Poloxamer-Based Biomaterial as a Pharmaceutical Strategy to Improve the Ivermectin Performance
Abstract
1. Introduction
2. Materials and Methods
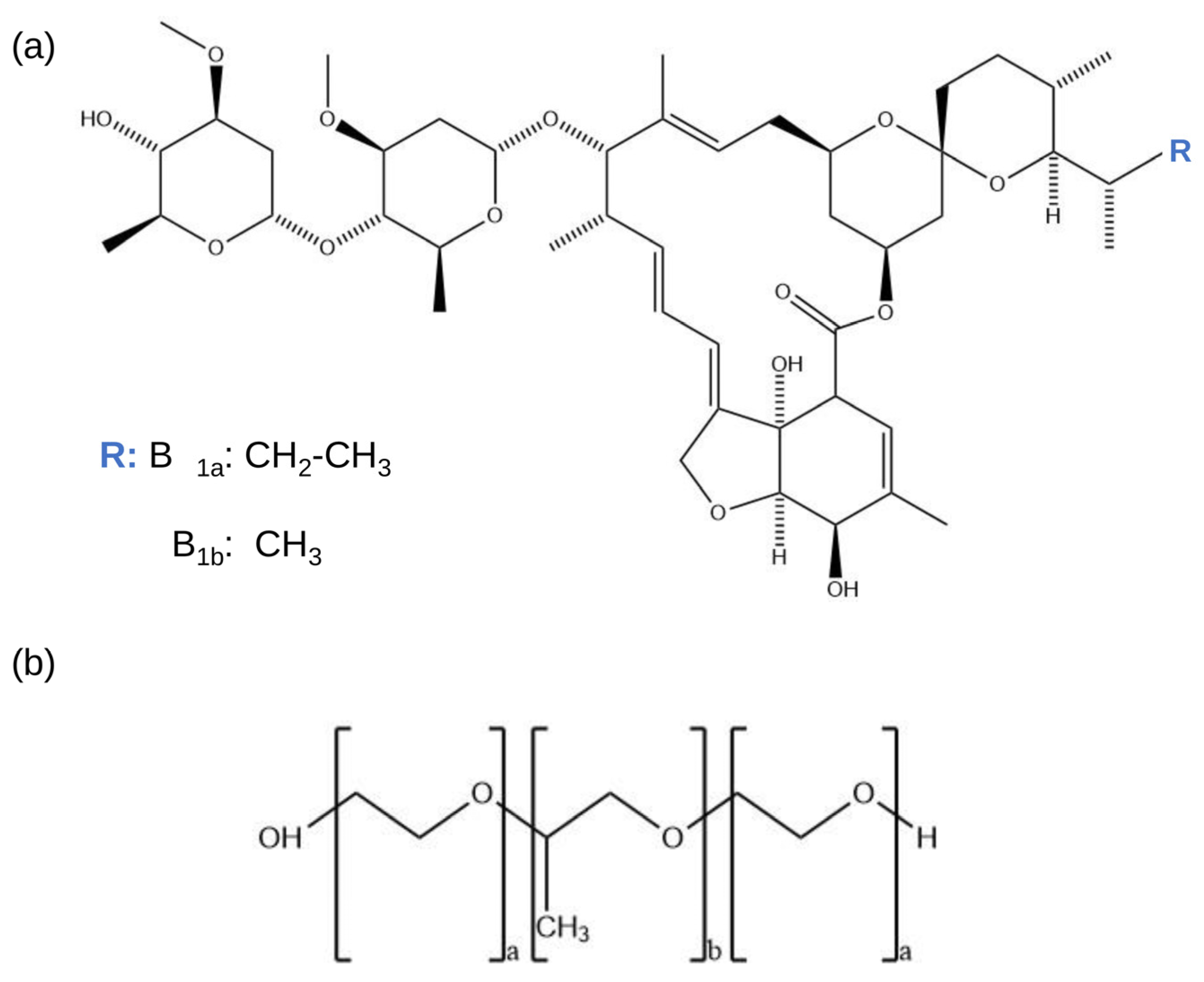
2.1. Chemicals and Reagents
2.2. Preformulation Studies
2.3. Solid Samples Preparation
2.3.1. Solid Dispersions by the Fusion Method
2.3.2. Physical Mixtures
2.4. Determination of Drug Content
2.5. Solid Dispersions Characterization
2.5.1. FT-IR Technique
2.5.2. XRPD Technique
2.5.3. SEM Technique
2.5.4. TEM Technique
2.5.5. Thermal Analysis Techniques
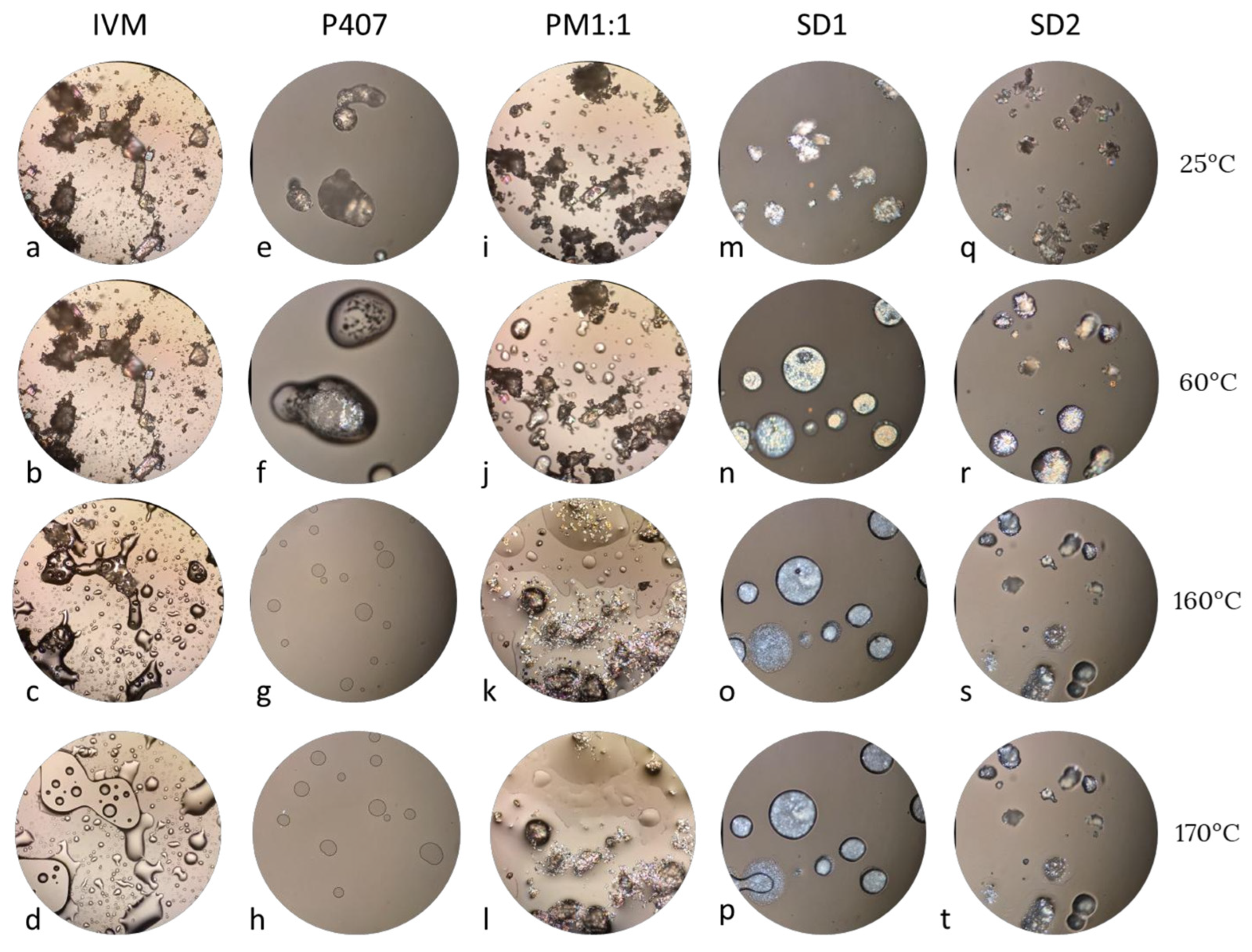
2.5.6. Hot Stage Microscopy
2.5.7. DLS Technique
2.6. Solubility Studies
2.7. Dissolution Study
2.8. In Vitro Release Profile
2.8.1. Dialysis Study
2.8.2. Chromatographic Method
2.9. Statistical Analysis
3. Results and Discussion
3.1. Preformulation of Solid Dispersions
3.2. Preparation of Solid Dispersions: Screening of Conditions
3.3. Solid Dispersions Characterization in Solid State
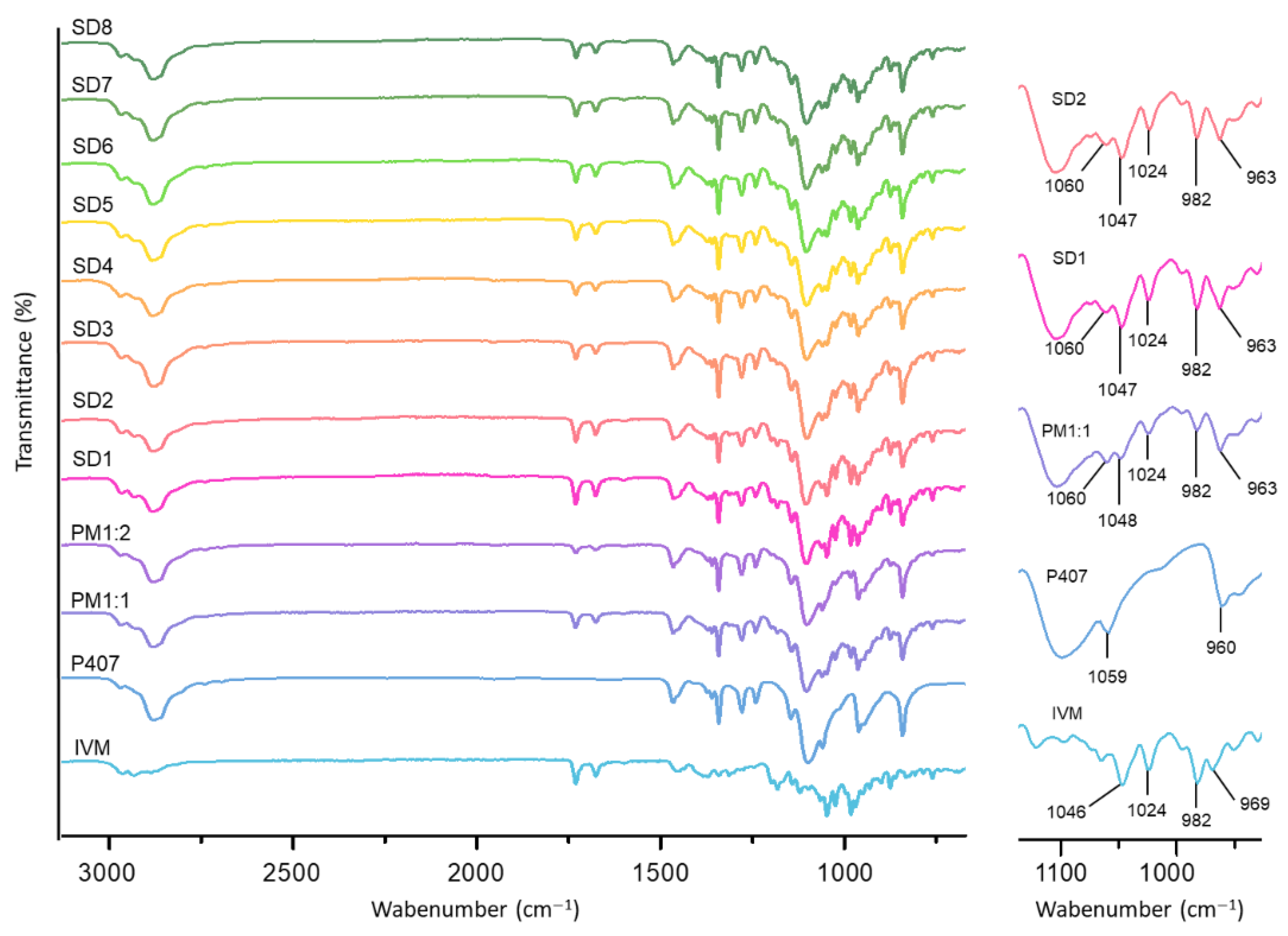
3.3.1. FT-IR
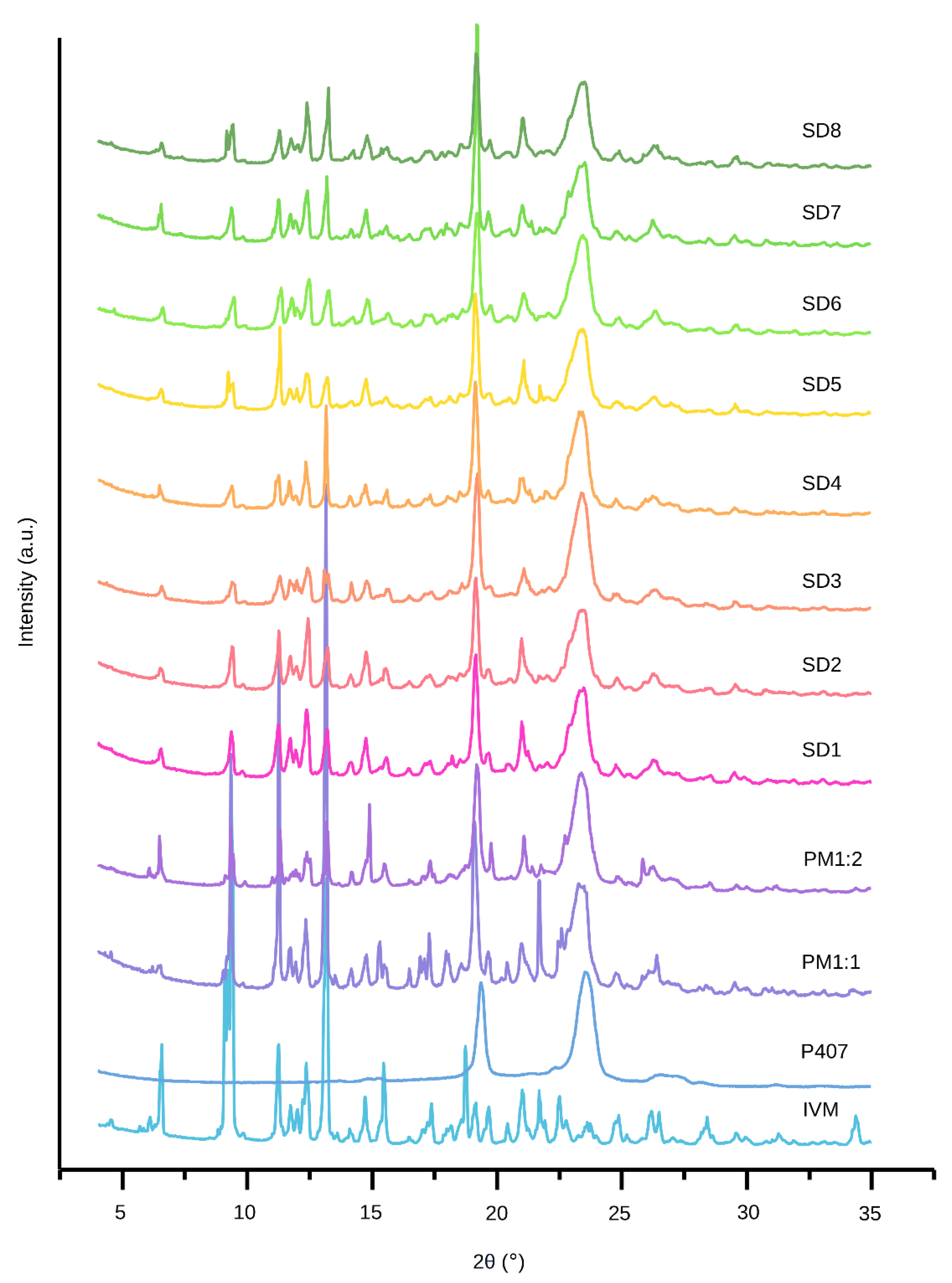
3.3.2. XRPD
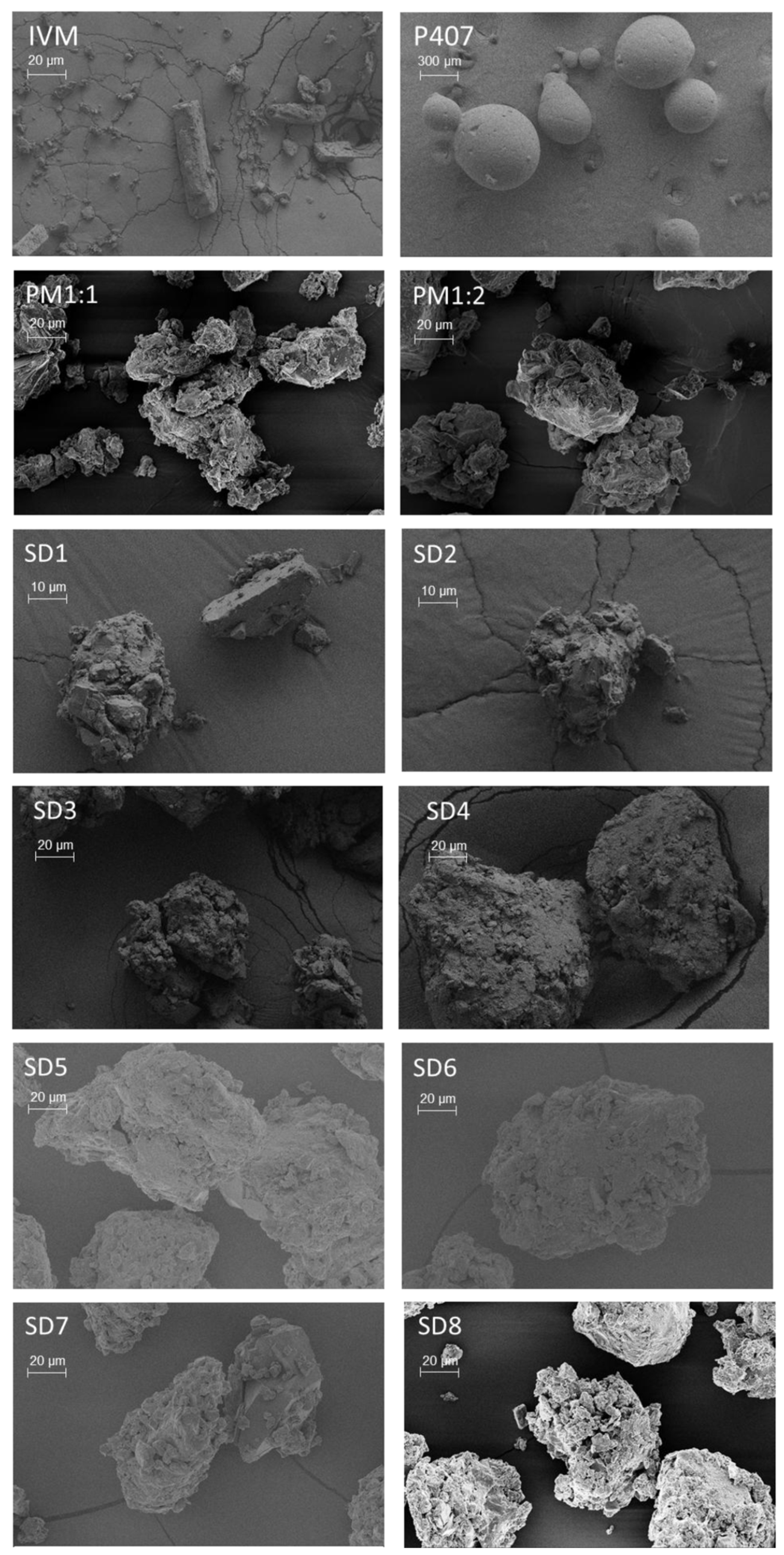
3.3.3. SEM
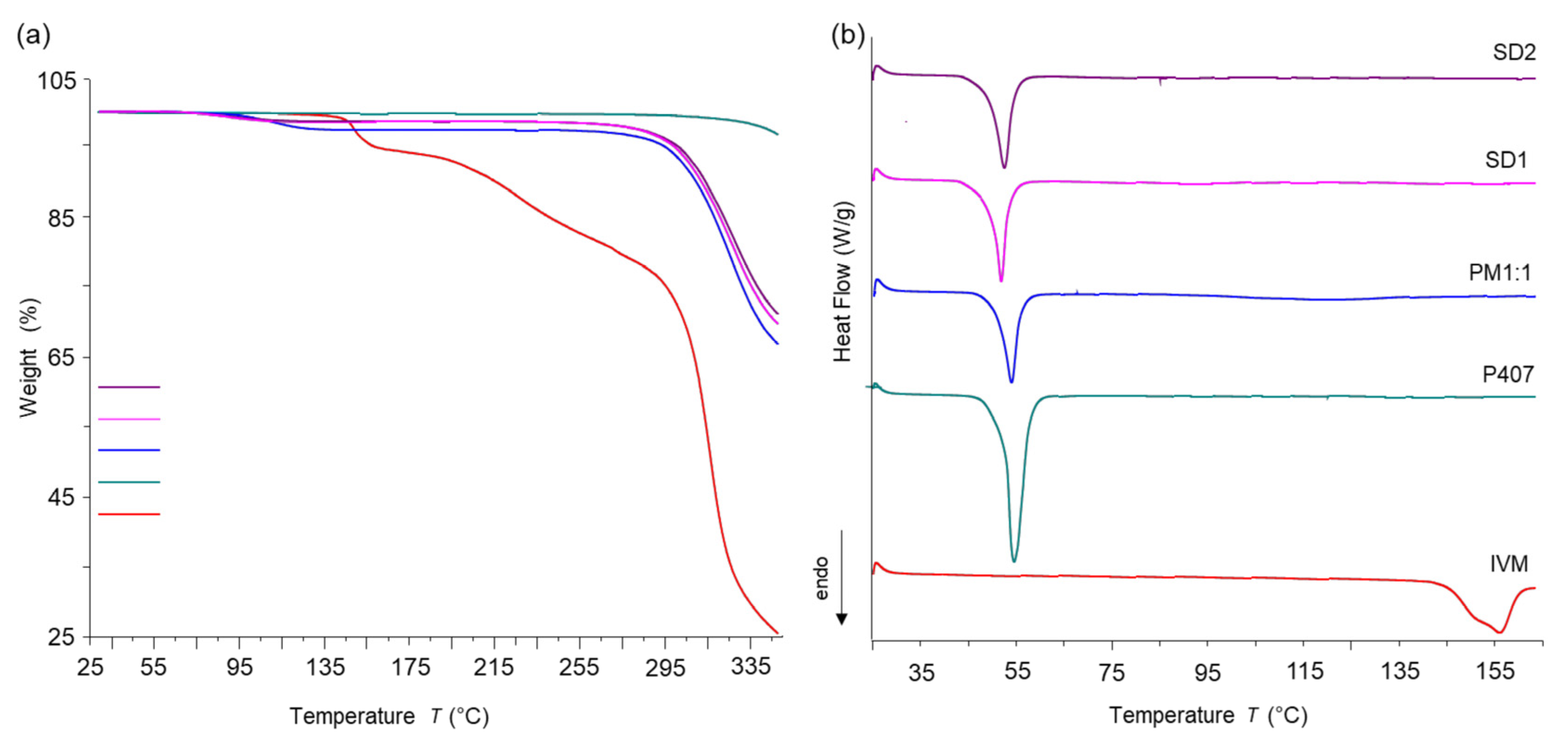
3.3.4. Thermal Analysis
3.4. Solid Dispersions Characterization After Being Dispersed in the Water
3.4.1. DLS
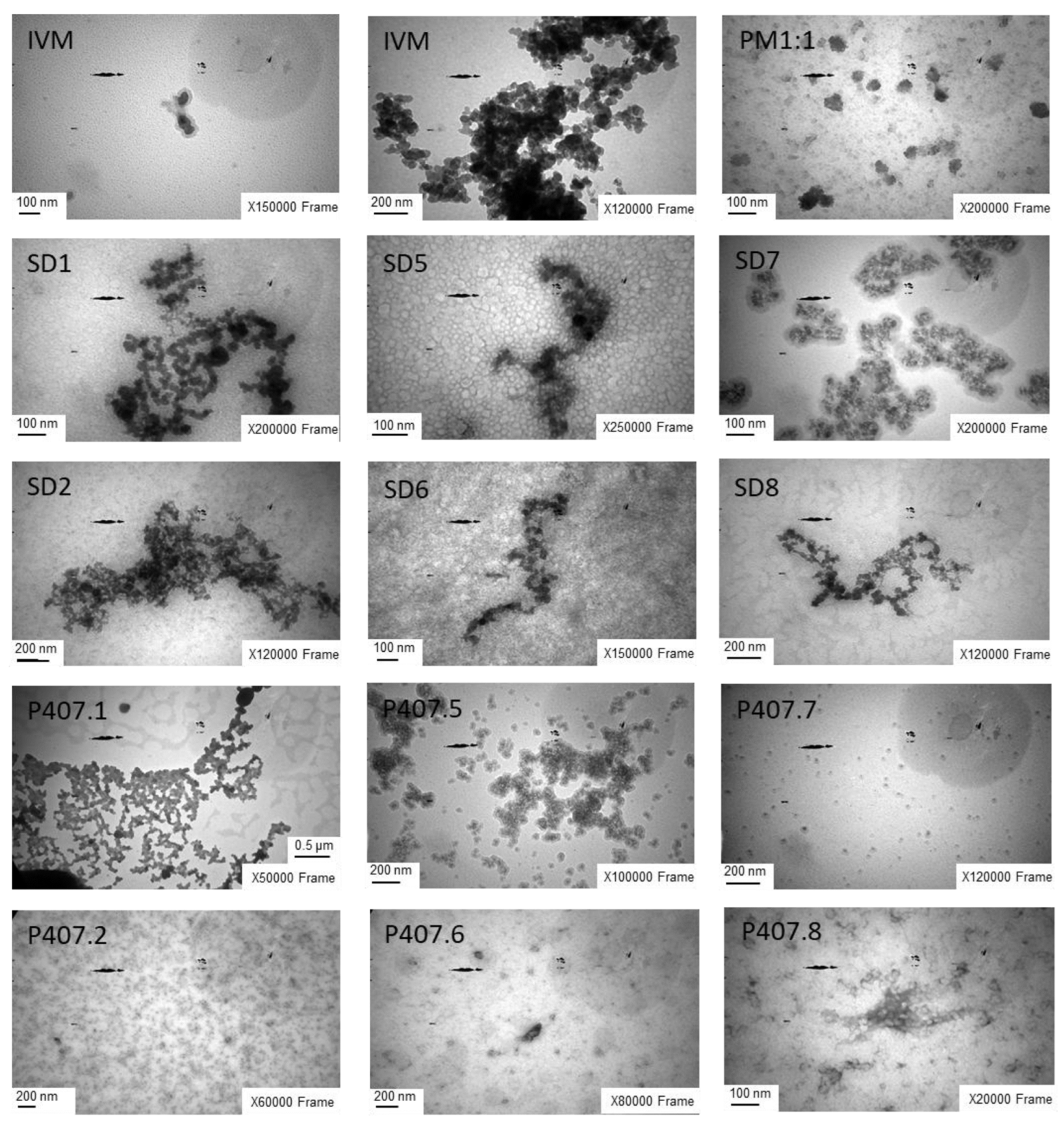
3.4.2. TEM
3.5. Studies in Solution
3.5.1. Saturation Solubility Studies
3.5.2. Dissolution Study
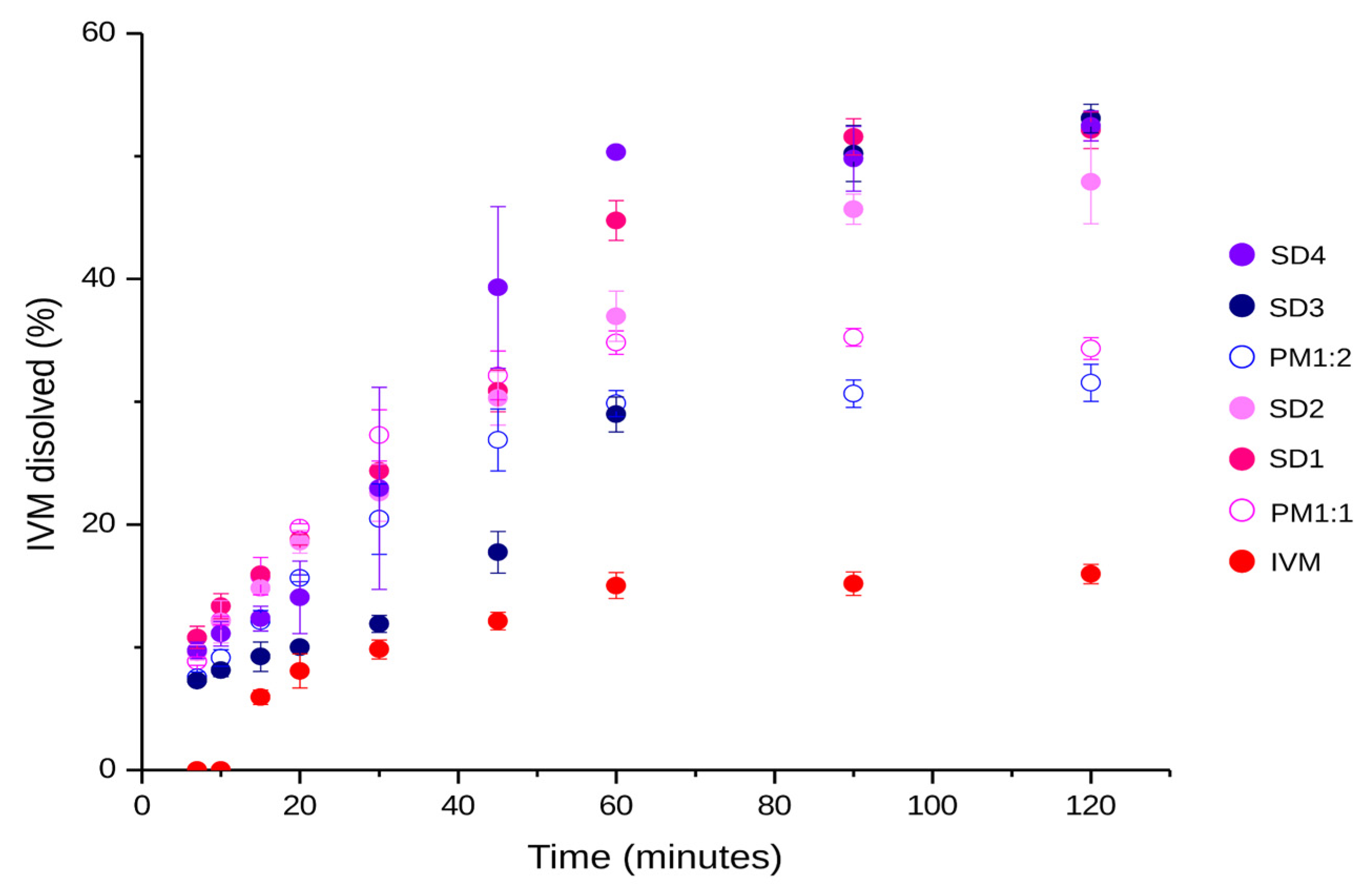
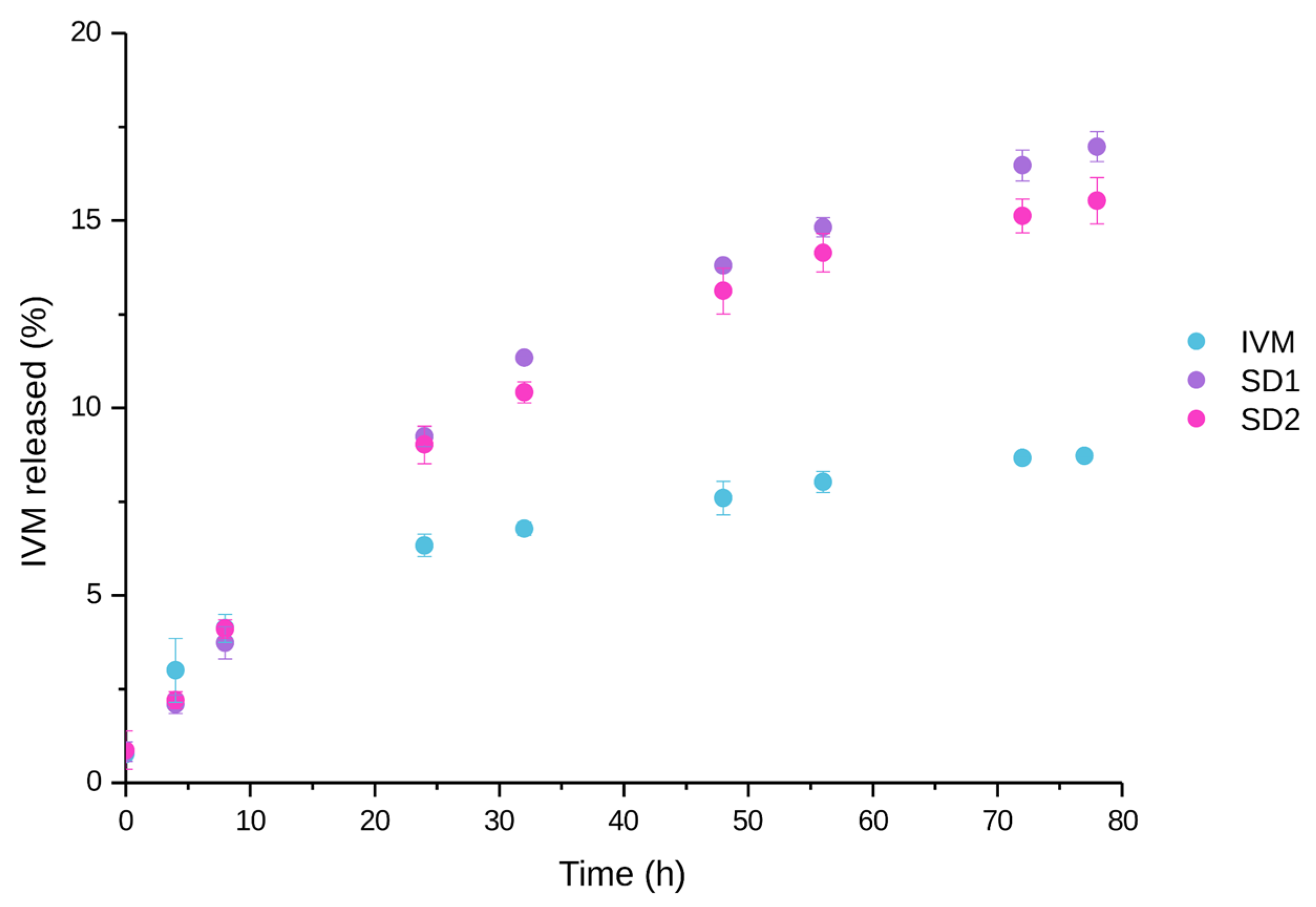
3.6. In Vitro Drug Release Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IVM | Ivermectin |
| P407 | Poloxamer 407 |
| P188 | Poloxamer 188 |
| PVP-k90 | Polyvinylpyrrolidone k90 |
| PVP-k30 | Polyvinylpyrrolidone k30 |
| PEG8000 | Polyethylene glycol 8000 |
| PEG6000 | Polyethylene glycol 6000 |
| PBS | Phosphate buffer solution |
| SDs | Solid dispersions |
| PM1:1 | Physical mixture 1:1 w/w |
| PM1:2 | Physical mixture 1:2 w/w |
| SGF | Simulated gastric fluid |
| XRPD | X-ray powder diffraction |
| FT-IR | Fourier-transform infrared spectroscopy |
| ATR | Attenuated total reflectance |
| SEM | Scanning electron microscopy |
| TEM | Transmission electron microscopy |
| DSC | Differential scanning calorimetry |
| TGA | Thermogravimetric analysis |
| DLS | Dynamic light scattering |
| PDI | Polydispersity index |
| ZP | Zeta potential |
| IC | Crystallinity index |
References
- Organización Panamericana de la Salud; Organización Mundial de la Salud. Enfermedades Infecciosas Desatendidas en las Américas: Historias de Éxito e Innovación para Llegar a los Más Necesitados; Organización Panamericana de la Salud: Washington, DC, USA, 2016; ISBN 978-92-75-11896-2. [Google Scholar]
- Ashour, D.S. Ivermectin: From theory to clinical application. Int. J. Antimicrob. Agents 2019, 54, 134–142. [Google Scholar] [CrossRef]
- Fent, G.M. Avermectin. In Encyclopedia of Toxicology, 3rd ed.; Philip, W., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2014; pp. 342–344. [Google Scholar] [CrossRef]
- Victoria, A. Ivermectina: Sus Múltiples Usos, Seguridad y Toxicidad. Rev. Chil. Dermatol. 2010, 26, 358–368. [Google Scholar]
- Camargo, J.A.; Sapin, A.; Nouvel, C.; Daloz, D.; Leonard, M.; Bonneaux, F.; Six, J.-L.; Maincent, P. Injectable PLA-based in situ forming implants for controlled release of Ivermectin a BCS Class II drug: Solvent selection based on physicochemical characterization. Drug Dev. Ind. Pharm. 2013, 39, 146–155. [Google Scholar] [CrossRef]
- Verma, S.; Patel, U.; Patel, R. Formulation and evaluation of ivermectin solid dispersion. J. Drug Deliv. Ther. 2017, 7, 15–17. [Google Scholar]
- Guo, D.; Dou, D.; Li, X.; Zhang, Q.; Bhutto, Z.A.; Wang, L. Ivermection-loaded solid lipid nanoparticles: Preparation, characterisation, stability and transdermal behaviour. Artif. Cells Nanomed. Biotechnol. 2018, 46, 255–262. [Google Scholar] [CrossRef]
- Giuliano, E.; Paolino, D.; Fresta, M.; Cosco, D. Drug-Loaded Biocompatible Nanocarriers Embedded in Poloxamer 407 Hydrogels as Therapeutic Formulations. Medicines 2018, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Campos, S.N.; Cid, A.G.; Romer, A.I.; Villegas, M.; Briones Nieva, C.A.; Gonzo, E.E.; Bermúdez, J.M. Solid dispersions as a technological strategy to improve the bio-performance of antiparasitic drugs with limited solubility. Proceedings 2021, 78, 13. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Ramsey, J.D.; Samadi, A.; Atoufi, Z.; Khodadadi Yazdi, M.; Ganjali, M.R.; Mohammadi Amirabad, L.; Zangene, E.; Farokhi, M.; Formela, K.; et al. Poloxamer: A versatile tri-block copolymer for biomedical applications. Acta Biomater. 2020, 110, 37–67. [Google Scholar] [CrossRef] [PubMed]
- Nugraha, D.H.; Anggadiredja, K.; Rachmawati, H. Mini-Review of Poloxamer as a Biocompatible Polymer for Advanced Drug Delivery. Braz. J. Pharm. Sci. 2022, 58, e21125. [Google Scholar] [CrossRef]
- Deshmane, S.V.; Biyani, K.R. Characterization of solid dispersion: A review. Int. J. Pharm. Allied Res. 2014, 4, 584–589. [Google Scholar]
- Simonazzi, A.; Davies, C.; Cid, A.G.; Gonzo, E.; Parada, L.; Bermúdez, J.M. Preparation and Characterization of Poloxamer 407 Solid Dispersions as an Alternative Strategy to Improve Benznidazole Bioperformance. J. Pharm. Sci. 2018, 107, 2829–2836. [Google Scholar] [CrossRef]
- Cid, A.G.; Simonazzi, A.; Palma, S.D.; Bermúdez, J.M. Solid dispersión technology as a strategy to improve the bioavailability of poorly soluble drugs. Ther. Deliv. 2019, 10, 363–382. [Google Scholar] [CrossRef]
- Malkawi, R.; Malkawi, W.I.; Al-Mahmoud, Y.; Tawalbeh, J. Current Trends on Solid Dispersions: Past, Present, and Future. Adv. Pharmacol. Pharm. Sci. 2022, 2022, 5916013. [Google Scholar] [CrossRef]
- Pironi, A.M.; Oliveira Eloy, J.; Rodero, C.F.; Gutierrez Antonio, S.; Alonso, J.D.; Chorilli, M. PVP solid dispersions containing Poloxamer 407 or TPGS for the improvement of ursolic acid release. Braz. J. Pharm. Sci. 2023, 59, e21217. [Google Scholar] [CrossRef]
- Starkloff, W.J.; Bucalá, V.; Palma, S.D.; Gonzalez Vidal, N.L. Design and in vitro characterization of ivermectin nanocrystals liquid formulation based on a top–down approach. Pharm. Dev. Technol. 2016, 22, 809–817. [Google Scholar] [CrossRef]
- Lopez-Vidal, L.; Parodi, P.; Actis, M.R.; Camacho, N.; Real, D.A.; Irazoqui, F.; Real, J.P.; Palma, S.D. Formulation and optimization of pH-sensitive nanocrystals for improved oral delivery. Drug. Deliv. Transl. Res. 2023, 14, 1301–1318. [Google Scholar] [CrossRef]
- Kassaee, S.N.; Ayoko, G.A.; Richard, D.; Wang, T.; Islam, N. Inhaled Ivermectin-Loaded Lipid Polymer Hybrid Nanoparticles: Development and Characterization. Pharmaceutics 2024, 16, 1061. [Google Scholar] [CrossRef] [PubMed]
- Freitas, C.S.; Lage, D.P.; Machado, A.S.; Vale, D.L.; Martins, V.T.; Cardoso, J.M.O.; Oliveira-da-Silva, J.A.; Reis, T.A.R.; Tavares, G.S.V.; Ramos, F.F.; et al. Exploring drug repositioning for leishmaniasis treatment: Ivermectin plus polymeric micelles induce immunological response and protection against tegumentary leishmaniasis. Cytokine 2023, 164, 156143. [Google Scholar] [CrossRef] [PubMed]
- Reis, T.A.R.; Oliveira-da-Silva, J.; Tavares, G.S.V.; Mendonça, D.V.C.; Freitas, C.S.; Costa, R.R.; Lage, D.P.; Martins, V.T.; Machado, A.S.; Ramos, F.F.; et al. Ivermectin presents effective and selective antileishmanial activity in vitro and in vivo against Leishmania infantum and is therapeutic against visceral leishmaniasis. Exp. Parasitol. 2021, 221, 108059. [Google Scholar] [CrossRef]
- Khandoker, S.S.; Nitesh, K.K.; Ashiqur, R.; Hasan, J.; Youssef, H.; Stephen, J.E.; Alfred, D.F.; Lokendra, P.; Lucian, A.L. Comparison and assessment of methods for cellulose crystallinity determination. Chem. Soc. Rev. 2023, 52, 6417–6446. [Google Scholar] [CrossRef]
- Mansour, S.M.; Shamma, R.N.; Ahmed, K.A.; Sabry, N.A.; Esmat, G.; Mahmoud, A.A.; Maged, A. Safety of inhaled ivermectin as a repurposed direct drug for treatment of COVID-19: A preclinical tolerance study. Int. Immunopharmacol. 2021, 99, 108004. [Google Scholar] [CrossRef] [PubMed]
- Langford, J.I.; Wilson, A.J.C. Scherrer after Sixty Years: A Survey and Some New Results in the Determination of Crystallite Size. J. Appl. Cryst. 1978, 11, 102–113. [Google Scholar] [CrossRef]
- USP 40-NF 35; The United States Pharmacopeia. United States Pharmacopeial Convention: Rockville, MD, USA, 2017.
- Muselík, J.; Komersová, A.; Kubová, K.; Matzick, K.; Skalická, B. A Critical Overview of FDA and EMA Statistical Methods to Compare In Vitro Drug Dissolution Profiles of Pharmaceutical Products. Pharmaceutics 2021, 13, 1703. [Google Scholar] [CrossRef]
- Costa, P.; Sousa Lobo, J.M. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Al Hossain, A.S.M.M.; Koly, F.J.; Roy, S.C.; Kumar, U.; Hasanuzzaman, M.D.; Islam, D. Preparation, Characterization and In vitro Evaluation of Solid Dispersion Formulation of Clopidogrel Using Hydrophilic Polymers. Dhaka Univ. J. Pharm. Sci. 2024, 23, 123–134. [Google Scholar] [CrossRef]
- Russo, J.; Fiegel, J.; Brogden, N.K. Rheological and Drug Delivery Characteristics of Poloxamer-Based Diclofenac Sodium Formulations for Chronic Wound Site Analgesia. Pharmaceutics 2020, 12, 1214. [Google Scholar] [CrossRef]
- Zhang, Y.; Huo, M.; Zhou, J.; Zou, A.; Weize Li, W.; Yao, C.; Xie, S. DDSolver: An Add-In Program for Modeling and Comparison of Drug Dissolution Profiles. AAPS J. 2010, 12, 263–271. [Google Scholar] [CrossRef]
- Na-Bangchang, K.; Banmairuroi, V.; Choemung, A. High-performance liquid chromatographic method for the determination of ivermectin in plasma. Southeast. Asian J. Trop. Med. Public Health 2006, 37, 848–858. [Google Scholar]
- HPLC Method for Ivermectin Determination. Available online: https://www.dikmatech.com/Application/show/id/805 (accessed on 5 June 2025).
- International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. ICH Harmonised Guideline: Validation of Analytical Procedures: Text and Methodology Q2(R2) 2023. Available online: https://www.ich.org/page/quality-guidelines (accessed on 8 July 2025).
- Bhujbal, S.V.; Mitra, B.; Jain, U.; Gong, Y.; Agrawal, A.; Karki, S.; Taylor, L.; Kumar, S.; Zhou, Q. Pharmaceutical amorphous solid dispersion: A review of manufacturing strategies. Acta Pharm. Sin. B 2021, 11, 2505–2536. [Google Scholar] [CrossRef]
- Lu, M.; Xiong, D.; Sun, W.; Yu, T.; Hu, Z.; Ding, J.; Cai, Y.; Yang, S.; Pan, B. Sustained release ivermectin-loaded solid lipid dispersion for subcutaneous delivery: In vitro and in vivo evaluation. Drug Deliv. 2017, 24, 622–631. [Google Scholar] [CrossRef] [PubMed]
- Magellan Laboratories Incorporated. Purification Process for Anti-Parasitic Fermentation Product. U.S. Patent No. US6265571B1, 24 July 2001. Available online: https://patents.google.com/patent/US6265571B1/ (accessed on 10 July 2025).
- Ly, H.Q.; Chen, Y.-J.; Nguyen, V.T.; Tseng, C.-L. Optimization of the poloxamer 407-conjugated gelatin to synthesize pH-sensitive nanocarriers for controlled paclitaxel delivery. J. Polym. Res. 2025, 32, 38. [Google Scholar] [CrossRef]
- Ponnusamy, C.; Sugumaran, A.; Krishnaswami, V.; Palanichamy, R.; Velayutham, R.; Natesan, S. Development and Evaluation of Polyvinylpyrrolidone K90 and Poloxamer 407 Self-Assembled Nanomicelles: Enhanced Topical Ocular Delivery of Artemisinin. Polymers 2021, 13, 3038. [Google Scholar] [CrossRef]
- Rolim, L.A.; dos Santos, F.C.M.; Chaves, L.L.; Gonçalves, M.L.C.M.; Freitas-Neto, J.L.; do Nascimento, A.L.S.; Soares-Sobrinho, J.L.; de Albuquerque, M.M.; de Lima, M.C.A.; Rolim-Neto, P.J. Preformulation study of ivermectin raw material. J. Therm. Anal. Calorim. 2013, 120, 807–816. [Google Scholar] [CrossRef]
- Real, D.; Orzan, L.; Leonardi, D.; Salomon, C.J. Improving the Dissolution of Triclabendazole from Stable Crystalline Solid Dispersions Formulated for Oral Delivery. AAPS Pharm. Sci. Tech. 2019, 21, 16. [Google Scholar] [CrossRef]
- Ghareeb, M.M.; Abdulrasool, A.A.; Hussein, A.A.; Noordin, M.I. Kneading Technique for Preparation of Binary Solid Dispersion of Meloxicam with Poloxamer 188. AAPS Pharm. Sci. Tech. 2009, 10, 1206–1215. [Google Scholar] [CrossRef]
- Velho, M.C.; Funk, N.L.; Deon, M.; Benvenutti, E.V.; Buchner, S.; Hinrichs, R.; Pilger, D.A.; Beck, R.C.R. Ivermectin-Loaded Mesoporous Silica and Polymeric Nanocapsules: Impact on Drug Loading, In Vitro Solubility Enhancement, and Release Performance. Pharmaceutics 2024, 16, 325. [Google Scholar] [CrossRef]
- Li, W.L.; Zhou, C.R.; Zhang, L. Investigation on the Decomposition Kinetics of Ivermectin. J. Therm. Anal. Calorim. 2015, 121, 797–806. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, Z.; Luo, L.; Li, K.; Zi, T.; Ren, J.; Pan, L.; Wang, Z.Y.; Wang, Z.H.; Liu, M.; et al. Impact of Poloxamer on Crystal Nucleation and Growth of Amorphous Clotrimazole. Pharmaceutics 2023, 15, 2164. [Google Scholar] [CrossRef] [PubMed]
- Simonazzi, A.; Cid, A.G.; Paredes, A.J.; Schofs, L.; Gonzo, E.E.; Palma, S.D.; Bermúdez, J.M. Development and in vitro evaluation of solid dispersions as strategy to improve albendazole biopharmaceutical behavior. Ther. Deliv. 2018, 9, 623–638. [Google Scholar] [CrossRef] [PubMed]
- El-Badry, M.; Hassan, M.A.; Ibrahim, M.A.; Elsaghir, H. Performance of poloxamer 407 as hydrophilic carrier on the binary mixtures with nimesulide. Farmacia 2013, 61, 1137–1150. [Google Scholar]
- Medarević, D.P.; Kachrimanis, K.; Mitrić, M.; Đuriš, J.; Đurić, Z.; Ibrić, S. Dissolution rate enhancement and physicochemical characterization of carbamazepine-poloxamer solid dispersions. Pharm. Dev. Technol. 2015, 21, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Barzegar-Jalali, M.; Ghanbarzadeh, S.; Adibkia, K.; Valizadeh, H.; Bibak, S.; Mohammadi, G.; Siahi-Shadbad, M.R. Development and characterization of solid dispersion of piroxicam for improvement of dissolution rate using hydrophilic carriers. BioImpacts 2014, 4, 141–148. [Google Scholar] [CrossRef]
- Gutiérrez-Saucedo, R.A.; Gómez-López, J.C.; Villanueva-Briseño, A.A.; Topete, A.; Soltero-Martínez, J.F.A.; Mendizábal, E.; Jasso-Gastinel, C.F.; Taboada, P.; Figueroa-Ochoa, E.B. Pluronic F127 and P104 Polymeric Micelles as Efficient Nanocarriers for Loading and Release of Single and Dual Antineoplastic Drugs. Polymers 2023, 15, 2249. [Google Scholar] [CrossRef]
- Hosny, K.M. Nanosized Cubosomal Thermogelling Dispersion Loaded with Saquinavir Mesylate to Improve Its Bioavailability: Preparation, Optimization, in vitro and in vivo Evaluation. Int. J. Nanomed. 2020, 16, 5113–5129. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and zeta potential—What they are and what they are not? J. Control. Release 2016, 325, 337–351. [Google Scholar] [CrossRef]
- Adeli, E. Preparation and evaluation of azithromycin binary solid dispersions using various polyethylene glycols for the improvement of the drug solubility and dissolution rate. Braz. J. Pharm. Sci. 2016, 52, 1–14. [Google Scholar] [CrossRef]
- Trucillo, P. Drug Carriers: A Review on the Most Used Mathematical Models for Drug Release. Processes 2022, 10, 1094. [Google Scholar] [CrossRef]
- Deb, P.K.; Kokaz, S.F.; Abed, S.N.; Paradkar, A.; Tekade, R.K. Pharmaceutical and Biomedical Applications of Polymers. In Advances in Pharmaceutical Product Development and Research, Basic Fundamentals of Drug Delivery, 1st ed.; Tekade, R.K., Ed.; Academic Press: Cambridge, MA, USA, 2019; Volume 6, pp. 203–267. [Google Scholar] [CrossRef]
| Kinetic Model | Equation |
|---|---|
| Zero-order | Q = k0 × t |
| First-order | ln(100 − Q) = ln Q0 − k1 × t |
| Higuchi | Q = kH × t1/2 |
| Korsemyer–Peppas | Q = kP × tn |
| Polymeric Excipient | Percentage (w/v) | IVM Solubility (µg/mL) | Solubility Increased (S/S0) |
|---|---|---|---|
| PEG6000 | 1 | 0.8 ± 0.2 | 0.3 |
| 2 | 2.4 ± 0.5 | 0.9 | |
| 5 | 4.3 ± 0.2 | 1.7 | |
| PEG8000 | 1 | 2.7 ± 0.2 | 1.0 |
| 2 | 1.9 ± 0.3 | 0.7 | |
| 5 | 5 ± 1 | 1.9 | |
| PVP-k30 | 1 | 25 ± 4 | 9.5 |
| 2 | 32 ± 3 | 12.4 | |
| 5 | 99 ± 5 | 38.0 | |
| PVP-k90 | 1 | 4.2 ± 0.3 | 1.6 |
| 2 | 20.0 ± 0.4 | 7.7 | |
| 5 | 9.3 ± 0.9 | 3.6 | |
| P188 | 1 | 16 ± 1 | 6.2 |
| 2 | 14 ± 3 | 5.5 | |
| 5 | 30 ± 1 | 11.7 | |
| P407 | 1 | (22 ± 1) × 102 | 833.8 |
| 2 | (73 ± 1) × 101 | 282.3 | |
| 5 | 147 ± 2 | 56.5 | |
| Sorbitol | 1 | 12.0 ± 0.1 | 4.6 |
| 2 | 3.4 ± 0.5 | 1.3 | |
| 5 | 1.6 ± 0.5 | 0.6 |
| Drug:P407 (w/w) | Cooling Ramp | Final Temperature (°C) | Drug Content (%) | |
|---|---|---|---|---|
| SD1 | 1:1 | Rapid | 0 | 99 ± 1 |
| SD2 | 1:1 | Rapid | 8.4 | 100.1 ± 0.3 |
| SD3 | 1:2 | Rapid | 0 | 99.5 ± 0.1 |
| SD4 | 1:2 | Rapid | 8.4 | 100.3 ± 0.8 |
| SD5 | 1:1 | Intermediate | 0 | 100 ± 1 |
| SD6 | 1:1 | Intermediate | 8.4 | 100.9 ± 0.4 |
| SD7 | 1:1 | Slow | 0 | 98.8 ± 0.2 |
| SD8 | 1:1 | Slow | 8.4 | 99.7 ± 0.7 |
| Samples | IVM | P407 | ||
|---|---|---|---|---|
| CI (%) | Crystallite Size (nm) | CI (%) | Crystallite Size (nm) | |
| IVM | 29.0 | 57.9 | - | - |
| P407 | - | - | 62.4 | 22.0 |
| PM1:1 | 16.1 | 57.3 | 24.3 | 23.3 |
| PM1:2 | 5.3 | 57.4 | 36.6 | 20.0 |
| SD1 | 5.2 | 44.5 | 29.3 | 20.1 |
| SD2 | 5.3 | 50.0 | 30.3 | 20.5 |
| SD3 | 5.2 | 43.4 | 39.5 | 21.6 |
| SD4 | 5.3 | 51.6 | 36.5 | 21.4 |
| SD5 | 5.6 | 50.2 | 32.8 | 20.9 |
| SD6 | 5.4 | 51.6 | 33.1 | 21.6 |
| SD7 | 5.3 | 56.3 | 33.5 | 21.8 |
| SD8 | 6.0 | 56.6 | 33.9 | 22.4 |
| Sample | Size (nm) | PDI | ZP |
|---|---|---|---|
| P407 | 26 ± 1 | 0.39 ± 0.04 | 5.7 ± 0.5 |
| P407.1 | 25 ± 1 | 0.34 ± 0.03 | 5.5 ± 0.7 |
| P407.2 | 25.2 ± 0.9 | 0.34 ± 0.05 | 5.1 ± 0.7 |
| SD1 | 32 ± 1 | 0.37 ± 0.03 | 22.1 ± 0.4 |
| SD2 | 32.9 ± 0.5 | 0.46 ± 0.04 | 19 ± 1 |
| SD3 | 31.7 ± 0.8 | 0.37 ± 0.05 | 22.4 ± 0.9 |
| SD4 | 32.2 ± 0.1 | 0.43 ± 0.05 | 21 ± 1 |
| Medium | H2O | SGF | ||
|---|---|---|---|---|
| Sample | Solubility (µg/mL) | Solubility Increased (S/S0) | Solubility (µg/mL) | Solubility Increased (S/S0) |
| IVM | 2.6 ± 0.4 | - | 4.80 ± 0.02 | - |
| PM1:1 | 4671 ± 53 | 1797 | 6521 ± 553 | 1359 |
| PM1:2 | 3619 ± 553 | 1392 | 6351 ± 724 | 1323 |
| SD1 | 4207 ± 54 | 1618 | 5267 ± 156 | 1097 |
| SD2 | 4135 ± 404 | 1590 | 5153 ± 98 | 1074 |
| SD3 | 4055 ± 563 | 1560 | 4120 ± 243 | 858 |
| SD4 | 3482 ± 433 | 1399 | 4203 ± 85 | 876 |
| SD5 | 1450 ± 190 | 558 | 5210 ± 105 | 1085 |
| SD6 | 602 ± 48 | 232 | 5052 ± 101 | 1053 |
| SD7 | 529 ± 40 | 203 | 4983 ± 256 | 1038 |
| SD8 | 490 ± 75 | 188 | 4296 ± 177 | 895 |
| Sample | Percentage of Dissolution | f2 |
|---|---|---|
| IVM | 15.0 ± 0.8 | - |
| PM1:1 | 35.5 ± 0.8 | 40.1 |
| PM1:2 | 31 ± 1 | 46.7 |
| SD1 | 53 ± 2 | 32.5 |
| SD2 | 49 ± 5 | 35.9 |
| SD3 | 54 ± 2 | 37.2 |
| SD4 | 53 ± 3 | 30.7 |
| Samples | Zero Order | First Order | Higuchi | Korsmeyer–Peppas | |||||
|---|---|---|---|---|---|---|---|---|---|
| k (mg/h) | r2 | k (mg/h) | r2 | k (mg/h) | r2 | k (mg/h) | r2 | n | |
| SD1 | 0.283 | 0.939 | 0.003 | 0.952 | 1.839 | 0.951 | 1.172 | 0.988 | 0.701 |
| SD2 | 0.297 | 0.921 | 0.003 | 0.936 | 1.922 | 0.965 | 1.062 | 0.990 | 0.659 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mezzano, B.A.; Bueno, M.S.; Fuertes, V.C.; Longhi, M.R.; Garnero, C. Poloxamer-Based Biomaterial as a Pharmaceutical Strategy to Improve the Ivermectin Performance. Pharmaceutics 2025, 17, 1101. https://doi.org/10.3390/pharmaceutics17091101
Mezzano BA, Bueno MS, Fuertes VC, Longhi MR, Garnero C. Poloxamer-Based Biomaterial as a Pharmaceutical Strategy to Improve the Ivermectin Performance. Pharmaceutics. 2025; 17(9):1101. https://doi.org/10.3390/pharmaceutics17091101
Chicago/Turabian StyleMezzano, Belén Alejandra, Maria Soledad Bueno, Valeria Cintia Fuertes, Marcela Raquel Longhi, and Claudia Garnero. 2025. "Poloxamer-Based Biomaterial as a Pharmaceutical Strategy to Improve the Ivermectin Performance" Pharmaceutics 17, no. 9: 1101. https://doi.org/10.3390/pharmaceutics17091101
APA StyleMezzano, B. A., Bueno, M. S., Fuertes, V. C., Longhi, M. R., & Garnero, C. (2025). Poloxamer-Based Biomaterial as a Pharmaceutical Strategy to Improve the Ivermectin Performance. Pharmaceutics, 17(9), 1101. https://doi.org/10.3390/pharmaceutics17091101








