2.1. Materials
DMY (purity ≥ 98%) was purchased from Hubei Chenxin Pharmaceutical Co., Ltd. (Wuhan, China), CIP (98% purity) was purchased from Sinopharm Group Chemical Reagents Co., Ltd. (Shanghai, China), methanol (HPLC) was purchased from Thermo Fisher Scientific (China) Co., Ltd. (Shanghai, China), and other reagents were purchased from Sinopharm Group Chemical Reagents Co., Ltd.
2.2. Methods
2.2.1. Preparation of DMY-CIP Cocrystal
Equimolar quantities (0.5 mmol each) of DMY and CIP were dissolved in 15 mL of 50% ethanol in a round-bottom flask. The mixture was magnetically stirred at 60 °C for 4 h. A portion of the solution was filtered into a screw-cap bottle, sealed with parafilm containing several pinholes, and allowed to evaporate at room temperature for single crystal growth. The remaining solution was transferred to an open petri dish for bulk cocrystal powder preparation. About one week later, transparent needle-shaped crystals were obtained.
2.2.2. Powder X-Ray Diffraction (PXRD)
Powder X-ray diffraction analysis was performed using a Rigaku MiniFlex 600 diffractometer (Rigaku Corporation, Tokyo, Japan) with Cu Kα radiation (λ = 1.54059 Å) at 40 kV and 150 mA. The samples were scanned from 3° to 50° (2θ) with a step size of 0.01° and a scanning rate of 10°/min. An appropriate amount of powder sample was ground and placed on a monocrystalline silicon sample stage, and the sample was flattened using a medicine spoon and weighing paper for testing. Data analysis was conducted using Origin 2021 software, and single-crystal powder diffraction patterns were simulated with Mercury 2021.3.0 software.
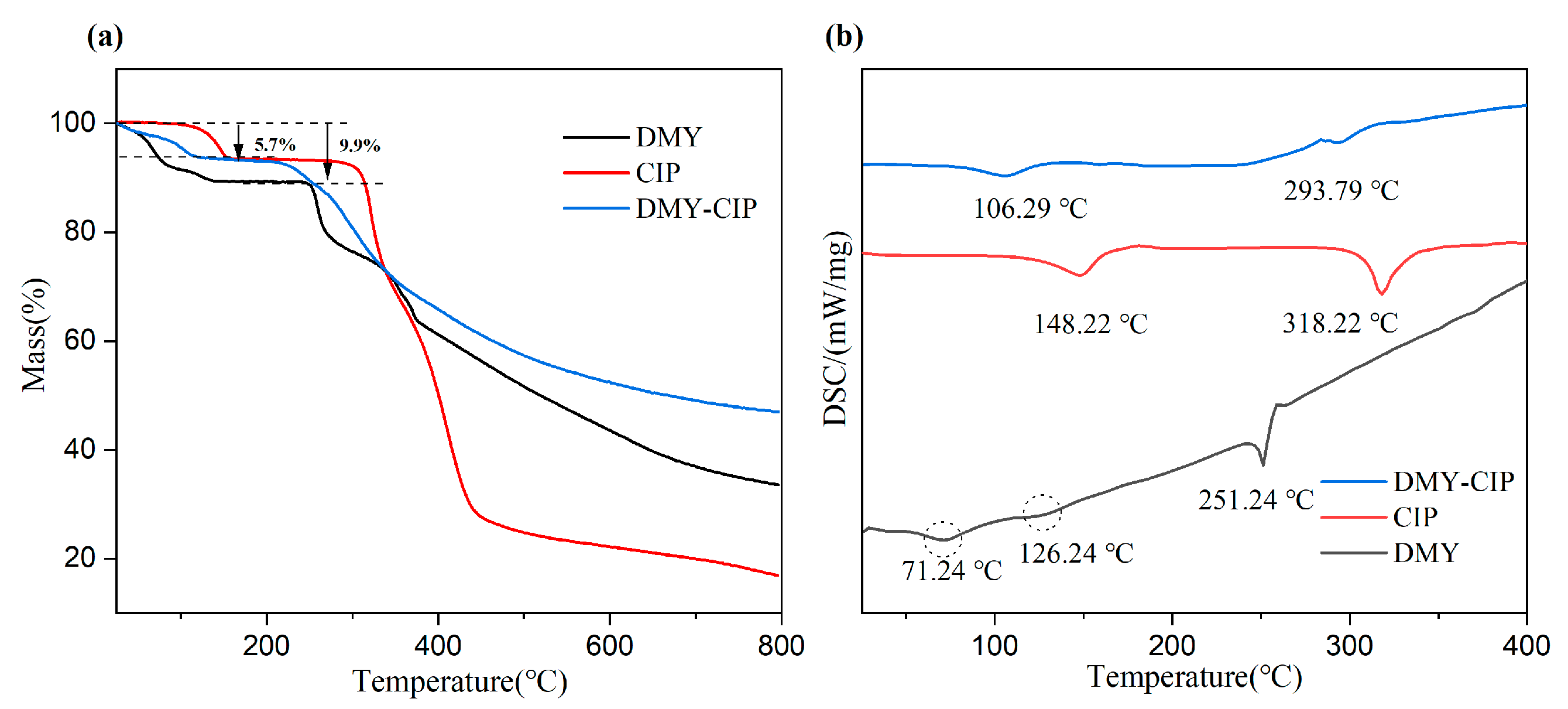
2.2.3. Thermogravimetric (TG) Analysis and Differential Scanning Calorimetry (DSC)
Thermal analysis was performed using a Netzsch STA449 F5 instrument (Netzsch-Gerätebau GmbH, Selb, Germany). Samples (2–5 mg) were weighed into alumina crucibles and heated from 30 °C to 800 °C at a constant heating rate of 10 °C/min under a dynamic nitrogen atmosphere. The protective gas (N1) and purge gas (N2), both high-purity nitrogen, were maintained at flow rates of 5 mL/min and 20 mL/min, respectively.
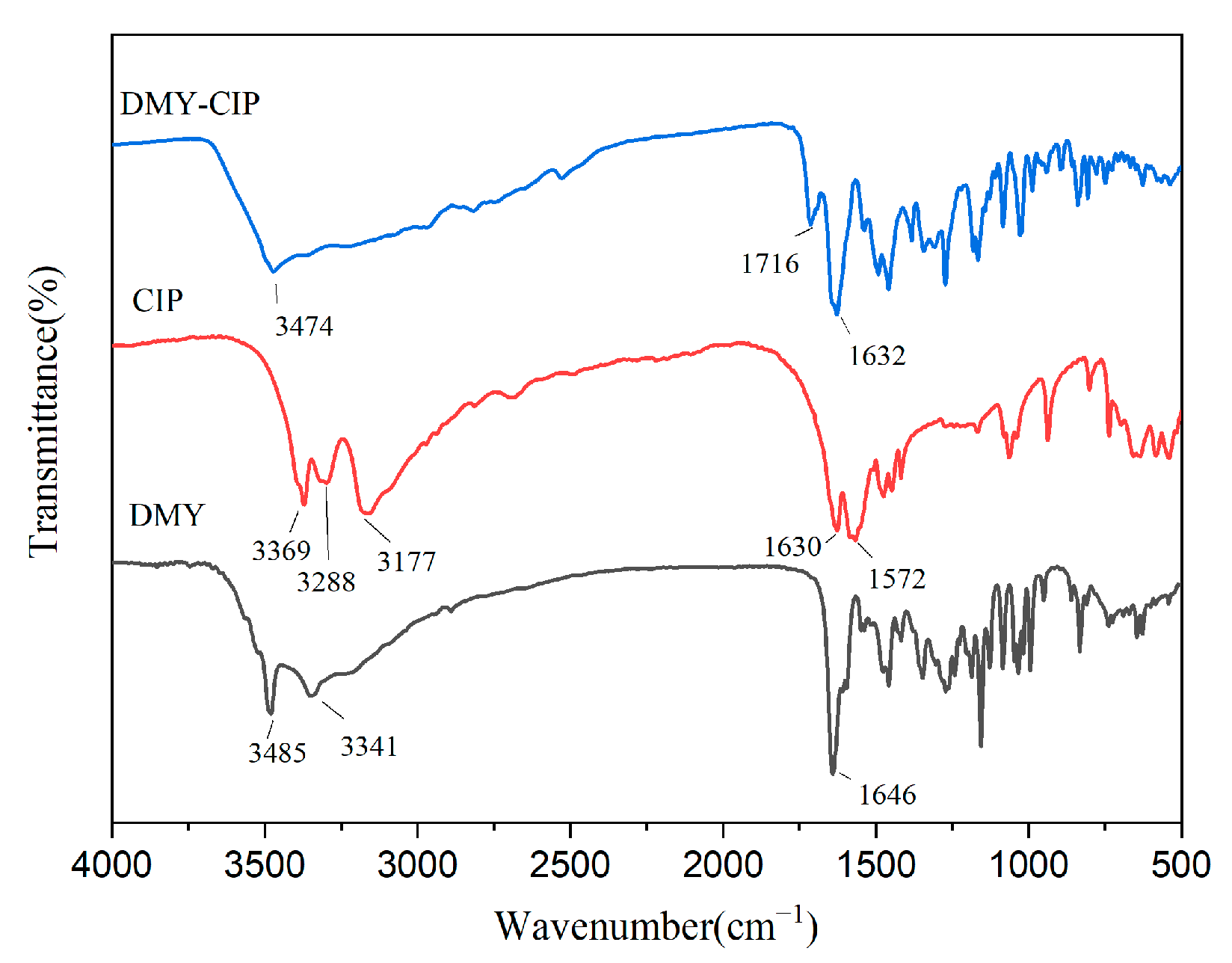
2.2.4. Fourier Transform Infrared Spectroscopy (FTIR)
Infrared spectra were acquired using a PerkinElmer Spectrum Two spectrometer (PerkinElmer Inc., Springfield, IL, USA) in the range of 4000–500 cm−1 with a resolution of 4 cm−1. Samples were prepared by thoroughly grinding with dried potassium bromide and pressing into sheets. The final spectra represent averages of 32 consecutive scans.
2.2.5. Single-Crystal X-Ray Diffraction (SCXRD)
Single-crystal X-ray diffraction data were collected at 293 K using a Bruker Apex Duo diffractometer (Bruker Corporation, Bremen, Germany) with Cu Kα radiation (
λ = 1.54178 Å). The structure was solved with the Olex2-1.5 solve structure solution program using Charge Flipping and refined with the SHELXL [
28] refinement package using Least Squares minimization.
2.2.6. High-Performance Liquid Chromatography (HPLC)
Chromatographic analysis was performed using an Agilent 1260 HPLC (Agilent Technologies Inc., Santa Clara, CA, USA) The chromatographic separation was performed on an Acclaim C18 reversed-phase column (5 μm, 120 Å, 4.6 × 250 mm). The mobile phase consisted of methanol–0.1% formic acid aqueous solution (40:60, v/v) at a flow rate of 1.0 mL/min. The column temperature was maintained at 30 °C, with detection wavelength set at 291 nm and injection volume of 10 μL. Samples were dissolved in 50% methanol prior to analysis. Under these conditions, complete resolution between DMY and CIP was achieved.
2.2.7. Solubility and Dissolution Experiments
Solubility experiments: Excess amounts of DMY and DMY-CIP powder were placed separately in screw-cap bottles containing 5 mL deionized water. The sealed bottles were equilibrated in a temperature-controlled magnetic stirrer at 20 °C, 25 °C, 30 °C, 35 °C, and 40 °C with continuous stirring at 200 rpm for 24 h. After equilibration, aliquots were filtered and appropriately diluted with 50% methanol for HPLC analysis of DMY content. All experiments were performed in triplicate. The remaining powder was filtered and dried at the end of the experiment and tested for PXRD.
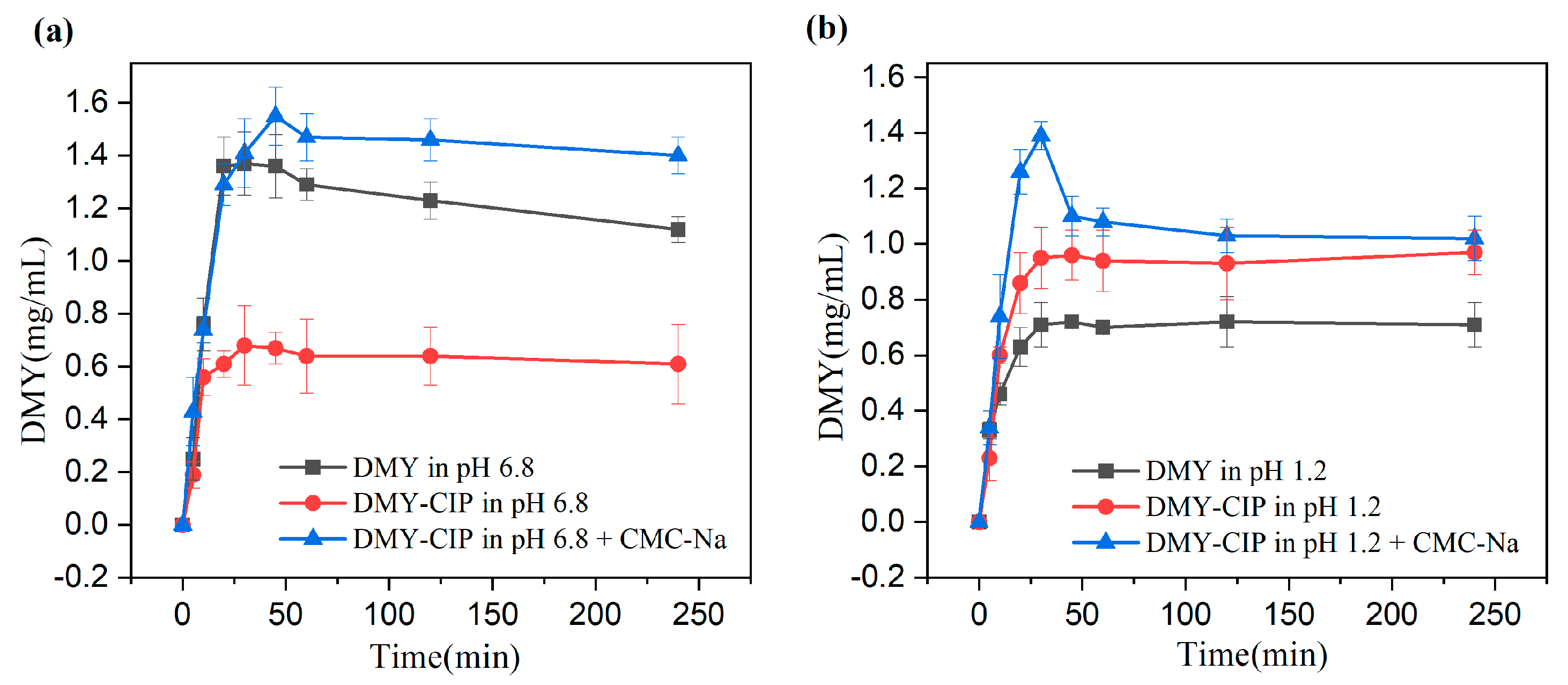
Dissolution Study: Owing to the excessively rapid dissolution of DMY and its cocrystal under sink conditions, which precludes a meaningful comparison of their dissolution behaviors, this study employed non-sink conditions to investigate the dissolution characteristics of different solid forms. The detailed experimental procedure was as follows: Two 20 mL aliquots each of hydrochloric acid buffer (pH 1.2) and phosphate buffer (pH 6.8) were prepared. To one aliquot of each buffer, a 0.5% CMC-Na solution was added to achieve a final concentration of 0.1% (w/v) CMC-Na. These prepared media were placed in round-bottom flasks, which were then equilibrated at 37 °C for 30 min in a thermostatic magnetic stirrer. An excess amount of DMY or DMY-CIP powder was subsequently introduced into each flask, and the dissolution test was initiated under constant stirring at 240 rpm and 37 °C. Samples were withdrawn at predetermined time intervals (5, 10, 20, 30, 45, 60 min, 2, and 4 h), immediately filtered through a 0.45 μm membrane filter, and the withdrawn volume was replaced with an equal volume of fresh pre-warmed medium. The filtered samples were appropriately diluted, and a 10 μL aliquot was injected for High-Performance Liquid Chromatography (HPLC) analysis. All experiments were performed in triplicate. After continuous stirring for 24 h, the residual solids were collected and subjected to crystal form identification by X-ray Powder Diffraction (XRPD).
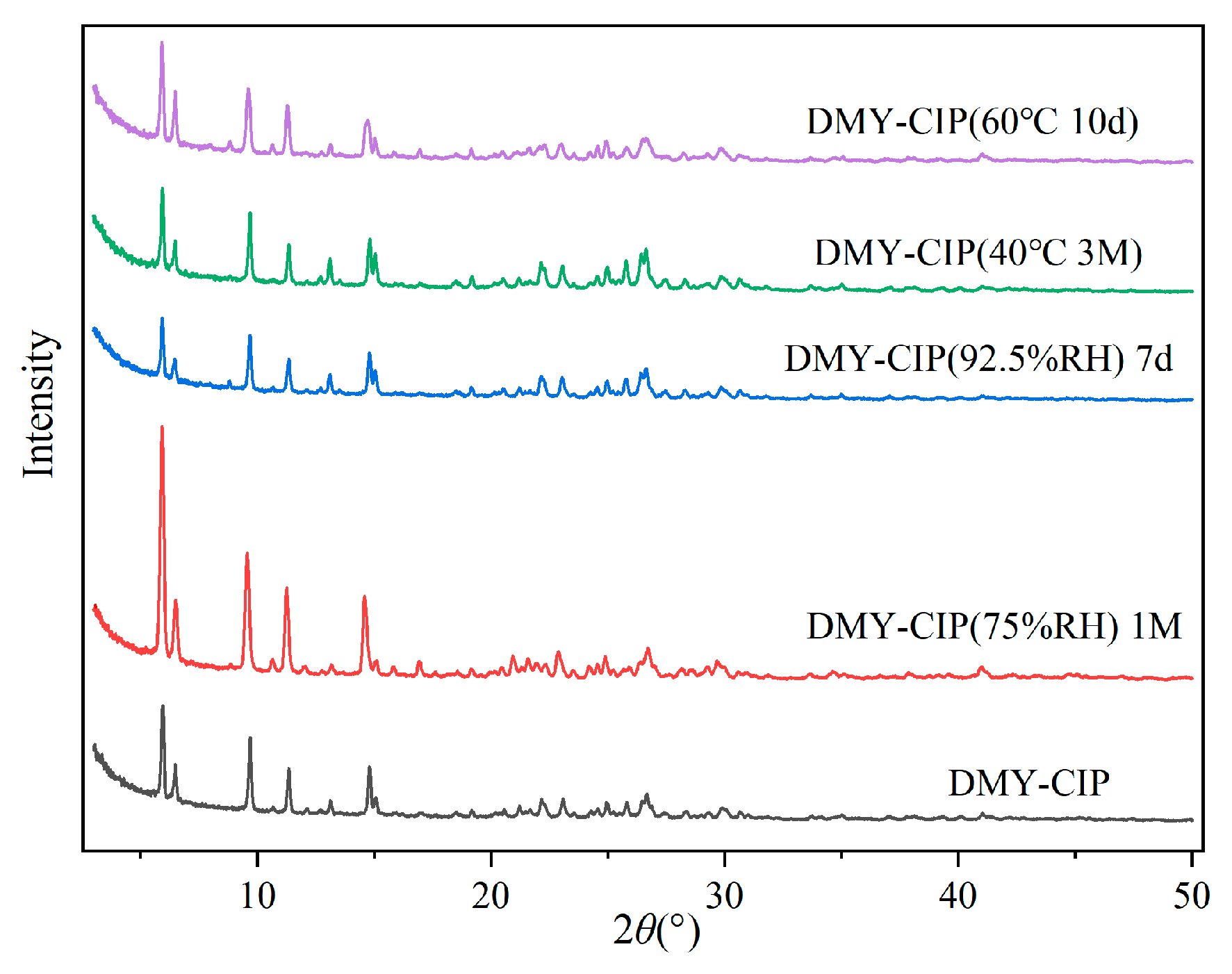
2.2.8. Stability Test
Stability evaluations were conducted by subjecting appropriate quantities of the raw material and cocrystal powder to various stress conditions: thermal stability was assessed by storage at 40 °C for 3 months and 60 °C for 10 days, while hygroscopic stability was examined under 75% RH for 1 month and 92.5% RH for 7 days. The hygroscopicity was quantitatively determined gravimetrically, with the percentage weight increase calculated as [(Wt − W0)/W0] × 100%, where W0 and Wt represent the initial and final weights, respectively. Following stability testing, samples were visually inspected for physical changes, analyzed by HPLC for content variation, and characterized by PXRD to evaluate crystalline form stability, thereby assessing both physical and chemical stability.
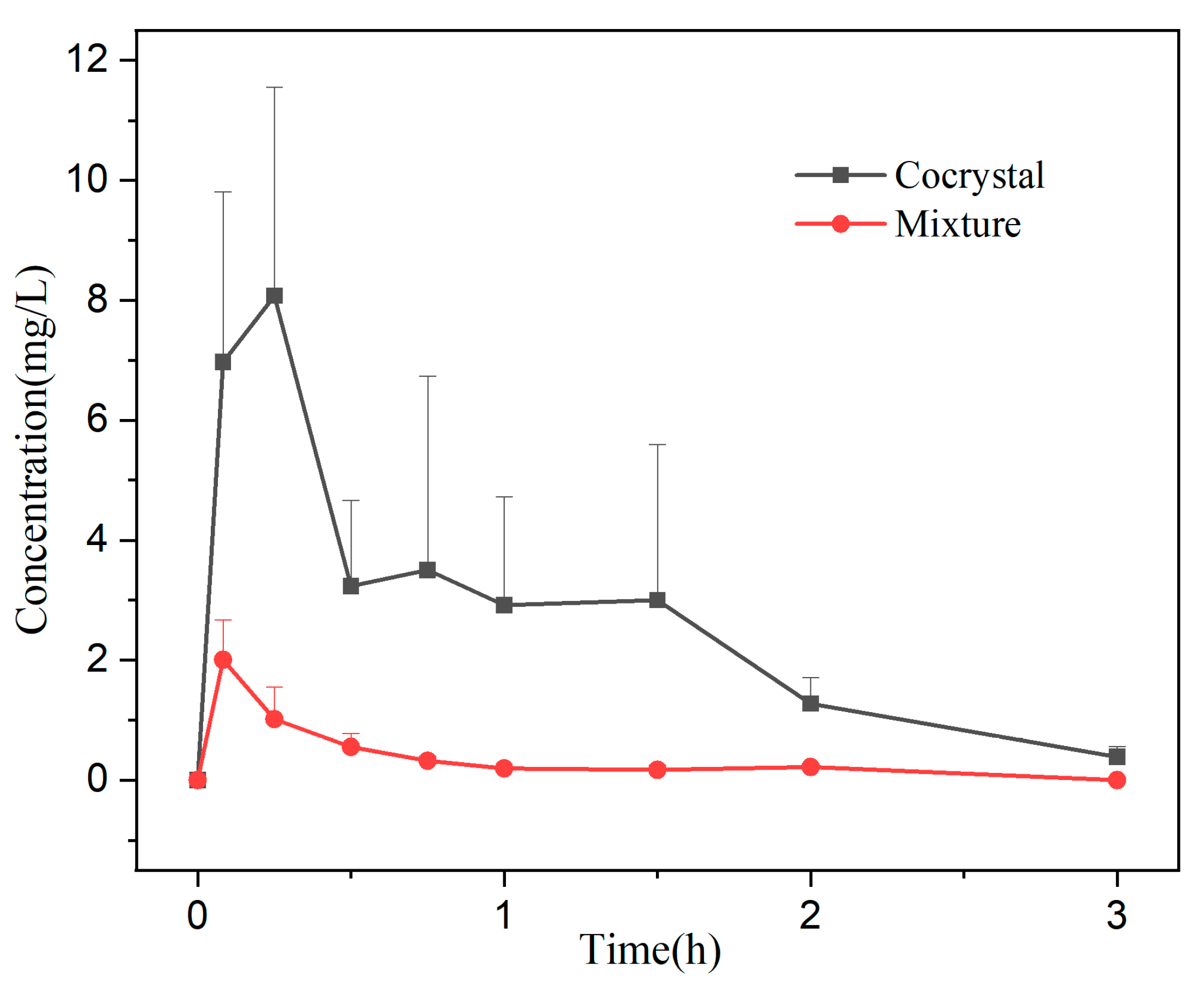
2.2.9. Bioavailability
The physical mixture and cocrystal samples were suspended in 0.5% CMC-Na solution to prepare 10 mg/mL gavage formulations as a gavage-administered preparation. Twelve male SD rats were randomly divided into two groups (
n = 6) after 5-day acclimatization feeding. The rats were fasted for 12 h before the experiment (free access to water) and were administered by gavage at a dose of 150 mg/kg within the safe [
29] and effective dose range. Blood samples (0.5 mL) were collected from the eye sockets at 0.083 h, 0.25 h, 0.5 h, 0.75 h, 1 h, 1.5 h, 2 h, and 3 h, respectively, in heparinized centrifuge tubes. The plasma was separated by centrifugation (10,000 rpm, 10 min) and saved at −4 °C until analysis.
The plasma was treated by liquid–liquid extraction: 200 μL of drug-containing plasma was accurately measured and placed in a 10 mL plugged centrifuge tube, followed by adding 30 μL of methanol, 10 μL of internal standard working solution (luteolin, 5 μg/mL), and 400 μL of 0.1% acetic acid solution, vortex mixing for 1 min. Four mL ethyl acetate was added as the extraction solvent, vortex extracted for 2 min, and centrifuged at 4200 rpm for 10 min. The supernatant was transferred and dried by nitrogen at 40 °C in a water bath. The residue was redissolved with a 150 μL mobile phase. After centrifugation, 20 μL supernatant was taken for HPLC analysis. The pharmacokinetic parameters were calculated by DAS 2.0 software based on a non-compartmental model.
2.2.10. Antibacterial Activity Assay
In this study, the in vitro antibacterial activity of DMY, CIP, physical mixtures, and cocrystals against Escherichia coli and Staphylococcus aureus was evaluated by the filter paper diffusion method. In this experiment, 6 mm-diameter sterile filter papers were used to load 10 μL 10% ethanol, dihydromyricetin solution (0.1 mg/mL), ciprofloxacin hydrochloride solution (0.1 mg/mL), mixed solution, and cocrystal solution (equivalent to DMY 0.1 mg/mL), respectively. The filter papers were pasted on an agar plate uniformly coated with 100 μL bacterial suspension (108 CFU/mL). After 18–24 h of culture at 37 °C, the diameter of the inhibition zone was accurately measured.
The MIC values were determined using the standard broth microdilution method in 96-well plates according to CLSI guidelines. All procedures, including preparation of drug solutions, bacterial suspensions, serial two-fold dilutions, and inoculation, were performed under aseptic conditions. Bacterial suspensions (approximately 106 CFU/mL) were added to wells containing serially diluted drug solutions, followed by incubation at 37 °C for 18–24 h. The MIC was initially assessed visually as the lowest drug concentration showing complete inhibition of visible growth (clear wells). For quantitative confirmation, the optical density at 600 nm (OD600) was measured using a microplate reader, with an OD variation threshold of <0.05 defining the MIC. All experiments were performed in triplicate, and the results were expressed as mean values.