Mechanisms of Self-Assembly of Giant Unilamellar Vesicles in the Army Liposome Formulation (ALF) Family of Vaccine Adjuvants
Abstract
1. Introduction
2. Materials and Methods
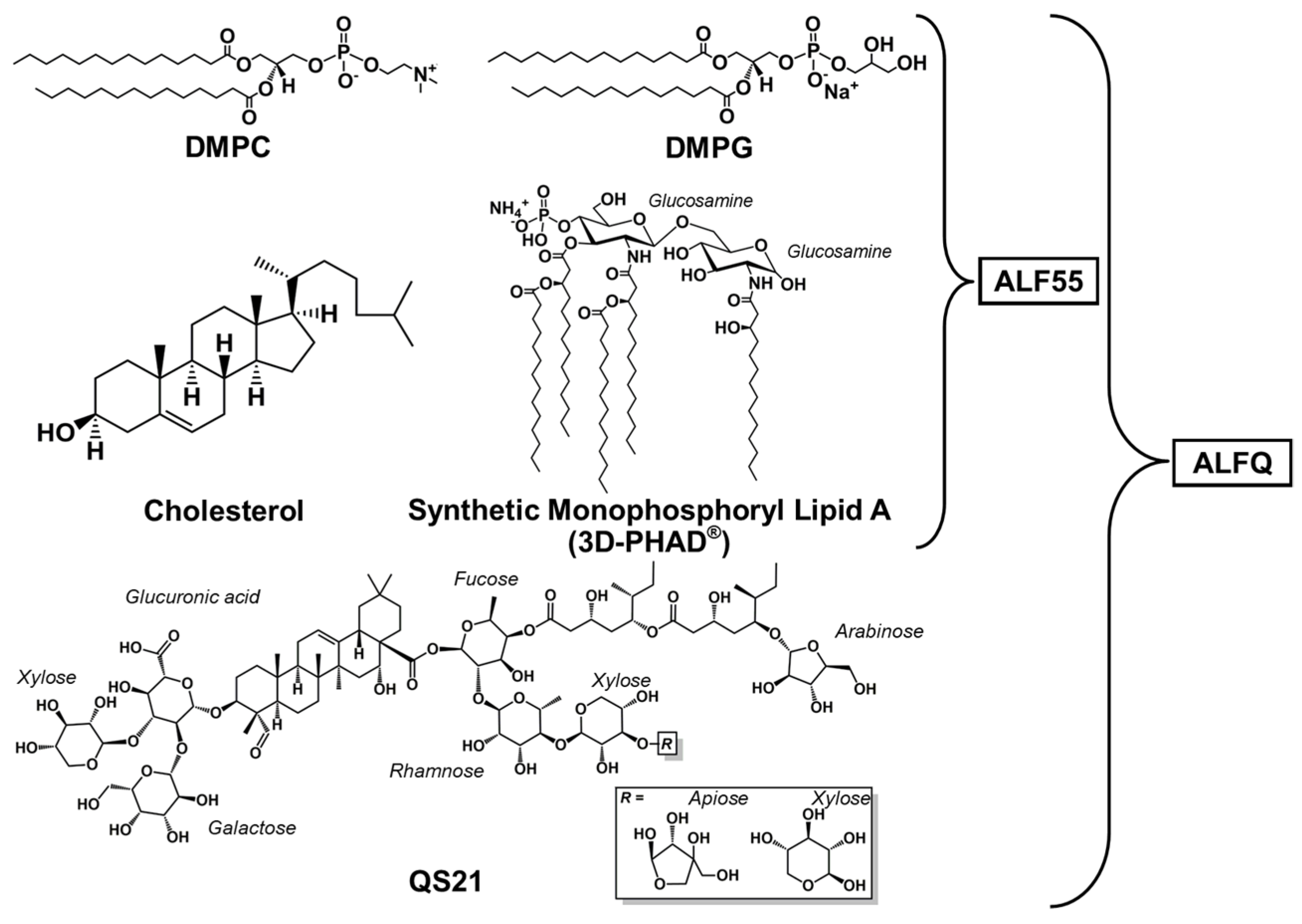
2.1. Materials and Reagents
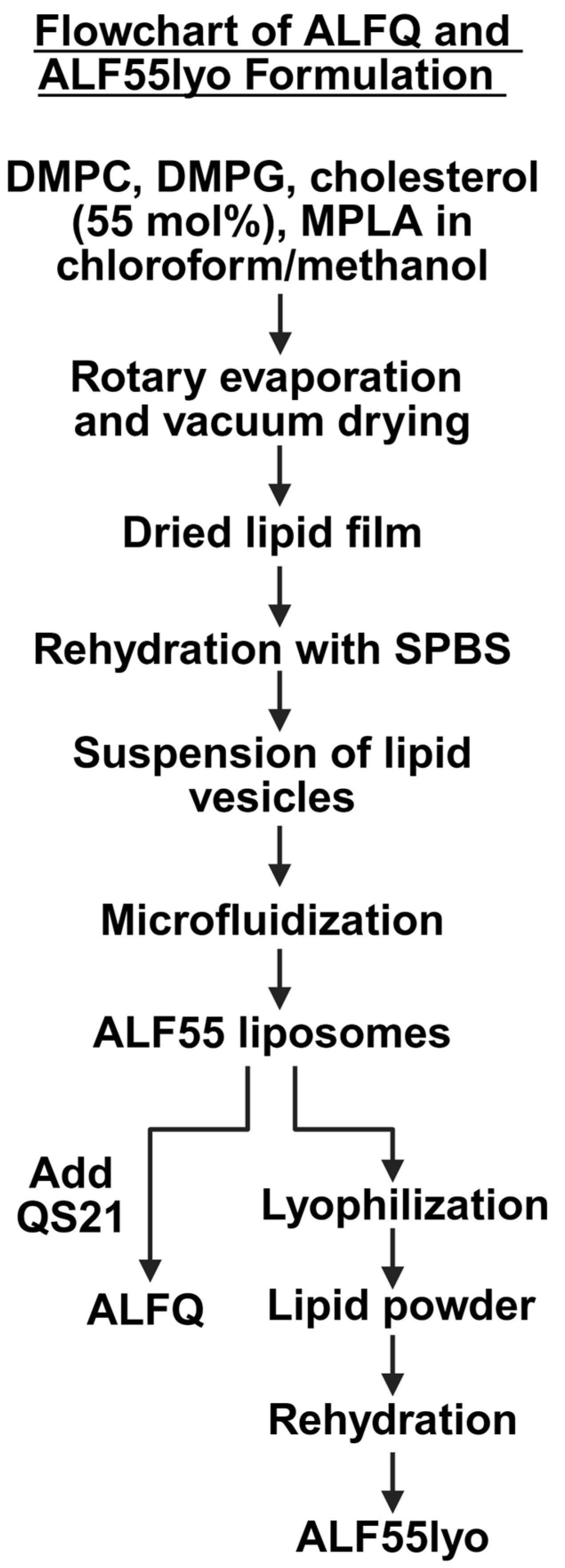
2.2. ALF55 and ALFQ Formulation Methods
2.3. Lyophilized ALF55 Formulation Methods
2.4. Microscopy
2.5. Isolation of SUVs from GUVs + TIMs in ALF55lyo
2.6. UPLC-MS/MS Analysis of Liposome Components
2.7. Dynamic Light Scattering
3. Results
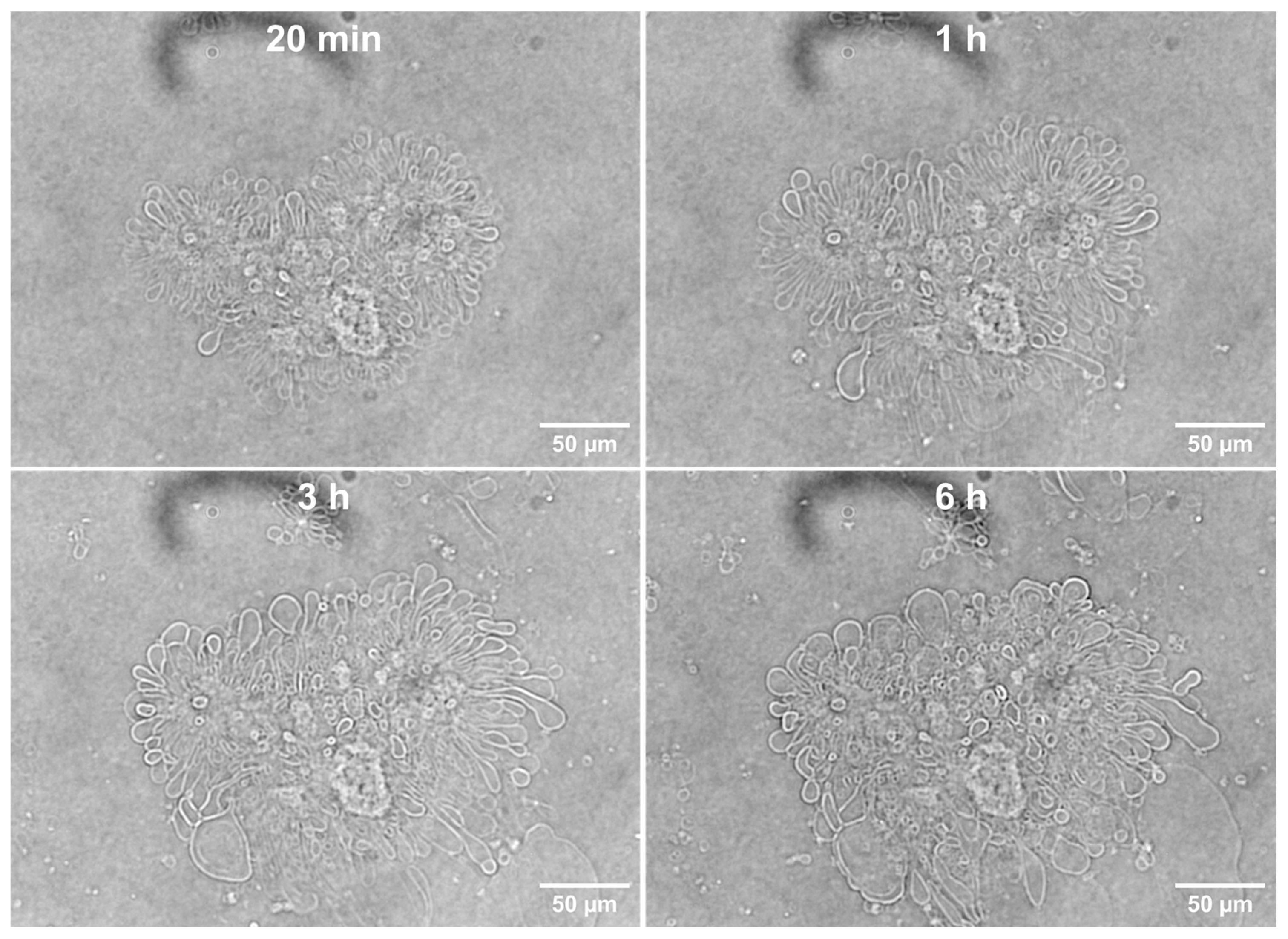
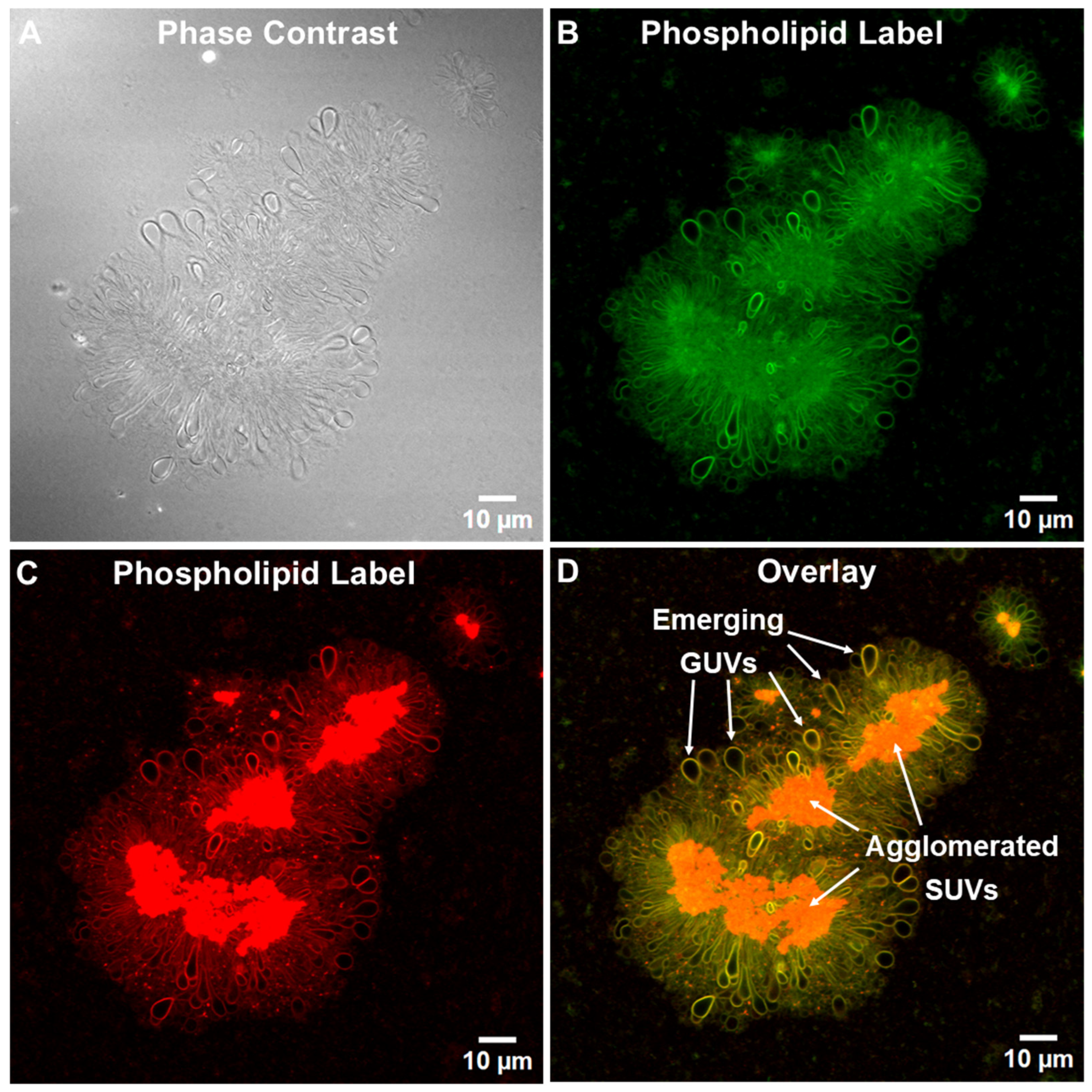
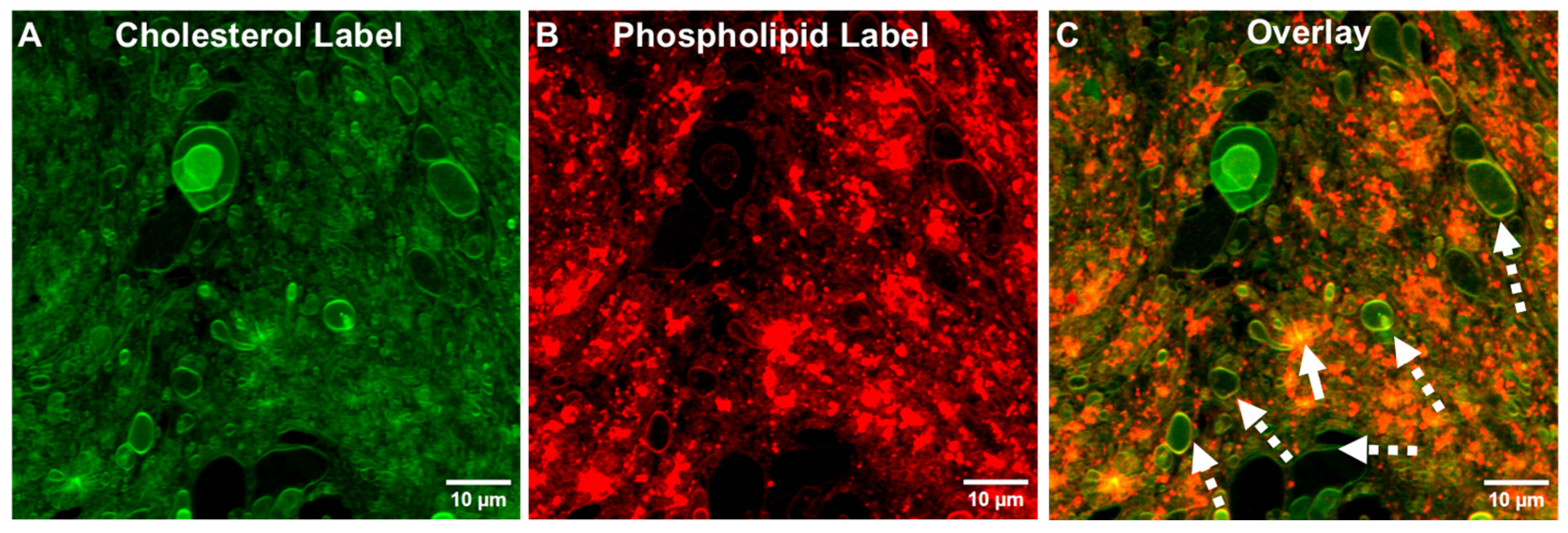
3.1. Tethered Incomplete Microspheres (TIMs) as a Presumed Intermediate Step and as a Byproduct in the Formation of GUVs
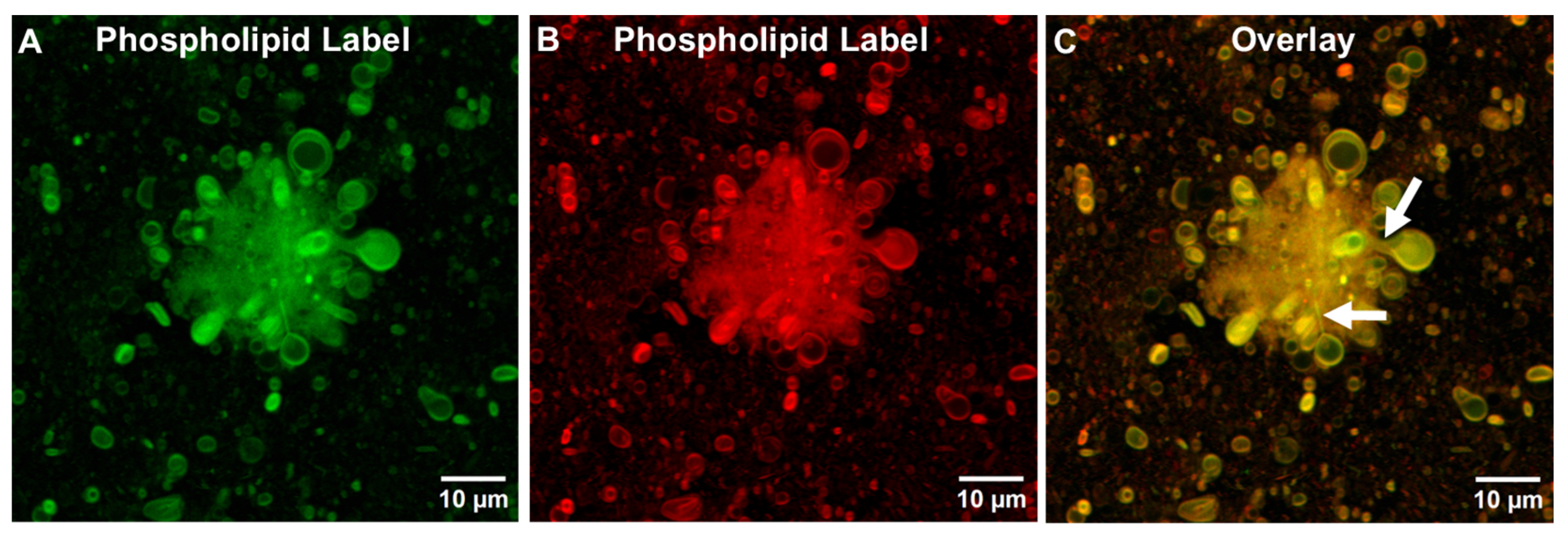
3.1.1. Initial Fusion Process Leading to Free-Floating GUVs in ALFQ
3.1.2. Detection of Phospholipids and Cholesterol in ALFQ TIMs
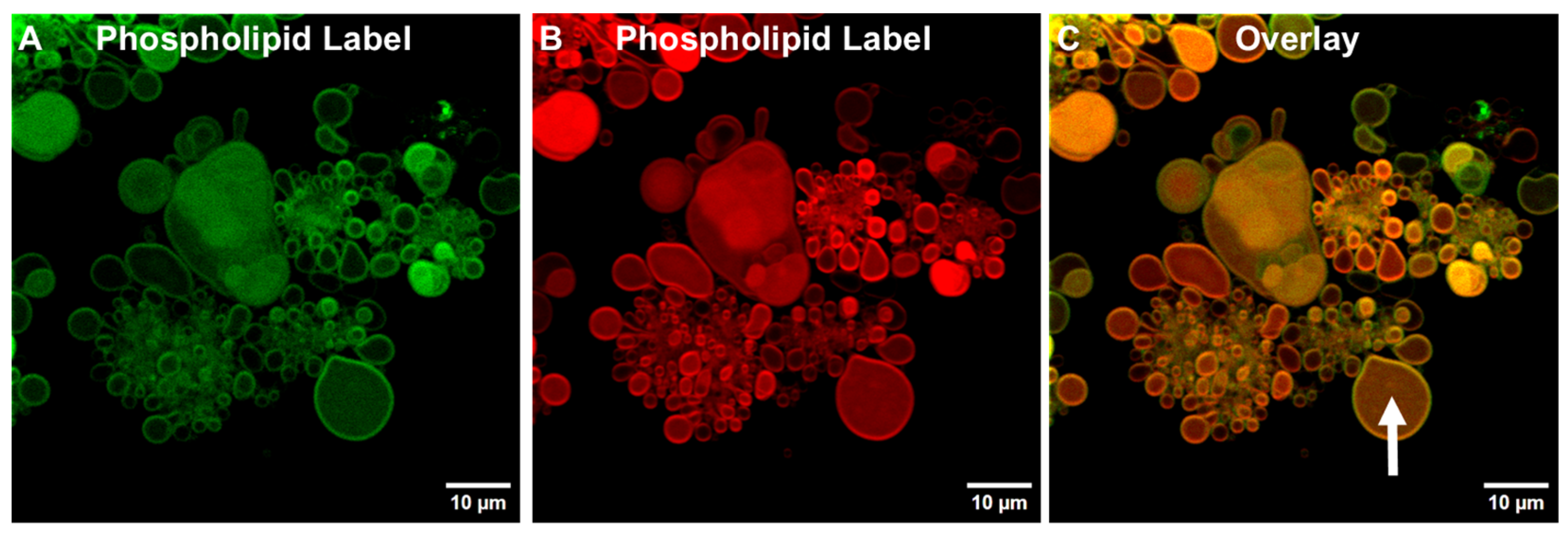
3.1.3. Detection of TIMs Containing Both Phospholipids and Cholesterol in Lyophilized ALF55 in the Absence of QS21 Saponin
3.1.4. Cholesterol Is Not Required for Generating TIMs and GUVs in ALFlyo
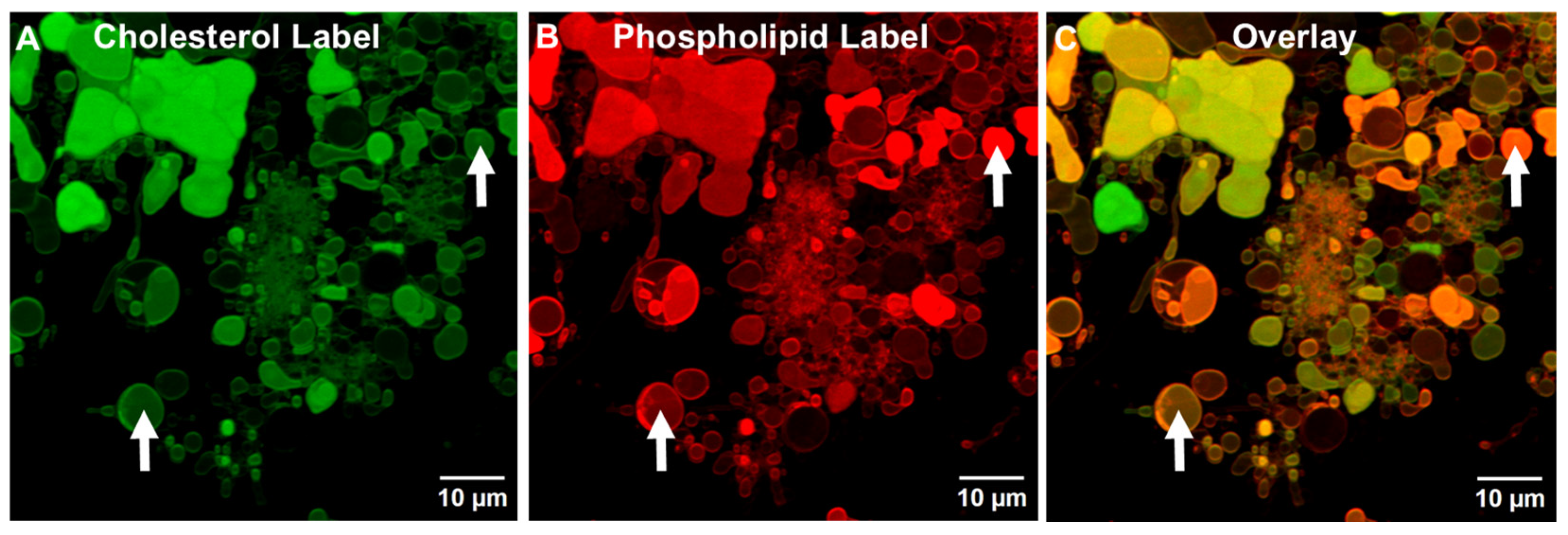
3.1.5. Differential Centrifugation of ALF55lyo
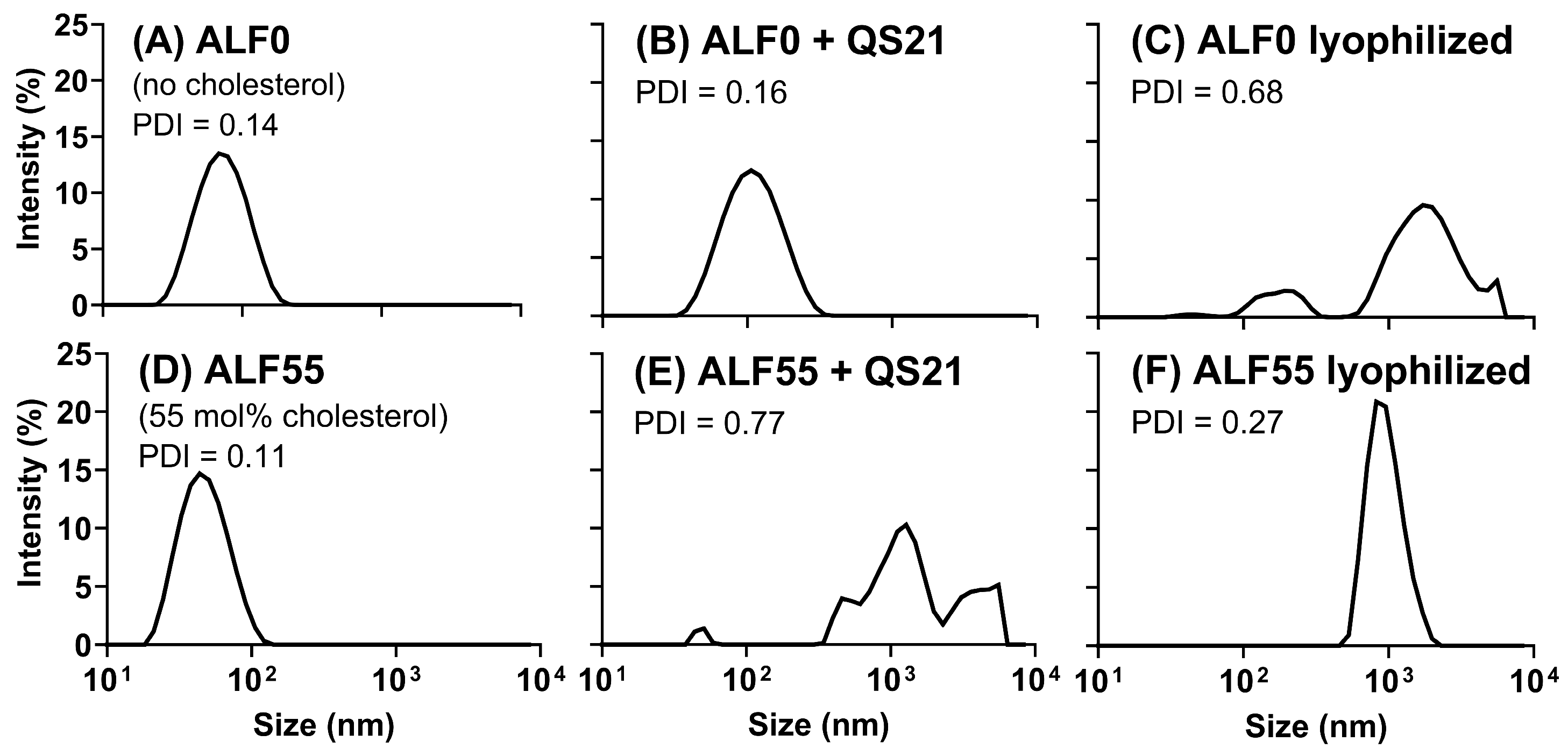
3.2. Comparative Influence of Cholesterol During the Generation of TIMs and GUVs in ALFQ and ALFlyo
3.3. Comparative Zeta Potentials of Liposomal Particles
4. Discussion
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Disclaimer
References
- Alving, C.R.; Peachman, K.K.; Matyas, G.R.; Rao, M.; Beck, Z. Army Liposome Formulation (ALF) Family of Vaccine Adjuvants. Expert. Rev. Vaccines 2020, 19, 279–292. [Google Scholar] [CrossRef]
- Abucayon, E.G.; Rao, M.; Matyas, G.R.; Alving, C.R. QS21-Initiated Fusion of Liposomal Small Unilamellar Vesicles to Form ALFQ Results in Concentration of Most of the Monophosphoryl Lipid A, QS21, and Cholesterol in Giant Unilamellar Vesicles. Pharmaceutics 2023, 15, 2212. [Google Scholar] [CrossRef]
- Janeway, C.A., Jr. Approaching the Asymptote? Evolution and Revolution in Immunology. Cold Spring Harb. Symp. Quant. Biol. 1989, 54 Pt 1, 1–13. [Google Scholar] [CrossRef]
- Steinman, R.M. Dendritic Cells: Understanding Immunogenicity. Eur. J. Immunol. 2007, 37 (Suppl. S1), S53–S60. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.L.; Peres, C.; Conniot, J.; Matos, A.I.; Moura, L.; Carreira, B.; Sainz, V.; Scomparin, A.; Satchi-Fainaro, R.; Preat, V.; et al. Nanoparticle Impact on Innate Immune Cell Pattern-Recognition Receptors and Inflammasomes Activation. Semin. Immunol. 2017, 34, 3–24. [Google Scholar] [CrossRef] [PubMed]
- Bonam, S.R.; Partidos, C.D.; Halmuthur, S.K.M.; Muller, S. An Overview of Novel Adjuvants Designed for Improving Vaccine Efficacy. Trends Pharmacol. Sci. 2017, 38, 771–793. [Google Scholar] [CrossRef]
- Lavelle, E.C.; McEntee, C.P. Vaccine adjuvants: Tailoring Innate Recognition to Send the Right Message. Immunity 2024, 57, 772–789. [Google Scholar] [CrossRef] [PubMed]
- Beutler, B.; Rietschel, E.T. Innate Immune Sensing and Its Roots: The Story of Endotoxin. Nat. Rev. Immunol. 2003, 3, 169–176. [Google Scholar] [CrossRef]
- Alving, C.R.; Rao, M.; Steers, N.J.; Matyas, G.R.; Mayorov, A.V. Liposomes Containing Lipid A: An Effective, Safe, Generic Adjuvant System for Synthetic Vaccines. Expert. Rev. Vaccines 2012, 11, 733–744. [Google Scholar] [CrossRef]
- Fries, L.F.; Gordon, D.M.; Richards, R.L.; Egan, J.E.; Hollingdale, M.R.; Gross, M.; Silverman, C.; Alving, C.R. Liposomal Malaria Vaccine in Humans: A Safe and Potent Adjuvant Strategy. Proc. Natl. Acad. Sci. USA 1992, 89, 358–362. [Google Scholar] [CrossRef]
- Bangham, A.D.; Horne, R.W.; Glauert, A.M.; Dingle, J.T.; Lucy, J.A. Action of Saponin on Biological Cell Membranes. Nature 1962, 196, 952–955. [Google Scholar] [CrossRef]
- Shen, L.; Luo, H.; Fan, L.; Tian, X.; Tang, A.; Wu, X.; Dong, K.; Su, Z. Potential Immunoregulatory Mechanism of Plant Saponins: A Review. Molecules 2024, 29, 113. [Google Scholar] [CrossRef] [PubMed]
- Roman, F.; Burny, W.; Ceregido, M.A.; Laupeze, B.; Temmerman, S.T.; Warter, L.; Coccia, M. Adjuvant System AS01: From Mode of Action to Effective Vaccines. Expert. Rev. Vaccines 2024, 23, 715–729. [Google Scholar] [CrossRef] [PubMed]
- Beck, Z.; Matyas, G.R.; Alving, C.R. Detection of Liposomal Cholesterol and Monophosphoryl Lipid A by QS-21 Saponin and Limulus Polyphemus Amebocyte Lysate. Biochim. Biophys. Acta 2015, 1848, 775–780. [Google Scholar] [CrossRef] [PubMed]
- Beck, Z.; Matyas, G.R.; Jalah, R.; Rao, M.; Polonis, V.R.; Alving, C.R. Differential Immune Responses to HIV-1 Envelope Protein Induced by Liposomal Adjuvant Formulations Containing Monophosphoryl Lipid A with or without QS21. Vaccine 2015, 33, 5578–5587. [Google Scholar] [CrossRef]
- Alving, C.R.; Rao, M.; Matyas, G.R. Similarities and Differences of Chemical Compositions and Physical and Functional Properties of Adjuvant System 01 and Army Liposome Formulation with QS21. Front. Immunol. 2023, 14, 1102524. [Google Scholar] [CrossRef]
- Hutter, J.N.; Robben, P.M.; Lee, C.; Hamer, M.; Moon, J.E.; Merino, K.; Zhu, L.; Galli, H.; Quinn, X.; Brown, D.R.; et al. First-In-Human Assessment of Safety and Immunogenicity of Low and High Doses of Plasmodium Falciparum Malaria Protein 013 (FMP013) Administered Intramuscularly with ALFQ Adjuvant in Healthy Malaria-naive Adults. Vaccine 2022, 40, 5781–5790. [Google Scholar] [CrossRef]
- Ober Shepherd, B.L.; Scott, P.T.; Hutter, J.N.; Lee, C.; McCauley, M.D.; Guzman, I.; Bryant, C.; McGuire, S.; Kennedy, J.; Chen, W.H.; et al. SARS-CoV-2 Recombinant Spike Ferritin Nanoparticle Vaccine Adjuvanted with Army Liposome Formulation Containing Monophosphoryl Lipid A and QS-21: A phase 1, Randomised, Double-Blind, Placebo-Controlled, First-in-Human Clinical Trial. Lancet Microbe 2024, 5, e581–e593. [Google Scholar] [CrossRef]
- Beck, Z.; Torres, O.B.; Matyas, G.R.; Lanar, D.E.; Alving, C.R. Immune Response to Antigen Adsorbed to Aluminum Hydroxide Particles: Effects of Co-Adsorption of ALF or ALFQ Adjuvant to the Aluminum-Antigen Complex. J. Control Release 2018, 275, 12–19. [Google Scholar] [CrossRef]
- Abucayon, E.G.; Barrientos, R.C.; Torres, O.B.; Sweeney, S.; Whalen, C.; Matyas, G.R. A Liquid Chromatography High-Resolution Tandem Mass Spectrometry Method to Quantify QS-21 Adjuvant and Its Degradation Products in Liposomal Drug Formulations. ACS Omega 2023, 8, 21016–21025. [Google Scholar] [CrossRef]
- Abucayon, E.G.; Sweeney, S.; Matyas, G.R. A Reliable Quantification of Cholesterol and 25-Hydroxycholesterol in Liposomal Adjuvant Formulation by Liquid Chromatography High-Resolution Tandem Mass Spectrometry. ACS Omega 2024, 9, 19637–19644. [Google Scholar] [CrossRef] [PubMed]
- Alving, C.R.; Beck, Z. Non-toxic Adjuvant Formulation Comprising a Monophosphoryl Lipid A (MPLA)-Containing Liposome Composition and A Saponin. US Patent 10,434,167, 8 October 2019. [Google Scholar]
- Cullis, P.R.; de Kruijff, B. Lipid Polymorphism and the Functional Roles of Lipids in Biological Membranes. Biochim. Biophys. Acta 1979, 559, 399–420. [Google Scholar] [CrossRef] [PubMed]
- Cullis, P.R.; de Kruijff, B.; Hope, M.J.; Nayar, R.; Schmid, S.L. Phospholipids and Membrane Transport. Can. J. Biochem. 1980, 58, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Cullis, P.R.; Hope, M.J.; Tilcock, C.P. Lipid Polymorphism and the Roles of Lipids in Membranes. Chem. Phys. Lipids 1986, 40, 127–144. [Google Scholar] [CrossRef]
- Keukens, E.A.; de Vrije, T.; Fabrie, C.H.; Demel, R.A.; Jongen, W.M.; de Kruijff, B. Dual Specificity of Sterol-Mediated Glycoalkaloid Induced Membrane Disruption. Biochim. Biophys. Acta 1992, 1110, 127–136. [Google Scholar] [CrossRef]
- Keukens, E.A.; de Vrije, T.; van den Boom, C.; de Waard, P.; Plasman, H.H.; Thiel, F.; Chupin, V.; Jongen, W.M.; de Kruijff, B. Molecular Basis of Glycoalkaloid Induced Membrane Disruption. Biochim. Biophys. Acta 1995, 1240, 216–228. [Google Scholar] [CrossRef]
- Tresset, G. The Multiple Faces of Self-Assembled Lipidic Systems. PMC Biophys. 2009, 2, 3. [Google Scholar] [CrossRef]
- Brandenburg, K.; Richter, W.; Koch, M.H.; Meyer, H.W.; Seydel, U. Characterization of the Nonlamellar Cubic and HII Structures of Lipid A From Salmonella Enterica Serovar Minnesota by X-ray Diffraction and Freeze-Fracture Electron Microscopy. Chem. Phys. Lipids 1998, 91, 53–69. [Google Scholar] [CrossRef]
- Seydel, U.; Labischinski, H.; Kastowsky, M.; Brandenburg, K. Phase Behavior, Supramolecular Structure, and Molecular Conformation of Lipopolysaccharide. Immunobiology 1993, 187, 191–211. [Google Scholar] [CrossRef]
- Santos, D.E.S.; De Nicola, A.; Dos Santos, V.F.; Milano, G.; Soares, T.A. Exploring the Molecular Dynamics of a Lipid-A Vesicle at the Atom Level: Morphology and Permeation Mechanism. J. Phys. Chem. B 2023, 127, 6694–6702. [Google Scholar] [CrossRef]
- Brandl, M.M.; Bachmann, D.; Drechsler, M.; Bauer, K.H. Liposome Preparation Using High-Pressure Homogenizers. In Liposome Technology, 2nd ed.; Gregoriadis, G., Ed.; CRC Press: Boca Raton, FL, USA, 1993; Volume 1, pp. 49–65. [Google Scholar]
- Microfluidics. How Microfluidizer Processors Achieve Superior Particle Size Reduction. Available online: https://www.microfluidics-mpt.com/microfluidics-technology/how-it-works?hsCtaTracking=0966ad92-635d-4f41-b29f-ea0408b9ced0%7C72a62a70-5c97-42ce-bc68-5eb2f213c958 (accessed on 22 June 2025).
- De Kruijff, B.; Cullis, P.R.; Radda, G.K. Outside-Inside Distributions and Sizes of Mixed Phosphatidylcholine-Cholesterol Vesicles. Biochim. Biophys. Acta 1976, 436, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Sarmento, M.J.; Hof, M.; Sachl, R. Interleaflet Coupling of Lipid Nanodomains–Insights From in Vitro Systems. Front. Cell Dev. Biol. 2020, 8, 284. [Google Scholar] [CrossRef]
- Augustin, J.M.; Kuzina, V.; Andersen, S.B.; Bak, S. Molecular Activities, Biosynthesis and Evolution of Triterpenoid Saponins. Phytochemistry 2011, 72, 435–457. [Google Scholar] [CrossRef]
- Antimisiaris, S.G. Preparation of DRV Liposomes. Methods Mol. Biol. 2023, 2622, 21–47. [Google Scholar] [CrossRef]
- Antimisiaris, S.G.; Jayasekera, P.; Gregoriadis, G. Liposomes as Aaccine Carriers. Incorporation of Soluble and Particulate Antigens in Giant Vesicles. J. Immunol. Methods 1993, 166, 271–280. [Google Scholar] [CrossRef]
- Gregoriadis, G. Liposomes as immunoadjuvants and vaccine carriers: Antigen entrapment. Immunomethods 1994, 4, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Gregoriadis, G.; McCormack, B.; Obrenovic, M.; Saffie, R.; Zadi, B.; Perrie, Y. Vaccine Entrapment in Liposomes. Methods 1999, 19, 156–162. [Google Scholar] [CrossRef] [PubMed]
| Component | Uncentrifuged ALF55lyo (mg) | Centrifuged ALF55lyo | |||
|---|---|---|---|---|---|
| Pellet (mg) | % Recovery | Supernatant (mg) | % Recovery | ||
| Total Phospholipid | 75.53 ± 0.57 | 38.15 ± 1.18 | 50.5 | 41.25 ± 1.35 | 54.6 |
| Cholesterol | 48.22 ± 3.66 | 26.21 ± 0.6 | 54.3 | 5.94 ± 0.07 | 12.3 |
| MPLA | 2.15 ± 0.08 | 1.54 ± 0.02 | 71.7 | 0.27 ± 0.00 | 12.7 |
| Liposome | Zeta Potential (mV) |
|---|---|
| ALF55 | −11.13 ± 0.76 |
| ALFQ | −12.73 ± 0.21 |
| ALF0 | −16.20 ± 1.51 |
| ALF0 + QS21 | −14.37 ± 0.84 |
| ALF0lyo | −15.03 ± 0.60 |
| ALF55lyo | −14.97 ± 0.96 |
| ALF55lyo pellet 1 | −14.20 ± 0.26 |
| ALF55lyo supernatant 1 | −12.40 ± 0.69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicolescu, C.; Komla, E.; Rao, M.; Matyas, G.R.; Alving, C.R. Mechanisms of Self-Assembly of Giant Unilamellar Vesicles in the Army Liposome Formulation (ALF) Family of Vaccine Adjuvants. Pharmaceutics 2025, 17, 1092. https://doi.org/10.3390/pharmaceutics17091092
Nicolescu C, Komla E, Rao M, Matyas GR, Alving CR. Mechanisms of Self-Assembly of Giant Unilamellar Vesicles in the Army Liposome Formulation (ALF) Family of Vaccine Adjuvants. Pharmaceutics. 2025; 17(9):1092. https://doi.org/10.3390/pharmaceutics17091092
Chicago/Turabian StyleNicolescu, Calin, Essie Komla, Mangala Rao, Gary R. Matyas, and Carl R. Alving. 2025. "Mechanisms of Self-Assembly of Giant Unilamellar Vesicles in the Army Liposome Formulation (ALF) Family of Vaccine Adjuvants" Pharmaceutics 17, no. 9: 1092. https://doi.org/10.3390/pharmaceutics17091092
APA StyleNicolescu, C., Komla, E., Rao, M., Matyas, G. R., & Alving, C. R. (2025). Mechanisms of Self-Assembly of Giant Unilamellar Vesicles in the Army Liposome Formulation (ALF) Family of Vaccine Adjuvants. Pharmaceutics, 17(9), 1092. https://doi.org/10.3390/pharmaceutics17091092








