Latest Advances in Inhalable Dry Powder Bacteriophage Therapy for Pulmonary Infections
Abstract
1. Introduction
2. Bacteriophages as a Therapy for Pulmonary Infections
2.1. Mechanism of Action of Lytic Phages in Targeting Pseudomonas aeruginosa
2.2. Advantages over Inhaled Antibiotics
2.3. Phages’ Ability to Evolve and Adapt Against MDR Bacteria
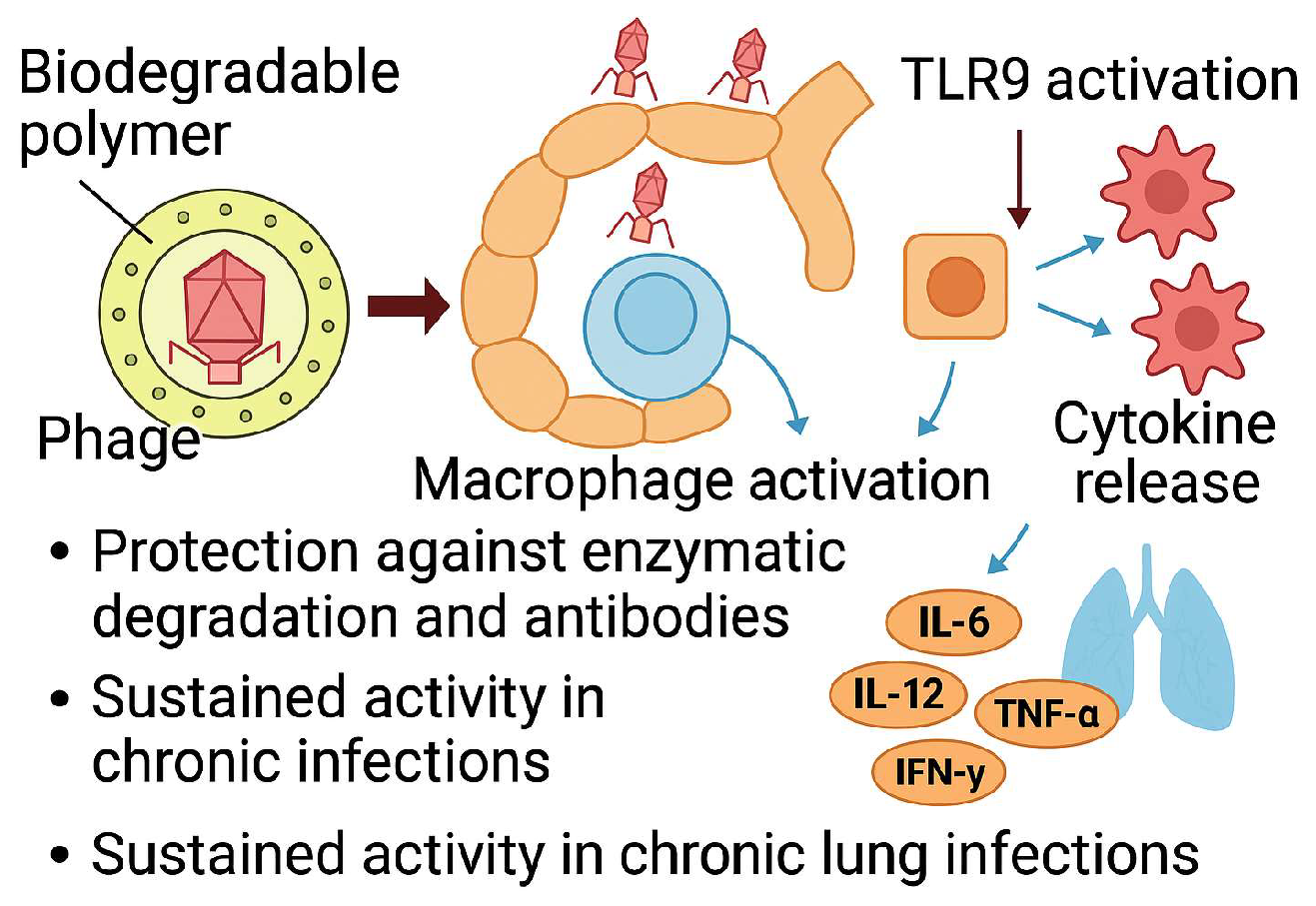
3. Dry Powder Inhaler Systems for Bacteriophage Therapy
| DPI Type | Phage | Technology | Advantages | Limitations | Reference |
|---|---|---|---|---|---|
| Carrier-based | PEV1, PEV20, PEV61 against P. aeruginosa | Lactose + leucine, spray dried | Stable up to 12 months, non-toxic to lung cells in vitro | Requires controlled-humidity storage | [41] |
| Carrier-free | Phage LUZ19 and Romulus | Spray drying with 4% trehalose solution | Forms respirable particles (1–5 µm) | Crystallization at high RH reduces viability | [49] |
| Nanoparticle-embedded | K. pneumoniae phage cocktail | Nanostructured lipid carrier | Mechanical protection, extended therapeutic effect | More complex formulation | [50] |
3.1. Drying Techniques for DPI Phage Formulations
3.2. Comparative Performance of DPI vs. Nebulized Formulations
4. Key Characteristics of Inhalable Phage Powders
5. Stability and Viability of Dry Powder Phages
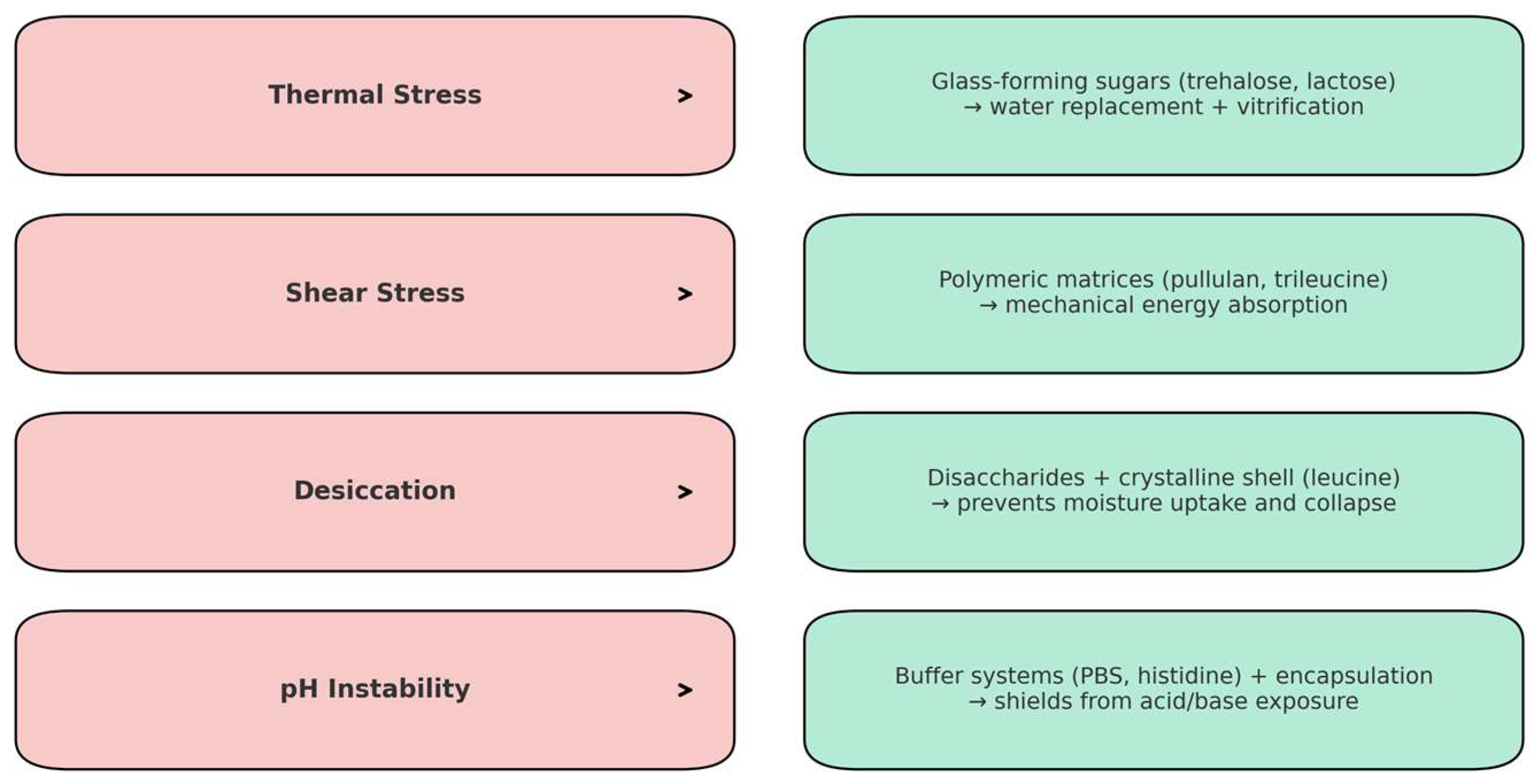
5.1. Mechanical Stress During Drying Process
5.2. Humidity Effects on Long-Term Storage
5.3. Thermal-Mediated Phage Instability
5.4. pH-Mediated Phage Instability
6. Stress Conditions for Bacteriophages During Delivery System Formulation Process
| Common Name of Phage | Scientific Name of Phage (Family) | Experimental Model | Formulation Type | Excipient Used | Effect on Phage Activity | Reference |
|---|---|---|---|---|---|---|
| PEV20 | Myoviridae | In vivo murine model with P. aeruginosa | Dry powder for inhalation | L-leucine and lactose | No activity loss, synergistic with ciprofloxacin | [6] |
| PEV20 | Myoviridae | In vivo murine model with P. aeruginosa | Dry powder for inhalation | L-leucine | No activity loss, synergistic with ciprofloxacin | [6] |
| PEV20 | Myoviridae | In vivo neutropenic mouse model | Dry powder for inhalation | L-leucine | 5.9 × 1010 CFU reduction, synergistic with ciprofloxacin | [97] |
| phiYY | Cystoviridae | In vivo clinical model of cystic fibrosis | Nebulization | Liquid solution | Stability limited by gastric pH | [99] |
| PEV1 | Podoviridae | In vivo murine lung infection model with P. aeruginosa | Spray-dried powder | Trehalose or lactose and leucine | <1 log10 loss with trehalose/lactose + leucine; without sugar or leucine, up to 8 log10 loss | [85] |
| PEV20 | Myoviridae | In vivo murine lung infection model with P. aeruginosa | Spray-dried powder | Trehalose or lactose and leucine | Similar to PEV1; lactose + leucine most effective (0.4–0.9 log10 loss) | [85] |
| PEV61 | Podoviridae | In vivo murine lung infection model with P. aeruginosa | Spray-dried powder | Trehalose or lactose and leucine | Similar to PEV1 and PEV20; best with lactose + leucine (0.3–0.4 log10 loss) | [85] |
| Phage 95 (ATCC 14211-B1) | Not specified (likely Podoviridae) | In vitro H441 lung epithelial model | Liposomal inhalable formulation | Hydrogenated phosphatidylcholine, cholesterol, DSPE-PEG, mannitol, sucrose | Viability improved; 0.64 log reduction after nebulization; 2-fold lower cellular uptake | [100] |
| PEV31 | Podoviridae | In vivo, BALB/c mice | Intratracheal suspension | Phosphate-buffered saline + 1 mM CaCl2 | Maintained infectivity initially; gradual decline in absence of bacteria; proliferation in presence of P. aeruginosa; slight inflammatory response at high dose | [101] |
| PEV2 | Podoviridae | In vitro aerosol model using Osmohaler | Spray-dried powder | Trehalose, mannitol, L-leucine | ~1.5 log loss overall; ~80% phage loss during aerosolization but higher total lung dose | [102] |
| PEV2 | Podoviridae | In vitro aerosol model using Osmohaler | Spray freeze drying | Trehalose, mannitol, L-leucine | ~2 log loss during atomization; ~20% phage loss during aerosolization, lower lung dose | [102] |
| LUZ19 | Podoviridae | In vitro | Spray-dried powder | D-trehalose + L-isoleucine | High concentration of L-isoleucine improves stability; activity loss < 1 × 1010 pfu/mg | [103] |
| 14-1 | Myoviridae | In vitro | Spray-dried powder | D-trehalose + L-isoleucine | High concentration of L-isoleucine reduces activity; activity loss < 1 × 1010 pfu/mg | [103] |
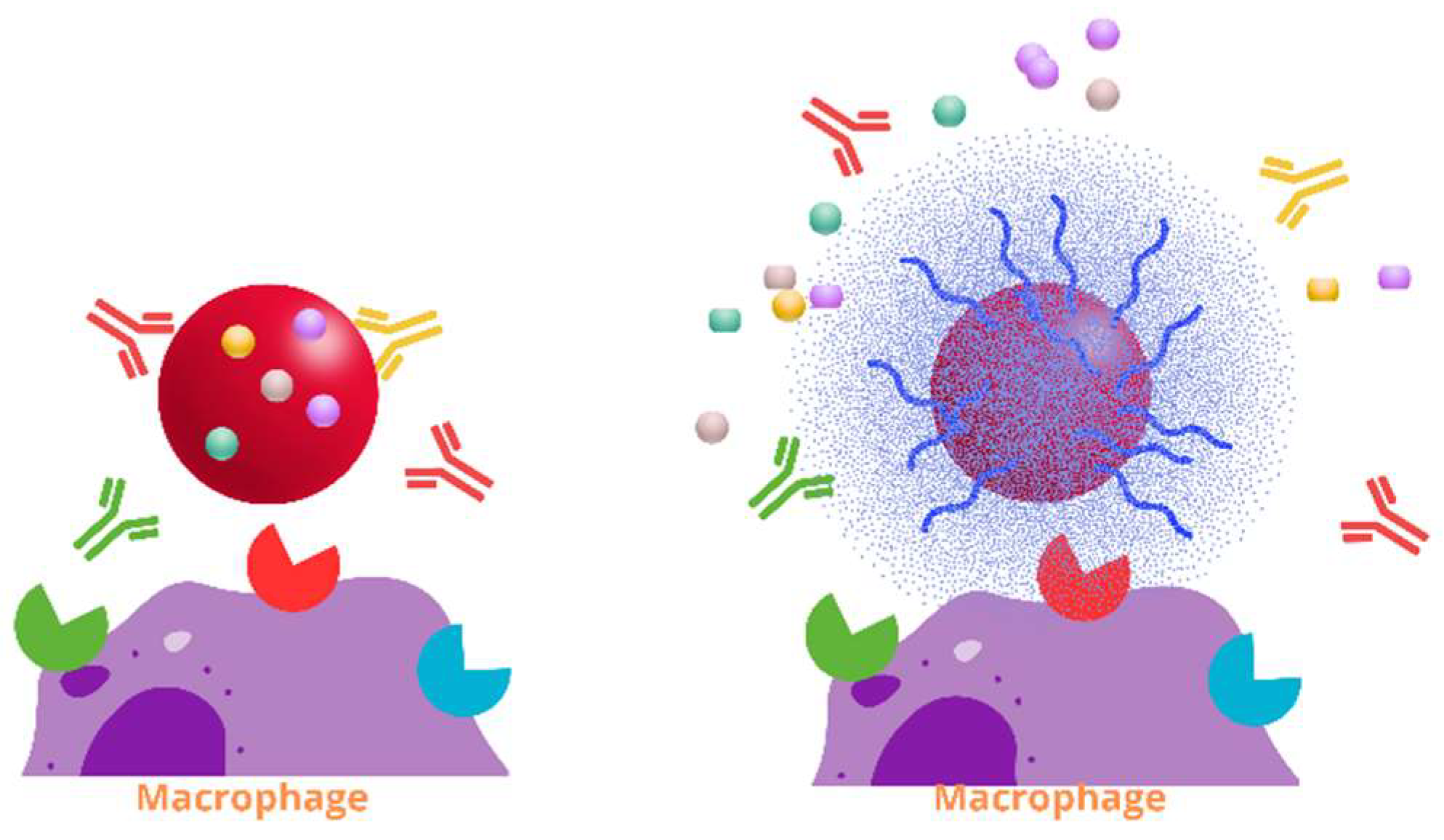
7. Pulmonary Immune Clearance Mechanisms for Phages
8. Regulatory and Quality Frameworks for Inhalable Phage Formulations
9. Preclinical and Clinical Evidence of Inhalable Dry Powder Phages
10. Challenges of Inhaled Phage Therapy
11. Future Perspectives
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Meaning |
| CFU | Colony-Forming Unit |
| PFU | Plaque-Forming Unit |
| RH | Relative Humidity |
| Tg | Glass Transition Temperature |
| SEM | Scanning Electron Microscopy |
| SFD | Spray Freeze Drying |
| COPD | Chronic Obstructive Pulmonary Disease |
| TEM | Transmission Electron Microscopy |
| TGA | Thermogravimetric Analysis |
| DPI | Dry Powder Inhaler |
| MDR | Multidrug Resistance |
| M. smegmatis | Mycobacterium smegmatis |
| PVA | Polyvinyl Alcohol |
| R-M | Restriction–Modification |
| CF | Cystic Fibrosis |
| PEV | Pseudomonas Phage |
| REases | Restriction Endonucleases |
| CRISPR-Cas | Clustered Regularly Interspaced Short Palindromic Repeats |
| CBASS | Cyclic Oligonucleotide-Based Anti-Phage Signaling System |
| pMDIs | Pressurized Metered Dose Inhalers |
| FPF | Fine Particle Fraction |
| VRF | Viable Respirable Fraction |
| TFF | Thin-Film Freezing |
| PVP | Polyvinylpyrrolidone |
| PLGA | Poly(Lactic-Co-Glycolic Acid) |
| TLR | Toll-Like Receptor |
| QC | Quality Control |
References
- Iszatt, J.J.; Larcombe, A.N.; Chan, H.-K.; Stick, S.M.; Garratt, L.W.; Kicic, A. Phage therapy for multi-drug resistant respiratory tract infections. Viruses 2021, 13, 1809. [Google Scholar] [CrossRef]
- Sharma, S.; Chatterjee, S.; Datta, S.; Prasad, R.; Dubey, D.; Prasad, R.K.; Vairale, M.G. Bacteriophages and its applications: An overview. Folia Microbiol. 2017, 62, 17–55. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, A.; Mudaliar, S.B.; Bhat, V.G.; Chakraborty, I.; Prasad, A.S.B.; Mazumder, N. Phage therapy: A novel approach against multidrug-resistant pathogens. 3 Biotech 2024, 14, 256. [Google Scholar] [CrossRef]
- Encinas-Basurto, D.; Eedara, B.B.; Mansour, H.M. Biocompatible biodegradable polymeric nanocarriers in dry powder inhalers (DPIs) for pulmonary inhalation delivery. J. Pharm. Investig. 2024, 54, 145–160. [Google Scholar] [CrossRef]
- Eedara, B.B.; Encinas-Basurto, D.; Manivannan, B.; Hayes, D., Jr.; Mansour, H.M. Inhalable Nanomedicines for the Treatment of Pulmonary Aspergillosis. In Nanomedicines for the Prevention and Treatment of Infectious Diseases; Springer: Berlin/Heidelberg, Germany, 2023; pp. 77–94. [Google Scholar]
- Lin, Y.; Chang, R.Y.K.; Britton, W.J.; Morales, S.; Kutter, E.; Li, J.; Chan, H.-K. Inhalable combination powder formulations of phage and ciprofloxacin for P. aeruginosa respiratory infections. Eur. J. Pharm. Biopharm. 2019, 142, 543–552. [Google Scholar] [CrossRef]
- Young, R. Phage lysis: Do we have the hole story yet? Curr. Opin. Microbiol. 2013, 16, 790–797. [Google Scholar] [CrossRef]
- Berry, J.; Rajaure, M.; Pang, T.; Young, R. The spanin complex is essential for lambda lysis. J. Bacteriol. 2012, 194, 5667–5674. [Google Scholar] [CrossRef] [PubMed]
- Kongari, R.; Rajaure, M.; Cahill, J.; Rasche, E.; Mijalis, E.; Berry, J.; Young, R. Phage spanins: Diversity, topological dynamics and gene convergence. BMC Bioinform. 2018, 19, 326. [Google Scholar] [CrossRef]
- Samanta, S.; Ranjan Sharma, A.; Saha, A.; Kumar Singh, M.; Das, A.; Bhattacharya, M.; Prasad Saha, R.; Lee, S.-S.; Chakraborty, C. The bacteriophage mu lysis system—A new mechanism of host lysis? BIOCELL 2021, 45, 1175–1186. [Google Scholar] [CrossRef]
- Vandenheuvel, D.; Singh, A.; Vandersteegen, K.; Klumpp, J.; Lavigne, R.; Van den Mooter, G. Feasibility of spray drying bacteriophages into respirable powders to combat pulmonary bacterial infections. Eur. J. Pharm. Biopharm. 2013, 84, 578–582. [Google Scholar] [CrossRef]
- Santamaría-Corral, G.; Senhaji-Kacha, A.; Broncano-Lavado, A.; Esteban, J.; García-Quintanilla, M. Bacteriophage–Antibiotic Combination Therapy against Pseudomonas aeruginosa. Antibiotics 2023, 12, 1089. [Google Scholar] [CrossRef]
- Maurice, N.M.; Bedi, B.; Sadikot, R.T. Pseudomonas aeruginosa Biofilms: Host Response and Clinical Implications in Lung Infections. Am. J. Respir. Cell Mol. Biol. 2018, 58, 428–439. [Google Scholar] [CrossRef] [PubMed]
- Høiby, N.; Moser, C.; Ciofu, O. Pseudomonas aeruginosa in the Frontline of the Greatest Challenge of Biofilm Infection-Its Tolerance to Antibiotics. Microorganisms 2024, 12, 2115. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Schneider-Futschik, E.K. Current and Emerging Inhaled Antibiotics for Chronic Pulmonary Pseudomonas aeruginosa and Staphylococcus aureus Infections in Cystic Fibrosis. Antibiotics 2023, 12, 484. [Google Scholar] [CrossRef]
- Langton Hewer, S.C.; Smyth, A.R.; Brown, M.; Jones, A.P.; Hickey, H.; Kenna, D.; Ashby, D.; Thompson, A.; Sutton, L.; Clayton, D.; et al. Intravenous or oral antibiotic treatment in adults and children with cystic fibrosis and Pseudomonas aeruginosa infection: The TORPEDO-CF RCT. Health Technol. Assess. 2021, 25, 1–128. [Google Scholar] [CrossRef]
- Islam, N.; Reid, D. Inhaled antibiotics: A promising drug delivery strategies for efficient treatment of lower respiratory tract infections (LRTIs) associated with antibiotic resistant biofilm-dwelling and intracellular bacterial pathogens. Respir. Med. 2024, 227, 107661. [Google Scholar] [CrossRef]
- Thomsen, K.; Høiby, N.; Jensen, P.Ø.; Ciofu, O.; Moser, C. Immune Response to Biofilm Growing Pulmonary Pseudomonas aeruginosa Infection. Biomedicines 2022, 10, 2064. [Google Scholar] [CrossRef]
- Høiby, N.; Henneberg, K.-Å.; Wang, H.; Stavnsbjerg, C.; Bjarnsholt, T.; Ciofu, O.; Johansen, U.R.; Sams, T. Formation of Pseudomonas aeruginosa inhibition zone during tobramycin disk diffusion is due to transition from planktonic to biofilm mode of growth. Int. J. Antimicrob. Agents 2019, 53, 564–573. [Google Scholar] [CrossRef]
- Wang, J.; Guo, Y.; Lu, W.; Liu, X.; Zhang, J.; Sun, J.; Chai, G. Dry powder inhalation containing muco-inert ciprofloxacin and colistin co-loaded liposomes for pulmonary P. Aeruginosa biofilm eradication. Int. J. Pharm. 2024, 658, 124208. [Google Scholar] [CrossRef]
- Yu, S.; Wang, S.; Zou, P.; Chai, G.; Lin, Y.-W.; Velkov, T.; Li, J.; Pan, W.; Zhou, Q.T. Inhalable liposomal powder formulations for co-delivery of synergistic ciprofloxacin and colistin against multi-drug resistant gram-negative lung infections. Int. J. Pharm. 2020, 575, 118915. [Google Scholar] [CrossRef] [PubMed]
- Hawas, S.; Verderosa, A.D.; Totsika, M. Combination Therapies for Biofilm Inhibition and Eradication: A Comparative Review of Laboratory and Preclinical Studies. Front. Cell. Infect. Microbiol. 2022, 12, 850030. [Google Scholar] [CrossRef]
- Sommerfeld Ross, S.; Gharse, S.; Sanchez, L.; Fiegel, J. Dry powder aerosols to co-deliver antibiotics and nutrient dispersion compounds for enhanced bacterial biofilm eradication. Int. J. Pharm. 2017, 531, 14–23. [Google Scholar] [CrossRef]
- Alhajj, N.; Yahya, M.F.Z.R.; O’Reilly, N.J.; Cathcart, H. Development and characterization of a spray-dried inhalable ternary combination for the treatment of Pseudomonas aeruginosa biofilm infection in cystic fibrosis. Eur. J. Pharm. Sci. 2024, 192, 106654. [Google Scholar] [CrossRef] [PubMed]
- Uyttebroek, S.; Chen, B.; Onsea, J.; Ruythooren, F.; Debaveye, Y.; Devolder, D.; Spriet, I.; Depypere, M.; Wagemans, J.; Lavigne, R.; et al. Safety and efficacy of phage therapy in difficult-to-treat infections: A systematic review. Lancet Infect. Dis. 2022, 22, e208–e220. [Google Scholar] [CrossRef] [PubMed]
- Hong, Q.; Chang, R.Y.K.; Assafiri, O.; Morales, S.; Chan, H.-K. Optimizing in vitro phage-ciprofloxacin combination formulation for respiratory therapy of multi-drug resistant Pseudomonas aeruginosa infections. Int. J. Pharm. 2024, 652, 123853. [Google Scholar] [CrossRef] [PubMed]
- Faruk, O.; Jewel, Z.A.; Bairagi, S.; Rasheduzzaman, M.; Bagchi, H.; Tuha, A.S.M.; Hossain, I.; Bala, A.; Ali, S. Phage treatment of multidrug-resistant bacterial infections in humans, animals, and plants: The current status and future prospects. Infect. Med. 2025, 4, 100168. [Google Scholar] [CrossRef]
- Kunisch, F.; Campobasso, C.; Wagemans, J.; Yildirim, S.; Chan, B.K.; Schaudinn, C.; Lavigne, R.; Turner, P.E.; Raschke, M.J.; Trampuz, A.; et al. Targeting Pseudomonas aeruginosa biofilm with an evolutionary trained bacteriophage cocktail exploiting phage resistance trade-offs. Nat. Commun. 2024, 15, 8572. [Google Scholar] [CrossRef]
- Le, S.; He, X.; Tan, Y.; Huang, G.; Zhang, L.; Lux, R.; Shi, W.; Hu, F. Mapping the tail fiber as the receptor binding protein responsible for differential host specificity of Pseudomonas aeruginosa bacteriophages PaP1 and JG004. PLoS ONE 2013, 8, e68562. [Google Scholar] [CrossRef]
- Supina, B.S.I.; Dennis, J.J. The Current Landscape of Phage–Antibiotic Synergistic (PAS) Interactions. Antibiotics 2025, 14, 545. [Google Scholar] [CrossRef]
- Kraus, S.; Fletcher, M.L.; Łapińska, U.; Chawla, K.; Baker, E.; Attrill, E.L.; O’Neill, P.; Farbos, A.; Jeffries, A.; Galyov, E.E.; et al. Phage-induced efflux down-regulation boosts antibiotic efficacy. PLoS Pathog. 2024, 20, e1012361. [Google Scholar] [CrossRef]
- Chan, B.K.; Sistrom, M.; Wertz, J.E.; Kortright, K.E.; Narayan, D.; Turner, P.E. Phage selection restores antibiotic sensitivity in MDR Pseudomonas aeruginosa. Sci. Rep. 2016, 6, 26717. [Google Scholar] [CrossRef]
- Gao, Z.; Feng, Y. Bacteriophage strategies for overcoming host antiviral immunity. Front. Microbiol. 2023, 14, 1211793. [Google Scholar] [CrossRef]
- Zhong, Y.; Lauschke, V.M. The phage anti-restriction induced system: New insights into bacterial immunity and bacteriophage escape strategies. Signal Transduct. Target Ther. 2024, 9, 269. [Google Scholar] [CrossRef]
- Rafiq, M.S.; Shabbir, M.A.; Raza, A.; Irshad, S.; Asghar, A.; Maan, M.K.; Gondal, M.A.; Hao, H. CRISPR-Cas System: A New Dawn to Combat Antibiotic Resistance. BioDrugs 2024, 38, 387–404. [Google Scholar] [CrossRef] [PubMed]
- Pons, B.J.; van Houte, S.; Westra, E.R.; Chevallereau, A. Ecology and evolution of phages encoding anti-CRISPR proteins. J. Mol. Biol. 2023, 435, 167974. [Google Scholar] [CrossRef]
- Bleriot, I.; Pacios, O.; Blasco, L.; Fernández-García, L.; López, M.; Ortiz-Cartagena, C.; Barrio-Pujante, A.; García-Contreras, R.; Pirnay, J.-P.; Wood, T.K.; et al. Improving phage therapy by evasion of phage resistance mechanisms. JAC Antimicrob. Resist. 2024, 6, dlae017. [Google Scholar] [CrossRef]
- Mayorga-Ramos, A.; Carrera-Pacheco, S.E.; Barba-Ostria, C.; Guamán, L.P. Bacteriophage-mediated approaches for biofilm control. Front. Cell. Infect. Microbiol. 2024, 14, 1428637. [Google Scholar] [CrossRef]
- Kovacs, C.J.; Rapp, E.M.; McKenzie, S.M.; Mazur, M.Z.; McHale, R.P.; Brasko, B.; Min, M.Y.; Burpo, F.J.; Barnhill, J.C. Disruption of Biofilm by Bacteriophages in Clinically Relevant Settings. Mil. Med. 2023, 189, e1294–e1302. [Google Scholar] [CrossRef] [PubMed]
- Murtazalieva, K.; Mu, A.; Petrovskaya, A.; Finn, R.D. The growing repertoire of phage anti-defence systems. Trends Microbiol. 2024, 32, 1212–1228. [Google Scholar] [CrossRef]
- Chang, R.Y.K.; Wallin, M.; Lin, Y.; Leung, S.S.Y.; Wang, H.; Morales, S.; Chan, H.-K. Phage therapy for respiratory infections. Adv. Drug Deliv. Rev. 2018, 133, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xie, Z.; Zhao, J.; Zhu, Z.; Yang, C.; Liu, Y. Prospects of Inhaled Phage Therapy for Combatting Pulmonary Infections. Front. Cell. Infect. Microbiol. 2021, 11, 758392. [Google Scholar] [CrossRef]
- Narang, A.S.; Mahato, R.I. Organ Specific Drug Delivery and Targeting to the Lungs; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar] [CrossRef]
- Pathak, V.; Chan, H.-K.; Zhou, Q.T. Formulation of Bacteriophage for Inhalation to Treat Multidrug-Resistant Pulmonary Infections. KONA Powder Part. J. 2025, 42, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Shetty, N.; Cipolla, D.; Park, H.; Zhou, Q.T. Physical stability of dry powder inhaler formulations. Expert Opin. Drug Deliv. 2019, 17, 77–96. [Google Scholar] [CrossRef] [PubMed]
- Mehta, T.; Najafian, S.; Patel, K.; Lacombe, J.; Chaudhuri, B. Optimization of Carrier-Based Dry Powder Inhaler Performance: A Review. Pharmaceutics 2025, 17, 96. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Miao, X.; Shan, Z.; Huang, Y.; Li, L.; Pan, X.; Yao, Q.; Li, G.; Wu, C. Studies on the spray dried lactose as carrier for dry powder inhalation. Asian J. Pharm. Sci. 2014, 9, 336–341. [Google Scholar] [CrossRef]
- Chang, R.Y.K.; Wallin, M.; Kutter, E.; Morales, S.; Britton, W.; Li, J.; Chan, H.-K. Storage stability of inhalable phage powders containing lactose at ambient conditions. Int. J. Pharm. 2019, 560, 11–18. [Google Scholar] [CrossRef]
- Vandenheuvel, D.; Meeus, J.; Lavigne, R.; Van den Mooter, G. Instability of bacteriophages in spray-dried trehalose powders is caused by crystallization of the matrix. Int. J. Pharm. 2014, 472, 202–205. [Google Scholar] [CrossRef]
- Chadha, P.; Katare, O.P.; Chhibber, S. Liposome loaded phage cocktail: Enhanced therapeutic potential in resolving Klebsiella pneumoniae mediated burn wound infections. Burns 2017, 43, 1532–1543. [Google Scholar] [CrossRef]
- Magramane, S.; Vlahović, K.; Gordon, P.; Kállai-Szabó, N.; Zelkó, R.; Antal, I.; Farkas, D. Inhalation Dosage Forms: A Focus on Dry Powder Inhalers and Their Advancements. Pharmaceuticals 2023, 16, 1658. [Google Scholar] [CrossRef]
- Pilcer, G.; Amighi, K. Formulation strategy and use of excipients in pulmonary drug delivery. Int. J. Pharm. 2010, 392, 1–19. [Google Scholar] [CrossRef]
- Nokhodchi, A.; Ghafourian, T. Future of Carrier-Free Dry Powder Inhaler Formulations. Pharm. Sci. 2024, 30, 279–281. [Google Scholar] [CrossRef]
- Kaur, S.; Kumari, A.; Kumari Negi, A.; Galav, V.; Thakur, S.; Agrawal, M.; Sharma, V. Nanotechnology Based Approaches in Phage Therapy: Overcoming the Pharmacological Barriers. Front. Pharmacol. 2021, 12, 699054. [Google Scholar] [CrossRef] [PubMed]
- Loh, B.; Gondil, V.S.; Manohar, P.; Khan, F.M.; Yang, H.; Leptihn, S. Encapsulation and Delivery of Therapeutic Phages. Appl. Environ. Microbiol. 2021, 87, e01979-20. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.-Y.; Liu, X.; Wang, C.-Y.; Wan, Q.-Q.; Tian, Y.-F.; Liu, S.-L.; Pang, D.-W.; Wang, Z.-G. Inhalable Polymeric Microparticles for Phage and Photothermal Synergistic Therapy of Methicillin-Resistant Staphylococcus aureus Pneumonia. Nano Lett. 2024, 24, 8752–8762. [Google Scholar] [CrossRef] [PubMed]
- Puapermpoonsiri, U.; Ford, S.; Van der Walle, C. Stabilization of bacteriophage during freeze drying. Int. J. Pharm. 2010, 389, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Temsaah, H.R.; Abdelkader, K.; Ahmed, A.E.; Elgiddawy, N.; Eldin, Z.E.; Elshebrawy, H.A.; Kasem, N.G.; El-Gohary, F.A.; Azmy, A.F. Chitosan nano-formulation enhances stability and bactericidal activity of the lytic phage HK6. BMC Biotechnol. 2025, 25, 3. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wang, Y.; Li, M.; Khanal, D.; Chan, H.-K. Can bacteriophage be stabilised by lipid encapsulation when nebulised for inhalation delivery against Pseudomonas aeruginosa? Int. J. Pharm. 2025, 678, 125670. [Google Scholar] [CrossRef] [PubMed]
- Southard, B.; Melton, K.; Sandoval, M.A.; Zaki, R.; Williams, R.O., III; Cui, Z. Development of an inhalable dry powder of mycobacteriophage D29 using thin-film freeze-drying. Int. J. Pharm. 2025, 682, 125919. [Google Scholar] [CrossRef]
- Agarwal, R.; Johnson, C.T.; Imhoff, B.R.; Donlan, R.M.; McCarty, N.A.; García, A.J. Inhaled bacteriophage-loaded polymeric microparticles ameliorate acute lung infections. Nat. Biomed. Eng. 2018, 2, 841–849. [Google Scholar] [CrossRef]
- Santos, D.; Maurício, A.C.; Sencadas, V.; Santos, J.D.; Fernandes, M.H.; Gomes, P.S. Spray Drying: An Overview. In Biomaterials—Physics and Chemistry—New Edition; InTech: London, UK, 2018. [Google Scholar] [CrossRef]
- Chang, R.Y.K.; Kwok, P.C.L.; Khanal, D.; Morales, S.; Kutter, E.; Li, J.; Chan, H.K. Inhalable bacteriophage powders: Glass transition temperature and bioactivity stabilization. Bioeng. Transl. Med. 2020, 5, e10159. [Google Scholar] [CrossRef]
- Raman, S.K.; Roy, T.; Verma, K.; Yadav, C.; Verma, S.; Deivreddy, V.S.R.; Sofi, H.S.; Bharti, R.; Sharma, R.; Bansode, H.; et al. Dry powder Inhalation of lytic mycobacteriophages for adjunct therapy in a mouse model of infection with Mycobacterium tuberculosis. Tuberculosis 2025, 152, 102631. [Google Scholar] [CrossRef]
- Vinner, G.K.; Rezaie-Yazdi, Z.; Leppanen, M.; Stapley, A.G.F.; Leaper, M.C.; Malik, D.J. Microencapsulation of Salmonella-Specific Bacteriophage Felix O1 Using Spray-Drying in a pH-Responsive Formulation and Direct Compression Tableting of Powders into a Solid Oral Dosage Form. Pharmaceuticals 2019, 12, 43. [Google Scholar] [CrossRef]
- Malik, D.J. Bacteriophage encapsulation using spray drying for phage therapy. Curr. Issues Mol. Biol. 2021, 40, 303–316. [Google Scholar] [CrossRef]
- Manohar, P.; Ramesh, N. Improved lyophilization conditions for long-term storage of bacteriophages. Sci. Rep. 2019, 9, 15242. [Google Scholar] [CrossRef] [PubMed]
- Ly, A.; Carrigy, N.B.; Wang, H.; Harrison, M.; Sauvageau, D.; Martin, A.R.; Vehring, R.; Finlay, W.H. Atmospheric spray freeze drying of sugar solution with phage D29. Front. Microbiol. 2019, 10, 488. [Google Scholar] [CrossRef] [PubMed]
- Moon, C.; Sahakijpijarn, S.; Williams, R.O. Thin-Film Freeze-Drying Process for Versatile Particles for Inhalation Drug Delivery. In Organ Specific Drug Delivery and Targeting to the Lungs; CRC Press: Boca Raton, FL, USA, 2022; pp. 271–308. [Google Scholar]
- Le Guellec, S.; Pardessus, J.; Bodier-Montagutelli, E.; L’hostis, G.; Dalloneau, E.; Piel, D.; Samaï, H.C.; Guillon, A.; Mujic, E.; Guillot-Combe, E. Administration of bacteriophages via nebulization during mechanical ventilation: In vitro study and lung deposition in macaques. Viruses 2023, 15, 602. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Cao, Y.; Chan, H.-K. Optimizing Performance of Inhalable Bacteriophage Powders using Human Serum Albumin (HSA). Int. J. Pharm. 2025, 678, 125709. [Google Scholar] [CrossRef]
- Vandenheuvel, D.; Lavigne, R.; Brüssow, H. Bacteriophage Therapy: Advances in Formulation Strategies and Human Clinical Trials. Annu. Rev. Virol. 2015, 2, 599–618. [Google Scholar] [CrossRef]
- Li, M.; Chang, R.Y.K.; Lin, Y.; Morales, S.; Kutter, E.; Chan, H.-K. Phage cocktail powder for Pseudomonas aeruginosa respiratory infections. Int. J. Pharm. 2021, 596, 120200. [Google Scholar] [CrossRef]
- Ergin, F. Effect of freeze drying, spray drying and electrospraying on the morphological, thermal, and structural properties of powders containing phage Felix O1 and activity of phage Felix O1 during storage. Powder Technol. 2022, 404, 117516. [Google Scholar] [CrossRef]
- Hassan, M.S.; Lau, R.W.M. Effect of Particle Shape on Dry Particle Inhalation: Study of Flowability, Aerosolization, and Deposition Properties. AAPS PharmSciTech 2009, 10, 1252. [Google Scholar] [CrossRef] [PubMed]
- Verreault, D.; Marcoux-Voiselle, M.; Turgeon, N.; Moineau, S.; Duchaine, C. Resistance of Aerosolized Bacterial Viruses to Relative Humidity and Temperature. Appl. Environ. Microbiol. 2015, 81, 7305–7311. [Google Scholar] [CrossRef]
- Leung, S.S.Y.; Parumasivam, T.; Gao, F.G.; Carter, E.A.; Carrigy, N.B.; Vehring, R.; Finlay, W.H.; Morales, S.; Britton, W.J.; Kutter, E.; et al. Effects of storage conditions on the stability of spray dried, inhalable bacteriophage powders. Int. J. Pharm. 2017, 521, 141–149. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.; Ghosh, D. The stabilizing excipients in dry state therapeutic phage formulations. AAPS PharmSciTech 2020, 21, 1–14. [Google Scholar] [CrossRef]
- Coleman, H.J.; Yang, Q.; Robert, A.; Padgette, H.; Funke, H.H.; Catalano, C.E.; Randolph, T.W. Formulation of three tailed bacteriophages by spray-drying and atomic layer deposition for thermal stability and controlled release. J. Pharm. Sci. 2024, 113, 3238–3245. [Google Scholar] [CrossRef] [PubMed]
- Malik, D.J.; Sokolov, I.J.; Vinner, G.K.; Mancuso, F.; Cinquerrui, S.; Vladisavljevic, G.T.; Clokie, M.R.; Garton, N.J.; Stapley, A.G.; Kirpichnikova, A. Formulation, stabilisation and encapsulation of bacteriophage for phage therapy. Adv. Colloid Interface Sci. 2017, 249, 100–133. [Google Scholar] [CrossRef]
- de Jonge, J.; Amorij, J.-P.; Hinrichs, W.L.; Wilschut, J.; Huckriede, A.; Frijlink, H.W. Inulin sugar glasses preserve the structural integrity and biological activity of influenza virosomes during freeze-drying and storage. Eur. J. Pharm. Sci. 2007, 32, 33–44. [Google Scholar] [CrossRef]
- Karunnanithy, V.; Abdul Rahman, N.H.B.; Abdullah, N.A.H.; Fauzi, M.B.; Lokanathan, Y.; Min Hwei, A.N.; Maarof, M. Effectiveness of Lyoprotectants in Protein Stabilization During Lyophilization. Pharmaceutics 2024, 16, 1346. [Google Scholar] [CrossRef]
- Carrigy, N.B.; Chang, R.Y.; Leung, S.S.Y.; Harrison, M.; Petrova, Z.; Pope, W.H.; Hatfull, G.F.; Britton, W.J.; Chan, H.-K.; Sauvageau, D.; et al. Anti-Tuberculosis Bacteriophage D29 Delivery with a Vibrating Mesh Nebulizer, Jet Nebulizer, and Soft Mist Inhaler. Pharm. Res. 2017, 34, 2084–2096. [Google Scholar] [CrossRef]
- Yan, W.; He, R.; Tang, X.; Tian, B.; Liu, Y.; Tong, Y.; To, K.K.W.; Leung, S.S.Y. The Influence of Formulation Components and Environmental Humidity on Spray-Dried Phage Powders for Treatment of Respiratory Infections Caused by Acinetobacter baumannii. Pharmaceutics 2021, 13, 1162. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.Y.; Wong, J.; Mathai, A.; Morales, S.; Kutter, E.; Britton, W.; Li, J.; Chan, H.-K. Production of highly stable spray dried phage formulations for treatment of Pseudomonas aeruginosa lung infection. Eur. J. Pharm. Biopharm. 2017, 121, 1–13. [Google Scholar] [CrossRef]
- Alfadhel, M.; Puapermpoonsiri, U.; Ford, S.J.; McInnes, F.J.; van der Walle, C.F. Lyophilized inserts for nasal administration harboring bacteriophage selective for Staphylococcus aureus: In vitro evaluation. Int. J. Pharm. 2011, 416, 280–287. [Google Scholar] [CrossRef]
- Zheng, H. Devitrification of lyoprotectants: A critical determinant for bacteriophages inactivation in freeze-drying and storage. Food Res. Int. 2023, 173, 113307. [Google Scholar] [CrossRef]
- Batalha, L.S.; Gontijo, M.T.P.; de Carvalho Teixeira, A.V.N.; Boggione, D.M.G.; Lopez, M.E.S.; Eller, M.R.; Mendonça, R.C.S. Encapsulation in alginate-polymers improves stability and allows controlled release of the UFV-AREG1 bacteriophage. Food Res. Int. 2021, 139, 109947. [Google Scholar] [CrossRef]
- Suboor, A. Development and Optimization of a Feasible Drying Formulation for Phage-Carrier Biopesticide Against Fire Blight. Master’s Thesis, University of Guelph, Guelph, ON, Canada, 2024. [Google Scholar]
- Zhang, Y.; Peng, X.; Zhang, H.; Watts, A.B.; Ghosh, D. Manufacturing and ambient stability of shelf freeze dried bacteriophage powder formulations. Int. J. Pharm. 2018, 542, 1–7. [Google Scholar] [CrossRef]
- Carrigy, N.B.; Liang, L.; Wang, H.; Kariuki, S.; Nagel, T.E.; Connerton, I.F.; Vehring, R. Spray-dried anti-Campylobacter bacteriophage CP30A powder suitable for global distribution without cold chain infrastructure. Int. J. Pharm. 2019, 569, 118601. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Quek, S.-Y.; Huang, K. Advanced strategies to overcome the challenges of bacteriophage-based antimicrobial treatments in food and agricultural systems. Crit. Rev. Food Sci. Nutr. 2024, 64, 12574–12598. [Google Scholar] [CrossRef] [PubMed]
- Colom, J.; Cano-Sarabia, M.; Otero, J.; Cortés, P.; Maspoch, D.; Llagostera, M. Liposome-encapsulated bacteriophages for enhanced oral phage therapy against Salmonella spp. Appl. Environ. Microbiol. 2015, 81, 4841–4849. [Google Scholar] [CrossRef] [PubMed]
- Tabare, E.; Dauchot, T.; Cochez, C.; Glonti, T.; Antoine, C.; Laforêt, F.; Pirnay, J.-P.; Delcenserie, V.; Thiry, D.; Goole, J. Eudragit® FS microparticles containing bacteriophages, prepared by spray-drying for oral administration. Pharmaceutics 2023, 15, 1602. [Google Scholar] [CrossRef]
- Vinner, G.K.; Richards, K.; Leppanen, M.; Sagona, A.P.; Malik, D.J. Microencapsulation of enteric bacteriophages in a pH-responsive solid oral dosage formulation using a scalable membrane emulsification process. Pharmaceutics 2019, 11, 475. [Google Scholar] [CrossRef]
- Jamaledin, R.; Sartorius, R.; Di Natale, C.; Onesto, V.; Manco, R.; Mollo, V.; Vecchione, R.; De Berardinis, P.; Netti, P. PLGA microparticle formulations for tunable delivery of a nano-engineered filamentous bacteriophage-based vaccine: In vitro and in silico-supported approach. J. Nanostruct. Chem. 2024, 14, 307–322. [Google Scholar] [CrossRef]
- Lin, Y.; Quan, D.; Chang, R.Y.K.; Chow, M.Y.T.; Wang, Y.; Li, M.; Morales, S.; Britton, W.J.; Kutter, E.; Li, J.; et al. Synergistic activity of phage PEV20-ciprofloxacin combination powder formulation-A proof-of-principle study in a P. aeruginosa lung infection model. Eur. J. Pharm. Biopharm. 2021, 158, 166–171. [Google Scholar] [CrossRef]
- Chan, H.-K.; Chang, R.Y.K. Inhaled Delivery of Anti-Pseudomonal Phages to Tackle Respiratory Infections Caused by Superbugs. J. Aerosol Med. Pulm. Drug Deliv. 2022, 35, 73–82. [Google Scholar] [CrossRef]
- Li, L.; Zhong, Q.; Zhao, Y.; Bao, J.; Liu, B.; Zhong, Z.; Wang, J.; Yang, L.; Zhang, T.; Cheng, M.; et al. First-in-human application of double-stranded RNA bacteriophage in the treatment of pulmonary Pseudomonas aeruginosa infection. Microb. Biotechnol. 2023, 16, 862–867. [Google Scholar] [CrossRef] [PubMed]
- Sawant, S.S.; Ahmed, M.U.; Gantala, N.-G.; Chiu, C.; Qu, L.; Zhou, Q. Development of Inhalable Bacteriophage Liposomes Against Pseudomonas aeruginosa. Pharmaceutics 2025, 17, 405. [Google Scholar] [CrossRef]
- Chow, M.Y.T.; Chang, R.Y.K.; Li, M.; Wang, Y.; Lin, Y.; Morales, S.; McLachlan, A.J.; Kutter, E.; Li, J.; Chan, H.-K. Pharmacokinetics and Time-Kill Study of Inhaled Antipseudomonal Bacteriophage Therapy in Mice. Antimicrob. Agents Chemother. 2020, 65. [Google Scholar] [CrossRef]
- Leung, S.S.Y.; Parumasivam, T.; Gao, F.G.; Carrigy, N.B.; Vehring, R.; Finlay, W.H.; Morales, S.; Britton, W.J.; Kutter, E.; Chan, H.-K. Production of Inhalation Phage Powders Using Spray Freeze Drying and Spray Drying Techniques for Treatment of Respiratory Infections. Pharm. Res. 2016, 33, 1486–1496. [Google Scholar] [CrossRef]
- Tabare, E.; Glonti, T.; Cochez, C.; Ngassam, C.; Pirnay, J.-P.; Amighi, K.; Goole, J. A Design of Experiment Approach to Optimize Spray-Dried Powders Containing Pseudomonas aeruginosaPodoviridae and Myoviridae Bacteriophages. Viruses 2021, 13, 1926. [Google Scholar] [CrossRef]
- Alipour-Khezri, E.; Skurnik, M.; Zarrini, G. Pseudomonas aeruginosa bacteriophages and their clinical applications. Viruses 2024, 16, 1051. [Google Scholar] [CrossRef] [PubMed]
- Vaezi, A.; Healy, T.; Ebrahimi, G.; Rezvankhah, S.; Shahraki, A.H.; Mirsaeidi, M. Phage therapy: Breathing new tactics into lower respiratory tract infection treatments. Eur. Respir. Rev. 2024, 33, 240029. [Google Scholar] [CrossRef] [PubMed]
- Myszor, I.T.; Gudmundsson, G.H. Modulation of innate immunity in airway epithelium for host-directed therapy. Front. Immunol. 2023, 14, 1197908. [Google Scholar] [CrossRef]
- Popescu, M.; Van Belleghem, J.D.; Khosravi, A.; Bollyky, P.L. Bacteriophages and the immune system. Annu. Rev. Virol. 2021, 8, 415–435. [Google Scholar] [CrossRef]
- Federici, S.; Nobs, S.P.; Elinav, E. Phages and their potential to modulate the microbiome and immunity. Cell. Mol. Immunol. 2021, 18, 889–904. [Google Scholar] [CrossRef]
- Cuomo, P.; Medaglia, C.; Casillo, A.; Gentile, A.; Fruggiero, C.; Corsaro, M.M.; Capparelli, R. Phage-resistance alters Lipid A reactogenicity: A new strategy for LPS-based conjugate vaccines against Salmonella Rissen. Front. Immunol. 2024, 15, 1450600. [Google Scholar] [CrossRef]
- Ling, K.-M.; Stick, S.M.; Kicic, A. Pulmonary bacteriophage and cystic fibrosis airway mucus: Friends or foes? Front. Med. 2023, 10, 1088494. [Google Scholar] [CrossRef]
- Echterhof, A.; Dharmaraj, T.; Khosravi, A.; McBride, R.; Miesel, L.; Chia, J.-H.; Blankenberg, P.M.; Lin, K.-Y.; Shen, C.-C.; Lee, Y.-L. The contribution of neutrophils to bacteriophage clearance and pharmacokinetics in vivo. JCI Insight 2024, 9, e181309. [Google Scholar] [CrossRef] [PubMed]
- Leong, E.W.; Ge, R. Lipid nanoparticles as delivery vehicles for inhaled therapeutics. Biomedicines 2022, 10, 2179. [Google Scholar] [CrossRef] [PubMed]
- Baldelli, A.; Liang, M. Design of respirable sprayed microparticles of encapsulated bacteriophages. Front. Drug Deliv. 2023, 3, 1209534. [Google Scholar] [CrossRef]
- Haddad, H.F.; Roe, E.F.; Collier, J.H. Expanding opportunities to engineer mucosal vaccination with biomaterials. Biomater. Sci. 2023, 11, 1625–1647. [Google Scholar] [CrossRef]
- Patel, B.; Gupta, N.; Ahsan, F. Particle engineering to enhance or lessen particle uptake by alveolar macrophages and to influence the therapeutic outcome. Eur. J. Pharm. Biopharm. 2015, 89, 163–174. [Google Scholar] [CrossRef]
- Pacheco, J.E.C.; Yalovenko, T.; Riaz, A.; Kotov, N.; Davids, C.; Persson, A.; Falkman, P.; Feiler, A.; Godaly, G.; Johnson, C.M. Inhalable porous particles as dual micro-nano carriers demonstrating efficient lung drug delivery for treatment of tuberculosis. J. Control. Release 2024, 369, 231–250. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, I.; Taniguchi, Y.; Tamura, Y.; Ochiai, K.; Makino, K. Effects of L-leucine on PLGA microparticles for pulmonary administration prepared using spray drying: Fine particle fraction and phagocytotic ratio of alveolar macrophages. Colloids Surf. A Physicochem. Eng. Asp. 2018, 537, 411–417. [Google Scholar] [CrossRef]
- Omidian, H.; Wilson, R.L. PLGA-Based Strategies for Intranasal and Pulmonary Applications. Pharmaceutics 2025, 17, 207. [Google Scholar] [CrossRef]
- Wdowiak, M.; Paczesny, J.; Raza, S. Enhancing the stability of bacteriophages using physical, chemical, and nano-based approaches: A review. Pharmaceutics 2022, 14, 1936. [Google Scholar] [CrossRef]
- Venkataraman, S.; Shahgolzari, M.; Yavari, A.; Hefferon, K. Bacteriophages as Targeted Therapeutic Vehicles: Challenges and Opportunities. Bioengineering 2025, 12, 469. [Google Scholar] [CrossRef]
- Fauconnier, A. Phage therapy regulation: From night to dawn. Viruses 2019, 11, 352. [Google Scholar] [CrossRef]
- Faltus, T. The medicinal phage-regulatory roadmap for phage therapy under EU pharmaceutical legislation. Viruses 2024, 16, 443. [Google Scholar] [CrossRef] [PubMed]
- Moraes de Souza, C.; Tanir, T.; Orellana, M.; Escalante, A.; Koeris, M.S. Manufacturing bacteriophages (part 2 of 2): Formulation, analytics and quality control considerations. Pharmaceuticals 2021, 14, 895. [Google Scholar] [CrossRef]
- Duyvejonck, H.; Merabishvili, M.; Pirnay, J.-P.; De Vos, D.; Verbeken, G.; Van Belleghem, J.; Gryp, T.; De Leenheer, J.; Van der Borght, K.; Van Simaey, L. Development of a qPCR platform for quantification of the five bacteriophages within bacteriophage cocktail 2 (BFC2). Sci. Rep. 2019, 9, 13893. [Google Scholar] [CrossRef] [PubMed]
- Bretaudeau, L.; Tremblais, K.; Aubrit, F.; Meichenin, M.; Arnaud, I. Good manufacturing practice (GMP) compliance for phage therapy medicinal products. Front. Microbiol. 2020, 11, 1161. [Google Scholar] [CrossRef]
- Mutti, M.; Corsini, L. Robust approaches for the production of active ingredient and drug product for human phage therapy. Osmotic Imbalances Ph Shift Due 2019, 10, 2289. [Google Scholar] [CrossRef]
- Chang, R.Y.K.; Chow, M.Y.T.; Wang, Y.; Liu, C.; Hong, Q.; Morales, S.; McLachlan, A.J.; Kutter, E.; Li, J.; Chan, H.-K. The effects of different doses of inhaled bacteriophage therapy for Pseudomonas aeruginosa pulmonary infections in mice. Clin. Microbiol. Infect. 2022, 28, 983–989. [Google Scholar] [CrossRef] [PubMed]
- Matinkhoo, S.; Lynch, K.H.; Dennis, J.J.; Finlay, W.H.; Vehring, R. Spray-dried Respirable Powders Containing Bacteriophages for the Treatment of Pulmonary Infections. J. Pharm. Sci. 2011, 100, 5197–5205. [Google Scholar] [CrossRef]
- Roach, D.R.; Leung, C.Y.; Henry, M.; Morello, E.; Singh, D.; Di Santo, J.P.; Weitz, J.S.; Debarbieux, L. Synergy between the Host Immune System and Bacteriophage Is Essential for Successful Phage Therapy against an Acute Respiratory Pathogen. Cell Host Microbe 2017, 22, 38–47.e4. [Google Scholar] [CrossRef]
- Sécher, T.; Mayor, A.; Heuzé-Vourc’h, N. Inhalation of Immuno-Therapeutics/-Prophylactics to Fight Respiratory Tract Infections: An Appropriate Drug at the Right Place! Front. Immunol. 2019, 10, 2760. [Google Scholar] [CrossRef]
- Cafora, M.; Deflorian, G.; Forti, F.; Ferrari, L.; Binelli, G.; Briani, F.; Ghisotti, D.; Pistocchi, A. Phage therapy against Pseudomonas aeruginosa infections in a cystic fibrosis zebrafish model. Sci. Rep. 2019, 9, 1527. [Google Scholar] [CrossRef]
- Golshahi, L.; Lynch, K.H.; Dennis, J.J.; Finlay, W.H. In vitro lung delivery of bacteriophages KS4-M and ΦKZ using dry powder inhalers for treatment of Burkholderia cepacia complex and Pseudomonas aeruginosa infections in cystic fibrosis. J. Appl. Microbiol. 2010, 110, 106–117. [Google Scholar] [CrossRef]
- Ciofu, O.; Tolker-Nielsen, T. Tolerance and resistance of Pseudomonas aeruginosa biofilms to antimicrobial agents—How P. aeruginosa can escape antibiotics. Front. Microbiol. 2019, 10, 913. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, H.-H.; Chen, Q.-H.; Wang, W.; Qu, Q.; Xu, W.-X.; Hu, Q.; Wu, X.-L.; Chen, Y.; Wan, Q.; Xu, T.-T. The efficacy of polymyxin B in treating stroke-associated pneumonia with carbapenem-resistant Gram-negative bacteria infections: A multicenter real-world study using propensity score matching. Front. Pharmacol. 2025, 16, 1413563. [Google Scholar] [CrossRef]
- McClure, W.R.; Cech, C.L. On the mechanism of rifampicin inhibition of RNA synthesis. J. Biol. Chem. 1978, 253, 8949–8956. [Google Scholar] [CrossRef] [PubMed]
- Campbell, E.A.; Korzheva, N.; Mustaev, A.; Murakami, K.; Nair, S.; Goldfarb, A.; Darst, S.A. Structural mechanism for rifampicin inhibition of bacterial RNA polymerase. Cell 2001, 104, 901–912. [Google Scholar] [CrossRef] [PubMed]
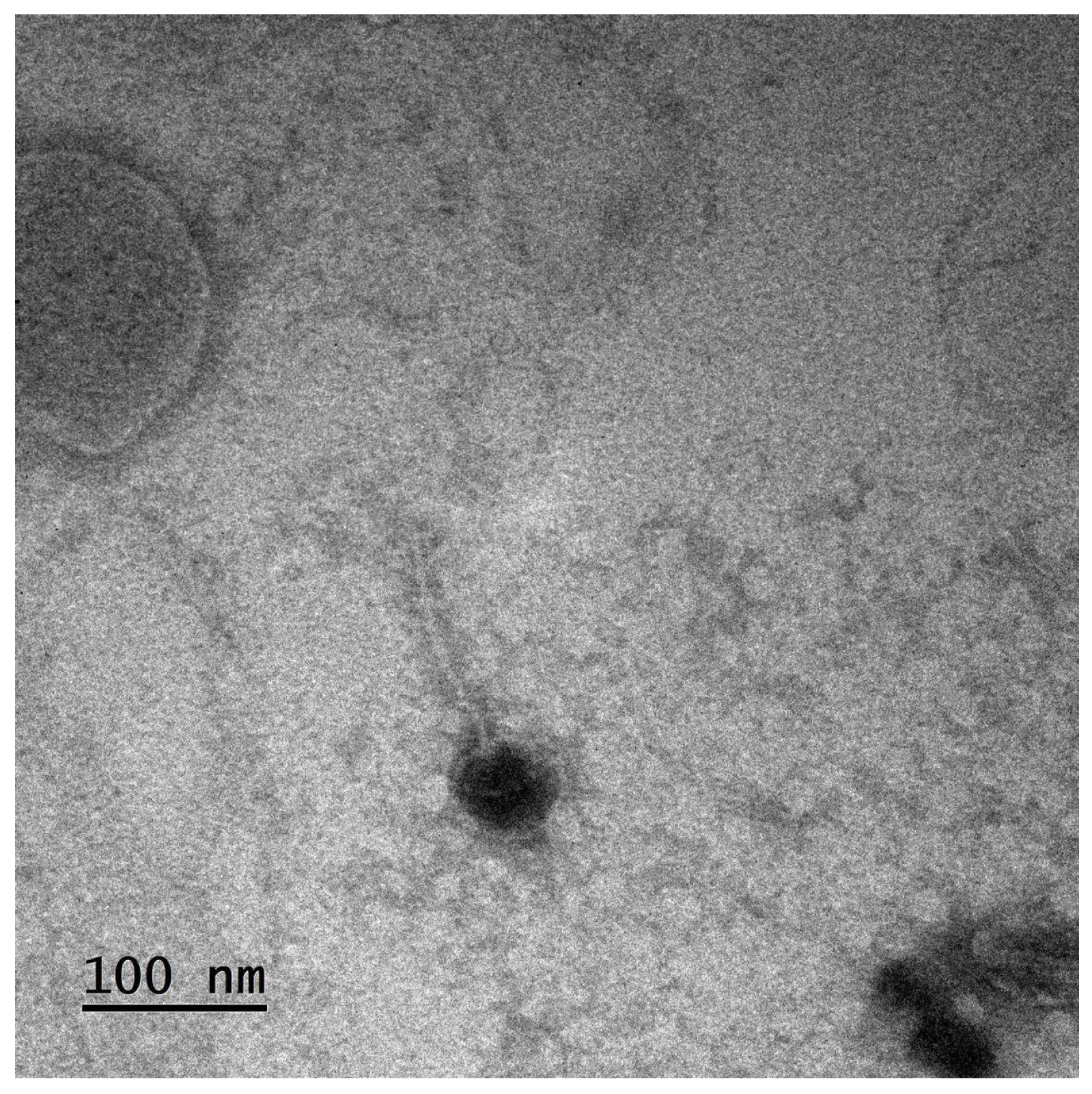
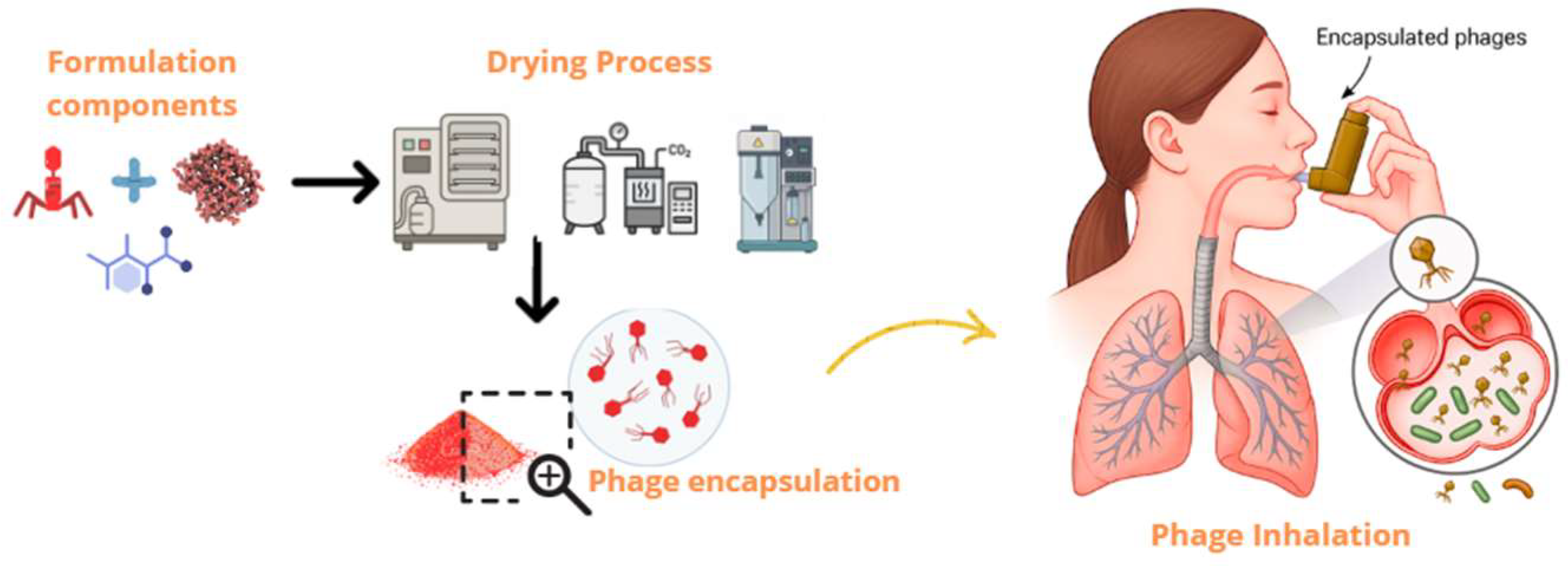
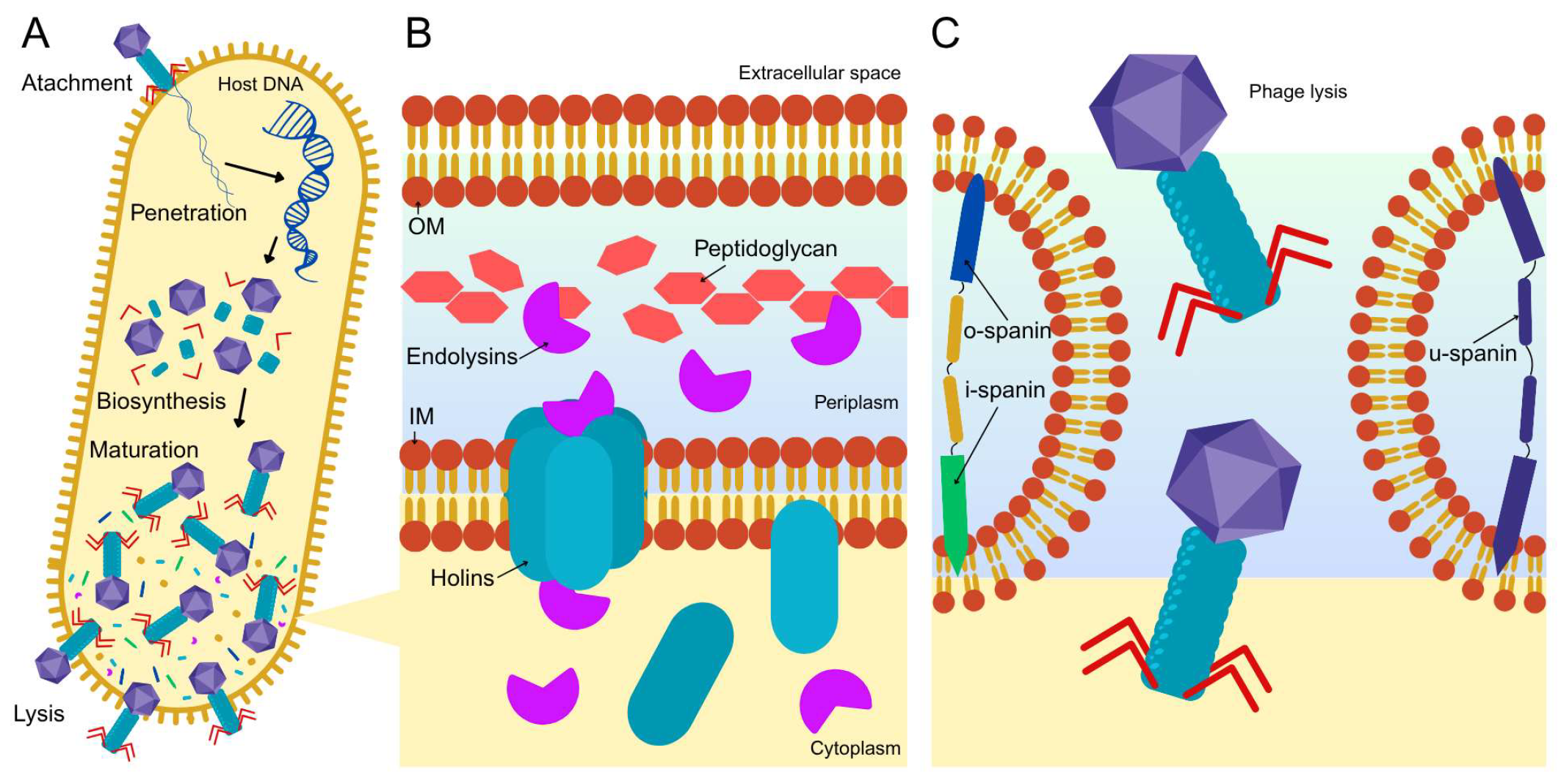
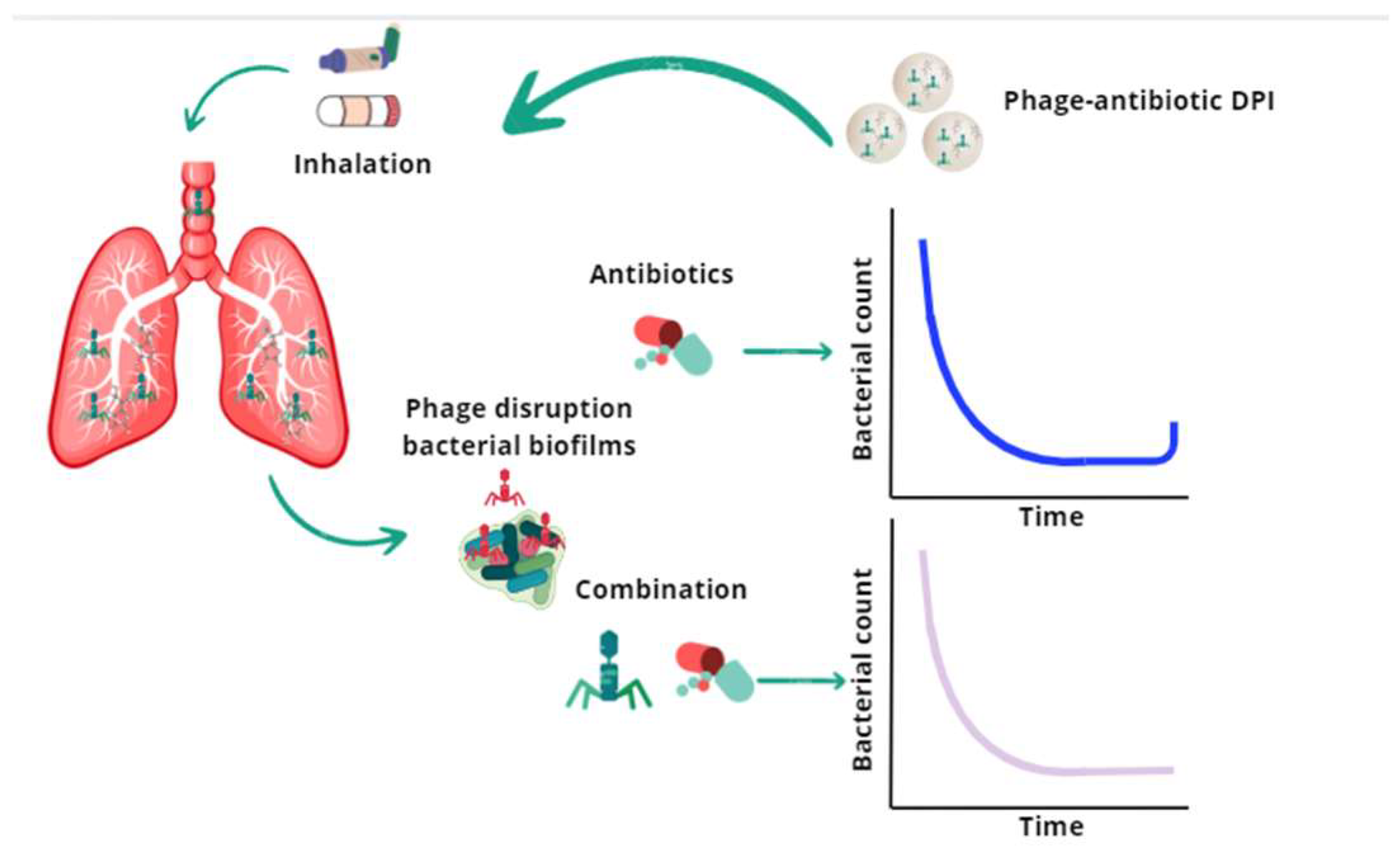
| Nanocarrier System | Particle Size | Phage Protection | Pulmonary Deposition Efficiency | Drying Method Compatibility | Reference |
|---|---|---|---|---|---|
| PLGA spheres | Geometric diameter: 10.67 µm Aerodynamic diameter: 4.62 µm | High (encapsulation preserves infectivity; minimal titer loss after freeze drying; stability ≥ 12 days at room temperature) | High (aerodynamic diameter in respirable range; lung retention confirmed after inhalation) | Vacuum freeze drying | [56] |
| PLGA spheres | Aerodynamic diameter: 3.3–3.8 µm | Lytic activity preserved | Cascade impaction results: geometric mean weight diameter: 6.6 µm | Stable immediately after freeze drying | [57] |
| Chitosan nanoparticles | Phage-loaded CS-NPs: 297 ± 18 nm | Encapsulation efficiency: ~97% Maintained infectivity under pH 3–12, at 25–80 °C | No direct lung model data | Not involved | [58] |
| Liposomes | PEV2: 301 ± 35.8 nm PEV40: 651 ± 14.3 nm | Titer reduction after nebulization | Viable respirable fraction (VRF) with vibrating mesh: ~70.3% (PEV2), 74.8% (PEV40) VRF with jet nebulizer: ~44% (PEV2), 28.2% (PEV40) | Not involved | [59] |
| PVP-K25 matrix powder | Mass median aerodynamic diameter: 2.84 µm | Preserved D29 viability post-TFF and during storage; active against intracellular M. smegmatis | Effective phage delivery to alveolar macrophages; targeted intracellular release | Thin-film freezing (TFF) | [60] |
| Drying Technique | Thermal Stress | Phage Recovery (%) | Particle Morphology | Aerodynamic Size | Cost/Scalability | References |
|---|---|---|---|---|---|---|
| Spray Drying | High (100–160 °C) | 50–90% | Spherical, dense, or collapsed | 1–5 µm | Low cost, high throughput | [24,63] |
| Lyophilization | Low (frozen) | >90% (with stabilizers) | Irregular, requires milling | 3–15 µm (post-milling) | High cost, slow | [57] |
| Spray Freeze Drying | Very low (−100 to −130 °C) | 60–85% | Porous, spherical | 2–6 µm | Medium–high, moderate scale-up | [64,65] |
| Thin-Film Freeze Drying | Minimal (−40 °C films) | >90% | Fragile, porous films | 1–5 µm (after milling) | High, emerging technique | [60] |
| Phage Studied | Powder Manufacturing Process | Excipients | Previous Phage Encapsulation | Notable Results | Reference |
|---|---|---|---|---|---|
| Phage cocktail (against Pseudomonas aeruginosa) | Freeze drying or lyophilization | Lactose | Yes, into PLGA microparticles | The study, conducted in a murine model, resulted in a significant reduction in the bacterial load in the lungs, indicating the potential efficacy of the treatment. | [61] |
| Phage PEV20 (against Pseudomonas aeruginosa) and ciprofloxacin | Spray drying | Leucine, with and without lactose | No | The formulations maintained antimicrobial synergy. | [6] |
| Phage cocktail PEV2, PEV1 and PEV20 (against Pseudomonas aeruginosa) | Spray drying | Lactose and leucine | No | The formulation of 80:20% lactose–leucine retained phage viability and achieved an FPF of up to 45%. Its in vitro efficacy against MDR strains was demonstrated. | [73] |
| Phage PEV2 (against Pseudomonas aeruginosa) | Spray drying | HSA–lactose | No | First study using HSA as an excipient. The formulation HSA–lactose 60:40% w/w demonstrated favorable results with lower titer loss (<log) and FPF > 50%. Future investigations need to be performed. | [71] |
| Mycobacteriophage (against Mycobacterium tuberculosis) | Freeze drying or lyophilization | Trehalose, leucine, and cyclodextrin | No | Although the formulation exhibited antibacterial activity in vitro and achieved FPF > 50%, it failed to produce a significant antibacterial effect in vivo. These findings underscore the challenges associated with translating in vitro efficacy into in vivo therapeutic outcomes. | [64] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Encinas-Basurto, D.; Martinez-Flores, P.D.; García, J.; Lopez-Mata, M.A.; García-González, G.; Rodea, G.E.; Eedara, B.B.; Mansour, H.M.; Juarez, J. Latest Advances in Inhalable Dry Powder Bacteriophage Therapy for Pulmonary Infections. Pharmaceutics 2025, 17, 1077. https://doi.org/10.3390/pharmaceutics17081077
Encinas-Basurto D, Martinez-Flores PD, García J, Lopez-Mata MA, García-González G, Rodea GE, Eedara BB, Mansour HM, Juarez J. Latest Advances in Inhalable Dry Powder Bacteriophage Therapy for Pulmonary Infections. Pharmaceutics. 2025; 17(8):1077. https://doi.org/10.3390/pharmaceutics17081077
Chicago/Turabian StyleEncinas-Basurto, David, Patricia Dolores Martinez-Flores, Joselyn García, Marco Antonio Lopez-Mata, Gerardo García-González, Gerardo E. Rodea, Basanth Babu Eedara, Heidi M. Mansour, and Josue Juarez. 2025. "Latest Advances in Inhalable Dry Powder Bacteriophage Therapy for Pulmonary Infections" Pharmaceutics 17, no. 8: 1077. https://doi.org/10.3390/pharmaceutics17081077
APA StyleEncinas-Basurto, D., Martinez-Flores, P. D., García, J., Lopez-Mata, M. A., García-González, G., Rodea, G. E., Eedara, B. B., Mansour, H. M., & Juarez, J. (2025). Latest Advances in Inhalable Dry Powder Bacteriophage Therapy for Pulmonary Infections. Pharmaceutics, 17(8), 1077. https://doi.org/10.3390/pharmaceutics17081077








