akt/GSK3β pathway↑, TGF-β pathway↓ | Wnt/β-catenin pathway↑, TGF-β pathway↓ | Wnt/β-catenin pathway↑, PI3K/Akt pathway↑, TGF-β pathway↓ | Wnt/β-catenin pathway↑, Sonic Hedgehog pathway↑, TGF-β pathway↓ | |
| Others | Promotes cell cycle progression from G0/G1 phase to S phase | p63↑, transient elevation of cytosolic Ca2+ levels | IGF-1↑, BMP4↓ | ALP↑, fibronectin↑, IGF-1↑, melanin synthesis↑ | ||
| Ref. | [87,88,89,90,91,92,132,133] | [93,94,95,96,97,98,99,100,101,102,103,104,105] | [106,107,108,109,110,111,112] | [113,114,115,116,117,118,119,120,121,122,123] | [124,125,126,127,128,129,130,131] | |
| Silybum Marianum | Rosemary | Forsythiasis | |
|---|---|---|---|
| Main Bioactive Components | Silibinin, apigenin, etc. | Rosmarinic acid, etc. | Forsythiaside-A, etc. |
| Study types | In Vitro (DPCs)/randomized, double-blind, placebo-controlled clinical trial (shampoo, rinse-off). | In Vivo (Wistar rats, C57BL/6 mice)/randomized, active-controlled clinical trial (topical) | In Vitro (DPCs, HaCaT)/In Vivo (C57BL/6 mouse) |
| Mechanism of Action | Pro-Proliferative, Pro-Angiogenic, Antioxidative, Anti-Aging, Wnt/β-Catenin pathway↑ 2, Akt pathway↑ | Anti-Androgenic, Wnt/β-Catenin pathway↑ | Anti-Apoptotic, TRPV3 pathway↓ |
| Major clinical outcomes | Hair count↑ | Hair count not significantly different from 2% minoxidil at 6 months | No clinical data |
| Ref. | [134,135] | [136,137,138,139] | [140,141] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diao, R.; Sun, M.; Zhang, N.; Liu, X.; Song, P. Novel Strategies for Androgenetic Alopecia Therapy: Integrating Multifunctional Plant Extracts with Nanotechnology for Advanced Cutaneous Drug Delivery. Pharmaceutics 2025, 17, 1220. https://doi.org/10.3390/pharmaceutics17091220
Diao R, Sun M, Zhang N, Liu X, Song P. Novel Strategies for Androgenetic Alopecia Therapy: Integrating Multifunctional Plant Extracts with Nanotechnology for Advanced Cutaneous Drug Delivery. Pharmaceutics. 2025; 17(9):1220. https://doi.org/10.3390/pharmaceutics17091220
Chicago/Turabian StyleDiao, Ruohan, Meiqi Sun, Ningxin Zhang, Xinqian Liu, and Ping Song. 2025. "Novel Strategies for Androgenetic Alopecia Therapy: Integrating Multifunctional Plant Extracts with Nanotechnology for Advanced Cutaneous Drug Delivery" Pharmaceutics 17, no. 9: 1220. https://doi.org/10.3390/pharmaceutics17091220
APA StyleDiao, R., Sun, M., Zhang, N., Liu, X., & Song, P. (2025). Novel Strategies for Androgenetic Alopecia Therapy: Integrating Multifunctional Plant Extracts with Nanotechnology for Advanced Cutaneous Drug Delivery. Pharmaceutics, 17(9), 1220. https://doi.org/10.3390/pharmaceutics17091220







