Once-Daily Versus Four-Times-Daily Intravenous Busulfan with Therapeutic Drug Monitoring as Conditioning for Hematopoietic Cell Transplantation in Children
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Patients and Transplant Characteristics
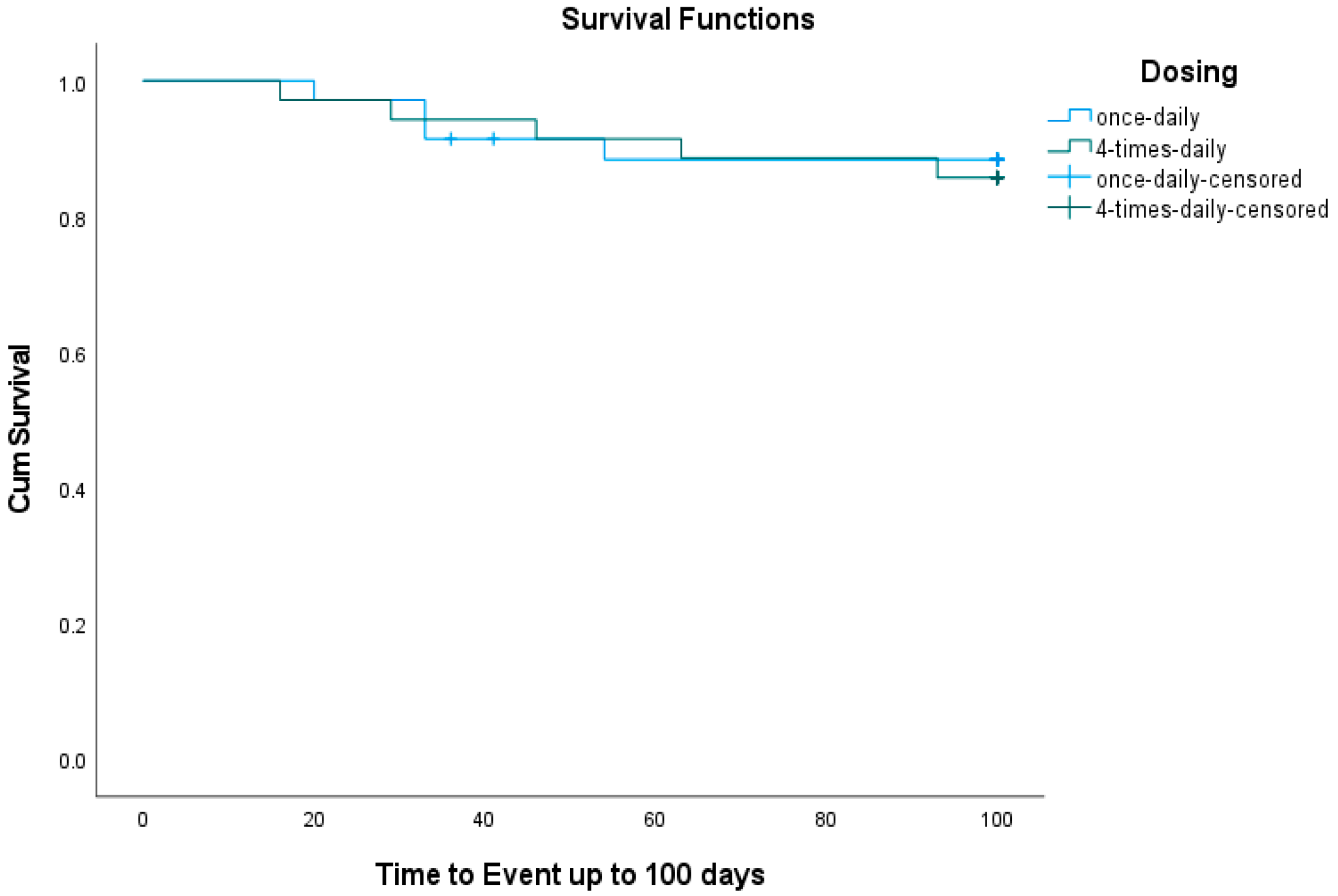
3.2. Event-Free Survival
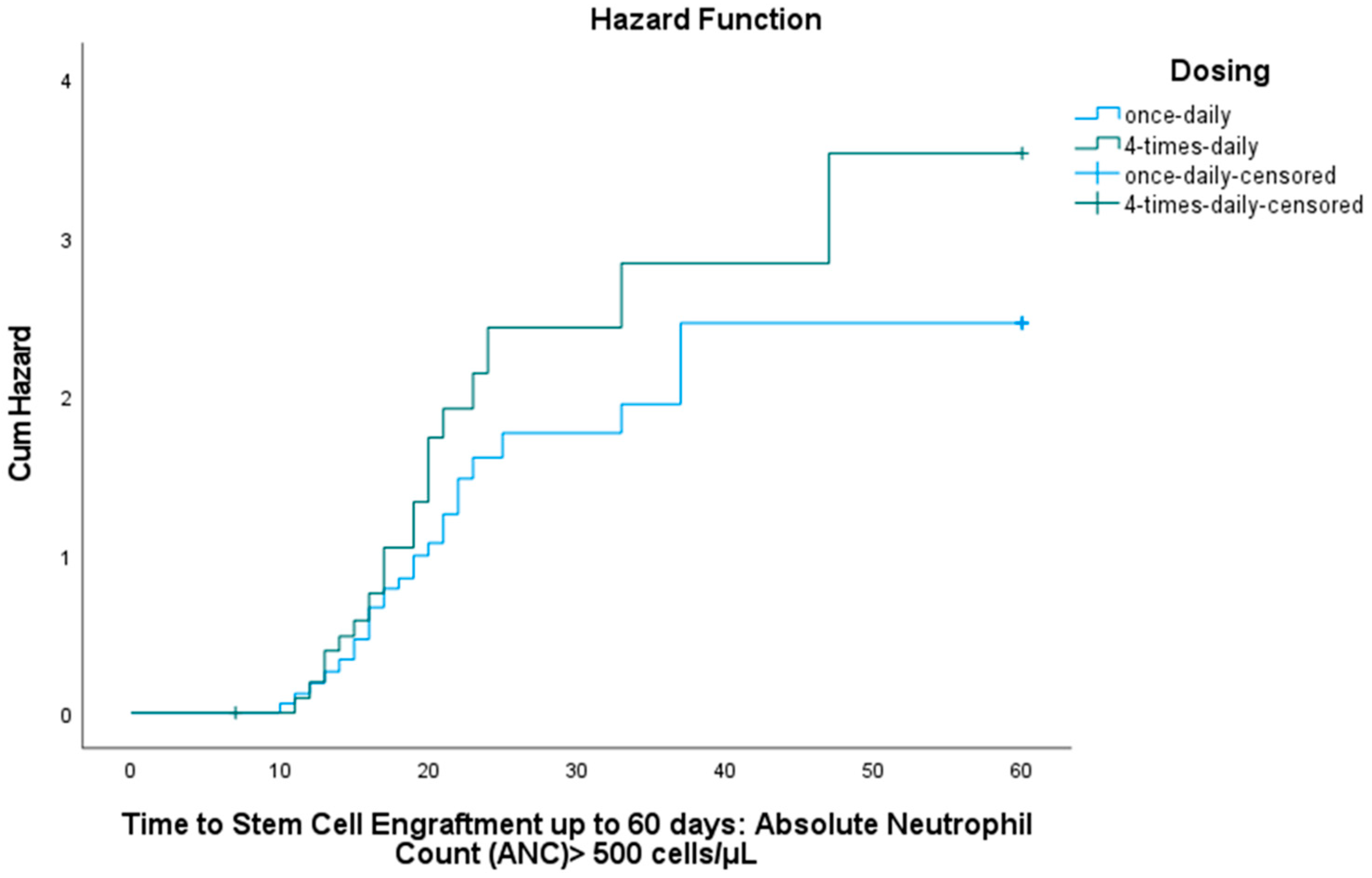
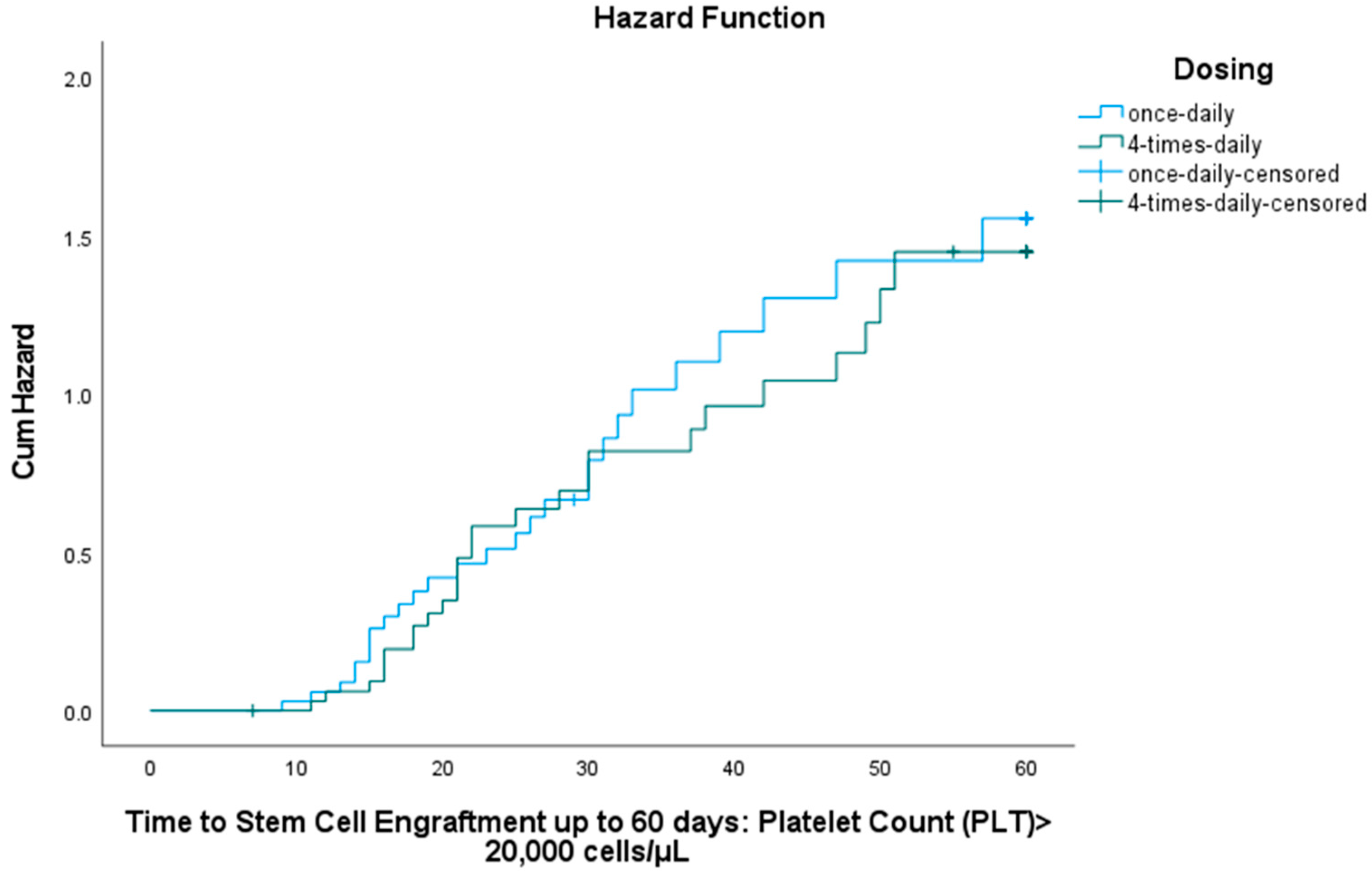
3.3. Stem Cell Engraftment
3.3.1. Neutrophil Engraftment
3.3.2. Platelet Engraftment
3.4. Incidence of Acute Graft-Versus-Host Disease (aGVHD)
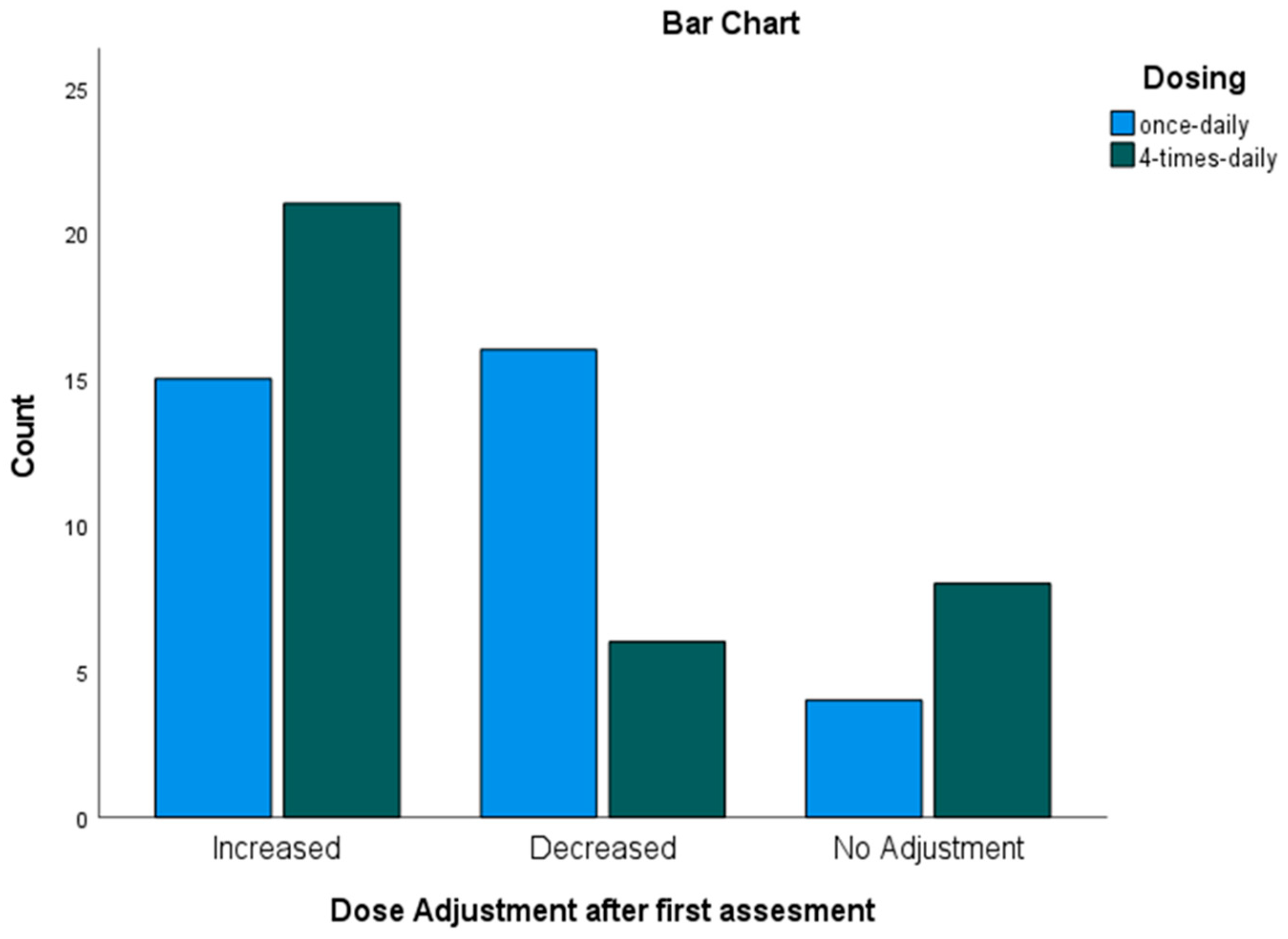
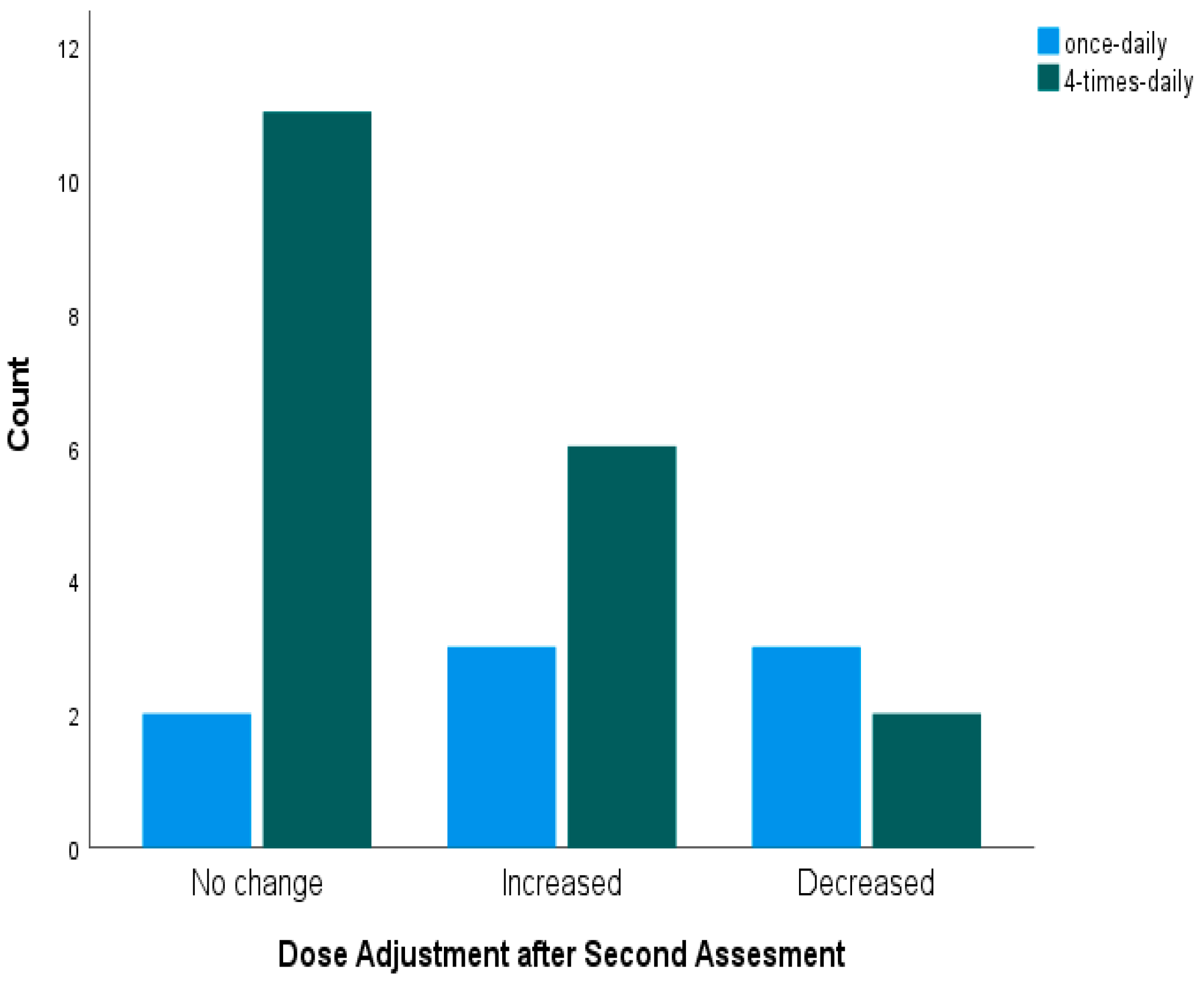
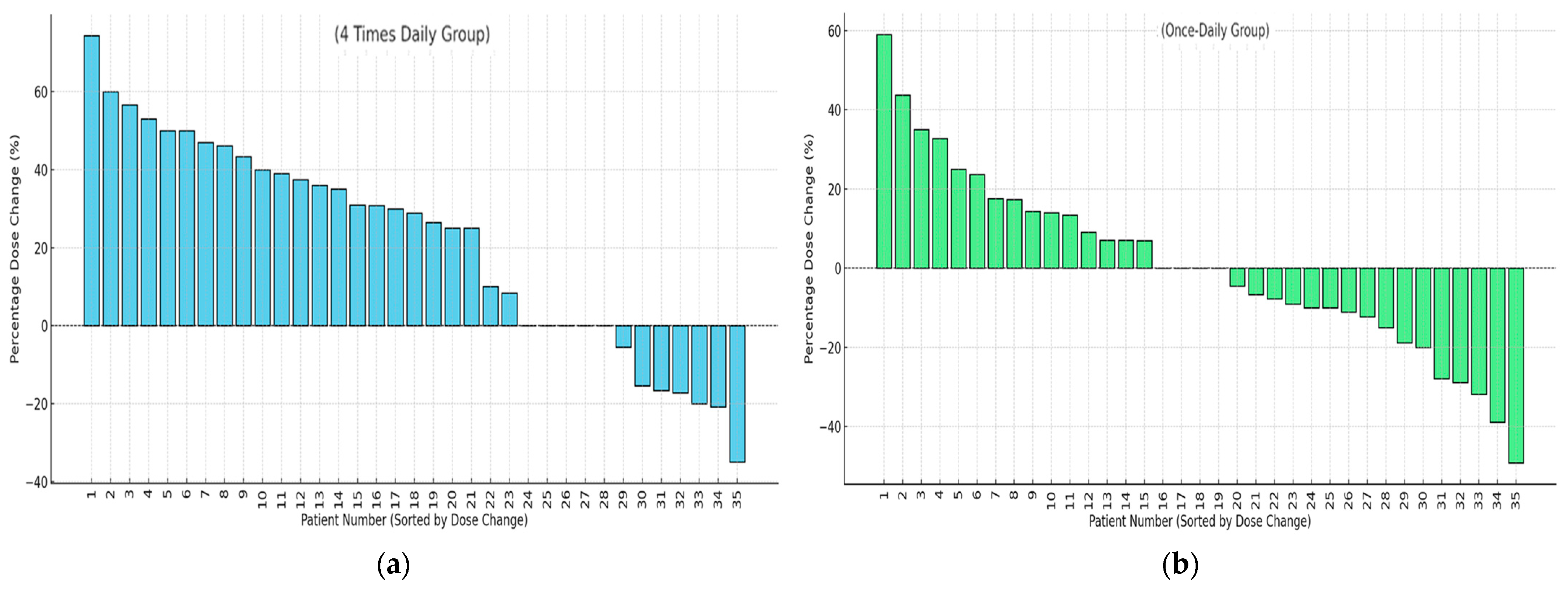
3.5. Dose Adjustments of Busulfan Based on MIPD
3.6. Treatment-Related Adverse Events
3.7. Drug-Drug Interactions
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Full Term/Definition |
| AUC | Area under the concentration–time curve |
| AUC0–24 | Area under the concentration–time curve from 0 to 24 h |
| ALK | Alkaline phosphatase |
| ALT | Alanine transaminase |
| AML | Acute myeloid leukemia |
| ANC | Absolute neutrophil count |
| aGVHD | Acute graft-versus-host disease |
| ATG | Anti-thymocyte globulin |
| Allo | Allogeneic |
| BM | Bone marrow |
| BU | Busulfan |
| BU1 | Busulfan once daily |
| BU4 | Busulfan four times daily |
| BU–FLU | Busulfan–Fludarabine |
| BU/MEL | Busulfan–Melphalan |
| BU/MEL/CYC | Busulfan–Melphalan–Cyclophosphamide |
| BU/TT | Busulfan–Thiotepa |
| BSA | Body surface area |
| Cmax | Maximum (peak) plasma concentration |
| CGD | Chronic granulomatous disease |
| CI | Confidence interval |
| CYC | Cyclophosphamide |
| CNS | Central nervous system |
| CSA | Cyclosporine |
| CSA–MMF | Cyclosporine with mycophenolate |
| DNA | Deoxyribonucleic acid |
| DDI | Drug–drug interaction |
| EFS | Event-free survival |
| EMA | European Medicines Agency |
| FDA | Food and Drug Administration |
| FLU | Fludarabine |
| GVHD | Graft-versus-host disease |
| H | Hour |
| HLH | Hemophagocytic lymphohistiocytosis |
| HLA | Human leukocyte antigen |
| HR | Hazard ratio |
| HSCT | Hematopoietic stem cell transplantation |
| IQR | Interquartile range |
| IV | Intravenous |
| JMML | Juvenile myelomonocytic leukemia |
| L/kg | Liter per kilogram |
| MEL | Melphalan |
| MDS | Myelodysplastic syndromes |
| MIPD | Model-informed precision dosing |
| mL/min/kg | Milliliter per minute per kilogram |
| m2 | Square meter |
| MMF | Mycophenolate mofetil |
| MPS 1 | Mucopolysaccharidosis type I |
| MTX | Methotrexate |
| N | Number of patients |
| NPD | Niemann–Pick disease |
| PBSC | Peripheral blood stem cells |
| PID | Primary immunodeficiency |
| PK | Pharmacokinetics |
| PO | Per os (by mouth) |
| PRN | Pro re nata (as needed) |
| Q6H | Every 6 h |
| RNA | Ribonucleic acid |
| SCID | Severe combined immunodeficiency |
| SD | Standard deviation |
| SOS | Sinusoidal obstruction syndrome |
| SPSS | Statistical Package for the Social Sciences |
| TT | Thiotepa |
| ULN | Upper limit of normal |
| VPA | Valproic acid |
Appendix A
Diseases Classification
- Inborn errors of immunity (severe combined immunodeficiency (SCID), primary immunodeficiency (PID), X-linked lymphoproliferative disease (XLP), chronic granulomatous disease (CGD), hemophagocytic lymphohistiocytosis (HLH), Wiskott–Aldrich syndrome).
- Metabolic disorders (osteopetrosis, alpha-mannosidosis, X-related adrenoleukodystrophy (ALD), metachromatic leukodystrophy (MLD), Krabbe leukodystrophy, mucopolysaccharidosis Type I (MPS 1), Niemann–Pick disease (NPD)).
- Non-malignant hematological disorders (pure red cell aplasia (PRCA), aplastic anemia, sideroblastic anemia, Diamond–Blackfan anemia (DBA), congenital amegakaryocytic thrombocytopenia (CAMT)).
References
- Lawson, R.; Staatz, C.E.; Fraser, C.J.; Ramachandran, S.; Teague, L.; Mitchell, R.; O’Brien, T.; Hennig, S. Population pharmacokinetic model for once-daily intravenous busulfan in pediatric subjects describing time-associated clearance. CPT Pharmacomet. Syst. Pharmacol. 2022, 11, 1002–1017. [Google Scholar] [CrossRef] [PubMed]
- Nabors, L.B.; Surboeck, B.; Grisold, W. Complications from Pharmacotherapy. Handb. Clin. Neurol. 2016, 134, 235–250. [Google Scholar] [PubMed]
- Gerson, S.L.; Caimi, P.F.; William, B.M.; Creger, R.J. Pharmacology and Molecular Mechanisms of Antineoplastic Agents for Hematologic Malignancies. In Hematology; Elsevier: Amsterdam, The Netherlands, 2018; pp. 849–912. [Google Scholar]
- Busulfan-StatPearls-NCBI Bookshelf. Available online: https://www.ncbi.nlm.nih.gov/books/NBK555986/ (accessed on 29 March 2025).
- Otsuka Pharmaceutical Development & Commercialization, Inc. BUSULFEX® Product Information; Otsuka Pharmaceutical Development & Commercialization, Inc.: Rockville, MD, USA, 2023. [Google Scholar]
- Highlights of Prescribing Information. Available online: www.fda.gov/medwatch (accessed on 29 March 2025).
- Palmer, J.; McCune, J.S.; Perales, M.-A.; Marks, D.; Bubalo, J.; Mohty, M.; Wingard, J.R.; Paci, A.; Hassan, M.; Bredeson, C.; et al. Personalizing Busulfan-Based Conditioning: Considerations from the American Society for Blood and Marrow Transplantation Practice Guidelines Committee. Biol. Blood Marrow Transplant. 2016, 22, 1915–1925. [Google Scholar] [CrossRef] [PubMed]
- Bartelink, I.H.; Lalmohamed, A.; van Reij, E.M.L.; Dvorak, C.C.; Savic, R.M.; Zwaveling, J.; Bredius, R.G.M.; Egberts, P.A.C.G.; Bierings, M.; Kletzel, M.; et al. Association of busulfan exposure with survival and toxicity after haemopoietic cell transplantation in children and young adults: A multicentre, retrospective cohort analysis. Lancet Haematol. 2016, 3, e526–e536. [Google Scholar] [CrossRef] [PubMed]
- Zao, J.H.; Schechter, T.; Liu, W.J.; Gerges, S.; Gassas, A.; Egeler, R.M.; Grunebaum, E.; Dupuis, L.L. Performance of Busulfan Dosing Guidelines for Pediatric Hematopoietic Stem Cell Transplant Conditioning. Biol. Blood Marrow Transplant. 2015, 21, 1471–1478. [Google Scholar] [CrossRef] [PubMed]
- Beschorner, W.E.; Pino, J.; Boitnott, J.K.; Tutschka, P.J.; Santos, G.W. Pathology of the liver with bone marrow transplantation. Effects of busulfan, carmustine, acute graft-versus-host disease, and cytomegalovirus infection. Am. J. Pathol. 1980, 99, 369. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC1903505/ (accessed on 29 March 2025). [PubMed]
- Domingos, V.; Nezvalova-Henriksen, K.; Dadkhah, A.; Moreno-Martinez, M.-E.; Hassine, K.B.; Pires, V.; Kröger, N.; Bauters, T.; Hassan, M.; Duncan, N.; et al. A practical guide to therapeutic drug monitoring in busulfan: Recommendations from the Pharmacist Committee of the European Society for Blood and Marrow Transplantation (EBMT). Bone Marrow Transplant. 2024, 59, 1641–1653. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, L.R.; Kanakry, C.G.; Zahurak, M.; Durakovic, N.; Bolaños-Meade, J.; Kasamon, Y.L.; Gladstone, D.E.; Matsui, W.; Borrello, I.; Huff, C.A.; et al. Therapeutic drug monitoring for either oral or intravenous busulfan when combined with pre- and post-transplantation cyclophosphamide. Leuk. Lymphoma 2016, 57, 666–675. [Google Scholar] [CrossRef] [PubMed]
- BUSULFAN INJECTION (Busulfan Injection) Highlights|Pfizer Medical Information-US. Available online: https://www.pfizermedicalinformation.com/busulfan/highlights (accessed on 29 March 2025).
- Jenke, A.; Freiberg-Richter, J.; Wilhelm, S.; Freund, M.; Renner, U.D.; Bornhäuser, M.; Schleyer, E.; Ehninger, G.; Schuler, U. Accidental busulfan overdose during conditioning for stem cell transplantation. Bone Marrow Transplant. 2005, 35, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.A.; Tran, H.T.; Quinlan, D.; Chaudhry, A.; Duggan, P.; Brown, C.; Stewart, D.; Ruether, J.D.; Morris, D.; Glück, S.; et al. Once-daily intravenous busulfan given with fludarabine as conditioning for allogeneic stem cell transplantation: Study of pharmacokinetics and early clinical outcomes. Biol. Blood Marrow Transplant. 2002, 8, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Zwaveling, J.; Den Hartigh, J.; Lankester, A.C.; Guchelaar, H.J.; Egeler, R.M.; Maarten Bredius, R.G. Once-daily intravenous busulfan in children prior to stem cell transplantation: Study of pharmacokinetics and early clinical outcomes. Anti Cancer Drugs 2006, 17, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Park, S.S.; Kwak, J.W.; Lim, Y.T. The Effectiveness of Once-daily Intravenous Busulfan as a Conditioning Regime for Hematopoietic Stem Cell Transplantation in Children with Acute Myelogenous Leukemia. Korean J. Hematol. 2009, 44, 1–7. [Google Scholar] [CrossRef]
- Bartelink, I.H.; Bredius, R.G.M.; Ververs, T.T.; Raphael, M.F.; van Kesteren, C.; Bierings, M.; Rademaker, C.M.A.; den Hartigh, J.; Uiterwaal, C.S.P.M.; Zwaveling, J.; et al. Once-Daily Intravenous Busulfan with Therapeutic Drug Monitoring Compared to Conventional Oral Busulfan Improves Survival and Engraftment in Children Undergoing Allogeneic Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2008, 14, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Bartelink, I.; Boelens, J.J.; Bredius, R.G.M.; Egberts, A.C.G.; Wang, C.; Bierings, M.B.; Shaw, P.J.; Nath, C.E.; Hempel, G.; Zwaveling, J.; et al. Body Weight-Dependent Pharmacokinetics of Busulfan in Paediatric Haematopoietic Stem Cell Transplantation Patients. Clin. Pharmacokinet. 2012, 51, 331–345. [Google Scholar] [CrossRef] [PubMed]
- McCune, J.S.; Bemer, M.J.; Barrett, J.S.; Baker, K.S.; Gamis, A.S.; Holford, N.H.G. Busulfan in infant to adult hematopoietic cell transplant recipients: A population pharmacokinetic model for initial and bayesian dose personalization. Clin. Cancer Res. 2014, 20, 754–763. [Google Scholar] [CrossRef] [PubMed]
- Micromedex Products: Please Login. Available online: https://www.micromedexsolutions.com/home/dispatch (accessed on 29 March 2025).
- Sign in-UpToDate. Available online: https://www.uptodate.com/login (accessed on 29 March 2025).
- Shao, D.-F.; Li, J.-H.; Hu, T.; Zhang, Z.-X.; Zhang, L.; Li, J.-J.; Cao, J.; Feng, S.-Q.; Tang, R.-H.; Zhong, D.-X.; et al. Clinical outcomes of individualized busulfan-dosing in hematopoietic stem cell transplantation in Chinese children undergoing with therapeutic drug monitoring. Bone Marrow Transplant. 2022, 57, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Cremers, S.; Schoemaker, R.; Bredius, R.; Den Hartigh, J.; Ball, L.; Twiss, I.; Vermeij, P.; Vossen, J. Pharmacokinetics of intravenous busulfan in children prior to stem cell transplantation. Br. J. Clin. Pharmacol. 2002, 53, 386–389. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Moon, S.; Rhee, S.J. Optimal Once-Daily Busulfan Administration in Pediatric Patients: A Simulation-Based Investigation of Intravenous Infusion Times. Drug Des. Dev. Ther. 2024, 18, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.-G.; Lee, J.-H.; Choi, S.-J.; Lee, J.-H.; Lee, Y.-S.; Seol, M.; Hur, E.-H.; Lee, S.H.; Bae, K.S.; Noh, G.J.; et al. Randomized comparison of four-times-daily versus once-daily intravenous busulfan in conditioning therapy for hematopoietic cell transplantation. Biol. Blood Marrow Transplant. 2007, 13, 1095–1105. [Google Scholar] [CrossRef] [PubMed]
| Patient Characteristics | BU4 n = 35 (%) | BU1 n = 35 (%) | p-Value |
|---|---|---|---|
| Age at HSCT, median (years)—(25th–75th Percentile (IQR)) | 3.85 (1.18–8.29 (7.11)) | 4.83 (3.08–6.6 (3.52)) | 0.304 |
| Follow up, median (days)—(25th–75th Percentile (IQR)) | 1169 (343–1587 (1244)) | 267 (174–537 (363)) | <0.001 |
| Age group (years) | 0.397 | ||
| <2 years | 12 (34.3%) | 7 (20%) | |
| 2–<6 years | 10 (28.6%) | 13 (37.1%) | |
| 6–18 years | 13 (37.1%) | 15 (42.9%) | |
| Sex | 0.131 | ||
| Male | 20 (57.1%) | 26 (74.3%) | |
| Female | 15 (42.9%) | 9 (25.7%) | |
| Body surface area, median (m2)—(25th–75th Percentile (IQR)) | 0.59 (0.44–0.92 (0.48)) | 0.72 (0.59–0.93 (0.34)) | 0.147 |
| Diagnosis | 0.794 * | ||
| Malignant Diseases: | 11 (31.4%) | 10 (28.6%) | |
| Leukemia | 5 | 6 | |
| Solid tumors | 6 | 4 | |
| Non-malignant Diseases: | 24 (68.6%) | 25 (71.4%) | |
| Inborn errors of immunity | 14 | 6 | |
| Metabolic disorders | 5 | 17 | |
| Non-malignant hematological disorders | 5 | 2 |
| Transplant Characteristics | BU4 n = 35 (%) | BU1 n = 35 (%) |
|---|---|---|
| Type of HSCT | ||
| Allogeneic | 30 (85.7%) | 31 (88.6%) |
| Autologous | 5 (14.3%) | 4 (11.4%) |
| Conditioning Protocol | ||
| BU-FLU | 20 (57.1%) | 20 (57.1%) |
| BU-MEL | 5 (14.3%) | 4 (11.4%) |
| BU-CYC-MEL | 5 (14.3%) | 5 (14.3%) |
| BU-FLU-CYC | 0 (0%) | 5 (14.3%) |
| BU-FLU-TT | 5 (14.3%) | 1 (2.9%) |
| Allogeneic Patients | AlloBU4 n = 30 (%) | AlloBU1 n = 31 (%) |
| Donor Source Cells | ||
| BM | 23 (76.7%) | 16 (51.6%) |
| PBSC | 6 (20%) | 5 (16.1%) |
| Cord | 1 (3.3%) | 10 (32.3%) |
| Type of Donor | ||
| Related donor | 16 (53.3%) | 10 (32.3%) |
| Unrelated donor | 14 (46.7%) | 21 (67.7%) |
| HLA Matching | ||
| Full matched donor | 23 (76.7%) | 23 (74.2%) |
| Haploidentical | 4 (13.3%) | 2 (6.5%) |
| Mismatched donor | 3 (10%) | 6 (19.4%) |
| Immunosuppressive Therapy | ||
| Yes: | 27 (90%) | 29 (93.5%) |
| ATG | 20 | 25 |
| Alemtuzumab | 7 | 4 |
| GVHD Prophylaxis | ||
| CSA-MMF | 17 (56.7%) | 22 (70.9%) |
| CSA-MTX | 6 (20%) | 6 (19.4%) |
| CSA only | 5 (16.7%) | 3 (9.7%) |
| CSA-MTX-MMF | 2 (6.7%) | 0 (0%) |
| Busulfan Adverse Events | BU4–n = 35 (%) | BU1–n = 35 (%) |
|---|---|---|
| Fever | 33 (94.3%) | 32 (91.4%) |
| Headache | 6 (17.1%) | 3 (8.6%) |
| Chills | 8 (22.9%) | 5 (14.3%) |
| Pain | 25 (71.4%) | 27 (77.1%) |
| Edema | 6 (17.1%) | 4 (11.4%) |
| Refractory Thrombocytopenia | 9 (25.7%) | 7 (20.0%) |
| Tachycardia | 5 (14.3%) | 3 (8.6%) |
| Hypertension | 7 (20.0%) | 5 (14.3%) |
| Thrombosis | 2 (5.7%) | 2 (5.7%) |
| Mucositis | 35 (100%) | 35 (100%) |
| Stomatitis | 12 (34.3%) | 14 (40.0%) |
| Nausea | 15 (42.9%) | 17 (48.6%) |
| Vomiting | 14 (40.0%) | 16 (45.7%) |
| Diarrhea | 18 (51.4%) | 20 (57.1%) |
| Constipation | 11 (31.4%) | 13 (37.1%) |
| Abdominal Pain | 6 (17.1%) | 8 (22.9%) |
| Abdominal Enlargement | 2 (5.7%) | 2 (5.7%) |
| Metabolic and Nutritional System | ||
| Hypomagnesemia | 21 (60.0%) | 20 (57.1%) |
| Hypokalemia | 18 (51.4%) | 17 (48.6%) |
| Hypocalcemia | 4 (11.4%) | 5 (14.3%) |
| Hyperbilirubinemia | 3 (8.6%) | 3 (8.6%) |
| Creatinine Increased | 7 (20.0%) | 6 (17.1%) |
| Respiratory System | ||
| Epistaxis | 4 (11.4%) | 4 (11.4%) |
| Dyspnea | 6 (17.1%) | 7 (20.0%) |
| Others | ||
| Rash | 9 (25.7%) | 10 (28.6%) |
| Seizures | 0 (0.0%) | 1 (2.9%) |
| Infections | ||
| Bacterial Infections | 10 (28.6%) | 8 (22.9%) |
| Fungal Infections | 6 (17.1%) | 4 (11.4%) |
| Viral Infections | 12 (34.3%) | 9 (25.7%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bazbaz, S.; Zaidman, I.; Even-Or, E.; Stepensky, P.; Sakran, R.; Kurnik, D.; Aldouby-Bier, G. Once-Daily Versus Four-Times-Daily Intravenous Busulfan with Therapeutic Drug Monitoring as Conditioning for Hematopoietic Cell Transplantation in Children. Pharmaceutics 2025, 17, 1081. https://doi.org/10.3390/pharmaceutics17081081
Bazbaz S, Zaidman I, Even-Or E, Stepensky P, Sakran R, Kurnik D, Aldouby-Bier G. Once-Daily Versus Four-Times-Daily Intravenous Busulfan with Therapeutic Drug Monitoring as Conditioning for Hematopoietic Cell Transplantation in Children. Pharmaceutics. 2025; 17(8):1081. https://doi.org/10.3390/pharmaceutics17081081
Chicago/Turabian StyleBazbaz, Safaa, Irina Zaidman, Ehud Even-Or, Polina Stepensky, Razan Sakran, Daniel Kurnik, and Gefen Aldouby-Bier. 2025. "Once-Daily Versus Four-Times-Daily Intravenous Busulfan with Therapeutic Drug Monitoring as Conditioning for Hematopoietic Cell Transplantation in Children" Pharmaceutics 17, no. 8: 1081. https://doi.org/10.3390/pharmaceutics17081081
APA StyleBazbaz, S., Zaidman, I., Even-Or, E., Stepensky, P., Sakran, R., Kurnik, D., & Aldouby-Bier, G. (2025). Once-Daily Versus Four-Times-Daily Intravenous Busulfan with Therapeutic Drug Monitoring as Conditioning for Hematopoietic Cell Transplantation in Children. Pharmaceutics, 17(8), 1081. https://doi.org/10.3390/pharmaceutics17081081






