Leaf Saponins of Quillaja brasiliensis as Powerful Vaccine Adjuvants
Abstract
1. Introduction
2. Saponins
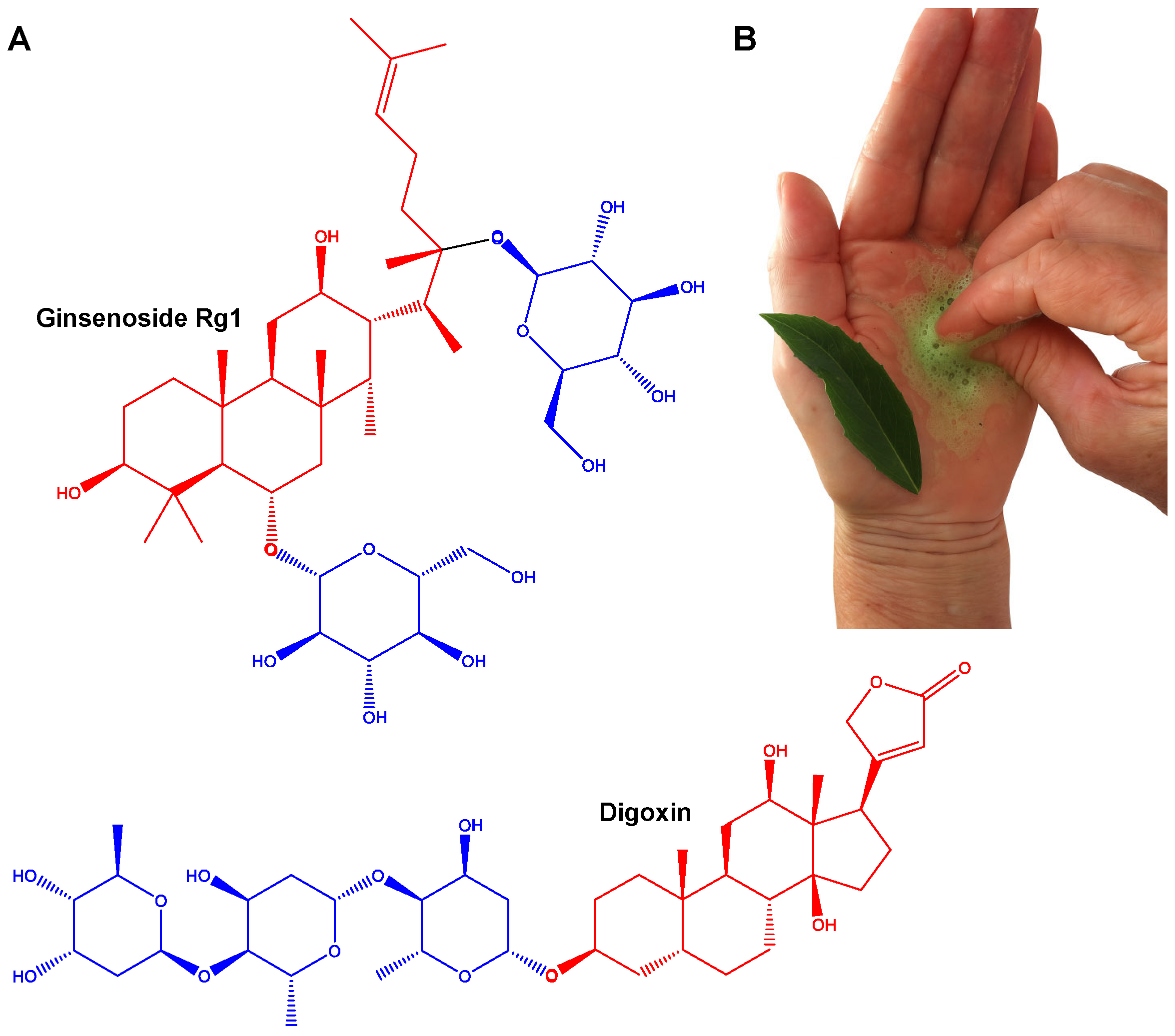
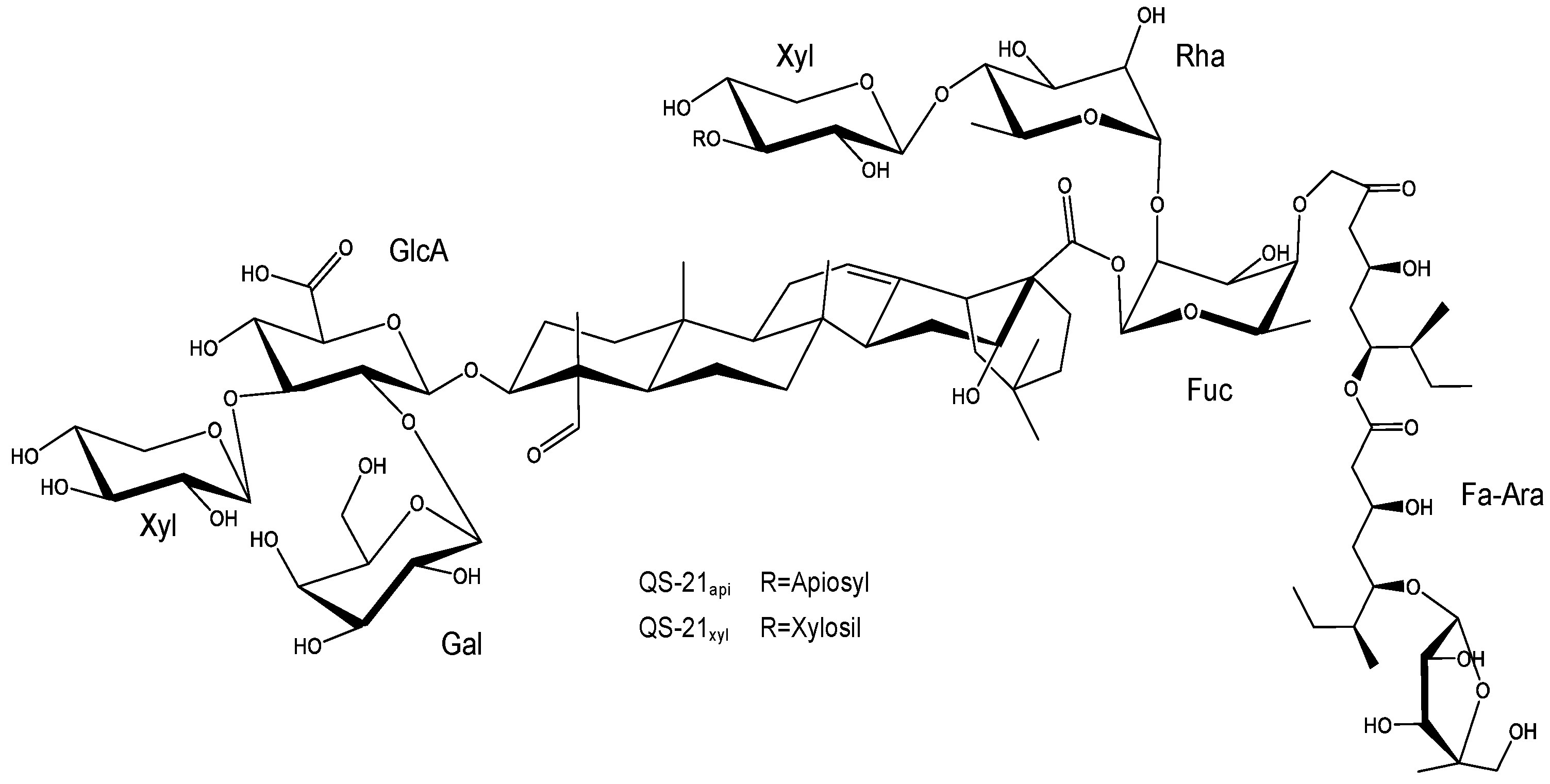
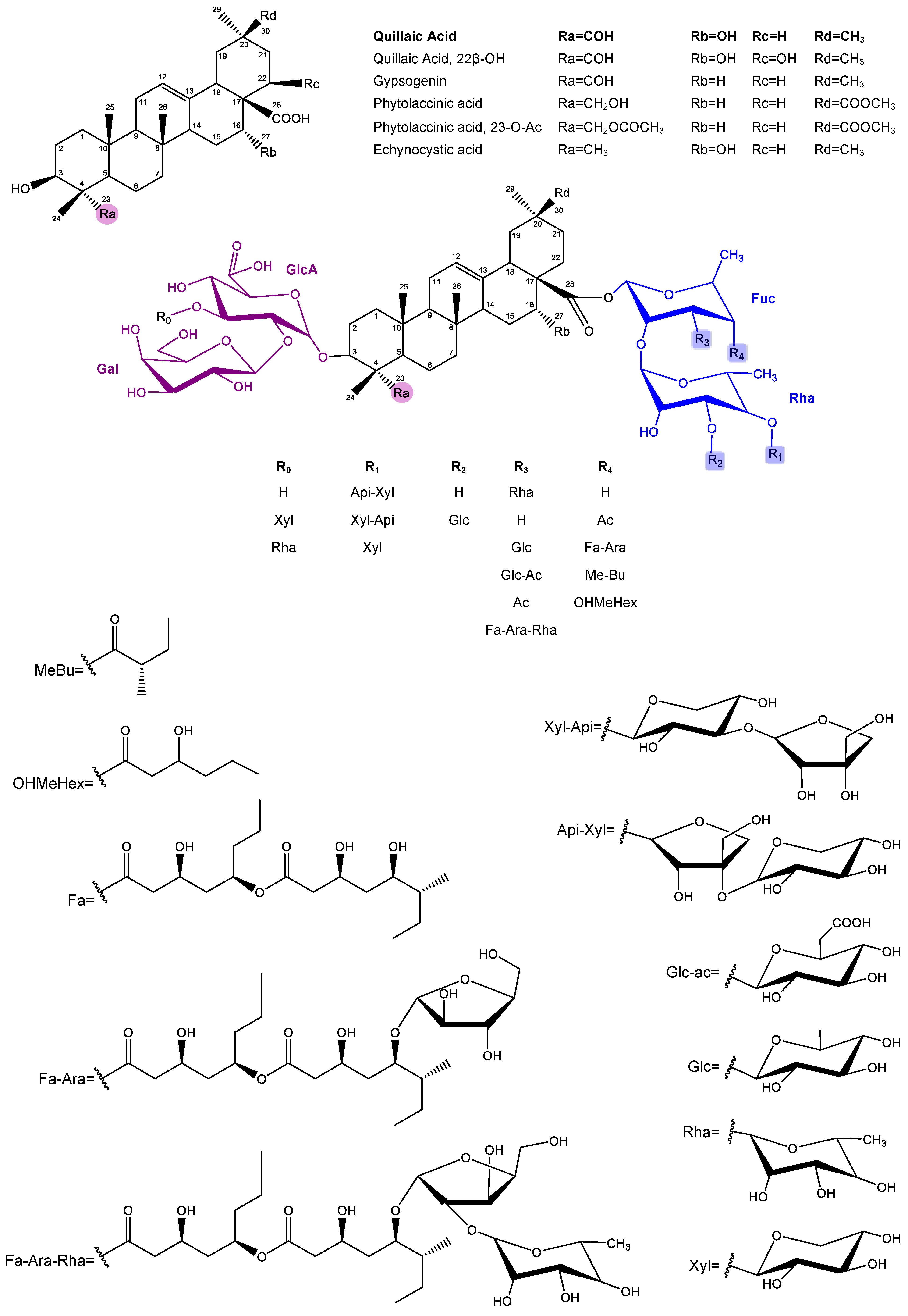
2.1. Characteristics, Structure, and Properties
2.2. Saponins as Immunoadjuvants
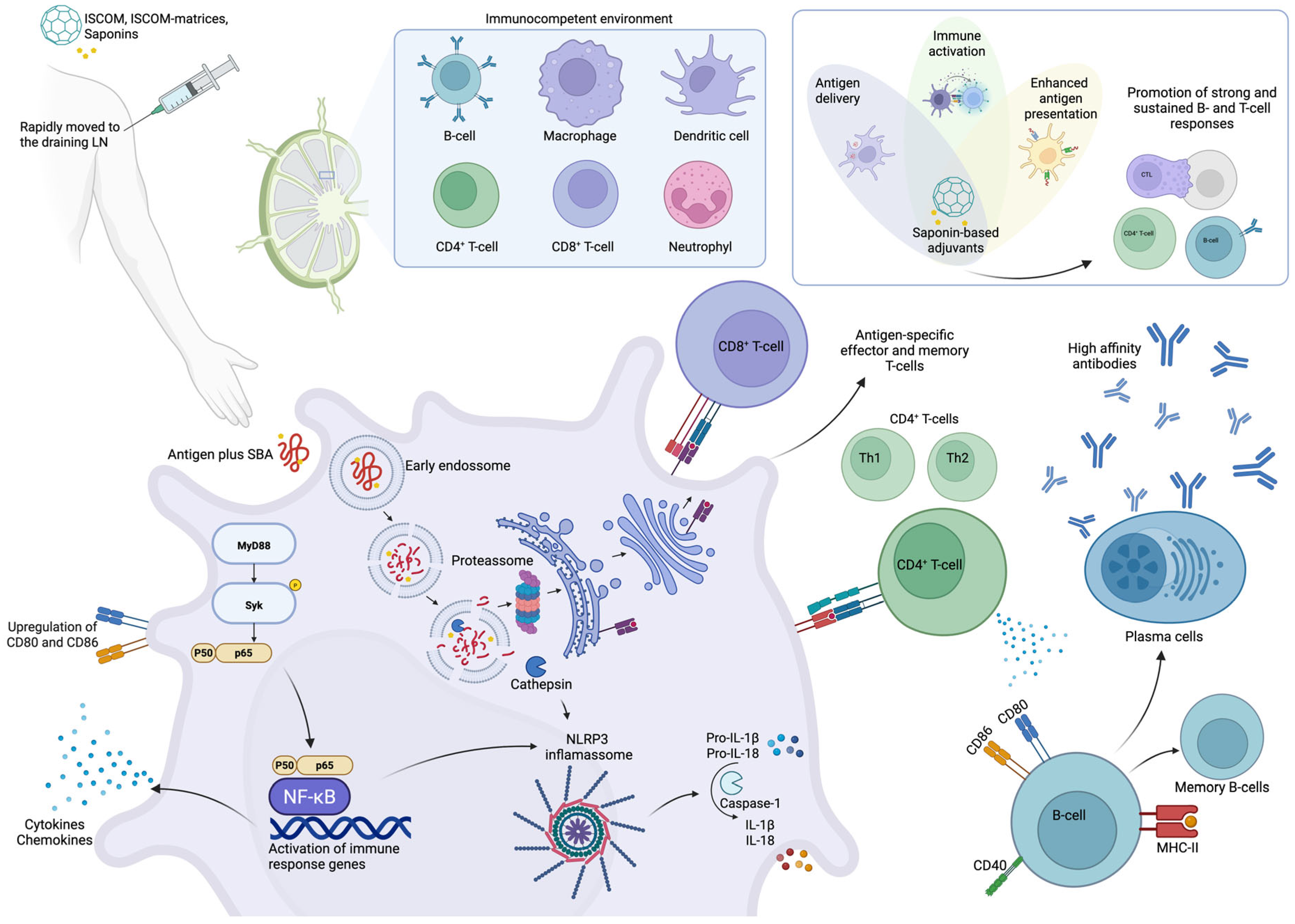
| AS01 | AS02 | Matrix-MTM | |
|---|---|---|---|
| Composition and delivery system | QS-21 + MPL in liposomes [27] | QS-21 + MPL in oil-in-water emulsion [27] | Saponin fraction from Q. saponaria in ISCOM-like nanoparticles [97] |
| Immune response | Th1 dominant response with CD8+ T cell activation and Ab responses [97] | Balanced T cell (Th1/Th2) and Ab responses [27] | Th1 dominant response with CD8+ T cell activation and Ab responses [97,118] |
| Clinical applications | Shingrix™ (Herpes Zoster); Mosquirix™ (Malaria); HIV, TB (candidate vaccines) [128] | Malaria, HIV, Hepatitis B (clinical trials) [128] | Nuvaxovid™ (NVX-CoV2373, COVID-19) [129]; Malaria [130] |
| Advantages | Efficient CD8+ activation; reduced QS-21 toxicity via liposomes | Broad immune stimulation; suitable for complex pathogens | Efficient CD8+ activation; self-assembling; reduced saponin toxicity via ISCOM-matrices |
| Limitations | Limited QS-21 supply; requires liposome formulation | Limited QS-21 supply; emulsion stability; potential reactogenicity | Limited saponin supply; formulation complexity |
2.3. Quillajaceae Saponin-Based Nanoparticulated Adjuvants
3. Saponins from Q. brasiliensis Leaves
3.1. Methods of Isolation, Structural Similarity, and Toxicity Concerns Among Q. saponaria Bark Saponins and Q. brasiliensis Leaves Saponins
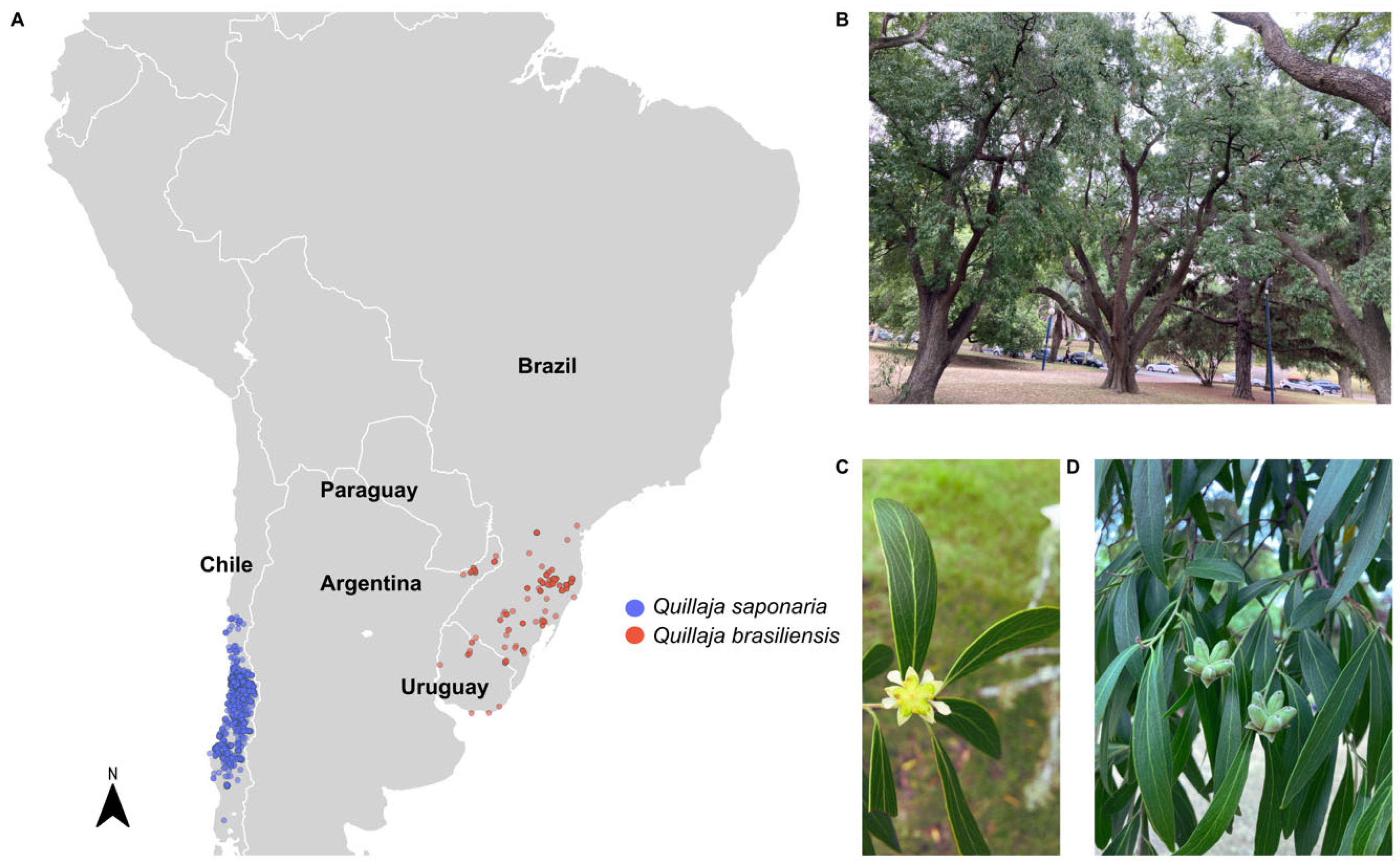
3.2. Q. Brasiliensis Saponins: A Potent Natural Immunoadjuvant
| Adjuvant/Route (μg/Dose) | Antigen | IgG | IgG1 | IgG2a/2c | IgG2b | IgG3 | IgA | NAb/HAI | DTH | Other Assays | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| AE-sc (400) | Herpesvirus (BoHV1, inactivated virus) | * | * | * | [168] | ||||||
| QB-90-sc (200, 150, 100 and 50) | * | * | * | ||||||||
| QB-90 a-sc (100) | Herpesvirus (BoHV5, inactivated virus) | ** | * | *** | *** | ** | *** | Upregulation of IFN-γ and IL-2 mRNA in splenocytes | [91] | ||
| AE-sc (400) | Poliovirus (Sabin 1, inactivated virus) | ** | ** | ** | * | ** | Upregulation of IFN-γ and IL-2 mRNA in splenocytes | [92] | |||
| QB-90-sc (50) | ** | ** | ** | * | ** | ||||||
| AE-sc (400, 200 and 100) | Pestivirus (BVDV, inactivated virus) | *** | *** | *** | *** | Splenocyte proliferation (including CD8+ T cells) and IFN-γ, IL-2, TNF, IL-10, and IL-17 cytokines secretion | [14,90] | ||||
| QB-90-sc (100, 50 and 10) | *** | *** | *** | *** | |||||||
| QB-90-sc (10) | Ovalbumin (OVA) | *** | ** | - | - | Splenocyte proliferation; IFN-γ, IL-2 secretion in IQB-90 vaccines | [86] | ||||
| IQB-90-sc (10) | *** | *** | ** | *** | |||||||
| QB-90-in (2) | - | - | - | - | - | ||||||
| IQB-90-in (2) | *** | *** | - | */*** b | - | ||||||
| AE-sc (400) | Rabies virus (inactivated virus) | * | * | * | * | Protection in a challenge assay provided by the adjuvanted anti-rabies vaccines | [172] | ||||
| QB-80-sc (100 and 50) | * | * | * | * | |||||||
| QB-90-sc (100 and 50) | * | * | * | * | |||||||
| Fraction B-sc (50) | * | * | * | ||||||||
| Fraction 3-sc (50) | * | * | * | ||||||||
| AE-sc (400 and 200) | Pestivirus (BVDV, inactivated virus) | **** | **** | *** | **** | Antigen-specific IFN-γ production in CD4+ and CD8+ T cells; dose sparing effect | [14] | ||||
| QB-80-sc (100, 50 and 10) | **** | **** | **** | *** | |||||||
| IMXQB-80-sc (2.5) | Zika virus (inactivated virus) | *** | **** | *** | *** | No differences between IMXQB-80 (2.5 μg) and QB-80 (10 μg) immunized animals | [175] | ||||
| QB-80-sc (10) | **** | *** | **** | *** | |||||||
| IQB80-sc (10 and 2) | Zika virus (recombinant E protein) | **** | ** | ** | **** | *** | *** | Antigen-specific splenocyte proliferation and antibody avidity increase | [94] | ||
| IQB90-sc (5) | Influenza (seasonal split vaccine) | **** | ** | ** | ** | **** | - | **** | ** | Protection in a challenge test provided by adjuvanted vaccines; dose sparing effect | [93] |
| IQB90-in (5) | **** | **** | **** | **** | **** | **** b | **** | ||||
| IMXQB-sc (5) | Influenza (seasonal split vaccine) | **** | *** | **** | *** | *** | * | Protection in a challenge test provided by adjuvanted vaccines; high levels of NAb and improved serum HI | [180] | ||
| IMXQB-in (2.5) | *** | *** | *** | *** | *** | *** | *** | ||||
| IMXQB-old mice sc (5) | Influenza (seasonal split vaccine) | ** | * | - | - | Protection in a challenge test provided by adjuvanted vaccines; IgG1, IgG2a, and IgA are maintained 120 days after priming | [181] | ||||
| IMXQB-old mice in (2.5) | *** | ** | * | **** | *** | ||||||
3.2.1. Humoral Response
3.2.2. Cell-Mediated Immune Response Induced by Q. brasiliensis Saponins
3.2.3. Mucosal Immunity Induced by Q. brasiliensis Saponins
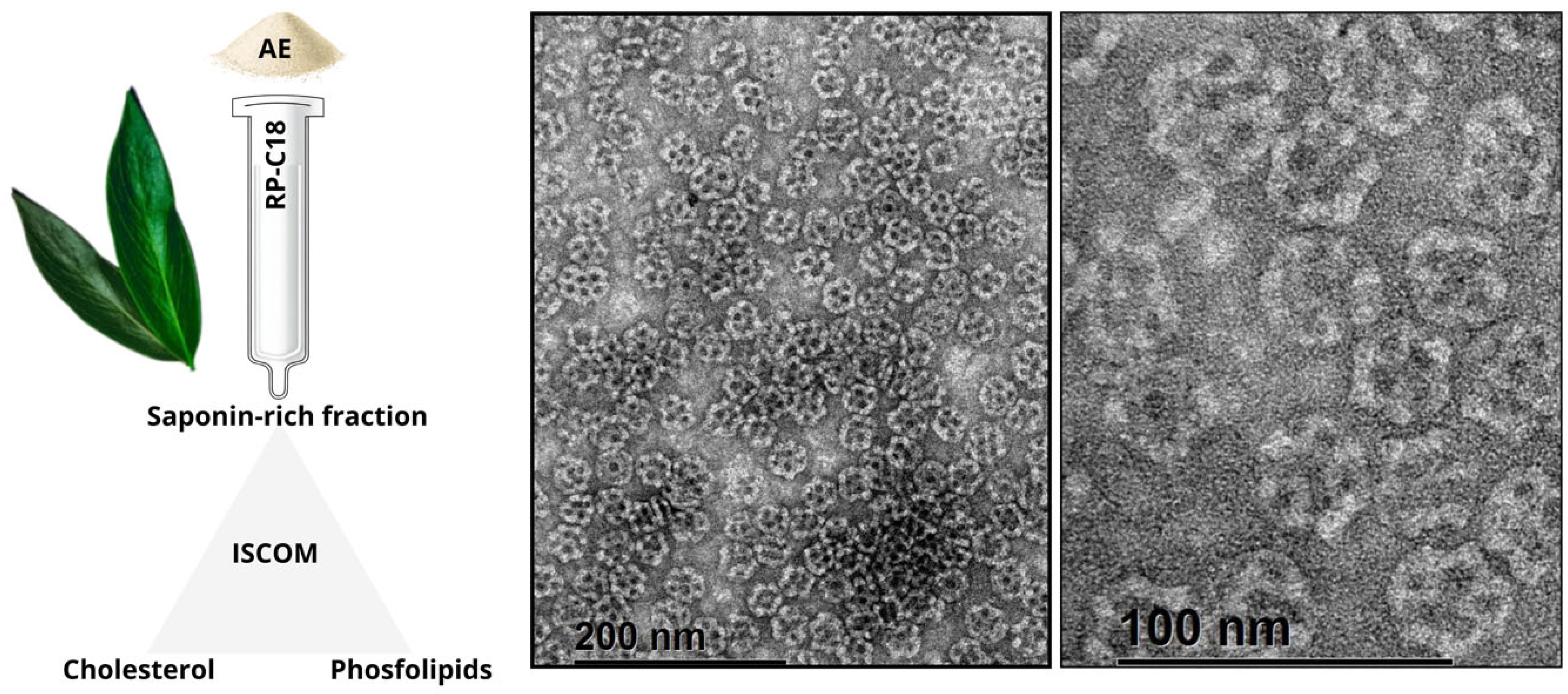
3.2.4. ISCOMs and ISCOM-Matrices Based on Q. brasiliensis Saponins
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Plotkin, S.A. Vaccines: Past, present and future. Nat. Med. 2005, 11, S5. [Google Scholar] [CrossRef]
- Rueckert, C.; Guzmán, C.A. Vaccines: From empirical development to rational design. PLoS Pathog. 2012, 8, e1003001. [Google Scholar] [CrossRef]
- Finco, O.; Rappuoli, R. Designing vaccines for the twenty-first century society. Front. Immunol. 2014, 5, 12. [Google Scholar] [CrossRef]
- De Gregorio, E.; Rappuoli, R. From empiricism to rational design: A personal perspective of the evolution of vaccine development. Nat. Rev. Immunol. 2014, 14, 505–514. [Google Scholar] [CrossRef]
- Meyer, H.; Ehmann, R.; Smith, G.L. Smallpox in the post-eradication Era. Viruses 2020, 12, 138. [Google Scholar] [CrossRef]
- Garçon, N.; Segal, L.; Tavares, F.; Van Mechelen, M. The safety evaluation of adjuvants during vaccine development: The AS04 experience. Vaccine 2011, 29, 4453–4459. [Google Scholar] [CrossRef]
- Ura, T.; Yamashita, A.; Mizuki, N.; Okuda, K.; Shimada, M. New vaccine production platforms used in developing SARS-CoV-2 vaccine candidates. Vaccine 2021, 39, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Krammer, F. SARS-CoV-2 vaccines in development. Nature 2020, 586, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Rappuoli, R.; Alter, G.; Pulendran, B. Transforming vaccinology. Cell 2024, 187, 5171–5194. [Google Scholar] [CrossRef] [PubMed]
- O’Hagan, D.T.; Valiante, N.M. Recent advances in the discovery and delivery of vaccine adjuvants. Nat. Rev. Drug Discov. 2003, 2, 727–735. [Google Scholar] [CrossRef]
- Ramon, G. Sur L’augmentation anormale de L’antitoxine chez les chevaux producteurs de serum antidiphtherique. Bull. Soc. Centr. Med. Vet. 1925, 101, 227–234. [Google Scholar]
- Schijns, V.; Fernández-Tejada, A.; Barjaktarović, Ž.; Bouzalas, I.; Brimnes, J.; Chernysh, S.; Gizurarson, S.; Gursel, I.; Jakopin, Ž.; Lawrenz, M.; et al. Modulation of immune responses using adjuvants to facilitate therapeutic vaccination. Immunol. Rev. 2020, 296, 169–190. [Google Scholar] [CrossRef]
- Roth, G.A.; Picece, V.C.T.M.; Ou, B.S.; Luo, W.; Pulendran, B.; Appel, E.A. Designing spatial and temporal control of vaccine responses. Nat. Rev. Mater. 2022, 7, 174–195. [Google Scholar] [CrossRef] [PubMed]
- Cibulski, S.; Rivera-Patron, M.; Suárez, N.; Pirez, M.; Rossi, S.; Yendo, A.C.; de Costa, F.; Gosmann, G.; Fett-Neto, A.; Roehe, P.M.; et al. Leaf saponins of Quillaja brasiliensis enhance long-term specific immune responses and promote dose-sparing effect in BVDV experimental vaccines. Vaccine 2018, 36, 55–65. [Google Scholar] [CrossRef]
- Hawken, J.; Troy, S.B. Adjuvants and inactivated polio vaccine: A systematic review. Vaccine 2012, 30, 6971–6979. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Shi, Y. Alum: An old dog with new tricks. Emerg. Microbes Infect. 2016, 5, e25. [Google Scholar] [CrossRef]
- Orr, M.T.; Khandhar, A.P.; Seydoux, E.; Liang, H.; Gage, E.; Mikasa, T.; Beebe, E.L.; Rintala, N.D.; Persson, K.H.; Ahniyaz, A.; et al. Reprogramming the adjuvant properties of aluminum oxyhydroxide with nanoparticle technology. npj Vaccines 2019, 4, 1. [Google Scholar] [CrossRef]
- Pulendran, B.; S. Arunachalam, P.; O’Hagan, D.T. Emerging concepts in the science of vaccine adjuvants. Nat. Rev. Drug Discov. 2021, 20, 454–475. [Google Scholar] [CrossRef]
- Kommareddy, S.; Sing, M.; O’Hagan, D.T. MF59: A Safe and Potent Adjuvant for Human Use. In Immunopotentiators in Modern Vaccines; Schijns, V.E.J.C., O’Hagan, D.T., Eds.; Academic Press: London, UK, 2016; pp. 249–263. ISBN 978-0-12-804019-5. [Google Scholar]
- O’Hagan, D.T.; Ott, G.S.; De Gregorio, E.; Seubert, A. The mechanism of action of MF59-An innately attractive adjuvant formulation. Vaccine 2012, 30, 4341–4348. [Google Scholar] [CrossRef] [PubMed]
- O’Hagan, D.T.; Ott, G.S.; Nest, G.V.; Rappuoli, R.; Giudice, G. Del The history of MF59® adjuvant: A phoenix that arose from the ashes. Expert Rev. Vaccines 2013, 12, 13–30. [Google Scholar] [CrossRef]
- Zheng, D.; Gao, F.; Zhao, C.; Ding, Y.; Cao, Y.; Yang, T.; Xu, X.; Chen, Z. Comparative effectiveness of H7N9 vaccines in healthy individuals. Hum. Vaccines Immunother. 2019, 15, 80–90. [Google Scholar] [CrossRef]
- O’Hagan, D.T. MF59 is a safe and potent vaccine adjuvant that enhances protection against influenza virus infection. Expert Rev. Vaccines 2007, 6, 699–710. [Google Scholar] [CrossRef]
- Mosca, F.; Tritto, E.; Muzzi, A.; Monaci, E.; Bagnoli, F.; Iavarone, C.; O’Hagan, D.; Rappuoli, R.; De Gregorio, E. Molecular and cellular signatures of human vaccine adjuvants. Proc. Natl. Acad. Sci. USA 2008, 105, 10501–10506. [Google Scholar] [CrossRef]
- Cunningham, A.L.; Sandgren, K.J.; Taylor, J. Current status of immunisation for herpes zoster. Hum. Vaccin. Immunother. 2025, 21, 2445384. [Google Scholar] [CrossRef]
- Alving, C.R.; Peachman, K.; Rao, M.; Reed, S.G. Adjuvants for human vaccines. Curr. Opin. Immunol. 2012, 24, 310–315. [Google Scholar] [CrossRef]
- Garçon, N.; Chomez, P.; Van Mechelen, M. GlaxoSmithKline Adjuvant Systems in vaccines: Concepts, achievements and perspectives. Expert Rev. Vaccines 2007, 6, 723–739. [Google Scholar] [CrossRef] [PubMed]
- Didierlaurent, A.M.; Laupèze, B.; Di Pasquale, A.; Hergli, N.; Collignon, C.; Garçon, N. Adjuvant system AS01: Helping to overcome the challenges of modern vaccines. Expert Rev. Vaccines 2017, 16, 55–63. [Google Scholar] [CrossRef]
- Roman, F.; Burny, W.; Ceregido, A.; Laupèze, B.; Temmerman, S.T.; Warter, L.; Coccia, M. Adjuvant system AS01: From mode of action to effective vaccines. Expert Rev. Vaccines 2024, 23, 715–729. [Google Scholar] [CrossRef] [PubMed]
- Morel, S.; Didierlaurent, A.; Bourguignon, P.; Delhaye, S.; Baras, B.; Jacob, V.; Planty, C.; Elouahabi, A.; Harvengt, P.; Carlsen, H.; et al. Adjuvant System AS03 containing α-tocopherol modulates innate immune response and leads to improved adaptive immunity. Vaccine 2011, 29, 2461–2473. [Google Scholar] [CrossRef] [PubMed]
- Loos, C.; Coccia, M.; Didierlaurent, A.M.; Essaghir, A.; Fallon, J.K.; Lauffenburger, D.; Luedemann, C.; Michell, A.; van der Most, R.; Zhu, A.L.; et al. Systems serology-based comparison of antibody effector functions induced by adjuvanted vaccines to guide vaccine design. npj Vaccines 2023, 8, 34. [Google Scholar] [CrossRef]
- Garçon, N.; Di Pasquale, A. From discovery to licensure, the Adjuvant System story. Hum. Vaccines Immunother. 2017, 13, 19–33. [Google Scholar] [CrossRef]
- Del Giudice, G.; Rappuoli, R.; Didierlaurent, A.M. Correlates of adjuvanticity: A review on adjuvants in licensed vaccines. Semin. Immunol. 2018, 39, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Tuo, W. QS-21: A Potent Vaccine Adjuvant. Nat. Prod. Chem. Res. 2016, 3, 3–4. [Google Scholar] [CrossRef]
- Atmar, R.L.; El Sahly, H.M. The Promise of New Vaccines Against Respiratory Viruses. Pediatrics 2024, 153, e2023065328. [Google Scholar] [CrossRef] [PubMed]
- Kensil, C.R. Saponins as vaccine adjuvants. Crit. Rev. Ther. Drug Carrier Syst. 1996, 13, 1–55. [Google Scholar]
- Villacres-Eriksson, M.; Behboudi, S.; Morgan, A.J.; Trinchieri, G.; Morein, B. Immunomodulation by Quillaja saponaria adjuvant formulations: In vivo stimulation of interleukin 12 and its effects on the antibody response. Cytokine 1997, 9, 73–82. [Google Scholar] [CrossRef]
- Amanat, F.; Krammer, F. SARS-CoV-2 Vaccines: Status Report. Immunity 2020, 52, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Mascola, J.R.; Fauci, A.S. Novel vaccine technologies for the 21st century. Nat. Rev. Immunol. 2020, 20, 87–88. [Google Scholar] [CrossRef]
- Coffman, R.L.; Sher, A.; Seder, R.A. Vaccine adjuvants: Putting innate immunity to work. Immunity 2010, 33, 492–503. [Google Scholar] [CrossRef]
- Turley, J.L.; Lavelle, E.C. Resolving adjuvant mode of action to enhance vaccine efficacy. Curr. Opin. Immunol. 2022, 77, 102229. [Google Scholar] [CrossRef]
- Ho, N.I.; Huis In ’t Veld, L.G.M.; Raaijmakers, T.K.; Adema, G.J. Adjuvants Enhancing Cross-Presentation by Dendritic Cells: The Key to More Effective Vaccines? Front. Immunol. 2018, 9, 2874. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Kawai, T. Toll-like receptor signaling pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef] [PubMed]
- Awate, S.; Babiuk, L.A.; Mutwiri, G. Mechanisms of action of adjuvants. Front. Immunol. 2013, 4, 114. [Google Scholar] [CrossRef] [PubMed]
- De Gregorio, E.; Caproni, E.; Ulmer, J.B. Vaccine adjuvants: Mode of action. Front. Immunol. 2013, 4, 214. [Google Scholar] [CrossRef]
- O’Hagan, D.T.; Fox, C.B. Are we entering a new age for human vaccine adjuvants? Expert Rev. Vaccines 2015, 14, 909–911. [Google Scholar] [CrossRef][Green Version]
- Sanders, M.T.; Brown, L.E.; Deliyannis, G.; Pearse, M.J. ISCOMTM-based vaccines: The second decade. Immunol. Cell Biol. 2005, 83, 119–128. [Google Scholar] [CrossRef]
- Den Brok, M.H.; Büll, C.; Wassink, M.; De Graaf, A.M.; Wagenaars, J.A.; Minderman, M.; Thakur, M.; Amigorena, S.; Rijke, E.O.; Schrier, C.C.; et al. Saponin-based adjuvants induce cross-presentation in dendritic cells by intracellular lipid body formation. Nat. Commun. 2016, 7, 13324. [Google Scholar] [CrossRef]
- Oyston, P.; Robinson, K. The current challenges for vaccine development. J. Med. Microbiol. 2012, 61, 889–894. [Google Scholar] [CrossRef]
- Kool, M.; Fierens, K.; Lambrecht, B.N. Alum adjuvant: Some of the tricks of the oldest adjuvant. J. Med. Microbiol. 2012, 61, 927–934. [Google Scholar] [CrossRef]
- Petrovsky, N. Comparative Safety of Vaccine Adjuvants: A Summary of Current Evidence and Future Needs. Drug Saf. 2015, 38, 1059–1074. [Google Scholar] [CrossRef]
- Schijns, V.E.J.C.; Lavelle, E.C. Trends in vaccine adjuvants. Expert Rev. Vaccines 2011, 10, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Pasquale, A.; Preiss, S.; Silva, F.; Garçon, N. Vaccine Adjuvants: From 1920 to 2015 and Beyond. Vaccines 2015, 3, 320–343. [Google Scholar] [CrossRef]
- Shah, R.R.; Hassett, K.J.; Brito, L.A. Overview of Vaccine Adjuvants: Introduction, History, and Current Status. In Vaccine Adjuvants: Methods and Protocols; Fox, C.B., Ed.; Methods in Molecular Biology; Springer Science + Business Media: New York, NY, USA, 2017; Volume 1494, pp. 1–13. ISBN 978-1-4939-6443-7. [Google Scholar]
- Garçon, N.; Hem, S.; Friede, M. Evolution of Adjuvants Across the Centuries. In Vaccine, 6th ed.; Plotkin, S., Orenstein, W., Offit, P., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2012; ISBN 9781455700905. [Google Scholar]
- Maisonneuve, C.; Bertholet, S.; Philpott, D.J.; De Gregorio, E. Unleashing the potential of NOD- and Toll-like agonists as vaccine adjuvants. Proc. Natl. Acad. Sci. USA 2014, 111, 12294–12299. [Google Scholar] [CrossRef] [PubMed]
- Brito, L.A.; Malyala, P.; O’Hagan, D.T. Vaccine adjuvant formulations: A pharmaceutical perspective. Semin. Immunol. 2013, 25, 130–145. [Google Scholar] [CrossRef]
- Yendo, A.C.A.; Colling, L.C.; Matsuura, H.N.; Vargas, L.R.B.; Martinelli, J.A.; Chitolina, G.Z.; Vainstein, M.H.; Fett-Neto, A.G. Quillaja lancifolia Immunoadjuvant Saponins Show Toxicity to Herbivores and Pathogenic Fungi. Plants 2025, 14, 1252. [Google Scholar] [CrossRef]
- Augustin, J.M.; Kuzina, V.; Andersen, S.B.; Bak, S. Molecular activities, biosynthesis and evolution of triterpenoid saponins. Phytochemistry 2011, 72, 435–457. [Google Scholar] [CrossRef] [PubMed]
- Wina, E.; Muetzel, S.; Becker, K. The impact of saponins or saponin-containing plant materials on ruminant production—A review. J. Agric. Food Chem. 2005, 53, 8093–8105. [Google Scholar] [CrossRef]
- Vincken, J.P.; Heng, L.; de Groot, A.; Gruppen, H. Saponins, classification and occurrence in the plant kingdom. Phytochemistry 2007, 68, 275–297. [Google Scholar] [CrossRef]
- Hosttetmann, K.; Marston, A. Saponins, 1995th ed.; Hostettmann, K., Marston, A., Eds.; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Oleszek, W.A. Chromatographic determination of plant saponins. J. Chromatogr. A 2002, 967, 147–162. [Google Scholar] [CrossRef]
- Sparg, S.G.; Light, M.E.; Van Staden, J. Biological activities and distribution of plant saponins. J. Ethnopharmacol. 2004, 94, 219–243. [Google Scholar] [CrossRef] [PubMed]
- Podolak, I.; Galanty, A.; Sobolewska, D. Saponins as cytotoxic agents: A review. Phytochem. Rev. 2010, 9, 425–474. [Google Scholar] [CrossRef] [PubMed]
- Lorent, J.H.; Quetin-Leclercq, J.; Mingeot-Leclercq, M.-P. The amphiphilic nature of saponins and their effects on artificial and biological membranes and potential consequences for red blood and cancer cells. Org. Biomol. Chem. 2014, 12, 8803–8822. [Google Scholar] [CrossRef]
- Sun, H.X.; Xie, Y.; Ye, Y.P. Advances in saponin-based adjuvants. Vaccine 2009, 27, 1787–1796. [Google Scholar] [CrossRef]
- Cheok, C.Y.; Salman, H.A.K.; Sulaiman, R. Extraction and quantification of saponins: A review. Food Res. Int. 2014, 59, 16–40. [Google Scholar] [CrossRef]
- Glauert, A.; Dingle, J.; Lucy, J. Action of saponin on biological cell membranes. Nature 1962, 195, 698. [Google Scholar] [CrossRef]
- De Groot, C.; Müller-Goymann, C.C. Saponin Interactions with Model Membrane Systems—Langmuir Monolayer Studies, Hemolysis and Formation of ISCOMs. Planta Med. 2016, 82, 1496–1512. [Google Scholar] [CrossRef]
- Böttger, S.; Hofmann, K.; Melzig, M.F. Saponins can perturb biologic membranes and reduce the surface tension of aqueous solutions: A correlation? Bioorg. Med. Chem. 2012, 20, 2822–2828. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, C.; Legault, J.; Girard-Lalancette, K.; Mshvildadze, V.; Pichette, A. Haemolytic activity, cytotoxicity and membrane cell permeabilization of semi-synthetic and natural lupane- and oleanane-type saponins. Bioorg. Med. Chem. 2009, 17, 2002–2008. [Google Scholar] [CrossRef]
- Press, J.B.; Reynolds, R.C.; May, R.D.; Marciani, D.J. Structure/function relationships of emmunostimulating saponins. Stud. Nat. Prod. Chem. 2000, 24, 131–174. [Google Scholar]
- Espinet, R.G. Nouveau vaccin antiaphteux a complexe glucoviral. Gac. Vet. 1951, 13, 268. [Google Scholar]
- Dalsgaard, K. Saponin adjuvants. 3. Isolation of a substance from Quillaja saponaria Molina with adjuvant activity in food-and-mouth disease vaccines. Arch. Gesamte Virusforsch. 1974, 44, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Dalsgaard, K. A study of the isolation and characterization of the saponin quil A: Evaluation of its adjuvant activity, with a special reference to the application in the vaccination of cattle against foot-and-mouth disease. Acta Vet. Scand. Suppl. 1978, 69, 7–40. [Google Scholar]
- Kensil, C.R.; Patel, U.; Lennick, M.; Marciani, D. Separation And Characterization Of Saponins with Adjuvant Activity From Quillaja saponaria Molina Cortex. J. Immunol. 1991, 146, 431–437. [Google Scholar] [CrossRef]
- Song, X.; Hu, S. Adjuvant activities of saponins from traditional Chinese medicinal herbs. Vaccine 2009, 27, 4883–4890. [Google Scholar] [CrossRef]
- Rajput, Z.I.; Hu, S.; Xiao, C.; Arijo, A.G. Adjuvant effects of saponins on animal immune responses. J. Zhejiang Univ. Sci. B 2007, 8, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Santos, W.R.; De Lima, V.M.F.; De Souza, E.P.; Bernardo, R.R.; Palatnik, M.; De Sousa, C.B.P. Saponins, IL12 and BCG adjuvant in the FML-vaccine formulation against murine visceral leishmaniasis. Vaccine 2002, 21, 30–43. [Google Scholar] [CrossRef]
- Kensil, C.R. QS-21 Adjuvant. In Vaccine Adjuvants Preparation Methods and Research Protocols; O’Hagan, D.T., Ed.; Humana Press Inc.: Totowa, NJ, USA, 2000; Volume 42, pp. 259–271. [Google Scholar]
- Morein, B.; Hu, K.-F.; Abusugra, I. Current status and potential application of ISCOMs in veterinary medicine. Adv. Drug Deliv. Rev. 2004, 56, 1367–1382. [Google Scholar] [CrossRef]
- Sun, H.X.; Xie, Y.; Ye, Y.P. ISCOMs and ISCOMATRIXTM. Vaccine 2009, 27, 4388–4401. [Google Scholar] [CrossRef]
- Sjölander, A.; Cox, J.C.; Barr, I.G. ISCOMs: An adjuvant with multiple functions. J. Leukoc. Biol. 1998, 64, 713–723. [Google Scholar] [CrossRef]
- Morein, B.; Bengtsson, K.L. Immunomodulation by iscoms, immune stimulating complexes. Methods 1999, 19, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Cibulski, S.P.; Mourglia-Ettlin, G.; Teixeira, T.F.; Quirici, L.; Roehe, P.M.; Ferreira, F.; Silveira, F. Novel ISCOMs from Quillaja brasiliensis saponins induce mucosal and systemic antibody production, T-cell responses and improved antigen uptake. Vaccine 2016, 34, 1162–1171. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, A.; Watt, M.; Gittleson, C. ISCOMATRIX vaccines: Safety in human clinical studies. Hum. Vaccin. 2010, 6, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, J.C.; Rodríguez, E.G. Vaccine adjuvants revisited. Vaccine 2007, 25, 3752–3762. [Google Scholar] [CrossRef]
- Kensil, C.R.; Wu, J.Y.; Anderson, C.A.; Wheeler, D.A.; Amsden, J. QS-21 and QS-7: Purified saponin adjuvants. Dev. Biol. Stand. 1998, 92, 41–47. [Google Scholar]
- Cibulski, S.P.; Silveira, F.; Mourglia-Ettlin, G.; Teixeira, T.F.; dos Santos, H.F.; Yendo, A.C.; de Costa, F.; Fett-Neto, A.G.; Gosmann, G.; Roehe, P.M. Quillaja brasiliensis saponins induce robust humoral and cellular responses in a bovine viral diarrhea virus vaccine in mice. Comp. Immunol. Microbiol. Infect. Dis. 2016, 45, 1–8. [Google Scholar] [CrossRef]
- Silveira, F.; Cibulski, S.P.; Varela, A.P.; Marqués, J.M.; Chabalgoity, A.; de Costa, F.; Yendo, A.C.A.; Gosmann, G.; Roehe, P.M.; Fernández, C.; et al. Quillaja brasiliensis saponins are less toxic than Quil A and have similar properties when used as an adjuvant for a viral antigen preparation. Vaccine 2011, 29, 9177–9182. [Google Scholar] [CrossRef]
- De Costa, F.; Yendo, A.C.A.; Cibulski, S.P.; Fleck, J.D.; Roehe, P.M.; Spilki, F.R.; Gosmann, G.; Fett-Neto, A.G. Alternative inactivated poliovirus vaccines adjuvanted with Quillaja brasiliensis or Quil-A saponins are equally effective in inducing specific immune responses. PLoS ONE 2014, 9, e105374. [Google Scholar] [CrossRef]
- Rivera-patron, M.; Baz, M.; Roehe, P.M.; Cibulski, S.P.; Silveira, F. ISCOM-Like Nanoparticles Formulated with Quillaja brasiliensis Saponins Are Promising Adjuvants for Seasonal Influenza. Vaccines 2021, 9, 1350. [Google Scholar] [CrossRef]
- Cibulski, S.; Varela, A.P.M.; Teixeira, T.F.; Cancela, M.P.; Sesterheim, P.; Souza, D.O.; Roehe, P.M.; Silveira, F. Zika Virus Envelope Domain III Recombinant Protein Delivered With Saponin-Based Nanoadjuvant From Quillaja brasiliensis Enhances Anti-Zika Immune Responses, Including Neutralizing Antibodies and Splenocyte Proliferation. Front. Immunol. 2021, 12, 632714. [Google Scholar] [CrossRef]
- Kensil, C.; Kammer, R. QS-21: A water-soluble triterpene glycoside adjuvant. Expert Opin. Investig. Drugs 1998, 7, 1475–1482. [Google Scholar] [CrossRef] [PubMed]
- Carnrot, C.; Carow, B.; Palm, A.-K.E.; Akpinar, E.; Helgesson, P.-H.; Osterman, I.L.; Bringeland, E.; Foreman, B.; Patel, N.; Bankefors, J.; et al. Biodistribution of the Saponin-Based Adjuvant Matrix-MTM Following Intramuscular Injection in Mice. Front. Drug Deliv. 2023, 3, 1279710. [Google Scholar] [CrossRef]
- Stertman, L.; Palm, A.-K.E.; Zarnegar, B.; Carow, B.; Lunderius Andersson, C.; Magnusson, S.E.; Carnrot, C.; Shinde, V.; Smith, G.; Glenn, G.; et al. The Matrix-MTM adjuvant: A critical component of vaccines for the 21st century. Hum. Vaccin. Immunother. 2023, 19, 2189885. [Google Scholar] [CrossRef]
- Wilson, N.S.; Duewell, P.; Yang, B.; Li, Y.; Marsters, S.; Koernig, S.; Latz, E.; Maraskovsky, E.; Morelli, A.B.; Schnurr, M.; et al. Inflammasome-Dependent and -Independent IL-18 Production Mediates Immunity to the ISCOMATRIX Adjuvant. J. Immunol. 2014, 192, 3259–3268. [Google Scholar] [CrossRef]
- Cibulski, S.P.; Rivera-Patron, M.; Mourglia-Ettlin, G.; Casaravilla, C.; Yendo, A.C.A.; Fett-Neto, A.G.; Chabalgoity, J.A.; Moreno, M.; Roehe, P.M.; Silveira, F. Quillaja brasiliensis saponin-based nanoparticulate adjuvants are capable of triggering early immune responses. Sci. Rep. 2018, 8, 13582. [Google Scholar] [CrossRef]
- Wang, P. Natural and Synthetic Saponins as Vaccine Adjuvants. Vaccines 2021, 9, 222. [Google Scholar] [CrossRef]
- Ghirardello, M.; Ruiz-de-Angulo, A.; Sacristan, N.; Barriales, D.; Jiménez-Barbero, J.; Poveda, A.; Corzana, F.; Anguita, J.; Fernández-Tejeda, A. Exploiting structure–activity relationships of QS-21 in the design and synthesis of streamlined saponin vaccine adjuvants. Chem.Comm. 2019, 1, 3–6. [Google Scholar] [CrossRef]
- Huis in ’t Veld, L.G.; Cornelissen, L.A.; van den Bogaard, L.; Ansems, M.; Ho, N.I.; Adema, G.J. Saponin-based adjuvant uptake and induction of antigen cross-presentation by CD11b+ dendritic cells and macrophages. npj Vaccines 2025, 10, 15. [Google Scholar] [CrossRef]
- Marciani, D.J. Elucidating the Mechanisms of Action of Saponin-Derived Adjuvants. Trends Pharmacol. Sci. 2018, 39, 573–585. [Google Scholar] [CrossRef] [PubMed]
- Marciani, D.J. Vaccine Adjuvants: From Empirical to a More Rational Drug Design. Explor. Res. Hypothesis Med. 2024, 9, 199–208. [Google Scholar] [CrossRef]
- Martin, L.B.B.; Kikuchi, S.; Rejzek, M.; Owen, C.; Reed, J.; Orme, A.; Misra, R.C.; El-Demerdash, A.; Hill, L.; Hodgson, H.; et al. Complete biosynthesis of the potent vaccine adjuvant QS-21. Nat. Chem. Biol. 2024, 20, 493–502. [Google Scholar] [CrossRef]
- Soltysik, S.; Wu, J.; Recchia, J.; Wheeler, D.A.; Newman-f, M.J.; Coughlin, R.T.; Kensil, C.R. Structure/function studies of QS-21 adjuvant: Assessment of triterpene aldehyde and glucuronic acid roles in adjuvant function. Vaccine 1995, 13, 1403–1410. [Google Scholar] [CrossRef] [PubMed]
- Newman, M.J.; Wu, J.Y.; Gardner, B.H.; Anderson, C.A.; Kensil, C.R.; Recchia, J.; Coughlin, R.T.; Powell, M.F. Induction of cross-reactive cytotoxic T-lymphocyte responses specific for HIV-1 gp120 using saponin adjuvant (QS-21) supplemented subunit vaccine formulations. Vaccine 1997, 15, 1001–1007. [Google Scholar] [CrossRef]
- Lacaille-Dubois, M.-D. Updated insights into the mechanism of action and clinical profile of the immunoadjuvant QS-21: A review. Phytomedicine 2019, 60, 152905. [Google Scholar] [CrossRef] [PubMed]
- Detienne, S.; Welsby, I.; Collignon, C.; Wouters, S.; Coccia, M.; Delhaye, S.; Van Maele, L.; Thomas, S.; Swertvaegher, M.; Detavernier, A.; et al. Central Role of CD169(+) Lymph Node Resident Macrophages in the Adjuvanticity of the QS-21 Component of AS01. Sci. Rep. 2016, 6, 39475. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, J. Covalent chemical events in immune induction: Fundamental and therapeutic aspects. Immunol. Today 1996, 17, 436–441. [Google Scholar] [CrossRef]
- Marciani, D.J. Vaccine adjuvants: Role and mechanisms of action in vaccine immunogenicity. Drug Discov. Today 2003, 8, 934–943. [Google Scholar] [CrossRef]
- Pifferi, C.; Fuentes, R.; Fernández-Tejada, A. Natural and synthetic carbohydrate-based vaccine adjuvants and their mechanisms of action. Nat. Rev. Chem. 2021, 5, 197–216. [Google Scholar] [CrossRef]
- Behboudi, S.; Morein, B.; Villacres-Eriksson, M. In vitro activation of antigen-presenting cells (APC) by defined composition of Quillaja saponaria Molina triterpenoids. Clin. Exp. Immunol. 1996, 105, 26–30. [Google Scholar] [CrossRef]
- Marty-Roix, R.; Vladimer, G.I.; Pouliot, K.; Weng, D.; Buglione-Corbett, R.; West, K.; MacMicking, J.D.; Chee, J.D.; Wang, S.; Lu, S.; et al. Identification of QS-21 as an inflammasome-activating molecular component of saponin adjuvants. J. Biol. Chem. 2016, 291, 1123–1136. [Google Scholar] [CrossRef]
- Wilson, N.S.; Yang, B.; Morelli, A.B.; Koernig, S.; Yang, A.; Loeser, S.; Airey, D.; Provan, L.; Hass, P.; Braley, H.; et al. ISCOMATRIX vaccines mediate CD8 T-cell cross-priming by a MyD88-dependent signaling pathway. Immunol. Cell Biol. 2012, 90, 540–552. [Google Scholar] [CrossRef]
- Morelli, A.B.; Becher, D.; Koernig, S.; Silva, A.; Drane, D.; Maraskovsky, E. ISCOMATRIX: A novel adjuvant for use in prophylactic and therapeutic vaccines against infectious diseases. J. Med. Microbiol. 2012, 61, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Cai, Y.; Jiang, Y.; He, X.; Wei, Y.; Yu, Y.; Tian, X. Vaccine adjuvants: Mechanisms and platforms. Signal Transduct. Target. Ther. 2023, 8, 283. [Google Scholar] [CrossRef]
- Olotu, A.; Fegan, G.; Wambua, J.; Nyangweso, G.; Leach, A.; Lievens, M.; Kaslow, D.C.; Njuguna, P.; Marsh, K.; Bejon, P. Seven-Year Efficacy of RTS,S/AS01 Malaria Vaccine among Young African Children. N. Engl. J. Med. 2016, 374, 2519–2529. [Google Scholar] [CrossRef]
- Kumarasamy, N.; Poongulali, S.; Bollaerts, A.; Moris, P.; Beulah, F.E.; Ayuk, L.N.; Demoitie, M.A.; Jongert, E.; Ofori-Anyinam, O. A randomized, controlled safety, and immunogenicity trial of the M72/AS01 candidate tuberculosis vaccine in HIV-positive indian adults. Medicine 2016, 95, e2459. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, A.; Tirpude, N.V.; Padwad, Y.; Hallan, V.; Kumar, S. Plant-derived immuno-adjuvants in vaccines formulation: A promising avenue for improving vaccines efficacy against SARS-CoV-2 virus. Pharmacol. Rep. 2022, 74, 1238–1254. [Google Scholar] [CrossRef]
- Leroux-Roels, G.; Van Belle, P.; Vandepapeliere, P.; Horsmans, Y.; Janssens, M.; Carletti, I.; Garçon, N.; Wettendorff, M.; Van Mechelen, M. Vaccine Adjuvant Systems containing monophosphoryl lipid A and QS-21 induce strong humoral and cellular immune responses against hepatitis B surface antigen which persist for at least 4 years after vaccination. Vaccine 2015, 33, 1084–1091. [Google Scholar] [CrossRef]
- Bonam, S.R.; Partidos, C.D.; Halmuthur, S.K.M.; Muller, S. An Overview of Novel Adjuvants Designed for Improving Vaccine Efficacy. Trends Pharmacol. Sci. 2017, 38, 771–793. [Google Scholar] [CrossRef] [PubMed]
- Gilabert-Oriol, R.; Weng, A.; Von Mallinckrodt, B.; Stöshel, A.; Nissi, L.; Melzig, M.F.; Fuchs, H.; Thakur, M. Electrophoretic mobility as a tool to separate immune adjuvant saponins from Quillaja saponaria Molina. Int. J. Pharm. 2015, 487, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Ragupathi, G.; Gardner, J.R.; Livingston, P.O.; Gin, D.Y. Natural and synthetic saponin adjuvant QS-21 for vaccines against cancer. Expert Rev. Vaccines 2011, 10, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Tejada, A.; Tan, D.S.; Gin, D.Y. Development of Improved Vaccine Adjuvants Based on the Saponin Natural Product QS-21 through Chemical Synthesis. Acc. Chem. Res. 2016, 49, 1741–1756. [Google Scholar] [CrossRef]
- Fernández-Tejada, A.; Walkowicz, W.E.; Tan, D.S.; Gin, D.Y. Semisynthesis of analogues of the saponin immunoadjuvant QS-21. In Methods in Molecular Biology; Humana Press Inc.: Totowa, NJ, USA, 2017; Volume 1494, pp. 45–71. [Google Scholar]
- Wilson, N.S.; Yang, B.; Morelli, A.B.; Koernig, S.; Yang, A.; Loeser, S.; Airey, D.; Albert, G.; Cho, I.; Robertson, A.; et al. Immune correlates analysis of the PREVENT-19 COVID-19 vaccine efficacy clinical trial. N. Engl. J. Med. 2020, 383, 2320–2332 NEJMoa2026920. [Google Scholar] [CrossRef]
- Yihunie, W.; Kebede, B.; Tegegne, A.; Getachew, M.; Abebe, D.; Aschale, Y.; Belew, H.; Bahiru, B.; Addis Tegegne, B. Systematic Review of Safety of RTS,S with AS01 and AS02 Adjuvant Systems Using Data from Randomized Controlled Trials in Infants, Children, and Adults. Clin. Pharmacol. 2023, 15, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Parums, D.V. Editorial: First Approval of the Protein-Based Adjuvanted Nuvaxovid (NVX-CoV2373) Novavax Vaccine for SARS-CoV-2 Could Increase Vaccine Uptake and Provide Immune Protection from Viral Variants. Med. Sci. Monit. 2022, 28, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Von Seidleinid, L. Roll out and prospects of the malaria vaccine R21/Matrix-M. PLOS Med. 2025, 22, e1004515. [Google Scholar] [CrossRef]
- Silva, A.L.; Peres, C.; Conniot, J.; Matos, A.; Moura, L.; Carreira, B.; Sainz, V.; Scomparin, A.; Satchi-Fainaro, R.; Préat, V.; et al. Nanoparticle impact on innate immune cell pattern-recognition receptors and inflammasomes activation. Semin. Immunol. 2017, 34, 3–24. [Google Scholar] [CrossRef]
- Zhao, L.; Seth, A.; Wibowo, N.; Zhao, C.X.; Mitter, N.; Yu, C.; Middelberg, A.P.J. Nanoparticle vaccines. Vaccine 2014, 32, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Pati, R.; Shevtsov, M.; Sonawane, A. Nanoparticle Vaccines Against Infectious Diseases. Front. Immunol. 2018, 9, 2224. [Google Scholar] [CrossRef]
- Xu, S.; Sun, C.; Qian, T.; Chen, Y.; Dong, X.; Wang, A.; Zhang, Q.; Ji, Y.; Jin, Z.; Liu, C.; et al. Animal vaccine revolution: Nanoparticle adjuvants open the future of vaccinology. J. Control. Release 2025, 383, 113827. [Google Scholar] [CrossRef]
- Facciolà, A.; Visalli, G.; Laganà, P.; La Fauci, V.; Squeri, R.; Pellicanò, G.F.; Nunnari, G.; Trovato, M.; Di Pietro, A. The new era of vaccines: The “nanovaccinology. ” Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 7163–7182. [Google Scholar] [CrossRef]
- Gregory, A.E.; Titball, R.; Williamson, D. Vaccine delivery using nanoparticles. Front. Cell. Infect. Microbiol. 2013, 3, 13. [Google Scholar] [CrossRef]
- Bezbaruah, R.; Chavda, V.P.; Nongrang, L.; Alom, S.; Deka, K.; Kalita, T.; Ali, F.; Bhattacharjee, B.; Vora, L. Nanoparticle-Based Delivery Systems for Vaccines. Vaccines 2022, 10, 1946. [Google Scholar] [CrossRef]
- Morein, B.; Sundquist, B.; Höglund, S.; Dalsgaard, K.; Osterhaus, A. Iscom, a novel structure for antigenic presentation of membrane proteins from enveloped viruses. Nature 1984, 308, 457–460. [Google Scholar] [CrossRef]
- Pearse, M.J.; Drane, D. ISCOMATRIXR adjuvant for antigen delivery. Adv. Drug Deliv. Rev. 2005, 57, 465–474. [Google Scholar] [CrossRef]
- Bigaeva, E.; Doorn, E.V.; Liu, H.; Hak, E. Meta-Analysis on Randomized Controlled Trials of Vaccines with QS-21 or ISCOMATRIX Adjuvant: Safety and Tolerability. PLoS ONE 2016, 11, e0154757. [Google Scholar] [CrossRef] [PubMed]
- Correia-Pinto, J.F.; Csaba, N.; Alonso, M.J. Vaccine delivery carriers: Insights and future perspectives. Int. J. Pharm. 2013, 440, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Sjölander, A.; Drane, D.; Maraskovsky, E.; Scheerlinck, J.P.; Suhrbier, A.; Tennent, J.; Pearse, M. Immune responses to ISCOM® formulations in animal and primate models. Vaccine 2001, 19, 2661–2665. [Google Scholar] [CrossRef]
- Lövgren Bengtsson, K.; Morein, B.; Osterhaus, A.D. ISCOM technology-based Matrix MTM adjuvant: Success in future vaccines relies on formulation. Expert Rev. Vaccines 2011, 10, 401–403. [Google Scholar] [CrossRef] [PubMed]
- Buglione-Corbett, R.; Pouliot, K.; Marty-Roix, R.; Li, W.; West, K.; Wang, S.; Morelli, A.B.; Lien, E.; Lu, S. Reduced MyD88 dependency of ISCOMATRIXTM adjuvant in a DNA prime-protein boost HIV vaccine. Hum. Vaccines Immunother. 2014, 10, 1078–1090. [Google Scholar] [CrossRef][Green Version]
- Mount, A.; Koernig, S.; Silva, A.; Drane, D.; Maraskovsky, E.; Morelli, A.B. Combination of adjuvants: The future of vaccine design. Expert Rev. Vaccines 2013, 12, 733–746. [Google Scholar] [CrossRef]
- Dey, A.K.; Srivastava, I.K. Novel adjuvants and delivery systems for enhancing immune responses induced by immunogens. Expert Rev. Vaccines 2011, 10, 227–251. [Google Scholar] [CrossRef]
- Toback, S.; Marchese, A.M.; Warren, B.; Ayman, S.; Zarkovic, S.; ElTantawy, I.; Mallory, R.M.; Rousculp, M.; Almarzooqi, F.; Piechowski-Jozwiak, B.; et al. Safety and immunogenicity of the NVX-CoV2373 vaccine as a booster in adults previously vaccinated with the BBIBP-CorV vaccine. Vaccine 2024, 42, 1777–1784. [Google Scholar] [CrossRef]
- Heath, P.T.; Galiza, E.P.; Baxter, D.N.; Boffito, M.; Browne, D.; Burns, F.; Chadwick, D.R.; Clark, R.; Cosgrove, C.; Galloway, J.; et al. Safety and Efficacy of NVX-CoV2373 Covid-19 Vaccine. N. Engl. J. Med. 2021, 385, 1172–1183. [Google Scholar] [CrossRef]
- Tian, J.H.; Patel, N.; Haupt, R.; Zhou, H.; Weston, S.; Hammond, H.; Logue, J.; Portnoff, A.D.; Norton, J.; Guebre-Xabier, M.; et al. SARS-CoV-2 spike glycoprotein vaccine candidate NVX-CoV2373 immunogenicity in baboons and protection in mice. Nat. Commun. 2021, 12, 372. [Google Scholar] [CrossRef]
- Levast, B.T.; Awate, S.; Babiuk, L.; Mutwiri, G.; Gerdts, V.; Van, S.; Littel-Van Den Hurk, D. Vaccine Potentiation by Combination Adjuvants. Vaccines 2014, 2, 297–322. [Google Scholar] [CrossRef]
- Garcia, A.; Lema, D. An Updated Review of Iscoms TM and Iscomatrix TM Vaccines. Curr. Pharm. Des. 2016, 22, 6294–6299. [Google Scholar] [CrossRef]
- Morelli, A.B.; Maraskovsky, E. ISCOMATRIX Adjuvant in the Development of Prophylactic and Therapeutic Vaccines. In Immunopotentiators in Modern Vaccines; Schijns, V.E.J.C., O’Hagan, D.T., Eds.; Academic Press: London, UK, 2016; pp. 311–332. ISBN 978-0-12-804019-5. [Google Scholar]
- Keech, C.; Glenn, G.M.; Albert, G.; Cho, I.; Robertson, A.; Reed, P.; Neal, S.; Plested, J.S.; Zhu, M.; Cloney-Clark, S.; et al. First-in-Human Trial of a SARS-CoV-2 Recombinant Spike Protein Nanoparticle Vaccine. medRxiv 2020. [Google Scholar] [CrossRef]
- Sjölander, A.; van’t Land, B.; Lövgren Bengtsson, K. Iscoms containing purified Quillaja saponins upregulate both Th1-like and Th2-like immune responses. Cell. Immunol. 1997, 177, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Huis in ’t Veld, L.G.M.; Ho, N.I.; Wassink, M.; den Brok, M.H.; Adema, G.J. Saponin-based adjuvant-induced dendritic cell cross-presentation is dependent on PERK activation. Cell. Mol. Life Sci. 2022, 79, 231. [Google Scholar] [CrossRef]
- Duewell, P.; Kisser, U.; Heckelsmiller, K.; Hoves, S.; Stoitzner, P.; Koernig, S.; Morelli, A.B.; Clausen, B.E.; Dauer, M.; Eigler, A.; et al. ISCOMATRIX Adjuvant Combines Immune Activation with Antigen Delivery to Dendritic Cells In Vivo Leading to Effective Cross-Priming of CD8+ T Cells. J. Immunol. 2011, 187, 55–63. [Google Scholar] [CrossRef]
- Davis, I.D.; Chen, W.; Jackson, H.; Parente, P.; Shackleton, M.; Hopkins, W.; Chen, Q.; Dimopoulos, N.; Luke, T.; Murphy, R.; et al. Recombinant NY-ESO-1 protein with ISCOMATRIX adjuvant induces broad integrated antibody and CD4(+) and CD8(+) T cell responses in humans. Proc. Natl. Acad. Sci. USA. 2004, 101, 10697–10702. [Google Scholar] [CrossRef] [PubMed]
- Reinke, S.; Thakur, A.; Gartlan, C.; Bezbradica, J.S.; Milicic, A. Inflammasome-Mediated Immunogenicity of Clinical and Experimental Vaccine Adjuvants. Vaccines 2020, 8, 554. [Google Scholar] [CrossRef] [PubMed]
- Gomes-da-Silva, J.; Filardi, F.L.R.; Barbosa, M.R.V.; Baumgratz, J.F.A.; Bicudo, C.E.M.; Cavalcanti, T.B.; Coelho, M.A.N.; Costa, A.F.; Costa, D.P.; Dalcin, E.C.; et al. Brazilian Flora 2020: Leveraging the power of a collaborative scientific network. Taxon 2022, 71, 178–198. [Google Scholar] [CrossRef]
- Luebert, F. Taxonomy and distribution of the genus Quillaja Molina (Quillajaceae). Feddes Repert. 2014, 124, 157–162. [Google Scholar] [CrossRef]
- San Martin, R.; Briones, R. Industrial Uses and Sustainable Supply of Quillaja saponaria (Roseaceae) saponins. Econ. Bot. 1999, 53, 302–311. [Google Scholar] [CrossRef]
- Schlotterbeck, T.; Castillo–Ruiz, M.; Cañon–Jones, H.; Martín, R.S. The Use ofLeaves from Young Trees ofQuillaja saponaria (Molina) Plantations as a New Source of Saponins. Econ. Bot. 2015, 69, 262–272. [Google Scholar] [CrossRef]
- Sander, V.A.; Corigliano, M.G.; Clemente, M. Promising Plant-Derived Adjuvants in the Development of Coccidial Vaccines. Front. Vet. Sci. 2019, 6, 20. [Google Scholar] [CrossRef]
- Snow, N. The Flowering Plants Handbook, A Practical Guide to Families and Genera of the World. (eBook edition). Syst. Bot. 2015, 40, 366–367. [Google Scholar] [CrossRef]
- de Costa, F.; Yendo, A.C.; Fleck, J.D.; Gosmann, G.; Fett-Neto, A. Immunoadjuvant and Anti-Inflammatory Plant Saponins: Characteristics and Biotechnological Approaches Towards Sustainable Production. Mini Rev. Med. Chem. 2011, 11, 857–880. [Google Scholar] [CrossRef] [PubMed]
- Magedans, Y.V.S.; Yendo, A.C.A.; Costa, F.D.; Gosmann, G.; Arthur, G. Foamy matters: An update on Quillaja saponins and their use as immunoadjuvants. Future Med. Chem. 2019, 11, 1485–1499. [Google Scholar] [CrossRef]
- Fleck, J.D.; Schwambach, J.; Almeida, M.E.; Yendo, A.C.A.; de Costa, F.; Gosmann, G.; Fett-Neto, A.G. Immunoadjuvant saponin production in seedlings and micropropagated plants of Quillaja brasiliensis. Vitr. Cell. Dev. Biol.-Plant 2009, 45, 715–720. [Google Scholar] [CrossRef]
- Fleck, J.D.; Kauffmann, C.; Spilki, F.; Lencina, C.L.; Roehe, P.M.; Gosmann, G. Adjuvant activity of Quillaja brasiliensis saponins on the immune responses to bovine herpesvirus type 1 in mice. Vaccine 2006, 24, 7129–7134. [Google Scholar] [CrossRef]
- Fleck, J.D.; de Costa, F.; Yendo, A.C.A.; Segalin, J.; Dalla Costa, T.C.T.; Fett-Neto, A.G.; Gosmann, G. Determination of new immunoadjuvant saponin named QB-90, and analysis of its organ-specific distribution in Quillaja brasiliensis by HPLC. Nat. Prod. Res. 2013, 27, 907–910. [Google Scholar] [CrossRef]
- Morais, V.; Suarez, N.; Silveira, F. Methods of saponin purification from Quillaja sp. for vaccine adjuvant production. Front. Nat. Prod. 2024, 3, 1524624. [Google Scholar] [CrossRef]
- Yendo, A.; de Costa, F.; Kauffmann, C.; Fleck, J.; Gosmann, G.; Fett-Neto, A. Purification of an Immunoadjuvant Saponin Fraction from Quillaja brasiliensis Leaves by Reversed-Phase Silica Gel Chromatography. In Vaccine Adjuvants; Fox, C.B., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2017; Volume 1494, pp. 87–93. ISBN 978-1-4939-6443-7. [Google Scholar]
- Yendo, A.C.A.; de Costa, F.; Cibulski, S.P.; Teixeira, T.F.; Colling, L.C.; Mastrogiovanni, M.; Soule, S.; Roehe, P.M.; Gosmann, G.; Ferreira, F.A.; et al. A rabies vaccine adjuvanted with saponins from leaves of the soap tree (Quillaja brasiliensis) induces specific immune responses and protects against lethal challenge. Vaccine 2016, 34, 2305–2311. [Google Scholar] [CrossRef] [PubMed]
- Kauffmann, C.; Machado, A.M.; Fleck, J.D.; Provensi, G.; Pires, V.S.; Guillaume, D.; Sonnet, P.; Reginatto, F.H.; Schenkel, E.P.; Gosmann, G. Constituents from leaves of Quillaja brasiliensis. Nat. Prod. Res. 2004, 18, 153–157. [Google Scholar] [CrossRef]
- Cibulski, S.; Amorim, T.; Joanda, D.S.; Raimundo, P.; Mangueira, Y.; Silva, L.; Norma, A.; Iris, S.; Paulo, M.; Roehe, M.; et al. ISCOM-Matrices Nanoformulation Using the Raw Aqueous Extract of Quillaja lancifolia (Q. brasiliensis). Bionanoscience 2022, 12, 1166–1171. [Google Scholar] [CrossRef] [PubMed]
- Cibulski, S.; Teixeira, T.F.; Varela, A.P.M.; de Lima, M.F.; Casanova, G.; Nascimento, Y.M.; Fechine Tavares, J.; da Silva, M.S.; Sesterheim, P.; Souza, D.O.; et al. IMXQB-80: A Quillaja brasiliensis saponin-based nanoadjuvant enhances Zika virus specific immune responses in mice. Vaccine 2020, 39, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Wallace, F.; Bennadji, Z.; Ferreira, F.; Olivaro, C. Structural characterisation of new immunoadjuvant saponins from leaves and the first study of saponins from the bark of Quillaja brasiliensis by liquid chromatography electrospray ionisation ion trap mass spectrometry. Phytochem. Anal. 2019, 30, 644–652. [Google Scholar] [CrossRef]
- Wallace, F.; Fontana, C.; Ferreira, F. Structure Elucidation of Triterpenoid Saponins Found in an Immunoadjuvant Preparation of Quillaja brasiliensis Using. Molecules 2022, 27, 2402. [Google Scholar] [CrossRef]
- Oda, K.; Matsuda, H.; Murakami, T.; Katayama, S.; Ohgitani, T.; Yoshikawa, M. Adjuvant and haemolytic activities of 47 saponins derived from medicinal and food plants. Biol. Chem. 2000, 381, 67–74. [Google Scholar] [CrossRef]
- Batista-Duharte, A.; Portuondo, D.; Carlos, I.Z.; Pérez, O. An approach to local immunotoxicity induced by adjuvanted vaccines. Int. Immunopharmacol. 2013, 17, 526–536. [Google Scholar] [CrossRef]
- Silveira, F.; Rivera-Patron, M.; Deshpande, N.; Sienra, S.; Checa, J.; Moreno, M.; Chabalgoity, J.A.; Cibulski, S.P.; Baz, M. Quillaja brasiliensis nanoparticle adjuvant formulation improves the efficacy of an inactivated trivalent influenza vaccine in mice. Front. Immunol. 2023, 14, 1163858. [Google Scholar] [CrossRef] [PubMed]
- Silveira, F.; García, F.; García, G.; Chabalgoity, J.A.; Rossi, S.; Baz, M. Intranasal Delivery of Quillaja brasiliensis Saponin-Based Nanoadjuvants Improve Humoral Immune Response of Influenza Vaccine in Aged Mice. Vaccines 2024, 12, 902. [Google Scholar] [CrossRef] [PubMed]
- Ruterbusch, M.; Pruner, K.B.; Shehata, L.; Pepper, M. In Vivo CD4+ T Cell Differentiation and Function: Revisiting the Th1/Th2 Paradigm. Annu. Rev. Immunol. 2020, 38, 705–725. [Google Scholar] [CrossRef] [PubMed]
- Hernández, A.; Yager, J.A.; Wilkie, B.N.; Leslie, K.E.; Mallard, B.A. Evaluation of bovine cutaneous delayed-type hypersensitivity (DTH) to various test antigens and a mitogen using several adjuvants. Vet. Immunol. Immunopathol. 2005, 104, 45–58. [Google Scholar] [CrossRef]
- Cher, D.J.; Mosmann, T.R. Two types of murine helper T cell clone. II. Delayed-type hypersensitivity is mediated by TH1 clones. J. Imunol. 1987, 138, 3688–3694. [Google Scholar] [CrossRef]
- Gupta, R.K.; Relyveld, E.H.; Lindblad, E.B.; Bizzini, B.; Ben-Efraim, S.; Gupta, C.K. Adjuvants-A balance between toxicity and adjuvanticity. Vaccine 1993, 11, 293–306. [Google Scholar] [CrossRef]
- Schijns, V.E.J.C. Activation and programming of adaptive immune responses by vaccine adjuvants Immune effector mechanisms. Vet. Sci. Tomorrow 2001, 3, 1–7. [Google Scholar]
- Baldridge, J.R.; Vard, J.R. Effective adjuvants for the induction of antigen-specific delayed-type hypersensitivity. Vaccine 1997, 15, 395–401. [Google Scholar] [CrossRef]
- Sewell, A.K. Why must T cells be cross-reactive? Nat. Rev. Immunol. 2012, 12, 669–677. [Google Scholar] [CrossRef]
- Kägi, D.; Ledermann, B.; Bürki, K.; Zinkernagel, R.M.; Hengartner, H. Lymphocyte-mediated cytotoxicity in vitro and in vivo: Mechanisms and significance. Immunol. Rev. 1995, 146, 95–115. [Google Scholar] [CrossRef]
- Mosmann, T.R.; Li, L.; Sad, S. Functions of CD8 T-cell subsets secreting different cytokine patterns. Semin. Immunol. 1997, 9, 87–92. [Google Scholar] [CrossRef]
- Guidotti, L.G.; Chisari, F. V Cytokine-mediated control of viral infections. Virology 2000, 273, 221–227. [Google Scholar] [CrossRef]
- Zaman, M.; Chandrudu, S.; Toth, I. Strategies for intranasal delivery of vaccines. Drug Deliv. Transl. Res. 2013, 3, 100–109. [Google Scholar] [CrossRef]
- Lavelle, E.C.; Ward, R.W. Mucosal vaccines-Fortifying the frontiers. Nat. Rev. Immunol. 2021, 22, 236–250. [Google Scholar] [CrossRef]
- Otczyk, D.C.; Cripps, A.W. Mucosal immunization: A realistic alternative. Hum. Vaccin. 2010, 6, 978–1006. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.; Nachbagauer, R.; Balmaseda, A.; Stadlbauer, D.; Ojeda, S.; Patel, M.; Rajabhathor, A.; Lopez, R.; Guglia, A.F.; Sanchez, N.; et al. Novel correlates of protection against pandemic H1N1 influenza A virus infection. Nat. Med. 2019, 25, 962–967. [Google Scholar] [CrossRef] [PubMed]
- Ito, R.; Ozaki, Y.A.; Yoshikawa, T.; Hasegawa, H.; Sato, Y.; Suzuki, Y.; Inoue, R.; Morishima, T.; Kondo, N.; Sata, T.; et al. Roles of anti-hemagglutinin IgA and IgG antibodies in different sites of the respiratory tract of vaccinated mice in preventing lethal influenza pneumonia. Vaccine 2003, 21, 2362–2371. [Google Scholar] [CrossRef]
- Holmgren, J.; Svennerholm, A.M. Vaccines against mucosal infections. Curr. Opin. Immunol. 2012, 24, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Patron, M.; Cibulski, S.P.; Miraballes, I.; Silveira, F. Formulation of IMXQB: Nanoparticles Based on Quillaja brasiliensis Saponins to be Used as Vaccine Adjuvants. In Plant Secondary Metabolism Engineering; Fett-Neto, A.G., Ed.; Methods in Molecular Biology; Humana: New York, NY, USA, 2022; Volume 2469, pp. 183–191. [Google Scholar]
- Drane, D.; Gittleson, C.; Boyle, J.; Maraskovsky, E. ISCOMATRIX adjuvant for prophylactic and therapeutic vaccines. Expert Rev. Vaccines 2007, 6, 761–772. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morais, V.; Suarez, N.; Cibulski, S.; Silveira, F. Leaf Saponins of Quillaja brasiliensis as Powerful Vaccine Adjuvants. Pharmaceutics 2025, 17, 966. https://doi.org/10.3390/pharmaceutics17080966
Morais V, Suarez N, Cibulski S, Silveira F. Leaf Saponins of Quillaja brasiliensis as Powerful Vaccine Adjuvants. Pharmaceutics. 2025; 17(8):966. https://doi.org/10.3390/pharmaceutics17080966
Chicago/Turabian StyleMorais, Víctor, Norma Suarez, Samuel Cibulski, and Fernando Silveira. 2025. "Leaf Saponins of Quillaja brasiliensis as Powerful Vaccine Adjuvants" Pharmaceutics 17, no. 8: 966. https://doi.org/10.3390/pharmaceutics17080966
APA StyleMorais, V., Suarez, N., Cibulski, S., & Silveira, F. (2025). Leaf Saponins of Quillaja brasiliensis as Powerful Vaccine Adjuvants. Pharmaceutics, 17(8), 966. https://doi.org/10.3390/pharmaceutics17080966






