Solid Lipid Nanoparticles and Nanostructured Lipid Carriers for Anticancer Phytochemical Delivery: Advances, Challenges, and Future Prospects
Abstract
1. Introduction
2. Phytochemicals with Antitumor Properties
2.1. Reseveratrol
2.2. Curcumin
2.3. Berberine
2.4. Camptothecin
2.5. Vincristine, Vinblastine, and Paclitaxel
2.6. Epigallocatechin-3-Gallate
2.7. Genistein
2.8. Quercetin
2.9. Thymoquinone
2.10. Betalains
2.11. Ursolic Acid
2.12. Sulforaphane
2.13. Plumbagin
2.14. Lycopene
2.15. β-Lapachone
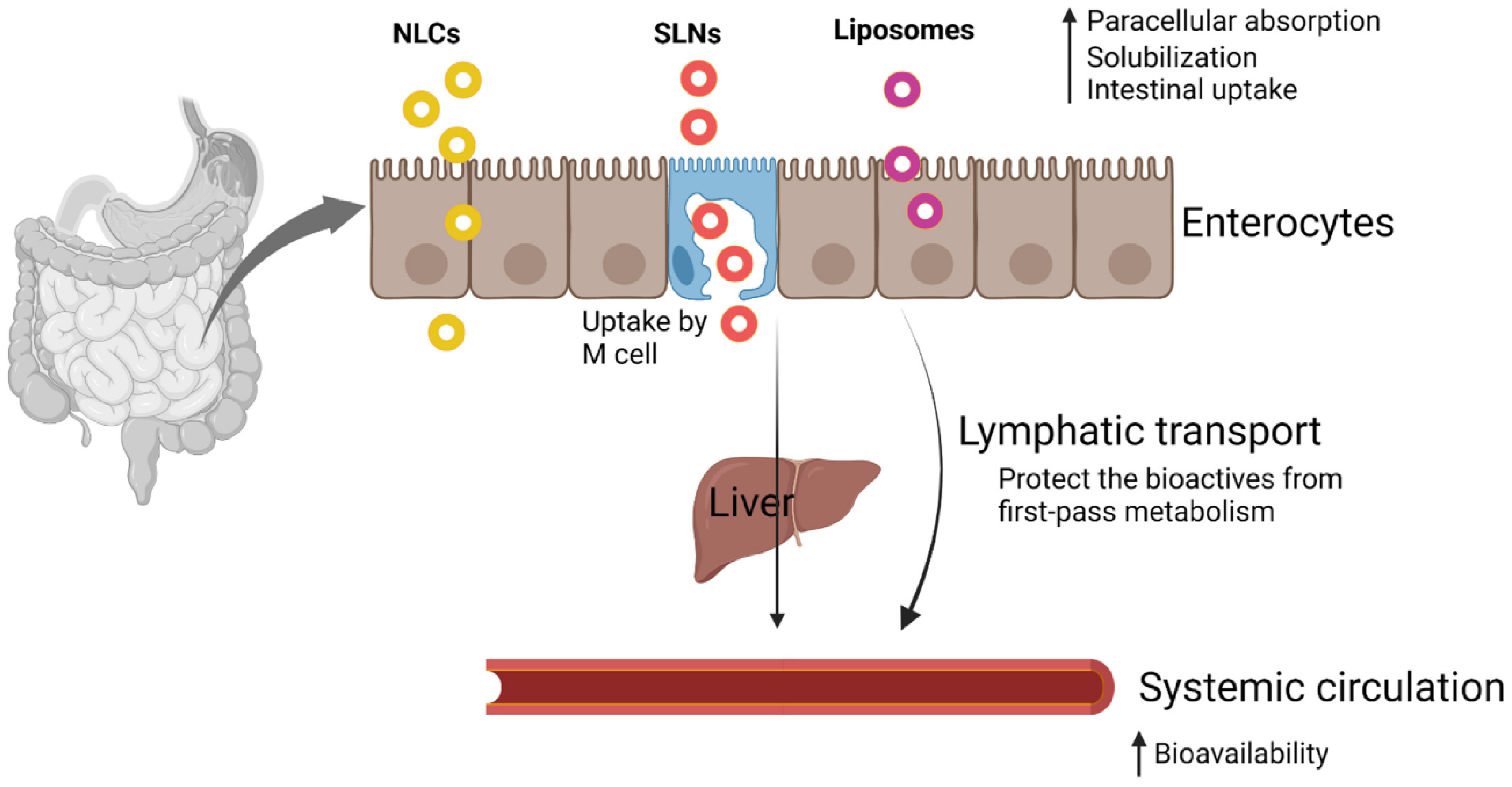
3. Oral Bioavailability and Stability Issues
4. Nanoparticle-Based Approaches for Phytochemical Delivery
5. Functionalization of Nanoparticles
6. Lipid-Based Nanoparticles
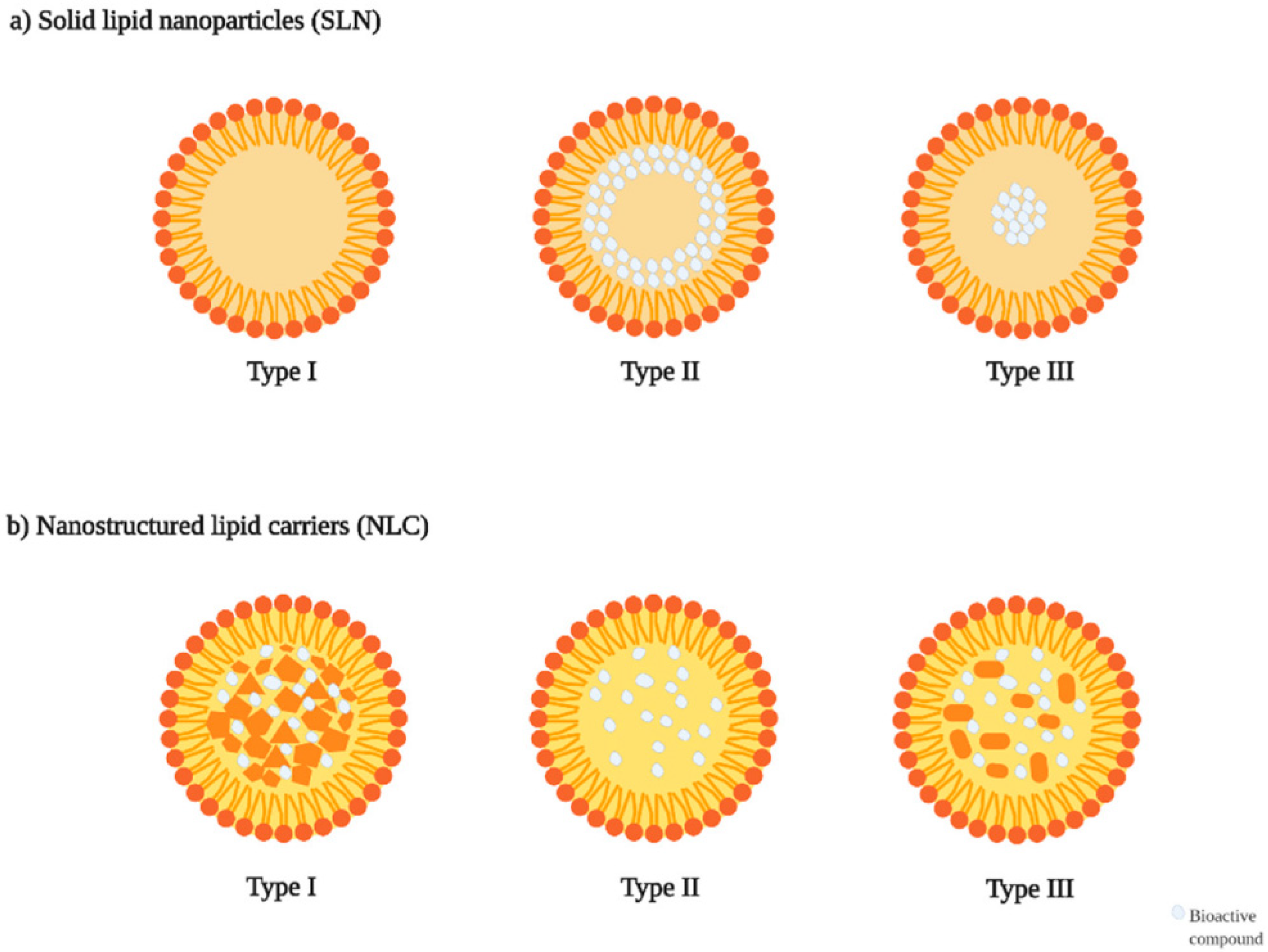
7. Solid Lipid Nanoparticles (SLNs)
7.1. Structural Components of SLNs
7.2. Role of SLNs for Anticancer Phytochemical Delivery
| Phytochemicals | Preparation Method | Composition | Therapeutic Application | Highlights | Reference |
|---|---|---|---|---|---|
| Allicin | High-pressure homogenization | Stearic acid, Tween 80 and lecithin | Lung cancer | The formulated allicin-loaded SLNs measured 67.01 nm in size with a surface charge of −29.29 mV. SLNs significantly reduced the viability of A549 cancer cells after 48 h, while normal HFF cells remained unaffected. Flow cytometry analysis indicated an increase in the subG1 peak and a notable upregulation of caspase-3 and caspase-9, with minimal impact on caspase-8, highlighting an intrinsic apoptotic pathway. Additionally, the SLNs exhibited strong antioxidant activity, effectively inhibiting ABTS and DPPH free radicals. | [182] |
| Lawsone | Hot homogenization | Precirol®, Tween 80, Poloxamer 407 | Lung carcinoma | The mean diameters of free SLNs and phyto-constituent SLNs were 97 ± 1.4 nm and 127 ± 3.1 nm, respectively. Developed SLNs exhibited high encapsulation efficiency (95.88 ± 3.29%) and drug loading capacity of 22.72 ± 1.39 mg/mL. Cytotoxicity assays showed that plain lawsone inhibited A549 cell growth with IC50 values of 17.99 ± 1.11, 13.37 ± 1.22, and 9.21 ± 1.15 μg/mL at 24, 48, and 72 h, respectively. The SLNs exhibited stronger cytotoxic effects after 48 h (IC50: 9.81 ± 1.3 μg/mL). SLNs (13.37 ± 1.22 μg/mL) induced ~52% apoptosis and necrosis after 48 h. qPCR results indicated Bcl-2 downregulation and caspase-9 upregulation, confirming apoptosis induction in A549 cells. | [183] |
| Lycopene | Hot homogenization and ultrasonication | Stearic acid, Poloxamer 407, Soy phosphatidylcholine/Soy lecithin. | Antioxidant and Anti-melanogenic | The particle size analysis of lycopene-loaded SLNs revealed an average size of 151.1 ± 2.3 nm. Electron microscopy examination confirmed that the nanoparticles were spherical, with an encapsulation efficiency of 85.76 ± 2.75%. Assessment of the anti-tyrosinase effects of SLNs demonstrated a significant reduction in cellular tyrosinase activity, melanin synthesis, and reactive oxygen species levels. Notably, SLNs effectively inhibited melanin production while exhibiting minimal toxicity toward melanoma cells. | [184] |
| Morus alba extract | High-pressure homogenization | -- | Cytotoxicity | The optimized SLNs exhibited favorable physicochemical properties and significantly enhanced cytotoxicity and apoptosis compared to extract alone (p < 0.05) using MDA-MB231 cell line. They effectively disrupted DNA replication and cell division by inhibiting the S (9.7 ± 1.7%) and G2/M (2.2 ± 0.6%) phases. The apoptosis rate was notably higher (p < 0.05 in SLNs (81.46%) than in extract alone (72.49%), confirming their superior therapeutic potential. | [185] |
| Thymoquinone | Oil-in-water microemulsion | -- | Brain malignancies | Thymoquinone-encapsulated Eudragit L100-coated SLNs released the highest drug content (78.215 ± 0.749%) at pH 5.5 after 22 h. Pharmacokinetic and biodistribution studies indicated that, 48 h post-administration, drug accumulated in various organs, including the brain (16.5 ± 1.5%), kidneys (21.167 ± 1.041%), heart (12.125 ± 0.781%), liver (16.375 ± 1.317%), lungs (13.5 ± 1.8%), and another unspecified tissue (17.15 ± 1.5%). Molecular modeling demonstrated that thymoquinone exhibited strong binding affinity to EGFR (−7.8 kcal/mol), comparable to the reference drug temozolomide. | [186] |
| Curcumin | High shear homogenization | Cholesterol, Poloxamer-188 | Breast cancer | The optimized formulation (Chol-CUR SLN) exhibited a uniform particle size of 166.4 ± 3.5 nm and a high encapsulation efficiency of 76.9 ± 1.9%. In vitro experiments on MDA-MB-231 human breast cancer cells demonstrated enhanced cellular uptake and significantly greater cytotoxicity for Chol-CUR SLNs compared to free curcumin. Additionally, exhibited markedly higher levels of apoptosis, indicating its improved therapeutic potential. | [187] |
7.3. Co-Loaded Phytochemicals in SLNs
8. Nanostructured Lipid Carriers (NLCs)
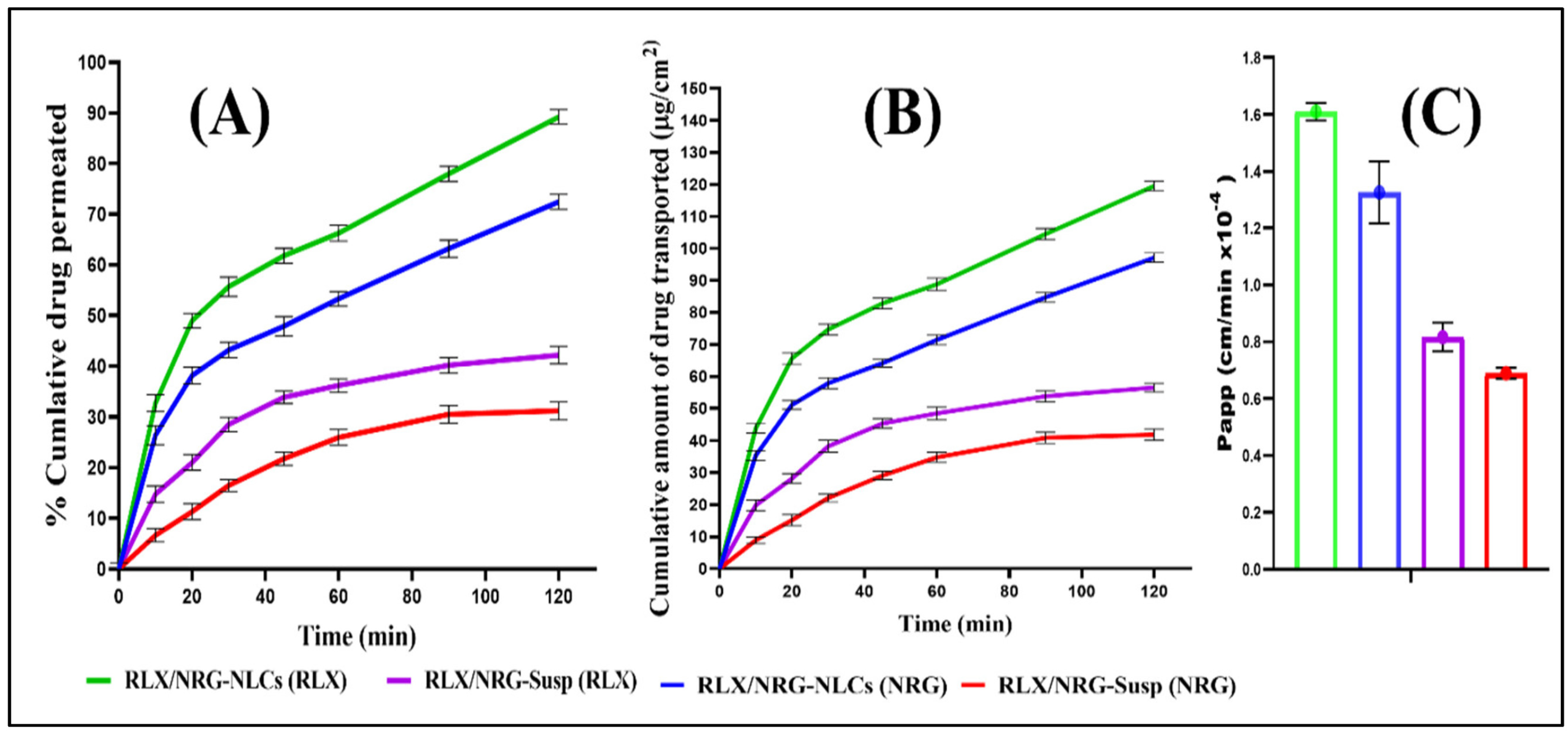
8.1. Role of NLCs for Anticancer Phytochemical Delivery
8.2. Co-Loaded Phytochemicals in NLCs
9. Preparation Methods
10. In Vitro Characterization Techniques
11. Clinical Trials, Patents and Regulatory Aspects
12. Advancements, Challenges, and Future Directions
13. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elmowafy, M.; Shalaby, K.; Elkomy, M.H.; Alsaidan, O.A.; Gomaa, H.A.M.; Abdelgawad, M.A.; Mostafa, E.M. Polymeric Nanoparticles for Delivery of Natural Bioactive Agents: Recent Advances and Challenges. Polymers 2023, 15, 1123. [Google Scholar] [CrossRef]
- Hossain, M.S.; Wazed, M.A.; Asha, S.; Amin, M.R.; Shimul, I.M. Dietary Phytochemicals in Health and Disease: Mechanisms, Clinical Evidence, and Applications—A Comprehensive Review. Food Sci. Nutr. 2025, 13, e70101. [Google Scholar] [CrossRef]
- Patra, S.; Gorai, S.; Pal, S.; Ghosh, K.; Pradhan, S.; Chakrabarti, S. A review on phytoestrogens: Current status and future direction. Phytother. Res. PTR 2023, 37, 3097–3120. [Google Scholar] [CrossRef]
- Yang, Y.; Ling, W. Health Benefits and Future Research of Phytochemicals: A Literature Review. J. Nutr. 2025, 155, 87–101. [Google Scholar] [CrossRef] [PubMed]
- AlSheikh, H.M.A.; Sultan, I.; Kumar, V.; Rather, I.A.; Al-Sheikh, H.; Tasleem Jan, A.; Haq, Q.M.R. Plant-Based Phytochemicals as Possible Alternative to Antibiotics in Combating Bacterial Drug Resistance. Antibiotics 2020, 9, 480. [Google Scholar] [CrossRef]
- Velmurugan, B.K.; Rathinasamy, B.; Lohanathan, B.P.; Thiyagarajan, V.; Weng, C.F. Neuroprotective Role of Phytochemicals. Molecules 2018, 23, 2485. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.M.A.; Akram, M.; Riaz, M.; Munir, N.; Rasool, G. Cardioprotective Potential of Plant-Derived Molecules: A Scientific and Medicinal Approach. Dose-Response 2019, 17, 1559325819852243. [Google Scholar] [CrossRef]
- Paudel, S.; Mishra, N.; Agarwal, R. Phytochemicals as Immunomodulatory Molecules in Cancer Therapeutics. Pharmaceuticals 2023, 16, 1652. [Google Scholar] [CrossRef]
- Chakraborty, N.; Banerjee, A.; Sarkar, A.; Ghosh, S.; Acharya, K. Mushroom polysaccharides: A potent immune-modulator. Biointerface Res. Appl. Chem. 2021, 11, 8915–8930. [Google Scholar]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Porfyris, O.; Detopoulou, P.; Adamantidi, T.; Tsoupras, A.; Papageorgiou, D.; Ioannidis, A.; Rojas Gil, A.P. Phytochemicals as Chemo-Preventive and Therapeutic Agents Against Bladder Cancer: A Comprehensive Review. Diseases 2025, 13, 103. [Google Scholar] [CrossRef] [PubMed]
- Woldeselassie, M.; Tamene, A. Therapeutic controversies over use of antioxidant supplements during cancer treatment: A scoping review. Front. Nutr. 2024, 11, 1480780. [Google Scholar] [CrossRef] [PubMed]
- Esteller, M.; Dawson, M.A.; Kadoch, C.; Rassool, F.V.; Jones, P.A.; Baylin, S.B. The Epigenetic Hallmarks of Cancer. Cancer Discov. 2024, 14, 1783–1809. [Google Scholar] [CrossRef]
- Kar, A.; Agarwal, S.; Singh, A.; Bajaj, A.; Dasgupta, U. Insights into molecular mechanisms of chemotherapy resistance in cancer. Transl. Oncol. 2024, 42, 101901. [Google Scholar] [CrossRef] [PubMed]
- Elshaer, M.; Chen, Y.; Wang, X.J.; Tang, X. Resveratrol: An overview of its anti-cancer mechanisms. Life Sci. 2018, 207, 340–349. [Google Scholar] [CrossRef]
- Harper, C.E.; Patel, B.B.; Wang, J.; Arabshahi, A.; Eltoum, I.A.; Lamartiniere, C.A. Resveratrol suppresses prostate cancer progression in transgenic mice. Carcinogenesis 2007, 28, 1946–1953. [Google Scholar] [CrossRef]
- Joshi, P.; Joshi, S.; Semwal, D.; Bisht, A.; Paliwal, S.; Dwivedi, J.; Sharma, S. Curcumin: An Insight into Molecular Pathways Involved in Anticancer Activity. Mini-Rev. Med. Chem. 2021, 21, 2420–2457. [Google Scholar] [CrossRef] [PubMed]
- Molla, S.; Hembram, K.C.; Chatterjee, S.; Nayak, D.; Sethy, C.; Pradhan, R.; Kundu, C.N. PARP inhibitor Olaparib Enhances the Apoptotic Potentiality of Curcumin by Increasing the DNA Damage in Oral Cancer Cells through Inhibition of BER Cascade. Pathol. Oncol. Res. POR 2020, 26, 2091–2103. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Du, X.; Ma, H.; Yao, J. The Anti-Cancer Mechanisms of Berberine: A Review. Cancer Manag. Res. 2020, 12, 695–702. [Google Scholar] [CrossRef]
- Piya, S.; Andreeff, M.; Borthakur, G. Targeting autophagy to overcome chemoresistance in acute myleogenous leukemia. Autophagy 2017, 13, 214–215. [Google Scholar] [CrossRef]
- Bacherikov, V.A. Total Synthesis, Mechanism of Action, and Antitumor Efficacy of Camptothecin and Some of its Analogues. Anticancer Agents Med. Chem. 2022, 22, 3438–3465. [Google Scholar] [CrossRef] [PubMed]
- Shamanna, R.A.; Lu, H.; Croteau, D.L.; Arora, A.; Agarwal, D.; Ball, G.; Aleskandarany, M.A.; Ellis, I.O.; Pommier, Y.; Madhusudan, S.; et al. Camptothecin targets WRN protein: Mechanism and relevance in clinical breast cancer. Oncotarget 2016, 7, 13269–13284. [Google Scholar] [CrossRef]
- Sadiq, S.C.; Joy, M.P.; Aiswarya, S.U.; Ajmani, A.; Keerthana, C.K.; Rayginia, T.P.; Isakov, N.; Anto, R.J. Unlocking nature’s pharmacy: An in-depth exploration of phytochemicals as potential sources of anti-cancer and anti-inflammatory molecules. Explor. Drug Sci. 2024, 2, 744–784. [Google Scholar] [CrossRef]
- Chimento, A.; De Luca, A.; D’Amico, M.; De Amicis, F.; Pezzi, V. The Involvement of Natural Polyphenols in Molecular Mechanisms Inducing Apoptosis in Tumor Cells: A Promising Adjuvant in Cancer Therapy. Int. J. Mol. Sci. 2023, 24, 1680. [Google Scholar] [CrossRef]
- Riaz, A.; Zara, R.; Bushra, G.; Kanwal, N.; Sadiqa, A.; Shareef, F.; Sarfraz, I.; Shah, M.A.; Ucak, I.; Bukhari, S.A. Cancer metabolism regulation by phytonutrients. In The Role of Phytonutrients in Metabolic Disorders; Elsevier: Amsterdam, The Netherlands, 2022; pp. 237–290. [Google Scholar]
- Jacob, S.; Kather, F.S.; Morsy, M.A.; Boddu, S.H.S.; Attimarad, M.; Shah, J.; Shinu, P.; Nair, A.B. Advances in Nanocarrier Systems for Overcoming Formulation Challenges of Curcumin: Current Insights. Nanomaterials 2024, 14, 672. [Google Scholar] [CrossRef]
- Bhat, S.S.; Prasad, S.K.; Shivamallu, C.; Prasad, K.S.; Syed, A.; Reddy, P.; Cull, C.A.; Amachawadi, R.G. Genistein: A Potent Anti-Breast Cancer Agent. Curr. Issues Mol. Biol. 2021, 43, 1502–1517. [Google Scholar] [CrossRef]
- Asgharian, P.; Tazekand, A.P.; Hosseini, K.; Forouhandeh, H.; Ghasemnejad, T.; Ranjbar, M.; Hasan, M.; Kumar, M.; Beirami, S.M.; Tarhriz, V.; et al. Potential mechanisms of quercetin in cancer prevention: Focus on cellular and molecular targets. Cancer Cell Int. 2022, 22, 257. [Google Scholar] [CrossRef] [PubMed]
- Majdalawieh, A.F.; Fayyad, M.W.; Nasrallah, G.K. Anti-cancer properties and mechanisms of action of thymoquinone, the major active ingredient of Nigella sativa. Crit. Rev. Food Sci. Nutr. 2017, 57, 3911–3928. [Google Scholar] [CrossRef]
- Alhmied, F.; Alammar, A.; Alsultan, B.; Alshehri, M.; Pottoo, F.H. Molecular Mechanisms of Thymoquinone as Anticancer Agent. Comb. Chem. High Throughput Screen. 2021, 24, 1644–1653. [Google Scholar] [CrossRef]
- Khwaza, V.; Oyedeji, O.O.; Aderibigbe, B.A. Ursolic Acid-Based Derivatives as Potential Anti-Cancer Agents: An Update. Int. J. Mol. Sci. 2020, 21, 5920. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.M.; Su, X.L. Anticancer effect of ursolic acid via mitochondria-dependent pathways. Oncol. Lett. 2019, 17, 4761–4767. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Jiang, X.; Meng, L.; Dong, X.; Shen, Y.; Xin, Y. Anticancer Activity of Sulforaphane: The Epigenetic Mechanisms and the Nrf2 Signaling Pathway. Oxid. Med. Cell. Longev. 2018, 2018, 5438179. [Google Scholar] [CrossRef] [PubMed]
- Kamal, M.M.; Akter, S.; Lin, C.N.; Nazzal, S. Sulforaphane as an anticancer molecule: Mechanisms of action, synergistic effects, enhancement of drug safety, and delivery systems. Arch. Pharmacal Res. 2020, 43, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Kavi Kishor, P.B.; Thaddi, B.N.; Guddimalli, R.; Nikam, T.D.; Sambasiva Rao, K.R.S.; Mukhopadhyay, R.; Singam, P. The Occurrence, Uses, Biosynthetic Pathway, and Biotechnological Production of Plumbagin, a Potent Antitumor Naphthoquinone. Molecules 2025, 30, 1618. [Google Scholar] [CrossRef]
- Moran, N.E.; Alexander, B.; Garg, S.; Marchant, N.; Hason, N.A. Relative Uptake of Tomato Carotenoids by In Vitro Intestinal and Prostate Cancer Cells. J. Nutr. 2024, 154, 3639–3651. [Google Scholar] [CrossRef]
- Gu, H.; Pan, C.D.; Xu, Q.D.; Lu, J.D.; Zhao, T.D.; Fu, K.D.; Yan, X.D.; Xu, Y.D.; Ye, J.D. Lycopene Suppresses Lung Cancer Progression via PI3K/AKT Pathway Inhibition and Apoptosis Induction: Mechanistic and Safety Insights from Preclinical Models. bioRxiv 2025. [Google Scholar] [CrossRef]
- Tumbath, S.; Jiang, L.; Li, X.; Zhang, T.; Zahid, K.R.; Zhao, Y.; Zhou, H.; Yin, Z.; Lu, T.; Jiang, S.; et al. β-Lapachone promotes the recruitment and polarization of tumor-associated neutrophils (TANs) toward an antitumor (N1) phenotype in NQO1-positive cancers. Oncoimmunology 2024, 13, 2363000. [Google Scholar] [CrossRef]
- Du, P.; Li, Y.; Han, A.; Wang, M.; Liu, J.; Piao, Y.; Chen, L. β-lapachone suppresses carcinogenesis of cervical cancer via interaction with AKT1. Front. Pharmacol. 2025, 16, 1509568. [Google Scholar] [CrossRef]
- Alqahtani, M.S.; Kazi, M.; Alsenaidy, M.A.; Ahmad, M.Z. Advances in Oral Drug Delivery. Front. Pharmacol. 2021, 12, 618411. [Google Scholar] [CrossRef]
- Yang, J.; He, X.; Zhao, D. Factors affecting phytochemical stability. In Handbook of Plant Food Phytochemicals: Sources, Stability and Extraction; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 332–374. [Google Scholar]
- Ahmad, R.; Srivastava, S.; Ghosh, S.; Khare, S.K. Phytochemical delivery through nanocarriers: A review. Colloids Surf. B Biointerfaces 2021, 197, 111389. [Google Scholar] [CrossRef]
- Gao, Q.; Feng, J.; Liu, W.; Wen, C.; Wu, Y.; Liao, Q.; Zou, L.; Sui, X.; Xie, T.; Zhang, J.; et al. Opportunities and challenges for co-delivery nanomedicines based on combination of phytochemicals with chemotherapeutic drugs in cancer treatment. Adv. Drug Deliv. Rev. 2022, 188, 114445. [Google Scholar] [CrossRef]
- Yu, F.; Ao, M.; Zheng, X.; Li, N.; Xia, J.; Li, Y.; Li, D.; Hou, Z.; Qi, Z.; Chen, X.D. PEG-lipid-PLGA hybrid nanoparticles loaded with berberine-phospholipid complex to facilitate the oral delivery efficiency. Drug Deliv. 2017, 24, 825–833. [Google Scholar] [CrossRef]
- Hassan, N.K.; Haider, M.; Jagal, J.; El-Labbad, E.; Elsayed, I.; Taha, M.S. Beta-Cyclodextrin-Capped Self-Assembled Zein Nanoparticles for Stable Quercetin Delivery. J. Drug Deliv. Sci. Technol. 2025, 111, 107174. [Google Scholar] [CrossRef]
- Mukherjee, D.; Krishnan, A. Therapeutic potential of curcumin and its nanoformulations for treating oral cancer. World J. Methodol. 2023, 13, 29–45. [Google Scholar] [CrossRef]
- Smoliga, J.M.; Baur, J.A.; Hausenblas, H.A. Resveratrol and health—A comprehensive review of human clinical trials. Mol. Nutr. Food Res. 2011, 55, 1129–1141. [Google Scholar] [CrossRef]
- Godos, J.; Romano, G.L.; Gozzo, L.; Laudani, S.; Paladino, N.; Dominguez Azpíroz, I.; Martínez López, N.M.; Giampieri, F.; Quiles, J.L.; Battino, M.; et al. Resveratrol and vascular health: Evidence from clinical studies and mechanisms of actions related to its metabolites produced by gut microbiota. Front. Pharmacol. 2024, 15, 1368949. [Google Scholar] [CrossRef] [PubMed]
- Afzal, O.; Rizwanullah, M.; Altamimi, A.S.; Alossaimi, M.A.; Kamal, M.; Ahmad, J. Harnessing natural polysaccharides-based nanoparticles for oral delivery of phytochemicals: Knocking down the barriers. J. Drug Deliv. Sci. Technol. 2023, 82, 104368. [Google Scholar] [CrossRef]
- Nicolescu, A.; Babotă, M.; Barros, L.; Rocchetti, G.; Lucini, L.; Tanase, C.; Mocan, A.; Bunea, C.I.; Crișan, G. Bioaccessibility and bioactive potential of different phytochemical classes from nutraceuticals and functional foods. Front. Nutr. 2023, 10, 1184535. [Google Scholar] [CrossRef]
- Hu, Y.; Lin, Q.; Zhao, H.; Li, X.; Sang, S.; McClements, D.J.; Long, J.; Jin, Z.; Wang, J.; Qiu, C. Bioaccessibility and bioavailability of phytochemicals: Influencing factors, improvements, and evaluations. Food Hydrocoll. 2023, 135, 108165. [Google Scholar] [CrossRef]
- Negi, P.S. Stability of phytochemicals at the point of sale. In Handbook of Plant Food Phytochemicals: Sources, Stability and Extraction; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 375–395. [Google Scholar]
- Wang, Y.J.; Pan, M.H.; Cheng, A.L.; Lin, L.I.; Ho, Y.S.; Hsieh, C.Y.; Lin, J.K. Stability of curcumin in buffer solutions and characterization of its degradation products. J. Pharm. Biomed. Anal. 1997, 15, 1867–1876. [Google Scholar] [CrossRef]
- Mrázková, M.; Sumczynski, D.; Orsavová, J. Influence of Storage Conditions on Stability of Phenolic Compounds and Antioxidant Activity Values in Nutraceutical Mixtures with Edible Flowers as New Dietary Supplements. Antioxidants 2023, 12, 962. [Google Scholar] [CrossRef]
- Lin, S.; Xiao, J. Impact of thermal processing on dietary flavonoids. Adv. Food Nutr. Res. 2024, 108, 1–34. [Google Scholar] [CrossRef]
- González-González, O.; Ramirez, I.O.; Ramirez, B.I.; O’Connell, P.; Ballesteros, M.P.; Torrado, J.J.; Serrano, D.R. Drug Stability: ICH versus Accelerated Predictive Stability Studies. Pharmaceutics 2022, 14, 2324. [Google Scholar] [CrossRef] [PubMed]
- Odriozola-Serrano, I.; Nogueira, D.P.; Esparza, I.; Vaz, A.A.; Jiménez-Moreno, N.; Martín-Belloso, O.; Ancín-Azpilicueta, C. Stability and Bioaccessibility of Phenolic Compounds in Rosehip Extracts during In Vitro Digestion. Antioxidants 2023, 12, 1035. [Google Scholar] [CrossRef]
- Llewellyn, C.A.; Airs, R.L.; Farnham, G.; Greig, C. Synthesis, Regulation and Degradation of Carotenoids Under Low Level UV-B Radiation in the Filamentous Cyanobacterium Chlorogloeopsis fritschii PCC 6912. Front. Microbiol. 2020, 11, 163. [Google Scholar] [CrossRef] [PubMed]
- Semitsoglou-Tsiapou, S.; Meador, T.B.; Peng, B.; Aluwihare, L. Photochemical (UV-vis/H2O2) degradation of carotenoids: Kinetics and molecular end products. Chemosphere 2022, 286, 131697. [Google Scholar] [CrossRef]
- Casella, P.; Iovine, A.; Mehariya, S.; Marino, T.; Musmarra, D.; Molino, A. Smart Method for Carotenoids Characterization in Haematococcus pluvialis red phase and Evaluation of Astaxanthin Thermal Stability. Antioxidants 2020, 9, 422. [Google Scholar] [CrossRef]
- Aslam, S.; Ahmad, M.; Riaz, M. Stability of carotenoids. In Carotenoids: Structure and Function in the Human Body; Springer: Cham, Switzerland, 2021; pp. 251–315. [Google Scholar]
- Zhou, F.; Peterson, T.; Fan, Z.; Wang, S. The Commonly Used Stabilizers for Phytochemical-Based Nanoparticles: Stabilization Effects, Mechanisms, and Applications. Nutrients 2023, 15, 3881. [Google Scholar] [CrossRef]
- Bhatia, N.K.; Tomar, V.R.; Kishor, S.; Deep, S. Effect of pH and temperature on physicochemical properties, aggregation behaviour and degradation kinetics of quercetin and baicalein in nearly aqueous media. J. Mol. Liq. 2022, 366, 120236. [Google Scholar] [CrossRef]
- Peng, H.; Shahidi, F. Oxidation and degradation of (epi)gallocatechin gallate (EGCG/GCG) and (epi)catechin gallate (ECG/CG) in alkali solution. Food Chem. 2023, 408, 134815. [Google Scholar] [CrossRef] [PubMed]
- Ardhi, A.; Raharjo, S.; Sudjarwo, W.A.A.; Schreiner, M. Oxidative stability of optimized nanostructured lipid carriers containing thymoquinone-rich oil. J. Am. Oil Chem. Soc. 2025, 102, 793–810. [Google Scholar] [CrossRef]
- Gabrič, A.; Hodnik, Ž.; Pajk, S. Oxidation of Drugs during Drug Product Development: Problems and Solutions. Pharmaceutics 2022, 14, 325. [Google Scholar] [CrossRef]
- Hnamte, M.; Pulikkal, A.K. Biocompatible polymeric nanoparticles as carriers for anticancer phytochemicals. Eur. Polym. J. 2024, 202, 112637. [Google Scholar] [CrossRef]
- Jacob, S.; Kather, F.S.; Boddu, S.H.S.; Rao, R.; Nair, A.B. Vesicular Carriers for Phytochemical Delivery: A Comprehensive Review of Techniques and Applications. Pharmaceutics 2025, 17, 464. [Google Scholar] [CrossRef]
- Eid, A.M.; Issa, L.; Arar, K.; Abu-Zant, A.; Makhloof, M.; Masarweh, Y. Phytochemical screening, antioxidant, anti-diabetic, and anti-obesity activities, formulation, and characterization of a self-nanoemulsion system loaded with pomegranate (Punica granatum) seed oil. Sci. Rep. 2024, 14, 18841. [Google Scholar] [CrossRef]
- Melim, C.; Magalhães, M.; Santos, A.C.; Campos, E.J.; Cabral, C. Nanoparticles as phytochemical carriers for cancer treatment: News of the last decade. Expert Opin. Drug Deliv. 2022, 19, 179–197. [Google Scholar] [CrossRef]
- Kumarasamy, R.V.; Natarajan, P.M.; Umapathy, V.R.; Roy, J.R.; Mironescu, M.; Palanisamy, C.P. Clinical applications and therapeutic potentials of advanced nanoparticles: A comprehensive review on completed human clinical trials. Front. Nanotechnol. 2024, 6, 1479993. [Google Scholar] [CrossRef]
- Ding, B.; Ahmadi, S.H.; Babak, P.; Bryant, S.L.; Kantzas, A. On the Stability of Pickering and Classical Nanoemulsions: Theory and Experiments. Langmuir ACS J. Surf. Colloids 2023, 39, 6975–6991. [Google Scholar] [CrossRef]
- Gorain, B.; Al-Dhubiab, B.E.; Nair, A.; Kesharwani, P.; Pandey, M.; Choudhury, H. Multivesicular liposome: A lipid-based drug delivery system for efficient drug delivery. Curr. Pharm. Des. 2021, 27, 4404–4415. [Google Scholar] [CrossRef] [PubMed]
- Shehata, T.M.; Nair, A.B.; Al-Dhubiab, B.E.; Shah, J.; Jacob, S.; Alhaider, I.A.; Attimarad, M.; Elsewedy, H.S.; Ibrahim, M.M. Vesicular emulgel based system for transdermal delivery of insulin: Factorial design and in vivo evaluation. Appl. Sci. 2020, 10, 5341. [Google Scholar] [CrossRef]
- Wang, F.; Wu, Y.; Zhang, J.; Wang, H.; Xie, X.; Ye, X.; Peng, D.; Chen, W. Induction of Cytochrome P450 Involved in the Accelerated Blood Clearance Phenomenon Induced by PEGylated Liposomes In Vivo. Drug Metab. Dispos. Biol. Fate Chem. 2019, 47, 364–376. [Google Scholar] [CrossRef]
- Morsy, M.A.; Nair, A.B. Prevention of rat liver fibrosis by selective targeting of hepatic stellate cells using hesperidin carriers. Int. J. Pharm. 2018, 552, 241–250. [Google Scholar] [CrossRef]
- Moammeri, A.; Chegeni, M.M.; Sahrayi, H.; Ghafelehbashi, R.; Memarzadeh, F.; Mansouri, A.; Akbarzadeh, I.; Abtahi, M.S.; Hejabi, F.; Ren, Q. Current advances in niosomes applications for drug delivery and cancer treatment. Mater. Today Bio 2023, 23, 100837. [Google Scholar] [CrossRef]
- Yaghmur, A.; Mu, H. Recent advances in drug delivery applications of cubosomes, hexosomes, and solid lipid nanoparticles. Acta Pharm. Sin. B 2021, 11, 871–885. [Google Scholar] [CrossRef]
- Jain, S.; Tripathi, S.; Tripathi, P.K. Invasomes: Potential vesicular systems for transdermal delivery of drug molecules. J. Drug Deliv. Sci. Technol. 2021, 61, 102166. [Google Scholar] [CrossRef]
- Matharoo, N.; Mohd, H.; Michniak-Kohn, B. Transferosomes as a transdermal drug delivery system: Dermal kinetics and recent developments. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2024, 16, e1918. [Google Scholar] [CrossRef]
- Chauhan, N.; Vasava, P.; Khan, S.L.; Siddiqui, F.A.; Islam, F.; Chopra, H.; Emran, T.B. Ethosomes: A novel drug carrier. Ann. Med. Surg. 2022, 82, 104595. [Google Scholar] [CrossRef] [PubMed]
- Seenivasan, R.; Halagali, P.; Nayak, D.; Tippavajhala, V.K. Transethosomes: A Comprehensive Review of Ultra-Deformable Vesicular Systems for Enhanced Transdermal Drug Delivery. AAPS PharmSciTech 2025, 26, 41. [Google Scholar] [CrossRef] [PubMed]
- Beach, M.A.; Nayanathara, U.; Gao, Y.; Zhang, C.; Xiong, Y.; Wang, Y.; Such, G.K. Polymeric Nanoparticles for Drug Delivery. Chem. Rev. 2024, 124, 5505–5616. [Google Scholar] [CrossRef] [PubMed]
- Pooresmaeil, M.; Namazi, H. Advances in development of the dendrimers having natural saccharides in their structure for efficient and controlled drug delivery applications. Eur. Polym. J. 2021, 148, 110356. [Google Scholar] [CrossRef]
- Havelikar, U.; Ghorpade, K.B.; Kumar, A.; Patel, A.; Singh, M.; Banjare, N.; Gupta, P.N. Comprehensive insights into mechanism of nanotoxicity, assessment methods and regulatory challenges of nanomedicines. Discov. Nano 2024, 19, 165. [Google Scholar] [CrossRef]
- Fan, D.; Cao, Y.; Cao, M.; Wang, Y.; Cao, Y.; Gong, T. Nanomedicine in cancer therapy. Signal Transduct. Target. Ther. 2023, 8, 293. [Google Scholar] [CrossRef]
- Sawicki, K.; Czajka, M.; Matysiak-Kucharek, M.; Fal, B.; Drop, B.; Męczyńska-Wielgosz, S.; Sikorska, K.; Kruszewski, M.; Kapka-Skrzypczak, L. Toxicity of metallic nanoparticles in the central nervous system. Nanotechnol. Rev. 2019, 8, 175–200. [Google Scholar] [CrossRef]
- Chiu, H.I.; Samad, N.A.; Fang, L.; Lim, V. Cytotoxicity of targeted PLGA nanoparticles: A systematic review. RSC Adv 2021, 11, 9433–9449. [Google Scholar] [CrossRef] [PubMed]
- Janaszewska, A.; Lazniewska, J.; Trzepiński, P.; Marcinkowska, M.; Klajnert-Maculewicz, B. Cytotoxicity of Dendrimers. Biomolecules 2019, 9, 330. [Google Scholar] [CrossRef]
- Mohanta, D.; Patnaik, S.; Sood, S.; Das, N. Carbon nanotubes: Evaluation of toxicity at biointerfaces. J. Pharm. Anal. 2019, 9, 293–300. [Google Scholar] [CrossRef]
- Jin, Z.; Al Amili, M.; Guo, S. Tumor Microenvironment-Responsive Drug Delivery Based on Polymeric Micelles for Precision Cancer Therapy: Strategies and Prospects. Biomedicines 2024, 12, 417. [Google Scholar] [CrossRef]
- Miller, T.; Breyer, S.; van Colen, G.; Mier, W.; Haberkorn, U.; Geissler, S.; Voss, S.; Weigandt, M.; Goepferich, A. Premature drug release of polymeric micelles and its effects on tumor targeting. Int. J. Pharm. 2013, 445, 117–124. [Google Scholar] [CrossRef]
- Goo, Y.T.; Grigoriev, V.; Korzun, T.; Sharma, K.S.; Singh, P.; Taratula, O.R.; Marks, D.L.; Taratula, O. Blood-Brain Barrier-Penetrating Nanocarriers Enable Microglial-Specific Drug Delivery in Hypothalamic Neuroinflammation. Adv. Healthc. Mater. 2025, 14, e2500521. [Google Scholar] [CrossRef]
- Hristova-Panusheva, K.; Xenodochidis, C.; Georgieva, M.; Krasteva, N. Nanoparticle-Mediated Drug Delivery Systems for Precision Targeting in Oncology. Pharmaceuticals 2024, 17, 677. [Google Scholar] [CrossRef] [PubMed]
- Majumder, J.; Minko, T. Multifunctional and stimuli-responsive nanocarriers for targeted therapeutic delivery. Expert Opin. Drug Deliv. 2021, 18, 205–227. [Google Scholar] [CrossRef]
- Graham, W.; Torbett-Dougherty, M.; Islam, A.; Soleimani, S.; Bruce-Tagoe, T.A.; Johnson, J.A. Magnetic Nanoparticles and Drug Delivery Systems for Anti-Cancer Applications: A Review. Nanomaterials 2025, 15, 285. [Google Scholar] [CrossRef]
- Howaili, F.; Özliseli, E.; Küçüktürkmen, B.; Razavi, S.M.; Sadeghizadeh, M.; Rosenholm, J.M. Stimuli-Responsive, Plasmonic Nanogel for Dual Delivery of Curcumin and Photothermal Therapy for Cancer Treatment. Front. Chem. 2020, 8, 602941. [Google Scholar] [CrossRef]
- Zandieh, M.A.; Farahani, M.H.; Daryab, M.; Motahari, A.; Gholami, S.; Salmani, F.; Karimi, F.; Samaei, S.S.; Rezaee, A.; Rahmanian, P.; et al. Stimuli-responsive (nano)architectures for phytochemical delivery in cancer therapy. Biomed. Pharmacother. 2023, 166, 115283. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Kataoka, K. Chemo-physical Strategies to Advance the in Vivo Functionality of Targeted Nanomedicine: The Next Generation. J. Am. Chem. Soc. 2021, 143, 538–559. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, J.; Wang, C.; Wang, T.; Zeng, Y.; Li, X.; Zuo, Y.; Chen, H.; Zhang, C.; Cao, Y.; et al. Aptamer-functionalized triptolide with release controllability as a promising targeted therapy against triple-negative breast cancer. J. Exp. Clin. Cancer Res. CR 2024, 43, 207. [Google Scholar] [CrossRef] [PubMed]
- Risinger, A.L.; Jackson, E.M.; Polin, L.A.; Helms, G.L.; LeBoeuf, D.A.; Joe, P.A.; Hopper-Borge, E.; Ludueña, R.F.; Kruh, G.D.; Mooberry, S.L. The taccalonolides: Microtubule stabilizers that circumvent clinically relevant taxane resistance mechanisms. Cancer Res. 2008, 68, 8881–8888. [Google Scholar] [CrossRef]
- Hussain, A.; Kumar, A.; Uttam, V.; Sharma, U.; Sak, K.; Saini, R.V.; Saini, A.K.; Haque, S.; Tuli, H.S.; Jain, A.; et al. Application of curcumin nanoformulations to target folic acid receptor in cancer: Recent trends and advances. Environ. Res. 2023, 233, 116476. [Google Scholar] [CrossRef]
- Halder, A.; Jethwa, M.; Mukherjee, P.; Ghosh, S.; Das, S.; Helal Uddin, A.B.M.; Mukherjee, A.; Chatterji, U.; Roy, P. Lactoferrin-tethered betulinic acid nanoparticles promote rapid delivery and cell death in triple negative breast and laryngeal cancer cells. Artif. Cells Nanomed. Biotechnol. 2020, 48, 1362–1371. [Google Scholar] [CrossRef]
- Mehrdadi, S. Drug Delivery of Solid Lipid Nanoparticles (SLNs) and Nanostructured Lipid Carriers (NLCs) to Target Brain Tumors. Adv. Pharm. Bull. 2023, 13, 512–520. [Google Scholar] [CrossRef]
- Wei, Q.Y.; He, K.M.; Chen, J.L.; Xu, Y.M.; Lau, A.T.Y. Phytofabrication of Nanoparticles as Novel Drugs for Anticancer Applications. Molecules 2019, 24, 4246. [Google Scholar] [CrossRef]
- Kim, J.H.; Dareowolabi, B.O.; Thiruvengadam, R.; Moon, E.Y. Application of Nanotechnology and Phytochemicals in Anticancer Therapy. Pharmaceutics 2024, 16, 1169. [Google Scholar] [CrossRef] [PubMed]
- Grewal, A.K.; Salar, R.K. Chitosan nanoparticle delivery systems: An effective approach to enhancing efficacy and safety of anticancer drugs. Nano TransMed 2024, 3, 100040. [Google Scholar] [CrossRef]
- Aldayel, T.S.; Badran, M.M.; Alomrani, A.H.; AlFaris, N.A.; Altamimi, J.Z.; Alqahtani, A.S.; Nasr, F.A.; Ghaffar, S.; Orfali, R. Chitosan-Coated Solid Lipid Nanoparticles as an Efficient Avenue for Boosted Biological Activities of Aloe perryi: Antioxidant, Antibacterial, and Anticancer Potential. Molecules 2023, 28, 3569. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, P.; Ramalingam, P.; Karthivashan, G.; Ko, Y.T.; Choi, D.K. Recent developments in solid lipid nanoparticle and surface-modified solid lipid nanoparticle delivery systems for oral delivery of phyto-bioactive compounds in various chronic diseases. Int. J. Nanomed. 2018, 13, 1569–1583. [Google Scholar] [CrossRef]
- Chavda, V.P.; Nalla, L.V.; Balar, P.; Bezbaruah, R.; Apostolopoulos, V.; Singla, R.K.; Khadela, A.; Vora, L.; Uversky, V.N. Advanced Phytochemical-Based Nanocarrier Systems for the Treatment of Breast Cancer. Cancers 2023, 15, 1023. [Google Scholar] [CrossRef]
- Fathi, F.; Machado, T.O.X.; Kodel, H.d.A.C.; Portugal, I.; Ferreira, I.O.; Zielinska, A.; Oliveira, M.; Souto, E.B. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) for the delivery of bioactives sourced from plants: Part II—Applications and preclinical advancements. Expert Opin. Drug Deliv. 2024, 21, 1491–1499. [Google Scholar] [CrossRef]
- Viegas, C.; Patrício, A.B.; Prata, J.M.; Nadhman, A.; Chintamaneni, P.K.; Fonte, P. Solid Lipid Nanoparticles vs. Nanostructured Lipid Carriers: A Comparative Review. Pharmaceutics 2023, 15, 1593. [Google Scholar] [CrossRef]
- Chutoprapat, R.; Kopongpanich, P.; Chan, L.W. A Mini-Review on Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: Topical Delivery of Phytochemicals for the Treatment of Acne Vulgaris. Molecules 2022, 27, 3460. [Google Scholar] [CrossRef]
- Ranjbar, S.; Emamjomeh, A.; Sharifi, F.; Zarepour, A.; Aghaabbasi, K.; Dehshahri, A.; Sepahvand, A.M.; Zarrabi, A.; Beyzaei, H.; Zahedi, M.M.; et al. Lipid-Based Delivery Systems for Flavonoids and Flavonolignans: Liposomes, Nanoemulsions, and Solid Lipid Nanoparticles. Pharmaceutics 2023, 15, 1944. [Google Scholar] [CrossRef]
- Jacob, S.; Nair, A.B.; Shah, J.; Gupta, S.; Boddu, S.H.S.; Sreeharsha, N.; Joseph, A.; Shinu, P.; Morsy, M.A. Lipid Nanoparticles as a Promising Drug Delivery Carrier for Topical Ocular Therapy—An Overview on Recent Advances. Pharmaceutics 2022, 14, 533. [Google Scholar] [CrossRef]
- Hald Albertsen, C.; Kulkarni, J.A.; Witzigmann, D.; Lind, M.; Petersson, K.; Simonsen, J.B. The role of lipid components in lipid nanoparticles for vaccines and gene therapy. Adv. Drug Deliv. Rev. 2022, 188, 114416. [Google Scholar] [CrossRef]
- Lam, K.; Schreiner, P.; Leung, A.; Stainton, P.; Reid, S.; Yaworski, E.; Lutwyche, P.; Heyes, J. Optimizing Lipid Nanoparticles for Delivery in Primates. Adv. Mater. 2023, 35, e2211420. [Google Scholar] [CrossRef]
- Yong, J.; Shu, H.; Zhang, X.; Yang, K.; Luo, G.; Yu, L.; Li, J.; Huang, H. Natural Products-Based Inhaled Formulations for Treating Pulmonary Diseases. Int. J. Nanomed. 2024, 19, 1723–1748. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, P. Lipid-Based Nanocarrier System for the Effective Delivery of Nutraceuticals. Molecules 2021, 26, 5510. [Google Scholar] [CrossRef]
- Kamboj, S.; Bala, S.; Nair, A.B. Solid lipid nanoparticles: An effective lipid based technology for poorly water soluble drugs. Int. J. Pharm. Sci. Rev. Res. 2010, 5, 78–90. [Google Scholar]
- Mishra, V.; Bansal, K.K.; Verma, A.; Yadav, N.; Thakur, S.; Sudhakar, K.; Rosenholm, J.M. Solid Lipid Nanoparticles: Emerging Colloidal Nano Drug Delivery Systems. Pharmaceutics 2018, 10, 191. [Google Scholar] [CrossRef] [PubMed]
- Scioli Montoto, S.; Muraca, G.; Ruiz, M.E. Solid Lipid Nanoparticles for Drug Delivery: Pharmacological and Biopharmaceutical Aspects. Front. Mol. Biosci. 2020, 7, 587997. [Google Scholar] [CrossRef]
- Khairnar, S.V.; Pagare, P.; Thakre, A.; Nambiar, A.R.; Junnuthula, V.; Abraham, M.C.; Kolimi, P.; Nyavanandi, D.; Dyawanapelly, S. Review on the Scale-Up Methods for the Preparation of Solid Lipid Nanoparticles. Pharmaceutics 2022, 14, 1886. [Google Scholar] [CrossRef]
- Borges, A.; Freitas, V.; Mateus, N.; Fernandes, I.; Oliveira, J. Solid Lipid Nanoparticles as Carriers of Natural Phenolic Compounds. Antioxidants 2020, 9, 998. [Google Scholar] [CrossRef] [PubMed]
- Akbari, J.; Saeedi, M.; Ahmadi, F.; Hashemi, S.M.H.; Babaei, A.; Yaddollahi, S.; Rostamkalaei, S.S.; Asare-Addo, K.; Nokhodchi, A. Solid lipid nanoparticles and nanostructured lipid carriers: A review of the methods of manufacture and routes of administration. Pharm. Dev. Technol. 2022, 27, 525–544. [Google Scholar] [CrossRef] [PubMed]
- Mirchandani, Y.; Patravale, V.B.; Brijesh, S. Solid lipid nanoparticles for hydrophilic drugs. J. Control. Release 2021, 335, 457–464. [Google Scholar] [CrossRef]
- Müller, R.H.; Mäder, K.; Gohla, S. Solid lipid nanoparticles (SLN) for controlled drug delivery—A review of the state of the art. Eur. J. Pharm. Biopharm. 2000, 50, 161–177. [Google Scholar] [CrossRef]
- Shah, R.; Eldridge, D.; Palombo, E.; Harding, I. Lipid Nanoparticles: Production, Characterization and Stability; Springer: Berlin/Heidelberg, Germany, 2015; Volume 1. [Google Scholar]
- Junyaprasert, V.B.; Morakul, B. Nanocrystals for enhancement of oral bioavailability of poorly water-soluble drugs. Asian J. Pharm. Sci. 2015, 10, 13–23. [Google Scholar] [CrossRef]
- Paliwal, R.; Paliwal, S.R.; Kenwat, R.; Kurmi, B.D.; Sahu, M.K. Solid lipid nanoparticles: A review on recent perspectives and patents. Expert Opin. Ther. Pat. 2020, 30, 179–194. [Google Scholar] [CrossRef]
- Pardeike, J.; Hommoss, A.; Müller, R.H. Lipid nanoparticles (SLN, NLC) in cosmetic and pharmaceutical dermal products. Int. J. Pharm. 2009, 366, 170–184. [Google Scholar] [CrossRef]
- zur Mühlen, A.; Schwarz, C.; Mehnert, W. Solid lipid nanoparticles (SLN) for controlled drug delivery—Drug release and release mechanism. Eur. J. Pharm. Biopharm. 1998, 45, 149–155. [Google Scholar] [CrossRef]
- Li, K.; Pi, C.; Wen, J.; He, Y.; Yuan, J.; Shen, H.; Zhao, W.; Zeng, M.; Song, X.; Lee, R.J.; et al. Formulation of the novel structure curcumin derivative-loaded solid lipid nanoparticles: Synthesis, optimization, characterization and anti-tumor activity screening in vitro. Drug Deliv. 2022, 29, 2044–2057. [Google Scholar] [CrossRef]
- Nair, A.B.; Shah, J.; Al-Dhubiab, B.E.; Jacob, S.; Patel, S.S.; Venugopala, K.N.; Morsy, M.A.; Gupta, S.; Attimarad, M.; Sreeharsha, N.; et al. Clarithromycin Solid Lipid Nanoparticles for Topical Ocular Therapy: Optimization, Evaluation and In Vivo Studies. Pharmaceutics 2021, 13, 523. [Google Scholar] [CrossRef] [PubMed]
- Gordillo-Galeano, A.; Mora-Huertas, C.E. Solid lipid nanoparticles and nanostructured lipid carriers: A review emphasizing on particle structure and drug release. Eur. J. Pharm. Biopharm. 2018, 133, 285–308. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.W.; Sweeney, C.; Dudhipala, N.; Lakhani, P.; Chaurasiya, N.D.; Tekwani, B.L.; Majumdar, S. Primaquine Loaded Solid Lipid Nanoparticles (SLN), Nanostructured Lipid Carriers (NLC), and Nanoemulsion (NE): Effect of Lipid Matrix and Surfactant on Drug Entrapment, in vitro Release, and ex vivo Hemolysis. AAPS PharmSciTech 2021, 22, 240. [Google Scholar] [CrossRef]
- Göke, K.; Bunjes, H. Drug solubility in lipid nanocarriers: Influence of lipid matrix and available interfacial area. Int. J. Pharm. 2017, 529, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.M.; Martins-Gomes, C.; Coutinho, T.E.; Fangueiro, J.F.; Sanchez-Lopez, E.; Pashirova, T.N.; Andreani, T.; Souto, E.B. Soft cationic nanoparticles for drug delivery: Production and cytotoxicity of solid lipid nanoparticles (SLNs). Appl. Sci. 2019, 9, 4438. [Google Scholar] [CrossRef]
- Moraes-Lacerda, T.; de Jesus, M.B. Mechanisms of solid lipid nanoparticles-triggered signaling pathways in eukaryotic cells. Colloids Surf. B Biointerfaces 2022, 220, 112863. [Google Scholar] [CrossRef] [PubMed]
- Nazarova, A.; Yakimova, L.; Filimonova, D.; Stoikov, I. Surfactant Effect on the Physicochemical Characteristics of Solid Lipid Nanoparticles Based on Pillar [5]arenes. Int. J. Mol. Sci. 2022, 23, 779. [Google Scholar] [CrossRef]
- Subroto, E.; Andoyo, R.; Indiarto, R. Solid Lipid Nanoparticles: Review of the Current Research on Encapsulation and Delivery Systems for Active and Antioxidant Compounds. Antioxidants 2023, 12, 633. [Google Scholar] [CrossRef]
- Rajpoot, K. Solid Lipid Nanoparticles: A Promising Nanomaterial in Drug Delivery. Curr. Pharm. Des. 2019, 25, 3943–3959. [Google Scholar] [CrossRef]
- Scalia, S.; Young, P.M.; Traini, D. Solid lipid microparticles as an approach to drug delivery. Expert Opin. Drug Deliv. 2015, 12, 583–599. [Google Scholar] [CrossRef]
- Talele, P.; Jadhav, A.; Tayade, S.; Sahu, S.; Sharma, K.K.; Shimpi, N. Hydroquinone loaded solid lipid nanoparticles comprised of stearic acid and ionic emulsifiers: Physicochemical characterization and in vitro release study. J. Mol. Liq. 2022, 368, 120590. [Google Scholar] [CrossRef]
- Gugleva, V.; Andonova, V. Recent Progress of Solid Lipid Nanoparticles and Nanostructured Lipid Carriers as Ocular Drug Delivery Platforms. Pharmaceuticals 2023, 16, 474. [Google Scholar] [CrossRef]
- Khunt, D.; Gayakvad, B.; Modi, V.; Misra, M.; Prajapati, B.; Patel, R.; Patel, R.; Harsoliya, M.; Chauhan, S. Solid lipid nanoparticles. In Lipid-Based Drug Delivery Systems; Jenny Stanford Publishing: Singapore, 2023; pp. 27–46. [Google Scholar]
- Elbrink, K.; Van Hees, S.; Holm, R.; Kiekens, F. Optimization of the different phases of the freeze-drying process of solid lipid nanoparticles using experimental designs. Int. J. Pharm. 2023, 635, 122717. [Google Scholar] [CrossRef]
- Biswas, U.K.; Bose, A.; Parmanik, A. Recent Advances in the Preparation, Properties, and Applications of Solid Lipid Nanoparticles in Drug Delivery. Pharm. Nanotechnol. 2024, 13, 512–527. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, L.; Santagati, L.M.; Sarpietro, M.G.; Castelli, F.; Panico, A.; Siciliano, E.A.; Lai, F.; Valenti, D.; Sinico, C. In Vitro Skin Permeation of Idebenone from Lipid Nanoparticles Containing Chemical Penetration Enhancers. Pharmaceutics 2021, 13, 1027. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.; Reddy, C.; Jacob, S. Delivery of a classical antihypertensive agent through the skin by chemical enhancers and iontophoresis. Ski. Res. Technol. 2009, 15, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.; Vyas, H.; Shah, J.; Kumar, A. Effect of permeation enhancers on the iontophoretic transport of metoprolol tartrate and the drug retention in skin. Drug Deliv. 2011, 18, 19–25. [Google Scholar] [CrossRef]
- Anroop, B.; Ghosh, B.; Parcha, V.; Kumar, A.; Khanam, J. Synthesis and comparative skin permeability of atenolol and propranolol esters. J. Drug Deliv. Sci. Technol. 2005, 15, 187–190. [Google Scholar] [CrossRef]
- Anroop, B.; Ghosh, B.; Parcha, V.; Khanam, J. Transdermal delivery of atenolol: Effect of prodrugs and iontophoresis. Curr. Drug Deliv. 2009, 6, 280–290. [Google Scholar] [CrossRef]
- Xu, Y.; Fourniols, T.; Labrak, Y.; Préat, V.; Beloqui, A.; des Rieux, A. Surface Modification of Lipid-Based Nanoparticles. ACS Nano 2022, 16, 7168–7196. [Google Scholar] [CrossRef]
- Wibel, R.; Braun, D.E.; Hämmerle, L.; Jörgensen, A.M.; Knoll, P.; Salvenmoser, W.; Steinbring, C.; Bernkop-Schnürch, A. In Vitro Investigation of Thiolated Chitosan Derivatives as Mucoadhesive Coating Materials for Solid Lipid Nanoparticles. Biomacromolecules 2021, 22, 3980–3991. [Google Scholar] [CrossRef]
- Abd-Elghany, A.E.; El-Garhy, O.; Fatease, A.A.; Alamri, A.H.; Abdelkader, H. Enhancing Oral Bioavailability of Simvastatin Using Uncoated and Polymer-Coated Solid Lipid Nanoparticles. Pharmaceutics 2024, 16, 763. [Google Scholar] [CrossRef]
- Ghayoumipour, N.; Ghafouri, H. Enhancing breast cancer treatment: Evaluating the efficacy of hyaluronic acid-coated tamoxifen-loaded solid lipid nanoparticles on MCF7 cells. Hum. Exp. Toxicol. 2025, 44, 9603271251322531. [Google Scholar] [CrossRef]
- Alfazani, T.S.; Elsupikhe, R.F.; Abuissa, H.M.; Baiej, K.M. Physical Characterization of Polyethylene Glycol Modified by Solid Lipid Nanoparticles for Targeted Drug Delivery. J. Nano Res. 2024, 85, 1–10. [Google Scholar] [CrossRef]
- Song, B.; Cho, C.-W. Applying polyvinyl alcohol to the preparation of various nanoparticles. J. Pharm. Investig. 2024, 54, 249–266. [Google Scholar] [CrossRef]
- Guorgui, J.; Wang, R.; Mattheolabakis, G.; Mackenzie, G.G. Curcumin formulated in solid lipid nanoparticles has enhanced efficacy in Hodgkin’s lymphoma in mice. Arch. Biochem. Biophys. 2018, 648, 12–19. [Google Scholar] [CrossRef]
- Ramalingam, P.; Ko, Y.T. Enhanced oral delivery of curcumin from N-trimethyl chitosan surface-modified solid lipid nanoparticles: Pharmacokinetic and brain distribution evaluations. Pharm. Res. 2015, 32, 389–402. [Google Scholar] [CrossRef]
- Patel, N.R.; Pattni, B.S.; Abouzeid, A.H.; Torchilin, V.P. Nanopreparations to overcome multidrug resistance in cancer. Adv. Drug Deliv. Rev. 2013, 65, 1748–1762. [Google Scholar] [CrossRef]
- Peetla, C.; Vijayaraghavalu, S.; Labhasetwar, V. Biophysics of cell membrane lipids in cancer drug resistance: Implications for drug transport and drug delivery with nanoparticles. Adv. Drug Deliv. Rev. 2013, 65, 1686–1698. [Google Scholar] [CrossRef]
- Baek, J.S.; Cho, C.W. Surface modification of solid lipid nanoparticles for oral delivery of curcumin: Improvement of bioavailability through enhanced cellular uptake, and lymphatic uptake. Eur. J. Pharm. Biopharm. 2017, 117, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Rahat, I.; Rizwanullah, M.; Gilani, S.J.; Bin-Jummah, M.N.; Imam, S.S.; Kala, C.; Asif, M.; Alshehri, S.; Sharma, S.K. Thymoquinone loaded chitosan-Solid lipid nanoparticles: Formulation optimization to oral bioavailability study. J. Drug Deliv. Sci. Technol. 2021, 64, 102565. [Google Scholar] [CrossRef]
- Aman, R.M.; Abu Hashim, I.I.; Meshali, M.M. Novel chitosan-based solid-lipid nanoparticles to enhance the bio-residence of the miraculous phytochemical “Apocynin”. Eur. J. Pharm. Sci. 2018, 124, 304–318. [Google Scholar] [CrossRef] [PubMed]
- Granja, A.; Nunes, C.; Sousa, C.T.; Reis, S. Folate receptor-mediated delivery of mitoxantrone-loaded solid lipid nanoparticles to breast cancer cells. Biomed. Pharmacother. 2022, 154, 113525. [Google Scholar] [CrossRef]
- Parvez, S.; Karole, A.; Mudavath, S.L. Transport mechanism of hydroxy-propyl-beta-cyclodextrin modified solid lipid nanoparticles across human epithelial cells for the oral absorption of antileishmanial drugs. Biochim. Biophys. Acta (BBA) Gen. Subj. 2022, 1866, 130157. [Google Scholar] [CrossRef]
- Mishchenko, E.; Gileva, A.; Markvicheva, E.; Koroleva, M.Y. Nanoemulsions and solid lipid nanoparticles with encapsulated doxorubicin and thymoquinone. Colloid J. 2023, 85, 736–745. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhang, L.; Hou, X. Potential roles and molecular mechanisms of phytochemicals against cancer. Food Funct. 2022, 13, 9208–9225. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, R.; Kulhari, H.; Pooja, D.; Gudem, S.; Bhargava, S.; Shukla, R.; Sistla, R. Encapsulation of biophenolic phytochemical EGCG within lipid nanoparticles enhances its stability and cytotoxicity against cancer. Chem. Phys. Lipids 2016, 198, 51–60. [Google Scholar] [CrossRef]
- Lu, Y.; Fang, D.; Guo, J.; Huang, H. Partial transformation from non-small cell lung cancer to small cell lung cancer: A case report and literatures review. Front. Oncol. 2025, 15, 1441182. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Xu, Z.; Liu, G.; Jiang, B.; de Bock, G.H.; Groen, H.J.M.; Vliegenthart, R.; Xie, X. Simultaneous Identification of EGFR, KRAS, ERBB2, and TP53 Mutations in Patients with Non-Small Cell Lung Cancer by Machine Learning-Derived Three-Dimensional Radiomics. Cancers 2021, 13, 1814. [Google Scholar] [CrossRef]
- Wang, B.; Wu, K.; Liu, R.; Huang, Y.; Chang, Z.; Gao, Y.; Liu, Y.; Chen, H.; Wang, Z.; Cui, Y.; et al. Phyllanthi Tannin Loaded Solid Lipid Nanoparticles for Lung Cancer Therapy: Preparation, Characterization, Pharmacodynamics and Safety Evaluation. Molecules 2023, 28, 7399. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhang, L.; Peng, H.; Li, Y.; Xiong, J.; Xu, Z. The formulation and delivery of curcumin with solid lipid nanoparticles for the treatment of on non-small cell lung cancer both in vitro and in vivo. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 4802–4808. [Google Scholar] [CrossRef]
- Suvarna, V.; Sawant, N.; Desai, N. A Review on Recent Advances in Mannose-Functionalized Targeted Nanocarrier Delivery Systems in Cancer and Infective Therapeutics. Crit. Rev. Ther. Drug Carr. Syst. 2023, 40, 43–82. [Google Scholar] [CrossRef] [PubMed]
- Chae, J.; Choi, Y.; Hong, J.; Kim, N.; Kim, J.; Lee, H.Y.; Choi, J. Anticancer and Antibacterial Properties of Curcumin-Loaded Mannosylated Solid Lipid Nanoparticles for the Treatment of Lung Diseases. ACS Appl. Bio Mater. 2024, 7, 2175–2185. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Zeng, M.; Li, K.; Pi, C.; Liu, Z.; Zhan, C.; Yuan, J.; Su, Z.; Wei, Y.; Wen, J.; et al. Solid lipid nanoparticle as an effective drug delivery system of a novel curcumin derivative: Formulation, release in vitro and pharmacokinetics in vivo. Pharm. Biol. 2022, 60, 2300–2307. [Google Scholar] [CrossRef]
- Rosière, R.; Van Woensel, M.; Gelbcke, M.; Mathieu, V.; Hecq, J.; Mathivet, T.; Vermeersch, M.; Van Antwerpen, P.; Amighi, K.; Wauthoz, N. New Folate-Grafted Chitosan Derivative To Improve Delivery of Paclitaxel-Loaded Solid Lipid Nanoparticles for Lung Tumor Therapy by Inhalation. Mol. Pharm. 2018, 15, 899–910. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Ma, X.; Chen, M.; He, J.; Zhang, W. Preparation, Characterization and In Vitro Anticancer Activity of Sulforaphene-Loaded Solid Lipid Nanoparticles. Foods 2024, 13, 3898. [Google Scholar] [CrossRef] [PubMed]
- Yeo, S.; Jung, S.; Kim, H.; Ahn, J.H.; Hwang, S.J. 4-Hexylresorcinol Loaded Solid Lipid Nanoparticles for Enhancing Anticancer Activity. Pharmaceuticals 2024, 17, 1296. [Google Scholar] [CrossRef]
- Kharazmi, F.; Neamati, A.; Tabrizi, M.H. Evaluation of the Anticancer Effects of Allicin-Loaded Solid Lipid Nanoparticles in the A549 Lung Cancer Cell Line. BioNanoScience 2024, 15, 49. [Google Scholar] [CrossRef]
- Rasouliyan, F.; Eskandani, M.; Jaymand, M.; Akbari Nakhjavani, S.; Farahzadi, R.; Vandghanooni, S.; Eskandani, M. Preparation, physicochemical characterization, and anti-proliferative properties of Lawsone-loaded solid lipid nanoparticles. Chem. Phys. Lipids 2021, 239, 105123. [Google Scholar] [CrossRef]
- Shahraki, O.; Daneshmand, S. Lycopene-loaded solid lipid nanoparticles: Preparation, characterization, ROS-scavenging, and in vitro anti-melanogenesis evaluations. Lett. Drug Des. Discov. 2023, 20, 1768–1774. [Google Scholar] [CrossRef]
- Palei, N.N.; Sabapati, M.; Vijayaraj, S.; Samajdar, S.; Dhar, A.K. Formulation of Morus alba extract loaded solid lipid nanoparticles: In silico, characterizations, and in vitro cytotoxicity study. Drug Dev. Ind. Pharm. 2025, 51, 14–28. [Google Scholar] [CrossRef]
- Senthamarai Pandi, J.; Pavadai, P.; Sundar, L.M.; Sankaranarayanan, M.; Panneerselvam, T.; Pandian, S.R.K.; Kunjiappan, S. Pharmacokinetics and Brain Tumor Delivery Studies of Thymoquinone-Encapsulated Eudragit L100-Coated Solid-Lipid Nanoparticles. J. Clust. Sci. 2025, 36, 26. [Google Scholar] [CrossRef]
- Rompicharla, S.V.K.; Bhatt, H.; Shah, A.; Komanduri, N.; Vijayasarathy, D.; Ghosh, B.; Biswas, S. Formulation optimization, characterization, and evaluation of in vitro cytotoxic potential of curcumin loaded solid lipid nanoparticles for improved anticancer activity. Chem. Phys. Lipids 2017, 208, 10–18. [Google Scholar] [CrossRef]
- Kumar, G.; Virmani, T.; Sharma, A.; Pathak, K. Codelivery of Phytochemicals with Conventional Anticancer Drugs in Form of Nanocarriers. Pharmaceutics 2023, 15, 889. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Zarrabi, A.; Hashemi, F.; Zabolian, A.; Saleki, H.; Bagherian, M.; Azami, N.; Bejandi, A.K.; Hushmandi, K.; Ang, H.L.; et al. Polychemotherapy with Curcumin and Doxorubicin via Biological Nanoplatforms: Enhancing Antitumor Activity. Pharmaceutics 2020, 12, 1084. [Google Scholar] [CrossRef]
- Wang, W.; Shanmugam, M.K.; Xiang, P.; Yam, T.Y.A.; Kumar, V.; Chew, W.S.; Chang, J.K.; Ali, M.Z.B.; Reolo, M.J.Y.; Peh, Y.X.; et al. Sphingosine 1-Phosphate Receptor 2 Induces Otoprotective Responses to Cisplatin Treatment. Cancers 2020, 12, 211. [Google Scholar] [CrossRef] [PubMed]
- Aktaş, I.; Özmen, Ö.; Tutun, H.; Yalçın, A.; Türk, A. Artemisinin attenuates doxorubicin induced cardiotoxicity and hepatotoxicity in rats. Biotech. Histochem. 2020, 95, 121–128. [Google Scholar] [CrossRef] [PubMed]
- George, B.P.; Chandran, R.; Abrahamse, H. Role of Phytochemicals in Cancer Chemoprevention: Insights. Antioxidants 2021, 10, 1455. [Google Scholar] [CrossRef] [PubMed]
- Ni, W.; Li, Z.; Liu, Z.; Ji, Y.; Wu, L.; Sun, S.; Jian, X.; Gao, X. Dual-Targeting Nanoparticles: Codelivery of Curcumin and 5-Fluorouracil for Synergistic Treatment of Hepatocarcinoma. J. Pharm. Sci. 2019, 108, 1284–1295. [Google Scholar] [CrossRef] [PubMed]
- Senthil Kumar, C.; Thangam, R.; Mary, S.A.; Kannan, P.R.; Arun, G.; Madhan, B. Targeted delivery and apoptosis induction of trans-resveratrol-ferulic acid loaded chitosan coated folic acid conjugate solid lipid nanoparticles in colon cancer cells. Carbohydr. Polym. 2020, 231, 115682. [Google Scholar] [CrossRef]
- Pi, C.; Zhao, W.; Zeng, M.; Yuan, J.; Shen, H.; Li, K.; Su, Z.; Liu, Z.; Wen, J.; Song, X.; et al. Anti-lung cancer effect of paclitaxel solid lipid nanoparticles delivery system with curcumin as co-loading partner in vitro and in vivo. Drug Deliv. 2022, 29, 1878–1891. [Google Scholar] [CrossRef]
- Rawal, S.; Gupta, P.; Bhatnagar, P.; Yadav, H.N.; Dinda, A.K. Solid Lipid Nanoformulation of Berberine Attenuates Doxorubicin Triggered in vitro Inflammation in H9c2 Rat Cardiomyocytes. Comb. Chem. High Throughput Screen. 2022, 25, 1695–1706. [Google Scholar] [CrossRef]
- Afarin, R.; Ahmadpour, F.; Hatami, M.; Monjezi, S.; Igder, S. Combination of Etoposide and quercetin-loaded solid lipid nanoparticles Potentiates apoptotic effects on MDA-MB-231 breast cancer cells. Heliyon 2024, 10, e31925. [Google Scholar] [CrossRef]
- Beloqui, A.; Solinís, M.; Rodríguez-Gascón, A.; Almeida, A.J.; Préat, V. Nanostructured lipid carriers: Promising drug delivery systems for future clinics. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 143–161. [Google Scholar] [CrossRef]
- Izza, N.m.; Watanabe, N.; Okamoto, Y.; Suga, K.; Wibisono, Y.; Kajimura, N.; Mitsuoka, K.; Umakoshi, H. Dependence of the Core–Shell Structure on the Lipid Composition of Nanostructured Lipid Carriers: Implications for Drug Carrier Design. ACS Appl. Nano Mater. 2022, 5, 9958–9969. [Google Scholar] [CrossRef]
- Souto, E.; Almeida, A.; Müller, R. Lipid nanoparticles (SLN®, NLC®) for cutaneous drug delivery: Structure, protection and skin effects. J. Biomed. Nanotechnol. 2007, 3, 317–331. [Google Scholar] [CrossRef]
- Viegas, C.; Seck, F.; Fonte, P. An insight on lipid nanoparticles for therapeutic proteins delivery. J. Drug Deliv. Sci. Technol. 2022, 77, 103839. [Google Scholar] [CrossRef]
- Mahor, A.K.; Singh, P.P.; Gupta, R.; Bhardwaj, P.; Rathore, P.; Kishore, A.; Goyal, R.; Sharma, N.; Verma, J.; Rosenholm, J.M. Nanostructured lipid carriers for improved delivery of therapeutics via the oral route. J. Nanotechnol. 2023, 2023, 4687959. [Google Scholar] [CrossRef]
- Rouco, H.; Diaz-Rodriguez, P.; Guillin, A.; Remuñán-López, C.; Landin, M. A Traffic Light System to Maximize Carbohydrate Cryoprotectants’ Effectivity in Nanostructured Lipid Carriers’ Lyophilization. Pharmaceutics 2021, 13, 1330. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, J.; Wang, M.; Sun, Z.; Chang, C.; Ying, Y.; Li, D.; Zheng, H. Effect of emulsifier type on camellia oil-based nanostructured lipid carriers for delivery of curcumin. Food Chem. 2025, 482, 144193. [Google Scholar] [CrossRef]
- Poonia, N.; Kharb, R.; Lather, V.; Pandita, D. Nanostructured lipid carriers: Versatile oral delivery vehicle. Future Sci. OA 2016, 2, Fso135. [Google Scholar] [CrossRef]
- Tan, S.L.J.; Billa, N. Improved Bioavailability of Poorly Soluble Drugs through Gastrointestinal Muco-Adhesion of Lipid Nanoparticles. Pharmaceutics 2021, 13, 1817. [Google Scholar] [CrossRef]
- Knoll, P.; Hörmann, N.; Nguyen Le, N.M.; Wibel, R.; Gust, R.; Bernkop-Schnürch, A. Charge converting nanostructured lipid carriers containing a cell-penetrating peptide for enhanced cellular uptake. J. Colloid Interface Sci. 2022, 628, 463–475. [Google Scholar] [CrossRef]
- Deshkar, S.S.; Jadhav, M.S.; Shirolkar, S.V. Development of Carbamazepine Nanostructured Lipid Carrier Loaded Thermosensitive Gel for Intranasal Delivery. Adv. Pharm. Bull. 2021, 11, 150–162. [Google Scholar] [CrossRef]
- Markovic, M.; Ben-Shabat, S.; Aponick, A.; Zimmermann, E.M.; Dahan, A. Lipids and Lipid-Processing Pathways in Drug Delivery and Therapeutics. Int. J. Mol. Sci. 2020, 21, 3248. [Google Scholar] [CrossRef]
- Yan, S.; Cheng, Y.; Li, L.; Zhong, C.; Chen, C.; Gao, X. Lipid-based formulations: A promising approach for poorly soluble drug delivery via the intestinal lymphatic system. J. Drug Deliv. Sci. Technol. 2023, 87, 104770. [Google Scholar] [CrossRef]
- Costa, R.; Costa Lima, S.A.; Gameiro, P.; Reis, S. On the Development of a Cutaneous Flavonoid Delivery System: Advances and Limitations. Antioxidants 2021, 10, 1376. [Google Scholar] [CrossRef] [PubMed]
- Gujar, K.; Wairkar, S. Nanocrystal technology for improving therapeutic efficacy of flavonoids. Phytomedicine 2020, 71, 153240. [Google Scholar] [CrossRef]
- Jandang, W.; Ampasavate, C.; Kiattisin, K. Natural Stabilizers and Nanostructured Lipid Carrier Entrapment for Photosensitive Compounds, Curcumin and Capsaicin. Pharmaceutics 2024, 16, 412. [Google Scholar] [CrossRef] [PubMed]
- Mura, P.; Maestrelli, F.; D’Ambrosio, M.; Luceri, C.; Cirri, M. Evaluation and Comparison of Solid Lipid Nanoparticles (SLNs) and Nanostructured Lipid Carriers (NLCs) as Vectors to Develop Hydrochlorothiazide Effective and Safe Pediatric Oral Liquid Formulations. Pharmaceutics 2021, 13, 437. [Google Scholar] [CrossRef]
- Gordillo-Galeano, A.; Ponce, A.; Mora-Huertas, C.E. In vitro release behavior of SLN, NLC, and NE: An explanation based on the particle structure and carried molecule location. J. Drug Deliv. Sci. Technol. 2022, 76, 103768. [Google Scholar] [CrossRef]
- Elmowafy, M.; Samy, A.; Raslan, M.A.; Salama, A.; Said, R.A.; Abdelaziz, A.E.; El-Eraky, W.; El Awdan, S.; Viitala, T. Enhancement of Bioavailability and Pharmacodynamic Effects of Thymoquinone Via Nanostructured Lipid Carrier (NLC) Formulation. AAPS PharmSciTech 2016, 17, 663–672. [Google Scholar] [CrossRef]
- Ashkar, A.; Sosnik, A.; Davidovich-Pinhas, M. Structured edible lipid-based particle systems for oral drug-delivery. Biotechnol. Adv. 2022, 54, 107789. [Google Scholar] [CrossRef] [PubMed]
- Araujo, V.H.S.; da Silva, P.B.; Szlachetka, I.O.; da Silva, S.W.; Fonseca-Santos, B.; Chorilli, M.; Ganassin, R.; de Oliveira, G.R.T.; da Rocha, M.C.O.; Fernandes, R.P. The influence of NLC composition on curcumin loading under a physicochemical perspective and in vitro evaluation. Colloids Surf. A Physicochem. Eng. Asp. 2020, 602, 125070. [Google Scholar] [CrossRef]
- Shimojo, A.A.M.; Fernandes, A.R.V.; Ferreira, N.R.E.; Sanchez-Lopez, E.; Santana, M.H.A.; Souto, E.B. Evaluation of the Influence of Process Parameters on the Properties of Resveratrol-Loaded NLC Using 22 Full Factorial Design. Antioxidants 2019, 8, 272. [Google Scholar] [CrossRef]
- Ahmadian, E.; Eftekhari, A.; Kavetskyy, T.; Khosroushahi, A.Y.; Turksoy, V.A.; Khalilov, R. Effects of quercetin loaded nanostructured lipid carriers on the paraquat-induced toxicity in human lymphocytes. Pestic. Biochem. Physiol. 2020, 167, 104586. [Google Scholar] [CrossRef]
- Chaudhari, V.S.; Gawali, B.; Saha, P.; Naidu, V.G.M.; Murty, U.S.; Banerjee, S. Quercetin and piperine enriched nanostructured lipid carriers (NLCs) to improve apoptosis in oral squamous cellular carcinoma (FaDu cells) with improved biodistribution profile. Eur. J. Pharmacol. 2021, 909, 174400. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Qiao, H.; Ni, J.M.; Shi, Y.B.; Qiang, Y. Preparation of isoliquiritigenin-loaded nanostructured lipid carrier and the in vivo evaluation in tumor-bearing mice. Eur. J. Pharm. Sci. 2013, 49, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Rahman, H.S.; Rasedee, A.; How, C.W.; Abdul, A.B.; Zeenathul, N.A.; Othman, H.H.; Saeed, M.I.; Yeap, S.K. Zerumbone-loaded nanostructured lipid carriers: Preparation, characterization, and antileukemic effect. Int. J. Nanomed. 2013, 8, 2769–2781. [Google Scholar] [CrossRef]
- Hosseini Torshizi, G.; Homayouni Tabrizi, M.; Karimi, E.; Younesi, A.; Larian, Z. Designing nanostructured lipid carriers modified with folate-conjugated chitosan for targeted delivery of osthole to HT-29 colon cancer cells: Investigation of anticancer, antioxidant, and antibacterial activities. Cancer Nanotechnol. 2024, 15, 7. [Google Scholar] [CrossRef]
- Karimi, N.; Ghanbarzadeh, B.; Hamishehkar, H.; Mehramuz, B.; Kafil, H.S. Antioxidant, antimicrobial and physicochemical properties of turmeric extract-loaded nanostructured lipid carrier (NLC). Colloid Interface Sci. Commun. 2018, 22, 18–24. [Google Scholar] [CrossRef]
- Sun, M.; Nie, S.; Pan, X.; Zhang, R.; Fan, Z.; Wang, S. Quercetin-nanostructured lipid carriers: Characteristics and anti-breast cancer activities in vitro. Colloids Surf. B Biointerfaces 2014, 113, 15–24. [Google Scholar] [CrossRef]
- Kamel, A.E.; Fadel, M.; Louis, D. Curcumin-loaded nanostructured lipid carriers prepared using Peceol™ and olive oil in photodynamic therapy: Development and application in breast cancer cell line. Int. J. Nanomed. 2019, 14, 5073–5085. [Google Scholar] [CrossRef]
- Santos Pimentel, L.; Sommerfeld, S.; Fernanda de Sousa Braga, P.; Flores Coleto, A.; Beatriz Fonseca, B.; Machado Bastos, L.; Ricardo Goulart, L.; Nunes de Morais Ribeiro, L. Antitumor activity of essential oils-based nanostructured lipid carriers on prostate cancer cells. Int. J. Pharm. 2024, 657, 124149. [Google Scholar] [CrossRef]
- Shete, M.B.; Deshpande, A.S.; Shende, P. Enhancement of in-vitro anti-oral cancer activities of silymarin using dispersion of nanostructured lipid carrier in mucoadhesive in-situ gel. Int. J. Pharm. 2023, 636, 122860. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, Y.; Dang, W.; Xing, B.; Yu, C.; Guo, P.; Pi, J.; Deng, X.; Qi, D.; Liu, Z. The anti-tumor and renoprotection study of E-[c(RGDfK)2]/folic acid co-modified nanostructured lipid carrier loaded with doxorubicin hydrochloride/salvianolic acid A. J. Nanobiotechnol. 2022, 20, 425. [Google Scholar] [CrossRef] [PubMed]
- Arshad, S.; Asim, M.H.; Mahmood, A.; Ijaz, M.; Irfan, H.M.; Anwar, F.; Ali, M.Y. Calycosin-loaded nanostructured lipid carriers: In-vitro and in-vivo evaluation for enhanced anti-cancer potential. J. Drug Deliv. Sci. Technol. 2022, 67, 102957. [Google Scholar] [CrossRef]
- Issa, A.Y.; Volate, S.R.; Wargovich, M.J. The role of phytochemicals in inhibition of cancer and inflammation: New directions and perspectives. J. Food Compos. Anal. 2006, 19, 405–419. [Google Scholar] [CrossRef]
- Khan, A.; Jahan, S.; Imtiyaz, Z.; Alshahrani, S.; Antar Makeen, H.; Mohammed Alshehri, B.; Kumar, A.; Arafah, A.; Rehman, M.U. Neuroprotection: Targeting Multiple Pathways by Naturally Occurring Phytochemicals. Biomedicines 2020, 8, 284. [Google Scholar] [CrossRef]
- Upadhyay, P.; Ghosh, A.; Sarangthem, V.; Singh, T.D. Nanocarrier mediated co-delivery of phytochemicals and chemo-drugs: An emerging strategy to combat lung cancer in a systemic way. Phytochem. Rev. 2024, 23, 485–527. [Google Scholar] [CrossRef]
- Alhalmi, A.; Amin, S.; Khan, Z.; Beg, S.; Al Kamaly, O.; Saleh, A.; Kohli, K. Nanostructured Lipid Carrier-Based Codelivery of Raloxifene and Naringin: Formulation, Optimization, In Vitro, Ex Vivo, In Vivo Assessment, and Acute Toxicity Studies. Pharmaceutics 2022, 14, 1771. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Li, G.; Zhang, H.; Chen, X.; Li, Y.; Yao, Q.; Xie, M. Sequential delivery of dual drugs with nanostructured lipid carriers for improving synergistic tumor treatment effect. Drug Deliv. 2020, 27, 983–995. [Google Scholar] [CrossRef]
- Palei, N.N.; Mounika, G.; Mohanta, B.C.; Rajangam, J. Quercetin and Morin dual drug loaded nanostructured lipid carriers: Formulation and in vitro cytotoxicity study on MCF7 breast cancer cells. J. Dispers. Sci. Technol. 2024, 45, 2146–2154. [Google Scholar] [CrossRef]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug Discov. 2019, 18, 41–58. [Google Scholar] [CrossRef]
- Abou-Elnour, F.S.; El-Habashy, S.E.; Essawy, M.M.; Abdallah, O.Y. Codelivery of ivermectin and methyl dihydrojasmonate in nanostructured lipid carrier for synergistic antileukemia therapy. Int. J. Pharm. 2024, 656, 124086. [Google Scholar] [CrossRef] [PubMed]
- Alhalmi, A.; Beg, S.; Almalki, W.H.; Alghamdi, S.; Kohli, K. Recent Advances in Nanotechnology-Based Targeted Therapeutics for Breast Cancer Management. Curr. Drug Metab. 2022, 23, 587–602. [Google Scholar] [CrossRef] [PubMed]
- Costa, B.; Amorim, I.; Gärtner, F.; Vale, N. Understanding Breast cancer: From conventional therapies to repurposed drugs. Eur. J. Pharm. Sci. 2020, 151, 105401. [Google Scholar] [CrossRef]
- Kaur, P.; Mishra, V.; Shunmugaperumal, T.; Goyal, A.K.; Ghosh, G.; Rath, G. Inhalable spray dried lipidnanoparticles for the co-delivery of paclitaxel and doxorubicin in lung cancer. J. Drug Deliv. Sci. Technol. 2020, 56, 101502. [Google Scholar] [CrossRef]
- Gomaa, E.; Fathi, H.A.; Eissa, N.G.; Elsabahy, M. Methods for preparation of nanostructured lipid carriers. Methods 2022, 199, 3–8. [Google Scholar] [CrossRef]
- Subroto, E.; Andoyo, R.; Indiarto, R.; Wulandari, E.; Wadhiah, E.F.N. Preparation of Solid Lipid Nanoparticle-Ferrous Sulfate by Double Emulsion Method Based on Fat Rich in Monolaurin and Stearic Acid. Nanomaterials 2022, 12, 3054. [Google Scholar] [CrossRef]
- Koroleva, M.; Portnaya, I.; Mischenko, E.; Abutbul-Ionita, I.; Kolik-Shmuel, L.; Danino, D. Solid lipid nanoparticles and nanoemulsions with solid shell: Physical and thermal stability. J. Colloid Interface Sci. 2022, 610, 61–69. [Google Scholar] [CrossRef]
- Munir, M.; Zaman, M.; Waqar, M.A.; Khan, M.A.; Alvi, M.N. Solid lipid nanoparticles: A versatile approach for controlled release and targeted drug delivery. J. Liposome Res. 2024, 34, 335–348. [Google Scholar] [CrossRef]
- Maddiboyina, B.; Jhawat, V.; Nakkala, R.K.; Desu, P.K.; Gandhi, S. Design expert assisted formulation, characterization and optimization of microemulsion based solid lipid nanoparticles of repaglinide. Prog. Biomater. 2021, 10, 309–320. [Google Scholar] [CrossRef]
- Nguyen, A.T.; Ife, A.F.; Goff, A.; Russo, D.; Palombo, E.A.; Shah, R.M.; Eldridge, D.S. Encapsulation of Rifampicin in Solid Lipid Nanoparticles by Microwave-Assisted Microemulsion Method. Part. Part. Syst. Charact. 2025, 42, 2400177. [Google Scholar] [CrossRef]
- Krishnasailaja, A.; Gazi, A.S. Formulation of methotrexate loaded solid lipid nanoparticles by micro emulsion technique. Curr. Nanomater. 2023, 8, 153–161. [Google Scholar] [CrossRef]
- Weerapol, Y.; Manmuan, S.; Chaothanaphat, N.; Limmatvapirat, S.; Sirirak, J.; Tamdee, P.; Tubtimsri, S. New Approach for Preparing Solid Lipid Nanoparticles with Volatile Oil-Loaded Quercetin Using the Phase-Inversion Temperature Method. Pharmaceutics 2022, 14, 1984. [Google Scholar] [CrossRef]
- Della Sala, F.; Borzacchiello, A.; Dianzani, C.; Muntoni, E.; Argenziano, M.; Capucchio, M.T.; Valsania, M.C.; Bozza, A.; Garelli, S.; Di Muro, M.; et al. Ultrasmall Solid-Lipid Nanoparticles via the Polysorbate Sorbitan Phase-Inversion Temperature Technique: A Promising Vehicle for Antioxidant Delivery into the Skin. Pharmaceutics 2023, 15, 1962. [Google Scholar] [CrossRef]
- Jain, U.; Jaiswal, H. Designing 5-fluorouracil-loaded lipid nanoparticles using double emulsion and solvent evaporation for skin cancer therapy. Onkol. Radioter. 2023, 17, 935–942. [Google Scholar]
- Chaudhary, S.A.; Patel, D.M.; Patel, J.K.; Patel, D.H. Solvent emulsification evaporation and solvent emulsification diffusion techniques for nanoparticles. In Emerging Technologies for Nanoparticle Manufacturing; Springer: Berlin/Heidelberg, Germany, 2021; pp. 287–300. [Google Scholar]
- Shukla, R.; Singh, A.; Singh, K.K. Vincristine-based nanoformulations: A preclinical and clinical studies overview. Drug Deliv. Transl. Res. 2024, 14, 1–16. [Google Scholar] [CrossRef] [PubMed]
- López, K.L.; Ravasio, A.; González-Aramundiz, J.V.; Zacconi, F.C. Solid Lipid Nanoparticles (SLN) and Nanostructured Lipid Carriers (NLC) Prepared by Microwave and Ultrasound-Assisted Synthesis: Promising Green Strategies for the Nanoworld. Pharmaceutics 2023, 15, 1333. [Google Scholar] [CrossRef]
- Park, H.; Kim, J.S.; Kim, S.; Ha, E.S.; Kim, M.S.; Hwang, S.J. Pharmaceutical Applications of Supercritical Fluid Extraction of Emulsions for Micro-/Nanoparticle Formation. Pharmaceutics 2021, 13, 1928. [Google Scholar] [CrossRef]
- Prasedya, E.S.; Frediansyah, A.; Martyasari, N.W.R.; Ilhami, B.K.; Abidin, A.S.; Padmi, H.; Fahrurrozi; Juanssilfero, A.B.; Widyastuti, S.; Sunarwidhi, A.L. Effect of particle size on phytochemical composition and antioxidant properties of Sargassum cristaefolium ethanol extract. Sci. Rep. 2021, 11, 17876. [Google Scholar] [CrossRef]
- Ibrahim, N.; Abbas, H.; El-Sayed, N.S.; Gad, H.A. Rosmarinus officinalis L. hexane extract: Phytochemical analysis, nanoencapsulation, and in silico, in vitro, and in vivo anti-photoaging potential evaluation. Sci. Rep. 2022, 12, 13102. [Google Scholar] [CrossRef] [PubMed]
- Andonova, V.; Peneva, P. Characterization Methods for Solid Lipid Nanoparticles (SLN) and Nanostructured Lipid Carriers (NLC). Curr. Pharm. Des. 2017, 23, 6630–6642. [Google Scholar] [CrossRef]
- Sarkar, T.; Sarkar, T.; Banerjee, S.; Goswami, M.; Das, P.; Bhakat, A.; Sarkar, K. Formulation of curcumin loaded solid lipid nano particles following response surface methodology to improve the anti-cancer activity in triple-negative breast cancer cells. Mater. Today Commun. 2025, 45, 112220. [Google Scholar] [CrossRef]
- de Barros, D.P.C.; Santos, R.; Reed, P.; Fonseca, L.P.; Oliva, A. Design of Quercetin-Loaded Natural Oil-Based Nanostructured Lipid Carriers for the Treatment of Bacterial Skin Infections. Molecules 2022, 27, 8818. [Google Scholar] [CrossRef]
- Bansal, G.; Suthar, N.; Kaur, J.; Jain, A. Stability Testing of Herbal Drugs: Challenges, Regulatory Compliance and Perspectives. Phytother. Res. PTR 2016, 30, 1046–1058. [Google Scholar] [CrossRef]
- Isleroglu, H.; Turker, I. Ultrasonic-assisted extraction and thermal stability of phytochemicals from fenugreek leaves. J. Appl. Res. Med. Aromat. Plants 2022, 30, 100390. [Google Scholar] [CrossRef]
- İnanç Horuz, T.; Maskan, M. Effect of the phytochemicals curcumin, cinnamaldehyde, thymol and carvacrol on the oxidative stability of corn and palm oils at frying temperatures. J. Food Sci. Technol. 2015, 52, 8041–8049. [Google Scholar] [CrossRef] [PubMed]
- Gambaro, R.; Chain, C.Y.; Scioli-Montoto, S.; Moreno, A.; Huck-Iriart, C.; Ruiz, M.E.; Cisneros, J.S.; Lamas, D.G.; Tau, J.; Gehring, S.; et al. Phytoactive-Loaded Lipid Nanocarriers for Simvastatin Delivery: A Drug Repositioning Strategy Against Lung Cancer. Pharmaceutics 2025, 17, 255. [Google Scholar] [CrossRef]
- Shaker, S.A.; Alshufta, S.M.; Gowayed, M.A.; El-Salamouni, N.S.; Bassam, S.M.; Megahed, M.A.; El-Tahan, R.A. Propolis-loaded nanostructured lipid carriers halt breast cancer progression through miRNA-223 related pathways: An in-vitro/in-vivo experiment. Sci. Rep. 2023, 13, 15752. [Google Scholar] [CrossRef] [PubMed]
- Thapa, A.; Das, P.; Dua, T.K.; Paul, P.; Nandi, G.; Dey, S.; Kumar, A.; Sahu, R. Assessment of in vitro antioxidant, antidiabetic, anti-inflammatory, and cytotoxicity properties and HPTLC-EDA based phytochemical screening of Macropanax undulatus leaves: An ethnomedicinal plant of Darjeeling Himalayan region. Pharmacol. Res. Nat. Prod. 2025, 7, 100220. [Google Scholar] [CrossRef]
- Ghalbi Ahangari, M.; Farimani, M.M.; Erfani, M.; Goudarzi, M. Technetium-99m radiolabeling through conjugation with l, l-ethylene dicysteine chelator of a trimethoxylated flavone and its bioevaluation in rat with induced C6 glioma tumor as a new cancer diagnostic agent. Radiochim. Acta 2024, 112, 327–337. [Google Scholar] [CrossRef]
- Sousa Carvalho, G.F.; Marques, L.K.; Sousa, H.G.; Silva, L.R.; Leão Ferreira, D.C.; Pires de Moura do Amaral, F.; Martins Maia Filho, A.L.; Figueredo-Silva, J.; Alves, W.D.S.; Oliveira, M.; et al. Phytochemical study, molecular docking, genotoxicity and therapeutic efficacy of the aqueous extract of the stem bark of Ximenia americana L. in the treatment of experimental COPD in rats. J. Ethnopharmacol. 2020, 247, 112259. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Zhao, H.; Wu, Y.; Huang, L.; Nie, W.; Liu, H.; Wu, G.; Pang, D.W.; Xie, H.Y. Phytochemical natural killer cells reprogram tumor microenvironment for potent immunotherapy of solid tumors. Biomaterials 2022, 287, 121635. [Google Scholar] [CrossRef]
- Gupta, S.; Tejavath, K.K. Nano phytoceuticals: A step forward in tracking down paths for therapy against pancreatic ductal adenocarcinoma. J. Clust. Sci. 2022, 34, 1–21. [Google Scholar] [CrossRef]
- Zheng, T. Improving absorption and bioactivities of phytochemicals using protein/polysaccharide nanoparticle delivery systems. Ph.D. Thesis, Rutgers The State University of New Jersey, School of Graduate Studies, New Brunswick, NJ, USA, 2022. [Google Scholar]
- Valleti, P.V.; Kumar, V.; Ramayanam, P.K.; Gopalappa, R.; Vijendra Dittekoppa, P.; Cm, A.; Sillanpaa, M.; Al-Farraj, S. Multifaceted Anticancer Potential of Gnidia glauca (Fresen.) Gilg Leaf Alkaloids: Impact on Multiple Cellular Targets. ACS Omega 2024, 9, 9615–9624. [Google Scholar] [CrossRef]
- Choudhari, J.; Nimma, R.; Nimal, S.K.; Totakura Venkata, S.K.; Kundu, G.C.; Gacche, R.N. Prosopis juliflora (Sw.) DC phytochemicals induce apoptosis and inhibit cell proliferation signaling pathways, EMT, migration, invasion, angiogenesis and stem cell markers in melanoma cell lines. J. Ethnopharmacol. 2023, 312, 116472. [Google Scholar] [CrossRef]
- Nandi, S.; Nag, A.; Khatua, S.; Sen, S.; Chakraborty, N.; Naskar, A.; Acharya, K.; Calina, D.; Sharifi-Rad, J. Anticancer activity and other biomedical properties of β-sitosterol: Bridging phytochemistry and current pharmacological evidence for future translational approaches. Phytother. Res. PTR 2024, 38, 592–619. [Google Scholar] [CrossRef]
- Rajendran, P.; Renu, K.; Ali, E.M.; Genena, M.A.M.; Veeraraghavan, V.; Sekar, R.; Sekar, A.K.; Tejavat, S.; Barik, P.; Abdallah, B.M. Promising and challenging phytochemicals targeting LC3 mediated autophagy signaling in cancer therapy. Immun. Inflamm. Dis. 2024, 12, e70041. [Google Scholar] [CrossRef]
- Rodríguez-Gómez, F.D.; Monferrer, D.; Penon, O.; Rivera-Gil, P. Regulatory pathways and guidelines for nanotechnology-enabled health products: A comparative review of EU and US frameworks. Front. Med. 2025, 12, 1544393. [Google Scholar] [CrossRef] [PubMed]
- Ickenstein, L.M.; Garidel, P. Lipid-based nanoparticle formulations for small molecules and RNA drugs. Expert Opin. Drug Deliv. 2019, 16, 1205–1226. [Google Scholar] [CrossRef] [PubMed]
- Assadpour, E.; Mahdi Jafari, S. A systematic review on nanoencapsulation of food bioactive ingredients and nutraceuticals by various nanocarriers. Crit. Rev. Food Sci. Nutr. 2019, 59, 3129–3151. [Google Scholar] [CrossRef]
- Abad, F.R.A.; Pourmadadi, M.; Abdouss, M. Development and characterization of a novel pH-Responsive nanocarrier for enhanced quercetin delivery and cytotoxicity in lung cancer. Inorg. Chem. Commun. 2024, 170, 113175. [Google Scholar] [CrossRef]
- Vargas, K.M.; Shon, Y.S. Hybrid lipid-nanoparticle complexes for biomedical applications. J. Mater. Chem. B 2019, 7, 695–708. [Google Scholar] [CrossRef]
- Mennati, A.; Rostamizadeh, K.; Manjili, H.K.; Fathi, M.; Danafar, H. Co-delivery of siRNA and lycopene encapsulated hybrid lipid nanoparticles for dual silencing of insulin-like growth factor 1 receptor in MCF-7 breast cancer cell line. Int. J. Biol. Macromol. 2022, 200, 335–349. [Google Scholar] [CrossRef]
- Gan, Y.; Xu, D.; Zhang, J.; Wang, Z.; Wang, S.; Guo, H.; Zhang, K.; Li, Y.; Wang, Y. Rana chensinensis Ovum Oil Based on CO2 Supercritical Fluid Extraction: Response Surface Methodology Optimization and Unsaturated Fatty Acid Ingredient Analysis. Molecules 2020, 25, 4170. [Google Scholar] [CrossRef]
- Piccolella, S.; Crescente, G.; Candela, L.; Pacifico, S. Nutraceutical polyphenols: New analytical challenges and opportunities. J. Pharm. Biomed. Anal. 2019, 175, 112774. [Google Scholar] [CrossRef] [PubMed]
- Antal, D.S.; Ardelean, F. Chapter 16—Challenges in the nanoscale delivery systems development in the pharmaceutical and nutraceutical markets. In Mitochondrial Dysfunction and Nanotherapeutics; de Oliveira, M.R., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 441–458. [Google Scholar]
- Watkins, R.; Wu, L.; Zhang, C.; Davis, R.M.; Xu, B. Natural product-based nanomedicine: Recent advances and issues. Int. J. Nanomed. 2015, 10, 6055–6074. [Google Scholar] [CrossRef]
- Soares, S.; Sousa, J.; Pais, A.; Vitorino, C. Nanomedicine: Principles, Properties, and Regulatory Issues. Front. Chem. 2018, 6, 360. [Google Scholar] [CrossRef] [PubMed]
- Agrahari, V.; Hiremath, P. Challenges associated and approaches for successful translation of nanomedicines into commercial products. Nanomedicine 2017, 12, 819–823. [Google Scholar] [CrossRef] [PubMed]

| Key Botanical Source | Phytochemical Name | Major Phytochemical Class | Key Physicochemical Properties | Formulation Challenges |
|---|---|---|---|---|
| Vitis vinifera | Resveratrol | Polyphenols | Molecular weight: 228.24 g/mol Log P: 3.1 (moderately lipophilic) Solubility: Less soluble in water, soluble in ethanol and other organic solvents | Poor aqueous solubility, rapid metabolism, sensitive to light, heat, oxygen, and pH |
| Curcuma longa | Curcumin | Polyphenols | Molecular weight: 368.38 g/mol Log P: 3.29 Solubility: Very poor water solubility, soluble in ethanol, DMSO, and acetone | Poor aqueous solubility, unstable under physiological pH, sensitive to light, heat, and oxidative conditions |
| Berberis aristata | Berberine | Alkaloids | Molecular weight: 336.36 g/mol Log P: 1.5 (moderate hydrophilicity) Solubility: Poor water solubility, soluble in acidic aqueous solutions and alcohols | Low water solubility, sensitive to light and heat, rapid metabolism and exhibits poor intestinal absorption |
| Catharanthus roseus | Vincristine/Vinblastine | Alkaloids | Molecular weight: 824.98 g/mol/811.04 g/mol Log P: 1.7/3.3 Solubility: Sparingly soluble in water, more soluble in acidic aqueous and organic solvents | Poor aqueous solubility, sensitive to elevated temperature and light, hydrolyses in alkaline pH |
| Taxus brevifolia | Paclitaxel | Alkaloids | Molecular weight: 853.91 g/mol Log P: 3.96 Solubility-Insoluble in water, soluble in organic solvents like Cremophor EL, ethanol, DMSO | Low water solubility, prone to degradation under extreme pH, light, and heat, high toxicity |
| Camellia sinensis | Epigallocatechin-3-gallate (EGCG) | Polyphenol | Molecular weight: 458.7 g/mol Log P: 0.1 Solubility: Soluble in water, acetone, ethanol, pyridine, tetrahydrofuran, and methanol | Unstable at neutral and alkaline pH, degrades upon exposure to light, heat, and oxygen, poor absorption |
| Glycine max | Genistein | Alkaloids | Molecular weight: 270.24 g/mol Log P: 2.62 (moderately lipophilic) Solubility: Poorly soluble in water, more soluble in organic solvents like ethanol and DMSO | Low aqueous solubility, sensitive to light, heat, oxygen, and pH, unstable under alkaline conditions |
| Camptotheca acuminata | Camptothecin | Alkaloids | Molecular weight: 348.35 g/mol Log P: 1.71 Solubility: Very poor water solubility, soluble in DMSO and ethanol | Low water solubility, unstable at neutral and basic pH |
| Quercus robur | Quercetin | Polyphenols | Molecular weight: 302.24 g/mol Log P: 1.82 Solubility: Poorly soluble in water, soluble in alcohol and alkaline solutions | Poor aqueous solubility, rapid metabolism, unstable in alkaline conditions, sensitive to light and oxidation |
| Nigella sativa | Thymoquinone | Terpenoids | Molecular weight: 164.20 g/mol Log P: ~2.54 Solubility: Insoluble in water, soluble in ethanol, methanol, chloroform, DMSO, and acetone | Poor aqueous solubility, Unstable in aqueous medium, sensitive to light, heat, and oxidation, and metabolic instability |
| Beta vulgaris | Betalains | Betacyanins and Betaxanthins | Molecular weight: 550 g/mol Log P: −3.1 Solubility: Highly soluble in water, insoluble in organic solvents | Poor stability under light, heat, and oxygen exposure, degradation at neutral or alkaline pH, limited lipophilicity, restricting incorporation into lipid-based systems, susceptibility to enzymatic and oxidative degradation during processing and storage |
| Rosmarinus officinalis | Ursolic acid | Triterpenoids | Molecular weight: 456.70 g/mol Log P: 7.09 Solubility: Very poor water solubility, soluble in organic solvents like ethanol, DMSO | Poor water solubility, very high lipophilicity, rapid metabolism, and poor absorption |
| Brassica oleracea | Sulforaphane | Isothiocyanates | Molecular weight: 177.28 g/mol Log P: 0.23 Solubility: Soluble in methanol, ethanol, DMSO, or ethyl acetate, water insoluble | Poor aqueous solubility, stability issues, short half-life, poor lipophilicity, rapid metabolism |
| Plumbago zeylanica | Plumbagin | Naphthoquinones | Molecular weight: 188.18 g/mol Log P: 2.26 Solubility: Low water solubility, soluble in organic solvents like ethanol and DMSO | Low water solubility, prone to oxidation, sensitive to light and air, and potential toxicity |
| Solanum lycopersicum | Lycopene | Carotenoids | Molecular weight: 536.89 g/mol Log P: 17.6 (extremely lipophilic) Solubility: Insoluble in water, soluble in oils, organic solvents such as chloroform, hexane | Low aqueous solubility, poor absorption, highly sensitive to light, heat, and oxygen |
| Tabebuia avellanedae | β-Lapachone | Quinones | Molecular weight: 242.23 g/mol Log P: 2.35 Solubility: Poorly soluble in water, soluble in DMSO, ethanol, and other organic solvents | Poor aqueous solubility, unstable in aqueous environment, sensitive to light and oxygen |
| Nanocarrier System | Composition | Encapsulation Efficiency | Stability | Biocompatibility | Targeting Ability | Scale-Up Potential | Applications |
|---|---|---|---|---|---|---|---|
| Solid Lipid Nanoparticles (SLNs) | Solid lipids, surfactants, cosurfactants | High for lipophilic drugs | Good | High | Moderate | Good | Drug and phytochemical delivery, cancer therapy |
| Nanostructured Lipid Carriers (NLCs) | Solid lipids, liquid lipids, surfactants, cosurfactants | High | Better than SLNs | High | High | Good | Drug and phytochemical delivery, chronic diseases |
| Nanoemulsions | Oil, water, surfactants, cosurfactants | Moderate to high | Low | High | Low | Good | Rapid drug delivery |
| Liposomes | Phospholipids, cholesterol, charged lipids (optional), hydration medium | Moderate | Moderate | High | Moderate | Challenging | Vaccines, drugs, and gene delivery |
| Niosomes | Non-ionic surfactants, cholesterol, charged inducers (optional), hydration medium | Moderate | Moderate | High | Moderate | Challenging | Topical and systemic delivery |
| Cubosomes | Lipids, stabilizers, aqueous phase | High | Good | High | High | Moderate | Dermal delivery |
| Ivosomes | Phospholipids, ionic liquids, stabilizers (optional), aqueous phase | Moderate | Limited data | Limited data | Moderate | Low | Emerging drug delivery |
| Ethosomes | Phospholipids, ethanol, water, optional additives (cholesterol, Propylene glycol, or isopropyl alcohol) | High | Low to moderate | High | High | Moderate | Transdermal drug delivery |
| Transfersomes | Phospholipids, edge activators (surfactants), optional additives (Ethanol or glycerol, Cholesterol), aqueous phase | High | Low | High | High | Moderate | Transdermal and systemic delivery |
| Transethosomes | Phospholipids, ethanol, edge activators (surfactants), optional additives (Cholesterol or Propylene glycol), aqueous phase | High | Low | High | High | Moderate | Transdermal and systemic delivery |
| Inorganic Nanoparticles | Metal and Metal oxides, Silica, Carbon nanotubes, Graphene oxide, Fullerenes, Quantum dots, etc. | Variable | High | Variable | High | Good | Bioimaging, biosensing, diagnostics, and cancer therapy |
| Polymeric Nanoparticles | Natural/synthetic polymers, crosslinking agents | High | Good | High | High | Good | Sustained release |
| Dendrimers | Branched synthetic polymers, surface modifiers | Very high | Good | Moderate to high | High | Moderate | Gene delivery, diagnostics |
| Polymeric micelles | Amphiphilic block copolymers (e.g., PEG-PLA, PEG-PCL) | Moderate to High | Good | Good | Good | Moderate | Poorly soluble anticancer drugs and phytochemicals, and diagnostics |
| Structural Component | Examples |
|---|---|
| Solid Lipids | Behenic acid, Beeswax, Carnauba wax, Cetyl palmitate, Glyceryl behenate (Compritol 888 ATO), Glyceryl caprate, Glyceryl monooleate, Glyceryl monostearate (Imwitor 900), Glyceryl palmitostearate (Precirol ATO 5), Hard fat, Hydrogenated vegetable oils, Labrafil M1944, Miglyol 812, Monostearin, Oleic acid, Palmitic acid, Paraffin, Polyethylene glycol (PEG) monostearate, Stearic acid, Tricaprin, Trilaurin, Trimyristin (Dynasan 114), Tripalmitin (Dynasan 116), Tri-stearin (Dynasan 118), Tristearin, Witepsol. |
| Emulsifiers | Butanol, Butyric acid, Cetylpyridinium chloride, Cremophor EL, Eumulgin SML 20, Lecithin, PEG-40 hydrogenated castor oil, Poloxamer 188, Poloxamer 407, Polysorbate 20, Polysorbate 60, Polysorbate 80, Polyvinyl alcohol, Sodium cholate, Sodium deoxycholate, Sodium dodecyl sulfate, Sodium glycocholate, Sodium oleate, Solutol HS 15, Span 20, Span 60, Span 80, Taurodeoxycholic acid sodium, Tyloxapol, Tween 20, Tween 60, Tween 80. |
| Co-Surfactants | Ethanol, Glycerol, PEG-400, Propylene glycol, Sodium glycocholate, Sodium taurocholate, Transcutol P. |
| Cryoprotectants | Glucose, Mannitol, Sorbitol, Sucrose, Trehalose. |
| Charge Modifiers | Dimethyldioctadecyl ammonium bromide, Dodecylamine (cationic), Sodium stearate (anionic), Stearylamine (cationic). |
| Penetration Enhancers | Ethanol, Linoleic acid, Menthol, Oleic acid. |
| Targeting Ligands | Aptamers, Folic acid, Monoclonal antibodies, Peptides, Transferrin. |
| Polymer Coatings | Chitosan, Hyaluronic acid, PEG, Polyvinyl alcohol. |
| Solid Lipid | Liquid Lipid | Surfactant |
|---|---|---|
| Beeswax | Caprylic/capric triglyceride | Lecithin |
| Cetyl palmitate | Carvacrol | PEG 400 |
| Glyceryl behenate (Compritol® 888 ATO) | Crodamolt glyceryl tricaprylate/caprate liquid | Pluronic F127 |
| Glyceryl monostearate | Long-chain triglycerides | Poloxamer 188 |
| Myristyl myristate | Medium chain triglyceride | Polysorbate 80 |
| Glyceryl palmitostearate (Precirol® ATO-5) | Miglyol 812N | Sodium lauryl sulfate |
| Stearic acid | Oleic acid | Span 80 |
| Tripalmitin | Squalene | Tween® 60 |
| Tristearin | Triglyceride esters | Tween® 80 |
| Name | Preparation Method | Composition | Therapeutic Application | Highlights | Reference |
|---|---|---|---|---|---|
| Cinnamon, Sage, and Thyme essential oils | Emulsification-ultrasonication | Shorea butter, poloxamer 188 | Prostate cancer | Three NLC formulations incorporating cinnamon, sage, or thyme essential oils, optimized using a 23 factorial design, demonstrated excellent structural integrity and good stability at 25 °C for over a year. The NLCs were biocompatible in vitro with normal prostate (PNT2) cells and in vivo in chicken embryos. In prostate cancer (PC3) cells, the NLCs inhibited proliferation and migration and altered cell morphology. In a chicken embryo xenograft model, they suppressed tumor growth and angiogenesis. | [228] |
| Silymarin | Hot melt emulsification | Compritol ATO 888/Miglyol 812 N, Tween 80 | Oral cancer | Silymarin, a poorly water-soluble compound, was successfully encapsulated in NLCs with a particle size of ~316 nm, a PDI of 0.341, and 71% encapsulation efficiency. The developed gel demonstrated sustained drug release and enhanced buccal retention. Compared to plain compound and NLCs, the gel showed a lower IC50 value against KB oral cancer cells, indicating greater cytotoxicity due to increased reactive oxygen species generation and apoptosis at the Sub-G0 phase. | [229] |
| Salvianolic acid | Emulsification-solvent evaporation method | Myrj 52, Lecithin, DSPE-PEG2000-E-[c(RGDfK)2]), Glycerol behenate, MCT 812, DSPE-PEG2000-Folate | Antitumour | Dual-targeted NLCs co-loaded with doxorubicin and salvianolic acid demonstrated high encapsulation efficiency (>80%) and small particle size (~18 nm). Surface modification with E-[c(RGDfK)2] and folic acid enabled effective targeting of various tumor cells. This formulation showed the strongest anti-tumor effects in vitro and in vivo. Polyphenolic acid mitigated doxorubicin-induced nephrotoxicity, reducing creatinine levels by 61.64% (free form) and 42.47% (NLCs). The E-[c(RGDfK)2]/FA modification further reduced kidney toxicity by 46.35% compared to the unmodified NLC-salvianolic acid/doxorubicin group. | [230] |
| Curcumin | High shear hot homogenization | Glyceryl monooleate, Geleol, Olive oil, Tween 80, Lecithin | Breast cancer | Curcumin-loaded NLCs prepared with glyceryl monooleate demonstrated faster drug release and significantly higher anticancer activity compared to NLCs prepared with Geleol™ and free curcumin under both light and dark conditions. The enhanced cellular uptake was attributed to their small particle size, spherical morphology, and negative zeta potential. Furthermore, glyceryl monooleate contributed to the inhibition of P-glycoprotein expression, thereby enhancing the cytotoxic effects of curcumin. | [227] |
| Calycosin | Nano-template engineering approach | Miglyol, stearic acid, Tween 80, Span 60, PEG 400, Sucrose stearate | Breast cancer | Calycosin-loaded NLCs exhibited nanoparticle size (100 nm), low PDI (0.27), negative zeta potential (−24.5 mV), spherical morphology, high encapsulation efficiency (89%), and sustained drug release. In vitro studies on MDA-MB-231 cells revealed enhanced apoptosis and dose- and time-dependent cytotoxicity, while in vivo evaluations confirmed significant antitumor activity through biochemical and immunohistochemical analyses. | [231] |
| Method | Procedure | Mechanism | Advantages | Limitations | Formulation/Processing Factors | Reference |
|---|---|---|---|---|---|---|
| Double Emulsion Method | A water-in-oil-in-water (w/o/w) multiple emulsion is formed by dispersing a primary water-in-oil (w/o) emulsion stabilized with a hydrophobic emulsifying agents into an external aqueous phase that contains a hydrophilic surfactant. Nanoparticles are then formed through continuous stirring and solvent evaporation. | Solvent evaporation results in emulsion solidification and lipid crystallization. | Best suited for hydrophilic and peptide-based drugs. Surface alteration of nanocarriers is feasible with water soluble polymers | Requirement of multiple steps, prone to instability (particle coalescence), and low encapsulation efficiency. | Type and concentration of surfactants, phase-volume ratio/Stirring rate, duration, and solvent evaporation conditions. | [244] |
| High-Pressure Homogenization (Hot and Cold Methods) | Hot: Molten lipids are blended with active ingredients and emulsified with a heated aqueous surfactant solution. This mixture is then subjected to high-pressure homogenization (400–800 bar), generating high-velocity streams (>25 m/s) and intense turbulence to form nanoparticles. Cold: Phytochemical is dispersed in molten lipids, rapidly cooled, and ground into microparticles (50–100 µm). These are then mixed with a cold aqueous stabilizer and homogenized at room temperature to maintain drug stability and minimize drug partitioning into the aqueous phase. | Combination of mechanical shear, cavitation, and turbulence disrupts larger particles, leading to the formation of a stable submicron dispersion. | Produces small particles (<500 nm), high stability, no organic solvents, ideal for thermostable phytochemicals, aseptic processing, ease of scale-up, and low risk of product contamination | High energy and temperature input can degrade thermolabile phytochemicals, aggregation risk, generation of supercooled melts, diverse colloidal structures, and phytochemical partitioning into the water phase | Type and concentration of surfactants, lipid, and stabilizers, phytochemical-to-lipid ratio/Homogenization pressure and number of cycles, pre-emulsification conditions, and cooling rate | [123,245] |
| Membrane Contractor Technique | Melted lipid phase forced through membrane pores maintained above its melting temperature. Formed droplets are then carried into an aqueous surfactant solution flowing tangentially to the membrane, followed by cooling to ambient conditions, results in the formation of SLNs. | Spontaneous emulsification is initiated at the interface of the membrane due to interfacial tension gradients | Continuous, scalable process, particle size can be controlled based on flux through the membrane | Many process parameters and formulation variables, risk of membrane clogging, and high cost | Type and concentration of surfactant, lipid melting point and viscosity/Membrane pore size, operating pressure, tangential flow rate of aqueous phase, and cooling rate | [246] |
| Microemulsion Method | Melted lipid is mixed with a surfactant and co-surfactant aqueous solution in a specific ratio, forming a microemulsion when dispersed and diluted with cold water (1:25 to 1:100) under stirring. | Negative surface free energy, driven by a significant decrease in interfacial tension and significant entropy gain during mixing, leads to a spontaneous and thermodynamically stable nano-sized dispersion | Thermodynamically stable, high encapsulation efficiency and low energy method. | Requires high surfactant/co-surfactant concentrations and a large water volume, which may require further processing steps to obtain a concentrated product. | Surfactant/Co-surfactant type and ratio, lipid concentration, oil to water ratio, drug-lipid-ratio/Stirring rate and duration | [247,248,249] |
| Phase Inversion Temperature Method | The emulsion is heated to the phase inversion temperature, where surfactant affinity for oil and water is balanced, then rapidly cooled to form small, stable droplets. | The method relies on temperature-dependent changes in nonionic surfactants, where heating to the critical temperature balances surfactant affinity for oil and water | Low energy process, requires limited amount of surfactant, uniform sized nanodroplets, highly stable SLNs and economical | Several temperature cycles required, stability affected by cooling rate. | Type and concentration of non-ionic surfactant, oil to water ratio, phytochemical to lipid ratio/Heating rate and phase inversion temperature, number of heating and cooling cycles | [250,251] |
| Solvent Emulsification/Evaporation | Lipids and phytochemicals are dissolved in an organic solvent, emulsified in an aqueous phase, and nanosized through high-speed homogenization. Vacuum evaporation (Rotavapor) removes the solvent, causing nanoparticle precipitation. | Emulsification followed by evaporation of organic solvent leads to precipitation of lipid nanoparticles. | Suitable for hydrophobic and thermolabile drugs, uniform size distribution. | Insolubility of lipids in organic solvents, residual solvent toxicity concerns, environmental issues, and required additional drying or ultrafiltration processing | Surfactant type and concentration, type of organic solvent, solvent to water ratio, type and concentration of lipid, phytochemical to lipid ratio/Emulsification technique and speed | [252,253] |
| Solvent Injection Method | Lipid and phytochemical are dissolved in a water-miscible organic solvent and injected rapidly into an aqueous surfactant solution under continuous mechanical agitation. Obtained coarse emulsion is nanosized via high-speed homogenization, followed by vacuum evaporation (Rotavapor) to remove the solvent, leading to nanoparticle precipitation. | Solvent diffusion from the lipid to the aqueous phase, combined with interfacial cavitation and vibration, results in the formation of nanosized lipid nanoparticles. | Simple and fast process, simple equipment, and avoids toxic organic solvents | Limited scalability, residual solvent removal needed. | Organic solvent type, surfactant and lipid type and concentration, phytochemical to lipid ratio/Injection rate, stirring rate and speed, aqueous phase temperature, solvent removal conditions | [254] |
| Sonocrystallization (Ultrasound-Assisted Method) | Lipid and phytochemical mixture is sonicated in an aqueous surfactant solution, causing cavitation, which leads to the formation of nanoparticles. | Ultrasound energy breaks lipid droplets into smaller particles. | Uniform nanoparticles, suitable for heat-sensitive drugs. | Potential probe contamination, not easily scalable. | Surfactant and lipid type and concentration, drug to lipid concentration/Sonication time and intensity, and crystallization conditions | [255] |
| Supercritical Fluid Technology | Lipid and phytochemical dissolved in supercritical CO2 under pressure expanded rapidly by spraying through a nozzle or atomizer to form nanoparticles. | Rapid expansion results in escape of gas, leads to particle precipitation. | Elimination of organic solvents, broad miscibility of lipids with gases, and the ability to produce SLNs in dry powder form. | Expensive equipment, high operational cost. | Type of supercritical fluid, lipid/drug solubility in supercritical fluid/Nozzle design and diameter, pressure and temperature, expansion rate, drying method | [180,256] |
| Assessment Category | Evaluation Parameters | Primary Tools/Methods | Significance | Reference |
|---|---|---|---|---|
| Particle size analysis | Size range (nm) and polydispersity index (PDI) | Dynamic light scattering (Zetasizer) | Nanoparticles with 10–200 nm size range are ideal for passive targeting of tumor tissue via the EPR effect. PDI < 0.3 indicates uniform particle size, reducing the risk of aggregation or phase separation during storage | [257] |
| Surface potential | Surface charge (mV) | Dynamic Light Scattering (Zetasizer) | Values > ±30 mV prevent aggregation and indicate good electrostatic stability | [258] |
| Surface morphology | Shape, surface texture, aggregation/Clustering Core–shell Structure (TEM) Crystallinity/amorphous nature (TEM) 3D Surface topography (AFM) | Scanning electron microscopy (SEM), Transmission electron microscopy (TEM), or Atomic force microscopy (AFM) | Influence biological performance, release behavior, and stability | [259] |
| Phytochemical encapsulation and loading efficiency | Encapsulation efficiency (%) = Total phytochemical-Free phytochemical/Total phytochemical) × 100 Loading capacity (%) = (Encapsulated phytochemical/Total weight of nanoparticles) × 100 | Ultraviolet spectroscopy (UV) High-performance liquid chromatography (HPLC) Centrifugation or ultrafiltration | High encapsulation efficiency and loading capacity ensure minimal phytochemical loss, maximized therapeutic output, and reduced toxicity | [111] |
| In vitro release studies | Cumulative phytochemical release, release kinetics | Franz diffusion system, dialysis setup with artificial membrane (cellulose or dialysis membrane) | Predicts in vivo behavior in physiological and tumor-specific environments. | [260] |
| Passive permeation | Flux, permeability coefficient, lag time | Franz diffusion cell, permeability chambers, side-by-side diffusion chambers with artificial (PAMPA, Caco-2) or biological membrane (excised tissues) | Assess the performance of phytochemical transport across biological membranes to help predict in vivo therapeutic outcomes | [261] |
| Long-Term Stability | Monitor formulation stability over time by assessing various in vitro characterization parameters | Stability chambers (temperature and humidity, oxidative stress chambers, photostability chambers | Retain their therapeutic potency, physicochemical integrity, and safety over time | [262,263,264] |
| Reactive Oxygen Species (ROS) generation | Fluorescence Intensity, % ROS-positive cells, mean fluorescence intensity, time and dose dependent ROS production, ROS source specificity | Microplate reader, flow cytometer, or fluorescence microscope after staining with ROS-sensitive dyes., 2′,7′-Dichlorodihydrofluorescein diacetate (DCFH-DA), use of N-Acetylcysteine (NAC) or Mitochondria-targeted hydroethidine (MitoSOX) | Provide mechanistic insight into ROS-mediated cytotoxicity, validate enhanced intracellular delivery, trigger apoptosis or autophagy, support combination therapy design, and early biobarker for therapeutic response | [265] |
| Protein/Gene expression analysis | Evaluation parameters include the ratio of gene/protein expression in treated cells versus control (fold change), relative quantification, band intensity, protein concentration, expression of key pathway markers including apoptosis (Bax, Bcl-2, caspase-3, PARP), autophagy (LC3-II, Beclin-1, p62/SQSTM1), cell cycle regulation (Cyclins, CDKs, p21, p53), oxidative stress (Nrf2, HO-1, SOD), drug resistance (P-gp, MRP1, ABC transporters) | qRT-PCR or RT-PCR, microarray analysis, RNA-Seq, Western blotting, ELISA, Immunofluorescence/Immunocytochemistry (IF/ICC), Flow cytometry, Mass spectrometry-based proteomics | Reveals molecular mechanism of action, confirms pathway-specific targeting, supports selectivity and safety, guides in vivo translation and biomarker identification | [266] |
| Cytotoxicity assay | IC50 value, percentage cell viability, time and dose-dependent cytotoxicity | MTT/XTT/MTS, Resazurin (Alamar Blue), Tryptan blue Exclusion, LDH release | Evaluate the anticancer potential by measuring cell viability and potency (e.g., IC50), and provide initial insights into cytotoxic effects | [267] |
| Pharmacokinetics and Biodistribution mapping | Drug concentration in tissues, tumor-to-organ ratio, plasma drug concentration (Cmax, Tmax, t½), biodistribution profile (graphical/heatmap), fluorescence or radioactivity intensity, organ accumulation index | Fluorescence imaging (DiR, FITC-labeled), radiolabel tracking, quantification (HPLC, LC-MS/MS), imaging (MRI, PET, SPECT) | Evaluates site-specific delivery, validates nanoformulation efficacy and safety, correlates therapeutic outcomes, and guides route and dosage optimization | [268] |
| Animal models (xenograft and synergistic) of cancer | Tumor volume and weight, tumor growth inhibition, survival rate/median survival time, tumor doubling time, histopathological and hematological examination | Digital vernier calipers, bioluminescence imaging, ultrasound/MRI/PET-CT, hematoxylin and eosin staining, immunohistochemistry, TUNEL assay, fluorescence/confocal microscopy, markers of organs such as liver (ALT, AST), and kidney (creatinine, BUN) | Validating therapeutic efficacy and safety, revealing molecular mechanisms, mapping biodistribution, preclinical validation before human clinical trials. | [269] |
| Cellular Uptake | Uptake efficiency, intracellular localization, mean fluorescence intensity, mechanisms of uptake, concentration and time dependent uptake | Fluorescent microscopy, flow cytometry, confocal imaging, clathrin-mediated, caveolae-mediated, or micropinocytosis inhibitors | Confirm effective phytochemical delivery, predicts therapeutic efficacy, guides formulation optimization and mechanistic understanding | [270,271,272] |
| Mitochondrial Membrane Potential (ΔΨm) Assay | Fluorescence intensity, red/green fluorescence ratio, percentage of cells with depolarized mitochondria, time and dose dependent response | JC-1, Rhodamine 123 dyes, flow cytometry | Early detection of apoptosis, assessment of mitochondrial health, mechanistic insights into cytotoxicity and screening of mitochondrial targeting compounds | [273] |
| Apoptosis/Necrosis Assay | Differentiate between apoptosis and necrosis via Annexin V-FITC/Propidium Iodide (PI) staining, Percentage of cells in each quadrant, and mean fluorescent intensity | Annexin V-FITC/PI staining, flow cytometry Caspase-3/7 activity assay, TUNEL assay, DAPI or Hoechst staining, Western blot for Bax, Bcl-2, cleaved PARP | Confirms efficacy of nanoformulations, mechanism of cell death, validates target action on cancer cells, supports dose optimization and safety | [274,275] |
| Autophagy Assay | LC3-II expression levels, LC3 puncta formation, Autophagic flux, p62/SQSTM1 levels, Acridine orange (AO) or monodansylcadaverine (MDC) staining, Beclin-1 expression | Immunofluorescence microscopy (LC3-GFP or LC3-mCherry), lysosomal inhibitors (e.g., bafilomycin A1, chloroquine), fluorescence microscopy, flow cytometry, RT-qPCR | Reveals autophagy’s role in cell survival or cell death, elucidates mechanism of action, predicts drug resistance or sensitization, guides combination strategies with autophagy enhancers or inhibitors | [276] |
| Application ID | Publication Date | Title | Summary of Invention |
|---|---|---|---|
| 201811376407.9 | 26 May 2020 | Preparation method of folic acid-targeted silymarin solid lipid nanoparticles | A folic acid-modified silymarin solid lipid nanoparticle system was developed to enhance lung tumor targeting. The formulation involves conjugating silymarin-loaded SLNs with folic acid–PEG3350–cephalin, enabling selective delivery to tumor cells. This targeted approach improves silymarin’s bioavailability, reduces toxicity, enhances therapeutic efficacy, and supports early patient recovery. The method is simple, economical, and environmentally friendly, making it suitable for clinical application. |
| 202010890499.3 | 18 December 2020 | Preparation method and application of quercetin (QT) and MicroRNA-150 co-loaded cationic solid lipid nanoparticles (SLNs) | Describes a method for preparing cationic SLNs co-loaded with quercetin and microRNA-150. The resulting nanoparticles exhibit good stability, biocompatibility, and effectively deliver both into HUVEC cells. |
| 202111029614 | 1 July 2021 | A formulation of hesperidin containing solid lipid nanoparticles through oral route and methods thereof | Hesperidin-loaded SLNs were developed using cold homogenization and ultrasonication to improve hesperidin’s poor solubility and low bioavailability. The formulation was evaluated for particle size, entrapment efficiency, drug content, diffusion, and morphology, aiming to enhance its effectiveness for oral delivery. |
| 17439617 | 19 May 2022 | Solid lipid nanoparticles of curcumin | Discloses a method for preparing curcumin-loaded SLNs with particle sizes ranging from 20 to 800 nm and exhibits high entrapment efficiency (50–100%). |
| 202210290513.5 | 28 June 2022 | Indirubin solid lipid nanoparticles and preparation method thereof | Indirubin-loaded solid lipid nanoparticles were developed using biocompatible materials to enhance the drug’s solubility, membrane permeability, and oral bioavailability. The formulation consists of indirubin (1–5%), lipid material (85–95%), and an emulsifier (3–10%), offering an improved delivery system for this traditional Chinese medicine compound. |
| 3207752 | 11 August 2022 | Process for preparing nanoformulation for delivery of berbamine | This invention describes a simple and efficient method for preparing berbamine-loaded solid lipid sustained-release nanoparticles. By adjusting the pH of the aqueous or lipid phase during formulation, the process achieves a high drug loading (12–50% w/w) and entrapment efficiency above 90%. |
| 202211064052 | 18 November 2022 | Resveratrol loaded nanoparticles and preparation method thereof | The formulation is prepared by dissolving resveratrol, soya phosphatidylcholine S-100, and tristearin in a chloroform-methanol mix, followed by emulsification, sonication, solvent evaporation, and purification through centrifugation. |
| 202221069827 | 30 December 2022 | Naringenin loaded solid lipid nanoparticles for oral delivery | Naringenin-loaded SLNs were developed using compritol to enhance its low oral bioavailability. Optimized using response surface methodology, the SLNs showed a particle size of 66.56 nm, good stability, and a sustained drug release of 94.97% over 24 h. Pharmacokinetic evaluation in rats demonstrated a 3.1-fold enhancement in bioavailability compared to naringenin suspension, indicating the formulation’s potential for improved oral delivery via intestinal lymphatic transport. |
| Application ID | Publication Date | Title | Summary of Invention |
|---|---|---|---|
| 201110029485.3 | 27 January 2011 | Resveratrol nanostructured lipid carrier and preparation method thereof | This invention presents an NLC formulation containing 0.1–1 wt% resveratrol, 2–20 wt% emulsifier, 2–30 wt% composite lipids (glyceryl triacetate, acetylated monoglyceride, and diisopropyl adipate), and water as the balance. The formulation offers enhanced water solubility, good stability, and is well-suited for use in cosmetic products. |
| 102016000602686 | 7 December 2016 | N-acetyl-L-cysteine modified curcumin nanostructured lipid carrier used for oral administration | This invention relates to an orally administered NLC modified with N-acetyl-L-cysteine for enhanced delivery of curcumin. The formulation includes curcumin, surfactants, lipid components, and N-acetyl-L-cysteine or its derivative. This NLC significantly improves curcumin’s water solubility, promotes its absorption, and enhances its oral bioavailability. |
| 201910145633.4 | 16 July 2019 | Nanostructured lipid carrier (NLC) for collaborative treatment of glioma as well as preparation method and application of NLC | This invention describes NLC formulation containing glyceryl monostearate, triglyceride, temozolomide, curcumin, poloxamer 188, and ethanol, developed via a microemulsion method. The NLC exhibits a uniform particle size (<100 nm), zeta potential of −8.54 ± 0.51 mV, and high entrapment efficiencies for temozolomide (91.53 ± 0.07%) and curcumin (88.64 ± 0.99%). |
| 20828766 | 12 October 2022 | Nanostructured drug delivery system as a multifunctional platform for therapy | This invention describes a functionalized lipid-based nanoplatform for targeted drug delivery, where one or more ligands are attached to the nanoparticle surface to enable specific targeting. The system encapsulates at least one active pharmaceutical ingredient and is designed to enhance the treatment of various diseases, particularly different types of cancer, including glioblastoma. |
| 202311053306 | 15 September 2023 | loaded nanostructured lipid carrier for breast cancer | This invention focuses on the formulation, optimization, and evaluation of quercetin-loaded NLCs. The quercetin-NLCs were successfully fabricated employing hot high-pressure homogenization and demonstrated enhanced drug absorption, protection of quercetin from degradation, extended circulation time, targeted uptake by cancer cells, and reduced systemic toxicity. |
| 202511011758 | 28 February 2025 | A transferrin-conjugated dual drug loaded nanostructured lipid carrier for glioblastoma and a method thereof | This invention describes transferrin-conjugated NLCs co-loaded with docetaxel and quercetin. The formulation is designed for intranasal delivery, enhancing bioavailability, cellular uptake, and therapeutic efficacy. It shows selective uptake by U87-MG glioblastoma cells and synergistic cytotoxicity. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jacob, S.; Rao, R.; Gorain, B.; Boddu, S.H.S.; Nair, A.B. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers for Anticancer Phytochemical Delivery: Advances, Challenges, and Future Prospects. Pharmaceutics 2025, 17, 1079. https://doi.org/10.3390/pharmaceutics17081079
Jacob S, Rao R, Gorain B, Boddu SHS, Nair AB. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers for Anticancer Phytochemical Delivery: Advances, Challenges, and Future Prospects. Pharmaceutics. 2025; 17(8):1079. https://doi.org/10.3390/pharmaceutics17081079
Chicago/Turabian StyleJacob, Shery, Rekha Rao, Bapi Gorain, Sai H. S. Boddu, and Anroop B. Nair. 2025. "Solid Lipid Nanoparticles and Nanostructured Lipid Carriers for Anticancer Phytochemical Delivery: Advances, Challenges, and Future Prospects" Pharmaceutics 17, no. 8: 1079. https://doi.org/10.3390/pharmaceutics17081079
APA StyleJacob, S., Rao, R., Gorain, B., Boddu, S. H. S., & Nair, A. B. (2025). Solid Lipid Nanoparticles and Nanostructured Lipid Carriers for Anticancer Phytochemical Delivery: Advances, Challenges, and Future Prospects. Pharmaceutics, 17(8), 1079. https://doi.org/10.3390/pharmaceutics17081079






