Hyaluronic Acid in Female Reproductive Health: Tailoring Molecular Weight to Clinical Needs in Obstetric and Gynecological Fields
Abstract
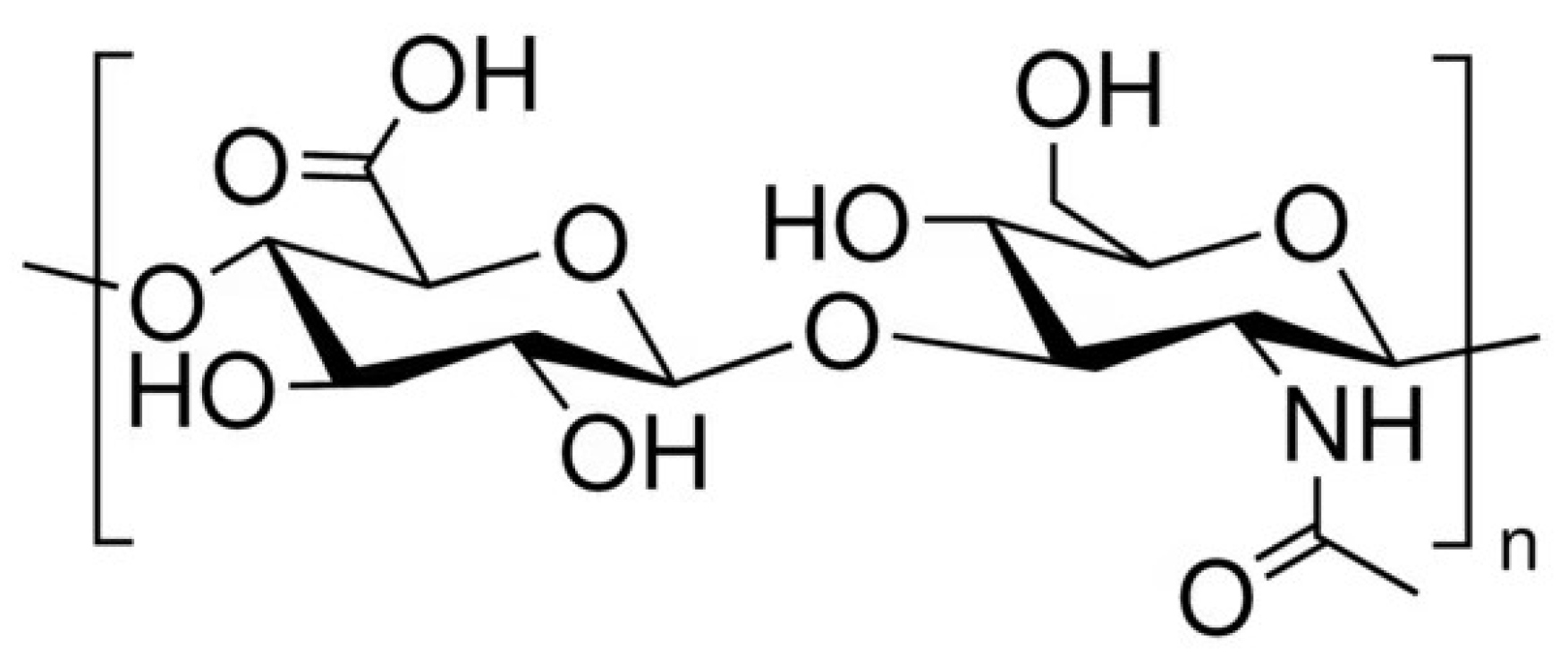
1. Introduction
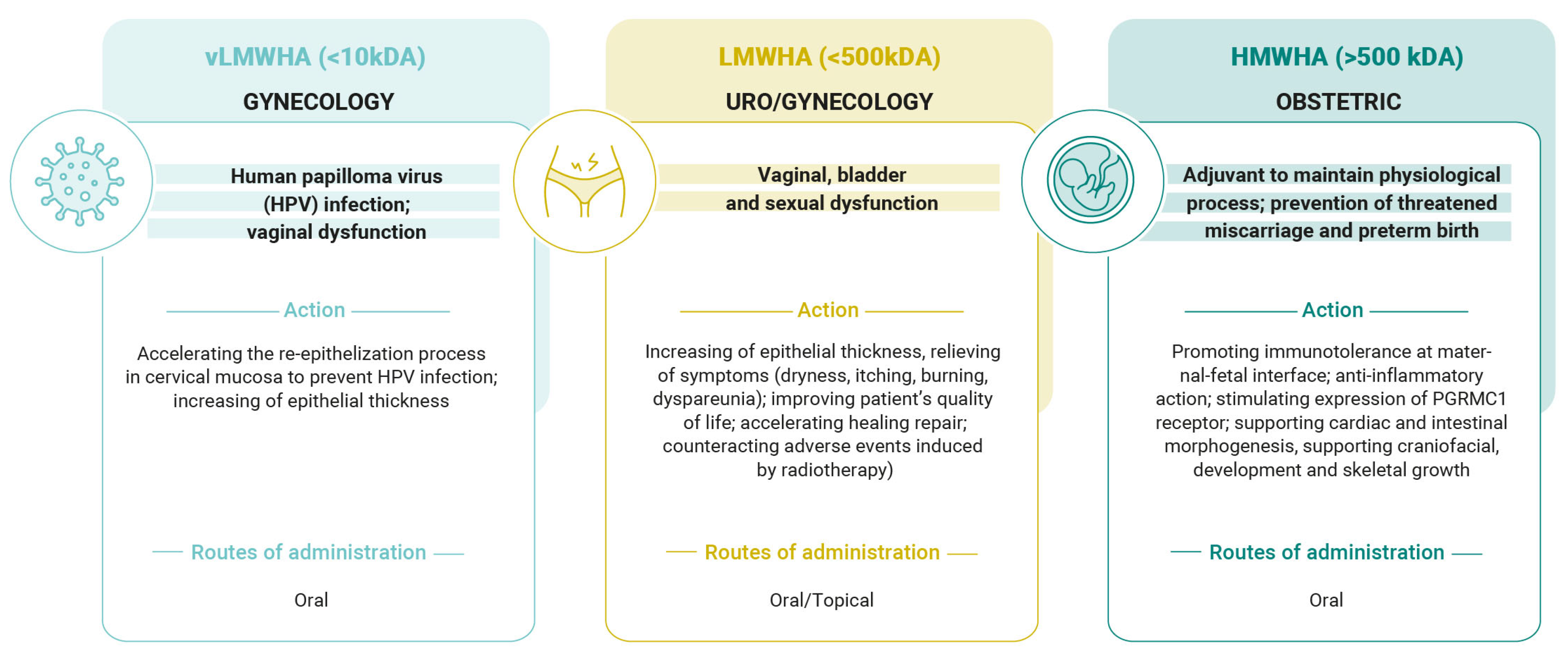
2. Supplementation of Low-Molecular-Weight (LMWHA) and Very Low-Molecular-Weight Hyaluronic Acid (vLMWHA) in Gynecological Disorders
2.1. LMWHA Is Useful in the Case of Genitourinary Syndrome of Menopause (GSM)
2.2. LMWHA Useful Against Pelvic Radiotherapy Discomfort
2.3. vLMWHA Is Useful in Human Papilloma Virus (HPV) Infection
| Study | Study | Patients | Treatment | Outcomes | Ref. |
|---|---|---|---|---|---|
| Costantino, D. et al., 2008 | Open-label, non-controlled clinical trial | Total n = 150 postmenopausal women | Vaginal suppositories (5 mg of LMWHA/suppository), one suppository for 28 days total | Decrease in vaginal dryness and itching, burning, and dyspareunia symptoms | [46] |
| La Galia et al., 2014 | Randomized, placebo- controlled clinical trial | Total n = 12 women with atrophic vaginitis (n = 6 treatment group; n = 6 placebo group) | Oral tablets (200 mg of vLMWHA/tablet), two tablets/day for 10 days; subsequently one tablet/day for three months | Increase in epithelium thickness and number of epithelial layers Decrease in itching, burning, and dyspareunia symptoms | [47] |
| Prestia, V.M. et al., 2020 | Non-controlled clinical trial | Total n = 50 menopausal women | One oral tablet (100 mg of LMWHA) and one suppository per day (5 mg of LMWHA) for 5 weeks; then one tablet per day for 10 months | Decrease in vaginal dryness and burning, itching, and dyspareunia perceptions | [48] |
| Chen, J. et al., 2013 | Multicenter, randomized, controlled, open-label, parallel-group clinical trial | Total n= 144 postmenopausal women (n = 72 treatment group; n = 72 control group) | Vaginal gel of LMWHA once every 3 days for a total of 10 applications over 30 days | Increase in vaginal dryness and itching, dyspareunia, and burning sensations | [52] |
| Jokar, A. et al., 2016 | Randomized, controlled clinical trial | Total n = 56 menopausal women (n = 28 treatment group; n = 28 = control group) | Vaginal cream (containing 5 mg of hyaluronic acid) for 8 weeks | Decrease in urinary incontinence, vaginal dryness, itching, and dyspareunia | [56] |
| Dinicola, S. et al., 2015 | Prospective, randomized clinical trial | Total n = 45 women in treatment with RT and BRT for cervical cancer (n = 23 treatment group; n = 22 control group) | Two vaginal suppositories (5 mg of LMWHA/suppository) for 4 months | Improvement in inflammation, cell atypia, fibrosis, mucositis, and bleeding | [59] |
| Delia, P. et al., 2019 | Randomized clinical trial | Total n = 180 women undergoing radiotherapy for CC (n = 88 treatment group; n = 89 control group) | Vaginal suppositories (5 mg of LMWHA/suppository) for 5 weeks | Lower intensity of pain; decrease in vaginal dryness, inflammation, and dyspareunia | [63] |
| Grandi, G. et al., 2022 | Case report | One patient with persistent HPV infection | Oral tabs (containing 50 mg of vLMWHA) | Increase in HPV lesions and viral clearance | [67] |
| Calcagno et al., 2024 | Case report | Total n = 5 patients with persistent HPV infection | Oral tabs (containing 50 mg of vLMWHA) | Increase in HPV lesions and viral clearance | [68] |
| Aragona et al., 2023 | Open-label, controlled clinical trial | Total n = 41 women with persistent HPV infection (n = 20 treatment group; n = 21 control group) | Oral tabs (containing 50 mg of vLMWHA) | Increase in HPV lesions and viral clearance | [69] |
| Tinelli, A. et al., 2024 | Open-label, controlled clinical trial | Total n = 163 women with HPV infection (n = 86 treatment group; n = 77 control group) | Oral tabs (containing 50 mg of vLMWHA) | Increase in HPV lesions and viral clearance | [70] |
| Porcaro, G. et al., 2025 | Non-controlled clinical trial | Total n = 106 women with HPV infection | Oral tabs (containing 50 mg of vLMWHA) | Increase in HPV lesions and viral clearance | [71] |
3. Role of High-Molecular-Weight Hyaluronic Acid (HMWHA) in Obstetric Field
3.1. HMWHA Supports Physiological Pregnancy
3.2. HMWHA Supports Embryonic Development
3.3. HMWHA Supports Endogenous Progesterone (P4)
3.4. HMWHA Helps in Counteracting Adverse Events in Pregnancy
| Study | Model and Design | Interventions | Findings | Ref. |
|---|---|---|---|---|
| Nakagawa, K. et al., 2012 | Fresh embryo transfer (fresh ET) | 0.5 mg/mL of hyaluronan or control medium | High concentration of HA supports the embryo during initial implantation into the endometrium | [75] |
| Nakamura, K. et al., 2004 | Mice (in vivo study) | 0.35% HMWHA in 0.5 ml solution versus vehicle | HMWHA inhibits pro-inflammatory factors (TNF-α or IFN-γ) | [77] |
| Wang, S. et al., 2019 | Trophoblast cells (Tros) | HMWHA (50 or 100 μg/mL) | HMWHA induces M2 polarization of macrophages at the maternal–fetal interface | [79] |
| Zhao, G. et al., 2014 | POI patients | Plasma endogenous levels of HMWHA | HMWHA correlates with PGRMC1 expression | [89] |
| Zhao, G. et al., 2014 | Granulosa cells (in vitro study) | HMWHA (100 µg/mL, 200 µg/mL, and 500 µg/mL) | HMWHA increases PGRMC1 expression in a time- and concentration-dependent manner | [89] |
| Cilaker Micili, S. et al., 2023 | Rats (in vivo study) | Low dose (2.5 mg) and high dose (5 mg) | HMWHA prevents PTB and decreases inflammatory cytokines (IL-1β and TNF-α) | [107] |
| Parente, E. et al., 2023 | Pregnant women (clinical study) | HMWHA (200 mg) in association with natural molecules versus control group | HMWHA prevents PTB and adverse events (pelvic pain, spontaneous contractions, miscarriages, and hospitalization) | [112] |
| Porcaro, G. et al., 2024 | Pregnant women (clinical study) | HMWHA (200 mg) in association with natural molecules in association with vaginal P4 versus control group | HMWHA induces SCH resorption faster and improves related symptoms (vaginal bleeding, abdominal pain, and uterine contractions) | [113] |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Meyer, K.; Palmer, J.W. The polysaccharide of the vitreous humor. J. Biol. Chem. 1934, 107, 629–634. [Google Scholar] [CrossRef]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The extracellular matrix at a glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef]
- Girish, K.; Kemparaju, K. The magic glue hyaluronan and its eraser hyaluronidase: A biological overview. Life Sci. 2007, 80, 1921–1943. [Google Scholar] [CrossRef]
- Sobolewski, K.; Bańkowski, E.; Chyczewski, L.; Jaworski, S. Collagen and glycosaminoglycans of Wharton’s jelly. Biol. Neonate 1997, 71, 11–21. [Google Scholar] [CrossRef]
- Robert, L.; Robert, A.-M.; Renard, G. Biological effects of hyaluronan in connective tissues, eye, skin, venous wall. Role in aging. Pathol. Biol. 2010, 58, 187–198. [Google Scholar] [CrossRef]
- Cyphert, J.M.; Trempus, C.S.; Garantziotis, S. Size Matters: Molecular Weight Specificity of Hyaluronan Effects in Cell Biology. Int. J. Cell Biol. 2015, 2015, 563818. [Google Scholar] [CrossRef]
- Fraser, J.R.; Laurent, T.C.; Laurent, U.B. Hyaluronan: Its nature, distribution, functions and turnover. J. Intern. Med. 1997, 242, 27–33. [Google Scholar] [CrossRef]
- Sturabotti, E.; Consalvi, S.; Tucciarone, L.; Macrì, E.; Di Lisio, V.; Francolini, I.; Minichiello, C.; Piozzi, A.; Vuotto, C.; Martinelli, A. Synthesis of Novel Hyaluronic Acid Sulfonated Hydrogels Using Safe Reactants: A Chemical and Biological Characterization. Gels 2022, 8, 480. [Google Scholar] [CrossRef]
- de Oliveira, J.D.; Carvalho, L.S.; Gomes, A.M.; Queiroz, L.R.; Magalhães, B.S.; Parachin, N.S. Genetic basis for hyper production of hyaluronic acid in natural and engineered microorganisms. Microb. Cell Fact. 2016, 15, 119. [Google Scholar] [CrossRef]
- Kaur, M.; Jayaraman, G. Hyaluronan production and molecular weight is enhanced in pathway-engineered strains of lactate dehydrogenase-deficient Lactococcus lactis. Metab. Eng. Commun. 2016, 3, 15–23. [Google Scholar] [CrossRef]
- Chien, L.J.; Lee, C.K. Enhanced hyaluronic acid production in Bacillus subtilis by coexpressing bacterial hemoglobin. Biotechnol. Prog. 2007, 23, 1017–1022. [Google Scholar] [CrossRef]
- Yu, H.; Stephanopoulos, G. Metabolic engineering of Escherichia coli for biosynthesis of hyaluronic acid. Metab. Eng. 2008, 10, 24–32. [Google Scholar] [CrossRef]
- Cheng, F.; Gong, Q.; Yu, H.; Stephanopoulos, G. High-titer biosynthesis of hyaluronic acid by recombinant Corynebacterium glutamicum. Biotechnol. J. 2016, 11, 574–584. [Google Scholar] [CrossRef]
- Volpi, N.; Schiller, J.; Stern, R.; Soltés, L. Role, metabolism, chemical modifications and applications of hyaluronan. Curr. Med. Chem. 2009, 16, 1718–1745. [Google Scholar] [CrossRef]
- Tammi, M.I.; Day, A.J.; Turley, E.A. Hyaluronan and homeostasis: A balancing act. J. Biol. Chem. 2002, 277, 4581–4584. [Google Scholar] [CrossRef]
- Itano, N.; Sawai, T.; Yoshida, M.; Lenas, P.; Yamada, Y.; Imagawa, M.; Shinomura, T.; Hamaguchi, M.; Yoshida, Y.; Ohnuki, Y.; et al. Three isoforms of mammalian hyaluronan synthases have distinct enzymatic properties. J. Biol. Chem. 1999, 274, 25085–25092. [Google Scholar] [CrossRef]
- Fallacara, A.; Baldini, E.; Manfredini, S.; Vertuani, S. Hyaluronic Acid in the Third Millennium. Polymers 2018, 10, 701. [Google Scholar] [CrossRef]
- Kotla, N.G.; Bonam, S.R.; Rasala, S.; Wankar, J.; Bohara, R.A.; Bayry, J.; Rochev, Y.; Pandit, A. Recent advances and prospects of hyaluronan as a multifunctional therapeutic system. J. Control Release 2021, 336, 598–620. [Google Scholar] [CrossRef]
- Balazs, E.A. Viscoelastic Properties of Hyaluronic Acid and Biological Lubrication. Univ. Mich. Med. Cent. J. 1968, 255–259. [Google Scholar] [PubMed]
- Day, A.J.; de la Motte, C.A. Hyaluronan Cross-Linking: A Protective Mechanism in Inflammation? Trends Immunol. 2005, 26, 637–643. [Google Scholar] [CrossRef]
- Atkins, E.D.; Sheehan, J.K. Structure for hyaluronic acid. Nat. New Biol. 1972, 235, 253–254. [Google Scholar] [CrossRef]
- Petrey, A.C.; de la Motte, C.A. Hyaluronan, a Crucial Regulator of Inflammation. Front. Immunol. 2014, 5, 101. [Google Scholar] [CrossRef]
- Tolg, C.; Messam, B.J.; McCarthy, J.B.; Nelson, A.C.; Turley, E.A. Hyaluronan Functions in Wound Repair That Are Captured to Fuel Breast Cancer Progression. Biomolecules 2021, 11, 1551. [Google Scholar] [CrossRef]
- Sudhakar, K.; Ji, S.M.; Kummara, M.R.; Han, S.S. Recent Progress on Hyaluronan-Based Products for Wound Healing Applications. Pharmaceutics 2022, 14, 2235. [Google Scholar] [CrossRef]
- Migliorini, F.; Maffulli, N.; Nijboer, C.H.; Pappalardo, G.; Pasurka, M.; Betsch, M.; Kubach, J. Comparison of Different Molecular Weights of Intra-Articular Hyaluronic Acid Injections for Knee Osteoarthritis: A Level I Bayesian Network Meta-Analysis. J. Biomed. 2025, 13, 175. [Google Scholar] [CrossRef]
- Kaul, A.; Short, W.D.; Keswani, S.G.; Wang, X. Immunologic Roles of Hyaluronan in Dermal Wound Healing. Biomolecules 2021, 11, 1234. [Google Scholar] [CrossRef]
- Shruti, T.; Rachana Kundur, R.; Siddhi, S.; Sugandhi, G.; Thangzuanlian, L.; Suma, S. Hyaluronan—A multipotent biomolecule in the field of medicine. J. Appl. Biol. Biotechnol. 2024, 12, 58–64. [Google Scholar]
- Humzah, D.; Molina, B.; Salti, G.; Cigni, C.; Bellia, G.; Grimolizzi, F. Intradermal Injection of Hybrid Complexes of High- and Low-Molecular-Weight Hyaluronan: Where Do We Stand and Where Are We Headed in Regenerative Medicine? Int. J. Mol. Sci. 2024 25, 3216. [CrossRef]
- Nappi, R.E.; Martella, S.; Albani, F.; Cassani, C.; Martini, E.; Landoni, F. Hyaluronic Acid: A Valid Therapeutic Option for Early Management of Genitourinary Syndrome of Menopause in Cancer Survivors? Healthcare 2022, 10, 1528. [Google Scholar] [CrossRef]
- Heymann, D.; Vidal, L.; Shoham, Z.; Kostova, E.; Showell, M.; Or, Y. The effect of hyaluronic acid in embryo transfer media in donor oocyte cycles and autologous oocyte cycles: A systematic review and meta-analysis. Hum. Reprod. 2022, 37, 1451–1469. [Google Scholar] [CrossRef]
- Dou, Y.; Yu, T.; Li, Z.; Wang, J.; Jiang, Y.; Liu, Y. Short- and Long-term Outcomes of Postoperative Intrauterine Application of Hyaluronic Acid Gel: A Meta-analysis of Randomized Controlled Trials. J. Minim. Invasive Gynecol. 2022, 29, 934–942. [Google Scholar] [CrossRef]
- Mazloomi, M.; Mohazzab, A.; Tahermanesh, K.; Fakehi, M.; Saeedzarandi, M.; Kooshari, Z.; Dehesh, P.; Ghaffari, S.R. The Efficacy of Hyaluronic Acid in Reducing Pelvic Adhesions in Patients Undergoing Gynecologic Laparoscopic Surgery: A Meta-Analysis of Randomized Clinical Trials. Health Sci. Rep. 2025, 8, e70887. [Google Scholar] [CrossRef]
- He, Y.; Tan, R.; Yu, H.; Mu, H.; Jin, H.; Dong, J.; Wang, W.; Wang, L.; Chen, S.; Wang, X. Comparative effectiveness and safety of 36 therapies or interventions for pregnancy outcomes with recurrent implantation failure: A systematic review and network meta-analysis. J. Assist. Reprod. Genet. 2023, 40, 2343–2356. [Google Scholar] [CrossRef]
- Buzzaccarini, G.; Marin, L.; Noventa, M.; Vitagliano, A.; Riva, A.; Dessole, F.; Capobianco, G.; Bordin, L.; Andrisani, A.; Ambrosini, G. Hyaluronic acid in vulvar and vaginal administration: Evidence from a literature systematic review. Climacteric 2021, 24, 560–571. [Google Scholar] [CrossRef]
- Balakrishnan, S.N.; Yamang, H.; Lorenz, M.C.; Chew, S.Y.; Than, L.T.L. Role of Vaginal Mucosa, Host Immunity and Microbiota in Vulvovaginal Candidiasis. Pathogens 2022, 11, 618. [Google Scholar] [CrossRef]
- Gold, J.M.; Shrimanker, I. Physiology, Vaginal; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Lacroix, G.; Gouyer, V.; Gottrand, F.; Desseyn, J.L. The Cervicovaginal Mucus Barrier. Int. J. Mol. Sci. 2020, 21, 8266. [Google Scholar] [CrossRef]
- Elstein, M. Cervical mucus: Its physiological role and clinical significance. Adv. Exp. Med. Biol. 1982, 144, 301–318. [Google Scholar]
- Aldunate, M.; Tyssen, D.; Johnson, A.; Zakir, T.; Sonza, S.; Moench, T.; Cone, R.; Tachedjian, G. Vaginal concentrations of lactic acid potently inactivate HIV. J. Antimicrob. Chemother. 2013, 68, 2015–2025. [Google Scholar] [CrossRef]
- Cruickshank, R. The conversion of the glycogen of the vagina into lactic acid. J. Pathol. Bacteriol. 1934, 39, 213–219. [Google Scholar] [CrossRef]
- Chen, X.; Lu, Y.; Chen, T.; Li, R. The Female Vaginal Microbiome in Health and Bacterial Vaginosis. Front. Cell Infect. Microbiol. 2021, 11, 631972. [Google Scholar] [CrossRef]
- Park, M.G.; Cho, S.; Oh, M.M. Menopausal Changes in the Microbiome-A Review Focused on the Genitourinary Microbiome. Diagnostics 2023, 13, 1193–1207. [Google Scholar] [CrossRef]
- The NAMS 2020 GSM Position Statement Editorial Panel. The 2020 genitourinary syndrome of menopause position statement of The North American Menopause Society. Menopause 2020, 27, 976–992. [Google Scholar] [CrossRef]
- Essendoubi, M.; Gobinet, C.; Reynaud, R.; Angiboust, J.F.; Manfait, M.; Piot, O. Human skin penetration of hyaluronic acid of different molecular weights as probed by Raman spectroscopy. Skin. Res. Technol. 2016, 22, 55–62. [Google Scholar] [CrossRef]
- Damodarasamy, M.; Johnson, R.S.; Bentov, I.; MacCoss, M.J.; Vernon, R.B.; Reed, M.J. Hyaluronan enhances wound repair and increases collagen III in aged dermal wounds. Wound Repair. Regen. 2014, 22, 521–526. [Google Scholar] [CrossRef]
- Costantino, D.; Guaraldi, C. Effectiveness, and safety of vaginal suppositories for the treatment of the vaginal atrophy in postmenopausal women: An open, non-controlled clinical trial. Eur. Rev. Med. Pharmacol. Sci. 2008, 12, 411–416. [Google Scholar]
- La Galia, T.; Micali, A.; Puzzolo, D.; Cancellieri, F. Oral Low-Molecular Weight Hyaluronic Acid in the Treatment of Atrophic Vaginitis. Int. J. Clin. Med. 2014, 5, 617–624. [Google Scholar] [CrossRef]
- Prestia, V.M.; Bertozzi, E.; Radice, M. Low-molecular weight hyaluronic acid for the treatment of vulvovaginal atrophy: An innovative clinical practice. Int. J. Med. Device Adjuv. Treat. 2020, 3, e260. [Google Scholar] [CrossRef]
- Prelevic, G.M.; Kocjan, T.; Markou, A. Hormone replacement therapy in postmenopausal women. Minerva Endocrinol. 2005, 30, 27–36. [Google Scholar]
- Beral, V.; Bull, D.; Reeves, G. Endometrial cancer and hormone-replacement therapy in the Million Women Study. Lancet 2005, 365, 1543–1551. [Google Scholar] [CrossRef]
- Pan, M.; Pan, X.; Zhou, J.; Wang, J.; Qi, Q.; Wang, L. Update on hormone therapy for the management of postmenopausal women. Biosci. Trends 2022, 16, 46–57. [Google Scholar] [CrossRef]
- Chen, J.; Geng, L.; Song, X.; Li, H.; Giordan, N.; Liao, Q. Evaluation of the efficacy and safety of hyaluronic acid vaginal gel to ease vaginal dryness: A multicenter, randomized, controlled, open-label, parallel-group, clinical trial. J. Sex. Med. 2013, 10, 1575–1584. [Google Scholar] [CrossRef]
- Chalkidou, A.; Oikonomou, E.; Lambrinos, D.; Bothou, A.; Kyriakou, D.; Nikolettos, K.; Marinos, G.; Iatrakis, G.; Zervoudis, S.; Nikolettos, N.; et al. The Comparative Study of the Administration of the Combination Preparation of Isoflavones and Hyaluronic Acid in Menopausal Women for the Treatment of the Symptoms of Menopause, Urogenital Atrophy and Oteoporosis in Relation to Existing Hormone Replacement Therapies. Mater. Sociomed. 2023, 35, 206–214. [Google Scholar] [CrossRef]
- Massarotti, C.; Asinaro, G.; Schiaffino, M.G.; Ronzini, C.; Vacca, I.; Lambertini, M.; Anserini, P.; Del Mastro, L.; Cagnacci, A. Vaginal oxygen plus hyaluronic acid on genito-urinary symptoms of breast cancer survivors. Climacteric 2023, 26, 129–134. [Google Scholar] [CrossRef]
- Faubion, S.S.; Larkin, L.C.; Stuenkel, C.A.; Bachmann, G.A.; Chism, L.A.; Kagan, R.; Kaunitz, A.M.; Krychman, M.L.; Parish, S.J.; Partridge, A.H.; et al. Management of genitourinary syndrome of menopause in women with or at high risk for breast cancer: Consensus recommendations from The North American Menopause Society and The International Society for the study of women’s sexual health. Menopause 2018, 25, 596–608. [Google Scholar] [CrossRef]
- Jokar, A.; Davari, T.; Asadi, N.; Ahmadi, F.; Foruhari, S. Comparison of the Hyaluronic Acid Vaginal Cream and Conjugated Estrogen Used in Treatment of Vaginal Atrophy of Menopause Women: A Randomized Controlled Clinical Trial. Int. J. Community Based Nurs. Midwifery 2016, 4, 69–78. [Google Scholar]
- Yang, H.; Song, L.; Zou, Y.; Sun, D.; Wang, L.; Yu, Z.; Guo, J. Role of Hyaluronic Acids and Potential as Regenerative Biomaterials in Wound Healing. ACS Appl. Bio Mater. 2021, 4, 311–324. [Google Scholar] [CrossRef]
- Majeed, H.; Gupta, V. Adverse Effects of Radiation Therapy. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Dinicola, S.; Pasta, V.; Costantino, D.; Guarald i, C.; Bizzarri, M. Hyaluronic acid and vitamins are effective in reducing vaginal atrophy in women receiving radiotherapy. Minerva Ginecol. 2015, 67, 523–531. [Google Scholar]
- Varytė, G.; Bartkevičienė, D. Pelvic Radiation Therapy Induced Vaginal Stenosis: A Review of Current Modalities and Recent Treatment Advances. Medicina 2021, 57, 336. [Google Scholar] [CrossRef]
- Bruner, D.W.; Lanciano, R.; Keegan, M.; Corn, B.; Martin, E.; Hanks, G.E. Vaginal stenosis and sexual function following intracavitary radiation for the treatment of cervical and endometrial carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 1993, 27, 825–830. [Google Scholar] [CrossRef]
- Miles, T.; Johnson, N. Vaginal dilator therapy for women receiving pelvic radiotherapy. Cochrane Database Syst. Rev. 2014, 2014, CD007291. [Google Scholar] [CrossRef]
- Delia, P.; Sansotta, G.; Pontoriero, A.; Iati, G.; De Salvo, S.; Pisana, M.; Potami, A.; Lopes, S.; Messina, G.; Pergolizzi, S. Clinical Evaluation of Low-Molecular-Weight Hyaluronic Acid-Based Treatment on Onset of Acute Side Effects in Women Receiving Adjuvant Radiotherapy after Cervical Surgery: A Randomized Clinical Trial. Oncol. Res. Treat. 2019, 42, 217–223. [Google Scholar] [CrossRef]
- Giuliano, A.R.; Nyitray, A.G.; Kreimer, A.R.; Pierce Campbell, C.M.; Goodman, M.T.; Sudenga, S.L.; Monsonego, J.; Franceschi, S. EUROGIN 2014 roadmap: Differences in human papillomavirus infection natural history, transmission and human papillomavirus-related cancer incidence by gender and anatomic site of infection. Int. J. Cancer 2015, 136, 2752–2760. [Google Scholar] [CrossRef]
- Egawa, N.; Egawa, K.; Griffin, H.; Doorbar, J. Human Papillomaviruses; Epithelial Tropisms, and the Development of Neoplasia. Viruses 2015, 7, 3863–3890. [Google Scholar] [CrossRef]
- Frega, A.; Gentili, C.; Proietti, S.; Lepore, E.; Unfer, V.; Fuso, A. Epigallocatechin gallate, folic acid, vitamin B12, and hyaluronic acid significantly increase apoptosis and p53 expression in HeLa cells. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 5240–5245. [Google Scholar] [CrossRef]
- Grandi, G.; Botticelli, L.; Fraia, P.D.; Babalini, C.; Masini, M.; Unfer, V. The Association of Four Natural Molecules-EGCG, Folic Acid, Vitamin B12, and HA-To Counteract HPV Cervical Lesions: A Case Report. J. Pers. Med. 2023, 13, 567. [Google Scholar] [CrossRef]
- Calcagno, M.; Incocciati, B.; Di Fraia, L.; Unfer, V. Counteracting HPV Cervical and Anal Infection through Dietary Supplementation of EGCG, Folic Acid, Vitamin B12 and Hyaluronic Acid: Clinical Case Reports. J. Clin. Med. 2024, 13, 3597. [Google Scholar] [CrossRef]
- Aragona, C.; Bezerra Espinola, M.S.; Bilotta, G.; Porcaro, G.; Calcagno, M. Evaluating the Efficacy of Pervistop(®), a New Combination Based on EGCG, Folic Acid, Vitamin B12 and Hyaluronic Acid on Patients with Human Papilloma Virus (HPV) Persistent Infections and Cervical Lesions: A Pilot Study. J. Clin. Med. 2023, 12, 2171. [Google Scholar] [CrossRef] [PubMed]
- Tinelli, A.; Gustapane, S.; Licchelli, M.; Coluccia, A.C.; Panese, G.; Proietti, S.; Gambioli, R. Treatment with Epigallocatechin Gallate, Folic Acid, Vitamin B12, and Hyaluronic Acid Decreases HPV Positivity in Women Attending Regional Screening in Puglia. Microorganisms 2024, 12, 1897. [Google Scholar] [CrossRef]
- Porcaro, G.; Pavone-Cossut, M.R.; Moretti, S.; Bilotta, G.; Aragona, C.; Unfer, V. Oral Treatment with EGCG, Folic Acid, Vitamin B12, and Hyaluronic Acid Improves HPV Clearance and Counteracts Its Persistence: A Clinical Study. Int. J. Mol. Sci. 2025, 26, 5251. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Wang, S.-C.; Sun, C.; Tao, Y.; Piao, H.L.; Wang, X.Q.; Du, M.R.; Li, D.-J. Hyaluronan-CD44 interaction promotes growth of decidual stromal cells in human first-trimester pregnancy. PLoS ONE 2013, 8, e74812. [Google Scholar] [CrossRef]
- Unfer, V.; Tilotta, M.; Kaya, C.; Noventa, M.; Török, P.; Alkatout, I.; Gitas, G.; Bilotta, G.; Laganà, A.S. Absorption, distribution, metabolism and excretion of hyaluronic acid during pregnancy: A matter of molecular weight. Expert. Opin. Drug Metab. Toxicol. 2021, 17, 823–840. [Google Scholar] [CrossRef]
- Jiang, D.; Liang, J.; Noble, P.W. Hyaluronan as an immune regulator in human diseases. Physiol. Rev. 2011, 91, 221–264. [Google Scholar] [CrossRef]
- Nakagawa, K.; Takahashi, C.; Nishi, Y.; Jyuen, H.; Sugiyama, R.; Kuribayashi, Y.; Sugiyama, R. Hyaluronan-enriched transfer medium improves outcome in patients with multiple embryo transfer failures. J. Assist. Reprod. Genet. 2012, 29, 679–685. [Google Scholar] [CrossRef][Green Version]
- Zhu, R.; Huang, Y.H.; Tao, Y.; Wang, S.C.; Sun, C.; Piao, H.L.; Wang, X.Q.; Du, M.R.; Li, D.J. Hyaluronan up-regulates growth and invasion of trophoblasts in an autocrine manner via PI3K/AKT and MAPK/ERK1/2 pathways in early human pregnancy. Placenta 2013, 34, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Yokohama, S.; Yoneda, M.; Okamoto, S.; Tamaki, Y.; Ito, T.; Okada, M.; Aso, K.; Makino, I. High, but not low, molecular weight hyaluronan prevents T-cell-mediated liver injury by reducing proinflammatory cytokines in mice. J. Gastroenterol. 2004, 39, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Azizieh, F.Y.; Raghupathy, R.G. Tumor necrosis factor-α and pregnancy complications: A prospective study. Med. Princ. Pract. 2015, 24, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Sun, F.; Han, M.; Liu, Y.; Zou, Q.; Wang, F.; Tao, Y.; Li, D.; Du, M.; Li, H.; et al. Trophoblast-derived hyaluronan promotes the regulatory phenotype of decidual macrophages. Reproduction 2019, 157, 189–198. [Google Scholar] [CrossRef]
- Camenisch, T.D.; Spicer, A.P.; Brehm-Gibson, T.; Biesterfeldt, J.; Augustine, M.L.; Calabro, A., Jr.; Kubalak, S.; Klewer, S.E.; McDonald, J.A. Disruption of hyaluronan synthase-2 abrogates normal cardiac morphogenesis and hyaluronan-mediated transformation of epithelium to mesenchyme. J. Clin. Investig. 2000, 106, 349–360. [Google Scholar] [CrossRef]
- Sivakumar, A.; Mahadevan, A.; Lauer, M.E.; Narvaez, R.J.; Ramesh, S.; Demler, C.M.; Souchet, N.R.; Hascall, V.C.; Midura, R.J.; Garantziotis, S.; et al. Midgut Laterality Is Driven by Hyaluronan on the Right. Dev. Cell 2018, 46, 533–551.e5. [Google Scholar] [CrossRef]
- Savin, T.; Kurpios, N.A.; Shyer, A.E.; Florescu, P.; Liang, H.; Mahadevan, L.; Tabin, C.J. On the growth and form of the gut. Nature 2011, 476, 57–62. [Google Scholar] [CrossRef]
- Applegate, K.E. Evidence-based diagnosis of malrotation and volvulus. Pediatr. Radiol. 2009, 39 (Suppl. S2), S161–S163. [Google Scholar] [CrossRef] [PubMed]
- Kurpios, N.A.; Ibañes, M.; Davis, N.M.; Lui, W.; Katz, T.; Martin, J.F.; Izpisúa Belmonte, J.C.; Tabin, C.J. The direction of gut looping is established by changes in the extracellular matrix and in cell:cell adhesion. Proc. Natl. Acad. Sci. USA 2008, 105, 8499–8506. [Google Scholar] [CrossRef] [PubMed]
- Casini, P.; Nardi, I.; Ori, M. Hyaluronan is required for cranial neural crest cells migration and craniofacial development. Dev. Dyn. 2012, 241, 294–302. [Google Scholar] [CrossRef]
- Yonemitsu, M.A.; Lin, T.Y.; Yu, K. Hyaluronic acid is required for palatal shelf movement and its interaction with the tongue during palatal shelf elevation. Dev. Biol. 2020, 457, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Li, Y.; Jakuba, C.; Sugiyama, Y.; Sayo, T.; Okuno, M.; Dealy, C.N.; Toole, B.P.; Takeda, J.; Yamaguchi, Y.; et al. Conditional inactivation of Has2 reveals a crucial role for hyaluronan in skeletal growth, patterning, chondrocyte maturation and joint formation in the developing limb. Development 2009, 136, 2825–2835. [Google Scholar] [CrossRef]
- Archer, C.W.; Dowthwaite, G.P.; Francis-West, P. Development of synovial joints. Birth Defects Res. C Embryo Today 2003, 69, 144–155. [Google Scholar] [CrossRef]
- Zhao, G.; Zhou, X.; Fang, T.; Hou, Y.; Hu, Y. Hyaluronic acid promotes the expression of progesterone receptor membrane component 1 via epigenetic silencing of miR-139-5p in human and rat granulosa cells. Biol. Reprod. 2014, 91, 116-1. [Google Scholar] [CrossRef]
- MacDonald, P.C.; Dombroski, R.A.; Casey, M.L. Recurrent secretion of progesterone in large amounts: An endocrine/metabolic disorder unique to young women? Endocr. Rev. 1991, 4, 372–401. [Google Scholar] [CrossRef]
- Mesiano, S. Roles of estrogen and progesterone in human parturition. Front. Horm. Res. 2001, 27, 86–104. [Google Scholar]
- Gellersen, B.; Fernandes, M.S.; Brosens, J.J. Non-genomic progesterone actions in female reproduction. Hum. Reprod. Update. 2009, 15, 119–138. [Google Scholar] [CrossRef]
- Thomas, P. Characteristics of membrane progestin receptor alpha (mPRalpha) and progesterone membrane receptor component 1 (PGMRC1) and their roles in mediating rapid progestin actions. Front. Neuroendocrinol. 2008, 29, 292–312. [Google Scholar] [CrossRef] [PubMed]
- Cahill, M.A. Progesterone receptor membrane component 1: An integrative review. J. Steroid Biochem. Mol. Biol. 2007, 105, 16–36. [Google Scholar] [CrossRef]
- Rohe, H.J.; Ahmed, I.S.; Twist, K.E.; Craven, R.J. PGRMC1 (progesterone receptor membrane component 1): A targetable protein with multiple functions in steroid signaling, P450 activation and drug binding. Pharmacol. Ther. 2009, 121, 14–19. [Google Scholar] [CrossRef]
- Peluso, J.J.; Liu, X.; Gawkowska, A.; Lodde, V.; Wu, C.A. Progesterone inhibits apoptosis in part by PGRMC1-regulated gene expression. Mol. Cell Endocrinol. 2010, 320, 153–161. [Google Scholar] [CrossRef]
- Mansouri, M.R.; Schuster, J.; Badhai, J.; Stattin, E.L.; Lösel, R.; Wehling, M.; Carlsson, B.; Hovatta, O.; Karlström, P.O.; Golovleva, I.; et al. Alterations in the expression, structure and function of progesterone receptor membrane component-1 (PGRMC1) in premature ovarian failure. Hum. Mol. Genet. 2008, 7, 3776–3783. [Google Scholar] [CrossRef]
- Wu, W.; Shi, S.-Q.; Huang, H.J.; Balducci, J.; Garfield, R.E. Changes in PGRMC1, a potential progesterone receptor, in human myometrium during pregnancy and labour at term and preterm. Mol. Hum. Reprod. 2011, 17, 233–242. [Google Scholar] [CrossRef]
- Feng, L.; Antczak, B.C.; Lan, L.; Grotegut, C.A.; Thompson, J.L.; Allen, T.K.; Murtha, A.P. Progesterone receptor membrane component 1 (PGRMC1) expression in fetal membranes among women with preterm premature rupture of the membranes (PPROM). Placenta 2014, 35, 331–333. [Google Scholar] [CrossRef]
- Choi, S.R.; Choi, H.E.; Jo, E.; Choi, H.Y.; Jung, S.; Jang, S.; Choi, S.J.; Hwang, S.O. Decreased expression of progesterone receptor membrane component 1 in fetal membranes with chorioamnionitis among women with preterm birth. Arch. Gynecol. Obstet. 2020, 301, 949–954. [Google Scholar] [CrossRef]
- Fouladi-Nashta, A.A.; Raheem, K.A.; Marei, W.F.; Ghafari, F.; Hartshorne, G.M. Regulation and roles of the hyaluronan system in mammalian reproduction. Reproduction 2017, 153, R43–R58. [Google Scholar] [CrossRef] [PubMed]
- Akgul, Y.; Holt, R.; Mummert, M.; Word, A.; Mahendroo, M. Dynamic changes in cervical glycosaminoglycan composition during normal pregnancy and preterm birth. Endocrinology 2012, 153, 3493–3503. [Google Scholar] [CrossRef] [PubMed]
- Kolar, S.L.; Kyme, P.; Tseng, C.W.; Soliman, A.; Kaplan, A.; Liang, J.; Nizet, V.; Jiang, D.; Murali, R.; Arditi, M.; et al. Group B Streptococcus Evades Host Immunity by Degrading Hyaluronan. Cell Host Microbe 2015, 18, 694–704. [Google Scholar] [CrossRef]
- Vornhagen, J.; Quach, P.; Boldenow, E.; Merillat, S.; Whidbey, C.; Ngo, L.Y.; Adams Waldorf, K.M.; Rajagopal, L. Bacterial Hyaluronidase Promotes Ascending GBS Infection and Preterm Birth. mBio 2016, 7, e00781-16. [Google Scholar] [CrossRef]
- Campo, G.M.; Avenoso, A.; Nastasi, G.; Micali, A.; Prestipino, V.; Vaccaro, M.; D’Ascola, A.; Calatroni, A.; Campo, S. Hyaluronan reduces inflammation in experimental arthritis by modulating TLR-2 and TLR-4 cartilage expression. Biochim. Biophys. Acta 2011, 1812, 1170–1181. [Google Scholar] [CrossRef]
- Lee, B.M.; Park, S.J.; Noh, I.; Kim, C.H. The effects of the molecular weights of hyaluronic acid on the immune responses. Biomater. Res. 2021, 25, 27. [Google Scholar] [CrossRef] [PubMed]
- Cilaker Micili, S.; Tarı, O.; Neri, I.; Proietti, S.; Unfer, V. Does high molecular weight-hyaluronic acid prevent hormone-induced preterm labor in rats? Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 3022–3032. [Google Scholar] [CrossRef] [PubMed]
- De Souza, A.B.; Chaud, M.V.; Santana, M.H.A. Hyaluronic acid behavior in oral administration and perspectives for nanotechnology-based formulations: A review. Carbohydr. Polym. 2019, 15, 115001. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M.; Maeshima, T.; Kubota, T.; Kurihara, H.; Masuda, Y.; Nomura, Y. Absorption of Orally Administered Hyaluronan. J. Med. Food 2016, 19, 1172–1179. [Google Scholar] [CrossRef]
- Oe, M.; Tashiro, T.; Yoshida, H.; Nishiyama, H.; Masuda, Y.; Maruyama, K.; Koikeda, T.; Maruya, R.; Fukui, N.; Nutr, J. Oral hyaluronan relieves knee pain: A review. Nutr. J. 2016, 15, 11. [Google Scholar] [CrossRef]
- Dawes, J.; Hodson, B.A.; Pepper, D.S. The absorption, clearance and metabolic fate of dermatan sulphate administered to man--studies using a radioiodinated derivative. Thromb. Haemost. 1989, 62, 945–949. [Google Scholar] [CrossRef]
- Parente, E.; Colannino, G.; Bilotta, G.; Espinola, M.S.B.; Proietti, S.; Oliva, M.M.; Neri, I.; Aragona, C.; Unfer, V. Effect of Oral High Molecular Weight Hyaluronic Acid (HMWHA), Alpha Lipoic Acid (ALA), Magnesium, Vitamin B6 and Vitamin D Supplementation in Pregnant Women: A Retrospective Observational Pilot Study. Clin. Pract. 2023, 13, 1123–1129. [Google Scholar] [CrossRef]
- Porcaro, G.; Laganà, A.S.; Neri, I.; Aragona, C. The Association of High-Molecular-Weight Hyaluronic Acid (HMWHA), Alpha Lipoic Acid (ALA), Magnesium, Vitamin B6, and Vitamin D Improves Subchorionic Hematoma Resorption in Women with Threatened Miscarriage: A Pilot Clinical Study. J. Clin. Med. 2024, 13, 706. [Google Scholar] [CrossRef] [PubMed]
- Mouri, M.I.; Hall, H.; Rupp, T.J. Threatened Abortion. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Sharma, B.; Deep, J.; Pandit, C.; Basnyat, B.; Khanal, B.; Raut, B.B.; Rajak, B.M.; Patel, D.; Laikangbam, R.; Basyal, R. Overview on current approach on recurrent miscarriage and threatened miscarriage. Clin. J. Obstet. Gynecol. 2020, 3, 151–157. [Google Scholar] [CrossRef]
- Xiang, L.; Wei, Z.; Cao, Y. Symptoms of an intrauterine hematoma associated with pregnancy complications: A systematic review. PLoS ONE 2014, 9, e111676. [Google Scholar] [CrossRef]
- Leite, J.; Ross, P.; Rossi, A.C.; Jeanty, P. Prognosis of very large first-trimester hematomas. J. Ultrasound Med. 2006, 25, 141–1445. [Google Scholar] [CrossRef]
- Zhang, Y.; Fan, Z.; Wang, J.; Guan, B.; Zhou, F.; Tang, Z.; Wu, W.; Huang, A. HPV vaccination, screening disparities, and the shifting landscape of cervical cancer burden: A global analysis of trends, inequalities, and policy implications. BMC Womens Health 2025, 25, 285. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Lyu, Y.; Li, J.; Li, Y.; Chi, C. Global, regional, and national burden of preterm birth, 1990-2021: A systematic analysis from the global burden of disease study 2021. EClinicalMedicine 2024, 76, 102840. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porcaro, G.; Mappa, I.; Leonforte, F.; Baldini, G.M.; Guarneri, M.F.; La Verde, M.; Sorrentino, F.; Laganà, A.S. Hyaluronic Acid in Female Reproductive Health: Tailoring Molecular Weight to Clinical Needs in Obstetric and Gynecological Fields. Pharmaceutics 2025, 17, 991. https://doi.org/10.3390/pharmaceutics17080991
Porcaro G, Mappa I, Leonforte F, Baldini GM, Guarneri MF, La Verde M, Sorrentino F, Laganà AS. Hyaluronic Acid in Female Reproductive Health: Tailoring Molecular Weight to Clinical Needs in Obstetric and Gynecological Fields. Pharmaceutics. 2025; 17(8):991. https://doi.org/10.3390/pharmaceutics17080991
Chicago/Turabian StylePorcaro, Giuseppina, Ilenia Mappa, Francesco Leonforte, Giorgio Maria Baldini, Maria Francesca Guarneri, Marco La Verde, Felice Sorrentino, and Antonio Simone Laganà. 2025. "Hyaluronic Acid in Female Reproductive Health: Tailoring Molecular Weight to Clinical Needs in Obstetric and Gynecological Fields" Pharmaceutics 17, no. 8: 991. https://doi.org/10.3390/pharmaceutics17080991
APA StylePorcaro, G., Mappa, I., Leonforte, F., Baldini, G. M., Guarneri, M. F., La Verde, M., Sorrentino, F., & Laganà, A. S. (2025). Hyaluronic Acid in Female Reproductive Health: Tailoring Molecular Weight to Clinical Needs in Obstetric and Gynecological Fields. Pharmaceutics, 17(8), 991. https://doi.org/10.3390/pharmaceutics17080991








