Etrog Citron (Citrus medica) as a Novel Source of Antiviral Agents: Overview of Its Bioactive Phytochemicals and Delivery Approaches
Abstract
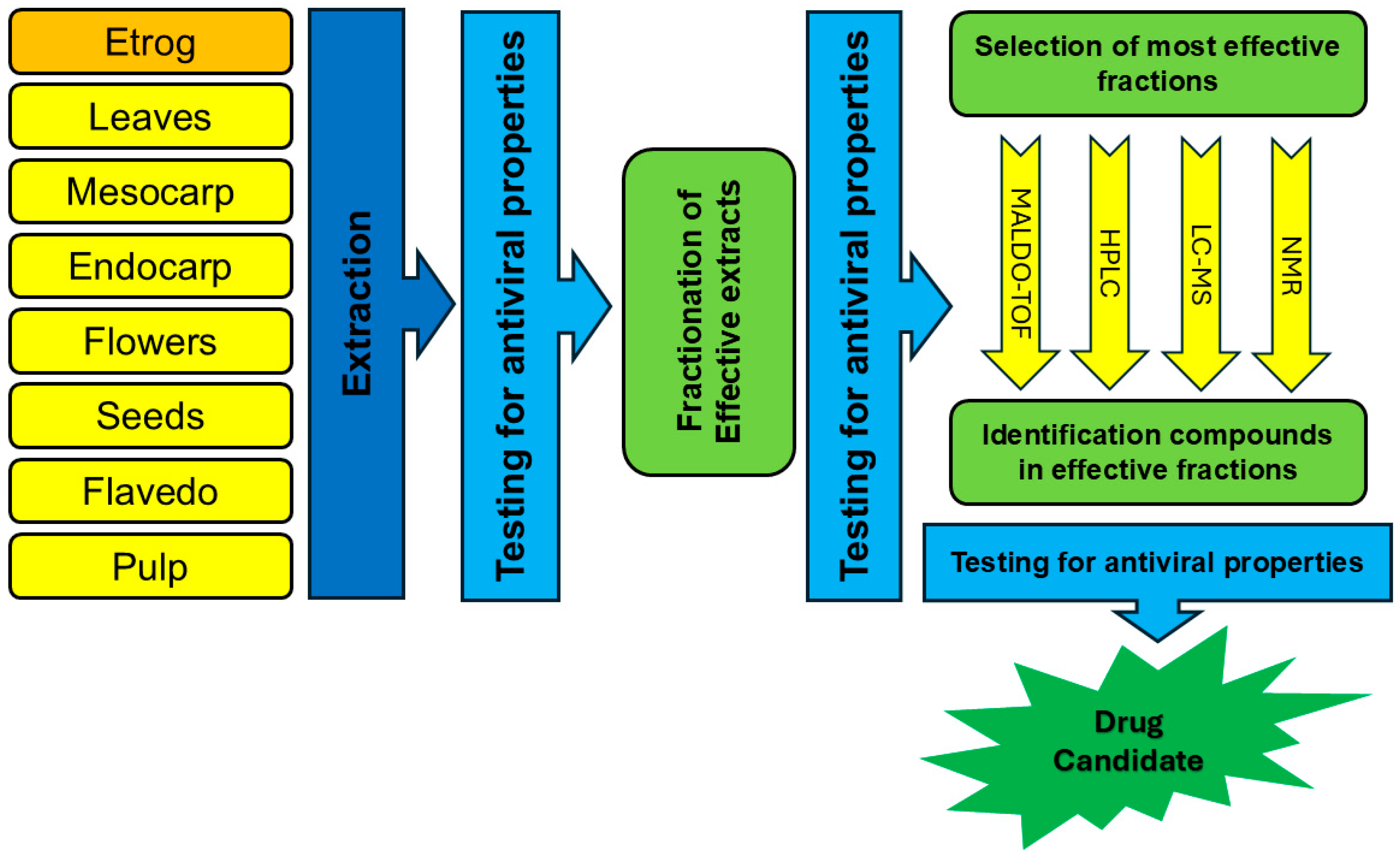
1. Introduction
2. Methods
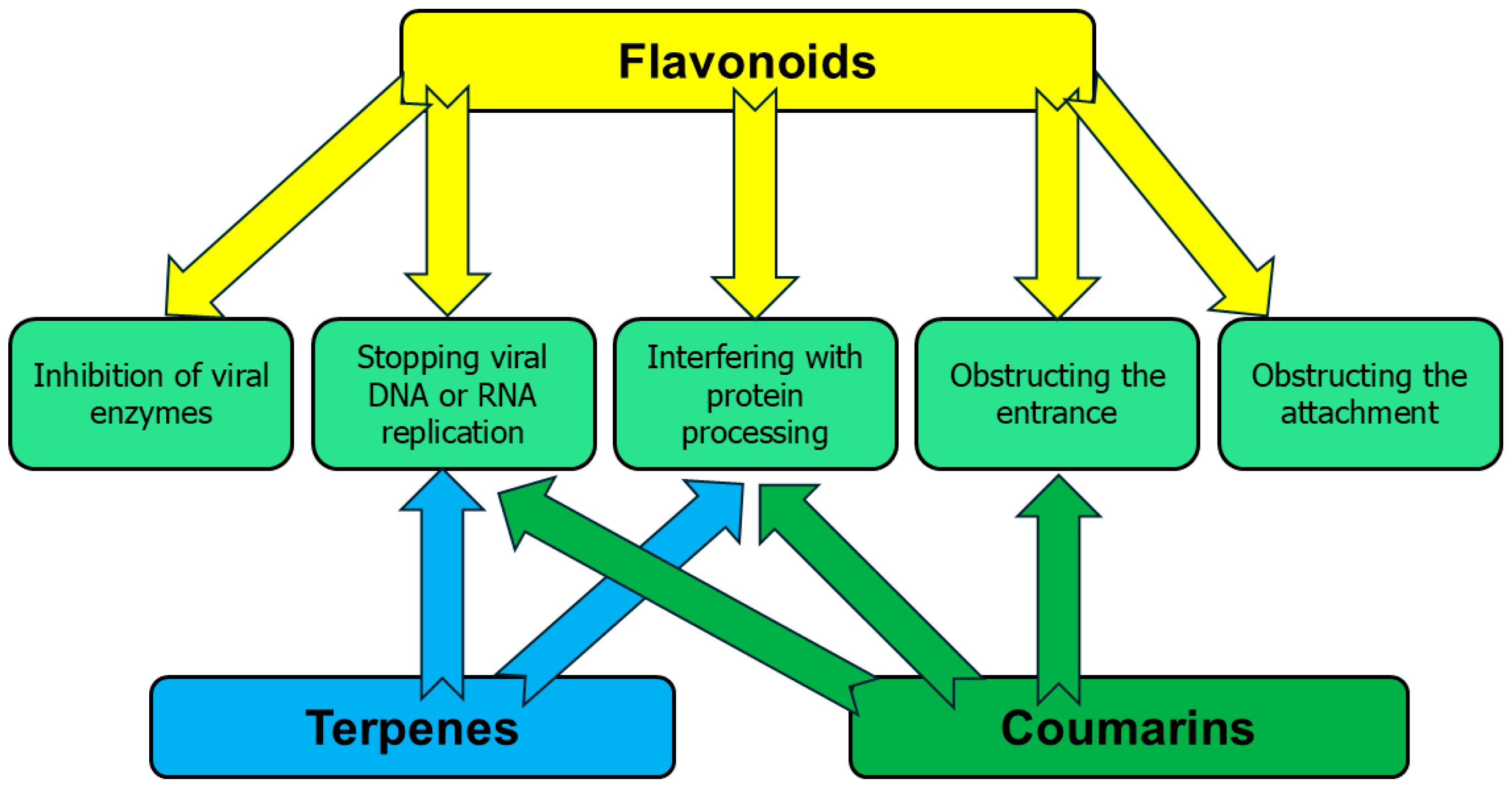
3. Antiviral Activity of Phytochemicals
| Compound | Structure | Part of the Plant | Virus | Mechanism | References |
|---|---|---|---|---|---|
| Apigenin | 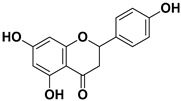 | Leaves, flowers, mesocarp, endocarp | BPXV EBV HCV SARS-CoV-2 | Inhibits synthesis of viral DNA, mRNA and proteins, Inhibition of HCV replication | [15,53,54,55] |
| Catechin | 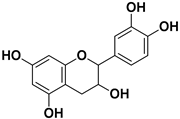 | Mesocarp, endocarp, seeds, flavedo, pulp | Dengue virus Influenza A (H1N1) | Inhibit a post-entry stage of the DENV replication cycle. Inhibits neuraminidase | [56,57] |
| Caffeic acid | 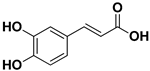 | Exocarp, mesocarp, endocarp, seeds | Ilhéus virus Severe fever with thrombocytopenia syndrome (SFTS) | Interacts with the envelope Unknown | [58,59] |
| Chlorogenic acid | 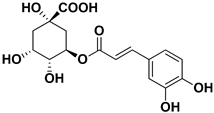 | Endocarp, mesocarp | Infectious Bursal Disease Virus (IBDV) | Unknown | [60] |
| Dihydroquercetin | 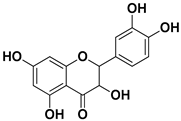 | Exocarp, endocarp, seeds | Coxsackie B4 virus | Unknown | [61] |
| Diosmin | 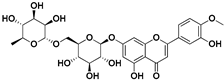 | Flowers, leaves, mesocarp, endocarp | Influenza A, Varicella zoster virus (VZV) | Unknown | [62,63] |
| Epicatechin |  | Flavedo, pulp | Singapore grouper iridovirus (SGIV) SARS-CoV-2 | Impact on the replication of SGIV, Disrupts the interaction of ACE2 and SARS-CoV-2 | [64,65] |
| Gallic acid |  | Exocarp, mesocarp, endocarp | Influenza A and B viruses | Unknown | [66] |
| Hesperidin | 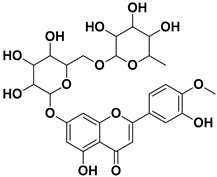 | Flavedo, exocarp, endocarp, mesocarp, seeds, flowers, leaves | SARS-CoV-2 | Inhibition of viral replication | [67] |
| Kaempferol 3-O-rutinoside | 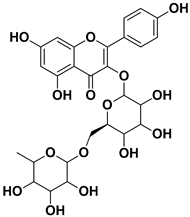 | Flavedo | HSV-1, HSV-2 SARS-CoV-2 | Unknown | [28,68] |
| Lonchocarpol A | 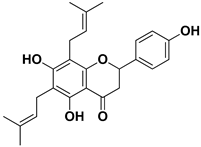 | Root, stem | HIV | Unknown | [69] |
| Naringin | 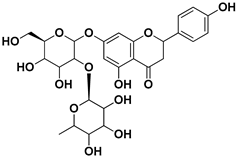 | Fructus, exocarp, mesocarp, endocarp, seeds, flavedo | Chikungunya virus | Anti-nsP2 protease activity | [70] |
| Neodiosmin | 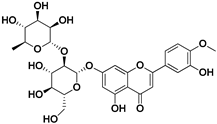 | Fructus | SARS-CoV-2 | Unknown | [71] |
| Nobiletin | 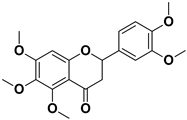 | Exocarp, mesocarp, endocarp, seeds | Chikungunya virus | Translation/replication stages and viral entry | [72] |
| Quercetin | 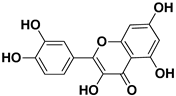 | Flowers, leaves, mesocarp, endocarp | SARS-CoV-2 HCV | Suppresses the early phases of infection, interacts with viral replication proteases Inhibits NS3 protease activity | [73,74] |
| Rutin (quercetin-3-rutinoside) |  | Flavedo | HSV-1, HSV-2 | Unknown | [28] |
| Sakuranetin | 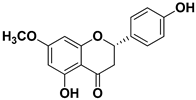 | Leaves | Human rhinoviruses, Influenza B/Lee/40 virus | Unknown Inhibition of viral RNA synthesis | [75,76] |
| Salicylic acid | 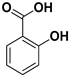 | Fructus | HSV-1, H1N1 influenza A, adenovirus type 5 | Unknown | [77] |
| Tangeritin |  | Exocarp, mesocarp, endocarp | RSV | Downregulates expression of RSV phosphoprotein | [78] |
| Vitexin | 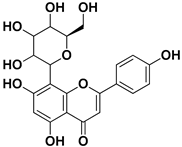 | Exocarp, endocarp, seeds | Influenza A (H1N1) virus HSV-1 HAV | Inflammation reduction Inhibition of viral replication | [79] |
| Compound | Structure | Part of the Plant | Virus | Mechanism | References |
|---|---|---|---|---|---|
| Citropten | 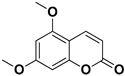 | Stem, root, barks, fresh fruit | HIV-1 | Unknown | [50] |
| Leptodactylone |  | Fresh fruit | SARS-CoV-2 | Unknown | [80] |
| Nordentatin | 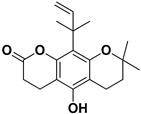 | Fresh fruit | HIV-1, Hepatitis B | Unknown | [81,82] |
| Scopoletin |  | Fresh fruit, fructus | SARS-CoV-2 | Inhibition of virus entry to the host cell | [83,84] |
| Umbelliferone |  | Fresh fruit | Viral hemorrhagic septicemia virus | Inhibition of VHSV replication, downregulation of Caspase 3 expression and inhibition of virus-induced apoptosis at later infection stages | [85] |
| Xanthyletin | 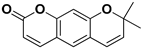 | Stem, root, barks, fresh fruit | HIV-1 | Unknown | [50] |
| Compound | Structure | Part of the Plant | Virus | Mechanism | References |
|---|---|---|---|---|---|
| α-pinene |  | Flavedo | Bronchitis virus | Inhibition of nucleocapsid protein | [86] |
| α-terpineol |  | Flavedo, fresh fruits, mesocarp | HSV-1 | Unknown | [87] |
| Carvacrol | 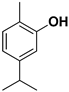 | Flavedo, fresh fruits, mesocarp | HSV-2 Feline calcivirus, Murine norovirus | Inhibits the HSV-2 proliferation Unknown Unknown | [88,89] |
| Citral | 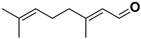 | Fructus, flavedo, mesocarp | Yellow fever virus | Unknown | [90] |
| Citronellol | 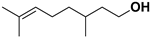 | Flavedo | HSV-1 | Unknown | [91] |
| Farnesol |  | Flavedo | HSV-1 | Unknown | [92] |
| Geraniol | 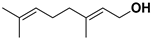 | Fructus, flavedo | HSV-1 | Changes the subcellular distribution of the oligonucleotides | [93] |
| Limonene | 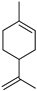 | Flavedo, fructus, oil glands, exocarp, mesocarp, seeds | Drug-resistant influenza virus | Inhibition intracellular replication | [94] |
| Limonin | 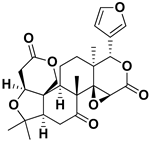 | Citron waste, fructus, bark | HIV-1 | Inhibition protease activity | [95] |
| Linalool | 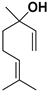 | Flavedo | AVD-II | Unknown | [96] |
| Nomilin |  | Fresh fructus | HIV-1 | Inhibition protease activity | [95] |
| Obacunone | 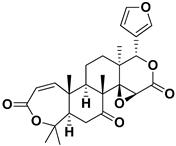 | Fresh fructus | SARS-CoV-2 | Disruption of target proteins | [97] |
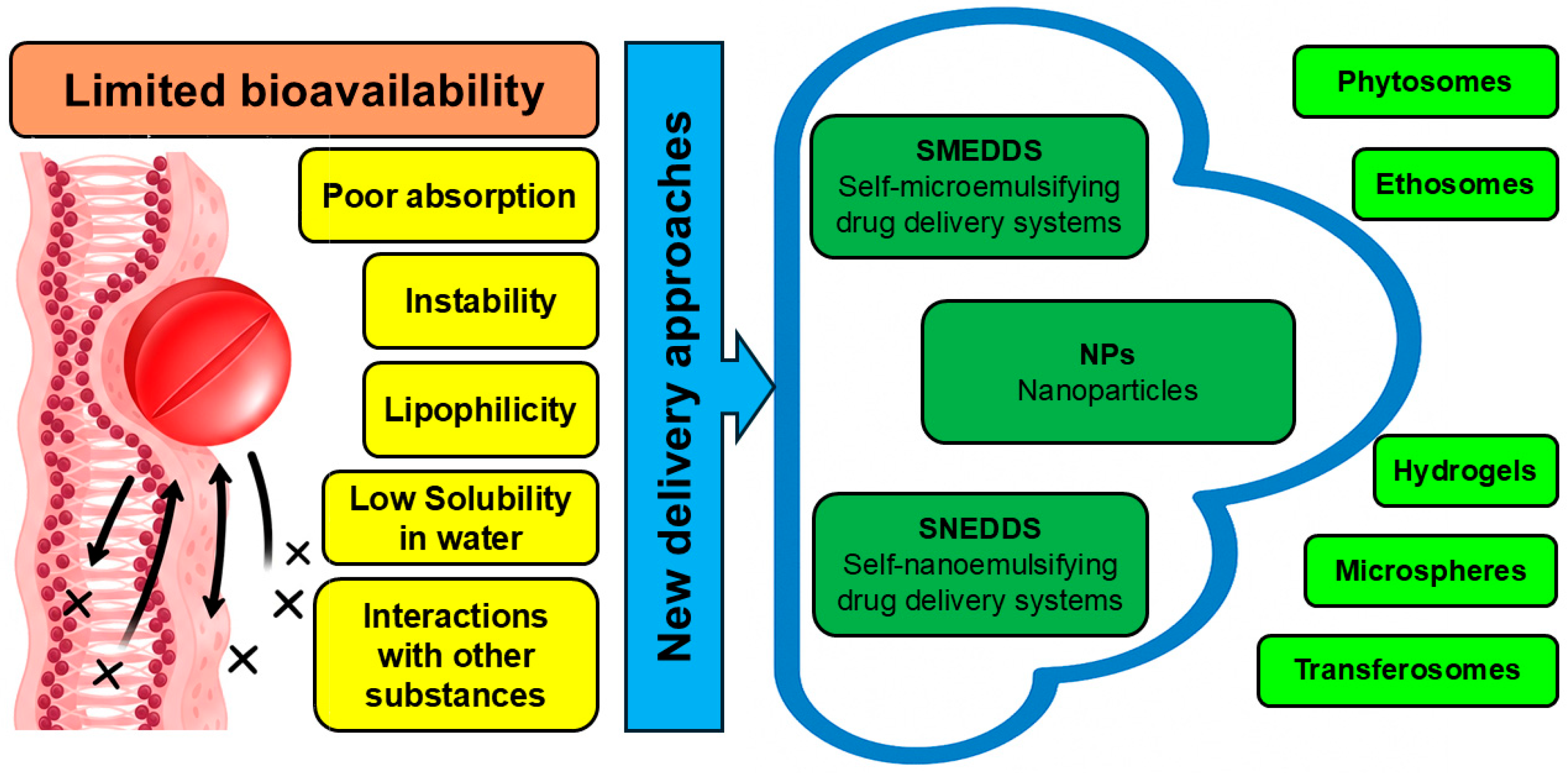
4. Applicability of Delivery Systems
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zitterl-Eglseer, K.; Marschik, T. Antiviral Medicinal Plants of Veterinary Importance: A Literature Review. Planta Medica 2020, 86, 1058–1072. [Google Scholar] [CrossRef]
- Dilip Bhandare, S. Exploration of Medicinal Plants as Potential Therapeutics against COVID-19: Molecular Insights and Drug Development Prospects with Other Significant Medicinal Information a Retrospective Exposition. J. Pharm. Pharmacol. 2025, 77, 18–31. [Google Scholar] [CrossRef]
- Lowe, H.; Steele, B.; Bryant, J.; Fouad, E.; Toyang, N.; Ngwa, W. Antiviral Activity of Jamaican Medicinal Plants and Isolated Bioactive Compounds. Molecules 2021, 26, 607. [Google Scholar] [CrossRef]
- Malekmohammad, K.; Rafieian-Kopaei, M.; Sardari, S.; Sewell, R.D.E. Effective Antiviral Medicinal Plants and Biological Compounds Against Central Nervous System Infections: A Mechanistic Review. Curr. Drug Discov. Technol. 2020, 17, 469–483. [Google Scholar] [CrossRef] [PubMed]
- Rana, J.N.; Gul, K.; Mumtaz, S. Isorhamnetin: Reviewing Recent Developments in Anticancer Mechanisms and Nanoformulation-Driven Delivery. Int. J. Mol. Sci. 2025, 26, 7381. [Google Scholar] [CrossRef]
- Rana, J.N.; Mumtaz, S. Prunin: An Emerging Anticancer Flavonoid. Int. J. Mol. Sci. 2025, 26, 2678. [Google Scholar] [CrossRef]
- Dahan, A.; Yarmolinsky, L.; Budovsky, A.; Khalfin, B.; Ben-Shabat, S. Therapeutic Potential of Ficus benjamina: Phytochemical Identification and Investigation of Antimicrobial, Anticancer, Pro-Wound-Healing, and Anti-Inflammatory Properties. Molecules 2025, 30, 1961. [Google Scholar] [CrossRef] [PubMed]
- Dahan, A.; Yarmolinsky, L.; Nakonechny, F.; Semenova, O.; Khalfin, B.; Ben-Shabat, S. Etrog Citron (Citrus medica) as a Novel Source of Antimicrobial Agents: Overview of Its Bioactive Phytochemicals and Delivery Approaches. Pharmaceutics 2025, 17, 761. [Google Scholar] [CrossRef] [PubMed]
- El Sayed, A.M.; El-Abd, E.A.W.; Afifi, A.H.; Hashim, F.A.; Kutkat, O.; Ali, M.A.; El Raey, M.A.; El Hawary, S.S. Comparative Metabolomics Analysis of Citrus medica Var. Sarcodactylis swingle and Limonia acidissima Linn. Fruits and Leaves Cultivated in Egypt in Context to Their Antiviral Effects. Heliyon 2024, 10, e32335. [Google Scholar] [CrossRef]
- Benedetto, N.; Carlucci, V.; Faraone, I.; Lela, L.; Ponticelli, M.; Russo, D.; Mangieri, C.; Tzvetkov, N.T.; Milella, L. An Insight into Citrus medica Linn.: A Systematic Review on Phytochemical Profile and Biological Activities. Plants 2023, 12, 2267. [Google Scholar] [CrossRef]
- Tundis, R.; Xiao, J.; Silva, A.S.; Carreiró, F.; Loizzo, M.R. Health-Promoting Properties and Potential Application in the Food Industry of Citrus medica L. and Citrus × clementina Hort. Ex Tan. Essential Oils and Their Main Constituents. Plants 2023, 12, 991. [Google Scholar] [CrossRef] [PubMed]
- Chhikara, N.; Kour, R.; Jaglan, S.; Gupta, P.; Gat, Y.; Panghal, A. Citrus medica: Nutritional, Phytochemical Composition and Health Benefits—A Review. Food Funct. 2018, 9, 1978–1992. [Google Scholar] [CrossRef]
- Ben-Shabat, S.; Yarmolinsky, L.; Porat, D.; Dahan, A. Antiviral Effect of Phytochemicals from Medicinal Plants: Applications and Drug Delivery Strategies. Drug Deliv. Transl. Res. 2020, 10, 354–367. [Google Scholar] [CrossRef]
- Wang, H.; Paulson, K.R.; Pease, S.A.; Watson, S.; Comfort, H.; Zheng, P.; Aravkin, A.Y.; Bisignano, C.; Barber, R.M.; Alam, T.; et al. Estimating Excess Mortality Due to the COVID-19 Pandemic: A Systematic Analysis of COVID-19-Related Mortality, 2020–21. Lancet 2022, 399, 1513–1536. [Google Scholar] [CrossRef]
- Farhat, A.; Hajer, B.H.; Bassem, K.; Youssef, B.H.; Philippe, M.; Slim, A.; Fendri, I. Apigenin Analogues as SARS-CoV-2 Main Protease Inhibitors: In-Silico Screening Approach. Bioengineered 2022, 13, 3350–3361. [Google Scholar] [CrossRef]
- Kausar, S.; Said Khan, F.; Ishaq Mujeeb Ur Rehman, M.; Akram, M.; Riaz, M.; Rasool, G.; Hamid Khan, A.; Saleem, I.; Shamim, S.; Malik, A. A Review: Mechanism of Action of Antiviral Drugs. Int. J. Immunopathol. Pharmacol. 2021, 35, 20587384211002620. [Google Scholar] [CrossRef]
- Huang, R.-L.; Tang, W.; Wang, C.; Yan, C.; Hu, Y.; Yang, H.-X.; Xiang, H.-Y.; Huang, X.-J.; Hu, L.-J.; Ye, W.-C.; et al. Antiviral C-Geranylated Flavonoids from Artocarpus Communis. Phytochemistry 2024, 225, 114165. [Google Scholar] [CrossRef]
- Du, H.; Cui, C.; Zhang, T.; Cai, Q.; Zhang, Y.; Hou, H. Mechanistic Insights into the Delivery and Pharmacodynamic Enhancement of Baicalin Nanoparticles in Traditional Chinese Plant Medicine-Based Antiviral Therapies. Ind. Crops Prod. 2025, 235, 121690. [Google Scholar] [CrossRef]
- Arias, B.Á.; Ramón-Laca, L. Pharmacological Properties of Citrus and Their Ancient and Medieval Uses in the Mediterranean Region. J. Ethnopharmacol. 2005, 97, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Sotoudeh-Anvari, A. The Applications of MCDM Methods in COVID-19 Pandemic: A State of the Art Review. Appl. Soft Comput. 2022, 126, 109238. [Google Scholar] [CrossRef] [PubMed]
- Ramadugu, C.; Keremane, M.L.; Hu, X.; Karp, D.; Federici, C.T.; Kahn, T.; Roose, M.L.; Lee, R.F. Genetic Analysis of Citron (Citrus medica L.) Using Simple Sequence Repeats and Single Nucleotide Polymorphisms. Sci. Hortic. 2015, 195, 124–137. [Google Scholar] [CrossRef]
- Yuan, Y.; Geng, X.; Wu, H.; Kumar, R.; Wang, J.; Xiao, J.; Tian, H. Chemical Composition, Antimicrobial Activities, and Microencapsulation by Complex Coacervation of Tea Tree Essential Oils. J. Food Process. Preserv. 2022, 46, e16585. [Google Scholar] [CrossRef]
- Gao, Z.; Zhong, W.; Chen, K.; Tang, P.; Guo, J. Chemical Composition and Anti-Biofilm Activity of Essential Oil from Citrus medica L. Var. sarcodactylis Swingle Against Listeria monocytogenes. Ind. Crops Prod. 2020, 144, 112036. [Google Scholar] [CrossRef]
- Ninfali, P.; Antonelli, A.; Magnani, M.; Scarpa, E.S. Antiviral Properties of Flavonoids and Delivery Strategies. Nutrients 2020, 12, 2534. [Google Scholar] [CrossRef]
- Ahmad, A.; Kaleem, M.; Ahmed, Z.; Shafiq, H. Therapeutic Potential of Flavonoids and Their Mechanism of Action Against Microbial and Viral Infections—A Review. Food Res. Int. 2015, 77, 221–235. [Google Scholar] [CrossRef]
- Di Petrillo, A.; Orrù, G.; Fais, A.; Fantini, M.C. Quercetin and Its Derivates as Antiviral Potentials: A Comprehensive Review. Phytother. Res. 2022, 36, 266–278. [Google Scholar] [CrossRef]
- Metwaly, A.M.; El-Fakharany, E.M.; Alsfouk, A.A.; Ibrahim, I.M.; Elkaeed, E.B.; Eissa, I.H. Integrated Study of Quercetin as a Potent SARS-CoV-2 RdRp Inhibitor: Binding Interactions, MD Simulations, and In Vitro Assays. PLoS ONE 2024, 19, e0312866. [Google Scholar] [CrossRef]
- Yarmolinsky, L.; Huleihel, M.; Zaccai, M.; Ben-Shabat, S. Potent Antiviral Flavone Glycosides from Ficus benjamina Leaves. Fitoterapia 2012, 83, 362–367. [Google Scholar] [CrossRef]
- Corcoran, M.P.; McKay, D.L.; Blumberg, J.B. Flavonoid Basics: Chemistry, Sources, Mechanisms of Action, and Safety. J. Nutr. Gerontol. Geriatr. 2012, 31, 176–189. [Google Scholar] [CrossRef]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.-H.; Jaremko, M. Important Flavonoids and Their Role as a Therapeutic Agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef] [PubMed]
- Beşler, Z.N.; Bayraktar, D.Z.; Koçak, M.C.; Kızıltan, G. Investigation of Potential Effects of Quercetin on COVID-19 Treatment: A Systematic Review of Randomized Controlled Trials. Clin. Sci. Nutr. 2024, 6, 107–117. [Google Scholar] [CrossRef]
- Dall’Acqua, S.; Miolo, G.; Innocenti, G.; Caffieri, S. The Photodegradation of Quercetin: Relation to Oxidation. Molecules 2012, 17, 8898–8907. [Google Scholar] [CrossRef]
- Zou, H.; Ye, H.; Kamaraj, R.; Zhang, T.; Zhang, J.; Pavek, P. A Review on Pharmacological Activities and Synergistic Effect of Quercetin with Small Molecule Agents. Phytomedicine 2021, 92, 153736. [Google Scholar] [CrossRef]
- Veckenstedt, A.; Güttner, J.; Béládi, I. Synergistic Action of Quercetin and Murine Alpha/Beta Interferon in the Treatment of Mengo Virus Infection in Mice. Antivir. Res. 1987, 7, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Kostova, I.; Raleva, S.; Genova, P.; Argirova, R. Structure-Activity Relationships of Synthetic Coumarins as HIV-1 Inhibitors. Bioinorg. Chem. Appl. 2006, 2006, 68274. [Google Scholar] [CrossRef]
- Torres, F.; Brucker, N.; Andrade, S.; Kawano, D.; Garcia, S.; Poser, G.; Eifler-Lima, V. New Insights into the Chemistry and Antioxidant Activity of Coumarins. Curr. Top. Med. Chem. 2014, 14, 2600–2623. [Google Scholar] [CrossRef]
- Pan, L.; Li, X.; Yan, Z.; Guo, H.; Qin, B. Phytotoxicity of Umbelliferone and Its Analogs: Structure–Activity Relationships and Action Mechanisms. Plant Physiol. Biochem. 2015, 97, 272–277. [Google Scholar] [CrossRef]
- Xiao, S.; Tian, Z.; Wang, Y.; Si, L.; Zhang, L.; Zhou, D. Recent Progress in the Antiviral Activity and Mechanism Study of Pentacyclic Triterpenoids and Their Derivatives. Med. Res. Rev. 2018, 38, 951–976. [Google Scholar] [CrossRef] [PubMed]
- Shintre, M.S.; Gaonkar, T.A.; Modak, S.M. Evaluation of an Alcohol-Based Surgical Hand Disinfectant Containing a Synergistic Combination of Farnesol and Benzethonium Chloride for Immediate and Persistent Activity Against Resident Hand Flora of Volunteers and with a Novel In Vitro Pig Skin Model. Infect. Control Hosp. Epidemiol. 2007, 28, 191–197. [Google Scholar] [CrossRef]
- Huang, Y.; Xie, F.-J.; Cao, X.; Li, M.-Y. Research Progress in Biosynthesis and Regulation of Plant Terpenoids. Biotechnol. Biotechnol. Equip. 2021, 35, 1799–1808. [Google Scholar] [CrossRef]
- Hagvall, L.; Karlberg, A.-T.; Christensson, J.B. Contact Allergy to Air-Exposed Geraniol: Clinical Observations and Report of 14 Cases. Contact Dermat. 2012, 67, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.A.; Sallem, O.W.; Abdelhassib, M.R.; Eldahshan, O.A. Potentiation of Anti-Helicobacter pylori Activity of Clarithromycin by Pelargonium graveolens Oil. Arab. J. Gastroenterol. 2021, 22, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.A.; Nagarajan, S.K.; Ramesh, V.; Palaniyandi, V.; Selvam, S.P.; Madhavan, T. Computational Evaluation of Major Components from Plant Essential Oils as Potent Inhibitors of SARS-CoV-2 Spike Protein. J. Mol. Struct. 2020, 1221, 128823. [Google Scholar] [CrossRef]
- Sivaraman, D.; Pradeep, P. Revealing Anti-Viral Potential of Bio-Active Therapeutics Targeting SARS-CoV2-Polymerase (RdRp) in Combating COVID-19: Molecular Investigation on Indian Traditional Medicines. arXiv 2020. [Google Scholar] [CrossRef][Green Version]
- Kumar, A.; Choudhir, G.; Shukla, S.K.; Sharma, M.; Tyagi, P.; Bhushan, A.; Rathore, M. Identification of Phytochemical Inhibitors Against Main Protease of COVID-19 Using Molecular Modeling Approaches. J. Biomol. Struct. Dyn. 2021, 39, 3760–3770. [Google Scholar] [CrossRef]
- Besli, N.; Ercin, N.; Carmena-Bargueño, M.; Sarikamis, B.; Kalkan Cakmak, R.; Yenmis, G.; Pérez-Sánchez, H.; Beker, M.; Kilic, U. Research into How Carvacrol and Metformin Affect Several Human Proteins in a Hyperglycemic Condition: A Comparative Study In Silico and In Vitro. Arch. Biochem. Biophys. 2024, 758, 110062. [Google Scholar] [CrossRef]
- Akermi, S.; Smaoui, S.; Chaari, M.; Elhadef, K.; Gentile, R.; Hait, M.; Roymahapatra, G.; Mellouli, L. Combined In Vitro/In Silico Approaches, Molecular Dynamics Simulations and Safety Assessment of the Multifunctional Properties of Thymol and Carvacrol: A Comparative Insight. Chem. Biodivers. 2024, 21, e202301575. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.; Manumol, M.; Alagarasu, K.; Parashar, D.; Cherian, S. Phytochemicals of Different Medicinal Herbs as Potential Inhibitors Against Dengue Serotype 2 Virus: A Computational Approach. Mol. Biotechnol. 2025, 3599–3612. [Google Scholar] [CrossRef]
- Dantas, L.B.R.; Alcântara, I.S.; Júnior, C.P.S.; de Oliveira, M.R.C.; Martins, A.O.B.P.B.; Dantas, T.M.; Ribeiro-Filho, J.; Coutinho, H.D.M.; Passos, F.R.S.; Quintans-Júnior, L.J.; et al. In Vivo and In Silico Anti-Inflammatory Properties of the Sesquiterpene Valencene. Biomed. Pharmacother. 2022, 153, 113478. [Google Scholar] [CrossRef]
- Chan, Y.-Y.; Li, C.-H.; Shen, Y.-C.; Wu, T.-S. Anti-Inflammatory Principles from the Stem and Root Barks of Citrus medica. Chem. Pharm. Bull. 2010, 58, 61–65. [Google Scholar] [CrossRef]
- Sarowska, J.; Wojnicz, D.; Jama-Kmiecik, A.; Frej-Mądrzak, M.; Choroszy-Król, I. Antiviral Potential of Plants Against Noroviruses. Molecules 2021, 26, 4669. [Google Scholar] [CrossRef]
- Obaid, R.J.; Mughal, E.U.; Naeem, N.; Sadiq, A.; Alsantali, R.I.; Jassas, R.S.; Moussa, Z.; Ahmed, S.A. Natural and Synthetic Flavonoid Derivatives as New Potential Tyrosinase Inhibitors: A Systematic Review. RSC Adv. 2021, 11, 22159–22198. [Google Scholar] [CrossRef]
- Khandelwal, N.; Chander, Y.; Kumar, R.; Riyesh, T.; Dedar, R.K.; Kumar, M.; Gulati, B.R.; Sharma, S.; Tripathi, B.N.; Barua, S.; et al. Antiviral Activity of Apigenin Against Buffalopox: Novel Mechanistic Insights and Drug-Resistance Considerations. Antivir. Res. 2020, 181, 104870. [Google Scholar] [CrossRef]
- Manvar, D.; Mishra, M.; Kumar, S.; Pandey, V.N. Identification and Evaluation of Anti Hepatitis C Virus Phytochemicals from Eclipta alba. J. Ethnopharmacol. 2012, 144, 545–554. [Google Scholar] [CrossRef]
- Wu, C.-C.; Fang, C.-Y.; Cheng, Y.-J.; Hsu, H.-Y.; Chou, S.-P.; Huang, S.-Y.; Tsai, C.-H.; Chen, J.-Y. Inhibition of Epstein-Barr Virus Reactivation by the Flavonoid Apigenin. J. Biomed. Sci. 2017, 24, 2. [Google Scholar] [CrossRef]
- Yi, B.; Chew, B.X.Z.; Chen, H.; Lee, R.C.H.; Fong, Y.D.; Chin, W.X.; Mok, C.K.; Chu, J.J.H. Antiviral Activity of Catechin Against Dengue Virus Infection. Viruses 2023, 15, 1377. [Google Scholar] [CrossRef] [PubMed]
- You, H.-L.; Huang, C.-C.; Chen, C.-J.; Chang, C.-C.; Liao, P.-L.; Huang, S.-T. Anti-Pandemic Influenza A (H1N1) Virus Potential of Catechin and Gallic Acid. J. Chin. Med. Assoc. 2018, 81, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Saivish, M.V.; Pacca, C.C.; da Costa, V.G.; de Lima Menezes, G.; da Silva, R.A.; Nebo, L.; da Silva, G.C.D.; de Aguiar Milhim, B.H.G.; da Silva Teixeira, I.; Henrique, T.; et al. Caffeic Acid Has Antiviral Activity against Ilhéus Virus In Vitro. Viruses 2023, 15, 494. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, M.; Shirasago, Y.; Tanida, I.; Kakuta, S.; Uchiyama, Y.; Shimojima, M.; Hanada, K.; Saijo, M.; Fukasawa, M. Structural Basis of Antiviral Activity of Caffeic Acid Against Severe Fever with Thrombocytopenia Syndrome Virus. J. Infect. Chemother. 2021, 27, 397–400. [Google Scholar] [CrossRef]
- Li, Y.; Yang, D.; Jia, Y.; He, L.; Li, J.; Yu, C.; Liao, C.; Yu, Z.; Zhang, C. Research Note: Anti-Inflammatory Effects and Antiviral Activities of Baicalein and Chlorogenic Acid against Infectious Bursal Disease Virus in Embryonic Eggs. Poult. Sci. 2021, 100, 100987. [Google Scholar] [CrossRef]
- Galochkina, A.V.; Anikin, V.B.; Babkin, V.A.; Ostrouhova, L.A.; Zarubaev, V.V. Virus-Inhibiting Activity of Dihydroquercetin, a Flavonoid from Larix sibirica, Against Coxsackievirus B4 in a Model of Viral Pancreatitis. Arch. Virol. 2016, 161, 929–938. [Google Scholar] [CrossRef]
- Yarmolinsky, L.; Nakonechny, F.; Budovsky, A.; Zeigerman, H.; Khalfin, B.; Sharon, E.; Yarmolinsky, L.; Ben-Shabat, S.; Nisnevitch, M. Antimicrobial and Antiviral Compounds of Phlomis viscosa Poiret. Biomedicines 2023, 11, 441. [Google Scholar] [CrossRef]
- AbuBakar, U.; Low, Z.X.; Aris, M.Z.M.; Lani, R.; Abidin, S.A.Z.; Abdullah-Zawawi, M.-R.; Hassandarvish, P.; Karsani, S.A.; Khairat, J.E. Antiviral Potential of Diosmin Against Influenza A Virus. Sci. Rep. 2025, 15, 17192. [Google Scholar] [CrossRef]
- Huang, L.; Kuang, J.; Yu, J.; Yu, Q.; Xu, W.; Liu, M.; Wei, Y.; Han, S.; Huang, Y.; Li, P. Antiviral Activity of Epicatechin Against Singapore Grouper Iridovirus In Vitro and In Vivo. Fish Shellfish. Immunol. 2025, 162, 110331. [Google Scholar] [CrossRef] [PubMed]
- Al-Shuhaib, M.B.S.; Hashim, H.O.; Al-Shuhaib, J.M.B. Epicatechin Is a Promising Novel Inhibitor of SARS-CoV-2 Entry by Disrupting Interactions between Angiotensin-Converting Enzyme Type 2 and the Viral Receptor Binding Domain: A Computational/Simulation Study. Comput. Biol. Med. 2022, 141, 105155. [Google Scholar] [CrossRef]
- Lee, J.-H.; Oh, M.; Seok, J.H.; Kim, S.; Lee, D.B.; Bae, G.; Bae, H.-I.; Bae, S.Y.; Hong, Y.-M.; Kwon, S.-O.; et al. Antiviral Effects of Black Raspberry (Rubus coreanus) Seed and Its Gallic Acid Against Influenza Virus Infection. Viruses 2016, 8, 157. [Google Scholar] [CrossRef]
- Huang, Y.; Zhou, W.; Sun, J.; Ou, G.; Zhong, N.-S.; Liu, Z. Exploring the Potential Pharmacological Mechanism of Hesperidin and Glucosyl Hesperidin against COVID-19 Based on Bioinformatics Analyses and Antiviral Assays. Am. J. Chin. Med. 2022, 50, 351–369. [Google Scholar] [CrossRef] [PubMed]
- Dubey, R.; Dubey, K. Molecular Docking Studies of Bioactive Nicotiflorin against 6W63 Novel Coronavirus 2019 (COVID-19). Comb. Chem. High Throughput Screen. 2021, 24, 874–878. [Google Scholar] [CrossRef] [PubMed]
- Meragelman, K.M.; McKee, T.C.; Boyd, M.R. Anti-HIV Prenylated Flavonoids from Monotes africanus. J. Nat. Prod. 2001, 64, 546–548. [Google Scholar] [CrossRef]
- Tripathi, P.K.; Singh, J.; Gaurav, N.; Garg, D.K.; Patel, A.K. In-Silico and Biophysical Investigation of Biomolecular Interaction between Naringin and NsP2 of the Chikungunya Virus. Int. J. Biol. Macromol. 2020, 160, 1061–1066. [Google Scholar] [CrossRef]
- Babaeekhou, L.; Ghane, M.; Abbas-Mohammadi, M. In Silico Targeting SARS-CoV-2 Spike Protein and Main Protease by Biochemical Compounds. Biologia 2021, 76, 3547–3565. [Google Scholar] [CrossRef]
- Lin, S.-C.; Chen, M.-C.; Li, S.; Lin, C.-C.; Wang, T.T. Antiviral Activity of Nobiletin Against Chikungunya Virus In Vitro. Antivir. Ther. 2017, 22, 689–697. [Google Scholar] [CrossRef]
- Chojnacka, K.; Witek-Krowiak, A.; Skrzypczak, D.; Mikula, K.; Młynarz, P. Phytochemicals Containing Biologically Active Polyphenols as an Effective Agent Against COVID-19-Inducing Coronavirus. J. Funct. Foods 2020, 73, 104146. [Google Scholar] [CrossRef]
- Bachmetov, L.; Gal-Tanamy, M.; Shapira, A.; Vorobeychik, M.; Giterman-Galam, T.; Sathiyamoorthy, P.; Golan-Goldhirsh, A.; Benhar, I.; Tur-Kaspa, R.; Zemel, R. Suppression of Hepatitis C Virus by the Flavonoid Quercetin Is Mediated by Inhibition of NS3 Protease Activity. J. Viral Hepat. 2012, 19, e81–e88. [Google Scholar] [CrossRef]
- Choi, H.-J. In Vitro Antiviral Activity of Sakuranetin Against Human Rhinovirus 3. Osong Public Health Res. Perspect. 2017, 8, 415–420. [Google Scholar] [CrossRef]
- Kwon, D.-H.; Ji, J.-H.; Yim, S.-H.; Kim, B.-S.; Choi, H.-J. Suppression of Influenza B Virus Replication by Sakuranetin and Mode of Its Action. Phytother. Res. 2018, 32, 2475–2479. [Google Scholar] [CrossRef]
- Jamshidinia, N.; Saadatpour, F.; Arefian, E.; Mohammadipanah, F. Augmented Antiviral Activity of Chlorhexidine Gluconate on Herpes Simplex Virus Type 1, H1N1 Influenza A Virus, and Adenovirus in Combination with Salicylic Acid. Arch. Virol. 2023, 168, 302. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.-J.; Wu, X.; Li, M.-M.; Li, G.-Q.; Yang, Y.-T.; Luo, H.-J.; Huang, W.-H.; Chung, H.Y.; Ye, W.-C.; Wang, G.-C.; et al. Antiviral Activity of Polymethoxylated Flavones from “Guangchenpi”, the Edible and Medicinal Pericarps of Citrus reticulata ‘Chachi’. J. Agric. Food Chem. 2014, 62, 2182–2189. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, N.M.; Al-Sayed, E.; Moghannem, S.; Azam, F.; El-Shazly, M.; Singab, A.N. Breaking Down the Barriers to a Natural Antiviral Agent: Antiviral Activity and Molecular Docking of Erythrina Speciosa Extract, Fractions, and the Major Compound. Chem. Biodivers. 2020, 17, e1900511. [Google Scholar] [CrossRef]
- Yang, Q.-Y.; Tian, X.-Y.; Fang, W.-S. Bioactive Coumarins from Boenninghausenia sessilicarpa. J. Asian Nat. Prod. Res. 2007, 9, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Sunthitikawinsakul, A.; Kongkathip, N.; Kongkathip, B.; Phonnakhu, S.; Daly, J.W.; Spande, T.F.; Nimit, Y.; Napaswat, C.; Kasisit, J.; Yoosook, C. Anti-HIV-1 Limonoid: First Isolation from Clausena excavata. Phytother. Res. 2003, 17, 1101–1103. [Google Scholar] [CrossRef]
- Su, C.-R.; Yeh, S.F.; Liu, C.M.; Damu, A.G.; Kuo, T.-H.; Chiang, P.-C.; Bastow, K.F.; Lee, K.-H.; Wu, T.-S. Anti-HBV and Cytotoxic Activities of Pyranocoumarin Derivatives. Bioorganic Med. Chem. 2009, 17, 6137–6143. [Google Scholar] [CrossRef]
- Baggieri, M.; Gioacchini, S.; Borgonovo, G.; Catinella, G.; Marchi, A.; Picone, P.; Vasto, S.; Fioravanti, R.; Bucci, P.; Kojouri, M.; et al. Antiviral, Virucidal and Antioxidant Properties of Artemisia Annua Against SARS-CoV-2. Biomed. Pharmacother. 2023, 168, 115682. [Google Scholar] [CrossRef]
- Sotoudeheian, M.; Mirahmadi, S.-M.-S.; Pirhayati, M.; Farahmandian, N.; Azarbad, R.; Toroudi, H.P. Targeting SIRT1 by Scopoletin to Inhibit XBB.1.5 COVID-19 Life Cycle. Curr. Rev. Clin. Exp. Pharmacol. 2025, 20, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Mendis, W.R.H.; Lim, J.-W.; Jung, S.-J.; Kang, S.Y. Antiviral Effects of Umbelliferone against Viral Hemorrhagic Septicemia Virus in Olive Flounder (Paralichthys olivaceus). Fish Shellfish. Immunol. 2024, 152, 109767. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wu, N.; Zu, Y.; Fu, Y. Comparative Anti-Infectious Bronchitis Virus (IBV) Activity of (-)-Pinene: Effect on Nucleocapsid (N) Protein. Molecules 2011, 16, 1044–1054. [Google Scholar] [CrossRef] [PubMed]
- Astani, A.; Reichling, J.; Schnitzler, P. Comparative Study on the Antiviral Activity of Selected Monoterpenes Derived from Essential Oils. Phytother. Res. 2010, 24, 673–679. [Google Scholar] [CrossRef]
- Wang, L.; Wang, D.; Wu, X.; Xu, R.; Li, Y. Antiviral Mechanism of Carvacrol on HSV-2 Infectivity Through Inhibition of RIP3-Mediated Programmed Cell Necrosis Pathway and Ubiquitin-Proteasome System in BSC-1 Cells. BMC Infect. Dis. 2020, 20, 832. [Google Scholar] [CrossRef]
- Sánchez, C.; Aznar, R.; Sánchez, G. The Effect of Carvacrol on Enteric Viruses. Int. J. Food Microbiol. 2015, 192, 72–76. [Google Scholar] [CrossRef]
- Gómez, L.A.; Stashenko, E.; Ocazionez, R.E. Comparative Study on In Vitro Activities of Citral, Limonene and Essential Oils from Lippia citriodora and L. Alba on Yellow Fever Virus. Nat. Prod. Commun. 2013, 8, 249–252. [Google Scholar] [CrossRef]
- Ornan, İ.E.; Özcelik, B.; Kartal, M.; Kan, Y. Antimicrobial and Antiviral Effects of Essential Oils from Selected Umbelliferae and Labiatae Plants and Individual Essential Oil Components. Turk. J. Biol. 2012, 36, 239–246. [Google Scholar] [CrossRef]
- Astani, A.; Reichling, J.; Schnitzler, P. Screening for Antiviral Activities of Isolated Compounds from Essential Oils. Evid. Based Complement. Altern. Med. 2011, 2011, 253643. [Google Scholar] [CrossRef]
- Shoji, Y.; Ishige, H.; Tamura, N.; Iwatani, W.; Norimatsu, M.; Shimada, J.; Mizushima, Y. Enhancement of Anti-Herpetic Activity of Antisense Phosphorothioate Oligonucleotides 5′ End Modified with Geraniol. J. Drug Target. 1998, 5, 261–273. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, L.; Li, R.; Yan, Y.; Yin, J.; Dai, Q.; Guo, X.; Li, W.; Li, Y.; Liu, M.; et al. In Vitro and In Vivo Antiviral Activity of Maqian (Zanthoxylum myriacanthum Var. Pubescens) Essential Oil and Its Major Constituents Against Strains of Influenza Virus. Ind. Crops Prod. 2022, 177, 114524. [Google Scholar] [CrossRef]
- Battinelli, L.; Mengoni, F.; Lichtner, M.; Mazzanti, G.; Saija, A.; Mastroianni, C.M.; Vullo, V. Effect of Limonin and Nomilin on HIV-1 Replication on Infected Human Mononuclear Cells. Planta Medica 2003, 69, 910–913. [Google Scholar] [CrossRef] [PubMed]
- Chiang, L.-C.; Ng, L.-T.; Cheng, P.-W.; Chiang, W.; Lin, C.-C. Antiviral Activities of Extracts and Selected Pure Constituents of Ocimum basilicum. Clin. Exp. Pharmacol. Physiol. 2005, 32, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Vardhan, S.; Sahoo, S.K. In Silico ADMET and Molecular Docking Study on Searching Potential Inhibitors from Limonoids and Triterpenoids for COVID-19. Comput. Biol. Med. 2020, 124, 103936. [Google Scholar] [CrossRef]
- Silva, L.M.; Hill, L.E.; Figueiredo, E.; Gomes, C.L. Delivery of Phytochemicals of Tropical Fruit By-Products Using Poly (Dl-Lactide-Co-Glycolide) (PLGA) Nanoparticles: Synthesis, Characterization, and Antimicrobial Activity. Food Chem. 2014, 165, 362–370. [Google Scholar] [CrossRef]
- Kanojiya, D.; Parmar, G.; Chauhan, B.; Gondalia, S.; Rakholiya, M. Phytosomes: A Contemporary Method for Delivering Novel Herbal Drugs. J. Nat. Remedies 2024, 24, 239–253. [Google Scholar] [CrossRef]
- Khan, J.; Alexander, A.; Ajazuddin; Saraf, S.; Saraf, S. Recent Advances and Future Prospects of Phyto-Phospholipid Complexation Technique for Improving Pharmacokinetic Profile of Plant Actives. J. Control. Release 2013, 168, 50–60. [Google Scholar] [CrossRef]
- Dipierro, F.; Khan, A.; Bertuccioli, A.; Maffioli, P.; Derosa, G.; Khan, S.; Khan, B.A.; Nigar, R.; Ujjan, I.; Devrajani, B.R. Quercetin Phytosome® as a Potential Candidate for Managing COVID-19. Minerva Gastroenterol. 2021, 67, 19–195. [Google Scholar] [CrossRef]
- Dinda, B.; Dinda, M.; Dinda, S.; Ghosh, P.S.; Das, S.K. Anti-SARS-CoV-2, Antioxidant and Immunomodulatory Potential of Dietary Flavonol Quercetin: Focus on Molecular Targets and Clinical Efficacy. Eur. J. Med. Chem. Rep. 2024, 10, 100125. [Google Scholar] [CrossRef]
- Kalita, B.; Patwary, B.N. Formulation and In Vitro Evaluation of Hesperidin-Phospholipid Complex and Its Antioxidant Potential. Curr. Drug Ther. 2020, 15, 28–36. [Google Scholar] [CrossRef]
- Saleh, A.; Abdelkader, D.H.; El-Masry, T.A.; Eliwa, D.; Alotaibi, B.; Negm, W.A.; Elekhnawy, E. Antiviral and Antibacterial Potential of Electrosprayed PVA/PLGA Nanoparticles Loaded with Chlorogenic Acid for the Management of Coronavirus and Pseudomonas Aeruginosa Lung Infection. Artif. Cells Nanomed. Biotechnol. 2023, 51, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Elimam, H.; El-Sawy, H.S.; Fayed, M.A.A.; Mahmoud, S.H.; Bakr, R.O.; Saleh, R.M.; Mostafa, A.; Elshal, M.F. Antiviral Potential of Rosuvastatin and Hesperidin in Combination with Favipiravir Liposomal Nanoformulations in Targeting the Main Protease (Mpro) of SARS-CoV-2: Molecular Docking, Molecular Dynamics and in-Vitro Studies. J. Drug Deliv. Sci. Technol. 2024, 97, 105799. [Google Scholar] [CrossRef]
- Khursheed, R.; Dua, K.; Vishwas, S.; Gulati, M.; Jha, N.K.; Aldhafeeri, G.M.; Alanazi, F.G.; Goh, B.H.; Gupta, G.; Paudel, K.R.; et al. Biomedical Applications of Metallic Nanoparticles in Cancer: Current Status and Future Perspectives. Biomed. Pharmacother. 2022, 150, 112951. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Liu, Z.-G.; Shen, W.; Gurunathan, S. Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef]
- Sarkar, J.; Das, S.; Aich, S.; Bhattacharyya, P.; Acharya, K. Antiviral Potential of Nanoparticles for the Treatment of Coronavirus Infections. J. Trace Elem. Med. Biol. 2022, 72, 126977. [Google Scholar] [CrossRef] [PubMed]
- Mizielińska, M.; Nawrotek, P.; Stachurska, X.; Ordon, M.; Bartkowiak, A. Packaging Covered with Antiviral and Antibacterial Coatings Based on ZnO Nanoparticles Supplemented with Geraniol and Carvacrol. Int. J. Mol. Sci. 2021, 22, 1717. [Google Scholar] [CrossRef]
- Abo-El-Yazid, Z.H.; Ahmed, O.K.; El-Tholoth, M.; Ali, M.A.-S. Green Synthesized Silver Nanoparticles Using Cyperus rotundus L. Extract as a Potential Antiviral Agent Against Infectious Laryngotracheitis and Infectious Bronchitis Viruses in Chickens. Chem. Biol. Technol. Agric. 2022, 9, 55. [Google Scholar] [CrossRef]
- Halder, A.; Das, S.; Ojha, D.; Chattopadhyay, D.; Mukherjee, A. Highly Monodispersed Gold Nanoparticles Synthesis and Inhibition of Herpes Simplex Virus Infections. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 89, 413–421. [Google Scholar] [CrossRef]
- Pilaquinga, F.; Bosch, R.; Morey, J.; Bastidas-Caldes, C.; Torres, M.; Toscano, F.; Debut, A.; Pazmiño-Viteri, K.; de Las Nieves Piña, M. High In Vitro activity of Gold and Silver Nanoparticles from Solanum mammosum L. Against SARS-CoV-2 Surrogate Phi6 and Viral Model PhiX174. Nanotechnology 2023, 34, 175705. [Google Scholar] [CrossRef]
- Ivanova, A.; Ivanova, K.; Fiandra, L.; Mantecca, P.; Catelani, T.; Natan, M.; Banin, E.; Jacobi, G.; Tzanov, T. Antibacterial, Antibiofilm, and Antiviral Farnesol-Containing Nanoparticles Prevent Staphylococcus Aureus from Drug Resistance Development. Int. J. Mol. Sci. 2022, 23, 7527. [Google Scholar] [CrossRef]
- Park, J.H.; Saravanakumar, G.; Kim, K.; Kwon, I.C. Targeted Delivery of Low Molecular Drugs Using Chitosan and Its Derivatives. Adv. Drug Deliv. Rev. 2010, 62, 28–41. [Google Scholar] [CrossRef]
- Pramanik, S.; Sali, V. Connecting the Dots in Drug Delivery: A Tour d’horizon of Chitosan-Based Nanocarriers System. Int. J. Biol. Macromol. 2021, 169, 103–121. [Google Scholar] [CrossRef] [PubMed]
- Emam, M.H.; Mahmoud, M.I.; El-Guendy, N.; Loutfy, S.A. Establishment of In-House Assay for Screening of Anti-SARS-CoV-2 Protein Inhibitors. AMB Express 2024, 14, 104. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Su, F.; Li, J.; Yu, B.; Xu, L.; Xiong, T.; Shao, K.; Yuan, X. Enhanced In Vivo Antiviral Activity against Pseudorabies Virus Through Transforming Gallic Acid into Graphene Quantum Dots with Stimulation of Interferon-Related Immune Responses. J. Mater. Chem. B 2023, 12, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Bosetti, R. Cost–Effectiveness of Nanomedicine: The Path to a Future Successful and Dominant Market? Nanomedicine 2015, 10, 1851–1853. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dahan, A.; Yarmolinsky, L.; Nakonechny, F.; Semenova, O.; Khalfin, B.; Fleisher-Berkovich, S.; Ben-Shabat, S. Etrog Citron (Citrus medica) as a Novel Source of Antiviral Agents: Overview of Its Bioactive Phytochemicals and Delivery Approaches. Pharmaceutics 2025, 17, 1173. https://doi.org/10.3390/pharmaceutics17091173
Dahan A, Yarmolinsky L, Nakonechny F, Semenova O, Khalfin B, Fleisher-Berkovich S, Ben-Shabat S. Etrog Citron (Citrus medica) as a Novel Source of Antiviral Agents: Overview of Its Bioactive Phytochemicals and Delivery Approaches. Pharmaceutics. 2025; 17(9):1173. https://doi.org/10.3390/pharmaceutics17091173
Chicago/Turabian StyleDahan, Arik, Ludmila Yarmolinsky, Faina Nakonechny, Olga Semenova, Boris Khalfin, Sigal Fleisher-Berkovich, and Shimon Ben-Shabat. 2025. "Etrog Citron (Citrus medica) as a Novel Source of Antiviral Agents: Overview of Its Bioactive Phytochemicals and Delivery Approaches" Pharmaceutics 17, no. 9: 1173. https://doi.org/10.3390/pharmaceutics17091173
APA StyleDahan, A., Yarmolinsky, L., Nakonechny, F., Semenova, O., Khalfin, B., Fleisher-Berkovich, S., & Ben-Shabat, S. (2025). Etrog Citron (Citrus medica) as a Novel Source of Antiviral Agents: Overview of Its Bioactive Phytochemicals and Delivery Approaches. Pharmaceutics, 17(9), 1173. https://doi.org/10.3390/pharmaceutics17091173













