Population PK Modeling of Denosumab Biosimilar MB09 and Reference Denosumab to Establish PK Similarity
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Subjects
2.2. Data Analysis
2.3. PK Modeling Development
2.3.1. Base PK Model
2.3.2. Variability Models
Model for Inter-Individual Variability
Model for Residual Variability
2.3.3. Covariate Analysis
2.4. Model Evaluation and Model-Based Bioequivalence
2.5. Simulations
3. Results
3.1. Subjects Characteristics
3.2. Modeling Results
3.2.1. PK Model Phase I Study
3.2.2. Comparison of Treatments
3.2.3. PK Model Both Studies
3.3. Model Evaluation Results
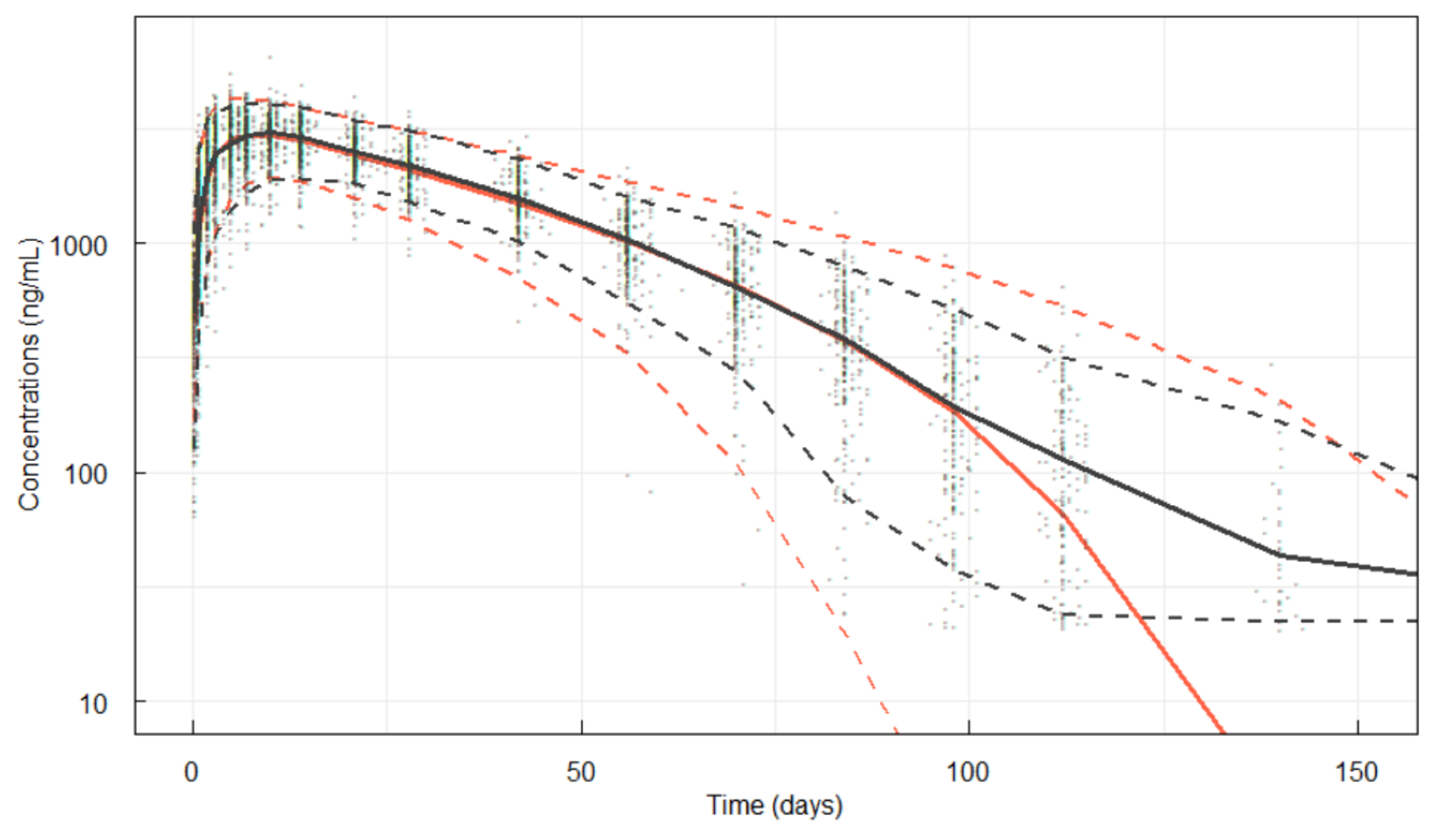
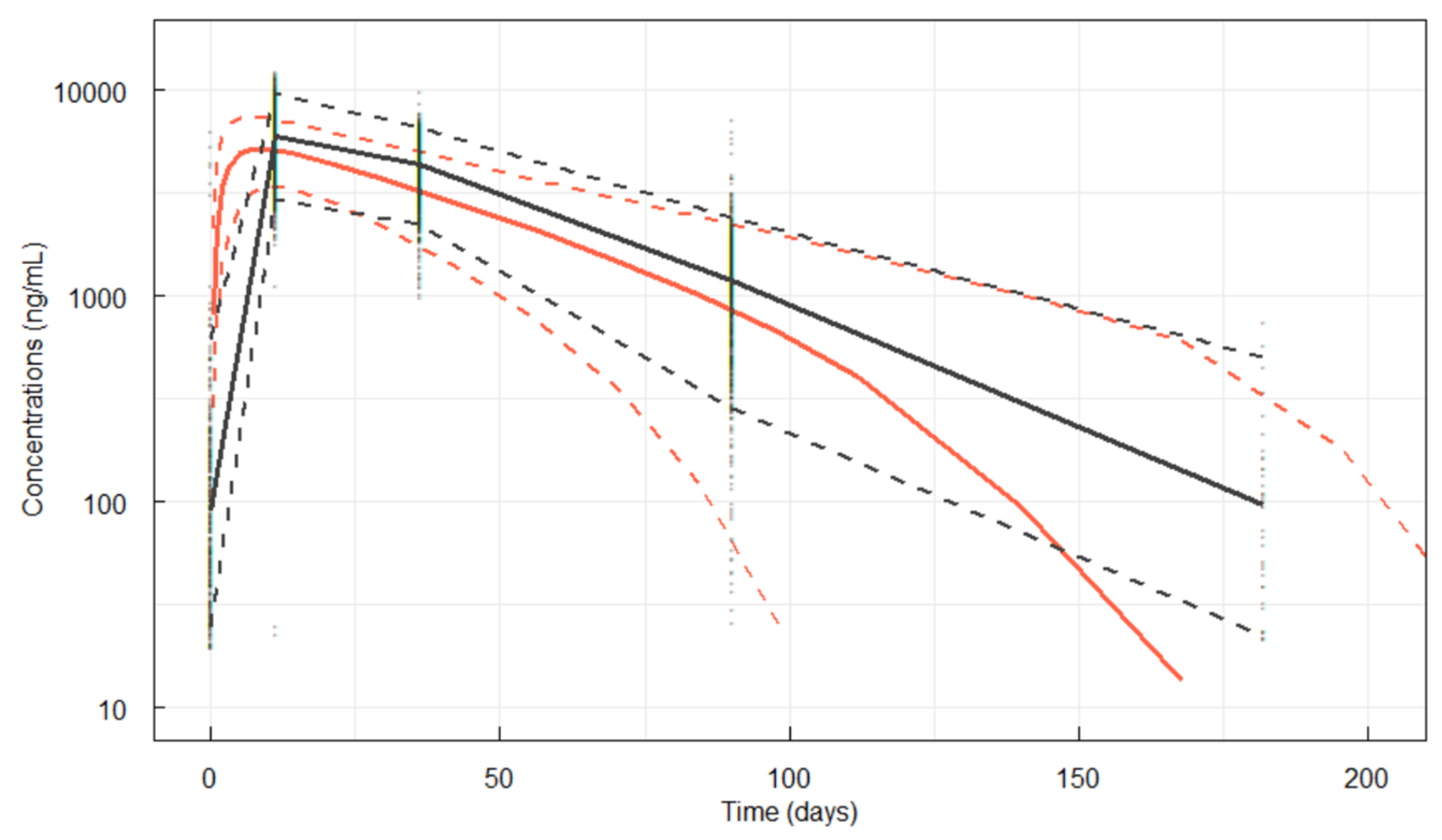
3.3.1. Visual Predictive Check
3.3.2. Sensitivity Analysis
3.4. Model-Based Bioequivalence
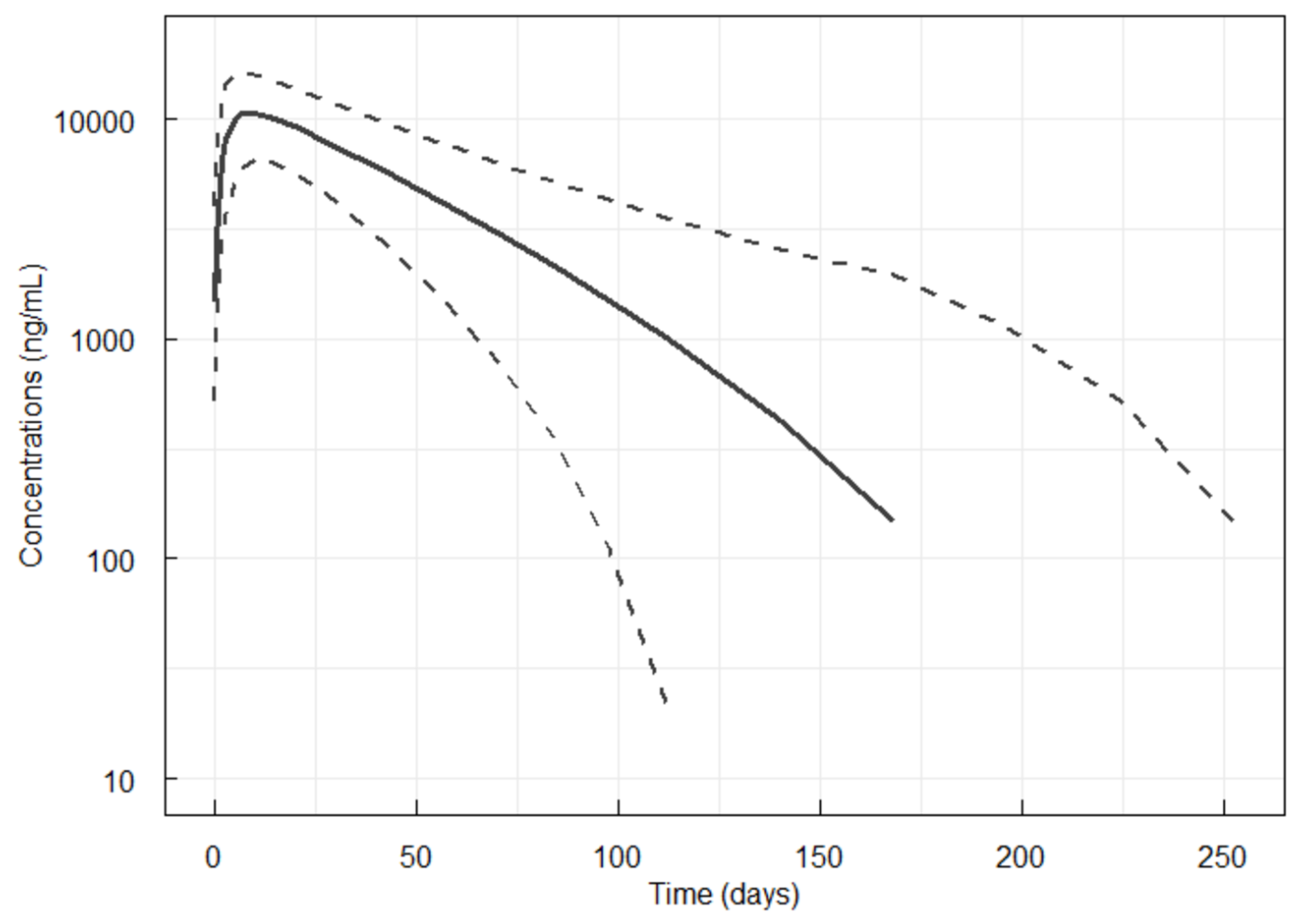
3.5. Simulations
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prolia-PI. Prolia-Highlights of Prescribing Information. 2025. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2025/125320Orig1s221lbl.pdf (accessed on 23 May 2025).
- Xgeva-PI. Xgeva-Highlights of Prescribing Information. 2020. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/125320s203lbl.pdf.pdf (accessed on 23 May 2025).
- Hanley, D.A.; Adachi, J.D.; Bell, A.; Brown, V. Denosumab: Mechanism of action and clinical outcomes. Int. J. Clin. Pract. 2012, 66, 1139–1146. [Google Scholar] [CrossRef]
- Tomaszewska-Kiecana, M.; Carapuça, E.; Florez-Igual, A.; Queiruga-Parada, J. MB09, a denosumab biosimilar candidate: Biosimilarity demonstration in a phase I study in healthy subjects. Clin. Transl. Sci. 2024, 17, e70013. [Google Scholar] [CrossRef] [PubMed]
- Prolia-SmPC. Prolia-Summary of Products Characteristics. 2025. Available online: https://www.ema.europa.eu/en/documents/product-information/prolia-epar-product-information_en.pdf (accessed on 23 May 2025).
- EMA—NAMs. Regulatory Acceptance of New Approach Methodologies (NAMs) to Reduce Animal Use Testing. 2025. Available online: https://www.ema.europa.eu/en/human-regulatory-overview/research-development/ethical-use-animals-medicine-testing/regulatory-acceptance-new-approach-methodologies-nams-reduce-animal-use-testing#main-content (accessed on 25 August 2025).
- Sutjandra, L.; Rodriguez, R.D.; Doshi, S.; Ma, M.; Peterson, M.C.; Jang, G.R.; Chow, A.T.; Pérez-Ruixo, J.J. Population pharmacokinetic meta-analysis of denosumab in healthy subjects and postmenopausal women with osteopenia or osteoporosis. Clin. Pharmacokinet. 2011, 50, 793–807. [Google Scholar] [CrossRef] [PubMed]
- Gibiansky, L.; Sutjandra, L.; Doshi, S.; Zheng, J.; Sohn, W.; Peterson, M.C.; Jang, G.R.; Chow, A.T.; Pérez-Ruixo, J.J. Population pharmacokinetic analysis of denosumab in patients with bone metastases from solid tumours. Clin. Pharmacokinet. 2012, 51, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Marathe, D.D.; Marathe, A.; Mager, D.E. Integrated model for denosumab and ibandronate pharmacodynamics in postmenopausal women. Biopharm. Drug Dispos. 2011, 32, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Bekker, P.J.; Holloway, D.L.; Rasmussen, A.S.; Murphy, R.; Martin, S.W.; Leese, P.T.; Holmes, G.B.; Dunstan, C.R.; DePaoli, A.M. A single-dose placebo-controlled study of AMG 162, a fully human monoclonal antibody to RANKL, in postmenopausal women. 2004. J. Bone Miner. Res. 2005, 20, 2275–2282. [Google Scholar] [CrossRef] [PubMed]
- Bauer, R.J. NONMEM Tutorial Part I: Description of Commands and Options, with Simple Examples of Population Analysis. CPT Pharmacomet. Syst. Pharmacol. 2019, 8, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Savic, R.M.; Karlsson, M.O. Importance of shrinkage in empirical bayes estimates for diagnostics: Problems and solutions. AAPS J. 2009, 11, 558–569. [Google Scholar] [CrossRef]
- Keizer, R.J.; Huitema, A.D.; Schellens, J.H.; Beijnen, J.H. Clinical pharmacokinetics of therapeutic monoclonal antibodies. Clin. Pharmacokinet. 2010, 49, 493–507. [Google Scholar] [CrossRef]
- Mould, D.R.; Green, B. Pharmacokinetics and pharmacodynamics of monoclonal antibodies: Concepts and lessons for drug development. BioDrugs 2010, 24, 23–39. [Google Scholar] [CrossRef] [PubMed]
- McLeay, S.C.; Morrish, G.A.; Kirkpatrick, C.M.; Green, B. The relationship between drug clearance and body size: Systematic review and meta-analysis of the literature published from 2000 to 2007. Clin. Pharmacokinet. 2012, 51, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Kendler, D.L.; Cosman, F.; Stad, R.K.; Ferrari, S. Denosumab in the Treatment of Osteoporosis: 10 Years Later: A Narrative Review. Adv. Ther. 2022, 39, 58–74. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.S.; Langdahl, B.; Plebanski, R.; Czerwinski, E.; Dokoupilova, E.; Supronik, J.; Rosa, J.; Mydlak, A.; Sapula, R.; Rowińska-Osuch, A.; et al. SB16 versus reference denosumab in postmenopausal women with osteoporosis: 18-month outcomes of a phase III randomized clinical trial. Bone 2025, 192, 117371. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Huang, Y.; Chen, Z.; Zeng, A.; Zhang, H.; Yu, Y.; Wei, S.; Li, Q.; Wang, X.; Wang, X.; et al. Efficacy and Safety of Denosumab Biosimilar QL1206 Versus Denosumab in Patients with Bone Metastases from Solid Tumors: A Randomized Phase III Trial. BioDrugs 2023, 37, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Jeka, S.; Dokoupilová, E.; Kivitz, A.; Żuchowski, P.; Vogg, B.; Krivtsova, N.; Sekhar, S.; Banerjee, S.; Schwebig, A.; Poetzl, J.; et al. Equivalence trial of proposed denosumab biosimilar GP2411 and reference denosumab in postmenopausal osteoporosis: The ROSALIA study. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2024, 39, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Hu, C.; Liu, Y.; Song, R.; Zhu, W.; Zhao, H.; Nino, A.; Zhang, F.; Liu, Y. Pharmacokinetics, pharmacodynamics, safety, and tolerability of single-dose denosumab in healthy Chinese volunteers: A randomized, single-blind, placebo-controlled study. PLoS ONE 2018, 13, e0197984. [Google Scholar] [CrossRef] [PubMed]
- Sohn, W.; Simiens, M.A.; Jaeger, K.; Hutton, S.; Jang, G. The pharmacokinetics and pharmacodynamics of denosumab in patients with advanced solid tumours and bone metastases: A systematic review. Br. J. Clin. Pharmacol. 2014, 78, 477–487. [Google Scholar] [CrossRef] [PubMed]

| Study | Study Description/Phase | Dose(s)/Frequency/Treatment | Scheduled Sampling Timepoints |
|---|---|---|---|
| MB09-A-01-19 PK study | A randomized, double-blind, three-arm, single-dose, parallel study to compare the pharmacokinetics, pharmacodynamics, safety and immunogenicity profile of MB09 (denosumab biosimilar) and EU/US-sourced Xgeva® in healthy male volunteers | 35 mg (n = 255) Single SC dose MB09 (TRT = 1) EU-sourced Xgeva (TRT = 2) US-sourced Xgeva (TRT = 3) | Serum: Rich PK Pre-dose, 8 and 16 h (±2 h), 24, 48, and 72 h (±4 h), Days 6, 8, and 11 (±1 day), Days 15, 22, and 29 (±2 days), Days 43, 57, 71, 85, 99, 113, 141, 169, 197, 225, and 253 (±3 days) |
| MB09-C-01-19 Efficacy study | A randomized, double-blind, parallel, multicenter, multinational study to compare the efficacy, pharmacokinetics, pharmacodynamics, safety and immunogenicity of MB09 (denosumab biosimilar) versus Prolia® (EU-sourced) in postmenopausal women with osteoporosis (SIMBA Study) | 60 mg (n = 555) 3 SC doses, every 6 months MB09 (TRT = 1) EU-sourced Prolia (TRT = 2) | Serum: Sparse PK Day 1 (0 predose), Day 11 and at Month 1, Month 3, Month 6 (predose) and Month 12 (predose) in the main period |
| MB09-A-01-19 Study (N = 255) | MB09-C-01-19 Study (N = 555) | |
|---|---|---|
| Age (years) | ||
| Mean (SD) | 39.5 (6.90) | 65.8 (5.94) |
| Age group, n (%) | ||
| ≥28 to <55 years | 254 (99.608) | 0 (0.0) |
| ≥55 to <68 years | 1 (0.392) | 342 (61.6) |
| ≥68 to ≤80 years | 0 (0.0) | 213 (38.4) |
| Smoking status, n (%) | ||
| Current Smoker | 0 (0.0) | 132 (23.8) |
| Former Smoker | 0 (0.0) | 74 (13.3) |
| Never Smoked | 0 (0.0) * | 349 (62.9) |
| Race, n (%) | ||
| White | 255 (100.0) | 551 (99.3) |
| American Indian or Alaska Native | 0 (0.0) | 4 (0.7) |
| Ethnicity, n (%) | ||
| Hispanic or Latino | 0 (0.0) | 23 (4.1) |
| Not Hispanic or Latino | 255 (100.0) | 532 (95.9) |
| Height (cm) | ||
| Mean (SD) | 178.66 (6.227) | 159.98 (6.186) |
| Weight (kg) | ||
| Mean (SD) | 82.97 (8.492) | 63.196 (8.7870) |
| Body mass index (kg/m2) | ||
| Mean (SD) | 25.98 (2.396) | 24.683 (3.0401) |
| Parameters (Units) | Final Estimate | % RSE | 95% CI | Inter-Individual Variability | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| KA (1/day) | 0.406 | 3.92% | 0.375 | 0.437 | 61% |
| V/F (L) | 9.33 | 1.21% | 9.11 | 9.55 | 13% |
| CL/F (L/day) | 0.123 | 3.55% | 0.114 | 0.132 | 37% |
| BW~CL | 1.32 | 16.2% | 0.901 | 1.74 | |
| Km (ng/mL) | 0.124 | 9.27% | 0.101 | 0.147 | |
| Vm (ng/day) | 0.139 | 5.28% | 0.125 | 0.153 | |
| Residual variability | 17% | ||||
| Parameters (Units) | Final Estimate | %RSE | 95% CI | Inter-Individual Variability | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| KA (1/day) | 0.349 | 2.7% | 0.330 | 0.368 | 60% |
| V/F (L) | 9.00 | 0.8% | 8.86 | 9.14 | 15.7% |
| CL/F (L/day) | 0.143 | 2.6% | 0.136 | 0.150 | 34.6% |
| BW~CL | 1.07 | 9.8% | 0.864 | 1.28 | |
| Km (ng/mL) | 0.162 | 18.8% | 0.102 | 0.222 | |
| Vm (ng/day) | 0.128 | 7.6% | 0.109 | 0.147 | |
| Study~V | −0.534 | 33.7% | −0.887 | −0.181 | |
| Residual variability | 19.5% | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Vidaurre, S.; Paravisini, A.; Queiruga-Parada, J. Population PK Modeling of Denosumab Biosimilar MB09 and Reference Denosumab to Establish PK Similarity. Pharmaceutics 2025, 17, 1146. https://doi.org/10.3390/pharmaceutics17091146
Sánchez-Vidaurre S, Paravisini A, Queiruga-Parada J. Population PK Modeling of Denosumab Biosimilar MB09 and Reference Denosumab to Establish PK Similarity. Pharmaceutics. 2025; 17(9):1146. https://doi.org/10.3390/pharmaceutics17091146
Chicago/Turabian StyleSánchez-Vidaurre, Sara, Alexandra Paravisini, and Javier Queiruga-Parada. 2025. "Population PK Modeling of Denosumab Biosimilar MB09 and Reference Denosumab to Establish PK Similarity" Pharmaceutics 17, no. 9: 1146. https://doi.org/10.3390/pharmaceutics17091146
APA StyleSánchez-Vidaurre, S., Paravisini, A., & Queiruga-Parada, J. (2025). Population PK Modeling of Denosumab Biosimilar MB09 and Reference Denosumab to Establish PK Similarity. Pharmaceutics, 17(9), 1146. https://doi.org/10.3390/pharmaceutics17091146







