From Current Therapeutics to Multitarget Ligands: A Review of Diabetes Pharmacological Treatments
Abstract
1. Diabetes and Insulin Resistance
1.1. A Multifactorial Scenario
1.2. Pathophysiology of Type 2 Diabetes Mellitus
2. Treatment of Type 2 Diabetes Mellitus
3. Metformin
4. Action of Insulin Secretion
4.1. Sulfonylureas
4.2. Glinides or Meglitinides
5. Insulin Sensitizers
Thiazolidinediones (Glytazones)
6. Incretin-Dependent Therapies
6.1. GLP-1 Receptor Agonists (GLP-1 RA)
| Drug Route | Dosage Regime | Pharmaceutical Form | |
|---|---|---|---|
| Short-Acting | |||
| Exenatide SC | 2/day
| 60 min before the two main meals. Interval ≥6 h | Pre-filled pen (glass) (10 and 5 μg) |
| Lixisenatide † SC | 1/day
| 1 h before the 1st meal of the day | Cartridge in pre-filled pen (20 and 10 μg) |
| Long-Acting | |||
| Dulaglutide SC | 1/week
| Anytime | Pre-filled pen (0.75, 1.5, 3, and 4.5 mg) |
| Exenatide XR SC | 1/week
| Anytime | Powder in vial + solvent in pre-filled syringe (2 mg) Cartrige in pre-filled pen (2 mg) |
| Liraglutide ‡ SC | 1/day
| Anytime | Pre-filled pen (6 mg/mL, doses of 0.6, 1.2, or 1.8 mg administered) |
| Semaglutide SC | 1/ week
| Anytime | Pre-filled pen (0.25, 0.5, 1, and 2 mg) |
| Semaglutide oral | 1/day
| 30 min before the 1st meal, drink, or other medication of the day | Oral tablets (3, 7, and 14 mg) |
6.2. Dipeptidyl Peptidase-4 Inhibitors (DPP-4i)
7. Action on Renal and Intestinal Glucose Absorption
7.1. α-Glucosidase Inhibitors (AGIs)
7.2. Sodium-Glucose-Linked Transporter Type 2 Inhibitors (SGLT2i)
8. Multitarget Ligand Strategies
8.1. Dual Glucose-Dependent Insulinotropic Polypeptide (GIP) and GLP-1 Receptor Agonist
8.2. GLP-1 and Glucagon Dual Agonists
8.3. GIP, GLP-1, and Glucagon Multiple-Agonists
8.4. SGLT-1/SGLT-2 Inhibitors
8.5. Dual Inhibitors of Aldose Reductase (AR) and Protein Tyrosine Phosphatase 1B (PTP1B)
8.6. PPAR-α/γ Dual Agonists
9. Insulin
9.1. Insulin and Antidiabetic Therapy
9.2. Basal Insulins
9.3. Prandial Insulins
9.4. Premixed Insulins
10. Conclusions
- Metformin is the preferred initial pharmacological agent for the treatment of T2D. Once treatment is started, metformin should be continued as long as it is tolerated and is not contraindicated. Subsequently and based on progress, other agents, including insulin, should be added to metformin.
- Long-term use of metformin may be associated with vitamin B12 deficiency, and periodic monitoring of vitamin B12 levels should be considered in patients treated with metformin, especially those with anemia or peripheral neuropathy.
- Early introduction of insulin should be considered if there is evidence of ongoing catabolism (weight loss), if there are symptoms of hyperglycemia, or when HbA1c levels or blood glucose levels are very high.
- Dual therapy should be considered in patients with newly diagnosed T2D who have HbA1c above their glycemic target.
- A patient-centered approach is essential when selecting pharmacological agents. This decision-making process should consider a range of factors, including the presence of comorbidities, such as atherosclerotic cardiovascular disease, heart failure, or chronic kidney disease; the risk of hypoglycemia; the potential impact on body weight; treatment cost; the likelihood of adverse effects; and individual patient preferences.
- Among T2D patients who have established cardiovascular disease, SGLT2 inhibitors or GLP-1 receptor agonists with demonstrated benefit in cardiovascular disease are recommended as part of the antihyperglycemic regimen.
- Among patients with cardiovascular disease at high risk of heart failure or who have coexisting heart failure, SGLT2 inhibitors are recommended.
- For patients with T2D and chronic kidney disease, the use of SGLT2 inhibitors or GLP-1 receptor agonists has been shown to reduce the risk of chronic kidney disease progression, cardiovascular events, or both; therefore, it should be considered.
- In most patients who require the greatest hypoglycemic effect of an injectable medication, GLP-1 receptor agonists are preferred over insulin.
- Treatment intensification should not be delayed for patients with T2M who do not meet treatment goals.
- The medication regimen should be reevaluated at regular intervals (every 3 to 6 months) and adjusted as necessary to incorporate new patient factors.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 2-h PG | 2 h plasma glucose |
| A1C or A1c or HbA1c | Glycosylated hemoglobin |
| AGI | α-Glucosidase inhibitors |
| AMPK | AMP-activated protein kinase |
| AMP | Adenosine monophosphate |
| AR | Aldose reductase |
| ASCVD | Atherosclerotic cardiovascular disease |
| AUC | Area under the curve |
| BMI | Body mass index |
| CAD | Diabetic ketoacidosis |
| CHMP | Committee for Medicinal Products for Human Use of EMA |
| CKD | Chronic kidney disease |
| CV | Cardiovascular |
| CVD | Cardiovascular disease |
| CVOT | Cardiovascular outcome trial |
| Cyt | Cytochrome |
| DAPA-HF | Dapagliflozin and Prevention of Adverse Events in Heart Failure |
| DDP-4 | Dipeptidyl peptidase-4 |
| DDP-4i | Dipeptidyl peptidase-4 inhibitor |
| DKA | Diabetic ketoacidosis |
| DKD | Diabetic kidney disease |
| DM | Diabetes mellitus |
| DNL | De novo lipogenesis |
| ECV | Cardiovascular disease |
| EMA | European Medicines Agency |
| ER | Extended release |
| eGFR | Estimated glomerular filtration rate |
| FDA | US Food and Drug Administration |
| FPG | Fasting plasma glucose |
| GADA | Glutamate decarboxylase autoantibodies |
| GI | Gastrointestinal |
| GIP | Glucose-dependent insulinotropic polypeptide |
| GLP-1 | Glucagon like peptide-1 |
| GLP-1 RA | Glucagon-like peptide-1 receptor agonist |
| GLUT | Glucose transporter protein |
| GSV | GLUT4 storage vesicles |
| HbA1c | Glycosylated hemoglobin |
| HDL | High-density lipoproteins |
| HF | Heart failure |
| HFrEF | Heart Failure with reduced ejection fraction |
| hHF | Hospitalization for heart failure |
| HIV | Human insufficiency virus |
| HOMA2-B | Homeostatic Model for Assessing β-Cell Functionality |
| HOMA2-IR | Homeostatic Model for Assessing Insulin Resistance |
| IDF | International Diabetes Federation |
| IFG | Impaired fasting glucose |
| IGT | Impaired glucose tolerance |
| IL-6 | Interleukin 6 |
| INSR | Insulin receptor |
| IR | Insulin resistance |
| IR | Immediate release |
| LADA | Latent autoimmune diabetes in adults |
| LDL | Low-density lipoproteins |
| LVEF | Left ventricular ejection fraction |
| LVH | Left ventricular hypertrophy |
| MACE | Major cardiovascular adverse effects |
| MARD | Moderate age-related diabetes |
| MR | Modified release |
| mRNA | Messenger RNA |
| MOD | Mild obesity-related diabetes |
| NCE | New chemical entity |
| NEFA | Fatty acids in esterified |
| NPH | Neutral protamine Hagedorn |
| OGTT | Oral glucose tolerance test |
| OXM | Oxyntomodulin |
| PIP | Phosphatidylinositol phosphate |
| PPAR | Peroxisome proliferator-activated receptors (PPAR) |
| PPB | Binding to plasma proteins |
| PTP1B | Protein tyrosine phosphatase 1B |
| RHI | Regular human insulin |
| RR | Risk ratio |
| SAID | Severe autoimmune diabetes |
| SGLT1 | Sodium-glucose linked transporter type 1 |
| SGLT2 | Sodium-glucose linked transporter type 2 |
| SGLT2i | Sodium-glucose linked transporter type 2 inhibitor |
| SIDD | Severe insulin-deficient diabetes |
| SIRD | Severe insulin-resistant diabetes |
| SmPC | Summary of product characteristics |
| SU | Sulfonylurea |
| t1/2 | Half-life of elimination |
| T1D | Type 1 diabetes |
| T2D | Type 2 diabetes or type 2 diabetes mellitus |
| Tmax | Time to maximum plasma drug concentration |
| TNF | Tumor necrosis factor |
| TZD | Thiazolidinedione |
| UACR | Urine albumin-creatinine ratio |
| VLDL | Very low-density lipoproteins |
| wk | Week |
| XR | Extended release |
References
- IDF Diabetes Federation. IDF Diabetes Atlas 11th Edition. 2025. Available online: http://www.rxlist.com/script/main/hp.asp (accessed on 1 July 2025).
- WHO (World Health Organization). Definition, Diagnosis and Classification of Diabetes Mellitus and Its complications. Report of a WHO Consultation. Part 1, Diagnosis and Classification of Diabetes Mellitus; WHO/NCD/NCS/99.2-World Health Organization. Department of Noncommunicable Disease Surveillance. WHO (World Health Organization): Geneva, Switzerland, 1999. Available online: https://iris.who.int/handle/10665/66040 (accessed on 18 August 2025).
- Davies, M.J.; Aroda, V.R.; Collins, B.S.; Gabbay, R.A.; Green, J.; Maruthur, N.M.; Rosas, S.E.; Del Prato, S.; Mathieu, C.; Mingrone, G.; et al. Management of Hyperglycemia in Type 2 Diabetes, 2022. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2022, 45, 2753–2786. [Google Scholar] [CrossRef]
- Leslie, R.D.; Palmer, J.; Schloot, N.C.; Lernmark, A. Diabetes at the crossroads: Relevance of disease classification to pathophysiology and treatment. Diabetologia 2015, 59, 13–20. [Google Scholar] [CrossRef]
- The American Diabetes Association (ADA). Standards Medical Care Diabetes 2022. Diabetes Care 2022, 45 (Suppl. 1), S1–S264. Available online: https://diabetesjournals.org/care/issue/45/Supplement_1 (accessed on 29 June 2025).
- The American Diabetes Association (ADA). Standards of Care in Diabetes 2025. Diabetes Care 2025, 48 (Suppl. 1), S1–S352. Available online: https://diabetesjournals.org/care/issue/48/Supplement_1 (accessed on 29 June 2025).
- WHO (World Health Organization). Classification of Diabetes Mellitus 2019; World Health Organization: Geneva, Switzerland, 2019; Available online: https://apps.who.int/iris/bitstream/handle/10665/325182/9789241515702-eng.pdf?sequence=1&isAllowed=y (accessed on 14 January 2025).
- The American Diabetes Association (ADA). Diagnosis and classification of Diabetes Mellitus. Diabetes Care 2014, 37 (Suppl. 1), S81–S90. [Google Scholar] [CrossRef]
- Ahlqvist, E.; Prasad, R.B.; Groop, L. 100 YEARS OF INSULIN: Towards improved precision and a new classification of diabetes mellitus. J. Endocrinol. 2022, 252, R59–R70. [Google Scholar] [CrossRef] [PubMed]
- The American Diabetes Association (ADA). ADA Standards of Care in Diabetes 2024. Diabetes Care 2024, 47 (Suppl. 1), 1–328. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.; Al-Mrabeh, A.; Sattar, N. Understanding the mechanisms of reversal of type 2 diabetes. Lancet Diabetes Endocrinol. 2019, 7, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Marshall, S.M. The pancreas in health and in diabetes. Diabetologia 2020, 63, 1962–1965. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Ghani, M.; DeFronzo, R.A. Is It Time to Change the Type 2 Diabetes Treatment Paradigm? Yes! GLP-1 RAs Should Replace Metformin in the Type 2 Diabetes Algorithm. Diabetes Care 2017, 40, 1121–1127. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Ferrannini, E.; Groop, L.; Henry, R.R.; Herman, W.H.; Holst, J.J.; Hu, F.B.; Kahn, C.R.; Raz, I.; Shulman, G.I.; et al. Type 2 diabetes mellitus. Nat. Rev. Dis. Primers 2015, 1, 15019. [Google Scholar] [CrossRef]
- Petersen, M.C.; Shulman, G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef]
- DeFronzo, R.A. From the Triumvirate to the Ominous Octet: A New Paradigm for the Treatment of Type 2 Diabetes Mellitus. Diabetes 2009, 58, 773–795. [Google Scholar] [CrossRef]
- Drucker, D.J.; Nauck, M.A. The incretin system: Glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006, 368, 1696–1705. [Google Scholar] [CrossRef]
- Meier, J.J.; Nauck, M.A. Incretins and the development of type 2 diabetes. Curr. Diab Rep. 2006, 6, 194–201. [Google Scholar] [CrossRef]
- Rieg, T.; Vallon, V. Development of SGLT1 and SGLT2 inhibitors. Diabetologia 2018, 61, 2079–2086. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Ghani, M.A.; DeFronzo, R.A. Inhibition of Renal Glucose Reabsorption: A Novel Strategy for Achieving Glucose Control in Type 2 Diabetes Mellitus. Endocr. Practice 2008, 14, 782–790. [Google Scholar] [CrossRef]
- Rizzo, M.R.; Di Meo, I.; Polito, R.; Auriemma, M.C.; Gambardella, A.; di Mauro, G.; Capuano, A.; Paolisso, G. Cognitive impairment and type 2 diabetes mellitus: Focus of SGLT2 inhibitors treatment. Pharmacol. Res. 2022, 176, 106062. [Google Scholar] [CrossRef]
- Scherer, T.; Sakamoto, K.; Buettner, C. Brain insulin signalling in metabolic homeostasis and disease. Nat. Rev. Endocrinol. 2021, 17, 468–483. [Google Scholar] [CrossRef]
- Peters, A.; Schweiger, U.; Pellerin, L.; Hubold, C.; Oltmanns, K.M.; Conrad, M.; Schultes, B.; Born, J.; Fehm, H.L. The selfish brain: Competition for energy resources. Neurosci. Biobehav. Rev. 2004, 28, 143–180. [Google Scholar] [CrossRef]
- Peters, A. The selfish brain: Competition for energy resources. Am. J. Hum. Biol. 2010, 23, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Vetcher, A.A.; Zhukov, K.V.; Gasparyan, B.A.; Borovikov, P.I.; Karamian, A.S.; Rejepov, D.T.; Kuznetsova, M.N.; Shishonin, A.Y. Different Trajectories for Diabetes Mellitus Onset and Recovery According to the Centralized Aerobic–Anaerobic Energy Balance Compensation Theory. Biomedicines 2023, 11, 2147. [Google Scholar] [CrossRef]
- Pozzilli, P.; Leslie, R.D.; Chan, J.; De Fronzo, R.; Monnier, L.; Raz, I.; Del Prato, S. The A1C and ABCD of glycaemia management in type 2 diabetes: A physician’s personalized approach. Diabetes/Metab. Res. Rev. 2010, 26, 239–244. [Google Scholar] [CrossRef]
- Dahlén, A.D.; Dashi, G.; Maslov, I.; Attwood, M.M.; Jonsson, J.; Trukhan, V.; Schiöth, H.B. Trends in Antidiabetic Drug Discovery: FDA Approved Drugs, New Drugs in Clinical Trials and Global Sales. Front. Pharmacol. 2022, 12, 807548. [Google Scholar] [CrossRef]
- Gonzalez-Lopez, C.; Wojeck, B.S. Role of metformin in the management of type 2 diabetes: Recent advances. Pol. Arch. Intern. Med. 2023, 133. [Google Scholar] [CrossRef]
- Verdonck, L.F.; Sangster, B.; van Heijst, A.N.P.; de Groot, G.; Maes, R.A.A. Buformin concentrations in a case of fatal lactic acidosis. Diabetologia 1981, 20, 45–46. [Google Scholar] [CrossRef]
- Fimognari, F.L.; Pastorelli, R.; Incalzi, R.A. Phenformin-induced lactic acidosis in an older diabetic patient: A recurrent drama (phenformin and lactic acidosis). Diabetes Care 2006, 29, 950–951. [Google Scholar] [CrossRef]
- Entezari, M.; Hashemi, D.; Taheriazam, A.; Zabolian, A.; Mohammadi, S.; Fakhri, F.; Hashemi, M.; Hushmandi, K.; Ashrafizadeh, M.; Zarrabi, A.; et al. AMPK signaling in diabetes mellitus, insulin resistance and diabetic complications: A pre-clinical and clinical investigation. Biomed. Pharmacother. 2022, 146, 112563. [Google Scholar] [CrossRef] [PubMed]
- He, L. Metformin and Systemic Metabolism. Trends Pharmacol. Sci. 2020, 41, 868–881. [Google Scholar] [CrossRef] [PubMed]
- Sciannimanico, S.; Grimaldi, F.; Vescini, F.; De Pergola, G.; Iacoviello, M.; Licchelli, B.; Guastamacchia, E.; Giagulli, V.A.; Triggiani, V. Metformin: Up to Date. Endocr. Metab. Immune Disord. Drug Targets 2020, 20, 172–181. [Google Scholar] [CrossRef]
- Hostalek, U.; Campbell, I. Metformin for diabetes prevention: Update of the evidence base. Curr. Med. Res. Opin. 2021, 37, 1705–1717. [Google Scholar] [CrossRef] [PubMed]
- Wasner, H.K. Metformin’s Mechanism of Action Is Stimulation of the Biosynthesis of the Natural Cyclic AMP Antagonist Prostaglandylinositol Cyclic Phosphate (Cyclic PIP). Int. J. Mol. Sci. 2022, 23, 2200. [Google Scholar] [CrossRef]
- Bell, D.S.H. Metformin-induced vitamin B12 deficiency can cause or worsen distal symmetrical, autonomic and cardiac neuropathy in the patient with diabetes. Diabetes Obes. Metab. 2022, 24, 1423–1428. [Google Scholar] [CrossRef]
- Di Mauro, S.; Filippello, A.; Scamporrino, A.; Purrello, F.; Piro, S.; Malaguarnera, R. Metformin: When Should We Fear Lactic Acidosis? Int. J. Mol. Sci. 2022, 23, 8320. [Google Scholar] [CrossRef]
- UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998, 352, 854–865. [Google Scholar] [CrossRef]
- ClinCalc DrugStats Database. The Top 300 of 2021. 2020. Available online: https://clincalc.com/DrugStats/Top300Drugs.aspx (accessed on 16 January 2024).
- Tomlinson, B.; Patil, N.G.; Fok, M.; Chan, P.; Lam, C.W.K. The role of sulfonylureas in the treatment of type 2 diabetes. Expert Opin. Pharmacother. 2021, 23, 387–403. [Google Scholar] [CrossRef]
- Thulé, P.M.; Umpierrez, G. Sulfonylureas: A New Look at Old Therapy. Curr. Diabetes Rep. 2014, 14, 1–8. [Google Scholar] [CrossRef]
- Davies, M.J.; D’Alessio, D.A.; Fradkin, J.; Kernan, W.N.; Mathieu, C.; Mingrone, G.; Rossing, P.; Tsapas, A.; Wexler, D.J.; Buse, J.B. Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018, 41, 2669–2701. [Google Scholar] [CrossRef] [PubMed]
- Malaisse, W.J. Pharmacology of the Meglitinide Analogs: New Treatment Options for Type 2 Diabetes Mellitus. Treat. Endocrinol. 2003, 2, 401–414. [Google Scholar] [CrossRef]
- Hu, S.; Boettcher, B.; Dunning, B. The mechanisms underlying the unique pharmacodynamics of nateglinide. Diabetologia 2003, 46, M37–M43. [Google Scholar] [CrossRef]
- Hu, S.; Wang, S.; Fanelli, B.; ABell, P.; EDunning, B.; Geisse, S.; Schmitz, R.; Boettcher, B.R. Pancreatic beta-cell K(ATP) channel activity and membrane-binding studies with nateglinide: A comparison with sulfonylureas and repaglinide. J. Pharmacol. Exp. Ther. 2000, 293, 444–452. [Google Scholar] [CrossRef]
- Guardado-Mendoza, R.; Prioletta, A.; Jiménez-Ceja, L.M.; Sosale, A.; Folli, F. The role of nateglinide and repaglinide, derivatives of meglitinide, in the treatment of type 2 diabetes mellitus. Arch. Med. Sci. 2013, 9, 936–943. [Google Scholar] [CrossRef]
- Wu, P.-C.; The NRPBKidney Consortium; Wu, V.-C.; Lin, C.-J.; Pan, C.-F.; Chen, C.-Y.; Huang, T.-M.; Wu, C.-H.; Chen, L.; Wu, C.-J. Meglitinides increase the risk of hypoglycemia in diabetic patients with advanced chronic kidney disease: A nationwide, population-based study. Oncotarget 2017, 8, 78086–78095. [Google Scholar] [CrossRef]
- Hurren, K.M.; Dunham, M.W. Are thiazolidinediones a preferred drug treatment for type 2 diabetes? Expert Opin. Pharmacother. 2021, 22, 131–133. [Google Scholar] [CrossRef]
- Eldor, R.; DeFronzo, R.A.; Abdul-Ghani, M. In vivo actions of peroxisome proliferator-activated receptors: Glycemic control, insulin sensitivity, and insulin secretion. Diabetes Care 2013, 36 (Suppl. 2), S162–S174. [Google Scholar] [CrossRef]
- Lebovitz, H.E. Thiazolidinediones: The Forgotten Diabetes Medications. Curr. Diabetes Rep. 2019, 19, 1–13. [Google Scholar] [CrossRef]
- Qaoud, M.T.; Al-masri, I.; Önkol, T. Peroxisome proliferator-activated receptors as superior targets to treat diabetic disease, design strategies—Review article. Turk. J. Pharm. Sci. 2022, 19, 353–370. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.H.; Prasad, P.; Saboorian, M.; Thiele, D.L.; Malet, P.F. CASE REPORT: Hepatic Injury Due to Troglitazone. Dig. Dis. Sci. 2000, 45, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Rosen, C.J. Revisiting the rosiglitazone story—lessons learned. N. Engl. J. Med. 2010, 363, 803–806. [Google Scholar] [CrossRef]
- Pouwels, K.B.; van Grootheest, K. The rosiglitazone decision process at FDA and EMA. What should we learn? Int. J. Risk Saf. Med. 2012, 24, 73–80. [Google Scholar] [CrossRef]
- Dormandy, J.A.; Charbonnel, B.; Eckland, D.J.A.; Erdmann, E.; Massi-Benedetti, M.; Moules, I.K.; Skene, A.M.; Tan, M.H.; Lefèbvre, P.J.; Murray, G.D.; et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial in macroVascular Events): A randomised controlled trial. Lancet 2005, 366, 1279–1289. [Google Scholar] [CrossRef]
- Levin, D.; Bell, S.; Sund, R.; Hartikainen, S.A.; Tuomilehto, J.; Pukkala, E.; Keskimäki, I.; Badrick, E.; Renehan, A.G.; Buchan, I.E.; et al. Pioglitazone and bladder cancer risk: A multipopulation pooled, cumulative exposure analysis. Diabetologia 2014, 58, 493–504. [Google Scholar] [CrossRef]
- Andersen, A.; Lund, A.; Knop, F.K.; Vilsbøll, T. Glucagon-like peptide 1 in health and disease. Nat. Rev. Endocrinol. 2018, 14, 390–403. [Google Scholar] [CrossRef]
- Deacon, C.F. Dipeptidyl peptidase 4 inhibitors in the treatment of type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2020, 16, 642–653. [Google Scholar] [CrossRef]
- Vilsbøll, T.; Krarup, T.; Deacon, C.F.; Madsbad, S.; Holst, J.J. Reduced Postprandial Concentrations of Intact Biologically Active Glucagon-Like Peptide 1 in Type 2 Diabetic Patients. Diabetes 2001, 50, 609–613. [Google Scholar] [CrossRef]
- Mathiesen, D.S.; Bagger, J.I.; Bergmann, N.C.; Lund, A.; Christensen, M.B.; Vilsbøll, T.; Knop, F.K. The Effects of Dual GLP-1/GIP Receptor Agonism on Glucagon Secretion—A Review. Int. J. Mol. Sci. 2019, 20, 4092. [Google Scholar] [CrossRef]
- Nauck, M.A.; Meier, J.J. Incretin hormones: Their role in health and disease. Diabetes Obes. Metab. 2018, 20 (Suppl. 1), 5–21. [Google Scholar] [CrossRef] [PubMed]
- Göke, R.; Fehmann, H.; Linn, T.; Schmidt, H.; Krause, M.; Eng, J.; Göke, B. Exendin-4 is a high potency agonist and truncated exendin-(9-39)-amide an antagonist at the glucagon-like peptide 1-(7-36)-amide receptor of insulin-secreting beta-cells. J. Biol. Chem. 1993, 268, 19650–19655. [Google Scholar] [CrossRef] [PubMed]
- Dickson, S.L.; Shirazi, R.H.; Hansson, C.; Bergquist, F.; Nissbrandt, H.; Skibicka, K.P. The Glucagon-Like Peptide 1 (GLP-1) Analogue, Exendin-4, Decreases the Rewarding Value of Food: A New Role for Mesolimbic GLP-1 Receptors. J. Neurosci. 2012, 32, 4812–4820. [Google Scholar] [CrossRef] [PubMed]
- Philis-Tsimikas, A.; Wysham, C.H.; Hardy, E.; Han, J.; Iqbal, N. Efficacy and tolerability of exenatide once weekly over 7 years in patients with type 2 diabetes: An open-label extension of the DURATION-1 study. J. Diabetes Its Complicat. 2019, 33, 223–230. [Google Scholar] [CrossRef]
- Deacon, C.F.; Mannucci, E.; Ahrén, B. Glycaemic efficacy of glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors as add-on therapy to metformin in subjects with type 2 diabetes—A review and meta analysis. Diabetes Obes. Metab. 2012, 14, 762–767. [Google Scholar] [CrossRef]
- Brunton, S.A.; Wysham, C.H. GLP-1 receptor agonists in the treatment of type 2 diabetes: Role and clinical experience to date. Postgrad. Med. 2020, 132, 3–14. [Google Scholar] [CrossRef]
- Lyseng-Williamson, K.A. Glucagon-Like Peptide-1 Receptor Analogues in Type 2 Diabetes: Their Use and Differential Features. Clin. Drug Investig. 2019, 39, 805–819. [Google Scholar] [CrossRef] [PubMed]
- ALevin, P.; Nguyen, H.; Wittbrodt, E.T.; Kim, S.C. Glucagon-like peptide-1 receptor agonists: A systematic review of comparative effectiveness research. Diabetes Metab. Syndr. Obes. Targets Ther. 2017, 10, 123–139. [Google Scholar] [CrossRef]
- Marx, N.; Federici, M.; Schütt, K.; Müller-Wieland, D.; Ajjan, R.A.; Antunes, M.J.; Christodorescu, R.M.; Crawford, C.; Di Angelantonio, E.; Eliasson, B.; et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur. Heart J. 2023, 44, 4043–4140. [Google Scholar] [CrossRef]
- Lincoff, A.M.; Brown-Frandsen, K.; Colhoun, H.M.; Deanfield, J.; Emerson, S.S.; Esbjerg, S.; Hardt-Lindberg, S.; Hovingh, G.K.; Kahn, S.E.; Kushner, R.F.; et al. Semaglutide and Cardiovascular Outcomes in Obesity without Diabetes. N. Engl. J. Med. 2023, 389, 2221–2232. [Google Scholar] [CrossRef]
- Lincoff, A.M.; Ryan, D.H. The SELECT trial of semaglutide in patients with overweight or obesity without diabetes: Establishing a new pathway to secondary prevention of cardiovascular disease. Eur. Heart J. Cardiovasc. Pharmacother. 2023, 10, 93–94. [Google Scholar] [CrossRef]
- EMA Semaglutide (Wegovy®). Summary of Product Characteristics. 2023. Available online: https://www.ema.europa.eu/en/documents/product-information/wegovy-epar-product-information_en.pdf (accessed on 16 January 2024).
- FDA Semaglutide (Wegovy®). Highlights of Prescribing Information. 2023. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/215256s007lbl.pdf (accessed on 16 January 2024).
- EMA Liraglutide (Saxenda®). Summary of Product Characteristics. 2023. Available online: https://www.ema.europa.eu/en/documents/product-information/saxenda-epar-product-information_en.pdf (accessed on 16 January 2024).
- Hinnen, D.; Kruger, D.F. Cardiovascular risks in type 2 diabetes and the interpretation of cardiovascular outcome trials. Diabetes Metab. Syndr. Obes. Targets Ther. 2019, 12, 447–455. [Google Scholar] [CrossRef]
- Basu, A.; Patel, D.; Winocour, P.; Ryder, B. Cardiovascular Impact of New Drugs (GLP-1 and Gliflozins): The ABCD Position Statement. Br. J. Diabetes 2021, 12, 1. Available online: https://bjd-abcd.com/index.php/bjd/article/view/711 (accessed on 26 February 2021). [CrossRef]
- Kristensen, S.L.; Rørth, R.; Jhund, P.S.; Docherty, K.F.; Sattar, N.; Preiss, D.; Køber, L.; Petrie, M.C.; McMurray, J.J.V. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: A systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019, 7, 776–785. [Google Scholar] [CrossRef]
- Yang, Z.; Lv, Y.; Yu, M.; Mei, M.; Xiang, L.; Zhao, S.; Li, R. GLP-1 receptor agonist-associated tumor adverse events: A real-world study from 2004 to 2021 based on FAERS. Front. Pharmacol. 2022, 13, 925377. [Google Scholar] [CrossRef]
- Marso, S.P.; Bain, S.C.; Consoli, A.; Eliaschewitz, F.G.; Jódar, E.; Leiter, L.A.; Lingvay, I.; Rosenstock, J.; Seufert, J.; Warren, M.L.; et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 1834–1844. [Google Scholar] [CrossRef]
- Vilsbøll, T.; Bain, S.C.; Leiter, L.A.; Lingvay, I.; Matthews, D.; Simó, R.; Helmark, I.C.; Wijayasinghe, N.; Larsen, M. Semaglutide, reduction in glycated haemoglobin and the risk of diabetic retinopathy. Diabetes Obes. Metab. 2017, 20, 889–897. [Google Scholar] [CrossRef]
- FDA/CEDERFDA Drug Safety and Availability. FDA Drug Safety Communication: FDA Adds Warnings About Heart Failure Risk to Labels of Type 2 Diabetes Medicines Containing Saxagliptin and Alogliptin. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-adds-warnings-about-heart-failure-risk-labels-type-2-diabetes (accessed on 10 October 2022).
- Liu, D.; Jin, B.; Chen, W.; Yun, P. Dipeptidyl peptidase 4 (DPP-4) inhibitors and cardiovascular outcomes in patients with type 2 diabetes mellitus (T2DM): A systematic review and meta-analysis. BMC Pharmacol. Toxicol. 2019, 20, 15. [Google Scholar] [CrossRef]
- Esaki, H.; Tachi, T.; Goto, C.; Sugita, I.; Kanematsu, Y.; Yoshida, A.; Saito, K.; Noguchi, Y.; Ohno, Y.; Aoyama, S.; et al. Renoprotective Effect of Dipeptidyl Peptidase-4 Inhibitors in Patients with Type 2 Diabetes Mellitus. Front. Pharmacol. 2017, 8, 835. [Google Scholar] [CrossRef]
- Pinto, L.C.; Rados, D.V.; Barkan, S.S.; Leitão, C.B.; Gross, J.L. Dipeptidyl peptidase-4 inhibitors, pancreatic cancer and acute pancreatitis: A meta-analysis with trial sequential analysis. Sci. Rep. 2018, 8, 782. [Google Scholar] [CrossRef]
- Moelands, S.V.; Lucassen, P.L.; Akkermans, R.P.; De Grauw, W.J.; A Van de Laar, F. Cochrane Metabolic and Endocrine Disorders Group Alpha-glucosidase inhibitors for prevention or delay of type 2 diabetes mellitus and its associated complications in people at increased risk of developing type 2 diabetes mellitus. Cochrane Database Syst. Rev. 2018, 2018, CD005061. [Google Scholar] [CrossRef]
- Hedrington, M.S.; Davis, S.N. Considerations when using alpha-glucosidase inhibitors in the treatment of type 2 diabetes. Expert Opin. Pharmacother. 2019, 20, 2229–2235. [Google Scholar] [CrossRef]
- Min, S.H.; Yoon, J.; Hahn, S.; Cho, Y.M. Efficacy and safety of combination therapy with an α-glucosidase inhibitor and a dipeptidyl peptidase-4 inhibitor in patients with type 2 diabetes mellitus: A systematic review with meta-analysis. J. Diabetes Investig. 2017, 9, 893–902. [Google Scholar] [CrossRef]
- Kasthuri, S.; Poongothai, S.; Anjana, R.M.; Selvakumar, J.; Muthukumar, S.; Kayalvizhi, S.; Tariq, S.; Honey, E.; Gupta, P.K.; Venkatesan, U.; et al. Comparison of Glycemic Excursion Using Flash Continuous Glucose Monitoring in Patients with Type 2 Diabetes Mellitus Before and After Treatment with Voglibose. Diabetes Technol. Ther. 2021, 23, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Dalsgaard, N.B.; Gasbjerg, L.S.; Hansen, L.S.; Hansen, N.L.; Stensen, S.; Hartmann, B.; Rehfeld, J.F.; Holst, J.J.; Vilsbøll, T.; Knop, F.K. The role of GLP-1 in the postprandial effects of acarbose in type 2 diabetes. Eur. J. Endocrinol. 2021, 184, 383–394. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, Q.; Li, L.; Kwong, J.S.W.; Jia, P.; Zhao, P.; Wang, W.; Zhou, X.; Zhang, M.; Sun, X. Alpha-glucosidase inhibitors and hepatotoxicity in type 2 diabetes: A systematic review and meta-analysis. Sci. Rep. 2016, 6, 32649. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Norton, L.; Abdul-Ghani, M. Renal, metabolic and cardiovascular considerations of SGLT2 inhibition. Nat. Rev. Nephrol. 2016, 13, 11–26. [Google Scholar] [CrossRef]
- Keller, D.M.; Ahmed, N.; Tariq, H.; Walgamage, M.; Walgamage, T.; Mohammed, A.; Chou, J.T.-T.; Kałużna-Oleksy, M.; Lesiak, M.; Straburzyńska-Migaj, E. SGLT2 Inhibitors in Type 2 Diabetes Mellitus and Heart Failure—A Concise Review. J. Clin. Med. 2022, 11, 1470. [Google Scholar] [CrossRef]
- Vallon, V.; Rose, M.; Gerasimova, M.; Satriano, J.; Platt, K.A.; Koepsell, H.; Cunard, R.; Sharma, K.; Thomson, S.C.; Rieg, T. Knockout of Na-glucose transporter SGLT2 attenuates hyperglycemia and glomerular hyperfiltration but not kidney growth or injury in diabetes mellitus. Am. J. Physiol. Physiol. 2013, 304, F156–F167. [Google Scholar] [CrossRef]
- Williams, D.M.; Nawaz, A.; Evans, M. Sodium-Glucose Co-Transporter 2 (SGLT2) Inhibitors: Are They All the Same? A Narrative Review of Cardiovascular Outcome Trials. Diabetes Ther. 2020, 12, 55–70. [Google Scholar] [CrossRef]
- Kalra, S. Sodium Glucose Co-Transporter-2 (SGLT2) Inhibitors: A Review of Their Basic and Clinical Pharmacology. Diabetes Ther. 2014, 5, 355–366. [Google Scholar] [CrossRef]
- Joshi, S.S.; Singh, T.; Newby, D.E.; Singh, J. Sodium-glucose co-transporter 2 inhibitor therapy: Mechanisms of action in heart failure. Heart 2021, 107, 1032–1038. [Google Scholar] [CrossRef]
- Zhou, Z.; Zheng, M.; Zuo, Z.; Wu, T. Comparison of cardiovascular outcomes of new antihyperglycemic agents in Type 2 Diabetes Mellitus: A meta-analysis. ESC Heart Fail. 2024, 11, 1647–1656. [Google Scholar] [CrossRef] [PubMed]
- Faluk, M.; Wardhere, A.; Thakker, R.; Khan, F.A. SGLT2 inhibitors in heart failure with preserved ejection fraction. Curr. Probl. Cardiol. 2024, 49, 102388. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Zhang, N.; Chen, R.; Zhao, J.-G.; Yu, P. Efficacy and safety of sodium–glucose cotransporter 2 inhibitors in type 2 diabetes: A meta-analysis of randomized controlled trials for 1 to 2 years. J. Diabetes Its Complicat. 2015, 29, 1295–1303. [Google Scholar] [CrossRef] [PubMed]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef] [PubMed]
- Neal, B.; Perkovic, V.; Mahaffey, K.W.; de Zeeuw, D.; Fulcher, G.; Erondu, N.; Shaw, W.; Law, G.; Desai, M.; Matthews, D.R.; et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 644–657. [Google Scholar] [CrossRef]
- Wiviott, S.D.; Raz, I.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Silverman, M.G.; Zelniker, T.A.; Kuder, J.F.; Murphy, S.A.; et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2019, 380, 347–357. [Google Scholar] [CrossRef]
- Cannon, C.P.; Pratley, R.; Dagogo-Jack, S.; Mancuso, J.; Huyck, S.; Masiukiewicz, U.; Charbonnel, B.; Frederich, R.; Gallo, S.; Cosentino, F.; et al. Cardiovascular Outcomes with Ertugliflozin in Type 2 Diabetes. New Engl. J. Med. 2020, 383, 1425–1435. [Google Scholar] [CrossRef]
- Tang, H.; Li, D.; Wang, T.; Zhai, S.; Song, Y. Effect of Sodium–Glucose Cotransporter 2 Inhibitors on Diabetic Ketoacidosis Among Patients with Type 2 Diabetes: A Meta-analysis of Randomized Controlled Trials. Diabetes Care 2016, 39, e123–e124. [Google Scholar] [CrossRef]
- Nagendra, L.; Dutta, D.; Girijashankar, H.B.; Khandelwal, D.; Lathia, T.; Sharma, M.; Bg, H. Safety and tolerability of sodium-glucose cotransporter-2 inhibitors in children and young adults: A systematic review and meta-analysis. Ann. Pediatr. Endocrinol. Metab. 2024, 29, 82–89. [Google Scholar] [CrossRef]
- Sung, J.; Padmanabhan, S.; Gurung, S.; Inglis, S.; Vicaretti, M.; Begg, L.; Cheung, N.W.; Girgis, C.M. SGLT2 inhibitors and amputation risk: Real-world data from a diabetes foot wound clinic. J. Clin. Transl. Endocrinol. 2018, 13, 46–47. [Google Scholar] [CrossRef]
- Papachristou, S.; Popovic, D.S.; Papanas, N. The new dual gastric inhibitory peptide/glucagon-like peptide 1 agonist tirzepatide in type 2 diabetes: Is the future bright? Diabetes Metab. Res. Rev. 2021, 37, e3503. [Google Scholar] [CrossRef]
- Frias, J.P.; Nauck, M.A.; Van, J.; Kutner, M.E.; Cui, X.; Benson, C.; Urva, S.; Gimeno, R.E.; Milicevic, Z.; Robins, D.; et al. Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: A randomised, placebo-controlled and active comparator-controlled phase 2 trial. Lancet 2018, 392, 2180–2193. [Google Scholar] [CrossRef] [PubMed]
- Lisco, G.; Disoteo, O.E.; De Geronimo, V.; De Tullio, A.; Giagulli, V.A.; Guastamacchia, E.; De Pergola, G.; Jirillo, E.; Triggiani, V. Is Tirzepatide the New Game Changer in Type 2 Diabetes? Endocrines 2024, 5, 72–86. [Google Scholar] [CrossRef]
- Rosenstock, J.; Wysham, C.; Frías, J.P.; Kaneko, S.; Lee, C.J.; Landó, L.F.; Mao, H.; Cui, X.; AKaranikas, C.A.; Thieu, V.T. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): A double-blind, randomised, phase 3 trial. Lancet 2021, 398, 143–155. [Google Scholar] [CrossRef]
- Dahl, D.; Onishi, Y.; Norwood, P.; Huh, R.; Bray, R.; Patel, H.; Rodríguez, Á. Effect of Subcutaneous Tirzepatide vs Placebo Added to Titrated Insulin Glargine on Glycemic Control in Patients With Type 2 Diabetes: The SURPASS-5 Randomized Clinical Trial. JAMA 2022, 327, 534–545. [Google Scholar] [CrossRef] [PubMed]
- Ludvik, B.; Giorgino, F.; Jódar, E.; Frias, J.P.; Landó, L.F.; Brown, K.; Bray, R.; Rodríguez, Á. Once-weekly tirzepatide versus once-daily insulin degludec as add-on to metformin with or without SGLT2 inhibitors in patients with type 2 diabetes (SURPASS-3): A randomised, open-label, parallel-group, phase 3 trial. Lancet 2021, 398, 583–598. [Google Scholar] [CrossRef]
- Del Prato, S.; Kahn, S.E.; Pavo, I.; Weerakkody, G.J.; Yang, Z.; Doupis, J.; Aizenberg, D.; Wynne, A.G.; Riesmeyer, J.S.; Heine, R.J.; et al. Tirzepatide versus insulin glargine in type 2 diabetes and increased cardiovascular risk (SURPASS-4): A randomised, open-label, parallel-group, multicentre, phase 3 trial. Lancet 2021, 398, 1811–1824. [Google Scholar] [CrossRef]
- Rosenstock, J.; Frías, J.P.; Rodbard, H.W.; Tofé, S.; Sears, E.; Huh, R.; Fernández Landó, L.; Patel, H. Tirzepatide vs Insulin Lispro Added to Basal Insulin in Type 2 Diabetes: The SURPASS-6 Randomized Clinical Trial. JAMA 2023, 330, 1631–1640. [Google Scholar] [CrossRef]
- Gastaldelli, A.; Cusi, K.; Landó, L.F.; Bray, R.; Brouwers, B.; Rodríguez, Á. Effect of tirzepatide versus insulin degludec on liver fat content and abdominal adipose tissue in people with type 2 diabetes (SURPASS-3 MRI): A substudy of the randomised, open-label, parallel-group, phase 3 SURPASS-3 trial. Lancet Diabetes Endocrinol. 2022, 10, 393–406. [Google Scholar] [CrossRef]
- Karagiannis, T.; Avgerinos, I.; Liakos, A.; Del Prato, S.; Matthews, D.R.; Tsapas, A.; Bekiari, E. Management of type 2 diabetes with the dual GIP/GLP-1 receptor agonist tirzepatide: A systematic review and meta-analysis. Diabetologia 2022, 65, 1251–1261. [Google Scholar] [CrossRef]
- Sattar, N.; McGuire, D.K.; Pavo, I.; Weerakkody, G.J.; Nishiyama, H.; Wiese, R.J.; Zoungas, S. Tirzepatide cardiovascular event risk assessment: A pre-specified meta-analysis. Nat. Med. 2022, 28, 591–598. [Google Scholar] [CrossRef]
- Sardar, M.B.; Nadeem, Z.A.; Babar, M. Tirzepatide: A Novel Cardiovascular Protective Agent in Type 2 Diabetes Mellitus and Obesity. Curr. Probl. Cardiol. 2024, 49, 102489. [Google Scholar] [CrossRef]
- Zweihary, A.M.A. Safety and Effectiveness of Tirzepatide Use in Obesity Without Type 2 Diabetes Mellitus. Cureus 2024, 16, e51788. [Google Scholar] [CrossRef]
- Scheen, A. Tirzepatide: Overview of clinical studies SURPASS in type 2 diabetes and SURMOUNT in obesity. Rev. Med. Liege 2024, 79, 812–820. [Google Scholar]
- Zeng, Q.; Xu, J.; Mu, X.; Shi, Y.; Fan, H.; Li, S. Safety issues of tirzepatide (pancreatitis and gallbladder or biliary disease) in type 2 diabetes and obesity: A systematic review and meta-analysis. Front. Endocrinol. 2023, 14, 1214334. [Google Scholar] [CrossRef]
- Caruso, I.; Di Gioia, L.; Di Molfetta, S.; Caporusso, M.; Cignarelli, A.; Sorice, G.P.; Laviola, L.; Giorgino, F. The real-world safety profile of tirzepatide: Pharmacovigilance analysis of the FDA Adverse Event Reporting System (FAERS) database. J. Endocrinol. Investig. 2024, 47, 2671–2678. [Google Scholar] [CrossRef]
- AParker, J.; AMcCullough, K.; Field, B.C.T.; Minnion, J.S.; Martin, N.M.; AGhatei, M.; Bloom, S.R. Glucagon and GLP-1 inhibit food intake and increase c-fos expression in similar appetite regulating centres in the brainstem and amygdala. Int. J. Obes. 2013, 37, 1391–1398. [Google Scholar] [CrossRef]
- Sadry, S.A.; Drucker, D.J. Emerging combinatorial hormone therapies for the treatment of obesity and T2DM. Nat. Rev. Endocrinol. 2013, 9, 425–433. [Google Scholar] [CrossRef]
- Cegla, J.; Troke, R.C.; Jones, B.; Tharakan, G.; Kenkre, J.; McCullough, K.A.; Lim, C.T.; Parvizi, N.; Hussein, M.; Chambers, E.S.; et al. Coinfusion of Low-Dose GLP-1 and Glucagon in Man Results in a Reduction in Food Intake. Diabetes 2014, 63, 3711–3720. [Google Scholar] [CrossRef] [PubMed]
- Day, J.W.; Ottaway, N.; Patterson, J.T.; Gelfanov, V.; Smiley, D.; Gidda, J.; Findeisen, H.; Bruemmer, D.; Drucker, D.J.; Chaudhary, N.; et al. A new glucagon and GLP-1 co-agonist eliminates obesity in rodents. Nat. Chem. Biol. 2009, 5, 749–757. [Google Scholar] [CrossRef]
- Pocai, A.; Carrington, P.E.; Adams, J.R.; Wright, M.; Eiermann, G.; Zhu, L.; Du, X.; Petrov, A.; Lassman, M.E.; Jiang, G.; et al. Glucagon-like peptide 1/glucagon receptor dual agonism reverses obesity in mice. Diabetes 2009, 58, 2258–2266. [Google Scholar] [CrossRef]
- Day, J.W.; Gelfanov, V.; Smiley, D.; Carrington, P.E.; Eiermann, G.; Chicchi, G.; Erion, M.D.; Gidda, J.; Thornberry, N.A.; Tschöp, M.H.; et al. Optimization of co-agonism at GLP-1 and glucagon receptors to safely maximize weight reduction in DIO-rodents. Biopolymers 2012, 98, 443–450. [Google Scholar] [CrossRef]
- Holst, J.J.; Rosenkilde, M.M. Oxyntomodulin—Past, present and future. Peptides 2025, 188, 171393. [Google Scholar] [CrossRef]
- Kueh, M.T.W.; Chong, M.C.; Miras, A.D.; le Roux, C.W. Oxyntomodulin physiology and its therapeutic development in obesity and associated complications. J. Physiol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Meek, C.L.; Lewis, H.B.; Reimann, F.; Gribble, F.M.; Park, A.J. The effect of bariatric surgery on gastrointestinal and pancreatic peptide hormones. Peptides 2016, 77, 28–37. [Google Scholar] [CrossRef]
- Laferrère, B. Bariatric surgery and obesity: Influence on the incretins. Int. J. Obes. Suppl. 2016, 6, S32–S36. [Google Scholar] [CrossRef]
- Bhat, V.K.; Kerr, B.D.; Vasu, S.; Flatt, P.R.; Gault, V.A. A DPP-IV-resistant triple-acting agonist of GIP, GLP-1 and glucagon receptors with potent glucose-lowering and insulinotropic actions in high-fat-fed mice. Diabetologia 2013, 56, 1417–1424. [Google Scholar] [CrossRef]
- Finan, B.; Douros, J.D.; Goldwater, R.; Hansen, A.M.K.; Hjerpsted, J.B.; Hjøllund, K.R.; Kankam, M.K.; Knerr, P.J.; Konkar, A.; Mowery, S.A.; et al. A once-daily GLP-1/GIP/glucagon receptor tri-agonist (NN1706) lowers body weight in rodents, monkeys and humans. Mol. Metab. 2025, 96, 102129. [Google Scholar] [CrossRef] [PubMed]
- Wanner, C.; Marx, N. SGLT2 inhibitors: The future for treatment of type 2 diabetes mellitus and other chronic diseases. Diabetologia 2018, 61, 2134–2139. [Google Scholar] [CrossRef]
- Pitt, B.; Bhatt, D.L.; Szarek, M.; Cannon, C.P.; Leiter, L.A.; McGuire, D.K.; Lewis, J.B.; Riddle, M.C.; Voors, A.A.; Metra, M.; et al. Effect of Sotagliflozin on Early Mortality and Heart Failure-Related Events: A Post Hoc Analysis of SOLOIST-WHF. JACC Heart Fail. 2023, 11, 879–889. [Google Scholar] [CrossRef]
- Iyer, N.; Hussein, S.; Singareddy, S.; Sn, V.P.; Jaramillo, A.P.; Yasir, M.; Nath, T.S. Sotagliflozin vs Dapagliflozin: A Systematic Review Comparing Cardiovascular Mortality. Cureus 2023, 15, e45525. [Google Scholar] [CrossRef]
- European Commission. Withdrawing, at the Holder’s Request, the Marketing Authorisation Granted by Decision C(2019)3337(final) for «Zynquista—Sotagliflozin», a Medicinal Product for Human Use. 2022. Available online: https://ec.europa.eu/health/documents/community-register/2022/20220322155285/dec_155285_en.pdf (accessed on 14 January 2025).
- Kousaxidis, A.; Petrou, A.; Lavrentaki, V.; Fesatidou, M.; Nicolaou, I.; Geronikaki, A. Aldose reductase and protein tyrosine phosphatase 1B inhibitors as a promising therapeutic approach for diabetes mellitus. Eur. J. Med. Chem. 2020, 207, 112742. [Google Scholar] [CrossRef] [PubMed]
- Maccari, R.; Del Corso, A.; Paoli, P.; Adornato, I.; Lori, G.; Balestri, F.; Cappiello, M.; Naß, A.; Wolber, G.; Ottanà, R. An investigation on 4-thiazolidinone derivatives as dual inhibitors of aldose reductase and protein tyrosine phosphatase 1B, in the search for potential agents for the treatment of type 2 diabetes mellitus and its complications. Bioorg. Med. Chem. Lett. 2018, 28, 3712–3720. [Google Scholar] [CrossRef]
- Singh, A.; Chaudhary, R. Potentials of peroxisome proliferator-activated receptor (PPAR) α, β/δ, and γ: An in-depth and comprehensive review of their molecular mechanisms, cellular Signalling, immune responses and therapeutic implications in multiple diseases. Int. Immunopharmacol. 2025, 155, 114616. [Google Scholar] [CrossRef]
- Cabrero, A.; Laguna, J.C.; Vazquez, M. Peroxisome Proliferator-Activated Receptors and the Control of Inflammation. Curr. Drug Targets Inflamm. Allergy 2002, 1, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Ogata, T.; Miyauchi, T.; Sakai, S.; Irukayama-Tomobe, Y.; Goto, K.; Yamaguchi, I. Stimulation of peroxisome-proliferator-activated receptor α (PPAR α) attenuates cardiac fibrosis and endothelin-1 production in pressure-overloaded rat hearts. Clin. Sci. 2002, 103, 284S–288S. [Google Scholar] [CrossRef] [PubMed]
- Tai, E.S.; Collins, D.; Robins, S.J.; O’connor, J.J.; Bloomfield, H.E.; Ordovas, J.M.; Schaefer, E.J.; Brousseau, M.E. The L162V polymorphism at the peroxisome proliferator activated receptor alpha locus modulates the risk of cardiovascular events associated with insulin resistance and diabetes mellitus: The Veterans Affairs HDL Intervention Trial (VA-HIT). Atherosclerosis 2006, 187, 153–160. [Google Scholar] [CrossRef]
- Home, P. Safety of PPAR agonists. Diabetes Care 2011, 34 (Suppl. 2), S215–S219. [Google Scholar] [CrossRef]
- Bortolini, M.; Wright, M.B.; Bopst, M.; Balas, B. Examining the safety of PPAR agonists—Current trends and future prospects. Expert Opin. Drug Saf. 2012, 12, 65–79. [Google Scholar] [CrossRef]
- Balakumar, P.; Mahadevan, N.; Sambathkumar, R. A Contemporary Overview of PPARα/γ Dual Agonists for the Management of Diabetic Dyslipidemia. Curr. Mol. Pharmacol. 2019, 12, 195–201. [Google Scholar] [CrossRef]
- Xie, Z.; Xin, J.; Huang, C.; Liao, C. Drugs targeting peroxisome proliferator-activated receptors. Drug Discov. Today 2025, 30, 104318. [Google Scholar] [CrossRef]
- Shubrook, J.H.; Pfotenhauer, K.M. Pharmacology—Insulin. Prim. Care Clin. Office Pract. 2022, 49, 301–313. [Google Scholar] [CrossRef]
- Cahn, A.; Miccoli, R.; Dardano, A.; Del Prato, S. New forms of insulin and insulin therapies for the treatment of type 2 diabetes. Lancet Diabetes Endocrinol. 2015, 3, 638–652. [Google Scholar] [CrossRef] [PubMed]
- Aeng, E.; Boucher, M.; Severn, M. Newer Drugs for Type 2 Diabetes: An Emerging Adjunctive Therapy to Insulin for Type 1 Diabetes? CADTH Iisue Emerg. Health Technol. 2018, 166, 21. [Google Scholar]
- Mata Cases, M. Tipos de Insulina. Diabetes Prácti. 2017, 8 (Supl Extr 4), 1–24. [Google Scholar] [CrossRef]
- Heller, S.; Raposo, J.F.; Tofé, S.; Hanif, W.; Schroner, Z.; Down, S.; Blevins, T. Breaking Barriers with Basal Insulin Biosimilars in Type 2 Diabetes. Clin. Diabetes 2022, 41, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Nkonge, K.M.; Nkonge, D.K.; Nkonge, T.N. Insulin Therapy for the Management of Diabetes Mellitus: A Narrative Review of Innovative Treatment Strategies. Diabetes Ther. 2023, 14, 1801–1831. [Google Scholar] [CrossRef]
- Pieber, T.R.; Asong, M.; Fluhr, G.; Höller, V.; Kristensen, N.R.; Larsen, J.H.; Ribel-Madsen, R.; Svehlikova, E.; Vinther, S.; Voortman, M.; et al. Pharmacokinetic and pharmacodynamic properties of once-weekly insulin icodec in individuals with type 2 diabetes. Diabetes Obes. Metab. 2023, 25, 3716–3723. [Google Scholar] [CrossRef]
- Heise, T.; Chien, J.; Beals, J.M.; Benson, C.; Klein, O.; Moyers, J.S.; Haupt, A.; Pratt, E.J. Pharmacokinetic and pharmacodynamic properties of the novel basal insulin Fc (insulin efsitora alfa), an insulin fusion protein in development for once-weekly dosing for the treatment of patients with diabetes. Diabetes Obes. Metab. 2023, 25, 1080–1090. [Google Scholar] [CrossRef] [PubMed]
- Saleem, F.; Sharma, A.; StatPearls Publishing. NPH Insulin—StatPearls—NCBI Bookshelf. 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK549860/ (accessed on 20 March 2024).
- Semlitsch, T.; Engler, J.; Siebenhofer, A.; Jeitler, K.; Berghold, A.; Horvath, K. (Ultra-)long-acting insulin analogues versus NPH insulin (human isophane insulin) for adults with type 2 diabetes mellitus. Cochrane Database Syst Rev. 2020, 11, CD005613. [Google Scholar]
- Horden, S. Insulin detemir: A review. Drugs Today 2006, 42, 505–517. [Google Scholar] [CrossRef]
- Kurtzhals, P. Pharmacology of Insulin Detemir. Endocrinol. Metab. Clin. N. Am. 2007, 36, 14–20. [Google Scholar] [CrossRef]
- Rezaei, S.; Taheri, A.; Taheri, S.; Kasirzadeh, S.; Fatemi, B.; Sorato, M.M. Efficacy and safety of insulin detemir versus glargine in patients with diabetes: A systematic review and meta-analysis. Expert Rev. Clin. Pharmacol. 2022, 15, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Drugbank Online. Insulin Detemir. 2022. Available online: https://go.drugbank.com/drugs/DB01307 (accessed on 5 October 2022).
- Keating, G.M. Insulin detemir: A review of its use in the management of diabetes mellitus. Drugs 2012, 72, 2255–2287. [Google Scholar] [CrossRef]
- Zhou, W.; Tao, J.; Zhou, X.; Chen, H. Insulin Degludec, a Novel Ultra-Long-Acting Basal Insulin versus Insulin Glargine for the Management of Type 2 Diabetes: A Systematic Review and Meta-Analysis. Diabetes Ther. 2019, 10, 835–852. [Google Scholar] [CrossRef] [PubMed]
- Drugbank Online. Insulin Degludec. 2022. Available online: https://go.drugbank.com/drugs/DB09564 (accessed on 5 October 2022).
- EMA/CHMP Insulin Icodec. CHMP Summary of Positive Opinion for Awiqli®. EMA/CHMP/107168/2024. 2024. Available online: https://www.ema.europa.eu/en/documents/smop-initial/chmp-summary-positive-opinion-awiqli_en.pdf (accessed on 14 January 2025).
- Moyers, J.S.; Hansen, R.J.; Day, J.W.; Dickinson, C.D.; Zhang, C.; Ruan, X.; Ding, L.; Brown, R.M.; Baker, H.E.; Beals, J.M. Preclinical Characterization of LY3209590, a Novel Weekly Basal Insulin Fc-Fusion Protein. J. Pharmacol. Exp. Ther. 2022, 382, 346–355. [Google Scholar] [CrossRef]
- Haahr, H.; Fita, E.G.; Heise, T. A Review of Insulin Degludec/Insulin Aspart: Pharmacokinetic and Pharmacodynamic Properties and Their Implications in Clinical Use. Clin. Pharmacokinet. 2016, 56, 339–354. [Google Scholar] [CrossRef]
- Heller, S.; Kurtzhals, P.; Verge, D.; Lindholm, A. Insulin aspart: Promising early results borne out in clinical practice. Expert Opin. Pharmacother. 2002, 3, 183–195. [Google Scholar] [CrossRef]
- Drugbank Online. Insulin Aspart. 2022. Available online: https://go.drugbank.com/drugs/DB01306 (accessed on 5 October 2022).
- ABecker, R.H.; Frick, A.D. Clinical Pharmacokinetics and Pharmacodynamics of Insulin Glulisine. Clin. Pharmacokinet. 2008, 47, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.K.; Ellis, S.L.; Ulrich, H. Insulin glulisine: A new rapid-acting insulin analogue for the treatment of diabetes. Expert Opin. Pharmacother. 2005, 6, 643–651. [Google Scholar] [CrossRef]
- Drugbank Online. Insulin Glulisine. 2022. Available online: https://go.drugbank.com/drugs/DB01309 (accessed on 11 October 2022).
- Islam, N.; Khanna, N.R.; Zito, P.M. Insulin Lispro; En: StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: http://www.ncbi.nlm.nih.gov/books/NBK507840/ (accessed on 20 May 2025).
- Selivanova, O.M.; Suvorina, M.Y.; Surin, A.K.; Dovidchenko, N.V.; Galzitskaya, O.V. Insulin and Lispro Insulin: What Is Common and Different in Their Behavior? Curr. Protein Pept. Sci. 2017, 18, 57–64. [Google Scholar] [CrossRef]
- Warren, M.; Bode, B.; Cho, J.I.; Liu, R.; Tobian, J.; Hardy, T.; Chigutsa, F.; Phillip, M.; Horowitz, B.; Ignaut, D. Improved postprandial glucose control with ultra rapid lispro versus lispro with continuous subcutaneous insulin infusion in type 1 diabetes: PRONTO-Pump-2. Diabetes Obes. Metab. 2021, 23, 1552–1561. [Google Scholar] [CrossRef]
- Drugbank Online. Insulin Lispro. 2022. Available online: https://go.drugbank.com/drugs/DB00046 (accessed on 6 October 2022).
- Odegard, P.S.; Capoccia, K.L. Inhaled Insulin: Exubera. Ann. Pharmacother. 2005, 39, 843–853. [Google Scholar] [CrossRef]
- Heinemann, L. The failure of exubera: Are we beating a dead horse? J. Diabetes Sci. Technol. 2008, 2, 518–529. [Google Scholar] [CrossRef]
- Mathieu, C.; Gale Ea, M. Inhaled insulin: Gone with the wind? Diabetologia 2008, 51, 1–5. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nuffer, W.; Trujillo, J.M.; Ellis, S.L. Technosphere insulin (Afrezza): A new, inhaled prandial insulin. Ann. Pharmacother. 2015, 49, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Mikhail, N. Place of technosphere inhaled insulin in treatment of diabetes. World J. Diabetes 2016, 7, 599–604. [Google Scholar] [CrossRef] [PubMed]
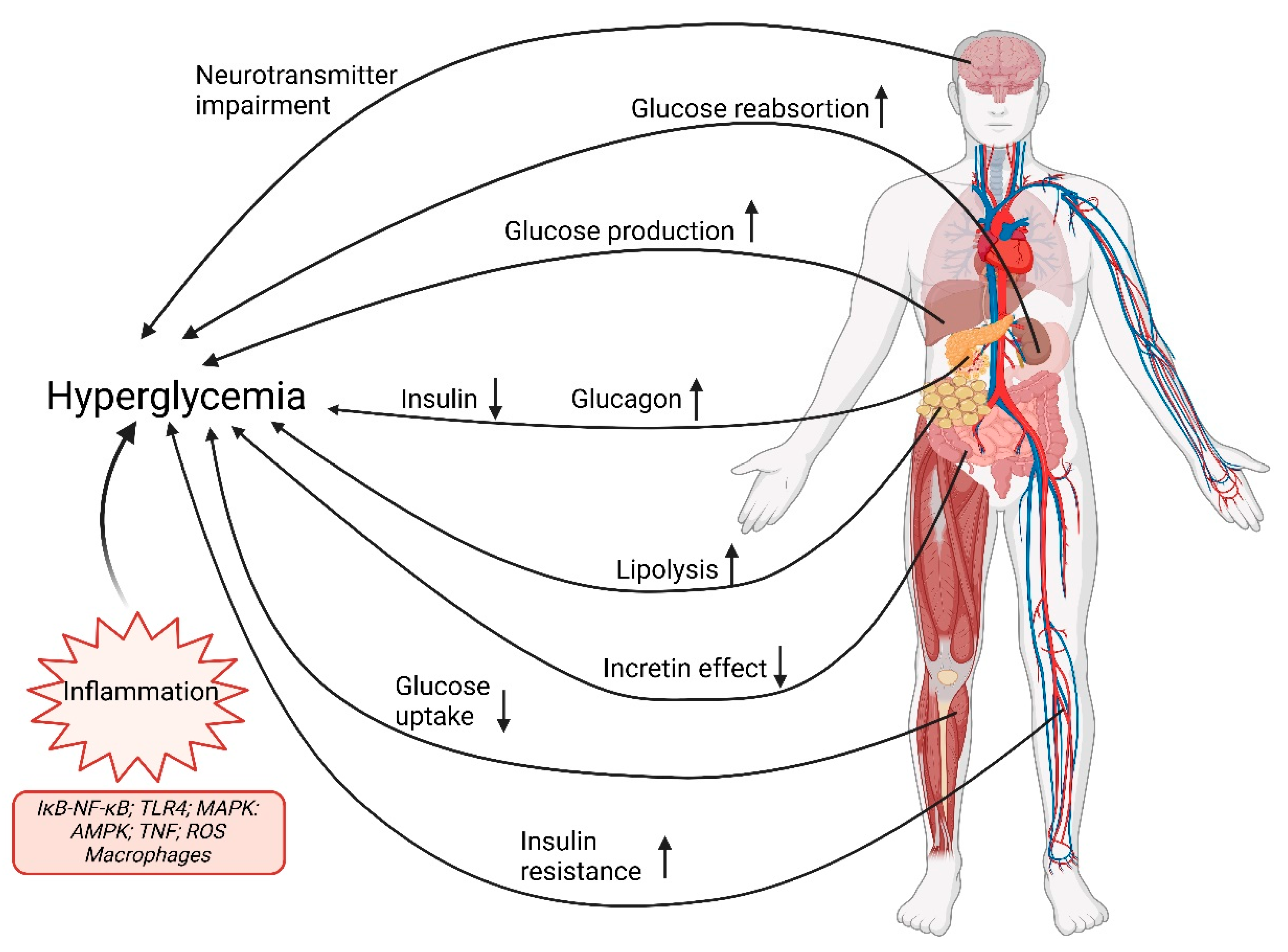
| Types of Diabetes | Description |
|---|---|
| Type 1 | Typically results from autoimmune destruction of pancreatic β cells, leading to a complete lack of insulin production. Frequently diagnosed in childhood or adolescence, though it can appear at any age. |
| Type 2 | Most prevalent form. Characterized by a gradual decline in β-cell function and insulin production, typically occurring in the context of insulin resistance and metabolic syndrome. Frequently linked to excess body weight and obesity. |
| Hybrid Forms of Diabetes | |
| Autoimmune diabetes of slow evolution in adults | Similar to type 1. It progresses slowly in adults. It has features of metabolic syndrome, has a single GAD (Glutamate Decarboxylase) autoantibody, and retains increased β-cell functionality. |
| Diabetes with a tendency to ketosis | Characterized by episodes of ketosis and initial insulin deficiency, though long-term insulin therapy may not be required. Ketosis tends to occur frequently, but the condition is not driven by autoimmune mechanisms. |
| Other Specific Types of Diabetes | |
| Monogenic diabetes | Caused by specific genetic mutations. Monogenic defects in the functionality of β cells. This form of diabetes presents diverse clinical profiles, each requiring tailored therapeutic approaches. Some cases emerge during the neonatal stage, while others appear in early adulthood. It is associated with monogenic mutations affecting insulin signaling, leading to pronounced insulin resistance in the absence of obesity. The condition arises when pancreatic β cells are unable to adequately compensate for the body’s insulin resistance. |
| Exocrine pancreas diseases | Cystic fibrosis and pancreatitis. |
| Endocrine disorders | Diseases with excess of insulin antagonist hormone secretion. |
| Induced by drugs/chemicals | Certain medications and chemical agents can interfere with insulin production or lead to the destruction of pancreatic β cells. |
| Infection-related diabetes | Viruses associated with the direct destruction of β cells. |
| Rare specific forms of autoimmune diabetes | Linked to uncommon autoimmune disorders that may contribute to the development of diabetes. |
| Other genetic syndromes related with diabetes | Numerous genetic syndromes and chromosomal anomalies are associated with an elevated risk of developing diabetes. |
| Unclassified diabetes | Cases of diabetes that do not clearly align with established classifications. It is intended as a provisional designation, particularly useful in the early stages after diagnosis, when a definitive categorization is not yet possible. |
| Hyperglycemia first detected during pregnancy | |
| In pregnancy | Type 1 or type 2 diabetes first diagnosed during pregnancy. |
| Gestational | Hyperglycemia below diagnostic levels for diabetes in pregnancy. |
| Parameter | Normal * | Prediabetes | T2D |
|---|---|---|---|
| Hemoglobin A1c (HbA1c) | <5.7% ‡ <6.0% § | 5.7–6.4% ‡ 6.0–6.4% § | ≥6.5% |
| Fasting plasma glucose (FPG) | 100 mg/dL ‡ <110 mg/dL § | 100–125 mg/dL ‡ 110–125 mg/dL § | ≥126 mg/dL |
| 2 h plasma glucose value during 75g oral glucose tolerance test (OGTT) | <140 mg/dL | 140–199 mg/dL | ≥200 mg/dL |
| Drug | Starting Dose (Maximum Daily Dose) | Tmax (h) | Duration of Action (h) | Metabolism | Elimination |
|---|---|---|---|---|---|
| First Generation | |||||
| Tolbutamide | 250 mg 1–3/day (3000 mg) | 3–4 | 6–12 | Inactive metabolites | Urine |
| Chloropropamide | 100 mg/day (750 mg) | 2–4 | ≥24 | Active metabolites | Urine |
| Tolazamida | 100 mg/day (1000 mg) | 3–4 | 12–24 | Active & inactive metabolites | Urine 85%, Feces 7% |
| Second Generation | |||||
| Glibenclamide (Glyburide) | 2.5 mg/day (20 mg in 1–2 doses) | 2–4 | 16–24 | Inactive or weakly active metabolites | Urine 50% Feces 50% |
| Glipizide | 2.5 mg/day (20 mg in 1–2 doses) | 1–3 (IR) 6– 12 (ER) | 12–24 | Inactive metabolites | Urine 90% Feces 10% |
| Gliquidone | 15 mg/day (180 mg in 2–3 doses) | 2–3 | 8–10 | Inactive metabolites | Urina 5% Feces 95% |
| Gliclazide | 40 mg/day (320 mg in 2 doses) 30–120 mg/day MR | 4–6 6–12 (MR) | 10–24 ≥24 (MR) | Inactive metabolites | Urine 60–70% Feces 10–20% |
| Glimepiride | 1 mg/day (6 mg) | 2–3 | ≥24 | Inactive and active metabolites | Urine 60% Feces 40% |
| Organ/Tissue | At Physiological Levels of GLP-1 in Clinical Studies | After Treatment with GLP-1 RA | In Preclinical Studies |
|---|---|---|---|
| Adipose | Increase Lipolysis Glucose uptake | ||
| Brain | Increase Satiety Decrease Appetite | ||
| Gastrointestinal | Decrease Gastric emptying Acid secretion | ||
| Heart | Increase Endothelial function | Increase Heart rate Myocardial contractility Diastolic function Cardiprotection Decrease Blood pressure | |
| Kidney | Increase Natriuresis | ||
| Muscle | Increase Glycogen synthesis Glucose oxidation | ||
| Pancreas | Increase Insulin secretion Decrease Glucagon secretion | Increase β cell proliferation |
| Drug | Relative Efficacy | MACE Composite Outcome [b] | |
|---|---|---|---|
| Reduction of HbA1c [a] | Reduction of Body Weight [a] | ||
| Dulaglutide 1/week [d] SC | ++ | ++ | Superior to placebo |
| Exenatide 2/day SC | + | +(+) | No CVOT |
| Exenatide XR 1/week SC | + | + | Not inferior to placebo |
| Lixisenatide 1/day SC | + | + | Not inferior to placebo |
| Liraglutide 1/day [c] SC | ++ | ++ | Superior to placebo |
| Semaglutide 1/week [c] SC | +++ | +++ | Superior to placebo |
| Oral semaglutide 1/day | ++(+) | ++(+) | Not inferior to placebo |
| DPP-4 Inhibitor | Dose | t1/2 | BA | PPB | Metabolism | Elimination |
|---|---|---|---|---|---|---|
| Alogliptin | 25 mg 1/day | 20 h | 100% | 20–30% | Minimal | Renal (>70%) |
| Linagliptin | 5 mg 1/day | ~12 h (effective) >100 h (terminal) | ~30% | >90% | Minimal | Hepatic (Renal < 7%) |
| Sitagliptin | 5 mg 1/day | 2.5 h (drug) 3 h (metabolite) | ~75% | N | Hydrolysis (CYP 3A4 and 3A5) to form active metabolite | Metabolism (drug) Renal (metabolite) |
| Sitagliptin | 100 mg 1/day | 12.5 h | ~87% | 18% | Minimal | Renal (>80%) |
| Vildagliptin | 50 mg 2/day | 2 h | 85% | ~9% | Hydrolysis (CYP independent) to form inactive metabolite | Metabolism (drug) Renal (metabolite) |
| Compound | CV Safety | Hospitalization Risk |
|---|---|---|
| Aligliptin | 0.96 vs. Placebo | 1.07 (p = 0.66) vs. Placebo |
| Linagliptin | 1.02 vs. Placebo 0.98 vs. Glimepiride | 0.90 (p = 0.26) vs. Placebo 1.21 (p = 0.18) vs. Glimepiride |
| Saxagliptin | 1 vs. Placebo | 1.27 (p < 0.007) vs. Placebo |
| Sitagliptin | 0.98 vs. Placebo | 1.00 (p = 0.98) vs. Placebo |
| Vildagliptin | No data | No data |
| Parameter | Canagliflozin | Dapagliflozin | Empagliflozin | Ertugliflozin |
|---|---|---|---|---|
| Oral dose (mg) | 100–300 | 5–10 | 10–25 | 5–15 |
| Half-life (hours) | 10.6–13.1 | 12.9 | 12.4 | 16.6 |
| Bioavailability (%) | 65 | 78 | 60 | 100 |
| Volume of distribution (L) | 83.5 | 118 | 73.8 | ~2000 times |
| Plasma protein binding (%) | 98 | 91 | 86.2 | 93.6 |
| Activity as SGLT2i vs. SGLT1i | ~250 times | ~1200 times | ~2500 times | ~2000 times |
| Drug | SD (mg) | MD (mg) | T2D | HF | ||
|---|---|---|---|---|---|---|
| eGFR Limit | Dose (mg) | eGFR Limit | Dose (mg) | |||
| Canagliflozin | 100 | 100–300 | 60 30–59 <30 | Max 300 Max 100 Max 100 in patients already treated with the drug. If not, do not initiate | ||
| Dapagliflozin | 10 | 10 | ≥45 <45 <25 | 10 Additional hypoglycemic treatment 10 mg in patients already treated with the drug; if not, contraindicated | ≥25 <25 | 10 mg 10 mg in patients already treated with the drug; if not, do not initiate |
| Empagliflozin | 10 | 10 | ≥60 45–59 <45 | Max 25 Max 20 mg, only if before treatment eGFR ≥60 Contraindicated | ≥20 <20 | 10 mg Contraindicate |
| Ertugliflozin | 5 | 5–15 | <60 <45 | Do not initiate Contraindicated | ||
| Insulin | Type | Concentration Dose | Action Onset | Time to Peak Action | Duration Action |
|---|---|---|---|---|---|
| First generation | |||||
| NPH | Intermediate | 40 and 100 UI/mL | 1–4 h | 4–12 h | 12–24 h |
| Detemir | Long | 100 UI/mL | 1–2 h | 4–8 h | 12–24 h |
| Glargine U100 | Long | 100 UI/mL | 1–4 h | 8–12 h | 5–24 h |
| Second generation | |||||
| Glargine U300 | Ultra-long | 300 UI/mL | 2–6 h | 12 h & no peak | 30–36 h |
| Degludec | Ultra-long | 100 and 200 UI/mL | 0.5–2 h | 9 h & no peak | >42 h |
| Third generation. Once weekly insulins. Under development | |||||
| Icodec [155] | Ultra-long | 700 UI/mL | 0.5–2 h | no peak | 196 h |
| Efsitora alfa [156] | Ultra-long | 35 units/mg | 3–5 days | 408 h | |
| Insulin | Type | Concentration Dose | Action Onset | Time to Peak Action | Duration Action |
|---|---|---|---|---|---|
| First generation | |||||
| RHI | Intermediate | 100 UI/mL | 0.5–1 h | 2.4–4 h | 5–8 h |
| Aspart | Rapid | 100 UI/mL | 5–20 min | 1–3 h | 3–5 h |
| Glulisine | Rapid | 100 UI/mL | 9–20 min | 1–3 h | 3–5 h |
| Lispro 100 UI | Rapid | 100 UI/mL | 9–20 min | 1–3 h | 3–5 h |
| Second generation | |||||
| Lispro 200 UI | Rapid | 200 UI/mL | 9–20 min | 1–3 h | 3–5 h |
| Third generation. Inhaled insulins | |||||
| Exubera | Rapid | 1 and 3 mg | 10–20 min | 2 h | 6 h |
| Tecnosphere | Rapid | 4, 8, and 12 UI | 12 min | 35–55 min | 1.5–4.5 h |
| Mixture | Concentration Dose | Action Onset | Time to Peak Action | Duration Action |
|---|---|---|---|---|
| First generation | ||||
| NPH + RHI | 100 UI | 0.5–1 h | 2–5 h | 10–16 h |
| Protaminated aspart + Aspart | 1000 UI | 5–30 min | 1–12 h | 15–18 h |
| Protaminated lispro + Lispro | 1000 UI | 10–15 min | 1–12 h | 10–16 h |
| Second generation | ||||
| RHI | 500 UI | <15 min | 4–8 h | 13–24 h |
| Third generation | ||||
| Degludec + Aspart | 100 UI | 10–20 min | 0.5–1.5 h | >24 h |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabré, F.; Centelles, J.J.; Cascante, M. From Current Therapeutics to Multitarget Ligands: A Review of Diabetes Pharmacological Treatments. Pharmaceutics 2025, 17, 1125. https://doi.org/10.3390/pharmaceutics17091125
Cabré F, Centelles JJ, Cascante M. From Current Therapeutics to Multitarget Ligands: A Review of Diabetes Pharmacological Treatments. Pharmaceutics. 2025; 17(9):1125. https://doi.org/10.3390/pharmaceutics17091125
Chicago/Turabian StyleCabré, Francesc, Josep J. Centelles, and Marta Cascante. 2025. "From Current Therapeutics to Multitarget Ligands: A Review of Diabetes Pharmacological Treatments" Pharmaceutics 17, no. 9: 1125. https://doi.org/10.3390/pharmaceutics17091125
APA StyleCabré, F., Centelles, J. J., & Cascante, M. (2025). From Current Therapeutics to Multitarget Ligands: A Review of Diabetes Pharmacological Treatments. Pharmaceutics, 17(9), 1125. https://doi.org/10.3390/pharmaceutics17091125









