1. Introduction
Cancer remains a leading global health burden, with limited therapeutic efficacy and debilitating complications [
1,
2,
3]. Conventional therapeutic approaches include surgery and chemotherapy, with evidence demonstrating that perioperative chemotherapy combined with surgery markedly improves local tumor control and overall survival compared to surgery alone [
4]. Notably, chemotherapy remains indispensable for treating metastatic tumors unsuitable for surgical resection [
5,
6]. Doxorubicin (DOX) is a first-line, broad-spectrum chemotherapeutic agent that exerts its antitumor effects primarily through interference with DNA and RNA synthesis and inhibition of topoisomerase II. [
7,
8,
9]. While demonstrating remarkable efficacy against various solid tumors (e.g., breast, lung, and ovarian cancers) and hematological malignancies (e.g., acute leukemia and lymphoma), DOX administration is hampered by severe toxicities [
10,
11]. The aqueous formulation exhibits dose-limiting cardiotoxicity, manifesting as arrhythmias and heart failure, creating a narrow therapeutic window that restricts clinical utility [
10]. The 1995 FDA approval of PEGylated liposomal DOX (Doxil
®) marked a milestone in nanomedicine. This formulation utilizes an ammonium sulfate gradient loading method to encapsulate DOX crystals within liposomes [
12], demonstrating dual therapeutic advantages: enhanced tumor accumulation through the Enhanced permeability and retention (EPR) effect coupled with reduced cardiac distribution, along with prolonged circulation half-life achieved via PEG-mediated evasion of reticuloendothelial system clearance [
13]. Despite these improvements, Doxil
® had long been criticized for unsatisfactory clinical translation, inefficient drug release due to rigid lipid bilayers, and potential biosafety risks of carrier materials [
14,
15]. These unresolved issues underscore the urgent need for novel drug delivery systems that optimize the balance between efficacy and safety in clinical chemotherapy.
To overcome the limitations of conventional carrier-based nanomedicines, carrier-free nanoassemblies composed of small-molecule drugs or prodrugs have emerged as a promising platform for cancer therapy [
16]. These systems retain the advantages of traditional nanomedicines while offering distinct benefits, including simple fabrication, high drug loading (>50%), and the elimination of carrier-related toxicity [
17,
18]. In particular, prodrug-based nanoassemblies integrate the precision of prodrug design with the efficiency of nanoscale delivery, representing a paradigm shift in drug development [
19]. An ideal prodrug strategy involves transient inactivation of the parent drug via chemical modification, followed by selective reactivation at disease sites, thereby minimizing off-target effects and enhancing therapeutic specificity [
20,
21]. Tumor-specific activation is enabled by the abnormal chemical milieu of tumors, such as elevated levels of reactive oxygen species (ROS) and glutathione (GSH) [
22,
23]. Accordingly, researchers have engineered cleavable linkers—such as disulfide bonds—that respond to these cues and trigger drug release within tumors [
24]. Beyond accelerating drug release, previous studies have shown that certain linkers also enhance the self-assembly of prodrugs by strengthening intermolecular interactions [
19,
25]. These insights have broadened the functional scope of chemical linkers in prodrug-based delivery and sparked growing interest in the design of novel linker chemistries. However, rationally designing linkers that are both tumor-responsive and conducive to nanoassembly remains a significant challenge.
Precise modulation of intermolecular forces is a key driver in the formation of prodrug nanoassemblies, enabling stable self-assembly and efficient delivery [
26,
27]. Strategically designed prodrug molecules can introduce structural defects—such as specific functional group arrangements or steric configurations—that facilitate spontaneous formation of ordered nanostructures [
19,
28]. This process is governed by non-covalent interactions, including π–π stacking, hydrophobic effects, and hydrogen bonding, which collectively stabilize the assembled architecture [
29,
30]. Emerging evidence suggests that tuning hydrophobic and hydrogen bonding interactions can precisely control the morphology, size, and stability of nanoassemblies, thereby regulating drug release kinetics and influencing therapeutic efficacy [
31,
32,
33]. For example, enhanced hydrophobicity may slow drug release and prolong circulation time, while engineered hydrogen bond networks can confer stimuli-responsive release behavior [
34,
35]. Despite the well-recognized role of π–π stacking in organic self-assembly systems [
36], its contribution to prodrug nanoassemblies remains largely unexplored. How π–π interactions influence assembly behavior, shape nanoparticle properties, and impact in vivo delivery and antitumor activity remains unclear. Addressing this gap is essential to advancing structure–function understanding and guiding the design of next-generation anticancer nanomedicines.
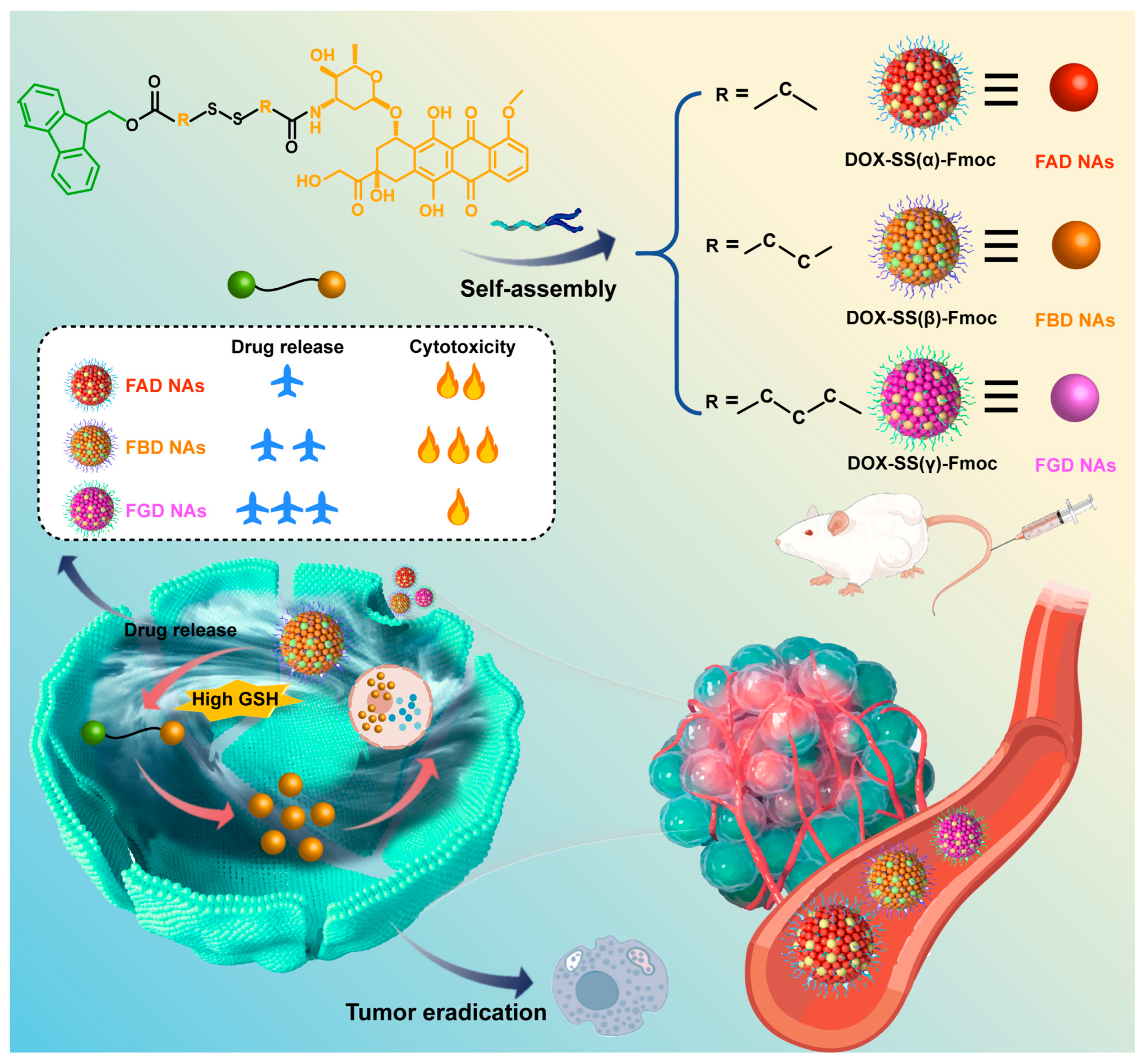
Guided by fundamental principles of molecular self-assembly, we engineered a next-generation DOX delivery system that couples π–π stacking with spatially programmed disulfide bonds (α, β, γ), achieving dual benefits of reduced carrier toxicity and controlled, efficient release. By leveraging the strong π–π stacking capability of Fmoc moieties to facilitate robust nanoassembly formation while systematically investigating the impact of disulfide bond positioning on both structural stability and redox-responsive drug release kinetics. As illustrated in
Scheme 1, we first successfully synthesized three structurally optimized prodrug variants, DOX-SS(α)-Fmoc (FAD), DOX-SS(β)-Fmoc (FBD), and DOX-SS(γ)-Fmoc (FGD), which spontaneously self-assembled into stable carrier-free nanoassemblies (FAD NAs, FBD NAs, FGD NAs). Following intravenous administration, these nanoassemblies maintained structural stability to ensure tumor-targeted delivery while minimizing off-target toxicity of DOX. Upon internalization by tumor cells, the high intracellular GSH levels triggered their disassembly to release DOX, thereby exerting potent antitumor effects. Through comprehensive investigations encompassing prodrug synthesis and structural characterization, nanoassembly preparation and physicochemical evaluation, cellular uptake and cytotoxicity profiling, pharmacokinetic and biodistribution analyses, as well as systematic assessment of antitumor efficacy and preliminary safety evaluation in tumor-bearing models, we demonstrated that FBD NAs exhibited particularly stable tumor reduction-responsive drug release behavior and superior antitumor activity. This integrated approach not only yields critical insights into structure-activity relationships but also establishes a promising optimized DOX delivery platform with significant therapeutic potential.
2. Materials and Methods
2.1. Experimental Materials
Chemical reagents including HBTU, DIPEA, DMAP, DOX‧HCl, and Fmoc were sourced from Anaiji Chemical Co., Ltd. (Shanghai, China). DSPE-PEG2K was acquired from AVTPharmaceutical Technology Co., Ltd. (Shanghai, China).All cell culture vessels (glass-bottom dishes, culture dishes, and plates) were obtained from NEST Biotechnology Co., Ltd. (Wuxi, China).
2.2. Design and Synthesis of Position-Specific Disulfide-Bridged DOX Prodrugs
We referred to the methods in the literature and synthesized three position-specific disulfide bridging DOX prodrugs (FAD, FBD, FGD) through a standardized three-step protocol [
33,
37]. First, dithiodiacids (2 mmol) were reacted with acetic anhydride (5 mL) at 25 °C for 2 h under N
2, followed by toluene-assisted drying. The ints were then coupled with Fmoc (2 mmol) and DMAP (0.2 mmol) in dichloromethane (10 mL) at 25 °C for 12 h, purified by silica column chromatography (CH
2Cl
2: MeOH, 500:1). Finally, the Fmoc intermediates (0.5 mmol) were conjugated with DOX·HCl (0.5 mmol) using HBTU/DIPEA in DMF (10 mL) at 30 °C for 48 h. All products were purified by preparative high-performance liquid chromatography (HPLC, acetonitrile:water, 70:30) with yields >50% and characterized by mass spectrometry (MS) and NMR. The MS characterization of prodrugs was carried out by high-resolution electrospray ionization mass spectrometry (HR-ESI-MS) in the positive ion mode. The molecular structure and purity of the prodrug were confirmed by precisely measuring the molecular ion peaks ([M + H]
+) and characteristic fragment peaks. The specific parameters are as follows: Scanning range:
m/
z 100–1500; drying temperature: 300 °C; capillary voltage: 3.5 kV; and collision energy: 20–40 eV (gradient optimized). The prodrugs were characterized by
1H NMR (400/600 MHz, DMSO-d
6 with 0.03% TMS) at 25 °C, analyzing chemical shifts (δ 0–15 ppm), peak patterns (e.g., aromatic protons at 7.5–8.5 ppm), and integration ratios (e.g., Fmoc 9H protons) with acquisition parameters of 32–64 scans, 12 ppm spectral width, and 2 s relaxation delay.
2.3. Preparation of DOX Prodrug NAs
The three prodrug-based nanoassemblies (FAD NAs, FBD NAs, and FGD NAs) were synthesized using a standardized nanoprecipitation protocol [
38,
39]. In brief, individual prodrugs (1 mg) were initially dissolved in a 1:1 (
v/
v) THF/ethanol cosolvent system (200 μL total volume). This organic solution was then rapidly injected into deionized water (1 mL) under continuous magnetic stirring at 1050 rpm. For PEGylated variants, DSPE-PEG
2K (20%
w/
w relative to prodrug) was incorporated into the organic phase prior to aqueous injection. Following nanoassembly formation, residual organic solvents were removed by rotary evaporation at 30 °C under reduced pressure in 5 min.
Prior to full-scale production, comprehensive parameter optimization was conducted. This systematic evaluation assessed the impact of different organic solvents (THF, ethanol, methanol, acetonitrile, and various combinations) and stirring speeds (650–1950 rpm) on nanoassembly characteristics. Based on extensive characterization data, the THF/ethanol (1:1) cosolvent system combined with 1050 rpm stirring was identified as the optimal formulation condition, yielding nanoassemblies with the most favorable physicochemical properties.
For quality control, the prepared nanoassemblies were characterized using dynamic light scattering (Malvern Zetasizer, NanoZS, Malvern Co., Worcestershire, UK). Samples were appropriately diluted (50 μL nanoassemblies in 950 μL deionized water) and equilibrated for 120 s prior to measurement. We evaluated three critical parameters (hydrodynamic diameter, PDI, and zeta potential) with triplicate measurements to verify reproducibility.
2.4. In Vitro Stability Study of NAs
The colloidal stability of PEGylated FAD NAs, FBD NAs, and FGD NAs (0.5 mg/mL) was systematically evaluated under three conditions: (1) in PBS containing 10% FBS (pH 7.4) at 37 °C with shaking (100 rpm), with particle size monitored over 24 h; (2) during 30-day storage at 4 °C; and (3) the formulations were mixed with 100 mg sucrose in 2 mL tubes, vortexed for complete dissolution, transferred to vials, and lyophilized (−80 °C pre-freeze for 24 h followed by 24 h primary drying) in a freeze-dryer. Reconstitution with 1 mL of deionized water yielded homogeneous nanosuspensions, with stability assessed via pre-/post-lyophilization particle size changes and powder morphology. In addition, the encapsulation rates of the three nanoassemblies during the stability experiment were determined by ultrafiltration and HPLC.
2.5. Evaluation of Intermolecular Interaction
We conducted a comprehensive evaluation of the driving forces underlying nanoassemblies. The computational analysis was performed using the Yinfu Cloud platform (Guangzhou Yinfu Information Technology Co., Ltd., Guangzhou, China), which involved the following: (1) constructing energy-minimized three-dimensional prodrug structures (in mol2 format) with precise disulfide bond positioning and (2) performing small-molecule docking through conformational searching to characterize hydrogen bonding, π–π stacking, and hydrophobic interactions. Then three chemical disruptors were used to probe intermolecular forces in NAs: 50 mM NaCl (electrostatic interactions), 50 mM urea (hydrogen bonds), and 50 mM SDS (hydrophobic interactions). For each test, 900 μL of disruptor solution was mixed with 100 μL of PEGylated prodrug nanoassemblies in 2 mL tubes, then incubated (37 °C, 100 rpm, 3 h). Particle size and PDI were measured pre- and post-incubation. Significant increases confirmed force-specific disruption.
2.6. Drug Release
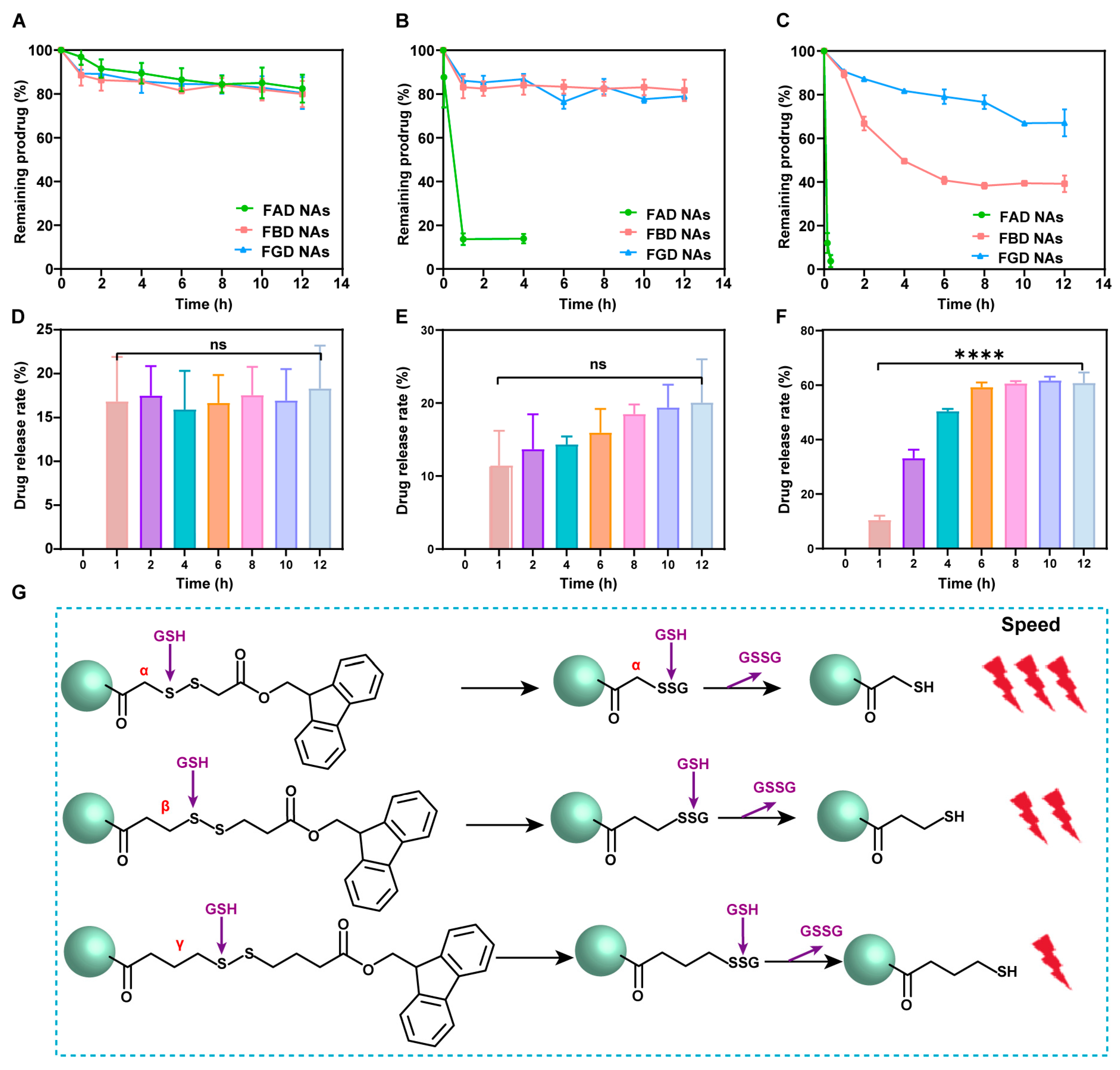
The release study was conducted using PBS (pH 7.4) containing 25% ethanol as the solvent, with three different dithiothreitol (DTT) concentrations (0, 10, and 100 μM). PEGylated FAD NAs, FBD NAs, and FGD NAs were introduced into 5 mL of release medium at a DOX-equivalent concentration of 200 μg/30 mL. The mixtures were then incubated in a constant-temperature shaker maintained at 37 °C with 100 rpm agitation. Aliquots were collected at specified time points: 0, 0.5, 1, 2, 4, 6, 8, 10, and 12 h for standard conditions; 0, 0.5, 1, and 4 h for FAD NAs in 10 μM DTT; and 0, 0.167, and 0.333 h for FAD NAs in 100 μM DTT. The released DOX prodrug was quantified by HPLC through peak area analysis.
2.7. Cell Culture
The mouse breast tumor cell line (4T1 cells), human non-small cell lung cancer (NSCLC) epithelial cell line (A549 cells), and murine fibroblasts (3T1 cells) were derived from the Cell Bank of the Chinese Academy of Sciences (Beijing, China). For cryopreservation, adherent cells at logarithmic growth phase (80% confluency) were trypsinized (0.25% trypsin, 1 min), centrifuged (1000 rpm, 5 min), and resuspended in freezing medium before storage at −80 °C. For recovery, frozen vials were rapidly thawed (37 °C, <1 min), centrifuged, and seeded in pre-warmed medium. Subculture followed the same trypsinization procedure, with a 1:5 split ratio into fresh medium. All cells were maintained at 37 °C/5% CO2 and passaged ≥3 times before experimental use to ensure stable morphology and proliferation.
2.8. Cellular Uptake
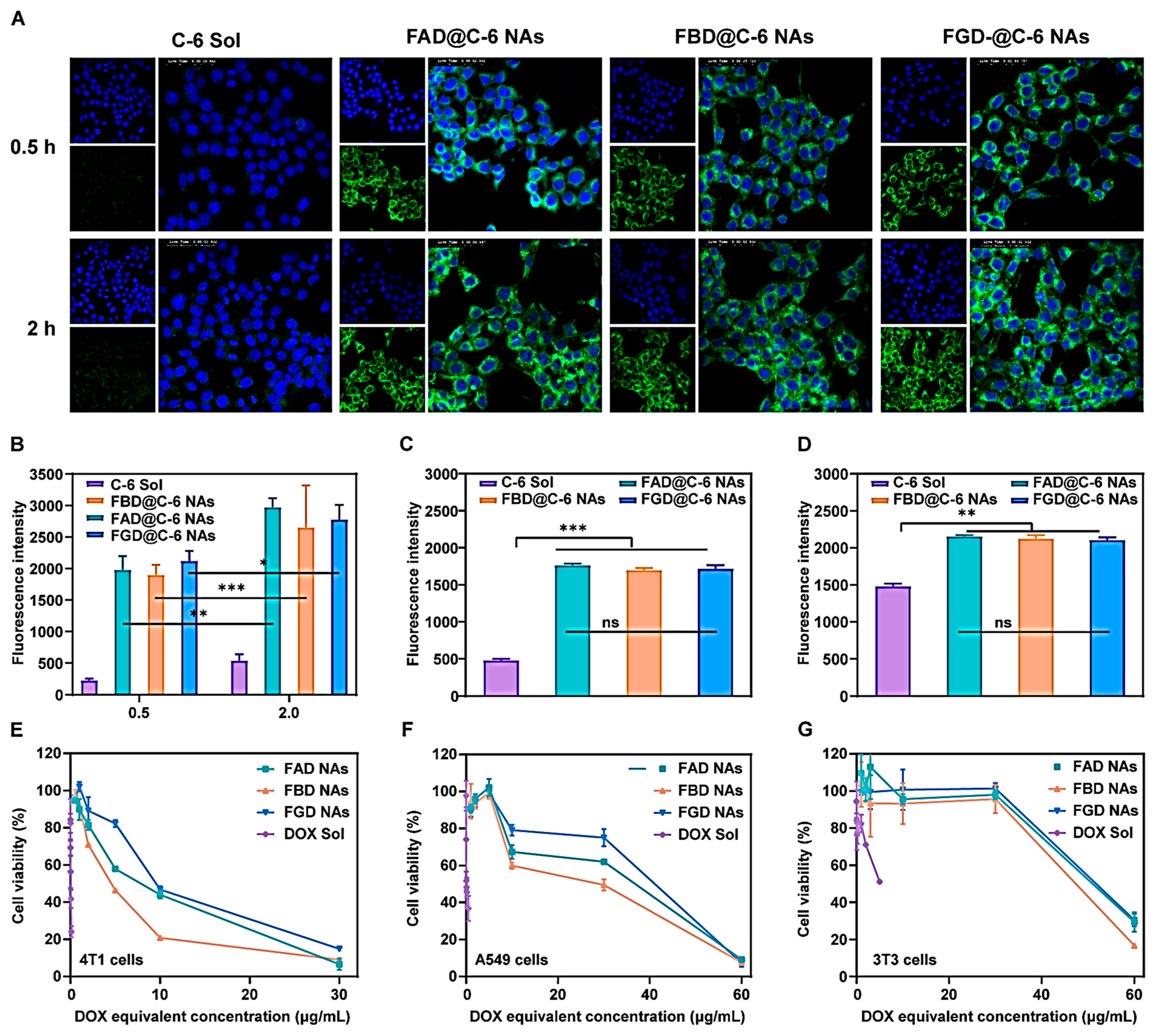
C-6-labeled nanoassemblies were prepared by co-dissolving DOX prodrug and C-6 (1.0 mg each, at a 9:1 molar ratio) in THF/ethanol (1:1, v/v), mixed with DSPE-PEG2K (20% w/w), then nanoprecipitated into deionized water under stirring (1050 rpm, 3 min). Subsequently, organic solvents were removed by rotary evaporation (30 °C) to obtain nanoassemblies (200 μg/mL DOX equivalent, 20 μg/mL C-6). For uptake studies, 4T1 cells were treated with C-6 formulations (200 ng/mL) for 0.5/2 h. Qualitative analysis involved fixation, Hoechst staining, and confocal microscopy by DAPI/FITC channels (CLSM, C2, Nikon, Tokyo, Japan).
After incubation periods of 0.5 or 2 h, 4T1 cells were processed for flow cytometric analysis (BD FACSCalibur, BD Biosciences, San Jose, CA, USA) following the same protocol used for confocal microscopy. Cells were trypsinized, centrifuged, and resuspended in PBS (pH 7.4), then filtered into flow cytometry tubes for quantitative fluorescence measurement. All procedures were performed under light-protected conditions to prevent fluorophore degradation.
2.9. In Vitro Cytotoxicity Investigation
The cytotoxic effects of DOX Sol, FAD NAs, FBD Nas, and FGD NAs on tumor cells (4T1 and A549 cells) and normal cells (3T3 cells) were evaluated by MTT assay. Briefly, cells were enzymatically detached with trypsin, pelleted by centrifugation (1000 rpm, 5 min), and adjusted to 104 cells/mL in complete medium. Cell suspensions (2000 cells/well) were seeded in 96-well plates and allowed to adhere for 15 h under standard culture conditions (37 °C, 5% CO2). Post-treatment, 20 μL of MTT reagent (5 mg/mL in PBS) was introduced per well. Following 4 h of metabolic conversion, the resulting formazan crystals were solubilized in 200 μL DMSO with orbital shaking (30 min, 300 rpm). Optical density was quantified at 490 nm using a microplate reader (ThermoFisher Scientific, Waltham, MA, USA), with cell viability expressed relative to untreated controls. Dose–response curves were generated via nonlinear regression to derive IC50 values. Strict aseptic techniques were maintained throughout all procedures.
2.10. Animal Housing
All experimental animals were purchased from Liaoning Changsheng Biotechnology Co., Ltd., with confirmed good health status, without genetic modification, and without any prior experimental procedures. Animal procedures were approved by the Institutional Animal Care and Use Committee of Shenyang Pharmaceutical University (Shenyang, China). The animal study protocol was approved by the Institutional Animal Ethics Committee (Approval Code: SYPU-2ACUC-S2024-0919-201; Approval Date: 19 September 2024). The study used adult male Sprague-Dawley (SD) rats (8–12 weeks old, ~200 g) and adult female BALB/c mice (6–8 weeks old, ~20 g). 20 SD rats and 68 BALB/c mice were housed under standard laboratory conditions with free access to food and water. Randomization of treatment order and cage positions was implemented, with blinded assessments to minimize handling and environmental biases.
2.11. Pharmacokinetics
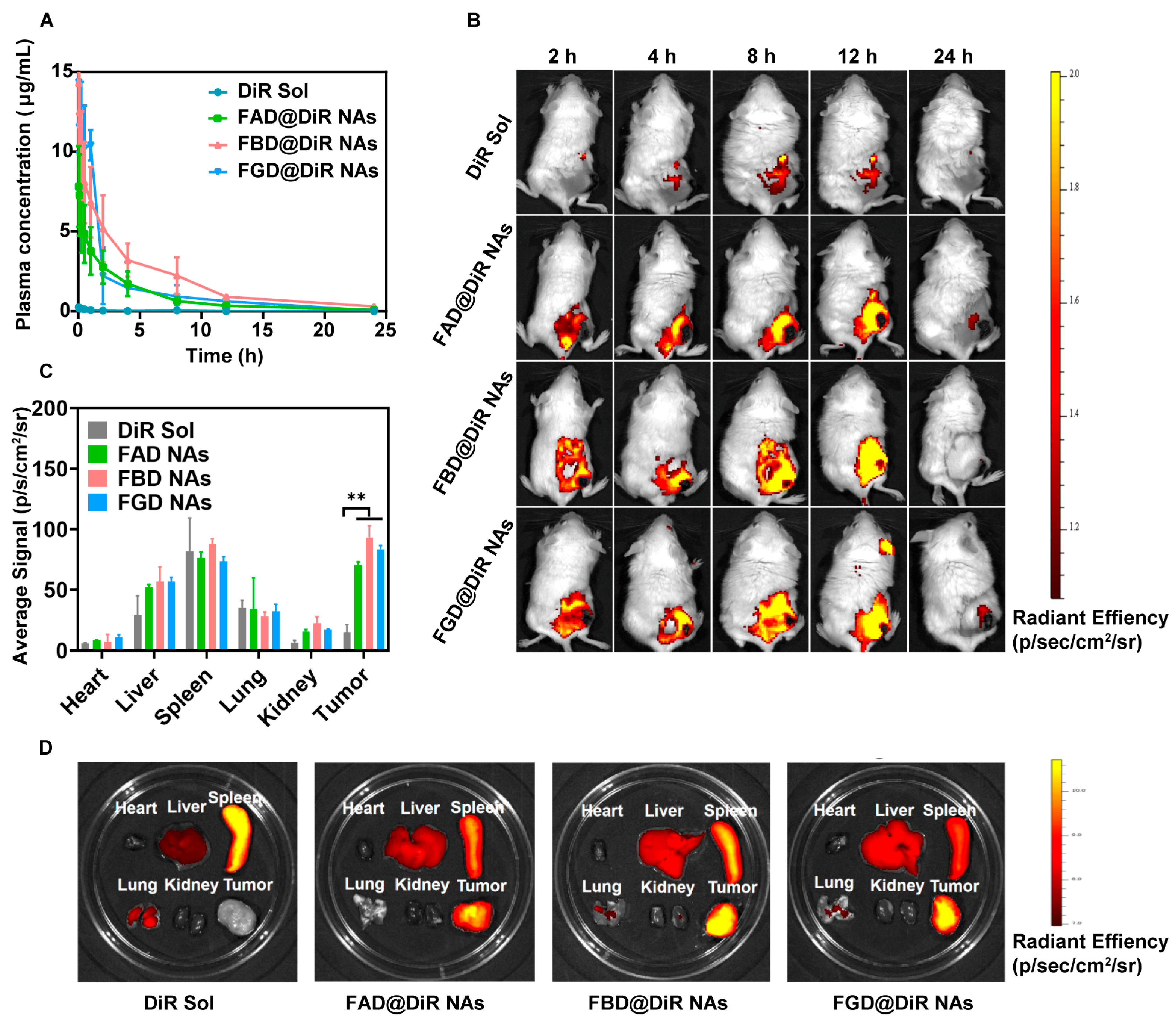
A total of 20 SD rats were randomly allocated into four treatment groups: DiR Sol, FAD@DiR NAs, FBD@DiR NAs, and FGD@DiR NAs (n = 5 per group; the experimental unit is a single animal). Test compounds—either DiR Sol or DiR-labeled prodrug nanoassemblies—were administered via tail vein injection at a standardized DiR-equivalent dose of 2 mg/kg. Serial blood samples were obtained through the ophthalmic venous plexus at predetermined time points. Following collection, whole blood was immediately centrifuged to isolate plasma fractions. DiR concentrations in plasma samples were subsequently quantified using fluorescence measurement with a microplate reader, with appropriate standard curves and quality controls included in each assay run. No animals, experimental units, or data points were excluded, as all animals maintained normal health status, body weight, and experimental data without abnormalities.
2.12. Biodistribution
For tumor model establishment, 4T1 cells suspended in PBS (pH 7.4) were subcutaneously inoculated into the right flank of female BALB/c mice. When tumor volumes reached approximately 400 mm3, the 20 tumor-bearing mice were randomly allocated into four treatment groups: DiR Sol, FAD@DiR NAs, FBD@DiR NAs, and FGD@DiR NAs (n = 5) and intravenously administered either DiR Sol or DiR-labeled prodrug nanoassemblies at a DiR-equivalent dose of 2 mg/kg. Whole-body fluorescence imaging was conducted using an IVIS Spectrum imaging system (PerkinElmer IVIS Spectrum, Waltham, MA, USA) at predetermined time intervals. At the predetermined peak fluorescence time point, mice were euthanized, followed by excision of tumors and major organs (heart, liver, spleen, lungs, and kidneys) for ex vivo fluorescence imaging analysis. No animals, experimental units, or data points were excluded, as all animals maintained normal health status, body weight, and experimental data without abnormalities.
2.13. In Vivo Anti-Tumor Efficiency of Three NAs
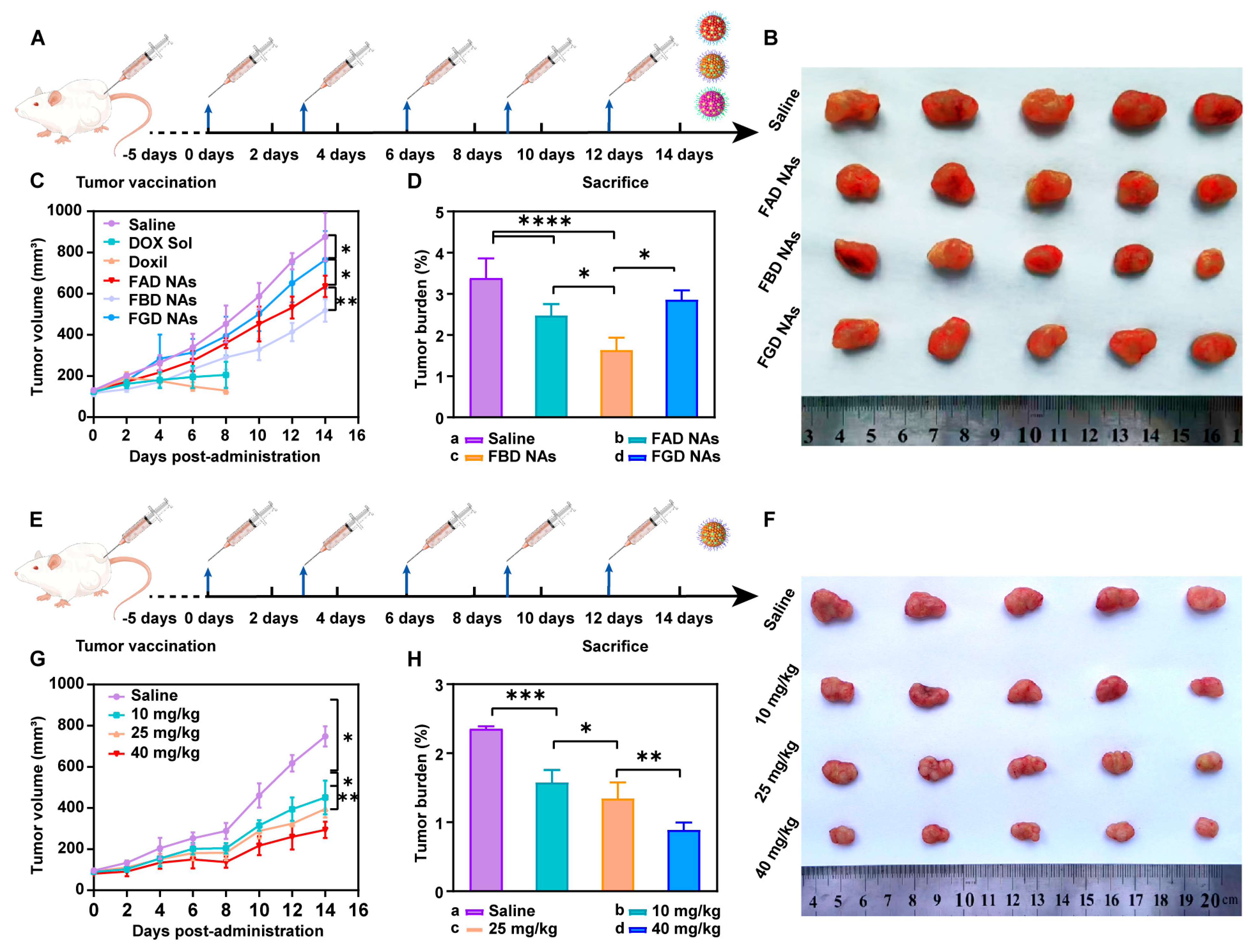
When 48 4T1 tumor-bearing BALB/c mice reached a tumor volume of ~100 mm3, they were randomly divided into six groups (n = 5): Saline, DOX Sol, Doxil, FAD NAs, FBD NAs, and FGD NAs. Treatments were administered intravenously (DOX-equivalent dose: 10 mg/kg) every 3 days for five cycles (Day 0–12). Tumor volumes were calculated (V = a × b2/2, where a and b are the long and short diameters, respectively), and growth curves were plotted. On Day 14 (48 h post-final dose), mice were euthanized, and tumors were excised, weighed, and fixed for histopathological analysis (H&E staining, Servicebio, Wuhan). Serum biochemical parameters were analyzed to assess hepatorenal function. The initial group size was n = 8. During the experiment, animals from certain groups that failed to meet health standards were excluded, ensuring a final count of n = 5 per group for analysis. We also performed in vivo efficacy studies of FBD NAs at varying doses.
2.14. Processing of Animal Experimental Data
Animals were randomly assigned to control and treatment groups to prevent selection bias (e.g., avoiding unintentionally assigning healthier animals to one group), balance confounding variables (e.g., weight, age, litter effects), and meet statistical assumptions (e.g., independence of observations). For basic comparisons (e.g., *t*-tests or one-way ANOVA), n = 5 per group may provide ~80% power to detect a large effect size (e.g., Cohen’s *d* > 1.0) if variability is low. Healthy animals meeting predefined age, weight, and baseline health criteria were included in the study, while those exhibiting severe adverse effects (>20% weight loss) or technical failures were excluded, with all criteria established a priori. Data points identified as outliers (>2SD) through predefined statistical methods were excluded, and technical errors were discarded. The study adhered to these pre-established inclusion/exclusion protocols throughout the experimental and analytical processes. Animals were randomly assigned to control and treatment groups to prevent selection bias (e.g., avoiding unintentionally assigning healthier animals to one group), balance confounding variables (e.g., weight, age, litter effects), and meet statistical assumptions (e.g., independence of observations). Only the randomization coordinator knew group assignments during allocation but was excluded from subsequent procedures, while all other personnel (treatment administrators, outcome assessors, and statisticians) remained blinded throughout the study, using coded identifiers and analyzing de-identified data until final results were completed.
2.15. Statistical Analysis
All statistical analyses were conducted using GraphPad Prism version 9.0 (GraphPad Software, San Diego, CA, USA). Continuous variables are expressed as mean ± standard deviation. Between-group differences were analyzed by two-tailed Student’s t-test or one-way ANOVA followed by Tukey’s post hoc test, respectively, for two groups and multiple groups. Statistical significance was defined as p < 0.05, with the following levels indicated: * p < 0.05, ** p < 0.01, and *** p < 0.001.
4. Conclusions and Discussion
In this study, we elucidate that the antitumor efficacy of precisely engineered prodrug nanoassemblies is intricately governed by the strategic placement of disulfide bonds in conjunction with π–π stacking interactions. These two factors synergistically modulate the drug release kinetics, thereby offering a solution to the long-standing delivery-release paradox that has plagued the field of nanomedicine. Our investigations reveal that robust π–π stacking interactions endow the prodrug nanoassemblies with exceptional self-driven assembly properties, facilitating their formation and stability. Concurrently, the systematic manipulation of disulfide bond locations within the prodrug architecture provides a precise means of controlling the drug liberation dynamics. Our experimental results clearly demonstrate position-dependent drug release kinetics, following the order of α > β > γ. Notably, the β-disulfide configuration emerges as the most clinically promising candidate, as it strikes an optimal balance between systemic stability and specific drug release, thus achieving the desired therapeutic efficacy.
This research represents an innovative integration of tumor reduction-responsive prodrug design with carrier-free nanoassembly technology. We have successfully developed three DOX prodrug nanoassemblies, namely FAD, FBD, and FGD NAs, which are distinguished by the different positions of disulfide bonds (α, β, and γ). To enhance the intermolecular interactions within these nanoassemblies, we incorporated 9-fluorenylmethanol as an aromatic conjugated side chain. Subsequently, we conducted a systematic evaluation of the impact of disulfide bond positioning on the properties of these nanoassemblies. The results indicate that the β-disulfide modified FBD NAs exhibit superior performance across multiple aspects. Firstly, they possess high stability, as evidenced by a particle size of approximately 110 nm, a zeta potential of −40 mV, and a drug loading capacity ranging from 45% to 50%. Secondly, they demonstrate controlled reduction-responsive release, with the release rate following the order of FAD > FBD > FGD. Thirdly, compared to their α- and γ-modified counterparts, FBD NAs exhibit superior cytotoxicity, more favorable pharmacokinetics (as indicated by the highest AUC0–24h value), and enhanced tumor accumulation. In 4T1 tumor-bearing mice, FBD NAs not only achieved significantly enhanced antitumor efficacy compared to FAD and FGD NAs but also maintained safety at a dose of 40 mg/kg (DOX equivalent), effectively addressing the toxicity limitations associated with conventional DOX formulations such as solutions and liposomes. These findings underscore the critical importance of disulfide bond positioning in prodrug design, with β-site modification uniquely optimizing the stability and release kinetics of the nanoassemblies. Overall, this study establishes a robust prodrug design strategy and a comprehensive structure-activity relationship framework, which hold great promise for advancing the clinical translation of tumor-targeted nanomedicines.