Nanotechnology-Enhanced Sunscreens: Balancing Efficacy, Safety, and Environmental Impact
Abstract
1. Introduction
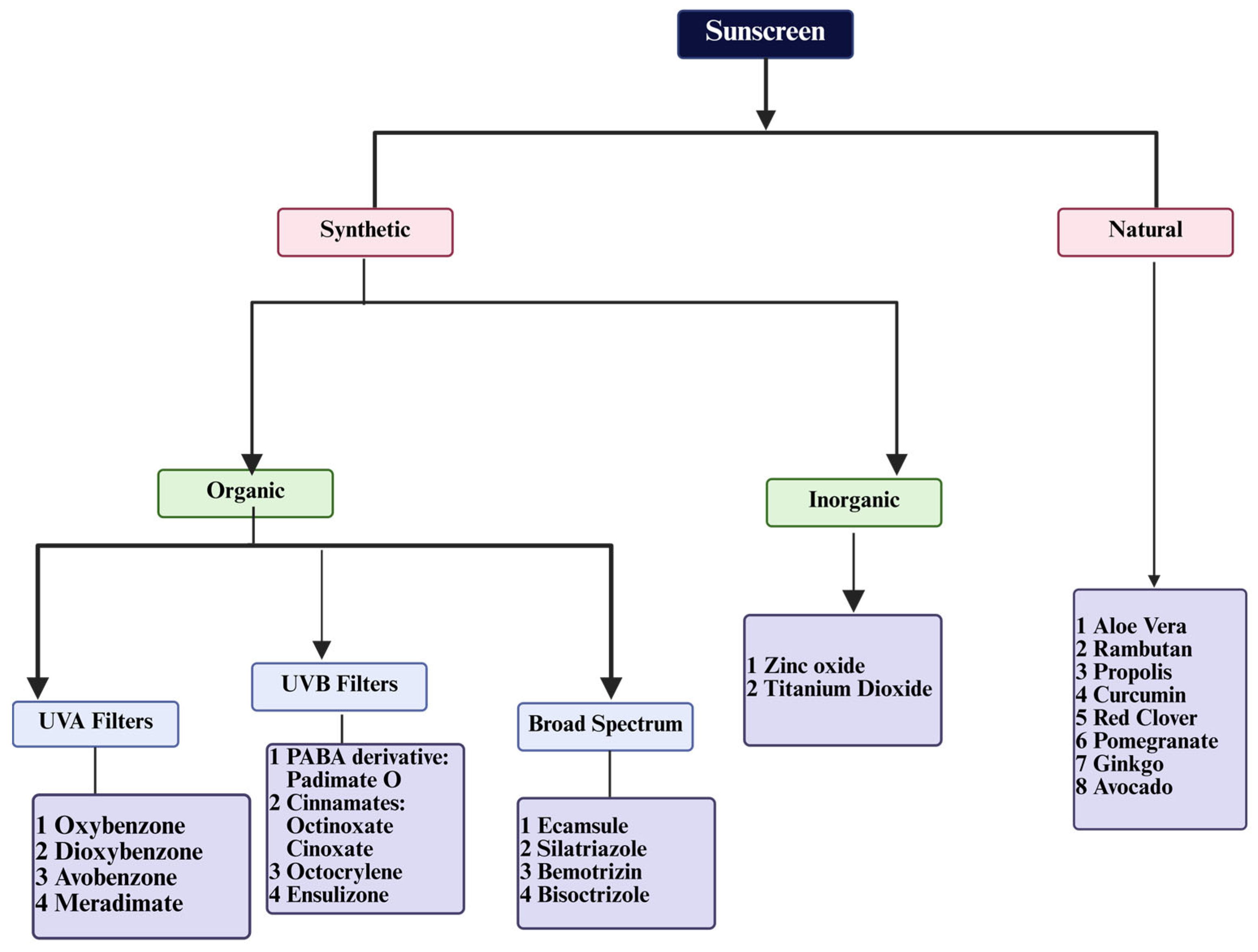
2. UV Filters
2.1. Organic Filters
2.2. Inorganic Filters
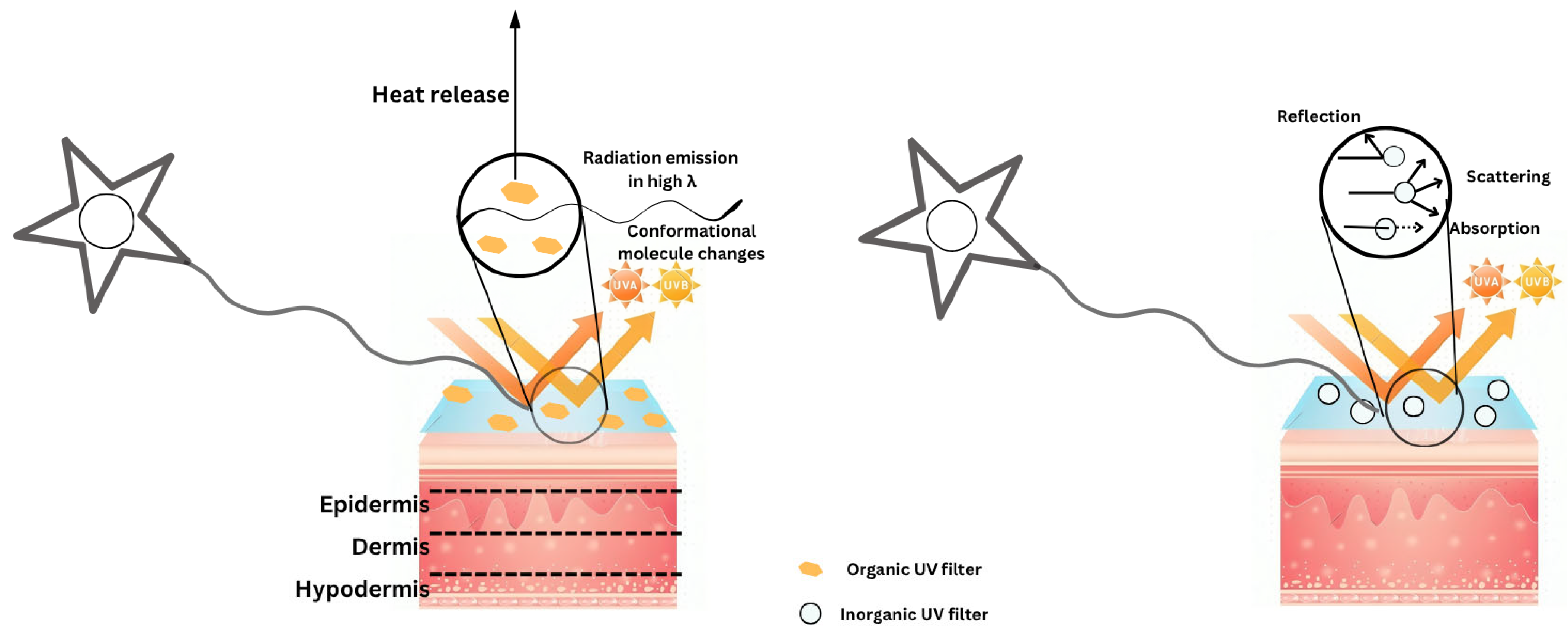
3. Mechanism of Photoprotection
- Butyloctyl salicylate—improves solubility and photostability of UV filters.
- Terephthalylidene dicamphor sulfonic acid (Mexoryl SX)—often acts synergistically with other filters to enhance UVA protection.
- Polyester-8—enhances UVB filter performance and boosts SPF while offering water resistance.
- Glyceryl stearate, hydroxyethyl acrylate/sodium acryloyldimethyl taurate copolymer, and silicone elastomers improve film uniformity and substantivity.
- Microfine zinc oxide or titanium dioxide, in combination with certain emollients or dispersing agents, improves skin adhesion and enhances broad-spectrum protection.
4. Eco-Friendly and Sustainable Ingredients in Sunscreen
4.1. Emerging Trends in Sustainable Sunscreens
4.1.1. Marine-Derived Nanomaterials
4.1.2. Biodegradable Nanocarriers
4.1.3. Blue-Light and Infrared Protection
4.1.4. Green Nanotechnology Approaches
4.1.5. Hybrid Natural-Synthetic Systems
4.1.6. Edible and Ingestible Sunscreens
5. Nanosystems
5.1. Nanosystems Containing Lipid
5.1.1. Lipid Nanoparticles
Solid Lipid Nanoparticles and Nanostructured Lipid Carriers
5.1.2. Vesicle-Based Systems
5.1.3. Liposome Structures
Niosomes
Transfersomes
Cubosomes
5.1.4. Nanoemulsions
5.2. Polymer-Based Nanosystems
5.2.1. Polymeric Nanoparticles
5.2.2. Nanofibers
5.3. Metal-Based Nanosystems
5.3.1. Silver Nanoparticles
5.3.2. Gold Nanoparticles
5.3.3. Titanium Oxide and Zinc Oxide Nanoparticles
5.3.4. Silica Nanoparticles
5.4. Additional Nanosystems
5.4.1. Dendrimers
5.4.2. Nanocrystals
5.4.3. Fullerenes
5.4.4. Nanodiamonds
5.4.5. Cyclodextrins
6. Patents [88,89,90]
7. Safety and Regulatory Aspects of Sunscreen Formulation:
8. Challenges
9. Future Perspectives
10. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chałupczak, N.V.; Lio, P.A. Sunscreens: Mechanisms and Safety in Depth. J. Drugs Dermatol. 2025, 24, 142–146. [Google Scholar] [CrossRef]
- Bojilova, R.; Mukhtarov, P.; Miloshev, N. Dependence of the Index of Biologically Active Ultraviolet Radiation on the Season and Time of Day. Atmosphere 2022, 13, 1455. [Google Scholar] [CrossRef]
- Shanshal, S.A.; Khaleel, S.M.; Hammoodi, S.H. Sun Exposure and Usage of Sun Protection: Knowledge, Perception and Practice among University Students. Malays. J. Med. Sci. 2024, 31, 208–221. [Google Scholar] [CrossRef]
- Akerlof, K.L.; Loevenich, J.; Melena, S.; Lipsky, C.A. Behaviorally segmented audiences for managing sunscreen chemical pollution risk in protected coastal natural resource areas. Risk Anal. 2024, 44, 349–365. [Google Scholar] [CrossRef] [PubMed]
- Hegde, A.R.; Kunder, M.U.; Narayanaswamy, M.; Murugesan, S.; Furtado, S.C.; Veerabhadraiah, B.B.; Srinivasan, B. Advancements in sunscreen formulations: Integrating polyphenolic nanocarriers and nanotechnology for enhanced UV protection. Environ. Sci. Pollut. Res. 2024, 31, 38061–38082. [Google Scholar] [CrossRef]
- Spiridonov, V.; Ćurić, M.; Novkovski, N. Solar Radiation: Driving Atmospheric Change. In Atmospheric Perspectives; Springer: Cham, Swizerland, 2025; pp. 137–170. [Google Scholar] [CrossRef]
- Ragunathan, V.; Kumaran, C. Superior UV Blocking, Biological and Suppressed Photocatalytic Properties of Melanoidins-Hybridized ZnO Nanocomposites. Arab. J. Sci. Eng. 2025, 50, 4177–4196. [Google Scholar] [CrossRef]
- Brzozowska, J.M.; Gotlib, J. Social Media Potential and Impact on Changing Behaviors and Actions in Skin Health Promotion: Systematic Review. J. Med. Internet Res. 2025, 27, e54241. [Google Scholar] [CrossRef] [PubMed]
- Sunena; Tomar, D.; Jawla, S. Clinical Applications of Sunscreens and Formulation Advancements. Curr. Drug Res. Rev. 2024, 16, 198–208. [Google Scholar] [CrossRef]
- Chavda, V.P.; Acharya, D.; Hala, V.; Daware, S.; Vora, L.K. Sunscreens: A comprehensive review with the application of nanotechnology. J. Drug Deliv. Sci. Technol. 2023, 86, 104720. [Google Scholar] [CrossRef]
- Tang, X.; Yang, T.; Yu, D.; Xiong, H.; Zhang, S. Current insights and future perspectives of ultraviolet radiation (UV) exposure: Friends and foes to the skin and beyond the skin. Environ. Int. 2024, 185, 108535. [Google Scholar] [CrossRef]
- Rajan, R. Interaction of Solar Radiation with the Human Skin. In Sunscreens for Skin of Color; Springer: Singapore, 2024; pp. 19–55. [Google Scholar] [CrossRef]
- Alnuqaydan, A.M. The dark side of beauty: An in-depth analysis of the health hazards and toxicological impact of synthetic cosmetics and personal care products. Front. Public Health 2024, 12, 1439027. [Google Scholar] [CrossRef]
- Gautam, K.; Anbumani, S. Understudied and underestimated impacts of organic UV filters on terrestrial ecosystems. Sci. Total Environ. 2024, 953, 176008. [Google Scholar] [CrossRef]
- Pniewska, A.; Kalinowska-Lis, U. A Survey of UV Filters Used in Sunscreen Cosmetics. Appl. Sci. 2024, 14, 3302. [Google Scholar] [CrossRef]
- Miranda, J.A.; Cruz, Y.F.; Girão, Í.C.; Souza, F.J.; Oliveira, W.N.; Alencar, É.D.; Amaral-Machado, L.; Egito, E.S. Beyond Traditional Sunscreens: A Review of Liposomal-Based Systems for Photoprotection. Pharmaceutics 2024, 16, 661. [Google Scholar] [CrossRef]
- Garcia, R.D.; Maltarollo, V.G.; Honório, K.M.; Trossini, G.H.G. Benchmark studies of UV–vis spectra simulation for cinnamates with UV filter profile. J. Mol. Model. 2015, 21, 150. [Google Scholar] [CrossRef]
- Irede, E.L.; Awoyemi, R.F.; Owolabi, B.; Aworinde, O.R.; Kajola, R.O.; Hazeez, A.; Raji, A.A.; Ganiyu, L.O.; Onukwuli, C.O.; Onivefu, A.P.; et al. Cutting-edge developments in zinc oxide nanoparticles: Synthesis and applications for enhanced antimicrobial and UV protection in healthcare solutions. RSC Adv. 2024, 14, 20992–21034. [Google Scholar] [CrossRef] [PubMed]
- Tonolli, P.N.; Baptista, M.S.; Chiarelli-Neto, O. Melanin, lipofuscin and the effects of visible light in the skin. J. Photochem. Photobiol. 2021, 7, 100044. [Google Scholar] [CrossRef]
- Couteau, C.; Dupont, C.; Paparis, E.; Coiffard, L.J.M. Demonstration of the dangerous nature of ‘homemade’ sunscreen recipes. J. Cosmet. Dermatol. 2021, 20, 1788–1794. [Google Scholar] [CrossRef]
- Špaglová, M.; Čermáková, P.; Jackuliaková, P.; Piešťanský, J. Role of Emulsifiers and SPF Booster in Sunscreen Performance: Assessing SPF, Rheological Behavior, Texture, and Stability. Cosmetics 2025, 12, 118. [Google Scholar] [CrossRef]
- Landry, K.S.; Young, E.; Avery, T.S.; Gropman, J. Efficacy of a Novel SPF Booster Based on Research Aboard the International Space Station. Cosmetics 2023, 10, 138. [Google Scholar] [CrossRef]
- Dini, I.; Laneri, S. The New Challenge of Green Cosmetics: Natural Food Ingredients for Cosmetic Formulations. Molecules 2021, 26, 3921. [Google Scholar] [CrossRef]
- Sasounian, R.; Martinez, R.M.; Lopes, A.M.; Giarolla, J.; Rosado, C.; Magalhães, W.V.; Velasco, M.V.R.; Baby, A.R. Innovative approaches to an eco-friendly cosmetic industry: A review of sustainable ingredients. Clean Technol. 2024, 6, 176–198. [Google Scholar] [CrossRef]
- Delannoy, A.; Baudier, P.; de Vassoigne, T. Perceptions of ethical cosmetics: Health behaviors during the COVID-19 pandemic. J. Consum. Behav. 2024, 23, 470–490. [Google Scholar] [CrossRef]
- Aguiar, J.B.; Martins, A.M.; Almeida, C.; Ribeiro, H.M.; Marto, J. Water sustainability: A waterless life cycle for cosmetic products. Sustain. Prod. Consum. 2022, 32, 35–51. [Google Scholar] [CrossRef]
- Damikouka, I.; Anastasopoulou, M.; Vgenopoulou, E. Sunscreens in the aquatic environment and potential solutions for mitigation of sunscreen pollution. Euro-Mediterr. J. Environ. Integr. 2024, 9, 1833–1850. [Google Scholar] [CrossRef]
- Shastri, D.H.; Gandhi, S.; Almeida, H. Enhancing Collaboration and Interdisciplinary Strategies for Navigating Innovative Technologies and Regulatory Approvals in the Cosmetic Industry. Curr. Cosmet. Sci. 2024, 3, 1–25. [Google Scholar] [CrossRef]
- Gieniusz, E.; Skrzydlewska, E.; Łuczaj, W. Current Insights into the Role of UV Radiation-Induced Oxidative Stress in Melanoma Pathogenesis. Int. J. Mol. Sci. 2024, 25, 11651. [Google Scholar] [CrossRef]
- Harinisri Ram, K.; Thamarai Selvi, B. Inventive Applications of Marine Resources in Cosmetic Production: A Review. In Multidisciplinary Applications of Marine Resources; Springer: Singapore, 2024; pp. 407–441. [Google Scholar]
- Ariede, M.B.; Junior, W.A.G.; Cândido, T.M.; de Aguiar, M.M.G.B.; Rosado, C.; Rangel-Yagui, C.d.O.; Pessoa, F.V.L.S.; Velasco, M.V.R.; Baby, A.R. Would Rutin Be a Feasible Strategy for Environmental-Friendly Photoprotective Samples? A Review from Stability to Skin Permeability and Efficacy in Sunscreen Systems. Cosmetics 2024, 11, 141. [Google Scholar] [CrossRef]
- Lawson, M.K. Improvement of Therapeutic Value of Quercetin with Chitosan Nanoparticle Delivery Systems and Potential Applications. Int. J. Mol. Sci. 2023, 24, 3293. [Google Scholar] [CrossRef] [PubMed]
- Maghsoudi, S.; Shahraki, B.T.; Rabiee, N.; Fatahi, Y.; Bagherzadeh, M.; Dinarvand, R.; Ahmadi, S.; Rabiee, M.; Tahriri, M.; Hamblin, M.R.; et al. The colorful world of carotenoids: A profound insight on therapeutics and recent trends in nano delivery systems. Crit. Rev. Food Sci. Nutr. 2022, 62, 3658–3697. [Google Scholar] [CrossRef]
- Mondal, S.; Bhattacharjee, B. Tailoring the optical properties of aloe vera functionalized zinc oxide nanoparticles in quest of an ameliorated sun-blocking agent: A semi-quantitative study on photo-protection, photo-stability, antioxidant and photo-catalytic properties. Emergent Mater. 2025, 1–26. [Google Scholar] [CrossRef]
- Pinto, F.; Fonseca, L.P.; de Barros, D.P.C. Dermal Delivery of Lipid Nanoparticles: Effects on Skin and Assessment of Absorption and Safety. Adv. Exp. Med. Biol. 2022, 1357, 83–114. [Google Scholar] [CrossRef]
- Kaurav, M.; Sahu, K.; Joshi, R.; Akram, W.; Raj, P.M.; Raj, R.; Minz, S. Oil and Fats as Raw Materials for Coating Industries. In Oils and Fats as Raw Materials for Industry; Wiley: Hoboken, NJ, USA, 2024; pp. 169–194. [Google Scholar]
- Poteraș, C.B.; Culețu, A.; Manolache, F.A. Nutritional Importance of Lentil, Lupin, Chickpea and Soy Legumes: A Review. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Food Sci. Technol. 2024, 81, 15–34. [Google Scholar] [CrossRef]
- Harutyunyan, S.; Stepanyan, T.; Khachatryan, G.; Goginyan, V. Biodiversity, spreading, and practical appliance of nodule bacteria in Armenia: Review. In Microbial Essentialism; Elsevier: Amsterdam, The Netherlands, 2024; pp. 419–442. [Google Scholar]
- Chabi, I.B.; Aïssi, M.V.; Zannou, O.; Kpoclou, Y.E.; Ayegnon, B.P.; Badoussi, M.E.; Ballogou, V.Y.; Goksen, G.; Mousavi Khaneghah, A.; Kayodé, A.P. New value chain Pentadesma nuts and butter from West Africa to international markets: Biological activities, health benefits, and physicochemical properties. Food Sci. Nutr. 2024, 12, 907–920. [Google Scholar] [CrossRef]
- Choungo Nguekeng, P.B.; Hendre, P.; Tchoundjeu, Z.; Kalousová, M.; Tchanou Tchapda, A.V.; Kyereh, D.; Masters, E.; Lojka, B. The Current State of Knowledge of Shea Butter Tree (Vitellaria paradoxa C.F.Gaertner.) for Nutritional Value and Tree Improvement in West and Central Africa. Forests 2021, 12, 1740. [Google Scholar] [CrossRef]
- Ezeokeke, E.E.; Ene, A.C.; Igwe, C.U. In Vivo Anti-Plasmodial Effect of Ethanol and Aqueous Extracts of Alchornea cordifolia. Biochem. Anal. Biochem. 2015, 4, 221. [Google Scholar]
- Baydar, S.Y.; Cakir-Koc, R.; KilInC, Y.B.; Ozdemir, B.; KaravelioGlu, Z. Fabrication and Characterization of Persea Gratissima Oil Loaded Chitosan Nanoparticles and Investigation of Its Neuroprotective Effects. Kocaeli J. Sci. Eng. 2021, 4, 128–135. [Google Scholar] [CrossRef]
- Souza, M.A.; Kunh, K.; Sanaiotto, O.; Provinelli, A.C.; Barufke, M.; Schindler, M.S.; Mazon, S.C.; Oliveira, J.V.; Brusco, I.; Scapinello, J.; et al. Anti-inflammatory and antinociceptive effects of Aloysia gratissima leaves essential oil: An in vivo study. J. Tradit. Complement. Med. 2024. [Google Scholar] [CrossRef]
- Ataollahi, M.; Akrami, E.; Kalani, M.; Zarei, M.; Chijan, M.R.; Sedigh-Rahimabadi, M.; Alipanah, H. Evaluation of anticoagulant and inflammatory effects of Tanacetum parthenium (L.) in a randomized controlled clinical trial. J. Herb. Med. 2022, 36, 100613. [Google Scholar] [CrossRef]
- Iqbal, J.; Khan, A.A.; Aziz, T.; Ali, W.; Ahmad, S.; Rahman, S.U.; Iqbal, Z.; Dablool, A.S.; Alruways, M.W.; Almalki, A.A.; et al. Phytochemical Investigation, Antioxidant Properties and In Vivo Evaluation of the Toxic Effects of Parthenium hysterophorus. Molecules 2022, 27, 4189. [Google Scholar] [CrossRef]
- Lieshchova, M.; Brygadyrenko, V. Effects of Origanum vulgare and Scutellaria baicalensis on the Physiological Activity and Biochemical Parameters of the Blood in Rats on a High-Fat Diet. Sci. Pharm. 2022, 90, 49. [Google Scholar] [CrossRef]
- Ślusarczyk, S.; Grzelka, K.; Jaśpińska, J.; Pawlikowska-Bartosz, A.; Pecio, Ł.; Stafiniak, M.; Rahimmalek, M.; Słupski, W.; Cieślak, A.; Matkowski, A. Changes in Growth and Metabolic Profile of Scutellaria baicalensis Georgi in Response to Sodium Chloride. Biology 2024, 13, 1058. [Google Scholar] [CrossRef]
- Santos, F.J.M.; Castillo, H.A.P.; QuinteroRamos, A.; Galán, G.Z.; Duran, R.; Borunda, E.O. Comparison of catalytic activity and antimicrobial properties of palladium nanoparticles obtained by Aloe barbadensis and Glycine max extracts, and chemical synthesis. Appl. Nanosci. 2022, 12, 2901–2913. [Google Scholar] [CrossRef]
- Swaraj, J.C.S. Medicinal and Nutritional Importance of Aloe barbadensis Miller in Human Health. In Medicinal Plants and Their Bioactive Compounds in Human Health; Springer: Singapore, 2024; Volume 1, pp. 91–105. [Google Scholar] [CrossRef]
- Santos, A.C.; Morais, F.; Simões, A.; Pereira, I.; Sequeira, J.A.D.; Pereira-Silva, M.; Veiga, F.; Ribeiro, A. Nanotechnology for the development of new cosmetic formulations. Expert Opin. Drug Deliv. 2019, 16, 313–330. [Google Scholar] [CrossRef]
- Akombaetwa, N.; Ilangala, A.B.; Thom, L.; Memvanga, P.B.; Witika, B.A.; Buya, A.B. Current advances in lipid nanosystems intended for topical and transdermal drug delivery applications. Pharmaceutics 2023, 15, 656. [Google Scholar] [CrossRef]
- Yu, Y.-Q.; Yang, X.; Wu, X.-F.; Fan, Y.-B. Enhancing Permeation of Drug Molecules Across the Skin via Delivery in Nanocarriers: Novel Strategies for Effective Transdermal Applications. Front. Bioeng. Biotechnol. 2021, 9, 646554. [Google Scholar] [CrossRef]
- Safta, D.A.; Bogdan, C.; Moldovan, M.-L. SLNs and NLCs for Skin Applications: Enhancing the Bioavailability of Natural Bioactives. Pharmaceutics 2024, 16, 1270. [Google Scholar] [CrossRef] [PubMed]
- Fathi, F.; Machado, T.O.X.; Kodel, H.d.A.C.; Portugal, I.; Ferreira, I.O.; Zielinska, A.; Oliveira, M.B.P.P.; Souto, E.B. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) for the delivery of bioactives sourced from plants: Part I—Composition and production methods. Expert Opin. Drug Deliv. 2024, 21, 1479–1490. [Google Scholar] [CrossRef] [PubMed]
- Biswas, U.K.; Sen, S.; Sharma, S.; Paul, M.; Nayak, A.K.; Bose, A. Nanostructured Lipid Carrier-Based Topical Gels as Novel Drug Delivery System: A Comprehensive Overview. Curr. Drug Deliv. 2024, 22, 1092–1111. [Google Scholar] [CrossRef]
- Kaul, S.; Gulati, N.; Verma, D.; Mukherjee, S.; Nagaich, U. Role of Nanotechnology in Cosmeceuticals: A Review of Recent Advances. J. Pharm. 2018, 3420204. [Google Scholar] [CrossRef]
- Ritwiset, A.; Krongsuk, S.; Johns, J.R. Molecular structure and dynamical properties of niosome bilayers with and without cholesterol incorporation: A molecular dynamics simulation study. Appl. Surf. Sci. 2016, 380, 23–31. [Google Scholar] [CrossRef]
- Arshad, N.; Shaheen, F.; Khan, I.N.; Naeem, S.; Riaz, M.; Siddique, M.I.; Ayesha, M.; Waqar, M.A. A comprehensive review on niosomes: Novel manufacturing techniques, factors influencing formation, applications and recent advances. Int. J. Polym. Mater. Polym. Biomater. 2024, 74, 1331–1348. [Google Scholar] [CrossRef]
- Musielak, E.; Krajka-Kuźniak, V. Liposomes and Ethosomes: Comparative Potential in Enhancing Skin Permeability for Therapeutic and Cosmetic Applications. Cosmetics 2024, 11, 191. [Google Scholar] [CrossRef]
- Kaur, P.; Verma, S.; Tomar, B.; Vyas, M.; Kakoty, V.; Saha, P.; Chandran, S.K. Exploring Applications of Flexible Vesicular Systems as Transdermal Drug Delivery. Curr. Drug Deliv. 2023, 21, 1062–1072. [Google Scholar] [CrossRef]
- Hameed, H.; Faheem, S.; Khan, M.A.; Hameed, A.; Ereej, N.; Ihsan, H. Ethosomes: A potential nanovesicular carrier to enhancing the drug delivery against skin barriers. J. Microencapsul. 2024, 41, 204–225. [Google Scholar] [CrossRef]
- Jahan, S.; Ali, A.; Sultana, N.; Qizilbash, F.F.; Ali, H.; Aqil, M.; Mujeeb, M.; Ali, A. An overview of phospholipid enriched-edge activator-based vesicle nanocarriers: New paradigms to treat skin cancer. J. Drug Target. 2024, 33, 17–41. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, A.; Elumalai, V.; Balasubramaniyam, S.; Elumalai, K. Transferosome Formulations as Innovative Carriers for Transdermal Drug Delivery: Composition, Properties, and Therapeutic Applications. Biomed. Mater. Devices 2025, 23, 1–27. [Google Scholar] [CrossRef]
- Madrid, R.R.; Mathews, P.D.; Pramanik, S.; Mangiarotti, A.; Fernandes, R.; Itri, R.; Dimova, R.; Mertins, O. Hybrid crystalline bioparticles with nanochannels encapsulating acemannan from Aloe vera: Structure and interaction with lipid membranes. J. Colloid Interface Sci. 2024, 673, 373–385. [Google Scholar] [CrossRef]
- Varshney, K.; Mazumder, R.; Rani, A.; Pandey, P.; Babu, M.R. Liquid Crystalline Lipid Nanoparticles: Emerging Trends and Applications in Skin Cancer. Pharm. Nanotechnol. 2024, 12. [Google Scholar] [CrossRef]
- Xu, X.; Tang, Q.; Gao, Y.; Chen, S.; Yu, Y.; Qian, H.; McClements, D.J.; Cao, C.; Yuan, B. Recent developments in the fabrication of food microparticles and nanoparticles using microfluidic systems. Crit. Rev. Food Sci. Nutr. 2024, 65, 2199–2213. [Google Scholar] [CrossRef] [PubMed]
- Romes, N.B.; Wahab, R.A.; Hamid, M.A. The role of bioactive phytoconstituents-loaded nanoemulsions for skin improvement: A review. Biotechnol. Biotechnol. Equip. 2021, 35, 711–730. [Google Scholar] [CrossRef]
- Bonifácio, B.V.; da Silva, P.B.; Aparecido dos Santos Ramos, M.; Maria Silveira Negri, K.; Maria Bauab, T.; Chorilli, M. Nanotechnology-based drug delivery systems and herbal medicines: A review. Int. J. Nanomed. 2013, 9, 1–15. [Google Scholar] [CrossRef]
- Qu, F.; Geng, R.; Liu, Y.; Zhu, J. Advanced nanocarrier- and microneedle-based transdermal drug delivery strategies for skin diseases treatment. Theranostics 2022, 12, 3372–3406. [Google Scholar] [CrossRef]
- Salvioni, L.; Morelli, L.; Ochoa, E.; Labra, M.; Fiandra, L.; Palugan, L.; Prosperi, D.; Colombo, M. The emerging role of nanotechnology in skincare. Adv. Colloid Interface Sci. 2021, 293, 102437. [Google Scholar] [CrossRef]
- Bilal, M.; Iqbal, H.M.N. New Insights on Unique Features and Role of Nanostructured Materials in Cosmetics. Cosmetics 2020, 7, 24. [Google Scholar] [CrossRef]
- Chaouat, C.; Balayssac, S.; Malet-Martino, M.; Belaubre, F.; Questel, E.; Schmitt, A.M.; Poigny, S.; Franceschi, S.; Perez, E. Green microparticles based on a chitosan/lactobionic acid/linoleic acid association. Characterisation and evaluation as a new carrier system for cosmetics. J. Microencapsul. 2017, 34, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Piccinino, D.; Capecchi, E.; Trifero, V.; Tomaino, E.; Marconi, C.; Del Giudice, A.; Galantini, L.; Poponi, S.; Ruggieri, A.; Saladino, R. Lignin Nanoparticles as Sustainable Photoprotective Carriers for Sunscreen Filters. ACS Omega 2022, 7, 37070–37077. [Google Scholar] [CrossRef] [PubMed]
- Dghoughi, A.; Raji, M.; Qaiss, A.e.K. Synthesis and sustainable applicability of triangular lignin nanoparticles in sunscreen formulations and UV shielding. New J. Chem. 2025, 49, 9145–9156. [Google Scholar] [CrossRef]
- Widsten, P.; Tamminen, T.; Liitiä, T. Natural Sunscreens Based on Nanoparticles of Modified Kraft Lignin (CatLignin). ACS Omega 2020, 5, 13438–13446. [Google Scholar] [CrossRef] [PubMed]
- Miletić, A.; Pavlić, B.; Ristić, I.; Zeković, Z.; Pilić, B. Encapsulation of Fatty Oils into Electrospun Nanofibers for Cosmetic Products with Antioxidant Activity. Appl. Sci. 2019, 9, 2955. [Google Scholar] [CrossRef]
- Zivic, F.; Grujovic, N.; Mitrovic, S.; Ahad, I.U.; Brabazon, D. Characteristics and Applications of Silver Nanoparticles. In Commercialization of Nanotechnologies—A Case Study Approach; Springer: Berlin/Heidelberg, Germany, 2018; pp. 227–273. [Google Scholar] [CrossRef]
- Budzianowska, A.; Bana’sbana’s, K.; Budzianowski, J.; Kikowska, M. Antioxidants to Defend Healthy and Youthful Skin—Current Trends and Future Directions in Cosmetology. Appl. Sci. 2025, 15, 2571. [Google Scholar] [CrossRef]
- Ahmad Kuthi, N.; Basar, N.; Chandren, S. Nanonutrition- and nanoparticle-based ultraviolet rays protection of skin. Adv. Nanotechnol.-Based Drug Deliv. Syst. 2022, 227–280. [Google Scholar] [CrossRef]
- Smijs, T.G.; Pavel, S. Titanium dioxide and zinc oxide nanoparticles in sunscreens: Focus on their safety and effectiveness. Nanotechnol. Sci. Appl. 2011, 4, 95–112. [Google Scholar] [CrossRef]
- Abu Hajleh, M.N.; Abu-Huwaij, R.; Al-Samydai, A.; Al-Halaseh, L.K.; Al-Dujaili, E.A. The revolution of cosmeceuticals delivery by using nanotechnology: A narrative review of advantages and side effects. J. Cosmet. Dermatol. 2021, 20, 3818–3828. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, V.; Yadav, P.; Porwal, M.; Sur, S.; Verma, A. Dendrimer as nanocarrier for drug delivery and drug targeting therapeutics: A fundamental to advanced systematic review. Int. J. Polym. Mater. Polym. Biomater. 2024, 73, 310–332. [Google Scholar] [CrossRef]
- Chary, P.S.; Shaikh, S.; Bhavana, V.; Rajana, N.; Vasave, R.; Mehra, N.K. Emerging role of nanocrystals in pharmaceutical applications: A review of regulatory aspects and drug development process. Appl. Mater. Today 2024, 40, 102334. [Google Scholar] [CrossRef]
- Dos Santos, Z.M.Q.; Zan, F.R.; Rech, V.C.; Ourique, A.F. Topical Nanotherapeutics for the Treatment of Acne Vulgaris. In Novel Nanocarriers for Skin Diseases; Academic Press: Cambridge, MA, USA, 2025; pp. 39–97. [Google Scholar]
- Chauhan, S.; Jain, N.; Nagaich, U. Nanodiamonds with powerful ability for drug delivery and biomedical applications: Recent updates on in vivo study and patents. J. Pharm. Anal. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Mohapatra, S.; Mishra, H.; Farooq, U.; Kumar, K.; Ansari, M.J.; Aldawsari, M.F.; Alalaiwe, A.S.; Mirza, M.A.; Iqbal, Z. Nanotechnology in Cosmetics and Cosmeceuticals—A Review of Latest Advancements. Gels 2022, 8, 173. [Google Scholar] [CrossRef] [PubMed]
- Jariani, P.; Shahnejat-Bushehri, A.-A.; Naderi, R.; Zargar, M.; Naghavi, M.R. Molecular and Phytochemical Characteristics of Flower Color and Scent Compounds in Dog Rose (Rosa canina L.). Molecules 2024, 29, 3145. [Google Scholar] [CrossRef]
- Espacenet—Patent search. Available online: https://worldwide.espacenet.com/ (accessed on 17 December 2024).
- Rajan, R. Sunscreen Metrics and Labelling Guidelines. In Sunscreens for Skin of Color; Springer: Singapore, 2024; pp. 129–158. [Google Scholar] [CrossRef]
- Sahu, A.; Srivastava, N.; Jena, D.; Singh, A.; Kumar, S.; Chauhan, I. Important Regulatory Guidelines on Natural Products. Curr. Tradit. Med. 2023, 10, 157–172. [Google Scholar] [CrossRef]
- Intellectual Property. Available online: https://iprsearch.ipindia.gov.in/publicsearch (accessed on 17 December 2024).
- Google Patents. Available online: https://patents.google.com/ (accessed on 17 December 2024).
- Rao, T.; Kesharwani, R.; Keservani, R.; Sharma, A. Formulations, Regulations, and Challenges of Nutraceuticals. 2024. Available online: https://books.google.com/books?hl=en&lr=&id=dBcEEQAAQBAJ&oi=fnd&pg=PT13&dq=In+Canada,+sunscreen+products+are+classified+as+either+prescription+drugs+or+natural+health+products+(NHPs)+depending+on+their+ingredients.+Products+containing+ingredients+like+titanium+dioxide&ots=P_YurZ9XPj&sig=9B6wAn3-lMWlUD5znozmRHftSag (accessed on 17 December 2024).
- Ekstein, S.F.; Hylwa, S. Sunscreens: A Review of UV Filters and Their Allergic Potential. Dermatitis 2023, 34, 176–190. [Google Scholar] [CrossRef]
- Lacourt, C.; Mukherjee, K.; Garthoff, J.; O’SUllivan, A.; Meunier, L.; Fattori, V. Recent and emerging food packaging alternatives: Chemical safety risks, current regulations, and analytical challenges. Compr. Rev. Food Sci. Food Saf. 2024, 23, e70059. [Google Scholar] [CrossRef]
- Salih, H.; Psomadakis, C.; George, S.M.C. Sunscreens: A narrative review. Ski. Health Dis. 2024, 4, e432. [Google Scholar] [CrossRef] [PubMed]
- Stras, A.; Grassmann, A.; Van Campenhout, P.; Deconinck, E.; Vanhaecke, T.; Desmedt, B. Analysis of preservatives and fragrances in topical medical devices: The need for more stringent regulation. Contact Dermat. 2024, 90, 594–606. [Google Scholar] [CrossRef] [PubMed]
- Osterwalder, U. Percutaneous Penetration. In Textbook of Cosmetic Dermatology; CRC Press: Boca Raton, FL, USA, 2024; pp. 151–166. [Google Scholar]
- Nizam, V.P.M.; Yerram, S.; Patnam, J.D.; CSA; Aglave, G.; Joga, R.; Raghuvanshi, R.S.; Srivastava, S. Ensuring Product Safety: A Comprehensive Retrospective Study of USFDA Drug Recalls (2019–2023). J. Pharm. Innov. 2024, 1, 19. [Google Scholar] [CrossRef]
- Nandini, O.B.; Subash Chandran, M.P.; Prasobh, G.R.; Varsha, V.R.; Najuma Shajahan Renjitha, R.S.A. World Journal of Pharmaceutical Research. Rev. Sunscreens 2024, 13, 111–129. [Google Scholar]
- Solaiman, S.M.; Algie, J.; Bakand, S.; Sluyter, R.; Sencadas, V.; Lerch, M.; Huang, X.-F.; Konstantinov, K.; Barker, P.J. Nano-sunscreens—A double-edged sword in protecting consumers from harm: Viewing Australian regulatory policies through the lenses of the European Union. Crit. Rev. Toxicol. 2019, 49, 122–139. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Pavelko, R.; Ford, H.; Barrows, S.; Donohue, S.; Stapel, M. Humanizing cancer: The role of anthropomorphism and perceived efficacy in melanoma prevention. Health Mark. Q. 2024, 41, 476–497. [Google Scholar] [CrossRef]
- Breakell, T.; Kowalski, I.; Foerster, Y.; Kramer, R.; Erdmann, M.; Berking, C.; Heppt, M.V. Ultraviolet Filters: Dissecting Current Facts and Myths. J. Clin. Med. 2024, 13, 2986. [Google Scholar] [CrossRef]
- Basir, S.A.; Hasmin, N.A.; Othman, M.R.; Munir, A.B. Nanocosmetics Labelling Framework for Malaysia with Reference to the EU. J. Consum. Policy 2024, 47, 165–195. [Google Scholar] [CrossRef]
- Thakur, N.; Thakur, N.; Kumar, A.; Thakur, V.K.; Kalia, S.; Arya, V.; Kumar, A.; Kumar, S.; Kyzas, G.Z. A critical review on the recent trends of photocatalytic, antibacterial, antioxidant and nanohybrid applications of anatase and rutile TiO2 nanoparticles. Sci. Total Environ. 2024, 914, 169815. [Google Scholar] [CrossRef]
- Pathak, K.; Pattnaik, S.; Porwal, A. Regulatory Concerns for Nanomaterials in Sunscreen Formulations. Appl. Clin. Res. Clin. Trials Regul. Aff. 2018, 5, 99–111. [Google Scholar] [CrossRef]
- Xiao, Y.; Ahmad, S.F.; Irshad, M.; Guo, H.; Mahmoud, H.A.; Awwad, E.M.; Khan, Y. Investigating the mediating role of ethical issues and healthcare between the metaverse and mental health in Pakistan, China, and Saudi Arabia. Humanit. Soc. Sci. Commun. 2024, 11, 441. [Google Scholar] [CrossRef]
- Goh, C.L.; Wu, Y.; Welsh, B.; Abad-Casintahan, M.F.; Tseng, C.J.; Sharad, J.; Jung, S.; Puangpet, P.; Chan, H.N.; Kon, K. Challenges and real-world solutions for adoption of holistic skincare routine (cleansing, treatment, moisturization, and photoprotection) in acne, rosacea, atopic dermatitis, and sensitive skin: An expert consensus. J. Cosmet. Dermatol. 2024, 23, 2516–2523. [Google Scholar] [CrossRef] [PubMed]
- Boman, A.; Miguel, M.; Andersson, I.; Slunge, D. The effect of information about hazardous chemicals in consumer products on behaviour—A systematic review. Sci. Total. Environ. 2024, 947, 174774. [Google Scholar] [CrossRef]
- Oargă, D.P.; Cornea-Cipcigan, M.; Cordea, M.I. Unveiling the mechanisms for the development of rosehip-based dermatological products: An updated review. Front. Pharmacol. 2024, 15, 1390419. [Google Scholar] [CrossRef]
- Eom, T.; Ozlu, B.; Ivanová, L.; Lee, S.; Lee, H.J.; Krajčovič, J.; Shim, B.S. Multifunctional Natural and Synthetic Melanin for Bioelectronic Applications: A Review. Biomacromolecules 2024, 25, 5489–5511. [Google Scholar] [CrossRef]
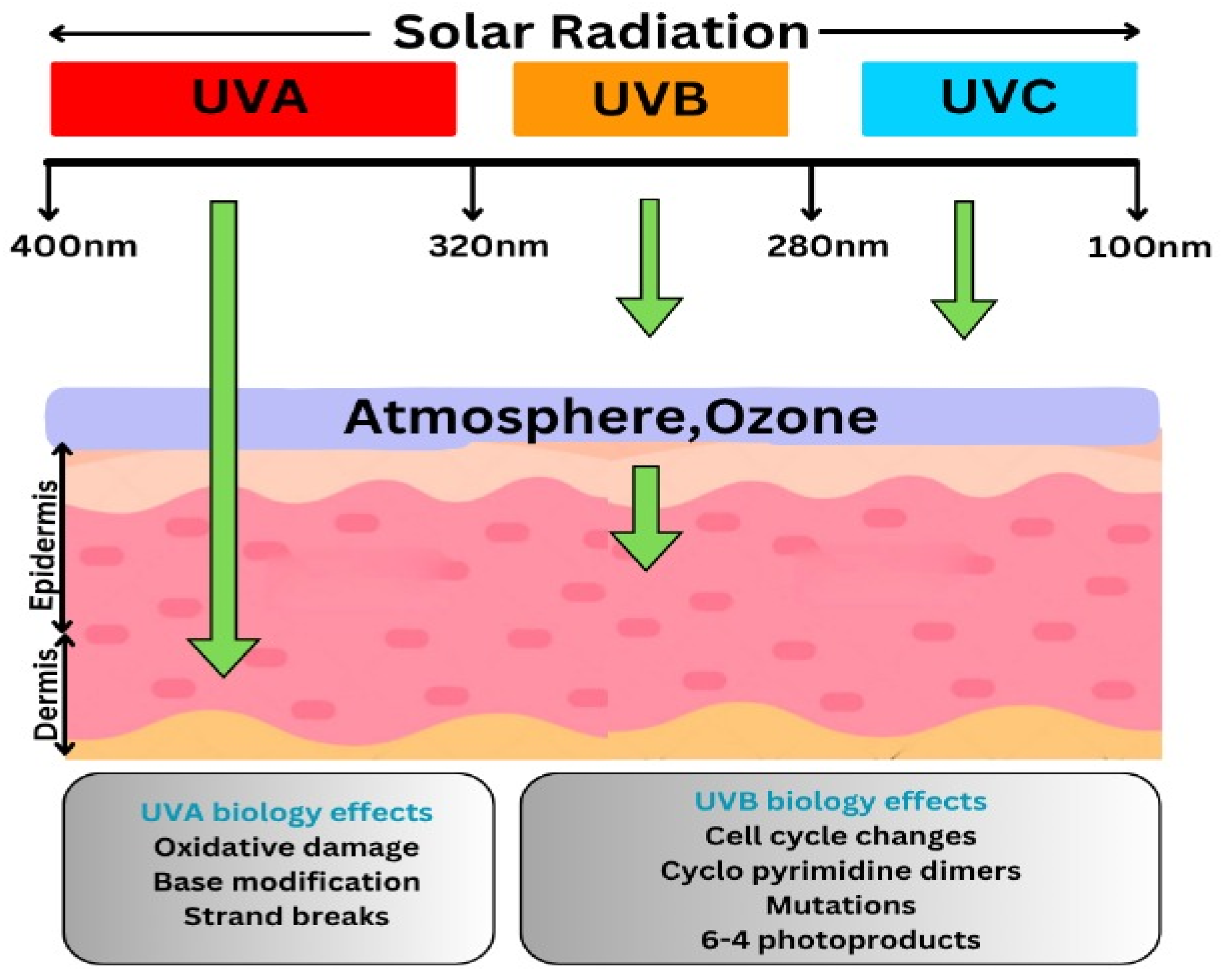
| Type of Ultraviolet Rays | Wavelength (nm) | Effects and Characteristics |
|---|---|---|
| Ultraviolet A (UVA) | 320–400 | Leads to premature skin aging, wrinkles, and cellular harm. Contributes to genetic mutations and plays a role in certain skin cancers. |
| Ultraviolet B (UVB) | 280–320 | Possesses slightly greater energy than UVA. Directly harms DNA in skin cells, leading to sunburn and a heightened risk of skin malignancies. |
| Ultraviolet C (UVC) | 200–280 | Extremely potent and hazardous. Naturally filtered out through ozone layer by preventing it from surface of earth. Man-made sources include welding torches, mercury vapor lamps, and UV disinfection bulbs, commonly used for eliminating microbes and pathogens. |
| Class | Usage (%) | INCI | Explanation | References |
|---|---|---|---|---|
| Helianthus annuus | 34 (7.7%) | Helianthus annuus Seed Oil | Extracted from sunflower seeds, Helianthus Annuus Seed Oil is derived from Helianthus annuus L., a member of the Compositae family. | [36] |
| Glycine max (oil) | 30 (6.8%) | Glycine soja oil. | Obtained through extraction or pressing, Glycine Soja Oil is derived from the soybean (Glycine soja, Leguminosae) and contains triglycerides of oleic, linoleic, and saturated fatty acids. | [37] |
| Glycine max (extract) | 3 (0.7%) | Glycine Soja Seed Extract | A natural extract obtained from soybean (Glycine soja, Leguminosae). | [38] |
| Vitellaria paradoxa | 16 (3.6%) | Butyrospermum parkii butter | Extract of the seedcake of the Shea Tree (Butyrospermum parkii, Sapotaceae). | [39] |
| Vitellaria paradoxa | 8 (1.8%) | Butyrospermum Parkii Butter Extract | Derived from the fruit of the Shea Tree (Butyrospermum parkii, Sapotaceae). | [40] |
| Vitellaria paradoxa | 2 (0.5%) | Butyrospermum Parkii Butter Seedcake Extract | Extract sourced from the Shea Tree (Butyrospermum parkii, Sapotaceae). | [41] |
| Persea gratissima | 11 (2.5%) | Persea Gratissima Fruit Extract | Extracted from the fruit of the avocado (Persea gratissima, Lauraceae). | [42] |
| Persea gratissima | 11 (2.5%) | Persea Gratissima Oil | Fixed oil obtained by pressing the dehydrated sliced flesh of the avocado pear (Persea gratissima, Lauraceae), consisting mainly of fatty acid glycerides. | [43] |
| Glycyrrhiza inflata | 21 (4.7%) | Glycyrrhiza Inflata Root Extract | Derived from the roots of Glycyrrhiza inflata (Leguminosae). | [43] |
| Tanacetum parthenium | 7 (1.6%) | Chrysanthemum parthenium extract | Extracted from the feverfew herb (Chrysanthemum parthenium, Asteraceae). | [44] |
| Tanacetum parthenium | 9 (2.0%) | Chrysanthemum parthenium Flower Extract | Sourced from the flowers of feverfew (Chrysanthemum parthenium, Asteraceae). | [45] |
| Scutellaria baicalensis | 8 (1.8%) | Scutellaria Baicalensis Extract | Extract derived from the whole plant Scutellaria baicalensis (Lamiaceae). | [46] |
| Scutellaria baicalensis | 8 (1.8%) | Scutellaria Baicalensis Root Extract | Obtained from the roots of Scutellaria baicalensis (Lamiaceae). | [47] |
| Aloe barbadensis | 2 (0.5%) | Aloe Barbadensis Leaf Extract | Derived from the leaves of aloe (Aloe barbadensis, Liliaceae). | [48] |
| Aloe barbadensis | 5 (1.1%) | Aloe Barbadensis Leaf Juice | Juice extracted from the leaves of Aloe barbadensis (Liliaceae). | [49] |
| Aloe barbadensis | 6 (1.4%) | Aloe Barbadensis Leaf Juice Powder | Powdered form obtained from the dried juice of Aloe barbadensis (Liliaceae). | [49] |
| Nanosystem | UV Protection | Skin Penetration | Environmental Risk | Biocompatibility |
|---|---|---|---|---|
| Liposomes | Moderate | High | Low | High |
| SLNs/NLCs | High | Moderate | Moderate | High |
| Dendrimers | Very High | Very High | Unknown | Variable |
| Nanoemulsions | Moderate | High | Moderate | High |
| Patent No. | Date of Patent | Work | Reference |
|---|---|---|---|
| 202441088941 | 22/11/2024 | Formulation design of herbal gel-based sunscreen containing silymarin from milk thistle and other ingredients to enhance UV protection and other applications. | [91] |
| 202441081252 | 25/10/2024 | A method of evaluating herbal sunscreen cream | [91] |
| 202411071590 | 22/09/2024 | Herbal-based sunscreen formulation and a method of preparation thereof | [91] |
| 202441056209 | 24/07/2024 | Herbal sunscreen formulation | [91] |
| 202441048923 | 26/06/2024 | Eco–friendly and biocomposite ZnO nanoparticle production for sunscreen and therapeutic formulations | [91] |
| 202411048040 | 21/06/2024 | Herbal sunscreen formulation comprising cucumber, tomato, and aloe vera extracts | [91] |
| 202412033703 | 28/04/2024 | Nano-lignin-based sunscreen composition, and a method of preparation thereof | [91] |
| 202411019446 | 16/03/2024 | Nano-sunscreen cream with ursolic acid-loaded solid lipid nanoparticles and method thereof | [91] |
| 202417013840 | 26/02/2024 | Sunscreen or daily care composition comprising bis-ethylhexyloxyphenol, methoxyphenyl triazine, and inorganic UV filters | [91] |
| 202421011132 | 22/03/2024 | Formulation and evaluation of sunscreen stick | [91] |
| 202441008561 | 23/02/2024 | Quercetin-loaded nanoliposomal UVA and UVB sunscreen cream formulation method | [91] |
| 202341090278 | 31/12/2023 | A green synthesis of sunscreen lotion and its product thereof | [91] |
| 202341086711 | 19/12/2023 | Sunscreen jacket | [91] |
| US2024385034A1 | 2024-11-21 | Protective band to prevent skin damage to drivers | [92] |
| WO2024233498A1 | 2024-11-14 | Water-in-oil emulsion metal oxide-based sunscreen formulations | [88] |
| KR102725430B1 | 2024-11-06 | Cosmetic composition for sunscreen containing lignin modified with a silane-based surface modifier | [88] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khobragade, R.; Chaudhary, A.A.; Ali, M.A.M.; Kale, M.; Raut, N.; Ghive, P.; Rudayni, H.A.; Nagpurkar, K.; Umekar, M.; Trivedi, R. Nanotechnology-Enhanced Sunscreens: Balancing Efficacy, Safety, and Environmental Impact. Pharmaceutics 2025, 17, 1080. https://doi.org/10.3390/pharmaceutics17081080
Khobragade R, Chaudhary AA, Ali MAM, Kale M, Raut N, Ghive P, Rudayni HA, Nagpurkar K, Umekar M, Trivedi R. Nanotechnology-Enhanced Sunscreens: Balancing Efficacy, Safety, and Environmental Impact. Pharmaceutics. 2025; 17(8):1080. https://doi.org/10.3390/pharmaceutics17081080
Chicago/Turabian StyleKhobragade, Ruchi, Anis Ahmad Chaudhary, Mohamed A. M. Ali, Mayur Kale, Neha Raut, Pratik Ghive, Hassan A. Rudayni, Krutika Nagpurkar, Milind Umekar, and Rashmi Trivedi. 2025. "Nanotechnology-Enhanced Sunscreens: Balancing Efficacy, Safety, and Environmental Impact" Pharmaceutics 17, no. 8: 1080. https://doi.org/10.3390/pharmaceutics17081080
APA StyleKhobragade, R., Chaudhary, A. A., Ali, M. A. M., Kale, M., Raut, N., Ghive, P., Rudayni, H. A., Nagpurkar, K., Umekar, M., & Trivedi, R. (2025). Nanotechnology-Enhanced Sunscreens: Balancing Efficacy, Safety, and Environmental Impact. Pharmaceutics, 17(8), 1080. https://doi.org/10.3390/pharmaceutics17081080








