Physiologically Based Pharmacokinetic Modeling to Assess Perpetrator and Victim Cytochrome P450 2C Induction Risk
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection of Clinical Inducers for In Vitro Characterization
2.2. Chemicals and Reagents
2.3. Preparation of Triculture Model
2.4. Determination of Compound Stability in the HTC Model
2.5. Delineating the CYP Induction Potential of Test Compounds Through Enzyme Activity and mRNA Levels
2.6. Estimation of EC50 and Emax Parameters from In Vitro Data
2.7. Workflow for PBPK and Mechanistic Static Modeling and Statistical Analysis
2.8. Equations
3. Results
3.1. Induction of CYPs in HTC
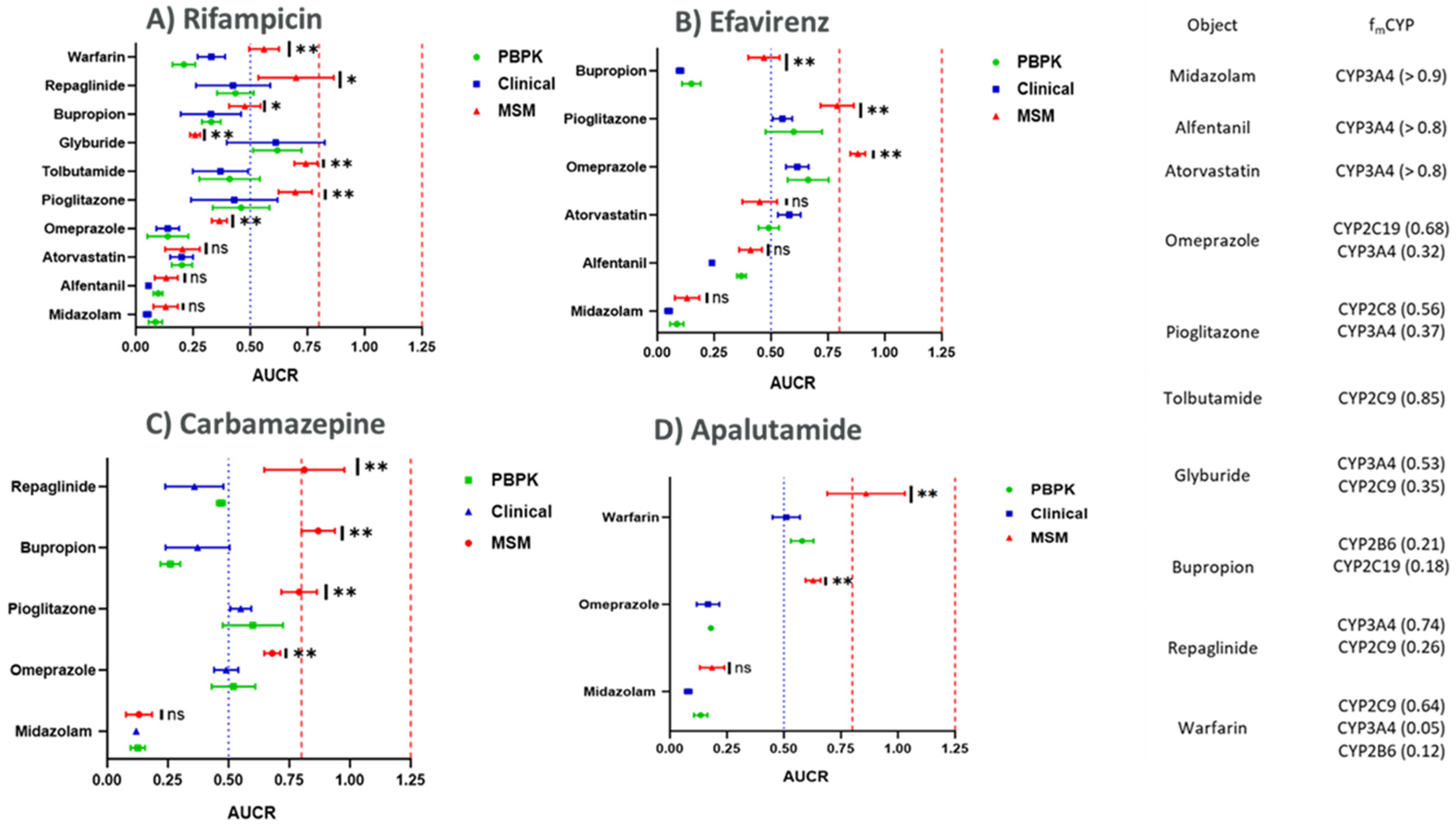
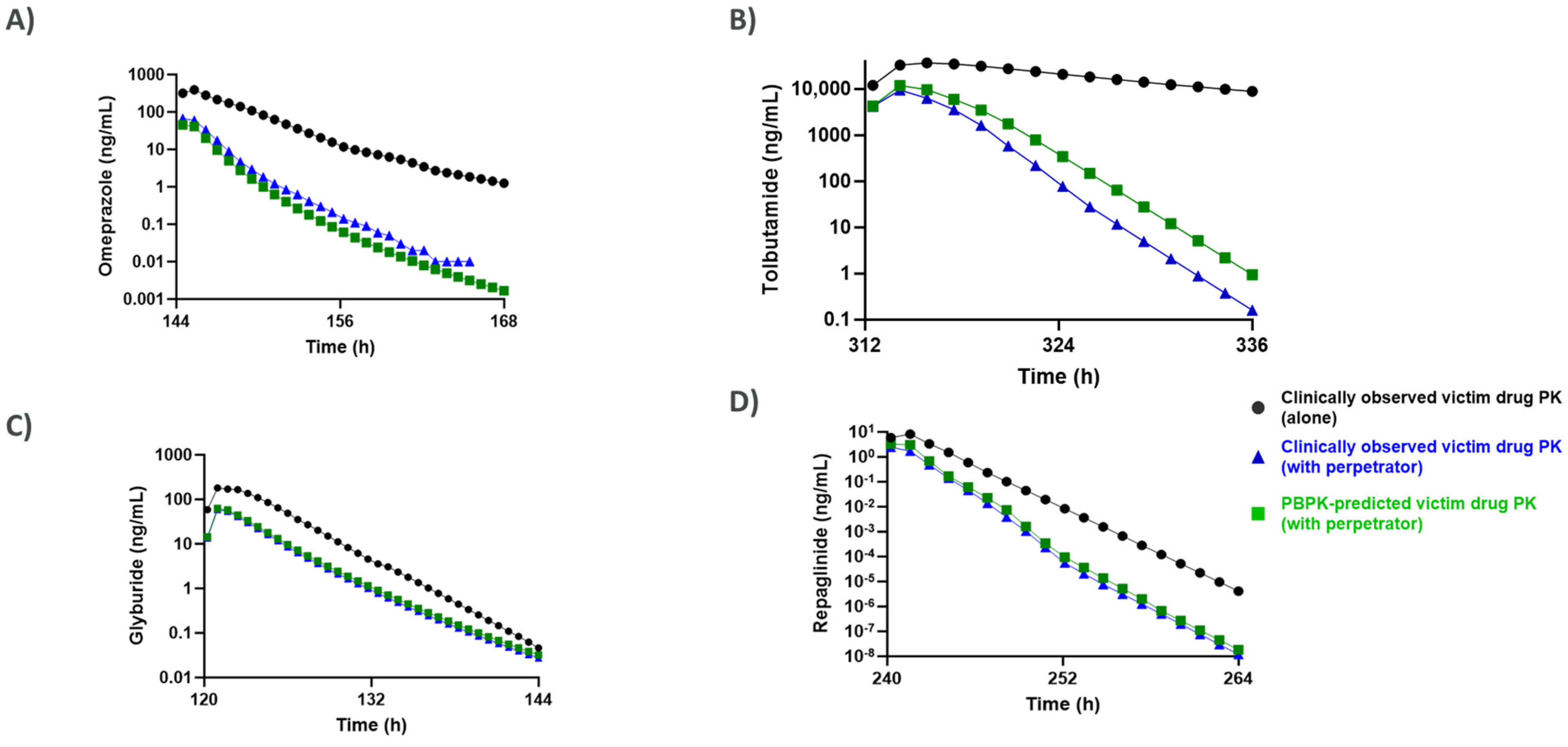
3.2. Comparison of CYP Induction-Mediated DDI Risk Assessment Using Mechanistic Static Modeling Approaches (MSM) and PBPK
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sinz, M.; Wallace, G.; Sahi, J. Current industrial practices in assessing CYP450 enzyme induction: Preclinical and clinical. AAPS J. 2008, 10, 391–400. [Google Scholar] [CrossRef]
- Shou, M.; Hayashi, M.; Pan, Y.; Xu, Y.; Morrissey, K.; Xu, L.; Skiles, G.L. Modeling, prediction, and in vitro in vivo correlation of CYP3A4 induction. Drug Metab. Dispos. 2008, 36, 2355–2370. [Google Scholar] [CrossRef] [PubMed]
- Einolf, H.J.; Chen, L.; Fahmi, O.A.; Gibson, C.R.; Obach, R.S.; Shebley, M.; Silva, J.; Sinz, M.W.; Unadkat, J.D.; Zhang, L.; et al. Evaluation of various static and dynamic modeling methods to predict clinical CYP3A induction using in vitro CYP3A4 mRNA induction data. Clin. Pharmacol. Ther. 2014, 95, 179–188. [Google Scholar] [CrossRef]
- Zhang, J.G.; Ho, T.; Callendrello, A.L.; Clark, R.J.; Santone, E.A.; Kinsman, S.; Xiao, D.; Fox, L.G.; Einolf, H.J.; Stresser, D.M. Evaluation of calibration curve–based approaches to predict clinical inducers and noninducers of CYP3A4 with Plated human hepatocytes. Drug Metab. Dispos. 2014, 42, 1379–1391. [Google Scholar] [CrossRef] [PubMed]
- Kenny, J.R.; Ramsden, D.; Buckley, D.B.; Dallas, S.; Fung, C.; Mohutsky, M.; Einolf, H.J.; Chen, L.; Dekeyser, J.G.; Fitzgerald, M.; et al. Considerations from the Innovation and Quality Induction Working Group in Response to Drug-Drug Interaction Guidances from Regulatory Agencies: Focus on CYP3A4 mRNA In Vitro Response Thresholds, Variability, and Clinical Relevance. Drug Metab. Dispos. 2018, 46, 1285–1303. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, H.; Kuramoto, S.; Ozeki, K. Evaluation of Methods to Assess CYP3A Induction Risk in Clinical Practice Using in Vitro Induction Parameters. Biol. Pharm. Bull. 2021, 44, 338–349. [Google Scholar] [CrossRef]
- Ramsden, D.; Fullenwider, C.L. Characterization of Correction Factors to Enable Assessment of Clinical Risk from In Vitro CYP3A4 Induction Data and Basic Drug-Drug Interaction Models. Eur. J. Drug Metab. Pharmacokinet. 2022, 47, 467–482. [Google Scholar] [CrossRef]
- International Council for Harmonisation. ICH Harmonised Guideline: M12 Clinical and Non-Clinical Standards for Drug Interaction Studies. 2024. Available online: https://www.ich.org/page/m12-drug-interactions-studies (accessed on 1 June 2024).
- Nagai, M.; Hosaka, T.; Satsukawa, M.; Yoshinari, K. Characterization of CYP2C Induction in Cryopreserved Human Hepatocytes and Its Application in the Prediction of the Clinical Consequences of the Induction. J. Pharm. Sci. 2018, 107, 2479–2488. [Google Scholar] [CrossRef]
- Hakkola, J.; Hukkanen, J.; Turpeinen, M.; Pelkonen, O. Inhibition and induction of CYP enzymes in humans: An update. Arch. Toxicol. 2020, 94, 3671–3722. [Google Scholar] [CrossRef]
- Kiyosawa, N.; Kwekel, J.C.; Burgoon, L.D.; Dere, E.; Williams, K.J.; Tashiro, C.; Chittim, B.; Zacharewski, T.R. Species-specific regulation of PXR/CAR/ER-target genes in the mouse and rat liver elicited by o, p’-DDT. BMC Genom. 2008, 9, 487. [Google Scholar] [CrossRef]
- Dixit, V.; Moore, A.; Tsao, H.; Hariparsad, N. Application of Micropatterned Cocultured Hepatocytes to Evaluate the Inductive Potential and Degradation Rate of Major Xenobiotic Metabolizing Enzymes. Drug Metab. Dispos. 2016, 44, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Hariparsad, N.; Ramsden, D.; Palamanda, J.; Dekeyser, J.G.; Fahmi, O.A.; Kenny, J.R.; Einolf, H.; Mohutsky, M.; Pardon, M.; Siu, Y.A.; et al. Considerations from the IQ Induction Working Group in Response to Drug-Drug Interaction Guidance from Regulatory Agencies: Focus on Downregulation, CYP2C Induction, and CYP2B6 Positive Control. Drug Metab. Dispos. 2017, 45, 1049–1059. [Google Scholar] [CrossRef] [PubMed]
- Aluri, K.C.; Slavsky, M.; Tan, Y.; Whitcher-Johnstone, A.; Zhang, Z.; Hariparsad, N.; Ramsden, D. Aminobenzotriazole inhibits and induces several key drug metabolizing enzymes complicating its utility as a pan CYP inhibitor for reaction phenotyping. Clin. Transl. Sci. 2024, 17, e13746. [Google Scholar] [CrossRef] [PubMed]
- Ramsden, D.; Fullenwider, C.L.; Santos, C.; LeCluyse, E.L. Quantitative clinical risk assessment of CYP2C, UDP-glucuronosyltransferase, P-glycoprotein induction, and complex drug-drug interactions using TruVivo human hepatocyte triculture platform. Drug Metab. Dispos. 2025, 53, 100052. [Google Scholar] [CrossRef]
- Miners, J.O.; Birkett, D.J. Cytochrome P4502C9: An enzyme of major importance in human drug metabolism. Br. J. Clin. Pharmacol. 1998, 45, 525–538. [Google Scholar] [CrossRef]
- Bidstrup, T.B.; Bjørnsdottir, I.; Sidelmann, U.G.; Thomsen, M.S.; Hansen, K.T. CYP2C8 and CYP3A4 are the principal enzymes involved in the human in vitro biotransformation of the insulin secretagogue repaglinide. Br. J. Clin. Pharmacol. 2003, 56, 305–314. [Google Scholar] [CrossRef]
- Weaver, J.R.; Odanga, J.J.; Wolf, K.K.; Piekos, S.; Biven, M.; Taub, M.; LaRocca, J.; Thomas, C.; Byer-Alcorace, A.; Chen, J.; et al. The morphology, functionality, and longevity of a novel all human hepatic cell-based tri-culture system. Toxicol. Vitr. 2023, 86, 105504. [Google Scholar] [CrossRef]
- Kaminsky, L.S.; Zhang, Z.-Y. Human P450 metabolism of warfarin. Pharmacol. Ther. 1997, 73, 67–74. [Google Scholar] [CrossRef]
- Ford, N.F. The Metabolism of Clopidogrel: CYP2C19 Is a Minor Pathway. J. Clin. Pharmacol. 2016, 56, 1474–1483. [Google Scholar] [CrossRef]
- Fahmi, O.A.; Maurer, T.S.; Kish, M.; Cardenas, E.; Boldt, S.; Nettleton, D. A combined model for predicting CYP3A4 clinical net drug-drug interaction based on CYP3A4 inhibition, inactivation, and induction determined in vitro. Drug Metab. Dispos. 2008, 36, 1698–1708. [Google Scholar] [CrossRef]
- Joković, D.; Milosavljević, F.; Stojanović, Z.; Šupić, G.; Vojvodić, D.; Uzelac, B.; Jukić, M.M.; Ćurčin, A.P. CYP2C19 slow metabolizer phenotype is associated with lower antidepressant efficacy and tolerability. Psychiatry Res. 2022, 312, 114535. [Google Scholar] [CrossRef]
- Lutz, J.D.; Kirby, B.J.; Wang, L.; Song, Q.; Ling, J.; Massetto, B.; Worth, A.; Kearney, B.P.; Mathias, A. Cytochrome P450 3A Induction Predicts P-glycoprotein Induction; Part 2: Prediction of Decreased Substrate Exposure After Rifabutin or Carbamazepine. Clin. Pharmacol. Ther. 2018, 104, 1191–1198. [Google Scholar] [CrossRef]
- Usman, M.; Zhen-Han, Z.; Ze-Na, C.; Jun-Ping, H.; Wen, Q.; Chang-Qing, Y.; Miyu, N.; Toshiyuki, S. Effect of iguratimod on diclofenac metabolism by CYP2C9 in rats and human recombinant CYP2C9 yeast cells. Braz. J. Pharm. Sci. 2019, 55, e17240. [Google Scholar] [CrossRef]
- Hariparsad, N.; Ramsden, D.; Taskar, K.; Badée, J.; Venkatakrishnan, K.; Reddy, M.B.; Cabalu, T.; Mukherjee, D.; Rehmel, J.; Bolleddula, J.; et al. Current Practices, Gap Analysis, and Proposed Workflows for PBPK Modeling of Cytochrome P450 Induction: An Industry Perspective. Clin. Pharmacol. Ther. 2022, 112, 770–781. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.Q.; Kimoto, E.; Callegari, E.; Obach, R.S. Mechanistic Modeling to Predict Midazolam Metabolite Exposure from In Vitro Data. Drug Metab. Dispos. 2016, 44, 781–791. [Google Scholar] [CrossRef]
- Kharasch, E.; Walker, A.; Hoffer, C.; Sheffels, P. Intravenous and oral alfentanil as in vivo probes for hepatic and first-pass cytochrome P450 3A activity: Noninvasive assessment by use of pupillary miosis. Clin. Pharmacol. Ther. 2004, 76, 452–466. [Google Scholar] [CrossRef]
- Klammers, F.; Goetschi, A.; Ekiciler, A.; Walter, I.; Parrott, N.; Fowler, S.; Umehara, K. Estimation of Fraction Metabolized by Cytochrome P450 Enzymes Using Long-Term Cocultured Human Hepatocytes. Drug Metab. Dispos. 2022, 50, 566–575. [Google Scholar] [CrossRef]
- Codaccioni, M.; Southall, R.L.; Dinh, J.; Johnson, T.N. Prediction of Pediatric Pharmacokinetics for CYP3A4 Metabolized Drugs: Comparison of the Performance of Two Hepatic Ontogeny Within a Physiologically Based Pharmacokinetic Model. J. Clin. Pharmacol. 2024, 64, 1083–1094. [Google Scholar] [CrossRef] [PubMed]
- Li, A.P.; Reith, M.; Rasmussen, A.; Gorski, J.; Hall, S.D.; Xu, L.; Kaminski, D.L.; Cheng, L.K. Primary human hepatocytes as a tool for the evaluation of structure—Activity relationship in cytochrome P450 induction potential of xenobiotics: Evaluation of rifampin, rifapentine and rifabutin. Chem. Interact. 1997, 107, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Backman, J.T.; Luurila, H.; Neuvonen, M.; Neuvonen, P.J. Rifampin markedly decreases and gemfibrozil increases the plasma concentrations of atorvastatin and its metabolites. Clin. Pharmacol. Ther. 2005, 78, 154–167. [Google Scholar] [CrossRef]
- Baldwin, R.M.; Ohlsson, S.; Pedersen, R.S.; Mwinyi, J.; Ingelman-Sundberg, M.; Eliasson, E.; Bertilsson, L. Increased omeprazole metabolism in carriers of the CYP2C19*17 allele; a pharmacokinetic study in healthy volunteers. Br. J. Clin. Pharmacol. 2008, 65, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, C.; Inui, N.; Hakamata, A.; Miyakawa, S.; Tanaka, S.; Uchida, S.; Namiki, N.; Odagiri, K.; Watanabe, H. Effect of co-administered inducer or inhibitor on omeprazole pharmacokinetics based on CYP2C19 genotype. J. Pharmacol. Sci. 2019, 139, 361–366. [Google Scholar] [CrossRef]
- Jaakkola, T.; Laitila, J.; Neuvonen, P.J.; Backman, J.T. Pioglitazone is metabolised by CYP2C8 and CYP3A4 in vitro: Potential for interactions with CYP2C8 inhibitors. Basic Clin. Pharmacol. Toxicol. 2006, 99, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.; Pieniaszek, H.J.; Gammaitoni, A.R.; Ahdieh, H. Oxymorphone extended release does not affect CYP2C9 or CYP3A4 metabolic pathways. J. Clin. Pharmacol. 2005, 45, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Perkins, E.J.; Posada, M.; Turner, P.K.; Chappell, J.; Ng, W.T.; Twelves, C. Physiologically Based Pharmacokinetic Modelling of Cytochrome P450 2C9-Related Tolbutamide Drug Interactions with Sulfaphenazole and Tasisulam. Eur. J. Drug Metab. Pharmacokinet. 2018, 43, 355–367. [Google Scholar] [CrossRef]
- Niemi, M.; Backman, J.T.; Neuvonen, M.; Neuvonen, P.J.; Kivistö, K.T. Effects of rifampin on the pharmacokinetics and pharmacodynamics of glyburide and glipizide. Clin. Pharmacol. Ther. 2001, 69, 400–406. [Google Scholar] [CrossRef]
- Varma, M.V.S.; Scialis, R.J.; Lin, J.; Bi, Y.-A.; Rotter, C.J.; Goosen, T.C.; Yang, X. Mechanism-based pharmacokinetic modeling to evaluate transporter-enzyme interplay in drug interactions and pharmacogenetics of glyburide. AAPS J. 2014, 16, 736–748. [Google Scholar] [CrossRef]
- Loboz, K.; Gross, A.; Williams, K.; Liauw, W.; Day, R.; Blievernicht, J.; Zanger, U.; Mclachlan, A. Cytochrome P450 2B6 activity as measured by bupropion hydroxylation: Effect of induction by rifampin and ethnicity. Clin. Pharmacol. Ther. 2006, 80, 75–84. [Google Scholar] [CrossRef]
- Sager, J.E.; Price, L.S.L.; Isoherranen, N. Stereoselective Metabolism of Bupropion to OH-bupropion, Threohydrobupropion, Erythrohydrobupropion, and 4′-OH-bupropion in vitro. Drug Metab. Dispos. 2016, 44, 1709–1719. [Google Scholar] [CrossRef]
- Niemi, M.; Backman, J.T.; Neuvonen, M.; Neuvonen, P.J.; Kivisto, K.T. Rifampin decreases the plasma concentrations and effects of repaglinide. Clin. Pharmacol. Ther. 2000, 68, 495–500. [Google Scholar] [CrossRef]
- Säll, C.; Houston, J.B.; Galetin, A. A comprehensive assessment of repaglinide metabolic pathways: Impact of choice of in vitro system and relative enzyme contribution to in vitro clearance. Drug Metab. Dispos. 2012, 40, 1279–1289. [Google Scholar] [CrossRef] [PubMed]
- Varma, M.V.S.; Lin, J.; Bi, Y.-A.; Rotter, C.J.; Fahmi, O.A.; Lam, J.L.; El-Kattan, A.F.; Goosen, T.C.; Lai, Y. Quantitative prediction of repaglinide-rifampicin complex drug interactions using dynamic and static mechanistic models: Delineating differential CYP3A4 induction and OATP1B1 inhibition potential of rifampicin. Drug Metab. Dispos. 2013, 41, 966–974. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, R.A. Interaction of sodium warfarin and rifampin. Studies in man. Ann. Intern. Med. 1974, 81, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Tornio, A.; Filppula, A.M.; Niemi, M.; Backman, J.T. Clinical Studies on Drug–Drug Interactions Involving Metabolism and Transport: Methodology, Pitfalls, and Interpretation. Clin. Pharmacol. Ther. 2019, 105, 1345–1361. [Google Scholar] [CrossRef]
- Fahmi, O.A.; Shebley, M.; Palamanda, J.; Sinz, M.W.; Ramsden, D.; Einolf, H.J.; Chen, L.; Wang, H. Evaluation of CYP2B6 Induction and Prediction of Clinical Drug–Drug Interactions: Considerations from the IQ Consortium Induction Working Group—An Industry Perspective. Drug Metab. Dispos. 2016, 44, 1720–1730. [Google Scholar] [CrossRef]
- Zilly, W.; Breimer, D.D.; Richter, E. Induction of drug metabolism in man after rifampicin treatment measured by increased hexobarbital and tolbutamide clearance. Eur. J. Clin. Pharmacol. 1975, 9, 219–227. [Google Scholar] [CrossRef]
- Gerbal-Chaloin, S.; Pascussi, J.M.; Pichard-Garcia, L.; Daujat, M.; Waechter, F.; Fabre, J.M.; Carrère, N.; Maurel, P. Induction of CYP2C genes in human hepatocytes in primary culture. Drug Metab. Dispos. 2001, 29, 242–251. [Google Scholar]
- Runge, D.; Köhler, C.; Kostrubsky, V.E.; Jäger, D.; Lehmann, T.; Runge, D.M.; May, U.; Stolz, D.B.; Strom, S.C.; Fleig, W.E.; et al. Induction of cytochrome P450 (CYP)1A1, CYP1A2, and CYP3A4 but not of CYP2C9, CYP2C19, multidrug resistance (MDR-1) and multidrug resistance associated protein (MRP-1) by prototypical inducers in human hepatocytes. Biochem. Biophys. Res. Commun. 2000, 273, 333–341. [Google Scholar] [CrossRef]
- Yajima, K.; Uno, Y.; Murayama, N.; Uehara, S.; Shimizu, M.; Nakamura, C.; Iwasaki, K.; Utoh, M.; Yamazaki, H. Evaluation of 23 lots of commercially available cryopreserved hepatocytes for induction assays of human cytochromes P450. Drug Metab. Dispos. 2014, 42, 867–871. [Google Scholar] [CrossRef]
- Zhang, J.; Kuehl, P.; Green, E.D.; Touchman, J.W.; Watkins, P.B.; Daly, A.; Hall, S.D.; Maurel, P.; Relling, M.; Brimer, C.; et al. The human pregnane X receptor: Genomic structure and identification and functional characterization of natural allelic variants. Pharmacogenetics 2001, 11, 555–572. [Google Scholar] [CrossRef]
- Järvinen, E.; Hammer, H.S.; Pötz, O.; Ingelman-Sundberg, M.; Stage, T.B. 3D Spheroid Primary Human Hepatocytes for Prediction of Cytochrome P450 and Drug Transporter Induction. Clin. Pharmacol. Ther. 2023, 113, 1284–1294. [Google Scholar] [CrossRef] [PubMed]


| Test Article | Hepatocyte Donor Designation | |||||
|---|---|---|---|---|---|---|
| Donor 1 | Donor 2 | Donor 3 | ||||
| EC50 (μM) | Emax Fold | EC50 (μM) | Emax Fold | EC50 (μM) | Emax Fold | |
| CYP3A4 | ||||||
| Apalutamide | 0.690 | 6.12 | 1.61 | 20.8 | 1.08 | 7.45 |
| Carbamazepine | 23.6 | 4.09 | 61.2 | 9.93 | 28.5 | 3.53 |
| Efavirenz | 2.61 | 5.32 | 4.38 | 11.3 | 3.04 | 5.31 |
| Rifampicin | 0.0655 | 5.60 | 0.128 | 13.4 | 0.0602 | 4.87 |
| CYP2C8 | ||||||
| Apalutamide | 1.68 | 8.36 | 1.22 | 4.55 | 1.26 | 7.20 |
| Carbamazepine | 35.6 | 3.21 | 20.0 | 2.26 | 40.2 | 5.07 |
| Efavirenz | 4.48 | 5.22 | 1.97 | 2.85 | 2.95 | 5.11 |
| Rifampicin | 0.504 | 6.89 | 0.260 | 4.51 | 0.307 | 7.36 |
| CYP2C9 | ||||||
| Apalutamide | 0.794 | 3.50 | 0.419 | 3.63 | 0.474 | 2.73 |
| Carbamazepine | 9.86 | 2.44 | NC | <2 | NC | <2 |
| Efavirenz | 0.455 | 2.46 | NC | <2 | NC | <2 |
| Rifampicin | 0.0876 | 3.23 | 0.0784 | 2.87 | 0.149 | 2.57 |
| CYP2C19 | ||||||
| Apalutamide | 4.01 | 8.42 | 5.22 | 7.70 | 6.04 | 14.6 |
| Carbamazepine | 124 | 5.11 | 186 | 4.48 | 182 | 7.22 |
| Efavirenz | 8.30 | 4.79 | NC | <2 | 2.91 | 3.56 |
| Rifampicin | 1.64 | 19.9 | 0.803 | 8.45 | 1.47 | 17.9 |
| CYP2B6 | ||||||
| Apalutamide | 4.44 | 10.2 | 2.98 | 6.71 | 2.96 | 9.06 |
| Carbamazepine | 46.9 | 5.74 | 41.9 | 4.84 | 59.0 | 6.93 |
| Efavirenz | 2.76 | 6.46 | 3.67 | 6.61 | 4.01 | 9.44 |
| Rifampicin | 0.415 | 6.71 | 0.53 | 5.91 | 0.399 | 8.44 |
| Test Article | Hepatocyte Donor Designation | |||||
|---|---|---|---|---|---|---|
| Donor 1 | Donor 2 | Donor 3 | ||||
| EC50 (μM) | Emax Fold | EC50 (μM) | Emax Fold | EC50 (μM) | Emax Fold | |
| CYP3A4 | ||||||
| Apalutamide | 0.686 | 2.96 | 0.709 | 3.03 | 1.50 | 10.6 |
| Carbamazepine | 6.40 | 2.31 | 2.70 | 3.38 | 17.8 | 4.44 |
| Efavirenz | 1.43 | 5.07 | 0.259 | 2.79 | 4.54 | 16.3 |
| Rifampicin | 0.0509 | 2.84 | 0.0652 | 3.45 | 0.0897 | 7.74 |
| CYP2C9 | ||||||
| Apalutamide | 13.1 | 2.79 | NC | <2 | 23.7 | 3.25 |
| Carbamazepine | 41.4 | 2.52 | NC | <2 | NC | <2 |
| Efavirenz | 4.29 | 2.40 | NC | <2 | NC | <2 |
| Rifampicin | 0.469 | 2.44 | NC | <2 | NC | <2 |
| CYP2C19 | ||||||
| Apalutamide | 0.455 | 3.38 | 1.03 | 3.62 | 0.865 | 9.66 |
| Carbamazepine | 8.14 | 2.48 | 2.05 | 3.63 | 18 | 4.54 |
| Efavirenz | 0.946 | 3.04 | 0.297 | 3.52 | 2.48 | 6.87 |
| Rifampicin | 0.0438 | 3.48 | 0.0816 | 4.09 | 0.105 | 9.32 |
| Perpetrator | Perpetrator Dose | Object | Object Dose | Object Metabolism CYP (fm) | References |
|---|---|---|---|---|---|
| Rifampicin | 600 mg (5 days) | Midazolam | 3 mg (after rifampicin on day 5) | CYP3A4 (>0.9) | [27,28] |
| 600 mg (5 days) | Alfentanil | 4 mg (after rifampicin on day 5) | CYP3A4 (≥0.8) | [27,29] | |
| 600 mg (5 days) | Atorvastatin (acid) | 40 mg (after rifampicin on day 5) | CYP3A4 (>0.9) | [30,31] | |
| 450 mg (6 days) | Omeprazole | 20 mg (after rifampicin on day 6) | CYP2C19 (0.68) CYP3A4 (0.32) | [32,33] | |
| 600 mg (6 days) | Pioglitazone | 30 mg (after rifampicin on day 5) | CYP2C8 (0.56) CYP3A4 (0.37) | [34] | |
| 600 mg (14 days) | Tolbutamide | 500 mg (after rifampicin on day 14) | CYP2C9 (0.85) Other CYPs (0.15) | [35,36] | |
| 600 mg (5 days) | Glyburide | 1.75 mg (12.5 h after final rifampicin dose) | CYP3A4 (0.53) CYP2C9 (0.35) CYP2C8 (0.12) | [37,38] | |
| 600 mg (10 days in the evening) | Bupropion | 150 mg (on day 8 of rifampicin dosing) | CYP2B6 (0.21) CYP2C19 (0.18) | [39,40] | |
| 600 mg (10 days) | Repaglinide | 0.5 mg (12.5 h after final rifampicin dose) | CYP3A4 (0.74) CYP2C8 (0.26) | [41,42,43] | |
| 600 mg (3 days) | Warfarin | 1.5 mg/kg (on day 4 after rifampicin dose) | CYP2C9 (0.64) CYP3A4 (0.05) CYP2B6 (0.12) | [44] |
| Substrate | Substrate Metabolism CYP (fm) | % AUC Reduction (PBPK Modeling) | % AUC Reduction (Mechanistic Static Modeling) | % AUC Reduction (Clinically Observed) | References |
|---|---|---|---|---|---|
| Midazolam | CYP3A4 (>0.9) | 91.4 | 87.1 | 94.7 | [27,28] |
| Alfentanil | CYP3A4 (≥0.8) | 90.3 | 86.7 | 94.5 | [29] |
| Atorvastatin (acid) | CYP3A4 (>0.8) | 79.9 | 79.6 | 80.2 | [30] |
| Omeprazole | CYP2C19 (0.68) CYP3A4 (0.32) | 86.4 | 64.2 | 86.1 | [32,33] |
| Pioglitazone | CYP2C8 (0.56) CYP3A4 (0.37) | 54.03 | 31.4 | 56.3 | [34] |
| Tolbutamide | CYP2C9 (0.85) Other CYPs (0.15) | 59.8 | 25.8 | 63.5 | [35,36] |
| Glyburide | CYP3A4 (0.53) CYP2C9 (0.35) CYP2C8 (0.12) | 38.2 | 74.1 | 38.9 | [37,38] |
| Bupropion | CYP2B6 (0.21) CYP2C19 (0.18) | 67.6 | 53.4 | 67.2 | [39,40] |
| Repaglinide | CYP3A4 (0.74) CYP2C8 (0.26) | 56.5 | 31.4 | 57.5 | [41,42,43] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Slavsky, M.; Karve, A.S.; Hariparsad, N. Physiologically Based Pharmacokinetic Modeling to Assess Perpetrator and Victim Cytochrome P450 2C Induction Risk. Pharmaceutics 2025, 17, 1085. https://doi.org/10.3390/pharmaceutics17081085
Slavsky M, Karve AS, Hariparsad N. Physiologically Based Pharmacokinetic Modeling to Assess Perpetrator and Victim Cytochrome P450 2C Induction Risk. Pharmaceutics. 2025; 17(8):1085. https://doi.org/10.3390/pharmaceutics17081085
Chicago/Turabian StyleSlavsky, Marina, Aniruddha Sunil Karve, and Niresh Hariparsad. 2025. "Physiologically Based Pharmacokinetic Modeling to Assess Perpetrator and Victim Cytochrome P450 2C Induction Risk" Pharmaceutics 17, no. 8: 1085. https://doi.org/10.3390/pharmaceutics17081085
APA StyleSlavsky, M., Karve, A. S., & Hariparsad, N. (2025). Physiologically Based Pharmacokinetic Modeling to Assess Perpetrator and Victim Cytochrome P450 2C Induction Risk. Pharmaceutics, 17(8), 1085. https://doi.org/10.3390/pharmaceutics17081085








