A Parent–Metabolite Middle-Out PBPK Model for Genistein and Its Glucuronide Metabolite in Rats: Integrating Liver and Enteric Metabolism with Hepatobiliary and Enteroluminal Transport to Assess Glucuronide Recycling
Abstract
1. Introduction
| Parameter | GT | References | GT-Glu | References |
|---|---|---|---|---|
| Fraction unbound in plasma (%) | 1 | [25] | 71 | [23] |
| Blood to plasma partition coefficient | 0.88 | [26] | 0.61 | [23] |
| pKa’s | 7.3, 10.18 and 11.68 | [13] | 3.82, 8.15 and 11.24 | ADMET predictor |
| Log P | 3.04 | [27] | 0.34 | ADMET predictor |
| Fraction unbound in liver microsome (%) | 71.8 | ADMET predictor | 100 | Assumed |
| Drug intrinsic solubility (mg/mL) | 0.024 | [28] | NA | |
| First order absorption rate constant ka (1/h) | 4.02 | [29] | NA | |
| In vitro Jmax,Mrp2 (ng/h/mg) a | NA | 111 | [30] | |
| Km,Mrp2 (ng/mL) a | NA | 468 | ||
| In vitro Jmax,Mrp3 (ng/h/mg) | NA | 486 | [31] | |
| Km,Mrp3 (ng/mL) | NA | 351 | ||
| In vitro Jmax,Bcrp (ng/h/mg) | NA | 2756 | [24] | |
| Km,Bcrp (ng/mL) | NA | 3054 | ||
| In vitro Jmax,Oatp1b2 (ng/h/mg) b | NA | 3240 | [9] | |
| Km,Oatp1b2 (ng/mL) b | NA | 13,512 |
2. Methods
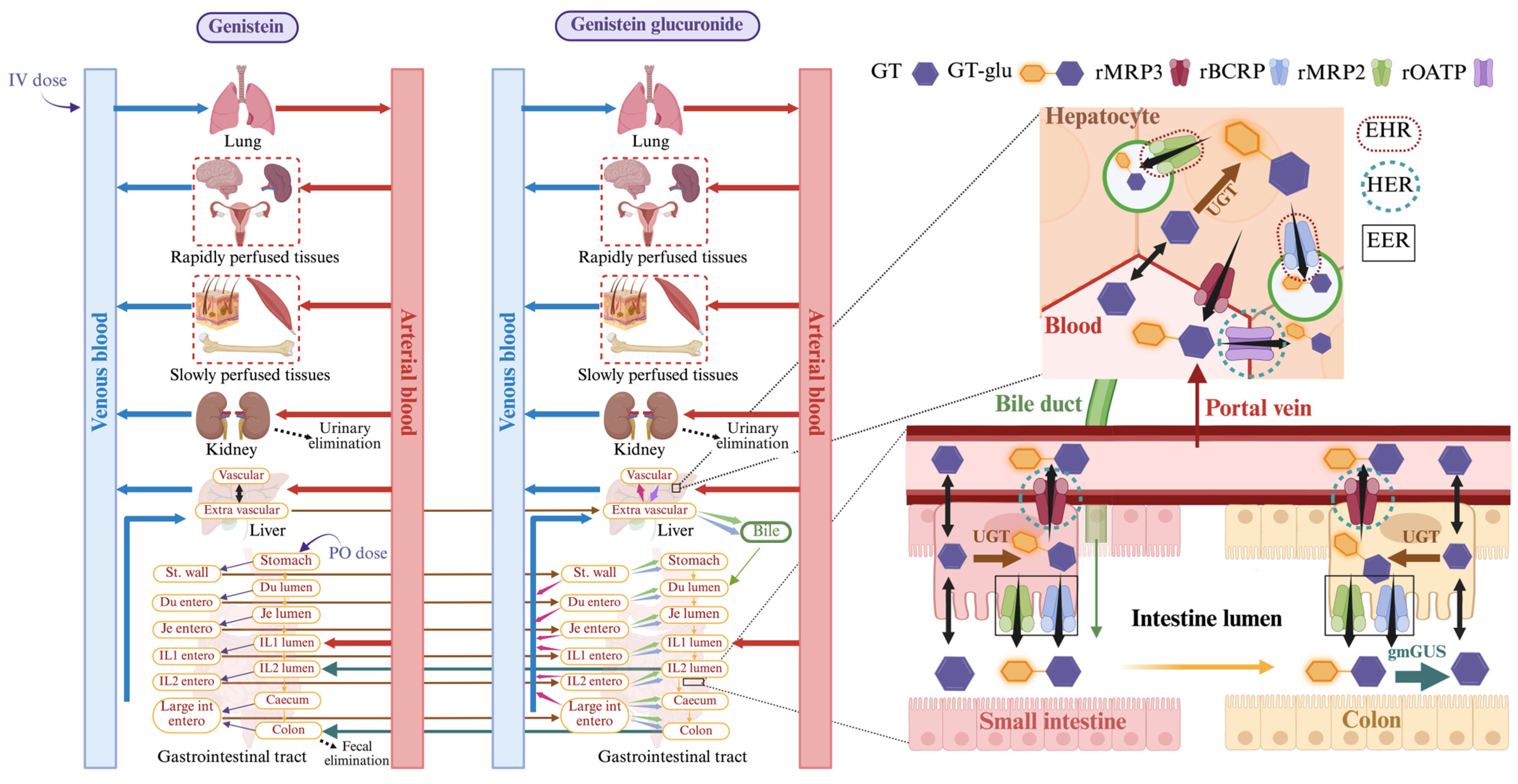
2.1. PBPK Model Building
2.1.1. Liver
2.1.2. Liver_Glu
2.1.3. Bile_Glu
2.1.4. Stomach
2.1.5. Duodenum
2.1.6. Jejunum, Ileum, Caecum, and Colon
2.1.7. Jejunum, Ileum, and Large Intestine Enterocytes
2.1.8. Stomach_Glu
2.1.9. Duodenum_Glu
2.1.10. Lumen and Enterocytes of Jejunum_Glu, Ileum_Glu, and Large Intestine_Glu
2.1.11. gmGUS Mediated Regeneration of Genistein in Distal Ileum and Colon
2.2. Model Fitting and Parameter Estimation
2.3. Sensitivity Analysis
3. Results
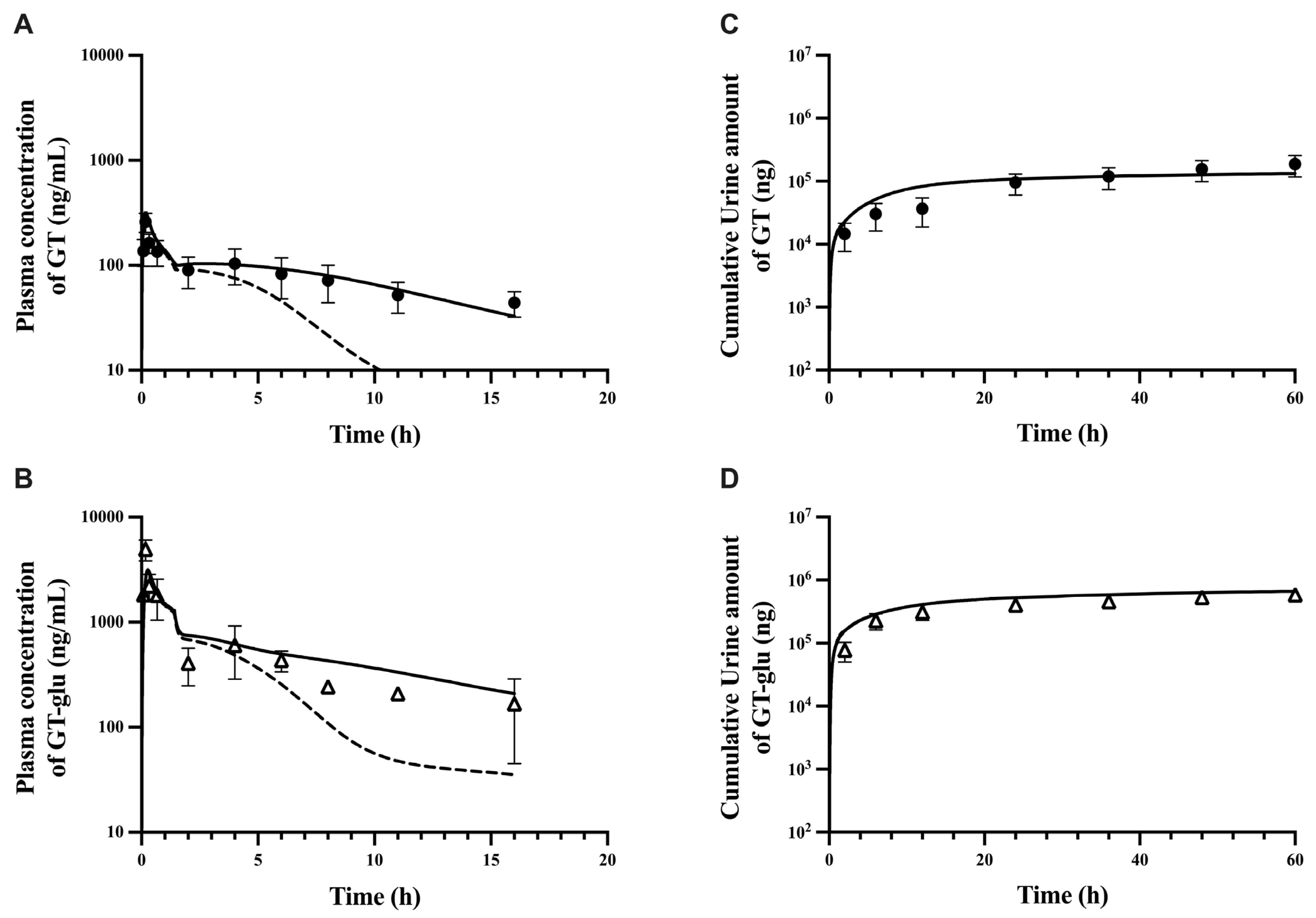
3.1. Parent–Metabolite Middle-Out PBPK Model Building and Parameter Estimation
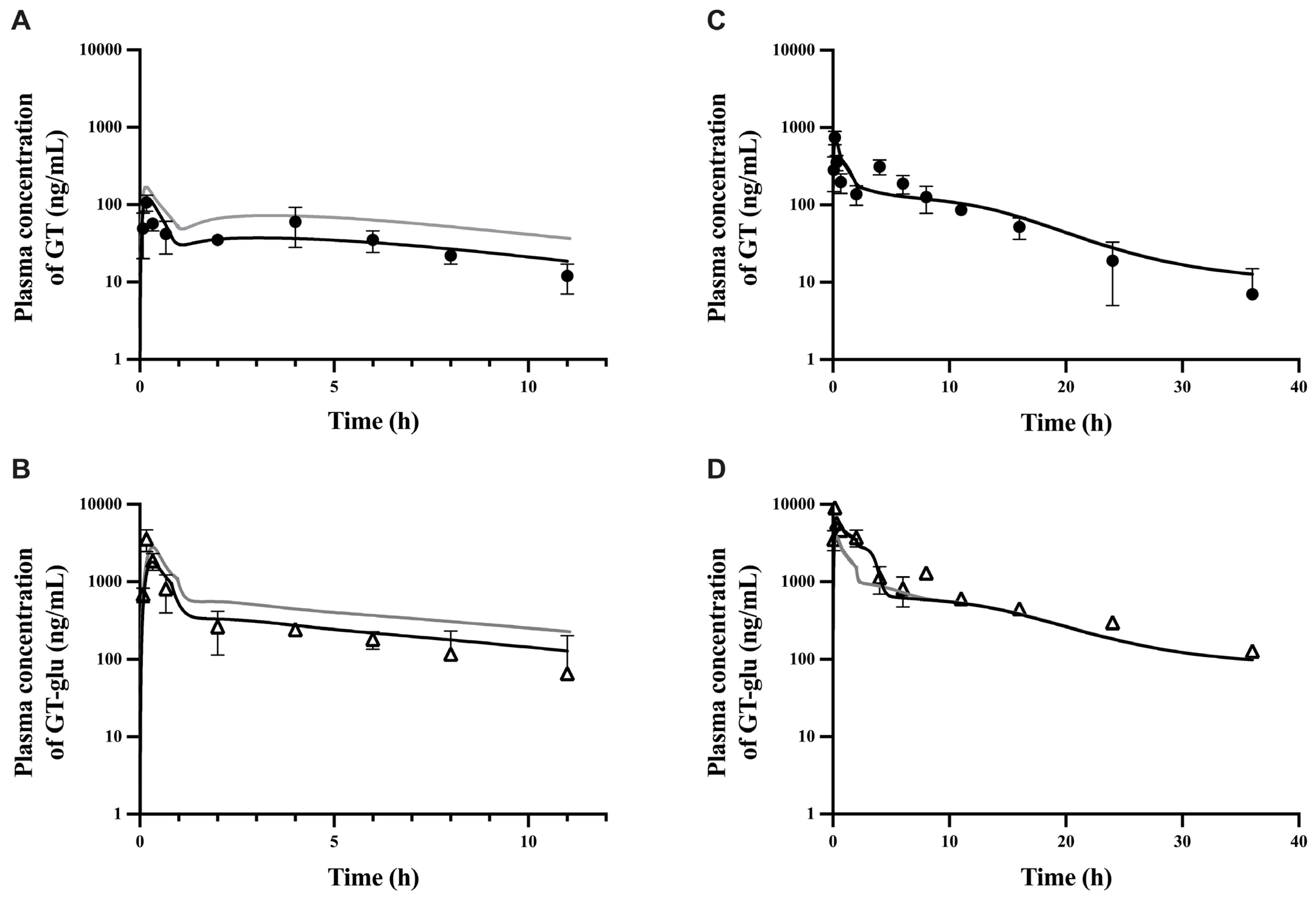
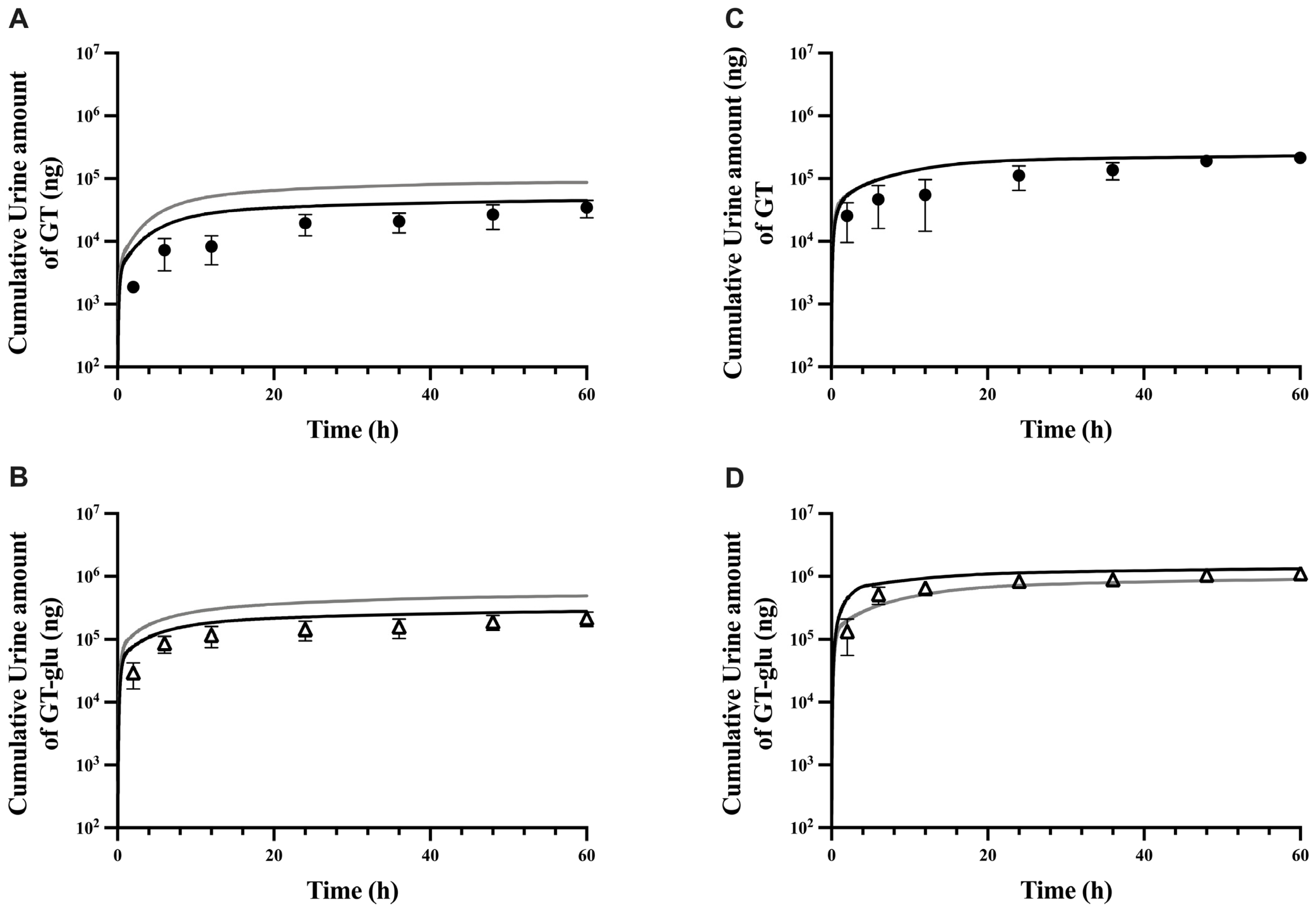
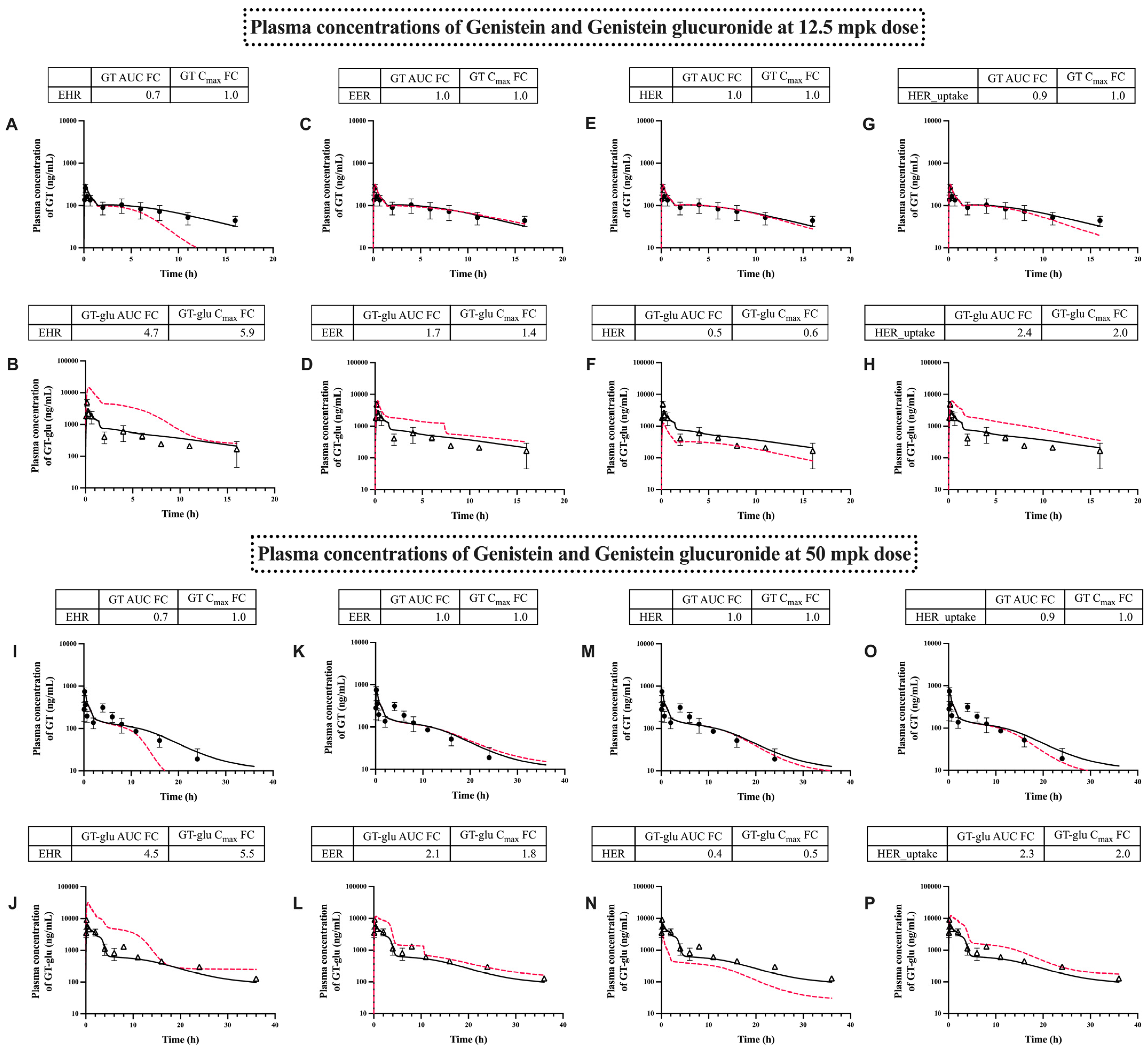
3.2. PBPK Model Performance
3.3. Model Sensitivity Evaluation
3.4. Transporter-Dependent Enterohepatic Recirculation of GT and GT-Glu
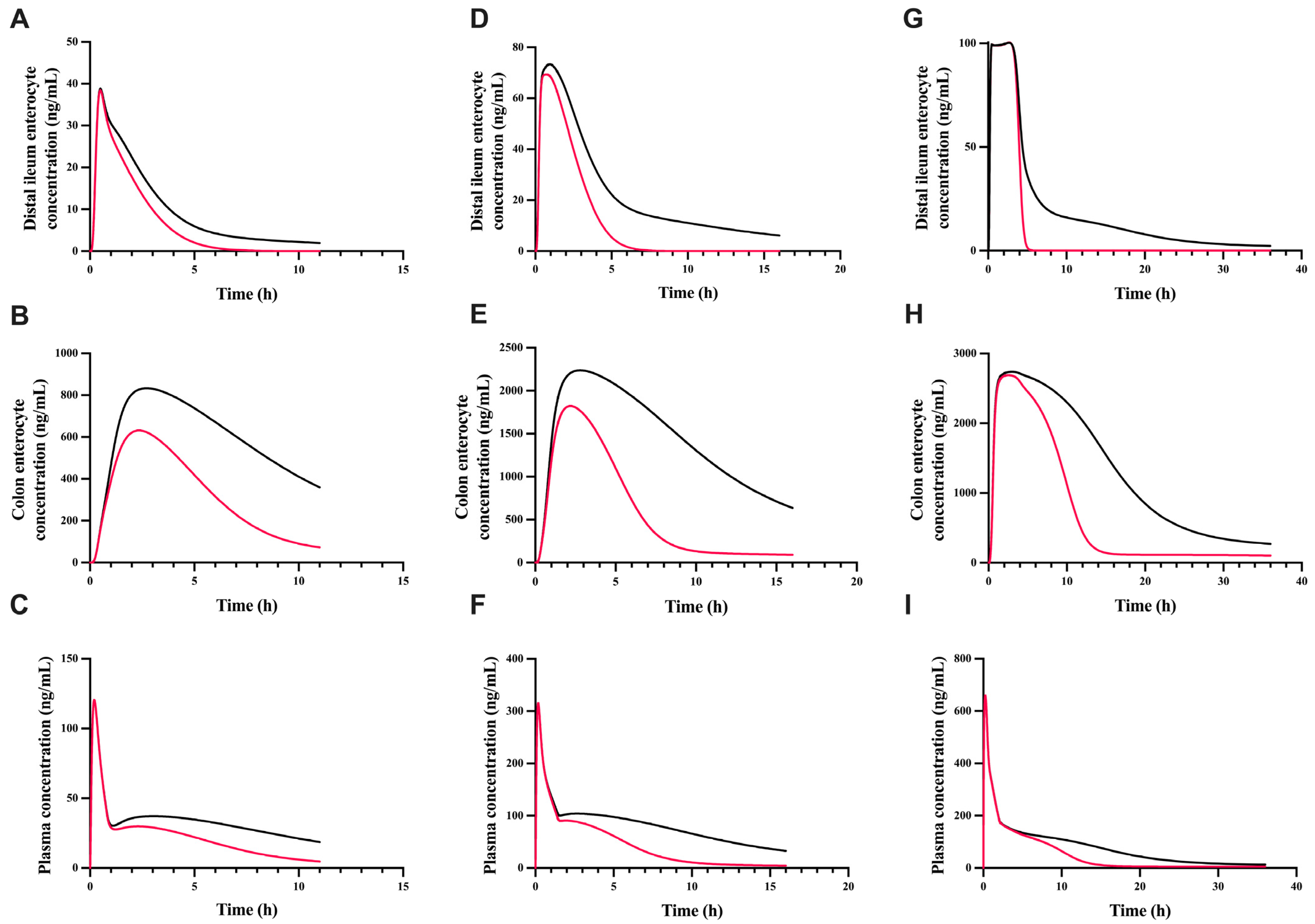
3.5. The Effect of gmGUS on the Plasma and Enterocyte Concentration of GT
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Wells, P.G.; Mackenzie, P.I.; Chowdhury, J.R.; Guillemette, C.; Gregory, P.A.; Ishii, Y.; Hansen, A.J.; Kessler, F.K.; Kim, P.M.; Chowdhury, N.R.; et al. Glucuronidation and the UDP-glucuronosyltransferases in health and disease. Drug Metab. Dispos. 2004, 32, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Shipkova, M.; Wieland, E. Glucuronidation in therapeutic drug monitoring. Clin. Chim. Acta 2005, 358, 2–23. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.M.; Holtzman, M.; Kim, T.; Kharasch, E.D. Buprenorphine metabolites, buprenorphine-3-glucuronide and norbuprenorphine-3-glucuronide, are biologically active. Anesthesiology 2011, 115, 1251–1260. [Google Scholar] [CrossRef] [PubMed]
- Frances, B.; Gout, R.; Campistron, G.; Panconi, E.; Cros, J. Morphine-6-glucuronide is more mu-selective and potent in analgesic tests than morphine. Prog. Clin. Biol. Res. 1990, 328, 477–480. [Google Scholar]
- Stone, A.N.; Mackenzie, P.I.; Galetin, A.; Houston, J.B.; Miners, J.O. Isoform selectivity and kinetics of morphine 3- and 6-glucuronidation by human udp-glucuronosyltransferases: Evidence for atypical glucuronidation kinetics by UGT2B7. Drug Metab. Dispos. 2003, 31, 1086–1089. [Google Scholar] [CrossRef]
- Gao, S.; Sun, R.; Singh, R.; Yu So, S.; Chan, C.T.Y.; Savidge, T.; Hu, M. The role of gut microbial beta-glucuronidase in drug disposition and development. Drug Discov. Today 2022, 27, 103316. [Google Scholar] [CrossRef]
- Tang, L.; Singh, R.; Liu, Z.; Hu, M. Structure and concentration changes affect characterization of UGT isoform-specific metabolism of isoflavones. Mol. Pharm. 2009, 6, 1466–1482. [Google Scholar] [CrossRef]
- Yang, Z.; Kulkarni, K.; Zhu, W.; Hu, M. Bioavailability and pharmacokinetics of genistein: Mechanistic studies on its ADME. Anticancer Agents Med. Chem. 2012, 12, 1264–1280. [Google Scholar] [CrossRef]
- Tu, Y.; Wang, L.; Rong, Y.; Tam, V.; Yin, T.; Gao, S.; Singh, R.; Hu, M. Hepatoenteric recycling is a new disposition mechanism for orally administered phenolic drugs and phytochemicals in rats. eLife 2021, 10, e58820. [Google Scholar] [CrossRef]
- Kurzer, M.S.; Xu, X. Dietary phytoestrogens. Annu. Rev. Nutr. 1997, 17, 353–381. [Google Scholar] [CrossRef]
- Hur, H.G.; Lay, J.O., Jr.; Beger, R.D.; Freeman, J.P.; Rafii, F. Isolation of human intestinal bacteria metabolizing the natural isoflavone glycosides daidzin and genistin. Arch. Microbiol. 2000, 174, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Hu, Y.; Zhang, B.; Teng, Z.; Gan, H.; Yang, Z.; Wang, Q.; Huan, M.; Mei, Q. Dose-dependent absorption, metabolism, and excretion of genistein in rats. J. Agric. Food Chem. 2008, 56, 8354–8359. [Google Scholar] [CrossRef] [PubMed]
- Mielczarek, C.; Pajak, W. Analysis of Acid-Base Properties of Flavonoid Genistein. J. Appl. Spectrosc. 2013, 80, 737–744. [Google Scholar] [CrossRef]
- Piskula, M.K.; Yamakoshi, J.; Iwai, Y. Daidzein and genistein but not their glucosides are absorbed from the rat stomach. FEBS Lett. 1999, 447, 287–291. [Google Scholar] [CrossRef]
- Yang, Z.; Zhu, W.; Gao, S.; Xu, H.; Wu, B.; Kulkarni, K.; Singh, R.; Tang, L.; Hu, M. Simultaneous determination of genistein and its four phase II metabolites in blood by a sensitive and robust UPLC-MS/MS method: Application to an oral bioavailability study of genistein in mice. J. Pharm. Biomed. Anal. 2010, 53, 81–89. [Google Scholar] [CrossRef]
- Andrade, J.E.; Twaddle, N.C.; Helferich, W.G.; Doerge, D.R. Absolute bioavailability of isoflavones from soy protein isolate-containing food in female BALB/c mice. J. Agric. Food Chem. 2010, 58, 4529–4536. [Google Scholar] [CrossRef]
- Coldham, N.G.; Zhang, A.-Q.; Key, P.; Sauer, M.J. Absolute bioavailability of [14 C] genistein in the rat; plasma pharmacokinetics of parent compound, genistein glucuronide and total radioactivity. Eur. J. Drug Metab. Pharmacokinet. 2002, 27, 249–258. [Google Scholar] [CrossRef]
- Chen, J.; Wang, S.; Jia, X.; Bajimaya, S.; Lin, H.; Tam, V.H.; Hu, M. Disposition of flavonoids via recycling: Comparison of intestinal versus hepatic disposition. Drug Metab. Dispos. 2005, 33, 1777–1784. [Google Scholar] [CrossRef]
- Soucy, N.V.; Parkinson, H.D.; Sochaski, M.A.; Borghoff, S.J. Kinetics of genistein and its conjugated metabolites in pregnant Sprague-Dawley rats following single and repeated genistein administration. Toxicol. Sci. 2006, 90, 230–240. [Google Scholar] [CrossRef]
- Wang, S.W.J.; Kulkarni, K.H.; Tang, L.; Wang, J.R.; Yin, T.J.; Daidoji, T.; Yokota, H.; Hu, M. Disposition of Flavonoids via Enteric Recycling: UDP-Glucuronosyltransferase (UGT) 1As Deficiency in Gunn Rats Is Compensated by Increases in UGT2Bs Activities. J. Pharmacol. Exp. Ther. 2009, 329, 1023–1031. [Google Scholar] [CrossRef]
- Boonpawa, R.; Spenkelink, A.; Punt, A.; Rietjens, I. In vitro-in silico-based analysis of the dose-dependent in vivo oestrogenicity of the soy phytoestrogen genistein in humans. Br. J. Pharmacol. 2017, 174, 2739–2757. [Google Scholar] [CrossRef] [PubMed]
- Zager, M.G.; Schlosser, P.M.; Tran, H.T. A delayed nonlinear PBPK model for genistein dosimetry in rats. Bull. Math. Biol. 2007, 69, 93–117. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schlosser, P.M.; Borghoff, S.J.; Coldham, N.G.; David, J.A.; Ghosh, S.K. Physiologically-based pharmacokinetic modeling of genistein in rats, Part I: Model development. Risk Anal. 2006, 26, 483–500. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Wei, Y.; Yin, T.; Xu, B.; Gao, S.; Hu, M. Transport-Glucuronidation Classification System and PBPK Modeling: New Approach To Predict the Impact of Transporters on Disposition of Glucuronides. Mol. Pharm. 2017, 14, 2884–2898. [Google Scholar] [CrossRef]
- Kodaira, H.; Kusuhara, H.; Fujita, T.; Ushiki, J.; Fuse, E.; Sugiyama, Y. Quantitative Evaluation of the Impact of Active Efflux by P-Glycoprotein and Breast Cancer Resistance Protein at the Blood-Brain Barrier on the Predictability of the Unbound Concentrations of Drugs in the Brain Using Cerebrospinal Fluid Concentration as a Surrogate. J. Pharmacol. Exp. Ther. 2011, 339, 935–944. [Google Scholar] [CrossRef]
- Katyayan, K.K.; Hui, Y.H. An evaluation of metabolite profiling of six drugs using dried blood spot. Xenobiotica 2019, 49, 1458–1469. [Google Scholar] [CrossRef]
- Rothwell, J.A.; Day, A.J.; Morgan, M.R. Experimental determination of octanol-water partition coefficients of quercetin and related flavonoids. J. Agric. Food Chem. 2005, 53, 4355–4360. [Google Scholar] [CrossRef]
- Liang, C.N.; Zhang, Y.; Jia, Y.; Wang, W.Z.; Li, Y.H.; Lu, S.K.; Jin, J.M.; Tang, S.Y. Engineering a Carbohydrate-processing Transglycosidase into Glycosyltransferase for Natural Product Glycodiversification. Sci. Rep. 2016, 6, 21051. [Google Scholar] [CrossRef]
- Steensma, A.; Noteborn, H.P.; Kuiper, H.A. Comparison of Caco-2, IEC-18 and HCEC cell lines as a model for intestinal absorption of genistein, daidzein and their glycosides. Environ. Toxicol. Pharmacol. 2004, 16, 131–139. [Google Scholar] [CrossRef]
- Wang, M.F.; Yang, G.Y.; He, Y.; Xu, B.B.; Zeng, M.; Ge, S.F.; Yin, T.J.; Gao, S.; Hu, M. Establishment and use of new MDCK II cells overexpressing both UGT1A1 and MRP2 to characterize flavonoid metabolism via the glucuronidation pathway. Mol. Nutr. Food Res. 2016, 60, 1967–1983. [Google Scholar] [CrossRef]
- Van De Wetering, K.; Feddema, W.; Helms, J.B.; Brouwers, J.F.; Borst, P. Targeted metabolomics identifies glucuronides of dietary phytoestrogens as a major class of MRP3 substrates in vivo. Gastroenterology 2009, 137, 1725–1735. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.A. Evaluation of a Generic Physiologically Based Pharmacokinetic Model for Lineshape Analysis. Clin. Pharmacokinet. 2008, 47, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Ramisetty, B.S.; Yang, S.; Dorlo, T.P.C.; Wang, M.Z. Determining tissue distribution of the oral antileishmanial agent miltefosine: A physiologically-based pharmacokinetic modeling approach. Antimicrob. Agents Chemother. 2024, 68, e0032824. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.P.; Delp, M.D.; Lindstedt, S.L.; Rhomberg, L.R.; Beliles, R.P. Physiological parameter values for physiologically based pharmacokinetic models. Toxicol. Ind. Health 1997, 13, 407–484. [Google Scholar] [CrossRef]
- Davies, B.; Morris, T. Physiological parameters in laboratory animals and humans. Pharm. Res. 1993, 10, 1093–1095. [Google Scholar] [CrossRef]
- McConnell, E.L.; Basit, A.W.; Murdan, S. Measurements of rat and mouse gastrointestinal pH, fluid and lymphoid tissue, and implications for in-vivo experiments. J. Pharm. Pharmacol. 2008, 60, 63–70. [Google Scholar] [CrossRef]
- Blouin, A.; Bolender, R.P.; Weibel, E.R. Distribution of organelles and membranes between hepatocytes and nonhepatocytes in the rat liver parenchyma. A stereological study. J. Cell Biol. 1977, 72, 441–455. [Google Scholar] [CrossRef]
- Chen, J.; Lin, H.; Hu, M. Metabolism of flavonoids via enteric recycling: Role of intestinal disposition. J. Pharmacol. Exp. Ther. 2003, 304, 1228–1235. [Google Scholar] [CrossRef]
- Young, A.; Levin, R. The Rat Distal Ileum Has a Reduced Absorptive and Secretory Capacity Compared with Proximal Ileum-Is It to Facilitate Its Chemosensing Function? Q. J. Exp. Physiol. Transl. Integr. 1989, 74, 561–563. [Google Scholar] [CrossRef]
- Musther, H.; Harwood, M.D.; Yang, J.; Turner, D.B.; Rostami-Hodjegan, A.; Jamei, M. The Constraints, Construction, and Verification of a Strain-Specific Physiologically Based Pharmacokinetic Rat Model. J. Pharm. Sci. 2017, 106, 2826–2838. [Google Scholar] [CrossRef]
- DeSesso, J.M.; Jacobson, C.F. Anatomical and physiological parameters affecting gastrointestinal absorption in humans and rats. Food Chem. Toxicol. 2001, 39, 209–228. [Google Scholar] [CrossRef] [PubMed]
- Kirman, C.R.; Hays, S.M.; Aylward, L.L.; Suh, M.; Harris, M.A.; Thompson, C.M.; Haws, L.C.; Proctor, D.M. Physiologically based pharmacokinetic model for rats and mice orally exposed to chromium. Chem. Biol. Interact. 2012, 200, 45–64. [Google Scholar] [CrossRef] [PubMed]
- Ebuzoeme, C.; Etim, I.; Ikimi, A.; Song, J.; Du, T.; Hu, M.; Liang, D.; Gao, S. Glucuronides Hydrolysis by Intestinal Microbial β-Glucuronidases (GUS) is Affected by Sampling, Enzyme Preparation, Buffer pH, and Species. Pharmaceutics 2021, 13, 1043. [Google Scholar] [CrossRef] [PubMed]
- Dawson, J.R.; Bridges, J.W. Intestinal microsomal drug metabolism. A comparison of rat and guinea-pig enzymes, and of rat crypt and villous tip cell enzymes. Biochem. Pharmacol. 1981, 30, 2415–2420. [Google Scholar] [CrossRef]
- Sakthivel, K.M.; Chandrasekaran, G. Protective Effect of Acacia Ferruginea against Ulcerative Colitis via Modulating Inflammatory Mediators, Cytokine Profile and NF-ΚB Signal Transduction Pathways. J. Environ. Pathol. Toxicol. Oncol. 2014, 33, 83–98. [Google Scholar] [CrossRef]
- Zhu, W.; Xu, H.Y.; Wang, S.W.J.; Hu, M. Breast Cancer Resistance Protein (BCRP) and Sulfotransferases Contribute Significantly to the Disposition of Genistein in Mouse Intestine. Aaps J. 2010, 12, 525–536. [Google Scholar] [CrossRef]
- Qin, Z.; Li, S.; Yao, Z.; Hong, X.; Wu, B.; Krausz, K.W.; Gonzalez, F.J.; Gao, H.; Yao, X. Chemical Inhibition and Stable Knock-down of Efflux Transporters Leads to Reduced Glucuronidation of Wushanicaritin in UGT1A1-Overexpressing HeLa Cells: The Role of Breast Cancer Resistance Protein (BCRP) and Multidrug Resistance-Associated Proteins (MRPs) in the Excretion of Glucuronides. Food Funct. 2017, 9, 1410–1423. [Google Scholar] [CrossRef]
- Sharma, S.; Singh, D.K.; Mettu, V.S.; Yue, G.; Ahire, D.; Basit, A.; Heyward, S.; Prasad, B. Quantitative Characterization of Clinically Relevant Drug-Metabolizing Enzymes and Transporters in Rat Liver and Intestinal Segments for Applications in PBPK Modeling. Mol. Pharm. 2023, 20, 1737–1749. [Google Scholar] [CrossRef]
- Horibe, S.; Minegaki, T.; Ohnishi, N.; Takara, K.; Yokoyama, T. Distribution of Abcg2 (BCRP) and Abcc2 (MRP2) mRNAs in rat small intestine and liver. EXCLI J. 2009, 8, 12–19. [Google Scholar]
- Jarvinen, E.; Deng, F.; Kiander, W.; Sinokki, A.; Kidron, H.; Sjostedt, N. The Role of Uptake and Efflux Transporters in the Disposition of Glucuronide and Sulfate Conjugates. Front. Pharmacol. 2021, 12, 802539. [Google Scholar] [CrossRef]
- Mottino, A.D.; Hoffman, T.; Jennes, L.; Vore, M. Expression and localization of multidrug resistant protein mrp2 in rat small intestine. J. Pharmacol. Exp. Ther. 2000, 293, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.A.; Jones, C.R.; Ungell, A.L.; Hatley, O.J. Predicting Drug Extraction in the Human Gut Wall: Assessing Contributions from Drug Metabolizing Enzymes and Transporter Proteins using Preclinical Models. Clin. Pharmacokinet. 2016, 55, 673–696. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Michiba, K.; Maeda, K.; Kusuhara, H. Quantitative Prediction of Pharmacokinetic Properties of Drugs in Humans: Recent Advance in in Vitro Models to Predict the Impact of Efflux Transporters in the Small Intestine and Blood–Brain Barrier. J. Pharmacol. Sci. 2021, 148, 142–151. [Google Scholar] [CrossRef] [PubMed]
- McArthur, J.B.; Chen, X. Glycosyltransferase Engineering for Carbohydrate Synthesis. Biochem. Soc. Trans. 2016, 44, 129–142. [Google Scholar] [CrossRef]
- Rodgers, T.; Rowland, M. Physiologically based pharmacokinetic modelling 2: Predicting the tissue distribution of acids, very weak bases, neutrals and zwitterions. J. Pharm. Sci. 2006, 95, 1238–1257. [Google Scholar] [CrossRef]
- Kwon, S.H.; Kang, M.J.; Huh, J.S.; Ha, K.W.; Lee, J.R.; Lee, S.K.; Lee, B.S.; Han, I.H.; Lee, M.S.; Lee, M.W.; et al. Comparison of oral bioavailability of genistein and genistin in rats. Int. J. Pharm. 2007, 337, 148–154. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Trame, M.N.; Lesko, L.J.; Schmidt, S. Sobol Sensitivity Analysis: A Tool to Guide the Development and Evaluation of Systems Pharmacology Models. CPT Pharmacomet. Syst. Pharmacol. 2015, 4, 69–79. [Google Scholar] [CrossRef]
- Schleipen, B.; Hertrampf, T.; Fritzemeier, K.H.; Kluxen, F.M.; Lorenz, A.; Molzberger, A.; Velders, M.; Diel, P. ERbeta-specific agonists and genistein inhibit proliferation and induce apoptosis in the large and small intestine. Carcinogenesis 2011, 32, 1675–1683. [Google Scholar] [CrossRef]
- Qi, W.; Weber, C.R.; Wasland, K.; Savkovic, S.D. Genistein inhibits proliferation of colon cancer cells by attenuating a negative effect of epidermal growth factor on tumor suppressor FOXO3 activity. BMC Cancer 2011, 11, 219. [Google Scholar] [CrossRef]
- MacLean, C.; Moenning, U.; Reichel, A.; Fricker, G. Closing the gaps: A full scan of the intestinal expression of p-glycoprotein, breast cancer resistance protein, and multidrug resistance-associated protein 2 in male and female rats. Drug Metab. Dispos. 2008, 36, 1249–1254. [Google Scholar] [CrossRef]
- Li, N.; Palandra, J.; Nemirovskiy, O.V.; Lai, Y. LC–MS/MS mediated absolute quantification and comparison of bile salt export pump and breast cancer resistance protein in livers and hepatocytes across species. Anal. Chem. 2009, 81, 2251–2259. [Google Scholar] [CrossRef] [PubMed]
- Gavins, F.K.; Dou, L.; Qin, Y.; Madla, C.M.; Murdan, S.; Basit, A.W.; Mai, Y.; Orlu, M. Prandial state and biological sex modulate clinically relevant efflux transporters to different extents in Wistar and Sprague Dawley rats. Biomed. Pharmacother. 2023, 160, 114329. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhang, Y.; Hua, F.; Lai, Y. Absolute difference of hepatobiliary transporter multidrug resistance-associated protein (MRP2/Mrp2) in liver tissues and isolated hepatocytes from rat, dog, monkey, and human. Drug Metab. Dispos. 2009, 37, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Rost, D.; Mahner, S.; Sugiyama, Y.; Stremmel, W. Expression and localization of the multidrug resistance-associated protein 3 in rat small and large intestine. Am. J. Physiol.-Gastrointest. Liver Physiol. 2002, 282, G720–G726. [Google Scholar] [CrossRef]
- Harbourt, D.E. An Investigation Into the Role of Glucuronidation on the Disposition and Toxicity of Mycophenolic Acid Using Targeted Quantitative Proteomics. Ph.D. Thesis, The University of North Carolina at Chapel Hill, Chapel Hill, NC, USA, 2009. [Google Scholar]
- Fallon, J.K.; Smith, P.C.; Xia, C.Q.; Kim, M.-S. Quantification of four efflux drug transporters in liver and kidney across species using targeted quantitative proteomics by isotope dilution NanoLC-MS/MS. Pharm Res-Dordr 2016, 33, 2280–2288. [Google Scholar] [CrossRef]
| Parameter | Estimated Values | Standard Error | % CV | 90% CI Range | Notes |
|---|---|---|---|---|---|
| Genistein | |||||
| Kp,scalar | 0.052 | 0.029 | 56% | 0.014–0.103 | Only applied to rapidly and slowly perfused tissues |
| PSli (mL/h) | 3.47 × 105 | 1.74 × 105 | 50% | 1.17 × 105–6.48 × 105 | |
| PSsp (mL/h) | 5.00 × 104 | 3.09 × 104 | 62% | 8.67 × 103–1.04 × 105 | |
| Suspension solubility factor | 36.15 | 10.45 | 29% | 18.08–54.28 | Used for all PO in stomach and duodenum lumens; 10 for jejunum for high PO |
| ka,ST (1/h) | 0.69 | 0.18 | 26% | 0.38–1.01 | |
| Frenal | 100 | Fixed | NA | NA | |
| ka,sys (1/h) | 500 | Fixed | NA | NA | |
| Intrinsic solubility correction factor | 2.0 | Fixed | NA | NA | Used for IV and mid- and high PO in all GI lumens |
| Genistein glucuronide | |||||
| ISEFMrp3 | 8.53 × 105 | 4.02 × 105 | 47% | 3.16 × 105–1.00 × 106 | |
| ISEFapical (Mrp2+Bcrp) | 8.65 × 105 | 6.26 × 105 | 72% | 2.88 × 104–1.70 × 106 | |
| ISEFUptake in liver | 9.35 × 103 | 4.45 × 103 | 48% | 3.40 × 103–1.00 × 104 | |
| Kp,scalar_Glu | 0.01 | Fixed | NA | NA | |
| Plasma Pharmacokinetics | Predicted (GT) | Observed (GT) | FE a | Predicted (GT-Glu) | Observed (GT-Glu) | FE a |
|---|---|---|---|---|---|---|
| 6.25 mg/kg | ||||||
| Cmax (ng/mL) | 120 | 107 | 1.1 | 1804 | 3568 | 0.5 |
| t1/2 (hour) | 5.5 | 3.2 | 1.7 | 6.2 | 3.4 | 1.8 |
| AUC0 to last (ng.h/mL) | 372 | 388 | 1.0 | 3455 | 3354 | 1.0 |
| %F | 13% | 16% | 0.8 | NA | NA | NA |
| 12.5 mg/kg | ||||||
| Cmax (ng/mL) | 315 | 260 | 1.2 | 3132 | 4941 | 0.6 |
| t1/2 (hour) | 10.5 | 9.7 | 1.1 | 13.2 | 15.0 | 0.9 |
| AUC0 to last (ng.h/mL) | 1318 | 1228 | 1.1 | 8989 | 7523 | 1.2 |
| %F | 22% | 26% | 0.9 | NA | NA | NA |
| 50 mg/kg | ||||||
| Cmax (ng/mL) | 659 | 747 | 0.9 | 5841 | 9050 | 0.6 |
| t1/2 (hour) | 10.4 | 6.7 | 1.5 | 13.0 | 11.3 | 1.2 |
| AUC0 to last (ng.h/mL) | 3185 | 2841 | 1.1 | 25,810 | 29,324 | 0.9 |
| %F | 13% | 15% | 0.8 | NA | NA | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramisetty, B.S.; Singh, R.; Hu, M.; Wang, M.Z. A Parent–Metabolite Middle-Out PBPK Model for Genistein and Its Glucuronide Metabolite in Rats: Integrating Liver and Enteric Metabolism with Hepatobiliary and Enteroluminal Transport to Assess Glucuronide Recycling. Pharmaceutics 2025, 17, 814. https://doi.org/10.3390/pharmaceutics17070814
Ramisetty BS, Singh R, Hu M, Wang MZ. A Parent–Metabolite Middle-Out PBPK Model for Genistein and Its Glucuronide Metabolite in Rats: Integrating Liver and Enteric Metabolism with Hepatobiliary and Enteroluminal Transport to Assess Glucuronide Recycling. Pharmaceutics. 2025; 17(7):814. https://doi.org/10.3390/pharmaceutics17070814
Chicago/Turabian StyleRamisetty, Bhargavi Srija, Rashim Singh, Ming Hu, and Michael Zhuo Wang. 2025. "A Parent–Metabolite Middle-Out PBPK Model for Genistein and Its Glucuronide Metabolite in Rats: Integrating Liver and Enteric Metabolism with Hepatobiliary and Enteroluminal Transport to Assess Glucuronide Recycling" Pharmaceutics 17, no. 7: 814. https://doi.org/10.3390/pharmaceutics17070814
APA StyleRamisetty, B. S., Singh, R., Hu, M., & Wang, M. Z. (2025). A Parent–Metabolite Middle-Out PBPK Model for Genistein and Its Glucuronide Metabolite in Rats: Integrating Liver and Enteric Metabolism with Hepatobiliary and Enteroluminal Transport to Assess Glucuronide Recycling. Pharmaceutics, 17(7), 814. https://doi.org/10.3390/pharmaceutics17070814








