Influence of Different Amino Acids on the Aerosolization, Stability and Cytotoxicity of Spray-Dried Cannabidiol Dry Powder for Inhalation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of CBD Spray-Dried Powder Formulations
2.3. Drug Content and HPLC Method for Quantification of CBD
2.4. Powder Physicochemical Characterizations
2.4.1. Residual Solvent Content
2.4.2. Particle Size and Morphology Analysis
2.4.3. Drug-Excipient Interaction
2.4.4. Crystallinity of Powders
2.5. In Vitro Aerosolization
2.6. Cellular Toxicity
2.7. Stability Study
2.8. Statistical Analysis
3. Results and Discussion
3.1. Yield and Physicochemical Characterizations of Spray-Dried CBD
3.2. Powder Morphology of Spray-Dried CBD
3.3. Drug Excipient Interaction of Spray-Dried CBD
3.4. Powder Crystallinity of Spray-Dried CBD
3.5. In Vitro Aerosolization of Spray-Dried CBD
3.6. Cellular Toxicity of Spray-Dried CBD
3.7. Stability Study of Spray-Dried CBD
4. Conclusion and Future Remarks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leinen, Z.J.; Mohan, R.; Premadasa, L.S.; Acharya, A.; Mohan, M.; Byrareddy, S.N. Therapeutic Potential of Cannabis: A Comprehensive Review of Current and Future Applications. Biomedicines 2023, 11, 2630. [Google Scholar] [CrossRef] [PubMed]
- Britch, S.C.; Babalonis, S.; Walsh, S.L. Cannabidiol: Pharmacology and therapeutic targets. Psychopharmacology 2021, 238, 9–28. [Google Scholar] [CrossRef]
- Yau, G.T.Y.; Tai, W.; Arnold, J.C.; Chan, H.K.; Kwok, P.C.L. Cannabidiol for the Treatment of Brain Disorders: Therapeutic Potential and Routes of Administration. Pharm. Res. 2023, 40, 1087–1114. [Google Scholar] [CrossRef] [PubMed]
- Abu-Sawwa, R.; Scutt, B.; Park, Y. Emerging Use of Epidiolex (Cannabidiol) in Epilepsy. J. Pediatr. Pharmacol. Ther. 2020, 25, 485–499. [Google Scholar] [CrossRef] [PubMed]
- EMA. Epidyolex. 2025. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/epidyolex (accessed on 27 July 2025).
- Guy, G.; Robson, P. A Phase I, open label, four-way crossover study to compare the pharmacokinetic profiles of a single dose of 20 mg of a cannabis based medicine extract (CBME) administered on 3 different areas of the buccal mucosa and to investigate the pharmacokinetics of CBME per oral in healthy male and female volunteers (GWPK0112). J. Cannabis Ther. 2004, 3, 79–120. [Google Scholar] [CrossRef]
- Huestis, M.A. Human cannabinoid pharmacokinetics. Chem. Biodivers. 2007, 4, 1770. [Google Scholar] [CrossRef]
- Devinsky, O.; Kraft, K.; Rusch, L.; Fein, M.; Leone-Bay, A. Improved Bioavailability with Dry Powder Cannabidiol Inhalation: A Phase 1 Clinical Study. J. Pharm. Sci. 2021, 110, 3946–3952. [Google Scholar] [CrossRef]
- Sanders, M. Inhalation therapy: An historical review. Prim. Care Respir. J. 2007, 16, 71–81. [Google Scholar] [CrossRef]
- Dolovich, M. New propellant-free technologies under investigation. J. Aerosol. Med. 1999, 12 (Suppl. 1), S9–S17. [Google Scholar] [CrossRef]
- Kanig, J.L. Pharmaceutical Aerosols. J. Pharm. Sci. 1963, 52, 513–535. [Google Scholar] [CrossRef]
- Cal, K.; Sollohub, K. Spray drying technique. I: Hardware and process parameters. J. Pharm. Sci. 2010, 99, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-Y.; Seville, P.C.; Williamson, I.J.; Birchall, J.C. The use of amino acids to enhance the aerosolisation of spray-dried powders for pulmonary gene therapy. J. Gene Med. 2005, 7, 343–353. [Google Scholar] [CrossRef]
- Li, H.Y.; Neill, H.; Innocent, R.; Seville, P.; Williamson, I.; Birchall, J.C. Enhanced dispersibility and deposition of spray-dried powders for pulmonary gene therapy. J. Drug Target. 2003, 11, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Timsina, M.P.; Martin, G.P.; Marriott, C.; Ganderton, D.; Yianneskis, M. Drug delivery to the respiratory tract using dry powder inhalers. Int. J. Pharm. 1994, 101, 1–13. [Google Scholar] [CrossRef]
- Rasenack, N.; Müller, B.W. Micron-Size Drug Particles: Common and Novel Micronization Techniques. Pharm. Dev. Technol. 2004, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Byron, P.R. Some Future Perspectives for Unit Dose Inhalation Aerosols. Drug Dev. Ind. Pharm. 1986, 12, 993–1015. [Google Scholar] [CrossRef]
- Kosović, E.; Sýkora, D.; Kuchař, M. Stability Study of Cannabidiol in the Form of Solid Powder and Sunflower Oil Solution. Pharmaceutics 2021, 13, 412. [Google Scholar] [CrossRef]
- Gutowski, T. Inhalable Cannabidiol: Formulation Considerations for Extremely Lipophilic, UV-Labile Substances. Ph.D. Thesis, Kiel University, Kiel, Germany, 2021. [Google Scholar]
- Tai, W.; Arnold, J.C.; Chan, H.-K.; Kwok, P.C.L. Spray freeze dried cannabidiol with dipalmitoylphosphatidylcholine (DPPC) for inhalation and solubility enhancement. Int. J. Pharm. 2024, 659, 124235. [Google Scholar] [CrossRef]
- Williams III, R.O.; Moon, C.; Koleng, J.J. Compositions of Cannabinoids for Delivery by Inhalation. Google Patents WO2021055672A1, 25 March 2021. Available online: https://patents.google.com/patent/WO2021055672A1/en (accessed on 18 February 2025).
- Tai, W.; Yau, G.T.Y.; Arnold, J.C.; Chan, H.-K.; Kwok, P.C.L. High-loading cannabidiol powders for inhalation. Int. J. Pharm. 2024, 660, 124370. [Google Scholar] [CrossRef]
- Komal, K.; Chen, S.; Hanton, L.R.; Glass, M.; Das, S.C. Preparation and in vitro characterization of inhalable cannabidiol dry powder for treating chronic obstructive pulmonary disease. Int. J. Pharm. 2025, 682, 125892. [Google Scholar] [CrossRef]
- Zijlstra, G.S.; Ponsioen, B.J.; Hummel, S.A.; Sanders, N.; Hinrichs, W.L.; de Boer, A.H.; Frijlink, H.W. Formulation and process development of (recombinant human) deoxyribonuclease I as a powder for inhalation. Pharm. Dev. Technol. 2009, 14, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Wahjudi, M.; Murugappan, S.; van Merkerk, R.; Eissens, A.C.; Visser, M.R.; Hinrichs, W.L.J.; Quax, W.J. Development of a dry, stable and inhalable acyl–homoserine–lactone–acylase powder formulation for the treatment of pulmonary Pseudomonas aeruginosa infections. Eur. J. Pharm. Sci. 2013, 48, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Amorij, J.P.; Saluja, V.; Petersen, A.H.; Hinrichs, W.L.J.; Huckriede, A.; Frijlink, H.W. Pulmonary delivery of an inulin-stabilized influenza subunit vaccine prepared by spray-freeze drying induces systemic, mucosal humoral as well as cell-mediated immune responses in BALB/c mice. Vaccine 2007, 25, 8707–8717. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez Furlán, L.T.; Pérez Padilla, A.; Campderros, M. Development of a functional beverage formulation with high protein content, inulin and Stevia. Int. J. Food Eng. 2011, 7, 1–16. [Google Scholar] [CrossRef]
- Van Drooge, D.; Hinrichs, W.; Frijlink, H. Incorporation of lipophilic drugs in sugar glasses by lyophilization using a mixture of water and tertiary butyl alcohol as solvent. J. Pharm. Sci. 2004, 93, 713–725. [Google Scholar] [CrossRef]
- Van Drooge, D.; Hinrichs, W.; Wegman, K.; Visser, M.; Eissens, A.; Frijlink, H. Solid dispersions based on inulin for the stabilisation and formulation of Δ9-tetrahydrocannabinol. Eur. J. Pharm. Sci. 2004, 21, 511–518. [Google Scholar] [CrossRef]
- Ógáin, O.N.; Li, J.; Tajber, L.; Corrigan, O.I.; Healy, A.M. Particle engineering of materials for oral inhalation by dry powder inhalers. I—Particles of sugar excipients (trehalose and raffinose) for protein delivery. Int. J. Pharm. 2011, 405, 23–35. [Google Scholar] [CrossRef]
- Adhikari, B.R.; Sinha, S.; Gordon, K.C.; Das, S.C. Amino acids improve aerosolization and chemical stability of potential inhalable amorphous Spray-dried ceftazidime for Pseudomonas aeruginosa lung infection. Int. J. Pharm. 2022, 621, 121799. [Google Scholar] [CrossRef]
- Chew, N.Y.; Shekunov, B.Y.; Tong, H.H.; Chow, A.H.; Savage, C.; Wu, J.; Chan, H.-K. Effect of amino acids on the dispersion of disodium cromoglycate powders. J. Pharm. Sci. 2005, 94, 2289–2300. [Google Scholar] [CrossRef]
- Momin, M.A.; Sinha, S.; Tucker, I.G.; Doyle, C.; Das, S.C. Dry powder formulation of kanamycin with enhanced aerosolization efficiency for drug-resistant tuberculosis. Int. J. Pharm. 2017, 528, 107–117. [Google Scholar] [CrossRef]
- Lu, W.; Rades, T.; Rantanen, J.; Chan, H.-K.; Yang, M. Amino acids as stabilizers for spray-dried simvastatin powder for inhalation. Int. J. Pharm. 2019, 572, 118724. [Google Scholar] [CrossRef]
- Zhang, C.; Jørgensen, F.S.; van de Weert, M.; Bjerregaard, S.; Rantanen, J.; Yang, M. Amino acids as stabilizers for lysozyme during the spray-drying process and storage. Int. J. Pharm. 2024, 659, 124217. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-F.; Xu, Y.; Qu, D.-S.; Li, H.-Y. The influence of amino acids on aztreonam spray-dried powders for inhalation. Asian J. Pharm. Sci. 2015, 10, 541–548. [Google Scholar] [CrossRef]
- Adhikari, B.R.; Bērziņš, K.; Fraser-Miller, S.J.; Gordon, K.C.; Das, S.C. Co-Amorphization of Kanamycin with Amino Acids Improves Aerosolization. Pharmaceutics 2020, 12, 715. [Google Scholar] [CrossRef] [PubMed]
- Seville, P.C.; Learoyd, T.P.; Li, H.Y.; Williamson, I.J.; Birchall, J.C. Amino acid-modified spray-dried powders with enhanced aerosolisation properties for pulmonary drug delivery. Powder Technol. 2007, 178, 40–50. [Google Scholar] [CrossRef]
- Jensen, K.T.; Löbmann, K.; Rades, T.; Grohganz, H. Improving co-amorphous drug formulations by the addition of the highly water soluble amino acid, proline. Pharmaceutics 2014, 6, 416–435. [Google Scholar] [CrossRef]
- Faghihi, H.; Vatanara, A.; Najafabadi, A.R.; Ramezani, V.; Gilani, K. The use of amino acids to prepare physically and conformationally stable spray-dried IgG with enhanced aerosol performance. Int. J. Pharm. 2014, 466, 163–171. [Google Scholar] [CrossRef]
- Mangal, S.; Nie, H.; Xu, R.; Guo, R.; Cavallaro, A.; Zemlyanov, D.; Zhou, Q.T. Physico-Chemical Properties, Aerosolization and Dissolution of Co-Spray Dried Azithromycin Particles with L-Leucine for Inhalation. Pharm. Res. 2018, 35, 28. [Google Scholar] [CrossRef]
- Simm, S.; Einloft, J.; Mirus, O.; Schleiff, E. 50 years of amino acid hydrophobicity scales: Revisiting the capacity for peptide classification. Biol. Res. 2016, 49, 31. [Google Scholar] [CrossRef]
- Pommié, C.; Levadoux, S.; Sabatier, R.; Lefranc, G.; Lefranc, M.P. IMGT standardized criteria for statistical analysis of immunoglobulin V-REGION amino acid properties. J. Mol. Recognit. 2004, 17, 17–32. [Google Scholar] [CrossRef]
- Matveev, Y.I.; Grinberg, V.Y.; Sochava, I.; Tolstoguzov, V. Glass transition temperature of proteins. Calculation based on the additive contribution method and experimental data. Food Hydrocoll. 1997, 11, 125–133. [Google Scholar] [CrossRef]
- Vehring, R. Pharmaceutical particle engineering via spray drying. Pharm. Res. 2008, 25, 999–1022. [Google Scholar] [CrossRef]
- Momin, M.A.M.; Shubhra, S.; Tucker, I.G.; and Das, S.C. Carrier-free combination dry powder inhaler formulation of ethionamide and moxifloxacin for treating drug-resistant tuberculosis. Drug Dev. Ind. Pharm. 2019, 45, 1321–1331. [Google Scholar] [CrossRef] [PubMed]
- Stewart, I.E.; Lukka, P.B.; Liu, J.; Meibohm, B.; Gonzalez-Juarrero, M.; Braunstein, M.S.; Lee, R.E.; Hickey, A.J. Development and Characterization of a Dry Powder Formulation for Anti-Tuberculosis Drug Spectinamide 1599. Pharm. Res. 2019, 36, 136. [Google Scholar] [CrossRef] [PubMed]
- Momin, M.A.M.; Rangnekar, B.; Sinha, S.; Cheung, C.-Y.; Cook, G.M.; Das, S.C. Inhalable Dry Powder of Bedaquiline for Pulmonary Tuberculosis: In Vitro Physicochemical Characterization, Antimicrobial Activity and Safety Studies. Pharmaceutics 2019, 11, 502. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Xing, Y.; Peng, H.; Liu, Z.; Zhou, Q.; Xue, Z.; Ma, Z.; Kebebe, D.; Zhang, B.; Liu, H. Physicochemical and Pharmacokinetic Evaluation of Spray-Dried Coformulation of Salvia miltiorrhiza Polyphenolic Acid and L-Leucine with Improved Bioavailability. J. Aerosol Med. Pulm. Drug Deliv. 2020, 33, 73–82. [Google Scholar] [CrossRef]
- Paudel, A.; Worku, Z.A.; Meeus, J.; Guns, S.; Van den Mooter, G. Manufacturing of solid dispersions of poorly water soluble drugs by spray drying: Formulation and process considerations. Int. J. Pharm. 2013, 453, 253–284. [Google Scholar] [CrossRef]
- Bodmeier, R.; McGinity, J. Solvent selection in the preparation of poly (DL-lactide) microspheres prepared by the solvent evaporation method. Int. J. Pharm. 1988, 43, 179–186. [Google Scholar] [CrossRef]
- Meeus, J.; Lenaerts, M.; Scurr, D.J.; Amssoms, K.; Davies, M.C.; Roberts, C.J.; Van den mooter, G. The Influence of Spray-Drying Parameters on Phase Behavior, Drug Distribution, and In Vitro Release of Injectable Microspheres for Sustained Release. J. Pharm. Sci. 2015, 104, 1451–1460. [Google Scholar] [CrossRef]
- Saingam, W.; Sakunpak, A. Development and validation of reverse phase high performance liquid chromatography method for the determination of delta-9-tetrahydrocannabinol and cannabidiol in oromucosal spray from cannabis extract. Rev. Bras. Farmacogn. 2018, 28, 669–672. [Google Scholar] [CrossRef]
- Meenach, S.A.; Vogt, F.G.; Anderson, K.W.; Hilt, J.Z.; McGarry, R.C.; Mansour, H.M. Design, physicochemical characterization, and optimization of organic solution advanced spray-dried inhalable dipalmitoylphosphatidylcholine (DPPC) and dipalmitoylphosphatidylethanolamine poly(ethylene glycol) (DPPE-PEG) microparticles and nanoparticles for targeted respiratory nanomedicine delivery as dry powder inhalation aerosols. Int. J. Nanomed. 2013, 8, 275–293. [Google Scholar] [CrossRef]
- Rangnekar, B.; Momin, M.A.; Eedara, B.B.; Sinha, S.; Das, S.C. Bedaquiline containing triple combination powder for inhalation to treat drug-resistant tuberculosis. Int. J. Pharm. 2019, 570, 118689. [Google Scholar] [CrossRef] [PubMed]
- Muralidharan, P.; Mallory, E.K.; Malapit, M.; Phan, H.; Ledford, J.G.; Hayes, D.; Mansour, H.M. Advanced design and development of nanoparticle/microparticle dual-drug combination lactose carrier-free dry powder inhalation aerosols. RSC Adv. 2020, 10, 41846–41856. [Google Scholar] [CrossRef] [PubMed]
- CrysAlisPRO. Oxford Diffraction/Agilent Technologies UK Ltd., Yarnton, England. 2014. Available online: https://rigaku.com/products/crystallography/x-ray-diffraction/crysalispro (accessed on 5 April 2025).
- Momin, M.A.; Adhikari, B.R.; Sinha, S.; Larson, I.; Das, S.C. Roflumilast powders for chronic obstructive pulmonary disease: Formulation design and the Influence of device, inhalation flow rate, and storage relative humidity on aerosolization. Pharmaceutics 2021, 13, 1254. [Google Scholar] [CrossRef] [PubMed]
- Marple, V.A.; Roberts, D.L.; Romay, F.J.; Miller, N.C.; Truman, K.G.; Van Oort, M.; Olsson, B.; Holroyd, M.J.; Mitchell, J.P.; Hochrainer, D. Next Generation Pharmaceutical Impactor (A New Impactor for Pharmaceutical Inhaler Testing). Part I: Design. J. Aerosol Med. 2003, 16, 283–299. [Google Scholar] [CrossRef]
- Brunaugh, A.D.; Wu, T.; Kanapuram, S.R.; Smyth, H.D.C. Effect of Particle Formation Process on Characteristics and Aerosol Performance of Respirable Protein Powders. Mol. Pharm. 2019, 16, 4165–4180. [Google Scholar] [CrossRef]
- Brunaugh, A.D.; Jan, S.U.; Ferrati, S.; Smyth, H.D.C. Excipient-Free Pulmonary Delivery and Macrophage Targeting of Clofazimine via Air Jet Micronization. Mol. Pharm. 2017, 14, 4019–4031. [Google Scholar] [CrossRef]
- Greenspan, L. Humidity Fixed Points of Binary Saturated Aqueous Solutions. J. Res. Natl. Bur. Stand A Phys. Chem. 1977, 81A, 89–96. [Google Scholar] [CrossRef]
- Kamruzzaman, M.; Cathcart, H.; McLoughlin, P.; O’Reilly, N.J. Evaluation of aspartame as a co-former in the preparation of co-amorphous formulations of dipyridamole using spray drying. Int. J. Pharm. 2024, 667, 124913. [Google Scholar] [CrossRef]
- Momin, M.A.M.; Tucker, I.G.; Das, S.C. High dose dry powder inhalers to overcome the challenges of tuberculosis treatment. Int. J. Pharm. 2018, 550, 398–417. [Google Scholar] [CrossRef]
- Zhu, K.; Tan, R.; Ng, W.; Shen, S.; Zhou, Q.; Heng, P. Analysis of the influence of relative humidity on the moisture sorption of particles and the aerosolization process in a dry powder inhaler. J. Aerosol Sci. 2008, 39, 510–524. [Google Scholar] [CrossRef]
- Shepard, K.; Dower, A.; Ekdahl, A.; Morgen, M.; Baumann, J.; Vodak, D. Solvent-Assisted Secondary Drying of Spray-Dried Polymers. Pharm. Res. 2020, 37, 156. [Google Scholar] [CrossRef] [PubMed]
- British Pharmacopoeia Commission. British Pharmacopoeia; Stationery Office: London, UK, 2015; Available online: https://www.pharmacopoeia.com/ (accessed on 19 February 2025).
- Heyder, J.; Gebhart, J.; Rudolf, G.; Schiller, C.F.; Stahlhofen, W. Deposition of particles in the human respiratory tract in the size range 0.005–15 μm. J. Aerosol Sci. 1986, 17, 811–825. [Google Scholar] [CrossRef]
- de Boer, A.H.; Gjaltema, D.; Hagedoorn, P.; Frijlink, H.W. Characterization of inhalation aerosols: A critical evaluation of cascade impactor analysis and laser diffraction technique. Int. J. Pharm. 2002, 249, 219–231. [Google Scholar] [CrossRef]
- Walz, M.; Hirth, T.; Weber, A. Investigation of chemically modified inulin as encapsulation material for pharmaceutical substances by spray-drying. Colloids Surf. A Physicochem. Eng. Asp. 2018, 536, 47–52. [Google Scholar] [CrossRef]
- Sou, T.; Kaminskas, L.M.; Nguyen, T.-H.; Carlberg, R.; McIntosh, M.P.; Morton, D.A.V. The effect of amino acid excipients on morphology and solid-state properties of multi-component spray-dried formulations for pulmonary delivery of biomacromolecules. Eur. J. Pharm. Biopharm. 2013, 83, 234–243. [Google Scholar] [CrossRef]
- Alhajj, N.; O’Reilly, N.J.; Cathcart, H. Designing enhanced spray dried particles for inhalation: A review of the impact of excipients and processing parameters on particle properties. Powder Technol. 2021, 384, 313–331. [Google Scholar] [CrossRef]
- Han, C.S.; Kang, J.H.; Kim, Y.J.; Kim, D.W.; Park, C.W. Inhalable Nano-Dimpled Microspheres Containing Budesonide-PLGA for Improved Aerodynamic Performance. Int. J. Nanomed. 2022, 17, 3405–3419. [Google Scholar] [CrossRef]
- Hassan, M.S.; Lau, R.W.M. Effect of particle shape on dry particle inhalation: Study of flowability, aerosolization, and deposition properties. Aaps. Pharmscitech. 2009, 10, 1252–1262. [Google Scholar] [CrossRef]
- Edwards, D.A. Delivery of biological agents by aerosols. Am. Inst. Chem. Eng. AIChE J. 2002, 48, 2. [Google Scholar] [CrossRef]
- Crowder, T.M.; Rosati, J.A.; Schroeter, J.D.; Hickey, A.J.; Martonen, T.B. Fundamental Effects of Particle Morphology on Lung Delivery: Predictions of Stokes’ Law and the Particular Relevance to Dry Powder Inhaler Formulation and Development. Pharm. Res. 2002, 19, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chang, S.-L.; Chang, T.-R.; You, Y.; Wang, X.-D.; Wang, L.-W.; Yuan, X.-F.; Tan, M.-H.; Wang, P.-D.; Xu, P.-W.; et al. Inclusion complexes of cannabidiol with β-cyclodextrin and its derivative: Physicochemical properties, water solubility, and antioxidant activity. J. Mol. Liq. 2021, 334, 116070. [Google Scholar] [CrossRef]
- Barth, A. The infrared absorption of amino acid side chains. Prog. Biophys. Mol. Biol. 2000, 74, 141–173. [Google Scholar] [CrossRef] [PubMed]
- Mudannayake, D.C.; Wimalasiri, K.M.S.; Silva, K.F.S.T.; Ajlouni, S. Comparison of Properties of New Sources of Partially Purified Inulin to Those of Commercially Pure Chicory Inulin. J. Food Sci. 2015, 80, C950–C960. [Google Scholar] [CrossRef]
- Wang, C.; Wang, J.; Sun, Y.; Freeman, K.; Mchenry, M.A.; Wang, C.; Guo, M. Enhanced stability and oral bioavailability of cannabidiol in zein and whey protein composite nanoparticles by a modified anti-solvent approach. Foods 2022, 11, 376. [Google Scholar] [CrossRef]
- Shekunov, B.Y.; Feeley, J.C.; Chow, A.H.L.; Tong, H.H.Y.; York, P. Physical Properties of Supercritically-Processed and Micronised Powders for Respiratory Drug Delivery. KONA Powder Part. J. 2002, 20, 178–187. [Google Scholar] [CrossRef]
- Tai, W.; Khanal, D.; Arnold, J.C.; Chan, H.K.; Kwok, P.C.L. Solubilising and Aerosolising Cannabidiol Using Methyl β-Cyclodextrin and Human Serum Albumin. AAPS PharmSciTech 2025, 26, 120. [Google Scholar] [CrossRef]
- Maloney Norcross, S.E.; Levin, L.P.K.; Hickey, A.J.; Hill, D.B. Biopolymeric Inhalable Dry Powders for Pulmonary Drug Delivery. Pharmaceuticals 2024, 17, 1628. [Google Scholar] [CrossRef]
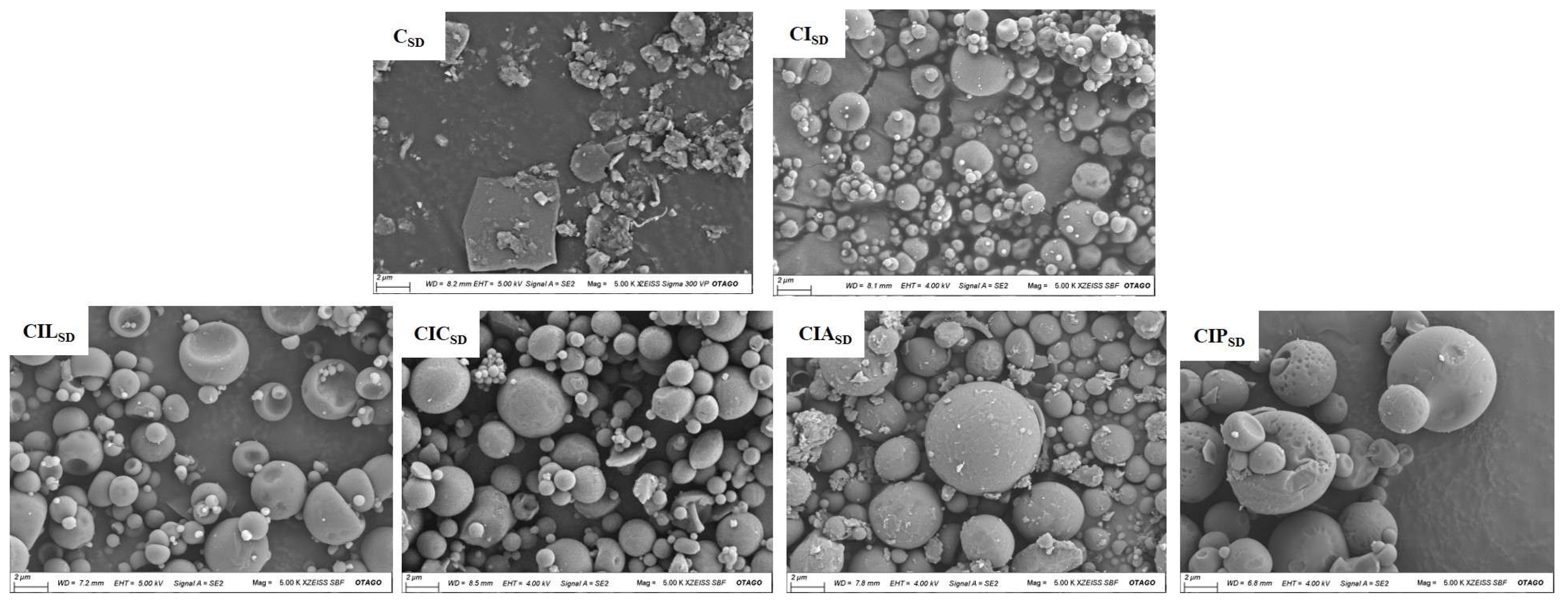
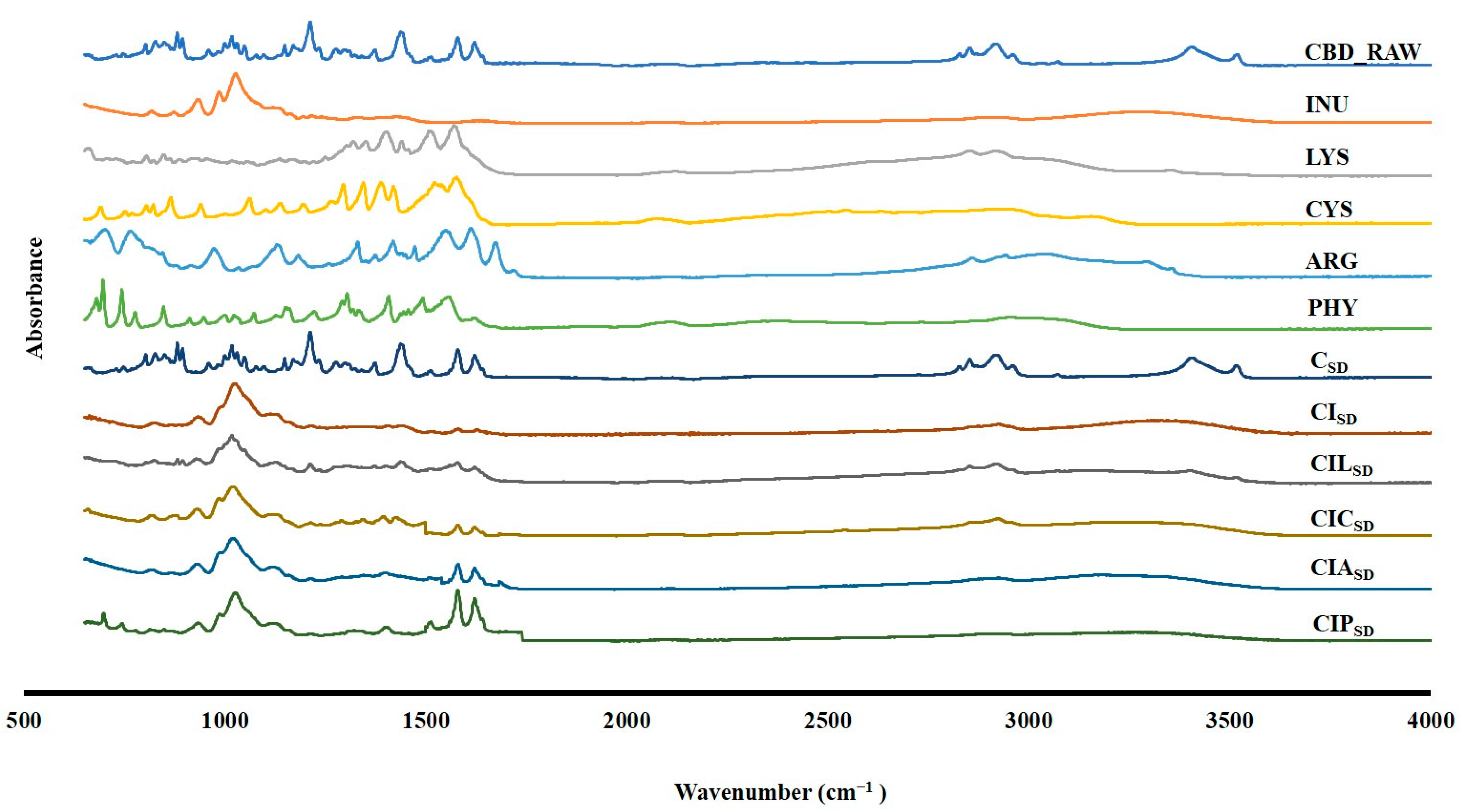
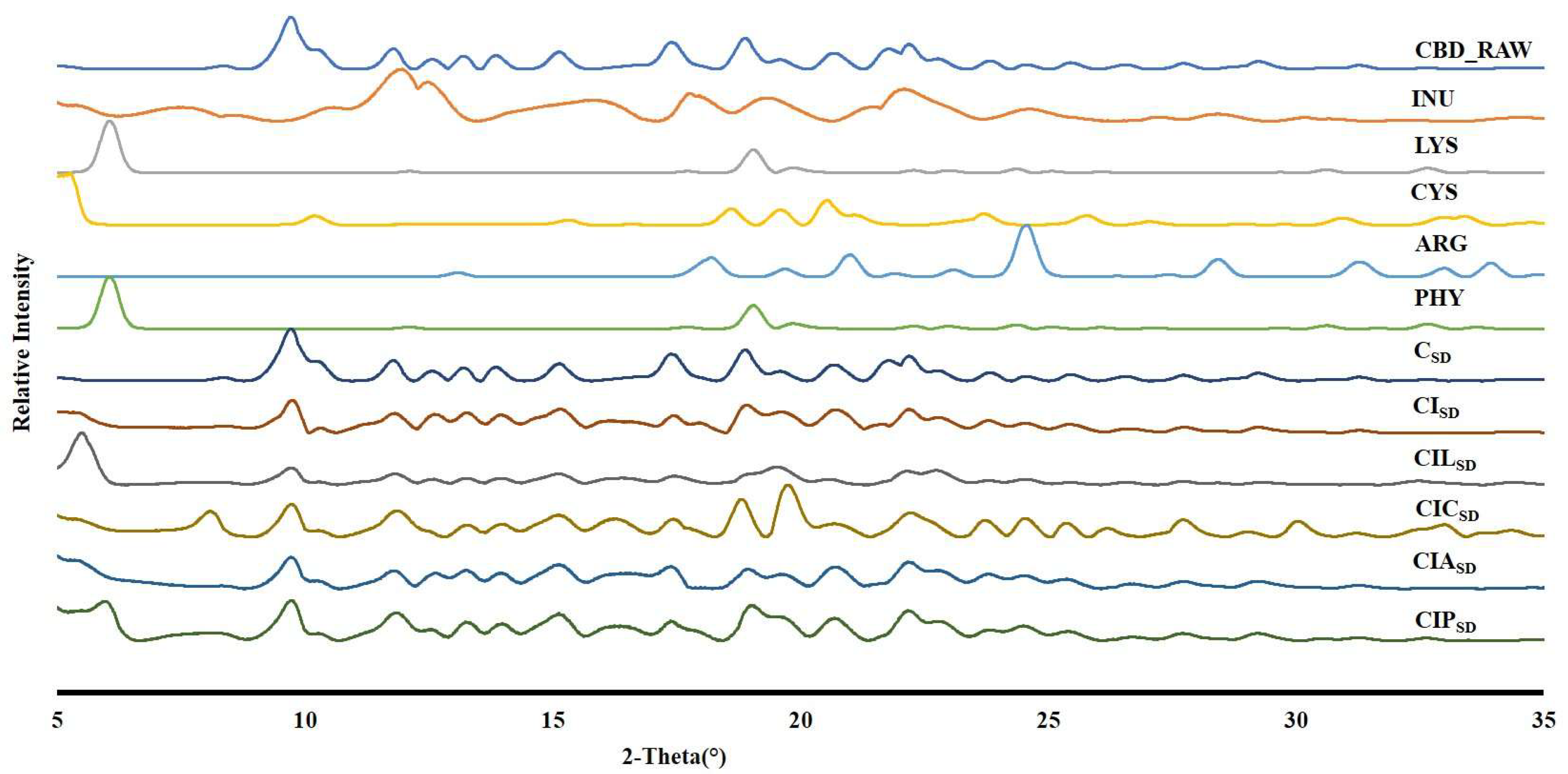
| Formulations | Concentration of CBD (% w/w) | Concentration of INU (% w/w) | Concentration of LYS (%w/w) | Concentration of CYS (%w/w) | Concentration of ARG (%w/w) | Concentration of PHY (%w/w) |
|---|---|---|---|---|---|---|
| CSD | 100 | - | - | - | - | - |
| CISD | 20 | 80 | - | - | - | - |
| CILSD | 20 | 60 | 20 | - | - | - |
| CICSD | 20 | 60 | - | 20 | - | - |
| CIASD | 20 | 60 | - | - | 20 | - |
| CIPSD | 20 | 60 | - | - | - | 20 |
| Formulations | Yield (%) | Particle Size (µm) n~300 | D50 (µm) | Drug Content (%) n = 3 | Residual Solvent Content (%) n = 2 |
|---|---|---|---|---|---|
| CSD | 5.4 | 1.7 ± 0.7 | 1.5 | 100.7 ± 1.7 | 0.12 ± 0.1 |
| CISD | 29.7 | 1.6 ± 0.4 | 1.5 | 90.2 ± 1.1 | 5.9 ± 0.2 |
| CILSD | 30.2 | 1.7 ± 0.7 | 1.5 | 94.5 ± 0.6 | 2.0 ± 0.0 |
| CICSD | 26.6 | 2.6 ± 0.8 | 2.5 | 98.3 ± 0.3 | 1.6 ± 0.3 |
| CIASD | 34.2 | 2.9 ± 1.1 | 2.7 | 98.7 ± 0.5 | 2.3 ± 0.0 |
| CIPSD | 55.6 | 2.2 ± 0.8 | 2.1 | 97.7 ± 0.8 | 1.8 ± 0.0 |
| Drug/Formulation/Raw Material | pIC50 |
|---|---|
| Spray-dried CBD with lysine (CILSD) | 4.3 ± 0.5 |
| Spray-dried CBD with cysteine (CICSD) | 4.3 ± 0.2 |
| Spray-dried CBD with arginine (CIASD) | 4.3 ± 0.3 |
| Spray-dried CBD with phenylalanine (CIPSD) | 4.3 ± 0.5 |
| Cannabidiol raw (CBD_raw) | 4.4 ± 0.3 |
| Inulin raw (INU) | >20 |
| Lysine raw (LYS) | >20 |
| Cysteine raw (CYS) | >20 |
| Arginine raw (ARG) | >20 |
| Phenylalanine raw (PHY) | >20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komal, K.; Hanton, L.R.; Glass, M.; Das, S.C. Influence of Different Amino Acids on the Aerosolization, Stability and Cytotoxicity of Spray-Dried Cannabidiol Dry Powder for Inhalation. Pharmaceutics 2025, 17, 1120. https://doi.org/10.3390/pharmaceutics17091120
Komal K, Hanton LR, Glass M, Das SC. Influence of Different Amino Acids on the Aerosolization, Stability and Cytotoxicity of Spray-Dried Cannabidiol Dry Powder for Inhalation. Pharmaceutics. 2025; 17(9):1120. https://doi.org/10.3390/pharmaceutics17091120
Chicago/Turabian StyleKomal, Komal, Lyall R. Hanton, Michelle Glass, and Shyamal C. Das. 2025. "Influence of Different Amino Acids on the Aerosolization, Stability and Cytotoxicity of Spray-Dried Cannabidiol Dry Powder for Inhalation" Pharmaceutics 17, no. 9: 1120. https://doi.org/10.3390/pharmaceutics17091120
APA StyleKomal, K., Hanton, L. R., Glass, M., & Das, S. C. (2025). Influence of Different Amino Acids on the Aerosolization, Stability and Cytotoxicity of Spray-Dried Cannabidiol Dry Powder for Inhalation. Pharmaceutics, 17(9), 1120. https://doi.org/10.3390/pharmaceutics17091120








