Significant Improvement in Bioavailability and Therapeutic Efficacy of Mebendazole Oral Nano-Systems Assessed in a Murine Model with Extreme Phenotypes of Susceptibility to Trichinella spiralis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Nanoparticle Synthesis
2.3. β-Cyclodextrin Citrate and Inclusion Complexes’ Synthesis
2.4. Particle Size Determination
2.5. Solubility and Dissolution Studies
2.6. Animal Model
2.7. Parasite
2.8. In Vitro Analysis of the Anthelmintic Activity of the MBZ Formulations
2.8.1. Preparation of T. spiralis Female Worms
2.8.2. Preparation of the Antiparasitic Solutions
2.8.3. In Vitro Parasitic Assay
2.9. Pharmacokinetic Analysis
2.9.1. HPLC Analysis
2.9.2. Pharmacokinetic Parameters—Relative Bioavailability
2.10. In Vivo Analysis of the Anthelmintic Activity of Pure MBZ and Its Formulations
2.10.1. Infection
2.10.2. Assessment of the New Formulations’ Therapeutic Efficacy
2.10.3. Assessment of Increasing Doses of Pure MBZ on Its Therapeutic Efficacy
2.11. Statistical Analysis
3. Results and Discussion
3.1. Particle Size, Solubility, and Dissolution Studies
3.2. In Vitro Assay
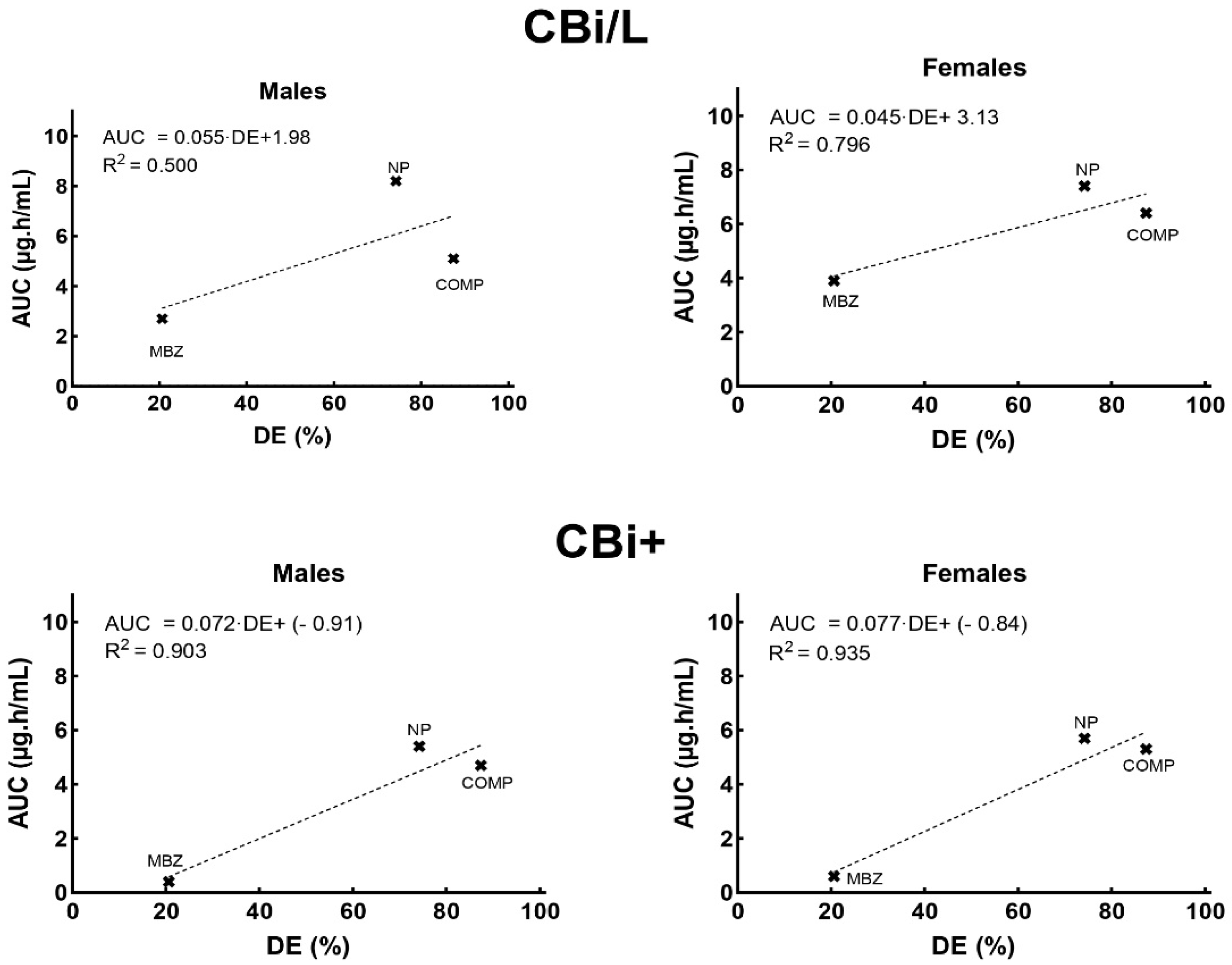
3.3. Pharmacokinetic Analysis
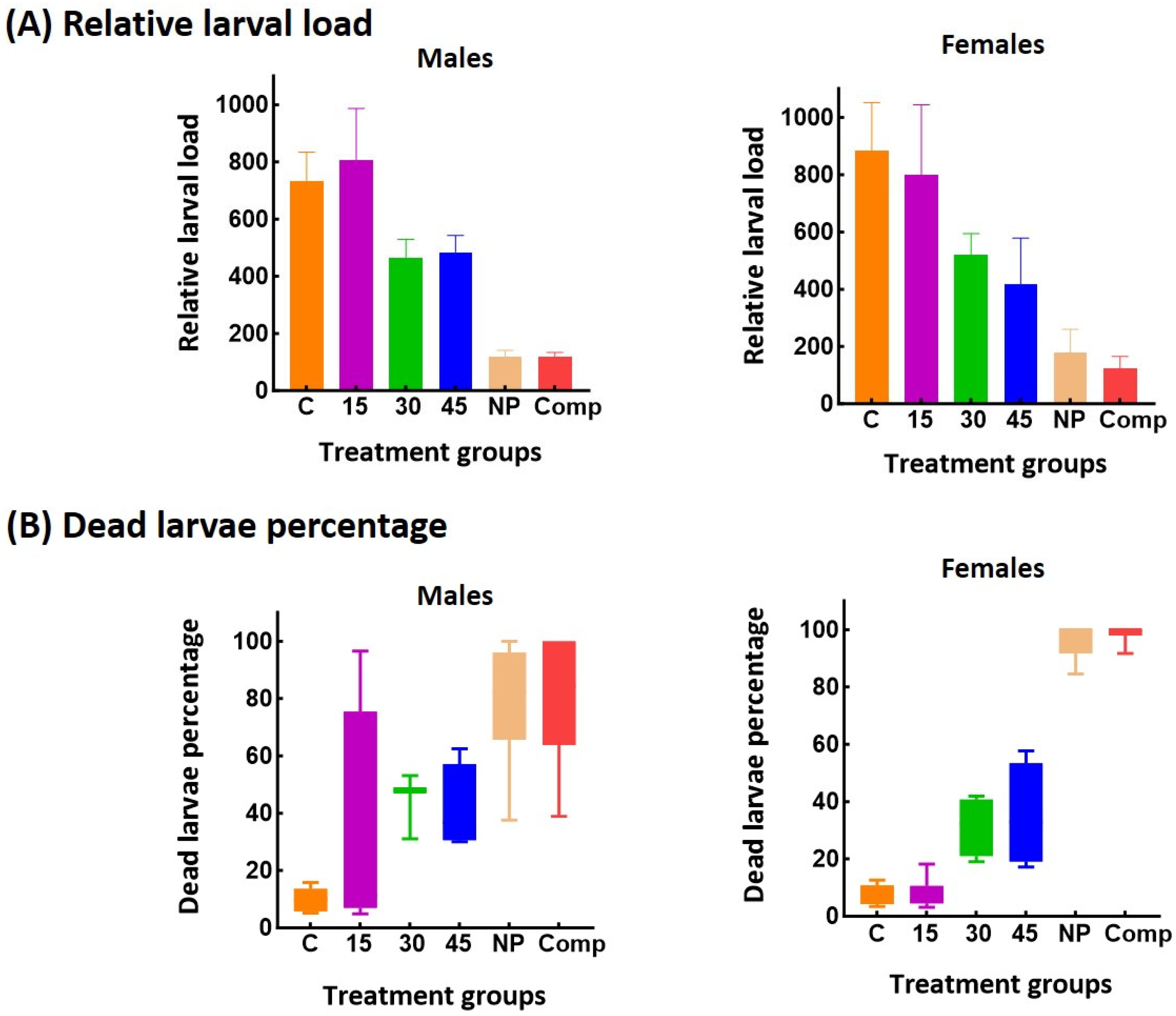
3.4. In Vivo Anthelmintic Activity of MBZ Formulations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A




Appendix B

References
- Rostami, A.; Gamble, H.R.; Dupouy-Camet, J.; Khazan, H.; Bruschi, F. Meat Sources of Infection for Outbreaks of Human Trichinellosis. Food Microbiol. 2017, 64, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Bruschi, F.; Murrell, K.D. 119—Trichinellosis. In Hunter’s Tropical Medicine and Emerging Infectious Diseases, 10th ed.; Ryan, E.T., Hill, D.R., Solomon, T., Aronson, N.E., Endy, T.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 882–884. [Google Scholar] [CrossRef]
- Calcagno, M.A.; Bourlot, I.; Taus, R.; Saracino, M.P.; Venturiello, S.M. Description of an Outbreak of Human Trichinellosis in an Area of Argentina Historically Regarded as Trichinella-Free: The Importance of Surveillance Studies. Vet. Parasitol. 2014, 200, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Carrillo, J.L.; Moreno-García, A.; Flores-De La Torre, J.A.; Muñoz-Escobedo, J.J.; López-Luna, A.; Maldonado-Tapia, C. Current Aspects in Trichinellosis. In Parasites and Parasitic Diseases; Bastidas, G., Ed.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Robertson, L.J. Parasites in Food: From a Neglected Position to an Emerging Issue. Adv. Food Nutr. Res. 2018, 86, 71–113. [Google Scholar] [CrossRef]
- Miguez, S.; Moreno, M.A.; Fariña, F.; Pasqualetti, M.; Ribicich, M. Understanding the global connection: Investigating the association between pork meat exports and trichinellosis. Vet. Parasitol. 2025, 338, 110509. [Google Scholar] [CrossRef]
- Cretu, C.M. Treatment. In Trichinella and Trichinellosis; Bruschi, F., Ed.; Academic Press: London, UK, 2021; pp. 417–429. [Google Scholar] [CrossRef]
- Palmeiri, M.S.; Bosch, F.; Ame, S.M.; Ali, S.M.; Hattendorf, J.; Keiser, J. Efficacy, Safety and Acceptability of a New Chewable Formulation versus the Solid Tablet of Mebendazole against Hookworm Infections in Children: An Open-Label, Randomized Controlled Trial. EClinicalMedicine 2020, 27, 100556. [Google Scholar] [CrossRef]
- Swanepoel, E.; Liebenberg, W.; de Villiers, M. Quality evaluation of generic drugs by dissolution test: Changing the USP dissolution medium to distinguish between active and non-active mebendazole polymorphs. Eur. J. Pharm. Biopharm. 2003, 55, 345–349. [Google Scholar] [CrossRef]
- Quelian, A.; Garbuio, P.; Hanashiro, T.; Ortega Markman, B.E.; Luiz, F.; Fonseca, A.; Ferreira Perazzo, F.; Cesar, P.; Rosa, P. Evaluation and Study of Mebendazole Polymorphs Present in Raw Materials and Tablets Available in the Brazilian Pharmaceutical Market. J. Appl. Pharm. Sci. 2014, 4, 1–7. [Google Scholar] [CrossRef]
- Navarrete-Vázquez, G.; Yépez, L.; Hernández-Campos, A.; Tapia, A.; Hernández-Luis, F.; Cedillo, R.; González, J.; Martínez-Fernández, A.; Martínez-Grueiro, M.; Castillo, R. Synthesis and Antiparasitic Activity of Albendazole and Mebendazole Analogues. Bioorg. Med. Chem. 2003, 11, 4615–4622. [Google Scholar] [CrossRef]
- Buchter, V.; Priotti, J.; Leonardi, D.; Lamas, M.C.; Keiser, J. Preparation, Physicochemical Characterization and In Vitro and In Vivo Activity Against Heligmosomoides Polygyrus of Novel Oral Formulations of Albendazole and Mebendazole. J. Pharm. Sci. 2020, 109, 1819–1826. [Google Scholar] [CrossRef]
- Frömming, K.-H.; Szejtli, J. Cyclodextrins in Pharmacy; Topics in Inclusion Science; Springer: Dordrecht, The Netherlands, 1994; Volume 5, ISBN 978-90-481-4242-2. [Google Scholar]
- Priotti, J.; García, A.; Leonardi, D.; Ferreira, M.J.; Lamas, M.C.; Nunes, T.G. Succinyl-β-Cyclodextrin: Influence of the Substitution Degree on Albendazole Inclusion Complexes Probed by NMR. Mater. Sci. Eng. C 2018, 92, 694–702. [Google Scholar] [CrossRef]
- Jafari, N.; Douglas, J.T.; Neuenswander, S.A.; Kelich, P.; Hageman, M.J. β-Cyclodextrin Derivatives Bind Aromatic Side Chains of the Cyclic Peptide Lanreotide. J. Pharm. Sci. 2024, 114, 878–886. [Google Scholar] [CrossRef]
- Pandikannan, S.; Shanmugasundaram, E.; Vellaisamy, K.; Muthuchamy, S.; Krishnan, N.K.; Mani, M.K.; Baskaralingam, V.; Sibiya, A.; Govindasamy, C.; Almutairi, K.M.; et al. Synthesis, Computational, and Spectral Investigation of Cinnamate Derivatives/β-Cyclodextrin Inclusion Complexes Studies for Biological Applications. J. Mol. Struct. 2025, 1327, 140970. [Google Scholar] [CrossRef]
- Mahmoud, M.N.A.; Khaled, M.M.; Ismail, M.A.; Medien, H.A.A.; Abdel-Samad, H.S.; Abdel-Shafi, A.A. Effect of Encapsulation in Cyclodextrin Nanocavities on the Photophysical Properties of Bithiophene-5-Carboxamidine Derivatives. J. Mol. Liq. 2025, 425, 127275. [Google Scholar] [CrossRef]
- García, A.; Priotti, J.; Codina, A.V.; Vasconi, M.D.; Quiroga, A.D.; Hinrichsen, L.I.; Leonardi, D.; Lamas, M.C. Synthesis and Characterization of a New Cyclodextrin Derivative with Improved Properties to Design Oral Dosage Forms. Drug Deliv. Transl. Res. 2019, 9, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Priotti, J.; Codina, A.V.; Leonardi, D.; Vasconi, M.D.; Hinrichsen, L.I.; Lamas, M.C. Albendazole Microcrystal Formulations Based on Chitosan and Cellulose Derivatives: Physicochemical Characterization and In Vitro Parasiticidal Activity in Trichinella Spiralis Adult Worms. AAPS PharmSciTech. 2017, 18, 947–956. [Google Scholar] [CrossRef]
- McRae, K.M.; Good, B.; Hanrahan, J.P.; McCabe, M.S.; Cormican, P.; Sweeney, T.; O’Connell, M.J.; Keane, O.M. Transcriptional Profiling of the Ovine Abomasal Lymph Node Reveals a Role for Timing of the Immune Response in Gastrointestinal Nematode Resistance. Vet. Parasitol. 2016, 224, 96–108. [Google Scholar] [CrossRef]
- McRae, K.M.; Good, B.; Hanrahan, J.P.; Glynn, A.; O’Connell, M.J.; Keane, O.M. Response to Teladorsagia Circumcincta Infection in Scottish Blackface Lambs with Divergent Phenotypes for Nematode Resistance. Vet. Parasitol. 2014, 206, 200–207. [Google Scholar] [CrossRef]
- McRae, K.M.; McEwan, J.C.; Dodds, K.G.; Gemmell, N.J. Signatures of Selection in Sheep Bred for Resistance or Susceptibility to Gastrointestinal Nematodes. BMC Genom. 2014, 15, 637. [Google Scholar] [CrossRef]
- Machado-Silva, J.R.; Neves, R.H.; Da Silva, L.O.; De Oliveira, R.M.F.; Da Silva, A.C. Do Mice Genetically Selected for Resistance to Oral Tolerance Provide Selective Advantage for Schistosoma Mansoni Infection? Exp. Parasitol. 2005, 111, 1–7. [Google Scholar] [CrossRef]
- Gurwitz, D.; McLeod, H.L. Genome-Wide Studies in Pharmacogenomics: Harnessing the Power of Extreme Phenotypes. Pharmacogenomics 2013, 14, 337–339. [Google Scholar] [CrossRef]
- García, A.; Leonardi, D.; Salazar, M.O.; Lamas, M.C. Modified β-Cyclodextrin Inclusion Complex to Improve the Physicochemical Properties of Albendazole. Complete in Vitro Evaluation and Characterization. PLoS ONE 2014, 9, e88234. [Google Scholar] [CrossRef] [PubMed]
- ICH Guideline. Guideline on Impurities: Guideline for Residual Solvents Step 5; EMA: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Berregi, I.; del Campo, G.; Caracena, R.; Miranda, J.I. Quantitative Determination of Formic Acid in Apple Juices by 1H NMR Spectrometry. Talanta 2007, 72, 1049–1053. [Google Scholar] [CrossRef] [PubMed]
- Telfah, A.; Al Bataineh, Q.M.; Al-Essa, K.; Al-Sawalmih, A.; Telfah, M.; Gogiashvili, M.; Bahti, A.; Majer, G.; Hergenröder, R. 1H and 13C NMR and FTIR Spectroscopic Analysis of Formic Acid Dissociation Dynamics in Water. J. Phys. Chem. B 2024, 128, 11417–11425. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.A. The concept of dissolution efficiency. J. Pharm. Pharmacol. 1975, 27, 48–49. [Google Scholar] [CrossRef]
- US Pharmacopeia (USP). Available online: https://www.usp.org/ (accessed on 6 August 2025).
- Hinrichsen, L.I.; Di Masso, R.J. Empleo de un modelo murino original de Argentina en la caracterización de fenotipos complejos. BAG J. Basic Appl. Genet. 2010, 21, 7. Available online: https://www.scielo.org.ar/scielo.php?script=sci_arttext&pid=S1852-62332010000200007&lng=es (accessed on 6 August 2025).
- Vasconi, M.D.; Bertorini, G.; Codina, A.V.; Indelman, P.; Di Masso, R.J.; Hinrichsen, L.I. Phenotypic Characterization of the Response to Infection with Trichinella Spiralis in Genetically Defined Mouse Lines of the CBi-IGE Stock. Open J. Vet. Med. 2015, 5, 111–122. [Google Scholar] [CrossRef]
- Codina, A.V.; Priotti, J.; Leonardi, D.; Vasconi, M.D.; Lamas, M.C.; Hinrichsen, L.I. Effect of Sex and Genotype of the Host on the Anthelmintic Efficacy of Albendazole Microcrystals, in the CBi-IGE Trichinella Infection Murine Model. Parasitology 2021, 148, 1545–1553. [Google Scholar] [CrossRef]
- Codina, A.V.; García, A.; Leonardi, D.; Vasconi, M.D.; Di Masso, R.J.; Lamas, M.C.; Hinrichsen, L.I. Efficacy of Albendazole: β-Cyclodextrin Citrate in the Parenteral Stage of Trichinella Spiralis Infection. Int. J. Biol. Macromol. 2015, 77, 203–206. [Google Scholar] [CrossRef]
- De Ruyck, H.; Daeseleire, E.; De Ridder, H.; Van Renterghem, R. Liquid Chromatographic-Electrospray Tandem Mass Spectrometric Method for the Determination of Mebendazole and Its Hydrolysed and Reduced Metabolites in Sheep Muscle. Anal. Chim. Acta 2003, 483, 111–123. [Google Scholar] [CrossRef]
- Nguyen, T.N.; Tran, P.; Choi, Y.E.; Park, J.S. Solid Dispersion of Mebendazole via Surfactant Carrier to Improve Oral Bioavailability and in Vitro Anticancer Efficacy. J. Pharm. Investig. 2023, 53, 443–455. [Google Scholar] [CrossRef]
- Modi, N.B. In Vitro-in Vivo Correlation. In Pharmaceutical Product Development: In Vitro-In Vivo Correlation; CRC Press: Boca Raton, FL, USA, 2007; pp. 107–123. [Google Scholar] [CrossRef]
- García, A.; Leonardi, D.; Vasconi, M.D.; Hinrichsen, L.I.; Lamas, M.C. Characterization of Albendazole-Randomly Methylated-β-Cyclodextrin Inclusion Complex and in Vivo Evaluation of Its Antihelmitic Activity in a Murine Model of Trichinellosis. PLoS ONE 2014, 9, e113296. [Google Scholar] [CrossRef]
- Randazzo, V.R.; Costamagna, S.R. Methylene Blue Test for the Determination of Viability of Free Larvae of Trichinella Spiralis. Rev. Argent. Microbiol. 2010, 42, 95–97. Available online: http://www.scielo.org.ar/scielo.php?script=sci_abstract&pid=S0325-75412010000200005&lng=en&nrm=iso&tlng=en (accessed on 6 August 2025).
- Sheskin, D.J. Handbook of Parametric and Non-Parametric Statistical Procedures, 5th ed.; Chapman & Hall/CRC: London, UK, 2011. [Google Scholar]
- Li, J.; Wang, Z.; Zhang, H.; Gao, J.; Zheng, A. Progress in the Development of Stabilization Strategies for Nanocrystal Preparations. Drug Deliv. 2021, 28, 19–36. [Google Scholar] [CrossRef]
- Nyamba, I.; Sombié, C.B.; Yabré, M.; Zimé-Diawara, H.; Yaméogo, J.; Ouédraogo, S.; Lechanteur, A.; Semdé, R.; Evrard, B. Pharmaceutical Approaches for Enhancing Solubility and Oral Bioavailability of Poorly Soluble Drugs. Eur. J. Pharm. Biopharm. 2024, 204, 114513. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Omar, T.; Zhou, Q.; Scicolone, J.; Callegari, G.; Dubey, A.; Muzzio, F. High-Dose Modified-Release Formulation of a Poorly Soluble Drug via Twin-Screw Melt Coating and Granulation. Int. J. Pharm. 2025, 670, 125090. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.; Guo, T.; Wang, Y.; Yang, X.; Liao, Y.; Tang, X.; Zhang, X. A Micrometer Sized Porous β-Cyclodextrin Polymer for Improving Bioavailability of Poorly Soluble Drug. Carbohydr. Polym. 2025, 350, 123042. [Google Scholar] [CrossRef] [PubMed]
- Brough, C.; Williams, R.O. Amorphous Solid Dispersions and Nano-Crystal Technologies for Poorly Water-Soluble Drug Delivery. Int. J. Pharm. 2013, 453, 157–166. [Google Scholar] [CrossRef]
- Mochizuki, T.; Kusuhara, H. Progress in the Quantitative Assessment of Transporter-Mediated Drug-Drug Interactions Using Endogenous Substrates in Clinical Studies. Drug Metab. Dispos. 2023, 51, 1105–1113. [Google Scholar] [CrossRef]
- Lin, J. Pharmacokinetic and Pharmacodynamic Variability: A Daunting Challenge in Drug Therapy. Curr. Drug Metab. 2007, 8, 109–136. [Google Scholar] [CrossRef]
- Gao, Y.; Sun, L.; Qiao, C.; Liu, Y.; Wang, Y.; Feng, R.; Zhang, H.; Zhang, Y. Cyclodextrin-Based Delivery Systems for Chemical and Genetic Drugs: Current Status and Future. Carbohydr. Polym. 2025, 352, 123174. [Google Scholar] [CrossRef]
- Delahousse, J.; Wagner, A.D.; Borchmann, S.; Adjei, A.A.; Haanen, J.; Burgers, F.; Letsch, A.; Quaas, A.; Oertelt-Prigione, S.; Oezdemir, B.C.; et al. Sex Differences in the Pharmacokinetics of Anticancer Drugs: A Systematic Review. ESMO Open 2024, 9, 104002. [Google Scholar] [CrossRef] [PubMed]
- Mauvais-Jarvis, F.; Berthold, H.K.; Campesi, I.; Carrero, J.J.; Dakal, S.; Franconi, F.; Gouni-Berthold, I.; Heiman, M.L.; Kautzky-Willer, A.; Klein, S.L.; et al. Sex- and Gender-Based Pharmacological Response to Drugs. Pharmacol. Rev. 2021, 73, 730–762. [Google Scholar] [CrossRef] [PubMed]
- Soldin, O.P.; Chung, S.H.; Mattison, D.R. Sex Differences in Drug Disposition. J. Biomed. Biotechnol. 2011, 2011, 187103. [Google Scholar] [CrossRef] [PubMed]
- Cisneros, E.P.; Morse, B.A.; Savk, A.; Malik, K.; Peppas, N.A.; Lanier, O.L. The Role of Patient-Specific Variables in Protein Corona Formation and Therapeutic Efficacy in Nanomedicine. J. Nanobiotechnol. 2024, 22, 714. [Google Scholar] [CrossRef]
- Sharifi, S.; Caracciolo, G.; Pozzi, D.; Digiacomo, L.; Swann, J.; Daldrup-Link, H.E.; Mahmoudi, M. The Role of Sex as a Biological Variable in the Efficacy and Toxicity of Therapeutic Nanomedicine. Adv. Drug Deliv. Rev. 2021, 174, 337–347. [Google Scholar] [CrossRef]
- Liu, J.; Lu, Y.F.; Corton, J.C.; Klaassen, C.D. Expression of Cytochrome P450 Isozyme Transcripts and Activities in Human Livers. Xenobiotica 2021, 51, 279–286. [Google Scholar] [CrossRef]
- Gerges, S.H.; El-Kadi, A.O.S. Sexual Dimorphism in the Expression of Cytochrome P450 Enzymes in Rat Heart, Liver, Kidney, Lung, Brain, and Small Intestine. Drug Metab. Dispos. 2023, 51, 81–94. [Google Scholar] [CrossRef]
- Kato, R.; Kamataki, T. Cytochrome P-450 as a Determinant of Sex Difference of Drug Metabolism in the Rat. Xenobiotica 1982, 12, 787–800. [Google Scholar] [CrossRef]
- Schwartz, J.B. The Influence of Sex on Pharmacokinetics. Clin. Pharmacokinet. 2003, 42, 107–121. [Google Scholar] [CrossRef]
- Rose, T.M.; Baranek, M.; Kaka, M.; Shwani, S. Natural Drugs: Trends, Properties, and Decline in FDA Approvals. J. Pharm. Sci. 2024, 114, 782–786. [Google Scholar] [CrossRef]
- Woolsey, S.J.; Mansell, S.E.; Kim, R.B.; Tirona, R.G.; Beaton, M.D. CYP3A Activity and Expression in Nonalcoholic Fatty Liver Disease. Drug Metab. Dispos. 2015, 43, 1484–1490. [Google Scholar] [CrossRef]
- Fredericks, J.; Hill, D.E.; Zarlenga, D.S.; Fournet, V.M.; Hawkins-Cooper, D.S.; Urban, J.F.; Kramer, M. Inactivation of Encysted Muscle Larvae of Trichinella Spiralis in Pigs Using Mebendazole. Vet. Parasitol. 2024, 327, 110140. [Google Scholar] [CrossRef]
- Hess, J.A.; Chandrasekar, P.H.; Mortiere, M.; Molinari, J.A. Comparative Efficacy of Ketoconazole and Mebendazole in Experimental Trichinosis. Antimicrob. Agents Chemother. 1986, 30, 953–954. [Google Scholar] [CrossRef] [PubMed]
- Mccracken, R.O.; Taylor, D.D. Mebendazole Therapy of Parenteral Trichinellosis. Science 1980, 207, 1220–1222. [Google Scholar] [CrossRef]
- Wesołowska, A. Sex-the Most Underappreciated Variable in Research: Insights from Helminth-Infected Hosts. Vet. Res. 2022, 53, 94. [Google Scholar] [CrossRef] [PubMed]
- Abou Rayia, D.M.; Saad, A.E.; Ashour, D.S.; Oreiby, R.M. Implication of Artemisinin Nematocidal Activity on Experimental Trichinellosis: In Vitro and in Vivo Studies. Parasitol. Int. 2017, 66, 56–63. [Google Scholar] [CrossRef]
- de la Torre-Iglesias, P.M.; García-Rodriguez, J.J.; Torrado, G.; Torrado, S.; Torrado-Santiago, S.; Bolás-Fernández, F. Enhanced Bioavailability and Anthelmintic Efficacy of Mebendazole in Redispersible Microparticles with Low-Substituted Hydroxypropylcellulose. Drug Des. Devel. Ther. 2014, 8, 1467–1479. [Google Scholar] [CrossRef] [PubMed]
- Maki, J.; Yanagisawa, T. Comparative Efficacy of Flubendazole and Mebendazole on Encysted Larvae of Trichinella Spiralis (USA Strain) in the Diaphragm of Mice and Rats. J. Helminthol. 1988, 62, 35–39. [Google Scholar] [CrossRef]
- Fornelio, A.C.; Caabeiro, F.R.; Gonzalez, A.J. The Mode of Action of Some Benzimidazole Drugs on Trichinella Spiralis. Parasitology 1987, 95 Pt 1, 61–70. [Google Scholar] [CrossRef]
- Beiting, D.P.; Bliss, S.K.; Schlafer, D.H.; Roberts, V.L.; Appleton, J.A. Interleukin-10 Limits Local and Body Cavity Inflammation during Infection with Muscle-Stage Trichinella Spiralis. Infect. Immun. 2004, 72, 3129–3137. [Google Scholar] [CrossRef]
- Pozio, E.; Sacchini, D.; Sacchi, L.; Tamburrini, A.; Alberici, F. Failure of Mebendazole in the Treatment of Humans with Trichinella Spiralis Infection at the Stage of Encapsulating Larvae. Clin. Infect. Dis. 2001, 32, 638–642. [Google Scholar] [CrossRef]
- Bruschi, F. (Ed.) Trichinella and Trichinellosis; Academic Press, Elsevier: London, UK, 2021. [Google Scholar]
- Martinez-Fernandez, A.R.; Samartin-Duran, M.L.; Toro Rojas, M.; Ubeira, F.M.; Rodriguez Cabeiro, F. Histopathological Modifications Induced Ny Mebendazole and Niridazole on Encysted Larvae of Trichinella Spiralis in CD-1 Mice. Wiad. Parazytol. 1987, 33, 525–535. [Google Scholar]



| Formulation | Particle Size # (µm) | Solubility in HCl 0.1 N (mg/mL) | Solubility Increase (Fold) | Dissolution Efficiency (%) | Dissolution Efficiency Increase (Fold) | IC50 (μM) |
|---|---|---|---|---|---|---|
| MBZ | 10.4 ± 6.9 | 0.025 ± 0.003 | - | 20.6 ± 0.1 | - | >200 |
| MBZ-PVA | 0.106 ± 0.004 (PI 0.083 ± 0.022) Zeta potential 15.63/−23.6 mV | 0.53 ± 0.04 | 21 | 74.2 ± 0.5 | 3.6 | >200 |
| MBZ-CD | 4.2 ± 2.1 | 2.015 ± 0.009 | 81 | 87.4 ± 0.3 | 4.2 | >200 |
| Formulations | Median Survival (hours) | Survival Proportion After 30 h (%) |
|---|---|---|
| MBZ a | Undefined | 79.3 |
| NP b | 30 | 47.6 |
| Comp (inclusion complex) a | Undefined | 68.2 |
| Parameter | Sex | Formulations | ||
|---|---|---|---|---|
| MBZ | NP | Comp | ||
| Cmax (µg/mL) # | ♂ | 1.3 ± 0.31 a | 2.3 ± 0.28 b | 1.7 ± 0.42 a, b |
| ♀ | 1.3 ± 0.26 a | 2.2 ± 0.08 b | 1.7 ± 0.20 a, b | |
| Tmax (h) | ♂ | 0.66 | 0.66 | 0.66 |
| ♀ | 0.66 | 1.33 | 1.33 | |
| AUC0–7 h (µg h/mL) # | ♂ | 2.7 ± 0.82 a | 8.2 ± 0.34 b | 5.1 ± 1.00 a, b |
| ♀ | 3.9 ± 0.63 a | 7.4 ± 1.16 a | 6.4 ± 1.24 a | |
| AUCr0–7 h (%) | ♂ | --- | 204 | 88 |
| ♀ | --- | 90 | 64 | |
| Parameter | Sex | Formulations | ||
|---|---|---|---|---|
| MBZ | NP | Comp | ||
| Cmax (µg/mL) # | ♂ | 0.3 ± 0.11 a | 1.5 ± 0.08 b | 1.1 ± 0.04 c |
| ♀ | 0.2 ± 0.02 a | 1.3 ± 0.09 b | 1.4 ± 0.08 b | |
| Tmax (h) | ♂ | 0.66 | 3 | 5 |
| ♀ | 0.66 | 1.33 | 1.33 | |
| AUC0–7 h (µg.h/mL) # | ♂ | 0.4 ± 0.20 a | 5.4 ± 0.18 b | 4.7 ± 0.50 b |
| ♀ | 0.6 ± 0.08 a | 5.7 ± 0.35 b | 5.3 ± 0.63 b | |
| AUCr0–7 h (%) | ♂ | --- | 1250 | 1175 |
| ♀ | --- | 850 | 783 | |
| CBi/L | CBi+ | ||||
|---|---|---|---|---|---|
| Male | Female | Male | Female | ||
| Variable | Relative larval load § | ||||
| Treatment | |||||
| Control | 95 ± 27.6 a | 101 ± 29.5 a | 554 ± 104.2 a | 952 ± 266.7 a | |
| MBZ | 102 ± 40.2 a | 222 ± 29.2 b | 807 ± 179.5 a | 800 ± 243.7 a | |
| NP | 10 ± 4.9 b | 42 ± 11.8 c | 121 ± 20.8 b | 180 ± 79.7 b | |
| Comp | 15 ± 5.5 b | 32 ± 5.4 c | 118 ± 16.4 b | 122 ± 43.1 b | |
| Variable | Dead larvae percentage # | ||||
| Treatment | |||||
| Control | 0 (0–3) a | 0 (0–4) a | 9 (6–12) a | 7 (3–10) a | |
| MBZ | 27 (7–75) b | 15 (6–25) b | 11 (7–75) a | 5 (5–11) a | |
| NP | 100 (0–100) b | 82 (38–100) c | 82 (66–96) b | 100 (92–100) b | |
| Comp | 67 (0–100) b | 88 (50–100) c | 84 (64–100) b | 100 (98–100) b | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Codina, A.V.; Indelman, P.; Hinrichsen, L.I.; Lamas, M.C. Significant Improvement in Bioavailability and Therapeutic Efficacy of Mebendazole Oral Nano-Systems Assessed in a Murine Model with Extreme Phenotypes of Susceptibility to Trichinella spiralis. Pharmaceutics 2025, 17, 1069. https://doi.org/10.3390/pharmaceutics17081069
Codina AV, Indelman P, Hinrichsen LI, Lamas MC. Significant Improvement in Bioavailability and Therapeutic Efficacy of Mebendazole Oral Nano-Systems Assessed in a Murine Model with Extreme Phenotypes of Susceptibility to Trichinella spiralis. Pharmaceutics. 2025; 17(8):1069. https://doi.org/10.3390/pharmaceutics17081069
Chicago/Turabian StyleCodina, Ana V., Paula Indelman, Lucila I. Hinrichsen, and María C. Lamas. 2025. "Significant Improvement in Bioavailability and Therapeutic Efficacy of Mebendazole Oral Nano-Systems Assessed in a Murine Model with Extreme Phenotypes of Susceptibility to Trichinella spiralis" Pharmaceutics 17, no. 8: 1069. https://doi.org/10.3390/pharmaceutics17081069
APA StyleCodina, A. V., Indelman, P., Hinrichsen, L. I., & Lamas, M. C. (2025). Significant Improvement in Bioavailability and Therapeutic Efficacy of Mebendazole Oral Nano-Systems Assessed in a Murine Model with Extreme Phenotypes of Susceptibility to Trichinella spiralis. Pharmaceutics, 17(8), 1069. https://doi.org/10.3390/pharmaceutics17081069





