Improving Alzheimer’s Disease and Parkinson’s Disease in Rats with Nanoemulsion and Byproducts Prepared from Cinnamon Leaves
Abstract
1. Introduction
2. Material and Methods
2.1. Processing of C. osmophloeum Leaves (Cinnamon Leaves) and Hydrosol
2.2. Proximate Analysis of Cinnamon Leaves
2.3. Determination of Functional Compounds in Cinnamon Leaves by UPLC-MS/MS
2.4. Preparation of Cinnamon Leaf Extract Nanoemulsion (CLEN)
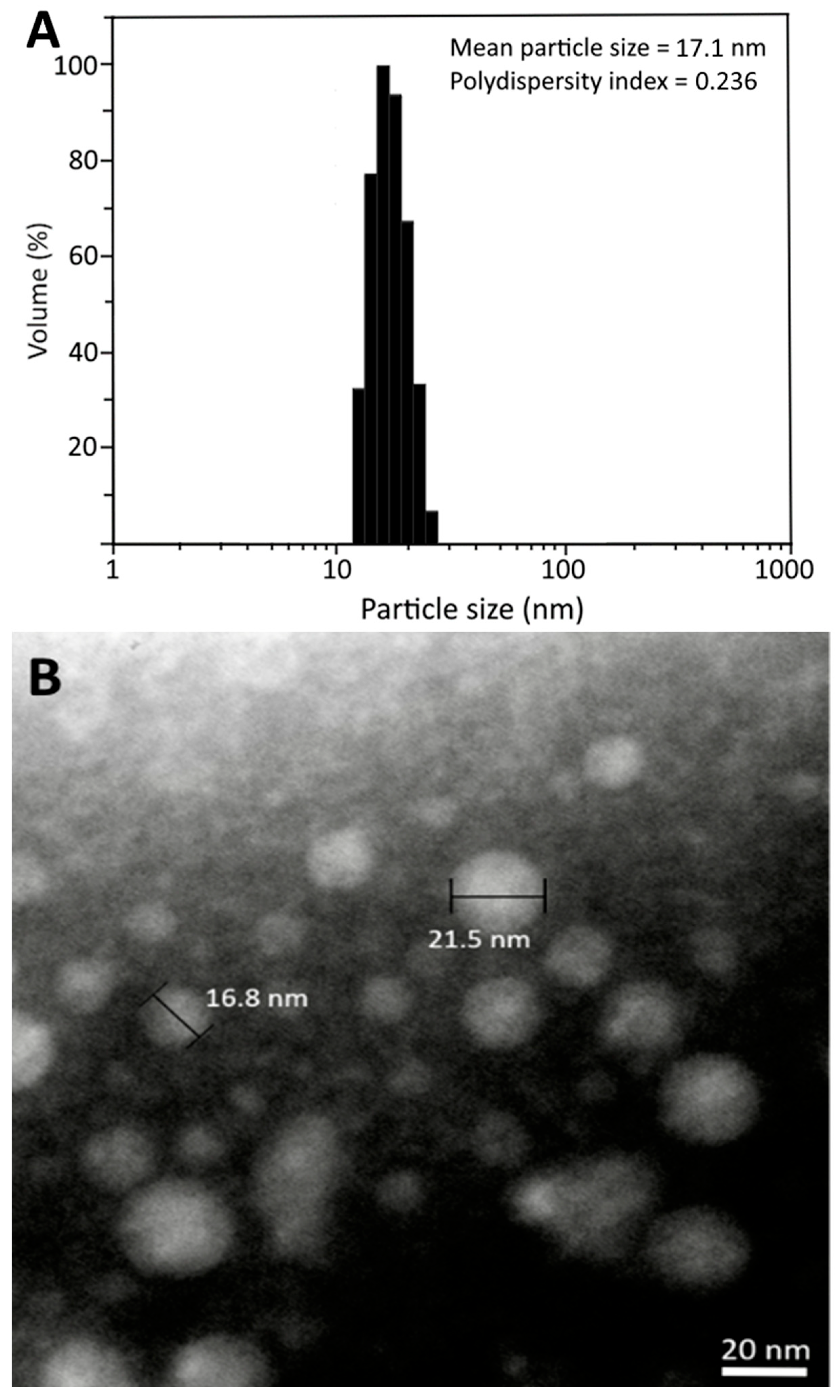
2.5. Characterization of CLEN
2.6. Encapsulation Efficiency of CA in CLEN
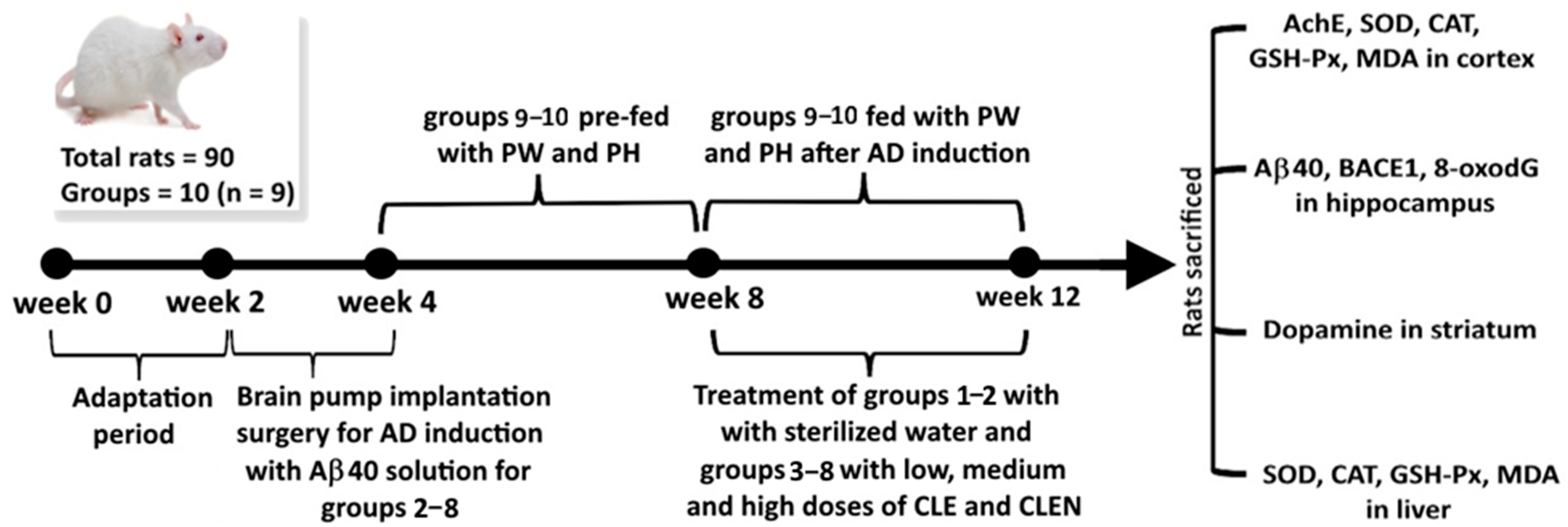
2.7. Animal Experiment
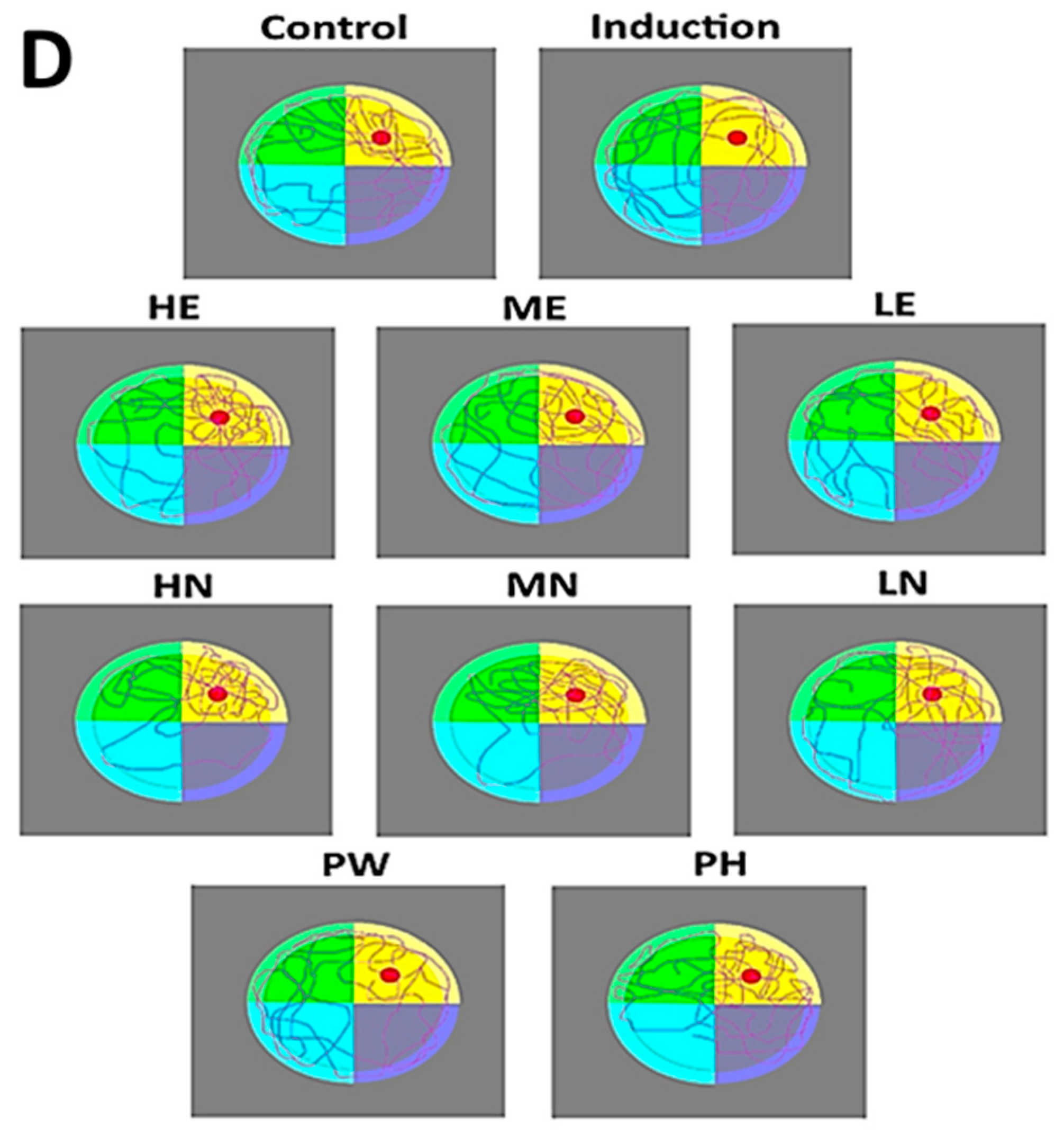
2.8. Morris Water Maze Test
- Control group (N): Fed with sterilized water.
- Induction group (I) (induced with Aβ40 solution): Fed with sterilized water.
- High-dose CLE (HE): Induced with Aß40 solution and fed with 90 mg/kg HE for 4 weeks.
- Medium-dose CLE (ME): Induced with Aß40 solution and fed with 60 mg/kg ME for 4 weeks.
- Low-dose CLE (LE): Induced with Aß40 solution and fed with 30 mg/kg LE for 4 weeks.
- High-dose CLEN (HN): Induced with Aß40 solution and fed with 90 mg/kg HN for 4 weeks.
- Medium-dose CLEN (MN): Induced with Aß40 solution and fed with 60 mg/kg MN for 4 weeks.
- Low-dose CLEN (LN): Induced with Aß40 solution and fed with 30 mg/kg LN for 4 weeks.
- Pre-fed cinnamon powder in water (0.5 g/10 mL) (PW) for 4 weeks: Induced with Aß40 solution and fed with 10 mL/kg for 4 weeks.
- Pre-fed cinnamon powder in hydrosol (0.5 g/10 mL) (PH) for 4 weeks: Induced with Aß40 solution and fed with 10 mL/kg for 4 weeks.
2.9. Measurement of Biochemical Parameters in the Rat Brain
2.10. Measurement of Acetylcholinesterase (AchE) in Rat Cortices
2.11. Measurement of Aβ40 in the Rat Hippocampus
2.12. Measurement of β-Secretase (BACE1) in the Rat Hippocampus
2.13. Measurement of 8-oxodG in the Rat Hippocampus
2.14. Measurement of Antioxidant Enzymes in the Cortices and Livers of Rats
2.14.1. Superoxide Dismutase (SOD)
2.14.2. Catalase (CAT)
2.14.3. Glutathione Peroxidase (GSH-Px)
2.15. Measurement of Dopamine Content in the Brain Striata of Rats
2.16. Measurement of Malondialdehyde (MDA) Content in the Cerebral Cortices and Livers of Rats
2.17. Statistical Analysis
3. Results and Discussion
3.1. Proximate Analysis of Fresh and Dried Cinnamon Leaves
3.2. Analysis of CA and the Other Compounds in Cinnamon Leaves and Hydrosol
3.3. Preparation of CLEN and Method Validation
3.4. Stability of CLEN
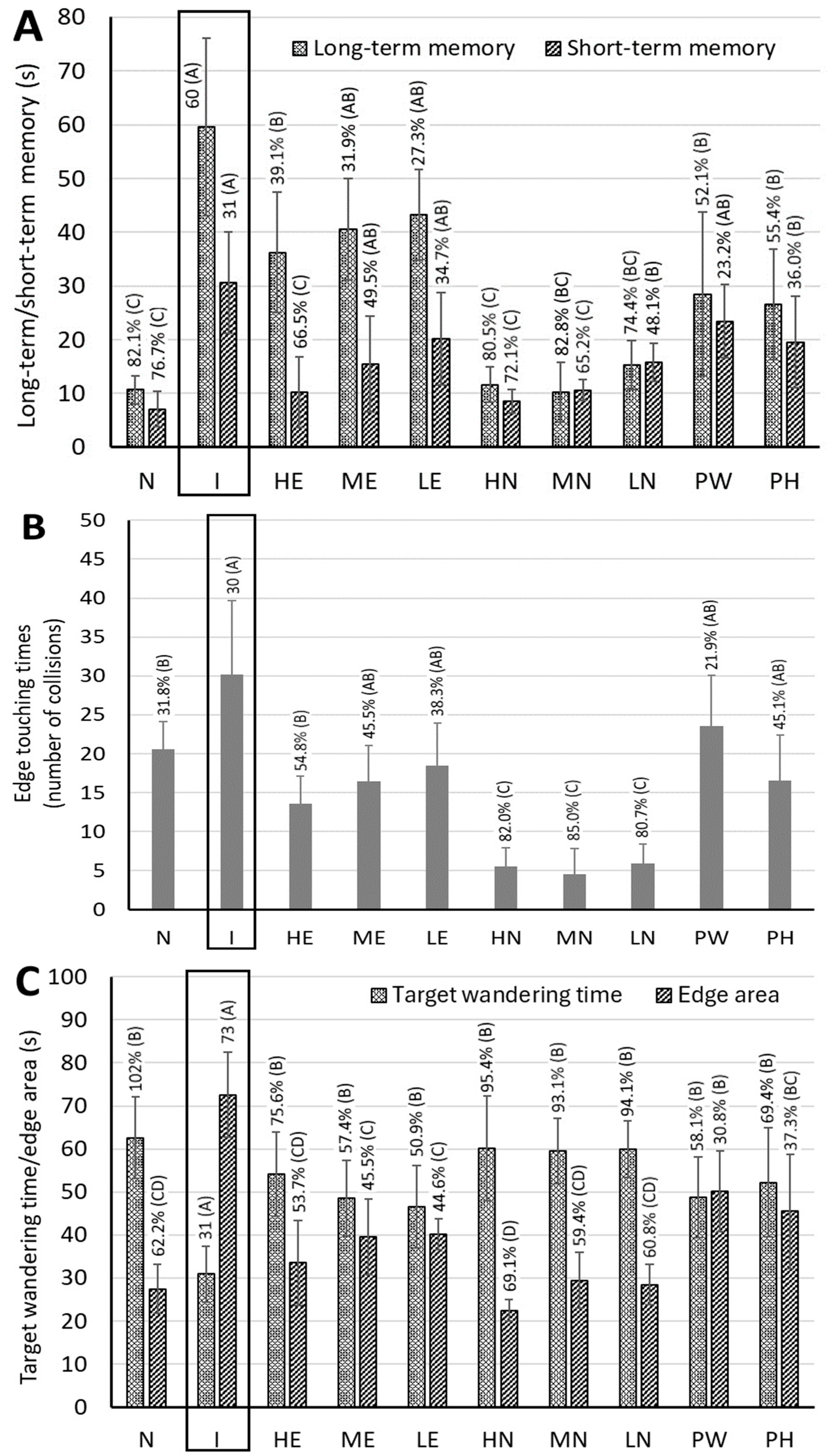
3.5. Memory Rest for AD Rats Fed with CLE, CLEN, and Powder
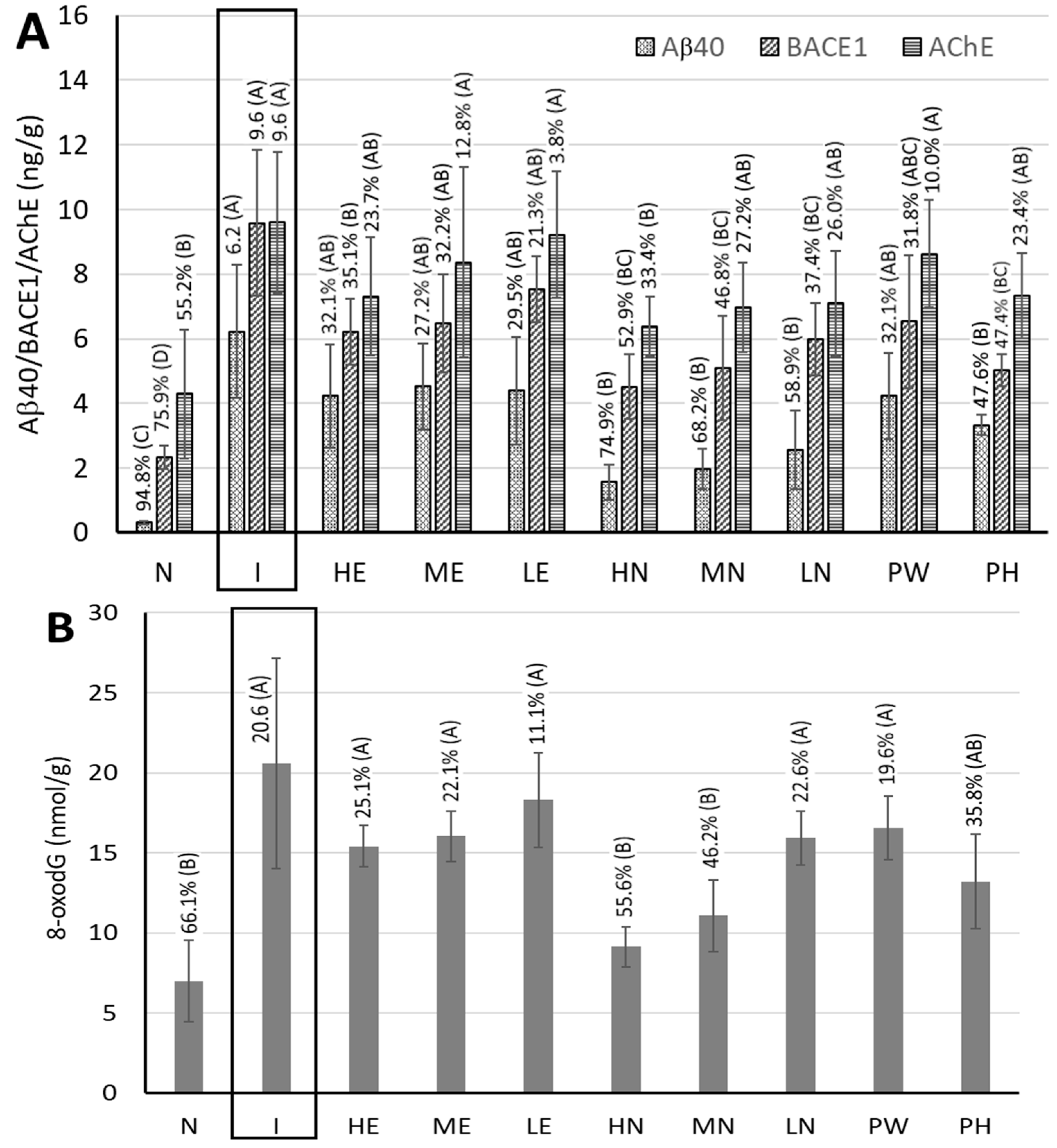
3.6. Measurement of Biological Activity Indicators in the Hippocampi of Rat Brains
3.7. Measurement of Acetylcholinesterase (AchE) Activity in the Cerebral Cortices of Rat Brains
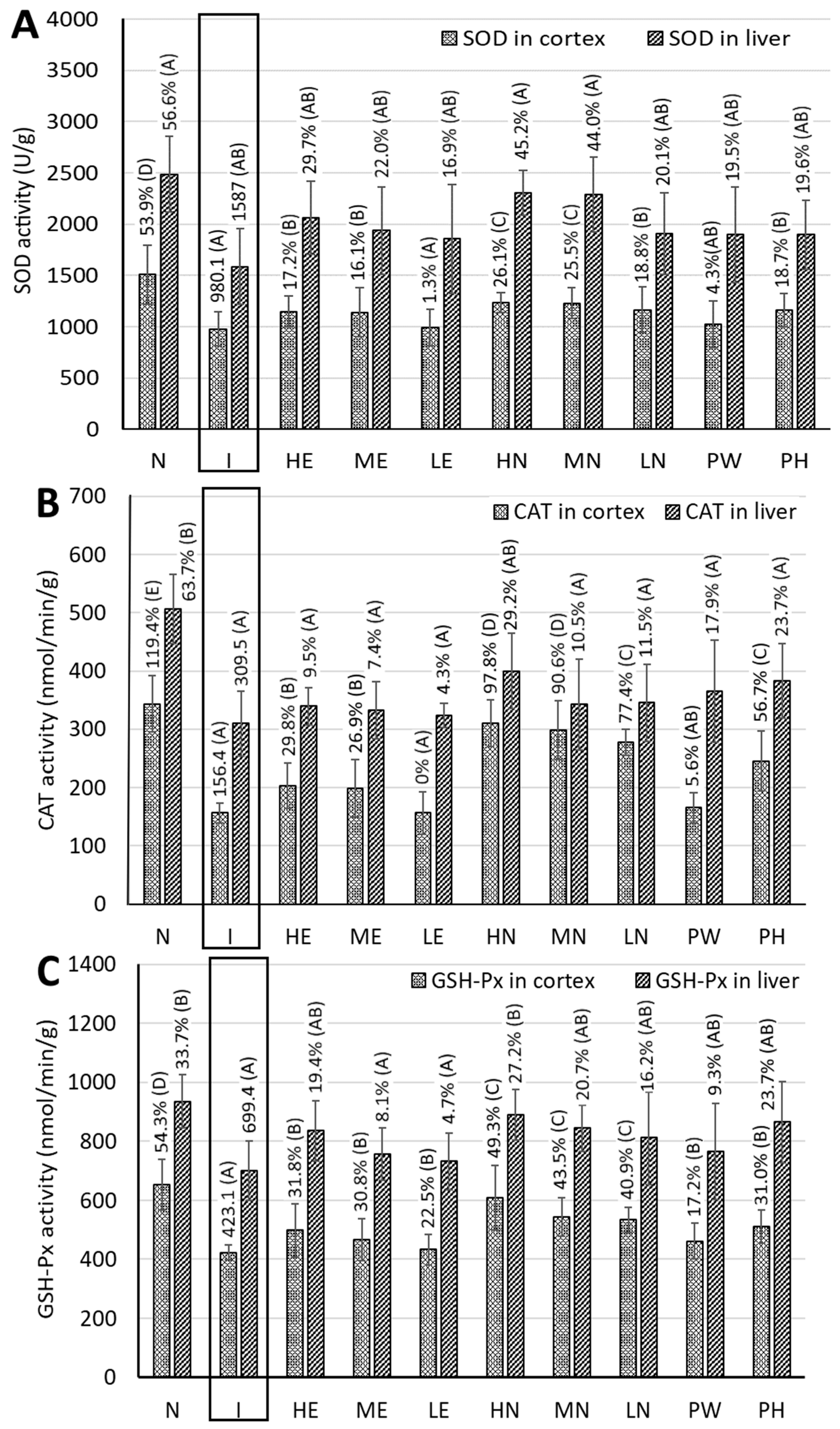
3.8. Measurement of Activities of Antioxidant Enzymes in the Cerebral Cortices of Rat Brains
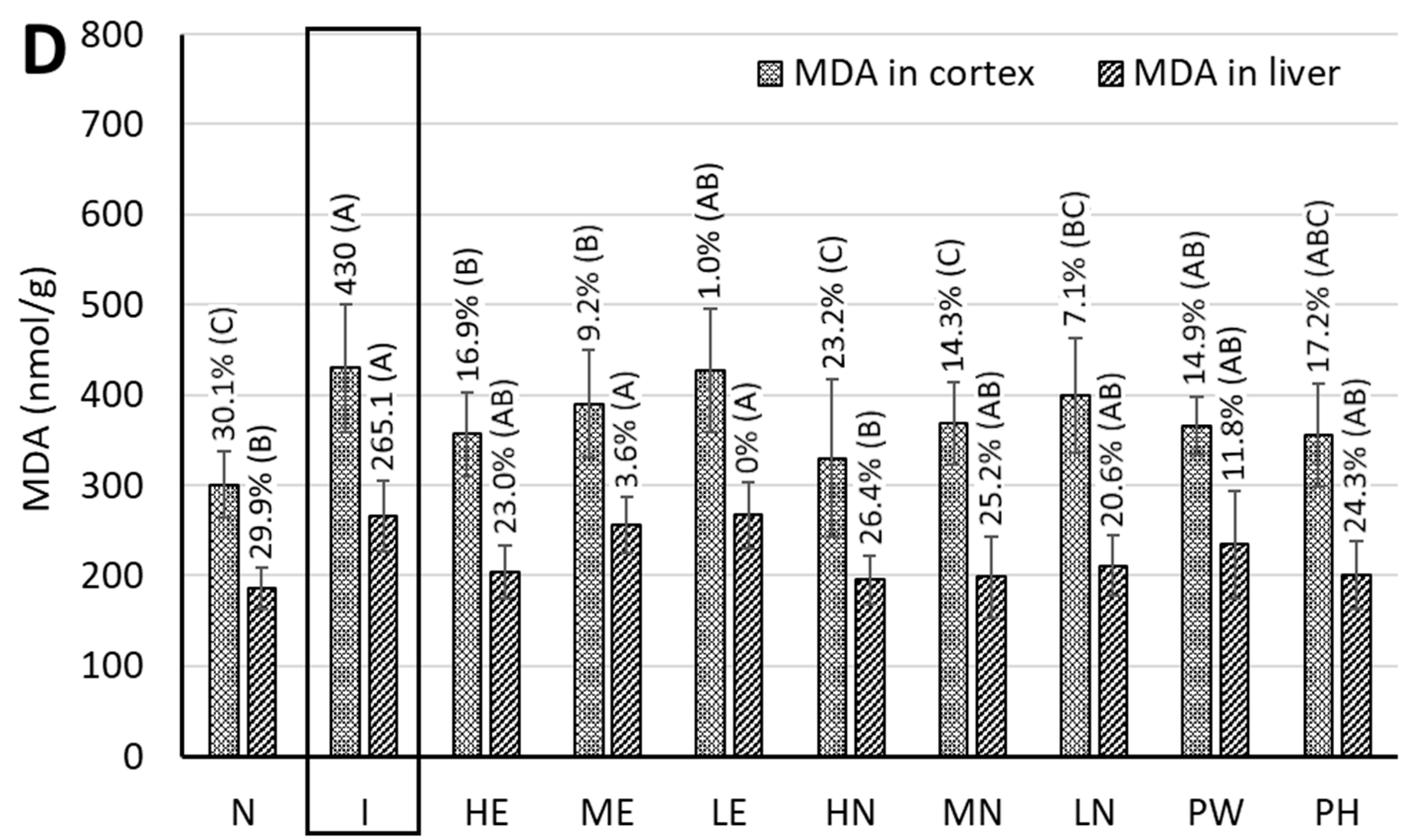
3.9. MDA Content in the Cortices of Rat Brains
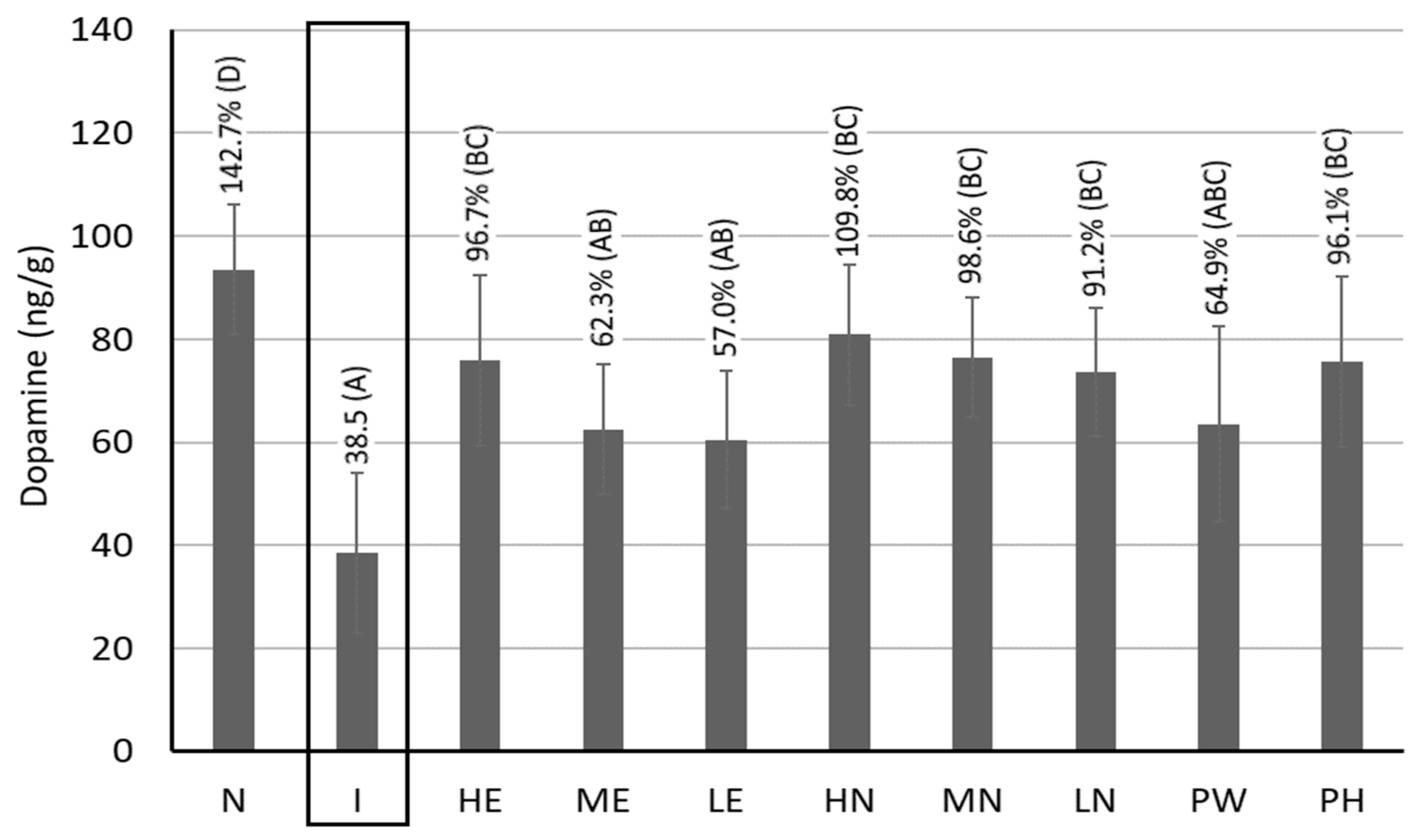
3.10. Dopamine Contents in the Brain Striata of Rats
3.11. Measurement of Antioxidant Enzyme Activities and MDA Content in Rat Livers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AchE | acetylcholinesterase |
| AD | Alzheimer’s disease |
| Akt | serine/threonine-specific protein kinase |
| ANOVA | analysis of variance |
| ApoE | apolipoprotein E |
| ATP | adenosine triphosphate |
| Aβ40 | amyloid beta protein 40 |
| BACE1 | beta-site amyloid precursor protein cleaving enzyme 1 (β-secretase 1) |
| BBB | blood–brain barrier |
| BCA | bicinchoninic acid |
| BDNF | brain-derived neurotrophic factor |
| BuChE | butyrylcholinesterase |
| bw | body weight |
| CA | cinnamaldehyde |
| cAMP | cyclic adenosine monophosphate |
| CLE | cinnamon leaf extract |
| CLEN | cinnamon leaf extract nanoemulsion |
| CAT | catalase |
| CNS | Chinese National Standard |
| DHA | docosahexaenoic acid |
| DJ-1 | parkinsonian disease protein 7/parkinsonism-associated deglycase |
| DLS | dynamic light scattering |
| DNA | deoxyribonucleic acid |
| EPA | eicosapentaenoic acid |
| ERK | extracellular signal-regulated kinase |
| ESI | electrospray ionization |
| GABRA1 | gamma aminobutyric acid A receptor alpha 1 |
| GABRA5 | gamma aminobutyric acid A receptor alpha 5 |
| GABRB2 | gamma aminobutyric acid A receptor beta 2 |
| GABRG2 | gamma aminobutyric acid A receptor gamma 2 |
| GLUT1 | glucose transporter type 1 |
| GSH-Px | glutathione peroxidase |
| GSK-3β | glycogen synthase kinase 3 beta |
| HE | high-dose extract |
| HLB | hydrophilic–lipophilic balance |
| HN | high-dose cinnamon leaf extract nanoemulsion |
| HPLC | high-performance liquid chromatography |
| HRP | horseradish peroxidase |
| IACUC | Institutional Animal Care and Use Committee |
| IL-1β | interleukin 1 beta |
| IRS-1 | insulin resistance substrate 1 |
| LDL | low-density lipoprotein |
| LE | low-dose extract |
| LN | low-dose cinnamon leaf extract nanoemulsion |
| LPS | lipopolysaccharide |
| MAO | monoamine oxidase |
| MAPK | mitogen-activated protein kinase |
| MDA | malondialdehyde |
| ME | medium-dose extract |
| MN | medium-dose cinnamon leaf extract nanoemulsion |
| MRM | multiple reaction monitoring |
| N | normal/control group |
| NF-κB | nuclear factor kappa beta |
| NO | nitric oxide |
| Nrf2 | nuclear factor erythroid 2-related factor 2 |
| NT-3 | neurotrophin 3 |
| 8-oxodG | 8-hydroxy-2′-deoxyguanosine |
| PBS | phosphate-buffered saline |
| PD | Parkinson’s disease |
| PDI | polydispersity index |
| PH | cinnamon powder in hydrosol |
| PI3K | phosphoinositide 3-kinase |
| PKA | protein kinase A |
| PW | cinnamon powder in water |
| ROS | reactive oxygen species |
| RSD | relative standard deviation |
| SABC | horseradish–streptavidin biotin conjugate |
| SOD | superoxide dismutase |
| STZ | streptozotocin |
| Tau | tubulin-associated unit protein |
| TCA | trans-cinnamaldehyde |
| TEM | transmission electron microscopy |
| TMB | 3,3′,5,5′-tetramethylbenzidine |
| TNF-α | tumor necrosis factor alpha |
| UPLC-MS/MS | ultra-performance liquid chromatography–tandem mass spectrometry |
| USFDA | United States Food and Drug Administration |
| UV | ultraviolet |
| Wnt | wingless-related integration site |
| 5XFAD | 5 familial Alzheimer’s disease mutation mice model |
References
- Gauthier, S.; Rosa-Neto, P.; Morais, J.; Webster, C. World Alzheimer report 2021: Journey through the diagnosis of dementia. Alzheimer’s Dis. Int. 2022. Available online: https://www.alzint.org/u/World-Alzheimer-Report-2021.pdf (accessed on 25 October 2024).
- Wang, Y.; Huang, Y.; Ma, A.; You, J.; Miao, J.; Li, J. Natural antioxidants: An effective strategy for the treatment of Alzheimer’s disease at the early stage. J. Agric. Food Chem. 2024, 72, 11854–11870. [Google Scholar] [CrossRef]
- Wakhloo, D.; Oberhauser, J.; Madira, A.; Mahajani, S. From cradle to grave: Neurogenesis, neuroregeneration and neurodegeneration in Alzheimer’s and Parkinson’s diseases. Neural Regen. Res. 2022, 17, 2606–2614. [Google Scholar] [CrossRef]
- Smith, M.A.; Rottkamp, C.A.; Nunomura, A.; Raina, A.K.; Perry, G. Oxidative stress in Alzheimer’s disease. BBA-Mol. Basis Dis. Biochim. Biophys. Acta 2000, 1502, 139–144. [Google Scholar] [CrossRef]
- Hu, K.; Wu, S.; Xu, J.; Zhang, Y.; Zhang, Y.; Wu, X.; Miao, J.; Yao, Y.; Zhu, S.; Chen, G.; et al. Pongamol alleviates neuroinflammation and promotes autophagy in Alzheimer’s disease by regulating the Akt/mTOR signaling pathway. J. Agric. Food Chem. 2024, 72, 13634–13645. [Google Scholar] [CrossRef] [PubMed]
- Pritam, P.; Deka, R.; Bhardwaj, A.; Srivastava, R.; Kumar, D.; Jha, A.K.; Jha, N.K.; Villa, C.; Jha, S.K. Antioxidants in Alzheimer’s disease: Current therapeutic significance and future prospects. Biology 2022, 11, 212. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.L. Oxidative stress and alleviating effect of natural antioxidants on Alzheimer’s disease. Prog. Biochem. Biophy. 2023, 50, 1144–1158. [Google Scholar]
- Wang, Y.C.; Wang, V.; Chen, B.H. Analysis of bioactive compounds in cinnamon leaves and preparation of nanoemulsion and by products for improving Parkinson’s disease in rats. Front. Nutr. 2023, 10, 1229192. [Google Scholar]
- Stahl, S.M. The new cholinesterase inhibitors for Alzheimer’s disease, part 2: Illustrating their mechanisms of action. J. Clin. Psych. 2000, 61, 813–814. [Google Scholar] [CrossRef]
- Williamson, J.M. Lecanemab Side Effects: What You Should Know. Medical News Today. 2023. Available online: https://www.medicalnewstoday.com/articles/drugs-leqembi-side-effects (accessed on 25 October 2024).
- Hussain, A.; Bloemer, J. Side effects of drugs used in the treatment of Alzheimer’s disease. Side Effects of Drugs Annual. In A Worldwide Yearly Survey of New Data in Adverse Drug Reactions; ScienceDirect’s: Amsterdam, The Netherlands, 2023; Chapter 3; Volume 45, p. 2732. [Google Scholar]
- Cleveland Clinic. Parkinson’s Disease Medications. 2023. Available online: https://my.clevelandclinic.org/health/treatments/parkinsons-disease-medications (accessed on 25 October 2024).
- Nakhaee, S.; Kooshki, A.; Hormozi, A.; Akbari, A.; Mehrpour, O.; Farrokhfall, K. Cinnamon and cognitive function: A systematic review of preclinical and clinical studies. Nutr. Neurosci. 2023, 27, 132–146. [Google Scholar] [CrossRef]
- Huang, Y.C.; Chen, B.H. A comparative study on improving Streptozotocin-induced type diabetes in rats by hydrosol, extract and nanoemulsion prepared from cinnamon leaves. Antioxidants 2023, 12, 29. [Google Scholar] [CrossRef]
- Yeh, T.F.; Lin, C.Y.; Chang, S.T. A potential low-coumarin cinnamon substitute: Cinnamomum osmophloeum leaves. J. Agric. Food Chem. 2014, 62, 1706–1712. [Google Scholar] [CrossRef] [PubMed]
- Caligore, D.; Giocondo, E.; Silvetti, M. The neurodegenerative elderly syndrome (NES) hypothesis: Alzheimer and Parkinson are two faces of the same disease. IBRO Neurosci. Rep. 2022, 13, 330–343. [Google Scholar] [CrossRef] [PubMed]
- Bantounou, M.A.; Shoaib, L.; Mazzoleni, A.; Modalavalasa, H.; Kumar, N.; Philip, S. The association between type 2 diabetes mellitus and Parkinson’s disease: A systematic review and meta-analysis. Brain Disord. 2024, 15, 100158. [Google Scholar] [CrossRef]
- Chauhan, A.; Dubey, S.; Jain, S. Association between type II diabetes mellitus and Alzheimer’s disease: Common molecular mechanism and therapeutic targets. Cell Biochem. Funct. 2024, 42, e4111. [Google Scholar] [CrossRef]
- Matsumoto, S.; Tsunematsu, T. Association between sleep, Alzheimer’s, and Parkinson’s disease. Biology 2021, 10, 1127. [Google Scholar] [CrossRef]
- Zochodne, D.; Kline, G.; Smith, E.E.; Hill, M.D. Diabetic Neurology; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Perl, D.P.; Olanow, C.W.; Calne, D. Alzheimer’s disease and Parkinson’s disease: Distinct entities or extremes of a spectrum of neurodegeneration? Ann. Neurol. 1998, 44 (Suppl. S1), S19–S31. [Google Scholar] [CrossRef]
- Sousa, J.A.; Bernardes, C.; Bernardo-Castro, S.; Balderiras, I.; Santana, I.; Sargento-Freitas, J. Reconsidering the role of blood-brain barrier in Alzheimer’s disease: From delivery to target. Front. Aging Neurosci. 2023, 15, 1102809. [Google Scholar] [CrossRef]
- Zlokovic, B.V. Neurovascular pathways to neurogeneration in Alzheimer’s disease and other disorders. Nat. Rev. Neurosci. 2011, 12, 723–738. [Google Scholar] [CrossRef]
- Lin, C.W.; Lin, P.Y.; Hsu, Y.W.; Pan, T.M.; Lee, C.L. Monascus-fermented metabolites repressed amyloid β-peptide-induced neurotoxicity and inflammatory response in in vitro and in vivo studies. J. Funct. Foods 2023, 204, 105509. [Google Scholar]
- Do, J.; Kim, N.; Jeon, S.H.; Gee, M.S.; Ju, Y.J.; Kim, J.H.; Oh, M.S.; Lee, J.K. Trans-cinnamaldehyde alleviates amyloid–Beta pathogenesis via the SIRTI-PGClα-PPARr pathway in 5XFAD transgenic mice. Int. J. Mol. Sci. 2020, 21, 4492. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.S.; Kang, H.Y.; Lee, J.K. Effect of treadmill exercise and trans-cinnamaldehyde against d-galactose- and aluminum chloride-induced cognitive dysfunction in mice. Brain Sci. 2020, 10, 793. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, H.N. Neuro-amelioration of cinnamaldehyde in aluminum-induced Alzheimer’s disease rat model. J. Histotechnol. 2020, 43, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Nistico, R.; Mehdawy, B.; Piccirilli, S.; Mercuri, N.B. Paraquat- and rotenone-induced models of Parkinson’s disease. Int. J. Immunopathol. Pharmacol. 2011, 24, 313–322. [Google Scholar] [CrossRef]
- Wu, B.; Liu, Y.; Li, H.; Zhu, L.; Zeng, L.; Zhang, Z.; Peng, W. Liver as a new target organ in Alzheimer’s disease: Insight from cholesterol metabolism and its role in amyloid-beta clearance. Neural Regen. Res. 2025, 20, 695–714. [Google Scholar] [CrossRef]
- Davila-Rodriguez, M.; Lopez-Malo, A.; Palou, E.; Ramirez-Corona, N.; Jimenez-Munguia, M.T. Antimicrobial activity of nanoemulsions of cinnamon, rosemary and oregano essential oils on fresh celery. LWT Food Sci. Technol. 2019, 112, 108247. [Google Scholar] [CrossRef]
- Ghosh, V.; Saranya, S.; Mukherjee, A.; Chandrasekaran, N. Cinnamon oil nanoemulsion formulation by ultrasonic emulsification: Investigation of its bactericidal activity. J. Nanosci. Nanotechnol. 2013, 13, 114–122. [Google Scholar] [CrossRef]
- Mukerjee, A.; Pandey, H.; Tripathi, A.K.; Singh, S.K. Development, characterization and evaluation of cinnamon oil- and usnic acid blended nanoemulsion to attenuate skin carcinogenicity in Swiss albino mice. Biocatal. Agric. Biotechnol. 2019, 20, 101227. [Google Scholar] [CrossRef]
- Olorunnado, S.E.; Odum, O.P.; Akinola, O.B. Evaluation of the neuroprotective potential of trans-cinnamaldehyde in female Wistar rat model of insulin resistance. J. Krishna Med. Sci. Univ. 2023, 12, 112–121. [Google Scholar]
- Tepe, A.S.; Ozaslan, M. Anti-Alzheimer, anti-diabetic, skin-whitening and antioxidant activities of the essential oil of Cinnamomum zeylanicum. Ind. Crops Prod. 2020, 145, 112069. [Google Scholar] [CrossRef]
- Perez-Taboada, I.; Alberquilla, S.; Martin, E.D.; Anand, R.; Vietti-Michelina, S.; Tebeka, N.N.; Cantley, J.; Cragg, S.J.; Mortalla, R.; Vallejo, M. Diabetes causes dysfunctional dopamine neurotransmission favoring nigrostriatal degeneration in mice. Mov. Disord. 2020, 35, 1636–1648. [Google Scholar] [CrossRef]
- Hong, C.-T.; Chen, K.-Y.; Wang, W.; Chiu, J.-Y.; Wu, D.; Chao, T.-Y.; Hu, C.-J.; Chau, K.-Y.D.; Bamodu, O.A. Insulin resistance promotes Parkinson’s disease through aberrant expression of α-synuclein, mitochondrial dysfunction and deregulation of the polo-like kinase 2 signaling. Cells 2020, 9, 740. [Google Scholar] [CrossRef]
- Charissé, D.; Erus, G.; Pomponio, R.; Gorges, M.; Schmidt, N.; Schneider, C.; Liepelt-Scarfone, I.; Riedel, O.; Reetz, K.; Schulz, J.B.; et al. Brain age and Alzheimer’s-like atrophy are domain-specific predictors of cognitive impairment in Parkinson’s disease. Neurobiol. Aging 2022, 109, 31–42. [Google Scholar] [CrossRef]
- Lovell, M.A.; Ehmann, W.D.; Butler, S.M.; Markesbery, W.R. Elevated thiobarbituric acid-reactive substances and antioxidant enzyme activity in the brain in Alzheimer’s disease. Neurology 1995, 4, 1594–1601. [Google Scholar] [CrossRef]
- Deol, P.; Fahrmann, J.; Sladek, F.M. Omega-6 and omega-3 oxylipins are implicated in soybean oil induced obesity in mice. Sci. Rep. 2017, 7, 12488. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, Y.; Song, S.; Khankari, N.K.; Brenna, J.T.; Shen, Y.; Ye, K. Associations of plasma omega-6 and omega-3 fatty acids with overall and 19 site-specific cancers: A population-based cohort study in UK Biobank. Int. J. Can. 2025, 156, 1154–1172. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, M.J.; Wurtman, R.J. Lecithin consumption increases acetylcholine concentrations in rat brain and adrenal gland. Science 1978, 202, 223–225. [Google Scholar] [CrossRef] [PubMed]
- Crispens, C.G.; Sorenson, J.R. Evaluation of the anticancer activities of Tweens 20, 40 and 60 in SJL/J mice. Antican. Res. 1991, 11, 407–408. [Google Scholar]
- Bagheri-Mohammadi, S.; Askari, S.; Alani, B.; Moosavi, M.; Ghasemi, R. Cinnamaldehyde regulates insulin and caspase-3 signaling pathways in the sporadic Alzheimer’s disease model: Involvement of hippocampal function via IRS-1, Akt and GSk-3β phosphorylation. J. Mol. Neurosci. 2022, 72, 2273–2291. [Google Scholar] [CrossRef]
- Tramutola, A.; Lanzillotta, C.; Di Domenico, F.; Head, E.; Butterfield, D.A.; Perluigi, M.; Barone, E. Brain insulin resistance triggers early onset Alzheimer’s disease in down syndrome. Neurobiol. Dis. 2020, 137, 104772. [Google Scholar] [CrossRef]
- Sajjadi, E.; Sajedianfard, J.; Hosseinzadeh, S.; Taherianfard, M. Effect of insulin and cinnamon extract on spatial memory and gene expression of GLUT1, 3, and 4 in streptozotocin-induced Alzheimer’s model in rats. Iran. J. Basic Med. Sci. 2023, 26, 680–687. [Google Scholar]
- Kazerouni, A.; Nazeri, M.; Karimzadeh, A.; SoukhakLari, R.; Moezi, L.; Pirsalami, F.; Moosavi, M. Sub-chronic oral cinnamaldehyde treatment prevents scopolamine-induced memory retrieval deficit and hippocampal Akt and MAPK dysregulation in male mice. Neurol. Res. 2020, 42, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Jana, A.; Modi, K.K.; Roy, A.; Anderson, J.A.; van Breeman, R.B.; Pahan, K. Up-regulation of neurotrophic factors by cinnamon and its metabolic sodium benzoate: Therapeutic implications for neurodegenerative disorders. J. Neuroimmune Pharmacol. 2013, 8, 739–755. [Google Scholar] [CrossRef] [PubMed]
- Pahan, P.; Pahan, K. Can cinnamon bring aroma in Parkinson’s disease treatment? Neural Regen. Res. 2015, 10, 30–32. [Google Scholar] [CrossRef]
- Smith, M.A.; Perry, G.; Richey, P.L.; Sayre, L.M.; Anderson, V.E.; Beal, M.F.; Kowall, N. Oxidative damage in Alzheimer’s disease. Nature 1996, 382, 120. [Google Scholar] [CrossRef]
- Karimirad, R.; Inbaraj, B.S.; Chen, B.H. Recent advances on the analysis and biological functions of cinnamaldehyde and its derivatives. Antioxidants 2025, 14, 765. [Google Scholar] [CrossRef]
- Wang, D.; Chen, F.; Han, Z.; Yin, Z.; Ge, X.; Lei, P. Relationship between amyloid-β deposition and blood-brain barrier dysfunction in Alzheimer’s disease. Front. Cell. Neurosci. 2021, 15, 695479. [Google Scholar] [CrossRef]
- Pardridge, W.M. The blood-brain barrier: Bottleneck in brain drug development. NeuroRX 2005, 2, 3–14. [Google Scholar] [CrossRef]
- Wunsch, A.; Mulac, D.; Langer, K. Lipoprotein imitating nanoparticles: Lecithin coating binds APoE and mediates non-lysosomal uptake leading to transcytosis over the blood-brain barrier. Int. J. Pharm. 2020, 589, 119821. [Google Scholar] [CrossRef]
- Betzer, O.; Shilo, M.; Opochinsky, R.; Barnoy, E.; Motiei, M.; Okun, E.; Yadid, G.; Popovtzer, R. The effect of nanoparticle size on the ability to cross the blood-brain barrier: An in vivo study. Nanomedicine 2017, 12, 1533–1546. [Google Scholar] [CrossRef]
- Ohta, S.; Kikuchi, E.; Ishijima, A.; Azuma, T.; Sakuma, I.; Ito, T. Investigating the optimum size of nanoparticle for their delivery into brain assisted by focused ultrasound-induced blood-brain barrier opening. Sci. Rep. 2020, 10, 18220. [Google Scholar] [CrossRef] [PubMed]
- Karthivashan, G.; Ganesan, P.; Park, S.Y.; Kim, J.S.; Choi, D.K. Therapeutic strategies and nano-drug delivery applications in management of ageing Alzheimer’s disease. Drug Deliv. 2018, 25, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Davoody, S.; Taei, A.A.; Khodabakshsh, P.; Dargahi, L. mTOR signaling and Alzheimer’s disease: What we know and where we are. CNS Neurosci. Therapeut. 2023, 30, e14463. [Google Scholar] [CrossRef] [PubMed]
- Long, H.Z.; Cheng, Y.; Zhou, Z.W.; Wen, D.D.; Gao, L.C. PI3K/AKT signal pathway: A target of natural products in the prevention and treatment of Alzheimer’s disease and Parkinson’s disease. Front. Pharmacol. 2021, 12, 648636. [Google Scholar] [CrossRef]
- AlRuwaili, R.; Al-Kuraishy, H.M.; Al-Gareebm, A.I.; Albuhadily, A.K.; Alexion, A.; Papadakis, M.; Abo-El Fetoh, M.E.; Batiha, G.E. Targeting of the PI3 K/AKT/GSK3β pathway in Parkinson’s disease: A therapeutic blueprint. Mol. Neurobiol. 2025, in press. [Google Scholar] [CrossRef]
- Luo, S.; Kang, S.S.; Wang, Z.H.; Liu, X.; Day, J.X.; Wu, Z.; Peng, J.; Xiang, D.; Springer, W.; Ye, K. Akt phosphorylates NQO1 and triggers its degradation, abolishing its antioxidative activities in Parkinson’s disease. J. Neurosci. 2019, 39, 7291–7305. [Google Scholar] [CrossRef]
- Chen, Y.; Zheng, X.; Wang, Y.; Song, J. Effect of PI3K/Akt/mTOR signaling pathway on JNK3 in parkinsonian rats. Exp. Ther. Med. 2018, 17, 1771–1775. [Google Scholar] [CrossRef]
- Sabrina, G.; Placido, B.; Emanuela, M. Triggering of inflammasome by impaired autophagy in response to acute experimental Parkinson’s disease involvement of PI3K/Akt/mTOR pathway. NeuroReport 2017, 28, 996–1007. [Google Scholar]
- Ruiz-Pozo, V.A.; Tamayo-Trujillo, T.; Cadena-Ullauri, S.; Frias-Toral, E.; Guevara- Ramirez, P.; Paz-Cruz, E.; Chapela, S.; Montalvan, M.; Morales-Lopez, T.; Simancas-Racines, D.; et al. The molecular mechanisms of the relationship between insulin resistance and Parkinson’s disease pathogenesis. Nutrients 2023, 15, 3585. [Google Scholar] [CrossRef]
- Wyss-Coray, T. Inflammation in Alzheimer’s disease: Driving force, bystander or beneficial response? Nat. Med. 2006, 12, 1005–1015. [Google Scholar]
- Zhang, L.; Zhang, Z.; Fu, Y.; Yang, P.; Qin, Z.; Chen, Y.; Xu, Y. Trans-cinnamaldehyde improves memory impairment by blocking microglial activation through the destabilization of iNOS mRNA in mice challenged with lipopolysaccharide. Neuropharmacology 2016, 110, 503–518. [Google Scholar] [CrossRef]
- Zhao, Y.; Deng, H.; Li, K.; Wang, L.; Wu, Y.; Dong, X.; Wang, X.; Chen, Y.; Xu, Y. Trans-cinnamaldehyde improves neuroinflammation-mediated NMDA receptor dysfunction and memory deficits through blocking NF-kB pathway in presenilin 1/2 conditional double knockout mice. Brain Behav. Immun. 2019, 82, 45–62. [Google Scholar] [CrossRef] [PubMed]
- Gabbouj, S.; Ryhanen, S.; Marttinen, M.; Wittrahm, R.; Takalo, M.; Kemppainen, S.; Martiskainen, H.; Tanila, H.; Haapasalo, A.; Hiltunen, M.; et al. Altered insulin signaling in Alzheimer’s disease brain-special emphasis on PI3K-Akt pathway. Front. Neurosci. 2019, 13, 629. [Google Scholar] [CrossRef] [PubMed]
- E1-ezz, D.A.; Maher, A.; Sallam, N.; E1-brairy, A.; Kenawy, S. Trans-cinnamaldehyde modulates hippocampal Nrf2 factor and inhibits amyloid beta aggregation in LPS-induced neuroinflammation mouse model. Neurochem. Res. 2018, 43, 2333–2342. [Google Scholar]
- Frydman-Marom, A.; Levin, A.; Farfara, D.; Benromano, T.; Scherzer-Attali, R.; Peled, S.; Vassar, R.; Segal, D.; Gazit, E.; Frenkel, D.; et al. Orally administered cinnamon extract reduces β-amyloid oligomerization and corrects cognitive impairment in Alzheimer’s disease animal models. PLoS ONE 2011, 6, e16564. [Google Scholar] [CrossRef]
- Qian, D.; Wang, Q.; Lin, S.; Li, Y.; Gu, X.; Xia, C.; Xu, Y.; Zhang, T.; Yang, L.; Wu, Q.; et al. Identification of potential targets of cinnamon for treatment against Alzheimer’s disease-related GABAergic synaptic dysfunction using network pharmacology. Sci. Rep. 2022, 12, 19959. [Google Scholar] [CrossRef]
- Momtaz, S.; Hassani, S.; Khan, F.; Ziaee, M.; Abdollahi, M. Cinnamon, a promising prospect towards Alzheimer’s disease. Pharmacol. Res. 2018, 130, 241–258. [Google Scholar] [CrossRef]
- Angelopoulou, E.; Paudel, Y.N.; Piperi, C.; Mishra, A. Neuroprotective potential of cinnamon and its metabolites in Parkinson’s disease: Mechanistic insights, limitations and novel therapeutic opportunities. J. Biochem. Mol. Toxicol. 2021, 35, e22720. [Google Scholar] [CrossRef] [PubMed]
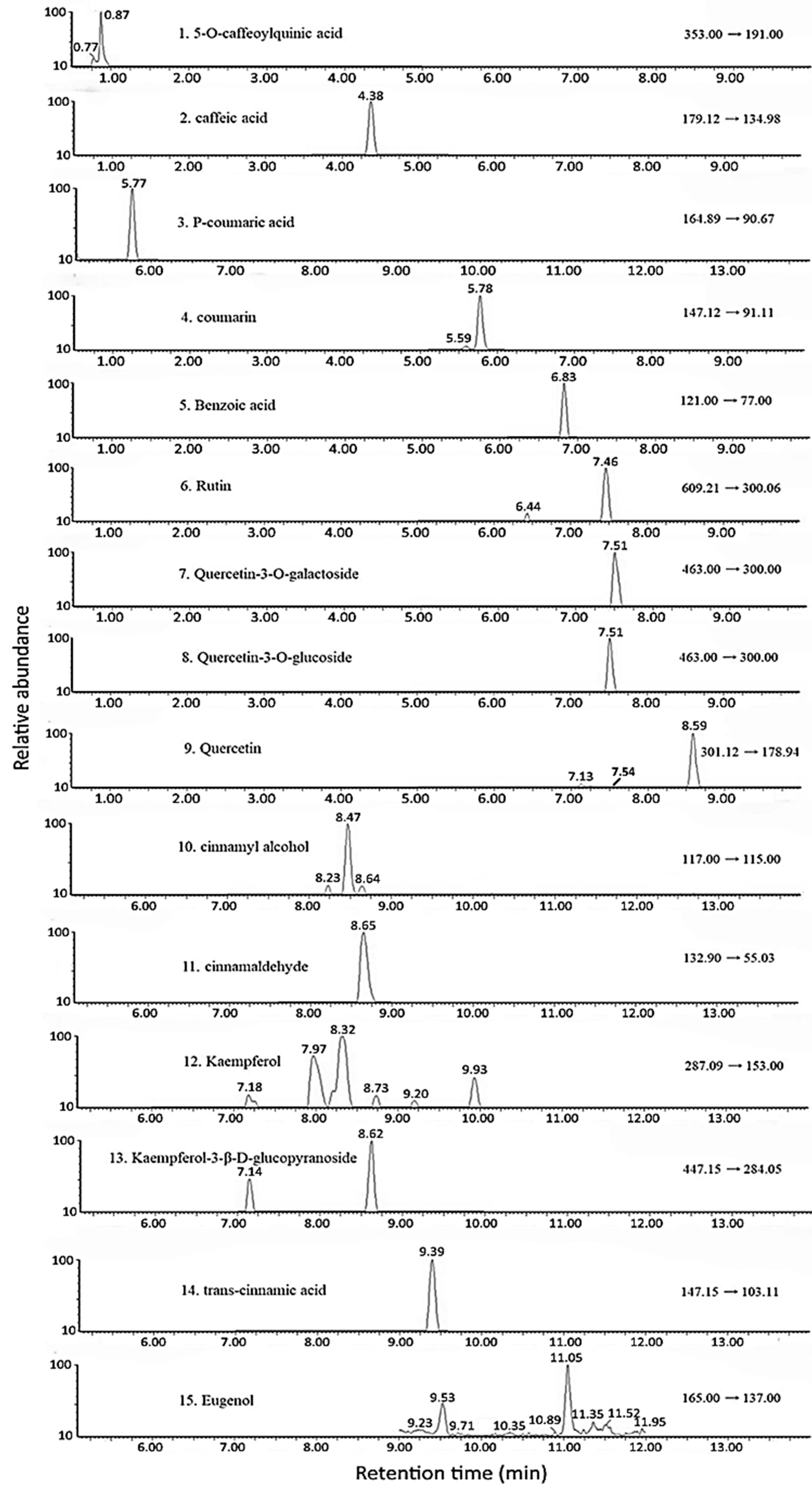
| Peak | Compound a | Retention Time (min) | MS/MS (m/z) | Content (μg/g) c | ||
|---|---|---|---|---|---|---|
| Precursor Ion | Product Ion | Powder | Hydrosol e | |||
| 1 | 5-O-Caffeoylquinic acid | 2.51 | 353 | 191 | 0.4 ± 0.1 | ND d |
| 2 | Caffeic acid | 4.38 | 179 | 134 | 2.4 ± 0.1 | ND |
| 3 | p-Coumaric acid | 5.77 | 164 | 90 | 4.7 ± 0.8 | ND |
| 4 | Coumarin | 5.78 | 147 | 91 | 2.6 ± 0.2 | ND |
| 5 | Benzoic acid | 6.83 | 121 | 77 | 67.7 ± 0.6 | 4.2 ± 0.3 |
| 6 | Rutin | 7.46 | 609 | 300 | 5.0 ± 0.2 | ND |
| 7 b | Quercetin-3-O-galactoside | 7.51 | 463 | 300 | 5.5 ± 0.1 | ND |
| 8 b | Quercetin-3-O-glucoside | 7.51 | 463 | 300 | - | ND |
| 9 | Quercetin | 7.54 | 301 | 151 | 20.9 ± 1.8 | ND |
| 10 | Cinnamyl alcohol | 8.47 | 117 | 115 | 92.2 ± 5.2 | 4.0 ± 0.4 |
| 11 | Cinnamaldehyde | 8.65 | 132 | 55 | 18,250.7 ± 334.86 | 1218.8 ± 92.4 |
| 12 | Kaempferol | 8.73 | 287 | 153 | 4.9 ± 06 | ND |
| 13 | Kaempferol-3-β-D-glucopyranoside | 8.62 | 447 | 284 | 19.9 ± 0.4 | ND |
| 14 | trans-Cinnamic acid | 9.39 | 147 | 103 | 464.9 ± 7.8 | 1.94 ± 0.11 |
| 15 | Eugenol | 11.05 | 165 | 137 | 220.1 ± 1.9 | 14.5 ± 0.2 |
| Day | Particle Size (nm) a,b | Polydispersity Index (PDI) a,b | Zeta Potential (mV) a,b |
|---|---|---|---|
| 0 | 17.3 ± 1.5 B | 0.177 ± 0.088 A | −42.1 ± 0.9 A |
| 15 | 16.9 ± 3.0 B | 0.160 ± 0.074 A | −42.8 ± 1.9 A |
| 30 | 19.1 ± 1.4 AB | 0.102 ± 0.047 A | −41.2 ± 2.0 A |
| 45 | 19.7 ± 2.1 AB | 0.110 ± 0.042 A | −41.9 ± 2.6 A |
| 60 | 20.7 ± 2.9 A | 0.063 ± 0.073 A | −41.0 ± 1.2 A |
| 75 | 22.1 ± 2.2 A | 0.099 ± 0.042 A | −39.6 ± 2.6 A |
| 90 | 23.2 ± 1.6 A | 0.169 ± 0.106 A | −40.0 ± 0.9 A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, B.-H.; Jen, C.-T.; Wang, C.-C.; Pan, M.-H. Improving Alzheimer’s Disease and Parkinson’s Disease in Rats with Nanoemulsion and Byproducts Prepared from Cinnamon Leaves. Pharmaceutics 2025, 17, 1200. https://doi.org/10.3390/pharmaceutics17091200
Chen B-H, Jen C-T, Wang C-C, Pan M-H. Improving Alzheimer’s Disease and Parkinson’s Disease in Rats with Nanoemulsion and Byproducts Prepared from Cinnamon Leaves. Pharmaceutics. 2025; 17(9):1200. https://doi.org/10.3390/pharmaceutics17091200
Chicago/Turabian StyleChen, Bing-Huei, Chen-Te Jen, Chia-Chuan Wang, and Min-Hsiung Pan. 2025. "Improving Alzheimer’s Disease and Parkinson’s Disease in Rats with Nanoemulsion and Byproducts Prepared from Cinnamon Leaves" Pharmaceutics 17, no. 9: 1200. https://doi.org/10.3390/pharmaceutics17091200
APA StyleChen, B.-H., Jen, C.-T., Wang, C.-C., & Pan, M.-H. (2025). Improving Alzheimer’s Disease and Parkinson’s Disease in Rats with Nanoemulsion and Byproducts Prepared from Cinnamon Leaves. Pharmaceutics, 17(9), 1200. https://doi.org/10.3390/pharmaceutics17091200











