Hyaluronic Acid Hampers the Inflammatory Response Elicited by Extracellular Vesicles from Activated Monocytes in Human Chondrocytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Isolation of Primary Chondrocytes
2.3. Cell Culture
2.4. Isolation of EVs
2.5. Treatment with CLHyA, EVs− or EVs+
2.6. The Determination of Cell Viability with the MTT Assay
2.7. Determination of IL-6 and MMP-13
2.8. Characterization of EVs− and EVs+ from THP-1 Cells
2.9. Quantification of Exosomal Markers in EVs and EVs+
2.10. Internalization of EVs−/EVs+ in COA and HC Cells
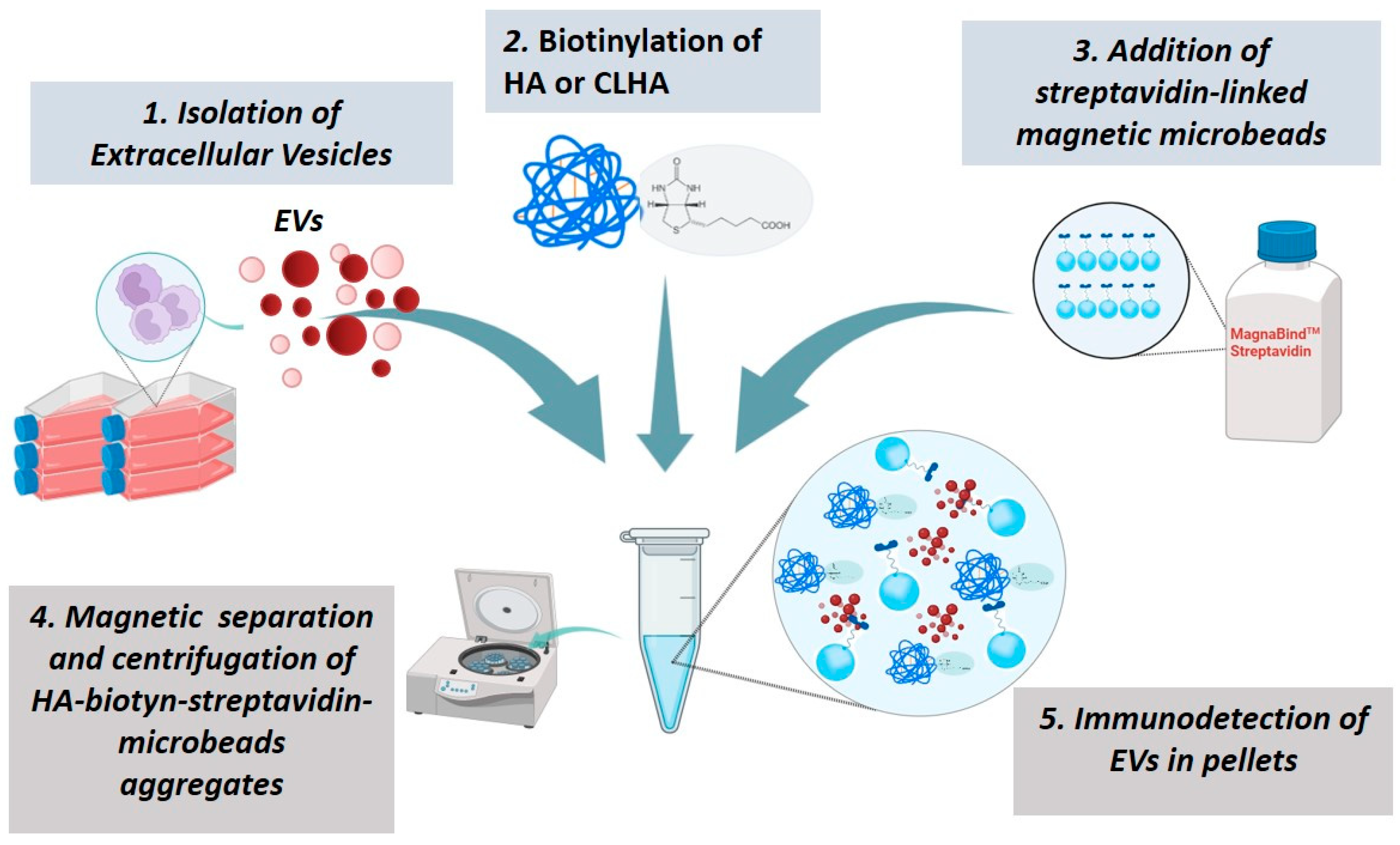
2.11. HyA or CLHyA Binding to EVs
2.12. Western Blotting Analysis
2.13. Statistical Analysis
3. Results
3.1. Effect of CLHyA on the Viability and Inflammatory State of COA and HC Chondrocytes
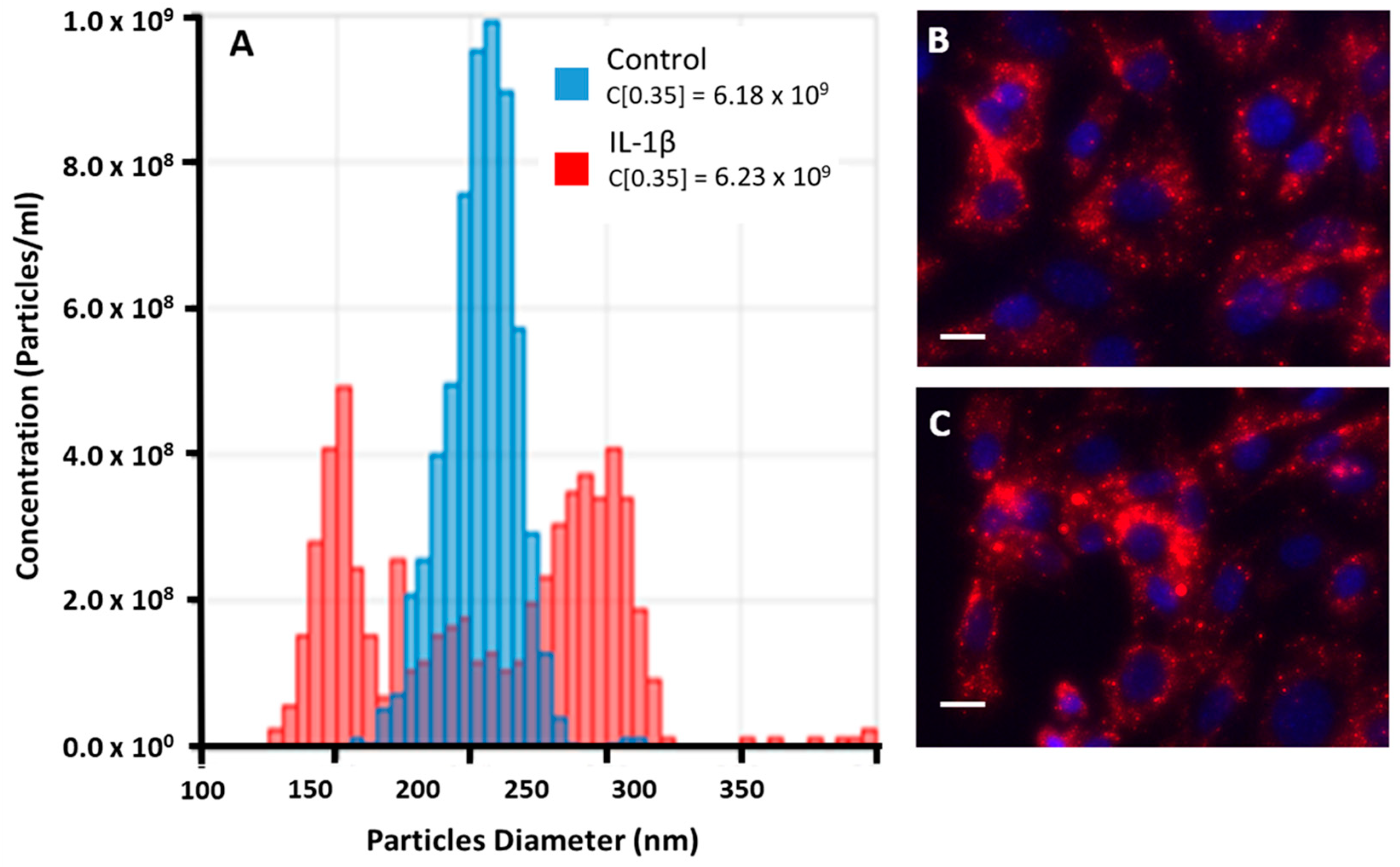
3.2. Analysis of EVs from Unstimulated or Stimulated THP-1 Cell Line
3.3. EVs− and EVs+ Cellular Internalization
3.4. The Effect of CLHyA on the Levels of IL-6 and MMP-13 in Chondrocytes Exposed to EVs− or EVs+
3.5. Interactions Between EVs+ and HyA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, H.A. Osteoarthritis-Insights from recent research. J. Rheum. Dis. 2022, 29, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Ashford, S.; Williard, J. Osteoarthritis: A review. Nurse Pract. 2014, 39, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Werb, Z.; Tremble, P.M.; Behrendtsen, O.; Crowley, E.; Damsky, C.H. Signal transduction through the fibronectin receptor induces collagenase and stromelysin gene expression. J. Cell Biol. 1989, 109, 877–889. [Google Scholar] [CrossRef] [PubMed]
- Homandberg, G.A.; Meyers, R.; Xie, D.L. Fibronectin fragments cause chondrolysis of bovine articular cartilage slices in culture. J. Biol. Chem. 1992, 267, 3597–3604. [Google Scholar] [CrossRef]
- Yasuda, T.; Poole, A.R. A fibronectin fragment induces type II collagen degradation by collagenase through an interleukin-1-mediated pathway. Arthritis Rheum. 2002, 46, 138–148. [Google Scholar] [CrossRef]
- Yasuda, T.; Poole, A.R.; Shimizu, M.; Nakagawa, T.; Julovi, S.M.; Tamamura, H.; Fujii, N.; Nakamura, T. Involvement of CD44 in induction of matrix metalloproteinases by a COOH-terminal heparin-binding fragment of fibronectin in human articular cartilage in culture. Arthritis Rheum. 2003, 48, 1271–1280. [Google Scholar] [CrossRef]
- Volpi, N.; Schiller, J.; Stern, R.; Soltes, L. Role, Metabolism, Chemical Modifications and Applications of Hyaluronan. Curr. Med. Chem. 2009, 16, 1718–1745. [Google Scholar] [CrossRef]
- Fraser, J.R.; Laurent, T.C.; Laurent, U.B. Hyaluronan: Its nature, distribution, functions and turnover. J. Intern. Med. 1997, 242, 27–33. [Google Scholar] [CrossRef]
- Knopf-Marques, H.; Pravda, M.; Wolfova, L.; Velebny, V.; Schaaf, P.; Vrana, N.E.; Lavalle, P. Hyaluronic Acid and Its Derivatives in Coating and Delivery Systems: Applications in Tissue Engineering, Regenerative Medicine and Immunomodulation. Adv. Healthc. Mater. 2016, 5, 2841–2855. [Google Scholar] [CrossRef]
- Ebid, R.; Lichtnekert, J.; Anders, H.J. Hyaluronan Is Not a Ligand but a Regulator of Toll-Like Receptor Signaling in Mesangial Cells: Role of Extracellular Matrix in Innate Immunity. ISRN Nephrol. 2014, 2014, 714081. [Google Scholar] [CrossRef]
- Maheu, E.; Bannuru, R.R.; Herrero-Beaumont, G.; Allali, F.; Bard, H.; Migliore, A. Why we should definitely include intra-articular hyaluronic acid as a therapeutic option in the management of knee osteoarthritis: Results of an extensive critical literature review. Semin. Arthritis Rheum. 2019, 48, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Migliore, A.; Frediani, B.; Gigliucci, G.; Anichini, S.E.; Cassol, M.; Crimaldi, S.; De Lucia, O.; Iolascon, G.; Foti, C. One-year follow-up showing effects of single intra-articular injection of hyaluronic acid (1,500–2,000 kDa) in symptomatic knee osteoarthritis. J. Biol. Regul. Homeost. Agents. 2018, 32, 1433–1441. [Google Scholar]
- Barbieri, E.; Capparucci, I.; Mannello, F.; Annibalini, G.; Contarelli, S.; Vallorani, L.; Gioacchini, A.M.; Ligi, D.; Maniscalco, R.; Gervasi, M.; et al. Efficacy of a Treatment for Gonarthrosis Based on the Sequential Intra-Articular Injection of Linear and Cross-Linked Hyaluronic Acids. Muscle Ligaments Tendons J. 2019, 9, 606. [Google Scholar] [CrossRef]
- Kreger, S.T.; Voytik-Harbin, S.L. Hyaluronan concentration within a 3D collagen matrix modulates matrix viscoelasticity, but not fibroblast response. Matrix Biol. 2009, 28, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, K.; Ochi, M.; Uchio, Y.; Adachi, N.; Matsusaki, M. Hyaluronic acid enhances proliferation and chondroitin sulfate synthesis in cultured chondrocytes embedded in collagen gels. J. Cell. Physiol. 1999, 179, 142–148. [Google Scholar] [CrossRef]
- Frean, S.P.; Abraham, L.A.; Lees, P. In vitro stimulation of equine articular cartilage proteoglycan synthesis by hyaluronan and carprofen. Res. Vet. Sci. 1999, 67, 183–190. [Google Scholar] [CrossRef]
- Takahashi, K.; Goomer, R.S.; Harwood, F.; Kubo, T.; Hirasawa, Y.; Amiel, D. The effects of hyaluronan on matrix metalloproteinase-3 (MMP-3), interleukin-1β(IL-1β), and tissue inhibitor of metalloproteinase-1 (TIMP-1) gene expression during the development of osteoarthritis. Osteoarthr. Cartil. 1999, 7, 182–190. [Google Scholar] [CrossRef]
- Goto, M.; Hanyu, T.; Yoshio, T.; Matsuno, H.; Shimizu, M.; Murata, N.; Shiozawa, S.; Matsubara, T.; Yamana, S.; Matsuda, T. Intra-articular injection of hyaluronate (SI-6601D) improves joint pain and synovial fluid prostaglandin E2 levels in rheumatoid arthritis: A multicenter clinical trial. Clin. Exp. Rheumatol. 2001, 19, 377–383. [Google Scholar]
- Withrow, J.; Murphy, C.; Liu, Y.; Hunter, M.; Fulzele, S.; Hamrick, M.W. Extracellular vesicles in the pathogenesis of rheumatoid arthritis and osteoarthritis. Arthritis Res. Ther. 2016, 18, 286. [Google Scholar] [CrossRef]
- Xie, F.; Liu, Y.; Chen, X.; Li, Q.; Zhong, J.; Dai, B.; Shao, X.; Wu, G. Role of MicroRNA, LncRNA, and Exosomes in the Progression of Osteoarthritis: A Review of Recent Literature. Orthop. Surg. 2020, 12, 708–716. [Google Scholar] [CrossRef]
- Gibbings, D.J.; Ciaudo, C.; Erhardt, M.; Voinnet, O. Multivesicular bodies associate with components of miRNA effector complexes and modulate miRNA activity. Nat. Cell. Biol. 2009, 11, 1143–1149. [Google Scholar] [CrossRef] [PubMed]
- Robbins, P.D.; Dorronsoro, A.; Booker, C.N. Regulation of chronic inflammatory and immune processes by extracellular vesicles. J. Clin. Investig. 2016, 126, 1173–1180. [Google Scholar] [CrossRef] [PubMed]
- Alcaraz, M.J.; Compañ, A.; Guillén, M.I. Extracellular Vesicles from Mesenchymal Stem Cells as Novel Treatments for Musculoskeletal Diseases. Cells 2019, 9, 98. [Google Scholar] [CrossRef] [PubMed]
- Alcaraz, M.J.; Guillén, M.I.; Ferrándiz, M.L. Emerging therapeutic agents in osteoarthritis. Biochem. Pharmacol. 2019, 165, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.C.; Song, S.J.; Zhang, Y.; Li, X. Role of Extracellular Vesicles in Autoimmune Pathogenesis. Front. Immunol. 2020, 1, 579043. [Google Scholar] [CrossRef]
- Kato, T.; Miyaki, S.; Ishitobi, H.; Nakamura, Y.; Nakasa, T.; Lotz, M.K.; Ochi, M. Exosomes from IL-1β stimulated synovial fibroblasts induce osteoarthritic changes in articular chondrocytes. Arthritis Res. Ther. 2014, 16, R163. [Google Scholar] [CrossRef]
- Domenis, R.; Zanutel, R.; Caponnetto, F.; Toffoletto, B.; Cifù, A.; Pistis, C.; Di Benedetto, P.; Causero, A.; Pozzi, M.; Bassini, F.; et al. Characterization of the Proinflammatory Profile of Synovial Fluid-Derived Exosomes of Patients with Osteoarthritis. Mediat. Inflamm. 2017, 2017, 4814987. [Google Scholar] [CrossRef]
- Carrabs, V. Role of Microvesicles in Gonarthrosis and Their Modulation by Hyaluronic Acid Administered in Viscosupplementation for the Rational Development of Innovative Medical Devices. Ph.D. Thesis, Biomolecular and Health Sciences, University of Urbino Carlo Bo, Urbino, Italy, 2023. [Google Scholar]
- Guillén, M.I.; Tofiño-Vian, M.; Silvestre, A.; Castejón, M.A.; Alcaraz, M.J. Role of peroxiredoxin 6 in the chondroprotective effects of microvesicles from human adipose tissue-derived mesenchymal stem cells. J. Orthop. Translat. 2021, 30, 61–69. [Google Scholar] [CrossRef]
- Tezel, A.; Fredrickson, G.H. The science of hyaluronic acid dermal fillers. J. Cosmet. Laser Ther. 2014, 16, 45. [Google Scholar] [CrossRef]
- Guescini, M.; Leo, G.; Genedani, S.; Carone, C.; Pederzoli, F.; Ciruela, F.; Guidolin, D.; Stocchi, V.; Mantuano, M.; Borroto-Escuela, D.; et al. Microvesicle and tunneling nanotube mediated intercellular transfer of g-protein coupled receptors in cell cultures. Exp. Cell. Res. 2012, 318, 603–613. [Google Scholar] [CrossRef]
- Necas, J.; Bartosikova, L.; Brauner, P.; Kolar, J. Hyaluronic acid (hyaluronan): A review. Vet. Med. 2008, 53, 397–411. [Google Scholar] [CrossRef]
- Rayahin, J.E.; Buhrman, J.S.; Zhang, Y.; Koh, T.J.; Gemeinhart, R.A. High and Low Molecular Weight Hyaluronic Acid Differentially Influence Macrophage Activation. ACS Biomater. Sci. Eng. 2015, 1, 481–493. [Google Scholar]
- Michalczyk, M.; Humeniuk, E.; Adamczuk, G.; Korga-Plewko, A. Hyaluronic Acid as a Modern Approach in Anticancer Therapy-Review. Int. J. Mol. Sci. 2022, 24, 103. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.G.; Williams, J.C.; Davis, B.K.; Jacobson, K.; Doerschuk, C.M.; Ting, J.P.Y.; Mackman, N. Monocytic microparticles activate endothelial cells in an IL-1β–dependent manner. Blood 2011, 118, 2366–2374. [Google Scholar] [CrossRef] [PubMed]
- Eitner, A.; Hofmann, G.O.; Schaible, H.G. Mechanisms of Osteoarthritic Pain. Studies in Humans and Experimental Models. Front. Mol. Neurosci. 2017, 10, 349. [Google Scholar] [CrossRef]
- Wiegertjes, R.; van de Loo, F.A.J.; Blaney Davidson, E.N. A roadmap to target interleukin-6 in osteoarthritis. Rheumatology 2020, 59, 2681–2694. [Google Scholar] [CrossRef]
- Eitner, A.; König, C.; Kohler, F.C.; Hofmann, G.O.; Wildemann, B.; Aurich, M.; Schaible, H.-G. Importance of IL-6 trans-signaling and high autocrine IL-6 production in human osteoarthritic chondrocyte metabolism. Osteoarthr. Cartil. 2024, 32, 561–573. [Google Scholar] [CrossRef]
- Tsuchida, A.I.; Beekhuizen, M.; Rutgers, M.; van Osch, G.J.; Bekkers, J.E.; Bot, A.G.; Geurts, B.; Dhert, W.J.; Saris, D.B.; Creemers, L.B. Interleukin-6 is elevated in synovial fluid of patients with focal cartilage defects and stimulates cartilage matrix production in an in vitro regeneration model. Arthritis Res. Ther. 2012, 14, R262. [Google Scholar] [CrossRef]
- Tchetina, E.V.; Squires, G.; Poole, A.R. Increased type II collagen degradation and very early focal cartilage degeneration is associated with upregulation of chondrocyte differentiation related genes in early human articular cartilage lesions. J. Rheumatol. 2005, 32, 876–886. [Google Scholar]
- Michael, J.W.P.; Schlüter-Brust, K.U.; Eysel, P. The Epidemiology, Etiology, Diagnosis, and Treatment of Osteoarthritis of the Knee. Dtsch. Arztebl. Int. 2010, 107, 152–162. [Google Scholar] [CrossRef]
- Wu, C.W.; Tchetina, E.V.; Mwale, F.; Hasty, K.; Pidoux, I.; Reiner, A.; Geurts, B.; Dhert, W.J.; Saris, D.B.; Creemers, L.B. Proteolysis Involving Matrix Metalloproteinase 13 (Collagenase-3) Is Required for Chondrocyte Differentiation That Is Associated with Matrix Mineralization. J. Bone Miner. Res. 2002, 17, 639–651. [Google Scholar] [CrossRef] [PubMed]
- Takaishi, H.; Kimura, T.; Dalal, S.; Okada, Y.; D’Armiento, J. Joint Diseases and Matrix Metalloproteinases: A Role for MMP-13. Curr. Pharm. Biotechnol. 2008, 9, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Fuerst, R.; Choi, J.Y.; Knapinska, A.M.; Cameron, M.D.; Ruiz, C.; Delmas, A.; Sundrud, M.S.; Fields, G.B.; Roush, W.R. Development of a putative Zn2+-chelating but highly selective MMP-13 inhibitor. Bioorg. Med. Chem. Lett. 2022, 76, 129014. [Google Scholar] [CrossRef]
- Naor, D.; Sionov, R.V.; Ish-Shalom, D. CD44: Structure, function, and association with the malignant process. Adv. Cancer Res. 1997, 71, 241–319. [Google Scholar]
- Stamenkovic, I.; Aruffo, A.; Amiot, M.; Seed, B. The hematopoietic and epithelial forms of CD44 are distinct polypeptides with different adhesion potentials for hyaluronate-bearing cells. EMBO J. 1991, 10, 343–348. [Google Scholar] [CrossRef]
- Bhattacharya, D.; Svechkarev, D.; Souchek, J.J.; Hill, T.K.; Taylor, M.A.; Natarajan, A.; Mohs, A.M. Impact of structurally modifying hyaluronic acid on CD44 interaction. J. Mater. Chem. B 2017, 5, 8183–8192. [Google Scholar] [CrossRef]
- Kobayashi, T.; Chanmee, T.; Itano, N. Hyaluronan: Metabolism and Function. Biomolecules 2020, 10, 1525. [Google Scholar] [CrossRef] [PubMed]
- Gee, K.; Lim, W.; Ma, W.; Nandan, D.; Diaz-Mitoma, F.; Kozlowski, M.; Kumar, A. Differential Regulation of CD44 Expression by Lipopolysaccharide (LPS) and TNF-α in Human Monocytic Cells: Distinct Involvement of c-Jun N-Terminal Kinase in LPS-Induced CD44 Expression. J. Immunol. 2002, 169, 5660–5672. [Google Scholar] [CrossRef]
- Božič, D.; Sitar, S.; Junkar, I.; Štukelj, R.; Pajnič, M.; Žagar, E.; Kralj-Iglič, V.; Kogej, K. Viscosity of Plasma as a Key Factor in Assessment of Extracellular Vesicles by Light Scattering. Cells 2019, 8, 1046. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrabs, V.; Guillén, M.I.; Ferrándiz, M.L.; Alcaraz, M.J.; Ferrini, F.; Agostini, R.; Guescini, M.; Fimognari, C.; Capparucci, I.; Barbieri, E.; et al. Hyaluronic Acid Hampers the Inflammatory Response Elicited by Extracellular Vesicles from Activated Monocytes in Human Chondrocytes. Pharmaceutics 2024, 16, 1386. https://doi.org/10.3390/pharmaceutics16111386
Carrabs V, Guillén MI, Ferrándiz ML, Alcaraz MJ, Ferrini F, Agostini R, Guescini M, Fimognari C, Capparucci I, Barbieri E, et al. Hyaluronic Acid Hampers the Inflammatory Response Elicited by Extracellular Vesicles from Activated Monocytes in Human Chondrocytes. Pharmaceutics. 2024; 16(11):1386. https://doi.org/10.3390/pharmaceutics16111386
Chicago/Turabian StyleCarrabs, Vittoria, Maria Isabel Guillén, María Luisa Ferrándiz, María José Alcaraz, Fabio Ferrini, Rachele Agostini, Michele Guescini, Carmela Fimognari, Italo Capparucci, Elena Barbieri, and et al. 2024. "Hyaluronic Acid Hampers the Inflammatory Response Elicited by Extracellular Vesicles from Activated Monocytes in Human Chondrocytes" Pharmaceutics 16, no. 11: 1386. https://doi.org/10.3390/pharmaceutics16111386
APA StyleCarrabs, V., Guillén, M. I., Ferrándiz, M. L., Alcaraz, M. J., Ferrini, F., Agostini, R., Guescini, M., Fimognari, C., Capparucci, I., Barbieri, E., & Sestili, P. (2024). Hyaluronic Acid Hampers the Inflammatory Response Elicited by Extracellular Vesicles from Activated Monocytes in Human Chondrocytes. Pharmaceutics, 16(11), 1386. https://doi.org/10.3390/pharmaceutics16111386







