Abstract
Periodontitis is one of the most common inflammatory diseases and it is linked to the presence of a dysbiotic subgingival microbiome. The purpose of this review is to evaluate the impact of antimicrobial photodynamic therapy (aPDT) on the subgingival microbiome. Herein, based on an extensive evaluation of randomized controlled trials (RCTs), the effects of aPDT as a supplement to non-surgical periodontal therapy (NSPT) were found to be the main focus of these works. Studies that focused on analyzing microbiological results were selected, yielding contradictory results. The observed microbiological changes were variable, even though some studies showed notable improvements in clinical indicators such as bleeding on probing (BOP), clinical attachment level (CAL), and probing depth (PD). Several studies found that aPDT did not significantly reduce important periodontal pathogens such Tannerella forsythia, Porphyromonas gingivalis, and Aggregatibacter actinomycetemcomitans. Nevertheless, after multiple aPDT sessions, other studies reported positive changes in the subgingival microbiome, with a rise in beneficial bacteria and a decrease in periodontopathogens. While aPDT seems to be a safe and well-tolerated adjuvant to non-surgical periodontal therapy, there is still conflicting evidence regarding how well it modulates the subgingival microbiota. Additional long-term research with larger sample sizes is required to evaluate the microbiological and clinical advantages of aPDT.
1. Introduction
Periodontal disease affects approximately 20–50% of individuals worldwide and is considered a significant public health concern. Its prevalence is projected to increase in the coming years, largely due to the aging global population [1].
Periodontitis is a chronic condition characterized by the accumulation of biofilms, which can lead to progressive destruction of the tissues surrounding the teeth, including the periodontal ligament and bone [2]. Complex and dynamic interactions between particular bacterial infections and harmful host immune responses are involved in the disease [2,3].
The oral microbiome is a diverse and dynamic community of microorganisms, including bacteria, fungi, viruses, protozoa, and archaea that reside in the oral cavity [1]. The complexity of the community can vary according to the niche within the oral cavity (teeth, gingival sulcus, tongue, lips, and palate). Oral microorganisms are organized in biofilms, surface-associated communities embedded in a self-produced extracellular polymeric substance (EPS) matrix that are highly structured physically and functionally [4,5].
Gram-negative bacteria from the red complex, Porphyromonas gingivalis, Tannerella forsythia, and Treponema denticola, are usually found in deep pockets in patients with periodontitis [2,6,7]. Although these bacteria are well-known periodontopathogens, new pathogens such as Desulfobulbus spp. (Gram-negative anaerobe), Filifactor alocis (Gram-positive anaerobe), and species of the phylum Candidatus Saccharibacteria have also recently been associated with periodontitis [8].
The development of periodontal disease has been linked to shifts in the relative abundance of microbial species, which disrupts the equilibrium of host–microbe interactions and triggers inflammatory changes [9]. Therefore, microbiome-targeted treatments to prevent periodontitis may be developed as a result of a better knowledge of the ecological processes and microbial interactions that facilitate progression to a dysbiotic state [6,10].
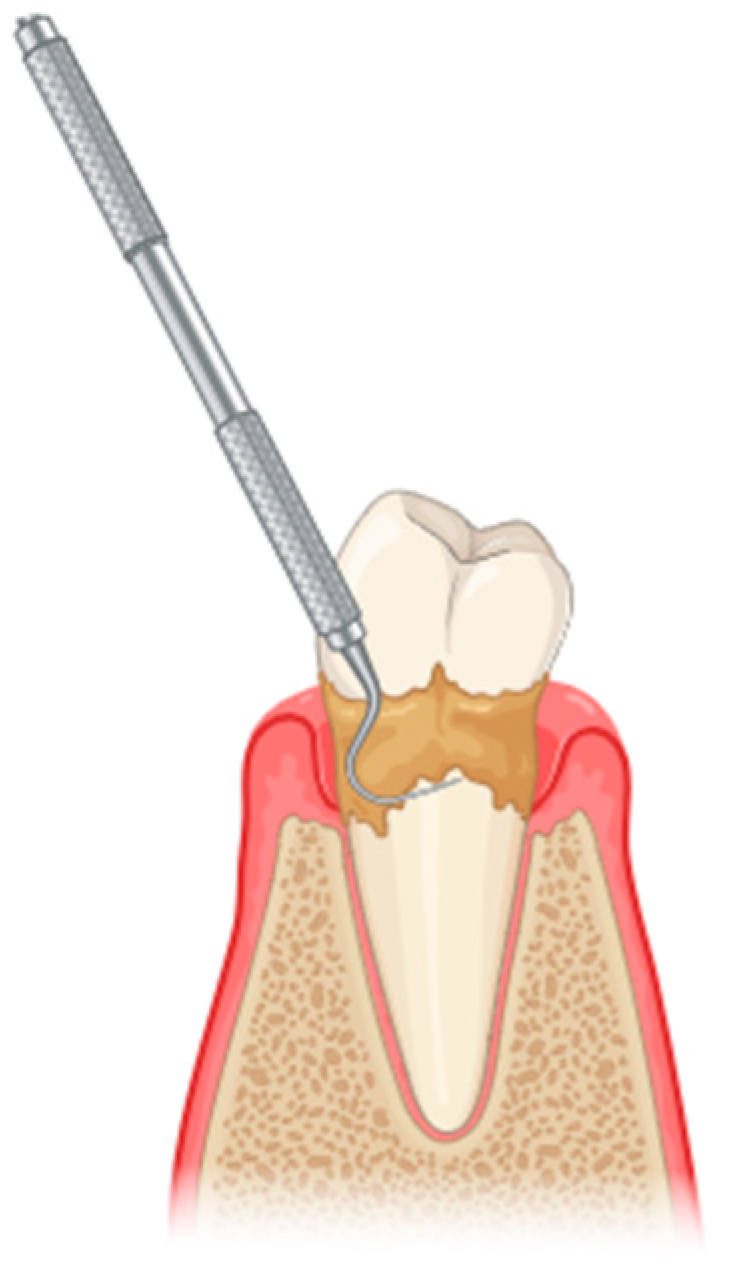
The initial treatment for periodontitis typically involves scaling and root planing (SRP), a procedure classified under non-surgical periodontal treatment (NSPT) (Figure 1). However, NSPT has several limitations, including anatomical challenges such as root concavities, deep pockets, furcation lesions, complex restorations contours, and limited tooth accessibility, among others [11]. Conventional mechanical periodontal therapy may be supplemented by several adjunctive methods [12]. The advent of laser technology in the healthcare domain, particularly in the context of the oral cavity, has allowed researchers and clinicians to conceptualize novel approaches to the treatment and management of oral diseases [13].
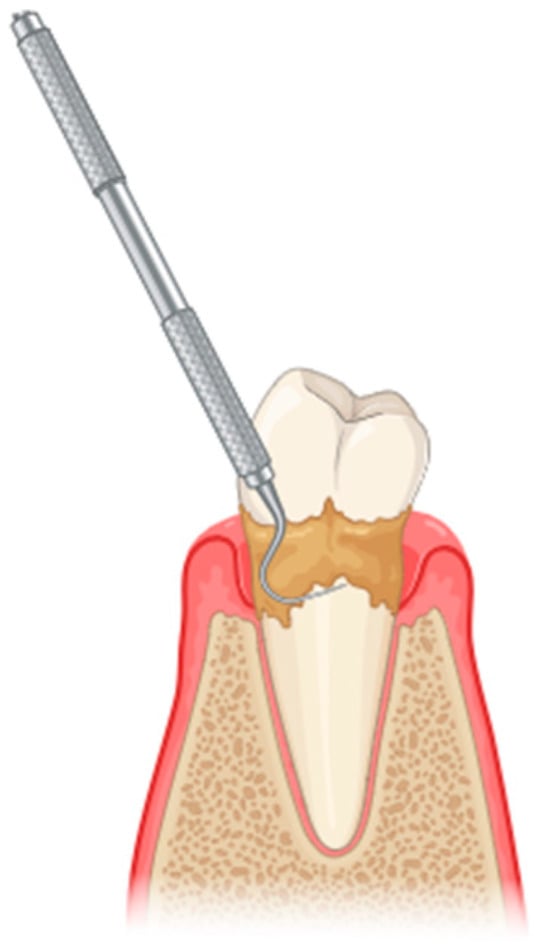
Figure 1.
Scaling and root planing to remove plaque and calculus from the tooth surfaces (created with BioRender.
Photodynamic therapy has been proposed as an adjuvant in non-surgical periodontal treatment, based on findings from multiple studies [14]. The antibacterial effects of diode laser irradiation are primarily attributed to the thermal mechanisms and absorption of incident energy by the pigmented cell membranes of many periodontopathogenic bacteria [15].
Oscar Raab, a medical student in Munich (Germany), accidently discovered the principle of photodynamic treatment (PDT) in 1900 while working on his PhD thesis examining the toxicity of the dye acridine red on Paramecium spp. [16]. Raab’s supervisors, Von Tappeiner and Lesionek, used this procedure on tumor cells and published their results in 1905. For over 25 years, photodynamic cancer therapy has been used in dermatological clinics and hospitals around the world to treat disorders such as basal cell carcinoma and actinic keratosis [17]. Several medical specialties, including dermatology, oncology, gynecology, and urology, use PDT more frequently as a result of its beneficial therapeutic effects [18]. In addition to the treatment of chronic inflammation, photodynamic therapy offers an attractive alternative for the treatment of drug-resistant bacterial infections [19].
Numerous studies have investigated the treatment of periodontitis and its effects on the microbial composition within periodontal pockets. To analyze the microbiome, metagenomic approaches such as 16S ribosomal RNA (rRNA) gene sequencing and shotgun metagenomic sequencing have been widely adopted as practical and efficient techniques. These methods enable the identification of both cultivated and previously uncultivated bacterial species [3]. High-throughput DNA sequencing technologies have further facilitated the comprehensive profiling of oral microbial communities, providing detailed insights into the structure and diversity of microbial communities associated with periodontal health and disease [6].
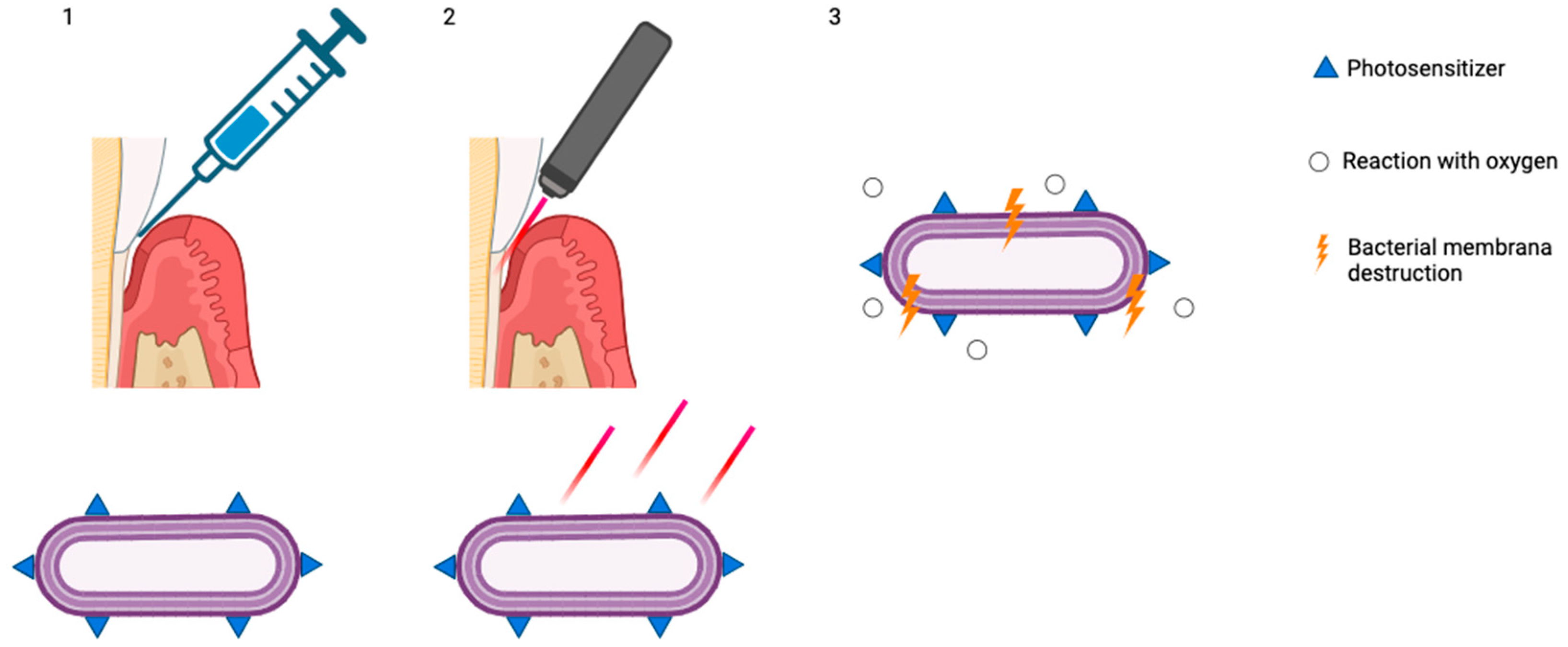
The use of aPDT as an adjunct to non-surgical periodontal treatment (Figure 2) remains a subject of ongoing debate in the scientific community.
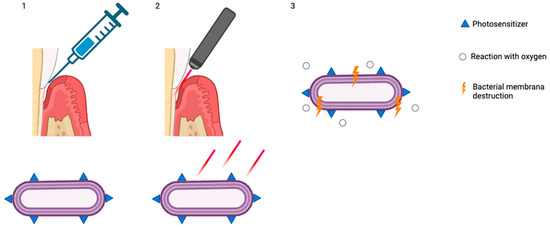
Figure 2.
(1) Accumulation of photosensitizers on the bacterial membrane; (2) exposure and stimulation with red light; (3) reaction with oxygen and bacterial membrane destruction (created with BioRender.
The studies in this review were gathered from a variety of databases, with the majority coming from PubMed and Scopus. A literature search was conducted using several keywords, including periodontitis, antimicrobial photodynamic therapy, and the oral microbiome. Randomized clinical trials published since 2009 were included.
2. In Vitro Efficacy of aPDT
In vitro tests have been carried out to find ways of enhancing the aPDT technique by adding other chemical compounds to the photosensitizer (Table 1). For instance, its combination with potassium iodide has shown efficacy in reducing microbial viability [20]. An in vitro study conducted by Bueno-Silva and collaborators evaluated the antimicrobial efficacy of aPDT plus methylene blue (MB) in reducing the metabolic activity and altering the microbial composition of multi-species oral biofilms. This study involved four groups: a control group (biofilm without treatment) and three test groups (LED: red light application without photosensitizer; MB: methylene blue application without light; and MB + LED: methylene blue application followed by red light irradiation). aPDT using MB and LED reduced the metabolic activity and total bacterial counts in multispecies subgingival biofilms. These results suggest that this approach may be an effective way of controlling polymicrobial oral biofilms [21].

Table 1.
Efficacy of aPDT—in vitro oral biofilm studies.
The ability of aPDT to eliminate specific bacteria in vitro, particularly Porphyromonas gingivalis, has been demonstrated, with the therapy achieving approximately 96% bacterial reduction in the study by Fekrazad et al. [30]. Oruba and his colleagues also conducted an in vitro study to evaluate the effectiveness of aPDT against P. gingivalis in gingival fibroblasts and keratinocytes [22]. Their results also revealed a high capacity for bacterial elimination, suggesting its therapeutic potential for treating periodontal infections [22]. Similar results have been found in other in vitro studies involving this microorganism [23,24,25,26].
The in vitro effects of aPDT on other species, such as Aggregatibacter actinomycetemcomitans, have also been studied [27,28,29]. De Sousa et al. observed a reduction in A. actinomycetemcomitans activity of more than 90%, indicating that aPDT is an effective strategy for controlling this important periodontopathogenic bacterium [29].
3. Antimicrobial Effects of aPDT During Active Periodontal Therapy
Several clinical studies have shown limited or no microbiological benefits when aPDT was used as an adjunct to SRP (Table 2 and Table 3). As an example, Pulikkotil et al. observed significant clinical improvements following SRP and aPDT, but found no significant reduction in A. actinomycetemcomitans compared to SRP alone [31]. Similar findings were reported in randomized controlled trials (RCTs) involving both smokers and non-smokers, where microbial biomarkers such as P. gingivalis, Prevotella intermedia, and Prevotella nigrescens remained unaffected by aPDT, despite minor clinical benefits such as reduced bleeding on probing (BoP) [32,33,34].

Table 2.
Effects of adjunctive aPDT in NSPT.

Table 3.
Methodological parameters in studies on aPTD during active periodontal therapy.
In contrast, some studies have demonstrated positive microbiological outcomes with aPDT [35,36,37,38,39]. A trial by Pinheiro et al. reported a 14.66% greater bacterial reduction in periodontal pockets when aPDT was combined with SRP, suggesting an increased decontamination efficacy [35]. Furthermore, an RCT comparing single vs. multiple aPDT sessions found that repeated aPDT applications induced significant shifts in subgingival microbial composition, including a decrease in anaerobic bacteria and an increase in aerobic, health-associated species. These results highlight that the antimicrobial effectiveness of aPDT may vary depending on the specific protocol used [36].
Different bacterial susceptibilities to treatment procedures were also observed. One split-mouth trial found that aPDT was more effective than SRP in reducing A. actinomycetemcomitans, whereas SRP was more effective against red complex pathogens such as P. gingivalis and T. forsythia. This complementarity suggests a potential synergistic effect when both methods are combined, though recolonization, particularly by T. forsythia, was observed in aPDT-treated sites [37]. Another study found that four sessions of adjunctive aPDT led to a substantial decrease in pathogens from the red and orange complexes, reinforcing the notion that multiple applications may be more effective [38].
Therefore, the existing literature on the microbiological effects of aPDT in periodontitis presents heterogeneous and, at times, conflicting findings. This inconsistency likely reflects variation in study design, baseline disease severity, aPDT protocols (e.g., photosensitizers, light sources, treatment frequency), and microbiological analysis techniques. As such, the current evidence base warrants cautious interpretation, and generalized conclusions should be avoided. Moreover, rather than seeking to resolve this heterogeneity across studies, this review highlights it as a critical issue that underscores the need for standardized protocols and harmonized microbiological endpoints in future trials.
4. aPDT in Periodontal Maintenance
In the context of supportive periodontal therapy (SPT), where microbial control is critical to prevent recurrence, the role of aPDT appears more promising (Table 4 and Table 5). Several studies reported significant short-term reductions in specific pathogens [40,41,42]. For example, a study involving patients in maintenance therapy showed decreased levels of Fusobacterium nucleatum and Eubacterium nodatum at three months post-aPDT, although recolonization by other species such as Eikenella corrodens occurred at six months, highlighting the transient nature of microbiological improvements [42]. Similarly, Corrêa et al. observed a significant short-term reduction in A. actinomycetemcomitans within 3 and 7 days post-treatment in sites treated with SRP + aPDT [41]. However, no significant differences were observed in P. gingivalis levels between groups, suggesting species-specific responses to aPDT [41]. Another trial with repeated aPDT applications in SPT patients demonstrated greater reductions in P. gingivalis, A. actinomycetemcomitans, BoP, and the periodontal inflamed surface area (PISA) index compared to SRP alone, suggesting cumulative microbiological and clinical benefits with repeated therapy [43].

Table 4.
Effects of aPDT on periodontal pathogens during SPT.

Table 5.
Methodological parameters in aPDT periodontal maintenance studies.
Grzech-Leśniak et al. demonstrated that multiple aPDT applications led to a significant decrease in several key periodontal pathogens, including T. forsythia and T. denticola, whereas SRP alone reduced only a subset of these species [44]. Interestingly, A. actinomycetemcomitans levels remained unchanged despite repeated aPDT applications, suggesting resilience or rapid recolonization by this pathogen [44]. In another RCT, Kolbe et al. evaluated aPDT as a monotherapy and found it to be as effective as SRP in reducing A. actinomycetemcomitans and P. gingivalis levels, proposing aPDT as a less invasive option for selected residual pockets [40].
On the other hand, not all studies support the adjunctive benefit of aPDT in the maintenance phase. Therefore, long-term effects remain a concern. A one year-long study involving multiple aPDT applications showed no significant differences in microbial profiles between the test and control groups across time points, indicating that aPDT, at least with the laser and photosensitizer protocols used, may not provide sustained microbiological benefits in residual pockets [45]. Similarly, a more recent RCT with 3-month follow-up found reductions in several key pathogens in both the SRP and SRP + aPDT groups, with no intergroup differences, once again casting doubt on the short-term extra microbiological advantages of aPDT [46].
The microbiological changes observed following aPDT extend beyond transient antimicrobial effects; they indicate a broader ecological restructuring of the subgingival biofilm. The rapid reduction in key periodontal pathogens, coupled with the disruption of biofilm architecture, facilitates recolonization by commensal species and promotes a rebalanced microbial ecosystem. However, the sustainability of these changes beyond six months remains uncertain, and aPDT should not be regarded as a shortcut to long-term remission. Unlike antibiotics, aPDT offers these advantages without contributing to antimicrobial resistance. Future research should incorporate advanced microbial profiling techniques and longer follow-up periods to determine whether these ecological shifts translate into sustained periodontal health.
5. Studies’ Limitations
In periodontal research specifically, the literature guidelines emphasize the importance of sample size calculation based on expected effect sizes, variances, and clinical endpoints; failing to achieve minimum thresholds compromises the validity of results and hinders reproducibility. Thus, while some studies observed reductions in key pathogens following aPDT [31,34,36,37,38,39,40,41,42,44,45,46], these findings must be interpreted with caution, particularly since insufficient sample sizes reduce precision, amplify random error, and undermine reliability of subgroup comparisons and microbiological trends.
Robust evaluation of the long-term efficacy and durability of aPDT requires large-scale randomized trials with extended follow-up, given that periodontitis is a chronic condition. However, several studies included in this review are limited by short follow-up durations (ranging from 1 to 6 months) [31,32,34,35,37,38,41,42,43,44,46] and small sample sizes (often fewer than 30 participants) [32,33,34,35,36,37,38], which present important methodological limitations. Only two studies [44,45] included more than 30 participants. Short-term follow-up may capture only transient improvements rather than sustained periodontal health, whereas the progression or recurrence of periodontitis typically unfolds over several months or years. Additionally, the inclusion of small cohorts raises concerns about statistical power and the generalizability of the findings. Limited sample sizes hinder the detection of modest yet clinically meaningful changes in microbial composition and reduce the reliability of subgroup analyses.
One of the most critical limitations is the wide variability in aPDT protocols across studies. Differences in photosensitizer types (curcumin, methylene blue, toluidine blue, and ICG), laser wavelengths (465–980 nm), energy dosimetry, number of applications (single vs. repeated sessions), and timing significantly impact the outcomes. Some photosensitizers have antibacterial properties, and this can lead to biased results in terms of the effectiveness of the therapy [47]. This lack of standardization makes cross-study comparisons difficult and may explain inconsistent microbiological results.
An important limitation of some aPDT studies is that certain photosensitizers display inherent antimicrobial activity, even in the absence of light. This ‘dark toxicity’ means that any reduction in microbial populations observed may not be due solely to photodynamic action, but could also be caused by the intrinsic antimicrobial effects of the photosensitiser itself. For instance, phenothiazine dyes such as methylene blue have cationic properties that allow them to disrupt microbial membranes even without light activation [40,48,49]. When studies fail to include adequate dark and light controls, such as untreated, photosensitiser-only, and light-only groups, it becomes difficult to distinguish between true photodynamic efficacy and non-light-dependent antimicrobial effects. Consequently, such experimental designs carry a risk of overestimating the contribution of light-mediated processes. Therefore, future aPDT research should rigorously implement dark/light control groups, and studies lacking these comparisons should be clearly identified to mitigate bias and ensure accurate attribution of antimicrobial outcomes.
Variability in microbial sampling techniques (e.g., paper points vs. curettes), detection methods (e.g., culture, qPCR, checkerboard DNA–DNA hybridization, metagenomics), and target species can contribute to the inconsistent findings reported in the literature. Most studies focus on a limited number of traditional periodontal pathogens (e.g., P. gingivalis, T. forsythia, A. actinomycetemcomitans), often overlooking the broader composition and structure of the oral microbiome. A narrow focus on a limited set of pathogens may underestimate the broader ecological impact of aPDT on the subgingival microbial community. While quantitative real-time PCR (qPCR) offers high sensitivity and specificity for predefined targets, such as Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans, its scope is inherently limited. In contrast, 16S rRNA gene sequencing provides a more comprehensive profile of complex biofilms, enabling the detection of low-abundance taxa and supporting in-depth ecological analyses [50,51]. These capabilities are essential for understanding community-level shifts, microbial diversity, and recolonization dynamics following aPDT. High-throughput sequencing should therefore be considered the reference standard in future aPDT studies, particularly those aiming to assess ecological outcomes. Additionally, the use of standardized reference databases and taxonomic frameworks enhances the comparability of findings across studies.
Some trials do not report double-blinding or the use of placebo treatments (e.g., photosensitizer or light without activation), potentially introducing bias in clinical and microbiological assessments.
6. Clinical Safety of aPDT in Periodontal Therapy
aPDT is considered a safe and well-tolerated addition to non-surgical periodontal treatment. According to the reviewed studies, there were no significant reports of tissue damage, allergic reactions to photosensitizers or systemic complications. Patients generally tolerate the procedure well, even when multiple sessions are required. When applied correctly, aPDT appears to be a safe adjunctive tool with minimal risks and good patient tolerance.
7. Conclusions
The use of aPDT as an adjunct to non-surgical periodontal treatment remains a subject of ongoing debate in the scientific community. While some evidence supports the microbiological benefits of aPDT, particularly when applied repeatedly or in specific contexts such as SPT, the overall data remains heterogeneous. Differences in treatment protocols, photosensitizers, light sources, and patient populations (e.g., smokers versus non-smokers, active treatment versus maintenance) can have a significant impact on outcomes.
Notably, aPDT appears to have species-specific effects and may reduce certain pathogens such as A. actinomycetemcomitans and F. nucleatum in the short term, which was not observed for P. gingivalis. The long-term stability of the microbial environment and the prevention of recolonization remain uncertain.
In order to better define the role of aPDT in affecting periodontal microbiota, future research should focus on identifying suitable target populations, optimizing application techniques, and demonstrating long-term effects.
Randomized controlled trials with follow-up periods of at least 12 months, adequately powered sample sizes, and protocol standardization (photosensitizer, wavelength, dosage) are needed to accurately evaluate the sustained microbial and clinical effects of aPDT. To improve the comparability and reliability of future aPDT research, it is recommended that standardized protocols for microbiome assessment be adopted. This includes the use of consistent sampling methods and the application of high-resolution sensitive molecular techniques such as 16S rRNA gene sequencing to enhance detection and quantification accuracy. Furthermore, transparent reporting of methodological protocols and validation procedures should be strongly encouraged. Establishing these best practices will support more robust and reproducible evaluations of aPDT efficacy on microbial communities.
Author Contributions
F.P.S. and L.J.B. conceptualization and preparation of the initial manuscript draft; F.P.S. and M.A.A. literature search; L.J.B. and R.C.A. provided overall supervision, guidance, and revision of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The authors thank FCT/MCTES for the financial support to CiiEM (10.54499/UIDB/04585/2020) through national funds.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
This article does not report any original data, and the research described is based on a review of existing literature.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| aPDT | Antimicrobial Photodynamic Therapy |
| NSPT | Non-surgical Periodontal Therapy |
| RCT | Randomized Controlled Trial |
| BOP | Bleeding on Probing |
| CAL | Clinical Attachment Level |
| PD | Probing Depth |
| SRP | Scaling and Root Planing |
| RNA | Ribonucleic Acid |
| DNA | Deoxyribonucleic Acid |
| MB | Methylene-Blue |
| LED | Light-emitting Diode |
| SPT | Supportive Periodontal Therapy |
| PISA | Periodontal Inflamed Surface Area |
| PCR | Polymerase Chain Reaction |
References
- Di Stefano, M.; Polizzi, A.; Santonocito, S.; Romano, A.; Lombardi, T.; Isola, G. Impact of Oral Microbiome in Periodontal Health and Periodontitis: A Critical Review on Prevention and Treatment. Int. J. Mol. Sci. 2022, 23, 5142. [Google Scholar] [CrossRef] [PubMed]
- Kwon, T.; Lamster, I.B.; Levin, L. Current Concepts in the Management of Periodontitis. Int. Dent. J. 2021, 71, 462–476. [Google Scholar] [CrossRef]
- Yu, P.; Tu, C.; Wara-aswapati, N.; Wang, C.; Tu, Y.; Hou, H.; Ueno, T.; Chen, I.; Fu, K.; Li, H.; et al. Microbiome of Periodontitis and Peri-implantitis before and after Therapy: Long-read 16S rRNA Gene Amplicon Sequencing. J. Periodontal Res. 2024, 59, 657–668. [Google Scholar] [CrossRef]
- Cugini, C.; Shanmugam, M.; Landge, N.; Ramasubbu, N. The Role of Exopolysaccharides in Oral Biofilms. J. Dent. Res. 2019, 98, 739–745. [Google Scholar] [CrossRef]
- Santacroce, L.; Passarelli, P.C.; Azzolino, D.; Bottalico, L.; Charitos, I.A.; Cazzolla, A.P.; Colella, M.; Topi, S.; Godoy, F.G.; D’Addona, A. Oral Microbiota in Human Health and Disease: A Perspective. Exp. Biol. Med. 2023, 248, 1288–1301. [Google Scholar] [CrossRef]
- Abusleme, L.; Hoare, A.; Hong, B.; Diaz, P.I. Microbial Signatures of Health, Gingivitis, and Periodontitis. Periodontol. 2000 2021, 86, 57–78. [Google Scholar] [CrossRef]
- Socransky, S.S.; Haffajee, A.D.; Cugini, M.A.; Smith, C.; Kent, R.L. Microbial Complexes in Subgingival Plaque. J. Clin. Periodontol. 1998, 25, 134–144. [Google Scholar] [CrossRef]
- Patini, R.; Staderini, E.; Lajolo, C.; Lopetuso, L.; Mohammed, H.; Rimondini, L.; Rocchetti, V.; Franceschi, F.; Cordaro, M.; Gallenzi, P. Relationship between Oral Microbiota and Periodontal Disease: A Systematic Review. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 5775–5788. [Google Scholar] [CrossRef] [PubMed]
- Iniesta, M.; Chamorro, C.; Ambrosio, N.; Marín, M.J.; Sanz, M.; Herrera, D. Subgingival Microbiome in Periodontal Health, Gingivitis and Different Stages of Periodontitis. J. Clin. Periodontol. 2023, 50, 905–920. [Google Scholar] [CrossRef] [PubMed]
- Seredin, P.; Goloshchapov, D.; Litvinova, T. Biomaterials and Agents: Pharmaceutical and Biomedical Applications in Dental Research. Pharmaceutics 2024, 16, 894. [Google Scholar] [CrossRef]
- Cobb, C.M.; Sottosanti, J.S. A Re-evaluation of Scaling and Root Planing. J. Periodontol. 2021, 92, 1370–1378. [Google Scholar] [CrossRef]
- Balan, P.; Belibasakis, G.; Ivanovski, S.; Bostanci, N.; Seneviratne, C.J. Community Dynamics of Subgingival Microbiome in Periodontitis and Targets for Microbiome Modulation Therapy. Crit. Rev. Microbiol. 2023, 49, 726–738. [Google Scholar] [CrossRef]
- Paschoal, M.A.B.; Belém, F.V.; Clementino, L.C.; Martins-Júnior, P.A. Application of Lasers in Dentistry: A Bibliometric Study of the Top 100 Most-Cited Papers. Braz. Oral Res. 2022, 36, e104. [Google Scholar] [CrossRef]
- Smiley, C.J.; Tracy, S.L.; Abt, E.; Michalowicz, B.S.; John, M.T.; Gunsolley, J.; Cobb, C.M.; Rossmann, J.; Harrel, S.K.; Forrest, J.L.; et al. Systematic Review and Meta-Analysis on the Nonsurgical Treatment of Chronic Periodontitis by Means of Scaling and Root Planing with or without Adjuncts. J. Am. Dent. Assoc. 2015, 146, 508–524.e5. [Google Scholar] [CrossRef]
- Katsikanis, F.; Strakas, D.; Vouros, I. The Application of Antimicrobial Photodynamic Therapy (aPDT, 670 Nm) and Diode Laser (940 Nm) as Adjunctive Approach in the Conventional Cause-Related Treatment of Chronic Periodontal Disease: A Randomized Controlled Split-Mouth Clinical Trial. Clin. Oral Investig. 2020, 24, 1821–1827. [Google Scholar] [CrossRef]
- Cieplik, F.; Deng, D.; Crielaard, W.; Buchalla, W.; Hellwig, E.; Al-Ahmad, A.; Maisch, T. Antimicrobial Photodynamic Therapy—What We Know and What We Don’t. Crit. Rev. Microbiol. 2018, 44, 571–589. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, M.; Maisch, T.; Nonell, S.; Plaetzer, K.; Almeida, A.; Tegos, G.P.; Hamblin, M.R. Photoantimicrobials—Are We Afraid of the Light? Lancet Infect. Dis. 2017, 17, e49–e55. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic Therapy—Mechanisms, Photosensitizers and Combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Kharkwal, G.B.; Sharma, S.K.; Huang, Y.-Y.; Dai, T.; Hamblin, M.R. Photodynamic Therapy for Infections: Clinical Applications: Photodynamic Therapy For Infections. Lasers Surg. Med. 2011, 43, 755–767. [Google Scholar] [CrossRef]
- Farias-da-Silva, F.F.; Benine-Warlet, J.; Groppo, F.C.; Steiner-Oliveira, C. Potentiation of Antimicrobial Photodynamic Therapy with Potassium Iodide and Methylene Blue: Targeting Oral Biofilm Viability. Photochem. Photobiol. Sci. 2024, 23, 2255–2263. [Google Scholar] [CrossRef]
- Bueno-Silva, B.; Parma-Garcia, J.; Frigo, L.; Suárez, L.J.; Macedo, T.T.; Uyeda, F.H.; Melo, M.A.R.D.C.; Sacco, R.; Mourão, C.F.; Feres, M.; et al. Antimicrobial Activity of Methylene Blue Associated with Photodynamic Therapy: In Vitro Study in Multi-Species Oral Biofilm. Pathogens 2024, 13, 342. [Google Scholar] [CrossRef] [PubMed]
- Oruba, Z.; Gawron, K.; Bereta, G.P.; Sroka, A.; Potempa, J.; Chomyszyn-Gajewska, M. Antimicrobial Photodynamic Therapy Effectively Reduces Porphyromonas gingivalis Infection in Gingival Fibroblasts and Keratinocytes: An In Vitro Study. Photodiagn. Photodyn. Ther. 2021, 34, 102330. [Google Scholar] [CrossRef]
- Moslemi, N.; Rouzmeh, N.; Shakerinia, F.; Bahador, A.; Soleimanzadeh Azar, P.; Kharazifard, M.J.; Paknejad, M.; Fekrazad, R. Photodynamic Inactivation of Porphyromonas gingivalis Utilizing Radachlorin and Toluidine Blue O as Photosensitizers: An In Vitro Study. J. Lasers Med. Sci. 2018, 9, 107–112. [Google Scholar] [CrossRef]
- Song, Y.; Lin, J.; Zhang, Z.; Xu, B.; Bi, L. Antimicrobial Effect of Photodynamic Therapy Using Sinoporphyrin Sodium and 390–400 Nm Light-Emitting Diode on Porphyromonas gingivalis In Vitro. Lasers Med. Sci. 2021, 36, 153–164. [Google Scholar] [CrossRef]
- Etemadi, A.; Azizi, A.; Pourhajibagher, M.; Chiniforush, N. In Vitro Efficacy of Antimicrobial Photodynamic Therapy With Phycocyanin and Diode Laser for the Reduction of Porphyromonas gingivalis. J. Lasers Med. Sci. 2022, 13, e55. [Google Scholar] [CrossRef] [PubMed]
- Shahmoradi, M.; Narimani, T.; Najafi, F.; Asadi, Y.; Fekrazad, R. Antimicrobial Photodynamic Therapy with Dendrosomal Curcumin and Blue Laser against Porphyromonas gingivalis. Photodiagn. Photodyn. Ther. 2023, 44, 103825. [Google Scholar] [CrossRef] [PubMed]
- Sales, L.S.; Miranda, M.L.; De Oliveira, A.B.; Ferrisse, T.M.; Fontana, C.R.; Milward, M.; Brighenti, F.L. Effect of the Technique of Photodynamic Therapy against the Main Microorganisms Responsible for Periodontitis: A Systematic Review of In-Vitro Studies. Arch. Oral Biol. 2022, 138, 105425. [Google Scholar] [CrossRef]
- Najafi, S.; Khayamzadeh, M.; Paknejad, M.; Poursepanj, G.; Kharazi Fard, M.J.; Bahador, A. An In Vitro Comparison of Antimicrobial Effects of Curcumin-Based Photodynamic Therapy and Chlorhexidine, on Aggregatibacter actinomycetemcomitans. J. Lasers Med. Sci. 2016, 7, 21–25. [Google Scholar] [CrossRef]
- De Sousa, G.R.; Soares, L.O.; Soares, B.M.; De Carvalho Cruz, R.; Uliana Junior, P.; Santiago, T.; Farias, L.M.; Magalhães, P.P.; Silveira, L.B.; Almeida Lopes, L.; et al. In Vitro Evaluation of Physical and Chemical Parameters Involved in aPDT of Aggregatibacter actinomycetemcomitans. Lasers Med. Sci. 2022, 37, 391–401. [Google Scholar] [CrossRef]
- Fekrazad, R.; Karamifar, K.; Bahador, A. Comparison of Antibacterial Effect of Photodynamic Therapy Using Indocyanine Green (Emundo) with 2% Metronidazole and 2% Chlorhexidine Gel on Porphyromonas Gingivalis (an In-Vitro Study). Photodiagnosis Photodyn. Ther. 2016, 15, 28–33. [Google Scholar] [CrossRef]
- Pulikkotil, S.; Toh, C.; Mohandas, K.; Leong, K. Effect of Photodynamic Therapy Adjunct to Scaling and Root Planing in Periodontitis Patients: A Randomized Clinical Trial. Aust. Dent. J. 2016, 61, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Christodoulides, N.; Nikolidakis, D.; Chondros, P.; Becker, J.; Schwarz, F.; Rössler, R.; Sculean, A. Photodynamic Therapy as an Adjunct to Non-Surgical Periodontal Treatment: A Randomized, Controlled Clinical Trial. J. Periodontol. 2008, 79, 1638–1644. [Google Scholar] [CrossRef]
- Queiroz, A.C.; Suaid, F.A.; De Andrade, P.F.; Novaes, A.B.; Taba, M.; Palioto, D.B.; Grisi, M.F.M.; Souza, S.L.S. Antimicrobial Photodynamic Therapy Associated to Nonsurgical Periodontal Treatment in Smokers: Microbiological Results. J. Photochem. Photobiol. B 2014, 141, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Borekci, T.; Meseli, S.E.; Noyan, U.; Kuru, B.E.; Kuru, L. Efficacy of Adjunctive Photodynamic Therapy in the Treatment of Generalized Aggressive Periodontitis: A Randomized Controlled Clinical Trial. Lasers Surg. Med. 2019, 51, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, S.L.; Donegá, J.M.; Seabra, L.M.S.; Adabo, M.D.; Lopes, T.; Do Carmo, T.H.D.; Ribeiro, M.C.; Bertolini, P.F.R. Capacity of Photodynamic Therapy for Microbial Reduction in Periodontal Pockets. Lasers Med. Sci. 2010, 25, 87–91. [Google Scholar] [CrossRef]
- Nie, M.; Huang, P.; Peng, P.; Shen, D.; Zhao, L.; Jiang, D.; Shen, Y.; Wei, L.; Bible, P.W.; Yang, J.; et al. Efficacy of Photodynamic Therapy as an Adjunct to Scaling and Root Planing on Clinical Parameters and Microbial Composition in Subgingival Plaque of Periodontitis Patients: A Split-mouth Randomized Clinical Trial. J. Periodontol. 2024, 95, 535–549. [Google Scholar] [CrossRef]
- Novaes, A.B.; Schwartz-Filho, H.O.; De Oliveira, R.R.; Feres, M.; Sato, S.; Figueiredo, L.C. Antimicrobial Photodynamic Therapy in the Non-Surgical Treatment of Aggressive Periodontitis: Microbiological Profile. Lasers Med. Sci. 2012, 27, 389–395. [Google Scholar] [CrossRef]
- Moreira, A.L.; Novaes, A.B.; Grisi, M.F.; Taba, M.; Souza, S.L.; Palioto, D.B.; De Oliveira, P.G.; Casati, M.Z.; Casarin, R.C.; Messora, M.R. Antimicrobial Photodynamic Therapy as an Adjunct to Non-Surgical Treatment of Aggressive Periodontitis: A Split-Mouth Randomized Controlled Trial. J. Periodontol. 2015, 86, 376–386. [Google Scholar] [CrossRef]
- Tabenski, L.; Moder, D.; Cieplik, F.; Schenke, F.; Hiller, K.-A.; Buchalla, W.; Schmalz, G.; Christgau, M. Antimicrobial Photodynamic Therapy vs. Local Minocycline in Addition to Non-Surgical Therapy of Deep Periodontal Pockets: A Controlled Randomized Clinical Trial. Clin. Oral Investig. 2017, 21, 2253–2264. [Google Scholar] [CrossRef]
- Kolbe, M.F.; Ribeiro, F.V.; Luchesi, V.H.; Casarin, R.C.; Sallum, E.A.; Nociti, F.H.; Ambrosano, G.M.B.; Cirano, F.R.; Pimentel, S.P.; Casati, M.Z. Photodynamic Therapy During Supportive Periodontal Care: Clinical, Microbiologic, Immunoinflammatory, and Patient-Centered Performance in a Split-Mouth Randomized Clinical Trial. J. Periodontol. 2014, 85, e277–e286. [Google Scholar] [CrossRef]
- Corrêa, M.G.; Oliveira, D.H.; Saraceni, C.H.C.; Ribeiro, F.V.; Pimentel, S.P.; Cirano, F.R.; Casarin, R.C.V. Short-term Microbiological Effects of Photodynamic Therapy in Non-Surgical Periodontal Treatment of Residual Pockets: A Split-mouth RCT. Lasers Surg. Med. 2016, 48, 944–950. [Google Scholar] [CrossRef]
- Chondros, P.; Nikolidakis, D.; Christodoulides, N.; Rössler, R.; Gutknecht, N.; Sculean, A. Photodynamic Therapy as Adjunct to Non-Surgical Periodontal Treatment in Patients on Periodontal Maintenance: A Randomized Controlled Clinical Trial. Lasers Med. Sci. 2009, 24, 681–688. [Google Scholar] [CrossRef]
- Costa, F.O.; Esteves Lima, R.P.; Costa, A.M.; Costa, A.A.; Mattos Pereira, G.H.; Cortelli, S.C.; Cortelli, J.R.; Magalhães Cyrino, R.; Aparecida Silva, T.; Miranda Cota, L.O. Adjunctive Effects of Photodynamic Therapy Using Indocyanine Green in Residual Pockets during Periodontal Maintenance Therapy: A Split-mouth Randomized Controlled Trial. J. Periodontol. 2023, 94, 1100–1111. [Google Scholar] [CrossRef]
- Grzech-Leśniak, K.; Gaspirc, B.; Sculean, A. Clinical and Microbiological Effects of Multiple Applications of Antibacterial Photodynamic Therapy in Periodontal Maintenance Patients. A Randomized Controlled Clinical Study. Photodiagn. Photodyn. Ther. 2019, 27, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, V.F.; Andrade, P.V.C.; Rodrigues, M.F.; Hirata, M.H.; Hirata, R.D.C.; Pannuti, C.M.; De Micheli, G.; Conde, M.C. Antimicrobial Photodynamic Effect to Treat Residual Pockets in Periodontal Patients: A Randomized Controlled Clinical Trial. J. Clin. Periodontol. 2015, 42, 440–447. [Google Scholar] [CrossRef]
- Chowdhury, U.R.; Kamath, D.; Rao, P. Indocyanine Green Based Antimicrobial Photodynamic Therapy as an Adjunct to Non-Surgical Periodontal Treatment in Periodontal Maintenance Patients: A Clinico-Microbiological Study. F1000Research 2024, 12, 949. [Google Scholar] [CrossRef]
- Abrahamse, H.; Hamblin, M.R. New Photosensitizers for Photodynamic Therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef] [PubMed]
- Gollmer, A.; Felgenträger, A.; Bäumler, W.; Maisch, T.; Späth, A. A Novel Set of Symmetric Methylene Blue Derivatives Exhibits Effective Bacteria Photokilling—A Structure—Response Study. Photochem. Photobiol. Sci. 2015, 14, 335–351. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, J.; Rahban, D.; Aghamiri, S.; Teymouri, A.; Bahador, A. Photosensitizers in Antibacterial Photodynamic Therapy: An Overview. Laser Ther. 2018, 27, 293–302. [Google Scholar] [CrossRef]
- Griffen, A.L.; Beall, C.J.; Campbell, J.H.; Firestone, N.D.; Kumar, P.S.; Yang, Z.K.; Podar, M.; Leys, E.J. Distinct and Complex Bacterial Profiles in Human Periodontitis and Health Revealed by 16S Pyrosequencing. ISME J. 2012, 6, 1176–1185. [Google Scholar] [CrossRef]
- Kim, Y.-T.; Jeong, J.; Mun, S.; Yun, K.; Han, K.; Jeong, S.-N. Comparison of the Oral Microbial Composition between Healthy Individuals and Periodontitis Patients in Different Oral Sampling Sites Using 16S Metagenome Profiling. J. Periodontal Implant Sci. 2022, 52, 394. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).