Selective Inhibition of Yersinia enterocolitica Type III Secretion by Lindera obtusiloba Extract and Cinnamtannin B1
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial and Culture Media
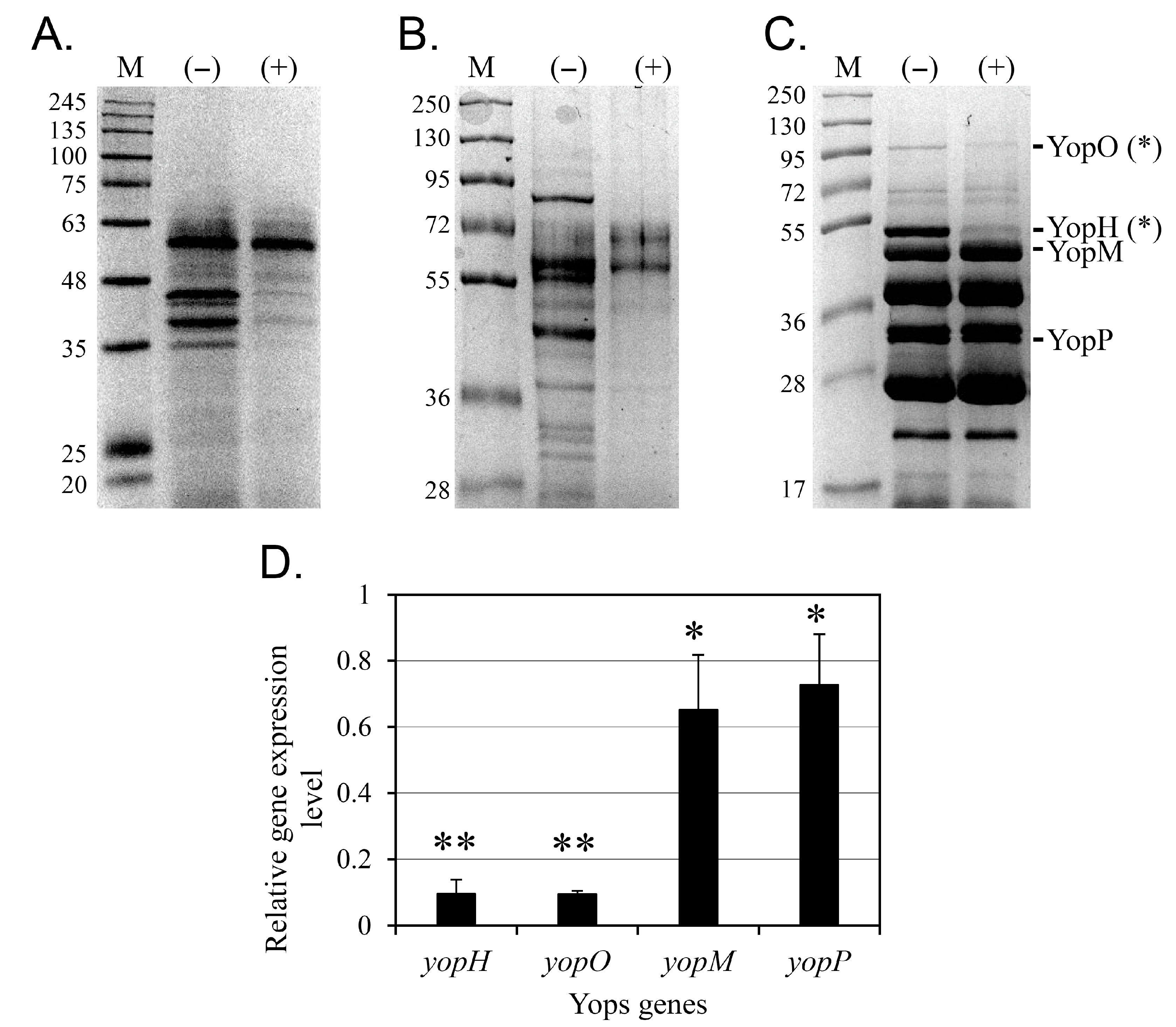
2.2. Analysis of T3SS-Secreted Proteins
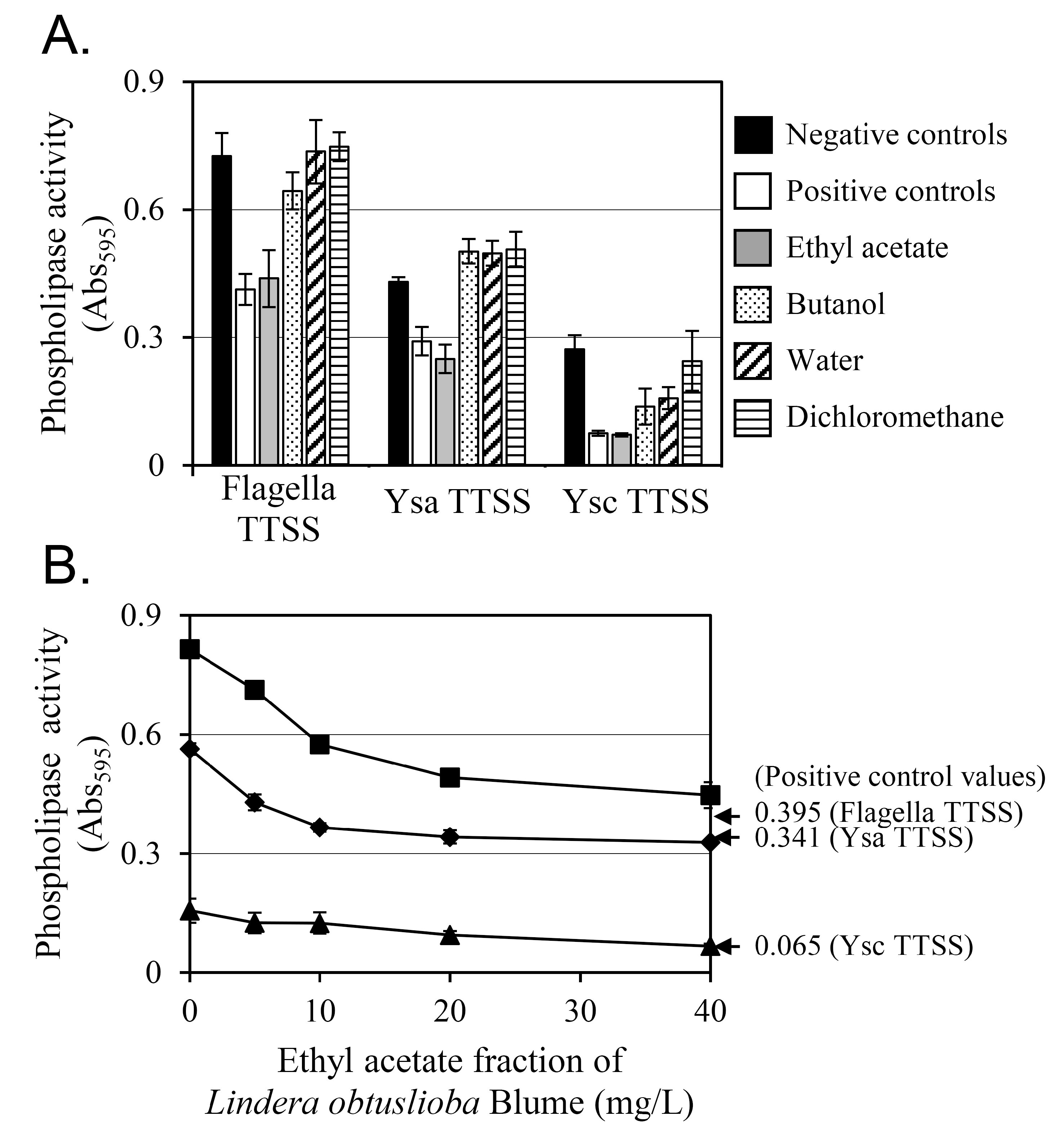
2.3. YplA Phospholipase Activity Assay
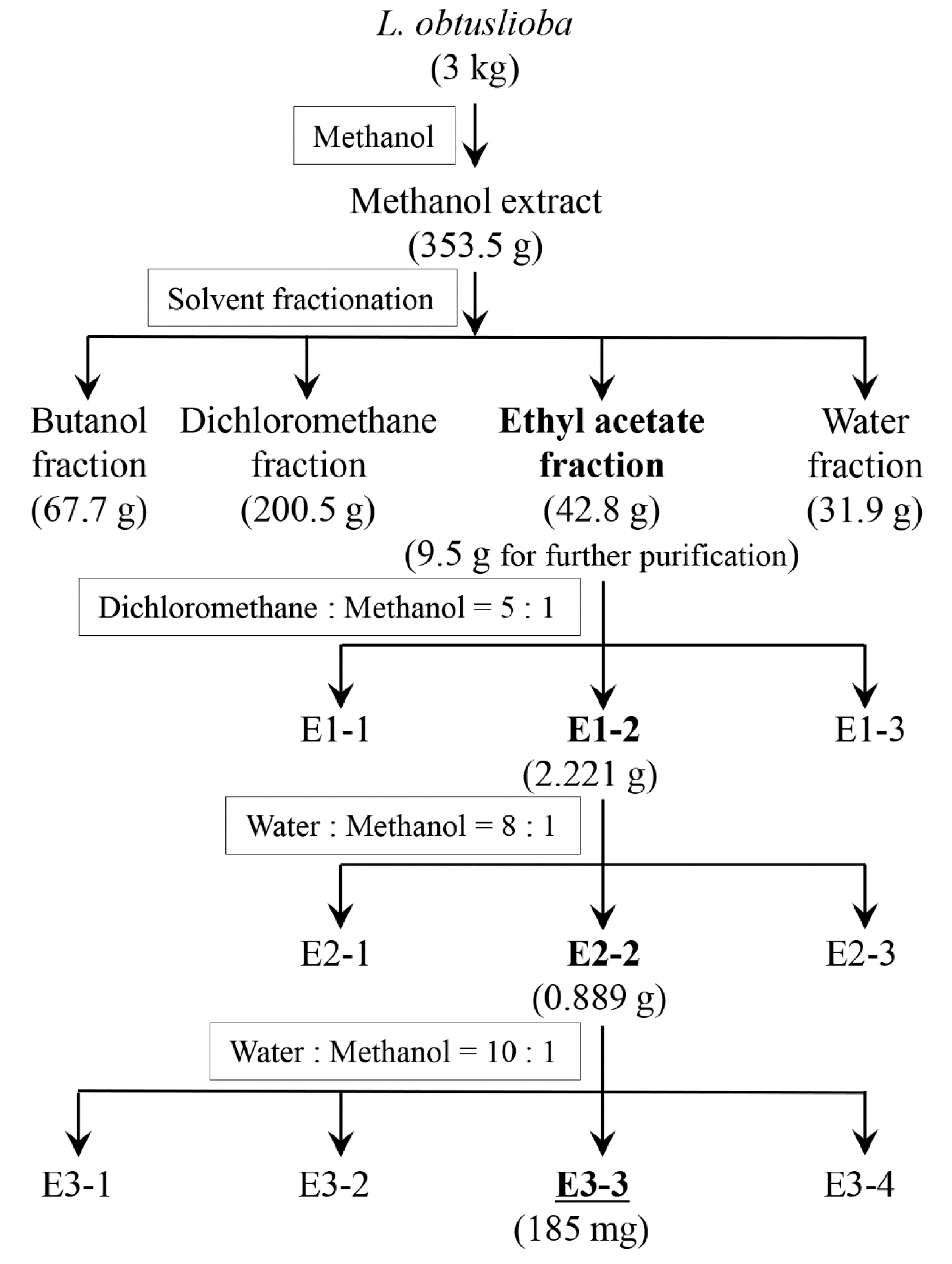
2.4. Preparation of the L. obtusiloba Extract
2.5. Isolation of Active Ingredients
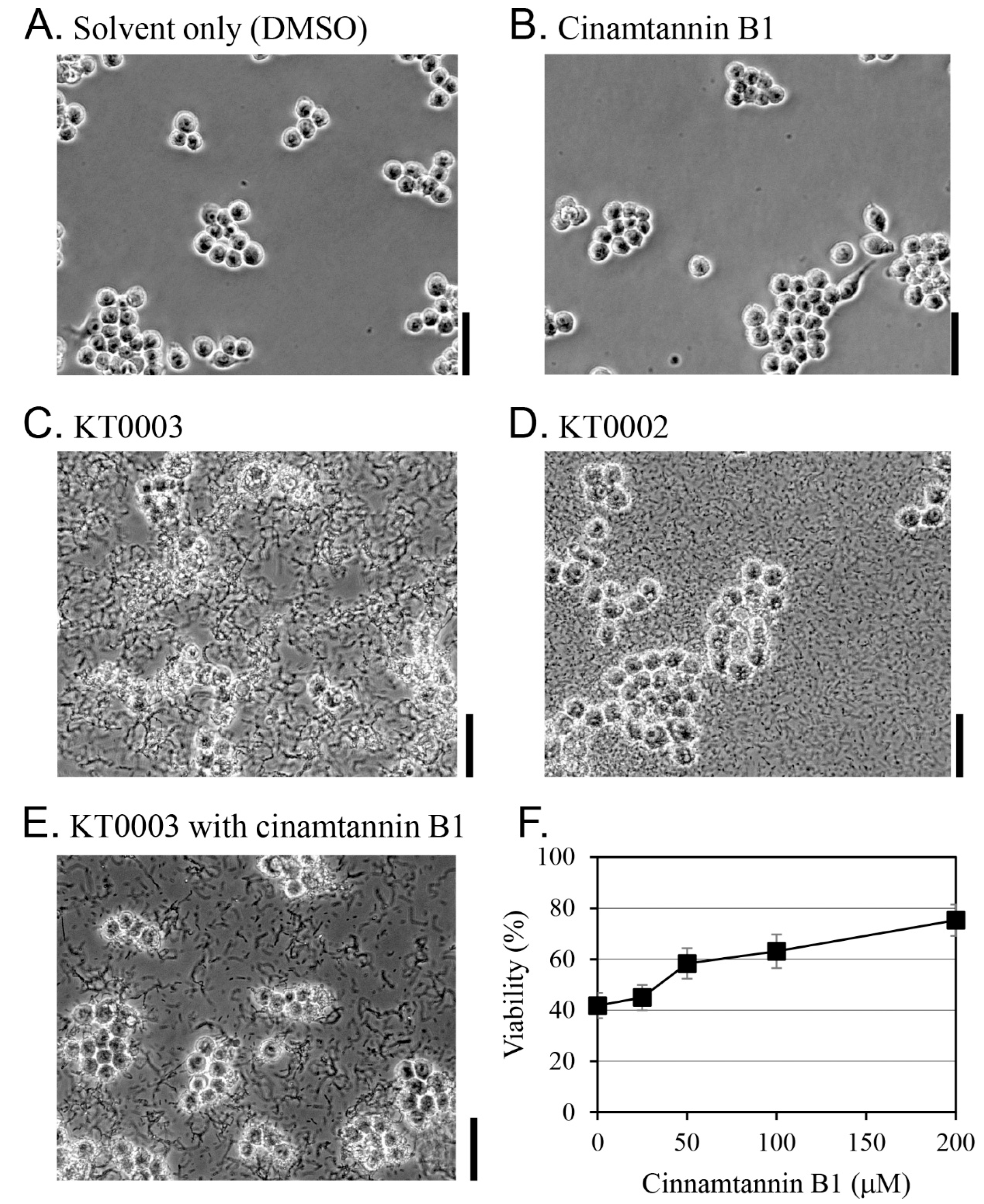
2.6. Morphological Changes to Macrophage Cells Following Y. enterocolitica Infection
2.7. Measuring Cell Cytotoxicity Due to Y. enterocolitica Infection
2.8. Gene Expression Analysis
2.9. Detection of Poly (ADP-Ribose) Polymerase (PARP) by Immunoblotting
3. Results
3.1. An Ethyl Acetate Fraction of L. obtusiloba Inhibited the Secretion of YplA
3.2. Cinnamtannin B1 Was Identified in the Ethyl Acetate Fraction of the L. obtusiloba Extract, Which Inhibited YplA Phospholipase Activity Secreted by T3SSs in Y. enterocolitica
3.3. Protein Secretion via the T3SS Is Inhibited by Cinnamtannin B1
3.4. Cinnamtannin B1 Prevents the Killing of RAW 264.7 Macrophages by Y. enterocolitica
3.5. Cinnamtannin B1 Inhibited the Y. enterocolitica-Induced Apoptosis of RAW 264.7 Macrophages
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATCC | American Type Culture Collection |
| DMEM | Dulbecco’s modified Eagle’s medium |
| DMSO | Dimethyl sulfoxide |
| Fops | Flagellar outer proteins |
| GDI | Guanine nucleotide dissociation inhibitor |
| KCCM | Korean Culture Center of Microorganisms |
| MOI | Multiplicity of infection |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| NMR | Nuclear magnetic resonance |
| PARP | Poly (ADP-ribose) polymerase |
| PBS | Phosphate-buffered saline |
| RT-qPCR | Reverse-transcription polymerase chain reaction |
| SDS-PAGE | Sodium dodecyl sulfate-polyacrylamide gel electrophoresis |
| SKU | Stock keeping unit |
| T3SS | Type III secretion system |
| Ysps | Yersinia secreted proteins |
| Yops | Yersinia outer proteins |
References
- Ventola, C. The antibiotic resistance crisis: Part 1: Causes and threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Silva, L.N.; Zimmer, K.R.; Macedo, A.J.; Trentin, D.S. Plant natural products targeting bacterial virulence factors. Chem. Rev. 2016, 116, 9162–9236. [Google Scholar] [CrossRef]
- Clatworthy, A.E.; Pierson, E.; Hung, D.T. Targeting virulence: A new paradigm for antimicrobial therapy. Nat. Chem. Biol. 2007, 3, 541–548. [Google Scholar] [CrossRef]
- Puhar, A.; Sansonetti, P.J. Type III secretion system. Curr. Biol. 2014, 24, R784–R791. [Google Scholar] [CrossRef] [PubMed]
- Beckham, K.S.H.; Roe, A.J. From screen to target: Insights and approaches for the development of anti-virulence compounds. Front. Cell. Infect. Microbiol. 2014, 4, 139. [Google Scholar] [CrossRef]
- Robins-Browne, R.M. Yersinia enterocolitica. In Food Microbiology; Elsevier: Amsterdam, The Netherlands, 2012; pp. 339–376. [Google Scholar]
- Zhou, S.; Wang, H.; Li, H.; Yang, Y.; Shi, D.; Yang, Z.; Yang, D.; Chen, T.; Li, J.; Jin, M. Emergence of polymyxin-resistant Yersinia enterocolitica strains in natural aquatic environments. Environ. Pollut. 2025, 364, 125341. [Google Scholar] [CrossRef]
- Young, G.M.; Schmiel, D.H.; Miller, V.L. A new pathway for the secretion of virulence factors by bacteria: The flagellar export apparatus functions as a protein-secretion system. Proc. Natl. Acad. Sci. USA 1999, 96, 6456–6461. [Google Scholar] [CrossRef] [PubMed]
- Haller, J.C.; Carlson, S.; Pederson, K.J.; Pierson, D.E. A chromosomally encoded type III secretion pathway in Yersinia enterocolitica is important in virulence. Mol. Microbiol. 2000, 36, 1436–1446. [Google Scholar] [CrossRef]
- Cornelis, G.R.; Boland, A.; Boyd, A.P.; Geuijen, C.; Iriarte, M.; Neyt, C.; Sory, M.-P.; Stainier, I. The Virulence plasmid of Yersinia, an antihost genome. Microbiol. Mol. Biol. Rev. 1998, 62, 1315–1352. [Google Scholar] [CrossRef] [PubMed]
- Young, B.M.; Young, G.M. Evidence for targeting of Yop effectors by the chromosomally encoded Ysa type III secretion system of Yersinia enterocolitica. J. Bacteriol. 2002, 184, 5563–5571. [Google Scholar] [CrossRef]
- Young, B.M.; Young, G.M. YplA is exported by the Ysc, Ysa, and flagellar type III secretion systems of Yersinia enterocolitica. J. Bacteriol. 2002, 184, 1324–1334. [Google Scholar] [CrossRef]
- Heesemann, J.; Gross, U.; Schmidt, N.; Laufs, R. Immunochemical analysis of plasmid-encoded proteins released by enteropathogenic Yersinia sp. grown in calcium-deficient media. Infect. Immun. 1986, 54, 561–567. [Google Scholar] [CrossRef]
- Michiels, T.; Wattiau, P.; Brasseur, R.; Ruysschaert, J.M.; Cornelis, G. Secretion of Yop proteins by Yersiniae. Infect. Immun. 1990, 58, 2840–2849. [Google Scholar] [CrossRef]
- Matsumoto, H.; Young, G.M. Translocated effectors of Yersinia. Curr. Opin. Microbiol. 2009, 12, 94–100. [Google Scholar] [CrossRef]
- Venecia, K.; Young, G.M. Environmental regulation and virulence attributes of the Ysa type III secretion system of Yersinia enterocolitica biovar 1B. Infect. Immun. 2005, 73, 5961–5977. [Google Scholar] [CrossRef] [PubMed]
- Mildiner-Earley, S.; Miller, V.L.; Walker, K.A. Environmental stimuli affecting expression of the Ysa type three secretion locus. In The Genus Yersinia: From Genomics to Function; Perry, R.D., Fetherston, J.D., Eds.; Springer: New York, NY, USA, 2007; pp. 211–216. [Google Scholar]
- Schmiel, D.H.; Wagar, E.; Karamanou, L.; Weeks, D.; Miller, V.L. Phospholipase A of Yersinia enterocolitica contributes to pathogenesis in a mouse model. Infect. Immun. 1998, 66, 3941–3951. [Google Scholar] [CrossRef] [PubMed]
- Warren, S.M.; Young, G.M. An amino-terminal secretion signal Is required for YplA export by the Ysa, Ysc, and flagellar type III secretion systems of Yersinia enterocolitica biovar 1B. J. Bacteriol. 2005, 187, 6075–6083. [Google Scholar] [CrossRef]
- Ham, Y.; Kim, T.-J. Inhibition of biofilm formation in Yersinia enterocolitica by edible plant extracts including Polygoni Multiflori Radix. J. Korean Wood Sci. Technol. 2022, 50, 448–457. [Google Scholar] [CrossRef]
- Ham, Y.; Xu, D.; Kim, T.-J. Effect of licorice extract on biofilm formation in two pathogenic bacteria: Aeromonas hydrophila and Yersinia enterocolitica. J. Korean Wood Sci. Technol. 2025, 53, 77–88. [Google Scholar] [CrossRef]
- Lee, B.-W.; Ha, J.-H.; Shin, H.-G.; Jeong, S.-H.; Kim, J.-H.; Lee, J.; Park, J.-Y.; Kwon, H.-J.; Jung, K.; Lee, W.-S.; et al. Lindera obtusiloba attenuates oxidative stress and airway inflammation in a murine model of ovalbumin-challenged asthma. Antioxidants 2020, 9, 563. [Google Scholar] [CrossRef]
- Lee, J.-A.; Gu, M.J.; Lee, Y.R.; Kim, Y.; Choi, I.; Kim, D.; Ha, S.K. Lindera obtusiloba Blume alleviates non-alcoholic fatty liver disease promoted by Nε-(carboxymethyl)lysine. Nutrients 2024, 16, 2330. [Google Scholar] [CrossRef]
- Lee, Y.J.; Lee, D.K.; Kim, J.S.; Park, K.J.; Cha, D.S.; Kim, D.K.; Kwon, J.; Oh, C.H.; Kim, K.S.; Jeon, H. Sedative and antinociceptive properties of Lindera obtusiloba. Nat. Prod. Sci. 2012, 18, 215–220. [Google Scholar]
- Haque, M.E.; Azam, S.; Balakrishnan, R.; Akther, M.; Kim, I.-S. Therapeutic potential of Lindera obtusiloba: Focus on antioxidative and pharmacological properties. Plants 2020, 9, 1765. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Do, J.-S.; Chung, H.-J. Antimicrobial activities of Lindera obtusiloba Blume and Zanthoxylum piperitum DC extracts. Korean J. Food Preserv. 2014, 21, 427–433. [Google Scholar] [CrossRef]
- Freise, C.; Ruehl, M.; Erben, U.; Neumann, U.; Seehofer, D.; Kim, K.Y.; Trowitzsch-Kienast, W.; Stroh, T.; Zeitz, M.; Somasundaram, R. A hepatoprotective Lindera obtusiloba extract suppresses growth and attenuates insulin like growth factor-1 receptor signaling and NF-kappaB activity in human liver cancer cell lines. BMC Complement. Altern. Med. 2011, 11, 39. [Google Scholar] [CrossRef]
- López, J.J.; Jardín, I.; Salido, G.M.; Rosado, J.A. Cinnamtannin B-1 as an antioxidant and platelet aggregation inhibitor. Life Sci. 2008, 82, 977–982. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Hao, L.; Li, L.; Liu, L.; Chen, G.; Jiang, W.; Xu, W.; Zhu, C.; Yao, G.; Fang, S. Cinnamtannin B-1 prevents ovariectomy-induced osteoporosis via attenuating osteoclastogenesis and ROS generation. Front. Pharmacol. 2020, 11, 1023. [Google Scholar] [CrossRef]
- Ksouri, A.; Klouz, A.; Bouhaouala-Zahar, B.; Moussa, F.; Bezzarga, M. Docking-based evidence for the potential of ImmunoDefender: A novel formulated essential oil blend incorporating synergistic antiviral bioactive compounds as promising Mpro inhibitors against SARS-CoV-2. Molecules 2023, 28, 4296. [Google Scholar] [CrossRef]
- Carriere, P.P.; Kapur, N.; Mir, H.; Ward, A.B.; Singh, S. Cinnamtannin B-1 inhibits cell survival molecules and induces apoptosis in colon cancer. Int. J. Oncol. 2018, 53, 1442–1454. [Google Scholar] [CrossRef]
- Jia, J.; Xia, J.; Liu, W.; Tao, F.; Xiao, J. Cinnamtannin B-1 inhibits the progression of osteosarcoma by regulating the miR-1281/PPIF axis. Biol. Pharm. Bull. 2023, 46, 67–73. [Google Scholar] [CrossRef]
- Kan, H.-L.; Wang, C.-C.; Cheng, Y.-H.; Yang, C.-L.; Chang, H.-S.; Chen, I.-S.; Lin, Y.-C. Cinnamtannin B1 attenuates rosacea-like signs via inhibition of pro-inflammatory cytokine production and down-regulation of the MAPK pathway. PeerJ 2020, 8, e10548. [Google Scholar] [CrossRef]
- Oh, Y.; Kim, T.-J. Temperature adaptive biofilm formation in Yersinia enterocolitica in response to pYV plasmid and calcium. Antibiotics 2025, 14, 857. [Google Scholar] [CrossRef]
- Berring, E.; Brancato, S.; Grant, K.; Schaper, E.; Kadavil, S.; Smagin, H.; Hatic, S.O.; Picking, W.; Serfis, A.B. Destabilization of phospholipid model membranes by YplA, a phospholipase A2 secreted by Yersinia enterocolitica. Chem. Phys. Lipids 2004, 131, 135–149. [Google Scholar] [CrossRef]
- Meysick, K.C.; Seidman, J.; Falconio, J.R. The Yersinia pseudotuberculosis YplA phospholipase differs in its activity, regulation and secretion from that of the Yersinia enterocolitica YplA. Microb. Pathog. 2009, 47, 24–32. [Google Scholar] [CrossRef]
- Ivanov, M.I.; Stuckey, J.A.; Schubert, H.L.; Saper, M.A.; Bliska, J.B. Two substrate-targeting sites in the Yersinia protein tyrosine phosphatase co-operate to promote bacterial virulence. Mol. Microbiol. 2005, 55, 1346–1356. [Google Scholar] [CrossRef]
- Fàbrega, A.; Roca, I.; Vila, J. Fluoroquinolone and multidrug resistance phenotypes associated with the overexpression of AcrAB and an orthologue of MarA in Yersinia enterocolitica. Int. J. Med. Microbiol. 2010, 300, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, K.; Watanabe, C.; Endang, H.; Umar, M.; Satake, T. Studies on the constituents of bark of Parameria laevigata MOLDENKE1). Chem. Pharm. Bull. 2001, 49, 551–557. [Google Scholar] [CrossRef]
- Jayaprakasha, G.K.; Ohnishi-Kameyama, M.; Ono, H.; Yoshida, M.; Jaganmohan Rao, L. Phenolic constituents in the fruits of Cinnamomum zeylanicum and their antioxidant activity. J. Agric. Food Chem. 2006, 54, 1672–1679. [Google Scholar] [CrossRef] [PubMed]
- Bancerz-Kisiel, A.; Lipczyńska-Ilczuk, K. Evaluation of the correlation between the mRNA expression levels of ystA and ymoA genes in Y. enterocolitica strains with different enterotoxic properties. Pathogens 2021, 10, 1136. [Google Scholar]
- Nakasone, N.; Higa, N.; Toma, C.; Ogura, Y.; Suzuki, T.; Yamashiro, T. Epigallocatechin gallate inhibits the type III secretion system of Gram-negative enteropathogenic bacteria under model conditions. FEMS Microbiol. Lett. 2017, 364, fnx111. [Google Scholar] [CrossRef] [PubMed]
- Ruckdeschel, K.; Deuretzbacher, A.; Haase, R. Crosstalk of signalling processes of innate immunity with Yersinia Yop effector functions. Immunobiology 2008, 213, 261–269. [Google Scholar] [CrossRef]
- Viboud, G.I.; Bliska, J.B. Yersinia outer proteins: Role in modulation of host cell signaling responses and pathogenesis. Annu. Rev. Microbiol. 2005, 59, 69–89. [Google Scholar] [CrossRef] [PubMed]
- Black, D.S.; Marie-Cardine, A.; Schraven, B.; Bliska, J.B. The Yersinia tyrosine phosphatase YopH targets a novel adhesion-regulated signalling complex in macrophages. Cell. Microbiol. 2000, 2, 401–414. [Google Scholar] [CrossRef]
- Cantwell, A.M.; Bubeck, S.S.; Dube, P.H. YopH inhibits early pro-inflammatory cytokine responses during plague pneumonia. BMC Immunol. 2010, 11, 29. [Google Scholar] [CrossRef]
- Matsuda, R.; Daniel, S.; Zhang, J.; Peterson, S.T.; Grayczyk, J.P.; Yost, W.; Apenes, N.; Kovalik, M.E.; Herrmann, B.; O’Neill, R.; et al. A TNF-IL-1 circuit controls Yersinia within intestinal pyogranulomas. J. Exp. Med. 2024, 221, e20230679. [Google Scholar] [CrossRef]
- Lee, W.L.; Singaravelu, P.; Wee, S.; Xue, B.; Ang, K.C.; Gunaratne, J.; Grimes, J.M.; Swaminathan, K.; Robinson, R.C. Mechanisms of Yersinia YopO kinase substrate specificity. Sci. Rep. 2017, 7, 39998. [Google Scholar] [CrossRef]
- Pha, K.; Wright, M.E.; Barr, T.M.; Eigenheer, R.A.; Navarro, L. Regulation of Yersinia protein kinase A (YpkA) linase activity by multisite autophosphorylation and identification of an N-terminal substrate-binding domain in YpkA. J. Biol. Chem. 2014, 289, 26167–26177. [Google Scholar] [CrossRef]
- Ke, Y.; Tan, Y.; Wei, N.; Yang, F.; Yang, H.; Cao, S.; Wang, X.; Wang, J.; Han, Y.; Bi, Y.; et al. Yersinia protein kinase A phosphorylates vasodilator-stimulated phosphoprotein to modify the host cytoskeleton. Cell. Microbiol. 2015, 17, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.L.; Grimes, J.M.; Robinson, R.C. Yersinia effector YopO uses actin as bait to phosphorylate proteins that regulate actin polymerization. Nat. Struct. Mol. Biol. 2015, 22, 248–255. [Google Scholar] [CrossRef] [PubMed]


| Target Gene | Primer Direction | Sequence (5′ to 3′) |
|---|---|---|
| gapA (The housekeeping gene) | Sense | CATCCCAGAACATCATCCC |
| Antisense | GCAGTCAGGTCAACAACT | |
| yopH | Sense | CTAACTCAAGAAGATACCGCTAA |
| Antisense | CTATTACCATTGCCGACACT | |
| yopM | Sense | CTTCTTGACTGCGATTTATGC |
| Antisense | AACTCTGCGGTAATTCTGG | |
| yopO | Sense | ATTCCAACGAAGCCAGAC |
| Antisense | GTTATCCGCCTTACATCAGT | |
| yopP | Sense | GAGAGAGATAGCCTGTTGAAAA |
| Antisense | ACTTATTGTGGGGTAAAGGATTT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoo, J.-H.; Kim, T.-J. Selective Inhibition of Yersinia enterocolitica Type III Secretion by Lindera obtusiloba Extract and Cinnamtannin B1. Pharmaceutics 2025, 17, 1217. https://doi.org/10.3390/pharmaceutics17091217
Yoo J-H, Kim T-J. Selective Inhibition of Yersinia enterocolitica Type III Secretion by Lindera obtusiloba Extract and Cinnamtannin B1. Pharmaceutics. 2025; 17(9):1217. https://doi.org/10.3390/pharmaceutics17091217
Chicago/Turabian StyleYoo, Jin-Hee, and Tae-Jong Kim. 2025. "Selective Inhibition of Yersinia enterocolitica Type III Secretion by Lindera obtusiloba Extract and Cinnamtannin B1" Pharmaceutics 17, no. 9: 1217. https://doi.org/10.3390/pharmaceutics17091217
APA StyleYoo, J.-H., & Kim, T.-J. (2025). Selective Inhibition of Yersinia enterocolitica Type III Secretion by Lindera obtusiloba Extract and Cinnamtannin B1. Pharmaceutics, 17(9), 1217. https://doi.org/10.3390/pharmaceutics17091217






