Local Chemotherapy of Skin Pre-Neoplastic Lesions and Malignancies from the Perspective of Current Pharmaceutics
Abstract
1. Introduction
2. Skin Anatomy, Histology, and Physiology in Brief
3. Dermal and Transdermal Drug Transport
4. Biopharmaceutical Aspects of Dermal and Transdermal Drug Delivery
4.1. Physiological Aspects
4.2. Pathophysiological Aspects: Pre-Cancer- and Cancer-Related Impairment of the Skin’s Barrier Function
4.3. Drug Aspects in Dermal Drug Delivery
4.4. Dermal Dosage Forms and Excipients
5. Nanotechnologies for Dermal Drug Delivery
5.1. Vesicular Drug Carriers
5.2. Other Lipid-Based Nanotechnologies
5.3. Polymeric Nanoparticles
5.4. Inorganic Nanoparticles
6. Local Chemotherapy of Skin Pre-Neoplastic Lesions and Malignancies
6.1. 5-Fluorouracil (5-FU)
6.2. Imiquimod (IMQ)
6.3. Tirbanibulin
6.4. Diclofenac
6.5. Ingenol Mebutate (IM)
6.6. Vitamin D3 and Analogs
6.7. Retinoids
6.8. Miscellaneous
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SCC | Squamous cell carcinoma |
| NMSCs | Non-melanoma skin cancers |
| BCC | Basal cell carcinoma |
| UV | Ultraviolet |
| ROS | Reactive oxygen species |
| RNS | Reactive nitrogen species |
| HPV | Human papillomavirus |
| AK | Actinic keratosis |
| BD | Bowen’s disease |
| SC | Stratum corneum |
| EPR | Enhanced permeability and retention effect |
| PDT | Photodynamic therapy |
| 5-FU | 5-Fluorouracil |
| API | Active pharmaceutical ingredient |
| DMSO | Dimethylsulfoxide |
| SLNs | Solid lipid nanoparticles |
| NLCs | Nanostructured lipid carriers |
| AgNPs | Silver nanoparticles |
| AuNPs | Gold nanoparticles |
| STING | Stimulator of Interferon Genes |
| BCS | Biopharmaceutical classification system |
| IMQ | Imiquimod |
| FDA | United States Food and Drug Administration |
| EMA | European Medicines Agency |
| ADP | Adenosine diphosphate ribose |
| COX-2 | Cyclooxygenase-2 |
| PGE2 | Prostaglandin E2 |
| IM | Ingenol mebutate |
| VD3A | Vitamin D3 analogs |
| VDR | Vitamin D receptor |
| RARs | Retinoic acid receptors |
| RXRs | Retinoid X receptors |
| CTCL | Cutaneous T-cell lymphoma |
| STAT3 | Signal Transducer and Activator of Transcription 3 |
| siRNA | Small interfering RNA |
References
- Roky, A.H.; Islam, M.M.; Ahasan, A.M.F.; Mostaq, M.S.; Mahmud, M.Z.; Amin, M.N.; Mahmud, M.A. Overview of skin cancer types and prevalence rates across continents. Cancer Pathog. Ther. 2024, 3, 89–100. [Google Scholar] [CrossRef]
- Nakamura, A.; Kataoka, K.; Takatsuka, S.; Takenouchi, T. Aging trends in skin cancer: A long-term observational study in Japan. JAAD Int. 2023, 13, 32–34. [Google Scholar] [CrossRef]
- Saginala, K.; Barsouk, A.; Aluru, J.S.; Rawla, P.; Barsouk, A. Epidemiology of Melanoma. Med. Sci. 2021, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Davis, L.E.; Shalin, S.C.; Tackett, A.J. Current state of melanoma diagnosis and treatment. Cancer Biol. Ther. 2019, 20, 1366–1379. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Gowda, B.H.J.; Ahmed, M.G.; Abourehab, M.A.S.; Chen, Z.-S.; Zhang, C.; Li, J.; Kesharwani, P. Advancements in nanoparticle-based treatment approaches for skin cancer therapy. Mol. Cancer 2023, 22, 10. [Google Scholar] [CrossRef]
- Attal, Z.G.; Shalata, W.; Soklakova, A.; Tourkey, L.; Shalata, S.; Abu Saleh, O.; Abu Salamah, F.; Alatawneh, I.; Yakobson, A. Advanced and Metastatic Non-Melanoma Skin Cancer: Epidemiology, Risk Factors, Clinical Features, and Treatment Options. Biomedicines 2024, 12, 1448. [Google Scholar] [CrossRef]
- Newlands, C.; Currie, R.; Memon, A.; Whitaker, S.; Woolford, T. Non-melanoma skin cancer: United Kingdom National Multidisciplinary Guidelines. J. Laryngol. Otol. 2016, 130, S125–S132. [Google Scholar] [CrossRef] [PubMed]
- Khayyati Kohnehshahri, M.; Sarkesh, A.; Mohamed Khosroshahi, L.; HajiEsmailPoor, Z.; Aghebati-Maleki, A.; Yousefi, M.; Aghebati-Maleki, L. Current status of skin cancers with a focus on immunology and immunotherapy. Cancer Cell Int. 2023, 23, 174. [Google Scholar] [CrossRef]
- Tang, X.; Yang, T.; Yu, D.; Xiong, H.; Zhang, S. Current insights and future perspectives of ultraviolet radiation (UV) exposure: Friends and foes to the skin and beyond the skin. Environ. Int. 2024, 185, 108535. [Google Scholar] [CrossRef]
- Norval, M. The mechanisms and consequences of ultraviolet-induced immunosuppression. Prog. Biophys. Mol. Biol. 2006, 92, 108–118. [Google Scholar] [CrossRef]
- Liu, H.M.; Cheng, M.Y.; Xun, M.H.; Zhao, Z.W.; Zhang, Y.; Tang, W.; Cheng, J.; Ni, J.; Wang, W. Possible Mechanisms of Oxidative Stress-Induced Skin Cellular Senescence, Inflammation, and Cancer and the Therapeutic Potential of Plant Polyphenols. Int. J. Mol. Sci. 2023, 24, 3755. [Google Scholar] [CrossRef] [PubMed]
- Rahman, I. Oxidative Stress in the Pathogenesis of Chronic Obstructive Pulmonary Disease. In Cigarette Smoke and Oxidative Stress; Springer: Berlin/Heidelberg, Germany, 2006; pp. 165–197. [Google Scholar] [CrossRef]
- Adnan, M.; Akhter, M.H.; Afzal, O.; Altamimi, A.S.A.; Ahmad, I.; Alossaimi, M.A.; Jaremko, M.; Emwas, A.-H.; Haider, T.; Haider, M.F. Exploring Nanocarriers as Treatment Modalities for Skin Cancer. Molecules 2023, 28, 5905. [Google Scholar] [CrossRef]
- Prohic, A. Precancerous Skin Lesions. In Dermatovenerology Textbook; Springer: Berlin/Heidelberg, Germany, 2024; pp. 497–506. [Google Scholar] [CrossRef]
- Hidalgo, L.; Saldías-Fuentes, C.; Carrasco, K.; Halpern, A.C.; Mao, J.J.; Navarrete-Dechent, C. Complementary and alternative therapies in skin cancer: A literature review of biologically active compounds. Dermatol. Ther. 2022, 35, e15842. [Google Scholar] [CrossRef]
- Jadhav, L.A.; Mandlik, S.K. Nanocarriers in skin cancer treatment: Emerging drug delivery approaches and innovations. Nano TransMed 2025, 4, 100068. [Google Scholar] [CrossRef]
- Chang, J.; Yu, B.; Saltzman, W.M.; Girardi, M. Nanoparticles as a Therapeutic Delivery System for Skin Cancer Prevention and Treatment. JID Innov. 2023, 3, 100197. [Google Scholar] [CrossRef]
- Maru, G.B.; Gandhi, K.; Ramchandani, A.; Kumar, G. The role of inflammation in skin cancer. Adv. Exp. Med. Biol. 2014, 816, 437–469. [Google Scholar] [CrossRef]
- Rangwala, S.; Tsai, K.Y. Roles of the immune system in skin cancer. Br. J. Dermatol. 2011, 165, 953–965. [Google Scholar] [CrossRef]
- Huang, Y.; Carlsson, L.; Jogeland, K.; Samuelsson, M.; Larsson, L.; Jonsborg, C. Management of complications after skin surgery relevant for melanoma in the trunk and extremities during the COVID-19 pandemic: A case series report. World J. Surg. Oncol. 2023, 21, 280. [Google Scholar] [CrossRef]
- Khan, S.U.; Ullah, M.; Saeed, S.; Saleh, E.A.M.; Kassem, A.F.; Arbi, F.M.; Wahab, A.; Rehman, M.; ur Rehman, K.; Khan, D.; et al. Nanotherapeutic approaches for transdermal drug delivery systems and their biomedical applications. Eur. Polym. J. 2024, 207, 112819. [Google Scholar] [CrossRef]
- Leong, M.Y.; Kong, Y.L.; Burgess, K.; Wong, W.F.; Sethi, G.; Looi, C.Y. Recent development of nanomaterials for transdermal drug delivery. Biomedicines 2023, 11, 1124. [Google Scholar] [CrossRef] [PubMed]
- Mearaj, S.; Kim, T.M.; Choi, J.W. Photoprotective and bioinspired cerium-encapsulating lignin nanocapsules for multifunctional UV protection applications. Chem. Eng. J. 2024, 501, 157668. [Google Scholar] [CrossRef]
- Smijs, T.G.; Pavel, S. Titanium dioxide and zinc oxide nanoparticles in sunscreens: Focus on their safety and effectiveness. Nanotechnol. Sci. Appl. 2011, 4, 95–112. [Google Scholar] [CrossRef]
- Yabe, S.; Sato, T. Cerium oxide for sunscreen cosmetics. J. Solid. State Chem. 2003, 171, 7–11. [Google Scholar] [CrossRef]
- Brito, S.; Baek, M.; Bin, B.-H. Skin Structure, Physiology, and Pathology in Topical and Transdermal Drug Delivery. Pharmaceutics 2024, 16, 1403. [Google Scholar] [CrossRef]
- Gallo, R.L. Human Skin Is the Largest Epithelial Surface for Interaction with Microbes. J. Investig. Dermatol. 2017, 137, 1213–1214. [Google Scholar] [CrossRef] [PubMed]
- McBride, A.; Bargmann, S.; Pond, D.; Limbert, G. Thermoelastic Modelling of the Skin at Finite Deformations. J. Therm. Biol. 2016, 62, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Dermitzakis, I.; Chatzi, D.; Kyriakoudi, S.A.; Evangelidis, N.; Vakirlis, E.; Meditskou, S.; Theotokis, P.; Manthou, M.E. Skin Development and Disease: A Molecular Perspective. Curr. Issues Mol. Biol. 2024, 46, 8239–8267. [Google Scholar] [CrossRef] [PubMed]
- Lukić, M.; Pantelić, I.; Savić, S.D. Towards Optimal pH of the Skin and Topical Formulations: From the Current State of the Art to Tailored Products. Cosmetics 2021, 8, 69. [Google Scholar] [CrossRef]
- Kuo, S.-H.; Shen, C.-J.; Shen, C.-F.; Cheng, C.-M. Role of pH Value in Clinically Relevant Diagnosis. Diagnostics 2020, 10, 107. [Google Scholar] [CrossRef]
- Sanchez, M.M.; Tonmoy, T.I.; Park, B.H.; Morgan, J.T. Development of a Vascularized Human Skin Equivalent with Hypodermis for Photoaging Studies. Biomolecules 2022, 12, 1828. [Google Scholar] [CrossRef]
- Elias, P.M. Structure and Function of the Stratum Corneum Extracellular Matrix. J. Investig. Dermatol. 2012, 132, 2131–2133. [Google Scholar] [CrossRef]
- Santiago, J.L.; Muñoz-Rodriguez, J.R.; Cruz-Morcillo, M.A.d.l.; Villar-Rodriguez, C.; Gonzalez-Lopez, L.; Aguado, C.; Nuncia-Cantarero, M.; Redondo-Calvo, F.J.; Perez-Ortiz, J.M.; Galan-Moya, E.M. Characterization of Permeability Barrier Dysfunction in a Murine Model of Cutaneous Field Cancerization Following Chronic UV-B Irradiation: Implications for the Pathogenesis of Skin Cancer. Cancers 2021, 13, 3935. [Google Scholar] [CrossRef]
- Ishitsuka, Y.; Roop, D.R. Loricrin: Past, Present, and Future. Int. J. Mol. Sci. 2020, 21, 2271. [Google Scholar] [CrossRef] [PubMed]
- Fujii, M. The Pathogenic and Therapeutic Implications of Ceramide Abnormalities in Atopic Dermatitis. Cells 2021, 10, 2386. [Google Scholar] [CrossRef] [PubMed]
- Alkilani, A.Z.; McCrudden, M.T.; Donnelly, R.F. Transdermal Drug Delivery: Innovative Pharmaceutical Developments Based on Disruption of the Barrier Properties of the Stratum Corneum. Pharmaceutics 2015, 7, 438–470. [Google Scholar] [CrossRef] [PubMed]
- Kougkolos, G.; Laudebat, L.; Dinculescu, S.; Simon, J.; Golzio, M.; Valdez-Nava, Z.; Flahaut, E. Skin Electroporation for Transdermal Drug Delivery: Electrical Measurements, Numerical Model and Molecule Delivery. J. Control Release 2024, 367, 235–247. [Google Scholar] [CrossRef]
- Ogórek, K.; Nowak, K.; Wadych, E.; Ruzik, L.; Timerbaev, A.R.; Matczuk, M. Are We Ready to Measure Skin Permeation of Modern Antiaging GHK–Cu Tripeptide Encapsulated in Liposomes? Molecules 2025, 30, 136. [Google Scholar] [CrossRef]
- Ramadon, D.; McCrudden, M.T.C.; Courtenay, A.J.; Donnelly, R.F. Enhancement Strategies for Transdermal Drug Delivery Systems: Current Trends and Applications. Drug Deliv. Transl. Res. 2022, 12, 758–791. [Google Scholar] [CrossRef]
- Jain, S.K.; Verma, A.; Jain, A.; Hurkat, P. Transfollicular Drug Delivery: Current Perspectives. Res. Rep. Transdermal Drug Deliv. 2016, 5, 1–17. [Google Scholar] [CrossRef]
- Meidan, V.M.; Bonner, M.C.; Michniak, B.B. Transfollicular Drug Delivery—Is it a Reality? Int. J. Pharm. 2005, 306, 1–14. [Google Scholar] [CrossRef]
- Rancan, F.; Vogt, A. Getting Under the Skin: What Is the Potential of the Transfollicular Route in Drug Delivery? Ther. Deliv. 2014, 5, 875–877. [Google Scholar] [CrossRef]
- Supe, S.; Takudage, P. Methods for Evaluating Penetration of Drug into the Skin: A Review. Ski. Res. Technol. 2020, 27, 299–308. [Google Scholar] [CrossRef]
- Bolzinger, M.-A.; Briançon, S.; Pelletier, J.; Chevalier, Y. Penetration of Drugs through Skin, A Complex Rate-controlling Membrane. Curr. Opin. Colloid. Interface Sci. 2012, 17, 156–165. [Google Scholar] [CrossRef]
- Farage, M.A.; Miller, K.W.; Elsner, P.; Maibach, H.I. Characteristics of the Aging Skin. Adv. Wound Care 2013, 2, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Russell-Goldman, E.; Murphy, G.F. The Pathobiology of Skin Aging: New Insights into an Old Dilemma. Am. J. Pathol. 2020, 190, 1356–1369. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Man, M.Q.; Li, T.; Elias, P.M.; Mauro, T.M. Aging-associated alterations in epidermal function and their clinical significance. Aging 2020, 12, 5551–5565. [Google Scholar] [CrossRef]
- White-Chu, E.F.; Reddy, M. Dry skin in the elderly: Complexities of a common problem. Clin. Dermatol. 2011, 29, 37–42. [Google Scholar] [CrossRef]
- Ribeiro, M.; Simões, M.; Vitorino, C.; Mascarenhas-Melo, F. Hydrogels in Cutaneous Wound Healing: Insights into Characterization, Properties, Formulation and Therapeutic Potential. Gels 2024, 10, 188. [Google Scholar] [CrossRef]
- Souto, E.B.; Fangueiro, J.F.; Fernandes, A.R.; Cano, A.; Sanchez-Lopez, E.; Garcia, M.L.; Severino, P.; Paganelli, M.O.; Chaud, M.V.; Silva, A.M. Physicochemical and Biopharmaceutical Aspects Influencing Skin Permeation and Role of SLN and NLC for Skin Drug Delivery. Heliyon 2022, 8, e08938. [Google Scholar] [CrossRef]
- Rawlings, A.V.; Matts, P.J. Stratum Corneum Moisturization at the Molecular Level: An Update in Relation to the Dry Skin Cycle. J. Investig. Dermatol. 2005, 124, 1099–1110. [Google Scholar] [CrossRef]
- Załęcki, P.; Rogowska, K.; Wąs, P.; Łuczak, K.; Wysocka, M.; Nowicka, D. Impact of Lifestyle on Differences in Skin Hydra-Tion of Selected Body Areas in Young Women. Cosmetics 2024, 11, 13. [Google Scholar] [CrossRef]
- Gidado, I.; Qassem, M.; Triantis, I.; Kyriacou, P. Review of Advances in the Measurement of Skin Hydration Based on Sensing of Optical and Electrical Tissue Properties. Sensors 2022, 22, 7151. [Google Scholar] [CrossRef]
- Goad, N.; Gawkrodger, D. Ambient Humidity and the Skin: The Impact of Air Humidity in Healthy and Diseased States. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 1285–1294. [Google Scholar] [CrossRef] [PubMed]
- Walters, K.; Lane, M. Dermal and Transdermal Drug Delivery Systems. In Dermal Drug Delivery; CRC Press: Boca Raton, FL, USA, 2020; pp. 1–60. [Google Scholar]
- David, P.; Singh, S.; Ankar, R.A. Comprehensive Overview of Skin Complications in Diabetes and Their Prevention. Cureus 2023, 15, e38961. [Google Scholar] [CrossRef]
- Giakoumaki, M.; Lambrou, G.I.; Vlachodimitropoulos, D.; Tagka, A.; Vitsos, A.; Kyriazi, M.; Dimakopoulou, A.; Anagnostou, V.; Karasmani, M.; Deli, H. Type I Diabetes Mellitus Suppresses Experimental Skin Carcinogenesis. Cancers 2024, 16, 1507. [Google Scholar] [CrossRef]
- Safer, J. Thyroid Hormone Action on Skin. Dermatoendocrinology 2011, 3, 211–215. [Google Scholar] [CrossRef]
- Gratton, R.; Del Vecchio, C.; Zupin, L.; Crovella, S. Unraveling the Role of Sex Hormones on Keratinocyte Functions in Human Inflammatory Skin Diseases. Int. J. Mol. Sci. 2022, 23, 3132. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, Y.H. Exploring Acne Treatments: From Pathophysiological Mechanisms to Emerging Therapies. Int. J. Mol. Sci. 2024, 25, 5302. [Google Scholar] [CrossRef]
- Gruber, J.V.; Stojkoska, V.; Riemer, J. Retinol Has a Skin Dehydrating Effect That Can Be Improved by a Mixture of Wa-Ter-Soluble Polysaccharides. Cosmetics 2020, 7, 80. [Google Scholar] [CrossRef]
- Kowalska, J.; Wrześniok, D. Skin-Related Adverse Reactions Induced by Oral Antidiabetic Drugs—A Review of Literature and Case Reports. Pharmaceuticals 2024, 17, 847. [Google Scholar] [CrossRef] [PubMed]
- Pyo, S.; Maibach, H. Skin Metabolism: Relevance of Skin Enzymes for Rational Drug Design. Ski. Pharmacol. Physiol. 2019, 32, 283–294. [Google Scholar] [CrossRef]
- Suresh, P.K. Enhanced Permeation Retention Effect-Modeling and Imaging Approaches for Nanoparticle-Mediated Anti-Cancer Diagnostics or Therapy. Lett. Drug Des. Discov. 2024, 21, 3633–3638. [Google Scholar] [CrossRef]
- Serzants, R.; Svalbe, B.; Cesnokova, I.; Stelfa, G.; Sizovs, A. Exploring the Limits of EPR-Driven Tumor Accumulation with Non-Opsonizing Nanomaterials. bioRxiv 2024. [Google Scholar] [CrossRef]
- Vijaykumar, V.; Saikiran, M.; Bharathy, V.; Ubaidulla, U. Formulation Challenges in Dermal Drug Delivery Systems: A Comprehensive Review of Physicochemical Properties and Advanced Delivery Strategies. Int. J. Drug Deliv. Technol. 2024, 14, 1124–1129. [Google Scholar] [CrossRef]
- Czyrski, G.S.; Hjort, M.K.F.; Rades, T.; Heinz, A. Comparing Effects of Terpene-Based Deep Eutectic Solvent and Solid Microneedles on Skin Permeation of Drugs with Varying Lipophilicity. Eur. J. Pharm. Biopharm. 2024, 205, 114576. [Google Scholar] [CrossRef]
- Kilian, D.; Lemmer, H.J.R.; Gerber, M.; Du Preez, J.L.; Plessis, J. Exploratory Data Analysis of the Dependencies between Skin Permeability, Molecular Weight and Log P. Die Pharm. 2016, 71, 311–319. [Google Scholar] [CrossRef]
- Goold, S.R.; Raddi, R.M.; Voelz, V.A. Expanded ensemble predictions of toluene–water partition coefficients in the SAMPL9 log P challenge. Phys. Chem. Chem. Phys. 2025, 27, 6005–6013. [Google Scholar] [CrossRef] [PubMed]
- Algotsson, J.; Jönsson, P.; Forsman, J.; Topgaard, D.; Söderman, O. Intermolecular Interactions Play a Role in the Distribution and Transport of Charged Contrast Agents in a Cartilage Model. PLoS ONE 2019, 14, e0215047. [Google Scholar] [CrossRef]
- Balouch, M.; Storchmannová, K.; Štěpánek, F.; Berka, K. Computational Prodrug Design Methodology for Liposome Formulability Enhancement of Small-Molecule APIs. Mol. Pharm. 2023, 20, 2119–2127. [Google Scholar] [CrossRef] [PubMed]
- Gavhane, S.A.; Somwanshi, S.B. Prodrug: Approach to Better Drug Delivery; IIP Series; IIP: Chikkamagaluru, India, 2024; pp. 56–61. [Google Scholar]
- Sahu, S.K.; Singh, A.; Verma, P.; Chaurasiya, S. Prodrug: A Brief Concept; IIP Series; IIP: Chikkamagaluru, India, 2024; pp. 247–256. [Google Scholar]
- Zeng, Y.; Ma, M.; Chen, Y.; Xie, H.; Ding, P.; Zhang, K. Enhancing Skin Delivery of Tranexamic Acid via Esterification: Synthesis and Evaluation of Alkyl Ester Derivatives. RSC Adv. 2024, 14, 34996–35004. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Ni, F.; Yao, J.; Huang, C.; Zhao, Y. Synthesis of Phosphoramidate Prodrugs of Phenolic Natural Products and Drugs by Ester Exchange. Synthesis 2022, 54, 3005–3014. [Google Scholar] [CrossRef]
- Suppasansatorn, P.; Wang, G.; Conway, B.R.; Wang, W.; Wang, Y. Skin Delivery Potency and Antitumor Activities of Temozolomide Ester Prodrugs. Cancer Lett. 2006, 244, 42–52. [Google Scholar] [CrossRef]
- Filonenko, E.; Kaprin, A.; Urlova, A.; Grigorievykh, N.; Ivanova-Radkevich, V. Topical 5-Aminolevulinic Acid-Mediated Photodynamic Therapy for Basal Cell Carcinoma. Photodiagnosis Photodyn. Ther. 2020, 30, 101644. [Google Scholar] [CrossRef] [PubMed]
- Gold, M. Therapeutic and Aesthetic Uses of Photodynamic Therapy Part Five of a Five-Part Series: ALA-PDT in Clinical Practice How One Clinician Performs This Procedure. J. Clin. Aesthet. Dermatol. 2009, 2, 32–35. [Google Scholar] [PubMed]
- Champeau, M.; Vignoud, S.; Mortier, L.; Mordon, S. Photodynamic Therapy for Skin Cancer: How to Enhance Drug Penetration? J. Photochem. Photobiol. B 2019, 197, 111544. [Google Scholar] [CrossRef] [PubMed]
- Winkler, A.; Müller-Goymann, C. Comparative Permeation Studies for Delta-Aminolevulinic Acid and Its n-Butylester through Stratum Corneum and Artificial Skin Constructs. Eur. J. Pharm. Biopharm. 2002, 53, 281–287. [Google Scholar] [CrossRef]
- Neittaanmäki-Perttu, N.; Grönroos, M.; Karppinen, T.T.; Tani, T.T.; Snellman, E. Hexyl-5-aminolaevulinate 0·2% vs. Methyl-5-aminolaevulinate 16% Daylight Photodynamic Therapy for Treatment of Actinic Keratoses: Results of a Randomized Double-blinded Pilot Trial. Br. J. Dermatol. 2016, 174, 427–429. [Google Scholar] [CrossRef]
- Fang, J.; Leu, Y.L. Prodrug Strategy for Enhancing Drug Delivery via Skin. Curr. Drug Discov. Technol. 2006, 3, 211–224. [Google Scholar] [CrossRef]
- Quigley, J.; Lloyd, D. A Topological Study of Prodrugs of 5-Fluorouracil. Int. J. Pharm. 2002, 231, 241–251. [Google Scholar] [CrossRef]
- Patrick, A.I.; Beall, H.D.; Gilroy, P.; Sloan, K.B. Effect of Vehicles on Topical Delivery of 5-Fluorouracil (5FU) by 1-Acyl-5FU Prodrugs. Int. J. Pharm. 1997, 154, 39–48. [Google Scholar] [CrossRef]
- Jacobson, E.; Kim, H.; Kim, M.; Wondrak, G.; Jacobson, M. Developing Topical Prodrugs for Skin Cancer Prevention. In Fundamentals of Cancer Prevention; Alberts, D., Hess, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 139–160. [Google Scholar]
- Sharma, A.; Sharma, D.; Baldi, A.; Jyoti, K.; Chandra, R.; Madan, J. Imiquimod-Oleic Acid Prodrug-Loaded Cream Reduced Drug Crystallinity and Induced Indistinguishable Cytotoxicity and Apoptosis in Mice Melanoma Tumour. J. Microencapsul. 2019, 36, 759–774. [Google Scholar] [CrossRef]
- Vaienti, S.; Calzari, P.; Nazzaro, G. Topical Treatment of Melanoma In Situ, Lentigo Maligna, and Lentigo Maligna Melanoma with Imiquimod Cream: A Systematic Review of the Literature. Dermatol. Ther. 2023, 13, 2187–2215. [Google Scholar] [CrossRef]
- Lein, A.; Oussoren, C. Dermal. In Practical Pharmaceutics; Springer International Publishing Switzerland: Cham, Switzerland, 2015; pp. 229–263. [Google Scholar]
- Ahuja, K.; An, M.; Lio, P. A Brief Review of Vehicles for Topical Therapies. Ski. Pharmacol. Physiol. 2025, 37, 104–108. [Google Scholar] [CrossRef]
- Herbig, M.E.; Evers, D.-H.; Gorissen, S.; Köllmer, M. Rational Design of Topical Semi-Solid Dosage Forms-How Far Are We? Pharmaceutics 2023, 15, 1822. [Google Scholar] [CrossRef]
- Kim, S.; Day, C.; Song, Y.; Holmes, A.; Garg, S. Innovative Topical Patches for Non-Melanoma Skin Cancer: Current Challenges and Key Formulation Considerations. Pharmaceutics 2023, 15, 2577. [Google Scholar] [CrossRef]
- Lei, Q.; He, D.; Ding, L.; Kong, F.; He, P.; Huang, J.; Guo, J.; Brinker, C.J.; Luo, G.; Zhu, W.; et al. Microneedle Patches Integrated with Biomineralized Melanin Nanoparticles for Simultaneous Skin Tumor Photothermal Therapy and Wound Healing. Adv. Funct. Mater. 2022, 32, 2113269. [Google Scholar] [CrossRef]
- Dragicevic, N.; Predic Atkinson, J.; Maibach, H.I. Chemical Penetration Enhancers: Classification and Mode of Action; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Špaglová, M.; Žigrayová, D.; Krchňák, D. Development Strategy of Dermal and Transdermal Formulation: Synergistic Effect of Chemical Penetration Enhancers. Acta Med. Bulg. 2024, 51, 171–181. [Google Scholar] [CrossRef]
- Borude, P.D. A Review Article on Permeability Enhancement. Int. J. Res. Appl. Sci. Eng. Technol. 2024, 12, 2301–2305. [Google Scholar] [CrossRef]
- Greve, T.M.; Andersen, K.B.; Nielsen, O.F. Penetration Mechanism of Dimethyl Sulfoxide in Human and Pig Ear Skin: An ATR-FTIR and near-FT Raman Spectroscopic in Vivo and in Vitro Study. Spectroscopy 2008, 22, 405–417. [Google Scholar] [CrossRef]
- Kulkarni, V.S.; Shaw, C. Use of Polymers and Thickeners in Semisolid and Liquid Formulations. In Essential Chemistry for Formulators of Semisolid and Liquid Dosages; Kulkarni, V.S., Shaw, C., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 43–69. [Google Scholar]
- Jampílek, J. Azone® and Its Analogues as Penetration Enhancers. In Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement; Springer: London, NY, USA, 2015; pp. 69–105. [Google Scholar]
- Singh, S.; Mazumder, R.; PADHI, S.; Mishra, R.; Kumar, V. Unlocking Skin Barriers: Applications and Properties of Natural Permeation Enhancers. J. Nat. Remedies 2024, 24, 2599–2624. [Google Scholar] [CrossRef]
- Hmingthansanga, V.; Singh, N.; Banerjee, S.; Manickam, S.; Velayutham, R.; Natesan, S. Improved Topical Drug Delivery: Role of Permeation Enhancers and Advanced Approaches. Pharmaceutics 2022, 14, 2818. [Google Scholar] [CrossRef] [PubMed]
- Dudhat, K. Emerging Trends in Transdermal Drug Delivery: Nanoparticle Formulations and Technologies for Enhanced Skin Penetration and Drug Efficiency. Pharm. Nanotechnol. 2024, 13, 1–21. [Google Scholar] [CrossRef]
- Lalotra, A.S.; Singh, V.; Khurana, B.; Agrawal, S.; Shrestha, S.; Arora, D. A Comprehensive Review on Nanotechnology-Based Innovations in Topical Drug Delivery for the Treatment of Skin Cancer. Curr. Pharm. Des. 2020, 26, 5720–5731. [Google Scholar] [CrossRef]
- Krishnan, V.; Mitragotri, S. Nanoparticles for Topical Drug Delivery: Potential for Skin Cancer Treatment. Adv. Drug Deliv. Rev. 2020, 153, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Nsairat, H.; Khater, D.; Sayed, U.; Odeh, F.; Al Bawab, A.; Alshaer, W. Liposomes: Structure, Composition, Types, and Clinical Applications. Heliyon 2022, 8, e09394. [Google Scholar] [CrossRef]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.; Sood, A.; Hua, S. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef] [PubMed]
- Guillot, A.J.; Martínez-Navarrete, M.; Garrigues, T.M.; Melero, A. Skin Drug Delivery Using Lipid Vesicles: A Starting Guideline for Their Development. J. Control. Release 2023, 355, 624–654. [Google Scholar] [CrossRef]
- Gugleva, V.; Ivanova, N.; Sotirova, Y.; Andonova, V. Dermal Drug Delivery of Phytochemicals with Phenolic Structure via Lipid-Based Nanotechnologies. Pharmaceuticals 2021, 14, 837. [Google Scholar] [CrossRef]
- Zhan, B.; Wang, J.; Li, H.; Xiao, K.; Fang, X.; Shi, Y.; Jia, Y. Ethosomes: A Promising Drug Delivery Platform for Transdermal Application. Chemistry 2024, 6, 993–1019. [Google Scholar] [CrossRef]
- Musielak, E.; Krajka-Kuźniak, V. Liposomes and Ethosomes: Comparative Potential in Enhancing Skin Permeability for Therapeutic and Cosmetic Applications. Cosmetics 2024, 11, 191. [Google Scholar] [CrossRef]
- Matharoo, N.; Mohd, H.; Michniak-Kohn, B. Transferosomes as a Transdermal Drug Delivery System: Dermal Kinetics and Recent Developments. WIREs Nanomed. Nanobiotechnol. 2024, 16, e1918. [Google Scholar] [CrossRef]
- Fernández-García, R.; Lalatsa, A.; Statts, L.; Bolás-Fernández, F.; Ballesteros, M.P.; Serrano, D.R. Transferosomes as Nanocarriers for Drugs across the Skin: Quality by Design from Lab to Industrial Scale. Int. J. Pharm. 2020, 573, 118817. [Google Scholar] [CrossRef]
- Muzzalupo, R.; Tavano, L. Niosomal Drug Delivery for Transdermal Targeting: Recent Advances. Res. Rep. Transdermal Drug Deliv. 2015, 4, 23–33. [Google Scholar] [CrossRef]
- Kaur, P.; Kriplani, P. Quality by Design for Niosome-Based Nanocarriers to Improve Transdermal Drug Delivery from Lab to Industry. Int. J. Pharm. 2024, 666, 124747. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.A.; Forster, C.; Feitosa, V.; Baby, A.R.; Léo, P.; Rangel-Yagui, C.O. Catalase-Loaded Polymersomes as a Promising Safe Ingredient to Active Photoprotection. J. Photochem. Photobiol. 2021, 7, 100056. [Google Scholar] [CrossRef]
- Fonseca, M.; Jarak, I.; Victor, F.; Domingues, C.; Veiga, F.; Figueiras, A. Polymersomes as the Next Attractive Generation of Drug Delivery Systems: Definition, Synthesis and Applications. Materials 2024, 17, 319. [Google Scholar] [CrossRef]
- Kotha, R.; Kara, D.; Roychowdhury, R.; Tanvi, K.; Rathnanand, M. Polymersomes Based Versatile Nanoplatforms for Controlled Drug Delivery and Imaging. Adv. Pharm. Bull. 2023, 13, 218–232. [Google Scholar] [CrossRef]
- Hua, C.; Qiu, L. Polymersomes for Therapeutic Protein and Peptide Delivery: Towards Better Loading Properties. Int. J. Nanomed. 2024, 19, 2317–2340. [Google Scholar] [CrossRef]
- Jain, S.; Tripathi, S.; Tripathi, P.K. Invasomes: Potential Vesicular Systems for Transdermal Delivery of Drug Molecules. J. Drug Deliv. Sci. Technol. 2021, 61, 102166. [Google Scholar] [CrossRef]
- Preeti, P.; Puri, D.; Singh, S. Invasomes: An Artificial Vesicle Nanocarrier to Enhance Transdermal Drug Delivery. Curr. Nanomed. 2024, 15, 1–15. [Google Scholar] [CrossRef]
- Afreen, U.; Shailaja, A.K. Pharmacosomes and Emulsomes: An Emerging Novel Vesicular Drug Delivery System. Glob. J. Anesthesia Pain Med. 2020, 3, 287–297. [Google Scholar] [CrossRef]
- Rana, P.; Mahajan, A.; Singh, D.; Singh, K. Pharmacosomes: A Versatile Delivery System for Problematic Molecules. Curr. Nanomed. 2021, 11, 82–90. [Google Scholar] [CrossRef]
- Gaikwad, S.S.; Morade, Y.Y.; Kothule, A.M.; Kshirsagar, S.J.; Laddha, U.D.; Salunkhe, K.S. Overview of Phytosomes in Treating Cancer: Advancement, Challenges, and Future Outlook. Heliyon 2023, 9, e16561. [Google Scholar] [CrossRef]
- Barani, M.; Sangiovanni, E.; Angarano, M.; Rajizadeh, M.; Mehrabani, M.; Piazza, S.; Gangadharappa, H.; Pardakhty, A.; Mehrbani, M.; Dell’Agli, M.; et al. Phytosomes as Innovative Delivery Systems for Phytochemicals: A Comprehensive Review of Literature. Int. J. Nanomed. 2021, 16, 6983–7022. [Google Scholar] [CrossRef]
- Nath, A.G.; Dubey, P.; Kumar, A.; Vaiphei, K.K.; Rosenholm, J.M.; Bansal, K.K.; Gulbake, A. Recent Advances in the Use of Cubosomes as Drug Carriers with Special Emphasis on Topical Applications. J. Lipids 2024, 2024, 2683466. [Google Scholar] [CrossRef]
- Marson, D.; Aulic, S.; Laurini, E.; Pricl, S. Chapter 9—Cubosomes: A Promising Vesicular System for Drug Delivery. In Systems of Nanovesicular Drug Delivery; Nayak, A.K., Hasnain, M.S., Aminabhavi, T.M., Torchilin, V.P., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 129–145. [Google Scholar]
- Jha, A.; Kumar, M.; Bharti, K.; Mishra, B. Chapter 17—Glycerosomes: A New Tool for Effective Drug Delivery. In Systems of Nanovesicular Drug Delivery; Nayak, A.K., Hasnain, M.S., Aminabhavi, T.M., Torchilin, V.P., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 277–291. [Google Scholar]
- Manca, M.L.; Zaru, M.; Manconi, M.; Lai, F.; Valenti, D.; Sinico, C.; Fadda, A.M. Glycerosomes: A New Tool for Effective Dermal and Transdermal Drug Delivery. Int. J. Pharm. 2013, 455, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, L.; Nourddine, H.; Saad, E.F.; Abdelali, D.; Hamid, R. Chitosan-Covered Liposomes as a Promising Drug Transporter: Nanoscale Investigations. RSC Adv. 2021, 11, 1503–1516. [Google Scholar] [CrossRef] [PubMed]
- Gaynanova, G.; Vasileva, L.; Kashapov, R.; Kuznetsova, D.; Kushnazarova, R.; Tyryshkina, A.; Vasilieva, E.; Petrov, K.; Zakharova, L.; Sinyashin, O. Self-Assembling Drug Formulations with Tunable Permeability and Biodegradability. Molecules 2021, 26, 6786. [Google Scholar] [CrossRef] [PubMed]
- Sebaaly, C.; Trifan, A.; Sieniawska, E.; Greige-Gerges, H. Chitosan-Coating Effect on the Characteristics of Liposomes: A Focus on Bioactive Compounds and Essential Oils: A Review. Processes 2021, 9, 445. [Google Scholar] [CrossRef]
- Colino, C.I.; Velez Gomez, D.; Alonso Horcajo, E.; Gutierrez-Millan, C. A Comparative Study of Liposomes and Chitosomes for Topical Quercetin Antioxidant Therapy. J. Drug Deliv. Sci. Technol. 2022, 68, 103094. [Google Scholar] [CrossRef]
- Esposto, B.S.; Jauregi, P.; Tapia-Blácido, D.R.; Martelli-Tosi, M. Liposomes vs. Chitosomes: Encapsulating Food Bioactives. Trends Food Sci. Technol. 2021, 108, 40–48. [Google Scholar] [CrossRef]
- Viegas, C.; Patrício, A.; Prata, J.; Nadhman, A.; Chintamaneni, P.; Fonte, P. Solid Lipid Nanoparticles vs. Nanostructured Lipid Carriers: A Comparative Review. Pharmaceutics 2023, 15, 1593. [Google Scholar] [CrossRef] [PubMed]
- Sotirova, Y. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: Current Perspectives in Wound Care. Scr. Sci. Pharm. 2023, 10, 24–34. [Google Scholar] [CrossRef]
- Akanda, M.; Mithu, M.S.H.; Douroumis, D. Solid Lipid Nanoparticles: An Effective Lipid-Based Technology for Cancer Treatment. J. Drug Deliv. Sci. Technol. 2023, 86, 104709. [Google Scholar] [CrossRef]
- Mall, J.; Naseem, N.; Haider, M.F.; Rahman, M.A.; Khan, S.; Siddiqui, S.N. Nanostructured Lipid Carriers as a Drug Delivery System: A Comprehensive Review with Therapeutic Applications. Intell. Pharm. 2024. [Google Scholar] [CrossRef]
- Souto, E.B.; Cano, A.; Martins-Gomes, C.; Coutinho, T.E.; Zielińska, A.; Silva, A.M. Microemulsions and Nanoemulsions in Skin Drug Delivery. Bioengineering 2022, 9, 158. [Google Scholar] [CrossRef]
- Musakhanian, J.; Osborne, D.W. Understanding Microemulsions and Nanoemulsions in (Trans)Dermal Delivery. AAPS PharmSciTech 2025, 26, 31. [Google Scholar] [CrossRef]
- Mohd-Nasir, H.; Abdul Aziz, Z.A.; Mohd Setapar, S.H. Chapter 14—In Vitro and in Vivo Safety Evaluation of Nanoemulsion as Skin Moisturizer. In Nanotechnology for the Preparation of Cosmetics Using Plant-Based Extracts; Mohd Setapar, S.H., Ahmad, A., Jawaid, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 345–354. [Google Scholar]
- Burger, C.; Shahzad, Y.; Brummer, A.; Gerber, M.; du Plessis, J. Traversing the Skin Barrier with Nano-Emulsions. Curr. Drug Deliv. 2017, 14, 458–472. [Google Scholar] [CrossRef]
- Lopes, L. Overcoming the Cutaneous Barrier with Microemulsions. Pharmaceutics 2014, 6, 52–77. [Google Scholar] [CrossRef]
- Baranovskii, V.Y.; Ganev, V.G.; Petkova, V.B.; Voicheva, K.C.; Dimitrov, M.V. Hydrogels Based on Polycarboxylic Acid-Agar-Agar Complexes. Colloid. J. 2012, 74, 645–648. [Google Scholar] [CrossRef]
- Parhi, R.; Sahoo, S.K.; Das, A. Applications of Polysaccharides in Topical and Transdermal Drug Delivery: A Recent Update of Literature. Braz. J. Pharm. Sci. 2022, 58, e20802. [Google Scholar] [CrossRef]
- Dimitrov, M.; Dotcheva, D.; Lambov, N. Preparation and Characterization of Polyethylene Oxide Hydrogels with Cytisine. Acta Pharm. Turc. 2004, 46, 49–54. [Google Scholar]
- Singpanna, K.; Pornpitchanarong, C.; Patrojanasophon, P. Nanocomposite Patches for Transdermal Drug Delivery: A Review. Sci. Eng. Health Stud. 2024, 24010005. [Google Scholar] [CrossRef]
- Despotopoulou, D.; Lagopati, N.; Pispas, S.; Gazouli, M.; Demetzos, C.; Pippa, N. The Technology of Transdermal Delivery Nanosystems: From Design and Development to Preclinical Studies. Int. J. Pharm. 2022, 611, 121290. [Google Scholar] [CrossRef]
- Anitha, P.; Bhargavi, J.; Sravani, G.; Aruna, B.; Ramkanth, S. Recent Progress of Dendrimers in Drug Delivery for Cancer Therapy. Int. J. Appl. Pharm. 2018, 10, 34. [Google Scholar] [CrossRef][Green Version]
- Nikzamir, M.; Hanifehpour, Y.; Akbarzadeh, A.; Panahi, Y. Applications of Dendrimers in Nanomedicine and Drug Delivery: A Review. J. Inorg. Organomet. Polym. Mater. 2021, 31, 2246–2261. [Google Scholar] [CrossRef]
- Pandita, D.; Madaan, K.; Kumar, S.; Poonia, N.; Lather, V. Dendrimers in Drug Delivery and Targeting: Drug-Dendrimer Interactions and Toxicity Issues. J. Pharm. Bioallied Sci. 2014, 6, 139. [Google Scholar] [CrossRef] [PubMed]
- Rout, S.; Bandaru, R.; Kenguva, G.; Hasan, N.; Alam, M.S.; Shukla, R.; Almalki, H.; Kesharwani, P.; Dandela, R. Dendrimers in Photodynamic Therapy. Nanomater. Photodyn. Ther. 2023, 281–305. [Google Scholar] [CrossRef]
- Kirkby, M.; Sabri, A.B.; Scurr, D.J.; Moss, G.P. Dendrimer-Mediated Permeation Enhancement of Chlorhexidine Digluconate: Determination of in Vitro Skin Permeability and Visualisation of Dermal Distribution. Eur. J. Pharm. Biopharm. 2021, 159, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Kokaz, S.F.; Deb, P.K.; Borah, P.; Bania, R.; Venugopala, K.N.; Nair, A.B.; Singh, V.; Al-Shar’i, N.A.; Hourani, W.; Gupta, G.; et al. Dendrimers: Properties and Applications in Biomedical Field. Nanoeng. Biomater. 2021, 215–243. [Google Scholar] [CrossRef]
- Sorroza-Martínez, K.; Ruiu, A.; González-Méndez, I.; Rivera, E. Design and Properties of Dendrimers for Pharmaceutical Applications. Dendrimer-Based Nanother. 2021, 15–31. [Google Scholar] [CrossRef]
- Dangova, M.; Ivanova, N.; Andonova, V. Nanocarriers-Assisted Nose-to-Brain Delivery of Levodopa: Current Progress and Prospects. Appl. Sci. 2025, 15, 331. [Google Scholar] [CrossRef]
- Liang, R.; Wei, M.; Evans, D.G.; Duan, X. Inorganic Nanomaterials for Bioimaging, Targeted Drug Delivery and Therapeutics. ChemInform 2015, 46, 14071–14081. [Google Scholar] [CrossRef]
- Nasirzadeh, K.; Nazarian, S.; Gheibi Hayat, S.M. Inorganic Nanomaterials; A Brief Overview of the Applications and Developments in Sensing and Drug Delivery. J. Appl. Biotechnol. Rep. 2016, 3, 395–402. [Google Scholar]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-Responsive Nanocarriers for Drug Delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef]
- Pham, S.H.; Choi, Y.; Choi, J. Stimuli-Responsive Nanomaterials for Application in Antitumor Therapy and Drug Delivery. Pharmaceutics 2020, 12, 630. [Google Scholar] [CrossRef]
- Kang, M.-S.; Kwon, M.S.; Jang, H.J.; Jeong, S.J.; Han, D.-W.; Kim, K.S. Biosafety of Inorganic Nanomaterials for Theranostic Applications. Emerg. Mater. 2022, 5, 1995–2029. [Google Scholar] [CrossRef]
- Mishra, M.; Prasad, K.; Ramakrishn, S. Nanomaterials in Drug Delivery—Promises and Limitations. Nano Med Mater. 2023, 3, 38. [Google Scholar] [CrossRef]
- Thu, H.E.; Haider, M.A.; Khan, S.; Sohail, M.F.; Hussain, Z. Nanotoxicity Induced by Nanomaterials: A Review of Factors Affecting Nanotoxicity and Possible Adaptations. OpenNano 2023, 14, 100190. [Google Scholar] [CrossRef]
- Ahmad, M.Z.; Abdel-Wahab, B.A.; Alam, A.; Zafar, S.; Ahmad, J.; Ahmad, F.J.; Midoux, P.; Pichon, C.; Akhter, S. Toxicity of Inorganic Nanoparticles Used in Targeted Drug Delivery and Other Biomedical Application: An Updated Account on Concern of Biomedical Nanotoxicology. J. Nanosci. Nanotechnol. 2016, 16, 7873–7897. [Google Scholar] [CrossRef]
- Mahamuni-Badiger, P.P.; Dhanavade, M.J. Challenges and Toxicity Assessment of Inorganic Nanomaterials in Biomedical Applications: Current Status and Future Roadmaps. J. Drug Deliv. Sci. Technol. 2023, 87, 104806. [Google Scholar] [CrossRef]
- Tsikourkitoudi, V.; Henriques-Normark, B.; Sotiriou, G.A. Inorganic Nanoparticle Engineering against Bacterial Infections. Curr. Opin. Chem. Eng. 2022, 38, 100872. [Google Scholar] [CrossRef]
- Pei, Z.; Lei, H.; Cheng, L. Bioactive Inorganic Nanomaterials for Cancer Theranostics. Chem. Soc. Rev. 2023, 52, 2031–2081. [Google Scholar] [CrossRef] [PubMed]
- Pacyga, K.; Pacyga, P.; Szuba, E.; Viscardi, S.; Topola, E.; Duda-Madej, A. Nanotechnology Meets Phytotherapy: A Cutting-Edge Approach to Treat Bacterial Infections. Int. J. Mol. Sci. 2025, 26, 1254. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, N.; Ermenlieva, N.; Simeonova, L.; Kolev, I.; Slavov, I.; Karashanova, D.; Andonova, V. Chlorhexidine–Silver Nanoparticle Conjugation Leading to Antimicrobial Synergism but Enhanced Cytotoxicity. Pharmaceutics 2023, 15, 2298. [Google Scholar] [CrossRef]
- Khan, S.S.; Ullah, I.; Ullah, S.; An, R.; Xu, H.; Nie, K.; Liu, C.; Liu, L. Recent Advances in the Surface Functionalization of Nanomaterials for Antimicrobial Applications. Materials 2021, 14, 6932. [Google Scholar] [CrossRef]
- Rizvi, M.; Gerengi, H.; Gupta, P. Functionalization of Nanomaterials: Synthesis and Characterization. Functionalized Nanomaterials for Corrosion Mitigation: Synthesis, Characterization, and Applications. Am. Chem. Soc. 2022, 1418, 1–26. [Google Scholar] [CrossRef]
- Gusmão, A.; de Albuquerque, P.B.S.; de Correia, A.C. Use of Metallic Nanoparticles Synthesized from Plant Extracts in Wound Healing—A Review. Appl. Nano 2024, 5, 205–226. [Google Scholar] [CrossRef]
- Shanmugasundaram, T.; Shanmugasundaram, T.; Radhakrishnan, M.; Gopikrishnan, V.; Kadirvelu, K.; Balagurunathan, R. In Vitro Antimicrobial and in Vivo Wound Healing Effect of Actinobacterially Synthesised Nanoparticles of Silver, Gold and Their Alloy. RSC Adv. 2017, 7, 51729–51743. [Google Scholar] [CrossRef]
- Jangid, H.; Singh, S.; Kashyap, P.K.; Singh, A. Advancing Biomedical Applications: An in-Depth Analysis of Silver Nanoparticles in Antimicrobial, Anticancer, and Wound Healing Roles. Front. Pharmacol. 2024, 15, 1438227. [Google Scholar] [CrossRef]
- Clichici, S.; Filip, A. In Vivo Assessment of Nanomaterials Toxicity. In Nanomaterials; Soloneski, S., Larramendy, M.L., Eds.; IntechOpen: Rijeka, Croatia, 2015; pp. 93–121. [Google Scholar]
- Dhanalekshmi, K.I.; Sangeetha, K.; Magesan, P.; Johnson, J.; Zhang, X.; Jayamoorthy, K. Photodynamic Cancer Therapy: Role of Ag- and Au-Based Hybrid Nano-Photosensitizers. J. Biomol. Struct. Dyn. 2020, 40, 4766–4773. [Google Scholar] [CrossRef]
- Augustine, R.; Hasan, A.; Primavera, R.; Wilson, R.J.; Thakor, A.S.; Kevadiya, B.D. Cellular Uptake and Retention of Nanoparticles: Insights on Particle Properties and Interaction with Cellular Components. Mater. Today Commun. 2020, 25, 101692. [Google Scholar] [CrossRef]
- Wang, X. The Comparison of Titanium Dioxide and Zinc Oxide Used in Sunscreen Based on Their Enhanced Absorption. Appl. Comput. Eng. 2023, 24, 237–245. [Google Scholar] [CrossRef]
- Wu, H. The Application of Metal Oxide Nanoparticles in Sunscreen and Their Potential Risks. Appl. Comput. Eng. 2023, 24, 210–215. [Google Scholar] [CrossRef]
- Vujovic, M.; Kostic, E. Titanium Dioxide and Zinc Oxide Nanoparticles in Sunscreens: A Review of Toxicological Data. J. Cosmet. Sci. 2019, 70, 223–234. [Google Scholar]
- Popov, A.; Zhao, X.; Zvyagin, A.; Lademann, J.; Roberts, M.; Sanchez, W.; Priezzhev, A.; Myllylä, R. ZnO and TiO2 Particles: A Study on Nanosafety and Photoprotection. Biophotonics Photonic Solut. Better Health Care II 2010, 7715, 77153G. [Google Scholar] [CrossRef]
- Osmond, M.J.; Mccall, M.J. Zinc Oxide Nanoparticles in Modern Sunscreens: An Analysis of Potential Exposure and Hazard. Nanotoxicology 2010, 4, 15–41. [Google Scholar] [CrossRef]
- Gulson, B.; McCall, M.J.; Bowman, D.M.; Pinheiro, T. A Review of Critical Factors for Assessing the Dermal Absorption of Metal Oxide Nanoparticles from Sunscreens Applied to Humans, and a Research Strategy to Address Current Deficiencies. Arch. Toxicol. 2015, 89, 1909–1930. [Google Scholar] [CrossRef]
- Warheit, D.B. Safety of titanium dioxide (E171) as a food additive for humans. Front. Toxicol. 2024, 19, 1333746. [Google Scholar] [CrossRef] [PubMed]
- Goette, D.K. Topical Chemotherapy with 5-Fluorouracil: A Review. J. Am. Acad. Dermatol. 1981, 4, 633–649. [Google Scholar] [CrossRef]
- du Vivier, A. Topical Cytostatic Drugs in the Treatment of Skin Cancer. Clin. Exp. Dermatol. 1982, 7, 89–92. [Google Scholar] [CrossRef]
- Kim, S.; Woo, Y.R.; Cho, S.H.; Lee, J.D.; Kim, H.S. Clinical Efficacy of 5-Fluorouracil and Bleomycin in Dermatology. J. Clin. Med. 2024, 13, 335. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.drugs.com/monograph/fluorouracil-topical.html (accessed on 4 April 2025).
- Bargman, H.; Hochman, J. Topical treatment of Bowen’s disease with 5-Fluorouracil. J. Cutan. Med. Surg. 2003, 7, 101–105. [Google Scholar] [CrossRef]
- Salim, A.; Leman, J.; McColl, J.; Chapman, R.; Morton, C. Randomized comparison of photodynamic therapy with topical 5-fluorouracil in Bowen’s disease. Br. J. Dermatol. 2003, 148, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Ryan, R.; Krementz, E.; Litwin, M. A Role for Topical 5-Fluorouracil Therapy in Melanoma. J. Surg. Oncol. 1988, 38, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Metterle, L.; Nelson, C.; Patel, N. Intralesional 5-fluorouracil (FU) as a treatment for nonmelanoma skin cancer (NMSC): A review. J. Am. Acad. Dermatol. 2016, 74, 552–557. [Google Scholar] [CrossRef]
- Maxfield, L.; Shah, M.; Schwartz, C.; Tanner, L.S.; Appel, J. Intralesional 5-fluorouracil for the treatment of squamous cell carcinomas. J. Am. Acad. Dermatol. 2021, 84, 1696–1697. [Google Scholar] [CrossRef]
- Luu, W.; McRae, M.Y. Intralesional 5-fluorouracil as a management for cutaneous squamous cell carcinomas: A rural Australian retrospective case series. Australas J. Dermatol. 2023, 64, 556–559. [Google Scholar] [CrossRef]
- Morse, L.G.; Kendrick, C.; Hooper, D.; Ward, H.; Parry, E. Treatment of squamous cell carcinoma with intralesional 5-Fluorouracil. Dermatol. Surg. 2003, 29, 1150–1153. [Google Scholar]
- Leonard, A.L.; Hanke, C.W. Treatment of giant keratoacanthoma with intralesional 5-fluorouracil. J. Drugs Dermatol. 2006, 5, 454–456. [Google Scholar]
- Peng, L.; Zhang, X.; Du, B.; Sun, L.-J.; Zhao, Y. Mechanism of 5-Fluorouracil Nanoparticles on Releasing Skin Squamous Cell Carcinoma through Regulation of Wnt/β-Catenin Expression. Mater. Express 2022, 12, 220–226. [Google Scholar] [CrossRef]
- Tian, J.; Tian, J.; Zhang, D.; Kurbatov, V.; Kurbatov, V.; Wang, Q.; Wang, Y.; Fang, D.; Wu, L.; Bosenberg, M.; et al. 5-Fluorouracil Efficacy Requires Anti-Tumor Immunity Triggered by Cancer-Cell-Intrinsic STING. EMBO J. 2021, 40, e106065. [Google Scholar] [CrossRef]
- Dai, X.-L.; Voronin, A.P.; Gao, W.; Perlovich, G.L.; Lu, T.-B.; Chen, J.-M. Intermolecular Interactions and Permeability of 5-Fluorouracil Cocrystals with a Series of Isomeric Hydroxybenzoic Acids: A Combined Theoretical and Experimental Study. CrystEngComm 2019, 21, 5095–5105. [Google Scholar] [CrossRef]
- Nikam, A.N.; Jacob, A.; Raychaudhuri, R.; Fernandes, G.; Pandey, A.; Rao, V.; Ahmad, S.F.; Pannala, A.S.; Mutalik, S. Topical Micro-Emulsion of 5-Fluorouracil by a Twin Screw Processor-Based Novel Continuous Manufacturing Process for the Treatment of Skin Cancer: Preparation and In Vitro and In Vivo Evaluations. Pharmaceutics 2023, 15, 2175. [Google Scholar] [CrossRef]
- Available online: https://pubmed.ncbi.nlm.nih.gov/?term=5-fluorouracil+skin+cancer&filter=datesearch.y_1 (accessed on 4 April 2025).
- Siddalingam, R.; Chidambaram, K. Topical Nano-Delivery of 5-Fluorouracil: Preparation and Characterization of Water-in-Oil Nanoemulsion. Trop. J. Pharm. Res. 2016, 15, 2311–2319. [Google Scholar] [CrossRef]
- Nawaz, A.; Latif, M.S.; Alnuwaiser, M.A.; Ullah, S.; Iqbal, M.; Alfatama, M.; Lim, V. Synthesis and Characterization of Chitosan-Decorated Nanoemulsion Gel of 5-Fluorouracil for Topical Delivery. Gels 2022, 8, 412. [Google Scholar] [CrossRef]
- Crisóstomo, L.C.; Carvalho, G.S.G.; Leal, L.K.A.M.; de Araújo, T.G.; Nogueira, K.A.B.; da Silva, D.A.; Ribeiro, F.d.O.S.; Petrilli, R.; Eloy, J.O. Sorbitan Monolaurate–Containing Liposomes Enhance Skin Cancer Cell Cytotoxicity and in Association with Microneedling Increase the Skin Penetration of 5-Fluorouracil. AAPS Pharmscitech 2022, 23, 212. [Google Scholar] [CrossRef] [PubMed]
- Calienni, M.N.; Prieto, M.J.; Couto, V.M.; de Paula, E.; del Alonso, S.V.; Montanari, J. 5-Fluorouracil-Loaded Ultradeformable Liposomes for Skin Therapy. AIP Conf. Proc. 2018, 1990, 020024. [Google Scholar] [CrossRef]
- Cosco, D.; Paolino, D.; Maiuolo, J.; Marzio, L.D.; Carafa, M.; Ventura, C.A.; Fresta, M. Ultradeformable Liposomes as Multidrug Carrier of Resveratrol and 5-Fluorouracil for Their Topical Delivery. Int. J. Pharm. 2015, 489, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Petrilli, R.; Eloy, J.O.; Saggioro, F.P.; Chesca, D.L.; de Souza, M.C.; Dias, M.V.S.; daSilva, L.L.P.; Lee, R.J.; Lopez, R.F.V. Skin Cancer Treatment Effectiveness Is Improved by Iontophoresis of EGFR-Targeted Liposomes Containing 5-FU Compared with Subcutaneous Injection. J. Control. Release 2018, 283, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Yaman, Ü.; Aslan, M.; Ozturk, S.; Ulubayram, K.; Eroğlu, İ. Surface Modified Nanoliposome Formulations Provide Sustained Release for 5-FU and Increase Cytotoxicity on A431 Cell Line. Pharm. Dev. Technol. 2020, 25, 1192–1203. [Google Scholar] [CrossRef]
- Khalid, W.; Shah, K.U.; Saeed, M.D.; Nawaz, A.; Rehman, F.U.; Shoaib, M.; Rehman, M.U.; Alasmari, A.; Alharbi, M.; Alasmari, F. 5-Fluorouracil-Loaded Hyaluronic Acid-Coated Niosomal Vesicles: Fabrication and Ex Vivo Evaluation for Skin Drug Delivery. ACS Omega 2023, 8, 45405–45413. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.R.; Wong, T.W. 5-Fluorouracil Ethosomes-Skin Deposition and Melanoma Permeation Synergism with Microwave. Artif. Cells Nanomed. Biotechnol. 2018, 46, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Puri, R.; Jain, S. Ethogel Topical Formulation for Increasing the Local Bioavailability of 5-Fluorouracil: A Mechanistic Study. Anti-Cancer Drugs 2012, 23, 923–934. [Google Scholar] [CrossRef]
- Hasan, N.; Imran, M.; Sheikh, A.; Tiwari, N.; Jaimini, A.; Kesharwani, P.; Jain, G.; Ahmad, F.J. Advanced Multifunctional Nano-Lipid Carrier Loaded Gel for Targeted Delivery of 5-Flurouracil and Cannabidiol against Non-Melanoma Skin Cancer. Environ. Res. 2023, 233, 116454. [Google Scholar] [CrossRef]
- Rajinikanth, P.S.; Chellian, J. Development and Evaluation of Nanostructured Lipid Carrier-Based Hydrogel for Topical Delivery of 5-Fluorouracil. Int. J. Nanomed. 2016, 11, 5067–5077. [Google Scholar] [CrossRef]
- Ali, A.; Madni, A.; Shah, H.A.; Jamshaid, T.; Jan, N.; Khan, S.; Khan, M.M.; Mahmood, M.A. Solid Lipid-Based Nanoparticulate System for Sustained Release and Enhanced in-Vitro Cytotoxic Effect of 5-Fluorouracil on Skin Melanoma and Squamous Cell Carcinoma. PLoS ONE 2023, 18, e0281004. [Google Scholar] [CrossRef]
- Khallaf, R.A.; Salem, H.F.; Abdelbary, A. 5-Fluorouracil Shell-Enriched Solid Lipid Nanoparticles (SLN) for Effective Skin Carcinoma Treatment. Drug Deliv. 2016, 23, 3452–3460. [Google Scholar] [CrossRef]
- Shenoy, V.S.; Gude, R.P.; Murthy, R.S.R. In Vitro Anticancer Evaluation of 5-Fluorouracil Lipid Nanoparticles Using B16F10 Melanoma Cell Lines. Int. Nano Lett. 2013, 3, 36. [Google Scholar] [CrossRef]
- Patel, G.; Yadav, B.K.N. Study of 5-Fluorouracil Loaded Chitosan Nanoparticles for Treatment of Skin Cancer. Recent Pat. Nanotechnol. 2020, 14, 210–224. [Google Scholar] [CrossRef] [PubMed]
- Patel, G.; Yadav, B.K.N. Formulation, Characterization and In Vitro Cytotoxicity of 5-Fluorouracil Loaded Polymeric Electrospun Nanofibers for the Treatment of Skin Cancer. Recent Pat. Nanotechnol. 2019, 13, 114–128. [Google Scholar] [CrossRef]
- Skok, K.; Zidarič, T.; Orthaber, K.; Pristovnik, M.; Kostevšek, N.; Žužek Rožman, K.; Šturm, S.; Gradišnik, L.; Maver, U.; Maver, T. Novel Methacrylate-Based Multilayer Nanofilms with Incorporated FePt-Based Nanoparticles and the Anticancer Drug 5-Fluorouracil for Skin Cancer Treatment. Pharmaceutics 2022, 14, 689. [Google Scholar] [CrossRef]
- Safwat, M.A.; Safwat, M.A.; Soliman, G.M.; Soliman, G.M.; Sayed, D.; Attia, M.A. Fluorouracil-Loaded Gold Nanoparticles for the Treatment of Skin Cancer: Development, in Vitro Characterization, and in Vivo Evaluation in a Mouse Skin Cancer Xenograft Model. Mol. Pharm. 2018, 15, 2194–2205. [Google Scholar] [CrossRef]
- Pourmanouchehri, Z.; Ebrahimi, S.; Limoee, M.; Jalilian, F.; Janfaza, S.; Vosoughi, A.; Behbood, L. Controlled Release of 5-Fluorouracil to Melanoma Cells Using a Hydrogel/Micelle Composites Based on Deoxycholic Acid and Carboxymethyl Chitosan. Int. J. Biol. Macromol. 2022, 206, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, O.; Faisal, S.M.; Ahmad, N. Bio-Mediated Synthesis of 5-FU Based Nanoparticles Employing Orange Fruit Juice: A Novel Drug Delivery System to Treat Skin Fibrosarcoma in Model Animals. Sci. Rep. 2019, 9, 12288. [Google Scholar] [CrossRef] [PubMed]
- Shojaei, S.; Doostan, M.; Mohammadi Motlagh, H.; Esnaashari, S.; Maleki, H. Development of 5-Fluorouracil/Etoposide Co-Loaded Electrospun Nanofibrous Scaffold for Localized Anti-Melanoma Therapy. J. Appl. Biomater. Funct. Mater. 2024, 22, 22808000241284439. [Google Scholar] [CrossRef]
- Nanda, J.; Bermudez, R. Imiquimod. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Garcia-Mouronte, E.; Berná-Rico, E.; de Nicolas-Ruanes, B.; Azcarraga-Llobet, C.; Bea-Ardebol, S. Imiquimod as Local Immunotherapy in the Management of Premalignant Cutaneous Conditions and Skin Cancer. Int. J. Mol. Sci. 2023, 24, 10835. [Google Scholar] [CrossRef] [PubMed]
- Wolff, F.; Loipetzberger, A.; Gruber, W.; Esterbauer, H.; Aberger, F.; Frischauf, A. Imiquimod Directly Inhibits Hedgehog Signalling by Stimulating Adenosine Receptor/Protein Kinase A-Mediated GLI Phosphorylation. Oncogene 2013, 32, 5574–5581. [Google Scholar] [CrossRef]
- Vidal, D.; Matias-Guiu, X.; Alomar, A. Open Study of the Efficacy and Mechanism of Action of Topical Imiquimod in Basal Cell Carcinoma. Clin. Exp. Dermatol. 2004, 29, 518–525. [Google Scholar] [CrossRef]
- Yokogawa, M.; Takaishi, M.; Nakajima, K.; Kamijima, R.; DiGiovanni, J.; Sano, S. Imiquimod Attenuates the Growth of UVB-Induced SCC in Mice through Th1/Th17 Cells. Mol. Carcinog. 2013, 52, 760–769. [Google Scholar] [CrossRef]
- Fan, Q.; Cohen, S.; John, B.; Riker, A. Melanoma in Situ Treated with Topical Imiquimod for Management of Persistently Positive Margins: A Review of Treatment Methods. Ochsner J. 2015, 15, 443–447. [Google Scholar]
- Ray, C.; Kluk, M.; Grin, C.; Grant-Kels, J. Successful Treatment of Malignant Melanoma in Situ with Topical 5% Imiquimod Cream. Int. J. Dermatol. 2005, 44, 428–434. [Google Scholar] [CrossRef]
- Patel, G.; Goodwin, R.; Chawla, M.; Laidler, P.; Price, P.; Finlay, A.; Motley, R. Imiquimod 5% Cream Monotherapy for Cutaneous Squamous Cell Carcinoma in Situ (Bowen’s Disease): A Randomized, Double-Blind, Placebo-Controlled Trial. J. Am. Acad. Dermatol. 2006, 54, 1025–1032. [Google Scholar] [CrossRef]
- Casadei, B.R.; Lotierzo, M.C.G.; Malheiros, B.; Barbosa, L.R.S. Drug Repurposing and Nanoparticles: New Strategies against Leishmaniasis. In Applications of Nanobiotechnology for Neglected Tropical Diseases; Academic Press: Cambridge, MA, USA, 2021; pp. 217–241. [Google Scholar]
- Berman, B. Safety, Efficacy, and Patient Acceptability of Imiquimod for Topical Treatment of Actinic Keratoses. Clin. Cosmet. Investig. Dermatol. 2011, 4, 35–40. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sorgi, D.; Sartori, A.; Germani, S.; Gentile, R.N.; Bianchera, A.; Bettini, R. Imiquimod Solubility in Different Solvents: An Interpretative Approach. Pharmaceutics 2024, 16, 282. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Abdella, S.; Abid, F.; Afinjuomo, F.; Youssef, S.; Holmes, A.; Song, Y.; Vaidya, S.; Garg, S. Development and Optimization of Imiquimod-Loaded Nanostructured Lipid Carriers Using a Hybrid Design of Experiments Approach. Int. J. Nanomed. 2023, 18, 1007–1029. [Google Scholar] [CrossRef] [PubMed]
- Al-Mayahy, M.H.; Marlow, M.; Scurr, D.J. The Complementary Role of ToF-SIMS in the Assessment of Imiquimod Permeated into the Skin from a Microemulsion Dosage Form. Al Mustansiriyah J. Pharm. Sci. 2019, 19, 196–210. [Google Scholar] [CrossRef]
- Ghezzi, M.; Pescina, S.; Delledonne, A.; Ferraboschi, I.; Sissa, C.; Terenziani, F.; Remiro, P.D.F.R.; Santi, P.; Nicoli, S. Improvement of Imiquimod Solubilization and Skin Retention via TPGS Micelles: Exploiting the Co-Solubilizing Effect of Oleic Acid. Pharmaceutics 2021, 13, 1476. [Google Scholar] [CrossRef]
- De Paula, D.; Martins, C.A.; Bentley, M.V.L.B. Development and Validation of HPLC Method for Imiquimod Determination in Skin Penetration Studies. Biomed. Chromatogr. 2008, 22, 1416–1423. [Google Scholar] [CrossRef]
- Layek, B.; Rahman Nirzhor, S.S.; Rathi, S.; Kandimalla, K.K.; Wiedmann, T.S.; Prabha, S. Design, Development, and Characterization of Imiquimod-Loaded Chitosan Films for Topical Delivery. AAPS PharmSciTech 2019, 20, 58. [Google Scholar] [CrossRef]
- Panoutsopoulou, E.; Zbytovská, J.; Vávrová, K.; Paraskevopoulos, G. Phospholipid-Based Microemulsions for Cutaneous Imiquimod Delivery. Pharmaceuticals 2022, 15, 515. [Google Scholar] [CrossRef]
- Telò, I.; Del Favero, E.; Cantù, L.; Frattini, N.; Pescina, S.; Padula, C.; Santi, P.; Sonvico, F.; Nicoli, S. Gel-like TPGS-Based Microemulsions for Imiquimod Dermal Delivery: Role of Mesostructure on the Uptake and Distribution into the Skin. Mol. Pharm. 2017, 14, 3281–3289. [Google Scholar] [CrossRef]
- Jadhav, S.T.; Salunkhe, V.R.; Bhinge, S.D. Nanoemulsion Drug Delivery System Loaded with Imiquimod: A QbD-Based Strategy for Augmenting Anti-Cancer Effects. Future J. Pharm. Sci. 2023, 9, 120. [Google Scholar] [CrossRef]
- Petrová, E.; Chvíla, S.; Balouch, M.; Štěpánek, F.; Zbytovská, J. Nanoformulations for Dermal Delivery of Imiquimod: The Race of “Soft” against “Hard”. Int. J. Pharm. 2023, 648, 123577. [Google Scholar] [CrossRef] [PubMed]
- Caimi, A.; Ramirez, C.; Perez, A.; Romero, E.; Morilla, M. In Vitro Anti-Melanoma Activity of Imiquimod in Ultradeformable Nanovesicles. Drug Dev. Ind. Pharm. 2022, 48, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Tampucci, S.; Guazzelli, L.; Burgalassi, S.; Carpi, S.; Carpi, S.; Chetoni, P.; Mezzetta, A.; Nieri, P.; Polini, B.; Pomelli, C.S.; et al. pH-Responsive Nanostructures Based on Surface Active Fatty Acid-Protic Ionic Liquids for Imiquimod Delivery in Skin Cancer Topical Therapy. Pharmaceutics 2020, 12, 1078. [Google Scholar] [CrossRef]
- Ma, M.; Wang, J.; Guo, F.; Lei, M.; Tan, F.; Li, N. Development of Nanovesicular Systems for Dermal Imiquimod Delivery: Physicochemical Characterization and in Vitro/in Vivo Evaluation. J. Mater. Sci. Mater. Med. 2015, 26, 192. [Google Scholar] [CrossRef]
- Carreño, G.F.; Alvarez-Figueroa, M.J.; González-Aramundiz, J.V. Dextran Nanocapsules with ω-3 in Their Nucleus: An Innovative Nanosystem for Imiquimod Transdermal Delivery. Pharmaceutics 2022, 14, 2445. [Google Scholar] [CrossRef]
- Argenziano, M.; Haimhoffer, Á.; Bastiancich, C.; Jicsinszky, L.; Caldera, F.; Trotta, F.; Scutera, S.; Alotto, D.; Fumagalli, M.; Musso, T.; et al. In Vitro Enhanced Skin Permeation and Retention of Imiquimod Loaded in β-Cyclodextrin Nanosponge Hydrogel. Pharmaceutics 2019, 11, 138. [Google Scholar] [CrossRef]
- Alvarez-Figueroa, M.J.; Narváez-Araya, D.; Armijo-Escalona, N.; Carrasco-Flores, E.A.; González-Aramundiz, J.V. Design of Chitosan Nanocapsules with Compritol 888 ATO® for Imiquimod Transdermal Administration. Evaluation of Their Skin Absorption by Raman Microscopy. Pharm. Res. 2020, 37, 195. [Google Scholar] [CrossRef] [PubMed]
- Dao, D.-P.D.; Sahni, V.N.; Sahni, D.R.; Balogh, E.A.; Grada, A.; Feldman, S.R. 1% Tirbanibulin Ointment for the Treatment of Actinic Keratoses. Ann. Pharmacother. 2021, 56, 494–500. [Google Scholar] [CrossRef]
- Schlesinger, T.E.; Stockfleth, E.; Grada, A.; Berman, B. Tirbanibulin for Actinic Keratosis: Insights into the Mechanism of Action. Clin. Cosmet. Investig. Dermatol. 2022, 15, 2495–2506. [Google Scholar] [CrossRef]
- Sajkiewicz, I.; Miga-Orczykowska, N.; Lemieszek, P.; Jasiuk, I.; Pustelniak, M.; Wójtowicz, J.; Krukar, K.; Rudnicka, K.; Łukaszewska, E.; Kister, K. Tirbanibulin as a Novel Treatment in Actinic Keratosis: A Literature Review. J. Educ. Health Sport. 2024, 70, 55328. [Google Scholar] [CrossRef]
- Atzeni, F.; Masala, I.; Sarzi-Puttini, P. A Review of Chronic Musculoskeletal Pain: Central and Peripheral Effects of Diclofenac. Pain Ther. 2018, 7, 163–177. [Google Scholar] [CrossRef]
- Müller-Decker, K. Cyclooxygenase-Dependent Signaling Is Causally Linked to Non-Melanoma Skin Carcinogenesis: Pharmacological, Genetic, and Clinical Evidence. Cancer Metastasis Rev. 2011, 30, 343–361. [Google Scholar] [CrossRef]
- Zhan, H.; Zheng, H. The Role of Topical Cyclo-Oxygenase-2 Inhibitors in Skin Cancer: Treatment and Prevention. Am. J. Clin. Dermatol. 2007, 8, 195–200. [Google Scholar] [CrossRef]
- Rodust, P.M.; Fecker, L.F.; Stockfleth, E.; Eberle, J. Activation of Mitochondrial Apoptosis Pathways in Cutaneous Squamous Cell Carcinoma Cells by Diclofenac/Hyaluronic Acid Is Related to Upregulation of Bad as Well as Downregulation of Mcl-1 and Bcl-w. Exp. Dermatol. 2012, 21, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Brinkhuizen, T.; Frencken, K.J.A.; Nelemans, P.J.; Hoff, M.L.S.; Kelleners-Smeets, N.W.J.; zur Hausen, A.; van der Horst, M.P.J.; Rennspiess, D.; Winnepenninckx, V.; van Steensel, M.A.M.; et al. The Effect of Topical Diclofenac 3% and Calcitriol 3 Μg/g on Superficial Basal Cell Carcinoma (sBCC) and Nodular Basal Cell Carcinoma (nBCC): A Phase II, Randomized Controlled Trial. J. Am. Acad. Dermatol. 2016, 75, 126–134. [Google Scholar] [CrossRef]
- Jarvis, B.; Figgitt, D. Topical 3% Diclofenac in 2.5% Hyaluronic Acid Gel: A Review of Its Use in Patients with Actinic Keratoses. Am. J. Clin. Dermatol. 2003, 4, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Pommergaard, H.-C.; Burcharth, J.; Rosenberg, J.; Raskov, H. Topical Combination of Diclofenac, Calcipotriol, and Difluoromethylornithine Has Beneficial Effects Comparable to 5-Fluorouracil for the Treatment of Non-Melanoma Skin Cancer in Mice. J. Chemother. 2014, 26, 105–110. [Google Scholar] [CrossRef]
- El-Khalawany, M.; Saudi, W.M.; Ahmed, E.Z.; Mosbeh, A.-S.; Sameh, A.; Rageh, M.A. The Combined Effect of CO2 Laser, Topical Diclofenac 3%, and Imiquimod 5% in Treating High-Risk Basal Cell Carcinoma. J. Cosmet. Dermatol. 2021, 21, 2049–2055. [Google Scholar] [CrossRef] [PubMed]
- Lucena, S.R.; Salazar, N.; Gracia-Cazaña, T.; Zamarrón, A.; González, S.; González, S.; Juarranz, A.; Gilaberte, Y. Combined Treatments with Photodynamic Therapy for Non-Melanoma Skin Cancer. Int. J. Mol. Sci. 2015, 16, 25912–25933. [Google Scholar] [CrossRef]
- Tampucci, S.; Carpi, S.; Digiacomo, M.; Polini, B.; Fogli, S.; Burgalassi, S.; Macchia, M.; Nieri, P.; Manera, C.; Monti, D. Diclofenac-Derived Hybrids for Treatment of Actinic Keratosis and Squamous Cell Carcinoma. Molecules 2019, 24, 1793. [Google Scholar] [CrossRef]
- Fidler, B.; Goldberg, T. Ingenol Mebutate Gel (Picato): A Novel Agent for the Treatment of Actinic Keratoses. Pharm. Ther. 2014, 39, 40–46. [Google Scholar]
- Gras, J. Ingenol Mebutate: A New Option for Actinic Keratosis Treatment. Drugs Today 2013, 49, 15. [Google Scholar] [CrossRef]
- Freiberger, S.N.; Cheng, P.F.; Iotzova-Weiss, G.; Neu, J.; Liu, Q.; Dziunycz, P.; Zibert, J.R.; Dummer, R.; Skak, K.; Levesque, M.P.; et al. Data from Ingenol Mebutate Signals via PKC/MEK/ERK in Keratinocytes and Induces Interleukin Decoy Receptors IL1R2 and IL13RA2. Mol. Cancer Ther. 2023, 14, 2132. [Google Scholar] [CrossRef] [PubMed]
- Challacombe, J.; Suhrbier, A.; Parsons, P. Neutrophils Are a Key Component of the Antitumor Efficacy of Topical Chemotherapy with Ingenol-3-Angelate. J. Immunol. 2006, 177, 8123–8132. [Google Scholar] [CrossRef]
- Velin, M.; Cardot-Leccia, N.; Cathelineau, A.C.; Duteil, L.; Queille-Roussel, C.; Passeron, T.; Bahadoran, P. Efficacy and Safety of 0.05% Ingenol Mebutate in the Treatment of Basal Cell Carcinoma: A Prospective Study. Ski. Health Dis. 2022, 3, e150. [Google Scholar] [CrossRef] [PubMed]
- Banzhaf, C.A.; Phothong, W.; Suku, M.H.; Ulrich, M.; Philipsen, P.A.; Mogensen, M.; Haedersdal, M. Basal Cell Carcinoma Treated with Combined Ablative Fractional Laser and Ingenol Mebutate—An Exploratory Study Monitored by Optical Coherence Tomography and Reflectance Confocal Microscopy. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Tan, I.J.; Pathak, G.; Silver, F.H. Topical Treatments for Basal Cell Carcinoma and Actinic Keratosis in the United States. Cancers 2023, 15, 3927. [Google Scholar] [CrossRef]
- An, J.H.; Shin, J.U.; Kim, H.J.; Lee, H.J.; Yoon, M.S.; Kim, D.-H. Comparison of the Treatment Outcomes of Photodynamic Therapy and Ingenol Mebutate in Bowen’s Disease: A Retrospective Observational Study. Ann. Dermatol. 2020, 32, 47–52. [Google Scholar] [CrossRef]
- Gupta, A.K.; Paquet, M. Mebutate: A Promising Treatment for Actinic Keratoses and Nonmelanoma Skin Cancers. J. Cutan. Med. Surgery 2013, 17, 173–179. [Google Scholar] [CrossRef]
- Alkhalaf, A.; Hofbauer, G.F.L. Ingenol Mebutate 150 Mg as Physician-Directed Treatment of Bowen’s Disease Under Occlusion. Dermatology 2016, 232, 17–19. [Google Scholar] [CrossRef] [PubMed]
- Jedlowski, P. Ingenol Mebutate Is Associated With Increased Reporting Odds for Squamous Cell Carcinoma in Actinic Keratosis Patients, a Pharmacovigilance Study of the FDA Adverse Event Reporting System (FAERS). J. Cutan. Med. Surg. 2022, 27, 39–43. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Lipner, S.R.; Lipner, S.R. Retrospective Analysis of Squamous Cell Carcinoma Associated with Ingenol Mebutate Reported to the US Food and Drug Administration. Dermatol. Ther. 2020, 33, e14114. [Google Scholar] [CrossRef]
- Ramsay, J.; Suhrbier, A.; Aylward, J.; Ogbourne, S.; Cozzi, S.; Poulsen, M.; Baumann, K.; Welburn, P.; Redlich, G.; Parsons, P. The Sap from Euphorbia Peplus Is Effective against Human Nonmelanoma Skin Cancers. Br. J. Dermatol. 2011, 164, 633–636. [Google Scholar] [CrossRef]
- Jørgensen, L.; McKerrall, S.J.; Kuttruff, C.A.; Ungeheuer, F.; Felding, J.; Baran, P.S. 14-Step Synthesis of (+)-Ingenol from (+)-3-Carene. Science 2013, 341, 878–882. [Google Scholar] [CrossRef]
- Tang, H.; Yusuff, N.; Wood, J.L. Progress Toward the Total Synthesis of Ingenol: Construction of the Complete Carbocyclic Skeleton. ChemInform 2001, 32. [Google Scholar] [CrossRef]
- Liang, X.; Grue-Sørensen, G.; Månsson, K.; Vedsø, P.; Soor, A.; Stahlhut, M.; Bertelsen, M.; Engell, K.M.; Högberg, T. Syntheses, Biological Evaluation and SAR of Ingenol Mebutate Analogues for Treatment of Actinic Keratosis and Non-Melanoma Skin Cancer. Bioorganic Med. Chem. Lett. 2013, 23, 5624–5629. [Google Scholar] [CrossRef]
- Bertelsen, M.; Stahlhut, M.; Grue-Sørensen, G.; Liang, X.; Christensen, G.B.; Skak, K.; Engell, K.M.; Högberg, T. Ingenol Disoxate: A Novel 4-Isoxazolecarboxylate Ester of Ingenol with Improved Properties for Treatment of Actinic Keratosis and Other Non-Melanoma Skin Cancers. Dermatol. Ther. 2016, 6, 599–626. [Google Scholar] [CrossRef] [PubMed]
- Trémezaygues, L.; Reichrath, J. Vitamin D Analogs in the Treatment of Psoriasis: Where Are We Standing and Where Will We Be Going? Dermatoendocrinology 2011, 3, 180–186. [Google Scholar] [CrossRef]
- Chakraborty, D.; Aggarwal, K. Comparative Evaluation of Efficacy and Safety of Calcipotriol versus Calcitriol Ointment, Both in Combination with Narrow-Band Ultraviolet B Phototherapy in the Treatment of Stable Plaque Psoriasis. Photodermatol. Photoimmunol. Photomed. 2023, 39, 512–519. [Google Scholar] [CrossRef]
- Rosenberg, A.; Tabacchi, M.; Ngo, K. Skin Cancer Precursor Immunotherapy for Squamous Cell Carcinoma Prevention. JCI Insight 2019, 4, e125476. [Google Scholar] [CrossRef]
- Azin, M.; Mahon, A.; Isaacman, S. Topical Calcipotriol plus 5-Fluorouracil Immunotherapy for Actinic Keratosis Treatment. JID Innov. 2022, 2, 100104. [Google Scholar] [CrossRef]
- Cunningham, T.; Tabacchi, M.; Eliane, J. Randomized Trial of Calcipotriol Combined with 5-Fluorouracil for Skin Cancer Precursor Immunotherapy. J. Clin. Investig. 2017, 127, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.; Nguyen, M.; Moore, S. Cyclic Calcipotriene 0.005% Foam and 1% 5-Fluorouracil Cream after Cryotherapy in Treatment of Hyperkeratotic Actinic Keratosis: A Retrospective Study. J. Am. Acad. Dermatol. 2021, 84, 1148–1150. [Google Scholar] [CrossRef]
- Dlott, A.; Spencer, S.; Di Pasqua, A. Calcipotriol and 5-Fluorouracil Combination Therapy for the Treatment of Actinic Keratosis in the Clinic: A Review Article. Clin. Drug Investig. 2024, 44, 733–737. [Google Scholar] [CrossRef] [PubMed]
- Sonego, B.; Zelin, E.; Bonin, S.; Pozzebon, T.; Bazzacco, G.; Corio, A.; Agozzino, M.; Caro Caposieno, D.R.; Zalaudek, I.; Di Meo, N. Calcipotriol as a Daylight Photodynamic Therapy Enhancer: A Case-Control Study. Dermatol. Rep. 2024, 17, 10077. [Google Scholar] [CrossRef]
- Grant, W.B. Roles of Solar UVB and Vitamin D in Reducing Cancer Risk and Increasing Survival. Anticancer Res. 2016, 36, 1357–1370. [Google Scholar] [PubMed]
- Feldman, D.; Krishnan, A.V.; Swami, S.; Giovannucci, E.; Feldman, B.J. The role of vitamin D in reducing cancer risk and progression. Nat. Rev. Cancer 2014, 14, 342–357. [Google Scholar] [CrossRef]
- Ordonez-Moran, P.; Larriba, M.J.; Pendas-Franco, N.; Aguilera, O.; Gonzalez-Sancho, J.M.; Munoz, A. Vitamin D and cancer: An update of in vitro and in vivo data. Front. Biosci. 2005, 10, 2723–2749. [Google Scholar] [CrossRef]
- Slominski, A.T.; Brożyna, A.A.; Skobowiat, C.; Zmijewski, M.A.; Kim, T.K.; Janjetovic, Z.; Oak, A.S.; Jozwicki, W.; Jetten, A.M.; Mason, R.S.; et al. On the role of classical and novel forms of vitamin D in melanoma progression and management. J. Steroid Biochem. Mol. Biol. 2018, 177, 159–170. [Google Scholar] [CrossRef]
- Podgorska, E.; Drzal, A.; Matuszak, Z.; Swakon, J.; Slominski, A.; Elas, M.; Urbanska, K. Calcitriol and Calcidiol Can Sensitize Melanoma Cells to Low–LET Proton Beam Irradiation. Int. J. Mol. Sci. 2018, 19, 2236. [Google Scholar] [CrossRef]
- Tatiya, A.; Patil, J.; Girase, T.; Patil, M.; Patel, K. An Overview on Management of Psoriasis Using Calcipotriene and Its Amalgamation as Nano Based Drug Delivery System. Mater. Proc. 2023, 14, 38. [Google Scholar] [CrossRef]
- Parveen, S.; Sartaj, A.; Iqubal, K.; Kumari, P.; Dang, S.; Aldosari, E.; Ali, J. Topical Co-Delivery of Tacrolimus and Calcipotriol Loaded Nanostructured Lipid Carrier: A Potential and Synergistic Approach in the Management of Psoriasis. J. Dispers. Sci. Technol. 2024, 46, 1301–1314. [Google Scholar] [CrossRef]
- Pradhan, M.; Alexander, A.; Singh, M.R.; Singh, D.; Saraf, S.; Saraf, S.; Yadav, K. Statistically Optimized Calcipotriol Fused Nanostructured Lipid Carriers for Effectual Topical Treatment of Psoriasis. J. Drug Deliv. Sci. Technol. 2021, 61, 102168. [Google Scholar] [CrossRef]
- Baldwin, H.; Webster, G.; Stein Gold, L.; Callender, V.; Cook-Bolden, F.E.; Guenin, E. 50 Years of Topical Retinoids for Acne: Evolution of Treatment. Am. J. Clin. Dermatol. 2021, 22, 315–327. [Google Scholar] [CrossRef]
- Ramchatesingh, B.; Martínez Villarreal, A.; Arcuri, D.; Lagacé, F.; Setah, S.A.; Touma, F.; Al-Badarin, F.; Litvinov, I.V. The Use of Retinoids for the Prevention and Treatment of Skin Cancers: An Updated Review. Int. J. Mol. Sci. 2022, 23, 12622. [Google Scholar] [CrossRef]
- Dogra, S.; Yadav, S. Acitretin in Psoriasis: An Evolving Scenario. Int. J. Dermatol. 2014, 53, 525–538. [Google Scholar] [CrossRef] [PubMed]
- Carr, D.R.; Trevino, J.J.; Donnelly, H.B. Retinoids for Chemoprophylaxis of Nonmelanoma Skin Cancer. Dermatol. Surg. 2011, 37, 129–145. [Google Scholar] [CrossRef]
- Weinstock, M.A.; Bingham, S.F.; Digiovanna, J.J.; Rizzo, A.E.; Marcolivio, K.; Hall, R.; Eilers, D.; Naylor, M.; Kirsner, R.; Kalivas, J.; et al. Tretinoin and the prevention of keratinocyte carcinoma (Basal and squamous cell carcinoma of the skin): A veterans affairs randomized chemoprevention trial. J. Investig. Dermatol. 2012, 132, 1583–1590. [Google Scholar] [CrossRef]
- Peck, G.L. Topical tretinoin in actinic keratosis and basal cell carcinoma. J. Am. Acad. Dermatol. 1986, 15, 829–835. [Google Scholar] [CrossRef]
- Sami, N.; Feld, S.d.l.; Wolverton, S.E. 46-Topical retinoids. In Comprehensive Dermatologic Drug Therapy, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 528–540.e4. [Google Scholar]
- Kang, S.; Goldfarb, M.T.; Weiss, J.S.; Metz, R.D.; Hamilton, T.A.; Voorhees, J.J.; Griffiths, C.E. Assessment of adapalene gel for the treatment of actinic keratoses and lentigines: A randomized trial. J. Am. Acad. Dermatol. 2003, 49, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Anaya-Ruiz, M.; Pérez-Santos, M. Drug Repurposing of Adapalene for Melanoma Treatment. Pharm. Pat. Anal. 2022, 11, 9–14. [Google Scholar] [CrossRef]
- Rusu, A.; Tanase, C.; Pascu, G.-A.; Todoran, N. Recent Advances Regarding the Therapeutic Potential of Adapalene. Pharmaceuticals 2020, 13, 217. [Google Scholar] [CrossRef]
- Wu, C.-S.; Chen, G.-S.; Lin, P.-Y.; Pan, I.-H.; Wang, S.-T.; Lin, S.H.; Yu, H.-S.; Lin, C.-C. Tazarotene Induces Apoptosis in Human Basal Cell Carcinoma via Activation of Caspase-8/t-Bid and the Reactive Oxygen Species-Dependent Mitochondrial Pathway. DNA Cell Biol. 2014, 33, 652–666. [Google Scholar] [CrossRef]
- Bianchi, L.; Orlandi, A.; Campione, E.; Angeloni, C.; Costanzo, A.; Spagnoli, L.G.; Chimenti, S. Topical Treatment of Basal Cell Carcinoma with Tazarotene: A Clinicopathological Study on a Large Series of Cases. Br. J. Dermatol. 2004, 151, 148–156. [Google Scholar] [CrossRef]
- Tang, J.Y.; Chiou, A.S.; Mackay-Wiggan, J.; Aszterbaum, M.; Chanana, A.M.; Lee, W.; Lindgren, J.; Acosta Raphael, M.; Thompson, B.J.; Bickers, D.R.; et al. Tazarotene: Randomized, Double-Blind, Vehicle-Controlled and Open-Label Concurrent Trials for Basal Cell Carcinoma Prevention and Therapy in Patients with Basal Cell Nevus Syndrome. Cancer Prev. Res. 2014, 7, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Cosio, T.; Di Prete, M.; Gaziano, R.; Lanna, C.; Orlandi, A.; Di Francesco, P.; Bianchi, L.; Campione, E. Trifarotene: A Current Review and Perspectives in Dermatology. Biomedicines 2021, 9, 237. [Google Scholar] [CrossRef]
- Mahajan, A.; Singh, L.; Singh, G.; Dhawan, R.; Kaur, M.; Malhi, P.; Thakur, K.; Kaur, L. An Evidence-Based Review on Bexarotene. Tumor Discov. 2023, 2, 0436. [Google Scholar] [CrossRef]
- Bubna, A. Alitretinoin in Dermatology-An Update. Indian J. Dermatol. 2015, 60, 520. [Google Scholar] [CrossRef] [PubMed]
- Sallam, M.A.; Prakash, S.; Krishnan, V.; Todorova, K.; Mandinova, A.; Mitragotri, S. Hyaluronic Acid Conjugates of Vorinostat and Bexarotene for Treatment of Cutaneous Malignancies. Adv. Ther. 2020, 3. [Google Scholar] [CrossRef]
- Warda, A.; Staniszewski, L.J.P.; Sabir, Z.L.; Livingston, S.; Sausedo, M.A.; Reshi, S.M.; Ron, E.; Applegate, M.T.; Haddad, D.; Khamisi, M.; et al. Development of Bexarotene Analogs for Treating Cutaneous T-Cell Lymphomas. Cells 2023, 12, 2575. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.; Date, A.; Joshi, M.; Patravale, V. Solid Lipid Nanoparticles (SLN) of Tretinoin: Potential in Topical Delivery. Int. J. Pharm. 2007, 345, 163–171. [Google Scholar] [CrossRef]
- Dragicevic, N.; Maibach, H. Combined Use of Nanocarriers and Physical Methods for Percutaneous Penetration Enhancement. Adv. Drug Deliv. Rev. 2018, 127, 58–84. [Google Scholar] [CrossRef] [PubMed]
- Hafeez, A.; Kazmi, I. Dacarbazine Nanoparticle Topical Delivery System for the Treatment of Melanoma. Sci. Rep. 2017, 7, 16517. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Sharma, S. Dacarbazine-Encapsulated Solid Lipid Nanoparticles for Skin Cancer: Physical Characterization, Stability, in-Vivo Activity, Histopathology, and Immunohistochemistry. Front. Oncol. 2023, 13, 1102269. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, C.O.; Silva-Carvalho, A.É.; de Mota, I.S.; Lopez, R.F.V.; Cunha-Filho, M.; Saldanha-Araújo, F.; Gratieri, T.; Gelfuso, G.M. Effect of Iontophoresis on Dacarbazine Cutaneous Delivery for Melanoma Topical Treatment. Int. J. Pharm. 2024, 665, 124730. [Google Scholar] [CrossRef]
- Vankudre, S.; Shirkoli, N.; Hawaldar, R. Enhanced Delivery of Dacarbazine Using Nanosponge Loaded Hydrogel for Targeted Melanoma Treatment: Formulation, Statistical Optimization and Pre-Clinical Evaluation. J. Pharm. Innov. 2025, 20, 27. [Google Scholar] [CrossRef]
- Li, C.; Han, X. Co-Delivery of Dacarbazine and All-Trans Retinoic Acid (ATRA) Using Lipid Nanoformulations for Synergistic Antitumor Efficacy Against Malignant Melanoma. Nanoscale Res. Lett. 2020, 15, 113. [Google Scholar] [CrossRef]
- Lei, M.; Wang, J.; Ma, M.; Yu, M.; Tan, F.; Li, N. Dual Drug Encapsulation in a Novel Nano-Vesicular Carrier for the Treatment of Cutaneous Melanoma: Characterization and in Vitro/in Vivo Evaluation. RSC Adv. 2015, 5, 20467–20478. [Google Scholar] [CrossRef]
- Yang, X.; Chang, L.; Lin, Q.; Wang, J.; Su, P.; Chen, X.; Yi, Z.; Dong, Y.; Luo, J. Microneedle Pretreatment and Cationic Nanoparticles for Transdermal Delivery of Doxorubicin against Melanoma. J. Drug Deliv. Sci. Technol. 2024, 93, 105417. [Google Scholar] [CrossRef]
- Slavkova, M.; Dimitrova, D.; Voycheva, C.; Popova, T.; Spassova, I.; Kovacheva, D.; Yordanov, Y.; Tzankova, V.; Tzankov, B. Composite Hydrogel with Oleic Acid-Grafted Mesoporous Silica Nanoparticles for Enhanced Topical Delivery of Doxorubicin. Gels 2024, 10, 356. [Google Scholar] [CrossRef]
- de A Huber, L.; Pereira, T.A.; Ramos, D.N.; Dias de Rezende, L.C.; Emery, F.d.S.; Sobral, L.M.; Leopoldino, A.M.; Lopez, R.F.V. Topical Skin Cancer Therapy Using Doxorubicin-Loaded Cationic Lipid Nanoparticles and Lontophoresis. J. Biomed. Nanotechnol. 2015, 11, 1975–1988. [Google Scholar] [CrossRef]
- Guo, M.; Zhou, G.; Liu, Z.; Liu, J.; Tang, J.; Xiao, Y.; Xu, W.; Liu, Y.; Chen, C. Direct Site-Specific Treatment of Skin Cancer Using Doxorubicin-Loaded Nanofibrous Membranes. Chin. Sci. Bull. 2017, 63, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Yasmeen, I.; Khan, M.A.; Ali, J.; Baboota, S.; Sartaj, A. Topical Delivery of Mannose Conjugated-Doxorubicin-Berberine Nanostructured Lipid Carrier Gel for Skin Cancer Amelioration: Formulation Optimization, in-Silico, in-Vitro, Ex-Vivo Assessment, and Dermatokinetic Analysis. J. Drug Deliv. Sci. Technol. 2024, 93, 105378. [Google Scholar] [CrossRef]
- Ahmed, K.S.; Shan, X.; Mao, J.; Qiu, L.; Chen, J. Derma Roller® Microneedles-Mediated Transdermal Delivery of Doxorubicin and Celecoxib Co-Loaded Liposomes for Enhancing the Anticancer Effect. Mater. Sci. Eng. C 2019, 99, 1448–1458. [Google Scholar] [CrossRef]
- Ferrari, G.; Pang, L.Y.; De Moliner, F.; Vendrell, M.; Reardon, R.; Higgins, A.J.; Chopra, S.; Argyle, D. Effective Penetration of a Liposomal Formulation of Bleomycin through Ex-Vivo Skin Explants from Two Different Species. Cancers 2022, 14, 1083. [Google Scholar] [CrossRef]
- Hamada, H.; Uesugi, D.; Ishihara, K.; Hosoda, R.; Shimoda, K.; Kuboki, A.; Uchida, N.; Kiriake, Y. Transdermal Delivery of Paclitaxel-Anionic Nanoparticles to Epidermis Layer, Pterostilbene, and Pterostilbene Glycoside, and Their Application for Treatment of Skin Cancer and Wrinkle. Int. J. Curr. Microbiol. Appl. Sci. 2024, 13, 1–7. [Google Scholar] [CrossRef]
- Tolentino, S.; Monteiro, M.M.; Saldanha-Araújo, F.; Cunha-Filho, M.; Gratieri, T.; Guerra, E.N.S.; Gelfuso, G.M. Bioadhesive Chitosan Films Loading Curcumin for Safe and Effective Skin Cancer Topical Treatment. Pharmaceutics 2025, 17, 18. [Google Scholar] [CrossRef]
- Jose, A.; Labala, S.; Ninave, K.M.; Gade, S.K.; Venuganti, V.V.K. Effective Skin Cancer Treatment by Topical Co-Delivery of Curcumin and STAT3 siRNA Using Cationic Liposomes. AAPS Pharmscitech 2018, 19, 166–175. [Google Scholar] [CrossRef]
- Samir, B.; El-Kamel, A.; Zahran, N.; Heikal, L. Resveratrol-Loaded Invasome Gel: A Promising Nanoformulation for Treatment of Skin Cancer. Drug Deliv. Transl. Res. 2024, 14, 3354–3370. [Google Scholar] [CrossRef] [PubMed]
- Kalam, M.A.; Ali, R.; Alhowyan, A.A.; Ahmad, A.; Iqbal, M.; Raish, M. Quercetin-Loaded Transliposomal Gel for Effective Management of Skin Cancer: In Vitro and Cell Line Efficacy Studies. J. Drug Deliv. Sci. Technol. 2024, 96, 105659. [Google Scholar] [CrossRef]
- Mangla, B.; Kaur, A.; Kumar, P.; Javed, S.; Ahsan, W.; Popli, H. Development of Nanobigel System Combining Apoptosis-Inducing Daidzein and Flaxseed Oil for the Synergistic Treatment of Skin Cancer. J. Drug Deliv. Sci. Technol. 2024, 98, 105843. [Google Scholar] [CrossRef]
- Ivanova, N.A. Anti-Melanoma Activity of Green-Produced Nanosilver-Chlorhexidine Complex. Pharmacia 2025, 72, 7. [Google Scholar] [CrossRef]
- Himalini, S.; Nallal, U.M.; Razia, M.; Chinnapan, S.; Chandrasekaran, M.; Ranganathan, V.; Gatasheh, M.; Hatamleh, A.; Al-Khattaf, F.; Kanimozhi, S. Antimicrobial, an-Ti-Melanogenesis and Anti-Tyrosinase Potential of Myco-Synthesized Silver Nanoparticles on Human Skin Melanoma SK-MEL-3 Cells. J. King Saud. Univ. Sci. 2022, 34, 101882. [Google Scholar] [CrossRef]
- Patil, S.; Khulbe, P.; Nitalikar, M.; Das, K.; BP, M.; Alshehri, S.; Khormi, A.; Almalki, M.; Hussain, S.; Rabbani, S.; et al. Development of Topical Silver Nano Gel Formulation of Bixin: Characterization, and Evaluation of Anticancer Activity. Saudi Pharm. J. 2024, 32, 102125. [Google Scholar] [CrossRef]
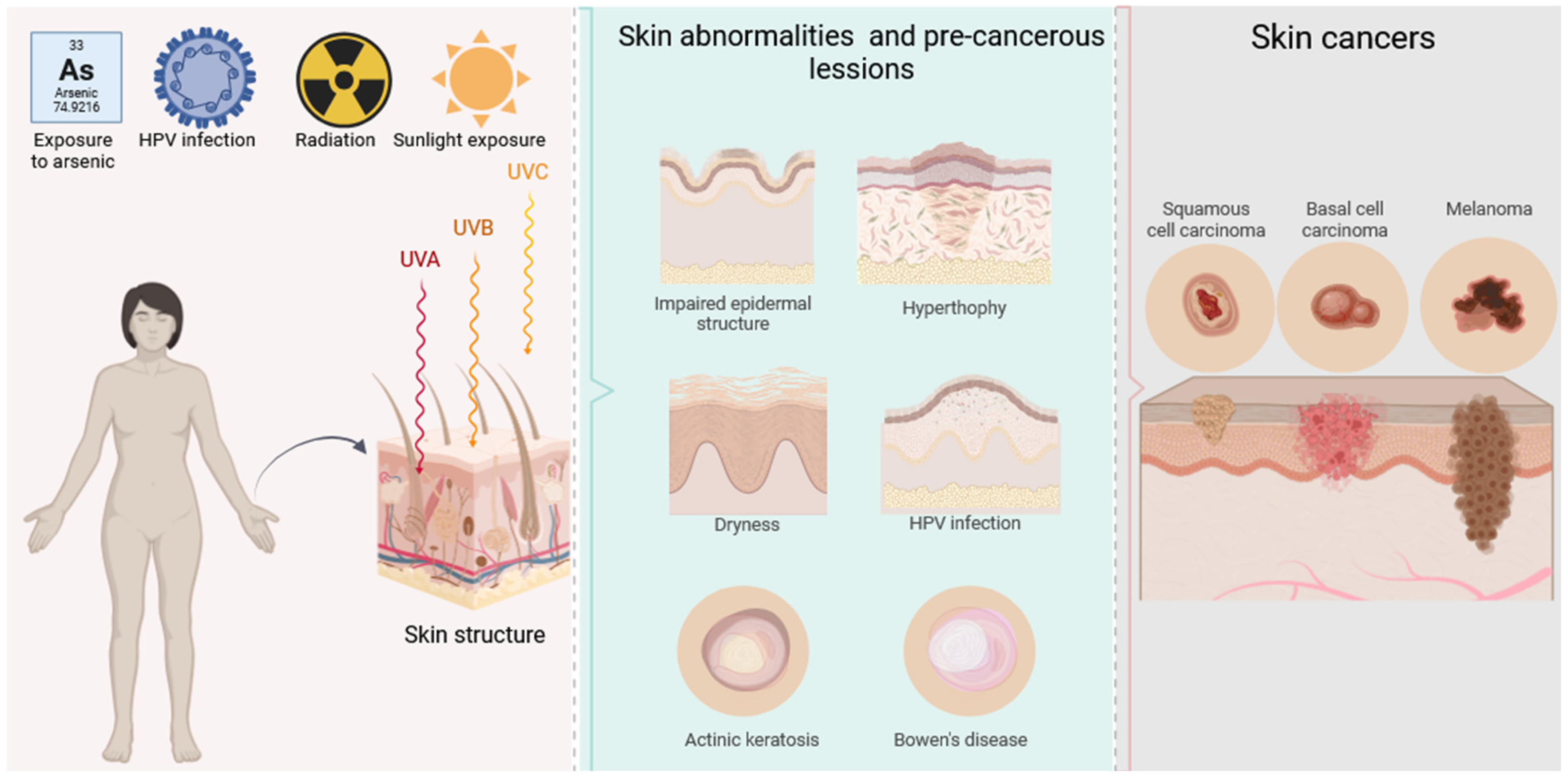
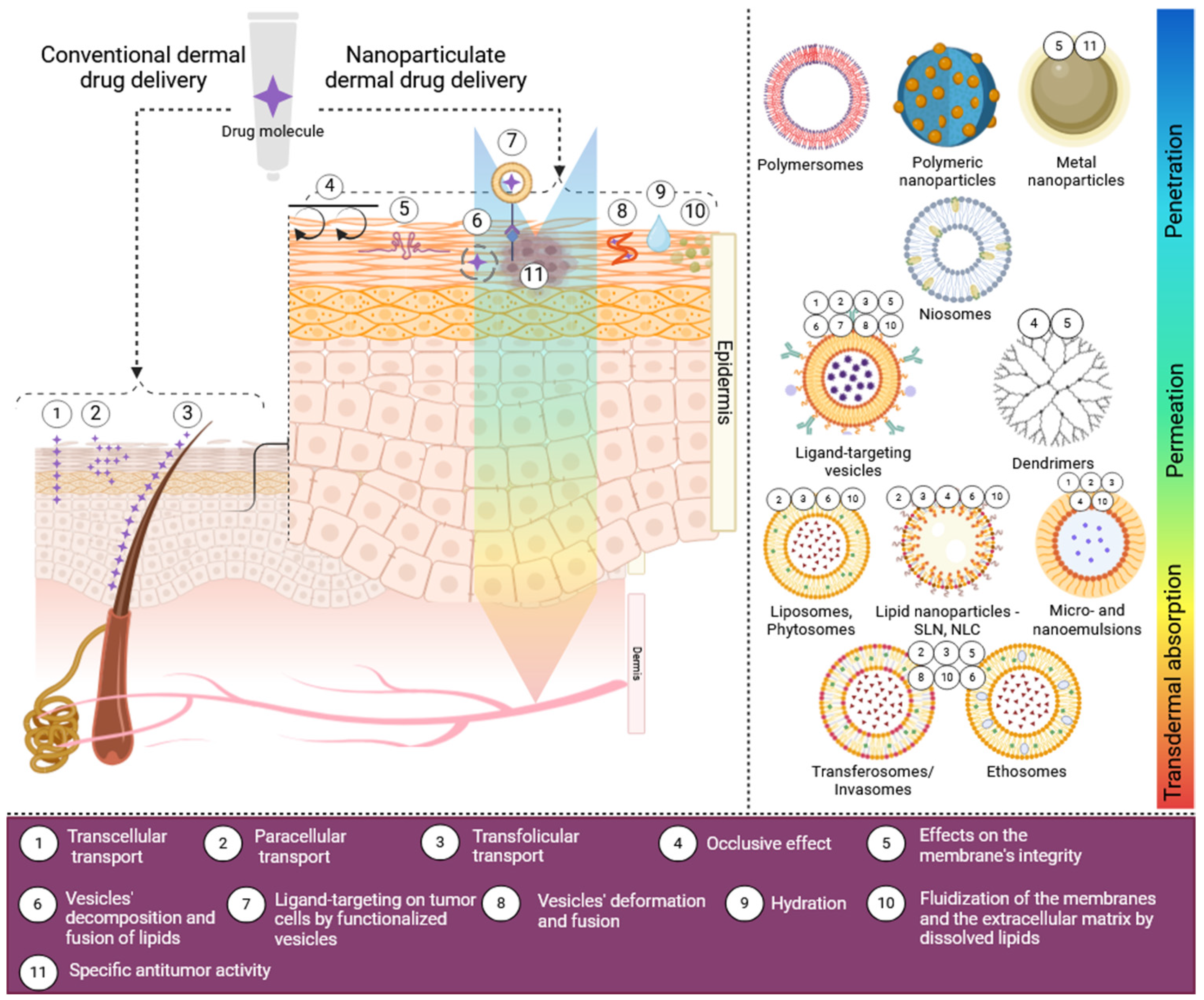
| Drug | Prodrug(s) | Activity | Reference(s) |
|---|---|---|---|
| Temozolomide | Temozolomide hexyl ester | Melanoma (in vitro and in vivo animal studies) | [77] |
| Proto-Porphyrin IX | 5-Aminolevulinic acid; Methyl-5-aminolevulinate; Butyl-5-aminolevulinate; Hexyl-5-aminolevulinate | In photodynamic therapy (PDT) of AK and BCC | [78,79,80,81,82] |
| 5-Fluorouracil (5-FU) | 1-Alkylaminocarbonyl 5-FU; N-Acyloxymethyl derivatives of 5-FU | AK BCC SCC | [83,84,85] |
| Nicotinic acid | Tetradecyl Nicotinate | Skin cancer prevention | [86] |
| Imiquimod | Imiquimod-oleic acid | AK BCC SCC Melanoma | [87,88] |
| Type of Vesicle | Characteristics | Advantages Over Liposomes and Overall | Advantages in Dermal Drug Delivery | References |
|---|---|---|---|---|
| Liposomes | Flexible uni- or multi-lamellar vesicles composed of phospholipids, water, and cholesterol | - | Enhanced drug permeation through skin layers | [105,106,107,108,109,110] |
| Ethosomes and transethosomes | Flexible nano-sized vesicles composed of phospholipids, water, and ethanol in high concentrations * | Better compatibility with chemically unstable APIs | Enhanced drug permeation through skin layers; highly suitable for transdermal drug delivery | [109,110] |
| Transferosomes | Highly deformable nano-sized vesicles composed of phospholipids, water/water—alcoholic mixture, and an edge activator(s) (most often surfactants) ** | High deformability | Enhanced drug permeation through skin layers; highly suitable for transdermal drug delivery | [111,112] |
| Niosomes | Nano-sized vesicles composed of nonionic surfactants, water, and cholesterol | Improved chemical stability; improved ability for drug stabilization; simpler preparation techniques and conditions; better reproducible technologies; lower production cost | Moderate enhancement of drug permeation through skin layers; suitable for topically active dermal formulations | [112,113,114] |
| Polymersomes | Nano-sized vesicles composed of amphiphilic block copolymers, surrounding an aqueous core | Increased loading capacity; increased chemical stability; improved ability for drug stabilization; suitable carriers for biomolecules such as peptides and proteins, incl. enzymes; adjustable release rate and suitability for controlled drug delivery | Generally, polymersomes are not suitable for dermal drug delivery due to their reduced penetrability; however, a useful application of these vesicles was recently discovered in the topical delivery of photoprotectors to the epidermis for skin cancer prevention [115] | [115,116,117,118] |
| Invasomes | Nano-sized vesicles similar to transferosomes but using terpenes as permeation enhancers | High deformability | Enhanced drug permeation through the skin layers | [119,120] |
| Pharmacosomes | Ultra-fine vesicular, micellar, or hexagonal aggregates carrying APIs that are covalently bound to the lipids | Higher entrapment efficiency; lowered risk of drug leakage | Improved control over drug liberation; reduced drug toxicity/adverse reactions | [121,122] |
| Phytosomes (herbosomes) | Nano-sized vesicles formed by the hydrogen binding of polyphenols to the hydrophilic choline heads of the liposomal phospholipids | Improved solubility, permeation, stability, and bioavailability of polyphenols | New perspectives and opportunities for the dermal drug delivery of polyphenols, incl. in skin cancer treatment | [123,124] |
| Cubosomes | Lyotropic nonlamellar liquid crystalline nano-sized vesicles usually comprise glyceryl monooleate, amphiphilic block copolymers, and/or other stabilizing agents | Higher entrapment efficiency; lowered risk of drug leakage | Bioadhesiveness; improved localized therapy of skin diseases; reduced drug toxicity/adverse reactions | [125,126] |
| Glycerosomes | Nano-sized vesicles composed of phospholipids, water, and glycerol ** | Higher entrapment efficiency; longer shelf life | Enhanced drug permeation through the skin layers | [127,128] |
| Chitosomes | Chitosan-covered liposomes | Higher entrapment efficiency; improved stability | Bioadhesiveness; Enhanced drug permeation through the skin layers; Possibly own antibacterial, antifungal, and anti-tumor activity | [129,130,131,132,133] |
| Formulation/Dosage Form | Nano-Carrier System | Therapeutic Combination | Result(s) | Reference(s) |
|---|---|---|---|---|
| Microemulsion | Microemulsion | n/a | Effectiveness in reducing carcinomatous areas in SCC-induced tissues; sustained drug release; significant skin permeation; minimal toxicity, enhanced patient compliance by reducing treatment duration and adverse reactions | [199] |
| Nanoemulsion | Nanoemulsion | n/a | Enhanced skin penetration and reduced irritation compared to conventional 5-FU 1% gel | [201] |
| Gel | Chitosan-functionalized nanoemulsion | n/a | Enhanced topical delivery of 5-FU; promoted retention of 5-FU in skin, and proven non-irritant properties of the nanoemulsion gel | [202] |
| Microneedle-assisted skin delivery | Liposomes | n/a | Enhanced 5-FU skin penetration and cytotoxicity against SCC; significant increase in 5-FU skin penetration by combining liposomes and microneedle technology | [203] |
| Liposomal suspension | Ultradeformable liposomes (transferosomes) | n/a | Enhanced 5-FU delivery to deeper skin layers | [204] |
| Liposomal suspension | Ultradeformable liposomes (transferosomes) | Resveratrol | Enhanced anti-tumor action of the drug combination of 5-FU and resveratrol against melanoma cells; improved skin permeation by ultradeformable liposomes | [205] |
| Liposomal suspension applied via subcutaneous injection or iontophoresis-assisted topical administration | Active-targeted immunoliposomes | Cetuximab | Reduced 5-FU permeation by iontophoresis as compared to injection and potential for reduced systemic side effects; increased 5-FU accumulation in viable epidermis; increased cellular uptake; effectively reduced proliferation of SCC cells | [206] |
| Liposomal suspension | Polymer-coated liposomes | n/a | Sustained release of 5-FU; increased cytotoxicity against epidermoid carcinoma cells as compared to uncoated liposomes | [207] |
| Niosomal suspension | Hyaluronic acid-coated niosomes | n/a | Improved targeting efficiency for 5-FU delivery, controlled drug release, and improved retention in skin | [208] |
| Microwave-assisted delivery of ethosomal suspension | Ethosomes | n/a | Enhanced skin penetration; increased cytotoxicity against human melanoma cells | [209] |
| Gel | Ethosomes | n/a | Enhanced local bioavailability of 5-FU; reduced skin irritation compared to marketed formulations | [210] |
| Gel | NLCs | Cannabidiol | Improved tumor remission and survival rate with reduced tumor volume in NMSCs; enhanced skin retention, excellent uptake, and deposition in skin layers | [211] |
| Gel | NLCs | n/a | Improved 5-FU permeation and skin retention; reduced skin irritation compared to plain 5-FU gel | [212] |
| Gel | SLNs | n/a | Enhanced permeability, sustained release, and cytotoxicity against melanoma and SCC; improved skin retention and permeation, and safety for topical applications | [213] |
| Gel | SLNs | n/a | Enhanced 5-FU skin penetration; reduced inflammatory reaction and reduced symptoms of angiogenesis in skin carcinoma-induced mice | [214] |
| SLNs suspension | SLNs | n/a | Improved cytotoxicity against melanoma cells compared to 5-FU solution; high entrapment efficiency | [215] |
| n/a | Chitosan nanoparticles | n/a | Enhanced encapsulation efficiency for 5-FU delivery; controlled drug release over 24 h; effectiveness against BCC | [216] |
| Nanofiber mat | Chitosan/Polyvinyl alcohol nanofibers | n/a | Effective treatment of BCC; high encapsulation efficiency and controlled release of 5-FU over 24 h | [217] |
| Nanofilms | Polyhydroxyethyl methacrylate/polyhydroxypropyl methacrylate/sodium deoxycholate nanoparticles | Iron–platinum nanoparticles | Controlled drug release with minimal side effects; anti-proliferative properties against BCC cells | [218] |
| Cream and gel | Gold nanoparticles | Gold nanoparticles | Reduced tumor volume in epidermoid carcinoma-bearing mice compared to free 5-FU, confirming enhanced anti-cancer efficacy and improved skin permeability | [219] |
| pH-responsive micellar hydrogel | Deoxycholic acid micelles | n/a | Improved anti-cancer activity against melanoma cells compared to 5-FU alone | [220] |
| Cream base | 5-FU nanocrystals | n/a | Improved efficiency against epidermoid cancer cells as compared to the micro-sized crystals of 5-FU | [221] |
| Nanofibrous scaffolds | Nanofibers | Etoposide | Sustained drug release, significant cytotoxicity, and apoptosis against melanoma cells | [222] |
| Formulation/Dosage Form | Nano-Carrier System | Result(s) | Reference(s) |
|---|---|---|---|
| Patch | NLCs | Improved drug deposition in deeper skin layers as compared to commercial cream; patches enhanced patient compliance for topical skin cancer treatment | [234] |
| Microemulsion | Microemulsion | Limited ability of the microemulsions to improve the delivery of IMQ over Aldara™ cream | [235] |
| Gel | Microemulsion * | α-Tocopheryl polyethylene glycol 1000 succinate and oleic acid enhanced IMQ solubility significantly; the micellar formulation improved skin retention and delivery of the drug | [236] |
| Microemulsion | Microemulsion | IMQ 1% microemulsion delivered similar drug quantities to the epidermis as the commercial product with a 5-fold higher dose of 5%; lower risk of systemic absorption compared to the established product | [239] |
| Gel-like microemulsion | Microemulsion | Enhanced IMQ delivery to the skin and by a gel-like formulation with a suitable viscosity for topical application viscosity | [240] |
| Nanoemulsion | Nanoemulsion | Improved IMQ solubility and drug release; enhanced cytotoxicity against epidermoid carcinoma cell line compared to commercial formulation | [241] |
| Nanosuspension/nanoemulsion | Liposomes, nanocrystals, nanoemulsions, lipid nanocapsules | Best results with respect to permeation enhancement were achieved by using an IMQ nanocrystal suspension or nanoemulsion, which demonstrated a three- and five-fold increased drug delivery, respectively, in comparison to the commercially available cream | [242] |
| Liposomal suspension | Ultradeformable liposomes (transferosomes) | In vitro anti-melanoma activity | [243] |
| Nanostructured formulation (likely of micro- or nanoemulsion type) * | pH-responsive micelles | In vitro anti-melanoma activity; improved skin retention in comparison to Imunocare® commercial product; pH-responsive drug release from micelles and possibility for selective drug release in tumor tissues | [244] |
| Ethosomal suspension | Ethosomes/transethosomes | Increased permeation rate of IMQ; improved retention and deposition into SC and the deeper epidermal and dermal layers as compared to Aldara™ cream; superior results were obtained with transethosomes (differing from the classic ethosomes by the presence of a permeation enhancer in their composition) | [245] |
| Nanosuspension | Polymeric nanoparticles (dextran nanocapsules) | Enhanced IMQ transdermal delivery, high encapsulation efficiency, and controlled drug release | [246] |
| Gel | β-Cyclodextrin-based nanosponges | Enhanced permeation and retention; sustained release of IMQ; greater inhibitory effect on fibroblast proliferation as compared to the pure drug | [247] |
| Nanosuspension * | Chitosan nanocapsules | Controlled IMQ release over 24 h; evaluated skin absorption; effective transdermal delivery | [248] |
| Therapeutic Agent/Combination | Mechanism of Action | Formulation/Dosage Form | Nanocarrier System | Result(s) | Reference(s) |
|---|---|---|---|---|---|
| Dacarbazine | Alkylating chemotherapeutic agent (usually for parenteral administration) | Cream | NLCs | Superior anti-proliferative activity of the lipid nanoparticles with dacarbazine in comparison to pure drug against melanoma cell lines | [315] |
| Gel | SLNs | Improved efficacy of the nano-formulation in the treatment of skin tumors induced in rats, as compared to free drug; stable formulation showing minimal side effects and potential in the topical treatment of melanoma | [316] | ||
| Iontophoresis-assisted application | n/a | Iontophoresis appeared as a promising method to enhance the topical delivery of dacarbazine, potentially offering a safer alternative for melanoma treatment by avoiding the adverse effects of systemic administration; the study highlighted the necessity of stabilizing the drug in advance | [317] | ||
| Gel | Nanosponges (polymeric) | Sustained drug release, effective permeation (73%), good biocompatibility, and superior inhibition of melanoma cell proliferation | [318] | ||
| Dacarbazine + tretionin | An alkylating chemotherapeutic agent combined with RARs/RAXs agonist | SLNs nanosuspension | SLNs | Effective inhibition of melanoma cell proliferation, induction of remarkable apoptosis, and inhibition of cell cycle progression and cell migration | [319] |
| Nanosuspension | Transethosomes | Superior skin permeation and enhanced cytotoxic effects against cutaneous melanoma, indicating potential for effective topical treatment of skin cancer | [320] | ||
| Doxorubicin | Anti-tumor antibiotic, interfering with DNA and RNA synthesis (usually for parenteral administration) | Microneedle-assisted delivery | Hybrid cationic nanoparticles | Targeted accumulation in subcutaneous melanoma tumors in mice, induction of apoptosis, and suppression of tumor growth | [321] |
| Gel | Oleic acid-grafted mesoporous silica nanoparticles | Improved permeation compared with the hydrogel containing free drug; improved doxorubicin’s cytotoxic effects toward epidermoid carcinoma cells | [322] | ||
| Iontophoresis-assisted application | SLNs | Increased epidermal penetration; superior cytotoxic activity against SCC cells compared to pure drug solution; significant improvement in tumor tissue restriction in vivo | [323] | ||
| Nanofibrous topical implantable delivery device | Core–shell implantable nanofibrous membranes | Controlled drug release, sufficient local concentration, allowing dose reduction and minimizing side effects; efficacy against melanoma tumors in vivo (in mice) | [324] | ||
| Doxorubicin + berberine | Anti-tumor antibiotic, interfering with DNA and RNA synthesis, combined with a naturally occurring isoquinoline alkaloid with proven anti-cancer activity and a complex mechanism of action | Gel | Mannose-conjugated NLCs | Improved permeation and skin deposition compared to conventional gel; potential for enhanced dual therapeutic approach for skin cancer amelioration based on in silico study | [325] |
| Doxorubicin + celecoxib | Anti-tumor antibiotic, interfering with DNA and RNA synthesis, combined with a non-steroidal anti-inflammatory drug, is effective in inhibiting skin cancer development | Microneedle-assisted delivery of liposomal gel | Liposomes | Enhanced in vivo anti-tumor efficacy of the combined liposomal gel against melanoma in comparison to single-drug liposomes; augmented skin penetration in the case of microneedle-assisted delivery | [326] |
| Bleomycin | Anti-tumor antibiotic, interfering with DNA synthesis (usually for parenteral administration) | Cream | Liposomes | Enhanced skin penetration by the liposomal formulation as compared to the free drug | [327] |
| Paclitaxel | Chemotherapeutic agents interfere with the normal function of microtubules during cell division | Anionic bicelles | Anionic bicelles | Effective penetration in SC; potential for treating skin cancer based on an in vivo study on mouse papillomas | [328] |
| Curcumin | A naturally occurring polyphenol with proven anti-proliferative and anti-tumor activity and a complex mechanism of action | Bioadhesive film (patch) | n/a | Enhanced drug penetration into the skin compared to a curcumin solution control; controlled drug release; effective against metastatic melanoma cells after topical application; superior tumor cell inhibition compared to a single dose of radiotherapy | [329] |
| Curcumin + anti-STAT3 siRNA | A naturally occurring polyphenol with proven anti-proliferative and anti-tumor activity, combined with gene therapy | Iontophoresis-assisted application | Liposomes | Inhibition of cancer cell growth in mouse melanoma cells, showing a statistically significant difference compared to treatments with either liposomal curcumin or STAT3 siRNA alone; iontophoretic administration demonstrated similar effectiveness in inhibiting tumor progression and STAT3 protein suppression compared with intra-tumoral administration | [330] |
| Resveratrol | A naturally occurring polyphenol with proven anti-proliferative and anti-tumor activity and a complex mechanism of action | Gel | Invasomes | High skin deposition and potency; high cellular uptake when tested on SCC; proven in vivo effectiveness in Ehrlich-induced mice models | [331] |
| Quercetin | A naturally occurring polyphenol with proven anti-proliferative and anti-tumor activity and a complex mechanism of action | Gel | Transferosomes | Enhanced skin permeation; lower cytotoxic concentrations against melanoma cells in comparison to quercetin conventional gel and solution | [332] |
| Diadzein + flaxseed oil | A naturally occurring polyphenol with proven anti-proliferative and anti-tumor activity, combined with a rich source of omega-3-polyunsaturated fatty acids source | Nanobigel | Nanobigel | Sustained release, improved drug permeation, and induction of apoptosis in epidermoid carcinoma cells | [333] |
| AgNPs + chlorhexidine | Silver nanoparticles with intrinsic anti-tumor activity, combined with a broad-spectrum antiseptic agent | Bioadhesive film (patch) | Green tea catechins-synthesized silver nanoparticles | Explicit and selective anti-melanoma activity of the nanosilver complex, fortified in the composition of the adhesive patch | [334] |
| AgNPs | Silver nanoparticles with intrinsic anti-tumor activity as a result of a complex mechanism of action | Nanosilver suspension | Myco-synthesized silver nanoparticles | Distinct anti-melanogenic activity | [335] |
| AgNPs + bixine | Silver nanoparticles with intrinsic anti-tumor activity, combined with a carotenoid with proven anti-proliferative and anti-tumor activity and a complex mechanism of action | Gel | Bixa orellana seed extract-synthesized silver nanoparticles | Moderate inhibitory activity against melanoma cancer cell lines; promising anti-cancer activity in vivo | [336] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanova, N. Local Chemotherapy of Skin Pre-Neoplastic Lesions and Malignancies from the Perspective of Current Pharmaceutics. Pharmaceutics 2025, 17, 1009. https://doi.org/10.3390/pharmaceutics17081009
Ivanova N. Local Chemotherapy of Skin Pre-Neoplastic Lesions and Malignancies from the Perspective of Current Pharmaceutics. Pharmaceutics. 2025; 17(8):1009. https://doi.org/10.3390/pharmaceutics17081009
Chicago/Turabian StyleIvanova, Nadezhda. 2025. "Local Chemotherapy of Skin Pre-Neoplastic Lesions and Malignancies from the Perspective of Current Pharmaceutics" Pharmaceutics 17, no. 8: 1009. https://doi.org/10.3390/pharmaceutics17081009
APA StyleIvanova, N. (2025). Local Chemotherapy of Skin Pre-Neoplastic Lesions and Malignancies from the Perspective of Current Pharmaceutics. Pharmaceutics, 17(8), 1009. https://doi.org/10.3390/pharmaceutics17081009






