Methods of Thermal Analysis as Fast and Reliable Tools for Identification and Quantification of Active Ingredients in Commercially Available Drug Products
Abstract
1. Introduction
2. Methods of Thermal Analysis
2.1. Differential Thermal Analysis (DTA)
2.2. Differential Scanning Calorimetry (DSC)
2.3. Thermogravimetric Analysis (TGA)
3. Thermal Analysis of Drug Products
4. Qualitative Analysis of Drug Products
4.1. Distinguishing Between Drug Products
4.2. Identification of Drug Product Ingredients
5. Quantification of APIs in Drug Products
5.1. DSC Measurements
5.2. TGA Measurements
6. Analysis of Non-Compliant Drug Products
7. General Remarks on Thermal Methods
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| API | Active pharmaceutical ingredients |
| DSC | Differential scanning calorimetry |
| DTA | Differential thermal analysis |
| TGA | Thermogravimetric analysis |
References
- World Health Organization. WHO Standards for Quality, Safety and Efficacy of Health Products: Stakeholder Feedback Report; World Health Organization: Geneva, Switzerland, 2023; Available online: https://iris.who.int/bitstream/handle/10665/372265/9789240076358-eng.pdf (accessed on 15 July 2025).
- ICH Official Web Site: ICH. Available online: https://www.ich.org (accessed on 15 July 2025).
- ICH Topic Q 6 A; Specifications: Test Procedures and Acceptance Criteria for New Drug Substances and New Drug Products: Chemical Substances. European Medicines Agency: London, UK, 2000. Available online: https://www.ema.europa.eu/en/ich-q6a-specifications-test-procedures-acceptance-criteria-new-drug-substances-new-drug-products-chemical-substances-scientific-guideline (accessed on 15 July 2025).
- WHO. Definition of Active Pharmaceutical Ingredient. Available online: https://da7648.approby.com/m/a6ae2665fe1326ef.pdf (accessed on 15 July 2025).
- Maleki, A.; Karimpour, A.R.; MalekiDizaj, S. The role of mechanical engineering in the development of nano drug delivery systems; A review. Int. J. Nano Dimens. 2018, 9, 1–6. [Google Scholar]
- World Health Organization. WHO Expert Committee on Specification for Pharmaceutical Preparations. Annex 2. Stability Testing of Active Pharmaceutical Ingredients and Finished Pharmaceutical Products; World Health Organization: Geneva, Switzerland, 2009; Report No. 953; Available online: https://www.who.int/publications/i/item/WHO_TRS_953 (accessed on 15 July 2025).
- Council of Europe. European Pharmacopoeia, 11th ed.; Council of Europe: Strasbourg, France, 2022; Volume 1. [Google Scholar]
- Yoshioka, S.; Stella, V.J. Stability of Drugs and Dosage Forms; Kluwer Academic Publisher: New York, NY, USA, 2002. [Google Scholar]
- Zając, M.; Jelińska, A. (Eds.) Quality Assessment of Drug Substances and Products; Karol Marcinkowski Medical University: Poznan, Poland, 2010; pp. 23–32. [Google Scholar]
- Ahmed, N.R. HPLC method for determination of paracetamol in pharmaceutical formulations and environmental water samples. Chem. Sci. Transact. 2019, 8, 237–243. [Google Scholar] [CrossRef]
- Gupta, D.; Bhardwaj, S.; Sethi, S.; Pramanik, S.; Das, D.K.; Kumar, R.; Singh, P.P.; Vashistha, V.K. Simultaneous spectrophotometric determination of drug components from their dosage formulations. Spectrochim. Acta Part A 2022, 270, 120819. [Google Scholar] [CrossRef]
- Tajik, S.; Dourandish, Z.; Nejad, F.G.; Afshar, A.A.; Beitollahi, H. Voltammetric determination of isoniazid in the presence of acetaminophen utilizing MoS2-nanosheet-modified screen-printed electrode. Micromachines 2022, 13, 369. [Google Scholar] [CrossRef]
- Wesolowski, M. Analysis of drug formulations by thermal decomposition. Thermochim. Acta 1992, 209, 223–251. [Google Scholar] [CrossRef]
- Saunders, M. Thermal analysis of pharmaceuticals. In Principles and Applications of Thermal Analysis; Gabbott, P., Ed.; Blackwell Publishing: Oxford, UK, 2008; pp. 286–329. [Google Scholar]
- Wesolowski, M. DSC of low molecular mass organic materials and pharmaceuticals. In Handbook of Differential Scanning Calorimetry: Techniques, Instrumentation, Inorganic, Organic and Pharmaceutical Substances; Menczel, J., Grebowicz, J., Eds.; Elsevier: Cambridge, MA, USA, 2023; pp. 485–658. [Google Scholar]
- Lever, T.; Haines, P.; Rouquerol, J.; Charsley, E.L.; Van Eckern, P.; Burlett, D.J. ICTAC nomenclature of thermal analysis (IUPAC Recommendations 2014). Pure Appl. Chem. 2014, 86, 545–553. [Google Scholar] [CrossRef]
- Wesolowski, M.; Leyk, E. Coupled and simultaneous thermal analysis techniques in the study of pharmaceuticals. Pharmaceutics 2023, 15, 1596. [Google Scholar] [CrossRef]
- Gabbott, P. (Ed.) Principles and Applications of Thermal Analysis; Blackwell Publishing: Oxford, UK, 2008. [Google Scholar]
- Menczel, J.D.; Grebowicz, J. (Eds.) Handbook of Differential Scanning Calorimetry: Techniques, Instrumentation, Inorganic, Organic and Pharmaceutical Substances; Elsevier: Cambridge, MA, USA, 2023. [Google Scholar]
- Rowe, R.C.; Sheskey, P.J.; Quinn, M.E. Handbook of Pharmaceutical Excipients, 6th ed.; Pharmaceutical Press: London, UK, 2009. [Google Scholar]
- Wesolowski, M. The influence of various tablet components on thermal decomposition of some pharmaceuticals. Microchim. Acta 1980, 73, 199–213. [Google Scholar] [CrossRef]
- Gucluyildiz, H.; Goodhart, F.W.; Ninger, F.C. Rapid determination of nitroglycerin volatility using thermogravimetric analysis. J. Pharm. Sci. 1977, 66, 265–266. [Google Scholar] [CrossRef]
- Wendlandt, W.W.; Collins, L.W. The identification of non-prescription internal analgesics by thermal analysis. Anal. Chim. Acta 1974, 71, 411–417. [Google Scholar] [CrossRef]
- Wendlandt, W.W. The thermal analysis of some non-prescription antacids. Thermochim. Acta 1974, 10, 93–99. [Google Scholar] [CrossRef]
- Collins, L.W.; Wendlandt, W.W. The thermal analysis of some non-prescription vitamin preparations. Thermochim. Acta 1975, 11, 253–260. [Google Scholar] [CrossRef]
- Brecht, D.; Uteschil, F.; Schmitz, O.J. Thermogravimetry coupled to an atmospheric pressure photo ionization quadrupole mass spectrometry for the product control of pharmaceutical formulations and the analysis of plasticizers in polymers. Talanta 2019, 198, 440–446. [Google Scholar] [CrossRef]
- Ramos, P. Application of thermal analysis to evaluate pharmaceutical preparations containing theophylline. Pharmaceuticals 2022, 15, 1268. [Google Scholar] [CrossRef]
- Ramos, P.; Raczak, B.K.; Silvestri, D.; Wacławek, S. Application of TGA/c-DTA for distinguishing between two forms of naproxen in pharmaceutical preparations. Pharmaceutics 2023, 15, 1689. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Z.; Bao, M.; Yang, S.; Cai, M.; Lan, L.; Sun, W.; Sun, G. Comprehensive quality evaluation of Huricha Liuwei Pills using multidimensional fingerprinting and multicomponent quantification. J. Pharm. Biomed. Anal. 2025, 263, 116925. [Google Scholar] [CrossRef]
- Pyramides, G.; Robinson, J.W.; Zito, S.W. The combined use of DSC and TGA for the thermal analysis of atenolol tablets. J. Pharm. Biomed. Anal. 1995, 13, 103–110. [Google Scholar] [CrossRef]
- Giron, D.; Goldbronn, C. Use of DSC and TG for identification and quantification of the dosage form. J. Therm. Anal. 1997, 48, 473–483. [Google Scholar] [CrossRef]
- Wesolowski, M.; Szynkaruk, P.; Makurat, E. DSC and IR as supporting tools for identification of methylxanthines in solid dosage forms of drugs. J. Therm. Anal. Calorim. 2012, 109, 807–815. [Google Scholar] [CrossRef]
- Tkachenko, M.L.; Zhnyakina, L.E.; Kosmynin, A.S. Physicochemical investigation of paracetamol—Caffeine solid mixtures. Pharm. Chem. J. 2003, 37, 430–432. [Google Scholar] [CrossRef]
- Bi, M.; Hwang, S.J.; Morris, K.R. Mechanism of eutectic formation upon compaction and its effects on tablet properties. Thermochim. Acta 2003, 404, 213–226. [Google Scholar] [CrossRef]
- Wesolowski, M.; Leyk, E.; Szynkaruk, P. Detection of magnesium compounds in dietary supplements and medicinal products by DSC, Infrared and Raman techniques. J. Therm. Anal. Calorim. 2014, 116, 671–680. [Google Scholar] [CrossRef]
- Miltyk, W.; Antonowicz, E.; Komsta, Ł. Recognition of tablet content by chemometric processing of differential scanning calorimetry curves—An acetaminophen example. Thermochim. Acta 2010, 507–508, 146–149. [Google Scholar] [CrossRef]
- Otto, M. Chemometrics. Statistics and Computer Application in Analytical Chemistry, 4th ed.; Wiley-VCH: New York, NY, USA, 2023. [Google Scholar]
- Cook, L.E.; Hildebrand, D.A. The thermogravimetry of sulfanilamide and related sulfa drugs. Thermochim. Acta 1974, 9, 129–133. [Google Scholar] [CrossRef]
- Khattab, F.I.; Amer, M.M.; Hassan, N.Y.M. Thermal analysis of pharmaceutical compounds. IV. Evaluation of sulphonamides by thermal analysis. J. Therm. Anal. 1982, 25, 367–375. [Google Scholar] [CrossRef]
- Becket, G.; Quah, S.B.; Hill, J.O. A DSC compositional analysis of some binary organic mixtures of pharmaceutical significance. J. Therm. Anal. 1993, 40, 537–542. [Google Scholar] [CrossRef]
- Blader Ceipidor, U.; Curini, R.; D’Ascenzo, G.; Tomassetti, M. Cholic acid determination from heats of fusion obtained using differential scanning calorimetry. Application to pharmaceutical products. Thermochim. Acta 1981, 46, 279–287. [Google Scholar] [CrossRef]
- Marino, A.; Bicchieri, M.; Curini, R.; D’Ascenzo, G.; Carunchio, V. Quantitative analysis of some bile acids by differential scanning calorimetry combined with TLC. Thermochim. Acta 1981, 46, 289–294. [Google Scholar] [CrossRef]
- D’Ascenzo, G.; Curini, R.; Marino, A.; De Angelis, G.; Carunchio, V. Differential scanning calorimetry as a tool for obtaining quantitative data on thin layer chromatography. Thermochim. Acta 1980, 39, 181–185. [Google Scholar] [CrossRef]
- Blader Ceipidor, U.; Curini, R.; D’Ascenzo, G.; Tomassetti, M. Analytical comparison of calorimetric, enzymatic and chemical methods for the quantitative determination of cholic acid. Thermochim. Acta 1981, 46, 269–278. [Google Scholar] [CrossRef]
- Bucci, R.; Magri, A.D.; Magri, A.L. Determination of diclofenac salts in pharmaceutical formulations. Fres. J. Anal. Chem. 1998, 362, 577–582. [Google Scholar] [CrossRef]
- Bucci, R.; Magri, A.D.; Magri, A.L. DSC in the chemical analysis of drugs. Determination of diclofenac in pharmaceutical formulations. J. Therm. Anal. Calorim. 2000, 61, 369–376. [Google Scholar] [CrossRef]
- Campanella, L.; Magri, A.L.; Tomassetti, M. Quantitative determination of acetaminophen in pharmaceutical formulations using differential scanning calorimetry. Comparison with spectrophotometric method. Drug. Dev. Ind. Pharm. 2007, 33, 830–840. [Google Scholar] [CrossRef] [PubMed]
- Talik, P.; Żuromska-Witek, B.; Hubicka, U.; Krzek, J. The use of the DSC method in quantification of active pharmaceutical ingredients in commercially available one component tablets. Acta Pol. Pharm.—Drug Res. 2017, 74, 1049–1055. [Google Scholar]
- Talik, P.; Czerniecka, E.; Hubicka, U.; Krzek, J. Quantification of active pharmaceutical ingredients in commercially available poly pharmaceutical tablets by means of DSC. Acta Pol. Pharm.—Drug Res. 2017, 74, 1057–1062. [Google Scholar]
- Leyk, E.; Komar, S.; Wesolowski, M. Determination of paracetamol in commercial suppositories using differential scanning calorimetry. Acta Pol. Pharm.–Drug Res. 2019, 76, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Noordin, M.I.; Chung, L.Y. A rapid micro quantification method of paracetamol in suppositories using differential scanning calorimetry. Drug Dev. Ind. Pharm. 2004, 30, 925–930. [Google Scholar] [CrossRef]
- Campanella, L.; Micieli, V.; Tomassetti, M.; Vecchio, S. Quantitative determination of acetylsalicylic acid in commercial drugs using DSC. Comparison with titration and UV spectrophotometric methods. J. Therm. Anal. Calorim. 2010, 102, 249–259. [Google Scholar] [CrossRef]
- Boiko, B.N.; Kolpakov, I.M. DSC monitoring of piracetam concentration stability during experimental storage. Pharm. Chem. J. 2011, 45, 309–312. [Google Scholar] [CrossRef]
- Boumrah, Y.; Bouzahia, I.; Bouanani, S.; Khimeche, K.; Dahmani, A. Thermodynamic and analytical studies of drugs binary systems of paracetamol mixed with pseudoephedrine.HCI, dextropropoxyphene.HCI and tramadol.HCI. Thermochim. Acta 2016, 634, 48–56. [Google Scholar] [CrossRef]
- Souza, S.P.M.C.; Morais, F.E.; Santos, E.V.; Martinez-Huitle, C.A.; Fernandes, N.S. Determination of calcium content in tables for treatment of osteoporosis using thermogravimetry (TG). J. Therm. Anal. Calorim. 2013, 111, 1965–1970. [Google Scholar] [CrossRef]
- Otero, R.L.S.; Galvão, R.K.H.; Araújo, M.C.; Cavalheiro, É.T.G. Thermogravimetric determination of L-ascorbic acid in non-effervescent formulations using multiple linear regression with temperature selection by the successive projections algorithm. Thermochim. Acta 2011, 526, 200–204. [Google Scholar] [CrossRef]
- Khanmohammadi, M.; Soleimani, M.; Morovvat, F.; Bagheri Garmarudi, A.; Khalafbeigi, M.; Ghasemi, K. Simultaneous determination of paracetamol and codeine phosphate in tablets by TGA and chemometrics. Thermochim. Acta 2012, 530, 128–132. [Google Scholar] [CrossRef]
- Gomes, A.P.B.; Correia, L.P.; Silva Simões, M.O.; Macědo, R.O. Development of thermogravimetric method to quantitative determination of mebendazole. J. Therm. Anal. Calorim. 2007, 87, 919–925. [Google Scholar] [CrossRef]
- Gomes, A.P.B.; Correia, L.P.; Silva Simǒes, M.O.; Macědo, R.O. Development of thermogravimetric method for quantitative determination of ketoconazole. J. Therm. Anal. Calorim. 2008, 91, 317–321. [Google Scholar] [CrossRef]
- Silva Portela, A.; Graças Almeida, M.; Gomes, A.P.B.; Correia, L.P.; Silva, P.C.D.; Neto, A.N.M.; Medeiros, A.C.D.; Simões, M.O.S. Vapor pressure curve determination of α-lipoic acid raw material and capsules by dynamic thermogravimetric method. Thermochim. Acta 2012, 544, 95–98. [Google Scholar] [CrossRef]
- Almaghrabi, M.; Alqurshi, A.; Jadhav, S.A.; Mazzacuva, F.; Cilibrizzi, A.; Raimi-Abraham, B.; Royall, P.G. Evaluating thermogravimetric analysis for the measurement of drug loading in mesoporous silica nanoparticles (MSNs). Thermochim. Acta 2023, 730, 179616. [Google Scholar] [CrossRef]
- Reddy, V.R.; Rajmohan, M.A.; Shilpa, R.L.; Raut, D.M.; Naveenkumar, K.; Suryanarayana, M.V.; Mathad, V.T. A novel quantification method of pantaprazole sodium monohydrate in sesquihydrate by thermogravimetric analyzer. J. Pharm. Biomed. Anal. 2007, 43, 1836–1841. [Google Scholar] [CrossRef]
- Medeiros, R.I.; Filho, N.R.A.; Leles, M.I.G. Development of forensic analytical chemistry method for examination of Merla by thermal analysis and high resolution gas chromatography. J. Therm. Anal. Calorim. 2009, 97, 337–342. [Google Scholar] [CrossRef]
- Curini, R.; Zamponi, S.; D’Ascenzo, F.; Angelis Curtis, S.; Marino, A.; Dezzi, A. Thermal analytical techniques applied to the narcotic field: Cocaine analysis. Thermochim. Acta 1989, 153, 11–26. [Google Scholar] [CrossRef]
- Boumrah, Y.; Bouanani, S.; Khimeche, K.; Dahmani, A. Analysis of synthetic drugs by differential scanning calorimetry. Case of amphetamine-type stimulants (ATS). J. Therm. Anal. Calorim. 2015, 120, 583–590. [Google Scholar] [CrossRef]
- Bibi, S.; Bremner, H.; Macdougall-Heasman, M.; Reid, R.; Simpson, K.; Tough, A.; Waddell, S.; Stewart, I.J.; Matthews, K.H. A preliminary investigation to group disparate batches of licit and illicit diazepam tablets using differential scanning calorimetry. Anal. Methods 2015, 7, 8597–8604. [Google Scholar] [CrossRef]
- Maria, J.; Noordin, M.I. Fast detection of sildenafil in adulterated commercial products using differential scanning calorimetry. J. Therm. Anal. Calorim. 2014, 115, 1907–1914. [Google Scholar] [CrossRef]
- Santos, M.K.; Mariotti, K.; Kahmann, A.; Anzanello, M.J.; Ferrão, M.F.; Gomes, A.; Limberger, R.P.; Ortiz, R. Comparison between counterfeit and authentic medicines: A novel approach using differential scanning calorimetry and hierarchical cluster analysis. J. Pharm. Biomed. Anal. 2019, 166, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.H.; Catelani, T.A.; Costa, E.D.M.; Garcia, J.S.; Trevisan, M.G. Coffee adulterant quantification by derivative thermogravimetry and chemometric analysis. J. Therm. Anal. Calorim. 2022, 147, 7353–7362. [Google Scholar] [CrossRef]
- ICH Harmonized Tripartite Guideline. Validation of Analytical Procedures: Text and Methodology Q2(R1). Available online: https://database.ich.org/sites/default/files/Q2%28R1%29%20Guideline.pdf (accessed on 15 August 2025).
- Chambi, J.T.; Fandaruff, C.; Cuffini, S.L. Identification and quantification techniques of polymorphic forms—A review. J. Pharm. Biomed. Anal. 2024, 242, 116038. [Google Scholar] [CrossRef]
- Herbrink, M.; Vromans, H.; Schellens, J.; Beijnen, J.; Nuijen, B. Thermal stability study of crystalline and novel spray-dried amorphous nilotinib hydrochloride. J. Pharm. Biomed. Anal. 2018, 148, 182–188. [Google Scholar] [CrossRef]
- Pan, Y.; Pang, W.; Lv, J.; Wang, J.; Yang, C.; Guo, W. Solid state characterization of azelnidipine-oxalic acid co-crystal and co-amorphous complexes: The effect of different azelnidipine polymorphs. J. Pharm. Biomed. Anal. 2017, 138, 302–315. [Google Scholar] [CrossRef]
- Dedroog, S.; Pas, T.; Vergauwen, B.; Huygens, C.; Van den Mooter, G. Solid-state analysis of amorphous solid dispersions: Why DSC and XRPD may not be regarded as stand-alone techniques. J. Pharm. Biomed. Anal. 2020, 178, 112937. [Google Scholar] [CrossRef]
- Baghel, S.; Cathcart, H.; O’Reilly, N. Understanding the generation and maintenance of supersaturation during the dissolution of amorphous solid dispersions using modulated DSC and 1H NMR. Int. J. Pharm. 2018, 536, 414–425. [Google Scholar] [CrossRef] [PubMed]
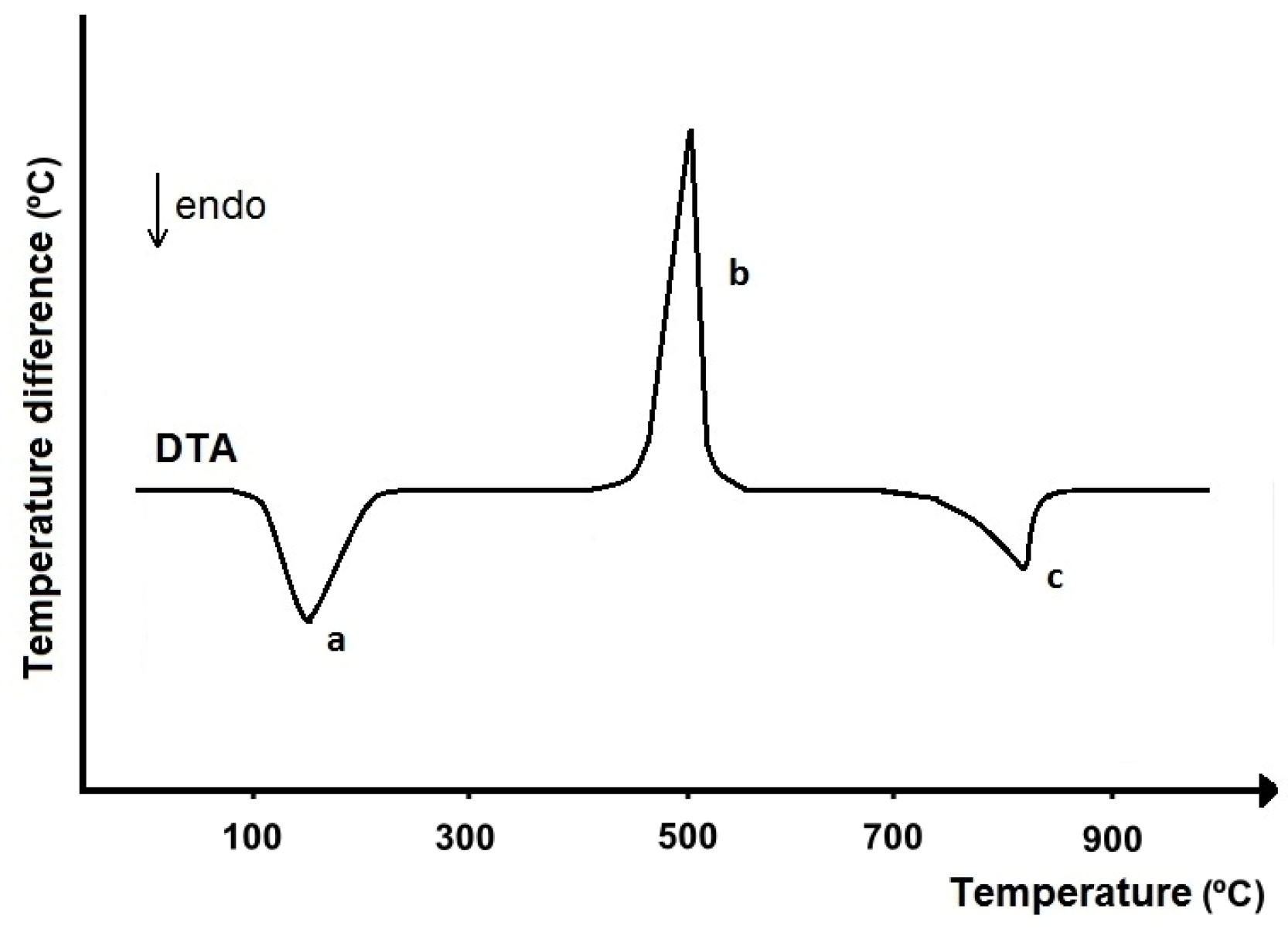

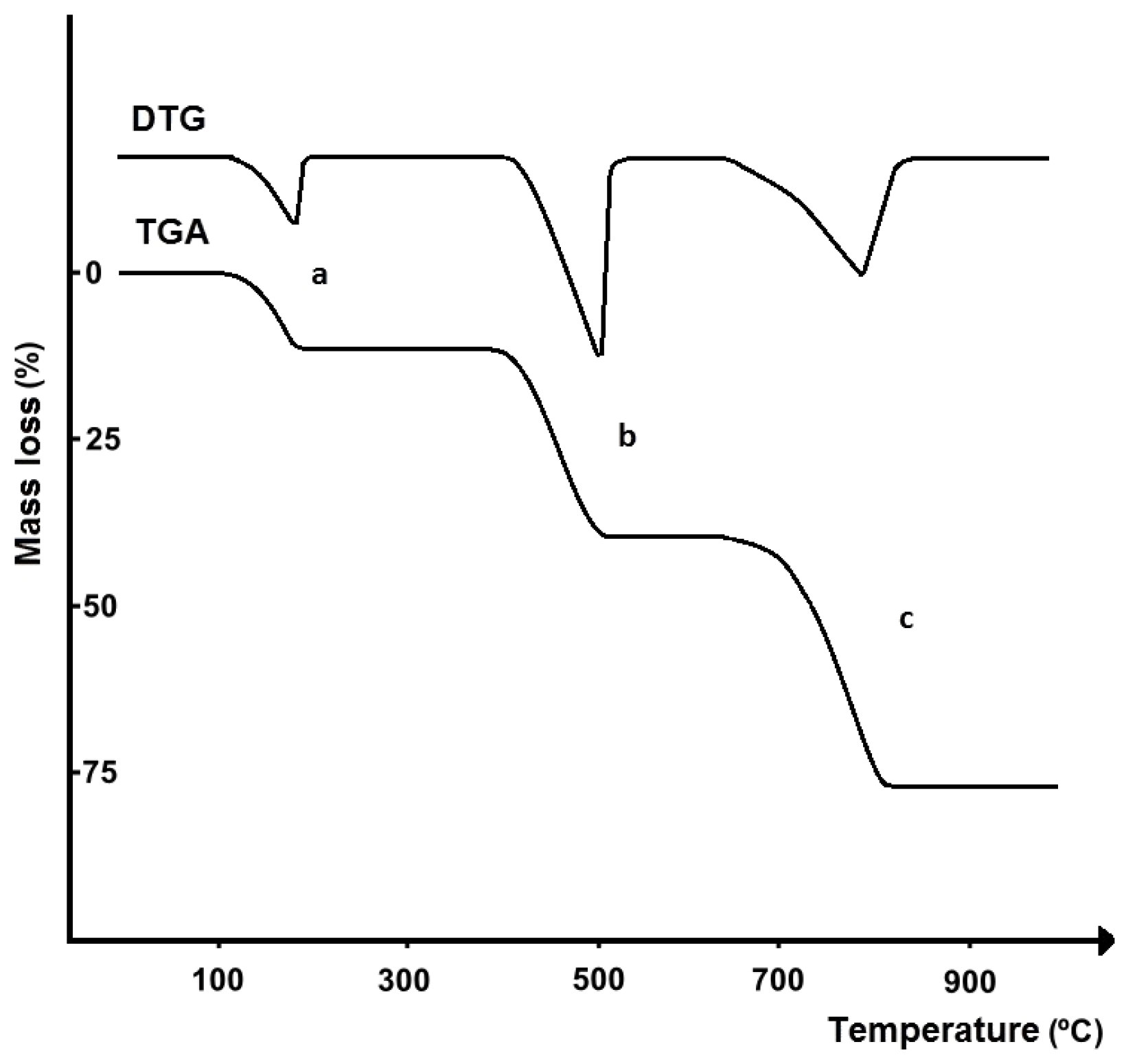
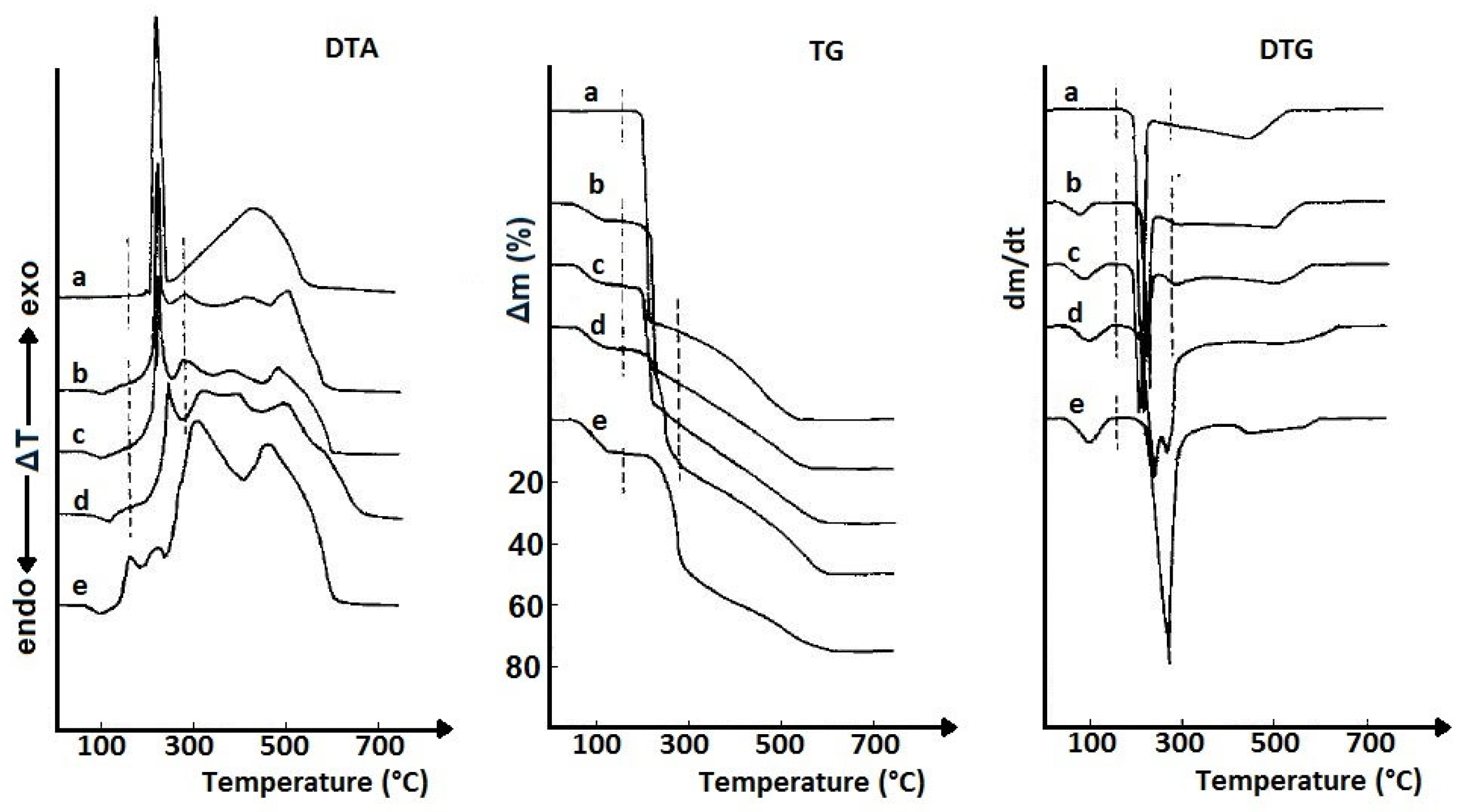

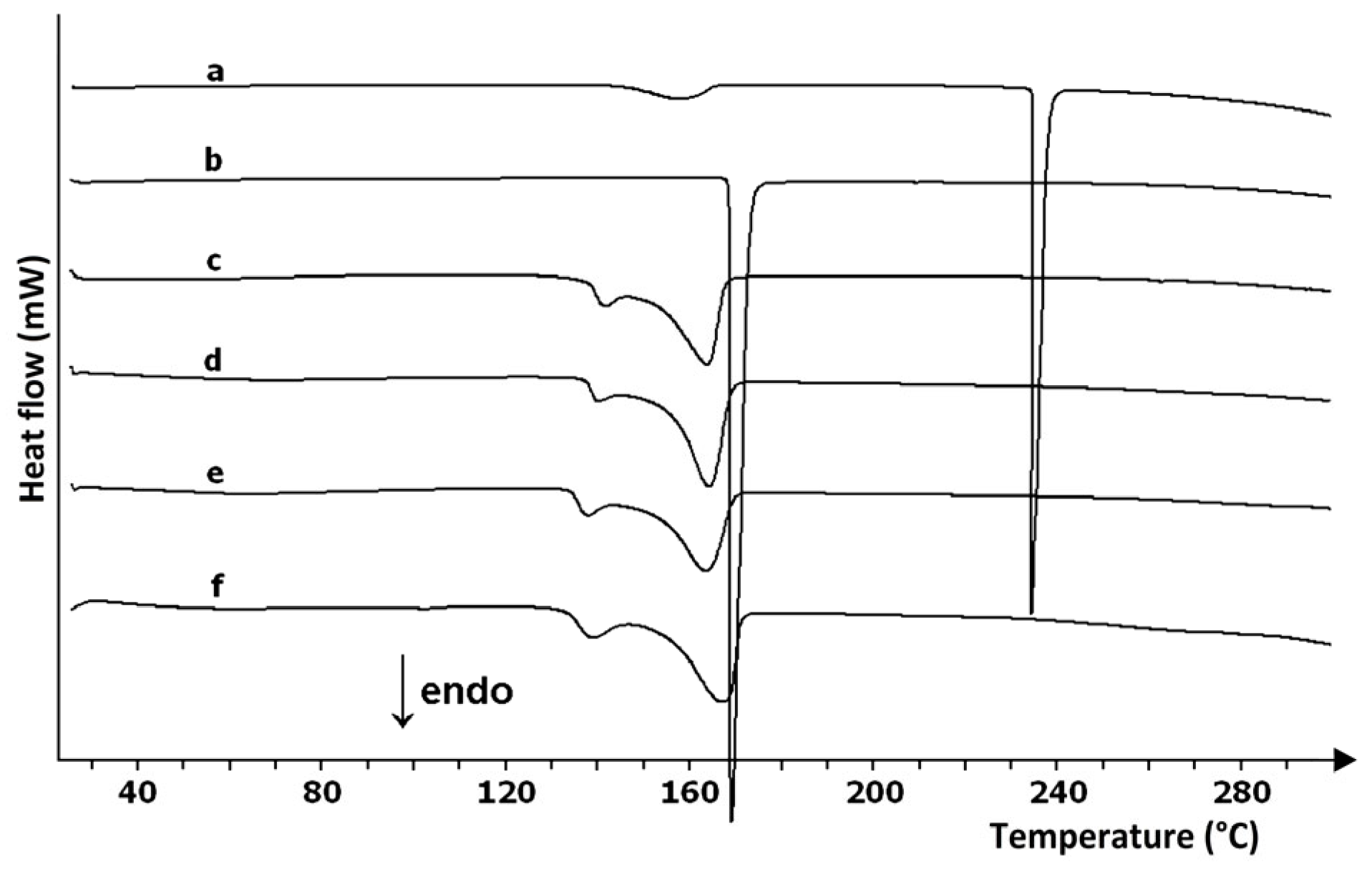

| Commercial Drug Products (Active Ingredients) | Thermal Analysis Devices | Measurement Conditions | Refs. |
|---|---|---|---|
| Tablets, capsules, powders (analgesics, antacids, vitamins) | Model 900 DTA (with DSC cell), Model 950 TGA (Du Pont) | DTA (DSC): 5–10 mg, 25–450 °C, 10 °C/min, nitrogen; TGA: 5–20 mg, 25–450 °C, 10 °C/min, nitrogen | [23,24,25] |
| Tablets (acetylsalicylic acid) | STA 7200 TG/DTG/DTA (Hitachi) | 4–6 mg, 45–550 °C, 10 °C/min, nitrogen 200 mL/min | [26] |
| Tablets (theophylline, aminophylline) | TG 209 F3 Tarsus (Netzsch) | 10 mg, 35–600 °C, 10 °C/min, nitrogen 40 mL/min | [27] |
| Tablets, coated tablets (naproxen, naproxen sodium) | TG 209 F3 Tarsus (Netzsch) | 10 mg, 35–600 °C, 10 °C/min, nitrogen 40 mL/min | [28] |
| Commercial Drug Products (Active Ingredients) | Thermal Analysis Devices | Measurement Conditions | Refs. |
|---|---|---|---|
| Tablets, granulates, capsules, powders, dragees (neuroleptics, expectorants, chemotherapeutics, vitamins, inorganic active ingredients) | Derivatograph OD-130 (MOM, Budapest) | 100 mg, 20–600 (1000) °C, 5 °C/min, furnace atmosphere | [13] |
| Ointments, suppositories (various active ingredients) | Derivatograph OD-130 (MOM, Budapest) | 200 mg, 20–600 (800) °C, 5 °C/min, furnace atmosphere | [13] |
| Tablets (atenolol) | DSC-60, TGA-50, TA-501 Thermal Analyzer (Shimadzu) | DSC: 0.8–1.2 mg, 20–335 °C, 10 °C/min, nitrogen 20 mL/min; TGA: 8–12 mg, 20–600 °C, 10 °C/min, nitrogen 20 mL/min | [30] |
| Tablets, pellets, capsules, suppositories, dosage forms in development (various active ingredients) | DSC-2, DSC-7, TGA-7 (Perkin-Elmer) | 20 °C/min, nitrogen | [31] |
| Tablets (coated and uncoated), pellets, granulates, capsules, dragees (methylxanthines) | DSC 822e (Mettler Toledo) | 4 mg, 25–300 °C, 10 °C/min, nitrogen 70 mL/min | [32] |
| Tablets (coated and uncoated), effervescent tablets, capsules, dietary supplements (magnesium salts) | DSC 822e (Mettler Toledo) | 4 mg, 25–300 °C, 10 °C/min, nitrogen 70 mL/min | [35] |
| Tablets, effervescent tablets, capsules, sachets (paracetamol) | “Jade” DSC calorimeter (Perkin-Elmer) | 3 mg, 30–300 °C, 5 °C/min, nitrogen 70 mL/min | [36] |
| Methods | Measurement Conditions | R | CV, % | Analytical Range |
|---|---|---|---|---|
| DSC, melting | Heat of melting from endothermic DSC peak in the range of 140–210 °C, dynamic nitrogen atmosphere | 0.996 | 4.5 | 1.00–6.70 mg |
| DSC, oxidation | Heat of oxidation from exothermic DSC peak in the range of 220–450 °C, dynamic oxygen atmosphere | 0.996 | 4.0 | 2.50–10.00 mmole/L |
| Chemical | Reaction with freshly distilled furfural at 65 °C, absorption measure of final solution at 340 nm | 0.998 | 4.4 | 0.120–1.250 mmole/L |
| Enzymatic | Reaction catalyzed by 3α-hydroxysteroid dehydrogenase, absorption measure of final solution at 750–760 nm | 0.999 | 2.4 | 1.00–5.00 mmole/L |
| Commercial Dosage Forms (Active Ingredients) | Thermal Analysis Devices | Measurement Conditions | Refs. |
|---|---|---|---|
| Capsules (chenodeoxycholic acid, ursodeoxycholic acid) | DSC-2 (Perkin-Elmer) | 4–5 mg, 2.5 °C/min, nitrogen 100 mL/min | [41] |
| Tablets, soluble tablets, suppositories, vials (diclofenac, diclofenac sodium) | DSC-7 series 1020 and TGS-2 (Perkin-Elmer) | DSC: 0.3–0.5 mg, 20–360 °C, 10 °C/min, nitrogen 50 mL/min; TGA: 1–2 mg, 20–840 °C, 10 °C/min, nitrogen 50 mL/min | [45,46] |
| No dosage form specified (paracetamol) | TG/DSC 625 (Stanton-Redcroft) | 4–6 mg, 20–400 °C, 2.5, 5, 10, 20 °C/min, argon | [47] |
| Tablets (paracetamol) | EXSTAR DSC 7020 (SII Nano Technology Inc.) | 4.9 mg, 30–190 °C, 10 °C/min, nitrogen 50 mL/min | [48] |
| Tablets (ibuprofen) | EXSTAR DSC 7020 (SII Nano Technology Inc.) | 5.0 mg, from 30 °C, 10 °C/min, nitrogen 50 mL/min | [49] |
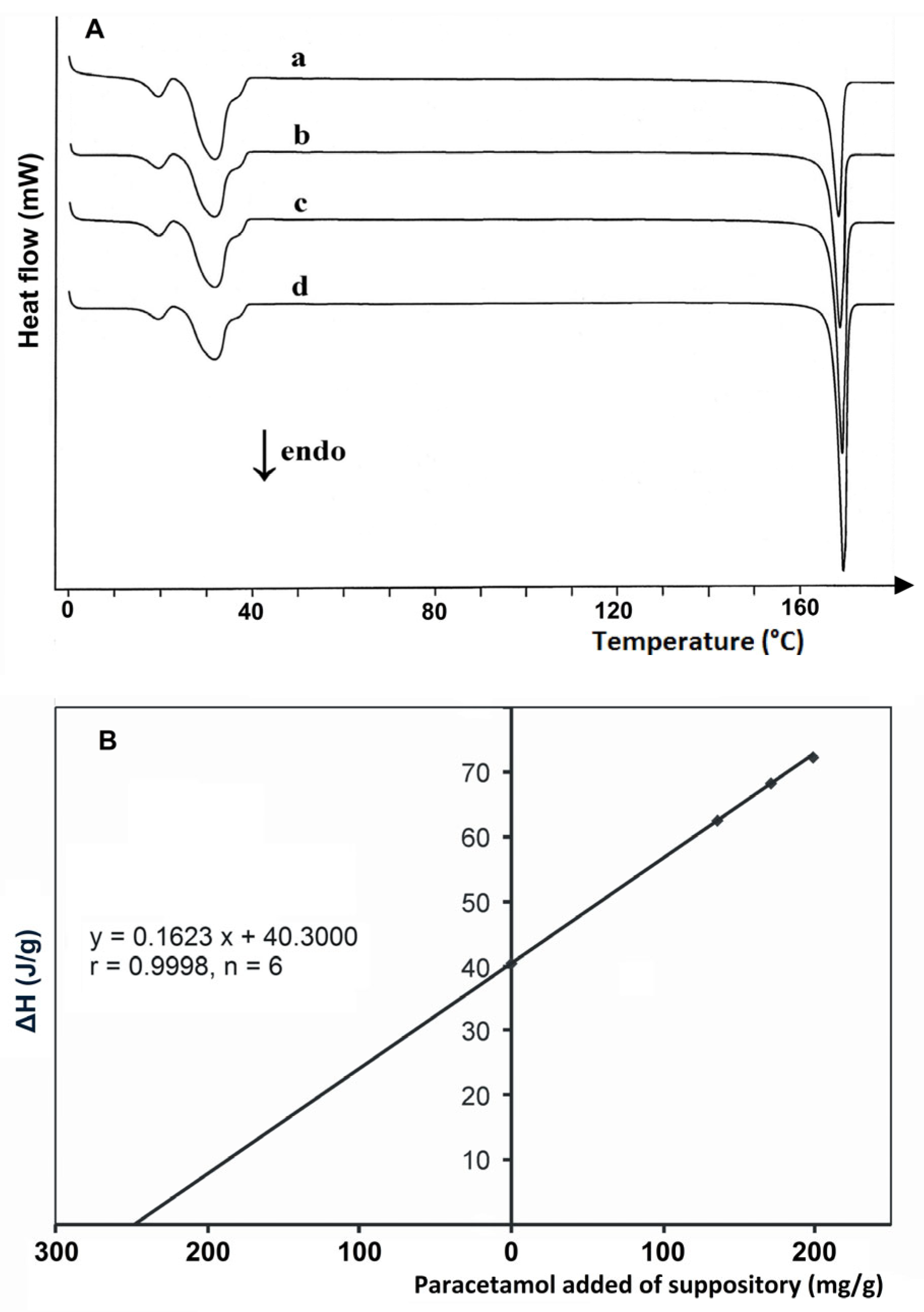
| Suppositories (paracetamol) | DSC 822e (Mettler Toledo) | 5.0 mg, 10–280 °C, 5 °C/min, nitrogen 80 mL/min | [50] |
| Suppositories (paracetamol) | DSC-6 (Perkin-Elmer) | 2.5–4.0 mg, 0–180 °C, 5 °C/min, nitrogen 20 mL/min | [51] |
| No dosage form specified (acetylsalicylic acid) | TG/DSC 625 (Stanton-Redcroft) | 7 mg, 20–600 °C, 10 °C/min, air 50 mL/min | [52] |
| Commercial Dosage Forms (Active Ingredients) | Thermal Analysis Devices | Measurement Conditions | Refs. |
|---|---|---|---|
| Tablets (calcium carbonate) | DTG 60 (Shimadzu) | 7 mg, 20–900 °C, 10 °C/min, air 50 mL/min | [55] |
| Non-effervescent tablets (L-ascorbic acid) | TGA-2950 (TA-Instruments) | 10 mg, 30–600 °C, 10 °C/min, oxygen 100 mL/min | [56] |
| Tablets (paracetamol, codeine phosphate) | Pyris Diamond TG/DTG (Perkin-Elmer) | 25–900 °C, 10 °C/min, nitrogen 100 mL/min | [57] |
| Tablets (mebendazole, ketoconazole) | DSC-50 and TGA-50H (Shimadzu) | 10, 20, 40, 60, 80 °C/min; DSC: 25–500 °C, nitrogen 50 mL/min; TGA: 8 mg, 25–900 °C, synthetic air 20 mL/min, nitrogen 50 mL/min | [58,59] |
| Capsules (α-lipolic acid) | TG/DTA model Q600 (TA-Instruments) | 5.0 mg, 25–900 °C, 10, 20, 40, 60, 80 °C/min, nitrogen 50 mL/min | [60] |
| Non-Compliant Products (Active Components) | Purpose of Research | Thermal Analysis Devices | Measurement Conditions | Ref. |
|---|---|---|---|---|
| Merla (cocaine) | Difference in chemical composition | DSC 822e and TGA/SDTA 851e (Mettler Toledo) | DSC: 5 mg, 25–600 °C, 10 °C/min, nitrogen 50 mL/min; TGA: 7 mg, 25–1400 °C, 50 °C/min, nitrogen 50 mL/min | [63] |
| Powders (cocaine) | Identification and quantification | DSC-2B and TG-S2 (Perkin-Elmer) | between 2.5 and 10 °C/min, nitrogen or air 50–100 mL/min | [64] |
| Powders (amphetamine-type drugs) | Quantification | DSC 8000 (Perkin-Elmer) | 2–5 mg, 10 °C/min, nitrogen 20 mL/min | [65] |
| Tablets (benzodiazepines, excipients) | Identification | DSC Q 100 (TA Instruments) | 5–10 mg, 20–250 °C, 10 °C/min, nitrogen 50 mL/min | [66] |
| Tablets, capsules, sachets, powders (sildenafil, excipients) | Detection | DSC 6000 (Perkin-Elmer) | 0.5–1.0 mg, 100–200 °C, 10 °C/min, nitrogen 20 mL/min | [67] |
| Tablets of Viagra and Cialis (sildenafil, tadalafil, excipients) | Discrimination between different tablets | DSC-60 (Shimadzu) | 1–2 mg, 30–330 °C, 10 °C/min, nitrogen 50 mL/min | [68] |
| Drug Products (e.g., Tablets, Capsules, Suppositories) | Thermal Methods (DSC, TGA) | Chromatographic Methods (HPLC) | Spectroscopic Methods (UV-Vis) |
|---|---|---|---|
| Time of analysis a | minutes | hours | hours |
| Labor-intensity of analysis | negligible b | high c | high c |
| Cost of analysis | low d | high e | high e |
| Transferring the sample into solution | not needed | necessary | necessary |
| Separation of analyte from matrix | not needed | necessary | necessary |
| Use of solvents and reagents | not needed | necessary | necessary |
| Problems with stability of analyte | no | yes | yes |
| Sample analysis in solid state | yes | no | no |
| Solid state properties of analyte f | yes | no | no |
| Green chemistry method | yes | no | no |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wesolowski, M. Methods of Thermal Analysis as Fast and Reliable Tools for Identification and Quantification of Active Ingredients in Commercially Available Drug Products. Pharmaceutics 2025, 17, 1099. https://doi.org/10.3390/pharmaceutics17091099
Wesolowski M. Methods of Thermal Analysis as Fast and Reliable Tools for Identification and Quantification of Active Ingredients in Commercially Available Drug Products. Pharmaceutics. 2025; 17(9):1099. https://doi.org/10.3390/pharmaceutics17091099
Chicago/Turabian StyleWesolowski, Marek. 2025. "Methods of Thermal Analysis as Fast and Reliable Tools for Identification and Quantification of Active Ingredients in Commercially Available Drug Products" Pharmaceutics 17, no. 9: 1099. https://doi.org/10.3390/pharmaceutics17091099
APA StyleWesolowski, M. (2025). Methods of Thermal Analysis as Fast and Reliable Tools for Identification and Quantification of Active Ingredients in Commercially Available Drug Products. Pharmaceutics, 17(9), 1099. https://doi.org/10.3390/pharmaceutics17091099






