Algal Metabolites as Novel Therapeutics Against Methicillin-Resistant Staphylococcus aureus (MRSA): A Review
Abstract
1. Introduction
2. Search Strategy and Selection Criteria
3. Detailed Study Related to Staphylococcus aureus
3.1. The Prevalent Traits of Staphylococcus aureus
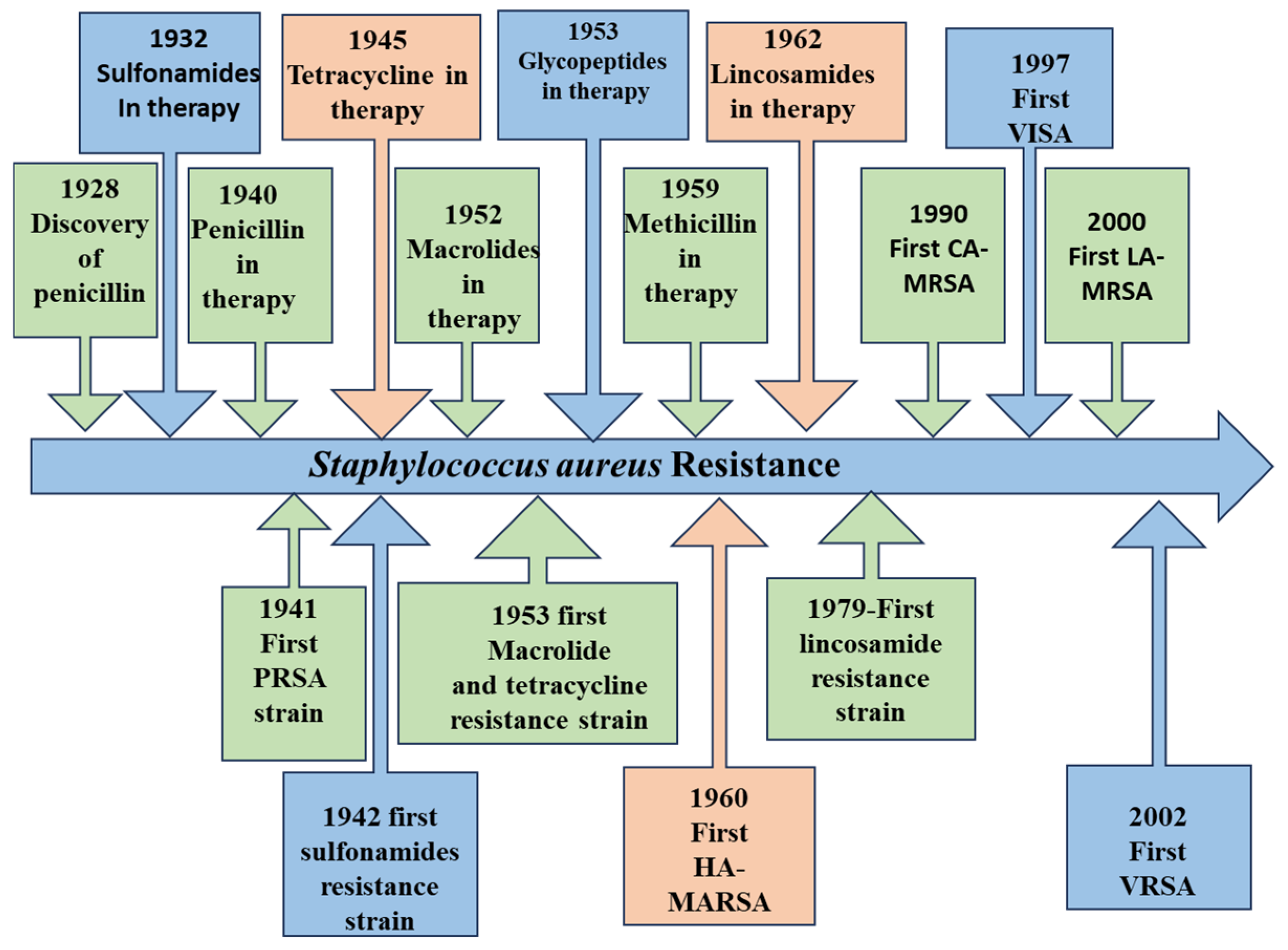
3.2. Staphylococcus aureus as a Superbug and Emergence of MRSA
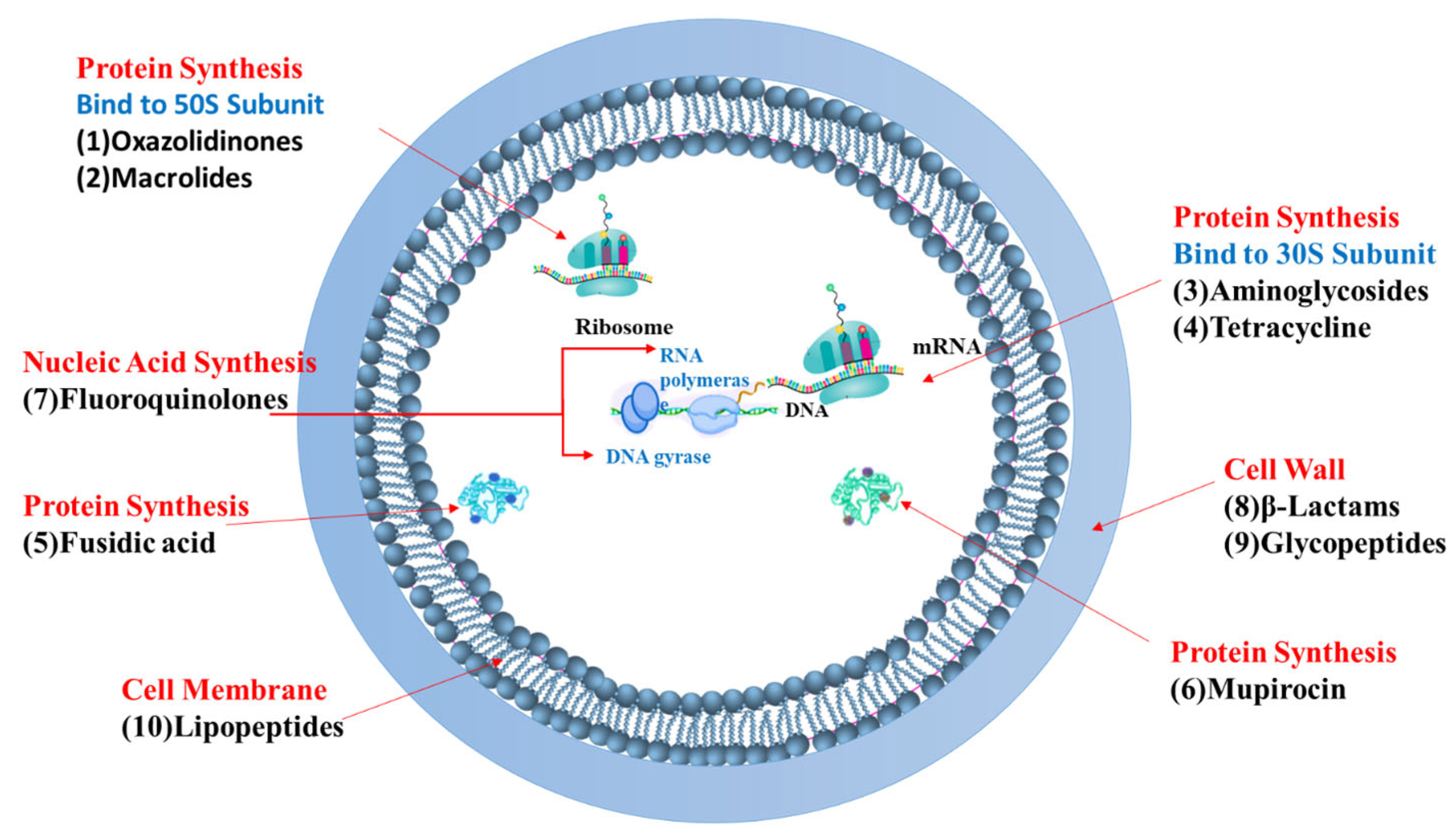
3.3. Mechanisms of Action of Current Antibiotics Against MRSA and Associated Challenges
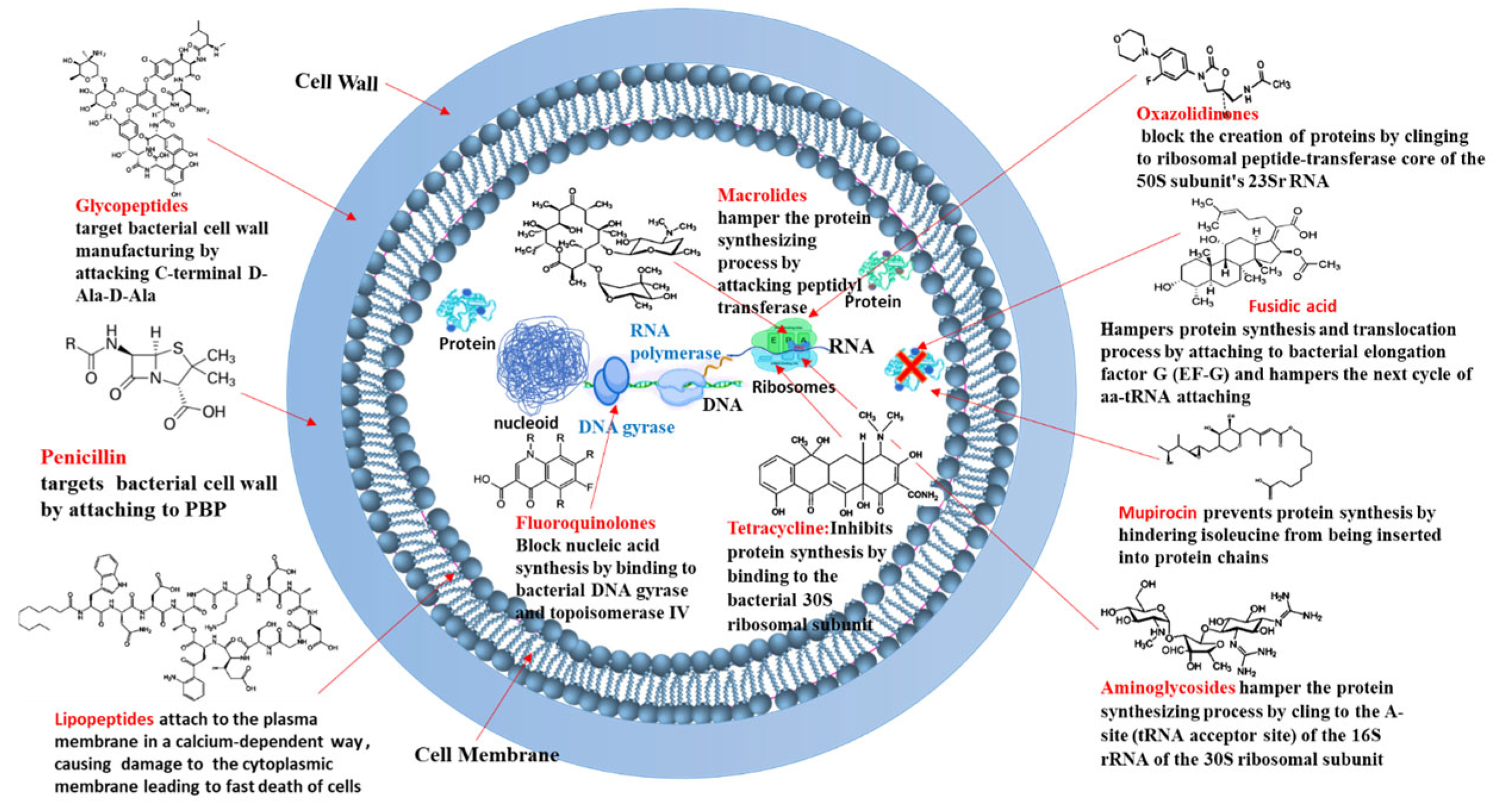
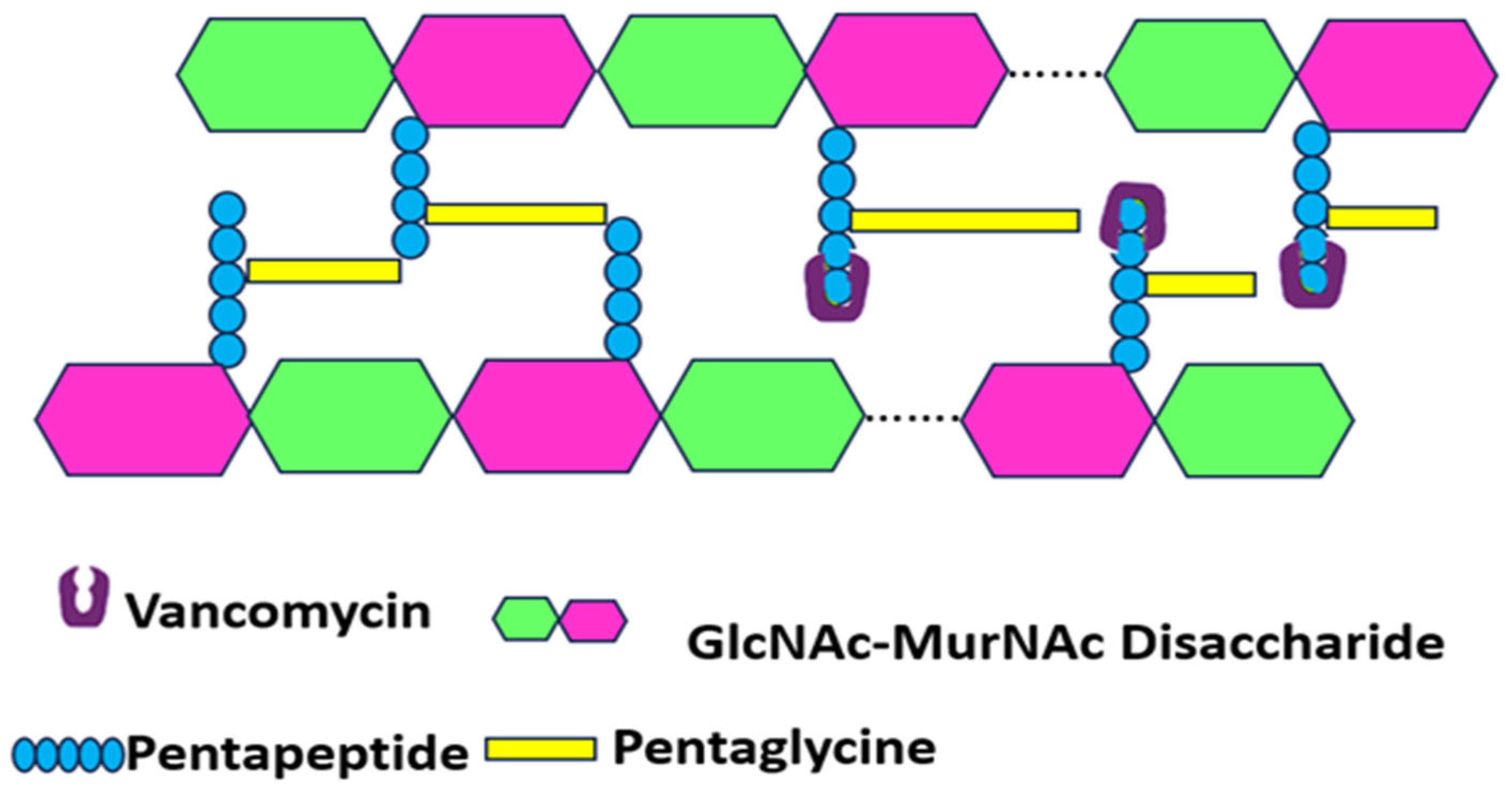
- Targeting Bacterial Cell Wall Synthesis: This is a common strategy. For instance, β-lactam antibiotics (e.g., penicillin and its derivatives, see Figure 3) disrupt peptidoglycan synthesis by inhibiting penicillin-binding proteins (PBPs), which are crucial for cross-linking peptidoglycan layers, leading to cell lysis [24,25]. Glycopeptides, such as vancomycin, bind directly to the D-alanyl-D-alanine termini of peptidoglycan precursors, thereby preventing transglycosylation and transpeptidation steps in cell wall assembly [21] (see Figure 4). Despite their initial effectiveness, the emergence of resistance, such as MRSA against methicillin and the rise in vancomycin-resistant strains (VRSA), limit their utility [26,27].
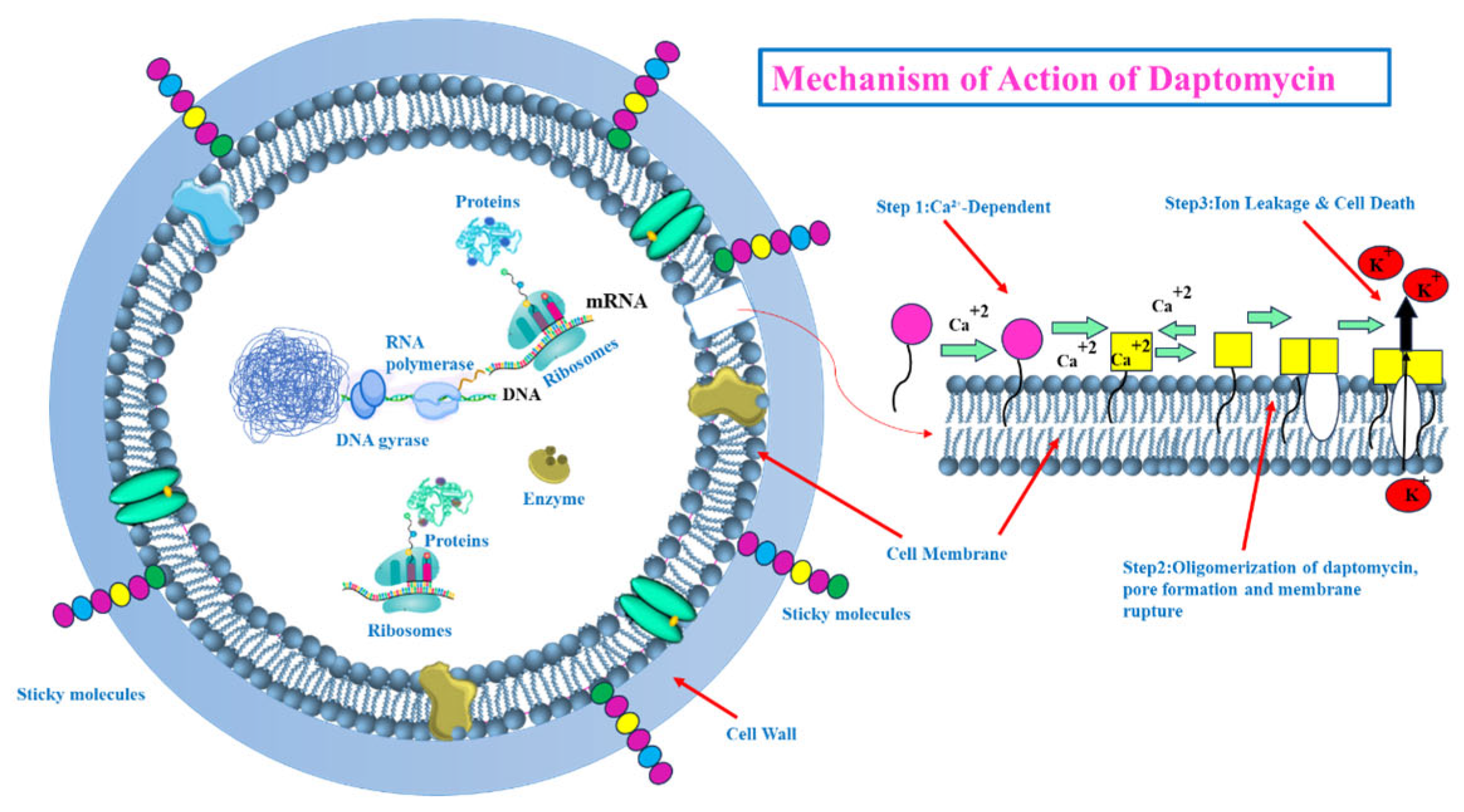
- Targeting the Bacterial Cell Membrane: Some antibiotics, like lipopeptides (e.g., daptomycin), compromise the integrity of the bacterial cell membrane. Daptomycin, in a calcium-dependent manner, inserts into the cell membrane, causing depolarization and pore formation, which leads to ion leakage and cell death [28,29] (see Figure 5). However, the efficacy of certain agents in this class can be affected by environmental factors, like daptomycin’s inactivation by pulmonary surfactants, restricting its use for specific infections [29].
- Inhibiting Bacterial Protein Synthesis: The bacterial ribosome (with its 30S and 50S subunits) is a critical target for many antibiotic classes (see Figure 3):
- ○
- Antibiotics like tetracyclines and aminoglycosides target the 30S ribosomal subunit. Tetracyclines typically block the binding of aminoacyl-tRNA to the A-site of the ribosome [30,31,32,33,34], while aminoglycosides bind to the A-site causing misreading of mRNA and production of aberrant proteins [35,36].
- ○
- ○
- Inhibiting Bacterial Nucleic Acid Replication: Fluoroquinolones interfere with DNA replication by inhibiting essential enzymes like DNA gyrase and topoisomerase IV (see Figure 3). These enzymes are vital for DNA uncoiling, replication, and segregation, and their inhibition leads to strand breaks and cell death [47,48,49,50,51].
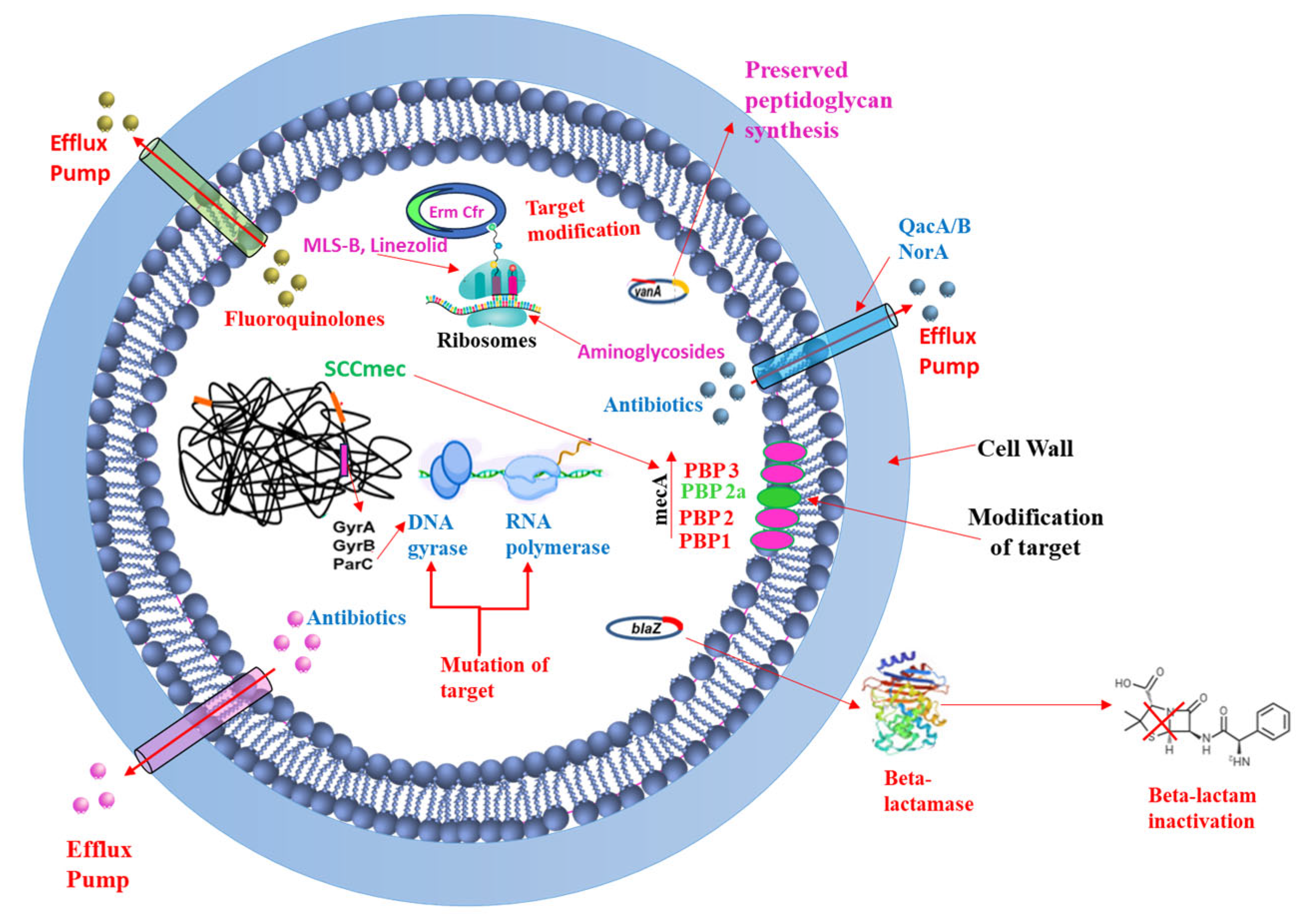
3.4. Staphylococcus aureus Resistance Mechanisms to Antimicrobial Agents
3.4.1. Efflux Pumps
3.4.2. Acquisition of Resistance Genes
3.4.3. Target Modification
3.4.4. Drug Inactivation
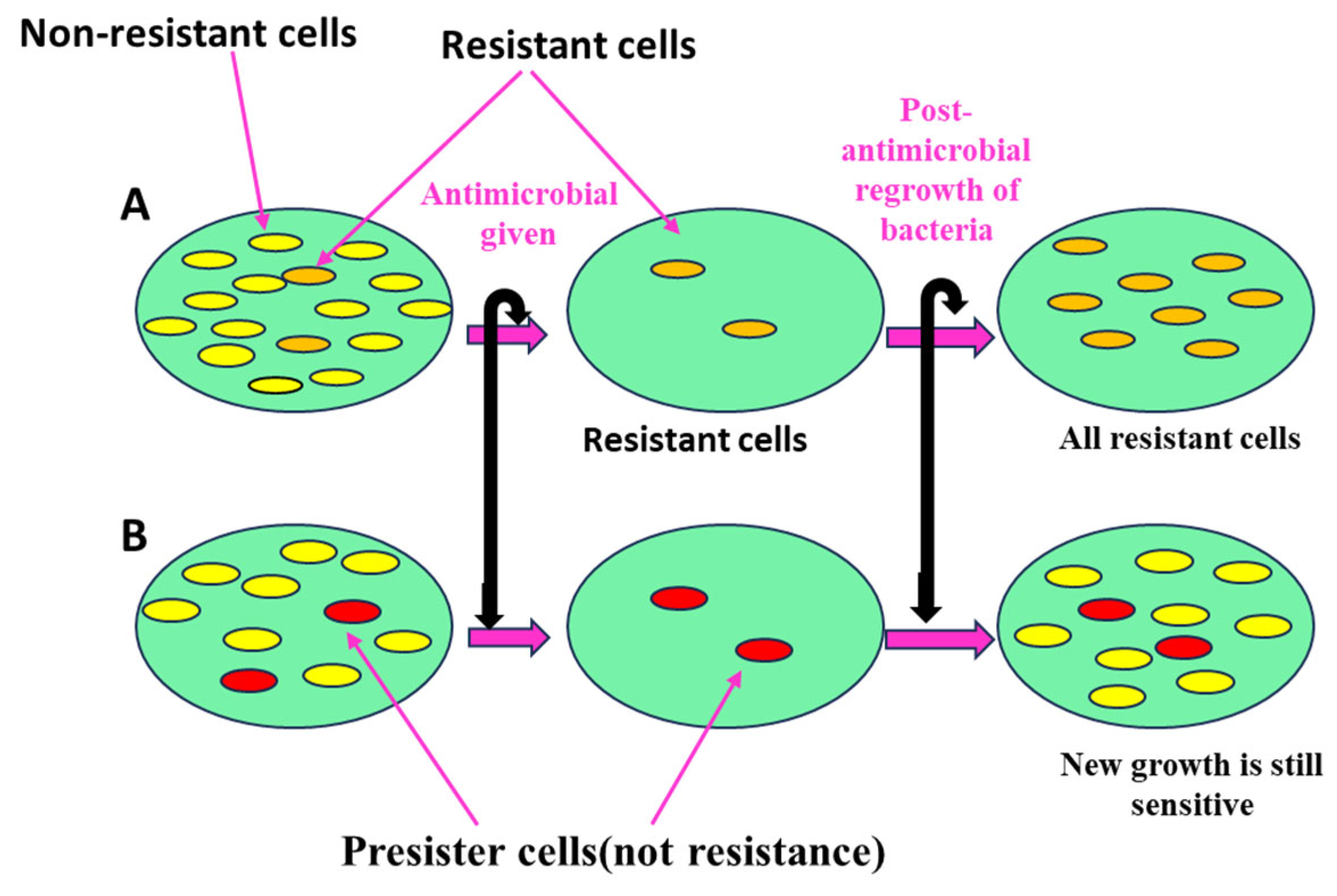
3.4.5. Persister Cells
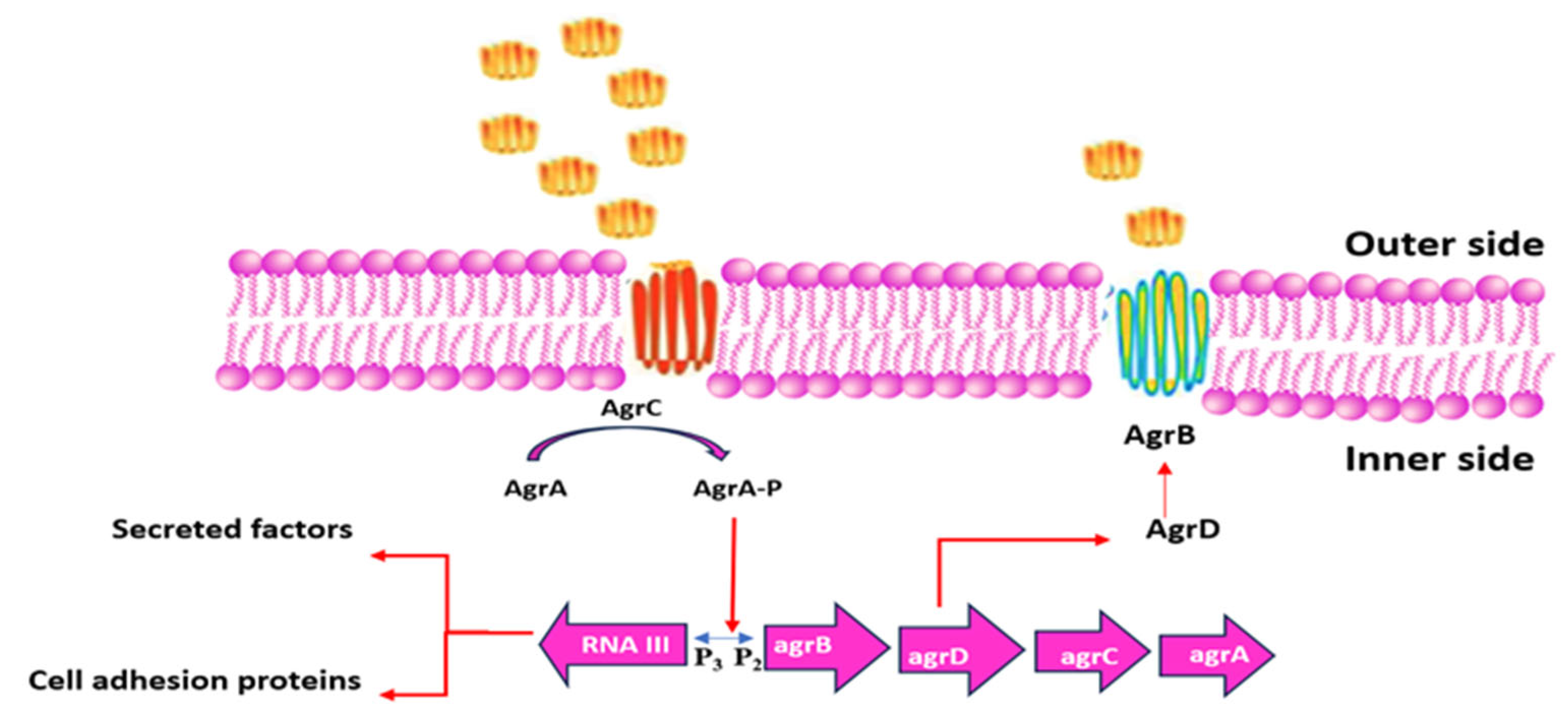
3.4.6. Quorum Sensing
3.4.7. Biofilm-Mediated Resistance
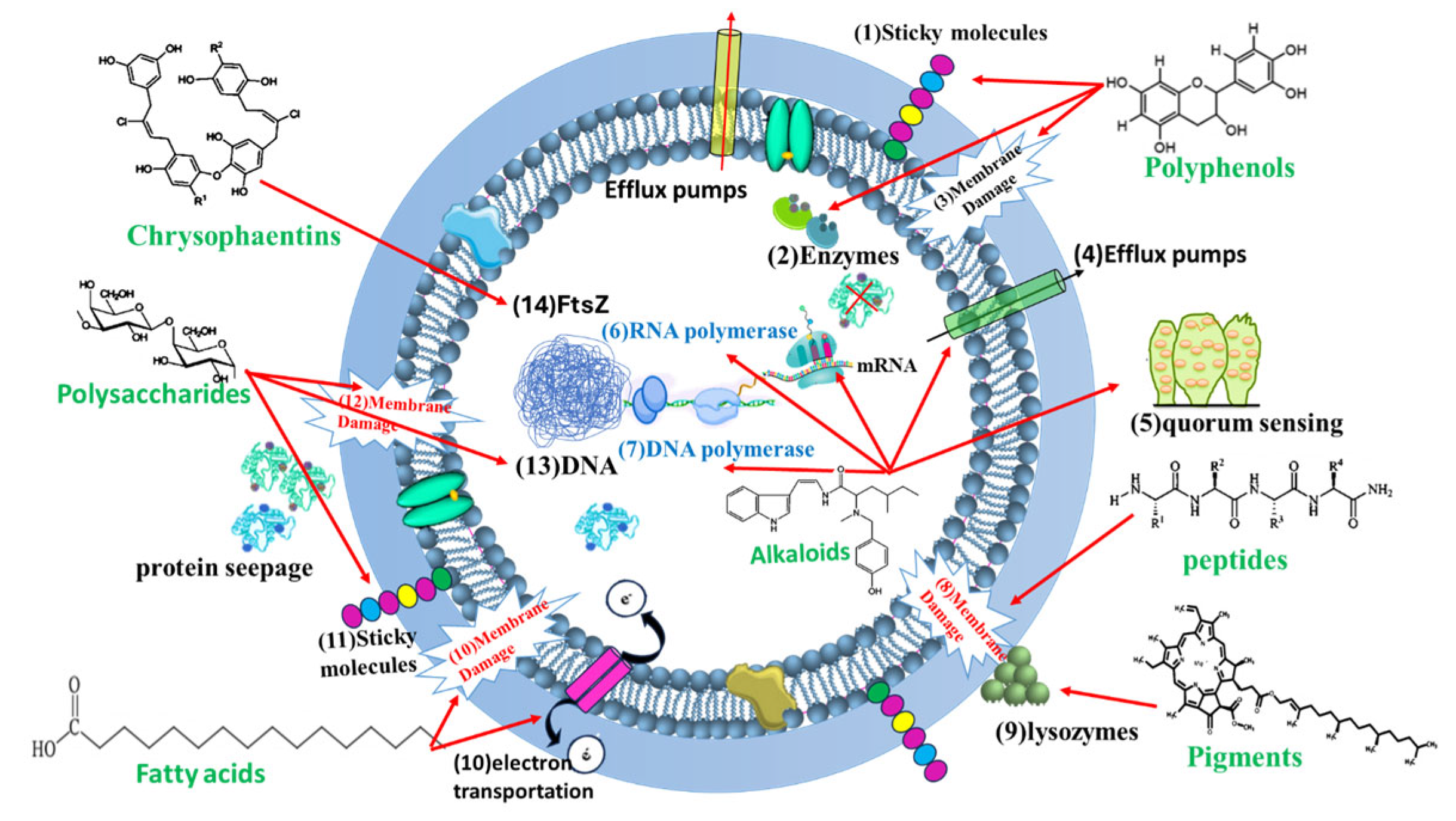
4. Algal Metabolites with Anti-MRSA Activity
- Specificity: While many of these compounds show promising in vitro activity against MRSA, it is crucial to remember that their efficacy and safety in vivo (animal or human) models need to be thoroughly investigated. In vitro activity does not always translate to clinical effectiveness.
- Mechanism of Action: The mechanisms described are based on current scientific understanding and may be incomplete or subject to revision as more research is conducted.
- Extraction and Purification: Obtaining pure compounds from algae can be challenging and costly. This is a significant factor in developing algal metabolites into viable therapeutics.
- Bioavailability: The ability of these compounds to be absorbed, distributed, metabolized, and excreted (ADME) in a living organism is crucial for their therapeutic potential. This needs to be studied extensively.
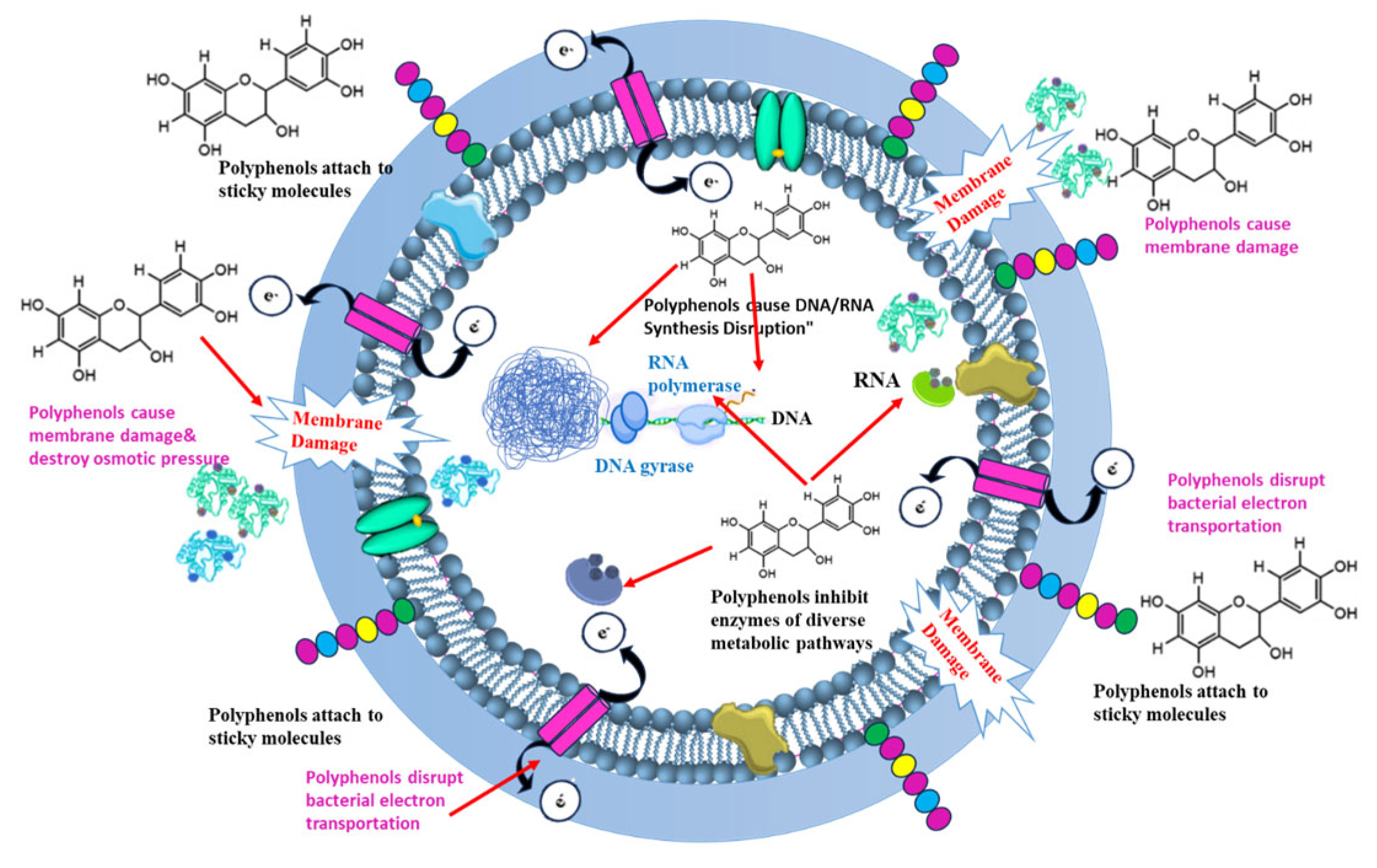
4.1. Polyphenols
4.1.1. Phlorotannins
- a.
- Biofilm Inhibition: Phlorotannins interfere with the formation and maturation of MRSA biofilms, reducing bacterial adherence and virulence.
- b.
- Quorum Sensing Interference: Phlorotannins can disrupt quorum sensing systems, which regulate gene expression and virulence in MRSA [83].
- c.
- d.
- Enzyme Inhibition: Phlorotannins can chelate metal ions, such as iron and zinc, that are essential for the activity of bacterial enzymes involved in metabolism and virulence. By binding these metal ions, phlorotannins effectively inhibit these enzymes, disrupting vital pathways [96]. Furthermore, they affect antioxidant enzyme activity [85].
- e.
4.1.2. Bromophenols
- a.
- Cell Membrane Disruption: Like phlorotannins, bromophenols can insert into the bacterial cell membrane, disrupting its integrity and leading to cell leakage.
- b.
- Quorum Sensing Inhibition: Bromophenols are known quorum sensing inhibitors, interfering with the bacterial communication system that controls the expression of virulence factors such as biofilm formation and toxin production. By inhibiting quorum sensing, bromophenols reduce the ability of MRSA to cause infection.
- c.
- Protein Inhibition: Bromophenols have been shown to inhibit bacterial proteins essential for survival and replication.
4.2. Alkaloids
4.2.1. Cyanobacterial Alkaloids
- a.
- Interference with Quorum Sensing: Some alkaloids can interfere with quorum sensing, a cell-to-cell communication system that MRSA uses to coordinate biofilm formation and virulence factor production. Berberine from cyanobacteria has been shown to inhibit gene regulation involved in biofilm formation [96].
- b.
- Disruption of Cell Membranes: Certain alkaloids may disrupt the integrity of bacterial cell membranes, leading to increased permeability and cell death. Alkaloids obtained from cyanobacteria possess the potential to suppress MRSA’s efflux pumps [98].
- c.
- Inhibition of Bacterial Enzymes: Some alkaloids may inhibit bacterial enzymes involved in cell wall synthesis, DNA replication, or protein synthesis.
- d.
- DNA intercalation: Some alkaloids can bind to and interfere with DNA production. Cyanobacterial alkaloids are capable of disrupting both MRSA’s transcription and translation processes by suppressing both DNA and RNA polymerases [98].
4.2.2. Other Marine Algal Alkaloids
- a.
- Disruption of Cell Membrane Function: Caulerpin is believed to primarily act by disrupting the cell membrane function of bacteria, including MRSA. This disruption can lead to increased membrane permeability, leakage of cellular contents, and, ultimately, cell death. The lipophilic nature of caulerpin allows it to easily insert into the lipid bilayer of bacterial membranes.
- b.
- Inhibition of Cell Division: Caulerpin may also inhibit bacterial cell division, preventing MRSA from replicating.
4.3. Pigments
- a.
- Oxidative Stress Induction: Pigments can generate reactive oxygen species (ROS), such as superoxide radicals and hydroxyl radicals, which damage bacterial cells. Sachindra et al. [107] demonstrated the antioxidant properties of fucoxanthin-rich extracts from Laminaria japonica and Undaria pinnatifida and highlighted their potential antibacterial activities.
- b.
- Cell Membrane Disruption: Some pigments can insert into the bacterial cell membrane, disrupting its structure and function.
- c.
- Photosensitization: When exposed to light, some pigments can generate singlet oxygen, a highly reactive form of oxygen that damages bacterial cells.
4.4. Polysaccharides
4.4.1. Ulvan
- a.
- Anti-Adhesive Properties: The negatively charged sulfate groups in ulvan interact with positively charged surface molecules on bacterial cells, hindering their adhesion to host tissues or surfaces.
- b.
- Biofilm Inhibition: By preventing bacterial adhesion, ulvan suppresses biofilm formation and may destabilize existing MRSA biofilms.
- c.
- Immune Modulation: Ulvan enhances host immunity by activating macrophages and neutrophils, increasing phagocytosis and cytokine production to combat infections.
- d.
- Direct Antibacterial Effects: Though secondary, ulvan may disrupt bacterial cell membranes, contributing to its antimicrobial action.
- e.
- Quorum Sensing Interference: Ulvan impedes bacterial communication, critical for biofilm development and virulence, thereby attenuating MRSA pathogenicity.
4.4.2. Carrageenan
4.4.3. Porphyran
- a.
- Inhibition of Bacterial Adhesion: The sulfated nature of porphyran is believed to play a key role in its antibacterial activity. The negatively charged sulfate groups can interact with positively charged molecules on the surface of bacterial cells, preventing their adhesion to host tissues or surfaces.
- b.
- Biofilm Inhibition: By preventing initial adhesion, porphyran can inhibit the formation of biofilms by MRSA. It may also disrupt existing biofilms.
- c.
- Immune Modulation: Some studies suggest that sulfated polysaccharides like porphyran can stimulate the immune system, enhancing the host’s ability to fight off bacterial infections. They may activate macrophages and other immune cells.
- d.
- Interference with Cell Wall Synthesis: Though less common, there is a potential that porphyran could interfere with enzymes involved in bacterial cell wall synthesis.
4.4.4. Fucoidan
4.4.5. Laminarin
4.5. Amino Acids and Peptides
- a.
- Membrane Disruption: Lipopeptides can insert into bacterial membranes and create pores, leading to leakage and cell death.
- b.
- Protein Synthesis Inhibition: Some peptides target ribosomes and block protein synthesis.
- c.
- Quorum Sensing Inhibition: Some peptides can interfere with quorum sensing signals, thus reducing virulence factor production.
4.6. Lectins
- a.
- Inhibition of Bacterial Adhesion: A key initial step in MRSA infection is the adhesion of bacterial cells to host tissues. Lectins can bind to carbohydrates on the surface of MRSA cells, preventing them from adhering to host cells.
- b.
- Agglutination of Bacterial Cells: Because lectins are often multivalent, they can bind to multiple bacterial cells simultaneously, causing them to agglutinate (clump together). This agglutination can prevent MRSA from colonizing and spreading.
- c.
- Interference with Biofilm Formation: Biofilms are structured communities of bacteria encased in a matrix of extracellular polymeric substances (EPS). Lectins can interfere with biofilm formation by causing the following:
- d.
- Preventing Initial Attachment: As mentioned above, lectins can prevent MRSA cells from initially attaching to surfaces, a crucial step in biofilm formation.
- e.
- Disrupting Biofilm Structure: Lectins can bind to carbohydrates within the biofilm matrix, disrupting its structure and stability.
- f.
- Immune Modulation: Some lectins can activate the immune system, enhancing the body’s ability to fight off MRSA infections. They can stimulate the activity of macrophages, neutrophils, and other immune cells.
- g.
- Direct Toxicity: Though less common, some lectins may have direct toxic effects on bacterial cells, leading to cell death.
- h.
- Disrupting cell wall synthesis: Some lectins can bind to the cell wall of bacteria, therefore disrupting cell wall growth.
4.7. Lipids and Fatty Acids
- a.
- Cell Membrane Disruption: PUFAs can disrupt the bacterial cell membrane, increasing its fluidity and permeability, ultimately leading to cell death.
- b.
- Interference with Fatty Acid Metabolism: Fatty acids can disrupt the bacterial lipid metabolism pathways essential for cell structure and function.
- c.
- Immune Modulation: Certain fatty acids can modulate the host immune response, enhancing the body’s ability to fight off MRSA infection.
4.7.1. Docosahexaenoic Acid (DHA)
4.7.2. Eicosapentaenoic Acid (EPA)
4.7.3. Palmitic Acid
4.7.4. Other Fatty Acids (e.g., Hexadecatrienoic Acid, Palmitoleic Acid)
4.8. Glycolipids
4.8.1. Sulfoquinovosyl Diacylglycerol (SQDG)
4.8.2. Monogalactosyl Diacylglycerol (MGDG)
4.8.3. Digalactosyl Diacylglycerol (DGDG)
- a.
- Disruption of Cell Membrane Integrity: The amphiphilic nature of glycolipids allows them to insert into the bacterial cell membrane.
- b.
- Increase Membrane Permeability: Glycolipids can increase the permeability of the cell membrane, leading to leakage of essential cellular components and disruption of ion gradients.
- c.
- Alter Membrane Fluidity: Glycolipids can alter the fluidity of the cell membrane, affecting the function of membrane proteins and transport systems.
- d.
- Inhibition of Bacterial Enzymes: Some glycolipids can inhibit bacterial enzymes involved in cell wall synthesis or other essential metabolic pathways.
- e.
- Interference with Biofilm Formation: Glycolipids can interfere with biofilm formation by preventing initial attachment as glycolipids can coat surfaces and prevent MRSA cells from initially attaching.
- f.
- Disrupting Biofilm Structure: Glycolipids can insert into the biofilm matrix, disrupting its structure and stability.
- g.
- Modulation of Host Immune Response: Some glycolipids may have immunomodulatory properties, enhancing the host’s ability to clear MRSA infections. They may stimulate the activity of immune cells such as macrophages.
4.9. Terpenoids
- a.
- Disruption of Cell Membrane Integrity: Many terpenoids are lipophilic and can insert themselves into the bacterial cell membrane, altering its fluidity, permeability, and function. This can lead to leakage of cellular contents, disruption of ion gradients, and, ultimately, cell death.
- b.
- Inhibition of Bacterial Enzymes: Some terpenoids can inhibit key bacterial enzymes involved in essential metabolic pathways or cell wall synthesis. For example, some terpenoids can inhibit peptidoglycan synthesis, a crucial step in bacterial cell wall formation.
- c.
- Efflux Pump Inhibition: Some terpenoids can inhibit bacterial efflux pumps, which are membrane proteins that pump antibiotics out of the bacterial cell. By inhibiting efflux pumps, terpenoids can increase the intracellular concentration of antibiotics, making MRSA more susceptible to these drugs.
- d.
- Protein Synthesis Inhibition: Terpenoids can interfere with bacterial protein synthesis, disrupting the production of essential proteins required for bacterial growth and survival.
4.9.1. Sargachromanol E
4.9.2. Dictyopterene A
4.9.3. Caulerprenyne
4.9.4. Sargaquinoic Acid
4.10. Saponins
- a.
- Disruption of Cell Membranes: The primary mechanism of action of saponins against bacteria, including MRSA, is the disruption of cell membranes. The amphipathic nature of saponins allows them to insert themselves into the lipid bilayer of bacterial cell membranes.
- b.
- Increase Membrane Permeability: Saponins can increase the permeability of the cell membrane, leading to leakage of essential cellular components (e.g., ions, proteins, nucleotides).
- c.
- Cause Membrane Disruption and Lysis: At higher concentrations, saponins can cause complete disruption and lysis (bursting) of the cell membrane.
- d.
- Interaction with Membrane Proteins: Saponins can also interact with membrane proteins, disrupting their function and leading to cell death.
- e.
- Inhibition of Bacterial Enzymes: Some saponins can inhibit bacterial enzymes involved in essential metabolic pathways, such as cell wall synthesis or DNA replication. However, this mechanism is less specifically studied for algal saponins specifically.
- f.
- Biofilm Inhibition: Several saponins have been shown to have anti-biofilm activity, preventing the formation of biofilms by bacteria. This can be particularly important for MRSA, which often forms biofilms that protect it from antibiotics and the host’s immune system.
- g.
- Immunomodulatory Effects: Some saponins can stimulate the immune system, enhancing the body’s ability to fight off bacterial infections. However, this mechanism is more relevant to in vivo (animal or human) studies.
4.11. Sterols (Fucosterol)
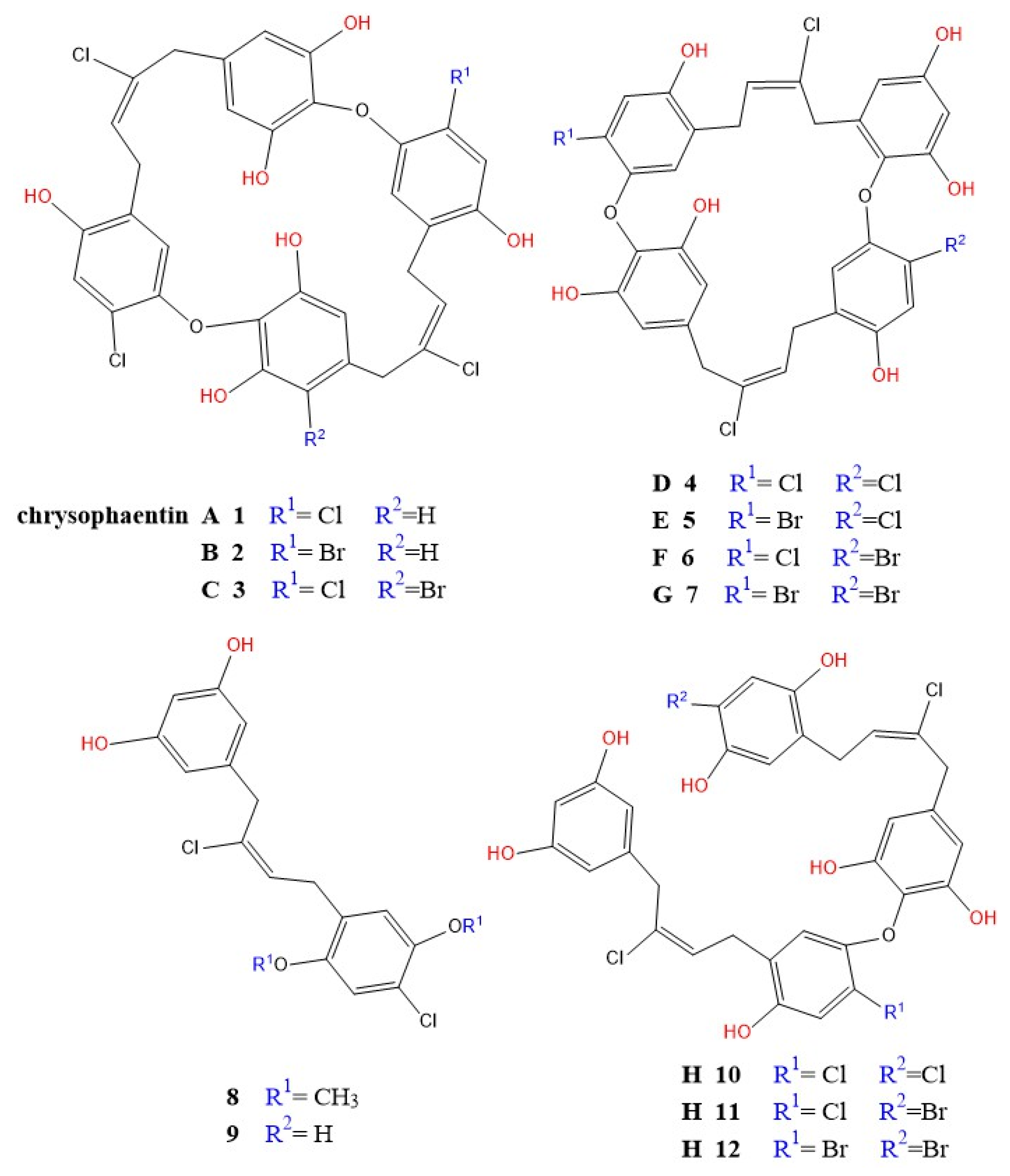
4.12. Chrysophaentins
4.13. Other Metabolites
Acrylic Acid
5. Recent Advances, Challenges, and Future Directions
5.1. Emerging Therapeutic Strategies
5.2. Key Developmental Hurdles
5.2.1. Bioavailability and Drug Delivery
5.2.2. Potential Toxicity
5.2.3. Scalability and Sustainable Production
5.2.4. Clinical Validation
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- WHO. Global Priority List of Antibiotic-Resistant Bacteria; World Health Organization: Geneva, Switzerland, 2023.
- Turner, N.A.; Sharma-Kuinkel, B.K.; Maskarinec, S.A.; Eichenberger, E.M.; Shah, P.P.; Carugati, M.; Holland, T.L.; Fowler, J.V.G. Methicillin-resistant Staphylococcus aureus: An overview of basic and clinical research. Nat. Rev. Microbiol. 2019, 17, 203–218. [Google Scholar] [CrossRef]
- Ventola, C.L. The antibiotic resistance crisis. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Kourtis, A.P.; Hatfield, K.M.; Baggs, J.; Mu, Y.; See, I.; Epson, E.; Nadle, J.; Bamberg, W.M.; Petit, S.; Kainer, M.A.; et al. Vital signs: Epidemiology and recent trends in MRSA infections. CDC Morb. Mortal. Wkly. Rep. 2019, 68, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Peacock, S.J.; Paterson, G.K. Mechanisms of methicillin resistance in Staphylococcus aureus. Annu. Rev. Biochem. 2015, 84, 577–601. [Google Scholar] [CrossRef]
- Otto, M. Staphylococcal biofilms. Microbiol. Spectr. 2018, 6, 207–228. [Google Scholar] [CrossRef] [PubMed]
- McGuinness, W.A.; Malachowa, N.; DeLeo, F.R. Vancomycin resistance in Staphylococcus aureus. Yale J. Biol. Med. 2017, 90, 269–281. [Google Scholar]
- Mayer, A.M.S.; Rodríguez, A.D.; Taglialatela-Scafati, O.; Fusetani, N. Marine pharmacology: A review of marine algal natural products with antibacterial activity. Mar. Drugs 2021, 19, 535. [Google Scholar]
- Sathya, R.; Manikandan, M.; Rajaram, R.; Ganesan, A.R.; Arulvasu, C.; Prabhu, D.; Dinesh, D.; Chinnadurai, V.; Rajasekar, T.; Thirumurugan, R. Antibacterial potential of algal terpenes against multidrug-resistant Staphylococcus aureus. Phytomedicine 2017, 36, 152–163. [Google Scholar]
- Pérez, M.J.; Falqué, E.; Domínguez, H. Marine bioactive compounds as therapeutics against MRSA. Biotechnol. Adv. 2016, 34, 832–846. [Google Scholar]
- Lane, A.L.; Stout, E.P.; Lin, A.S.; Prudhomme, J.; Le Roch, K.; Fairchild, C.R.; Franzblau, S.G.; Hay, M.E.; Aalbersberg, W.; Kubanek, J. Antimalarial bromophycolides J-Q from the Fijian red alga Callophycus serratus. J. Org. Chem. 2009, 74, 2736–2742. [Google Scholar] [CrossRef]
- Trabelsi, N.; Ben Hlima, H.; Aifa, S.; Renda, G.; Geraci, C.; Mangano, S.; Michaud, P.; Abdelkafi, S.; Feki, H. Ulvan polysaccharides as inhibitors of S. aureus biofilm formation. Carbohydr. Polym. 2020, 240, 116291. [Google Scholar]
- Khan, B.A.; Muzammil, S.; Chen, W.T.; Kanthaswamy, S.; Bhattacharya, T.; Murtaza, G.; ul-Haq, I. The Growing Threat of Methicillin-Resistant Staphylococcus aureus (MRSA): A Review of the Therapeutic Role of Marine Natural Products. Molecules 2024, 29, 243. [Google Scholar] [CrossRef]
- Cheng, A.; De Dent, A.C.; Schneewind, O.; Missiakas, D. A play in four acts: Staphylococcus aureus abscess formation. Trends Microbiol. 2011, 19, 225–232. [Google Scholar] [CrossRef]
- Gardete, S.; Tomasz, A. Mechanisms of vancomycin resistance in Staphylococcus aureus. J. Clin. Invest. 2014, 124, 2836–2840. [Google Scholar] [CrossRef]
- Raineri, E.J.M.; Altulea, D.; van Dijl, J.M. Staphylococcal trafficking and infection-from “nose to gut” and back. FEMS Microbiol. Rev. 2022, 46, fuab041. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.; Cerquetella, M.; Attili, A.R. Amphixenosic aspects of Staphylococcus aureus infection in man and animals. Curr. Top. Microbiol. Immunol. 2017, 409, 297–323. [Google Scholar] [PubMed]
- Fleming, A. On the antibacterial action of cultures of a penicillium, with special reference to their use in the isolation of B. influenzae. Bull. World Health Organ. 2001, 79, 780–790. [Google Scholar] [CrossRef]
- Lowy, F.D. Antimicrobial resistance: The example of Staphylococcus aureus. J. Clin. Investig. 2003, 111, 1265–1273. [Google Scholar] [CrossRef] [PubMed]
- Jokinen, E.; Laine, J.; Huttunen, R.; Rahikka, P.; Huhtala, H.; Vuento, R.; Vuopio, J.; Syrjänen, J. Comparison of outcome and clinical characteristics of bacteremia caused by methicillin-resistant, penicillin-resistant and penicillin-susceptible Staphylococcus aureus strains. Infect. Dis. 2017, 49, 493–500. [Google Scholar] [CrossRef]
- Maddiboyina, B.; Roy, H.; Ramaiah, M.; Sarvesh, C.N.; Kosuru, S.H.; Nakkala, R.K.; Nayak, B.S. Methicillin-resistant Staphylococcus aureus: Novel treatment approach breakthroughs. Bull. Natl. Res. Centre. 2023, 47, 95. [Google Scholar] [CrossRef]
- Sarkar, P.; Haldar, J. Glycopeptide Antibiotics: Mechanism of Action and Recent Developments. In Antibiotic Drug Resistance; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2019; pp. 73–95. [Google Scholar]
- Miklasi’nska-Majdanik, M. Mechanisms of Resistance to Macrolide Antibiotics among Staphylococcus aureus. Antibiotics 2021, 10, 1406. [Google Scholar] [CrossRef] [PubMed]
- Walsh, C.T.; Wencewicz, T.A. Antibiotics: Challenges, Mechanisms, Opportunities; ASM Press: Washington, DC, USA, 2016; p. 477. Available online: http://www.asmscience.org/content/book/10.1128/9781555819316 (accessed on 29 August 2018).
- Foster, T.J. Antibiotic resistance in Staphylococcus aureus. Current status and future prospects. FEMS Microbiol. Rev. 2017, 41, 430–449. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Peng, H.; Rao, X. Molecular Events for Promotion of Vancomycin Resistance in Vancomycin Intermediate Staphylococcus aureus. Front. Microbiol. 2016, 7, 1601. [Google Scholar] [CrossRef]
- Lin, L.C.; Chang, S.C.; Ge, M.C.; Liu, T.P.; Lu, J.J. Novel single-nucleotide variations associated with vancomycin resistance in vancomycin-intermediate Staphylococcus aureus. Infect. Drug Resist. 2018, 11, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Zeng, D.; Debabov, D.; Hartsell, T.L.; Cano, R.J.; Adams, S.; Schuyler, J.A.; McMillan, R.; Pace, J.L. Approved Glycopeptide Antibacterial Drugs: Mechanism of Action and Resistance. Cold Spring Harb. Perspect. Med. 2016, 6, a026989. [Google Scholar] [CrossRef]
- Heidary, M.; Khosravi, A.D.; Khoshnood, S.; Nasiri, M.J.; Soleimani, S.; Goudarzi, M. Daptomycin. J. Antimicrob. Chemother. 2018, 73, 1–11. [Google Scholar] [CrossRef]
- Steenbergen, J.N.; Alder, J.; Thorne, G.M.; Tally, F.P. Daptomycin: A lipopeptide antibiotic for the treatment of serious Gram-positive infections. J. Antimicrob. Chemother. 2005, 55, 283–288. [Google Scholar] [CrossRef]
- Duggar, B.M. Aureomycin: A product of the continuing search for new antibiotics. Ann. NY Acad. Sci. 1948, 51, 177–181. [Google Scholar] [CrossRef]
- Nikaido, H.; Thanassi, D.G. Penetration of lipophilic agents with multiple protonation sites into bacterial cells: Tetracyclines and fluoroquinolones as examples. Antimicrob. Agents Chemother. 1993, 37, 1393–1399. [Google Scholar] [CrossRef]
- Schnappinger, D.; Hillen, W. Tetracyclines: Antibiotic action, uptake, and resistance mechanisms. Arch. Microbiol. 1996, 165, 359–369. [Google Scholar] [CrossRef]
- Zhanel, G.G.; Karlowsky, J.A.; Rubinstein, E.; Hoban, D.J. Tigecycline: A novel glycylcycline antibiotic. Expert. Rev. Anti-Infect. Ther. 2006, 4, 9–25. [Google Scholar] [CrossRef]
- Chopra, I.; Hawkey, P.M.; Hinton, M. Tetracyclines, molecular and clinical aspects. J. Antimicrob. Chemother. 1992, 29, 245–277. [Google Scholar] [CrossRef]
- Magnet, S.; Blanchard, J.S. Molecular insights into aminoglycoside action and resistance. Chem. Rev. 2005, 105, 477–498. [Google Scholar] [CrossRef] [PubMed]
- Vestergaard, M.; Frees, D.; Ingmer, H. Antibiotic Resistance and the MRSA Problem. Microbiol. Spectr. 2019, 7, 747–765. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Mukherjee, M.M.; Varela, M.F. Modulation of bacterial multidrug resistance efflux pumps of the major facilitator superfamily. Int. J. Bacteriol. 2013, 2013, 204141. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Bayer, A.; Cosgrove, S.E.; Daum, R.S.; Fridkin, S.K.; Gorwitz, R.J.; Kaplan, S.L.; Karchmer, A.W.; Levine, D.P.; Murray, B.E.; et al. Infectious Diseases Society of America. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2011, 52, e18–e55. [Google Scholar] [CrossRef]
- Emeryk, A.; Henryk, M.; Pirozynski, M.; Klatka, J.; Służewski, W.; Antczak, A.; Bartkowiak-Emeryk, M.; Kowalska, M.; Dutkowska, A. Macrolide antibiotics in respiratory diseases. Recommendations of the Polish Expert Group—AD 2015. Pneumonol. I Alergol. Pol. 2016, 84, 62–80. [Google Scholar] [CrossRef]
- Dinos, G.P. The macrolide antibiotic renaissance. Br. J. Pharmacol. 2017, 174, 2967–2983. [Google Scholar] [CrossRef]
- Vázquez-Laslop, N.; Mankin, A.S. How Macrolide Antibiotics Work. Trends Biochem. Sci. 2018, 43, 668–684. [Google Scholar] [CrossRef]
- Skarżyńska, M.; Zając, M.; Wasyl, D. Antibiotics and bacteria: Mechanisms of action and resistance strategies. Adv. Microbiol. 2020, 59, 49–62. [Google Scholar] [CrossRef]
- Guo, Y.; Song, G.; Sun, M.; Wang, J.; Wang, Y. Prevalence and Therapies of Antibiotic-Resistance in Staphylococcus aureus. Front. Cell. Infect. Microbiol. 2020, 10, 107. [Google Scholar] [CrossRef]
- Hashemian, S.M.R.; Farhadi, T.; Ganjparvar, M. Linezolid: A review of its properties, function, and use in critical care. Drug Des. Dev. Ther. 2018, 12, 1759–1767. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.N. Ribosome-targeting antibiotics and mechanisms of bacterial resistance. Nat. Rev. Microbiol. 2014, 12, 35–48. [Google Scholar] [CrossRef]
- Godtfredsen, W.; Roholt, K.; Tybring, L. Fucidin: A new orally active antibiotic. Lancet 1962, 1, 928–931. [Google Scholar] [CrossRef]
- Howden, B.P.; Grayson, M.L. Dumb and dumber—The potential waste of a useful antistaphylococcal agent: Emerging fusidic acid resistance in Staphylococcus aureus. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2006, 42, 394–400. [Google Scholar] [CrossRef]
- Bodley, J.W.; Zieve, F.J.; Lin, L.; Zieve, S.T. Formation of the ribosome-G factor-GDP complex in the presence of fusidic acid. Biochem. Biophys. Res. Commun. 1969, 37, 437–443. [Google Scholar] [CrossRef]
- Singh, A.; Kaur, K.; Mohana, P.; Kaur, A.; Heer, S.; Arora, S.; Bedi, N.; Bedi, P.M. Mechanistic insights of drug resistance in Staphylococcus aureus with special reference to newer antibiotics. In Insights Into Drug Resistance in Staphylococcus aureus; IntechOpen: London, UK, 2021; p. 61. [Google Scholar] [CrossRef]
- Brar, R.K.; Jyoti, U.; Patil, R.K.; Patil, H.C. Fluoroquinolone antibiotics: An overview. Adesh Univ. J. Med. Sci. Res. 2020, 2, 26–30. [Google Scholar] [CrossRef]
- Lekshmi, M.; Ammini, P.; Adjei, J.; Sanford, L.M.; Shrestha, U.; Kumar, S.; Varela, M.F. Modulation of antimicrobial efflux pumps of the major facilitator superfamily in Staphylococcus aureus. AIMS Microbiol. 2018, 4, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.S.; Viveiros, M.; Amaral, L.; Couto, I. Multidrug efflux pumps in Staphylococcus aureus: An update. Open Microbiol. J. 2013, 7, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Poole, K. Mechanisms of bacterial biocide and antibiotic resistance. J. Appl. Micro-Biol. 2002, 92 (Suppl. S1), 55S–64S. [Google Scholar] [CrossRef]
- Nakaminami, H.; Takadama, S.; Okita, M.; Sasaki, M.; Noguchi, N. Fast-acting bactericidal activity of olanexidine gluconate against qacA/B-positive methicillin-resistant Staphylococcus aureus. J. Med. Microbiol. 2019, 68, 957–960. [Google Scholar] [CrossRef]
- Du, D.; Wang-Kan, X.; Neuberger, A.; Veen, H.W.V.; Pos, K.M.; Piddock, L.J.V.; Luisi, B.F. Multidrug efflux pumps: Structure, function and regulation. Nat. Rev. Microbiol. 2018, 16, 523–539. [Google Scholar] [CrossRef] [PubMed]
- Reygaert, W.C. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol. 2018, 4, 482–501. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.L. General principles of antibiotic resistance in bacteria. Drug Discov. Today 2014, 11, 33–39. [Google Scholar] [CrossRef]
- Blázquez, J.; Couce, A.; Rodríguez-Beltrán, J.; Rodríguez-Rojas, A. Antimicrobials as promoters of genetic variation. Curr. Opin. Microbiol. 2012, 15, 561–569. [Google Scholar] [CrossRef]
- Joshi, A.; Patil, R.H. Metal nanoparticles as inhibitors of enzymes and toxins of multidrug-resistant Staphylococcus aureus. Infect. Med. 2023, 2, 294–307. [Google Scholar] [CrossRef] [PubMed]
- Badshah, S.L.; Ullah, A. New developments in non-quinolone-based antibiotics for the inhibition of bacterial gyrase and topoisomerase IV. Eur. J. Med. Chem. 2018, 152, 393–400. [Google Scholar] [CrossRef]
- Tremblay, S.; Lau, T.T.; Ensom, M.H. Addition of rifampin to vancomycin for methicillin-resistant Staphylococcus aureus infections: What is the evidence? Ann. Pharmacother. 2013, 47, 1045–1054. [Google Scholar] [CrossRef]
- Yang, S.J.; Kreiswirth, B.N.; Sakoulas, G.; Yeaman, M.R.; Xiong, Y.Q.; Sawa, A.; Bayer, A.S. Enhanced expression of dltABCD is associated with development of daptomycin nonsusceptibility in a clinical endocarditis isolate of Staphylococcus aureus. J. Infect. Dis. 2009, 200, 1916–1920. [Google Scholar] [CrossRef]
- Cox, G.; Wright, G.D. Intrinsic antibiotic resistance: Mechanisms, origins, challenges and solutions. Int. J. Med. Microbiol. 2013, 303, 287–292. [Google Scholar] [CrossRef]
- Khameneh, B.; Iranshahy, M.; Soheili, V.; Fazly-Bazzaz, B.S. Review on plant antimicrobials: A mechanistic viewpoint. Antimicrob. Resist. Infect. Control. 2019, 8, 118. [Google Scholar] [CrossRef]
- Jubair, N.; Rajagopal, M.; Chinnappan, S.; Abdullah, N.B.; Fatima, A. Review on the Antibacterial Mechanism of Plant-Derived Compounds against Multidrug-Resistant Bacteria (MDR). Evid.-Based Complement. Altern. Med. Ecam 2021, 2021, 3663315. [Google Scholar] [CrossRef] [PubMed]
- Belaynehe, K.M.; Shin, S.W.; Hong-Tae, P.; Yoo, H.S. Occurrence of aminoglycoside-modifying enzymes among isolates of Escherichia coli exhibiting high levels of amino-glycoside resistance isolated from Korean cattle farms. FEMS Microbiol. Lett. 2017, 364, fnx129. [Google Scholar] [CrossRef]
- Fisher, R.A.; Gollan, B.; Helaine, S. Persistent bacterial infections and persister cells. Nat. Rev. Microbiol. 2017, 15, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Wood, T.K.; Knabel, S.J.; Kwan, B.W. Bacterial persister cell formation and dormancy. Appl. Env. Microbiol. 2013, 79, 7116–7121. [Google Scholar] [CrossRef]
- Keren, I.; Kaldalu, N.; Spoering, A.; Wang, Y.; Lewis, K. Persister cells and tolerance to antimicrobials. FEMS Microbiol. Lett. 2004, 230, 13–18. [Google Scholar] [CrossRef]
- Rajput, A.; Kaur, K.; Kumar, M. SigMol: Repertoire of quorum sensing signaling molecules in prokaryotes. Nucleic Acids Res. 2016, 44, D634–D639. [Google Scholar] [CrossRef]
- Perez-Perez, M.; Jorge, P.; Perez Rodriguez, G.; Pereira, M.O.; Lourenco, A. Quorum sensing inhibition in Pseudomonas aeruginosa biofilms: New insights through network mining. Biofouling 2017, 33, 128–142. [Google Scholar] [CrossRef]
- Platt, T.G.; Fuqua, C. What’s in a name? The semantics of quorum sensing. Trends Microbiol. 2010, 18, 383–387. [Google Scholar] [CrossRef]
- Kalia, V.C.; Purohit, H.J. Quenching the quorum sensing system: Potential antibacterial drug targets. Crit. Rev. Microbiol. 2011, 37, 121–140. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Pan, Y.; Shen, J.; Xu, Y. The efficacy and safety of tigecycline for the treatment of bloodstream infections: A systematic review and meta-analysis. Ann. Clin. Microbiol. Antimicrob. 2017, 16, 24. [Google Scholar] [CrossRef] [PubMed]
- Craft, K.M.; Nguyen, J.M.; Berg, L.J.; Townsend, S.D. Methicillin-resistant Staphylococcus aureus (MRSA): Antibiotic-resistance and the biofilm phenotype. Med. Chem. Commun. 2019, 10, 1231–1241. [Google Scholar] [CrossRef]
- Kanwar, I.; Sah, A.K.; Suresh, P.K. Biofilm-mediated Antibiotic-resistant Oral Bacterial Infections: Mechanism and Combat Strategies. Curr. Pharm. Des. 2017, 23, 2084–2095. [Google Scholar] [CrossRef]
- Mah, T.F. Biofilm-specific antibiotic resistance. Future Microbiol. 2012, 7, 1061–1072. [Google Scholar] [CrossRef]
- Manasherob, R.; Mooney, J.A.; Lowenberg, D.W.; Bollyky, P.L.; Amanatullah, D.F. Tolerant Small-colony Variants Form Prior to Resistance Within a Staphylococcus aureus Biofilm Based on Antibiotic Selective Pressure. Clin. Orthop. Relat. Res. 2021, 479, 1471–1481. [Google Scholar] [CrossRef]
- Klomjit, A.; Praiboon, J.; Tiengrim, S.; Chirapart, A.; Thamlikitkul, V. Phytochemical Composition and Antibacterial Activity of Brown Seaweed, Padina australis against Human Pathogenic Bacteria. J. Fish. Environ. 2021, 45, 8–22. [Google Scholar]
- Baweja, P.; Kumar, S.; Sahoo, D.; Levine, I. Biology of Seaweeds. In Seaweed in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2016; pp. 41–106. ISBN 9780128027936. [Google Scholar]
- Manandhar, B.; Paudel, P.; Seong, S.H.; Jung, H.A.; Choi, J.S. Characterizing eckol as a therapeutic aid: A systematic review. Mar. Drugs 2019, 17, 361. [Google Scholar] [CrossRef]
- Kim, S.K.; Pangestuti, R.; Kim, Y.J. Biological activities of phlorotannins: An overview. J. Funct. Foods 2012, 4, 691–700. [Google Scholar]
- Lee, S.H.; Kim, S.K. Biological Phlorotannins of Eisenia Bicyclis. In Marine Algae Extracts: Processes, Products, and Applications; John Wiley and Sons: Hoboken, NJ, USA, 2015; pp. 453–464. [Google Scholar]
- Shannon, E.; Abu-Ghannam, N. Antibacterial Derivatives of Marine Algae: An Overview of Pharmacological Mechanisms and Applications. Mar. Drugs 2016, 14, 81. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, B.; Nayak, R.; Bhuyan, P.P.; Patra, S.; Behera, C.; Sahoo, S.; Ki, J.S.; Quarta, A.; Ragusa, A.; Jena, M. Algal Phlorotannins as Novel Antibacterial Agents with Reference to the Antioxidant Modulation: Current Advances and Future Directions. Mar. Drugs 2022, 20, 403. [Google Scholar] [CrossRef] [PubMed]
- Lavoie, S.; Sweeney-Jones, A.M.; Mojib, N.; Dale, B.; Gagaring, K.; McNamara, C.W.; Kubanek, J. Antibacterial oligomeric polyphenols from the green alga Cladophora socialis. J. Org. Chem. 2019, 84, 5035–5045. [Google Scholar] [CrossRef]
- Nagayama, K.; Iwamura, Y.; Shibata, T.; Hirayama, M.; Nakamura, T. Antimicrobial activity of phlorotannins from the brown alga Ecklonia kurome. Mar. Biol. 2002, 141, 721–726. [Google Scholar]
- Cotas, J.; Leandro, A.; Monteiro, P.; Pacheco, D.; Figueirinha, A.; Gonçalves, A.M.M.; da Silva, G.J.; Pereira, L. Seaweed Phenolics: From Extraction to Applications. Mar. Drugs 2020, 18, 384. [Google Scholar] [CrossRef] [PubMed]
- Carpine, R.; Sieber, S. Antibacterial and antiviral metabolites from cyanobacteria: Their application and their impact on human health. Curr. Res. Biotechnol. 2021, 3, 65–81. [Google Scholar] [CrossRef]
- Whitfield, F.B.; Helidoniotis, F.; Shaw, K.J.; Svoronos, D. Distribution of bromophenols in species of marine algae from eastern Australia. J. Agric. Food Chem. 1999, 47, 2367–2373. [Google Scholar] [CrossRef]
- Xu, N.; Fan, X.; Yan, X.; Li, X.; Niu, R.; Tseng, C.K. Antibacterial bromophenols from the marine red alga Rhodomela confervoides. Phytochemistry 2003, 62, 1221–1224. [Google Scholar] [CrossRef]
- Ganesan, P.; Kumar, C.; Bhaskar, N. Antioxidant properties of methanol extract and its solvent fractions obtained from selected brown marine algae from India. Bioresour. Technol. 2011, 102, 1519–1526. [Google Scholar]
- Rajasulochana, P.; Krishnamoorthy, P.; Dhamotharan, R. Isolation, identification of bromophenol compound and antibacterial activity of Kappaphycus sp. Int. J. Pharm. Biol. Sci. 2012, 3, 173–186. [Google Scholar]
- Guven, K.C.; Bora, A.; Sunam, G. Hordenine from the alga Phyllophora nervosa. Phytochemistry 1970, 9, 1893–1897. [Google Scholar] [CrossRef]
- Bhowmick, S.; Mazumdar, A.; Moulick, A.; Adam, V. Algal metabolites: An inevitable substitute for antibiotics. Biotechnol. Adv. 2020, 43, 107571. [Google Scholar] [CrossRef] [PubMed]
- Yap, W.F.; Tay, V.; Tan, S.H.; Yow, Y.Y.; Chew, J. Decoding antioxidant and antibacterial potentials of Malaysian green seaweeds: Caulerpa racemosa and Caulerpa lentillifera. Antibiotics 2019, 8, 152. [Google Scholar] [CrossRef]
- Kar, J.; Ramrao, D.P.; Zomuansangi, R.; Lalbiaktluangi, C.; Singh, S.M.; Joshi, N.C.; Kumar, A.; Kaushalendra, M.S.; Yadav, M.K.; Singh, P.K. Revisiting the role of cyanobacteria-derived metabolites as antimicrobial agent: A 21st century perspective. Front. Microbiol. 2022, 13, 1034471. [Google Scholar] [CrossRef] [PubMed]
- Mo, S.; Krunic, A.; Chlipala, G.; Orjala, J. Antimicrobial ambiguine isonitriles from the cyanobacterium Fischerella ambigua. J. Nat. Prod. 2009, 72, 894–899. [Google Scholar] [CrossRef] [PubMed]
- Al-Mousawi, N.J.; Al-Assadi, I.J.; Al-Aarajy, M.J. Isolation, Identification and Antibacterial Activity of Alkaloid Compound N-Methylcytisine from Cyanobacterium Hapalosiphon aureus. Biomed. Chem. Sci. 2023, 2, 119–124. [Google Scholar] [CrossRef]
- Janarthanan, M.; Senthil, K.M. The properties of bioactive substances obtained from seaweeds and their applications in textile industries. J. Ind. Text. 2018, 48, 361–401. [Google Scholar] [CrossRef]
- Pesando, D.; Dubreuil, F.; Gnassia-Barelli, M.; Cabioch, J.; Jupin, H. Cellular effects of caulerpicin, a toxin extracted from Caulerpa taxifolia (Chlorophyceae). Bot. Mar. 1984, 27, 167–171. [Google Scholar]
- Torres, Y.R.; Berlinck, R.G.; Nascimento, G.G.; Fortier, S.C.; Pessoa, C.; de Moraes, M.O. Antibacterial activity against resistant bacteria and cytotoxicity of four alkaloid toxins isolated from the marine sponge Arenosclera brasiliensis. Toxicon 2002, 40, 885–891. [Google Scholar] [CrossRef]
- Stengel, D.B.; Connan, S.; Popper, Z.A. Algal chemodiversity and bioactivity: Sources of natural variability and implications for commercial application. Biotechnol. Adv. 2011, 29, 483–501. [Google Scholar] [CrossRef]
- Pangestuti, R.; Kim, S.K. Seaweed Proteins, Peptides, and Amino Acids. In Seaweed Sustainability; Elsevier: Amsterdam, The Netherlands, 2015; pp. 125–140. [Google Scholar] [CrossRef]
- Goud, M.J.P.; Seshikala, D.; Charya, M.S. Antibacterial activity and biomolecular composition of certain fresh water microalgae collected from River Godavari (India). Sci. World J. 2007, 2, 19–23. [Google Scholar] [CrossRef]
- Sachindra, N.M.; Sato, E.; Maeda, H.; Hosokawa, M.; Niimi, S.; Maoka, T. Comparative evaluation of antioxidant properties of fucoxanthin-rich extracts from Laminaria japonica and Undaria pinnatifida. J. Agric. Food Chem. 2007, 55, 9185–9192. [Google Scholar]
- Aryee, A.N.; Agyei, D.; Akanbi, T.O. Recovery and utilization of seaweed pigments in food processing. Curr. Opin. Food Sci. 2018, 19, 113–119. [Google Scholar] [CrossRef]
- Karpiński, T.M.; Adamczak, A. Fucoxanthin—An antibacterial carotenoid. Antioxidants 2019, 8, 239. [Google Scholar] [CrossRef] [PubMed]
- Afzal, S.; Yadav, A.K.; Poonia, A.K.; Choure, K.; Yadav, A.N.; Pandey, A. Antimicrobial therapeutics isolated from algal sources: Retrospect and prospect. Biologia 2023, 78, 291–305. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.; Silva, S.A.; Carpena, M.; Garcia-Oliveira, P.; Gullón, P.; Barroso, M.F.; Prieto, M.A.; Simal-Gandara, J. Macroalgae as a Source of Valuable Antimicrobial Compounds: Extraction and Applications. Antibiotics 2020, 9, 642. [Google Scholar] [CrossRef]
- Benedetti, Y.; Masci, G.; Balestri, F.; Giovannini, G.; Angiolella, L.; Mastromei, G.; Tredici, M.R. Evaluation of the Anti-Adhesive Potential of Spirulina platensis Extract on Human Cells Challenged with Escherichia coli O157: H7. Mar. Drugs 2023, 21, 582. [Google Scholar]
- Abu-Ghannam, N.; Rajauria, G. Antimicrobial activity of compounds isolated from algae. In Functional Ingredients from Algae for Foods and Nutraceuticals; Elsevier: Amsterdam, The Netherlands, 2013; pp. 287–306. [Google Scholar] [CrossRef]
- Hussein, G.; Sankawa, U.; Goto, H.; Matsumoto, K.; Watanabe, H. Astaxanthin, a carotenoid with potential in human health and nutrition. J. Nat. Prod. 2006, 69, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Deyab, M.A.; Abou-Dobara, M.I. Antibacterial activity of some marine algal extracts against most nosocomial bacterial infections. Egypt. J. Exp. Biol. (Bot.) 2013, 9, 281–286. [Google Scholar]
- Bruce, D.L.; Duff, D.C.B.; Antia, N.J. The identification of two antibacterial products of the marine planktonic alga Isochrysis galbana. Microbiology 1967, 48, 293–298. [Google Scholar] [CrossRef]
- Bendich, A. Physiological role of antioxidants in the immune system. J. Am. Coll. Nutr. 1993, 12, 599–607. [Google Scholar] [CrossRef]
- Jachlewski, T.G.; Åberg, A.K.; Bröms, J.E.; Hammarström, L.; Holst, O. Ulvan inhibits the adhesion of pathogenic bacteria to human intestinal cells. Food Chem. Toxicol. 2015, 83, 240–246. [Google Scholar]
- Kidgell, J.T.; Magnusson, M.; Buschmann, A.H.; Queiroz, O. Extraction, fractionation and applications of ulvan. Rev. Aquac. 2019, 11, 686–704. [Google Scholar]
- Chatterjee, S.; Lee, L.Y.; Kawaguchi, A.; Abed, N.; Savage, P.B.; McDaniel, S.W.; Rinehart, K.L.; Kato, I.; Yother, J. Inhibition of bacterial adhesion by sulfated polysaccharides: A structure-activity relationship study. Glycobiology 2013, 23, 585–595. [Google Scholar]
- Goggin, H.; Jardine, F.; Thornton, J.; Ryall, B.; Martin, K.; Marchant, R.; Banat, I.M.; Burgess, J.G. The effect of carrageenan on biofilm formation by Staphylococcus aureus. J. Appl. Microbiol. 2015, 118, 1460–1469. [Google Scholar]
- Rajoka; Riaz, M.S.; Muhammad, H.; Wu, Y.K.; Zhao, L.; Arfat, Y.A.; Memon, A.S.; Zhu, J.; Xu, X.; Shao, D.; et al. Iota-Carrageenan Oligosaccharides Exhibit Promising Antibacterial Activity and Synergistic Antibiotic Effects Against Clinical Multidrug-Resistant Bacteria. Front. Microbiol. 2021, 12, 629158. [Google Scholar]
- Pérez, M.J.; Falqué, E.; Domínguez, H. Antimicrobial Action of Compounds from Marine Seaweed. Mar. Drugs 2016, 14, 52. [Google Scholar] [CrossRef]
- Berriquillo, M.A.; Kim, S.; Je, J. Fucoidan inhibits biofilm formation and enhances the activity of antibiotics against methicillin-resistant Staphylococcus aureus. Int. J. Biol. Macromol. 2019, 140, 311–319. [Google Scholar]
- Aydın, M.; Aksoy, A.; Yılmaz, M.; Gök, V.; Aksoy, L.; Özkan, A.; Özkan, E.E.; Yücel, G.; Yücel, M.; Aksoy, M. Inhibitory effects of fucoidan on the adhesion of Helicobacter pylori to gastric cells. J. Funct. Foods 2018, 47, 157–164. [Google Scholar]
- Wijesekara, I.; Pangestuti, R.; Kim, S. Fucoidan reduces the expression of virulence genes and biofilm formation in Vibrio harveyi. Appl. Environ. Microbiol. 2017, 83, e00698-17. [Google Scholar]
- Zhao, D.; Wang, J.; Liu, Y.; Li, D.; Zhang, Q.; Wang, S.; Liu, Y.; Zhang, H.; Wang, Q.; Zhang, H.; et al. Fucoidan from Fucus vesiculosus enhances the maturation and function of human dendritic cells. Int. Immunopharmacol. 2014, 18, 309–315. [Google Scholar]
- Lee, H.J.; Kim, Y.M.; Kim, H.J.; Jeon, Y.J.; Kim, S. Fucoidan inhibits bacterial efflux pumps and enhances the activity of antibiotics against Staphylococcus aureus. Int. J. Antimicrob. Agents 2018, 52, 321–328. [Google Scholar]
- Abou Zeid, A.H.; Aboutabl, E.A.; Sleem, A.A.; El-Rafie, H.M. Water soluble polysaccharides extracted from Pterocladia capillacea and Dictyopteris membranacea and their biological activities. Carbohydr. Polym. 2014, 113, 62–66. [Google Scholar] [CrossRef]
- Admassu, H.; Gasmalla, M.A.A.; Yang, R.; Zhao, W. Bioactive peptides derived from seaweed protein and their health benefits: Antihypertensive, antioxidant, and antidiabetic properties. J. Food Sci. 2018, 83, 6–16. [Google Scholar] [CrossRef]
- Rioux, L.E.; Turgeon, S.L.; Beaulieu, M.; Bouchard, D. Prebiotic potential of laminarin from the brown seaweed Laminaria digitata. J. Appl. Phycol. 2010, 22, 633–641. [Google Scholar]
- Besednova, N.N.; Zaporozhets, T.S.; Somova, L.M.; Kuznetsova, T.A. Review: Prospects for the use of extracts and polysaccharides from marine algae to prevent and treat the diseases caused by Helicobacter pylori. Helicobacter 2015, 20, 89–97. [Google Scholar] [CrossRef]
- Berri, M.; Olivier, M.; Holbert, S.; Dupont, J.; Demais, H.; Le Goff, M.; Collen, P.N. Ulvan from Ulva armoricana (Chlorophyta) activates the PI3K/Akt signalling pathway via TLR4 to induce intestinal cytokine production. Algal Res. 2017, 28, 39–47. [Google Scholar] [CrossRef]
- García-Beltrán, J.M.; Arizcun, M.; Chaves-Pozo, E. Antimicrobial Peptides from Photosynthetic Marine Organisms with Potential Application in Aquaculture. Mar. Drugs 2023, 21, 290. [Google Scholar] [CrossRef] [PubMed]
- Suyama, W.; Suzuki, M.; Adachi, K.; Fujii, K.; Yokoyama, A. A new antibacterial cyclic depsipeptide, cyanopeptolin S, from the cyanobacterium Microcystis aeruginosa NIES-298. Tetrahedron Lett. 2003, 44, 825–828. [Google Scholar]
- Babica, P.; Bláha, L.; Maršálek, B. Exploring the structural diversity and biological activity of microcystins. Chem.-Biol. Interact. 2006, 164, 74–99. [Google Scholar]
- Falanga, A.; Lombardi, L.; Franci, G.; Vitiello, M.; Iovene, M.R.; Morelli, G.; Galdiero, M.; Galdiero, S. Marine Antimicrobial Peptides: Nature Provides Templates for the Design of Novel Compounds against Pathogenic Bacteria. Int. J. Mol. Sci. 2016, 17, 785. [Google Scholar] [CrossRef] [PubMed]
- Rojas, V.; Rivas, L.; Cárdenas, C.; Guzmán, F. Cyanobacteria and eukaryotic microalgae as emerging sources of antibacterial peptides. Molecules 2020, 25, 5804. [Google Scholar] [CrossRef]
- Ng, J.H.; Ilag, L.L. Cryptic protein fragments as an emerging source of peptide drugs. IDrugs Investig. Drugs J. 2006, 9, 343–346. [Google Scholar]
- Guzmán, F.; Wong, G.; Román, T.; Cárdenas, C.; Alvárez, C.; Schmitt, P.; Albericio, F.; Rojas, V. Identification of Antimicrobial Peptides from the Microalgae Tetraselmis suecica (Kylin) Butcher and Bactericidal Activity Improvement. Mar. Drugs 2019, 17, 453. [Google Scholar] [CrossRef]
- Beaulieu, L.; Bondu, S.; Doiron, K.; Rioux, L.E.; Turgeon, S.L. Characterization of antibacterial activity from protein hydrolysates of the macroalga Saccharina longicruris and identification of peptides implied in bioactivity. J. Funct. Foods 2015, 17, 685–697. [Google Scholar] [CrossRef]
- Ishida, K.; Matsuda, H.; Murakami, M.; Yamaguchi, K.; Kawaguchipeptin, B. An antibacterial cyclic undecapeptide from the cyanobacterium Microcystis aeruginosa. J. Nat. Prod. 1997, 60, 724–726. [Google Scholar] [CrossRef] [PubMed]
- Kabara, J.J.; Swieczkowski, D.M.; Conley, A.J.; Truant, J.P. Fatty acids and derivatives as antimicrobial agents. Antimicrob. Agents Chemother. 1972, 2, 23–28. [Google Scholar] [CrossRef]
- Sampaio, A.H.; Rogers, D.J.; Barwell, C.J.; Saker-Sampaio, S.; Nascimento, K.S.; Nagano, C.S.; Farias, W.R.L. New affinity procedure for the isolation and further characterization of the blood group B specific lectin from the red marine alga Ptilota plumosa. J. Appl. Phycol. 2002, 14, 489–495. [Google Scholar] [CrossRef]
- Kasanah, N.; Amelia, W.; Mukminin, A.; Triyanto, I.A. Antibacterial activity of Indonesian red algae Gracilaria edulis against bacterial fish pathogens and characterization of active fractions. Nat. Prod. Res. 2019, 33, 3303–3307. [Google Scholar] [CrossRef] [PubMed]
- Desbois, A.P.; Mearns-Spragg, A.; Smith, V.J. A fatty acid from the diatom Phaeodactylum tricornutum is antibacterial against diverse bacteria including multi-resistant Staphylococcus aureus (MRSA). Mar. Biotechnol. 2009, 11, 45–52. [Google Scholar] [CrossRef]
- Gutiérrez-Del-Río, I.; Brugerolle de Fraissinette, N.; Castelo-Branco, R.; Oliveira, F.; Morais, J.; Redondo-Blanco, S.; Villar, C.J.; Iglesias, M.J.; Soengas, R.; Cepas, V.; et al. Chlorosphaerolactylates A-D: Natural Lactylates of Chlorinated Fatty Acids Isolated from the Cyanobacterium Sphaerospermopsis sp. LEGE 00249. J. Nat. Prod. 2020, 83, 1885–1890. [Google Scholar] [CrossRef]
- Kim, Y.G.; Lee, J.H.; Raorane, C.J.; Oh, S.T.; Park, J.G.; Lee, J. Herring Oil and Omega Fatty Acids Inhibit Staphylococcus aureus Biofilm Formation and Virulence. Front. Microbiol. 2018, 9, 1241. [Google Scholar] [CrossRef]
- Anjali, K.P.; Sangeetha, B.M.; Devi, G.; Raghunathan, R.; Dutta, S. Bioprospecting of seaweeds (Ulva lactuca and Stoechospermum marginatum): The compound characterization and functional applications in medicine-a comparative study. J. Photochem. Photobiol. B Biol. 2019, 200, 111622. [Google Scholar] [CrossRef]
- Ullah, I.; Ali, S.; Alqahtani, A.S.; Nadeem, M.S.; Imran, M.; Patel, M.; Albogami, S. Molecular mechanisms of omega-3 fatty acids in combating infectious diseases and associated complications. Front. Immunol. 2021, 12, 701994. [Google Scholar]
- Simopoulos, A.P. Omega-3 fatty acids in inflammation and autoimmune diseases. J. Am. Coll. Nutr. 2002, 21, 495–505. [Google Scholar] [CrossRef]
- Spiegel, C.; Steixner, S.J.M.; Coraça-Huber, D.C. Antibiofilm Activity of Omega-3 Fatty Acids and Its Influence on the Expression of Biofilm Formation Genes on Staphylococcus aureus. Antibiotics 2022, 11, 932. [Google Scholar] [CrossRef]
- Domínguez, H.; Pateiro, M.; Lorenzo, J.M.; Gullón, B. Oxidation stability of omega-3 fatty acids: An overview. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1159–1179. [Google Scholar]
- Balcão, V.M.; Vila-Nova, N.S.; Albuquerque, T.M.R.; Falcão-Silva, V.S. Biofilms and Fatty Acids: A Structural and Functional Link. Front. Microbiol. 2021, 12, 738321. [Google Scholar]
- Al-Fatlawi, A.A.; Abdul-Hussein, R.S.; Al-Janabi, A.H.; Hassan, A.K. Synergistic antimicrobial activity of omega-3 polyunsaturated fatty acids and conventional antibiotics against Staphylococcus aureus. J. Pure Sci. 2020, 16, 334. [Google Scholar]
- Viana, I.; Silva, J.; Gibbs, P.A.; Teixeira, P. Antibacterial activity of saturated fatty acids against Staphylococcus aureus in food model systems. Food Control 2015, 54, 120–125. [Google Scholar]
- Cherng, J.Y.; Liu, C.C.; Shen, C.R.; Lin, H.H.; Shih, M.F. Beneficial effects of Chlorella-11 peptide on blocking LPS-induced macrophage activation and alleviating thermal injury-induced inflammation in rats. Int. J. Immunopathol. Pharmacol. 2010, 23, 811–820. [Google Scholar] [CrossRef]
- Le, P.N.T.; Desbois, A.P. Antibacterial Effect of Eicosapentaenoic Acid against Bacillus cereus and Staphylococcus aureus: Killing Kinetics, Selection for Resistance, and Potential Cellular Target. Mar. Drugs 2017, 15, 334. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Srebnik, M.; Hanani, N. Bioactive compounds from algae. Eur. J. Lipid Sci. Technol. 2002, 104, 754–776. [Google Scholar]
- Heo, S.J.; Jeon, Y.J. Screening of biological activities of Korean marine plants and their potent antibacterial activity against methicillin-resistant Staphylococcus aureus (MRSA). J. Korean Fish. Soc. 2009, 42, 417–423. [Google Scholar]
- Mitani, T.; Furuya, M.; Nagayama, K.; Tanaka, Y.; Sakagami, Y. Sargaquinoic acid, an isoprenoid quinone from the brown alga Sargassum micracanthum as a novel inhibitor of eukaryotic DNA polymerase α. J. Nat. Prod. 2003, 66, 930–934. [Google Scholar]
- Hughes, C.C.; Fenical, W. Antibacterials from the sea. Chem. A Eur. J. 2010, 16, 12512–12525. [Google Scholar] [CrossRef] [PubMed]
- Aminin, D.L.; Menchinskaya, E.S.; Pisliagin, E.A.; Silchenko, A.S.; Avilov, S.A.; Kalinin, V.I. Holothurins as potential anticancer and anti-infectious agents. Mar. Drugs 2015, 13, 2761–2815. [Google Scholar]
- Plaza, A.; Keffer, J.L.; Bifulco, G.; Lloyd, J.R.; Bewley, C.A. Chrysophaentins A-H, antibacterial bisdiarylbutene macrocycles that inhibit the bacterial cell division protein FtsZ. J. Am. Chem. Soc. 2010, 132, 9069–9077. [Google Scholar] [CrossRef]
- Keffer, J.L.; Hammill, J.T.; Lloyd, J.R.; Plaza, A.; Wipf, P.; Bewley, C.A. Geographic variability and anti-staphylococcal activity of the chrysophaentins and their synthetic fragments. Mar. Drugs 2012, 10, 1103–1125. [Google Scholar] [CrossRef]
- Li, X.; Ma, S. Advances in the discovery of novel antimicrobials targeting the assembly of bacterial cell division protein FtsZ. Eur. J. Med. Chem. 2015, 95, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kusuma, K.D.; Payne, M.; Ung, A.T.; Bottomley, A.L.; Harry, E.J. FtsZ as an Antibacterial Target: Status and Guidelines for Progressing This Avenue. ACS Infect. Dis. 2019, 5, 1279–1294. [Google Scholar] [CrossRef]
- Waterman, P.G.; Mole, S. Analysis of Phenolic Plant Metabolites, 1st ed.; Wiley-Blackwell: Hoboken, NJ, USA, 1994; pp. 150–153, ISBN-10: 0632029692; ISBN-13: 978-0632029693. [Google Scholar]
- Li, Y.X.; Wijesekara, I.; Li, Y.; Kim, S.K. Phlorotannins as bioactive agents from brown algae. Process Biochem. 2011, 46, 2219–2224. [Google Scholar] [CrossRef]
- Salehi, B.; Sharifi-Rad, J.; Seca, A.M.L.; Pinto, D.C.G.A.; Michalak, I.; Trincone, A.; Mishra, A.P.; Nigam, M.; Zam, W.; Martins, N. Current Trends on Seaweeds: Looking at Chemical Composition, Phytopharmacology, and Cosmetic Applications. Molecules 2019, 24, 4182. [Google Scholar] [CrossRef]
- Okuda, T.; Ito, H. Tannins of Constant Structure in Medicinal and Food Plants-Hydrolyzable Tannins and Polyphenols Related to Tannins. Molecules 2011, 16, 2191–2217. [Google Scholar] [CrossRef]
- Besednova, N.N.; Andryukov, B.G.; Zaporozhets, T.S.; Kryzhanovsky, S.P.; Kuznetsova, T.A.; Fedyanina, L.N.; Makarenkova, I.D.; Zvyagintseva, T.N. Algae Polyphenolic Compounds and Modern Antibacterial Strategies: Current Achievements and Immediate Prospects. Biomedicines 2020, 8, 342. [Google Scholar] [CrossRef] [PubMed]
- Thomas, N.V.; Kim, S.K. Potential pharmacological applications of polyphenolic derivatives from marine brown algae. Environ. Toxicol. Pharmacol. 2011, 32, 325–335. [Google Scholar] [CrossRef]
- Mashjoor, S.; Yousefzadi, M.; Esmaeili, M.A.; Rafiee, R. Cytotoxicity and antimicrobial activity of marine macro algae (Dictyotaceae and Ulvaceae) from the Persian Gulf. Cytotechnology 2016, 68, 1717–1726. [Google Scholar] [CrossRef] [PubMed]
- Poole, J.; Diop, A.; Rainville, L.C.; Barnabé, S. Bioextracting polyphenols from the brown seaweed Ascophyllum nodosum from Québec’s north shore coastline. Ind. Biotechnol. 2019, 15, 212–218. [Google Scholar] [CrossRef]
- Machu, L.; Misurcova, L.; Ambrozova, J.V.; Orsavova, J.; Mlcek, J.; Sochor, J.; Jurikova, T. Phenolic content and antioxidant capacity in algal food products. Molecules 2015, 20, 1118–1133. [Google Scholar] [CrossRef]
- Gómez-Guzmán, M.; Rodríguez-Nogales, A.; Algieri, F.; Gálvez, J. Potential role of seaweed polyphenols in cardiovascular-associated disorders. Mar. Drugs 2018, 16, 250. [Google Scholar] [CrossRef]
- Imbs, T.I.; Zvyagintseva, T.N. Phlorotannins are polyphenolic metabolites of brown algae. Russ. J. Mar. Biol. 2018, 44, 263–273. [Google Scholar] [CrossRef]
- Gupta, S.; Abu-Ghannam, N. Recent developments in the application of seaweeds or seaweed extracts as a means for enhancing the safety and quality attributes of foods. Innov. Food Sci. Emerg. Technol. 2011, 12, 600–609. [Google Scholar] [CrossRef]
- Nagayama, K.; Iwamura, Y.; Shibata, T.; Hirayama, I.; Nakamura, T. Bactericidal activity of phlorotannins from the brown alga Ecklonia kurome. J. Antimicrob. Chemother. 2002, 50, 889–893. [Google Scholar] [CrossRef]
- Wang, W.; Wang, M.Y.; Tan, C.H.; Lan, W.J.; Wang, C.Y.; Li, H.J. A new anti-MRSA bromophenol from the brown alga Leathesia marina and its synergistic effect with oxacillin. Phytochemistry 2024, 221, 113898. [Google Scholar] [CrossRef]
- Alghazeer, R.; Whida, F.; Abduelrhman, E.; Gammoudi, F.; Naili, M. In vitro antibacterial activity of alkaloid extracts from green, red and brown macroalgae from western coast of Libya. Afr. J. Biotechnol. 2013, 12, 7086–7091. [Google Scholar]
- Mo, S.; Krunic, A.; Santarsiero, B.D.; Franzblau, S.G.; Orjala, J. Hapalindole-related alkaloids from the cultured cyanobacterium Fischerella ambigua. Phytochemistry 2010, 71, 2116–2123. [Google Scholar] [CrossRef] [PubMed]
- França, P.H.; Barbosa, D.P.; da Silva, D.L.; Ribeiro, Ê.A.; Santana, A.E.; Santos, B.V.; Barbosa-Filho, J.M.; Quintans, J.S.S.; Barreto, R.S.S.; Quintans-Júnior, L.J.; et al. Indole alkaloids from marine sources as potential leads against infectious diseases. BioMed Res. Int. 2014, 2014, 375423. [Google Scholar] [CrossRef] [PubMed]
- Vijayabaskar, P.; Vaseela, N.; Thirumaran, G. Potential antibacterial and antioxidant properties of a sulfated polysaccharide from the brown marine algae Sargassum swartzii. Chin. J. Nat. Med. 2012, 10, 421–428. [Google Scholar] [CrossRef]
- Kadam, S.U.; O’Donnell, C.P.; Rai, D.K.; Hossain, M.B.; Burgess, C.M.; Walsh, D.; Tiwari, B.K. Laminarin from Irish Brown Seaweeds Ascophyllum nodosum and Laminaria hyperborea: Ultrasound Assisted Extraction, Characterization and Bioactivity. Mar. Drugs 2015, 13, 4270–4280. [Google Scholar] [CrossRef]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as nutritional and functional food sources: Revisiting our understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef]
- Choi, S.M.; Jang, E.J.; Cha, J.D. Synergistic effect between fucoidan and antibiotics against clinic methicillin-resistant Staphylococcus aureus. Adv. Biosci. Biotechnol. 2015, 6, 275–285. [Google Scholar] [CrossRef]
- Luria, S.E. On the mechanism of enzyme inhibition by acrylic acid. J. Cell. Comp. Physiol. 1945, 26, 75–83. [Google Scholar]
- Awai, K.; Hikosaka, K.; Sakurai, A.; Ohta, H.; Sato, N. Antimicrobial activity of monogalactosyl diacylglycerol from marine algae. J. Appl. Phycol. 2011, 23, 1025–1031. [Google Scholar]
- Mishra, V.; Kaur, N.; Arora, N.; Jaglan, S.; Saini, R.V. Digalactosyldiacylglycerol: A review of its extraction, characterization, and biological activities. J. Food Biochem. 2020, 44, e13156. [Google Scholar]
- Funayama, S.; Sakai, R.; Anraku, Y.; Kondo, M.; Imaizumi, N. Antimicrobial glycolipids from the marine diatom Chaetoceros simplex. J. Nat. Prod. 2006, 69, 87–91. [Google Scholar]
- Cho, M.; Lee, H.; Kandasamy, S.; Lee, J.; You, S. Antibacterial activity of sargachromanol E from Sargassum yezoense against methicillin-resistant Staphylococcus aureus. Bioorganic Med. Chem. Lett. 2011, 21, 6403–6407. [Google Scholar]
- Hayes, M. (Ed.) Marine Bioactive Compounds: Sources, Characterization and Applications; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar] [CrossRef]
- Sieburth, J.M. Acrylic acid, an “antibiotic” principle in Phaeocystis blooms in Antarctic waters. Science 1960, 132, 676–677. [Google Scholar] [CrossRef] [PubMed]
- Ianora, A.; Bentley, M.G.; Caldwell, G.S.; Casotti, R.; Cembella, A.D.; Engström-Öst, J.; Halsband, C.; Sonnenschein, E.C.; Legrand, C.; Llewellyn, C.A.; et al. The relevance of marine chemical ecology to ecosystem function. Mar. Drugs 2011, 9, 1625–1648. [Google Scholar] [CrossRef]
- Smetacek, V.; Zingone, A. Green and golden seaweed tides on the rise. Nature 2013, 504, 84. [Google Scholar] [CrossRef]
- Freese, E.; Sheu, C.W.; Galliers, E. Function of lipophilic acids as antimicrobial food additives. Nature 1973, 241, 321–325. [Google Scholar] [CrossRef]
- Cherrington, C.A.; Hinton, M.; Mead, G.C.; Chopra, I. Organic acids: Chemistry, antibacterial activity. Adv. Microb. Physiol. 1991, 32, 87–108. [Google Scholar] [CrossRef]
- Lambert, R.J.; Stratford, M. Weak-acid preservatives: Modelling microbial inhibition. J. Appl. Microbiol. 1999, 86, 157–164. [Google Scholar] [CrossRef]
- Anthony, N.J.; Giba, G.A.; St-Cyr, G.; Pinto, D.J.P. Inhibition of alanine racemase by acrylate. Biochemistry 1997, 36, 15303–15310. [Google Scholar]
- Rodriguez, A.C.; Pereira, D.S.; Alves, M.P.; Costa, J.V.; Berlinck, R.G.S. A Diterpenoid from Laurencia L. as a Novel Inhibitor of Staphylococcus aureus Sortase A. J. Nat. Prod. 2025, 88, 512–519. [Google Scholar] [CrossRef]
- Costa, F.S.; de Lima, J.M.; Oliveira, G.F.; de Sousa, M.P.; Polito, M.L.T.M. A Low-Molecular-Weight Sulfated Polysaccharide from Ulva lactuca Potentiates Linezolid Activity against MRSA Biofilms. Int. J. Biol. Macromol. 2025, 285, 131745. [Google Scholar] [CrossRef]
- Martínez, K.A.; Lauritano, C.; Druka, D.; Romano, G.; Grohmann, T.; Jaspars, M.; Martín, J.; Díaz, C.; Cautain, B.; de la Cruz, M.; et al. Amphidinol 22, a New Cytotoxic and Antifungal Amphidinol from the Dinoflagellate Amphidinium carterae. Mar. Drugs 2019, 17, 385. [Google Scholar] [CrossRef]
- El-Sayed, H.; Morad, M.Y.; Sonbol, H.; Hammam, O.A.; Abd El-Hameed, R.M.; Ellethy, R.A.; Ibrahim, A.M.; Hamada, M.A. Myco-Synthesized Selenium Nanoparticles as Wound Healing and Antibacterial Agent: An In Vitro and In Vivo Investigation. Microorganisms 2023, 11, 2341. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.; da Silva, S.M.C.; Lemos, M.F.L.; Cardoso, S.M. Overcoming the Delivery Challenges of Marine-Derived Bioactive Compounds: The Role of Nano-Based Drug Delivery Systems. Mar. Drugs 2024, 22, 118. [Google Scholar] [CrossRef]
- Burýšková, B.; Bláha, L.; Babica, P. Toxic potential of cyanobacterial metabolites: A review of recent trends (2020–2025). Toxicon 2025, 252, 107981. [Google Scholar] [CrossRef]
- Greenwell, H.C.; Laurens, L.M.L. Macro- and micro-algae biorefineries: A perspective on the challenges and opportunities for sustainable production of biofuels and bioproducts. Curr. Opin. Biotechnol. 2024, 89, 103074. [Google Scholar] [CrossRef]
- Zio, F.D.; Angelo, I.D.; Stefano, M.D. From Sea to Skin: Marine Compounds in the Treatment of MRSA-Infected Wounds. Pharmaceutics 2024, 16, 84. [Google Scholar] [CrossRef]
- Al-Rajh, A.A.; Al-Ghamdi, H.A.; Al-Ghamdi, F.A.; Habila, M.A.; ALOthman, Z.A. Green Synthesis of Silver Nanoparticles Using Sargassum wightii Seaweed Extract and Their Potent Antibacterial and Anti-Biofilm Activities Against MRSA. J. Infect. Public Health 2024, 17, 148–157. [Google Scholar] [CrossRef]
| Chemical Group | Specific Compound/Sub-Class | Algal Source(s) | Mechanism of Action Against MRSA | Key Findings and MIC Values | Developmental Considerations | Reference(s) |
|---|---|---|---|---|---|---|
| Polyphenols | Phlorotannins (e.g., Eckol) | Brown algae (Padina australis, Ecklonia kurome, Eisenia bicyclis) | Cell membrane disruption; inhibition of methicillin-resistance genes; biofilm and quorum sensing (QS) interference; enzyme and nucleic acid inhibition. | MIC values as low as 32–64 µg/mL against MRSA (E. bicyclis). | Efficacy is influenced by the degree of polymerization. | [80,81,82,83,84,85,86,87,88,89] |
| Polyphenols | Bromophenols | Red algae (Rhodomela larix, Kappaphycus sp.) | Cell membrane disruption; quorum sensing (QS) inhibition; protein inhibition. | Effective against MRSA and other Staphylococcus species. | Widespread across macroalgal taxa with diverse structures. | [90,91,92,93,94,95] |
| Alkaloids | Cyanobacterial Alkaloids (Hapalindoles, N-methylcytisin) | Cyanobacteria (Hapalosiphon sp., Fischerella sp., Nostoc sp.) | QS interference; efflux pump suppression; DNA intercalation/polymerase suppression. | N-methylcytisin showed potent action against S. aureus at 150 µg/mL. | Research on specific anti-MRSA activity is less extensive than other classes. | [96,97,98,99,100] |
| Alkaloids | Caulerpin | Green algae (Caulerpa taxifolia) | Disruption of cell membrane function; inhibition of cell division. | Strong antibacterial effects reported, particularly against resistant strains like MRSA. | A bisindole alkaloid with a lipophilic nature allowing membrane insertion. | [101,102] |
| Pigments | Carotenoids, Chlorophylls, Phycobiliproteins | Green, red, brown algae and cyanobacteria (Spirulina, Haematococcus, etc.) | Direct action (membrane disruption, cell wall interference) and indirect action (ROS generation, immunomodulation, biofilm inhibition). | Fucoxanthin is effective against S. aureus; Astaxanthin inhibits biofilm. | Many act as antioxidants, a host-mediated benefit, rather than being directly bactericidal. | [96,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117] |
| Polysaccharides (Sulfated) | Fucoidan, Laminarin, Ulvan, Carrageenan | Brown algae (Laminaria), green algae (Ulva), red algae (Kappaphycus) | Anti-adhesion and biofilm disruption; immune modulation (activating macrophages); efflux pump interference (Fucoidan). | Fucoidan shows synergistic effects with ampicillin; MIC against MRSA ranges from 64 to 512 µg/mL. | Activity depends on molecular weight and degree of sulfation; Some have primarily indirect effects. | [98,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132] |
| Peptides and Amino Acids | Antimicrobial Peptides (AMPs), Cyanopeptolins | Cyanobacteria (Microcystis), green algae (Tetraselmis) | Direct membrane disruption via pore formation; inhibition of protein synthesis or proteases; QS inhibition. | SP-1 peptide from Spirulina is non-toxic to blood cells. | High toxicity is a major concern for some compounds like microcystins. | [96,98,133,134,135,136,137,138,139,140,141,142,143] |
| Lectins | Griffithsin, Ptilota plumosa lectin | Red algae (Griffithsia sp.), green algae (Codium) | Inhibition of bacterial adhesion; agglutination of bacterial cells; biofilm formation interference. | Research on algal lectins specifically against MRSA is limited; mechanisms are often extrapolated. | - | [144] |
| Lipids and Fatty Acids | PUFAs (DHA, EPA) and SFAs (Palmitic acid) | Diatoms (Phaeodactylum), green Algae (Chlorella vulgaris) | Cell membrane disruption by altering fluidity; interference with fatty acid metabolism; biofilm and QS inhibition. | PUFAs are generally more potent than SFAs but face challenges with oxidation and bioavailability. | - | [96,145,146,147,148,149,150,151,152,153,154,155,156,157,158] |
| Terpenoids | Sargachromanol E, Bromophycolides | Brown algae (Sargassum), red algae (Callophycus) | Disruption of cell membrane; inhibition of DNA polymerase and other enzymes; efflux pump inhibition. | Bromophycolides show potent activity with IC50 values in the low µM range against MRSA. | An incredibly diverse class with a wide range of activities depending on structure. | [11,84,96,150,159,160,161,162,163] |
| Saponins | (e.g., Holothurins used as model) | Green andbrown algae (Enteromorpha, Sargassum) | Primary: strong cell membrane disruption leading to lysis; biofilm inhibition. | Research on algal saponins is very limited; can be toxic at high concentrations. | - | [164] |
| Sterols | Fucosterol | Brown algae (Fucus vesiculosus) | Disrupts bacterial cell membrane; inhibits biofilm formation; decreases cellular metabolic activity. | Can enhance the activity of conventional antibiotics. | - | [93] |
| Chrysophaentins | Chrysophaentins A-H | Marine alga Chrysophaeum taylori | Novel Mechanism: inhibits the FtsZ protein (a bacterial GTPase), preventing Z-ring formation and halting cell division. | Potent activity with MIC of 1.5 µg/mL against MRSA. | Represents a significant advancement with a novel target, reducing the chance of cross-resistance. | [96,165,166,167] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibraheem, I.B.M.; Alharbi, R.M.; Abdel-Raouf, N.; Al-Enazi, N.M.; Alsamhary, K.I.; Ali, H.M. Algal Metabolites as Novel Therapeutics Against Methicillin-Resistant Staphylococcus aureus (MRSA): A Review. Pharmaceutics 2025, 17, 989. https://doi.org/10.3390/pharmaceutics17080989
Ibraheem IBM, Alharbi RM, Abdel-Raouf N, Al-Enazi NM, Alsamhary KI, Ali HM. Algal Metabolites as Novel Therapeutics Against Methicillin-Resistant Staphylococcus aureus (MRSA): A Review. Pharmaceutics. 2025; 17(8):989. https://doi.org/10.3390/pharmaceutics17080989
Chicago/Turabian StyleIbraheem, Ibraheem Borie M., Reem Mohammed Alharbi, Neveen Abdel-Raouf, Nouf Mohammad Al-Enazi, Khawla Ibrahim Alsamhary, and Hager Mohammed Ali. 2025. "Algal Metabolites as Novel Therapeutics Against Methicillin-Resistant Staphylococcus aureus (MRSA): A Review" Pharmaceutics 17, no. 8: 989. https://doi.org/10.3390/pharmaceutics17080989
APA StyleIbraheem, I. B. M., Alharbi, R. M., Abdel-Raouf, N., Al-Enazi, N. M., Alsamhary, K. I., & Ali, H. M. (2025). Algal Metabolites as Novel Therapeutics Against Methicillin-Resistant Staphylococcus aureus (MRSA): A Review. Pharmaceutics, 17(8), 989. https://doi.org/10.3390/pharmaceutics17080989






